Abstract
Intestinal absorption of immunoglobulins is critical for health and survival of newborn calves because there is no transfer of immunoglobulins in utero. The objective of this study was to determine if feeding beef cows Se-enriched alfalfa hay during the last trimester of gestation improves passive transfer of ovalbumin (OVA), a surrogate protein marker for IgG absorption. Control cows (n = 15) were fed non-Se-fortified alfalfa hay (5.3 mg Se/head daily) plus a mineral supplement containing inorganic Se (3 mg Se/head daily). Med-Se (n = 15) and High-Se cows (n = 15) were fed Se-biofortified alfalfa hay (27.6 and 57.5 mg Se/head daily, respectively); both groups received mineral supplement without added Se. Calves were randomly assigned to receive orally administered OVA at 12, 24, or 36 h of age. Calves that received their oral dose of OVA at 12 h of age had higher serum OVA concentrations across the first 48 h of life if born to High-Se cows compared to calves born to Control cows (P = 0.05), with intermediate values for calves born to Med-Se cows. Our results, using OVA as a model for passive transfer, suggest that if calves do not receive adequate colostrum to reach maximum pinocytosis, then supranutritional Se supplementation in beef cattle may improve passive transfer in their calves, if calves receive colostrum within the first 12 h of age.
Abbreviations: ICP-MS, inductively coupled argon plasma emission spectroscopy; Ig, immunoglobulin; MW, molecular weight; OVA, ovalbumin; TMB, 3, 3’, 5, 5’-tetramethyl benzidine; TrxR, thioredoxin reductase; T-TBS, Tween-Tris buffered saline; WB, whole blood
Keywords: Newborn beef calves, Ovalbumin, Passive transfer, Pregnant beef cows, Selenium-enriched alfalfa hay
1. Introduction
In cattle, as in other bovid species, immunoglobulins (Ig) are transferred from colostrum of the dam to the newborn calf by passive transfer of Ig across intestinal epithelium as there is no transfer of Ig across the syndesmochorial placenta of the cow in utero [1]. Calves that fail to suckle adequately possess low concentrations of Ig in their serum. Therefore, intestinal absorption of a sufficient amount of Ig is a key factor in neonatal health and survival. Passive immunity provided by maternal Ig protects neonates from enteric disease, diarrhea, and respiratory disease for several weeks after birth [1]. If failure of passive transfer occurs, calves are predisposed to infection, sepsis, and death [2,3].
Immunoglobulin IgG1 is the predominant IgG subclass in colostrum of cows and is derived from serum of the dam [4,5]. Calves are born with a digestive tract that facilitates absorption of colostral proteins instead of degrading them [6]. The level of proteolytic activity is low in the abomasum and small intestine, and further decreased by trypsin inhibitors in colostrum [7].
Three distinct processes must occur in gut epithelia of the neonate for IgG1 absorption to be successful: pinocytosis of large proteins, transcellular transfer of proteins across the enterocyte, and exocytosis of proteins into the lymphatic system [8]. Epithelial cells of the small intestine absorb colostral IgG1 via pinocytosis. Proteins pass through enterocytes in vacuoles and are released via exocytosis into lacteals [2,3]. From the lacteals, colostral proteins enter the systemic circulation, which allows newborn calves to receive maternal IgG1 by passive transfer [2,3]. Closure of the intestine to absorption is defined as cessation of absorption of macromolecules from gut to blood in neonates [9]. It is thought that the process of closure occurs in a retrograde manner, in that the basal cell membrane ceases to release the vacuolated proteins, transport ceases, and then uptake by the pinocytosis tubular system ceases [[10], [11], [12], [13]].
The period during which the intestine is permeable to IgG1 varies in calves, but is highest immediately after birth, declines after 6 h, and drops to relatively low levels by 24 h, signaling gut closure to passive transport [14]. Peak serum IgG1 concentrations are normally reached 32 h postpartum [8]. After absorption ceases, serum concentrations of passively acquired IgG1 begin to decline through normal catabolic processes [15].
We have previously shown that supplementing dairy cows with supranutritional Se-yeast during the dry period, or spiking colostrum with Na-selenite, improved IgG status in their calves [16]; Kamada et al. [17] showed similar results with spiking colostrum with Na-selenite. The underlying mechanism for improved passive transfer is unknown. Selenium has been postulated to act directly on intestinal epithelial cells to activate pinocytosis [17]. Another hypothesis is that supranutritional concentrations of Se delay enterocyte replacement by a more mature population of intestinal epithelial cells, which have been shown to be incapable of passive transfer [18,19].
Based on our previous findings in dairy cows, the objective of this study was to determine if feeding Se-replete beef cows Se-enriched alfalfa (Medicago sativa) hay during the last trimester of gestation delays turnover and replacement of fetal intestinal epithelial cells in their calves, thus extending the period of time during which large proteins can be transferred from intestinal lumen to neonatal blood. We previously reported that feeding Se-biofortified hay to these pregnant beef cows improved Se status in cows and their offspring, as well as the IgG1 concentrations in colostrum compared to cows receiving control hay [20]. In this paper, to test the hypothesis that passive transfer is enhanced in calves born to cows fed Se-biofortified alfalfa hay in the last trimester of pregnancy compared to calves born to Control cows fed the USDA-approved upper limit of Na-selenite in salt [21], we administered 25 g oral ovalbumin (OVA) to calves at 12, 24, or 36 h after birth, and measured serum OVA concentrations to estimate the time of gut closure. We chose OVA with 44,300 Da molecular weight (MW) as a surrogate protein for IgG (the bovine IgG molecule is approximately 150,000 Da MW) because human albumin with 68,000 Da MW and human IgG with 163,000 Da MW have been previously shown to be absorbed equally in relation to total dose in calves [10]. If passive transfer was prolonged by enhanced Se intake in dams, we expected OVA concentrations in serum of newborn calves to be higher at all dosing time points compared to Control calves.
2. Materials and methods
2.1. Animal ethics statement and study design
The experimental protocol was reviewed and approved by the Oregon State University Animal Care and Use Committee (ACUP Number: 4629) and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). This was a prospective clinical trial of 8–13 wk (average 10 wk) duration involving 45 pregnant Angus and Angus-cross beef cows. Multiple bulls had been utilized initially to establish pregnancy by artificial insemination; several bulls were then used to cover non-pregnant cows. The study was conducted at the Hogg Animal Metabolism barn on the Oregon State University campus (Corvallis, OR, USA).
The cows ranged in age from 2 to 14 yr (mean ± SD, 4.0 yr ± 2.4) and originated from the Oregon State University beef ranch. All cows had calved at least once previously. Body weights at 7 mo of pregnancy ranged from 420 to 757 kg (mean ± SD, 613.2 kg ± 95.5), and BCS ranged from 6 to 7 (1 to 9 scale). Routine farm management practices included the following vaccinations during Fall 2014: Bovine Rhinotracheitis-Virus Diarrhea-Parainfluenza 3-Respiratory Syncytial Virus Vaccine, modified live virus, Leptospira Canicola-Grippotyphosa-Hardjo-Icterohaemorrhagiae-Pomona bacterin (Pyramid® 10, Boehringer Ingelheim, St. Joseph, MO), Clostridium Chauvoei-Septicum-Novyi-Haemolyticum-Sordelli-Tetani-Perfringens Types C & D bacterin-toxoid (Cavalry 9; Merck Animal Health, Madison, NJ), and Bovine Rota-Coronavirus vaccine, killed virus-Clostridium Perfringens Type C-Escherichia coli bacterin-toxoid (Scourguard 4kc; Zoetis Inc., Kalamazoo, MI). Cows were also dewormed with Ivermectin (Ivomec®; Merial, Duluth, GA).
The study design consisted of 3 treatment groups, with 3 pens of 5 cows each (n = 15) per treatment. Before calving, cows were blocked by BW, cow age, and projected calving date and assigned to treatment group using a randomized complete block design. Ear tags were used to identify cows. Cows were housed in dry barn lots with continuous access to water, feed bunks, and shelter. Pens provided 10 m2/cow of concrete flooring in open lots that were strip cleaned once weekly, 5 m2/cow of shavings in loafing area, and 98 cm of feeder space/cow as concrete bunks. All measurements exceeded recommended beef housing requirements [22].
All cows received hay at a rate of 2.5% BW/d, of which 70% was alfalfa hay (17% CP; 58.7% TDN) and 30% was grass hay [6.8% CP; predominantly Schedonorus phoenix (Scop.) Holub and Agrostis capillaris] to achieve a ration CP of 13.9% (Table S1). Cows were fed alfalfa hay once daily in the evening. Alfalfa hay was readily consumed such that by the next morning feed bunks were empty. There was no wastage as alfalfa hay was kept swept into reach. Grass hay was fed in the morning. Mineral supplement was added as a top dressing on the alfalfa hay in the evenings. Control cows received mineral supplement containing 120 mg/kg Se (US FDA regulations) from Na-selenite, at a calculated amount to provide 3 mg Se/head daily. Treatment cows received the same amount of mineral supplement top dressing that did not contain Na-selenite. The mineral supplement (DM basis) was in loose granular format and contained 57.0 to 64.0 g/kg calcium; 30.0 g/kg phosphorus; 503 to 553 g/kg sodium chloride; 50.0 g/kg magnesium; 50 mg/kg cobalt; 2500 mg/kg copper; 200 mg/kg manganese; 200 mg/kg iodine; 6500 mg/kg zinc (Wilbur-Ellis Company, Clackamas, OR). Prior to the start of this experiment, cows had free-choice access to the same mineral supplement containing 120 mg/kg Se from Na-selenite.
After calving, calves were left with their mothers in individual pens for 48 h. Ear tags were used to identify calves. Body weights of all calves were recorded within 2 h of calving, and after each nursing for 48 h. Calves were placed in a large weigh sling (Nasco; Fort Atkinson, WI) and weighed using a MAX digital weight indicator (Western Scale Co.; Port Coquitlam, B.C., Canada) for precise mobile weighing.
Within each treatment group an equal number of calves were randomly assigned to receive an oral dose of OVA at 12, 24, or 36 h after birth. Calves were given their oral dose of OVA (25 g albumin from chicken egg-white powder dissolved in 100 mL water; Sigma, St. Louis, MO; 62–88% purity) using a dosing syringe.
2.2. Selenium-fortified alfalfa hay
The soil was enriched with Se by mixing inorganic Na-selenate (RETORTE Ulrich Scharrer GmbH, Röthenbach, Germany) with water and spraying it onto the soil surface of an alfalfa field at application rates of 0, 45.0, or 89.9 g Se/ha immediately after the second cutting of hay in July 2014. The application rates were chosen based on a previous study [23]. Third-cutting alfalfa hay was harvested 40 d after Se application and then analyzed for nutrient and Se content (Table S1). Alfalfa yield was approximately 4.45 t/ha. A Penn State forage sampler was used to take 25 cores from random bales in each hay source (0, 45.0, or 89.9 g Se/ha). Samples were collected prior to beginning the feeding trial for each alfalfa hay source. Core samples were blended and representative samples were selected for analysis. Alfalfa hay samples were submitted to commercial laboratories for analysis. Hay samples were prepared for Se analysis as described [24], and Se was determined using inductively coupled argon plasma emission spectroscopy (ICP-MS; ELAN 6000, Perkin Elmer, Shelton, CT). Quantification of Se was performed by the standard addition method, using a four-point standard curve. A quality-control sample (in similar matrix) was analyzed after every five samples, and analysis was considered acceptable if the Se concentration of the quality-control sample fell within ± 5% of the standard/reference value for the quality control.
2.3. Colostrum and blood collection for selenium analyses
Colostrum was collected from cows prior to the first nursing. Whole blood samples were collected from the jugular vein of calves within 2 h of birth (before colostrum feeding) into evacuated 2 mL EDTA tubes (final EDTA concentration 2 g/L; Becton Dickinson, Franklin Lakes, NJ). Blood samples were also collected at 12, 24, 36, and 48 h after birth. Colostrum and blood samples were frozen at −20 °C until further analyses were performed.
Selenium concentrations in colostrum and whole blood (WB) were determined by a commercial laboratory (Utah Veterinary Diagnostic Laboratory, Logan, UT) using an inductively coupled argon plasma emission spectrometry (ELAN 6000, Perkin Elmer, Shelton, CT) method as previously described [23]. Results are reported as ng/mL Se.
2.4. Colostrum and blood collection for IgG1 analyses
Jugular venous blood was also collected from calves within 2 h of birth and at 12, 24, 36, and 48 h after birth into evacuated tubes without EDTA (10 mL; Becton Dickinson) for subsequent harvesting of serum. The tubes were centrifuged at 850 x g for 10 min at room temperature; serum was collected, transferred into 2.0 mL screw cap self-standing micro tubes (ISC BioExpress, Kaysville, UT) and stored at -20 °C until further analyses were performed. Concentrations of IgG1 in colostrum and calf serum were quantified using a direct sandwich ELISA procedure as previously reported [20]. Results are reported as mg/mL IgG1.
2.5. Blood collection for OVA analyses
The blood collection times (12, 24, 36, and 48 h after birth) ensured that blood was collected at uniform time intervals after oral dosing of calves with OVA. Concentrations of OVA in calf serum were quantified using a direct sandwich ELISA procedure. The protocol was adapted from a commercially developed assay (Bethyl Laboratories, Montgomery, TX). In brief, 96-well microplates (Thermo Scientific™ Pierce™, Fisher Scientific, Pittsburgh, PA) were coated with 100 μL of antiserum containing 0.04 μg of anti-chicken egg albumin antibody produced in rabbit (whole antiserum; 4 mg/mL; Sigma) diluted in 0.05 M carbonate-bicarbonate buffer (pH 9.6) and incubated for 1 h at room temperature. After incubation, plates were washed 3 times in Tween-Tris buffered saline (T-TBS; 50 mM Tris, 0.14 M NaCl, 0.05% Tween-20; pH 8.0). The plates were then incubated for 30 min with 100 μl T-TBS, and then washed again 3 times with T-TBS. For the standard, albumin from chicken egg white (Sigma) was diluted in T-TBS from 50 ng to 0.1 ng. Calf serum samples from all time points were diluted in T-TBS at 1:50, 1:200, and 1:800. The standards and samples were plated in duplicate at 100 μL per well, and allowed to incubate for 1 h at room temperature. After incubation, plates were washed 3 times in T-TBS. Anti-ovalbumin antibody complexed with horseradish peroxidase [rabbit anti-Ovalbumin (OVA) antibody (HRP); 1 mg/mL; antibodies-online Inc., Atlanta, GA) was added at 1 μg in 100 μL/well and allowed to incubate for 1 h at room temperature. Plates were then washed 3 times in T-TBS, and 100 μL of 3, 3’, 5, 5’-tetramethyl benzidine (TMB; Sigma) was added to each well. Plates were kept in the dark at room temperature and read at 650 nm until an absorbance of at least 0.650 optical density was reached in the most concentrated standard well. The TMB reaction was then stopped by adding 100 μL of 2 N H2SO4 and the plate was read at 450 nm. Results are reported as milligrams OVA per deciliter plasma. The inter-assay CV for OVA was 8.6%.
2.6. Statistical analysis
Statistical analyses were performed using SAS, version 9.4 (SAS, Inc; Cary NC) software. The experimental design was a dose-response trial with control and two treatment levels of Se-biofortified alfalfa hay fed to cows. For outcome variables (except for colostrum), repeated measures over time were collected in calves. For OVA measurements, we additionally accounted for time of OVA administration. We tested for homogeneity of variance among treatments and for normal distribution of residuals. The effects of agronomic Se fortification on colostral Se and IgG1 concentrations were measured using PROC GLM. Fixed effects in the models were Se application rate (Control, Med-Se, High-Se), calf gender, and age group of cow (2–3 years, > 3 years). For repeated measures within animals (calf WB-Se concentrations, serum IgG1 concentrations, serum OVA concentrations), we modeled repeated measurements within animals using an unstructured variance-covariance structure. The unstructured variance-covariance matrix provided the most parsimonious matrix based on the lowest value by the Aikaike Information Criterion. Fixed effects in the models were Se application rate (Control, Med-Se, High-Se), blood sampling time, and the interaction of blood sampling time and Se application rate. All statistical tests were two-sided. Data are reported as least-square means ± SEM. Statistical significance was declared at P ≤ 0.05 and a statistical tendency was declared at 0.05 < P ≤ 0.10.
3. Results
3.1. Fertilizing with sodium selenate increases selenium content of alfalfa hay based on the rate of selenium application
As previously reported [20], fertilizing fields with increasing amounts of Na-selenate increased the Se concentration of third-cutting alfalfa hay from 0.34 mg Se/kg DM (non-fertilized control) to 2.42 and 5.17 mg Se/kg DM for Na-selenate application rates of 45.0 and 89.9 g Se/ha, respectively. Calculated Se intake from dietary sources was 8.3 (including 3 mg Se/head daily from Na-selenite), 27.6, and 57.5 mg Se/head daily for cows consuming 70% alfalfa hay and 30% grass hay with Se concentrations of 0.34 to 2.42 and 5.17 mg Se/kg in the alfalfa hay DM, respectively.
3.2. Feeding selenium-biofortified alfalfa hay to cows increases selenium concentrations in colostrum and newborn calf whole blood
As previously reported and shown in Fig. S1, colostral Se concentrations collected from cows within 2 h of parturition increased linearly with agronomic Se biofortification (P < 0.001). Colostrum Se-concentrations were 119, 504, and 1336 ng/mL respectively for cows assigned for Control, Med-Se, and High-Se treatments (SEM = 13 ng/mL). In calves, WB-Se concentrations at birth also increased linearly (P < 0.001) with agronomic Se biofortification from 138 ± 37 for Control to 279 ± 37 for Med-Se, and 429 ± 37 ng/mL for High-Se treatments. During the first 48 h, WB-Se concentrations decreased in calves to 113 ± 32 for Control, 251 ± 32 for Med-Se, and 391 ± 32 ng/mL for High-Se treatments. There were significant time (decreased from birth; P = 0.001), treatment (P = 0.003), but not time by treatment interaction effects (P = 0.57).
3.3. Feeding pregnant cows selenium-biofortified alfalfa hay increases IgG1 concentrations in colostrum with no change in newborn calf serum IgG1 concentrations between treatment groups
As previously reported and shown in Fig. S2, IgG1 concentrations of colostrum collected from cows within 2 h of parturition increased linearly with agronomic Se biofortification (P < 0.001). Control cows had 99 mg IgG1/mL (mean), Med-Se cows had 168 mg IgG1/mL, and High-Se cows had 204 mg IgG1/mL (SEM = 18).
Most calves in the study consumed their first colostrum within 2 to 3 h of birth and calves consumed an average of 2.63 L (range: 0.95 to 5.22 L; 33 of 45 calves consumed more than 2 L) within the first 24 h. Serum IgG1 concentrations in calves differed within the first 48 h of life (P < 0.001). Before colostrum ingestion, IgG1 was not detectable, but by 12 h, IgG1 was detectable in all calves. The IgG1 concentrations increased from 12 h to 24 h (P < 0.001), remained similar at 36 h (P = 0.99), and tended to decrease at 48 h (P = 0.06). There was no effect of Se treatment (P = 0.38) nor Se treatment by time interaction (P = 0.40).
3.4. If OVA is administered orally to newborn calves within the first 12 h of life, there is greater passive transfer in calves whose dams received selenium-biofortified alfalfa hay during pregnancy
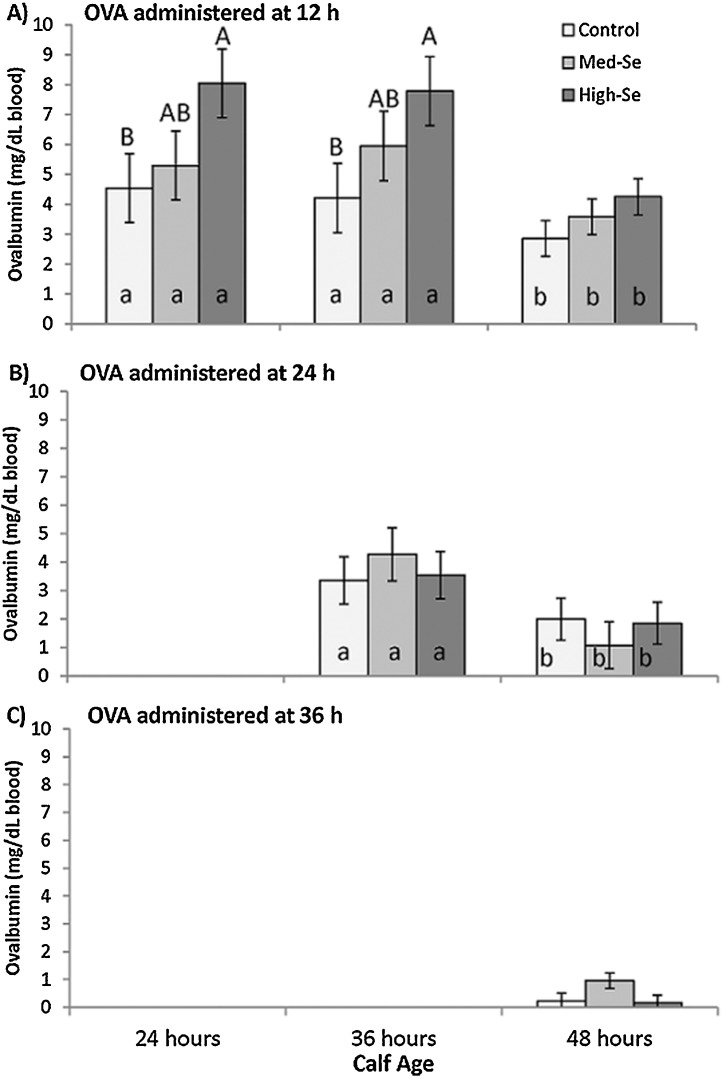
As shown in Fig. 1 A, calves that received their oral dose of OVA at 12 h of age had higher serum OVA concentrations across the first 48 h of life if born to High-Se cows compared to calves born to Control cows (P = 0.05), with intermediate values for calves born to Med-Se cows. Calves born to High-Se dams that had OVA administered at 12 h of age attained on average serum OVA concentrations of 8.1 mg/dL. Similar values were measured at 36 h of age, with decreasing serum OVA concentrations (to 4.3 mg/dL) observed in this group at 48 h of age.
Fig. 1.
Effect of Se supplementation of pregnant beef cows with Se-enriched alfalfa hay on serum ovalbumin (OVA) concentrations in their calves after calves received oral OVA at 12, 24, or 36 h of age. Dams consumed 2.5% body weight/day alfalfa hay grown in fields not fertilized with Se (0 g Se/ha; Control), or harvested from fields fertilized with Na-selenite at application rates of 45.0 (Med-Se) or 89.9 (High-Se) g Se/ha for 10 wk ± 16 d prior to calving. Control cows (n = 15) were fed non-Se-fortified alfalfa hay (5.3 mg Se/head daily) plus a mineral supplement containing inorganic Se (3 mg Se/head daily). Med-Se (n = 15) and High-Se cows (n = 15) were fed Se-biofortified alfalfa hay (27.6 and 57.5 mg Se/head daily, respectively); both groups received mineral supplement without added Se. Within each dam treatment group, an equal number of calves were randomly assigned to receive an oral dose of OVA (25 g albumin from chicken egg-white powder dissolved in 100 mL water) at A) 12 h after birth, B) 24 h after birth, or C) 36 h after birth. Serum OVA concentrations were measured at subsequent 12 h intervals. Values that differ at P < 0.05 between treatments have different letters (A–B), and values that differ within a treatment have different letters (a–b).
No significant Se treatment by time interaction was detected (P = 0.26). Concentrations of OVA remained similar between 24 and 36 h of age (P = 0.94), but then were lower at 48 h (P < 0.001) compared to 24 and 36 h
As shown in Fig. 1B, all calves that received their oral dose of OVA at 24 h of age had on average OVA serum concentrations of 3.6 mg/dL at 36 h regardless of Se status at birth (P = 0.78), and all concentrations decreased significantly by 48 h (P < 0.001) with no Se treatment by time interactions (P = 0.46).
As shown in Fig. 1C, all calves that received their oral dose of OVA at 36 h of age had similar serum OVA concentrations at 48 h of age, which were close to the detection limit in most calves (13 out of 15 calves had OVA serum concentrations < 1 mg/dL). No significant Se treatment differences were detected (P = 0.12).
The OVA concentrations at 48 h were dependent on calf age when the OVA dose was administered: calves that received OVA at 12 h had the highest OVA concentrations (3.57 ± 0.33 mg/dL), calves that had OVA administered at 24 h had intermediate OVA concentrations (1.64 ± 0.34 mg /dL), and calves that had OVA administered at 36 h had the lowest OVA concentrations (0.45 ± 0.33 mg/dL). The beneficial effect of dams receiving High-Se on OVA concentrations in calves was limited to calves that received OVA at 12 h of age, as they had the highest OVA concentrations of all calf groups at 24, 36, and 48 h of age.
4. Discussion
Using OVA as a surrogate protein marker for IgG1 in newborn calves, we evaluated the hypothesis that supranutritional Se supplementation of the dam delays turnover and replacement of fetal epithelial cells by a more mature population of intestinal epithelial cells that are incapable of passive transfer. Calves born to High-Se dams that had OVA administered orally at 12 h of age had the highest serum OVA concentrations at 24 and 36 h of age, whereas no differences between treatment groups were observed in calves that had OVA administered orally at either 24 or 36 h of age. We suggest that an enhanced Se status at birth improves passive transfer at or before 12 h of age, but the beneficial effect disappears if the surrogate protein marker is administered when calves are older (24 and 36 h of age).
Pinocytosis is a non-selective process by which macromolecules, including OVA, are taken up by fetal intestinal epithelial cells. Once taken up by pinocytosis, macromolecules are repackaged by the Golgi apparatus and transferred across the basal membrane of the cell into the lymphatic system. Movement of macromolecules out of gut epithelial cells in neonatal calves continues after pinocytosis stops, consistent with the observation that maximum concentration of serum IgG1 occurs after gut closure [2,3]. After fetal epithelial cells are replaced with cells of the adult phenotype, all of the processes associated with absorption of large molecules cease. From our data, it is not possible to determine which of these processes in passive transfer is affected by Se supplementation of dams. Our results are consistent with either or both increased pinocytosis and a delay in turnover of fetal epithelial cells in calves born to High-Se dams.
We previously reported that concentrations of IgG1 in colostrum increased in a linear relationship to the amount of Se ingested by the dam [20]. The mechanism by which High-Se supplementation increases colostral IgG1 concentration is unknown, and multiple factors may be involved. It is possible that increased concentrations of selenoproteins acting on mammary gland epithelium alter the number of specific nutrient transporters or growth and vascularization of mammary tissues as is hypothesized for intestinal tissues [25]. Because timing of colostrum formation also has an effect on IgG1 concentrations, High-Se supplementation may also increase the duration of colostrum synthesis attributable to endocrine regulation or genetic variation of the transporter(s) [5].
Because IgG concentrations were higher in colostrum of High-Se dams, their calves may have consumed more IgG1; however, this was not reflected in short-term (≤ 48 h) higher serum IgG1 concentrations in calves [20]. We previously hypothesized that calves reached the physiologic limitation for mass of Ig that can be passively transferred from a given volume of colostrum [26]. In Besser et al. [26], calves consumed more than 2 L of colostrum, a threshold volume that Stott et al. [27] proposed as necessary to maximize pinocytosis of all available fetal enterocytes. This suggests that reaching thresholds for mass of IgG1 consumed or volume of colostrum consumed are the primary factors in achieving effective passive transfer in Se-replete calves [27]. Our new results, using OVA as a model for passive transfer, suggest that if calves do not receive adequate colostrum to reach maximal pinocytosis, then High-Se treatment of dams may improve passive transfer if calves receive colostrum within the first 12 h of age.
These results are consistent with the findings of Stott et al. [27] who showed that age at initial feeding had little influence on passive transfer up to 12 h postpartum, and that in general the highest amount of passive transfer, based on serum Ig concentrations, occurred during the first 4 h after initial feeding of colostrum and was dependent on the amount of colostrum fed. Calves fed 2 L did not have a further increase in passive transfer if a second feeding was administered 12 h later. Stott et al. [27] showed that peak serum IgG concentrations occurred during the first 16 h after the time of initial colostrum ingestion. Immunoglobulin G1, albumin, and the neonatal form of the Ig receptor, FcRn, may be secreted into colostrum as a large complex [28]. Despite its name, this receptor does not play a role in absorption of IgG1 in the neonate. The main role of this receptor is to recycle IgG1 back into the intestinal lumen [28], thus prolonging the availability of IgG1 for absorption by pinocytosis.
Our hypothesis that passive transfer is enhanced in calves born to cows fed Se-biofortified alfalfa hay in the last trimester of pregnancy was confirmed if calves received colostrum within the first 12 h of age. Maternal nutritional plane and Se supply during gestation in sheep have been shown to affect total proliferating small intestinal cells in their lambs at 20 days of age [25]. Lambs from ewes fed 100% of NRC nutritional requirements as well as high dietary Se had greater percent proliferation of small intestinal cells compared to lambs from ewes receiving adequate Se [25]. Total small intestinal cells were also affected by nutritional plane during gestation [25]. Thus, it is possible that small intestinal growth and development can be affected by maternal Se nutrition in cattle during the third trimester of gestation, thereby altering passive transfer capacity in small intestinal epithelial cells of newborn calves.
Gut closure is a gradual process, beginning around 12 h post-partum and ending around 24 h post-partum [1,2]. Moretti et al. [29] observed vacuoles containing colostral Ig in jejunal epithelial samples taken from goat kids at 0 and 18 h of age, but not in samples taken at 36 and 96 h of age. The later samples were collected after both pinocytosis and transcellular transfer of large molecules ended. Turnover of gut epithelial cells from fetal to adult phenotypes is probably the most important component of this gradual change.
In some species such as the sheep and pig, the time of gut closure is affected by the time of first colostrum feeding, such that delaying the first feeding delays the time of gut closure [1]. This also appears true in calves, although the difference in time of gut closure in calves given an early feeding and calves given a later feeding was only 2–3 h [8]. Thus, the time of the initial feeding is important. Serum concentrations of IgG1 in calves given colostrum at 12 h or later were not as high as those fed before 12 h [27]. Wallace et al. [20] noted a trend of increasing concentrations of IgG1 in calf serum between 12 and 24 h for all treatment groups, but no further increase after 24 h.
We chose to provide Control cows with the maximum FDA recommended level of Se (3 mg Se/head daily) from Na-selenite, as this is common practice in Se-deficient areas in the United States. Current FDA recommendations limit the amount of dietary Se supplementation to 0.3 mg/kg (as fed), or 3 mg per cow daily without taking into account the amount of Se already present in feed sources or the chemical form of Se [21]. Consequently, calculated Se intake from dietary sources was 8.3 mg Se/head (including 3 mg Se/head daily from Na-selenite) for Control cows. This is reflected by the increase in whole blood Se concentrations in Control cows from151 ± 4 ng/mL (mean ± SEM) at baseline to 193 ± 14 ng/mL at parturition [20]. In comparison, treatment groups receiving Se-biofortified hay consumed 3.3 (27.6 mg Se/head daily) and 6.9-fold (57.5 mg Se/head daily) higher dietary levels of Se compared with Control cows. We have previously shown in ruminant species (sheep, dairy cattle, and beef cattle) that there are production and immune function benefits of supplementing organic Se at supranutritional rates (i.e., lower concentrations for extended time periods, and higher concentrations for shorter periods) in Se deficient regions [30]. No adverse effects were observed in this study or our previous studies using Na-selenite supplementation at current FDA recommended levels or supranutritional organic Se supplementation.
Our data suggests that increases in calf WB-Se concentrations were attained in utero as concentrations were increased at birth prior to ingestions of colostrum. Although Se concentrations in colostrum were enriched in cows that received supranutritional Se supplementation, subsequent ingestion of colostrum did not further increase WB-Se concentrations in their calves [20].
We also observed a significant decrease in serum OVA concentrations over time within treatment groups. Because of its lower MW compared to IgG1, OVA is likely cleared from the blood by the kidneys over 24 to 36 h [31]. Thus, IgG1 serum concentrations remained nearly the same at 48 h compared to 12 h, whereas OVA concentrations had decreased significantly by 48 h.
5. Conclusion
In summary, we have demonstrated that calves born to High-Se dams and fed OVA at 12 h of age had higher serum OVA concentrations at 24 and 36 h of age compared to Control calves. This suggests that supranutritional Se supplementation of dams can improve passive transfer by increasing the amount of protein transferred. It is possible that neonatal small intestinal development can be affected by maternal Se nutrition in cattle during the third trimester of gestation, thereby altering passive transfer capacity in newborn calves. Enhancing the Se status of the neonatal calf can be achieved by feeding the dam Se-biofortified alfalfa hay during the last 8 weeks of pregnancy. In addition, feeding Se-fortified alfalfa hay to the pregnant beef cow increases IgG1 concentrations in colostrum. Based on these results from OVA administration, we suggest that early feeding (≤ 12 h of age) will also increase IgG1 concentrations in calves from High-Se dams receiving similar volumes/concentrations of IgG1 colostrum compared to Control calves.
Author contributions
JAH and GJP conceived and designed the experiments; KDA, WRV, BPD, GB, JAH performed the experiments; GB, JAH, BPD analyzed the data; all authors contributed to data interpretation and manuscript writing.
Conflict of interest
None
Acknowledgements
Funded by a grant from the Agriculture Research Foundation (JAH and GJP, Principal Investigators), Oregon State University, Corvallis, Oregon, United States of America.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jtemb.2018.05.014.
Contributor Information
K. Denise Apperson, Email: appersok@oregonstate.edu.
William R. Vorachek, Email: william.vorachek@oregonstate.edu.
Brian P. Dolan, Email: Brian.Dolan@oregonstate.edu.
Gerd Bobe, Email: Gerd.Bobe@oregonstate.edu.
Gene J. Pirelli, Email: gene.pirelli@oregonstate.edu.
Jean A. Hall, Email: Jean.Hall@oregonstate.edu.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Bush L.J., Staley T.E. Absorption of colostral immunoglobulins in newborn calves. J. Dairy Sci. 1980;63(4):672–680. doi: 10.3168/jds.S0022-0302(80)82989-4. [DOI] [PubMed] [Google Scholar]
- 2.Patt J.A. Factors affecting duration of intestinal permeability to macromolecules in newborn animals. Biol. Rev. 1977;52(4):411–429. [Google Scholar]
- 3.Weaver D.M., Tyler J.W., VanMetre D.C., Hostetler D.E., Barrington G.M. Passive transfer of colostral immunoglobulins in calves. J. Vet. Intern. Med. 2000;14(6):569–577. doi: 10.1892/0891-6640(2000)014<0569:ptocii>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Butler J.E. Bovine immunoglobulins: an augmented review. Vet. Immunol. Immunopathol. 1983;4(1-2):43–152. doi: 10.1016/0165-2427(83)90056-9. [DOI] [PubMed] [Google Scholar]
- 5.Baumrucker C.R., Burkett A.M., Magliaro-Macrina A.L., Dechow C.D. Colostrogenesis: mass transfer of immunoglobulin G1 into colostrum. J. Dairy Sci. 2010;93(7):3031–3038. doi: 10.3168/jds.2009-2963. [DOI] [PubMed] [Google Scholar]
- 6.Hardy R.N. Proteolytic activity during the absorption of [131I]γ-gamma-globulin in the new-born calf. J. Physiol. 1969;205(2):453–470. doi: 10.1113/jphysiol.1969.sp008977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quigley J.D., Martin K.R., Dowlen H.H. Concentrations of trypsin-inhibitor and immunoglobulins in colostrum of Jersey cows. J. Dairy Sci. 1995;78(7):1573–1577. doi: 10.3168/jds.S0022-0302(95)76780-7. [DOI] [PubMed] [Google Scholar]
- 8.Stott G.H., Marx D.B., Menefee B.E., Nightengale G.T. Colostral immunoglobulin transfer in calves i. Period of absorption. J. Dairy Sci. 1979;62(10):1632–1638. doi: 10.3168/jds.S0022-0302(79)83472-4. [DOI] [PubMed] [Google Scholar]
- 9.Lecce J.G., Morgan D.O. Effect of dietary regimen on cessation of intestinal absorption of large molecules (closure) in the neonatal pig and lamb. J. Nutr. 1962;78:263–268. doi: 10.1093/jn/78.3.263. [DOI] [PubMed] [Google Scholar]
- 10.Staley T.E., Corley L.D., Bush L.J., Jones E.W. The ultrastructure of neonatal calf intestine and absorption of heterologous proteins. Anat. Rec. 1972;172(3):559–579. doi: 10.1002/ar.1091720310. [DOI] [PubMed] [Google Scholar]
- 11.Staley T.E., Jones E.W., Corley L.D. Fine structure of duodenal absorptive cells in the newborn pig before and after feeding of colostrum. Am. J. Vet. Res. 1969;30(4):567–581. [PubMed] [Google Scholar]
- 12.Staley T.E., Jones E.W., Marshall A.E. The jejunal absorptive cell of the newborn pig: an electron microscopic study. Anat. Rec. 1968;161(4):497–515. doi: 10.1002/ar.1091610412. [DOI] [PubMed] [Google Scholar]
- 13.Staley T.E., Jones E.W., Bush L.J. Maternal transport of immunoglobulins to the calf. J. Dairy Sci. 1971;54(9):1323. [PubMed] [Google Scholar]
- 14.Quigley J.D., 3rd, Drewry J.J. Nutrient and immunity transfer from cow to calf pre- and postcalving. J. Dairy Sci. 1998;81(10):2779–2790. doi: 10.3168/jds.S0022-0302(98)75836-9. [DOI] [PubMed] [Google Scholar]
- 15.Chappuis G. Neonatal immunity and immunisation in early age: lessons from veterinary medicine. Vaccine. 1998;16(14–15):1468–1472. doi: 10.1016/S0264-410X(98)00110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall J.A., Bobe G., Vorachek W.R., Estill C.T., Mosher W.D., Pirelli G.J., Gamroth M. Effect of supranutritional maternal and colostral selenium supplementation on passive absorption of immunoglobulin G in selenium-replete dairy calves. J. Dairy Sci. 2014;97(7):4379–4391. doi: 10.3168/jds.2013-7481. [DOI] [PubMed] [Google Scholar]
- 17.Kamada H., Nonaka I., Ueda Y., Murai M. Selenium addition to colostrum increases immunoglobulin G absorption by newborn calves. J. Dairy Sci. 2007;90(12):5665–5670. doi: 10.3168/jds.2007-0348. [DOI] [PubMed] [Google Scholar]
- 18.Broughton C.W., Lecce J.G. Electron-microscopic studies of the jejunal epithelium from neonatal pigs fed different diets. J. Nutr. 1970;100(4):445–449. doi: 10.1093/jn/100.4.445. [DOI] [PubMed] [Google Scholar]
- 19.Smeaton T.C., Simpson-Morgan M.W. Epithelial cell renewal and antibody transfer in the intestine of the foetal and neonatal lamb. Aust. J. Exp. Biol. Med. Sci. 1985;63(Pt 1):41–51. doi: 10.1038/icb.1985.5. [DOI] [PubMed] [Google Scholar]
- 20.Wallace L.G., Bobe G., Vorachek W.R., Dolan B.P., Estill C.T., Pirelli G.J., Hall J.A. Effects of feeding pregnant beef cows selenium-enriched alfalfa hay on selenium status and antibody titers in their newborn claves. J. Anim. Sci. 2017;95:2408–2420. doi: 10.2527/jas.2017.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FDA . 2016. Title 21 - Food and Drugs: Food Additive Permitted in Feed and Drinking Water of Animals. Section 21CFR.920 Selenium. [Google Scholar]
- 22.4th ed. Midwest Plan Service, Iowa State University; Ames, IA, USA: 1987. MWPS-6, Beef Housing and Equipment Handbook. [Google Scholar]
- 23.Hall J.A., Bobe G., Hunter J.K., Vorachek W.R., Stewart W.C., Vanegas J.A., Estill C.T., Mosher W.D., Pirelli G.J. Effect of feeding selenium-fertilized alfalfa hay on performance of weaned beef calves. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis T.Z., Stegelmeier B.L., Panter K.E., Cook D., Gardner D.R., Hall J.O. Toxicokinetics and pathology of plant-associated acute selenium toxicosis in steers. J. Vet. Diagn. Invest. 2012;24(2):319–327. doi: 10.1177/1040638711435407. [DOI] [PubMed] [Google Scholar]
- 25.Meyer A.M., Neville T.L., Reed J.J., Taylor J.B., Reynolds L.P., Redmer D.A., Hammer C.J., Vonnahme K.A., Caton J.S. Maternal nutritional plane and selenium supply during gestation impact visceral organ mass and intestinal growth and vascularity of neonatal lamb offspring. J. Anim. Sci. 2013;91(6):2628–2639. doi: 10.2527/jas.2012-5953. [DOI] [PubMed] [Google Scholar]
- 26.Besser T.E., Garmedia A.E., McGuire T.C., Gay C.C. Effect of colostral immunoglobulin G1 and immunoglobulin M concentrations on immunoglobulin absorption in calves. J. Dairy Sci. 1985;68(8):2033–2037. doi: 10.3168/jds.S0022-0302(85)81065-1. [DOI] [PubMed] [Google Scholar]
- 27.Stott G.H., Marx D.B., Menefee B.E., Nightengale G.T. Colostral immunoglobulin transfer in calves II. The rate of absorption. J. Dairy Sci. 1979;62(11):1766–1773. doi: 10.3168/jds.S0022-0302(79)83495-5. [DOI] [PubMed] [Google Scholar]
- 28.Baumrucker C.R., Bruckmaier R.M. Colostrogenesis: IgG(1) transcytosis mechanisms. J. Mammary Gland Biol. Neoplasia. 2014;19(1):103–117. doi: 10.1007/s10911-013-9313-5. [DOI] [PubMed] [Google Scholar]
- 29.Moretti D.B., Nordi W.M., Lima A.L., Pauletti P., Susin I., Machado-Neto R. Goat kids’ intestinal absorptive mucosa in period of passive immunity acquisition. Livest. Sci. 2012;144(1-2):1–10. [Google Scholar]
- 30.Brummer F.A., Pirelli G., Hall J.A. Selenium supplementation strategies for livestock in Oregon, Oregon State University Extension Service. EM. 2014;9094:1–9. [Google Scholar]
- 31.Deutsch H.F., Smith V.R. Intestinal permeability to proteins in the newborn herbivore. Am. J. Physiol. 1957;191(2):271–276. doi: 10.1152/ajplegacy.1957.191.2.271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.