Abstract
The role of fluoroquinolones (FQs) as empirical therapy for community-acquired pneumonia (CAP) remains controversial in countries with high tuberculosis (TB) endemicity owing to the possibility of delayed TB diagnosis and treatment and the emergence of FQ resistance in Mycobacterium tuberculosis. Although the rates of macrolide-resistant Streptococcus pneumoniae and amoxicillin/clavulanic acid-resistant Haemophilus influenzae have risen to alarming levels, the rates of respiratory FQ (RFQ) resistance amongst these isolates remain relatively low. It is reported that ca. 1–7% of CAP cases are re-diagnosed as pulmonary TB in Asian countries. A longer duration (≥7 days) of symptoms, a history of night sweats, lack of fever (>38 °C), infection involving the upper lobe, presence of cavitary infiltrates, opacity in the lower lung without the presence of air, low total white blood cell count and the presence of lymphopenia are predictive of pulmonary TB. Amongst patients with CAP who reside in TB-endemic countries who are suspected of having TB, imaging studies as well as aggressive microbiological investigations need to be performed early on. Previous exposure to a FQ for >10 days in patients with TB is associated with the emergence of FQ-resistant M. tuberculosis isolates. However, rates of M. tuberculosis isolates with FQ resistance are significantly higher amongst multidrug-resistant M. tuberculosis isolates than amongst susceptible isolates. Consequently, in Taiwan and also in other countries with TB endemicity, a short-course (5-day) regimen of a RFQ is still recommended for empirical therapy for CAP patients if the patient is at low risk for TB.
Keywords: Community-acquired pneumonia, Fluoroquinolones, Tuberculosis, Fluoroquinolone resistance, Multidrug-resistant Mycobacterium tuberculosis
1. Introduction
Community-acquired pneumonia (CAP) is one of the leading causes of death worldwide. The mortality rate has increased significantly over the past 10 years not only in Taiwan but also in other countries in the Asia-Pacific region [1], [2], [3]. The key causative pathogens of CAP are Streptococcus pneumoniae, Haemophilus influenzae and atypical pathogens [1], [2], [3]. According to the antimicrobial treatment guidelines of the Infectious Diseases Society of America, the American Thoracic Society, the European Respiratory Society and the Infectious Diseases Society of Taiwan [1], [2], [3], the drugs of choice for CAP in outpatients are penicillin-related agents if urine cultures are positive for pneumococcal antigen, and macrolide- or tetracycline-related agents if urine cultures are negative for pathogens. However, the rate of penicillin, macrolide and tetracycline resistance amongst S. pneumoniae isolates is high in Taiwan [4]. The antibiotic options for inpatients with CAP are β-lactams or respiratory fluoroquinolones (RFQs) (levofloxacin, moxifloxacin and gemifloxacin). In the Intensive Care Unit (ICU), a β-lactam antibiotic combined with a macrolide or with a fluoroquinolone (FQ) is appropriate [1], [2], [3]. However, the increasing resistance of key pathogens to β-lactam antibiotics poses great challenges to physicians in Taiwan. RFQs can be used in the treatment of CAP in outpatients [5], inpatients and patients in the ICU. RFQs have been shown to have excellent activity against key causative pathogens of CAP as well as atypical pathogens; however, use of RFQs for empirical treatment of CAP might mask the diagnosis of tuberculosis (TB), leading to delayed treatment and FQ resistance amongst subsequently isolated Mycobacterium tuberculosis strains.
This article briefly reviews the common microbial causes of CAP, the resistance rates amongst key pathogens, and the proper administration of FQs in the treatment of CAP. The incidence of and mortality associated with TB and the status of multidrug-resistant M. tuberculosis (MDR-TB) in Taiwan are also described. In addition, we review the controversies surrounding the empirical use of FQs to treat patients with CAP, treatment options for patients with a delayed TB diagnosis, and the emergence of FQ resistance amongst M. tuberculosis isolates.
2. Community-acquired pneumonia
2.1. Aetiology of community-acquired pneumonia in Taiwan
Lauderdale et al. collected 168 isolates from 468 patients from December 2001 to April 2002 in Taiwan and found that the most common cause of CAP amongst adult patients in Taiwan was S. pneumoniae (24%), followed by atypical pathogens (Mycoplasma pneumoniae, Chlamydophila pneumoniae and Legionella pneumophila), H. influenzae and Klebsiella pneumoniae [6]. The aetiology of CAP was undetermined in ca. 40% of CAP cases [6], [7]. Staphylococcus aureus was the causative pathogen in 2% of CAP cases, and the overall mortality rate of patients with CAP was 8.3% [6].
2.2. Antimicrobial susceptibility profiles amongst respiratory pathogens
Lin et al. found that amongst all S. pneumoniae strains that caused bacteraemia, only 29.2% were susceptible to penicillin, 15.1% to erythromycin, 18% to tetracycline and 33.7% to clindamycin [8]. However, 96.4% were susceptible to cefotaxime, 97.3% to levofloxacin and 98.4% to moxifloxacin. Amongst non-bacteraemic strains, only 23.8% were susceptible to penicillin, 5% to erythromycin, 30% to tetracycline and 30% to clindamycin. However, the rates of susceptibility amongst S. pneumoniae isolates to cefotaxime, levofloxacin and moxifloxacin were each 100% [8].
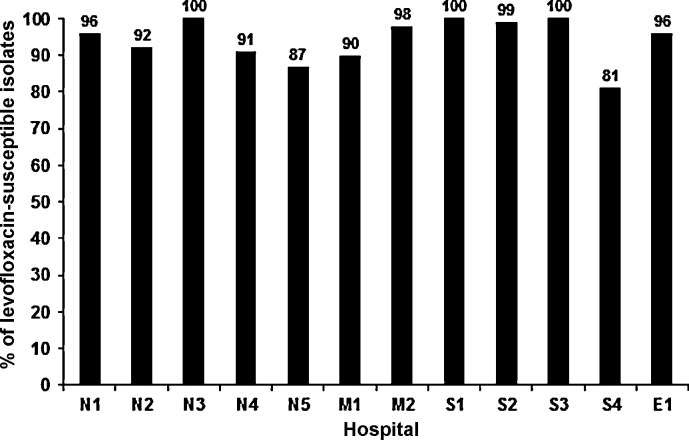
The rate of non-susceptibility of S. pneumoniae to levofloxacin in a medical centre in Taiwan was 1.2% in 2005, peaked at 4.2% in 2007 and then gradually decreased to 3% in 2010 [9]. For moxifloxacin, the non-susceptible rate was 1.3% in 2005, 4% in 2008 and then gradually decreased to 1% in 2009 and 2010. Hsieh et al. also showed that the prevalence of FQ-resistant S. pneumoniae isolates in Taiwan was low, even though FQs are widely used in that country [9]. Amongst the FQ-non-susceptible isolates in that study, serotype 9V (20%) was the most common, followed by 19F (6.8%), 23F (3.9%) and 14F (1.8%) [10]. These serotypes are all vaccine-type S. pneumoniae. Fig. 1 shows the proportion of levofloxacin resistance amongst S. pneumoniae isolated from 12 major teaching hospitals located in different parts of Taiwan in 2010. The majority (81–100%) of the S. pneumoniae isolates were susceptible to levofloxacin [9].
Fig. 1.
Proportion of levofloxacin-susceptible Streptococcus pneumoniae isolates obtained from 12 major teaching hospitals in different parts of Taiwan, 2010. N1–N5, five hospitals in North Taiwan; M1–M2, two hospitals in central Taiwan; S1–S4, four hospitals in southern Taiwan; and E1, one hospital in eastern Taiwan.
The susceptibility rate of H. influenzae to amoxicillin/clavulanic acid (AMC) decreased markedly from 95% in 2002 to 88% in 2009 in a medical centre in Taiwan [11]. AMC should be administered with caution to patients with CAP. In addition, Jean and Hsueh showed that the rate of extended-spectrum β-lactamase (ESBL)-producing K. pneumoniae strains in Taiwan was 26% [4]. Wang et al. found that the resistance rates to AMC, cefuroxime, cefaclor, ceftazidime, ceftriaxone and levofloxacin amongst K. pneumoniae isolates associated with community-acquired respiratory tract infection were all ≤10% [12]. Amongst 101 community-acquired meticillin-resistant S. aureus (CA-MRSA) isolated in a medical centre in Taiwan, 96% were susceptible to levofloxacin and moxifloxacin [13].
Regarding atypical pathogens in Taiwan, the rates of susceptibility to levofloxacin were reported to be 93.9% for M. pneumoniae, 85.7% for C. pneumoniae and 100% for L. pneumophila [14].
2.3. Role of respiratory fluoroquinolones in the treatment of community-acquired pneumonia
In Taiwan, patients with CAP who were previously healthy and have not used antibiotics in the 3 months prior to disease onset are normally given a macrolide or doxycycline as outpatient treatment [3]. However, the rates of non-susceptibility to penicillin and erythromycin amongst clinical isolates of S. pneumoniae have increased markedly in recent years [4]. Therefore, caution should be exercised before administering macrolides for CAP unless atypical pathogens are highly suspected. For patients with co-morbidities, a RFQ or a β-lactam antibiotic plus macrolide is suggested [3]. For inpatients with co-morbidities, especially in the ICU, a β-lactam antibiotic plus either azithromycin or a FQ is suggested [1], [2], [3]. For atypical pathogens, FQs are as effective as macrolides. The length of stay in hospital and the time to clinical stability favour the use of FQs [15]. Drago et al. showed that the combination of levofloxacin with ceftriaxone produced the highest rate of synergy (54%), mainly against macrolide-resistant isolates, whereas clarithromycin combined with AMC was shown to be antagonistic in 22% of isolates [16]. No antagonism was noted between FQ and β-lactam antibiotics [16]. The prevalence of levofloxacin-resistant S. pneumoniae increased markedly during the period 2001–2007 in Hong Kong, especially amongst the elderly [17]. The most common aetiology of levofloxacin resistance was suboptimal use of a FQ in which small doses (100–200 mg) of ofloxacin and levofloxacin were administered two or three times daily. Accordingly, appropriate doses of a RFQ (levofloxacin, 750 mg/day; moxifloxacin, 400 mg/day; and gemifloxacin, 320 mg/day) are recommended [1], [2], [3].
3. Tuberculosis and community-acquired pneumonia
3.1. Tuberculosis in Taiwan
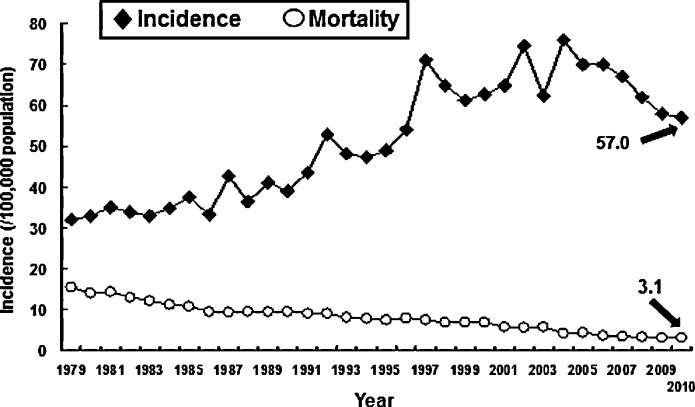
The incidence of TB was 75 cases per 100 000 population in 2002, but decreased to 62 cases per 100 000 population in 2003 because of the severe acute respiratory syndrome (SARS) outbreak (Fig. 2 ). In 2004, the incidence rebounded to 76 cases per 100 000 population (16 784 new cases) [18]. In 2005, the Taiwan Centers for Disease Control (CDC) performed a ‘Stop TB Program’ to try to reduce the incidence by one-half. From 2005 to 2010 the TB incidence rate decreased from 70 to 57 per 100 000 population and the mortality rate decreased from 4.3 to 3 per 100 000 population (Fig. 2). The overall number of patients who died decreased from 970 in 2005 to 700 in 2010. The long-term trend in TB mortality also decreased from 294 per 100 000 population in 1947 to 3 per 100 000 in 2010.
Fig. 2.
Incidence of and mortality rates (per 100 000 population) associated with tuberculosis in Taiwan, 1979–2010.
3.2. Proportion of tuberculosis amongst patients with community-acquired pneumonia
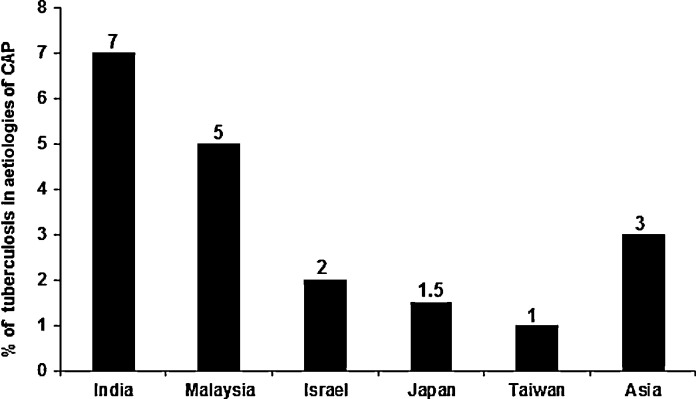
Fig. 3 demonstrates the proportions of TB amongst patients initially diagnosed as having CAP in different countries. The rates ranged from 1–3% in Taiwan to 7% in India [6], [7], [19], [20]. The majority (>50%) of those patients with CAP due to TB were of advanced age (>65 years) and had various co-morbidities [6].
Fig. 3.
Proportion of Mycobacterium tuberculosis as the causative agent of community-acquired pneumonia (CAP) in several countries.
3.3. Antimicrobial resistance and multidrug resistance amongst Mycobacterium tuberculosis isolates
The Taiwan CDC reported that there was a marked difference in antimicrobial resistance rates between patients with incident TB and patients with recurrent TB [21]; the rates of resistance were, respectively, 9% and 18% to isoniazid, 2% and 10% to rifampicin, 2% and 7% to ethambutol, 8% and 12% to streptomycin and 14% and 23% to any first-line drug during 2009–2010 (http://www.cdc.gov.tw). The rates of resistance to first-line drugs tended to decrease during 2000–2010 except for the rate of resistance to streptomycin, which remained stable. The rate of incident MDR-TB was 1% in 2010. The rate of resistance to any drug class as well as the incidence of MDR-TB also showed a downward trend during that decade [22].
3.4. Fluoroquinolone resistance amongst Mycobacterium tuberculosis isolates in Taiwan
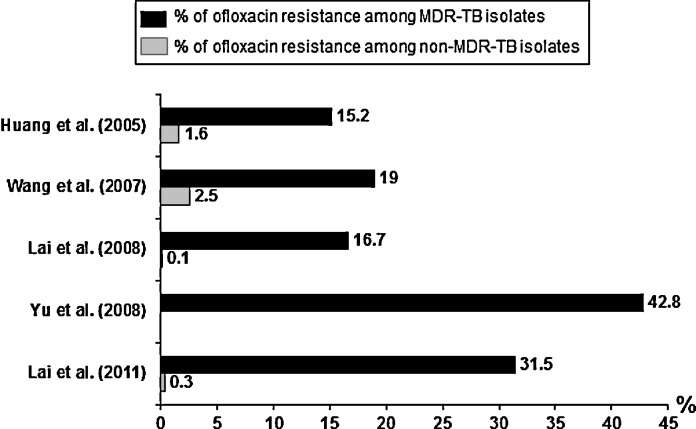
During the period 2000–2006, the FQ resistance rate amongst non-MDR-TB isolates in Taiwan was 0.1%, that amongst isolates from patients with previous anti-TB treatment was 7.9% and that amongst MDR-TB isolates was 16.7% [23]. Wang et al. evaluated FQ susceptibility as well as genetic mutations amongst isolates from patients who had been exposed to FQs from January 2004 to December 2005 [24]. They found that multiple drug resistance had the strongest correlation with FQ resistance (19% of isolates) [24]. Neither previous use of FQs nor the duration of FQ exposure was correlated with FQ susceptibility. Amongst the FQ-resistant isolates, 35.7% had a gyrA mutation (D94G and A90V) and 7.1% had a gyrB mutation (N538) [24].
Amongst the 215 MDR-TB isolates from Taiwan reported by Yu et al., 42.8% were resistant to ofloxacin [21]. The rate of extensively drug-resistant TB (XDR-TB) was 10.3% in 2004 (12/116 isolates) and 10.1% in 2005 (10/99 isolates) [21]. According to the annual report from Taiwan CDC, the rate of FQ-resistant MDR-TB isolates was 29.1% (144/494) from July 2009 to March 2009 (http://www.cdc.gov.tw). In total, 43 MDR-TB isolates were also resistant to capreomycin, amikacin or kanamycin. The rate of XDR-TB amongst MDR-TB isolates was 8.7%. Nearly all FQ-resistant M. tuberculosis isolates were found amongst MDR-TB isolates (Fig. 4 ) [20], [21], [22], [23], [24], [25], [26]. The most common sites from which FQ-resistant M. tuberculosis isolates were obtained were the genitourinary tract (5.1%), pulmonary tract (1.5%) and pleural cavity (1.0%) [22].
Fig. 4.
Proportion of quinolone resistance amongst multidrug-resistant Mycobacterium tuberculosis (MDR-TB) and non-MDR-TB isolates in Taiwan [21], [22], [23], [24], [26].
3.5. Empirical use of fluoroquinolones amongst community-acquired pneumonia patients and delayed tuberculosis diagnosis and treatment
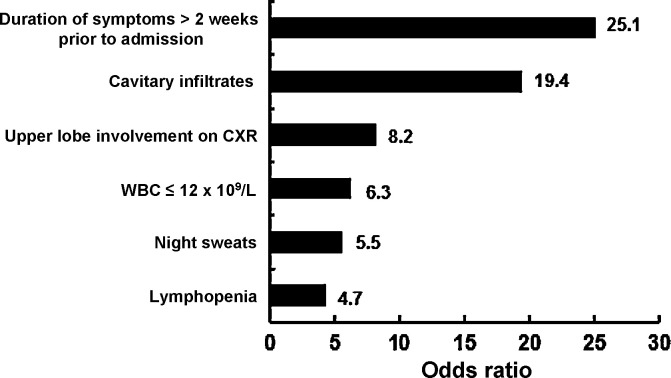
Dooley et al. was the first to point out the possibility that empirical treatment with a FQ might delay the diagnosis of TB [27]. Amongst patients who received empirical FQ treatment, the median time between symptom onset and receipt of anti-TB medication was 21 days compared with a median time of 5 days amongst those who did not receive FQs [27], [28]. However, the sample size in their study was small. Of the 17 patients who did not receive FQs, 7 received anti-TB therapy and 3 patients did not receive antibiotic treatment. The initial acid-fast bacillus smear was positive in 8 of those patients. TB was suspected in at least one-half of the patients who did not receive FQs, so antibiotics were not prescribed and anti-TB treatment was given directly. Interestingly, of those patients who received FQ monotherapy, 83% experienced improvement in the symptoms of TB, and clinical improvement occurred an average of 3 days after the initiation of therapy. Other studies have also reported that the delay in initiation of anti-TB medication was longer amongst patients who received FQs than amongst patients who did not receive FQ-based antibiotics (43.1 ± 40.0 days vs. 18.7 ± 16.9 days; P = 0.04) [28], [29], [30]. Median healthcare delay for patients who received antibiotics for non-TB diagnoses/indications prior to TB diagnosis was 39 days versus 15 days (P < 0.01) for patients who had initially received TB therapy [26]. Not only did administration of a FQ result in a delayed diagnosis of TB (median 29 days), but administration of other antibiotics (such as β-lactams, macrolides or carbapenems) had a similar effect (median 31 days). In the study by Golub et al., only 57% of patients initially diagnosed as having pneumonia had a chest radiograph [31]. Interestingly, 34% of the chest radiographs suggested a diagnosis of TB; however, the patients were still subsequently prescribed empirical antibiotics. More importantly, both antibiotic use and not having a chest radiograph taken during the first healthcare visit were independently related to longer healthcare delays in the overall cohort. Mathur et al. showed that ca. 44–55% of active TB patients were given an incorrect diagnosis at the initial presentation, mainly because of atypical radiographic manifestations [32]. A study from Malaysia clearly demonstrated that a duration of symptoms of >2 weeks before hospital admission [odds ratio (OR) = 25.1; P < 0.001), history of night sweats (P = 0.038), a chest radiograph showing upper lobe involvement (P = 0.012) or cavitary infiltrates (P = 0.002), a total white blood cell count of ≤12 × 109/L on admission (P = 0.029) and lymphopenia (P = 0.040)] was significantly associated with culture-positive pulmonary TB (Fig. 5 ) [19]. In addition, lower lung field TB (LLFTB) is difficult to differentiate from pneumonia and is often misdiagnosed because of atypical findings on chest radiographs [33], [34]. In Taiwan, ca. 20% of pulmonary TB patients have LLFTB [33]. Multivariate analysis conducted by Lin et al. showed that prolonged duration of symptoms ≥7 days (OR = 4.57; P = 0.038), lack of fever >38 °C (OR = 9.04; P = 0.001) and the absence of air bronchograms (OR = 12.08; P = 0.007) were significant predictors of LLFTB in patients with LLF pneumonia [35]. A calculated probability of >0.36 suggests LLFTB with a sensitivity of 81.8% and a specificity of 86.1% [35].
Fig. 5.
Clinical and laboratory predictors of patients with tuberculosis who were initially diagnosed as having community-acquired pneumonia [19]. CXR, chest radiography; WBC, white blood cell count.
3.6. Empirical use of fluoroquinolones (FQs) amongst community-acquired pneumonia patients and the emergence of FQ resistance in Mycobacterium tuberculosis
Recently, Lai et al. reported that the FQ resistance rate in M. tuberculosis was only 1.3% in Taiwan during 2005–2010 [25]. Prior to that time period, FQ resistance increased markedly from 7.7% in 1995–1997 to 22.2% in 1998–2000 [26]. Lai et al. found that approximately two-thirds of ofloxacin-resistant M. tuberculosis isolates were MDR and, surprisingly, the rates of FQ resistance were highest amongst adults aged 34–44 years [25]. Only 22.2% of FQ-resistant TB isolates (8/36) were susceptible to all first-line anti-TB agents. Their results were similar to those reported by van den Boogaard et al., who showed that the rate of FQ-resistant TB was low in TB patients and was not related to previous brief FQ exposure [36]. Park et al. evaluated the impact of short-term exposure to FQ on ofloxacin resistance in human immunodeficiency virus (HIV)-negative patients with TB and found that the rate of ofloxacin-resistant M. tuberculosis was low and that most cases of ofloxacin resistance were associated with MDR-TB [37]. They also found that the frequency of ofloxacin-resistant M. tuberculosis was low amongst patients who were exposed to FQs for a short period of time [37]. These findings favour the application of FQs in the regimen for CAP or TB in patients with shorter disease durations.
4. Solutions
In Taiwan, only 1–2% of patients with CAP receive a final diagnosis of TB. It is important to differentiate between CAP and TB in the initial presentation. If the lesion is located in the upper lung field, clinical specimen collection or rapid nucleic acid amplification could shorten the delay to TB diagnosis rather than restrict the empirical treatment with FQs in CAP. If the lesion is in the lower lobe, risk factors including advanced age, prolonged duration of the lesion, lack of fever and absence of air bronchograms should raise the suspicion of LLFTB.
Although use of FQs in Taiwan is high, the incidence of TB, the mortality rate associated with TB and the rate of drug resistance have decreased. The rate of FQ resistance in S. pneumoniae is low and the rate of susceptibility to FQs is high amongst H. influenzae, K. pneumoniae (including ESBL-producing strains), atypical pathogens and CA-MRSA in Taiwan.
Because of the high incidence of CAP caused by atypical pathogens in Taiwan, coverage of atypical pneumonia must be considered in the empirical treatment of CAP both in outpatients and inpatients. Macrolides should be used with caution because of high rates of resistance to that antimicrobial class in S. pneumoniae. FQs have a good synergistic effect with other antimicrobial agents, with the exception of a macrolide combined with a β-lactam, which might show some antagonist properties.
5. Conclusions
Empirical treatment of CAP with a FQ might mask active TB, delay treatment and contribute to the development of FQ resistance. FQ resistance in M. tuberculosis is related to FQ duration and the timing of exposure. Exposure to a FQ for >10 days and exposure for >60 days before TB diagnosis were both shown to be associated with a significant risk of developing FQ resistance. Consequently, in Taiwan as well as in other countries with endemicity of TB, a short-course (5-day) regimen of a FQ (levofloxacin, moxifloxacin and gemifloxacin) is still recommended for empirical therapy for CAP patients if the patient is at low risk for TB. Furthermore, FQ resistance is less likely to occur amongst M. tuberculosis strains isolated from patients with short-term exposure (<10 days) to FQ.
Funding: No funding sources.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Mandell L.A., Wunderink R.G., Anzueto A., Bartlett J.G., Campbell G.D., Dean N.C. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl. 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodhead M., Blasi F., Ewig S., Huchon G., Ieven M., Ortqvist A. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26:1138–1180. doi: 10.1183/09031936.05.00055705. [DOI] [PubMed] [Google Scholar]
- 3.Infectious Diseases Society of Taiwan; Taiwan Society of Pulmonary and Critical Medicine; Medical Foundation in Memory of Dr. Deh-Lin Cheng; Foundation of Professor Wei-Chuan Hsieh for Infectious Diseases Research and Education; CY Lee's Research Foundation for Pediatric Infectious Diseases and Vaccines Guidelines on antimicrobial therapy of pneumonia in adults in Taiwan, revised 2006. J Microbiol Immunol Infect. 2007;40:279–283. [PubMed] [Google Scholar]
- 4.Jean S.S., Hsueh P.R. Antimicrobial drug resistance in Taiwan. J Formos Med Assoc. 2011;110:4–13. doi: 10.1016/S0929-6646(11)60002-8. [DOI] [PubMed] [Google Scholar]
- 5.Wispelwey B., Schafer K.R. Fluoroquinolones in the management of community-acquired pneumonia in primary care. Expert Rev Anti Infect Ther. 2010;8:1259–1271. doi: 10.1586/eri.10.110. [DOI] [PubMed] [Google Scholar]
- 6.Lauderdale T.L., Chang F.Y., Ben R.J., Yin H.C., Ni Y.H., Tsai J.W. Etiology of community acquired pneumonia among adult patients requiring hospitalization in Taiwan. Respir Med. 2005;99:1079–1086. doi: 10.1016/j.rmed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Yen M.Y., Hu B.S., Chen Y.S., Lee S.S., Lin Y.S., Wann S.R. A prospective etiologic study of community-acquired pneumonia in Taiwan. J Formos Med Assoc. 2005;104:724–730. [PubMed] [Google Scholar]
- 8.Lin S.H., Lai C.C., Tan C.K., Liao W.H., Hsueh P.R. Outcomes of hospitalized patients with bacteraemic and non-bacteraemic community-acquired pneumonia caused by Streptococcus pneumoniae. Epidemiol Infect. 2011;139:1307–1316. doi: 10.1017/S0950268810002402. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh Y.C., Chang L.Y., Huang Y.C., Lin H.C., Huang L.M., Hsueh P.R. Circulation of international clones of levofloxacin non-susceptible Streptococcus pneumoniae in Taiwan. Clin Microbiol Infect. 2010;16:973–978. doi: 10.1111/j.1469-0691.2009.02951.x. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh Y.C., Lin P.Y., Chiu C.H., Huang Y.C., Chang K.Y., Liao C.H. National survey of invasive pneumococcal diseases in Taiwan under partial PCV7 vaccination in 2007: emergence of serotype 19A with high invasive potential. Vaccine. 2009;27:5513–5518. doi: 10.1016/j.vaccine.2009.06.091. [DOI] [PubMed] [Google Scholar]
- 11.Chung K.P., Huang Y.T., Lee L.N., Yu C.J., Lai C.C., Hsueh P.R. Alarmingly decreasing rates of amoxicillin–clavulanate susceptibility among clinical isolates of Haemophilus influenzae from 2001 to 2009 in a medical center in Taiwan. J Infect. 2011;62:185–187. doi: 10.1016/j.jinf.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Wang H., Chen M., Xu Y., Sun H., Yang Q., Hu Y. Antimicrobial susceptibility of bacterial pathogens associated with community-acquired respiratory tract infections in Asia: report from the Community-Acquired Respiratory Tract Infection Pathogen Surveillance (CARTIPS) study, 2009–2010. Int J Antimicrob Agents. 2011;38:376–383. doi: 10.1016/j.ijantimicag.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y.H., Liu C.Y., Lu J.J., King C.H., Hsueh P.R. In vitro activity of nemonoxacin (TG-873870), a novel non-fluorinated quinolone, against clinical isolates of Staphylococcus aureus, enterococci and Streptococcus pneumoniae with various resistance phenotypes in Taiwan. J Antimicrob Chemother. 2009;64:1226–1229. doi: 10.1093/jac/dkp370. [DOI] [PubMed] [Google Scholar]
- 14.van Rensburg D.J., Perng R.P., Mitha I.H., Bester A.J., Kasumba J., Wu R.G. Efficacy and safety of nemonoxacin versus levofloxacin for community-acquired pneumonia. Antimicrob Agents Chemother. 2010;54:4098–4106. doi: 10.1128/AAC.00295-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin A.T., Peyrani P., Wiemken T., Arnold F. Macrolides versus quinolones in Legionella pneumonia: results from the Community-Acquired Pneumonia Organization international study. Int J Tuberc Lung Dis. 2010;14:495–499. [PubMed] [Google Scholar]
- 16.Drago L., Nicola L., Rodighiero V., Larosa M., Mattina R., De Vecchi E. Comparative evaluation of synergy of combinations of β-lactams with fluoroquinolones or a macrolide in Streptococcus pneumoniae. J Antimicrob Chemother. 2011;66:845–849. doi: 10.1093/jac/dkr016. [DOI] [PubMed] [Google Scholar]
- 17.Ho P.L., Cheng V.C., Chow K.H. Decreasing prevalence of levofloxacin-resistant Streptococcus pneumoniae in Hong Kong, 2001 to 2007. J Antimicrob Chemother. 2009;63:836–838. doi: 10.1093/jac/dkp038. [DOI] [PubMed] [Google Scholar]
- 18.Lo H.Y., Chou P., Yang S.L., Lee C.Y., Kuo H.S. Trends in tuberculosis in Taiwan, 2002–2008. J Formos Med Assoc. 2011;110:501–510. doi: 10.1016/S0929-6646(11)60076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liam C.K., Pang Y.K., Poosparajah S. Pulmonary tuberculosis presenting as community-acquired pneumonia. Respirology. 2006;11:786–792. doi: 10.1111/j.1440-1843.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- 20.Singh A. Fluoroquinolones should not be the first-line antibiotics to treat community-acquired pneumonia in areas of tuberculosis endemicity. Clin Infect Dis. 2007;45:133. doi: 10.1086/518702. [DOI] [PubMed] [Google Scholar]
- 21.Yu M.C., Wu M.H., Jou R., Extensively drug-resistant tuberculosis, Taiwan Emerg Infect Dis. 2008;14:849–850. doi: 10.3201/eid1405.071398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai C.C., Liu W.L., Tan C.K., Huang Y.C., Chung K.P., Lee M.R. Differences in drug resistance profiles of Mycobacterium tuberculosis isolates causing pulmonary and extrapulmonary tuberculosis in a medical centre in Taiwan, 2000–2010. Int J Antimicrob Agents. 2011;38:125–129. doi: 10.1016/j.ijantimicag.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Lai C.C., Tan C.K., Huang Y.T., Chou C.H., Hung C.C., Yang P.C. Extensively drug-resistant Mycobacterium tuberculosis during a trend of decreasing drug resistance from 2000 through 2006 at a medical center in Taiwan. Clin Infect Dis. 2008;47:e57–e63. doi: 10.1086/591702. [DOI] [PubMed] [Google Scholar]
- 24.Wang J.Y., Lee L.N., Lai H.C., Wang S.K., Jan I.S., Yu C.J. Fluoroquinolone resistance in Mycobacterium tuberculosis isolates: associated genetic mutations and relationship to antimicrobial exposure. J Antimicrob Chemother. 2007;59:860–865. doi: 10.1093/jac/dkm061. [DOI] [PubMed] [Google Scholar]
- 25.Lai C.C., Tan C.K., Huang Y.T., Liao C.H., Hsueh P.R. Fluoroquinolone-resistant tuberculosis at a medical centre in Taiwan, 2005–10. J Antimicrob Chemother. 2011;66:2437–2438. doi: 10.1093/jac/dkr302. [DOI] [PubMed] [Google Scholar]
- 26.Huang T.S., Kunin C.M., Shin-Jung Lee S., Chen Y.S., Tu H.Z., Liu Y.C. Trends in fluoroquinolone resistance of Mycobacterium tuberculosis complex in a Taiwanese medical centre: 1995–2003. J Antimicrob Chemother. 2005;56:1058–1062. doi: 10.1093/jac/dki353. [DOI] [PubMed] [Google Scholar]
- 27.Dooley K.E., Golub J., Goes F.S., Merz W.G., Sterling T.R. Empiric treatment of community-acquired pneumonia with fluoroquinolones, and delays in the treatment of tuberculosis. Clin Infect Dis. 2002;34:1607–1612. doi: 10.1086/340618. [DOI] [PubMed] [Google Scholar]
- 28.Chen T.C., Lu P.L., Lin C.Y., Lin W.R., Chen Y.H. Fluoroquinolones are associated with delayed treatment and resistance in tuberculosis: a systematic review and meta-analysis. Int J Infect Dis. 2011;15:e211–e216. doi: 10.1016/j.ijid.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Yoon Y.S., Lee H.J., Yoon H.I., Yoo C.G., Kim Y.W., Han S.K. Impact of fluoroquinolones on the diagnosis of pulmonary tuberculosis initially treated as bacterial pneumonia. Int J Tuberc Lung Dis. 2005;9:1215–1219. [PubMed] [Google Scholar]
- 30.Wang J.Y., Hsueh P.R., Jan I.S., Lee L.N., Liaw Y.S., Yang P.C. Empirical treatment with a fluoroquinolone delays the treatment for tuberculosis and is associated with a poor prognosis in endemic areas. Thorax. 2006;61:903–908. doi: 10.1136/thx.2005.056887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golub J.E., Bur S., Cronin W.A., Gange S., Sterling T.R., Oden B. Impact of empiric antibiotics and chest radiograph on delays in the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2005;9:392–397. [PubMed] [Google Scholar]
- 32.Mathur P., Sacks L., Auten G., Sall R., Levy C., Gordin F. Delayed diagnosis of pulmonary tuberculosis in city hospitals. Arch Intern Med. 1994;154:306–310. [PubMed] [Google Scholar]
- 33.Chang S.C., Lee P.Y., Perng R.P. The value of roentgenographic and fiber bronchoscopic findings in predicting outcome of adults with lower lung field tuberculosis. Arch Intern Med. 1991;151:1581–1583. [PubMed] [Google Scholar]
- 34.Wang J.Y., Lee L.N., Hsueh P.R. Factors changing the manifestation of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2005;9:777–783. [PubMed] [Google Scholar]
- 35.Lin C.H., Chen T.M., Chang C.C., Tsai C.H., Chai W.H., Wen J.H. Unilateral lower lung field opacities on chest radiography: a comparison of the clinical manifestations of tuberculosis and pneumonia. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2011.03.028. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.van den Boogaard J., Semvua H.H., van Ingen J., Mwaigwisya S., van der Laan T., van Soolingen D. Low rate of fluoroquinolone resistance in Mycobacterium tuberculosis isolates from northern Tanzania. J Antimicrob Chemother. 2011;66:1810–1814. doi: 10.1093/jac/dkr205. [DOI] [PubMed] [Google Scholar]
- 37.Park I.N., Hong S.B., Oh Y.M., Lim C.M., Lee S.D., Lew W.J. Impact of short-term exposure to fluoroquinolones on ofloxacin resistance in HIV-negative patients with tuberculosis. Int J Tuberc Lung Dis. 2007;11:319–324. [PubMed] [Google Scholar]