Abstract
Transfusion-related acute lung injury (TRALI) is a life-threatening complication of acute respiratory distress occurring within 6 hours of blood transfusion. TRALI is one of the leading causes of transfusion-related fatalities and specific therapies are unavailable. Neutrophils are recognized as the major pathogenic cells, whereas T regulatory cells and dendritic cells appear to be important for protection against TRALI. The pathogenesis, however, is complex and incompletely understood. It is frequently postulated that the complement system plays an important role in the TRALI pathogenesis. In this article, we assess the evidence regarding the involvement of complement in TRALI from both human and animal studies. We hypothesize about the potential connection between the complement system and neutrophils in TRALI. Additionally, we draw parallels between TRALI and other acute pulmonary disorders of acute lung injury and acute respiratory distress syndrome regarding the involvement of complement. We conclude that, even though a role for complement in the TRALI pathogenesis seems plausible, studies investigating the role of complement in TRALI are remarkably limited in number and also present conflicting findings. Different types of TRALI animal models, diverse experimental conditions, and the composition of the gastrointestinal microbiota may perhaps all be factors which contribute to these discrepancies. More systematic studies are warranted to shed light on the contribution of the complement cascade in TRALI. The underlying clinical condition of the patient, which influences the susceptibility to TRALI, as well as the transfusion factor (antibody-mediated vs non–antibody-mediated), will be important to take into consideration when researching the contribution of complement. This should significantly increase our understanding of the role of complement in TRALI and may potentially result in promising new treatment strategies.
Keywords: Transfusion-related acute lung injury, TRALI, Complement, C5a, C5Ar, NETs
1.1. Transfusion-Related Acute Lung Injury
Transfusion-related acute lung injury (TRALI) is a syndrome of acute respiratory distress occurring within 6 hours of blood transfusion [1]. TRALI is characterized by the acute onset of noncardiogenic pulmonary edema [1]. It is one of the leading causes of transfusion-related fatalities, and specific therapies are lacking [1,2]. TRALI is particularly prevalent in critically ill patients, with incidences reported up to 15% and with a reported survival rate as low as 53% compared to 83% in acute lung injury control patients [3].
The pathogenesis of TRALI is complicated and incompletely understood [1]. Generally, a 2-hit model is considered to represent the TRALI pathophysiology [4,5]. The first hit is related to the underlying clinical condition of the patient (eg, inflammation), whereas the second hit is conveyed by factors in the transfused blood product. These second hit factors may be antibodies (around 60%-80% of all TRALI cases) such as anti–human leukocyte antigen (HLA) class I or II or anti-human neutrophil antigen (HNA) antibodies [1,6], or antibody-independent factors such as biological response modifiers (eg, lipids) [1,7]. TRALI mainly occurs when both hits are combined, although cases have been described which report the onset of TRALI in healthy subjects (eg, [8]). Animal models of TRALI have significantly contributed to our current understanding of the pathophysiology [9]. Based on autopsy reports of TRALI patients, TRALI animal models, and in vitro TRALI experiments, neutrophils (PMNs) are considered to be the key pathogenic cells in antibody and non–antibody-mediated TRALI (in the transfused recipient) [1,10]. PMNs have been suggested to exert their pathogenic effects in TRALI through direct activation, production of reactive oxygen species (ROS), or the formation of neutrophil extracellular traps (NETs) [1,10]. It was suggested, using a murine TRALI model, that anti–major histocompatibility complex (MHC) class I antibodies may bind to the pulmonary endothelium and subsequently sequester PMNs via their FcγRs, resulting in PMN activation and TRALI induction [11]. In addition, using a murine model of anti-MHC class I antibody-mediated TRALI, PMNs and ROS were found to be critically required for TRALI induction as demonstrated by in vivo PMN depletion and by using C57BL/6 gp91phox knockout mice [12]. Furthermore, platelets were shown to induce NET formation in TRALI mice, suggesting that NETs may thereby induce lung injury via direct toxicity to pulmonary endothelial cells [13]. Additionally, NETs were shown to be formed upon direct priming of PMNs by anti-HNA-3a antibodies [14]. Regarding anti-HNA-3a–mediated TRALI, PMNs were also demonstrated to interact with von Willebrand factor via CTL-2, the carrier of the HNA-3 antigen, which enabled antibody-induced signaling via CD11b/CD18, resulting in PMN activation and agglutination [15]. Next, monocytes/macrophages may exert pathogenic effects in TRALI [1,16]. In addition, red blood cells (RBCs) from the transfusion product or from the transfused recipient have been suggested to elicit pathogenic effects in TRALI rat and mouse models [1]. Interestingly, targeting recipient red blood cells with the red blood specific antibody Ter119 was shown to prevent the occurrence of TRALI in a murine model [17]. On the other hand, CD4+ T regulatory cells [12,18] and dendritic cells [12] have been shown to be the major cells protecting against TRALI, as demonstrated using a murine model of TRALI. Much more research is required to understand the specific nature of the pathogenic immune responses occurring in TRALI. The complexity in dissecting the pathophysiology of TRALI is further illustrated by controversies which have arisen, such as the involvement of recipient platelets [19,20]. One of the other controversies appears to be the involvement of the complement system in TRALI, which will be the focus of the current article.
1.2. The Complement System
The complement system forms an important part of the innate immune system. It eradicates microbial pathogens, apoptotic cells, and immune complexes while leaving healthy cells intact [21]. Activation of the complement system needs to be effectively controlled to prevent damage to the host, for which several complement regulatory proteins are present. During a blood transfusion, foreign blood cells enter the body, and in the presence of alloantibodies to antigenic polymorphisms on the recipient tissue, the complement system can be activated which can result in adverse transfusion reactions [22]. The complement system is composed of around 50 proteins which either reside in the blood or are membrane-bound. The complement system can be activated via three different routes, namely, the classical pathway (CP), the lectin pathway (LP), and the alternative pathway (AP) [21]. Several diseases are linked to unwanted over-activation of the complement system. The CP is activated via allo- and autoantibodies bound to RBCs that are formed following RBC transfusion or present in autoimmune hemolytic anemia [22,23]. In addition, mutations resulting in altered function or lack of complement regulatory proteins are often associated with overactivation of the AP as seen in atypical hemolytic uremic syndrome and paroxysmal nocturnal hemoglobinuria [24,25]. In contrast to the CP and AP, however, diseases related to the LP are relatively poorly defined. Studies have indicated that inhibition of the LP might be therapeutically relevant in ischemia reperfusion injury, although the mechanism remains poorly understood [26,27]. Furthermore, a large part of the population is deficient in Mannose binding lectin (MBL), the key player in initiation of the LP, but when the MBL pathway is activated, the effects that occur are relatively mild and predominately result in (more) severe infections in neonates [28].
Antibodies are the main drivers of the CP. Interestingly, complement was shown to be most effectively activated by IgG hexamers assembled at the cell surface [29]. In addition, the CP can also be activated via binding of C-reactive protein (CRP) to phosphocholine sites exposed on disrupted plasma membranes [[30], [31], [32]]. Binding of C1q to IgG, IgM, or CRP results in activation of C1s and C1r, which in turn cleave C4 and C2. The cleavage products, C4b and C2a, form the C3 convertase of the CP (C4b2a). The C3 convertase can cleave C3 into C3b and C3a. C3b covalently binds to the surface, whereas C3a is a chemoattractant for immune cells. Binding of C3b to the surface eventually leads to the formation of C5 convertases (C4b2aC3b) which cleave C5 and generate the potent chemoattractant C5a and the C5b which forms the basis of the membrane attack complex (MAC), which is composed of C5b-9.
Activation of the LP occurs when carbohydrate moieties on microbial pathogens are bound by MBL or ficolins. This binding results in activation of the MBL-associated serine proteases (MASPs) which in turn cleave C4 and C2, forming a C3 convertase (C4b2a) analogous to activation via the CP [21]. The AP is an important player in the immune system, as it is believed that complement activation heavily relies on the amplification loop provided by the AP [33,34]. Although the AP can be initiated spontaneously at a low rate due to turnover of C3 into C3(H2O) in plasma, its main function lies in amplifying the C3b deposition on the (cellular) surface. When Factor B (FB) binds to a C3b molecule, it is cleaved by Factor D (FD), resulting in the formation of the AP C3 convertase C3bBb. This C3 convertase can also cleave C3, and incorporation of another C3b molecule results in formation of AP C5 convertases (C3bBbC3b) which, similar to CP/LP C5 convertases, results in C5 cleavage and generation of C5a and the MAC [21].
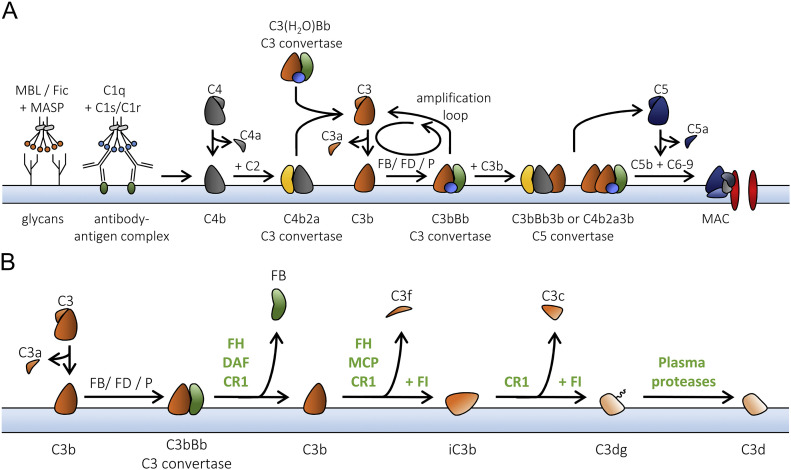
Several fluid-phase and membrane-bound complement regulators inhibit the activation of the complement system. For example, the fluid phase regulators C1 inhibitor (C1-INH) and C4b binding protein (C4bp) inhibit activation via the CP and LP. On the other hand, the fluid-phase regulators Factor H (FH) and FH-like 1 (FHL-1) inhibit the AP [35]. In addition, the more recently discovered FH-related proteins (FHRs) are believed to fine tune the regulatory capacity of FH; however, in vivo evidence is lacking, and in vitro evidence is often contradicting [36]. Most cells express several membrane-bound regulators like complement receptor (CR) 1, CD46 (also known as membrane cofactor protein [MCP]), CD55 (also known as decay-accelerating factor (DAF), and CD59. Finally, the fluid phase regulators vitronectin and clusterin are able to prevent formation of the MAC, whereas Factor I (FI) is a glycoprotein that downregulates the complement system by cleavage of deposited C4b and C3b into inactive forms C3d and C4d in the presence of co-factors such as FH, C4bp, CR1, and MCP [35]. The complement system is schematically summarized in Figure 1A.
Fig 1.
A, The complement system. The complement system is initiated by the binding of recognition molecules from the lectin or the classical pathway (LP and CP). In the LP, MBL or ficolins (Fic) are complexed with MASP proteins and recognize glycan moieties on a pathogenic surface. The C1 complex, composed of C1q and 2 molecules of C1r and C1s each, recognizes antibody-antigen complexes. After activation of the MASP proteins or C1r/C1s, C4 is cleaved by C1s into C4b, which deposits on the surface via its thioester. The subsequent cleavage of C2 by C1s allows for the formation of the C4b2a C3 convertase—able to cleave C3 into C3b. Via the AP, a C3 convertase, can also form in the fluid phase, composed of C3(H2O) and Bb (the large product upon FB cleavage by FD), or as C3bBb deposited on the surface. Both these AP convertases are stabilized with properdin (P). The alternative pathway functions as an amplification loop, increasing the deposition of C3b. When the number of deposited C3b molecules increases, C5 becomes the preferred substrate for the convertases, initiating the terminal pathway. C5b, together with C6, C7, C8, and several molecules of C9, forms a lytic pore known as the membrane-attack complex (MAC).
B, The breakdown of deposited C3b. C3b that is attached to a surface can be regulated via multiple mechanisms. FH, DAF (CD55), and complement receptor 1 (CR1) are able to compete with FB to prevent formation or accelerate the decay of the AP C3 convertase. In addition, FH, MCP (CD46), and CR1 function as cofactors for the serine protease FI to cleave C3b into its inactive form, iC3b, releasing the C3f fragment. CR1 also aids FI to cleave iC3b into C3dg. Other plasma proteases will cleave off the last C3g fragment, leaving the last antigenic fragment, C3d, attached to the surface.
As TRALI is frequently triggered by antibodies present in the transfusion product [1,6] and CRP also plays an important pathogenic role in TRALI [37] and is increased in TRALI patients [38], the involvement of complement, and specifically the CP, in TRALI appears to be plausible.
2. Evidence for the Involvement of Complement in TRALI
Although it has been suggested that the complement system plays a role in TRALI, the number of studies directly investigating this appears to be surprisingly limited. Regarding human studies, Ambruso et al reported (published abstract only) the activation of complement in 2 anti-HLA class II-mediated TRALI patient samples, which was absent in pretransfusion samples [39]. Interestingly, the authors found complement to be activated in the product transfused during the onset of TRALI symptoms, suggesting that the activated complement components in the transfusion product played a role in the TRALI reaction. In a prospective, randomized, double-blind, crossover study, Palfi et al looked into the C3d concentrations in pre- and posttransfusion plasma samples of intensive care patients receiving plasma from multiparous donors compared to those receiving plasma from nontransfused nulliparous women [40]. C3d results from the breakdown of deposited C3b (Fig 1B). It was concluded that plasma from multiparous blood donors may impair pulmonary function in intensive care unit patients, but the authors did not find any differences in C3d levels. Notably, however, only 5 posttransfusion reactions occurred (in 4 cases after the transfusion of plasma from multiparous donors) in 100 patients, of which there were 1 typical TRALI reaction and 4 mild cases of TRALI. Lucas et al found complement fixing anti-HNA-1a IgM antibodies in the serum of 1 female donor (out of the 3 donors of the pooled platelet concentrate) that may have triggered TRALI due to interdonor incompatibility for HNA-1a [41]. Dry et al performed an autopsy of a TRALI patient and did not observe the presence of macrophages in the alveolar air spaces [42], which has been described to occur 24-48 hours after infusion of activated complement in rabbit lungs [43]. This lack of intra-alveolar macrophages, however, may not yet have occurred because the TRALI patient died 2 hours after initial clinical signs of pulmonary edema.
Studies using animal models of TRALI have also assessed a contribution of (components of) the complement system. Müller and colleagues used a murine model of TRALI based on lipopolysaccharide (LPS) priming and infusion of an anti-MHC class I antibody [44]. This resulted in increased levels of C3a and C5a in bronchoalveolar lavage fluid (BALF). C1-INH attenuated the pulmonary levels of C3a associated with improved lung injury scores; however, there was no effect on C5a levels in the BALF. In addition, despite the administration of the C1-INH, high levels of pulmonary and systemic inflammatory cytokines persisted, including macrophage inflammatory protein (MIP)-2, the murine homolog of IL-8. Furthermore, Strait and colleagues found an important role for C5a in murine TRALI using the anti-MHC class I antibody (34-1-2s) in BALB/c mice [45]. Mice which were deficient in C3, C5, or C5a receptor (C5aR) were protected from TRALI. The requirement for C5a for TRALI induction was suggested to be related to the fact that only adult male mice were susceptible to 34-1-2s–mediated TRALI, whereas adult female mice were apparently resistant [45]. Female mice have only approximately 25% as much plasma C5 as males, and the subtype of C5 in male mice is not present in females, which may at least in part explain these sex-specific differences in TRALI susceptibility [46]. This was further supported by data demonstrating that infusion of female mice with plasma from male WT or male C3-deficient mice enabled TRALI, whereas infusion with plasma from C5-deficient males failed to induce TRALI [45]. Looney and coworkers, on the other hand, described the occurrence of TRALI in C5aR-deficient BALB/c mice 2 hours after TRALI induction with 34-1-2s injection, suggesting C5a to be dispensable in murine TRALI [11]. Differences between the study of Strait et al [45] and Looney et al [11] may be explained by the timing of experimental endpoints after 34-1-2s injection, which was 30 minutes in the study by Strait et al [45], whereas this was 2 hours in the study of Looney et al [11]. Additionally, the observed differences may be due to the nature of the animal housing which may affect the composition of the gastrointestinal microbiota and thereby the susceptibility to TRALI [19,47]. Furthermore, in a study by Sachs et al using an ex vivo rat model of anti-CD177–mediated TRALI, complement was not found to be required for TRALI induction (TRALI induction was more dependent on the density of the cognate antigen), as TRALI occurred in a complement-free environment [48]. In an earlier ex vivo TRALI study by Seeger and colleagues using rabbit lungs, rabbit plasma was suggested to have served as a source of complement and induced TRALI together with anti-5b (anti-HNA-3a) antibodies and PMNs [49]. In this study, however, there was no direct investigation regarding the contribution of complement.
All the studies mentioned above, which provide evidence supporting or against the involvement of complement in TRALI, are summarized in Table 1 .
Table 1.
Evidence supporting or against the involvement of complement in TRALI
| Study | Animal/human | Evidence supporting complement involvement in TRALI | Evidence against complement involvement in TRALI |
|---|---|---|---|
| Ambruso et al, Blood 2006 (abstract) [39] | Human | Complement activation was observed in 2 patient samples during the anti-HLA class II–mediated TRALI reaction as opposed to those patient samples collected before the transfusion. Complement was activated in the product transfused during the onset of TRALI symptoms. | |
| Palfi et al, Transfusion 2001 [40] | Human | No difference in C3d concentrations in pre- and posttransfusion plasma samples of intensive care patients receiving plasma from multiparous donors compared to those receiving plasma from donors with no history of transfusion or pregnancy in a prospective, randomized, double-blind, crossover study in which 5 posttransfusion reactions occurred. | |
| Lucas et al, Vox Sang 2000 [41] | Human | Complement fixing anti-HNA-1a IgM antibodies in sera of 1 female donor (out of the 3 donors of the pooled platelet concentrate) initiated a TRALI reaction due to interdonor incompatibility for HNA-1a. | |
| Dry et al, Am J Clin Pathol 1999 [42] | Human | Autopsy of TRALI patient did not demonstrate the presence of macrophages within alveolar air spaces as has been described to occur 24 to 48 h after activated complement infusion in rabbit lungs [42]. | |
| Müller, Vox Sang 2014 [44] | Mouse | Increased C3a and C5a in BALF upon LPS priming and anti-MHC class I antibody-mediated TRALI induction in mice. C1-inhibitor attenuated pulmonary levels of C3a associated with improved lung injury scores. | No effect on C5a levels in BALF plus persistence of high levels of pulmonary and systemic inflammatory cytokines (including MIP-2) with C1-inhibitor (C1-INH). |
| Strait et al, J Exp Med 2011 [45] | Mouse | C5a in particular was found to be important in inducing anti-MHC class I antibody (34-1-2s)–mediated murine TRALI. Mice deficient in C3, C5, or C5a receptor (C5aR) did not develop any TRALI 30 min after TRALI induction. | |
| Looney et al, J Clin Invest 2006 [11] | Mouse | Occurrence of TRALI in C5aR-deficient BALB/c mice 2 h after 34-1-2s injection. | |
| Sachs et al, Blood 2006 [48] | Rat | Complement was not found to be required for TRALI induction in an ex vivo rat lung model, where TRALI induction by CD177-specific antibodies was found to occur in a complement-free environment. | |
| Seeger et al, Blood 1990 [49] | Rabbit | Rabbit plasma may have served as a source of complement and induced severe lung edema and increased lung vascular permeability in an ex vivo rabbit lung model together with anti-5b (anti-HNA-3a) antibodies and PMNs. |
3. The Potential Connection Between the Complement System and PMNs in Immune Disorders
PMNs are the key pathogenic cells in TRALI, exerting their effects through direct activation, ROS production, or release of NETs [1,10]. Interestingly, the innate immune system is able to activate PMNs via different effector molecules. IgG bound to its target is recognized by PMNs via their Fcγ receptors (FcγRs) [50,51]. C3b and its degradation products can be recognized by several CRs, and C3a and C5a, which are generated during complement activation, are potent chemoattractants [21,52]. Recognition of these substrates results in PMN activation, ROS production, and NET formation [50,51,53].
Immune complexes are capable of binding to activating and inhibitory FcγRs which are expressed by innate immune effector cells including PMNs [54]. The ROS response mediated by IgG is influenced by the combination of FcγRIIa and FcγRIIIb isoforms expressed on PMNs [55]. PMNs that are activated by IgG elicit a robust ROS response [56]. It has been shown that blocking of PMN-FcγRIIIb is sufficient to inhibit immune complex– mediated ROS production in a setting of autoimmune arthritis [57]. Next to activation via immune complexes, ROS production in PMNs can also occur after activation with C5a [21,58]. Furthermore, the importance of blocking C5a and the C5aR in complement-mediated PMN activation and ROS production is also shown in an in vivo anti-neutrophil cytoplasmic autoantibody model of glomerulonephritis, which suggests that the C5a receptor mediates PMN activation and ROS formation in anti-neutrophil cytoplasmic autoantibody–induced glomerulonephritis [59]. Importantly, it has been shown that production of ROS is of high importance to induce NETs [60].
Several autoimmune diseases are characterized by the presence of immune complexes, and in vivo and in vitro studies have shown that immune complexes can induce the release of NETs via binding to FcγRs [61,62]. In addition, these immune complexes can activate the complement system via the CP. The importance of C3 in NET formation was first shown in mice where it was found that PMNs of C3-deficient mice did not form NETs [63]. Also, Guglietta et al reported C3aR-dependent NET formation in a setting of small intestinal tumorigenesis [64]. In that study, circulating LPS was found to upregulate C3aRs on PMNs resulting in C3aR-dependent NET formation, induction of coagulation, and stimulation of protumorigenic neutrophilia. In addition, it was shown that pathogens that were opsonized with both IgG and C3b induced more NET formation than bacteria opsonized with IgG alone [65]. Furthermore, it is not only C3 that is involved in the release of NETs because NET induction by antibodies and immune complexes is greatly enhanced when PMNs are first primed by C5a [66,67].
In addition, studies have shown the link between activation of FcγRs and the C5aR [51,68]. For instance, using the K/BxN mouse serum transfer-induced arthritis mouse model expressing human FcγRIIa on PMNs but lacking their own activating FcγR (γ-chain–deficient mice), it was shown that cross talk between PMN-FcγRIIa and PMN-C5aR promoted inflammatory arthritis in mice [68].
Taken together, the complement system is a potent system to activate PMNs, and induce ROS production and NET formation, all processes also described to occur in TRALI [1,10].
4. Complement Involvement in Acute Lung Injury and ARDS
In further assessment of the potential contribution of complement in TRALI, parallels may be drawn with other forms of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS).
Activation of the complement system plays an important role in ALI caused by highly pathogenic viruses such as influenza virus A (H5N1 and H7N9) and severe acute respiratory syndrome [69]. It has been shown that infection of the human host with highly pathogenic viruses such as influenza A results in complement activation and formation of C5a [69,70]. In addition, BALF and serum of mice and humans infected with fatal H1N1 virus contained increased levels of C5a [71,72]. Furthermore, it has been shown that ALI induced by H5N1 played a relevant role by excessive complement activation including C5a generation [73]. Several murine in vivo studies using H1N1 and H5N1 as ALI inducers have shown beneficial effects of blocking C5a or C5aR in ALI [69,[72], [73], [74], [75]]. In addition, C5a can also directly impact vascular permeability by activating endothelial cells [22], which are also activated in TRALI [76]. Russkamp et al observed alveolar inflammation with increased recruitment of Ly6-G(+)CD11b(+) leukocytes to the alveolar air spaces with severe alveolar-capillary barrier dysfunction upon intratracheal administration of C5a in mice [74]. The authors demonstrated a role for the CC-chemokine receptor 5 in their model of C5a-mediated ALI. Furthermore, as discussed above, C5a plays an important role in ROS generation and formation of NETs. Similar to TRALI, ROS and NETs have also been shown to play a role in ALI [69,[77], [78], [79]]. Notably, it was suggested that intravascular activation of PMNs by C5a may be related to the generation of ROS, resulting in the onset of ALI [78]. These studies underline the importance of C5a in mediating ALI and may support further investigations into the role of C5a in TRALI.
ARDS is a serious and frequent complication of multiple medical and surgical interventions, with pneumonia, sepsis, and aspiration of gastric contents being common risk factors, and like TRALI, PMNs appear to play a strong role in the pathogenesis of ARDS [10]. ARDS occurs within 1 week of a known clinical insult or presents with new/worsening respiratory symptoms in case of an unknown clinical event. The role of the complement system in ARDS is not extensively studied. C5a was not detectable in 38 polytrauma patients at risk to develop ARDS, but monitoring of C3a and C3 in plasma was suggested to identify polytrauma patients at high risk for ARDS at an early stage of the disease [80]. In contrast, a different study of 61 patients at risk for ARDS development, due to a major nonthoracic trauma or fungemia, gram-negative bacteremia, or hypotension lasting more than 2 hours, found C5a to be a useful predictor for ARDS [81]. Yet, another study by Schein and colleagues investigated 59 patients in septic shock and found no indicative effect of complement activation whatsoever for the development of ARDS [82]. The discrepancy between these studies may be related to the different ARDS etiologies. It appears that the complement system may perhaps play a role in ARDS, but the heterogeneous etiologies of ARDS add to the complexity and should be carefully considered when studying the exact role of the different complement proteins in various types of ARDS. This may also be true for assessing the role of complement in TRALI, as the underlying clinical condition of the patient also influences the susceptibility to TRALI. Additionally, the transfusion factor (antibody-mediated vs non–antibody-mediated) should be taken into account.
5. Conclusions
It is frequently assumed that the complement system is part of the TRALI pathogenesis, which hypothetically seems plausible as outlined in this article. Studies systematically investigating the role of complement in TRALI, however, appear to be surprisingly limited in number. Moreover, the reported studies demonstrate conflicting data regarding complement involvement in TRALI. This apparent controversy may at least in part be explained by the different types of TRALI animal models and the varying experimental conditions. This may possibly include the manufacturing process used to generate the 34-1-2s antibody, which may have differed between studies, and this may consequently have differentially impacted the antibody-Fc glycosylation composition and thereby the interaction with Fc receptors or complement. Additionally, the controversy may possibly be due to the composition of the gastrointestinal microbiota [47], which can be influenced by changes in environmental animal housing conditions (specific pathogen-free vs barrier-free housing) [19,47]. Barrier-free mice were shown to be hypersusceptible to TRALI, whereas specific pathogen-free mice were resistant to TRALI (unless primed with LPS), and fecal transfer from barrier-free mice to specific pathogen-free mice could restore the susceptibility to TRALI [47], indicating a role for the gastrointestinal microbiota in TRALI. Overall, this reveals a need for systematic in-depth investigations into the potential contribution of various complement cascade components in inducing TRALI. This can be performed using both TRALI animal models (including the use of complement component knockout mice and the testing of available specific complement cascade inhibitors) as well as TRALI patient samples. The underlying clinical condition of the patient, which influences the susceptibility to TRALI, as well as the transfusion factor (antibodies vs biological response modifiers) should be taken into account (the latter has not been investigated). This will shed light on the relevance of the complement system in TRALI which may open up new therapeutic avenues to explore in combatting TRALI; for example, eculizumab, an anti-C5 blocking antibody or anti-FH.07 antibody which targets FH, or C1-INH.
Acknowledgments
Acknowledgments
This work was supported by Sanquin (grant PPOC-18-08).
Declaration of Competing Interest
None.
References
- 1.Semple JW, Rebetz J, Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood. 2019;133:1840–1853. doi: 10.1182/blood-2018-10-860809. [DOI] [PubMed] [Google Scholar]
- 2.Semple JW, McVey MJ, Kim M, Rebetz J, Kuebler WM, Kapur R. Targeting transfusion-related acute lung injury: the journey from basic science to novel therapies. Crit Care Med. 2018;46:e452–e458. doi: 10.1097/CCM.0000000000002989. [DOI] [PubMed] [Google Scholar]
- 3.Vlaar AP, Binnekade JM, Prins D, Van Stein D, Hofstra JJ, Schultz MJ, et al. Risk factors and outcome of transfusion-related acute lung injury in the critically ill: a nested case-control study. Crit Care Med. 2010;38:771–778. doi: 10.1097/CCM.0b013e3181cc4d4b. [DOI] [PubMed] [Google Scholar]
- 4.Silliman CC, Paterson AJ, Dickey WO, Stroneck DF, Popovsky MA, Caldwell SA, et al. The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion. 1997;37:719–726. doi: 10.1046/j.1537-2995.1997.37797369448.x. [DOI] [PubMed] [Google Scholar]
- 5.Silliman CC. The two-event model of transfusion-related acute lung injury. Crit Care Med. 2006;34:S124–S131. doi: 10.1097/01.CCM.0000214292.62276.8E. [DOI] [PubMed] [Google Scholar]
- 6.Peters AL, Van SD, Vlaar AP. Antibody-mediated transfusion-related acute lung injury; from discovery to prevention. Br J Haematol. 2015;170:597–614. doi: 10.1111/bjh.13459. [DOI] [PubMed] [Google Scholar]
- 7.Peters AL, van Hezel ME, Juffermans NP, Vlaar AP. Pathogenesis of non-antibody mediated transfusion-related acute lung injury from bench to bedside. Blood Rev. 2015;29:51–61. doi: 10.1016/j.blre.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Dooren MC, Ouwehand WH, Verhoeven AJ, von dem Borne AE, Kuijpers RW. Adult respiratory distress syndrome after experimental intravenous gamma-globulin concentrate and monocyte-reactive IgG antibodies. Lancet. 1998;352:1601–1602. doi: 10.1016/s0140-6736(05)61049-5. [DOI] [PubMed] [Google Scholar]
- 9.Fung,Y.L and Tung, J.P. How different animal models help us understand TRALI. ISBT Sci Ser 2018;13:197-205.
- 10.Rebetz J, Semple JW, Kapur R. The pathogenic involvement of neutrophils in acute respiratory distress syndrome and transfusion-related acute lung injury. Transfus Med Hemother. 2018;45:290–298. doi: 10.1159/000492950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. J Clin Invest. 2006;116:1615–1623. doi: 10.1172/JCI27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapur R, Kim M, Aslam R, McVey MJ, Tabuchi A, Luo A, et al. T regulatory cells and dendritic cells protect against transfusion-related acute lung injury via IL-10. Blood. 2017;129:2557–2569. doi: 10.1182/blood-2016-12-758185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–6343. doi: 10.1182/blood-2012-01-405183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayat B, Tjahjono Y, Berghofer H, Werth S, Deckmyn H, De Meyer SF, et al. Choline transporter-like protein-2: new von Willebrand Factor-binding partner involved in antibody-mediated neutrophil activation and transfusion-related acute lung injury. Arterioscler Thromb Vasc Biol. 2015;35:1616–1622. doi: 10.1161/ATVBAHA.115.305259. [DOI] [PubMed] [Google Scholar]
- 16.Kapur R, Kasetty G, Rebetz J, Egesten A, Semple JW. Osteopontin mediates murine transfusion-related acute lung injury via stimulation of pulmonary neutrophil accumulation. Blood. 2019;134(1):74–84. doi: 10.1182/blood.2019000972. [DOI] [PubMed] [Google Scholar]
- 17.Crow AR, Kapur R, Koernig S, Campbell IK, Jen CC, Mott PJ, et al. Treating murine inflammatory diseases with an anti-erythrocyte antibody. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau8217. [DOI] [PubMed] [Google Scholar]
- 18.He R, Li L, Kong Y, Tian L, Tian X, Fang P, et al. Preventing murine transfusion-related acute lung injury by expansion of CD4(+) CD25(+) FoxP3(+) Tregs using IL-2/anti-IL-2 complexes. Transfusion. 2019;59:534–544. doi: 10.1111/trf.15064. [DOI] [PubMed] [Google Scholar]
- 19.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119:3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hechler B, Maitre B, Magnenat S, Heim V, El Mdawar MB, Gachet C, et al. Platelets are dispensable for antibody-mediated transfusion-related acute lung injury in the mouse. J Thromb Haemost. 2016;14:1255–1267. doi: 10.1111/jth.13335. [DOI] [PubMed] [Google Scholar]
- 21.Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part I—molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stowell SR, Winkler AM, Maier CL, Arthur CM, Smith NH, Girard-Pierce KR, et al. Initiation and regulation of complement during hemolytic transfusion reactions. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/307093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berentsen S. Role of complement in autoimmune hemolytic anemia. Transfus Med Hemother. 2015;42:303–310. doi: 10.1159/000438964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parente R, Clark SJ, Inforzato A, Day AJ. Complement factor H in host defense and immune evasion. Cell Mol Life Sci. 2017;74:1605–1624. doi: 10.1007/s00018-016-2418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeZern AE, Brodsky RA. Paroxysmal nocturnal hemoglobinuria: a complement-mediated hemolytic anemia. Hematol Oncol Clin North Am. 2015;29:479–494. doi: 10.1016/j.hoc.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2011;108:7523–7528. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobo J, Kocsis A, Gal P. Be on target: strategies of targeting alternative and lectin pathway components in complement-mediated diseases. Front Immunol. 2018;9:1851. doi: 10.3389/fimmu.2018.01851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auriti C, Prencipe G, Moriondo M, Bersani I, Bertaina C, Mondi V, et al. Mannose-binding lectin: biologic characteristics and role in the susceptibility to infections and ischemia-reperfusion related injury in critically ill neonates. J Immunol Res. 2017;2017 doi: 10.1155/2017/7045630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343:1260–1263. doi: 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berman S, Gewurz H, Mold C. Binding of C-reactive protein to nucleated cells leads to complement activation without cytolysis. J Immunol. 1986;136:1354–1359. [PubMed] [Google Scholar]
- 31.Biro A, Rovo Z, Papp D, Cervenak L, Varga L, Fust G, et al. Studies on the interactions between C-reactive protein and complement proteins. Immunology. 2007;121:40–50. doi: 10.1111/j.1365-2567.2007.02535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braig D, Nero TL, Koch HG, Kaiser B, Wang X, Thiele JR, et al. Transitional changes in the CRP structure lead to the exposure of proinflammatory binding sites. Nat Commun. 2017;8 doi: 10.1038/ncomms14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harboe M, Garred P, Karlstrom E, Lindstad JK, Stahl GL, Mollnes TE. The down-stream effects of mannan-induced lectin complement pathway activation depend quantitatively on alternative pathway amplification. Mol Immunol. 2009;47:373–380. doi: 10.1016/j.molimm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004;138:439–446. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt CQ, Lambris JD, Ricklin D. Protection of host cells by complement regulators. Immunol Rev. 2016;274:152–171. doi: 10.1111/imr.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez-Corral P, Pouw RB, Lopez-Trascasa M, Jozsi M. Self-damage caused by dysregulation of the complement alternative pathway: relevance of the factor H protein family. Front Immunol. 2018;9:1607. doi: 10.3389/fimmu.2018.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapur R, Kim M, Shanmugabhavananthan S, Liu J, Li Y, Semple JW. C-reactive protein enhances murine antibody-mediated transfusion-related acute lung injury. Blood. 2015;126:2747–2751. doi: 10.1182/blood-2015-09-672592. [DOI] [PubMed] [Google Scholar]
- 38.Kapur R, Kim M, Rondina MT, Porcelijn L, Semple JW. Elevation of C-reactive protein levels in patients with transfusion-related acute lung injury. Oncotarget. 2016;7:78048–78054. doi: 10.18632/oncotarget.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ambruso DR, Silliman CC, Kelher M, Thurman G, Giclas P. Complement activation in transfusion related acute lung injury (TRALI) Blood (Abstract) 2006;954 [Google Scholar]
- 40.Palfi M, Berg S, Ernerudh J, Berlin G. A randomized controlled trial oftransfusion-related acute lung injury: is plasma from multiparous blood donors dangerous? Transfusion. 2001;41:317–322. doi: 10.1046/j.1537-2995.2001.41030317.x. [DOI] [PubMed] [Google Scholar]
- 41.Lucas G, Rogers S, Evans R, Hambley H, Win N. Transfusion-related acute lung injury associated with interdonor incompatibility for the neutrophil-specific antigen HNA-1a. Vox Sang. 2000;79:112–115. doi: 10.1159/000031222. [DOI] [PubMed] [Google Scholar]
- 42.Dry SM, Bechard KM, Milford EL, Churchill WH, Benjamin RJ. The pathology of transfusion-related acute lung injury. Am J Clin Pathol. 1999;112:216–221. doi: 10.1093/ajcp/112.2.216. [DOI] [PubMed] [Google Scholar]
- 43.Larsen GL, McCarthy K, Webster RO, Henson J. Henson PM. A differential effect of C5a and C5a des Arg in the induction of pulmonary inflammation. Am J Pathol. 1980;100:179–192. [PMC free article] [PubMed] [Google Scholar]
- 44.Muller MC, Stroo I, Wouters D, Zeerleder SS, Roelofs JJ, Boon L, et al. The effect of C1-inhibitor in a murine model of transfusion-related acute lung injury. Vox Sang. 2014;107:71–75. doi: 10.1111/vox.12128. [DOI] [PubMed] [Google Scholar]
- 45.Strait RT, Hicks W, Barasa N, Mahler A, Khodoun M, Köhl J, et al. MHC class I-specific antibody binding to nonhematopoietic cells drives complement activation to induce transfusion-related acute lung injury in mice. J Exp Med. 2011;208:2525–2544. doi: 10.1084/jem.20110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baba A, Fujita T, Tamura N. Sexual dimorphism of the fifth component of mouse complement. J Exp Med. 1984;160:411–419. doi: 10.1084/jem.160.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapur R, Kim M, Rebetz J, Hallström B, Björkman JT, Takabe-French A, et al. Gastrointestinal microbiota contributes to the development of murine transfusion-related acute lung injury. Blood Adv. 2018;2:1651–1663. doi: 10.1182/bloodadvances.2018018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sachs UJ, Hattar K, Weissmann N, Bohle RM, Weiss T, Sibelius U, et al. Antibody-induced neutrophil activation as a trigger for transfusion-related acute lung injury in an ex vivo rat lung model. Blood. 2006;107:1217–1219. doi: 10.1182/blood-2005-04-1744. [DOI] [PubMed] [Google Scholar]
- 49.Seeger W, Schneider U, Kreusler B, von Witzleben E, Walmrath D, Grimminger F, et al. Reproduction of transfusion-related acute lung injury in an ex vivo lung model. Blood 1990;76:1438-1444. [PubMed]
- 50.Akerley WL, III, Guyre PM, Davis BH. Neutrophil activation through high-affinity Fc gamma receptor using a monomeric antibody with unique properties. Blood. 1991;77:607–615. [PubMed] [Google Scholar]
- 51.Karsten CM, Kohl J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology. 2012;217:1067–1079. doi: 10.1016/j.imbio.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 52.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 53.Aleman OR, Mora N, Cortes-Vieyra R, Uribe-Querol E, Rosales C. Differential use of human neutrophil Fcgamma receptors for inducing neutrophil extracellular trap formation. J Immunol Res. 2016;2016 doi: 10.1155/2016/2908034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 55.van der Heijden J, Nagelkerke S, Zhao X, Geissler J, Rispens T, van den Berg TK, et al. Haplotypes of FcgammaRIIa and FcgammaRIIIb polymorphic variants influence IgG-mediated responses in neutrophils. J Immunol. 2014;192:2715–2721. doi: 10.4049/jimmunol.1203570. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol. 2017;7:373. doi: 10.3389/fcimb.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jakus Z, Simon E, Frommhold D, Sperandio M, Mocsai A. Critical role of phospholipase Cgamma2 in integrin and Fc receptor-mediated neutrophil functions and the effector phase of autoimmune arthritis. J Exp Med. 2009;206:577–593. doi: 10.1084/jem.20081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khameneh HJ, Ho AW, Laudisi F, Derks H, Kandasamy M, Sivasankar B, et al. C5a regulates IL-1beta production and leukocyte recruitment in a murine model of monosodium urate crystal-induced peritonitis. Front Pharmacol. 2017;8:10. doi: 10.3389/fphar.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20:289–298. doi: 10.1681/ASN.2008050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stoiber W, Obermayer A, Steinbacher P, Krautgartner WD. The role of reactive oxygen species (ROS) in the formation of extracellular traps (ETs) in humans. Biomolecules. 2015;5:702–723. doi: 10.3390/biom5020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen K, Nishi H, Travers R, Tsuboi N, Martinod K, Wagner DD, et al. Endocytosis of soluble immune complexes leads to their clearance by FcgammaRIIIB but induces neutrophil extracellular traps via FcgammaRIIA in vivo. Blood. 2012;120:4421–4431. doi: 10.1182/blood-2011-12-401133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aleyd E, Al M, Tuk CW, van der Laken CJ. van EM. IgA Complexes in plasma and synovial fluid of patients with rheumatoid arthritis induce neutrophil extracellular traps via FcalphaRI. J Immunol. 2016;197:4552–4559. doi: 10.4049/jimmunol.1502353. [DOI] [PubMed] [Google Scholar]
- 63.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guglietta S, Chiavelli A, Zagato E, Krieg C, Gandini S, Ravenda PS, et al. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat Commun. 2016;7 doi: 10.1038/ncomms11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palmer LJ, Damgaard C, Holmstrup P, Nielsen CH. Influence of complement on neutrophil extracellular trap release induced by bacteria. J Periodontal Res. 2016;51:70–76. doi: 10.1111/jre.12284. [DOI] [PubMed] [Google Scholar]
- 66.Behnen M, Leschczyk C, Moller S, Batel T, Klinger M, Solbach W, et al. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcgammaRIIIB and Mac-1. J Immunol. 2014;193:1954–1965. doi: 10.4049/jimmunol.1400478. [DOI] [PubMed] [Google Scholar]
- 67.Wang H, Wang C, Zhao MH, Chen M. Neutrophil extracellular traps can activate alternative complement pathways. Clin Exp Immunol. 2015;181:518–527. doi: 10.1111/cei.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsuboi N, Ernandez T, Li X, Nishi H, Cullere X, Mekala D, et al. Regulation of human neutrophil Fcgamma receptor IIa by C5a receptor promotes inflammatory arthritis in mice. Arthritis Rheum. 2011;63:467–478. doi: 10.1002/art.30141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang R, Xiao H, Guo R, Li Y, Shen B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect. 2015;4:e28. doi: 10.1038/emi.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, et al. H5N1 virus attachment to lower respiratory tract. Science. 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 71.Ohta R, Torii Y, Imai M, Kimura H, Okada N, Ito Y. Serum concentrations of complement anaphylatoxins and proinflammatory mediators in patients with 2009 H1N1 influenza. Microbiol Immunol. 2011;55:191–198. doi: 10.1111/j.1348-0421.2011.00309.x. [DOI] [PubMed] [Google Scholar]
- 72.Garcia CC, Weston-Davies W, Russo RC, Tavares LP, Rachid MA, Alves-Filho JC, et al. Complement C5 activation during influenza A infection in mice contributes to neutrophil recruitment and lung injury. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun S, Zhao G, Liu C, Wu X, Guo Y, Yu H, et al. Inhibition of complement activation alleviates acute lung injury induced by highly pathogenic avian influenza H5N1 virus infection. Am J Respir Cell Mol Biol. 2013;49:221–230. doi: 10.1165/rcmb.2012-0428OC. [DOI] [PubMed] [Google Scholar]
- 74.Russkamp NF, Ruemmler R, Roewe J, Moore BB, Ward PA, Bosmann M. Experimental design of complement component 5a-induced acute lung injury (C5a-ALI): a role of CC-chemokine receptor type 5 during immune activation by anaphylatoxin. FASEB J. 2015;29:3762–3772. doi: 10.1096/fj.15-271635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bosmann M, Ward PA. Protein-based therapies for acute lung injury: targeting neutrophil extracellular traps. Expert Opin Ther Targets. 2014;18:703–714. doi: 10.1517/14728222.2014.902938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morsing KSH, Peters AL, van Buul JD, Vlaar APJ. The role of endothelium in the onset of antibody-mediated TRALI. Blood Rev. 2018;32:1–7. doi: 10.1016/j.blre.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 77.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Till GO, Ward PA. Oxygen radicals in complement and neutrophil-mediated acute lung injury. J Free Radic Biol Med. 1985;1:163–168. doi: 10.1016/0748-5514(85)90021-2. [DOI] [PubMed] [Google Scholar]
- 79.Lefrancais E, Mallavia B, Zhuo H, Calfee CS, Looney MR. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight. 2018;3 doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zilow G, Sturm JA, Rother U, Kirschfink M. Complement activation and the prognostic value of C3a in patients at risk of adult respiratory distress syndrome. Clin Exp Immunol. 1990;79:151–157. doi: 10.1111/j.1365-2249.1990.tb05171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hammerschmidt DE, Weaver LJ, Hudson LD, Craddock PR, Jacob HS. Association of complement activation and elevated plasma-C5a with adult respiratory distress syndrome. Pathophysiological relevance and possible prognostic value. Lancet. 1980;1:947–949. doi: 10.1016/s0140-6736(80)91403-8. [DOI] [PubMed] [Google Scholar]
- 82.Schein RM, Bergman R, Marcial EH, Schultz D, Duncan RC, Arnold PI, et al. Complement activation and corticosteroid therapy in the development of the adult respiratory distress syndrome. Chest. 1987;91:850–854. doi: 10.1378/chest.91.6.850. [DOI] [PubMed] [Google Scholar]