Abstract
We carried out a study to determine if the high-neurovirulence GDVII strain of Theiler’s murine encephalomyelitis virus (TMEV) and the demyelinating, low-neurovirulence BeAn strain induced apoptosis in murine astrocytes. Astrocytes, the major glial cell population of the central nervous system, were semipermissive for GDVII virus replication. Programmed cell death, demonstrated by apoptosis-specific caspase-3 protease activity, was maximal 8 h after GDVII infection at an m.o.i. of 1. Purified TMEV capsid proteins VP1, VP2, and VP3 did not induce apoptosis but antibodies to VP1 and VP2 inhibited it. Antibody inhibition of caspase-3 activity as well as flow cytometry experiments implicated TNF-related apoptosis-inducing ligand (TRAIL) and TNF-α-receptor (TNF-R) in apoptosis signaling. Converselly, TNF-α and the TRAIL-receptor were not upregulated. Furthermore, the number of functional TNF-α receptors, but not their affinity, was increased in apoptotic GDVII virus-infected astrocytes, as confirmed in binding experiments with 125I-labeled recombinant murine TNF-α. In vivo studies showed that most of the cells loaded with the virus when injected in the brains of SJL mice were neurons but very few showed TUNEL costaining. Conversely, many of the apoptotic cells found were also positive for GFAP staining.
Introduction
Programmed cell death or apoptosis is a highly conserved and controlled process that eliminates unwanted or damaged cells in multicellular organisms (Vaux and Korsmeyer, 1999). Induction of apoptosis in several cell types by viruses has been reported, including turkey spleen cells by avian adenovirus type II (Rautenschlein et al., 2000), mouse neuroblastoma cells by Langat flavivirus (Prikhod’ko et al., 2001), feline fibroblasts by feline immunodeficiency virus (Mizuno et al., 2001), HeLa cells by reovirus (Connolly et al., 2001), and Vero cells by avian coronavirus (Liu et al., 2001). The “altruistic suicide” of central nervous system (CNS) cells infected by viruses such as the alphaviruses, Semliki forest virus, and Sindbis virus, has also been demonstrated (Allsopp and Fazakerley, 2000).
Theiler’s murine encephalomyelitis virus (TMEV) is a picornavirus that persistently infects the murine CNS (Theiler, 1937). GDVII and BeAn viruses, representing the high- and low-neurovirulence groups, respectively, have been studied so far. Intracerebral inoculation of BeAn virus induces a chronic demyelinating disease in susceptible strains of mice that is reminiscent of human multiple sclerosis, whereas inoculation of GDVII virus causes an acute encephalitis with rapid demise (within 1 week) Dal Canto and Lipton 1976, Lehrich et al 1976, Lipton 1975.
BeAn virus induces apoptosis in cultured microgia but not in astrocytes (Zheng et al., 2001). Here we report that, consistent with the extensive cell death triggered within brain, GDVII virus is an inducer of apoptosis mainly in semipermissive astrocytes, although it also infects neurons upon intracerebral injection of mice. The apoptotic mechanism involves tumor necrosis factor (TNF) receptors and the TNF-related apoptosis-inducing ligand (TRAIL), the same family of “cell suicide” inducers implicated in BeAn induction of apoptosis in other cellular systems Jelachich et al 1995, Jelachich et al 1999, Jelachich and Lipton 2001.
To demonstrate the pathological relevance of our in vitro results, we further established that intracerebral injection of GDVII virus induced apoptosis mainly in cerebral astrocytes around the injection site.
Results
Cytopathic effect and virus production in infected astrocyte cultures
As shown previously (Zheng et al., 2001), astrocyte cultures did not exhibit cytopathic effect (CPE) or loss of the normal polygonal flat morphology when infected with BeAn virus. Mock-infected cells maintained a flattered morphology with adherence to plastic. By contrast, GDVII virus infection induced CPE within 18–24 h in astrocyte monolayers. Although the percentage of infected cells is almost 100% in both primary and secondary cultures, as determined by the infectious center assay, the foci were more evident in secondary trypsinized cultures reaching 70–80% confluence than in primary, contact-inhibited cultures (not shown). Analysis of virus production by titration of infected astrocyte supernatants on BHK-21 cells demonstrated maximal titers of 5–30 × 105 PFU/ml in BeAn-infected astrocytes equivalent to the production of 0.2–1.2 PFU/cell. Titers two orders of magnitude higher (107), or 7–33 PFU/cell, were found in GDVII-infected cells (Fig. 1). Nonspecific binding of virus to the plastic of culture flasks without cells was not detected and the presence of residual virus remaining from the inoculum was ruled out (Fig. 1, circles). Despite the low PFU output from BeAn-infected cells supernatants, our previous analysis by flow cytometry documented BeAn virus replication in the cytoplasm of astrocytes (Rubio and Martin-Clemente, 1999), and another recent study reported that BeAn virus is localized within the astrocytic cells, with little virus released into the supernatants (Zheng et al., 2001).
Fig. 1.
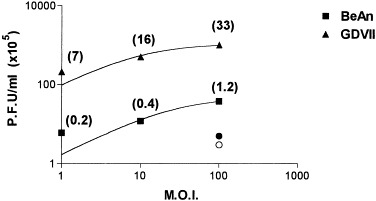
Semilog graph showing virus production in supernatants of BeAn- and GDVII virus-infected astrocytes measured by plaque assay on BHK-21 cell monolayers. Cells (3 × 103) were infected at m.o.i.s of 1, 10, and 100 for 45 min at room temperature. Residual virus remaining from the inoculum were washed three times and cultures were replenished with complete medium. The supernatants were centrifuged and tested 24 h postinfection. Circles (•, ○) indicate titers detected at a m.o.i. of 100 just after the washes were completed and numbers in parentheses indicate PFU/cell.
GDVII virus-induced apoptosis in astrocyte cultures
DNA laddering analysis has shown that BeAn virus does not induce apoptosis in astrocytes (Zheng et al., 2001). The ability of GDVII virus to induce apoptosis was assessed based on changes in caspase-3 activity, an enzyme with substrate specificity for the amino acid sequence Asp-Glu-Va1-Asp (Nicholson et al., 1995), since this enzyme is the main executioner caspase in a cascade of proteolytic cleavage events in dying cells. Astrocyte cultures infected at m.o.i.s of 1, 10, and 100 showed a significant increase in caspase-3 activity in comparison with mock-infected cultures (P < 0.05) (Fig. 2). This induction was specific since caspase-3 activity in cells infected at an m.o.i. of 10 and treated with 50 μM Z-VAD-FMK, the irreversible pan-caspase inhibitor, was not appreciably greater than that in mock-infected cultures. Caspase-3-specific activity was calculated based on a calibration curve using known amounts of chromophore p-nitroaniline (pNA), released from the sustrate upon cleavage by the enzyme. Specific activity was calculated as picomoles of pNA liberated per hour per microgram of cell lysate extracts using the formula provided with the CaspACE kit. Mean specific activities were 96.5 ± 4.9 pmol/μg for GDVII-infected astrocytes and 24.3 ± 1.6 pmol/μg for untreated cells. Maximal apoptosis was detected at an m.o.i. of 1. A well-defined positive control is provided by treating cultures with the strong apoptosis inducer Staurosporine (Fig. 2, +).
Fig. 2.
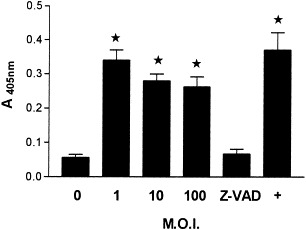
Caspase-3 activity in GDVII-infected astrocyte cultures. Astrocytes were mock-infected (0), or infected at m.o.i.s of 1, 10, or 100 for 24 h. Cells infected at an m.o.i. of 10 were simultaneously treated with the irreversible pan-caspase inhibitor Z-VAD-FMK at a final concentration of 50 μM (Z-VAD). Cell extracts were tested for caspase-3 activity according to assay conditions of the CaspACE kit. Each enzymatic reaction contained 50 μg of total protein of cell extracts. A positive control was obtained by treating cultures with 200 nM Staurosporine (+). Error bars indicated SD of three independent experiments. *, indicated significant differences calculated by Student’s test, P < 0.05.
Analysis of the kinetics of caspase-3 induction in astrocyte cultures infected at an m.o.i. of 10 for periods ranging from 0 to 48 h revealed rapid induction of activity (4 h) that peaked after 8 h and decreased by 48 h (Fig. 3). We found around 30% of dead cells when the cultures were observed under phase-contrast microscopy at optimal conditions for apoptosis induction (i.e., 8 h at an m.o.i. of 1).
Fig. 3.
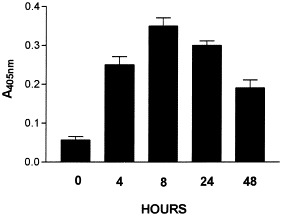
Kinetics of caspase-3 induction in astrocyte cultures infected at an m.o.i. of 10 with GDVII virus for 0 to 48 h.
To exclude the possibility that cytotoxic factors in the BHK-21 cellular extract used as a source of virus induced the observed apoptosis, we tested various dilutions (from 10−2 to 10−5) of rabbit anti-TMEV antiserum, containing antibodies to the VP-1 and VP-2 capsid proteins (Clatch et al., 1987), for their ability to block apoptosis. Incubation with this antiserum completely abrogated the induction of caspase-3 activity (Fig. 4), indicating that apoptosis is dependent upon binding of the GDVII virus to receptors on astrocytes. This binding is presumably sterically inhibited upon binding of antibody to the virus. Preimmune rabbit serum incubated with virus at 10−2 dilution had no inhibitory effect on the apoptosis-inducing capacity of GDVII virus (Fig. 4).
Fig. 4.
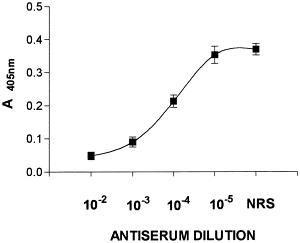
Inhibition of the GDVII virus-induced apoptotic cell death by anti-TMEV antiserum. Purified virus was preincubated for 30 min at 37°C with indicated dilutions of rabbit antiserum or normal rabbit serum (NRS) diluted 10−2 and added to the astrocytic cultures. Caspase-3 activity was analyzed 24 h postinfection. Results represent mean values ± SD of triplicate samples.
Purified virion proteins do not induce apoptosis
Capsid proteins from CsCl-purified TMEV were isolated by reverse-phase high-pressure liquid chromatography (HPLC) on C-8 columns. VP1, VP2, and VP3 purified native protein were added to astrocyte monolayers in amounts corresponding to three-fold greater than the virus inoculum at an m.o.i. of 10, since the structural proteins are present in equimolar amounts in the TMEV capsid (Rozhon et al., 1985). None of the purified proteins induced significant caspase-3 activity (Fig. 5). The slight induction by VP2 might reflect some residual intact virus contamination in this particular preparation. Because the TMEV attachment site resides in a structure composed of both VP1 and VP3 proteins (Tyler, 1987), these two purified proteins were added together, as well as the three isolated proteins, without any appreciable induction of apoptosis (not shown). The lack of apoptosis inducing capability of the low-virulence BeAn strain in astrocytic cells is once more demonstrated (Fig. 5, BeAn).
Fig. 5.
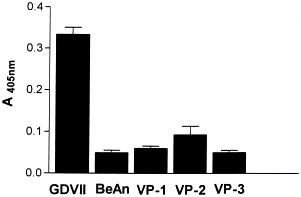
Apoptosis in astrocytes cultures to which TMEV VP1, VP2, or VP3 native proteins, purified by high-pressure liquid chromatography, were absorbed. Monolayer cells were treated three-fold with the amount of pure proteins, equivalent to an m.o.i. of 10, which was also used for infection with intact GDVII and BeAn virions.
Upregulated TRAIL and TNF-R in infected astrocytes
BeAn TMEV induces low-level secretion of TNF-α (Sierra and Rubio, 1993) and upregulates tumor necrosis factor-α-receptor (TNF-R) expression by 210% in astrocyte cultures (Aranguez et al., 1995). To determine whether GDVII virus-induced apoptosis involved signaling through TNF family members, the relative changes in expression of TNF-α and TRAIL and their respective receptors were examined in GDVII virus-infected cultures. Flow cytometry revealed no increase in TNF-α and TRAIL-R in infected versus mock-infected cultures (not shown). Conversely, cytoplasmic TRAIL and surface TNF-R were both upregulated in GDVII-infected cultures (Fig. 6, thick lines). Negative controls were provided by cultures mock-infected or infected with the nonneurovirulent strain BeAn (shaded profiles), or treated with control antibodies (thin lines). The increase in TRAIL and TNF-R was more evident in primary confluent astrocytes that in trypsinized, actively growing cultures (not shown).
Fig. 6.
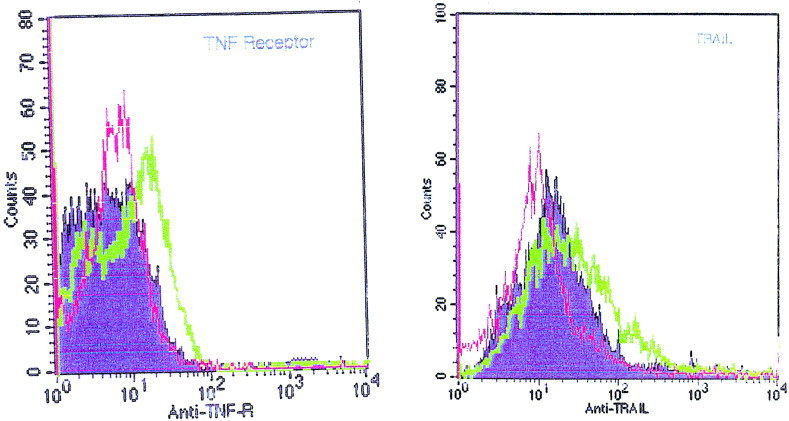
Expression of cytoplasmic TRAIL protein and cell surface TNF-R in astrocytes as determined by flow cytometry. Cells were mock-infected or infected with the nonneurovirulent strain BeAn (shaded profiles), or infected at an m.o.i. of 10 with GDVII virus for 8 h (thick lines). Stainings obtained with normal goat IgG or rabbit serum instead of primary antibodies are shown (thin lines) as negative controls. The font sizes are different in the two panels (80 and 100 counts).
TNF-α binding parameters in astrocyte cultures
Murine astrocytes exhibit a single binding site for TNF-α, and binding is specific and saturable since it was completely displaced by a 200-fold excess of unlabeled TNF (Aranguez et al., 1995). We compared the binding capacity of mock-infected astrocytes with GDVII-infected cultures (m.o.i. of 10) at 4 h when CPE was not detectable but caspase-3 was already induced (Fig. 3). As determined by Scatchard analysis, the Kd of 125I-TNF binding in GDVII virus-infected astrocytes differed only slightly from that of mock-infected cells (2.75 × 10−10 M vs 3.0 × 10−10 M). Nevertheless, 6385 receptors/cell were detected in infected cultures compared to 3258 receptor sites/cell in mock-infected cultures (Fig. 7). This 196% increase in the number of binding sites is essentially similar to a 208% increase previously reported for BeAn virus-infected astrocytes (Aranguez et al., 1995). The increase is consistent with the upregulated expression of TNF-R demonstrated by flow cytometry (Fig. 6).
Fig. 7.
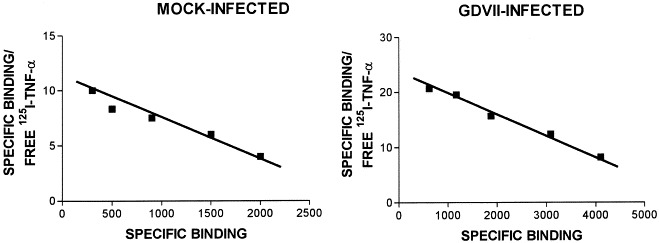
Scatchard analysis of specific 125I-TNF-α binding to mock-infected or GDVII virus-infected astrocytes. The ratio bound/free against bound 125I-TNF-α was plotted. The linear plot indicates a simple bimolecular reaction with a single type of receptor.
Inhibition of apoptosis by antibodies
Analysis of caspase-3 induction in astrocyte cultures infected with GDVII virus at an m.o.i. of 10 in the presence of purified antibodies or antisera against TNF, TNF-R, TRAIL, and TRAIL-R revealed inhibition of enzyme activity only in the presence of anti-TNF-R and anti-TRAIL ligand (Fig. 8). However, the extent of inhibition never exceeded 40 to 60%, even at concentrations of antibody of 10 μg/ml, suggesting that both TRAIL and TNF-R provide different death-inducing signals. Normal rabbit serum or normal purified goat IgG (Fig. 8, −) does not induce any significant inhibition of caspase-3 activity. In one experiment, both antibodies were combined but no synergistic effect was found (not shown).
Fig. 8.
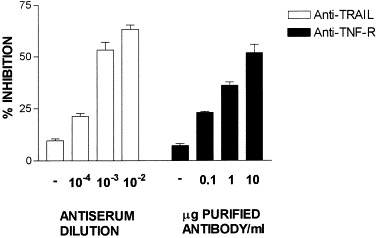
Inhibition of GDVII-induced cell death by anti-TRAIL and anti-TNF-R antibodies. Cells were infected, washed, and cultured for 24 h in the presence of either increasing concentrations of affinity-purified antibody or decreasing dilutions of antiserum. Caspase-3 activity was further determined as described under Materials and Methods. Negative controls were provided by normal rabbit serum (diluted 10−2) and normal purified goat IgG (10 μg/ml) (−). Results are given as mean percentage inhibition ± SD of infected cells based on absorbance at 405 nm in triplicate samples.
Identification of apoptotic astrocytes
Efficient TMEV growth has been detected previously in cultures of astrocytes Rubio and Martin-Clemente 1999, Zheng et al 2001. Immunochemical staining of astrocytes in chamber slides for the astrocyte-specific GFAP marker revealed extensive cytoplasmic staining (Fig. 9A). TUNEL staining delineated relatively few nuclei (Fig. 9B), indicating that only some cells, that must be responsible of the caspase-3 activity, undergo apoptosis. Quantitative analysis of TUNEL-and GFAP-stained cells (Fig. 9C) using the analytical imaging station revealed a mean of 19% costained cells per field.
Fig. 9.
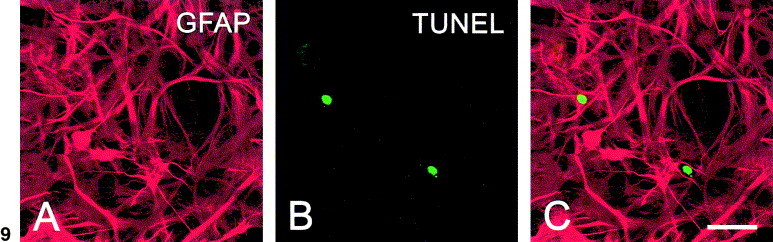
Confocal images of astrocyte cultures infected for 24 h at an m.o.i. of 10 with GDVII. Staining for GFAP (A), TUNEL (B), or merged images (C) were shown. Scale bar, 30 μm.
Induction of apoptosis in the mouse brain
To determine whether astrocytes as well as other brain cells undergo apoptosis after infection, the cortex, septum, nucleus accumbens, and anterior hypothalamus were examined 4–7 days after intracerebral inoculation of GDVII virus (2 × 106 PFU). TMEV has been shown to infect astrocytes after intracerebral inoculation (Rubio and Martin-Clemente, 1999). Cytoplasmic GFAP (Fig. 10A) and nuclear TUNEL staining (Fig. 10B) on serial sections through the nucleus accumbens revealed colocalization of the two stains (Fig. 10C), indicating that GDVII virus induces apoptosis in astrocytes in vivo.
Fig. 10.
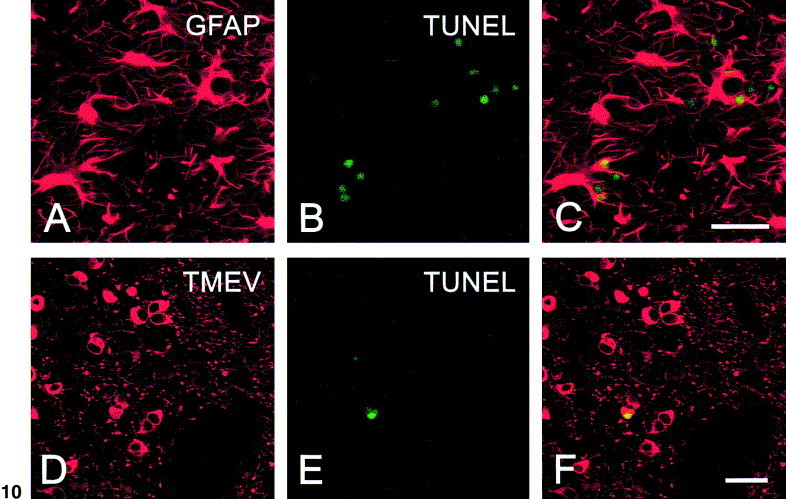
Sections of brain stained for GFAP (A), TUNEL (B and E), or replicating TMEV (D) at the level of the nucleus accumbens region 4 days after intracerebral inoculation of virus. In C and F, merged images of respective rows shows colocalization of staining in some cells. Scale bar in C and F, 30 μm.
Consistent with previous results (Tsunoda et al., 1997), most of the infected cells in the brain were neurons based in morphological criteria as cells with big nuclei and round, nongranulated cell bodies with few processes. Both soma and neuropil were clearly labeled, as shown in the nucleus accumbens (Fig. 10D). This is not unexpected since previous studies have quantitated 15 × 103 receptors per neuron compared with 2.5 × 103 receptors per cell on astrocytes and oligodendrocytes (Rubio et al., 1990). Nevertheless, TUNEL staining was found in few infected neuronal nuclei, where the typical apoptotic chromatin condensation around the margin could be observed (Fig. 10E). Fig. 10F shows a stained nucleus, merged with the neuronal soma.
Control sections that omitted TdT for TUNEL, anti-TMEV, or anti-GFAP antibody were negative (not shown). Some remaining necrosis was found around the injection site in brains of control 6-week-old mice injected with 20 μl of DMEM, but no TUNEL staining was detected. No significant apoptotic events were detectable in healthy normal brains from age-matched SJL animals.
Discussion
Recent neurobiological evidence suggests a role for astrocytes, the major glial population of the CNS, beyond that of structural and trophic support of neurons (Nedergaard, 1994). Here we show that astrocytes undergo apoptosis when infected with the high-neurovirulence GDVII strain of TMEV as demonstrated by the induction of cysteine protease caspase-3 activity. The specific detection of the active form of caspase-3 is considered to accurately detect apoptosis, free of interpretation bias and artifacts (Alnemri et al., 1996).
Our results are in accord with previous studies demonstrating that the GDVII virus is 50-fold more potent than the low-neurovirulence demyelinating BeAn strain in inducing apoptosis in BSC-1 African green monkey kidney cells (Jelachich and Lipton, 1996), and that BeAn induced apoptosis in microglia but not in mouse astrocytes, as measured by DNA laddering (Zheng et al., 2001). We also showed that, consistent with its neuropathogenic behavior in vivo, GDVII replicated and induced a cytophatic effect in astrocyte cultures.
Production of caspase-3 by astrocytes is a relatively early event, with maximal production at 8 h postinfection, slowly decreasing thereafter. The cell-permeable pan-caspase-specific inhibitor Z-VAD-FMK provided the control for the activity measured. The dose-dependent inhibition of caspase-3 by an anti-TMEV antiserum containing antibodies to capsid proteins VP1 and VP2 demonstrated that the effect is not due to cytotoxic factors contained in the crude extract used as a source of virus.
Several viral proteins have been reported to induce or enhance apoptosis, including hepatitis C virus core protein (Zhu et al., 2001), hepatitis B HBx protein (Su et al., 2001), the 17-kDa protein from bursal disease virus (Yao and Vakharia, 2001), the adenovirus E4orf4 protein (Livne et al., 2001), the SV40 small t antigen (Gjoerup et al., 2001), and the matrix protein from vesicular stomatitis virus (Kopecky et al., 2001). Nevertheless, purified TMEV capsid proteins do not induce apoptosis in astrocytes consistent with the failure of TMEV VP2, unlike Coxackievirus VP2, to induce apoptosis in other cells (Henke et al., 2000). The two VP2 proteins differ in their high-variable regions located between amino acids 140 and 200 (Henke et al., 2001).
Apoptosis is usually triggered through pathways comprising “death” ligands and their cognate receptors Pan et al 1997, Pitti et al 1996. Flow cytometry, used to detect altered expression of TNF-TRAIL family members in GDVII-infected astrocytes, indicated a significant upregulation in TNF-R and TRAIL expression. A considerable background production of TRAIL in untreated astrocytes was also detected. Those results were more evident in primary than in secondary trypsinized cultures. Very low levels of TNF-α (up to 200 U/ml) were previously detected by ELISA in the supernatants of astrocytes infected with BeAn virus (Sierra and Rubio, 1993). TRAIL upregulation after infection with cytomegalovirus, reovirus, measles virus, and TMEV has been recently reported Clarke et al 2000, Jelachich and Lipton 2001, Sedger et al 1999, Vidalain et al 2000.
Binding experiments with 125I-labeled recombinant murine TNF-α revealed an increase from 3258 specific and saturable TNF-R sites detected in uninfected astrocytes to 6385 binding sites per GDVII-infected cell, with the Kd remaining almost unchanged. This 196% increase is similar to that previously reported to be induced by BeAn (Aranguez et al., 1995), despite the inability of BeAn to induce apoptosis. Thus, upregulation of TNF-R might not be the crucial factor for apoptosis signaling in this system.
Several studies have described inhibition of apoptosis by antibodies against TNF-α and TRAIL Bermudez et al 1999, Clarke et al 2000, Kaplan et al 2000, Kayagaki et al 1999. In our hands, antibodies to TRAIL and TNF-R at concentrations from 10 to 0.1 μg/ml inhibited GDVII-induced caspase-3 activity by 60 and 50%, respectively (Fig. 8). However, TRAIL has been reported to be unable to bind TNF-R1 (Pitti et al., 1996), so that the possible interactions between the two signals in our system remain unclear. Astrocytes were the cellular source of signals since potential contamination by microglia, oligodendrocytes, neurons, or meningeal fibroblast was carefully ruled out in our cultures. Thus, astrocytes appear to generate signals for their own programmed death as reported for reovirus infection (Clarke et al., 2000).
Almost all astrocytes were infected with GDVII virus in culture and approximately 20% of such cells became apoptotic, as determined by TUNEL staining (Fig. 9). In the infected CNS, TUNEL staining of brain sections revealed apoptotic cells, many of which were also positive for GFAP staining (Fig. 10A–C). Thus, infection with GDVII virus caused programmed cell death in astrocytic cells also in the in vivo situation. By contrast, most of the cells loaded with GDVII virus were neurons, but very few showed TUNEL costaining (Fig. 10D–F).
The inability of BeAn virus to induce apoptosis upon infection might be related to the persistence of the virus and its demyelinating effect on the CNS of susceptible mice. Despite the fact that we have studied the nonpersisting, nondemyelinating strain of TMEV, our results might bear on the pathogenesis of neurodegenerative disorders, especialy since TRAIL has been reported to be upregulated in peripheral immune cells of patients with multiple sclerosis (Huang at al., 2000).
Materials and methods
Mice
SJL mice 4 to 6 weeks old were purchased from the Jackson Laboratory, Bar Harbor, MA, USA, and maintained on standard laboratory feed and water ad libitum in the Instituto Cajal animal care center.
Viruses
Baby hamster kidney cells (BHK-21) were grown at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS) and penicillin–streptomycin (Gibco-BRL, Paisley, Scotland). For these studies, a strain of TMEV isolated in 1957 from a feral mouse in Belem, Brazil, called BeAn 8386, and the high-neurovirulence strain GDVII were used. After plaque purification and several passages in BHK-21 cells, cell cultures were infected for 48 h at 33°C, sonicated, and centrifuged to remove cell debris and viral particles were purified. The virus titer (108 PFU/ml) was determined by a standard plaque assay with 1% Noble agar (Difco Laboratories, Detroit, MI) and staining with 0.2% crystal violet in 20% methanol.
Astrocyte cultures
Astrocyte cultures were prepared by mechanical dissociation of the cerebral cortex from newborn SJL mice (Rubio et al., 1990). The cortex was isolated under a dissecting microscope and cleaned of choroid plexus and meninges. Cell suspensions were filtered through 135-μm pore-size mesh into DMEM containing 10% FCS and penicillin–streptomycin. After centrifugation, cells were filtered through a 40 μm nylon cell strainer (Falcon–Becton–Dickinson, Le Pont De Claix, France) and cultured in 25-cm2 tissue culture flasks (Costar, Cambridge, MA) at 37°C. The medium was changed after 4 days in culture and subsequently two times a week for the entire culture period. Cultures were enriched in astrocytes by the removal of less adherent microglia and oligodendrocytes by shaking overnight at 37°C and 250 rpm in a table-top shaker (Thermo Forma, Marietta, OH). Cellular confluence was observed 10 days after plating, producing around 3 × 106 cells per flask, showing a polygonal flat morphology. A mean of 98% astrocytes was confirmed by indirect immunofluorescence staining of methanol-fixed cultures using rabbit antiglial fibrillar acidic protein (GFAP) antiserum (Dakopatts, Glostrup, Denmark). The lack of noticeable mature oligodendrocytes and microglial/macrophage cells was determined using a guinea pig antimyelin basic protein (MBP) antiserum prepared as described elsewhere (Rubio et al., 1990) and monoclonal anti-Mac-1 antibody (Serotec, Oxford, UK). Secondary fluorescein-labeled antibodies were purchased from Sigma Chemical Co. (St. Louis, MO).
Viral proteins purification
The purification of TMEV virion particles was performed by isopycnic centrifugation in CsCl gradients (Lipton and Friedman, 1980). Individual VP1, VP2, and VP3 virion proteins were purified by reverse-phase HPLC with and Aquapore RP-300 column (Browlee Labotories, Santa Clara, CA) (Rubio and Martin-Clemente, 1999). Elution peaks were dialyzed, retaining all their immunological properties. Purity was checked by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) (Laemmli, 1970).
Neutralization by antibodies
Antiserum was produced in New Zealand White rabbits by subcutaneous and intramuscular injections at multiple sites. Four injections containing 300 μg of CsCl gradient-purified TMEV virus emulsified with complete Freund adjuvant (Difco) were given at 2-week intervals. The rabbits were bled 10 days after the last booster and antiserum was frozen at −20°C until used. The specificity of the antibody response was checked by Western immunoblotting using purified TMEV virion proteins separated by SDS–PAGE and showed that the antiserum contained antibodies against VP1 and VP2 capsid proteins. GDVII virus samples at different concentrations in culture medium were incubated with increasing dilution of the rabbit anti-TMEV antiserum for 30 min at 37°C. Thereafter, the above incubation mixtures were used for infection of astrocyte cultures and further determination of caspase-3 induction.
Affinity-isolated goat antibodies to mouse TNF-α and to soluble TNF-RI were purchased from Sigma. Rabbit polyclonal antiserum to TRAIL and rabbit-purified IgG to TRAIL-RI were acquired from Alexis Corp. (San Diego, CA). Different amounts of antibodies were added to the infected cultures and caspase-3 was determined 24 h later.
Caspase-3 assay
Caspase-3 (CPP32) activity was determined in astrocyte cell lysates using the CaspACE assay system kit from Promega (Madison, WI) according to the manufacturer’s protocol. Briefly, infected cell cultures were washed with ice-cold phosphate-buffered saline (PBS), lysed with cell lysis buffer, centrifuged at 15,000 g for 20 min after 2 cycles of freeze-thaw, and assessed for caspase-3 activity in 96-well polystyrene plates (Nunc-Immuno plates, Nunc, Roskilde, Denmark) based on absorbance at 405 nm. Apoptosis-positive controls were obtained by incubating cultures with 200 nM Staurosporine (Sigma) for 6 h.
Flow cytometry
Mock- and GDVII-infected cultures (m.o.i. of 10) were trypsinized, washed, and allowed to recover for 2 h at 37°C in complete medium. After washing cells with PBS containing 5% FCS and 0.2% sodium azide (Sigma), membranes were permeabilized with 0.3% Saponin (Sigma) for 5 min at room temperature. Cells were incubated with primary purified antibody or antisera (diluted 1:200) for TNF-α, TNF-RI, TRAIL, and TRAIL-RI (listed under Neutralization by antibodies) for 30 min at 4°C. After two washes, cells were incubated with the secondary FITC-conjugated goat anti-rabbit or donkey anti-goat antibodies (Cappel-Organon, Durham, NC) diluted 1:200. After two more washes, the cells were fixed in 1% paraformaldehyde and analyzed in a FACSCalibur (Becton–Dickinson, Palo Alto, CA). Data were evaluated using CELL Quest 3.1f, supplied with the instrument.
Tnf-α radioiodination
Recombinant mouse TNF-α, free of protein stabilizers, was purchased from Innogenetics (Antwerp, Belgium) and labeled with 125I (Amersham) using the chloramine T method (Greenwood et al., 1963). 125I-rTNF-α and free iodine were separated on disposable PD-10 Sephdex G-25M columns (Pharmacia Biotech, Uppsala, Sweden). Specific activity was 203 Ci/mmol and the protein was 96% trichloroacetic acid (TCA) precipitable. The iodinated protein migrated as a single peak with an apparent molecular mass of 17 kDa in SDS–polyacrylamide slab gels.
Binding assay
Confluent astrocyte monolayers in multiwell (six-well) plates (Falcon–Becton–Dickinson) were used in the binding experiments. Cells were incubated with different amounts of 125I-TNF-α with or without unlabeled TNF-α, as stated in the text. The buffer used was PBS containing 0.1% bovine serum albumin (Gibco-BRL). After washing three times, cells were detached from the plastic surface with 2% SDS at 60°C and counted in a LKB-Wallac 1282 Compugamma counter (Sollentuna, Sweden).
Statistical analysis
The dissociation constant (Kd) and maximum binding (B max) were calculated from the Scatchard plot of the binding assay data using the GraphPad Prism version 3.00 program. Data are given as mean values (±SD) of triplicate determinations from three independent experiments.
Intracerebral mice inoculations
Six-week-old SJL mice were anesthetized with Fluorthane and injections were made with a 25-μ1 Hamilton syringe at a site 1 mm right lateral and 2 mm rostral of the bregma. Twenty microliters of a suspension of GDVII virus (2 × 106 PFU) was infused at a rate of 1 μl every 5 s and the needle was maintained for an additional 5 s. Four to seven days after injections, brains were removed and samples were processed by immunochemistry. Acute encephalitis killed remaining, infected animals within a week after inoculation. Control injected mice received 20 μl DMEM.
Immunocytochemistry
Animals in all cases were perfused though the heart 4 days after intracerebral virus injection with 4% paraformaldehyde in PBS. After perfusion, brains were removed, immersed in the same fixative for 3 h, and left overnight in PBS. Vibratome sections of 30–40 μm were processed free-floating for immunohistochemistry. Sections or astrocyte monolayers in culture chambers (Lab-Tek Chamber slide, Nunc, Neperville, IL) were incubated with primary antibody (rabbit anti-TMEV or rabbit anti-GFAP, diluted 1:1000) followed by goat anti-rabbit Cy-3-conjugated antibody (Amersham) diluted 1:1000. After several rinses in PBS, sections were stained for TUNEL and examined in a Leica TCS NT confocal laser scanning microscope equipped with an argon/krypton-mixed gas laser with an excitation peak of 647 nm for Cy-3. Specificity was controlled by omission of the primary antibodies. Quantitative analysis was performed using an Analytical Imaging Station (Imaging Research Inc., Canada).
TUNEL assay
Apoptotic cells were detected using the TUNEL method. Samples were processed for TUNEL using a fluorescein in situ cell death detection kit following the manufacturer’s instructions (Boehringer Mannheim, Mannheim, Germany). Stained cells were visualized by flourescence microscopy in the confocal laser microscope at an excitation peak of 488 nm. Method specificity was controlled by omission of terminal deoxynucleotidyl transferase (TdT) in the first step of the labeling.
References
- Allsopp T.E., Fazakerley J.K. Altruistic cell suicide and the specialized case of the virus-infected nervous system. Trends Neurosci. 2000;23:284–290. doi: 10.1016/s0166-2236(00)01591-5. [DOI] [PubMed] [Google Scholar]
- Alnemri E.S., Livingston D.J., Nicholson D.W., Salvesen G., Thornberry N.A., Wong W.W., Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- Aranguez E., Torres C., Rubio N. The receptor for tumor necrosis factor on murine astrocytes: characterization, intracellular degradation, and regulation by cytokines and Theiler’s murine encephalomyelitis virus. Glia. 1995;13:185–194. doi: 10.1002/glia.440130305. [DOI] [PubMed] [Google Scholar]
- Bermudez L.E., Parker A., Petrofsky M. Apoptosis of mycobacterium avium-infected macrophages is mediated by both tumour necrosis factor (TNF) and Fas, and involves the activation of caspases. Clin. Exp. Immunol. 1999;116:94–99. doi: 10.1046/j.1365-2249.1999.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P., Meintzer S.M., Gibson S., Widmann C., Garrington T.P., Johnson G.L., Tyler K.L. Reovirus-induced apoptosis is mediated by TRAIL. J. Virol. 2000;74:8135–8139. doi: 10.1128/jvi.74.17.8135-8139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatch R.J., Pevear D.C., Rozhon E., Roos R.P., Miller S.D., Lipton H.L. Characterization and specificity of humoral immune response to Theiler’s murine encephalomyelitis virus capsid proteins. J. Gen. Virol. 1987;68:3191–3196. doi: 10.1099/0022-1317-68-12-3191. [DOI] [PubMed] [Google Scholar]
- Connolly J.L., Barton E.S., Dermody T.S. Reovirus binding to cell surface sialic acid potentiates virus-induced apoptosis. J. Virol. 2001;75:4029–4039. doi: 10.1128/JVI.75.9.4029-4039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M.C., Lipton H.L. Primary demyelination in Theiler’s virus infection. Lab. Invest. 1976;33:626–637. [PubMed] [Google Scholar]
- Gjoerup O., Zaveri D., Roberts T.M. Induction of p53-independent apoptosis by simian virus 40 small t antigen. J. Virol. 2001;75:9142–9155. doi: 10.1128/JVI.75.19.9142-9155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood F.C., Hunter W.M., Glover J.W. The preparation of (131I) labelled human growth hormone of high specific radioactivity. J. Biochem. 1963;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke A., Launhardt H., Klement K., Stelzner A., Zell R., Munder T. Apoptosis in coxsackievirus B3-caused diseases: interaction between the capsid protein VP2 and the proapoptotic protein Siva. J. Virol. 2000;74:4284–4290. doi: 10.1128/jvi.74.9.4284-4290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke A., Nestler M., Strunze S., Saluz H.P., Hortchansky P., Menzel M.U., Zell R., Stelzner A., Munder T. The apoptotic capability of coxsackievirus B3 is influenced by the efficient interaction between the capsid protein VP2 and the proapoptotic host protein Siva. Virology. 2001;289:15–22. doi: 10.1006/viro.2001.1082. [DOI] [PubMed] [Google Scholar]
- Huang W.-X., Huang M.P., Gomes M.A., Hillert J. Apoptosis mediators fasL and TRAIL are upregulated in peripheral blood mononuclear cells in MS. Neurology. 2000;55:928–934. doi: 10.1212/wnl.55.7.928. [DOI] [PubMed] [Google Scholar]
- Jelachich M., Lipton H.L. Theiler’s murine encephalomyelitis virus kills restrictive but not permissive cells by apoptosis. J. Virol. 1996;70:6856–6861. doi: 10.1128/jvi.70.10.6856-6861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelachich M.L., Bandyopadhyay P., Blum K., Lipton H.L. Theiler’s virus growth in murine macrophage cell lines depends on the state of differentiation. Virology. 1995;209:437–444. doi: 10.1006/viro.1995.1276. [DOI] [PubMed] [Google Scholar]
- Jelachich M.L., Bramlage C., Lipton H.L. Differentiation of M1 myeloid precursor cells into macrophages results in binding and infection by Theiler’s murine encephalomyelitis virus and apoptosis. J. Virol. 1999;73:3227–3235. doi: 10.1128/jvi.73.4.3227-3235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelachich M.L., Lipton H.L. Theiler’s murine encephalomyelitis virus induces apoptosis in gamma interferon-activated M1 diffentiated myelomonocytic cells through a mechanism involving tumor necrosis factor alpha (TNF-α) and TNF-α-related apoptosis-inducing ligand. J. Virol. 2001;75:5930–5938. doi: 10.1128/JVI.75.13.5930-5938.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M.J., Ray D., Mo R.R., Yun R.L., Richardson B.C. TRAIL (Apo2 ligand) and TWEAK (Apo3 ligand) mediate CD4+ T cell killing of antigen-presenting macrophages. J. Immunol. 2000;164:2897–2904. doi: 10.4049/jimmunol.164.6.2897. [DOI] [PubMed] [Google Scholar]
- Kayagaki N., Yamaguchi N., Nakayama M., Takeda K., Akiba H., Tsutsui H., Okamura H., Nakanishi K., Okumura K., Yagita H. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J. Immunol. 1999;163:1906–1913. [PubMed] [Google Scholar]
- Kopecky S.A., Willingham M., Lyles D.S. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol. 2001;75:12169–12181. doi: 10.1128/JVI.75.24.12169-12181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrich J.C., Arnason B.G.W., Hochberg F. Demyelinative myelopathy in mice induced by the DA virus. J. Neurol. Sci. 1976;29:149–160. doi: 10.1016/0022-510x(76)90167-2. [DOI] [PubMed] [Google Scholar]
- Lipton H.L. Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect. Immunol. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H.L., Friedman A. Purification of Theiler’s murine encephalomyelitis virus and analysis of the structural virion polypeptides: correlation of the polypeptide profile with virulence. J. Virol. 1980;33:1165–1172. doi: 10.1128/jvi.33.3.1165-1172.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Xu Y., Liu D.X. Induction of caspase-dependent apoptosis in cultured cells by the avian coronavirus infectious bronchitis virus. J. Virol. 2001;75:6402–6409. doi: 10.1128/JVI.75.14.6402-6409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livne A., Shtrichman R., Kleinberger T. Caspase activation by adenovirus E4orf4 protein is cell line specific and is mediated by the death receptor pathway. Virology. 2001;75:789–798. doi: 10.1128/JVI.75.2.789-798.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Goto Y., Baba K., Masuda K., Ohno K., Tsujimoto H. TNF-·-induced cell death in feline immunodeficiency virus-infected cells is mediated by the caspase cascade. Virology. 2001;287:446–455. doi: 10.1006/viro.2001.1042. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;262:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Nicholson D.W., Ali A., Thornberry N.A., Vaillancourt J.P., Ding C.K., Gallant M., Gareau Y., Griffin P.R., Labelle M., Lazebnik Y.A., Munday N.A., Raju S.M., Smulson M.E., Yamin T.-T., Yu V.L., Miller D.K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Pan G., O’Rourke K., Chinnaiyan A.M., Gentz R., Ebner R., Ni J., Dixit V.M. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- Pitti R.M., Marsters S.A., Ruppert S., Donahue J., Moore A., Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Prikhod’ko G.G., Prikhod’ko E.A., Cohen J.I., Pletnev A.G. Infection with langat flavivirus or expression of the envelope protein induces apoptotic cell death. Virology. 2001;286:328–335. doi: 10.1006/viro.2001.0980. [DOI] [PubMed] [Google Scholar]
- Rautenschlein S., Suresh M., Sharma J.M. Pathogenic avian adenovirus type II induces apoptosis in turkey spleen cells. Arch. Virol. 2000;145:1671–1683. doi: 10.1007/s007050070083. [DOI] [PubMed] [Google Scholar]
- Rozhon E.J., Kratochvil J.D., Lipton H.L. Comparison of structual and nonstructural proteins of virulent and less virulent Theiler’s virus isolates using two-dimensional gel electrophoresis. Virus Res. 1985;2:11–28. doi: 10.1016/0168-1702(85)90056-5. [DOI] [PubMed] [Google Scholar]
- Rubio N., De Felipe C., Torres C. Theiler’s murine encephalomyelitis virus-binding activity on neural and non-neural cell lines and tissues. J. Gen. Virol. 1990;71:2867–2872. doi: 10.1099/0022-1317-71-12-2867. [DOI] [PubMed] [Google Scholar]
- Rubio N., Martin-Clemente B. Theiler’s murine encephalomyelitis virus infection induces early expression of c-fos in astrocytes. Virology. 1999;258:21–29. doi: 10.1006/viro.1999.9684. [DOI] [PubMed] [Google Scholar]
- Sedger L.M., Shows D.M., Blanton R.A., Peschon J.J., Cosman R.G., Wiley S.R. IFN-gamma mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J. Immunol. 1999;163:920–926. [PubMed] [Google Scholar]
- Sierra A., Rubio N. Theiler’s murine encephalomyelitis virus induces tumour necrosis factor-α in murine astrocyte cell cultures. Immunology. 1993;78:399–404. [PMC free article] [PubMed] [Google Scholar]
- Su F., Theodosis C.h.N., Schneider R.J. Role of NF-kB and myc proteins in apoptosis induced by hepatitis B virus HBx protein. Virology. 2001;75:215–225. doi: 10.1128/JVI.75.1.215-225.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler M. Spontaneous encephalomyelitis of mice, a new virus disease. J. Exp. Med. 1937;65:705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I., Kurtz C.I.B., Fujinami R.S. Apoptosis in acute and chronic central nervous system disease induced by Theiler’s murine encephalomyelitis virus. Virology. 1997;228:388–393. doi: 10.1006/viro.1996.8382. [DOI] [PubMed] [Google Scholar]
- Tyler K.L. Host and viral factors that influence viral neurotrophism. I. Viral cells attachment proteins and target cell receptors. Trends Neurol. Sci. 1987;10:455–460. [Google Scholar]
- Vaux D.L., Korsmeyer S.J. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Vidalain P.O., Azocar O., Lamouille B., Astier A., Rabourdin-Combe C., Servet-Delprat C. Measles virus induces functional TRAIL production by human dendritic cells. J. Virol. 2000;74:556–559. doi: 10.1128/jvi.74.1.556-559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K., Vakharia V.N. Induction of apoptosis in vitro by the 17-kDa nonstructural protein of infectious bursal disease virus: possible role in viral pathogenesis. Virology. 2001;285:50–58. doi: 10.1006/viro.2001.0947. [DOI] [PubMed] [Google Scholar]
- Zheng L., Calenoff A.M., Dal Canto M.C. Astrocytes, not microglia, are the main cells responsible for viral persistence in Theiler’s murine encephalomyelitis virus infection leading to demyelination. J. Neuroimmunol. 2001;118:256–267. doi: 10.1016/s0165-5728(01)00338-1. [DOI] [PubMed] [Google Scholar]
- Zhu N., Ware C.F., Lai M.M.C. Hepatitis C virus core protein enhances FADD-mediated apoptosis and suppresses TRADD signaling of tumor necrosis factor receptor. Virology. 2001;283:178–187. doi: 10.1006/viro.2001.0896. [DOI] [PubMed] [Google Scholar]


