Abstract
Upper room (UR)-ultraviolet germicidal (UVGI) systems, one of several disinfection applications of UV, target airborne infectious diseases in rooms of buildings such as healthcare facilities. Previous studies have introduced many experiments showing the germicidal effect of UR-UVGI systems. In this study, a novel numerical method of estimating the germicidal effect of UR-UVGI systems for air exhaled by ward patients was introduced. The method adopts and modifies the concept of ventilation efficiency because the germicidal effect depends upon how the air containing airborne infectious particles flows and stays within UV-radiated area. A case study based on a four-patient ward showed that UV doses were correlated with the age of the air exhaled by a source patient, as expected. Moreover, the UV doses were considerably affected by the position of the UR-UVGI system. Inactivation rates of the influenza virus estimated using the UV doses, were in the range of 48–74%, and those of Mycobacterium tuberculosis were 68–90% in the breathing area of a neighboring patient. The results indicate not directly the decreased concentration of airborne infectious particles, but the possibility of inactivation caused by the UR-UVGI system, which is useful for system optimization.
Keywords: Ultraviolet germicidal irradiation, Ventilation efficiency, Exhaled air, CFD
1. Introduction
The ongoing likelihood of a global influenza epidemic and the threat of bioterrorism require more sophisticated building hygiene systems to prevent their spread during the initial stage. As one of the countermeasures against these threat, ultraviolet germicidal irradiation (UVGI) systems have attracted attention. The use of UV radiation for germicidal purposes has a long history, but its practical application was only considered after the identification of UV radiation emission from discharge lamps in the early 20th century [1]. A number of infectious microorganisms and viruses that have been prevalent at the time, such as influenza and tuberculosis, were shown to be effectively inactivated by UV radiation [2], [3]. For example, Wells had performed several initial experiments showing the germicidal effect of UV radiation in combating airborne infectious microorganisms as done by other researchers [4] [5]. His book, published in 1954, was one of the first comprehensive sources that described the characteristics of airborne infectious diseases and the effective usage of UV radiation [6].
UVGI systems used to disinfect indoor air are mainly divided into two types, upper room (UR)-UVGI and in-duct (ID)-UVGI systems [7]. A ID-UVGI system can be an effective countermeasure when infectious microorganisms pass through air conditioning units or ducts during the contagion stage. On the other hand, a UR-UVGI system installed in a room begins to inactivate infectious airborne microorganisms, as soon as they are emitted from one or more sources and exposed to UV rays in the room, due to proximity. This inactivation can prevent the dispersion of airborne microorganisms not only in the room but also to other rooms. The position of the UR-UVGI systems is restricted to locations safe for occupants, such as the upper part of the room, and several horizontal louvers are usually placed in front of UVC lamps to ensure horizontal UVC rays only radiate the upper part of the room, because UVGI systems use the ultraviolet C-band (UVC) with wavelengths in the range 200–280 nm. This wavelength range has a germicidal effect on a wide range of microorganisms, but is also known to possibly cause skin redness and eye irritation when overexposed to humans [8]. Therefore, it is important to identify factors such as the airflow transferring air from the lower space to the upper space and the distribution of UVC intensity from the UR-UVGI system. Experimental methods using less harmful microorganisms in full-scale chambers have been employed to evaluate the germicidal efficacy of UR-UVGI [9], [10], [11], but it is costly and slow to evaluate and design UR-UVGI systems using experimental methods at the design stage. Therefore, numerical methods have been applied by several studies [12], [13].
In this study, we introduce a novel method using computation fluid dynamics (CFD) simulations to calculate the UV dose for the air exhaled from a source such as a patient. The germicidal effect of the UR-UVGI system can be estimated using the calculated UV dose. An existing method for calculating the ventilation efficiency was reviewed first to elucidate how the new method for calculating UV dose was modified from the existing method. The UV dose of the air constantly exhaled from a patient can be calculated using this method in a room with a steady state airflow distribution. The newly proposed method was illustrated with a ward model, and the relationship between the calculated UV doses and the ventilation efficiency was discussed.
2. Methodology
2.1. Ventilation efficiency and the UV dose for the air exhaled by occupants
The ventilation efficiency can be expressed as the ability of ventilation systems to remove pollution originating from a source in a room [14]. The overall ventilation efficiency has customarily been measured using a trace gas method. Sandberg introduced the concept of the age of air, the mean time elapsed before fresh air introduced into a room reaches a specific point to evaluate local ventilation efficiency [15]. However, measuring the age of air is very tedious work, even in an small enclosed room. Therefore, Kato et al. [16] introduced a numerical simulation method to estimate the local age of air in a room with steady state flow and diffusion fields. The scale of ventilation efficiency 3 (SVE3) corresponding to the local age of air, among several terms introduced by Kato, defines the mean traveling time required by the air mass from a supply opening to reach a specific point in a room. The SVE3 can be calculated using the contaminant concentration at a point under conditions of uniform contaminant generation throughout a room and fresh air intake from supply openings. Because the SVE3 is normalized by the nominal ventilation time, the SVE3 of ventilation systems with different air exchange rates can be compared with each other.
A volume of air at a point consists of air from each supply opening when there are multiple supply openings. Kato et al. [17] introduced the SVE3∗ (new SVE3) as an index that indicates the age of air from one of two or more supply openings. In the process of calculating the SVE3∗, if the air from the specific supply opening is assumed to be a contaminant, the mass ratio of the contaminant at a given point is defined as the contribution ratio of the supply opening at that point. The contribution ratio was previously defined as the SVE4 by Kato et al. [18]. The contribution ratio, r [-], can be defined by the following scalar transport equation (Fig. 1 ).
| (1) |
where, uj is air velocity [m/s], νt is the turbulent viscosity [m2/s] and σ is the turbulence Schmidt number [-]. Each term in this equation indicates the transient, the convection and the diffusion effects from the left.
Fig. 1.
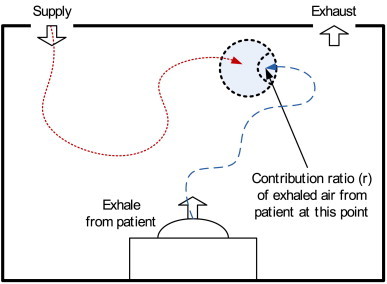
Contribution ratio (SVE4) of air exhaled by a patient.
Because the UV dose, D [J/m2] at a point can be expressed by multiplying the UV intensity, I [W/m2] at that point with the residence time [s], the UV dose can be obtained by treating the UV intensity as the contaminant source [kg/m3s] of the same value at the same spatial point and by solving the following transport equation of the contaminant scalar.
| (2) |
The concentration [kg/m3] solved by Eq. (2) would be the UV dose. Sung et al. suggested a method using this concept to calculate the UV dose of the air supplied to a ward through a supply opening [13].
In this study, the concept of the SVE3∗ was applied to calculate the UV dose of the exhaled air from a patient. The mouth of a patient was treated as another supply opening, like those of the air conditioning or ventilation systems, to find the contribution ratio of the exhaled air from the patient (Fig. 2 ). In addition, it was assumed that the mouth constantly exhales only, like other supply openings, to calculate the contribution ratio, which can be obtained in steady state flow and diffusion fields (Fig. 3 ).
Fig. 2.
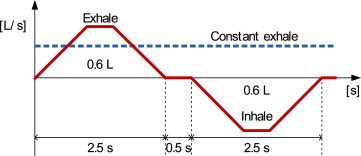
Constant exhale model for a patient.
Fig. 3.
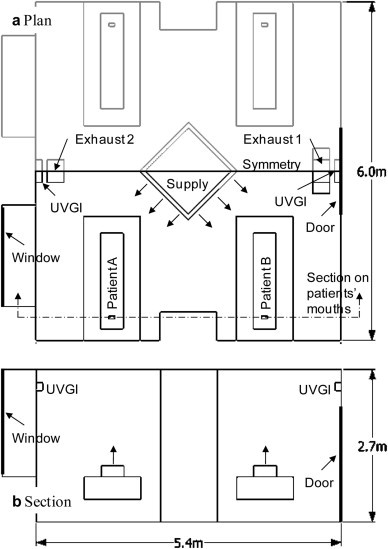
Model of the four-patient ward.
The following scalar transport equation of the UV intensity as a contaminant source weighted by the contribution ratio is thus solved.
| (3) |
The UV dose in Eq. (2) can be issued by dividing the result of Eq. (3) by the contribution ratio calculated by Eq. (1). This process is the same as calculating the SVE3∗, besides assuming a non-uniform contaminant emission which actually means UV intensity distribution in the upper area of a room.
2.2. Germicidal effect of UVGI system
The germicidal effect of a UVGI system depends on the UV dose and the type of microorganisms concerned. Their relationship has been expressed in terms of an exponential decay equation [19].
| (4) |
where, SR is the survival rate and k is the UV rate constant [m2/J] that indicates the UV susceptibility of each microorganism or virus. A higher UV rate constant means that a microorganism or a virus is more easily inactivated by UV rays. The UV rate constant varies with the type of microorganism and virus; however, in general, it is known to be higher for viruses and bacteria than fungi [7], as shown in Table 1 . Therefore, UR-UVGI systems are not appropriate for the removal of airborne fungal spores, accounting for the low UV rate constants of fungal spores and short exposure time to UV. However, ID-UVGI systems are considered effective for surface disinfection of fungal spores because long exposure time is available in air conditioning systems. The UV rate constant can also vary with the environmental conditions when microorganisms and viruses are exposed to UV. Most bacteria and viruses are more susceptible to UV in air than in water [24], which enables UR-UVGI systems to inactivate them more effectively in air. Additionally, it is known that humidity can affect the UV susceptibilities of viruses and bacteria in air [25], [26], but the effect was neglected in this study because their relationship is not clearly identified yet.
Table 1.
UV rate constant for representative airborne viruses and microbes.
| Microbe | UV rate constant [m2/J] | Reference | |
|---|---|---|---|
| Virus | Coronavirus | 0.377 | Walker et al., 2007 [20] |
| Influenza A | 0.27 | McDevitt et al. [21] | |
| Bacteria | Mycobacterium tuberculosis | 0.472 | Riley et al., 1976 [22] |
| Staphylococcos aureus | 0.960 | Luckiesh et al., 1949 [23] | |
| Fungi | Aspergillus niger | 0.00058 | |
| Cladosporium herbarum | 0.0037 | ||
2.3. Case study
A simulated ward with dimensions 5.4 × 6.0 × 2.7 m was used to illustrate the method introduced in the previous chapter (Fig. 3). The four-patient ward has a squared supply opening with fresh air flowing toward each patient at the center of the ceiling as shown in Fig. 3 and has one or two exhaust openings, depending on the case. The supply air has a velocity of 4.18 m/s, corresponding to a ventilation rate of about 11 times per hour. The UR-UVGI system is installed on the wall at a height of 2.4 m either above the door or beside the window, depending on the case, to radiate the UVC only to the upper area of the ward. The spatial distribution of the UVC intensity radiated from UR-UVGI systems measured by Sung et al. [13] was used in this study. Patients, simplified as boxes with dimensions 0.4 × 0.2 × 1.7 m, were placed on the beds either near door or the window and were constantly exhaling contaminants at an airflow of 0.24 l/s from their mouths. The temperature of the exhaled air was set to 32 °C, and the heat generation of each patient’s body was set to 24 W. Because the ward has absolute symmetry, only half of it was modeled for the calculation. Distributions of the airflow and the UV dose were calculated using a commercial CFD code, STAR-CD V.3.26 (CD-Adapco JAPAN Co., Ltd.), and the standard k-ε model was used as a turbulence model. The other boundary conditions of CFD simulation are shown in Table 2 .
Table 2.
Boundary conditions of CFD simulation.
| Boundary conditions | |
|---|---|
| Supply | kin = 3/2 × (Uin × 0.05)2, εin = Cu × kin3/2/lin Uin: inlet velocity, Cu = 0.09, lin: length of opening/7 |
| Exhaust | Free slip, mass balanced |
| Wall | Standard wall function |
| Symmetry | Free slip |
| Radiation | View factor method |
| Thermal loads | Patients: 48 W (24 W/patient × 2 patients) on the beds |
| Lighting: 329 W from the ceiling surface | |
| Windows: 492 W from the window surface |
In this case study, the UR-UVGI system was placed at different wall positions to determine the influence of the position of the UR-UVGI system on the UV dose. If the air containing microorganisms or viruses stays longer near the UR-UVGI, where the UVC intensity is comparatively strong, the overall UV dose would be higher. Therefore the position of the UR-UVGI system is an important factor for the germicidal performance of the system in addition to the distribution of the airflow. The residence time of air in a UV-irradiated area is also affected by the composition of the exhaust air openings and the position of a source patient.
In eight cases, the UV dose is calculated based on the positions of a UR-UVGI system and a source patient and the composition of the exhaust air openings, as shown in Table 3 .
Table 3.
Simulation cases.
| Case | Exhaust opening | Source | UR-UVGI | |
|---|---|---|---|---|
| 1 | A | One on the ceiling near the door | Patient A | Two cases of door side (door) and window side (window) installation |
| B | Patient B | |||
| 2 | A | Two on the ceiling near the door and the window | Patient A | |
| B | Patient B | |||
3. Results
3.1. Contribution ratio and the age of the air exhaled air by patients
Fig. 4 shows the contribution ratio of the air exhaled by a patient on the vertical section corresponding to patients’ mouths, indicating how the air at any point is composed both of air exhaled by the patient and of air delivered by the ventilation system. In other words, the contribution ratios indicate the ratio of exhaled air to all of the air from outside of the ward. In all cases, the contribution ratios near the source patient are highest. However, the overall contribution ratios are of the order of −3 because the flow rate of air exhaled by the patient (0.864 [m3/h]) is very small compared to that from the ventilation system (500 [m3/h]). The air from the patient tends to spread to the exhaust openings, so that the contribution ratios near the neighboring patient are lower in Cases 1-B and 2-B than in Cases 1-A and 2-A. Most of the air from patient B seems to be exhausted through the nearby exhaust opening before spreading to the neighboring patient A in Cases 1-B and 2-B. Conversely, dividing the exhaust opening caused a small rise in the contribution ratios near the neighboring patient as shown in Cases 1-B and 2-B, although this trend is not noticeably observed in Cases 1-A and 1-B.
Fig. 4.
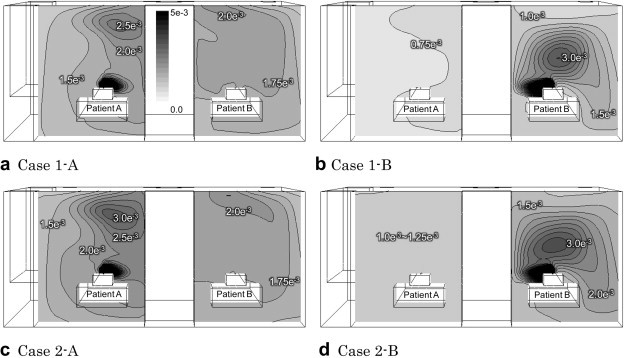
Contribution ratio (r) of air exhaled by a patient (breathing area section).
Fig. 5 shows the distribution of the SVE3∗, which is the age of the exhaled air from a source patient. Overall, the distributions of the SVE3∗ were different from those of the contribution ratios in all cases because the contribution ratio at a location just defines the average mass ratio of the air from a patient, whereas the SVE3∗ is determined by the route and the duration time of the air from the patient to the location. The SVE3∗ values in the area of a neighboring patient indicate are comparatively low in the cases where patient A is a source, which means the air from patient A can reach the area of patient B more quickly than in reverse cases. The air from patient B flows predominately toward a nearby exhaust opening in Cases 1-B and 2-B, and therefore the contribution ratios near patient A are comparatively small. The time elapsed for the additional small amount of the air from patient B to reach patient A could be long, irrespective of the contribution ratios. Besides, the air from patient A tends to flow toward the one exhaust opening near patient B in Case 1-A, so the SVE3∗ near patient B is less than that in Case 2-A where the exhaust opening is divided into two.
Fig. 5.
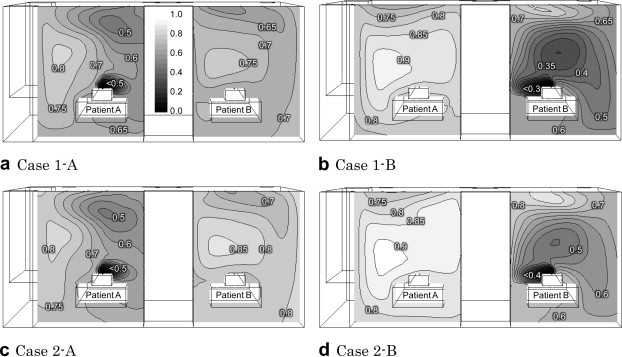
SVE3∗ of air exhaled by a patient (breathing area section).
3.2. UV dose and the inactivation ratio of the air exhaled by patients
The UV dose distributions of the air exhaled by a source patient for each case are shown in Fig. 6 . The values at each location represent how much air exhaled from a source patient has been irradiated by the UR-UVGI system installed in the upper area before the air reaches that particular location.
Fig. 6.
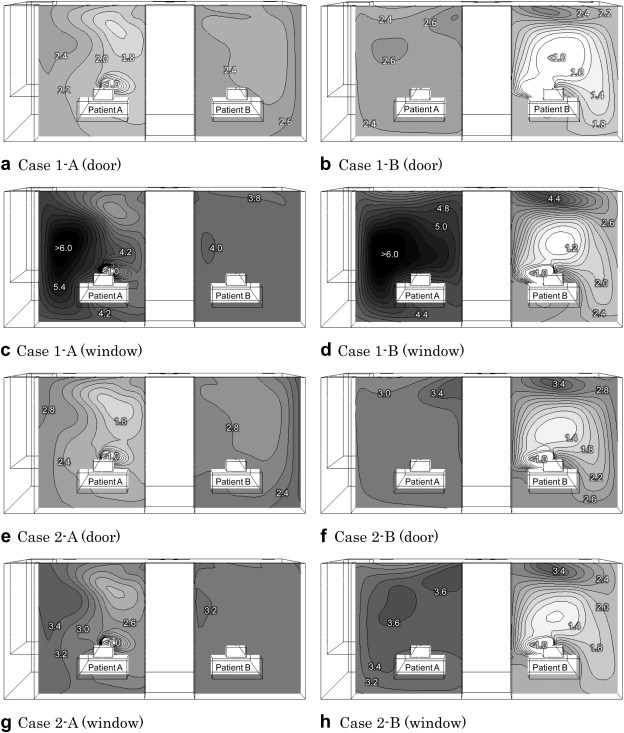
UV dose (J/m2) distributions of air exhaled by a patient (breathing area section).
As shown in Fig. 6, the UV doses in most cases were low near the source patient and high near the neighboring patient, which means that the air near the source patient was not affected by the UR-UVGI system as much as the air near the neighboring patient. Relating this trend to the SVE3∗ distribution, the air immediately after exhalation from a source patient has less opportunity to be irradiated by UV rays because the SVE3∗, the age of the exhaled air, was short near the source patient. Among the eight cases, Cases 1-A (window) and 1-B (window) where the UR-UVGI system is installed on the opposite side of the one exhaust opening show the highest UV dose distributions. Of the two cases, Case 1-B (windows) shows the highest UV dose distribution near the neighboring patient.
Fig. 7 shows the average UV doses of the air exhaled by a source patient in the breathing zones (0.15 × 0.1 × 0.1 m directly over the mouth) of neighboring patients and the exhaust air. In all cases, the UV doses of the air exhaled from patient B were higher than those from patient A in the breathing zones of the neighboring patient, regardless of the position of the UR-UVGI system and the number of exhaust openings. However, the UV doses of the air exhaled from patient A were higher in the exhaust opening when only one exhaust opening was operated. The reason is because most of the air exhaled from patient B is assumed to be promptly exhausted through a nearby exhaust opening before being sufficiently irradiated by the UR-UVGI system. This trend was also observed in the cases where two exhaust openings were operated. Moreover, the UV doses of air exhaled from patient B were higher at the exhaust opening 2, located near patient B. In other words, the UV doses at the exhaust opening near the source patient were comparatively low in all cases.
Fig. 7.
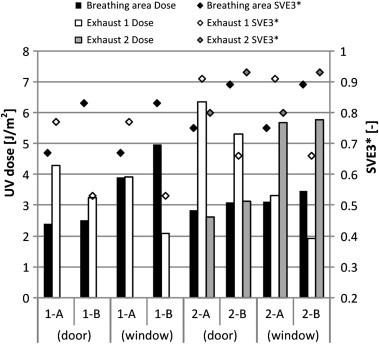
UV dose and SVE3∗ of air exhaled by a patient.
The SVE3∗s were also plotted in Fig. 7 to compare with the UV doses. The SVE3∗s, when compared in terms of a source patient, were correlated with the UV doses in all cases. This outcome implies that the UV doses are proportional to the age of air to a certain degree, because the exhaled air from a source patient may have a greater chance of being irradiated by the UV rays, provided that the air has a longer residence time. Besides, the position of the UR-UVGI system affected the UV doses, regardless of the SVE3∗.
Inactivation rates of the air exhaled by source patients were calculated using Eq. (4), where influenza A virus or Mycobacterium tuberculosis is assumed to be included in the exhaled air, as shown in Table 4 . The UV doses were averaged in the entire room, the breathing zone of the neighboring patient and the exhaust opening; the UV rate constants shown in Table 1 were used. Because the UV rate constant of M. tuberculosis is higher than that of influenza A virus, the inactivation rate of M. tuberculosis is also generally higher. As explained regarding UV doses in previous phases, Cases 1-A (window) and 1-B (window) show the highest inactivation rates, up to 90%, in the breathing zone of the neighboring patient. Inactivation rates at the exhaust openings range from 43% to 95%, which means that M. tuberculosis could be disinfected by up to 95% and that influenza A virus could be disinfected by up to 82%, when they were exhausted.
Table 4.
Estimated inactivation rates [%] of influenza virus and M. tuberculosis.
| Case | Room average | Breathing zone of the neighboring patient | Exhaust air |
||
|---|---|---|---|---|---|
| Average | Exhaust 1 | Exhaust 2 | |||
| 1-A (door) | 46/65 | 48/68 | 69/87 | 69/87 | – |
| 1-B (door) | 43/63 | 49/69 | 58/78 | 58/78 | – |
| 1-A (window) | 67/85 | 65/84 | 65/84 | 65/84 | – |
| 1-B (window) | 62/82 | 74/90 | 43/63 | 43/63 | – |
| 2-A (door) | 52/72 | 53/74 | 70/88 | 82/95 | 51/71 |
| 2-B (door) | 52/72 | 57/77 | 68/86 | 76/92 | 57/77 |
| 2-A (window) | 55/76 | 57/77 | 70/88 | 59/79 | 78/93 |
| 2-B (window) | 51/71 | 61/80 | 65/84 | 40/60 | 79/93 |
4. Discussion
The method of calculating the UV doses of air exhaled by a source patient proposed in this study modified the process of calculating the SVE3∗ from several concepts of ventilation efficiency. As a result, the UV doses were correlated with the SVE3∗. Although the trend of the UV doses can be partially predicted using the SVE3∗, the UV dose needs to be calculated using the proposed method to consider other factors possibly affecting the UV dose distribution, such as the position or the number of UR-UVGI systems. Both the SVE3∗ and the UV doses of the air exhaled from more than two source patients can be calculated using this method, though only the UV doses for one source patient were calculated in this study.
The most important point about this method is that the UV dose calculated indicates how much the air exhaled from a source patient has been UV irradiated. The UV dose distribution does not directly represent the inactivated concentration, but the inactivation rate eventually estimated from the UV dose. Even if the UV dose value is low at a location, it does not always indicate a high concentration of infectious contaminant because the concentration could have been low even when the UR-UVGI system was not being operated. Nevertheless, this method is useful to estimate the germicidal effect of UR-UVGI system itself, even when the concentration of the source microorganism is not confirmed.
Finally, several assumptions were made in this study to calculate the UV doses of the air exhaled by a source patient. For instance, airborne infectious particles were treated as a passive scalar contaminant that flows along the airflow, and the contaminants were assumed to be contained in the constant exhalation of source patient. The assumptions were adopted partially for the purpose of using the concept of ventilation efficiency. However, Fabian et al. showed that the influenza virus could be included in fine particles generated during breathing and that most of the particles were below 1 μm, which were rarely affected by drag force or gravity [26]. Moreover, McDevitt performed a series of experiment using an atomizer for illustrating constant exhaled breath [27].
5. Conclusions
The germicidal effect of a UR-UVGI system is related with the air movement in a room because the air carries airborne infectious contaminants from sources. A new modified method using the concept of ventilation efficiency was introduced in this study. The results showed that the UV doses of the air exhaled by a source patient were correlated with SVE3∗, the age of the exhaled air. The UV doses and the SVE3∗ are affected by the air movement, which is mostly affected by how the ventilation system is operated. However, a longer residence time of contaminated air is not always desirable because a longer age of the air does not guarantee a higher UV dose. A longer age of air without enough UV radiation in the lower area of the room could be contrarily harmful to the occupants. The UV doses, therefore, need to be estimated.
In addition to the air movement, the position of the UR-UVGI system was another important factor for the germicidal effect in the case model study. The residence time of the air near the UR-UVGI system, where UV radiation was the strongest, was eventually decided by the air movement, not just by the position of the system. The effects of the position of the UR-UVGI system and the air movement on the UV doses were clearly illustrated using the method in the case study. The method is applicable to estimating the germicidal performance of UR-UVGI system itself, regardless of the source concentration and species.
Acknowledgments
The authors would like to express special appreciation to Tokyo Electric Power Company and NIHON SEKKEI, Inc. for their financial support for this research.
References
- 1.Hockberger P.E. A history of ultraviolet photobiology for humans, animals and microorganisms. Photochemistry and Photobiology. 2002;76:561–579. doi: 10.1562/0031-8655(2002)0760561AHOUPF2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 2.Hollaender A., Oliphant J.W. The inactivating effect of monochromatic ultraviolet radiation of influenza virus. Journal of Bacteriology. 1944;48(4):447–454. doi: 10.1128/jb.48.4.447-454.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley R.L., Mills C.C., O’Grady F., Sultan L.U., Wittstadt F., Shivpuri D.N. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. American Review of Respiratory Disease. 1962;85:511–525. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 4.Wells W.F., Brown H.W. Recovery of influenza virus suspended in air and its destruction by ultraviolet radiation. American Journal of Epidemiology. 1936;24(2):407–413. [Google Scholar]
- 5.Wells W.F., Fair G.M. Viability of B. coli exposed to ultra-violet radiation in air. Science. 1935;82(2125):280–281. doi: 10.1126/science.82.2125.280-a. [DOI] [PubMed] [Google Scholar]
- 6.Wells W.F. Harvard University Press; 1955. Airborne contagion and air hygiene. [Google Scholar]
- 7.IUVA . IUVA Draft Guideline IUVA-G01A; 2006. General guideline for UVGI air and surface disinfection system. [Google Scholar]
- 8.WHO Environmental health criteria monograph ultraviolet radiation. Retrieved April 15, 2011 from http://www.inchem.org/documents/ehc/ehc/ehc160.htm.
- 9.Xu P., Peccia J., Fabian P., Martyny J., Fennelly K., Hernandez M. Efficacy of ultraviolet germicidal irradiation of upper-room air in inactivating airborne bacterial spores and mycobacteria in full-scale studies. Atmospheric Environment. 2003;37:405–419. [Google Scholar]
- 10.First M., Rudnick S.N., Banahan K.F., Vincent R.L., Brickner P.W. Fundamental factors affecting upper-room ultraviolet germicidal irradiation – Part I. Experimental. Journal of Occupational and Environmental Hygiene. 2007;4(5):321–331. doi: 10.1080/15459620701271693. [DOI] [PubMed] [Google Scholar]
- 11.Ko G., First M.W., Burge H.A. The characterization of upper-room ultraviolet germicidal irradiation in inactivating airborne microorganisms. Environmental Health Perspectives. 2002;110(1):95–101. doi: 10.1289/ehp.0211095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noakes C., Sleigh P., Fletcher L., Beggs C. Use of CFD modelling to optimise the design of upper-room UVGI disinfection systems for ventilated rooms. Indoor Built Environment. 2006;15(4):347–356. [Google Scholar]
- 13.Sung M., Kato S. Method to evaluate UV dose of upper-room UVGI system using the concept of ventilation efficiency. Building and Environment. 2010;45(7):1626–1631. doi: 10.1016/j.buildenv.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandberg M. What is ventilation efficiency? Building and Environment. 1981;16:123–135. [Google Scholar]
- 15.Sandberg M., Sjoberg M. The use of moments for assessing air quality in ventilated rooms. Building and Environment. 1983;18:181–197. [Google Scholar]
- 16.Kato S. New ventilation efficiency scales based on spatial distribution of contaminant concentration aided by numerical simulation. ASHRAE Transaction. 1988;94:309–330. [Google Scholar]
- 17.Kato S., Yang J.H. Study on inhaled air quality in a personal air-conditioning environment using new scales of ventilation efficiency. Building and Environment. 2008;43:494–507. [Google Scholar]
- 18.Kato S, Murakami S, Kobayashi H. New scale for evaluating ventilation efficiency as affected by supply and exhaust openings based on spatial distribution of contaminant. ISRACVE ’93, 1993.
- 19.Kowalski W., Bahnfleth W. Effective UVGI system design through improved modeling. ASHRAE Transaction. 2000;106(2) NM-00-11-1. [Google Scholar]
- 20.Walker C.M., Ko G.P. Effect of ultraviolet germicidal irradiation on viral aerosols. Environmental Science & Technology. 2007;41:5460–5465. doi: 10.1021/es070056u. [DOI] [PubMed] [Google Scholar]
- 21.McDevitt J, Rudnick S and Radonovich L. Characterization of UVC light sensitivity of influenza virus aerosols. AAAR 29th Annual Conference, 2010: 389.
- 22.Riley R.L., Knight M., Middlebrook G. Ultraviolet susceptibility of BCG and virulent tubercle bacilli. American Review of Respiratory Disease. 1976;113:413–418. doi: 10.1164/arrd.1976.113.4.413. [DOI] [PubMed] [Google Scholar]
- 23.Luckiesh M., Taylor A.H., Knowles T., Leppelmeier E.T. Inactivation of molds by germicidal ultraviolet energy. Journal of Franklin Institute. 1949;248:311–325. [Google Scholar]
- 24.Kowalski W. Springer; 2010. Ultraviolet germicidal irradiation handbook. [Google Scholar]
- 25.Macher J.M., Alevantis L.E., Chang Y.L., Liu K.S. Effect of ultraviolet germicidal lamps on airborne microorganisms in an outpatient waiting room. Applied Occupational & Environmental Hygiene. 1992;7(8):505–513. [Google Scholar]
- 26.McDevitt J.J., Milton D.K., Rudnick S.N., First M.W. Inactivation of poxviruses by upper-room UVC light in a simulated hospital room environment. PLoS ONE. 2008;3(9) doi: 10.1371/journal.pone.0003186. e3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabian P., McDevitt J.J., DeHaan W.H., Fung R.O.P., Cowling B.J., Chan K.H. Influenza virus in human exhaled breath: an observational study. PLoS ONE. 2008;3(7) doi: 10.1371/journal.pone.0002691. e2691. [DOI] [PMC free article] [PubMed] [Google Scholar]


