HISTORICAL PERSPECTIVE AND SCOPE
In Schaffer's 1965 issue, Diseases of the Newborn,103 no mention is made of necrotizing enterocolitis (NEC). In the 1971 issue,104 it is discussed only briefly. Although references are made to an entity resembling NEC in the 19th and early 20th centuries,43, 89, 95 it generally was not recognized as a disease that affects primarily premature infants until the 1950s and 1960s,12, 80, 99, 107 which attests to its relatively infrequent incidence or recognition prior to modern neonatal intensive care.
During the past two decades, improvements in mechanical ventilatory support coupled with prevention and treatment for pulmonary immaturity have significantly improved the survival rate of low birth weight neonates. Concurrently, the incidence of NEC has increased in most centers and has emerged as the most common gastrointestinal emergency in neonates, affecting 2000 to 4000 newborns in the United States each year.21, 22, 65, 102, 116, 125 Of these, 10% to 50% die, resulting in approximately 1000 infant deaths per year.36 This number is close to the number of all US children fewer than 15 years of age who die of leukemia, or all children and adolescents fewer than 20 years who die of meningitis. The survivors of the acute episode of NEC frequently suffer with the effects of short bowel syndrome,63, 64 which is a major cause of prolonged hospitalization and high medical expenses.
Comprehensive information about the clinical aspects of NEC can be found in numerous textbooks and previously written reviews. * In this review, the clinical presentation and treatment of NEC is given only brief attention. The major focus is to provide an in-depth discussion of putative pathophysiologic events leading to NEC. An understanding of these events may hold the key for future prevention of this devastating disease.
CLINICAL PRESENTATION
Necrotizing enterocolitis is characterized by the following symptomatology and pathology: abdominal distention and tenderness, pneumatosis intestinalis, occult or frank blood in stools, intestinal gangrene, bowel perforation, sepsis, and shock. Based on clinical presentation, Bell and colleagues11 originally described three levels of NEC, with stage 1 being suggestive, stage 2 being definitive, and stage 3 being severe (Table 1) . Although stage 1 is nonspecific and may, in many instances, reflect feeding intolerance, sepsis, or gastrointestinal hemorrhage due to stress or other factors, stages 2 and 3 frequently are associated with considerable morbidity and mortality.
Table 1.
MODIFIED BELL STAGING CRITERIA FOR NECROTIZING ENTEROCOLITIS
| Stage | Classification< | Systemic Signs | Intestinal Signs | Radiologic Signs |
|---|---|---|---|---|
| IA | Suspected NEC | Temperature instability, apnea, bradycardia, lethargy | Increased pregavage residuals, midabdominal distention, emesis, guaiac-positive stool | Normal or intestinal dilation, mild ileus |
| IB | Suspected NEC | Same as above | Bright red blood from rectum | Same as above |
| IIA | Proven NEC—mildly ill | Same as above | Same as above, plus absent bowel sounds, with or without abdominal tenderness | Intestinal dilation, ileus, pneumatosis intestinalis |
| IIB | Proven NEC—moderately ill | Same as above, plus mild metabolic acidosis and mild thrombocytopenia | Same as above, plus absent bowel sounds, definite tenderness, with or without abdominal cellulitis or right lower quadrant mass | Same as IIB, plus definite ascites |
| IIIA | Advanced NEC—severely ill, bowel intact | Same as IIB, plus hypotension bradycardia, severe apnea, combined respiratory and metabolic acidosis, disseminated intravascular coagulation, and neutropenia | Same as above, plus signs of generalized peritonitis, marked tenderness, and distention of abdomen | Same as IIB, plus definite ascites |
| IIIB | Advanced NEC—severely ill, bowel perforated | Same as IIIA | Same as IIIA | Same as IIB, plus pneumoperitoneum |
| NEC = necrotizing enterocolitis. | ||||
RADIOLOGIC FINDINGS
During the early stages of illness, the radiographic findings are nonspecific and include dilated bowel loops, generalized bowel distention, and bowel-wall thickening. A single persistent dilated loop should cause suspicion but is not a definitive sign of NEC or perforation.
Pneumatosis intestinalis, or gas in the bowel wall, in the appropriate clinical setting is usually diagnostic of NEC (Figs. 1 and 2) When pneumatosis intestinalis extends into the portal circulation (Fig. 3) , it frequently is associated with severe disease. Pneumoperitoneum is frequently diagnostic of intestinal perforation with NEC. The air within the peritoneal cavity floats to the most nondependent area, which is just beneath the anterior upper abdominal wall in the supine patient. This air frequently outlines the falciform ligament (Fig. 4). Left lateral decubitus films can provide more information than supine films. Free air floats to the right surface of the peritoneal cavity and appears as a lucent area lateral to the liver edge. Careful serial reviews of left lateral decubitus films commonly are used to follow the progression of NEC and to determine whether perforation has occurred. Usually, pneumoperitoneum is considered an indication for surgery, whereas pneumatosis alone, without clinical deterioration of hematologic signs or acid-base status, usually is treated medically.
Figure 1.
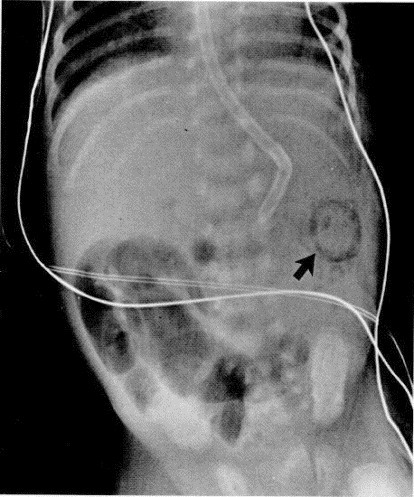
Several dilated air-filled loops of bowel in the right lower quadrant indicate a focal ileus. The arrow points to submucosal air in the splenic flexure.
Figure 2.
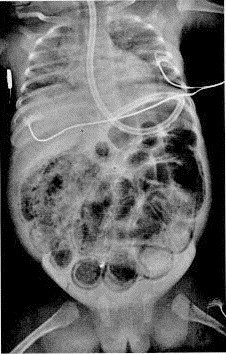
Film of the chest and abdomen shows massive air-distended bowel with diffuse intramural air.
Figure 3.
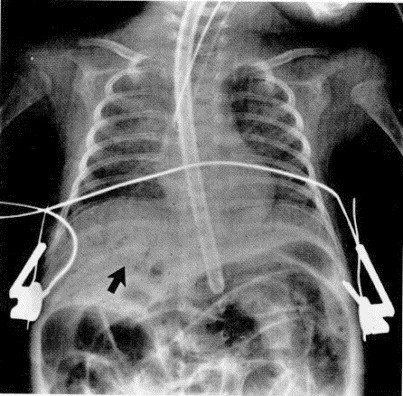
Air in the portal system (arrow).
Figure 4.
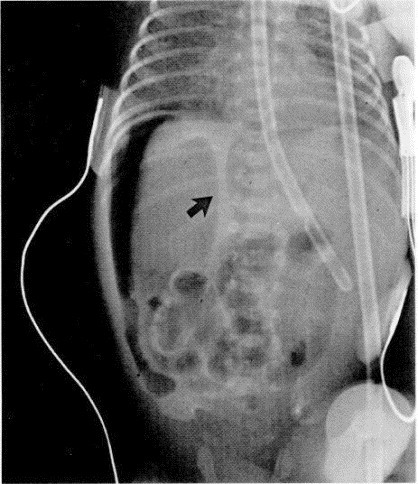
Left lateral decubitus film of the abdomen shows free intraperitoneal air from a bowel perforation. Free air is shown on both sides of the falciform ligament (arrow).
LABORATORY FEATURES
No individual laboratory features are diagnostic of NEC. Laboratory analysis primarily is used to confirm clinical diagnostic impressions and to judge progression of severity of disease.108 Peripheral hematologic studies may reveal abnormally high or low white blood cell counts with a shift toward immature precursors. Progressively decreasing absolute granulocyte count and thrombocytopenia suggest increasing severity of disease. If these are seen together with acidosis and severe electrolyte abnormalities, then the severity of NEC likely is progressing toward the necessity for surgical intervention.
PATHOLOGIC FINDINGS
The pathologic findings of NEC have been described by examination of the most severely affected patients who either died or who had intestinal perforation requiring resection of gangrenous bowel.5, 65 Gross examination reveals involvement predominantly in the terminal ileum and proximal colon. In severe cases, however, the bowel from the stomach to the rectum may be involved. Histologic analysis reveals mucosal edema, hemorrhage, coagulation necrosis, and mucosal ulceration. The pathologic changes of NEC suggest a multifactorial cause, with bacterial overgrowth, inflammation, ischemia, and necrotic tissue all having a role.
CURRENT THERAPIES
Therapy for NEC is highly dependent on its severity. Bell's staging criteria11 can be highly useful as guidelines. In stage 1, there is only a strong suspicion of the disease. Because of the potentially devastating progression of NEC, precautions need to be taken. In this scenario, clinical judgment based on the patient's condition should guide whether and how long the patient should be taken off enteral feedings, whether and how long intravenous antibiotics should be used, and how aggressively the patient is monitored with radiographs and laboratory tests. Once a definitive diagnosis is made but the patient has not progressed to surgical NEC (usually stage 3), the bowel should be decompressed using a large-bore orogastric tube with low intermittent suction. Careful attention needs to be placed on managing fluids and electrolytes because considerable third spacing of fluids with losses of sodium and protein may occur. Potassium fluxes (high or low serum potassium) with acid-base imbalances are likely to occur. Renal failure is a frequent concomitant finding. Systemic antibiotic therapy, usually with ampicillin and gentamicin, are started after obtaining blood cultures. In cases in which resistant Staphylococcus epidermidis is suspected, vancomycin is used instead of ampicillin. When perforation is suspected, or has occurred, clindamycin or metronidazole is used to treat anaerobic infection. Intensive support of respiratory and circulatory status is provided, as are frequent monitoring of the abdominal status with left lateral decubitus radiographs and frequent monitoring of acid-base and hematologic status. A persistent acidosis with continued deterioration of platelet and white blood cell counts may be an indication for surgery even in the absence of overt perforation on radiography.
A review of surgical therapy of NEC is beyond the scope of this review. It should be remembered, however, that NEC is a disease that requires a carefully coordinated approach among neonatologists and pediatric surgeons. Because NEC can be such a rapidly progressive disease, the pediatric surgery section should be notified immediately whenever a diagnosis of NEC is made, even when only medical intervention is necessary at the time. This will allow for more rapid mobilization for surgery if and when it progresses to a surgical emergency.
RISK FACTORS AND PUTATIVE PATHOPHYSIOLOGY
The pathophysiology of NEC is not clearly understood. Although classically described as a disorder of sick premature infants in the neonatal intensive care unit (NICU), clinical experience shows that this disease occurs in several different settings and hosts. Although occasionally occurring in term infants and sick preterm infants who are not on enteral feeding and on ventilatory support, many cases of NEC occur in premature infants receiving intermediate or stepdown neonatal intensive care.29, 112 Extremely premature infants (<28 weeks' gestation) are at risk for the development of NEC for a protracted period. The risk is high until the infant has achieved a postconceptual age of 35 to 36 weeks and sometimes later depending on coincident gastrointestinal problems.126, 127
The current thinking about the pathophysiology of NEC is based on epidemiologic studies from which several important risk factors have been dissected. The putative risk factors that predominate are prematurity, aggressive enteral feedings, infectious agents, and hypoxicischemic insults.
PREMATURITY
The primary risk factor for NEC is prematurity because approximately 90% of cases occur in premature infants.57, 65, 126 This disease rarely is seen in older children and adults. The cause of this age restriction remains unclear, but an immature mucosal barrier and immune response, in addition to impaired circulatory dynamics, are thought to make premature neonates particularly susceptible.62, 83 Immaturities of the developing gastrointestinal tract include immunologic factors, poor motility, reduced digestive absorptive function, increased membrane fluidity, low levels of protective mucus, and reduced regenerative capabilities, resulting in increased potential for tissue damage. Differences in gastrointestinal maturity that predispose premature infants to NEC include the following:
Immunologic factors
Decreased IgA secretory component
Decreased intestinal T lymphocytes
Poor antibody response
Lumenal factors
Lower H+ ion output in stomach
Low proteolytic enzyme activity
Immature intestinal barrier
Mucin blanket composition
Microvillus membrane biophysical properties and composition
Higher permeability
Lower and less organized motility
Immunologic Factors
Necrotizing enterocolitis occurs most frequently in the terminal ileum and colon.66 Large numbers of lymph follicles (Peyer's patches) are present in these regions.88 An observation in rabbits of decreased IgA secretory component overlying the Peyer's patches of the ileum,93 increased macromolecular uptake in this area60, 87, 129 and translocation of bacteria in this region13, 14, 56 are interesting in view of the frequent localization of NEC to this part of the intestine. Infant animals have also been shown to have decreased numbers of intestinal T lymphocytes,50 indicating a compromise in cellular immunity early in life. The underdeveloped T-lymphocyte function may compromise immunologic surveillance and recognition of alterations in the membrane of an epithelial cell infected by a bacterial or viral agent. This compromises the ability to destroy the infected cell before the infectious agent can cross the basement membrane underlying the epithelium.59
Infants of less than 35 weeks' gestation have demonstrated a relatively poor response in the production of antibodies when compared with those of more than 35 weeks' gestation.97 As the microbial agents or their toxins are allowed to enter the intestine, the premature newborn likely has a limited capability to respond immunologically.73, 97
Immature Lumenal Factors
Numerous immaturities in lumenal digestion exist in premature infants. One of the first lines of defense against ingested pathogens and toxins is gastric acid.119 Gastric-hydrogen ion output is low in the human neonate when compared with adults.48, 83 This places these infants at an increased risk for colonization of enteric pathogens. Accordingly, acidification of feedings was demonstrated to decrease the incidence of NEC in one study.27
Proteolytic enzyme activity is also low. Enterokinase, the brushborder enzyme in the duodenum, converts inactive trypsinogen to active trypsin. The relatively low enterokinase activity4, 132 and subsequently low tryptic activity might suppress the hydrolysis of various toxins that have the ability to damage the intestine. The occurrence of pigbel, a disease similar to NEC, seen in New Guinea, supports the importance of proteases as lumenal protective agents. Many highlanders of New Guinea subsist on a diet high in antiproteases. During festivals, massive quantities of uncooked meat are consumed after a prolonged fast. Enterocolitis develops, thought to be due to Clostridium beta toxin,82 which passes into the intestine without the benefit of hydrolysis by endogenous proteases. The ensuing necrotic lesions in the gastrointestinal tract together with the propensity to perforate are similar to the intestinal pathology of NEC.
Immature Intestinal Epithelial Barrier
The intestinal mucin blanket also seems to be scant and have a different composition in newborn infant2, 57, 102 when compared with adults, which makes the immature intestine more permeable to high molecular weight molecules.39 This is also likely to facilitate bacterial adherence to the epithelium.2, 57, 101
The microvillus membrane composition and biophysical properties have been found to change with increasing maturation. Neonatal rabbits'90 and rats'51 microvillus membranes have higher lipid-protein ratios than those of adults. The fluidity (organization) of the microvillus membrane also changes as the animal matures and is shifted toward a more mature pattern by glucocorticoids administered to the mother antenatally or the infant postnatally.85, 91
Immature neonates have higher intestinal permeability than older children and adults. Preterm infants, especially those born at less than 33 weeks of gestation have higher serum concentrations of β-lactoglobulin than term neonates given equivalent milk feedings.98 The permeability of the preterm human intestine to intact carbohydrate is greater in infants than in children or adults.7 Using measurements of lactulose and rhamnose in the urine after ingesting milk containing these carbohydrates, some investigators have found that preterm neonates of 31 to 36 weeks' gestational age had an enhanced permeability of lactulose in the first week of life that changed to a more mature pattern in the second week. Infants born at a gestational age of 26 to 29 weeks had a more mature period of permeability at birth, followed by a temporary period of enhanced permeability at 3 to 4 weeks of age. Overall, there seems to be a developmental pattern of decreased permeability with maturation. Breast-feeding is associated with a lower permeability to lactulose than formula-feeding,120 which suggests that human milk contains factors that stimulate maturation of the small intestine. The specific nature of these factors remains speculative.
In addition to being relatively permeable to the uptake of macromolecules, the intestine of the newborn is also more permeable to the uptake of intact bacteria. When a breakdown in the mucosal barrier occurs, lumenal bacteria may translocate across the bowel wall into the blood or mesenteric lymph nodes.47, 121 Translocation is likely to be responsible for many of the positive bacterial cultures obtained from the blood of neonates with NEC.34
Motility
The motility of the small intestine in premature infants is considerably lower and less organized than that in term infants.121 This is caused by an intrinsic immaturity of the enteric nervous system that may cause delayed transit and subsequent bacterial overgrowth and distention. Although it is not clear specifically what role immature motility has in the pathogenesis of NEC, it likely contributes to the milieu in which the interaction of nutrients, immature host defenses, and other factors initiate the cascade of events culminating in NEC.15
AGGRESSIVE ENTERAL FEEDINGS
Necrotizing enterocolitis rarely, if ever, occurs in utero.21, 65, 72 The fetus swallows up to 150 mL/kg/d of sterile amniotic fluid, which contains various nutrients, growth factors, and immunoglobulins in concentrations very different than those of formula or human milk.94, 106 Although amniotic fluid provides a small amount of nutrients that can be utilized by the fetus, the majority of nutrition for the fetus comes from the placental circulation. In postnatal life, the previously sterile intestine becomes colonized with bacteria and is frequently stressed with relatively concentrated feedings. What accounts for the exclusive onset of NEC in the postnatal state? Perhaps, some of the components of amniotic fluid are highly protective to the fetal gastrointestinal (GI) tract. The fact that the GI tract of the fetus is not colonized with bacteria or exposed to a high volume of lumenal nutrients also likely has a role.
Although NEC occasionally occurs in infants who have never been fed,3 it most frequently occurs in premature infants on enteral feedings and especially those whose enteral intakes are being aggressively increased. The advancement of formula feedings at rates greater than 20 kcal/kg/d has been found to be associated with an increase in the incidence of NEC.3, 18, 20, 29, 118, 130 Despite the importance of aggressive enteral feedings in the pathogenesis of NEC, the finding in several studies that "minimal enteral feeding" or priming the GI tract by a very slow intake using food as a trophic agent to stimulate GI mucosal development has not been associated with an increased incidence of NEC.71, 76, 79, 111 Rather, there has been an improved tolerance to subsequent enteral feedings, lower incidence of cholestasis, and higher levels of potentially trophic gut hormones.
The possibility that human milk provides protection against NEC is supported by a multicenter trial designed to evaluate the effect of early diet on the incidence of NEC.77 The study population, which was divided into infants fed only formula, those fed with formula plus expressed human milk, and those fed with expressed human milk alone, showed an incidence of 7.2%, 2.5%, and 1.2%, respectively. The theoretic basis of this is that human milk contains several known growth factors,96 hormones,67 macrophages, leukocytes, lymphocytes, immunoglobulins,75 and enzymes.110 Whether a similar protective effect would be incurred by banked donor human milk, which loses many potentially protective factors, remains speculative.
INFECTIOUS AGENTS
Prematurity and the presence of bacteria in the GI tract seem to be the most consistent risk factors associated with the development of NEC. Whether bacteria are primary inciting factors, merely permissive agents, or both in the pathogenic cascade leading to NEC remains unanswered.
Several lines of evidence support th0e thesis that infection is necessary for the development of NEC.10, 19, 72, 100 This includes epidemiologic evidence for outbreaks suggestive of an infective process, the frequent isolation of infectious agents with NEC, and the decreased incidence of NEC resulting from preventive measures. Because many of the bacteria isolated from infants with NEC (from stool, blood, and peritoneal fluid) are organisms commonly found in the intestine, it is impossible to tell whether the presence of these bacteria is a causative factor or whether they are merely bystanders to a separate pathologic process. The latter is unlikely because of the large number of infants with NEC who exhibit overt pneumatosis intestinalis. If one uses Bell's criteria for the diagnosis of "proven" NEC (stage 2), the radiologic finding of pneumatosis is a necessary component. The gas in pneumatosis is thought to be derived primarily from bacterial fermentation.43, 44, 61
Kosloske68, 69, presents bacteria as central factors in the "unifying hypothesis for pathogenesis and prevention of necrotizing enterocolitis." Bacteria commonly isolated from infants with NEC are gram-negative rods, including Klebsiella spp., Escherichia coli Enterobacter spp., and Pseudomonas spp.28 These bacteria can be isolated from blood, peritoneal fluid, intestinal tissues, and feces.28, 81, 100, 122, 131 Other microorganisms associated with NEC are the bacteria Clostridium difficile and Staphylococcus epidermidis and the viruses coronavirus and rotavirus. The major arguments against etiologic roles for C difficile and S epidermis in NEC are the high rate of isolation of these bacteria or identification of their toxins in healthy, matched neonates and the lack of consistent recovery or detection of the bacteria or toxins in patients with NEC.69
Bacterial toxins also have been proposed as causes of NEC105 however, some potent toxins, such as Clostridium toxin, are isolated commonly from asymptomatic infants.37 Because many different infectious agents have been associated with NEC, these agents may possess common virulence factors that may predispose susceptible hosts to the cascade of events involved in the pathophysiologic cascade of NEC. For example, many members of the family Enterobacteriaceae possess genes encoding Shiga-like toxin and cholera-like toxin.1, 53, 115 Whether these genes are expressed by NEC pathogens and whether they have any role in disease are still unknown. Other possible common virulence factors may involve metabolic traits that predispose the host to other factors in the pathogenic cascade, such as a high capability to ferment lactose.26
A majority of very premature babies in the NICU are started on broad-spectrum IV antibiotic therapy shortly after birth during a "rule out sepsis" work-up. This can markedly alter the normal flora with which the neonate would become colonized.9, 55 Rather than becoming colonized with Lactobacillus and other "normal" gut flora, resistant species indigenous to the NICU may colonize in the baby's intestine. Whether or how much of a role this has in the pathogenesis of NEC is not known. Recent studies also have shown that NEC-associated bacteria have a greater propensity than non-NEC-associated bacteria of the same species to prevent adherence of gram-positive bacteria to the GI tract and to cause disease in an animal model during coinfection with grampositive isolates from the homologous child.92 This could lead to an intestinal milieu consisting of a very large number of organisms more conducive to the development of NEC than would otherwise reside there. Other studies have shown that certain Klebsiella species that have a genetic predisposition (plasmid) for active fermentation of lactose also have the ability to cause NEC in isolated loops of rabbit small intestine.26
Infection-Associated Inflammatory Mediators
Similar to sepsis and adult respiratory distress syndrome, NEC seems to involve a final common pathway that includes the endogenous production of inflammatory mediators involved in the development of intestinal injury. Endotoxin lipopolysaccharide, platelet-activating factor (PAF), tumor necrosis factor (TNF), and other cytokines together with prostaglandins and leukotrienes are thought to be involved in the final common pathway of NEC pathogenesis.25, 52
Bacteria possess endotoxins that instigate the inflammatory cascade by activating PAF, TNF, and interleukin 1. PAF injected into the aorta of adult rats has been found to cause necrosis of the bowel24 that can be prevented by pretreatment with PAF-acetylhydrolase70 and can be exacerbated by a nitric oxide synthase inhibitor.78 The generation of these inflammatory mediators also has been shown to be decreased by the pretreatment with glucocorticoids,24 which also have been shown to decrease the incidence of NEC if administered to the mother prior to the birth of her infant and in infants who are at high risk for the development of NEC.6
HYPOXIA-ISCHEMIA
Early theories of the pathogenesis of NEC suggested circulatory perturbations to be the most important determinants of pathogenesis.74, 113 The physiologic support for linking ischemia to NEC relates a shunting of blood from the intestine during times of perinatal asphyxia. This was generated by the fact that many of the babies at that time who developed NEC also had antecedent episodes of perinatal distress.54, 74, 113 Laboratory investigations in several animal models also demonstrated that a lack of perfusion to the bowel was an important antecedent to bowel necrosis.30, 31, 117 This, coupled with the knowledge of a known redistribution of blood from the intestine to preserve blood flow to the brain, heart, and kidneys (in diving mammals, the "diving reflex"), led to the logical conclusion that hypoxia-ischemia is a major predisposing factor in the pathogenesis of NEC.109 Other compelling evidence supporting ischemia as a major causative factor for NEC included histopathologic data that clearly indicated that ischemia occurs in the disease process.5
The clinical data that supported a hypoxic-ischemic role in the pathogenesis of NEC were largely anecdotal. The physiologic studies and clinical reports linking circulatory disturbances to the etiology of NEC recently have been disputed by both physiologic and clinical data.86 Subsequent epidemiologic case-control studies showed that hypoxicischemic insults were not relevant risk factors in the development of NEC.29, 33, 65, 114 The fact that so many cases of NEC occur in the intermediate or stepdown intensive care unit in babies who have never had known antecedent stresses, such as low Apgar scores, need for ventilatory support, umbilical catheters, polycythemia, or significant apneic episodes mitigates against hypoxia-ischemia as a major causative factor.
Even though both physiologic and clinical observations argue against a primary circulatory aberration as the cause of NEC, the histologic appearance of the disease, which includes coagulation necrosis suggesting ischemia as a component of the pathway, is compelling.5 Hypoxia/ischemia to the bowel actually may be a secondary event that is mediated by other factors. It is tenable that inflammatory mediators released after endotoxin or bacterial invasion across the mucosa may lead to vasoconstriction and hypoxic-ischemic insults. The vascular control systems are known to be quite immature in these infants and could make the premature intestine more susceptible to the development of local tissue hypoxia.86 Resting vascular resistance is known to be low in premature infants. The endothelium of the small intestine is responsible for the production of nitric oxide, a potent mediator known to maintain low resting vascular resistance. If endothelial injury occurs, subsequent damage to the nitric oxide system likely could have a detrimental effect on local vascular tone, which could, at least theoretically, result in some of the tissue injury seen in NEC that is compatible with an ischemic insult.78 Thus, other triggers, such as bacterial toxins or chemical irritation, could set off a cascade of events that could lead to endothelial disruption, altered nitric oxide production, and subsequently vascular compromise.86 The specific relationship of endotoxin, PAF, and nitric oxide together with other mediators remains to be elucidated. Whether elucidation of this pathway might result in a potential treatment or prevention for NEC is discussed later.
PREVENTION
Several measures are commonly used to prevent NEC outbreaks. The first includes careful epidemic precautions when an outbreak of NEC is suspected. These precautions are predicated on an increased awareness of NEC being present in the NICU in more than one infant and a suspicion that microbial agents are involved in the pathogenesis of the outbreak. Preventive measures include strict infection-control measures to prevent fecal and oral spread; cohorting of patients, contacts, and personnel; and using a decreased threshold for early intervention, such as placing babies nulla per os (NPO), providing antibiotics while cultures are pending, and delaying or slowing enteral feedings while an outbreak is suspected. Although oral antimicrobial agents have been used for prophylaxis in contacts to interrupt the outbreak,8, 40, 49 the possibility of emergence of resistant organisms limits their routine long-term use.41
Although glucocorticoids are not routinely used for the prophylaxis of NEC as they are being used for the prevention of respiratory distress syndrome (RDS), the data are highly suggestive of their potential benefit.6, 32, 51, 58 The mechanism for this is unclear but may involve a maturational effect on the microvillus membrane,85, 91 an increase in the activities of several enzymes involved in digestion and absorption of nutrients,83 or a decrease in mucosal inflammation. In reality, many of the babies at highest risk for NEC are also at highest risk for RDS and thus may actually be receiving prophylaxis antenatally with glucocorticoids via the mother. The decreased incidence of NEC in babies of mothers who are treated with antenatal steroids6, 32 should provide an additional indication for the administration of corticosteroids to mothers in preterm labor. Whether the routine use of postnatal glucocorticoid prophylaxis for NEC would be effective without causing other risks to the infant, such as increasing the incidence of sepsis or catabolism, is not known, and further studies are needed to determine the risks versus the benefits of such prophylaxis.
Providing human milk to premature infants has been shown to decrease the incidence of NEC in one large multicenter study.77 Fresh human milk is composed of numerous immunoprotective factors, such as immunoglobulins, lysozyme, lactoferrin, macrophages, lymphocytes, and neutrophils.23 The possibility of banked or refrigerated donor milk providing benefit is less clear because the cellular components and immunoglobulins are compromised by the pasteurization or freezing process. Another potentially beneficial component of human milk recently discovered is PAF acetyl hydrolase (PAF-AH-102). This enzyme inhibits the activity of PAF, which is likely to be a significant mediator in the pathophysiologic cascade of NEC. Because of the likely protective effect against NEC and other infections in addition to the nutritional and psychosocial benefits afforded by human milk, it is the author's opinion that premature infants should be provided with his or her own mother's fresh milk whenever feasible.
The finding that an IgG-IgA preparation obtained from human serum provided protection against NEC in premature infants in a randomized trial is compelling.42 Whether this preparation acts to directly prevent bacterial invasion into the gut or to counteract the release of cytokines from monocytes128 is not known. This study should be repeated and confirmed at a larger scale, preferably in a multicenter format prior to recommending routine use of this modality for the prevention of NEC.
As previously discussed, the aggressive institution of enteral feedings in preterm infants is a frequent risk factor associated with NEC. This has, in many cases, caused neonatologists to institute an overly cautious approach to enteral feedings whereby the baby is placed NPO for several weeks after birth and nourished only by the parenteral route. This approach is known to cause atrophy of the intestinal mucosa and may be highly detrimental in that the onset of NEC may be delayed only after feedings are finally instituted. An alternative approach with several different names, including "minimal enteral nutrition," "intestinal priming," and "hypocaloric feedings," has been studied in a controlled randomized manner38, 79 involving use of enteral nutrition to provide topical nutrition to the small intestine during the first week or two of life while providing most of the nutritional needs for the infant by the parenteral route. It is usually reserved for infants weighing less than 1500 grams. "Priming" is not contraindicated while the infant is on ventilatory support or using umbilical catheters. The enteral feedings are increased so that the infant is on full enteral intake in the third to fourth week of life. This approach has been found to improve tolerance to subsequent enteral intake, stimulate early secretion of several intestinal hormones that may be trophic in the intestinal mucosa, and improve motility.16 Despite demonstrating these advantages, the numbers from the individual studies were too small to determine whether this approach caused a significant decrease in the incidence of NEC.
The osmolarity, carbohydrate composition, and pH of the enteral intake also have been implicated in the pathogenesis of NEC. Formulas with high osmolarity have been shown to increase the incidence of NEC in human infants17 and cause mucosal injury in animal models.35 Many oral medications, such as vitamin preparations, use hyperosmolar vehicles123, 124 that potentially could cause osmotic injury to the bowel. The carbohydrate found in human milk and most commercial infant formulas is lactose. There is some controversy as to whether the premature small intestine has the capability to hydrolyze lactose to the same degree as that of the term infant.83 Also, the lactose not hydrolyzed by the intestine may be hydrolyzed by bacteria, with the subsequent fermentative generation of hydrogen gas and short-chain fatty acids.61 The presence of certain bacteria that have an especially efficient capability to ferment lactose using beta-galactosidase could lead to a high concentration of lumenal short-chain fatty acids, which could alter the pH of the small bowel and cause mucosal breakdown, with the invasion of bacteria into the mucosa and production of pneumatosis intestinalis.26 In the upper GI tract, the situation is different than in the distal. Many premature infants are hypochlorhydric and do not respond as quickly as older children or adults to a meal with brisk production of gastric acid and peptic proteases.48, 83 This acid environment is an effective barrier to many potentially pathogenic microorganisms. A prospective doubleblind study that compared the incidence of NEC in a group of infants fed a regular formula to infants fed a formula supplemented with acid to achieve a pH between 3 and 4 showed a lower incidence of NEC in the acid-supplemented group.27 These studies should be confirmed before considering routine use of this method.
Another method that has been attempted as prophylaxis against NEC included the provision of oral aminoglycosides (kanamycin or gentamicin). Several of the studies suggested a significant decrease in the incidence of NEC in the groups given oral antibiotics,8, 40, 49 compared with controls. The long-term use of these antibiotics unfortunately was associated with the emergence of resistant strains of Staphylococcus, Klebsiella, and E coli.41 These findings have discouraged the routine use of long-term oral antibiotic therapy for the prophylaxis of NEC.
(Table 2) shows the pathophysiologic features together with the corresponding preventative measures that have been attempted or are being used in the prevention of NEC.
Table 2.
PREVENTION BASED ON PATHOPHYSIOLOGY
| Pathophysiologic Feature | Preventive Measures |
|---|---|
| Microbial infection | Oral antibiotics Formula acidification Epidemiologic control Human milk IgG-IgA |
| Immature intestine | Glucocorticoids Human milk Intestinal priming |
FUTURE APPROACHES FOR PREVENTION
As the pathophysiologic cascade for NEC becomes more defined and the tools for investigative science improve, the likelihood of finding effective prophylaxis for NEC increases. The potential for prophylaxis of NEC can be found at several levels. It is reasonable to assume that interventions aimed at the more proximal events of the cascade offer a greater likelihood for effective prophylaxis. Areas for possible intervention include the following:
Identification of a common microbial agent or common bacterial virulence factors
Maturation of the intestine using trophic agents
Interference in the host-response to the inciting factors
At this time, a single microbial agent known to cause NEC has not been identified. Some as yet unidentified microbes may be involved. Viruses such as coronavirus and rotavirus have been implicated in the pathogenesis of NEC but have not been consistently found. As previously discussed, it is highly likely that bacteria are involved, not necessarily as triggering agents, but as agents that enable the pathophysiologic cascade of NEC to proceed. The fact that so many premature neonates are placed on broad-spectrum antibiotics shortly after birth to rule out sepsis changes the gut flora toward resistant organisms that are likely to possess virulence factors very different than those in the normal flora. Perhaps strong consideration should not be given to pre-emptively treating all premature babies with antibiotics for 3 days pending culture results as is so commonly done. Obviously, the risk of sepsis needs to be carefully weighed against the likelihood of altering the bacterial microenvironment in each individual baby. Some bacteria cultured from babies with NEC have been found to have the capability to inhibit adherence of normal gut flora to CaCo-2 cells in culture.92 Other studies have shown that certain bacteria isolated from neonates with NEC have a highly efficient capability to ferment lactose in the presence of hydrolytic products of casein.26 These bacteria in turn were found to have the capability to produce severe intestinal damage in isolated rabbit intestinal loops. When isolated from the original bacterium and cloned into an otherwise nonpathogenic bacterium, the previously nonpathogenic bacterium becomes a rapid lactose fermenter and is able to cause disease in the isolated rabbit intestinal loops. There are likely to be numerous other virulence traits possessed by NEC-associated bacteria that have not yet been identified. Identification of these traits, proof of their pathogenicity, and subsequent production of neither active nor passive immunity against them offer the likelihood of specific prophylaxis against NEC.
The finding that human milk is likely to be protective against NEC has stimulated the search for various immunologic and nonimmunologic components of human milk that could be responsible. A specific nutrient, such as glutamine or nucleotides, may be involved. Glutamine is one of the most important metabolic substrates for the GI tract. This amino acid has been found to attenuate various forms of enterocolitis in animal models and to decrease permeability to lactulose and mannitol in adults. It is thought to become an essential amino acid during times of stress. It is not normally supplied to these babies in appreciable quantities by either the enteral or parenteral route because most premature neonates are not provided with appreciable enteral nutrition, and neonatal TPN solutions do not contain glutamine. Other growth factors and hormones that are known to directly improve GI function and maturity, such as epidermal growth factor, insulin-like growth factor 1, and thyroid hormone, also offer a fertile area for future investigation for the prevention of NEC.
Understanding the specific steps in the inflammatory cascade resulting in NEC also should offer opportunities for further intervention. Inhibitors of PAF, stimulators or inhibitors of nitric oxide synthase, cytokines, Prostaglandins, and leukotrienes may have a role in the future prevention of NEC. Although intervening in the inflammatory cascade seems to offer hope, the disease may be so far advanced that rescue at this stage is no longer likely, and prevention may be impossible. Intervention into more proximal events in the pathologic cascade by altering the lumenal milieu by immunizing against microbial virulence factors or maturing the intestinal barrier should offer the most promise for prophylaxis against NEC.
ACKNOWLEDGMENTS
The author thanks Jonathan Williams, MD, Division of Pediatric Radiology, University of Florida, for the radiographs; Pat Pike for her expert editorial assistance; and Matthew Knight, MD, for his critical review of the manuscript.
Footnotes
Address reprint requests to Josef Neu, MD, Department of Pediatrics, University of Florida College of Medicine, 1600 SW Archer Road, Room HD513, Gainesville, FL 32610
References 11, 21, 22, 65, 84, 112, 116, and 125.
References
- 1.Acheson D.W. Enterotoxins in acute infective diarrhoea. J Infect. 1992;24:225–245. doi: 10.1016/s0163-4453(05)80028-3. [DOI] [PubMed] [Google Scholar]
- 2.Allen A. Structure and function of gastrointestinal mucus. In: Johnson L.R., editor. Physiology of the Gastrointestinal Tract, Vol 1. Raven Press; New York: 1981. pp. 617–639. [Google Scholar]
- 3.Anderson D.M., Kliegman R.M. The relationship of neonatal alimentation practices to the occurrence of endemic necrotizing enterocolitis. Am J Perinatol. 1991;8:62. doi: 10.1055/s-2007-999344. [DOI] [PubMed] [Google Scholar]
- 4.Antonowicz I., Lebenthal E. Developmental pattern of small intestinal enterokinase and disaccharidase activities in the human fetus. Gastroenterology. 1977;72:1299–1303. [PubMed] [Google Scholar]
- 5.Ballance W.A., Dahms B.B., Shenker N. Pathology of neonatal necrotizing enterocolitis: A ten year experience. J Pediatr. 1990;117(suppl):6–13. doi: 10.1016/s0022-3476(05)81124-2. [DOI] [PubMed] [Google Scholar]
- 6.Bauer C.R., Morrison J.C., Poole W.K. A decreased incidence of necrotizing enterocolitis after prenatal glucocorticoid therapy. Pediatrics. 1984;73:682–688. [PubMed] [Google Scholar]
- 7.Beach R.C., Menzies I.S., Clayden G.S. Gastrointestinal permeability changes in the preterm neonate. Arch Dis Child. 1982;57:141–145. doi: 10.1136/adc.57.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell M.J., Kosloske A.M., Benton C. Neonatal necrotizing enterocolitis: Prevention of perforation. J Pediatr Surg. 1973;8:601. doi: 10.1016/0022-3468(73)90397-7. [DOI] [PubMed] [Google Scholar]
- 9.Bell M.J., Rudinsky M., Brotherton T. Gastrointestinal microecology in the critically ill neonate. J Pediatr Surg. 1984;19:745. doi: 10.1016/s0022-3468(84)80362-0. [DOI] [PubMed] [Google Scholar]
- 10.Bell M.J., Shackelford R.D., Feigin J. Epidemiologic and bacteriologic evaluation of neonatal necrotizing enterocolitis. J Pediatr Surg. 1979;14:1–4. doi: 10.1016/s0022-3468(79)80567-9. [DOI] [PubMed] [Google Scholar]
- 11.Bell M.J., Ternberg J.L., Feigin R.D. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berdon W.E. Necrotizing enterocolitis in premature infants. Radiology. 1964;83:879. doi: 10.1148/83.5.879. [DOI] [PubMed] [Google Scholar]
- 13.Berg R.D. Inhibition of Escherichia coli translocation from the gastrointestinal tract by normal cecal flora in gnotobiotic or antibiotic-decontaminated mice. Infect Immun. 1980;29:1073–1081. doi: 10.1128/iai.29.3.1073-1081.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg R.D., Garlington A.W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in the gnotobiotic or antibiotic-decontaminated mice. Infect Immun. 1979;23:403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berseth C.L. Gestational evolution of small intestinal motility in preterm and term infants. J Pediatr. 1989;1:138. doi: 10.1016/s0022-3476(89)80302-6. [DOI] [PubMed] [Google Scholar]
- 16.Berseth C.L., Nordyke C. Enteral nutrients promote postnatal maturation of motor activity in preterm infants. Am J Physiol. 1993;264:g1046. doi: 10.1152/ajpgi.1993.264.6.G1046. [DOI] [PubMed] [Google Scholar]
- 17.Book L.S., Herbst J.J., Atherton S.O. Necrotizing enterocolitis in low birth weight infants fed elemental formula. J Pediatr. 1975;87:602. doi: 10.1016/s0022-3476(75)80835-3. [DOI] [PubMed] [Google Scholar]
- 18.Book L.S., Herbst J.J., Jung A.L. Comparison of fast-and slow-feeding rate schedules to the development of necrotizing enterocolitis. J Pediatr. 1976;89:463. doi: 10.1016/s0022-3476(76)80552-5. [DOI] [PubMed] [Google Scholar]
- 19.Book L.S., Overall J.C., Jr, Herbst J.J. Clustering of necrotizing enterocolitis. Interruption by infection-control measures. N Engl J Med. 1977;297:984–986. doi: 10.1056/NEJM197711032971805. [DOI] [PubMed] [Google Scholar]
- 20.Brown E.G., Sweet A.Y. Preventing necrotizing enterocolitis in neonates. JAMA. 1978;240:2452. [PubMed] [Google Scholar]
- 21.Brown E.G., Sweet A.Y. Monographs in Neonatology. Grune & Stratton; New York: 1980. Neonatal necrotizing enterocolitis. [Google Scholar]
- 22.Brown E.G., Sweet A.Y. Neonatal necrotizing enterocolitis. Pediatr Clin North Am. 1982;29:1149–1170. doi: 10.1016/s0031-3955(16)34252-3. [DOI] [PubMed] [Google Scholar]
- 23.Buescher E.S. Host defense mechanisms of human milk and their relations to enteric infections and necrotizing enterocolitis. Clin Perinatol. 1994;21:247–262. [PubMed] [Google Scholar]
- 24.Caplan M.S., Hsueh W. Necrotizing enterocolitis: Role of platelet activating factor, endotoxin, and tumor necrosis factor. J Pediatr. 1990;117(suppl):47–51. doi: 10.1016/s0022-3476(05)81130-8. [DOI] [PubMed] [Google Scholar]
- 25.Caplan M.S., MacKendrick W. Inflammatory mediators and intestinal injury. Clin Perinatol. 1994;21:235–246. [PubMed] [Google Scholar]
- 26.Carbonaro C.A., Clark D.A., Elseviers D. A bacterial pathogenicity determinant associated with necrotizing enterocolitis. Microbiol Pathol. 1988;5:427–436. doi: 10.1016/0882-4010(88)90004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrion V., Egan E.A. Prevention of necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 1990;11:317. doi: 10.1097/00005176-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Chan K.L., Saing H., Yung R.W.H. A study of pre-antibiotic bacteriology in 125 patients with necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:45–48. doi: 10.1111/j.1651-2227.1994.tb13242.x. [DOI] [PubMed] [Google Scholar]
- 29.Covert R.F., Neu J., Elliott M.J. Factors associated with age of onset of necrotizing enterocolitis. Am J Perinatol. 1989;6:455–460. doi: 10.1055/s-2007-999639. [DOI] [PubMed] [Google Scholar]
- 30.Crissinger K., Granger D. Mucosal injury induced by ischemia and reperfusion in the piglet intestine: Influences of age and feeding. Gastroenterology. 1989;97:920. doi: 10.1016/0016-5085(89)91498-4. [DOI] [PubMed] [Google Scholar]
- 31.Crissinger K., Tso P., Burney D. The role of lipids in ischemia/reperfusion-induced changes in mucosal permeability in developing piglets. Gastroenterology. 1992;102:1693. doi: 10.1016/0016-5085(92)91732-j. [DOI] [PubMed] [Google Scholar]
- 32.Crowley P., Chalmers I., Keirse M.J.N.C. The effects of corticosteroid administration before delivery: Overview of the evidence from controlled trials. Br J Obstet Gynaecol. 1990;97:11. doi: 10.1111/j.1471-0528.1990.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 33.DeCurtis M., Paone M., Vetrano G. A case control study of necrotizing enterocolitis occurring over 8 years in a neonatal intensive care unit. Eur J Pediatr. 1987;146:398. doi: 10.1007/BF00444947. [DOI] [PubMed] [Google Scholar]
- 34.Deitch E.A. Role of bacterial translocation in necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:33–36. doi: 10.1111/j.1651-2227.1994.tb13239.x. [DOI] [PubMed] [Google Scholar]
- 35.deLemos R. Report of the Sixty-eighth Ross Conference on Pediatric Research: Necrotizing Enterocolitis in the Newborn Infant. Ross Laboratories; Columbus, OH: 1975. The role of hyperosmolar formulas in necrotizing enterocolitis-animal studies; p. 75. [Google Scholar]
- 36.Department of Health, Education, and Welfare . Vital Statistics of the United States, Part B. US Printing Office; Washington, DC: 1975. [Google Scholar]
- 37.Donta S.T., Myers M.G. Clostridium difficile toxin in asymptomatic neonates. J Pediatr. 1982;100:431. doi: 10.1016/s0022-3476(82)80454-x. [DOI] [PubMed] [Google Scholar]
- 38.Dunn L., Hulman S., Weiner J. Beneficial effects of early hypocaloric enteral feeding on neonatal gastrointestinal function: Preliminary report of a randomized trial. J Pediatr. 1988;112:622. doi: 10.1016/s0022-3476(88)80185-9. [DOI] [PubMed] [Google Scholar]
- 39.Edwards P.A.W. Is mucus a selective barrier to macromolecules? Br Med Bull. 1978;34:55–56. doi: 10.1093/oxfordjournals.bmb.a071459. [DOI] [PubMed] [Google Scholar]
- 40.Egan E.A., Mantilla G., Nelson R.M. A prospective controlled trial of oral kanamycin in the prevention of neonatal necrotizing enterocolitis. J Pediatr. 1976;89:467. doi: 10.1016/s0022-3476(76)80553-7. [DOI] [PubMed] [Google Scholar]
- 41.Egan E.A., Nelson R.M., Mantilla G. Additional experience with routine use of oral kanamycin prophylaxis for necrotizing enterocolitis in infants under 1500 grams. J Pediatr. 1977;90:331. doi: 10.1016/s0022-3476(77)80683-5. [DOI] [PubMed] [Google Scholar]
- 42.Eibl M.M., Wolf H.M., Furnkranz H. Prevention of necrotizing enterocolitis in low birth weight infants by IgA-IgG feeding. N Engl J Med. 1988;319:1. doi: 10.1056/NEJM198807073190101. [DOI] [PubMed] [Google Scholar]
- 43.Engel R.R. Necrotizing enterocolitis in the newborn: Report of the 68th Ross Conference on Pediatric Research. Ross Laboratories; Columbus, Ohio: 1974. [Google Scholar]
- 44.Engel R.R., Virning N.L., Hunt C.E. Origin of mural gas in necrotizing enterocolitis. Pediatr Res. 1973;7:292. [Google Scholar]
- 45.Engelhardt E.L., Neu J., Sankar M.B. Changes in phospholipid and cholesterol concentrations of the rat microvillus membrane during maturation. J Pediatr Gastroenterol Nutr. 1989;9:89–93. [PubMed] [Google Scholar]
- 46.Genersich A. Bauchfellentzundung beim Neugebornen in Forge von Perforation des Ileums. Arch Pathol Anat. 1891;126:485. [Google Scholar]
- 47.Glode M.P., Sutton A., Moxon E.R. Pathogenesis of neonatal Escherichia coli meningitis: Induction of bacteremia and meningitis in infant rats fed E. coli K1. Infect Immun. 1977;16:75–80. doi: 10.1128/iai.16.1.75-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grand R.J., Watkins J.B., Torti F.M. Development of the human gastrointestinal tract. Gastroenterology. 1976;70:790–810. [PubMed] [Google Scholar]
- 49.Grylock L.J., Sconlon J.W. Oral gentamicin therapy in the prevention of neonatal necrotizing enterocolitis. Am J Dis Child. 1978;132:1192. doi: 10.1001/archpedi.1978.02120370040010. [DOI] [PubMed] [Google Scholar]
- 50.Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell: Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978;148:1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halac E., Halac J., Begue E.F. Prenatal and postnatal corticosteroid therapy to prevent necrotizing enterocolitis: A controlled trial. J Pediatr. 1990;117:132. doi: 10.1016/s0022-3476(05)72461-6. [DOI] [PubMed] [Google Scholar]
- 52.Harris M.C., Costarino A.T., Jr, Sullivan S. Cytokine elevations in critically ill infants with sepsis and necrotizing enterocolitis. J Pediatr. 1994;124:105–111. doi: 10.1016/s0022-3476(94)70264-0. [DOI] [PubMed] [Google Scholar]
- 53.Harris R.C. Review of selected bacterial enterotoxins and their role in gastroenteritis. Ann Clin Lab Sci. 1988;18:102–108. [PubMed] [Google Scholar]
- 54.Hopkins G., Gould V., Stevenson J. Necrotizing enterocolitis in premature infants. Am J Dis Child. 1970;120:229. doi: 10.1001/archpedi.1970.02100080113009. [DOI] [PubMed] [Google Scholar]
- 55.Hoy C., Millar M.R., MacKay P. Quantitative changes in faecal microflora preceding necrotizing enterocolitis in premature neonates. Arch Dis Child. 1990;65:1057. doi: 10.1136/adc.65.10_spec_no.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inman L.R., Cantey J.R. Specific adherence of Escherichia coli (strain RDEC-1) to membranous (M) cells of the Peyer's patch in Escherichia coli diarrhea in the rabbit. J Clin Invest. 1983;71:1–8. doi: 10.1172/JCI110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Israel E.J. Neonatal necrotizing enterocolitis, a disease of the immature intestinal mucosal barrier. Acta Paediatr Suppl. 1994;396:27–32. doi: 10.1111/j.1651-2227.1994.tb13238.x. [DOI] [PubMed] [Google Scholar]
- 58.Israel E.J., Schiffrin E.F., Carter E.A. Prevention of necrotizing enterocolitis in the rat with prenatal cortisone. Gastroenterology. 1990;99:1333. doi: 10.1016/0016-5085(90)91158-3. [DOI] [PubMed] [Google Scholar]
- 59.Janeway C.A., Jr, Jones B., Hayday A. Specificity and function of T cells bearning gamma/delta receptors. Immunol Today. 1988;9:73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- 60.Keljo D.J., Hamilton J.R. Quantitative determination of macromolecular transport across intestinal Peyer's patches. Am J Physiol. 1983;244:g637–g644. doi: 10.1152/ajpgi.1983.244.6.G637. [DOI] [PubMed] [Google Scholar]
- 61.Kien C.L. Colonic fermentation of carbohydrate in the premature infant: possible relevance to necrotizing enterocolitis. J Pediatr. 1990;117(suppl):52–58. doi: 10.1016/s0022-3476(05)81131-x. [DOI] [PubMed] [Google Scholar]
- 62.Kliegman R.M. Models of the pathogenesis of necrotizing enterocolitis. J Pediatr. 1990;117(suppl):2–5. doi: 10.1016/S0022-3476(05)81123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kliegman R.M., Fanaroff A.A. Neonatal necrotizing enterocolitis: A nine-year experience. II. Outcome assessment. Am J Dis Child. 1981;135:608–611. doi: 10.1001/archpedi.1981.02130310014006. [DOI] [PubMed] [Google Scholar]
- 64.Kliegman R.M., Fanaroff A.A. Neonatal necrotizing enterocolitis: A nine-year experience. Am J Dis Child. 1981;135:603–607. doi: 10.1001/archpedi.1981.02130310009005. [DOI] [PubMed] [Google Scholar]
- 65.Kliegman R.M., Fanaroff A.A. Necrotizing enterocolitis. N Engl J Med. 1984;310:1093–1103. doi: 10.1056/NEJM198404263101707. [DOI] [PubMed] [Google Scholar]
- 66.Kliegman R.M., Walsh M.C. Neonatal necrotizing enterocolitis: Pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17:219–288. doi: 10.1016/0045-9380(87)90031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koldovsky O., Thornburg W. Hormones in milk. J Pediatr Gastroenterol Nutr. 1987;6:172. [PubMed] [Google Scholar]
- 68.Kosloske A.M. Pathogenesis and prevention of necrotizing enterocolitis: A hypothesis based on personal observation and a review of the literature. Pediatrics. 1984;74:1086–1092. [PubMed] [Google Scholar]
- 69.Kosloske A.M. A unifying hypothesis for pathogenesis and prevention of necrotizing enterocolitis. J Pediatr. 1990;117(suppl):68–74. doi: 10.1016/S0022-3476(05)81134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurukawa M., Lee E.L., Johnston J.M. Platelet-activating factor-induced ischemic bowel necrosis: The effect of platelet-activating factor acetylhydrolase. Pediatr Res. 1993;34:237–241. doi: 10.1203/00006450-199308000-00027. [DOI] [PubMed] [Google Scholar]
- 71.La Gamma E.F., Ostertag S.G., Birenbaum H. Failure of delayed oral feedings to prevent necrotizing enterocolitis. Am J Dis Child. 1985;139:385. doi: 10.1001/archpedi.1985.02140060067031. [DOI] [PubMed] [Google Scholar]
- 72.Larson H.E. Neonatal necrotizing enterocolitis: A neonatal infection. J Hosp Infect. 1988;1A(suppl):334–339. doi: 10.1016/0195-6701(88)90208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawrence G., Bates J., Gaul A. Pathogenesis of neonatal necrotizing enterocolitis. Lancet. 1982;1:137–139. doi: 10.1016/s0140-6736(82)90383-x. [DOI] [PubMed] [Google Scholar]
- 74.Lloyd J. The etiology of gastrointestinal perforations in the newborn. J Pediatr Surg. 1969;4:77. doi: 10.1016/0022-3468(69)90186-9. [DOI] [PubMed] [Google Scholar]
- 75.Lucas A. Human milk and infant feeding. In: Battaglia F., Boyd R., editors. Perinatal Medicine. Butterworths; London: 1983. pp. 172–200. [Google Scholar]
- 76.Lucas A., Bloom S.R., Aynsley-Green A. Gut hormones and "minimal enteral feeding.". Acta Paediatr Scand. 1986;75:719. doi: 10.1111/j.1651-2227.1986.tb10280.x. [DOI] [PubMed] [Google Scholar]
- 77.Lucas A., Cole T.J. Breast milk and neonatal necrotizing enterocolitis. Lancet. 1990;336:1519. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 78.MacKendrick W, Caplan M, Hsueh W: Endogenous nitric oxide protects against platelet-activating factor-induced bowel injury in the rat. Pediatr Res 34:222-228 [DOI] [PubMed]
- 79.Meetze W.H., Valentine C., McGuigan J.E. Gastrointestinal priming prior to full enteral nutrition in very low birth weight infants. J Pediatr Gastroenterol Nutr. 1992;15:163. doi: 10.1097/00005176-199208000-00011. [DOI] [PubMed] [Google Scholar]
- 80.Mizrahi A. Necrotizing enterocolitis in premature infants. J Pediatr. 1965;66:697. doi: 10.1016/s0022-3476(65)80003-8. [DOI] [PubMed] [Google Scholar]
- 81.Mollitt D.L., Tepas J.J., String D.L. Does patient age or intestinal pathology influence the bacteria found in cases of necrotizing enterocolitis? South Med J. 1991;84:879–882. doi: 10.1097/00007611-199107000-00014. [DOI] [PubMed] [Google Scholar]
- 82.Murrell T.G.C., Roth L., Egerton J. Pigbel: Enteritis necroticans-a study in diagnosis and management. Lancet. 1966;1:217–222. doi: 10.1016/s0140-6736(66)90048-1. [DOI] [PubMed] [Google Scholar]
- 83.Neu J. Functional development of the fetal gastrointestinal tract. Seminar Perinatol. 1989;12:224–235. [PubMed] [Google Scholar]
- 84.Neu J: Necrotizing enterocolitis. In Rudolph AM (ed): Rudolph's Pediatrics, ed 19, 1991
- 85.Neu J., Ozaki C.K., Angilides K.J. Glucocorticoid-mediated alteration of fluidity of brush-border membrane in rat small intestine. Pediatr Res. 1986;20:79–82. doi: 10.1203/00006450-198601000-00022. [DOI] [PubMed] [Google Scholar]
- 86.Nowicki P.T., Nankervis C.A. The role of the circulation in the pathogenesis of necrotizing enterocolitis. Clin Perinatol. 1994;21:219–234. [PubMed] [Google Scholar]
- 87.Owen R.L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: An ultrastructural study. Gastroenterology. 1977;72:440–451. [PubMed] [Google Scholar]
- 88.Owen R.L., Jones A.L. Epithelial cell specialization within human Peyer's patches: An ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974;66:189–203. [PubMed] [Google Scholar]
- 89.Paltauf A. Die spontane Dickdarmruptur be Foeten und Neugeborenen. Virchows Arch (Pathol Anat) 1888;111:461. [Google Scholar]
- 90.Pang K.Y., Bresson J.L., Walker W.A. Development of the gastrointestinal mucosal barrier. III. Evidence for structural differences in microvillus membranes from newborn and adult rabbits. Biochim Biophys Acta. 1983;727:201. doi: 10.1016/0005-2736(83)90385-1. [DOI] [PubMed] [Google Scholar]
- 91.Pang K.Y., Bresson J.L., Walker W.A. Development of the gastrointestinal mucosal barrier. VII. In utero maturation of the microvillus surface by cortisone. Am J Physiol. 1985;249:g85. doi: 10.1152/ajpgi.1985.249.1.G85. [DOI] [PubMed] [Google Scholar]
- 92.Panigrahi P., Gupta S., Gewolb I.H. Occurrence of necrotizing enterocolitis may be dependent on patterns of bacterial adherence and intestinal colonization: Studies in Caco2 tissue culture and weanling rabbit models. Pediatr Res. 1994;36:115–121. doi: 10.1203/00006450-199407001-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pappo J., Owen R.L. Absence of secretory component expression by epithelial cells overlying rabbit gut-associated lymphoid tissue. Gastroenterology. 1988;95:1173–1177. doi: 10.1016/0016-5085(88)90347-2. [DOI] [PubMed] [Google Scholar]
- 94.Pritchard J.A. Fetal swallowing and amniotic fluid volume. Obstet Gynecol. 1966;28:606. [PubMed] [Google Scholar]
- 95.Quasir K. Uber eine besonders schwer verlaufende Form von Enteritis beim Saugling. "Enterocolitis ulcerosa necroticans," II. Klinische Studien. Oesterr Z. Kinderh. 1952;8:136. [PubMed] [Google Scholar]
- 96.Read L.C. Milk growth factors. In: Cockburn F., editor. Fetal and Neonatal Growth. John Wiley & Sons; Chicester, UK: 1988. p. 131. [Google Scholar]
- 97.Rieger C.H.L., Rothberg R.M. Development of the capacity to produce specific antibody to an ingested food antigen in the premature infant. J Pediatr. 1975;87:515–518. doi: 10.1016/s0022-3476(75)80811-0. [DOI] [PubMed] [Google Scholar]
- 98.Robertson D.M., Paganelli R., Dinwiddie R. Milk antigen absorption in the preterm and term neonate. Arch Dis Child. 1982;57:141–145. doi: 10.1136/adc.57.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rossier A., Sarrut S., Delplanque J. L'enterocolite ulcero-necrotique du premature. Ann Paediatr. 1959;35:1428. [PubMed] [Google Scholar]
- 100.Rotbart H.A., Levin M.J. How contagious is necrotizing enterocolitis? Pediatr Infect Dis. 1983;2:406–413. doi: 10.1097/00006454-198309000-00019. [DOI] [PubMed] [Google Scholar]
- 101.Rozee K.R., Cooper D., Lam K. Microbial flora of the mouse ileum mucous layer and epithelial surface. Appl Environ Microbiol. 1982;43:1451–1463. doi: 10.1128/aem.43.6.1451-1463.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Santulli T.V., Schullinger J.N., Heird W.C. Acute necrotizing enterocolitis in infancy: A review of 64 cases. Pediatrics. 1975;55:376. [PubMed] [Google Scholar]
- 103.Schaffer A.J., editor. Diseases of the Newborn. ed 2. WB Saunders; Philadelphia: 1965. [Google Scholar]
- 104.Schaffer A.J., Avery M.E., editors. Disease of the Newborn. WB Saunders; Philasdelphia: 1971. [Google Scholar]
- 105.Scheifle D.W. Role of bacterial toxins in neonatal necrotizing enterocolitis. J Pediatr. 1990;117(suppl):44. doi: 10.1016/s0022-3476(05)81129-1. [DOI] [PubMed] [Google Scholar]
- 106.Schleivert P., Johnson W., Galask R.P. Amniotic fluid antibacterial mechanisms: Newer concepts. Semin Perinatol. 1977;1:59. [PubMed] [Google Scholar]
- 107.Schmid K.O. Uber eine besonders schwer verlafende Form von Enteritis beim Saugling, "Enterocolitis ulcerosa necroticans." I. Pathologisch-Anatomische Studien. Oesterr Z Kinderh. 1952;8:114. [PubMed] [Google Scholar]
- 108.Schober P.H., Nassiri J. Risk factors and severity indices in necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:49–52. doi: 10.1111/j.1651-2227.1994.tb13243.x. [DOI] [PubMed] [Google Scholar]
- 109.Scholander P. The master switch of life. Sci Am. 1963;209:92. doi: 10.1038/scientificamerican1263-92. [DOI] [PubMed] [Google Scholar]
- 110.Shahani K.M., Kwan A.J., Friend B.A. Role and significance of enzymes in human milk. Am J Clin Nutr. 1980;33:1861. doi: 10.1093/ajcn/33.8.1861. [DOI] [PubMed] [Google Scholar]
- 111.Slagle T.A., Gross S.J. Effect of early enteral substrate on subsequent feeding tolerance. J Pediatr. 1988;113:526. doi: 10.1016/s0022-3476(88)80646-2. [DOI] [PubMed] [Google Scholar]
- 112.Stall B.J., Kliegman R.M., editors. Necrotizing enterocolitisClin Perinatol. 1994;21:2. [Google Scholar]
- 113.Stevenson J., Graham C., Oliver T. Neonatal necrotizing enterocolitis. Am J Surg. 1969;118:260. doi: 10.1016/0002-9610(69)90129-9. [DOI] [PubMed] [Google Scholar]
- 114.Stoll B., Kanto W., Glass R. Epidemiology of necrotizing enterocolitis: A case control study. J Pediatr. 1980;96:447. doi: 10.1016/s0022-3476(80)80696-2. [DOI] [PubMed] [Google Scholar]
- 115.Tesh V.L., O'Brien A.D. The pathogenic mechanisms of Shiga toxin and the Shiga-like toxins. Mol Microbiol. 1991;5:1817–1822. doi: 10.1111/j.1365-2958.1991.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 116.Touloukian R.J. Neonatal necrotizing enterocolitis: An update on etiology, diagnosis, and treatment. Surg Clin N Am. 1976;56:281–298. doi: 10.1016/s0039-6109(16)40877-7. [DOI] [PubMed] [Google Scholar]
- 117.Touloukian R., Posch J., Spencer R. The pathogenesis of ischemic gastroenterocolitis of the neonate: Selective gut mucosal ischemia in asphyxiated neonatal piglets. J Pediatr Surg. 1972;7:194. doi: 10.1016/0022-3468(72)90496-4. [DOI] [PubMed] [Google Scholar]
- 118.Uauy R.D., Fanaroff A.A., Korones S.B. Necrotizing enterocolitis in very low birth weight infants: Biodemographic and clinical correlates. J Pediatr. 1991;119:630. doi: 10.1016/s0022-3476(05)82418-7. [DOI] [PubMed] [Google Scholar]
- 119.Udall J.N. Maturation of intestinal host defense: an update. Nutr Res. 1981;1:399–418. [Google Scholar]
- 120.Weaver L.T., Laker M.F., Nelson R. Milk feeding and changes in intestinal permeability and morphology in the newborn. J Pediatr Gastroenterol Nutr. 1987;6:351–358. doi: 10.1097/00005176-198705000-00008. [DOI] [PubMed] [Google Scholar]
- 121.Wells C.L., Maddaus M.A., Simmons R.L. Proposed mechanism for the translocation of intestinal bacteria. Rev Infect Dis. 1988;10:958–979. doi: 10.1093/clinids/10.5.958. [DOI] [PubMed] [Google Scholar]
- 122.Westra-Meijer C.M.M., Degener J.E., Dzoljic-Danilovic G. Quantitative study of the aerobic and anaerobic faecal flora in neonatal necrotizing enterocolitis. Arch Dis Child. 1983;58:523–528. doi: 10.1136/adc.58.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.White K.C., Harkavy K.L. Hypertonic formula resulting from added oral medications. Am J Dis Child. 1982;136:931. doi: 10.1001/archpedi.1982.03970460061013. [DOI] [PubMed] [Google Scholar]
- 124.Willis D.M., Chabot J., Radde I.C. Unsuspected hyperosmolarity of oral solutions contributing to necrotizing enterocolitis in very low birth weight infants. Pediatrics. 1983;71:19. [PubMed] [Google Scholar]
- 125.Wilson R., Kanto W.P., Jr, McCarthy B.J. Epidemiologic characteristics of necrotizing enterocolitis: A population-based study. Am J Epidemiol. 1981;114:880–887. doi: 10.1093/oxfordjournals.aje.a113258. [DOI] [PubMed] [Google Scholar]
- 126.Wilson R., Kanto W.P., Jr, McCarthy B.J. Age at onset of necrotizing enterocolitis. Am J Dis Child. 1982;136:814. doi: 10.1001/archpedi.1982.03970450056013. [DOI] [PubMed] [Google Scholar]
- 127.Wilson R., Kanto W.P., Jr, McCarthy B.J. Age at onset of necrotizing enterocolitis: An epidemiologic analysis. Pediatr Res. 1982;16:82. doi: 10.1203/00006450-198201001-00017. [DOI] [PubMed] [Google Scholar]
- 128.Wolf H.M., Eibl M.M. The anti-inflammatory effect of oral immunoglobulin (IgA-IgG) preparation and its possible relevance for the prevention of necrotizing enterocolitis. Acta Paediatr Suppl. 1994;296:37–40. doi: 10.1111/j.1651-2227.1994.tb13240.x. [DOI] [PubMed] [Google Scholar]
- 129.Wolf J.L., Rubin D.H., Finberg R. Intestinal M cells: A pathway for entry of reovirus into the host. Science. 1981;212:471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]
- 130.Wright L.L., Uauy R.D., Younes N. Rapid advances in feeding increase the risk of necrotizing enterocolitis in very low birth weight infants. Pediatr Res. 1993;33:313A. [Google Scholar]
- 131.Yoshioka H., Iseki K., Fujita K. Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics. 1983;72:317–321. [PubMed] [Google Scholar]
- 132.Zoppi G., Andreotti G., Pajno-Ferrara F. Exocrine pancreas function in premature and full term neonates. Pediatr Res. 1972;6:880–886. doi: 10.1203/00006450-197212000-00005. [DOI] [PubMed] [Google Scholar]


