Abstract
We report the isolation and characterization of GP73, a novel 73 kDa human Golgi protein. The GP73 cDNA was cloned by differential screening of a cDNA library derived from the liver of a patient with adult giant-cell hepatitis (GCH), a rare form of hepatitis with presumed viral etiology. In vitro transcription–translation studies indicate that GP73 is an integral membrane protein, and immunolocalization experiments using epitope-tagged GP73 demonstrate that the protein is localized to the Golgi apparatus. Northern blot analysis of RNA from multiple human tissues reveals a single GP73 mRNA transcript with a size of approximately 3.0 kb. Immunohistochemical studies using rabbit polyclonal antisera directed against recombinant GP73 demonstrate that the protein is preferentially expressed by epithelial cells in many human tissues. In normal livers, GP73 is consistently present in biliary epithelial cells, whereas hepatocytes show little or no signal. In contrast, livers of patients with GCH display strong GP73 immunoreactivity in multinucleated hepatocytes. GP73 mRNA and protein are expressed in highly differentiated HepG2 hepatoma cells after infection with adenovirus in vitro. We conclude that GP73 represents a novel, epithelial cell-specific integral membrane Golgi protein that can be upregulated in response to viral infection.
Keywords: Adenovirus, Epithelial cells, Golgi apparatus, Hepatitis, Liver, Nucleotide sequence, Transmembrane protein
1. Introduction
The Golgi apparatus plays a central role in the exocytic pathway of eukaryotic cells. Over the past decades, marked progress has been made in the understanding of the complex structure of this organelle, and in the mechanisms by which proteins are transported within its compartments (Berger, 1997). A growing number of Golgi-associated or Golgi-resident proteins has been identified in recent years. Many of these molecules are involved in the processing of proteins synthesized in the rough endoplasmic reticulum (Roth, 1997), or in the transport of protein cargo within the Golgi apparatus (Duden and Schekman, 1997). However, in many cases the function of newly identified Golgi resident proteins remains unknown, and the regulation of their expression is incompletely understood (Farquhar and Hauri, 1997). The identification of novel Golgi proteins and the elucidation of their function is likely to contribute to a better understanding of this organelle in normal and diseased tissues.
One line of investigation into the function of the Golgi proteins has been to study the perturbation of this organelle by certain viral infections (Gonatas, 1997, Petterson, 1991). Examples for virus-mediated interference with normal Golgi function are abundant in the literature, and include herpes simplex (Alconada et al., 1999, Campadelli et al., 1993), cytomegalovirus (Fish et al., 1996) and poliovirus (Sandoval and Carrasco, 1997) infections.
In this study, we report the isolation and characterization of GP73, a novel Golgi membrane protein that is predominantly expressed by epithelial cells. GP73 is overexpressed in adult giant-cell hepatitis (GCH), an uncommon form of hepatitis with a presumed viral etiology (Phillips et al., 1991), and is induced in hepatoma cells after adenovirus infection in vitro. Our findings raise new hypotheses regarding the etiology of GCH, and about the potential role of the Golgi apparatus in viral hepatitis. Furthermore, they introduce GP73 as a novel cellular response protein to viral infection.
2. Materials and methods
2.1. Isolation of GP73 cDNA
A partial GP73 cDNA clone was identified in a search for unique mRNAs in the liver of a patient with acute adult GCH, a distinct form of hepatitis with a presumed paramyxoviral etiology (Phillips et al., 1991). GCH is characterized by the presence of large, hepatocyte-derived syncitial giant cells, and by a fulminant course in many affected patients. Based on the histological and ultrastructural features of the disease, a viral etiology has been postulated (Fimmel et al., 1998, Phillips et al., 1991). A directional cDNA library was prepared from two percutaneous liver biopsies of the index patient (ZAP Express, Stratagene, La Jolla, CA) (Fimmel et al., 1998). A normal adult liver cDNA library was obtained commercially (Stratagene, La Jolla, CA). GCH-specific cDNA clones were identified by differential hybridization. Briefly, a cDNA probe generated from the mass-excised GCH library was enriched for disease-specific clones by subtractive hybridization with complementary T3 RNA derived from the normal liver library. GCH-enriched and normal liver-derived, 32P-labeled cDNA probes were used to screen duplicate plaque lifts representing the GCH phage library. Clones with differential signals were analyzed by DNA sequencing. Using this methodology, we identified several novel clones that represented differentially expressed mRNAs. One clone was of particular interest because of its extensive sequence identity to a previously described partial cDNA that was identified in cultured amniotic cells in response to infection with the Newcastle disease virus (‘Human Newcastle disease virus-inducible mRNA’, GenBank accession no. U25276). The full-length GP73 cDNA was isolated by rapid amplification of 5′ cDNA ends (RACE)-PCR (Ausubel et al., 1987), and sequenced in both directions by automated dideoxy chain termination sequencing. Sequence analyses were performed using Blast algorithms and other software programs available through the Worldwide Web (Altschul et al., 1990). The GenBank accession number for GP73 is AF23056.
2.2. In vitro transcription–translation of GP73
In order to determine the membrane topology of GP73, we performed in vitro translation studies using a combined, single tube transcription–translation system (TNT T3 Coupled Reticulo-cyte Lysate System, Promega, Madison, WI) (Blobel and Dobberstein, 1975). Reactions were performed with the full-length GP73 cDNA in pBK-CMV in the presence of 10 mCi/ml 35S-methionine. A control reaction was performed using the cDNA encoding pre-β-lactamase, a secretory protein. Parallel reactions were performed in the presence of optimized amounts of canine pancreatic microsomal membranes (Promega, Madison, WI). Aliquots of the reactions were treated with Na2CO3 (pH 11.5) for 30 min at 0°C. This treatment removes peripheral or secreted proteins from the microsomes but leaves integral membrane proteins intact (Gilmore and Blobel, 1985). Next, the alkaline-treated samples were centrifuged at 80 000g for 15 min at 4°C to pelletize the microsomes. The pellets were then washed with PBS and resuspended in 1×sodium dodecyl sulfate (SDS) loading buffer in the presence of 2-mercaptoethanol. Alternatively, aliquots of the translation reaction were incubated with Peptide N-glycosidase F (PNGase F) (33 U/ml) for 1 h at 37°C. PNGase F treatment cleaves the innermost GlcNAc and asparagine residues of high mannose, hybrid, and complex oligosaccharides from N-linked glycoproteins (Maley et al., 1989). Following PNGase F treatment, the samples were mixed with an equal volume of 2×SDS loading buffer with 2-mercaptoethanol. Treated and untreated reaction products were separated by SDS–polyacrylamide gel electrophoresis (SDS–PAGE), and visualized by autoradiography.
2.3. Immunolocalization of epitope-tagged GP73 in 293 cells
The intracellular localization of the GP73 protein was studied in 293 cells, a human embryonal kidney cell line (ATCC CRL 1573). We obtained 293 cells that had been stably transfected with the pVgRXR plasmid, which encodes the ecdysone receptor and Zeocin resistance genes [EcR293, (No et al., 1996)]. The cells were maintained in 125 μg/ml Zeocin. An inducible GP73 expression plasmid was prepared by blunt-ended ligation of the full-length GP73 uninterrupted open reading frame (ORF) into an EcoRV site located within the multi-cloning site of pIND(SP1)/V5/His (Invitrogen). The resultant plasmid contained a minimal heat-shock promoter and five copies of the ecdysone/glucocorticoid response element upstream of the GP73 ORF, followed by an in-frame V5 epitope tag and a polyhistidine tag. EcR-293 cells were transfected with the GP73 expression plasmid by calcium phosphate coprecipitation. Individual clones displaying ponasterone A-inducible GP73 expression were identified immunocytochemically by antibody staining for the V5 epitope as described below. Select double-transfected clones were maintained in media containing 500 μg/ml G418 and 250 μg/ml Zeocin (Invitrogen, Carlsbad, CA). After induction with ponasterone A (5 μM) for 16 h, the cells were fixed in 4% paraformaldehyde for 15 min, and permeabilized with 0.2% Triton X-100 for 5 min. Aliquots of fixed cells were sequentially incubated with mouse mAbs directed against V5 or against giantin, a known Golgi integral membrane protein (Linstedt et al., 1997). Antibody binding was detected using fluorescein isothiocyanate (FITC)- or Cy3-conjugated sheep anti-mouse IgG antibodies at the manufacturer's recommended dilutions (Sigma Pharmaceuticals, St. Louis, MO). In control experiments, the order of primary antibody addition did not affect the fluorescent signals. Furthermore, staining with single antibodies gave identical signals to that observed with combinations of both primary and secondary antibodies (not shown). Control reactions were performed in which the primary antibodies were omitted. The cells were viewed under a Zeiss confocal microscope (Zeiss, New York, NY).
2.4. GP73 RNA measurements
A commercial northern blot containing mRNA samples from multiple normal human tissues was used to determine the GP73 mRNA transcript size (Multiple Choice Northern Blot, Origene Technologies, Rockville, MD). An antisense mRNA probe directed against bp 2574–3042 of the GP73 cDNA was prepared by T7 RNA polymerase transcription in the presence of 32P-UTP (MAXIscript T7 RNA Kit, Ambion, Austin, TX), and was hybridized to the northern blot under high stringency conditions. A multiple-tissue RNA dot blot was used to quantify GP73 mRNA abundance in 50 normal human tissues (RNA Master Blot, Clontech Laboratories, Palo Alto, CA). A cDNA probe directed against bp 690–3042 of the GP73 cDNA was generated by random-labeling in the presence of 32P-dATP, using a commercial kit (Prime-it II, Stratagene, La Jolla, CA). This probe was hybridized to the multiple-tissue RNA blot under high stringency conditions. Subsequently, the blot was stripped, and rehybridized to a control probe directed against human ubiquitin cDNA, following the manufacturer's specifications (Clontech). The hybridization signals were quantified by densitometry. The GP73 signals were corrected for their respective ubiquitin signals, and expressed relative to the signal obtained in normal liver tissue. For RNA measurements in cultured cells, total cellular RNA was isolated using a rapid, guanidine-based method (RNA Stat 60, Tel-Test, Friendswood, TX). 20 μg aliquots of RNA were hybridized to the GP73 antisense cRNA probe (see above) by ribonuclease protection assay (RPA), using a commercial kit (RPAIII, Ambion, Austin, TX). The RPA control probe was directed against a 316 bp fragment of the human GAPDH cDNA (Ambion).
2.5. Generation of polyclonal antisera against GP73
A 1083 bp fragment of the GP73 cDNA, corresponding to the extracellular portion of the protein (amino acids 41–400) was amplified by polymerase chain reaction (PCR), using a primer pair that creates an N-terminal BamHI restriction site (5′-CATAGGGATCCCTCCAGACACGGATCATGGAGCTGGAAGGC-3′), and a C-terminal HindIII restriction site (5′-GAGAGAAGCTTTCAGAGTGTATGATTCCGCTTTTCACGCTG-3′). The PCR fragment was cloned into the bacterial expression vector pQE9 (Qiagen, Chatsworth, CA), using the BamHI and HindIII restriction sites within the multicloning site. The vector features an N-terminal 6× Histidine tag for the purification of the recombinant protein from bacterial lysates. The GP73–His fusion protein was expressed in XL1 Blue bacteria, and purified by Ni-NTA affinity chromatography, following the manufacturer's protocols (Ni-NTA agarose, Qiagen, Chatsworth, CA). Rabbit polyclonal antisera were obtained against recombinant GP73 protein, following standard immunological methods (Harlow and Lane, 1999). Animal maintenance, antigen injections, and bleeds were performed by a commercial vendor (Pocono Rabbit Farm & Laboratory, Inc., Canadensie, PA). Western blotting studies were performed on lysates of HepSK-1 cells, a cell line that spontaneously expresses GP73 mRNA (Fimmel, unpublished data), and on 293 cells that had been transfected with the GP73 expression plasmid. Cultured cells were treated with lysis buffer (1% SDS, 10% glycerol, 20 mM DTT, 62.5 mM Tris–Cl pH 6.8), and 15 μg aliquots of cellular protein were separated by SDS–PAGE. After transfer to PVDF membranes, western blot analysis was performed using rabbit GP73 antiserum. Control reactions were performed using pre-immune sera from the same animal. The reaction products were visualized with a horseradish peroxidase-labeled secondary antibody, and visualized by enhanced chemiluminescence (ECL, Amersham Pharmacia Biotech Corporation, Arlington Heights, IL).
2.6. Immunohistochemical studies of GP73 expression in human tissues
Paraformaldehyde-fixed liver tissue samples from normal subjects and patients with GCH were obtained from collaborators. The samples from normal human organs were obtained commercially (Novagen, Madison, WI). All tissues were fixed in 4% paraformaldehyde, and sectioned at 5 mm thickness. The slides were deparaffinized, pretreated with peroxidase block (0.03% hydrogen peroxide), and subjected to indirect immunostaining using the rabbit GP73 antibody (1:1000) and a commercial detection kit (DAKO Envision+, DAKO Corporation, Carpinteria, CA). The detection system is based on an HRP-labeled polymer that is conjugated to the secondary, mouse anti-rabbit antibody. The reaction products were detected using the chromogen 3-amino-9-ethylcarbazole (AEC). Slides were counterstained with Meyer's hematoxylin, and viewed with a Zeiss microscope.
2.7. Effect of adenovirus infection on GP73 expression in HepG2 cells
HepG2 cells were purchased from the ATCC, and grown on 60 mm dishes in a 1:1 mixture of Dulbecco's Modified Eagle Medium and Ham's F12 with 10% FBS. The cells were maintained at 37°C in a humidified atmosphere of 5% CO2–95% air, and used at approximately 75% confluence. The cells were infected with the recombinant adenovirus rec700 at 100 PFU/cell for 24 h (Wold et al., 1986). Control cells were mock-infected. The cells were maintained in serum-free media for the first hour of infection. Viral replication, a feature of late infection, was inhibited by the addition of Ara C (20 μg/ml) (Carlin et al., 1989). Measurements of GP73 mRNA and protein were performed as described in 2.4, 2.5.
3. Results
3.1. Sequence of human GP73
The full-length GP73 cDNA comprises 3042 bp and contains a single ORF of 1200 bp (Fig. 1A ). Two in-frame methionine codons (bp 151 and bp 178) spaced ten codons apart provide potential sites for translational initiation (Fig. 1A, underlined ATG codons) (Kozak, 1987). Both codons are contained within likely consensus sites for eukaryotic translational initiation, and would yield proteins of 400 or 391 amino acids respectively. The 3′ UTR contains an in-frame stop codon at bp 1351 (marked by asterisks) and three potential polyadenylation sites at bp 1448, 2950, and 3003. The 400 aa protein has a predicted molecular mass of 45 kDa. It contains a high percentage of acidic amino acids (Asp 5.5%, Glu 12.2%), and has a pI=4.72. A comparison of the GP73 cDNA sequence with the GenBank expressed sequence tags (EST) data base showed extensive areas of identity at the cDNA level with the tentative human clone 168297 (THC 168297) that has been localized to BAC WI-6758 on chromosome 9. Compared with the full-length cDNA, the THC lacks crucial 5′ coding information. BLAST and FASTA analyses of the GP73 cDNA revealed an additional human EST sequence with significant homology but not identity at the cDNA level (gb AC003976, p=10−23). This putative homolog has so far not been characterized. The predicted GP73 protein is predominantly hydrophilic but has a hydrophobic N-terminus, as determined by Kyte–Doolittle hydrophobicity analysis (Kyte and Doolittle, 1982) (Fig. 1B). The N-terminus is predicted to encode a single membrane-spanning domain (underlined in Fig. 1A), with a potential signal peptidase cleavage site between amino acids 28 and 29 (VLG-FN) (Nielsen et al., 1997). The C-terminus is predicted to be extracellularly located, and contains no additional transmembrane stretches. The N-terminus contains an N-myristoylation consensus sequence [GLGNGRRS, Fig. 1A, tilde (∼) symbol]. This feature is of potential interest since several cellular N-myristoyl proteins play important roles in signal transduction and in protein phosphorylation/dephosphorylation reactions (Rudnick et al., 1993). No nuclear, mitochondrial, or lysosomal targeting sequences are predicted (Schutze et al., 1994). Five potential glycosylation sites are present in the C-terminus of the protein (Fig. 1A, ^ symbol) (Hansen et al., 1995, Hansen et al., 1998). Several coiled coil domains are found immediately following the transmembrane domain (Fig. 1C). Coiled coils are involved in protein–protein interactions (Lupas, 1996, Lupas et al., 1991), suggesting that GP73 may interact with other proteins through its extracellular domain.
Fig. 1.

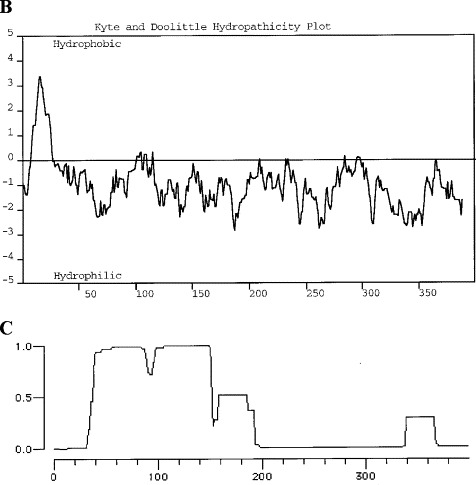
(A) Nucleotide and deduced amino acid sequence of GP73 (GenBank accession number AF236056). Two potential starting methionines are underlined. The presumptive transmembrane domain is underlined. Several O- or N-linked glycosylation consensus sites are marked by a caret (^). The stop codon is marked by asterisks. (B) Hydropathicity analysis of GP73. The hydropathicity analysis was carried out using the algorithm by Kyte and Doolittle. A hydrophobic stretch is present in the N-terminal portion of the protein, corresponding to a predicted membrane-spanning domain. The remainder of the protein is predicted to be hydrophilic. (C) Coiled coil analysis of GP73. The analysis was performed according to the algorithm by Lupas. The graph plots the likelihood for a given amino acid to be part of a coiled coil domain.
3.2. In vitro transcription–translation of GP73
Hydropathicity analysis of the deduced GP73 protein predicts a single, N-terminal hydrophobic segment long enough to span the plasma membrane (Fig. 1B). In order to determine the membrane topology of GP73, in vitro transcription–translation studies were performed in the absence or presence of canine microsomes (Fig. 2 ). The addition of microsomes allows membrane proteins to be translocated, glycosylated, and to have signal peptides cleaved (Blobel et al., 1985). Translation in the absence of microsomes yielded a predominant protein band of approximately 53 kDa (Fig. 2, lane 3). Close inspection reveals a doublet, compatible with two translation products with similar molecular weights. This indicates that both putative translation start sites are being used (see Fig. 1A). The molecular size of GP73 is larger than would be expected from the size of its ORF. A similar electrophoretic behavior has previously been reported for other highly acidic proteins, including presenilin (Daigle and Li, 1993). It has been suggested that the retarded migration of these proteins may be due to their unusually acidic nature, which might interfere with their denaturation by SDS during electrophoresis. Transcription–translation in the presence of microsomes was followed by the appearance of two predominant products with upshifted molecular weights of 58 and 62 kDa, suggesting the occurrence of post-translational modification of the core protein (Fig. 2, lane 4). We hypothesized that this molecular size shift might be due to the glycosylation of the protein in the microsomes. In order to test this hypothesis, we treated an aliquot of the reaction product with peptide:N-glycosidase F (PNGase F, New England Biolabs, Beverly, MA) after solubilization of the microsomes. As shown in lane 6, PNGaseF treatment reduced the molecular size to that observed in the absence of microsomes. These data demonstrate that GP73 is glycosylated, in agreement with the predictions made from the analysis of its cDNA sequence (see Fig. 1A). The identical size of the proteins in lanes 3 and 6 argues against the proteolytic removal of a leader peptide, which would have resulted in a 2–3 kDa reduction of their apparent molecular weights. Integral membrane proteins can be distinguished from secretory and peripheral membrane proteins by treating the microsomes at high pH. This treatment will extract vesicle contents (e.g. secreted proteins) and dissociate peripheral membrane proteins, but will not dissociate integral membrane proteins (Gilmore and Blobel, 1985). As shown in lane 5, alkaline treatment (pH 11.5) of the microsomes did not remove the GP73 protein, although this treatment did completely remove the control secretory protein, β-lactamase (lane 1: β-lactamase cDNA translated in the presence of microsomes, molecular weight 29 kDa; lane 2: alkaline treatment of an aliquot of material from lane 1). We conclude that GP73 is an integral membrane protein with a type II topology.
Fig. 2.
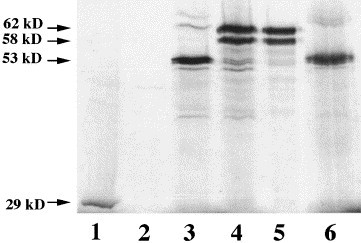
In vitro transcription–translation of GP73. 35S-labeled GP73 or β-lactamase proteins were transcribed and translated in a reticulocyte in vitro system in the absence or presence of canine microsomes. The reaction products were separated by SDS–PAGE, and visualized by autoradiography. Molecular sizes are marked by arrows. Lane 1: control reaction using β-lactamase cDNA. Lane 2: alkaline extract of material contained in lane 1. Lane 3: transcription–translation of GP73 cDNA in the absence of microsomes. Lane 4: transcription–translation of GP73 in the presence of microsomes. Lane 5: alkaline extract (pH 11.5) of material contained in lane 4. Lane 6: PNGase F treatment of solubilized microsomes contained in lane 4.
3.3. GP73 protein expression in transfected 293 cells.
GP73 immunoreactivity in transfected cells was present in tubulo-vesicular structures with a diameter of approximately 2–3 μm and a length of 3–5 μm (Fig. 3A ). A comparison of the GP73 immunoreactivity pattern with the transmission microscopic images of the same cells demonstrated a juxtanuclear distribution (Fig. 3C). Occasionally, the stained structures encircled the nucleus without overlapping with the nuclear membrane. This pattern is characteristic of a Golgi distribution in many cultured cells (Louvard et al., 1982). The signal for GP73 colocalized with that for giantin, a type II Golgi membrane protein that is located in the cis- and medial-Golgi subcompartments (Linstedt and Hauri, 1993) (Fig. 3B). The localization of was not affected by cycloheximide treatment or by varying the ponasterone A concentrations ranging from 0 to 5 μM (data not shown). These results suggest that the Golgi localization of GP73 was not due to a delay in the intracellular transport of the overexpressed protein or to the inhibition of protein trafficking out of the Golgi apparatus. Staining of nonpermeabilized cells revealed no GP73 immunoreactivity, suggesting that the protein was not expressed at the cell surface (data not shown). Taken together, these observations show that GP73 is a resident Golgi protein.
3.
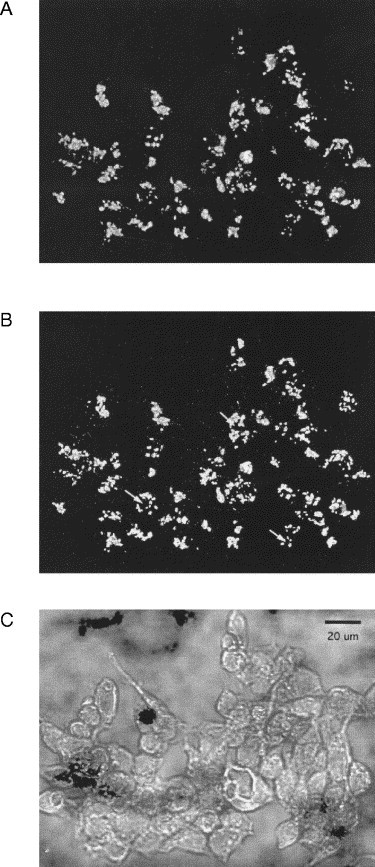
Subcellular localization of GP73 protein. 293 cells were stably transfected with an ecdysone-inducible expression vector containing the full-length GP73 ORF and an in-frame V5 epitope. Cells were induced with 5 μM ponasterone A for 16 h, and analyzed by indirect immunofluorescence (63× objective, water immersion, numerical aperture 1.25) using antibodies directed epitope-tagged GP73 and against the Golgi marker protein giantin. (A) GP73 immunoreactivity, (B) giantin immunoreactivity, (C) transmission microscopy of cells stained in (A) and (B). The nuclei of selected cells are marked by arrows in (B) to demonstrate the perinuclear localization of the GP73 and giantin signals. The 20 μm bar in (C) indicates the magnification.
3.4. GP73 mRNA measurements
Northern blot analysis of GP73 mRNA revealed a single transcript with a molecular size of approximately 3.0 kb in multiple human tissues (Fig. 4 ). High levels of expression were found in colon and stomach. Low levels were present in testis and spleen. Of note, placenta and liver tissues did not reveal any signal above background. In order to analyze the expression pattern of GP73 mRNA in normal human tissues in detail, we probed a panel of mRNA samples from 50 normal human tissues (Fig. 5A ). A wide range of mRNA steady-state levels was observed (Fig. 5B). Particularly high levels were present in the colon, stomach, prostate, and trachea. In contrast, no or minimal expression was present in muscle, lymphoid tissues, and white blood cells. A small but reproducible signal was present in normal liver. A 20-fold difference in expression levels was observed between tissues with the lowest (skeletal muscle) and highest (colon) expression. The wide range of mRNA levels suggests that GP73 expression may be regulated in a tissue- and/or cell-specific manner.
Fig. 4.
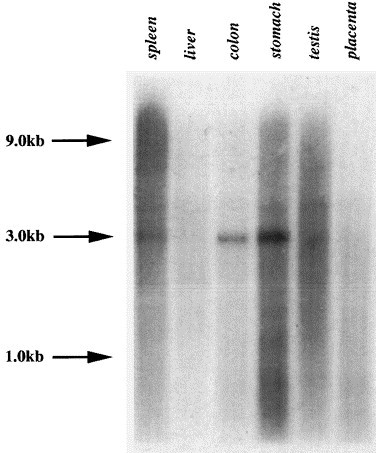
Northern blot analysis of GP73 expression in human tissues. Poly(A+) mRNA (2 μg/lane) from normal human tissues was separated by northern blotting, and probed with a 32P-labelled GP73 cRNA fragment as described in Section 2.4. The positions of the 1.0, 3.0, and 9.0 kb molecular weight markers are indicated by arrows.
Fig. 5.
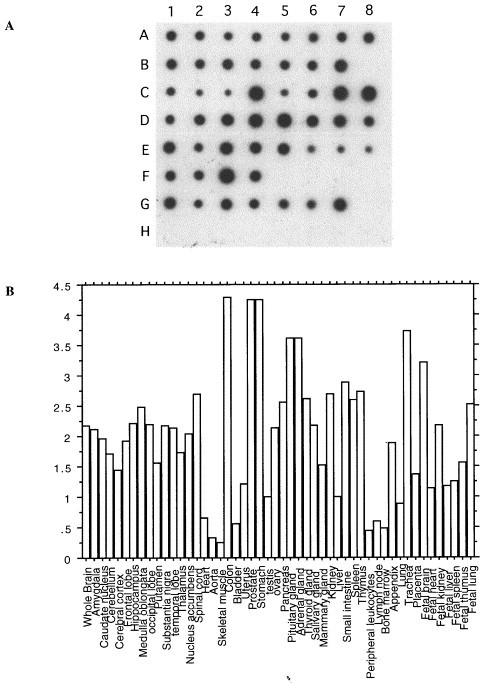
mRNA dot blot analysis of GP73 expression in multiple human tissues. (A) RNA dot blots: mRNA from 50 normal human tissues was spotted onto a nitrocellulose membrane, and probed with a cDNA probe directed against GP73 as described in Section 2. The samples are arranged as follows: A1–8 (whole brain, amygdala, caudate nucleus, cerebellum, cerebral cortex, frontal lobe, hippocampus, medulla oblongata); B1–7 (occipital lobe, putamen, substantia nigra, temporal lobe, thalamus, nucleus acumbens, spinal cord); C1–8 (heart, aorta, skeletal muscle, colon, bladder, uterus, prostate, stomach); D1–8 (testis, ovary, pancreas, pituitary gland, adrenal gland, thyroid gland, salivary gland, mammary gland); E1–8 (kidney, liver, small intestine, spleen, thymus, peripheral leukocyte, lymph node, bone marrow); F1–4 (appendix, lung, trachea, placenta); G1–7 (fetal brain, fetal heart, fetal kidney, fetal liver, fetal spleen, fetal thymus, fetal lung). H1–7 contain control samples that are not expected to yield hybridization signals (100 ng yeast total RNA, 100 ng yeast tRNA, 100 ng Escherichia coli rRNA, 100 ng E. coli DNA, 100 ng Poly r(A), 100 ng human Cot1DNA, 100 ng human DNA, 500 ng human DNA). (B) Quantification of the results obtained in (A). The autoradiogram shown in (A) was evaluated by densitometry, as described in Section 2. GP73 signals (corrected for the corresponding ubiquitin signals) are expressed relative to the signal obtained in normal liver.
3.5. GP73 polyclonal antibodies
The rabbit polyclonal antisera raised against recombinant GP73 specifically recognized a major protein band with an estimated molecular size of approximately 73 kDa (Fig. 6 ). Similar to the in vitro transcription–translation studies, the apparent size of the GP73 protein was substantially larger than would be expected based on its cDNA sequence, most likely due to a combination of glycosylation and abnormal migration of this highly acidic protein. The GP73 signal was markedly upregulated in 293 cells after ponasterone A induction, a finding that confirms the specificity of the GP73 antisera.
Fig. 6.
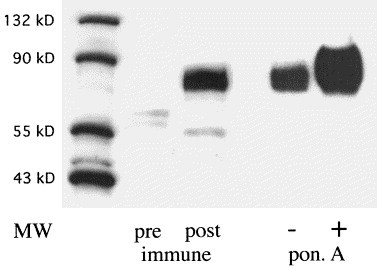
Western blot analysis of GP73 expression in HepSK-1 and GP73-transfected 293 cells. Cellular proteins were extracted from cultured HepSK-1 and GP73-transfected 293 cells, separated by SDS–PAGE (15 μg/lane), transferred to a PVDF membrane, and probed with a polyclonal rabbit antibody (dilution 1:5000) raised against GP73 (aa 41–400). The positions of molecular weight (MW) markers are indicated. Pre-, post-immune: lysates of HepSK-1 cells were probed with prei-mmune sera or post-immune sera from week 8 after injection respectively. PonA: 293 cells were incubated in the absence (−) or presence (+) of 5 μM ponasterone A for 16 h, and cellular lysates were probed with post-immune sera. Signals were detected using an HRP-conjugated secondary antibody (1:1500), and visualized by ECL. The immune sera specifically recognize a broad protein band of approximately 73 kDa (GP73). GP73 expression is strongly induced after ponasterone A induction in 293 cells.
3.6. Immunohistochemical localization of GP73 protein in human tissues
Immunohistochemical staining of human autopsy tissues revealed that GP73 was predominantly expressed by cells of the epithelial lineage (Fig. 7 ). In the colon, GP73 immunoreactivity was confined to the columnar epithelial cells of the intestinal glands. The staining was particularly strong in cells located at the surface of the glands, which contains the most highly differentiated colonocytes (Fig. 7A). In the lung, a distinct GP73 signal was found in the ciliated columnar epithelium of the bronchioles (Fig. 7B). In the kidney, a strong signal was present in simple cuboidal epithelial cells of the proximal and distable tubules and in collecting ducts, whereas little or no immunoreactivity was found in the glomeruli (Fig. 7C). In the prostate, GP73 expression was restricted to the glandular epithelial cells (Fig. 7D). In normal liver, GP73 was consistently expressed in biliary epithelial cell (Fig. 7E).
Fig. 7.
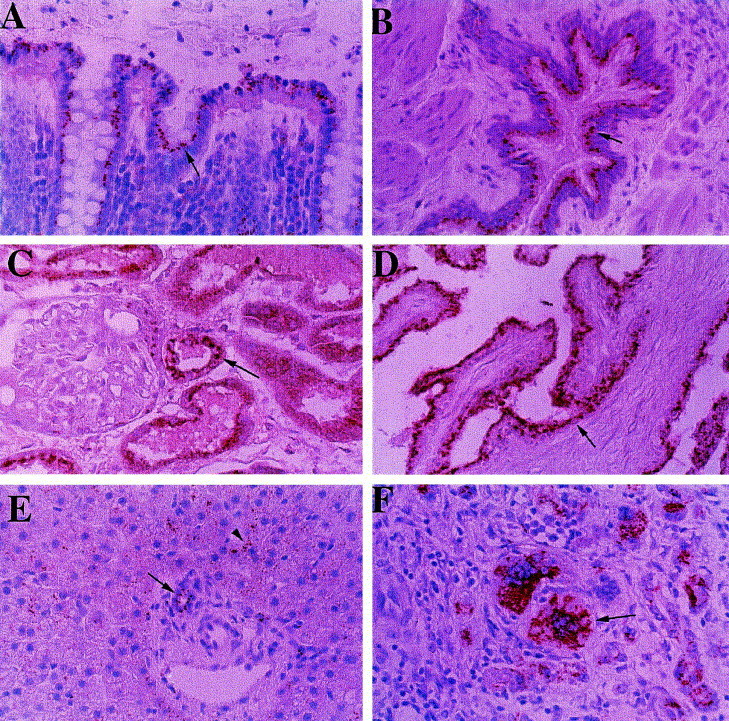
Expression of GP73 protein in normal human tissues. Tissue- and cell-specific GP73 expression was studied in normal human autopsy tissues and in the liver of a patient with GCH by indirect immunohistochemistry, as noted in Section 2. GP73 immunoreactivity is represented by the red signal, with a blue background provided by the hematoxylin counterstain. (A) Colon, (B) lung, (C) kidney, (D) prostate, (E) normal liver, (F) liver with GCH (original magnification ×400). GP73 is predominantly expressed in cells of the epithelial lineage (indicated by arrows), including colonic enterocytes (A), bronchial epithelial cells (B), proximal and distal tubular epithelial cells (C), and prostate epithelial cells (D). In normal liver, GP73 is expressed in biliary epithelial cells (arrow) and in occasional hepatocytes (arrowhead) (E). In GCH, strong staining is present in heptocyte-derived multinucleated syncitia (arrow).
Expression in hepatocytes was either absent or weak, and was limited to scattered cells in the periportal area. In liver tissues from patients with GCH, GP73 was strongly expressed in hepatocyte-derived syncitial giant cells (Fig. 7F). This signal was much stronger than that observed in normal biliary epithelial cells or hepatocytes. Taken together, these studies suggest that GP73 is predominantly expressed by cells of the epithelial lineage, and that GCH is characterized by the upregulation of GP73 expression in diseased hepatocytes.
3.7. Effect of adenovirus infection on GP73 expression in HepG2 cells
The finding of GP73 expression in GCH raised the question whether GP73 expression could be induced in hepatocyte-derived cell lines by virus infection in vitro. Adenovirus infection of cultured HepG2 cells provides a useful in vitro model to study the interaction between viral infection and host cell gene expression. We measured GP73 expression in HepG2 cells that were infected with the recombinant adenovirus rec700 (Fig. 8 ). Under control conditions (mock infection), no GP73 mRNA or protein were detectable in cellular extracts. In contrast, GP73 mRNA became detectable after adenovirus infection. Of note, GP73 protein expression was particularly strong in virus-infected cells in the presence of Ara C. Since Ara C prevents viral DNA replication and the expression of late adenoviral genes, this finding suggests that GP73 expression is a feature of early adenovirus infection. The absence of GP73 protein in adenovirus-infected cells in the absence of Ara C (despite the presence of GP73 mRNA) is most likely due to the suppression of cellular protein synthesis in late infection. Our data suggest that GP73 expression is an authentic feature of the cellular response to adenovirus infection.
Fig. 8.
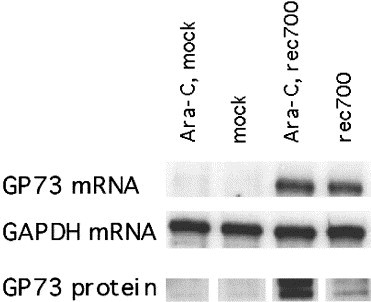
Expression of GP73 mRNA and protein in adenovirus-infected HepG2 cells. HepG2 cells were infected with rec700 adenovirus or mock-infected. Aliquots of cells were infected in the presence of Ara C (20 μg/ml) to inhibit viral DNA replication and the expression of late viral genes. GP73 mRNA was measured by ribonuclease protection assay as described in Section 2.4. GAPDH mRNA levels were measured to document that comparable amounts of mRNA (20 μg) had been loaded in each lane. GP73 protein levels were measured by western blotting on 15 μg aliquots of cellular proteins, as described in Section 2.5.
4. Discussion
GP73 displays the typical features of a type II transmembrane protein: a short cytoplasmic N-terminus followed by a single transmembrane domain and a longer, lumenal C-terminal domain (Munro, 1998). Our immunocytochemical data demonstrate that GP73 localizes to the Golgi apparatus, as evidenced by its colocalization with the Golgi marker protein giantin (Linstedt and Hauri, 1993). In view of the known distribution of giantin in the cis- and medial-Golgi cisternae, we postulate that GP73 is contained in the same subcompartments. Based on our initial sequence information, we considered alternative cellular localizations of GP73. In particular, the presence of a putative signal peptidase cleavage site adjacent to the transmembrane domain raised the possibility that GP73 might be a secreted protein (Nielsen et al., 1997). However, our in vitro translation data clearly demonstrate that GP73 becomes alkali-resistant upon incorporation into microsomal membranes, a feature that is typical for integral membrane proteins and incompatible with secreted or peripheral membrane proteins. The possibility that GP73 might be targeted to the plasma membrane was also considered. However, our immunocytochemical data consistently demonstrated the absence of any plasma membrane staining, suggesting that GP73 is not localized to this membrane. Unlike many of the known Golgi type II membrane proteins, GP73 is not predicted to have any oligosaccharide-modifying properties, and a computer-based motif analysis failed to predict any specific enzymatic properties of the molecule (Paulson and Colley, 1989). In this regard, GP73 resembles another recently described type II Golgi protein, GPP130/GIMPc (Linstedt et al., 1997). The two proteins share several structural features, including a highly acidic amino acid composition, and the presence of a coiled coil domain. Golgi proteins with acidic pK A values have been postulated to play a role in calcium sequestration. Coiled coil domains may provide interactions with other proteins in the Golgi lumen, including homo- or hetero-dimerizations (Farquhar and Hauri, 1997). The identification of such interacting proteins in future experiments may provide clues about the function of GP73.
GP73 is expressed in most normal human tissues, suggesting that it may have housekeeping functions. However, the striking variability of its mRNA and protein levels in normal human tissues suggests the presence of regulatory controls and, possibly, multiple functions. In support of a cell-specific role of GP73, we found that the protein was preferentially expressed in cells of the epithelial lineage, including colonic, bronchial, renal, prostate, and hepatic biliary epithelial cells. GP73 expression was absent in normal hepatocytes, the second major epithelial cell type in the liver. Hepatocytes are derived from a common epithelial progenitor cell with biliary epithelial cells during normal embryological development (Germain et al., 1988). The dramatic upregulation of GP73 protein expression in hepatocyte syncitia may indicate an alteration of hepatocyte differentiation in human GCH.
Our data raise a number of questions regarding the etiology of GCH. Based on indirect morphological and serological evidence, a paramyxoviral infection has been implicated as the etiological agent in many cases of the disease (Fimmel et al., 1998, Phillips et al., 1991), but no specific viral agent has been identified to date. Many of the morphological alterations of human GCH are reminiscent of the changes observed in models of murine coronavirus hepatitis. Morphologically, this infection is characterized by the disruption of the normal intracellular vesicle targeting process through the interaction of viral proteins with resident Golgi proteins (Lavi et al., 1996, Opstelten et al., 1996). Interestingly, mice rendered immunodeficient by homozygous deficiency of the transcription factor STAT1 develop syncitial GCH upon infection with coronavirus (Durbin et al., 1996), suggesting that the distinct phenotype of human GCH may be due to the combination of an impaired immunological response and a viral infection. Based on the similarities between human and murine GCH, we speculate that the Golgi apparatus may be an important target for the putative viral agent in this disease. Our data provide a rationale for a search for viral proteins that might interact with GP73 in this disease.
Our in vitro data obtained in adenovirus-infected HepG2 cells demonstrate that the de novo expression of GP73 is a feature of the cellular response to viral infection. This response may not be limited to one particular virus, since a partial GP73 cDNA was first isolated based on its overexpression in an in vitro model of Newcastle virus infection (gb U25276). The functional implications of increased GP73 expression in viral infection remain to be determined. In principle, GP73 expression might affect viral replication, intracellular trafficking of viral proteins, or the maturation of viral particles. Alternatively, it might lead to changes in host cell function, for example by altering cellular proliferation or differentiation. These possibilities can now be addressed using suitable in vitro models. Our findings in the adenovirus infection model suggest that early adenoviral gene functions may be required for the regulation of GP73 expression. Of note, the early adenoviral E1A protein has previously been reported to induce an ‘epithelial phenotype’ in mesenchymally derived tumor cell lines (Frisch, 1994), a feature that is reminiscent of its expression in hepatocyte syncitia. In future studies, we plan to utilize the availability of mutant adenoviral strains with defects in E1A and other early genes to map viral genes that are necessary for GP73 expression. Furthermore, it will be of interest to determine whether GP73 expression is upregulated in response to infection with known hepatotropic viruses, such as hepatitis B and C viruses. If positive, such studies might support the use of GP73 as a serum or tissue marker of viral disease.
Acknowledgments
We thank A. Linstedt for providing us with the giantin antibody, and J. Freeman for performing laser confocal microscopy. R. Lee, M. Peters, J. Rabkin, J. Awad, T. Wright, and the Minnesota Tissue Repository (director: H. Sharp) provided human liver tissues. R. Cagan and J. Joly helped with advice and encouragement throughout the project.
Received by J.A. Engler
References
- Alconada A., Bauer U., Sodeik B., Hoflack B. Intracellular traffic of herpes simplex virus glycoprotein E: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 1999;73:377–387. doi: 10.1128/jvi.73.1.377-387.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K. Current Protocols in Molecular Biology. Wiley; New York: 1987. [Google Scholar]
- Berger E.G. The Golgi apparatus: from discovery to contemporary studies. In: Berger E.G., Roth J., editors. The Golgi Apparatus. Birkhäuser; 1997. pp. 1–36. [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J. Cell Biol. 1975;67:852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli G., Brandimarti R., DiLazzaro C., Ward P.L., Roizman B., Torrisi M.R. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc. Natl. Acad. Sci. USA. 1993;90:2798–2802. doi: 10.1073/pnas.90.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin C.R., Tollefson A.E., Brady H.A., Hoffman B.L., Wold W.S.M. Epidermal growth factor receptor is down-regulated by a 10,400 MW protein encoded by the E3 region of adenovirus. Cell. 1989;57:135–144. doi: 10.1016/0092-8674(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Daigle I., Li C. apl-1, a Caenorhabditis elegans gene encoding a protein related to the human β-amyloid protein precursor. Proc. Natl. Acad. Sci. USA. 1993;90:12 045–12 049. doi: 10.1073/pnas.90.24.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duden R., Schekman R. Insights into Golgi function through mutants in yeast and animal cells. In: Berger E.G., Roth J., editors. The Golgi Apparatus. Birkhäuser; 1997. pp. 219–246. [Google Scholar]
- Durbin J.E., Hackenmiller R., Simon M.C., Levy D.E. Targeted disruption of the mouse STAT1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- Farquhar M.G., Hauri H.-P. Protein sorting and vesicular traffic in the Golgi apparatus. In: Berger E.G., Roth J., editors. The Golgi Apparatus. Birkhäuser; 1997. pp. 63–130. [Google Scholar]
- Fimmel C.J., Guo L., Compans R., Brunt E.M., Hickman S., Perrillo R.R., Mason A.L. Further characterization of a viral agent associated with syncitial giant cell hepatitis. Am. J. Gastroenterol. 1998;93:1931–1937. doi: 10.1111/j.1572-0241.1998.00548.x. [DOI] [PubMed] [Google Scholar]
- Fish K.N., Britt W., Nelson J.A. A novel mechanism for persistence of human cytomegalovirus in macrophages. J. Virol. 1996;70:1855–1862. doi: 10.1128/jvi.70.3.1855-1862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch S.M. E1a induces the expression of epithelial characteristics. J. Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.127.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R., Blobel G. Translocation of secretory proteins across the microsomal membrane occurs through an environment accessible to aqueous perturbants. Cell. 1985;42:497–505. doi: 10.1016/0092-8674(85)90107-2. [DOI] [PubMed] [Google Scholar]
- Gonatas N.K. The Golgi in disease. In: Berger E.G., Roth J., editors. The Golgi Apparatus. Birkhäuser; 1997. pp. 247–274. [Google Scholar]
- Germain L., Blouin M.-J., Marceau N. Biliary epithelial and hepatocytic cell lineage relationships in embryonic rat liver as determined by the differential expression of cytokeratins, α-fetoprotein, albumin and cell surface-exposed components. Cancer Res. 1988;48:4909–4918. [PubMed] [Google Scholar]
- Hansen J.E., Lund O., Engelbrecht J., Bohr H., Nielsen J.O., Hansen J.-E.S., Brunak S. Prediction of O-glycosylation of mammalian proteins: specificity patterns of UDP-GalNAc:-polypeptide N-acetylgalactosaminyltransferase. Biochem. J. 1995;308:801–813. doi: 10.1042/bj3080801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J.E., Lund O., Tolstrup N., Gooley A.A., Williams K.L., Brunak S. NetOglyc: prediction of mucin type O-glycosylation sites based on sequence context and surface accessibility. Glycoconjugate J. 1998;15:115–130. doi: 10.1023/a:1006960004440. [DOI] [PubMed] [Google Scholar]
- Harlow E., Lane D. Using Antibodies. A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1999. [Google Scholar]
- Kozak M. An analysis of 5-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8132. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lavi E., Wang Q., Weiss S.R., Gonatas N.K. Syncitia formation induced by coronavirus infection is associated with fragmentation and rearrangement of the Golgi apparatus. Virology. 1996;221:325–334. doi: 10.1006/viro.1996.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt A.D., Hauri H.-P. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell. 1993;4:579–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt A.D., Mehta A., Suhan J., Reggio H., Hauri H.-P. Sequence and overexpression of GPP130/GIMPc: evidence for saturable pH-sensitive targeting of a type II early Golgi membrane protein. Mol. Biol. Cell. 1997;8:1073–1087. doi: 10.1091/mbc.8.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvard D., Reggio H., Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J. Cell Biol. 1982;92:92–107. doi: 10.1083/jcb.92.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A. Coiled coils: new structures and new functions. TIBS. 1996;21:375–391. [PubMed] [Google Scholar]
- Lupas A., VanDyke M., Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Maley F., Trimble R.B., Tarentino A.L., Plummer T.H., Jr Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal. Biochem. 1989;180:195–203. doi: 10.1016/0003-2697(89)90115-2. [DOI] [PubMed] [Google Scholar]
- Munro S. Localization of proteins to the Golgi apparatus. Trends Cell Biol. 1998;8:11–15. doi: 10.1016/S0962-8924(97)01197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H., Engelbrecht J., Brunak S., von Heijine G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- No D., Yao T.-Po., Evans R.M. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc. Natl. Acad. Soc. USA. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opstelten D.-J.E., Raamsman M.J.B., Wolfs K., Horzinek M.C., Rotter P.J.M. Envelope glycoprotein interactions in coronavirus assembly. J. Cell Biol. 1996;131:339–349. doi: 10.1083/jcb.131.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J.C., Colley K.J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J. Biol. Chem. 1989;264:17 615–17 618. [PubMed] [Google Scholar]
- Petterson R.F. Protein localization and virus assembly at intracellular membranes. Curr. Top. Microbiol. Immunol. 1991;170:67–106. doi: 10.1007/978-3-642-76389-2_3. [DOI] [PubMed] [Google Scholar]
- Phillips M.J., Blendis L.M., Poucell S., Patterson J., Petric M., Roberts E., Levy G.A., Superina R.A., Greig P.D., Cameron R., Langer B., Purcell R.H. Syncitial giant-cell hepatitis. Sporadic hepatitis with distinctive pathological features, a severe clinical course and paramyxoviral features. N. Engl. J. Med. 1991;324:455–460. doi: 10.1056/NEJM199102143240705. [DOI] [PubMed] [Google Scholar]
- Roth J. Topology of glycosylation in the Golgi apparatus. In: Berger E.G., Roth J., editors. The Golgi Apparatus. Birkhäuser; 1997. pp. 131–162. [Google Scholar]
- Rudnick D.A., McWherter C.A., Gokel G.W., Gordon J.I. MyristoylCoa:Protein N-myristoyltransferase. Adv. Enzymol. 1993;67:375–430. doi: 10.1002/9780470123133.ch5. [DOI] [PubMed] [Google Scholar]
- Sandoval I.V., Carrasco L. Poliovirus infection and expression of the poliovirus protein 2B provoke the disassembly of the Golgi complex, the organelle target for the antipoliovirus drug Ro-090179. J. Virol. 1997;71:4679–4693. doi: 10.1128/jvi.71.6.4679-4693.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze M.-P., Peterson P.A., Jackson M.R. An N-terminal double-arginine motif maintains type II membrane proteins in the endoplasmic reticulum. EMBO J. 1994;13:1696–1705. doi: 10.1002/j.1460-2075.1994.tb06434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W.S.M., Deutscher S.L., Takemori N., Bhat B.M., Magie S.C. Evidence that AGUAUAUGA and CCAAGAUGA initiate translation in the same mRNA in region E3 of adenovirus. Virology. 1986;148:168–180. doi: 10.1016/0042-6822(86)90412-5. [DOI] [PubMed] [Google Scholar]


