Abstract
Background
Acute disseminated encephalomyelitis is an immune-mediated disease that produces multiple inflammatory lesions in the brain and spinal cord.
Methods
This study retrospectively evaluated 15 children with acute disseminated encephalomyelitis in children and adolescents from a single institution in Adana, Turkey.
Results
The patients presented in a seasonal distribution, with 73.3%: (11/15) presenting in winter or spring. The majority of patients (13/15, 86.7%) had a history of acute febrile illness 2 to 40 days before presentation, and five children had serologic evidence of specific triggers: mycoplasma (2 children), influenza-A (H1N1) (1 child), or Epstein-Barr virus. All children were treated with a standard protocol of 3 to 5 days of intravenous administration of methylprednisolone and intravenous immunoglobulin for patients who continued to deteriorate. Oseltamivir and clarithromycin were administered in patients with influenza-A (H1N1) and mycoplasma according to the serology. In 13 patients, all neurologic signs and symptoms resolved after treatment. Only one patient was left with severe neurologic sequelae and another child had recurrent attacks and was ultimately diagnosed with possible multiple sclerosis.
Conclusions
The present series demonstrates that acute disseminated encephalomyelitis in children occurs predominantly in winter or spring and often follows an upper respiratory tract illness for those along the southern coast of Anatolia (Mediterranean region). Early treatment with immunomodulative agents is recommended and is likely to result in a favorable outcome or full recovery. This study also suggests benefit from antiviral and antibiotic treatment initiated as soon as possible after the onset of illness.
Keywords: Alzheimer's disease, acute disseminated encephalomyelitis, multiple sclerosis, children, adolescents, Turkey
Introduction
Acute disseminated encephalomyelitis (ADEM) is a first clinical event with a presumed inflammatory or demyelinating cause, with acute or subacute onset that affects multifocal areas of the brain and spinal cord. The clinical presentation must be polysymptomatic and must include encephalopathy in the form of alteration in consciousness or behavioral change. Systemic symptoms, such as fever, malaise, myalgia, headache, nausea, and vomiting, are common precursors to the neurologic symptoms 1, 2. The etiology and pathophysiology of ADEM are not fully understood, but ADEM usually follows an infection of the upper respiratory tract or immunization in children and young adults. An autoimmune response to myelin basic protein, triggered by infection or immunization, is considered among the most likely etiologic factors 3, 4, 5.
The typical neuroradiologic findings of ADEM are subcortical and central white matter lesions and lesions at the cortical gray–white matter junction of both cerebral hemispheres, the cerebellum, the brainstem, and the spinal cord. Periventricular white matter and gray matter of the cortex, thalamus, and basal ganglia may also be involved 6.
Wider use of magnetic resonance imaging (MRI) in pediatric medicine is increasing awareness of ADEM, but little research has been done to document the clinical and radiologic features in children across different regions. ADEM has been reported in many countries from America and Europe, however, knowledge about its clinical features, microbiology, neuroimaging, and treatment in Turkey is incomplete. We evaluated consecutive children and adolescents diagnosed with ADEM from a single institution in Adana, Turkey, to examine the relationships between clinical features, microbiology, neuroimaging, and treatment outcomes in this region.
Materials and Methods
We retrospectively evaluated 15 consecutive children with ADEM and adolescents at the pediatric neurology division of Baskent University, Adana Hospital (Adana, Turkey) between June 2008 and June 2012. All children had been diagnosed using reliable clinical, laboratory, and neuroimaging techniques according to the International Pediatric Multiple Sclerosis (MS) Study Group criteria 2, 5. Patients presenting with a clinically isolated demyelinating syndrome (e.g., optic neuritis or transverse myelitis) or with clinical or radiologic evidence for dissemination in time and space suggesting MS were excluded 2, 5. Extensive workup for bacterial and viral infections was performed for all patients. Immunologic investigations were simultaneously performed in cerebrospinal fluid (CSF) and serum samples. Besides those, investigation of CSF lactate for mitochondrial disorder and angiotensin converting enzyme for neurosarcoidosis were performed for all patients 7, 8, 9. At the time of diagnosis, C-reactive protein, erythrocyte sedimentation rate, complete blood count, CSF analyses (cell counts and phenotypes, protein and glucose content, polymerase chain reaction tests for herpes simplex virus, oligoclonal bands, immunoglobulin-G, lactate, angiotensin converting enzyme), serum serologic tests for bacterial (salmonella, brucella, borreliosis, and mycoplasma pneumoniae) and viral infections (cytomegalovirus, Epstein-Barr virus, and herpes simplex virus), and serum chemistry (serum angiotensin converting enzyme levels and immunoglobulin-G) were obtained. All physical and neurologic examinations performed in the hospital and during outpatient follow-up were performed by the same pediatric neurologist (IE).
Brain computed tomography (CT) was performed as part of the initial examination in some patients and brain MRIs were acquired in all patients. Brain MRI scans were performed in all patients using a 1.5-T MR scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany). Imaging studies included axial contrast-enhanced T1-weighted spin echo with fat suppression images, axial and sagittal precontrast spin echo T1-weighted images, T2-weighted coronal and axial turbo spin echo image, axial fluid-attenuated inversion recovery images, and T1-weighted axial, sagittal, and coronal contrast-enhanced spin echo images. Spinal MRIs were acquired in cases where there was a clinical suspicion of spinal cord involvement. All patients underwent follow-up MRI several weeks after their neurologic symptoms resolved.
All children were treated with a standard protocol consisting of 3 to 5 days' treatment with intravenous methylprednisolone 30 mg/kg followed by oral prednisolone 1 mg/kg for 2 weeks and then tapering over the next 2 weeks along with symptomatic treatment. Intravenous immunoglobulin (2 gm/kg divided over 2 days) was given for patients who continued to deteriorate. Seizures were managed with intravenous phenytoin. Antiviral and antibiotic therapy was given according to the patients' clinical status at admission.
All of the patients were regularly followed at the outpatient clinic at 4 weeks after discharge and every 3 to 6 months thereafter until preparation of this article, depending on clinical conditions.
Statistical analyses
Statistical analyses were performed using SPSS for Windows version 14.0 (Statistical Package for Social Sciences, SPSS Inc, Chicago, IL). Descriptive statistics regarding age, sex, symptoms, neurologic findings, results of neuroimaging, history of prodromal infection at the time of diagnosis, and prognosis were calculated.
Results
From June 2008 to June 2012, nine boys and six girls (age range, 0.5 to 16 years; median age, 3.5 ± 4.40 years) diagnosed with ADEM were treated at Adana Hospital (Adana, Turkey). Five children (33.3%) were below the age of 3 years, three children (20%) were in the 3- to 6-year age group, five children (33.3%) were in the 6- to 12-year age group, and two children (13.3%) were in the 12- to 18-year age group. The follow-up time after diagnosis ranged from 0.6 to 4 years (median, 1.8 ± 1.11 years). The cases occurred in a seasonal distribution with 73.3% (11/15) presenting in winter or spring. Only four cases (26.7%) occurred in summer, and no cases were encountered in late fall (Table 1 ).
Table 1.
Demographic findings, preceding symptoms, and clinical findings of study patients
| Patient No. | Age (y)/Sex | History of Acute Febrile Illness | Development of ADEM After Febrile Illness (no. days) | Season | Presenting Signs and Symptoms | Specific Triggers | Neurologic Manifestation |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyramidal | Brainstem | Cerebellar | Visual | Sensory | Myelitis | |||||||
| 1 | 7/boy | + | 3 | Winter | Coma, gait disturbances | − | − | + | − | − | − | |
| 2 | 7/boy | + | 2 | Winter | Coma, fever, seizure, gait disturbances | Influenza-A (H1N1) | + | − | − | − | − | − |
| 3 | 12/girl | + | 25 | Spring | Fever, headache, vomiting, gait disturbances, irritability | − | − | − | − | + | + | |
| 4 | 2/boy | + | 4 | Winter | Stupor, seizure, gait disturbances, fever, | − | − | − | − | + | + | |
| 5 | 0.5/girl | − | − | Winter | Lethargy, cranial neuropathy, truncal ataxia | Mycoplasma | − | − | + | − | − | − |
| 6 | 3/boy | − | − | Winter | Stupor, seizure | − | − | − | − | − | − | |
| 7 | 2/boy | + | Summer | Cranial neuropathy, gait disturbances, fever, irritability | + | + | + | − | − | − | ||
| 8 | 6/girl | + | 15 | Spring | Lethargy, gait disturbances, headache | + | − | − | − | + | + | |
| 9 | 3/boy | + | 3 | Spring | Stupor, fever, gait disturbances | − | − | + | − | + | + | |
| 10 | 3,5/girl | + | 15 | Winter | Lethargy, fever, gait disturbances, vomiting | EBV | − | − | + | + | + | + |
| 11 | 16/girl | + | 30 | Summer | Headache, confusion | + | − | + | − | − | − | |
| 12 | 7/girl | + | 15 | Summer | Gait disturbances, excessive irritability | EBV | − | − | + | − | − | − |
| 13 | 1.5/boy | + | 4 | Summer | Stupor, fever, gait disturbances, | − | − | + | − | − | − | |
| 14 | 0.5/boy | + | 4 | Winter | Stupor, fever, | − | − | − | − | − | − | |
| 15 | 8/boy | + | 40 | Winter | Seizure, gait disturbances, headache, confusion | Mycoplasma | − | − | + | − | + | + |
Abbreviations:
ADEM = Acute disseminated encephalomyelitis
EBV = Epstein-Barr virus
Eighty-six percent of patients (13/15) had a history of acute febrile illness 2 to 40 days before presentation, with an average of 7 days between onset of acute febrile illness and the appearance of neurologic symptoms. Of these 13 patients, 10 (76.9%) had an upper respiratory tract infection and three (23.1%) had acute gastroenteritis. One of the patients with preceding upper respiratory tract infection also received a combined vaccine (diphtheria-tetanus-acellular, pertussis-inactivated, polio, Haemophilus influenzae B) 6 weeks before admission. No preceding illness could be identified in the two other children (13.3%). Five children had serologic evidence of specific triggers: mycoplasma (2 children); influenza-A (H1N1) (1 child), Epstein-Barr virus (2 children) (Table 1).
The most common presenting symptom was gait disturbance (12/15, 80%), followed by altered consciousness (10/15, 66.7%), fever (7/15, 46.7%), headache (4/15, 26.7%), seizures (4/15, 26.7%), meningismus (4/15, 26.7%), and vomiting (3/15, 20%). Of the 10 children presenting with altered consciousness, three exhibited lethargy, five stupor, and two were in a coma. Five of the patients had behavioral changes, three of them exhibited irritability, and two displayed confusion. Details of the clinical presentation are presented in Table 1.
The leukocytes ranged from 2000 to 25,700/mm3 (median, 10,000 ± 6873/mm3). Leukocytes were normal in 10 patients, while two had leukopenia and three had leukocytosis. Erythrocyte sedimentation rate ranged from 3 to 73 mm/hr (median, 29 ± 21.2). CSF examination showed pleocytosis (>20/μl) in five patients (33.3%) and protein elevation in four patients (>40 mg/dL, median, 25 ± 24.5, range 11-110 mg/dL). Oligoclonal bands in CSF were measured in all patients, but only one patient showed intrathecal synthesis (case 4, Table 2 ).
Table 2.
Laboratory findings
| Patient No. | Leukocytes (number/mm3) | ESR (mm/hr) | CSF Findings |
||
|---|---|---|---|---|---|
| Pleocytosis | Protein Concentration (mg/dL) | Oligoclonal Bands | |||
| 1 | 8700 | 19 | + | 25 | − |
| 2 | 2000 | 29 | − | 15 | − |
| 3 | 25,700 | 73 | − | 44 | − |
| 4 | 4500 | 19 | − | 35 | + |
| 5 | 11,200 | 53 | − | 23 | − |
| 6 | 13,800 | 15 | − | 14 | − |
| 7 | 9800 | 9 | + | 27 | − |
| 8 | 9300 | 30 | + | 24 | − |
| 9 | 14,600 | 13 | − | 17 | − |
| 10 | 23,800 | 47 | − | 16 | − |
| 11 | 7900 | 3 | − | 50 | − |
| 12 | 8000 | 46 | − | 11 | − |
| 13 | 10,800 | − | + | 110 | − |
| 14 | 10,000 | − | − | 32 | − |
| 15 | 22,300 | 55 | + | 44 | − |
Abbreviations:
ESR = Erythrocyte sedimentation rate
CSF = Cerebrospinal fluid
Electroencephalograms were recorded in eight patients, three of which showed generalized slowing (cases 1, 2, and 10), and one showed focal epileptiform discharges (case 6). The electroencephalogram was normal in four patients.
Head CT scans were acquired in 14 of 15 patients but revealed lesions in only three (cases 10, 14, and 15) (Table 3 ), while MRI revealed cerebral lesions in all 15 patients (summarized in Table 3). Figures 1 and 2 present the abnormal MRI findings in case 15 and case 10, respectively. MRI revealed lesions in the frontal (6/15, 40%), temporal (2/15, 13.3%), parietal (5/15, 33.3%), and occipital subcortical white matter (1/15, 6.7%), basal ganglia (3/15, 20%), periventricular white matter (2/15, 13.3%), thalamus (3/15, 20%), and internal capsule (1/15, 6.7 %). However, only four (26.7%) presented with cerebral lesions alone. Six patients had lesions in the brainstem (40%), six in the spinal cord (40%), and five in the cerebellum (33.3%). Seven patients exhibited gadolinium enhancement on MRI.
Table 3.
Neuroimaging results
| Patient No. | CT | MRI |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerebral Lesion |
Extra Cerebral Lesion |
Control MRI | |||||||||||
| Frontal Lobe | Temporal Lobe | Parietal Lobe | Occipital Lobe | Basal Ganglion | Periventricular White Matter | Subcortical | Thalamus | Cerebellum | Brainstem | Spinal Cord | |||
| 1 | Normal | + | − | − | − | + | − | − | − | − | − | − | CR |
| 2 | Normal | − | − | − | − | + | − | − | − | + | − | − | PR |
| 3 | Normal | + | − | + | − | − | − | + | − | − | − | + | CR |
| 4 | Normal | − | − | − | − | − | − | − | + | − | − | + | CR |
| 5 | Normal | − | − | − | − | − | − | − | + | − | + | − | CR |
| 6 | Normal | − | − | − | − | + | − | − | − | − | − | − | CR |
| 7 | Normal | + | + | + | − | − | − | + | − | + | + | + | CR |
| 8 | Normal | − | − | − | − | − | + | − | − | − | − | + | CR |
| 9 | Normal | − | − | − | − | − | − | − | − | − | + | + | CR |
| 10 | Abnormal | + | − | + | − | − | + | + | + | + | + | − | MNL |
| 11 | Normal | + | − | − | − | − | − | + | − | − | − | − | CR |
| 12 | Absent | − | − | − | − | − | − | − | − | + | + | − | CR |
| 13 | Normal | − | − | − | − | − | − | − | − | + | + | − | CR |
| 14 | Abnormal | − | − | + | + | − | − | + | − | − | − | − | CR |
| 15 | Abnormal | + | + | + | − | − | − | + | − | − | − | + | PR |
Abbreviations:
CR = Complete resolution
CT = Computerized tomography
MNL = Multiple new lesions
MRI = Magnetic resonance imaging
PR = Partial resolution
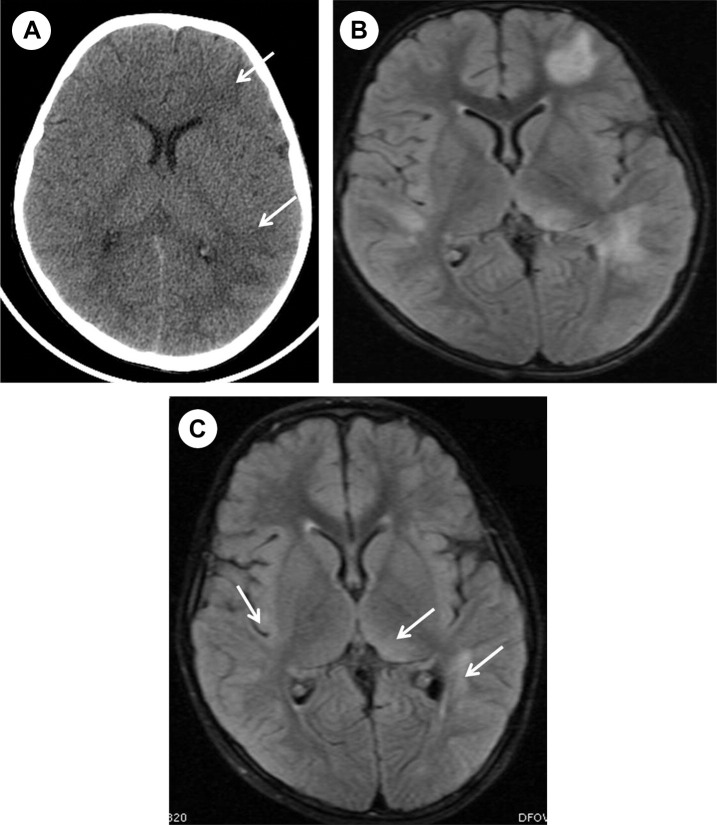
Figure 1.
(A) Computed tomography shows hypodensity in the left periventricular white matter (arrows), and (B) fluid-attenuated inversion recovery MRI sequence shows multiple hyperintense lesions in the periventricular white matter and left thalamic. (C) Follow-up brain MRI obtained 1 month later shows size decrease and no appearance of new lesions (arrows).
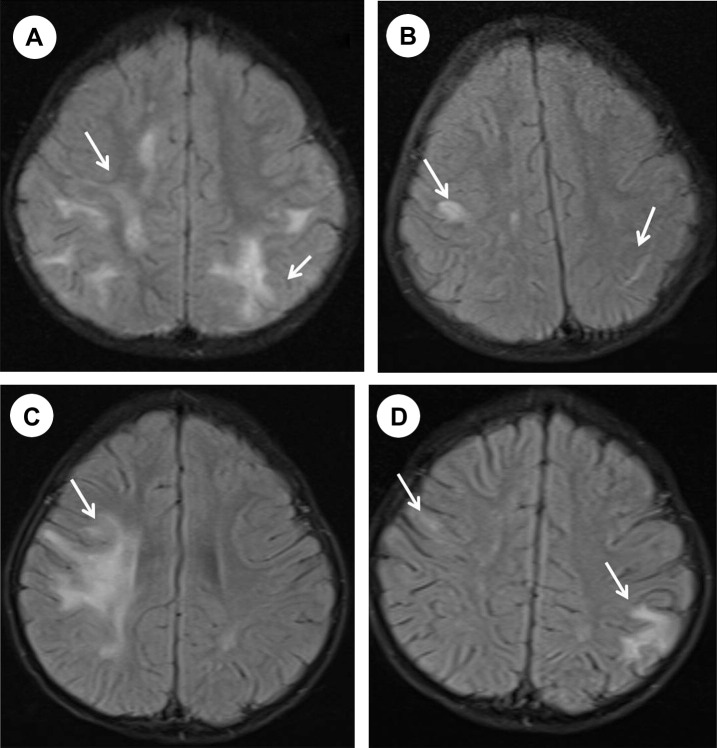
Figure 2.
(A) The first MRI scan shows multiple hyperintense lesions in the subcortical and deep white matter. (B) One year later, a follow-up MRI shows partial resolution of the lesions. (C) One month after the scan shown in part B, brain MRI shows new lesions in the right frontoparietal subcortical and deep white matter. (D) Follow-up brain MRI image obtained 6 months later shows partial resolution of the these lesions and new lesion in the left parietal subcortical white matter.
Follow-up MRI showed complete resolution in 12 patients and partial resolution in two other patients (cases 2 and 15, Table 3 and Fig 1). Case 10 had multiple new lesions in brain and spinal cord during follow-up and was eventually diagnosed with MS (Table 3, Fig 2).
Ten patients were treated with a standard protocol of 3 to 5 days of intravenous methylprednisolone (30 mg/kg) followed by oral prednisolone (1 mg/kg) for 2 weeks, tapering off within 2 weeks along with symptomatic treatment. Three patients received both the standard steroid protocol and intravenous immunoglobulin-G (IVIG) therapy. Patients showing continued deterioration were also administered IVIG (2 g/kg) divided over 2 days. One patient (case 10) was treated with the standard 5-day treatment of intravenous methylprednisolone (30 mg/kg) plus IVIG, followed by oral prednisolone (1 mg/kg) for 3 months and tapered off within the next 3 months. Due to recurrent attacks, one girl was put on daily prednisolone. Since new attacks occurred daily under prednisolone, she was also prescribed azathioprine and then daily azathioprine alone. In case 5, because clinical findings were improved spontaneously when MRI was applied, neither IVIG nor steroid treatment was given. Oseltamivir was administered in one patient with influenza-A (case 2) and clarithromycin was administered in patients with mycoplasma (cases 5 and 15).
Follow-up evaluation ranged from 0.6 to 4 years (median, 1.8 ± 1.11 years). When the study was concluded in June 2012, only one child (case 10) was still experiencing recurrent attacks. Investigations to exclude vasculitis, vasculopathy, and chronic infection were then conducted. However, serology for these disorders was negative. Visual-evoked potential recordings were also performed. The results were compatible with demyelination in visual pathways, and she had multiple new symptomatic or asymptomatic lesions on MRI and was diagnosed as possible MS. The other cases showed no clinical deterioration or recurrent neurological symptoms during follow-up, but one patient (case 2) recovered with severe neurologic sequelae 7. The one patient with positive oligoclonal bands in CSF had no new attacks during 2.6 years of follow-up.
Discussion
ADEM is more common in children than in adults 4 and most common in younger children 10. In the present case series from a single institution, age at presentation ranged from 6 months to 16 years. In this case, 13 of 15 patients (86.6%) were younger than 12 years old. ADEM is more common in boys 4, 10, and nine of 15 cases in this series (60%) were boys. Previous studies have also reported seasonal variation, with ADEM more prevalent in the winter months 4, 10. It is tempting to speculate that the fluctuation in disease occurrence could be caused by environmental factors (e.g., infectious agents). Thus, demographic and epidemiologic characteristics are consistent with most previous case series from other regions.
Seasonal variations of ADEM are usually attributed to the cyclical manifestations of various infectious diseases. In the current series, 11 of 15 (73.3%) presented in winter or spring, consistent with seasonal infections as significant triggers for ADEM. Indeed, ADEM is often preceded by a viral or bacterial infection, usually in the form of a nonspecific upper respiratory infection. In three previous studies, an antecedent infection was identified in 72-77% of ADEM patients 5, 6, 10. In this series, 13 of 15 children (86%) presenting with the clinical and radiologic features of ADEM had a preceding acute febrile illness (10 upper respiratory tract infections and three cases of acute gastroenteritis). Thirteen of 15 children (86%) with ADEM may have been caused by seasonal variation in the infectious triggers. In general, ADEM symptoms present 7-14 days after a viral infection or immunization 5, 11. In this study, an average of 7 days separated the onset of acute febrile illness and the appearance of neurologic symptoms. The epidemiology of ADEM is similar across studies and patient populations, particularly the propensity of ADEM to appear following an upper respiratory tract illness.
Numerous viral pathogens have been associated with ADEM, including measles, rubella, varicella, influenza, Epstein-Barr virus, Coxsackie virus, coronavirus, human immunodeficiency virus, herpes simplex virus, cytomegalovirus, and West Nile virus. Other organisms associated with ADEM include hemolytic streptococcus (group A), mycoplasma pneumonia, and leptospirosis 4, 5, 10, 11. In this study, serologic evidence for specific triggers was found in five children: mycoplasma in two (in winter), Epstein-Barr virus in two (one in summer and one in winter), and influenza-A (H1N1) (in winter) in one. However, the vast majority of patients had no microbiologic diagnosis despite numerous laboratory investigations. Early recognition of pathogens is important because rapid treatment for this pathogen could have some positive effect 7. Oseltamivir and clarithromycin were administered in patients with influenza-A and mycoplasma according to the serology. This study shows the benefits of antiviral and antibiotic treatment initiated as soon as possible after the onset of illness.
Fever, vomiting, and meningismus are often seen at the time of initial ADEM presentation and may persist during hospitalization 6, 12. In this study, nearly half of the patients had fever and one third had meningismus and vomiting. These children were treated initially with cefotaxime or another appropriate antibiotic and acyclovir until a diagnosis could be established. Encephalopathy is a characteristic feature of ADEM and may progress rapidly in association with multifocal neurologic deficits. The level of consciousness at presentation ranges from subtle lethargy to coma 5, 13. Encephalopathy in the form of alteration in consciousness was noted in 10 of the patients in this study and three exhibited lethargy, five exhibited stupor, and two were in a coma. Encephalopathy in the form of behavioral change was noted in five of the patients in this study, three of the patients had irritability, and two had mild confusion. Leake et al. 14 reviewed the cases of 42 children and adolescents with ADEM and noted that the majority exhibited multiple neurologic abnormalities, including lethargy, ataxia, an inability to walk, aphasia (slow, slurred, or decreased speech), cranial neuropathies, and abnormal reflexes (95% with hyperreflexia). The presenting symptoms in the current series (Table 1) were gait disturbance (12 cases), altered consciousness (10 cases), behavioral changes (five cases), fever (seven cases), headache (four cases), seizures (four cases), meningismus (four cases), and vomiting (three cases).
Analysis of CSF revealed pleocytosis or increased protein concentrations in the majority of patients with ADEM. However, the CSF can also be normal. Oligoclonal bands are seen in some patients with ADEM but are a nonspecific finding more often associated with MS 6, 10. In this study, lymphocytic pleocytosis was detected in five patients and mild CSF protein elevation in four patients (Table 2). Tenembaum et al. 8 reported that in a series of 84 children with 54 CSF samples analyzed, none showed oligoclonal bands. However, CSF-restricted oligoclonal immunoglobulin-G bands were detectable in as many as 58% of patients with ADEM, according to recent reviews 15, 16. In the present study, only one case had oligoclonal banding. Previous studies associated the presence of oligoclonal bands in CSF with a higher probability of MS 6, 17, 18, 19. However, the single patient in the current study experienced no new attacks during 2.6 years of follow-up (although the possibly of MS in the future cannot be excluded).
ADEM is classically considered an acute monophasic illness, although not all symptoms and deficits occur contemporaneously. New deficits within 3 months of onset are considered to be part of the same acute episode. Additional ADEM episodes occur rarely and happen in two main contexts: recurrent ADEM and multiphasic ADEM 2. The frequency of relapsing ADEM or ADEM progressing to MS has varied significantly among studies 14, 18, 20, 21, 22. One patient in the present study was considered to have possible MS based on clinical and radiologic recurrence. International consensus definitions for MS require two subsequent non-ADEM demyelinating events in children with an initial ADEM event 2. Banwell et al. 23 reported that 50-70% of pediatric MS cases had a polyfocal presentation and 30-50% had a monofocal presentation. Although MS is more commonly preceded by clinically isolated syndrome, there have been cases in which the initial presentation met criteria for ADEM. At what point a patient presenting with ADEM who has a subsequent event not associated with encephalopathy should be reclassified as MS remains unclear 2. On the other hand, a review of 1540 childhood cases of MS showed that the first attack was more likely to be characterized by polyfocal features and encephalopathy (ADEM-like) 23. A child with possible MS in the present study had altered mental status (lethargy) as well, so altered mental status was of no predictive value for subsequent MS in this series. Intrathecal synthesis of oligoclonal bands was detected in over 90% of children with MS 24. However, oligoclonal bands can be absent initially and are only detected later in the MS disease course 23, and the one case with possible MS in the current study did not exhibit oligoclonal bands.
MRI is a more sensitive modality for diagnosis of ADEM than CT 14, 20. Of the 14 patients who had head CT scans, only three exhibited lesions consistent with ADEM (Table 3) compared with 100% by MRI. This implies that ADEM is likely to be diagnosed with greater frequency as MRI becomes available in more regions and is used more often in pediatric medicine. The presentations of ADEM as revealed by MRI are variable. Lesions associated with ADEM are typically bilateral but may be asymmetric and tend to have poorly defined margins. Almost all patients in the current series had multiple lesions in the deep and subcortical white matter, while the periventricular white matter was generally spared. The thalamus and basal ganglia are frequently affected, and lesions in these locations are often symmetrical. Brainstem and spinal cord abnormalities on MRI are common in ADEM 8, 11, 13, 25, 26, 27, 28, 29. Only four patients featured isolated cerebral lesions, whereas the other 11 showed additional involvement of the brainstem (six cases), spinal cord (six cases), cerebellum (five cases), and thalamus (three cases). These lesion distributions, including common cerebral lesions in frontal and parietal subcortical white matter, are generally consistent with previous observations. Tenembaum et al. 8 divided ADEM MRI findings into four groups: small lesions (<5 mm), large confluent white matter lesions, symmetric bilateral lesions with thalamic involvement, and large demyelinating acute hemorrhagic encephalomyelitis. Liao et al. 30 suggest that ADEM is a broad spectrum disease and that patients showing bilateral large confluent lesions, focal demyelination lesions, or pure bilateral basal ganglia or bilateral thalamic lesions on MRI have classic ADEM and have a lower probability of progression to MS compared with patients with multiple small ovoid lesions. By this definition, all of the patients in the current study should be considered classic ADEM cases. Follow-up imaging after ADEM is typified by complete or near-complete lesion resolution and by the absence of new lesion formation. By contrast, follow-up imaging of MS usually demonstrates new, often asymptomatic, lesions 8, 10, 23, 31. Fourteen of the patients in the current study had complete or partial resolution of the lesions as evidenced by follow-up MRI. However, one patient had five new symptomatic or asymptomatic lesions (Table 3).
ADEM in children is generally managed with intravenous corticosteroids at doses of 20-30 mg/kg/day (maximum 1 g) for 3-5 days, which often results in dramatic clinical improvement 5, 32, 33. Children who fail to respond to corticosteroids may benefit from IVIG at 2 g/kg divided over 2-5 days 34. In the present study, 10 patients were treated with a standard protocol of intravenous methylprednisolone followed by oral prednisolone, while three were treated with both standard steroid and IVIG and one (case 10) with standard steroid, IVIG, and daily azathioprine.
Conclusions
The present study demonstrates that ADEM in children occurs predominantly in winter or spring and often follows an upper respiratory tract illness for those along the southern coast of Anatolia (Mediterranean region). ADEM has a wide range of neurologic presentations in children and should be considered in a child who develops multifocal neurologic abnormalities with encephalopathy following a febrile infectious illness. Early treatment with immunomodulative agents is recommended and is likely to result in a favorable outcome or even full recovery. On the other hand, this study shows the benefits of antiviral and antibiotic treatment initiated as soon as possible after the onset of illness. In 13 of 15 patients in this series, all neurologic signs and symptoms resolved after treatment. Only one patient recovered with severe neurologic sequelae and the remaining patient, who experienced recurrent attacks, was ultimately diagnosed with possible MS. It is still difficult to accurately predict progression to MS. Therefore, prolonged follow-up is required to establish a final diagnosis. Comprehensive long-term monitoring of such patients may provide additional information useful for etiologic insight and prognosis in this region.
References
- 1.Garg R.K. Acute disseminated encephalomyelitis. Postgrad Med J. 2003;79:11–17. doi: 10.1136/pmj.79.927.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krupp L.B., Banwell B., Tenembaum S., International Pediatric MS Study Group Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(16 Suppl 2):S7–S12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz S., Mohr A., Knauth M., Wildemann B., Storch-Hagenlocher B. Acute disseminated encephalomyelitis: A follow-up study of 40 adult patients. Neurology. 2001;56:1313–1318. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- 4.Murtthy S.N., Faden H.S., Cohen M.E., Bakshi R. Acute disseminated encephalomyelitis in children. Pediatrics. 2002;110(2 Pt 1):e21. doi: 10.1542/peds.110.2.e21. [DOI] [PubMed] [Google Scholar]
- 5.Tenembaum S., Chitnis T., Ness J., Hahn J.S., International Pediatric MS Study Group Acute disseminated encephalomyelitis. Neurology. 2007;68(16 Suppl 2):23–36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 6.Hynson J.L., Kornberg A.J., Coleman L.T., Shield L., Harvey A.S., Kean M.J. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;22:1308–1312. doi: 10.1212/wnl.56.10.1308. [DOI] [PubMed] [Google Scholar]
- 7.Ozkale Y., Erol I., Ozkale M., Demir S., Alehan F. Acute disseminated encephalomyelitis associated with influenza A H1N1 infection. Pediatr Neurol. 2012;47:62–64. doi: 10.1016/j.pediatrneurol.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenembaum S., Chamoles N., Fejerman N. Acute disseminated encephalomyelitis: A long term follow-up study of 84 pediatric patients. Neurology. 2002;59:1224–1231. doi: 10.1212/wnl.59.8.1224. [DOI] [PubMed] [Google Scholar]
- 9.Erol I., Alehan F., Horvath R., Schneiderat P., Talim B. Demyelinating disease of central and peripheral nervous systems associated with a A8344G mutation in tRNALys. Neuromuscul Disord. 2009;19:275–278. doi: 10.1016/j.nmd.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Dale R.C., de Sousa C., Chong W.K., Cox T.C., Harding B., Neville B.G. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123:2407–2422. doi: 10.1093/brain/123.12.2407. [DOI] [PubMed] [Google Scholar]
- 11.Stonehouse M., Gupte G., Wassmer E., Whitehouse W.P. Acute disseminated encephalomyelitis: recognition in the hands of general paediatricians. Arch Dis Child. 2003;88:122–124. doi: 10.1136/adc.88.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brass S.D., Caramanos Z., Santos C., Dilenge M.E., Lapierre Y., Rosenblatt B. Multiple sclerosis vs acute disseminated encephalomyelitis in childhood. Pediatr Neurol. 2003;29:227–231. doi: 10.1016/s0887-8994(03)00235-2. [DOI] [PubMed] [Google Scholar]
- 13.Davis L.E., Booss J. Acute disseminated encephalomyelitis in children: A changing picture. Pediatr Infect Dis J. 2003;22:829–831. doi: 10.1097/01.inf.0000087847.37363.78. [DOI] [PubMed] [Google Scholar]
- 14.Leake J.A., Albani S., Kao A.S. Acute disseminated encephalomyelitis in childhood: Epidemiologic, clinical and laboratory features. Pediatr Infect Dis J. 2004;23:756–764. doi: 10.1097/01.inf.0000133048.75452.dd. [DOI] [PubMed] [Google Scholar]
- 15.Menge T., Hemmer B., Nessler S. Acute disseminated encephalomyelitis. Arch Neurol. 2005;62:1673–1680. doi: 10.1001/archneur.62.11.1673. [DOI] [PubMed] [Google Scholar]
- 16.Wingerchuk D.M., Lucchinetti C.F. Comparative immunopathogenesis of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis. Curr Opin Neurol. 2007;20:343–350. doi: 10.1097/WCO.0b013e3280be58d8. [DOI] [PubMed] [Google Scholar]
- 17.de Seze J., Debouverie M., Zephir H. Acute fulminant demyelinating disease: A descriptive study of 60 patients. Arch Neurol. 2007;64:1426–1432. doi: 10.1001/archneur.64.10.1426. [DOI] [PubMed] [Google Scholar]
- 18.Neuteboom R.F., Boon M., Catsman Berrevoets C.E. Prognostic factors after a first attack of inflammatory CNS demyelination in children. Neurology. 2008;71:967–973. doi: 10.1212/01.wnl.0000316193.89691.e1. [DOI] [PubMed] [Google Scholar]
- 19.Atzori M., Battistella P.A., Perini P. Clinical and diagnostic aspects of multiple sclerosis and acute monophasic encephalomyelitis in pediatric patients: a single centre prospective study. Mult Scler. 2009;15:363–370. doi: 10.1177/1352458508098562. [DOI] [PubMed] [Google Scholar]
- 20.Mikaeloff Y., Adamsbaum C., Husson B. MRI prognostic factors for relapse after acute CNS inflammatory demyelination in childhood. Brain. 2004;127:1942–1947. doi: 10.1093/brain/awh218. [DOI] [PubMed] [Google Scholar]
- 21.Singhi P.D., Ray M., Singhi S., Kumar Khandelwal N. Acute disseminated encephalomyelitis in North Indian children: Clinical profile and follow-up. J Child Neurol. 2006;21:851–857. doi: 10.1177/08830738060210100201. [DOI] [PubMed] [Google Scholar]
- 22.Suppiej A., Vittorini R., Fontanin M. Acute disseminated encephalomyelitis in children: Focus on relapsing patients. Pediatr Neurol. 2008;39:12–17. doi: 10.1016/j.pediatrneurol.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Banwell B., Ghezzi A., Bar-Or A., Mikaeloff Y., Tardieu M. Multiple sclerosis in children: Clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol. 2007;6:887–902. doi: 10.1016/S1474-4422(07)70242-9. [DOI] [PubMed] [Google Scholar]
- 24.Pohl D., Rostasy K., Reiber H., Hanefeld F. CSF characteristics in early-onset multiple sclerosis. Neurology. 2004;63:1966–1967. doi: 10.1212/01.wnl.0000144352.67102.bc. [DOI] [PubMed] [Google Scholar]
- 25.Baum P.A., Barkovich A.J., Koch T.K., Berg B.O. Deep gray matter involvement in children with acute disseminated encephalomyelitis. AJNR Am J Neuroradiol. 1994;15:1275–1283. [PMC free article] [PubMed] [Google Scholar]
- 26.Kesselring J., Miller D., Robb S. Acute disseminated encephalomyelitis: MRI findings and the distinction from multiple sclerosis. Brain. 1990;113:291–302. doi: 10.1093/brain/113.2.291. [DOI] [PubMed] [Google Scholar]
- 27.Caldemeyer K.S., Smith R.R., Harris T.M., Edwards M.K. MRI in acute disseminated encephalomyelitis. Neuroradiology. 1994;36:216–220. doi: 10.1007/BF00588134. [DOI] [PubMed] [Google Scholar]
- 28.Atlas S.W., Grossman R.I., Goldberg H.I. MR diagnosis of acute disseminated encephalomyelitis. J Comput Assist Tomogr. 1986;10:798–801. doi: 10.1097/00004728-198609000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Kimura S., Nezu A., Ohtsuki N., Kobayashi T., Osaka H., Uehara S. Serial magnetic resonance imaging in children with postinfectious encephalitis. Brain Dev. 1996;18:461–465. doi: 10.1016/s0387-7604(96)00046-0. [DOI] [PubMed] [Google Scholar]
- 30.Liao M.F., Huang C.C., Lyu R.K. Acute disseminated encephalomyelitis that meets modified McDonald criteria for dissemination in space is associated with a high probability of conversion to multiple sclerosis in Taiwanese patients. Eur J Neurol. 2011;18:252–259. doi: 10.1111/j.1468-1331.2010.03114.x. [DOI] [PubMed] [Google Scholar]
- 31.Callen D.J., Shroff M.M., Branson H.M. Role of MRI in the differentiation of ADEM from MS in children. Neurology. 2009;72:968–973. doi: 10.1212/01.wnl.0000338630.20412.45. [DOI] [PubMed] [Google Scholar]
- 32.Pohl D., Waubant E., Banwell B. Treatment of pediatric multiple sclerosis and variants. Neurology. 2007;68(16 Suppl 2):54–65. doi: 10.1212/01.wnl.0000259407.40023.ab. [DOI] [PubMed] [Google Scholar]
- 33.Alper G., Schor N.F. Toward the definition of acute disseminated encephalitis of childhood. Curr Opin Pediatr. 2004;16:637–640. doi: 10.1097/01.mop.0000136120.83277.72. [DOI] [PubMed] [Google Scholar]
- 34.Hahn J.S., Siegler D.J., Enzmann D. Intravenous gammaglobulin therapy in recurrent acute disseminated encephalomyelitis. Neurology. 1996;46:1173–1174. doi: 10.1212/wnl.46.4.1173. [DOI] [PubMed] [Google Scholar]