Abstract
In this study, by partial sequence analysis of the genome segments encoding VP5* and VP7, we characterized a novel bovine group A rotavirus, namely, Tak2, that was detected from adult cattle diarrhea in Tochigi Prefecture, Japan. The nucleotide (nt) and deduced amino acid (aa) sequences of the genome segments encoding VP5* and half of the amino terminal portion of VP7 of Tak2 revealed a low identity with those of group A rotaviruses carrying previously published P and G type specificities (VP5*: nt identity, 61.6%–67.6% and aa identity, 58.0%–71.4%; half of the amino terminal portion of VP7: nt identity, 57.8%–73.5% and aa identity, 61.2%–70.9%). Additionally, phylogenetic analysis of the nt sequences of the genome segments encoding VP5* and half of the amino terminal portion of VP7 revealed that Tak2 formed a branch separate from the established P and G types. These results suggested that Tak2 could possess novel P and G types yet not reported among group A rotaviruses.
Keywords: Adult cattle diarrhea, Bovine group A rotavirus, P type, G type, VP4, VP7
Bovine group A rotavirus (BoRV-A) is an important pathogen that causes neonatal calf diarrhea (Ishizaki et al., 1996, Fukai et al., 1998, De Verdier Klingenberg et al., 1999, Falcone et al., 1999, Alfieri et al., 2004). Viruses classified as group A rotavirus (RV-A), including BoRV-A, possess two independent neutralization antigens, namely, VP4 and VP7, on the outer capsid, that specify the P type (for protease-sensitive protein) and G type (for glycoprotein), respectively (Estes, 2001, Kapikian et al., 2001). To date, six P types (P6[1], P7[5], P8[11], P11[14], P[17], and P[21]) and 8 G types (G1, G3, G5, G6, G7, G8, G10 and G15) have been reported among BoRV-A (Matsuda et al., 1990, Blackhall et al., 1992, Brüssow et al., 1992, Isegawa et al., 1994, Fukai et al., 1999, Fukai et al., 2005, Rao et al., 2000, Alfieri et al., 2004). Types G2 and G11 in BoRV-A have been detected using PCR-derived cDNA probes (Hussein et al., 1993). Reports on the distribution patterns of these types reveal that P7[5], P8[11], G6, and G10 are the most common (Fukai et al., 1998, Falcone et al., 1999, Gulati et al., 1999, Okada and Matsumoto, 2002, Alfieri et al., 2004). G8 ranks third among the most common G type in BoRV-A, and several G8 strains reported to date have been detected and/or isolated sporadically, or their prevalence has been reported (Sato et al., 1997, Falcone et al., 1999, Fukai et al., 1999, Okada and Matsumoto, 2002, Fodha et al., 2005).
Bovine group B and C rotaviruses cause diarrhea in adult cattle (Parwani et al., 1996, Chang et al., 1997, Tsunemitsu et al., 1999a, Tsunemitsu et al., 1999b, Hayashi et al., 2001, Mawatari et al., 2004); in the case of BoRV-A, however, there were only few reports associating it with diarrhea in adult cattle (Sato et al., 1997). There were several reports of adult diarrhea caused by RV-A in humans (Hrdy, 1987, Iturriza-Gómara et al., 2000, Del Refugio Gonzalez-Losa et al., 2001, Griffin et al., 2002). We discovered the prevalence of adult cattle diarrhea in Tochigi Prefecture, Japan, in 2004, and detected BoRV-As from these diarrheic specimens. The P and G types in these diarrheic specimens could not be identified using RT-PCR. The purpose of this study was to genetically characterize the detected BoRV-A by the partial sequence analysis of the genome segments encoding the proteins VP4 and VP7.
The prevalence of adult cattle diarrhea was noted in a dairy farm in Tochigi Prefecture, Japan, in October 2004. This dairy farm raised approximately 90 dairy cattle, and approximately 8 adult cattle suffered from watery diarrhea for nearly 9 days. In contrast, no calves raised on this farm suffered from diarrhea over this period. Unfortunately, we did not record the incidence of diarrhea in adult cattle and calves raised on this farm after the observation period. The diarrheic specimens were obtained from four adult cattle aged 3, 4, 5, and 8 years, and RV-A was detected from these specimens by using the commercial kit Rotascreen (Denka Seiken, Tokyo, Japan) according to the manufacturer's instructions. BoRV-A was attempted to isolate from the diarrheic specimens in MA-104 cells in the presence of trypsin as described previously (Matsuda et al., 1990). Indirect immunofluorescence assay using anti-BoRV-A hyperimmune guinea pig serum, which has been described previously, was used to detect BoRV-As in MA-104 cells inoculated with the diarrheic specimens, culture supernatants, and infected cell culture lysates (Knowlton et al., 1991). Viral genomic double-stranded RNA (dsRNA) was extracted from the diarrheic specimens by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The extracted dsRNA was then analyzed by polyacrylamide gel electrophoresis (PAGE) and silver staining, as described previously (Fukai et al., 1998). The dsRNA was also used for first-stranded cDNA synthesis by MuLV-reverse transcriptase (Applied Biosystems, Foster City, CA, USA), and the cDNA was amplified by PCR using the ReadyMix Taq PCR Reaction Mix (Sigma–Aldrich, Saint Louis, MO, USA) as described previously (Fukai et al., 1998). In order to perform P typing, Bov4Com5, Bov4Com3, P1, P5, P10, con2, con3, pNCDV, pUK, and pB223 primers were synthesized as described by Isegawa et al. (1993) and Gouvea et al. (1994a). To perform G typing, sBeg9, End9(UK), DT6, HT8, and ET10 primers were synthesized as described by Gouvea et al., 1990, Gouvea et al., 1993, Gouvea et al., 1994b. Subsequently, the genome segments encoding the VP7s of the detected BoRV-As were amplified by PCR, using the primers VP7-MS (sense, 5′-CRGARYTAGATATGTCAGAA-3′, 491-510) and VP7-MA (antisense, 5′-AAYGTTATGTCCATYGGATT-3′, 563–544). Among the four diarrheic specimens obtained, the PCR-amplified products of the specimen termed Tak2 were selected and sequenced. The products were ligated into the pMOSBlue vector (Amersham Biosciences, Piscataway, NJ, USA), and both strands were sequenced using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems) on an ABI PRISM 3100 DNA analyzer (Applied Biosystems). The nucleotide (nt) and deduced amino acid (aa) sequences of the genome segments encoding VP4 and VP7 of Tak2 were compared with the corresponding RV-A sequences, which have been published previously. Phylogenetic trees were constructed by the neighbor-joining method (Saitou and Nei, 1987) using Clustal W (Thompson et al., 1994), and the bootstrap probabilities of each node were calculated using 1000 replicates. The final tree was obtained using the TreeView program, version 1.6.6 (Page, 1996). The other causative agents of adult cattle diarrhea, such as bovine group B and C rotaviruses, bovine coronavirus, bovine viral diarrhea virus, Clostridium spp., Salmonella spp. and Coccidium spp. were examined as described previously (Chinsangaram et al., 1994, Vilcek et al., 1994, Tsunemitsu et al., 1999a, Tsunemitsu et al., 1999b, Asai et al., 2002, Choi et al., 2003, Mawatari et al., 2004).
RV-A was detected from all the collected diarrheic specimens by the commercial kit and indirect immunofluorescence assay, while the other causative agents of adult cattle diarrhea were not detected from these specimens. However, marked fluorescence and cytopathic effect were not observed in the MA-104 cells inoculated with the culture supernatants and infected cell culture lysates of the second and third passages (data not shown); thus, BoRV-A could not be isolated from these specimens. In addition, the dsRNAs of all diarrheic specimens could not be detected successfully using PAGE (data not shown).
In P typing, no amplified signal was observed in the second PCR using Bov4Com5, P1, P5, and P10 primers or in the first and second PCR using con2, con3, pNCDV, pUK, and pB223 primers, although weak signal was observed in the first PCR using Bov4Com5 and Bov4Com3 primers (data not shown). For the genome segment encoding VP4, an 817-bp fragment in the partial sequence corresponding to its VP5* region was determined. The sequence reported here was deposited in the DDBJ/EMBL/GenBank databases (accession no. AB259664). When the partial VP4 sequence of Tak2 was compared with those of the currently identified 24 P genotypes, it was found that these sequences share low identities ranging between 61.6% and 67.6% at the nt level and 58.0% and 71.4% at the aa level with the established 24 P genotypes (Table 1 ). On the other hand, for the P[22] and P[23] types, these sequences could not be compared with those of Tak2 because only the sequences corresponding to their VP8* regions have been published (Martella et al., 2003, McNeal et al., 2005). Phylogenetic analysis revealed that Tak2 formed a branch separate from the 24 established P genotypes (Fig. 1 ).
Table 1.
Comparison of the nt and aa sequence identities of the genome segment encoding VP5* of Tak2 with strains representing the established twenty-four P types
| Strain | Species of origin | P genotype | P serotype | Tak2 |
Accession no. | |
|---|---|---|---|---|---|---|
| nt identity (%) | aa identity (%) | |||||
| NCDV-Lincoln | Bovine | 1 | 6 | 65.7 | 67.8 | AB119636 |
| SA11 | Simian | 2 | 5B | 66.7 | 69.2 | D16346 |
| RRV | Simian | 3 | 5B | 65.7 | 70.0 | M18736 |
| L26 | Human | 4 | 1B | 65.3 | 67.8 | M58292 |
| UK | Bovine | 5 | 7 | 65.2 | 68.9 | M22306 |
| ST3 | Human | 6 | 2A | 63.3 | 66.7 | L33895 |
| OSU | Porcine | 7 | 9 | 66.4 | 68.9 | X13190 |
| Wa | Human | 8 | 1A | 65.7 | 65.9 | L34161 |
| AU-1 | Human | 9 | 3 | 64.4 | 67.0 | D10970 |
| 69M | Human | 10 | 4 | 66.0 | 71.1 | M60600 |
| B223 | Bovine | 11 | 8 | 61.6 | 58.0 | D13394 |
| H2 | Equine | 12 | 4 | 67.6 | 71.4 | D13397 |
| MDR-13 | Porcine | 13 | Not tested | 65.2 | 65.9 | L07886 |
| Sun9 | Bovine | 14 | 11 | 66.7 | 67.0 | AB158430 |
| Lp14 | Ovine | 15 | Not tested | 64.8 | 67.8 | L11599 |
| Eb | Murine | 16 | 10 | 64.0 | 68.5 | L18992 |
| 993-83 | Bovine | 17 | Not tested | 63.2 | 63.4 | D16352 |
| L338 | Equine | 18 | Not tested | 65.2 | 68.5 | D13399 |
| 4F | Porcine | 19 | 12 | 66.5 | 68.1 | L10359 |
| EHP | Murine | 20 | 13 | 63.1 | 66.7 | U08424 |
| Hg18 | Bovine | 21 | Not tested | 66.4 | 68.9 | AF237665 |
| TUCH | Simian | 24 | Not tested | 65.9 | 67.0 | AY596189 |
| Dhaka6 | Human | 25 | Not tested | 64.4 | 65.8 | AY773004 |
| 134/04-15 | Porcine | 26 | Not tested | 65.0 | 64.8 | DQ061053 |
Fig. 1.
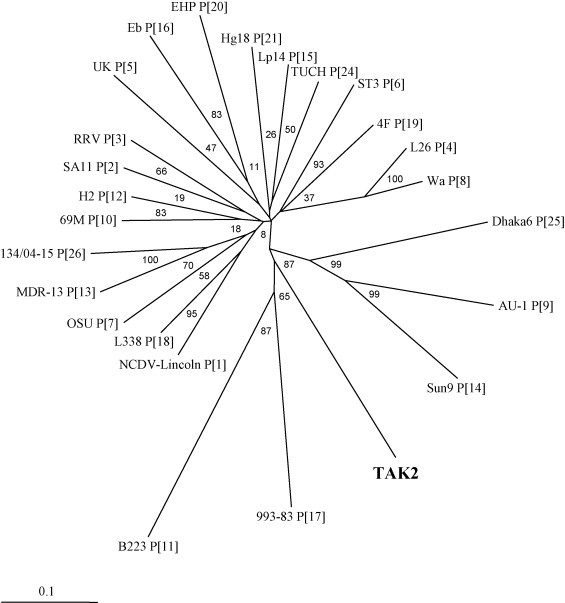
The phylogenetic tree constructed by the neighbor-joining method, using the nucleotide sequences of the genome segments encoding VP5* of the reference strains and Tak2, which is indicated in boldface. The accession numbers of the reference strains are listed in Table 1. The percentage bootstrap values are shown at the branch nodes. The scale bar represents 10% nucleotide difference. P genotypes are indicated after the strain names.
In G typing, no amplified signal was observed in the first and second PCR using sBeg9, End9(UK), DT6, HT8, and ET10 primers (data not shown). Therefore, two primers were synthesized according to the nt sequences of the genome segments encoding the VP7 of previously published BoRV-As, and amplification of the genome segment encoding the VP7 of Tak2 was attempted by PCR. Among the first PCR performed using sBeg9 and End9(UK) and the second PCR using sBeg9 and VP7-MA as well as VP7-MS and End9(UK), approximately 600 bp of the product was detected only in the second PCR performed using sBeg9 and VP7-MA (data not shown). Unfortunately, no amplified signal was observed in the second PCR using VP7-MS and End9(UK) (data not shown). For the genome segment encoding VP7, 522 bp of the partial sequence corresponding to approximately half of the amino terminal portion was determined. The sequence reported here was deposited in the DDBJ/EMBL/GenBank databases (accession no. AB259665). The partial VP7 sequence of Tak2 was compared with those of strains representing the 15 previously established G types (Table 2 ). The percentages of nt and aa identities with all the established 15 G types were low, ranging from 57.8% to 73.5% and 61.2% to 70.9%, respectively. Phylogenetic analysis revealed that Tak2 formed a branch separate from all the 15 established G types (Fig. 2 ).
Table 2.
Comparison of the nt and aa sequence identities of the genome segment encoding the half of the amino terminal side of VP7 of Tak2 with strains representing the established fifteen G types
| Strain | Species of origin | G sero/genotype | Tak2 |
Accession no. | |
|---|---|---|---|---|---|
| nt identity (%) | aa identity (%) | ||||
| Wa | Human | 1 | 71.1 | 70.3 | K02033 |
| S2 | Human | 2 | 69.7 | 64.8 | M11164 |
| HO-5 | Equine | 3 | 73.0 | 70.9 | AB046464 |
| HOCHI | Human | 4 | 67.8 | 63.0 | AB012078 |
| OSU | Porcine | 5 | 71.6 | 67.9 | X04613 |
| NCDV-Lincoln | Bovine | 6 | 72.4 | 68.5 | M12394 |
| PO-13 | Pigeon | 7 | 57.8 | 67.9 | D82979 |
| Sun9 | Bovine | 8 | 72.4 | 65.5 | AB158431 |
| 116E | Human | 9 | 69.3 | 66.7 | L14072 |
| KK3 | Bovine | 10 | 73.5 | 66.7 | D01056 |
| YM | Porcine | 11 | 69.7 | 67.3 | M23194 |
| L26 | Human | 12 | 67.8 | 64.2 | M58290 |
| L338 | Equine | 13 | 71.7 | 67.3 | D13549 |
| CH3 | Equine | 14 | 69.0 | 65.5 | D25229 |
| Hg18 | Bovine | 15 | 71.7 | 61.2 | AF237666 |
Fig. 2.

The phylogenetic tree constructed by the neighbor-joining method, using the nucleotide sequences of the genome segments encoding half of the amino terminal portion of VP7 of reference strains and Tak2, which is indicated in boldface. The accession numbers of the reference strains are listed in Table 2. The percentage bootstrap values are shown at the branch nodes. The scale bar represents 10% nucleotide difference. The G types are indicated after the strain names.
RV-As are classified within the same P genotype if the relevant aa sequences share >89% identity (Gorziglia et al., 1990). Considering this, our results suggested that BoRV-A Tak2 might belong to a novel P genotype, although only partial sequence of the genome segment encoding VP4 of Tak2 was determined, and the sequence could not be compared with those of the P[22] and P[23] types. However, phylogenetic analysis of the aa sequences of the VP8* of 26 P genotypes in RV-As revealed that the P[22] and P[23] types were closely related to the P[13] and P[24] types, respectively (Martella et al., 2006). In contrast, Tak2 was observed to share a common origin with the P[11] and P[17] types, which appeared to have a distinct genetic origin as compared to the VP4s belonging to the other P genotypes in previous study (Rao et al., 2000). These results suggested that Tak2 could be a novel P genotype. Despite numerous efforts, the amplification of the PCR product, including the complete genome segment encoding VP4, of all four diarrheic specimens contained Tak2 and the determination of those sequences have been unsuccessful to date. The present study suggests that Tak2 is a BoRV-A with a novel P genotype specificity, which has not been reported among BoRV-A thus far.
RV-As with different G types share <85% aa sequence conservation in the VP7, while strains sharing >90% aa identity are considered to belong to the same G type (Green et al., 1988, Green et al., 1989, Kapikian et al., 2001). Based on sequence and phylogenetic analysis of the partial sequence of the VP7 gene, strain Tak2 could not be assigned to any of the previously established G types. Accordingly, although the full-length VP7 gene remains to be determined, the data suggest that strain Tak2 may be a BoRV-A with a novel G type specificity, never reported among animal RV-A so far.
The feces of the animals were screened against a broad panel of viruses, bacteria and parasites, but the presence of other undetected pathogens, such as caliciviruses, parvoviruses, Escherichia coli, Yersinia spp., Campylobacter spp., or Giardia spp. was not investigated. Accordingly, it is not possible to associate the outbreak of diarrhea in adult cattle with the novel RV-A strain Tak2. However, BoRV-A in adult cattle with diarrhea have been already detected (Sato et al., 1997). The factors responsible for symptomatic infections by BoRV-A in adult cattle are unknown. Immunity raised against BoRV-A carrying unusual P and G type specificities, such as Tak2, is estimated to be low because P7[5], P8[11], G6, and G10 are the most common P and G types in BoRV-A, according to previous studies (Fukai et al., 1998, Falcone et al., 1999, Gulati et al., 1999, Okada and Matsumoto, 2002, Alfieri et al., 2004). Accordingly, the lack of homotypic immunity (specific for Tak2-like RV-As) may be one of the factors that have helped the virus to infect and cause disease in adult animals. The isolation of BoRV-A from the specimens of adult cattle diarrhea in this study needs to be repeated, and serological surveillance on these isolates in cattle is necessary in the future. However, the fact that no calf raised in this farm was affected with diarrhea is inconsistent with the above hypothesis. These questions warrant further studies to evaluate the pathogenic attitude of the strain Tak2.
Since 2000, RV-As carrying new P and G type specificities have been detected and/or isolated in quick succession, and almost all of those RV-As were of animal origin (Rao et al., 2000, Liprandi et al., 2003, Martella et al., 2003, Martella et al., 2006, McNeal et al., 2005, Rahman et al., 2005). The discovery of BoRV-A Tak2, which may have a unique combination of novel VP7 and VP4 types, is an example of the enormous diversity among the circulating RV-A strains. Complete genome sequencing of all 11 segments may help to understand the origin of the unusual BoRV-A strain Tak2. The evolution of RV-As is the result of interspecies transmissions, reassortment of genome segments between diverse strains, genome rearrangements and accumulation of point mutations. The recent discovery of novel P and G types has important implications in future Human RV-A vaccine strategies. Since domestic animals, including cattle, may serve as an important reservoir of novel RV-A strains, epidemiological RV-A surveillance needs to be performed regularly.
References
- Alfieri A.F., Alfieri A.A., Barreiros M.A., Leite J.P., Richtzenhain L.J. G and P genotypes of group A rotavirus strains circulating in calves in Brazil, 1996–1999. Vet. Microbiol. 2004;99:167–173. doi: 10.1016/j.vetmic.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Asai T., Otagiri Y., Osumi T., Namimatsu T., Hirai H., Sato S. Isolation of Salmonella from diarrheic feces of pigs. J. Vet. Med. Sci. 2002;64:159–160. doi: 10.1292/jvms.64.159. [DOI] [PubMed] [Google Scholar]
- Blackhall J., Bellinzoni R., Mattion N., Estes M.K., LaTorre J.L., Magnusson G. A bovine rotavirus serotype 1: serological characterization of the virus and nucleotide sequence determination of the structural glycoprotein VP7 gene. Virology. 1992;189:833–837. doi: 10.1016/0042-6822(92)90617-x. [DOI] [PubMed] [Google Scholar]
- Brüssow H., Nakagomi O., Gerna G., Eichhorn W. Isolation of an avianlike group A rotavirus from a calf with diarrhea. J. Clin. Microbiol. 1992;30:67–73. doi: 10.1128/jcm.30.1.67-73.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.O., Parwani A.V., Smith D., Saif L.J. Detection of group B rotaviruses in fecal samples from diarrheic calves and adult cows and characterization of their VP7 genes. J. Clin. Microbiol. 1997;35:2107–2110. doi: 10.1128/jcm.35.8.2107-2110.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsangaram J., Akita G.Y., Osburn B.I. Detection of bovine group B rotaviruses in feces by polymerase chain reaction. J. Vet. Diagn. Invest. 1994;6:302–307. doi: 10.1177/104063879400600304. [DOI] [PubMed] [Google Scholar]
- Choi Y.K., Kang M.S., Yoo H.S., Lee D.Y., Lee H.C., Kim D.Y. Clostridium perfringens type A myonecrosis in a horse in Korea. J. Vet. Med. Sci. 2003;65:1245–1247. doi: 10.1292/jvms.65.1245. [DOI] [PubMed] [Google Scholar]
- Del Refugio Gonzalez-Losa M., Polanco-Marin G.G., Manzano-Cabrera L., Puerto-Solis M. Acute gastroenteritis associated with rotavirus in adults. Arch. Med. Res. 2001;32:164–167. doi: 10.1016/s0188-4409(00)00270-8. [DOI] [PubMed] [Google Scholar]
- De Verdier Klingenberg K., Nilsson M., Svensson L. Rotavirus G-type restriction, persistence, and herd type specificity in Swedish cattle herds. Clin. Diagn. Lab. Immunol. 1999;6:181–185. doi: 10.1128/cdli.6.2.181-185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M.K. Rotaviruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. fourth ed. Lippincott Raven; Philadelphia: 2001. pp. 1747–1785. [Google Scholar]
- Falcone E., Tarantino M., Di Trani L., Cordiori P., Lavazza A., Tollis M. Determination of bovine rotavirus G and P serotypes in Italy by PCR. J. Clin. Microbiol. 1999;37:3879–3882. doi: 10.1128/jcm.37.12.3879-3882.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodha I., Boumaiza A., Chouikha A., Dewar J., Armah G., Geyer A., Trabelsi A., Steele A.D. Detection of group A rotavirus strains circulating in calves in Tunisia. J. Vet. Med. B. 2005;52:49–50. doi: 10.1111/j.1439-0450.2004.00810.x. [DOI] [PubMed] [Google Scholar]
- Fukai K., Sakai T., Kamata H. Distribution of G serotypes and P genotypes of bovine group A rotavirus isolated in Japan. Aust. Vet. J. 1998;76:418–422. doi: 10.1111/j.1751-0813.1998.tb12393.x. [DOI] [PubMed] [Google Scholar]
- Fukai K., Sakai T., Hirose M., Itou T. Prevalence of calf diarrhea caused by bovine group A rotavirus carrying G serotype 8 specificity. Vet. Microbiol. 1999;66:301–311. doi: 10.1016/s0378-1135(99)00021-8. [DOI] [PubMed] [Google Scholar]
- Fukai K., Yamada K., Inoue K. Serological characterization of novel P11[14],G8 bovine group A rotavirus, Sun9, isolated in Japan. Virus Res. 2005;114:167–171. doi: 10.1016/j.virusres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Larralde G., Kapikian A.Z., Chanock R.M. Antigenic relationships among human rotaviruses as determined by outer capsid protein VP4. Proc. Natl. Acad. Sci. U.S.A. 1990;87:7155–7159. doi: 10.1073/pnas.87.18.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Glass R.I., Woods P., Taniguchi K., Clark H.F., Forrester B., Fang Z.Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Ramirez C., Li B., Santos N., Saif L.J., Clark H.F., Hoshino Y. Restriction endonuclease analysis of the VP7 genes of human and animal rotaviruses. J. Clin. Microbiol. 1993;31:917–923. doi: 10.1128/jcm.31.4.917-923.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Santos N., Timenetsky M.C. VP4 typing of bovine and porcine group A rotaviruses by PCR. J. Clin. Microbiol. 1994;32:1333–1337. doi: 10.1128/jcm.32.5.1333-1337.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Santos N., Timenetsky M.C. Identification of bovine and porcine rotavirus G types by PCR. J. Clin. Microbiol. 1994;32:1338–1340. doi: 10.1128/jcm.32.5.1338-1340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K.Y., Sears J.F., Taniguchi K., Midthun K., Hoshino Y., Gorziglia M., Nishikawa K., Urasawa S., Kapikian A.Z., Chanock R.M., Flores J. Prediction of human rotavirus serotype by nucleotide sequence analysis of the VP7 protein gene. J. Virol. 1988;62:1819–1823. doi: 10.1128/jvi.62.5.1819-1823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K.Y., Hoshino Y., Ikegami N. Sequence analysis of the gene encoding the serotype-specific glycoprotein (VP7) of two new human rotavirus serotypes. Virology. 1989;168:429–433. doi: 10.1016/0042-6822(89)90289-4. [DOI] [PubMed] [Google Scholar]
- Griffin D.D., Fletcher M., Levy M.E., Ching-Lee M., Nogami R., Edwards L., Peters H., Montague L., Gentsch J.R., Glass R.I. Outbreaks of adult gastroenteritis traced to a single genotype of rotavirus. J. Infect. Dis. 2002;185:1502–1505. doi: 10.1086/340218. [DOI] [PubMed] [Google Scholar]
- Gulati B.R., Nakagomi O., Koshimura Y., Nakagomi T., Pandey R. Relative frequencies of G and P types among rotaviruses from Indian diarrheic cow and buffalo calves. J. Clin. Microbiol. 1999;37:2074–2076. doi: 10.1128/jcm.37.6.2074-2076.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Nagai M., Hayakawa Y., Takeuchi K., Tsunemitsu H. Outbreak of diarrhoea and milk drop in cows infected with bovine group B rotavirus. Vet. Rec. 2001;149:331–332. doi: 10.1136/vr.149.11.331. [DOI] [PubMed] [Google Scholar]
- Hrdy D. Epidemiology of rotaviral infection in adults. Rev. Infect. Dis. 1987;9:461–469. doi: 10.1093/clinids/9.3.461. [DOI] [PubMed] [Google Scholar]
- Hussein H.A., Parawani A.V., Rosen B.I., Lucchelli A., Saif L.J. Detection of rotavirus serotypes G1, G2, G3, and G11 in feces of diarrheic calves by using polymerase chain reaction-derived cDNA probes. J. Clin. Microbiol. 1993;31:2491–2496. doi: 10.1128/jcm.31.9.2491-2496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isegawa Y., Nakagomi O., Nakagomi T., Ishida S., Uesugi S., Ueda S. Determination of bovine rotavirus G and P serotypes by polymerase chain reaction. Mol. Cell. Probes. 1993;7:277–284. doi: 10.1006/mcpr.1993.1041. [DOI] [PubMed] [Google Scholar]
- Isegawa Y., Nakagomi O., Brüssow H., Minamoto N., Nakagomi T., Ueda S. A unique VP4 gene allele carried by unusual bovine rotavirus strain, 993/83. Virology. 1994;198:366–369. doi: 10.1006/viro.1994.1043. [DOI] [PubMed] [Google Scholar]
- Ishizaki H., Sakai T., Shirahata T., Taniguchi K., Urasawa T., Urasawa S., Goto H. The distribution of G and P types within isolates of bovine rotavirus in Japan. Vet. Microbiol. 1996;48:367–372. doi: 10.1016/0378-1135(95)00168-9. [DOI] [PubMed] [Google Scholar]
- Iturriza-Gómara M., Green J., Brown D.W., Ramsay M., Desselberger U., Gray J.J. Molecular epidemiology of human group A rotavirus infections in the United Kingdom between 1995 and 1998. J. Clin. Microbiol. 2000;38:4394–4401. doi: 10.1128/jcm.38.12.4394-4401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A.Z., Hoshino Y., Chanock R.M. Rotaviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. fourth ed. Lippincott Raven; Philadelphia: 2001. pp. 1787–1833. [Google Scholar]
- Knowlton D.R., Spector D.M., Ward R.L. Development of an improved method for measuring neutralizing antibody to rotavirus. J. Virol. Methods. 1991;33:127–134. doi: 10.1016/0166-0934(91)90013-p. [DOI] [PubMed] [Google Scholar]
- Liprandi F., Gerder M., Bastidas Z., López J.A., Pujol F.H., Ludert J.E., Joelsson D.B., Ciarlet M. A novel type of VP4 carried by a porcine rotavirus strain. Virology. 2003;315:373–380. doi: 10.1016/s0042-6822(03)00534-8. [DOI] [PubMed] [Google Scholar]
- Martella V., Ciarlet M., Camarda A., Pratelli A., Tempesta M., Greco G., Cavalli A., Elia G., Decaro N., Terio V., Bozzo G., Camero M., Buonavoglia C. Molecular characterization of the VP4, VP6, VP7, and NSP4 genes of lapine rotaviruses in Italy: emergence of a novel VP4 genotype. Virology. 2003;314:370–385. doi: 10.1016/s0042-6822(03)00418-5. [DOI] [PubMed] [Google Scholar]
- Martella V., Ciarlet M., Bányai K., Lorusso E., Cavalli A., Corrente M., Elia G., Arista S., Camero M., Desario C., Decaro N., Lavazza A., Buonavoglia C. Identification of a novel VP4 genotype carried by a serotype G5 porcine rotavirus strain. Virology. 2006;346:301–311. doi: 10.1016/j.virol.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Nakagomi O., Offit P.A. Presence of three P types (VP4 serotypes) and two G types (VP7 serotypes) among bovine rotavirus strains. Arch. Virol. 1990;115:199–207. doi: 10.1007/BF01310530. [DOI] [PubMed] [Google Scholar]
- Mawatari T., Taneichi A., Kawagoe T., Hosokawa M., Togashi K., Tsunemitsu H. Detection of a bovine group C rotavirus from adult cows with diarrhea and reduced milk production. J. Vet. Med. Sci. 2004;66:887–890. doi: 10.1292/jvms.66.887. [DOI] [PubMed] [Google Scholar]
- McNeal M.M., Sestak K., Choi A.H., Basu M., Cole M.J., Aye P.P., Bohm R.P., Ward R.L. Development of a rotavirus-shedding model in rhesus macaques, using a homologous wild-type rotavirus of a new P genotype. J. Virol. 2005;79:944–954. doi: 10.1128/JVI.79.2.944-954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Matsumoto Y. Bovine rotavirus G and P types and sequence analysis of the VP7 gene of two G8 bovine rotaviruses from Japan. Vet. Microbiol. 2002;84:297–305. doi: 10.1016/s0378-1135(01)00445-x. [DOI] [PubMed] [Google Scholar]
- Page R.D.M. Treeview: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Parwani A.V., Lucchelli A., Saif L.J. Identification of group B rotaviruses with short genome electropherotypes from adult cows with diarrhea. J. Clin. Microbiol. 1996;34:1303–1305. doi: 10.1128/jcm.34.5.1303-1305.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M., Matthijnssens J., Nahar S., Podder G., Sack D.A., Azim T., Van Ranst M. Characterization of a novel P[25],G11 human group A rotavirus. J. Clin. Microbiol. 2005;43:3208–3212. doi: 10.1128/JCM.43.7.3208-3212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C.D., Gowda K., Reddy B.S.Y. Sequence analysis of VP4 and VP7 genes of nontypeable strains identified a new pair of outer capsid proteins representing novel P and G genotypes in bovine rotavirus. Virology. 2000;276:104–113. doi: 10.1006/viro.2000.0472. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sato M., Nakagomi T., Tajima K., Ezura K., Akashi H., Nakagomi O. Isolation of serotype G8, P[1] bovine rotavirus from adult cattle with diarrhea. J. Clin. Microbiol. 1997;35:1266–1268. doi: 10.1128/jcm.35.5.1266-1268.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemitsu H., Smith D.R., Saif L.J. Experimental inoculation of adult dairy cows with bovine coronavirus and detection of coronavirus in feces by RT-PCR. Arch. Virol. 1999;144:167–175. doi: 10.1007/s007050050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemitsu H., Morita D., Takaku H., Nisimori T., Imai K., Saif L.J. First detection of bovine group B rotavirus in Japan and sequence of its VP7 gene. Arch. Virol. 1999;144:805–815. doi: 10.1007/s007050050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek S., Herring A.J., Herring J.A., Nettleton P.F., Lowings J.P., Paton D.J. Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch. Virol. 1994;136:309–323. doi: 10.1007/BF01321060. [DOI] [PubMed] [Google Scholar]


