Abstract
Background
Acute respiratory infections (ARIs) last for less than 30 days and are the most common acute diseases affecting people. Exercise has been shown to improve health generally, but it is uncertain whether exercise may be effective in reducing the occurrence, severity, and duration of ARIs. This is an update of our review published in 2015.
Objectives
To evaluate the effectiveness of exercise for altering the occurrence, severity, or duration of acute respiratory infections.
Search methods
We searched CENTRAL (2020, Issue 2), MEDLINE (1948 to March week 1, 2020), Embase (1974 to 05 March 2020), CINAHL (1981 to 05 March 2020), LILACS (1982 to 05 March 2020), SPORTDiscus (1985 to 05 March 2020), PEDro (searched 05 March 2020), OTseeker (searched 05 March 2020), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov (searched 05 March 2020).
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs (method of allocation that is not truly random, e.g. based on date of birth, medical record number) of exercise for ARIs in the general population.
Data collection and analysis
Two review authors independently extracted data from the included trials using a standard form. One review author entered data, which a second review author checked. We contacted trial authors to request missing data. There were sufficient differences in the populations trialed and in the nature of the interventions to use the random‐effects model (which makes fewer assumptions than the fixed‐effect model) in the analysis.
Main results
We included three new trials for this update (473 participants) for a total of 14 trials involving 1377 adults, published between 1990 and 2018. Nine trials were conducted in the USA, and one each in Brazil, Canada, Portugal, Spain, and Turkey.
Sample sizes ranged from 16 to 419 participants, aged from 18 to 85 years. The proportion of female participants ranged from 52% to 100%. Follow‐up duration ranged from 1 to 36 weeks (median = 12 weeks).
Moderate‐intensity aerobic exercise (walking, bicycling, treadmill, or a combination) was evaluated in 11 trials, and was most commonly prescribed at least three times a week for 30 to 45 minutes.
There was no difference between exercise and no exercise in the number of ARI episodes per person per year (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.77 to 1.30; 4 trials; 514 participants; low‐certainty evidence); proportion of participants who experienced at least one ARI over the study period (RR 0.88, 95% CI 0.72 to 1.08; 5 trials; 520 participants; low‐certainty evidence); and the number of symptom days per episode of illness (mean difference (MD) −0.44 day, 95% CI −2.33 to 1.46; 6 trials; 557 participants; low‐certainty evidence). Exercise reduced the severity of ARI symptoms measured on the Wisconsin Upper Respiratory Symptom Survey (WURSS‐24) (MD −103.57, 95% CI −198.28 to −8.87; 2 trials; 373 participants; moderate‐certainty evidence) and the number of symptom days during follow‐up period (MD −2.24 days, 95% CI −3.50 to −0.98; 4 trials; 483 participants; low‐certainty evidence).
Excercise did not have a significant effect on laboratory parameters (blood lymphocytes, salivary secretory immunoglobulin, and neutrophils), quality of life outcomes, cost‐effectiveness, and exercise‐related injuries. There was no difference in participant dropout between the intervention and control groups.
Overall, the certainty of the evidence was low, downgraded mainly due to limitations in study design and implementation, imprecision, and inconsistency.
Seven trials were funded by public agencies; five trials did not report funding; and two trials were funded by private companies.
Authors' conclusions
Exercise did not reduce the number of ARI episodes, proportion of participants experiencing at least one ARI during the study, or the number of symptom days per episode of illness. However, exercise reduced the severity of ARI symptoms (two studies) and the number of symptom days during the study follow‐up period (four studies). Small study size, risk of bias, and heterogeneity in the populations studied contributed to the uncertainty of the findings. Larger trials that are designed to avoid risk of bias associated with participant selection, blinding of outcomes assessors, and with adequate reporting of all outcomes proposed for measurement in trials, would help to provide more robust evidence.
Keywords: Adult; Aged; Aged, 80 and over; Female; Humans; Male; Middle Aged; Exercise; Acute Disease; Bicycling; Randomized Controlled Trials as Topic; Respiratory Tract Infections; Respiratory Tract Infections/epidemiology; Respiratory Tract Infections/prevention & control; Severity of Illness Index; Time Factors; Walking
Plain language summary
Exercise versus no exercise for the occurrence, severity, and duration of acute respiratory infections
Review question
Does exercise change the occurrence, severity, or duration of acute respiratory infections such as the common cold, influenza, cough, and sore throat?
Background
Exercise has been shown to improve health generally. We reviewed the evidence to find out whether exercise is also effective at changing the occurrence, severity, or duration of acute respiratory infections (colds and coughs) that last less than a month.
Search date
We searched for studies up to 5 March 2020.
Study characteristics
We added evidence from three trials (473 participants) for this update, for a total of 14 trials involving 1377 participants, aged 18 to 85 years. Exercise was supervised and prescribed at least three times a week, with 30 to 45 minutes of moderate‐intensity activities in most trials.
Study funding sources
Seven studies were funded by public agencies; five studies did not report funding; and two studies were funded by private companies.
Key results
It is unclear whether exercise changed the number of acute respiratory infections a person experiences each year; the number of people who experienced at least one acute respiratory infection during the study period; or the number of days with symptoms during each episode of illness. Exercise reduced symptom severity and the number of days symptoms lasted whilst participants were enrolled in the trial. We are uncertain whether exercise has an important effect on other outcomes, such as effects measured by laboratory tests (blood and salivary samples), quality of life, cost‐effectiveness, or injuries related to exercise.
Certainty of evidence
Overall, the certainty of the evidence was low, which means that further research is likely to impact our conclusions. There is a need for better designed studies to allow a clearer understanding as to whether exercise reduces the occurrence, severity, or duration of acute respiratory infections.
Summary of findings
Summary of findings for the main comparison. Exercise versus no exercise for the occurrence, severity, and duration of acute respiratory infections.
| Exercise versus no exercise for the occurrence, severity, and duration of acute respiratory infections | ||||||
| Patient or population: healthy people Setting: university health centre Intervention: bicycle, treadmill, or walk Comparison: no exercise | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control intervention for acute respiratory infections (total) | Risk with exercise | |||||
| Number of ARI episodes per person per year Self‐reported Follow‐up: adjusted for 1 year | Median risk in study population | Rate ratio 1.00 (0.77 to 1.30) | 514 (4 trials) | ⊕⊕⊝⊝ lowa,c |
||
| 39 per 100 | 39 per 100 (30 to 51) | |||||
|
Proportion of participants who experienced at least 1 ARI over the study period Follow‐up: 12 to 52 weeks |
Median risk in study population | RR 0.88 (0.72 to 1.08) | 520 (5 trials) | ⊕⊕⊝⊝ lowa,c |
This outcome combines a wide range of follow‐up periods. | |
| 61 per 100 | 54 per 100 (44 to 66) | |||||
|
Severity of ARI symptoms Follow‐up: 8 weeks Self‐reported The lower the score the lower the symptoms |
Mean 342 points | Mean 236 points | MD 103.57 lower (198.28 lower to 8.87 lower) | 373 (2 trials) | ⊕⊕⊝⊝ lowa,b | This scale is an area under the curve (WURSS‐24 score by days of illness). The scale is composed of 19 items. All items are scored on an 8‐point Likert scale from 0 (absent or no impairment) through 1 (very mild), 3 (mild), 5 (moderate), and 7 (severe). The scale assesses symptoms over the last 24 hours. Global severity calculated as the area under the curve using WURSS‐24 scores for y‐axis and duration of ARI illness as x‐axis. |
|
Number of symptom days during follow‐up period Follow‐up: 12 weeks |
Mean 7.66 days | Mean 6.34 days | MD 2.24 lower (3.5 lower to 0.98 lower) | 483 (4 trials) | ⊕⊕⊝⊝ lowa,b | |
| Number of symptom days per episode of illness | Mean 7.93 days | Mean 7.42 days | MD 0.44 lower (2.33 lower to 1.46 higher) | 557 (6 trials) | ⊕⊕⊝⊝ lowa,b | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARI: acute respiratory infection; CI: confidence interval; MD: mean difference; RR: risk ratio; WURSS‐24: Wisconsin Upper Respiratory Symptom Survey | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLimitations in study design and implementation. Risk of selection bias and lack of blinding; allocation concealment not reported in the trials. bImprecision of results. The confidence interval is very wide because of a small number of participants. cInconsistency of results. Heterogeneity between trials may be due to differences in populations, intensity and duration of the intervention, and length of follow‐up period.
Background
Description of the condition
Acute respiratory infections (ARIs) are the most common acute diseases affecting people worldwide (Bellos 2010; Del Mar 2000; Shi 2019), and can range from the common cold to pneumonia. In the UK, consultation for ARI symptoms is very common in primary care (15% to 20% of all general practitioner consultations) (Lim 2012; Renati 2016; Thomas 2000).
Respiratory infections occur due to invasion by micro‐organisms (e.g. virus, bacteria, fungi) in any part of the respiratory tract (Dasaraju 1996). These infections are classified according to the anatomical areas: upper or lower respiratory tract infection (Dasaraju 1996; Lim 2012; Renati 2016; Thomas 2000). Upper respiratory tract infections are generally considered to occur in the airway above the glottis or vocal cords and include tonsillitis, pharyngitis, laryngitis, sinusitis, otitis media, certain types of influenza, and the common cold. Lower respiratory tract infections occur in the trachea, bronchial tubes, bronchioles, and lungs. Lower respiratory tract infection is often more serious and can include bronchitis, bronchiolitis, and pneumonia. ARIs are defined as infections of the respiratory tract lasting less than 30 days (WHO 1990).
ARIs have been estimated to be associated with an annual death rate of 4.25 million people globally; every person is anticipated to experience a total of one to two years with ARIs (Del Mar 2000; Marciniuk 2014; WHO 2002; WLF 2010). Viral ARIs have been previously estimated to cost USD 40 billion annually in direct and indirect costs (Fendrick 2003). The annual economic burden of influenza alone was most recently estimated at USD 11.2 billion annually in the USA (Putri 2018).
Preventive strategies for ARIs in the community include stopping smoking, handwashing, avoiding contact with infected people, good nutrition, and vaccines (Cohen 1993; Jefferson 2011; Roth 2008).
Description of the intervention
Treatment for respiratory infection depends on the cause and severity of the infection. Rest is generally indicated, as well as the use of analgesics, antipyretics, and adequate hydration (Dasaraju 1996; Lim 2012). Antibiotics are indicated only for suspected bacterial infection, which is more common in people who have high fever, when the infection persists for more than 7 to 10 days, or for pneumonia (Pugh 2015). Antifungals can also be used if fungi are suspected to be the cause of infection (Lim 2012).
Exercise has been shown to improve health generally, and may be effective in reducing the occurrence, severity, and duration of ARIs (Barrett 2012; Barrett 2018; Chubak 2006; Nieman 2010; Obasi 2013). We defined exercise as "a planned and structured program of motor actions to improve or maintain components of physical fitness" (Carpersen 1985). The types of exercise prescribed can vary by mode, dose, setting, the person who delivers the intervention, and any accompanying behavioural strategies (e.g. counselling, pamphlets) (Campbell 2007).
How the intervention might work
People who regularly exercise enjoy improvements in general health and better maximal oxygen uptake (VO₂ max), muscular strength, flexibility, and body composition (Warburton 2006). Specific effects on ARIs could theoretically include decreased deterioration of immune system, improved natural immune function, nasal mucosal immunity, decreased inflammatory cytokines, and stress resistance (Chubak 2006; Engels 2004; Manzaneque 2004; Nieman 2010; Nieman 2019).
Why it is important to do this review
Exercise is a low‐cost and readily available intervention that most people could implement. We found no prior systematic review evaluating trial evidence about the effectiveness of exercise for altering the occurrence, severity, or duration of ARIs. Observational studies have shown an association between exercise and decreased rates of ARIs (Chubak 2006; Nieman 2010; Nieman 2019). However, this might be attributable to several biases. This is an update of our previous Cochrane Review (Grande 2015).
Objectives
To evaluate the effectiveness of exercise for altering the occurrence, severity, or duration of acute respiratory infections.
Methods
Criteria for considering studies for this review
Types of studies
We included both randomised controlled trials (RCTs) and quasi‐RCTs (trials with methods of allocation that are not truly random, e.g. based on date of birth, medical record number) of exercise versus no exercise for the occurrence, severity, and duration of acute respiratory infections (ARIs) in the general population.
Types of participants
People of all ages, including those with chronic respiratory conditions. We considered the definition of ARIs used by the trial authors.
Types of interventions
We included trials that used exercise in at least one group compared to no exercise or no intervention. We documented all reported details of the intervention duration, frequency of sessions, and season of the exercise programme. We included two comparisons:
exercise versus no exercise; and
exercise versus usual care.
Types of outcome measures
Primary outcomes
Number of ARI episodes per person per year.
Proportion of participants who experienced at least one ARI over the study period.
Severity of ARI symptoms.
Number of symptom days in the follow‐up period (12 weeks).
Number of symptom days per episode of illness.
Secondary outcomes
Laboratory‐assessed immune parameters.
Quality of life.
Cost to the patient.
Exercise‐related injuries.
Adherence to the group intervention (in the no‐exercise group this means dropout from the control group).
Search methods for identification of studies
Electronic searches
We searched CENTRAL (2019, Issue 2), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (1948 to March week 1, 2020), Embase (1974 to 05 March 2020), CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1981 to 05 March 2020), LILACS (Latin American and Caribbean Health Science Information database) (1982 to 05 March 2020), SPORTDiscus (1985 to 05 March 2020), PEDro (Physiotherapy Evidence Database) (5 March 2020), and OTseeker (5 March 2020).
We used the search strategy described in Appendix 1 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE (Lefebvre 2011). We adapted the search strategy to search Embase (Appendix 2), CINAHL (Appendix 3), LILACS (Appendix 4), SPORTDiscus (Appendix 5), PEDro (Appendix 6), and OTseeker (Appendix 7). We did not apply any language or publication restrictions.
Searching other resources
We searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTP) (apps.who.int/trialsearch) and US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov) for completed and ongoing trials (5 March 2020). We checked the reference lists of all primary studies and review articles for additional references. We emailed experts in the field regarding any unpublished data that might be included in the review.
Data collection and analysis
Selection of studies
Two review authors (AJG, JK) independently screened the titles and abstracts of studies identified by the database searches. We retrieved the full‐text articles of potentially relevant studies. Two review authors (AJG, JK) independently screened the full‐text articles and identified studies for inclusion in the review, and recorded reasons for exclusion of ineligible studies. Any disagreements were resolved by discussion or by referring to a third review author (VS) when required. We recorded the study screening and selection process in sufficient detail to generate a PRISMA flow diagram (Figure 1) and Characteristics of excluded studies tables.
1.
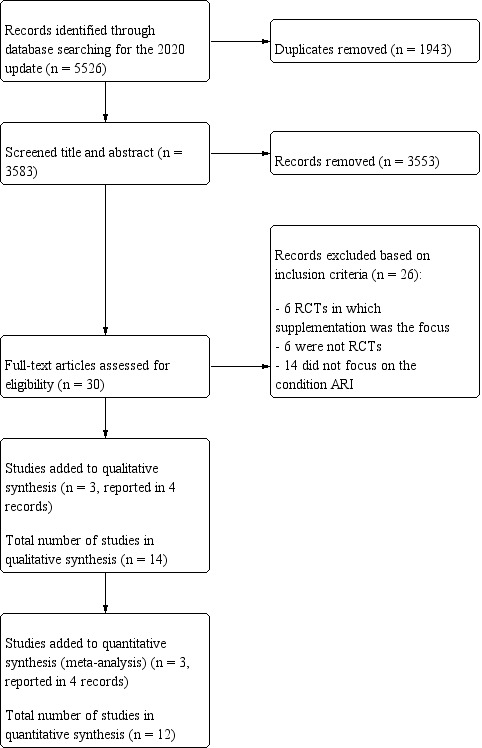
Study flow diagram (2020 update).
Data extraction and management
Two review authors (AJG, JK) independently extracted data from the included trials using an online form developed for this purpose. Two review authors (AJG, JK) entered the extracted data into Review Manager 5 (Review Manager 2014).
Assessment of risk of bias in included studies
Two review authors (AJG, JK) independently assessed the risk of bias for each included trial using the 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Any disagreements were resolved by discussion or by referral to a third review author (VS) if required. We assessed risk of bias according to the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias (other sources of bias related to particular trial designs (cross‐over and cluster‐randomised) or specific circumstances.
We classified risk of bias as low, high, or unclear (Higgins 2011b).
Measures of treatment effect
We used risk ratios (RR) for dichotomous data, for example proportion of people who experienced at least one ARI over the study period, exercise‐related injuries, and adherence to the group intervention.
We combined continuous outcomes using the same scales by using mean difference (MD) (e.g. severity of ARI; number of symptom days in the follow‐up period (12 weeks); number of symptom days per episode; laboratory parameters; quality of life; and financial cost to the patient (USD)). Where measurement scales differed, we used standardised mean difference (SMD) (e.g. laboratory parameters ‐ immunoglobulin A (IgA), neutrophils).
We used rate ratios to compare rates between groups (e.g. number of ARI episodes per person per year).
Where required, we converted medians to means and interquartile ranges to standard deviations (Hozo 2005).
Unit of analysis issues
We considered the individual participant to be the unit of analysis. Where RCTs included more than two arms, for example outdoor exercise versus indoor exercise versus control, we combined both exercise groups, and the controls were entered once.
Dealing with missing data
We emailed corresponding trial authors to obtain data that were missing from published papers and required for analyses. We checked for consistency between randomised and analysed individuals to verify the intention‐to‐treat (ITT) analysis for each outcome.
Assessment of heterogeneity
We assessed heterogeneity using the I² statistic to describe the percentage of variability in effect. We considered heterogeneity as: I² = 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% represents considerable heterogeneity. (The importance of the observed value of I² depends on magnitude and direction of effects and strength of evidence for heterogeneity (e.g. P value from the Chi² test, or a confidence interval for I²) (Higgins 2011a).)
Assessment of reporting biases
In the case of mismatches between trial protocols and reports, we contacted trial authors to obtain clarification. We planned to construct a funnel plot if 10 or more trials were meta‐analysed.
Data synthesis
We pooled data where appropriate for meta‐analysis (two or more trials) using a random‐effects model due to anticipated heterogeneity between the interventions trialed and populations included using Review Manager 5 (Review Manager 2014). Where data could not be pooled, we described results narratively.
Summary of findings table and assessment of the certainty of the evidence
We created Table 1 for the following outcomes: number of ARI episodes per person per year; proportion of participants who experienced at least one ARI over the study period; severity of ARI symptoms; number of symptom days in the follow‐up period (12 weeks); and number of symptom days per episode of illness. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence as it related to the trials which contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), employing GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions and to downgrade evidence certainty using footnotes, and made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to conduct the following subgroup analyses.
Participant variables
Age groups (children, adults, and the elderly).
Setting of exercise (hospital, community centre).
Independent or non‐supervised programmes.
Associated chronic conditions (asthma, diabetes, hypertension, chronic obstructive pulmonary disease (COPD)).
Menopausal women.
Exercise variables
Type of exercise (resistance, endurance, stretching).
Frequency of exercise (number of sessions per week).
Intensity of exercise: light (1.6 to 2.9 metabolic equivalents), moderate (3 to 5.9 metabolic equivalents), or vigorous (≥ 6 metabolic equivalents).
Sensitivity analysis
We planned to analyse included trials in meta‐analyses to verify whether the impact of risk of bias affected the overall treatment effect of exercising. We explored which trials contributed to changes in heterogeneity.
Summary of findings and assessment of the certainty of the evidence
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
In the 2015 review, we identified 7186 records from the initial search by combining all prespecified databases, handsearching reference lists, grey literature, and trial register search results. After removal of duplicates 4853 records remained, of which 4821 records were excluded following title and abstract screening by two review authors. We retrieved the full‐text articles for the remaining 32 records; 18 of these did not meet our inclusion criteria and were excluded, and 11 trials (14 reports) fulfilled our eligibility criteria and were included in the review.
For this update, we searched eight databases and two trials registers, which yielded 5526 records (Electronic searches). After removal of duplicates, we screened 3583 records by title and abstract, and obtained 30 records for full‐text review. We excluded 26 reports (see Characteristics of excluded studies), and included three trials (4 reports) (Figure 1), which were added to the 11 trials (14 reports) from our 2015 review (Grande 2015), for a total of 14 included trials (15 reports).
Included studies
We included three new trials (N = 473 participants) for this update (Barrett 2018; Dias 2014; Silva 2018), for a total of 14 trials involving 1377 adults published between 1990 and 2018 (Characteristics of included studies; Table 2; Table 3) (Barrett 2012; Barrett 2018; Chubak 2006; Ciloğlu 2005; Dias 2014; Klentrou 2002; Manzaneque 2004; Nieman 1990; Nieman 1993; Nieman 1997; Silva 2018; Sloan 2013; Weidner 1998; Weidner 2003). Nine trials were conducted in the USA and one each in Brazil, Canada, Portugal, Spain, and Turkey. Sample sizes ranged from 16 to 419 participants aged between 18 and 85 years. The proportion of female participants varied between 52% and 100%. Duration of follow‐up showed a median 12 weeks (minimum 1 week; maximum 36 weeks). The most prescribed exercise type for the intervention was aerobic (walking, bicycling, treadmill, or a combination of these was evaluated in 11 of 14 trials), at least three times a week. Duration was 30 to 45 minutes at moderate intensity. Participants were supervised in 90% of the trials.
1. Characteristics of included studies ‐ baseline.
| Study ID | Location | N randomised/ withdrawals | % female | Age | Participant information |
| Barrett 2012 | USA | 154/5 | 81.8 | 50 years or over | Healthy, sedentary |
| Barrett 2018 | USA | 419/29 | 76 | 49.6 ± 11.6 years | Healthy, sedentary |
| Chubak 2006 | USA | 115/0 | 100 | 50 years or over | Overweight/obese, non‐smoking, sedentary postmenopausal women |
| Ciloğlu 2005 | Turkey | 90/0 | 100 | 45 to 65 years | Sedentary postmenopausal women |
| Dias 2014 | Brazil | 16/0 | 100 | 58.67 ± 6.12 years | Sedentary postmenopausal women |
| Klentrou 2002 | Canada | 20/0 | Not reported | 25 to 50 years | Sedentary men or women |
| Manzaneque 2004 | Spain | 29/3 | 51.7 | 18 to 21 years | Healthy, sedentary |
| Nieman 1990 | USA | 50/14 | 100 | 25 to 45 years | Mildly obese, sedentary women |
| Nieman 1993 | USA | 32/2 | 100 | 67 to 85 years | Healthy, sedentary |
| Nieman 1997 | USA | 102/11 | 100 | 25 to 70 years | Obese, sedentary women |
| Silva 2018 | Portugal | 38/12 | 73 | 71.00 ± 4.05 years | Healthy, sedentary |
| Sloan 2013 | USA | 32/0 | 100 | 54.1 ± 5.3 years | Healthy postmenopausal women |
| Weidner 1998 | USA | 50/0 | 52 | 19 to 29 years | Moderately fit, maximal oxygen uptake value corresponding to > 40th percentile for age and gender |
| Weidner 2003 | USA | 25/0 | 68.1 | 19 to 29 years | Sedentary |
2. Characteristics of exercise interventions for ARI.
| Study ID | Location | Type of exercise | Setting/frequency | Intensity | Duration | Supervision | Comparison group | Outcomes in meta‐analysis |
| Barrett 2012 | USA | Bicycle, treadmill, and brisk walk | UW Health Sports Medicine Center 1 group contact (2.5 hours per week) + 45 minutes home practice per day | 12 to 16 Borg Scale | 8 weeks | Supervised by exercise physiologist | Meditation and non‐exercise | Number of ARI episodes, proportion of people with ARI, ARI severity, number of symptoms per day, laboratory parameters ‐ neutrophils, quality of life, cost to patient, adherence to intervention |
| Barrett 2018 | USA | Bicycle, treadmill, and brisk walk | UW Health Sports Medicine Center 1 group contact (2.5 hours per week) + 45 minutes home practice per day | 12 to 16 Borg Scale | 8 weeks | Supervised by experienced exercise instructors | Non‐exercise | Number of ARI episodes, proportion of people with ARI, ARI severity, number of symptom days, laboratory parameters ‐ neutrophils, quality of life, cost to patient, adherence to intervention |
| Chubak 2006 | USA | Bicycle, treadmill, or walk | Study facilities 5 days per week; plus 2 days per week at home | 45 minutes of moderate‐intensity exercise | 12 months | Supervised by exercise physiologist in the first 3 months | Once‐weekly, 45‐minute stretching sessions | Adherence; ARI episodes |
| Ciloğlu 2005 | Turkey | Walking on a treadmill or an outdoor track | Indoor and outdoor exercises at university 5 days per week | 30 minutes each time respectively at 60% MHR | 12 weeks | Supervised | Outdoor exercise and non‐exercise | Adherence; ARI symptoms |
| Dias 2014 | Brazil | Resistance training | Gymnasium, group exercise, 3 days per week | Each weight was determined subjectively. | 9 weeks | Supervised training | Non‐exercise | Adherence |
| Klentrou 2002 | Canada | Bicycles, treadmills, stair climbers, or combined | Research centre, group exercise, 3 days per week | 30 minutes at 75% HR reserve + 15 minutes of stretching | 12 weeks | Supervised | Non‐exercise | Adherence; ARI symptoms; salivary secretion immunoglobulin |
| Manzaneque 2004 | Spain | Qigong (traditional Chinese exercise) | Research centre, group exercise, 5 days per week | 8 distinct movements repeated 8 times for 30 minutes each session | 4 weeks | Qualified Qigong instructor | Non‐exercise | Adherence |
| Nieman 1990 | USA | Walking | Research centre, group exercise, 5 days per week | 45 minutes at 60% reserve heart rate | 15 weeks | Supervised | Non‐exercise | Adherence; ARI episodes; ARI symptoms; lymphocytes |
| Nieman 1993 | USA | Brisk walking | Research centre, group activity, 5 days per week | 30 to 40 minutes at 60% target heart rate | 12 weeks | Supervised | Calisthenics | Adherence; ARI episodes; lymphocytes |
| Nieman 1997 | USA | Walking | Research centre, indoor group activity, 5 days per week | 45 minutes at 60% to 80% reserve heart rate | 12 weeks | Supervised | Non‐exercise | Adherence; ARI symptoms; lymphocytes |
| Silva 2018 | Portugal | Resistance training and aerobic training | Research centre, indoor group activity, 3 days per week | 90 min (10 min of warm‐up, 30 min of aerobic training, and 45 min of resistance training, ending with a 5‐minute cool‐down) | 36 weeks | Supervised training by physical education | Non‐exercise | Number of ARI episodes, proportion of people with ARI, severity of ARI, number of symptoms days per episode, adherence to intervention group |
| Sloan 2013 | USA | Walking | Home‐based exercise 5 days per week | 30 minutes at 75% of individual HR max | 16 weeks | Not supervised | Non‐exercise | ARI episodes; adherence; ARI symptoms; salivary secretion immunoglobulin |
| Weidner 1998 | USA | Bicycle, walking, or jogging | Research centre, group activity, 6 days of exercise | 30 minutes at 70% of target heart rate | 10 days | Supervised training | Non‐exercise | Adherence; ARI symptoms |
| Weidner 2003 | USA | Bicycle, walking, or jogging | Research centre, group activity, 5 days per week | 30 minutes at 70% of target heart rate | 7 days | Supervised | Non‐exercise | Adherence |
ARI: Acute Respiratory Infection HR: Heart Rate MHR: Maximal Heart Rate
Design
Of the 14 included trials, 12 were RCTs; Nieman 1997 was a factorial RCT; and Weidner 2003 was a quasi‐RCT.
Sample sizes
Sample sizes ranged from 16 participants, in Dias 2014, to 419 participants, in Barrett 2018.
Settings
Of the 14 included trials, 12 were conducted in university health centres and exercise laboratories; one study was conducted at a gymnasium (Dias 2014); and one was exclusively home‐based (Sloan 2013). Nine trials were conducted in the USA (Barrett 2012; Barrett 2018; Chubak 2006; Nieman 1990; Nieman 1993; Nieman 1997; Sloan 2013; Weidner 1998; Weidner 2003), and one each in Brazil (Dias 2014), Canada (Klentrou 2002), Portugal (Silva 2018), Spain (Manzaneque 2004), and Turkey (Ciloğlu 2005).
Participants
Participants' age ranged from 18 years, Manzaneque 2004, to 85 years, Nieman 1993. The proportion of female participants ranged from 51.7%, Manzaneque 2004, to 100% (Chubak 2006; Ciloğlu 2005; Dias 2014; Nieman 1990; Nieman 1993; Nieman 1997; Sloan 2013). Four studies included postmenopausal women (Chubak 2006; Ciloğlu 2005; Dias 2014; Sloan 2013). Twelve studies included sedentary participants (Barrett 2012; Barrett 2018; Chubak 2006; Ciloğlu 2005; Dias 2014; Klentrou 2002; Manzaneque 2004; Nieman 1990; Nieman 1993; Nieman 1997; Silva 2018; Weidner 2003).
Interventions
The interventions varied: 13 studies conducted interventions in groups, and researchers recommended exercises to do at home. One study was based in participants' homes. The most common intervention was aerobic exercise (walking, bicycling, treadmill, or a combination) (n = 11 trials) (Barrett 2012; Barrett 2018; Ciloğlu 2005; Klentrou 2002; Nieman 1990; Nieman 1993; Nieman 1997; Sloan 2013; Weidner 1998; Weidner 2003). One trial tested Qigong (a traditional Chinese exercise and healing technique) (Manzaneque 2004), and two investigated resistance training (Dias 2014; Silva 2018). Nine trials tested exercise for five days per week (Barrett 2012; Barrett 2018; Chubak 2006; Ciloğlu 2005; Manzaneque 2004; Nieman 1990; Nieman 1993; Nieman 1997; Sloan 2013); three times per week (Dias 2014; Klentrou 2002; Silva 2018); or for five consecutive days (Weidner 1998; Weidner 2003). Twelve trials tested exercise for 30 to 40 minutes at each training session (Barrett 2012; Barrett 2018; Chubak 2006; Ciloğlu 2005; Klentrou 2002; Manzaneque 2004; Nieman 1990; Nieman 1993; Nieman 1997; Sloan 2013; Weidner 1998; Weidner 2003). Study duration ranged from seven days, in Weidner 2003, to 12 months, in Chubak 2006. One trial did not include supervision during exercise (Sloan 2013).
Comparisons
We planned to conduct two comparisons: exercise versus no exercise and exercise versus usual care. No included studies compared exercise to usual care.
Outcome measures
Primary outcomes
Six trials reported the number of ARI episodes per person per year (Barrett 2012; Barrett 2018; Chubak 2006; Nieman 1990; Sloan 2013; Silva 2018).
Five trials reported the proportion of participants who experienced at least one ARI during the study period (Barrett 2012; Barrett 2018; Chubak 2006; Nieman 1993; Silva 2018).
Barrett 2012 and Barrett 2018 reported severity of ARI symptoms.
Four trials reported the number of symptom days in the follow‐up period (Barrett 2012; Barrett 2018; Klentrou 2002; Nieman 1997).
Six trials reported the number of symptom days per episode of illness (Barrett 2012; Barrett 2018; Ciloğlu 2005; Nieman 1990; Silva 2018; Sloan 2013).
Secondary outcomes
Reported laboratory‐assessed immune parameters were lymphocytes (Nieman 1990; Nieman 1993; Nieman 1997), immunoglobulin A (IgA), (Ciloğlu 2005; Klentrou 2002; Manzaneque 2004; Sloan 2013), and neutrophils (Barrett 2012; Barrett 2018; Manzaneque 2004; Nieman 1997).
Two trials reported quality of life (Barrett 2012; Barrett 2018).
Two trials reported cost to patients (Barrett 2012; Barrett 2018).
One trial reported exercise‐related injuries (Nieman 1993).
Eleven trials reported on adherence to the group intervention (Barrett 2012; Barrett 2018; Chubak 2006; Ciloğlu 2005; Dias 2014; Klentrou 2002; Manzaneque 2004; Nieman 1990; Nieman 1993; Silva 2018; Sloan 2013).
Study funding sources
Five studies did not report funding sources (Ciloğlu 2005; Dias 2014; Manzaneque 2004; Nieman 1990; Weidner 2003). Four studies were funded by the National Institutes of Health (Barrett 2012; Barrett 2018; Sloan 2013; Weidner 1998). Two studies were supported by the Cybex Corporation through the American College of Sports Medicine Foundation (Nieman 1993; Nieman 1997). Chubak 2006 was funded by the National Cancer Institute. Klentrou 2002 was supported by Brock University. Silva 2018 was supported by the European Regional Development Fund (FEDER) through the Competitive Factors Thematic Operational Programme (COMPETE) and Foundation for Science and Technology (FCT), Portugal, under the projects PEst‐C/QUI/UI0062/2013 (Research Unit 62/94 QOPNA) and PTDC/QUI‐QUI/117803/2010 (Future asthma management helped by non‐invasive sampling: contributes for the definition of a rapid and non‐invasive diagnostic tool). Project NORTE‐01‐0145‐FEDER‐000010 – Health, Comfort and Energy in the Built Environment (HEBE), co‐financed by Programa Operacional Regional do Norte (NORTE2020) through FEDER. (See Characteristics of included studies).
Excluded studies
We excluded 26 studies in this update (see Characteristics of excluded studies). We excluded six RCTs because the intervention was not relevant to our review (focus was exercise supplementation) (Dubnov‐Raz 2015; Komano 2018; Qieqeshlaq 2016; Shing 2007; Strasser 2016; Witard 2014); six reports because they were not RCTs or quasi‐RCTs (Kunz 2015; Kurowski 2014; Lee 2015; Morgado 2018; Nieman 2014; Rocco 2018); and 14 studies that did not evaluate the impact of the intervention on ARIs (Boukelia 2017; Colburn 2018; Couto 2014; Crabtree 2015; Edwards 2015; Fujimaki 2017; Johansson 2017; Killer 2015; Kulnik 2014; Langeskov‐Christensen 2015; Morris 2016; Oliveira 2016; van Middendorp 2016; Vaz Fragoso 2016).
Risk of bias in included studies
The risk of bias for each included trial is presented in Figure 2 and Figure 3.
2.
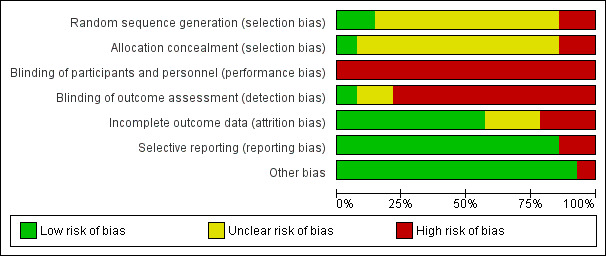
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
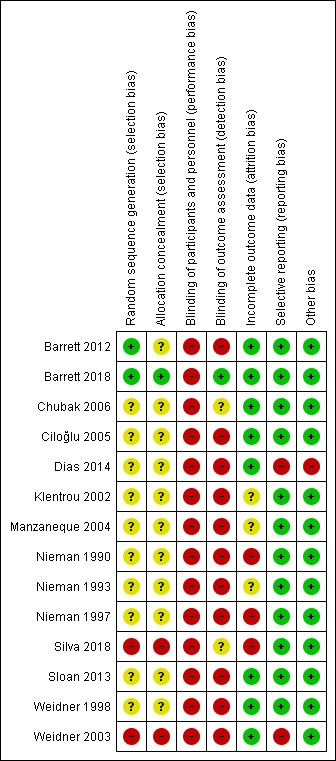
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two trials clearly reported randomisation procedures (Barrett 2012; Barrett 2018), whilst the remaining 12 included trials provided insufficient information to permit assessment and were therefore rated as at unclear risk of bias. Two trials did not adequately randomise participants who were alternately assigned to groups and were therefore judged as at high risk of bias (Silva 2018; Weidner 2003). We classified allocation concealment as unclear in 11 included trials due to insufficient information (Barrett 2012; Chubak 2006; Ciloğlu 2005; Dias 2014; Klentrou 2002; Manzaneque 2004; Nieman 1990; Nieman 1993; Nieman 1997; Sloan 2013; Weidner 1998). Two studies were quasi‐randomised ("participants were alternately assigned to each group" and "allocation was done due to convenience") and were assessed as at high risk of bias for this domain (Silva 2018; Weidner 2003).
Blinding
The nature of the interventions meant that blinding of participants was not possible, therefore we classified all included trials as at high risk of bias for this domain. Outcome assessor blinding was unclear due to poor reporting, except for Chubak 2006, which collected outcome information by telephone interview, and Nieman 1990, which blinded participants to the study objectives. Silva 2018 provided insufficient information about blinding and was assessed as at unclear risk of bias. Barrett 2018 reported that assessors were blinded until the end of the trial and was judged as at low risk of bias for this domain.
Incomplete outcome data
We classified eight trials as at low risk of attrition bias because few participants were lost to follow‐up, or reported missing data on colds or other upper respiratory tract infections and adjusted data analysis (Barrett 2012; Barrett 2018; Chubak 2006; Ciloğlu 2005; Dias 2014; Sloan 2013; Weidner 1998; Weidner 2003). We assessed three studies as at high risk of bias for this domain due to substantial losses to follow‐up (> 30%) and because intention‐to‐treat analyses were not used (Nieman 1990; Nieman 1997; Silva 2018). We assessed three trials as at unclear risk of bias due to lack of information on participant dropouts or missing information on statistical correction for dropouts (Klentrou 2002; Manzaneque 2004; Nieman 1993).
Selective reporting
We judged 12 trials as at low risk of bias for selective reporting (Barrett 2012; Barrett 2018; Chubak 2006; Ciloğlu 2005; Klentrou 2002; Manzaneque 2004; Nieman 1990; Nieman 1993; Nieman 1997; Silva 2018; Sloan 2013; Weidner 2003). Four of these trials were registered before commencement and the outcomes reported were consistent with those prespecified in the protocols (Barrett 2012; Barrett 2018; Chubak 2006; Silva 2018). Eight studies were not registered, but we did not detect reporting bias between methods and results sections (Ciloğlu 2005; Klentrou 2002; Manzaneque 2004; Nieman 1990; Nieman 1993; Nieman 1997; Sloan 2013; Weidner 2003). Two trials did not report on all outcomes proposed in methods sections and were thus judged to be at high risk of bias for selective reporting (Dias 2014; Weidner 2003).
Other potential sources of bias
We judged one trial as at high risk of bias due to poor reporting (Dias 2014). It was not possible to extract data because the trial authors reported only means and P values in the results (Dias 2014). The sample size was small, and the trial was underpowered. We assessed all of the other included trials as free from other sources of bias.
Effects of interventions
See: Table 1
Primary outcomes
1. Number of ARI episodes per person per year
Six included trials (N = 582) reported this outcome (Barrett 2012; Barrett 2018; Chubak 2006; Nieman 1990; Sloan 2013; Silva 2018). However, we excluded Nieman 1990 and Sloan 2013 from meta‐analysis due to lack of clarity regarding the data. Exercise did not significantly differ from non‐exercise in terms of number of ARI episodes (rate ratio (RR) 1.00, 95% confidence interval (CI) 0.77 to 1.30; 514 participants; 4 trials; Analysis 1.1). Heterogeneity was low to moderate (Chi² test = 5.30; df = 3; P = 0.15; I² = 43%). We downgraded the certainty of the evidence to low due to limitations in design and implementation (risk of selection bias and lack of blinding, allocation concealment not reported in the trials) and inconsistency of results (heterogeneity between trials may be due to differences in populations, intensity and duration of the intervention, and length of the follow‐up period).
1.1. Analysis.
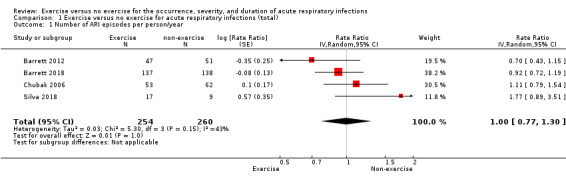
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 1 Number of ARI episodes per person/year.
2. Proportion of participants who experienced at least one ARI over the study period
Five trials (N = 520) reported this outcome (Barrett 2012; Barrett 2018; Chubak 2006; Nieman 1993; Silva 2018). The difference between groups in the proportion of participants who experienced at least one ARI over the study period was not significant (RR 0.88, 95% CI 0.72 to 1.08; Analysis 1.2). Heterogeneity was low (Chi² test = 5.70; df = 4; P = 0.22; I² = 30%). We downgraded the certainty of the evidence to low due to limitations in study design and implementation (risk of selection bias and lack of blinding, allocation concealment not reported in the trials) and inconsistency of results (heterogeneity between trials may be due to differences in populations, intensity and duration of the intervention, and length of the follow‐up period).
1.2. Analysis.
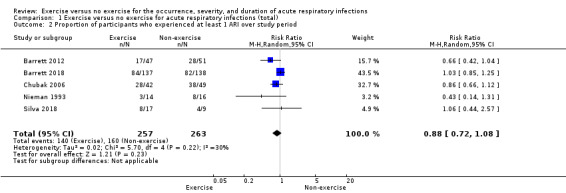
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 2 Proportion of participants who experienced at least 1 ARI over study period.
3. Severity of ARI symptoms
Two trials (N = 373) reported this outcome using the Wisconsin Upper Respiratory Symptom Survey (WURSS‐24) score (Barrett 2012; Barrett 2018). In WURSS‐24, the lower the score, the lower the symptoms over the duration of ARI episodes. There was a significant difference between the exercise group and the non‐exercise group in total WURSS‐24 score, favouring the exercise group (mean difference (MD) −103.57 points, 95% CI −198.28 to −8.87; Analysis 1.3). We downgraded the certainty of the evidence to low due to limitations in study design and implementation (lack of blinding) and imprecision of results (the CI was very wide due to the small number of participants).
1.3. Analysis.
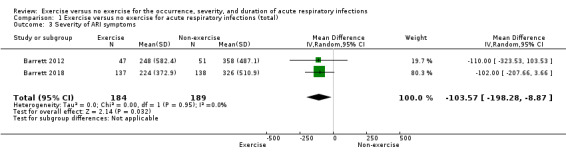
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 3 Severity of ARI symptoms.
4. Number of symptom days in the follow‐up period (12 weeks)
Four trials (N = 483) reported this outcome (Barrett 2012; Barrett 2018; Klentrou 2002; Nieman 1997). Nieman 1997 had a factorial design; we combined exercise and exercise and diet data and compared these with diet alone and control data. The exercise group had on average two fewer symptom days; there was a statistically significant difference between participants in the exercise group and those in the non‐exercise group (MD −2.24, CI −3.50 to −0.98; Analysis 1.4). Heterogeneity was low (Chi² test = 3.18; df = 3; P = 0.36; I² = 6%). We downgraded the certainty of the evidence to low due to limitations in study design and implementation (risk of selection bias and lack of blinding, allocation concealment not reported in the trials).
1.4. Analysis.
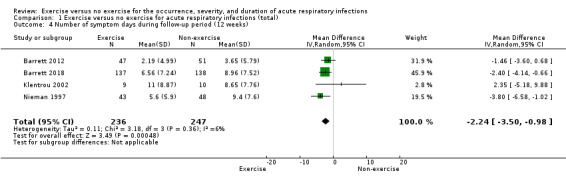
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 4 Number of symptom days during follow‐up period (12 weeks).
5. Number of symptom days per episode of illness
Six trials (N = 557) reported this outcome (Barrett 2012; Barrett 2018; Ciloğlu 2005; Nieman 1990; Silva 2018; Sloan 2013). There was no statistically significant difference between participants in the exercise group and those in the control group in number of symptom days per episode of illness (MD −0.44 days, 95% CI −2.33 to 1.46; Analysis 1.5). Heterogeneity was substantial (Chi² test = 42.02; df = 5; P < 0.001; I² = 88%). We downgraded the certainty of the evidence to low due to limitations in study design and implementation (risk of selection bias and lack of blinding, allocation concealment not reported in the trials) and inconsistency of results (heterogeneity between trials may be due to differences in populations, intensity and duration of the intervention, and length of the follow‐up period). As sufficient data were available, we conducted a post hoc analysis of this outcome specifically in the postmenopausal population (Analysis 1.13). The difference between groups was statistically significant in the number of symptom days per episode of illness, favouring the exercise group (MD −1.07, 95% CI −1.74 to −0.41).
1.5. Analysis.
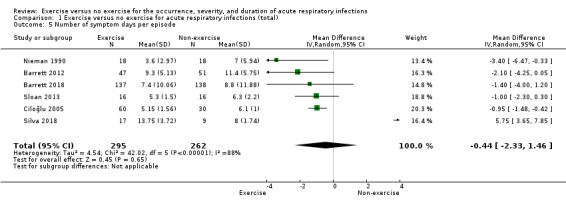
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 5 Number of symptom days per episode.
1.13. Analysis.
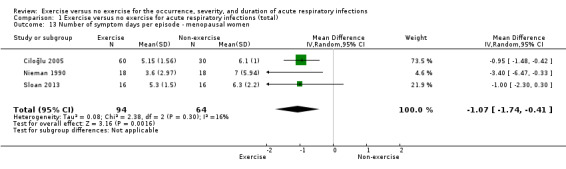
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 13 Number of symptom days per episode ‐ menopausal women.
Secondary outcomes
1.1. Laboratory‐assessed immune parameters ‐ lymphocytes
Three trials (N = 157) reported this outcome (Nieman 1990; Nieman 1993; Nieman 1997). There was a very small mean increase in the exercise group compared with the non‐exercise group, but this difference was not statistically significant (MD 0.11 µL, 95% CI −0.10 to 0.31; Analysis 1.6). Heterogeneity was low (Chi² = 2.24; df = 2; P = 0.33; I² = 11%). We downgraded the certainty of the evidence to moderate due to limitations in study design and implementation (lack of blinding).
1.6. Analysis.
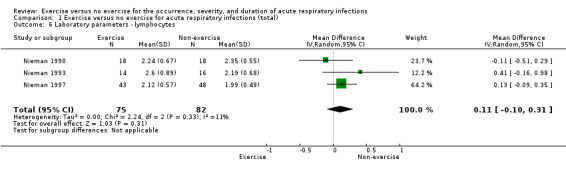
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 6 Laboratory parameters ‐ lymphocytes.
1.2 Laboratory‐assessed immune parameters ‐ immunoglobulin A (IgA)
Four trials (N = 166) reported IgA (Ciloğlu 2005; Klentrou 2002; Manzaneque 2004; Sloan 2013). There was no significant difference between participants in the exercise and non‐exercise groups (standardised mean difference (SMD) 0.07 µmol/L, 95% CI −0.37 to 0.52; Analysis 1.7). There was a moderate level of heterogeneity (Chi² test = 5.14; df = 3; P = 0.16; I² = 42%). We downgraded the certainty of the evidence to low due to limitations in study design and implementation (lack of blinding).
1.7. Analysis.
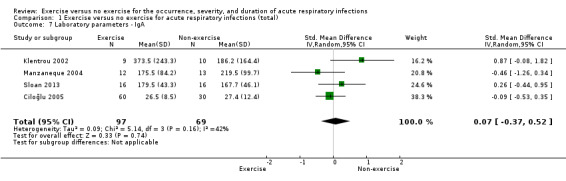
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 7 Laboratory parameters ‐ IgA.
1.3 Laboratory‐assessed immune parameters ‐ neutrophils
Four trials (N = 489) reported this outcome (Barrett 2012; Barrett 2018; Manzaneque 2004; Nieman 1997). There was no significant difference between participants in the exercise and non‐exercise groups (SMD −0.24 mm³, 95% CI −0.54 to 0.06; Analysis 1.8). Heterogeneity was moderate (Chi² test = 6.47; df = 3; P = 0.10; I² = 54%). We downgraded the certainty of the evidence to low due to limitations in study design and implementation (lack of blinding).
1.8. Analysis.
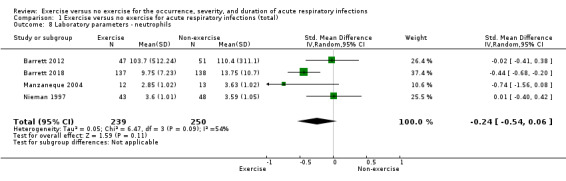
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 8 Laboratory parameters ‐ neutrophils.
2. Quality of life
Two trials analysed quality of life (N = 373), which was reported in two domains (physical and mental health) (Barrett 2012; Barrett 2018). The physical health domain (MD 1.37 points, 95% CI −2.31 to 5.05) and the mental health domain (MD 3.37 points, 95% CI −0.59 to 7.34) were not statistically significant (Analysis 1.9). We downgraded the certainty of the evidence to moderate due to limitations in study design and implementation (lack of blinding).
1.9. Analysis.
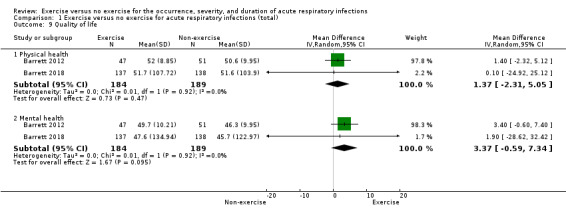
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 9 Quality of life.
3. Cost to the patient
Two trials (N = 373) reported this outcome (Barrett 2012; Barrett 2018). There was no significant difference between participants in the exercise and non‐exercise groups (MD −53.46 USD, 95% CI −128.17 to 21.24; Analysis 1.10). We downgraded the certainty of the evidence to moderate due to limitations in study design and implementation (lack of blinding).
1.10. Analysis.
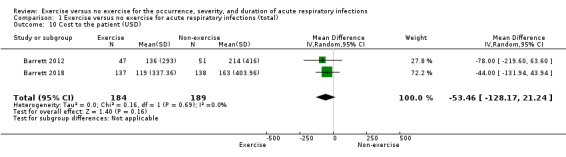
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 10 Cost to the patient (USD).
4. Exercise‐related injuries
One trial reported this outcome (N = 30) (Nieman 1993). There was no significant difference between participants in the exercise and non‐exercise groups (RR 5.67, 95% CI 0.29 to 108.91; Analysis 1.11). We downgraded the certainty of the evidence to low due to limitations in study design and implementation (lack of blinding) and imprecision of results (the CI is very wide due to the small number of participants).
1.11. Analysis.
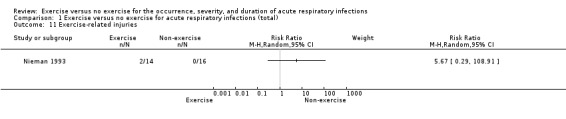
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 11 Exercise‐related injuries.
5. Adherence to the group intervention
Eleven trials (N = 828) reported this outcome (Barrett 2012; Barrett 2018; Chubak 2006; Ciloğlu 2005; Dias 2014; Klentrou 2002; Manzaneque 2004; Nieman 1990; Nieman 1993; Silva 2018; Sloan 2013). There was no significant difference between participants in the exercise and non‐exercise groups (RR 0.95, 95% CI 0.89 to 1.02; Analysis 1.12). This outcome had moderate to substantial levels of heterogeneity (Chi² test = 26.52; df = 10; P = 0.003; I² = 62%). We downgraded the certainty of the evidence to low due to limitations in study design and implementation (lack of blinding) and inconsistency of results (heterogeneity between trials may be due to differences in populations, intensity and duration of the intervention, and length of the follow‐up period).
1.12. Analysis.
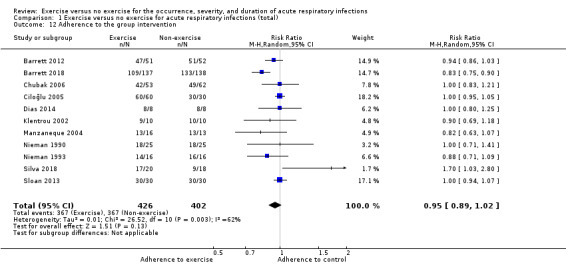
Comparison 1 Exercise versus no exercise for acute respiratory infections (total), Outcome 12 Adherence to the group intervention.
Subgroup analysis
Considering that immune system functions are impacted by aging, thus increasing the risk of acute and chronic diseases (Ghosh 2014), we performed a post hoc subgroup analysis for the number of symptom days per episode in menopausal women. Analysis of the type, frequency, and intensity of exercise could not be completed in the postmenopausal women subgroup due to paucity of data.
Sensitivity analysis
We analysed the trials included in the meta‐analyses to verify whether the impact of risk of bias affected the overall treatment effect of exercising. We explored which trials contributed to changes in heterogeneity. We excluded trials with three or more 'Risk of bias' domains rated as high to explore sensitivity.
We found important levels of heterogeneity for the number of symptoms day per episode. We investigated the source of heterogeneity and found that removing Silva 2018 from the analysis decreased heterogeneity (I² = 88% to I² = 0%). This was due to the study's small sample size, dropout rate greater than 20%, and wide confidence interval compared to the other five trials included in the meta‐analysis (Barrett 2012; Barrett 2018; Ciloğlu 2005; Nieman 1990; Sloan 2013).
Barrett 2018 contributed to increased heterogeneity (I² = 62% when included; I² = 8% when excluded from analysis) for the outcome adherence to the group intervention. This was due to many losses from the exercise group compared to the control group.
We conducted sensitivity analysis by assessing the impact of removing trials that were rated as at high risk of bias in three or more domains.
We assessed the impact of excluding Nieman 1997 (three domains rated as at high risk of bias) from the outcome number of symptom days in the follow‐up period (12 weeks). However, excluding this study did not substantially change the effect size estimate (MD changed from −2.24 to −1.89; the difference between groups remained significant).
We assessed the impact of excluding Nieman 1990 (three domains rated as at high risk of bias) from the outcome number of symptom days per episode of illness. Excluding this study from the analysis shifted the MD from −0.44 to 0.02, but the difference between groups remained not significant.
Removal of Nieman 1990 and Nieman 1997 (both with three domains rated as at high risk of bias) for the outcome laboratory‐assessed immune parameters ‐ lymphocytes shifted the MD from 0.11 to 0.41, but the difference between groups remained not significant.
Removal of Nieman 1997 (three domains rated as at high risk of bias) for analysis of the outcome laboratory‐assessed immune parameters ‐ neutrophils shifted the SMD from −0.24 to −0.33, but the difference between groups remained not significant.
Exclusion of Dias 2014 and Silva 2018 (both with four domains rated as at high risk of bias) and Nieman 1990 (three domains rated as at high risk of bias) for analysis of adherence to the group intervention shifted the RR from 0.95 to 1.00, but the difference between groups remained not significant.
Discussion
Summary of main results
We included three new trials for this update that contributed a total of 473 participants (Barrett 2018; Dias 2014; Silva 2018). The updated review includes a total of 14 trials involving 1377 adults.
We aimed to determine the effectiveness of exercise to alter the occurrence, severity, or duration of acute respiratory infections (ARIs). Analysis of three primary outcomes showed no significant differences between those who exercised and those who did not: number of ARI episodes per person per year; proportion of participants who experienced at least one ARI over the study period; and number of symptom days per episode. Severity of ARI symptoms and number of symptom days during the follow‐up period were significantly lower amongst people who exercised.
There were no significant differences between the exercise and non‐exercise groups for laboratory parameters (e.g. lymphocytes, immunoglobulin A (IgA), and neutrophils), quality of life, cost to the patient, exercise‐related injuries, and adherence to the group intervention.
Overall, the certainty of the evidence was low, downgraded mainly due to limitations in study design and implementation, imprecision, and inconsistency.
Overall completeness and applicability of evidence
Fourteen trials randomised 1377 participants who commenced the intervention; 80 participants were lost to follow‐up. We searched eight databases with no language restrictions and searched two trials registers. Despite including 14 trials, only six studies contributed data for one outcome, whilst all other outcomes analyses included data from fewer studies. Data were limited in terms of outcomes being assessed across studies, and patient‐centred outcomes were not considered in most of the included trials. Data were sufficient to perform meta‐analyses for most outcomes; no data were available to assess exercise‐related injuries in a meta‐analysis. In most of the included studies reporting with regard to selection bias was poor, and most studies were assessed as at high risk of performance and detection bias.
Quality of the evidence
Two trials clearly described randomisation processes (Barrett 2012; Barrett 2018), but 12 trials did not report randomisation. It was not possible to discern whether randomisation was conducted appropriately from the reported characteristics of the study groups.
The nature of the exercise interventions meant that it was not possible to blind participants. Whilst outcome assessors could be blinded, only two trials provided sufficient information to confirm that this was done.
Three trials appeared to be at high risk of attrition bias (Nieman 1990; Nieman 1997; Silva 2018). Intention‐to‐treat analysis or another statistical strategy could have been used to adjust for loss to follow‐up.
Overall, we assessed the certainty of the evidence as low due to lack of blinding, risk of selection bias (allocation concealment not reported in the trials), and imprecision (the confidence interval was very wide due to the small number of participants).
Several trials appeared to have focused on pathophysiological processes rather than pragmatic outcomes of interest to patients (Ciloğlu 2005; Manzaneque 2004; Nieman 1990; Nieman 1993; Nieman 1997; Sloan 2013; Weidner 1998; Weidner 2003). This is not a problem of selective reporting so much as a different research objective in these trials compared to the objectives of our review.
Potential biases in the review process
The three main limitations of this review were lack of reporting to permit 'Risk of bias' assessment, clinical variability, and lack of consistent criteria for ARI classification. Most trials were not registered, presenting another potential source of bias, although we found no ongoing trials in clinical trials registries.
Agreements and disagreements with other studies or reviews
We found one narrative systematic review of physical activity and the risk of ARI amongst athletes. It reported on 30 studies published up to 2009, and included 8575 athletes and 1798 non‐athletes (Moreira 2009). The authors highlighted the heterogeneity problem of exercise intensity, duration, and the wide variety of participants included in the primary studies, as well as different types of study designs. The authors also identified problems related to risk of bias similar to those we identified. We are in agreement with their call for studies of better methodological rigour (Moreira 2009). The authors of Moreira 2009 also speculated that there is a J‐shaped curve that describes the relationship between physical activity and risk of ARI (that both low and high levels of physical activity increase the risk of ARI, whilst moderate levels of physical activity reduce the risk) (Moreira 2009). Our review could not test this hypothesis because we included only trials that tested moderate exercise.
Another systematic review using Cochrane methods evaluated exercise for preventing the common cold, including four randomised controlled trials with a total of 281 participants (Lee 2014). Lee 2014 reported that prevention of the common cold and mean illness days were significantly lower in the exercise groups. A key difference between Lee 2014 and our review appears to be the inclusion criteria: Lee 2014 considered regular exercise more than five times per week, and moderate intensity was defined as greater than 60% of maximal heart rate. Our search was broader and more comprehensive (including more trials and more outcomes). We considered all intensities of exercise and did not include a frequency cut‐off. We believe it is important to explore exercise features first, and if possible, conduct more specific analyses.
Authors' conclusions
Implications for practice.
Based on the available evidence, we could not determine whether exercise impacts the occurrence, severity, or duration of acute respiratory infections (ARIs). Meta‐analyses suggested that the number of symptom days in the follow‐up may be reduced by exercise (Analysis 1.4; 4 trials), and that exercise reduced the severity of ARI symptoms (Analysis 1.3; 2 trials). However, the evidence was of low certainty, and there was considerable risk of bias in most trials.
Implications for research.
Despite epidemiological data appearing to support a reduced occurrence of ARIs with increased physical activity, more and better randomised controlled trials are needed to answer the question about the effects of exercise on the occurrence, severity, and duration of ARIs. Studies are also needed that compare exercise to usual care; we found no studies making this comparison in our literature searches. Greater methodological rigour and clearer reporting (in compliance with the relevant reporting guidelines) are needed for future research (patient selection, blinding of outcome assessors, reporting of all outcomes analysed, and registration of study protocols).
What's new
| Date | Event | Description |
|---|---|---|
| 5 March 2020 | New search has been performed | We included three new trials in this update (Barrett 2018; Dias 2014; Silva 2018), for a total of 14 included trials. Exercise reduced the severity of acute respiratory infection symptoms (2 trials; moderate‐certainty evidence) and decreased the number of symptom days (during the follow‐up period, 12 weeks) (4 trials; low‐certainty evidence). We recruited two new authors, and three of the original authors withdrew from this update. |
| 5 March 2020 | New citation required and conclusions have changed | Conclusions changed for three primary outcomes. Severity of acute respiratory infection symptoms outcome (previously no significant difference between groups) now shows a significantly lower Wisconsin Upper Respiratory Symptom Survey (WURSS‐24) score in the exercise group (mean difference −103.6). The number of symptom days in the follow‐up period is now significantly less (−2.24 days) in the exercise group. The number of symptom days per episode of illness was previously significantly lower in the exercise group; there is now no significant difference between groups for this outcome. |
Acknowledgements
We wish to thank Tammy Hoffmann, Elaine M Beller, and Chris B Del Mar, who were authors of the protocol and the first publication of this review. We also thank the following people for commenting on the draft protocol: Emma Lake, Marcial Fallas, Jonathan Peake, Sree Nair, and Hans van der Wouden. We wish to thank the following people for peer reviewing the draft review: Noorin Bhimani, Nancy Banasiak, David Nieman Terry Neeman, the Contact Editor, Hans van der Wouden, and the Sign‐off Editor, Michelle Guppy. We would also like to thank the following peer reviewers: Nicolette Bishop, Terry Neeman, Janet Wale, and other peer reviewers who wished to remain anonymous.
Appendices
Appendix 1. MEDLINE (Ovid)
1 respiratory tract infections/ or bronchitis/ or common cold/ or influenza, human/ or laryngitis/ or exp pharyngitis/ or exp pneumonia/ or exp sinusitis/ 2 (respiratory adj2 (infect* or illness or symptom* or acute or virus*)).tw. 3 (common cold* or colds or coryza).tw. 4 ((acute or viral or virus* or bacter*) adj2 rhinit*).tw. 5 (influenza* or flu or ili).tw. 6 (pharyngit* or laryngit* or tonsillit* or sore thoat*).tw. 7 (throat* adj3 (infect* or inflam*)).tw. 8 (nasopharyngit* or rhinopharyngit*).tw. 9 Cough/ 10 cough*.tw. 11 (sinusit* or rhinosinusit* or nasosinusit*).tw. 12 (bronchit* or pneumon* or bronchopneumon* or pleuropneumon*).tw. 13 (ari or urti or lrti).tw. 14 or/1‐13 15 exp Exercise/ 16 exp Exercise Movement Techniques/ 17 exp Exercise Therapy/ 18 Physical Fitness/ 19 physical endurance/ or exercise tolerance/ 20 Physical Exertion/ 21 exp Sports/ 22 Dancing/ 23 (exercis* or sport* or fitness* or gym* or aerobic*).tw. 24 ((weight* or strength* or enduranc* or circuit*) adj5 (program* or train* or session*)).tw. 25 (physical* adj5 (fit* or activ* or movement* or train* or condition* or program*)).tw. 26 (activ* adj2 life*).tw. 27 (run* or walk* or jog* or sprint* or treadmill* or row* or swim* or bicycl* or cycl* or danc* or yoga or tai chi or tai ji or qigong or qi gong).tw. 28 or/15‐27 29 14 and 28
Appendix 2. Embase (Elsevier) search strategy
#37 #25 AND #36 27022 #36 #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 1390178 #35 run*:ab,ti OR walk*:ab,ti OR jog*:ab,ti OR sprint*:ab,ti OR treadmill*:ab,ti OR row*:ab,ti OR swim*:ab,ti OR bicycl*:ab,ti OR cycl*:ab,ti OR danc*:ab,ti OR yoga:ab,ti OR 'tai chi':ab,ti OR 'tai ji':ab,ti OR qigong:ab,ti OR 'qi gong':ab,ti AND [embase]/lim 976799 #34 (activ* NEAR/2 life*):ab,ti AND [embase]/lim 5574 #33 (physical* NEAR/5 (fit* OR activ* OR movement* OR train* OR condition* OR program*)):ab,ti AND [embase]/lim 74609 #32 ((weight* OR strength* OR enduranc* OR circuit*) NEAR/5 (program* OR train* OR session*)):ab,ti AND [embase]/lim 18505 #31 exercis*:ab,ti OR sport*:ab,ti OR fitness*:ab,ti OR gym*:ab,ti OR aerobic*:ab,ti AND [embase]/lim 267006 #30 'sport'/exp AND [embase]/lim 58241 #29 'training'/de OR 'endurance'/de OR 'exercise tolerance'/de OR 'physical capacity'/de AND [embase]/lim 78874 #28 'physical activity'/exp OR 'physical activity, capacity and performance'/de AND [embase]/lim166092 #27 'kinesiotherapy'/exp AND [embase]/lim 29817 #26 'exercise'/exp AND [embase]/lim 140065 #25 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 427944 #24 (throat* NEAR/3 (infect* OR inflam*)):ab,ti AND [embase]/lim 750 #23 cough*:ab,ti AND [embase]/lim 34249 #22 'coughing'/de AND [embase]/lim 46685 #21 influenza*:ab,ti OR 'flu'/de OR ili:ab,ti AND [embase]/lim 75233 #20 'influenza'/exp AND [embase]/lim 38070 #19 ((acute OR viral OR virus* OR bacter*) NEAR/2 rhinitis):ab,ti AND [embase]/lim 263 #18 'common cold':ab,ti OR 'common colds':ab,ti OR colds:ab,ti OR coryza:ab,ti AND [embase]/lim 3569 #17 'common cold'/de OR 'common cold symptom'/de AND [embase]/lim 4712 #16 sinusit*:ab,ti OR rhinosinusit*:ab,ti OR nasosinusit*:ab,ti AND [embase]/lim 13953 #15 'sinusitis'/exp AND [embase]/lim 21736 #14 nasopharyngit*:ab,ti OR rhinopharyngit*:ab,ti AND [embase]/lim 774 #13 pharyngit*:ab,ti OR laryngit*:ab,ti OR tonsillit*:ab,ti OR 'sore throat':ab,ti OR 'sore throats':ab,ti AND [embase]/lim 10891 #12 'sore throat'/de AND [embase]/lim 7948 #11 'tonsillitis'/exp AND [embase]/lim 7170 #10 'laryngitis'/de AND [embase]/lim 2091 #9 'pharyngitis'/exp AND [embase]/lim 15830 #8 bronchit*:ab,ti AND [embase]/lim 15281 #7 'bronchitis'/exp AND [embase]/lim 33544 #6 pneumon*:ab,ti OR bronchopneumon*:ab,ti OR pleuropneumon*:ab,ti AND [embase]/lim 124552 #5 'pneumonia'/exp AND [embase]/lim 141952 #4 'respiratory tract inflammation'/de OR 'inflammation of the lungs, bronchi and pleura'/de AND [embase]/lim 5088 #3 ari:ab,ti OR urti:ab,ti OR lrti:ab,ti AND [embase]/lim 2690 #2 (respiratory NEAR/2 (infect* OR illness OR symptom* OR acute OR virus*)):ab,ti AND [embase]/lim 70124 #1 'respiratory tract infection'/de OR 'upper respiratory tract infection'/de OR 'viral upper respiratory tract infection'/de OR 'lower respiratory tract infection'/de AND [embase]/lim 49071
Appendix 3. CINAHL (EBSCO) search strategy
S38 S28 AND S37 398 S37 S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 181,452 S36 (MH "Quantitative Studies") 8,454 S35 TI placebo* OR AB placebo* 19,960 S34 (MH "Placebos") 6,623 S33 TI random* OR AB random* 99,149 S32 TI ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) 14,511 S31 TI clinic* W1 trial* OR AB clinic* W1 trial* 27,568 S30 PT clinical trial 49,810 S29 (MH "Clinical Trials+") 112,137 S28 S14 AND S27 1,874 S27 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 169,592 S26 TI (run* or walk* or jog* or sprint* or treadmill* or row* or swim* or bicycl* or cycl* or danc* or yoga or tai chi or tai ji or qigong or qi gong) OR AB (run* or walk* or jog* or sprint* or treadmill* or row* or swim* or bicycl* or cycl* or danc* or yoga or tai chi or tai ji or qigong or qi gong) 60,655 S25 TI activ* N5 life* OR AB activ* N5 life* 4,190 S24 TI (physical* N5 (fit* or activ* or movement* or train* or condition* or program*)) OR AB (physical* N5 (fit* or activ* or movement* or train* or condition* or program*)) 24,749 S23 TI ( (weight* or strength* or enduranc* or circuit*) N5 (program* or train* or session*)) OR AB ((weight* or strength* or enduranc* or circuit*) N5 (program* or train* or session*)) 7,466 S22 TI (exercis* or sport* or fitness* or gym* or aerobic*) OR AB (exercis* or sport* or fitness* or gym* or aerobic*) 64,226 S21 (MH "Dancing+") 1,535 S20 (MH "Sports+") 33,190 S19 (MH "Exertion") OR (MH "Exercise Intensity") 8,146 S18 (MH "Physical Endurance+") 5,631 S17 (MH "Physical Fitness+") 7,255 S16 (MH "Therapeutic Exercise+") 25,339 S15 (MH "Exercise+") 47,369 S14 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 42,221 S13 TI (ari or urti or lrti) OR AB (ari or urti or lrti) 333 S12 TI (bronchit* or pneumon* or bronchopneumon* or pleuropneumon*) OR AB (bronchit* or pneumon* or bronchopneumon* or pleuropneumon*) 10,910 S11 TI (sinusit* or rhinosinusit* or nasosinusit*) OR AB (sinusit* or rhinosinusit* or nasosinusit*) 1,859 S10 TI cough* OR AB cough* 4,012 S9 (MH "Cough") 2,172 S8 TI (nasopharyngit* or rhinopharyngit*) OR AB (nasopharyngit* or rhinopharyngit*) 70 S7 TI (throat* N3 (infect* or inflam*)) OR AB (throat* N3 (infect* or inflam*)) 85 S6 TI (pharyngit* or laryngit* or tonsillit* or sore throat*) OR AB (pharyngit* or laryngit* or tonsillit* or sore thoat*) 728 S5 TI (influenza* or flu or ili) OR AB (influenza* or flu or ili) 10,739 S4 TI ((acute or viral or virus* or bacter*) N2 rhinit*) OR AB ((acute or viral or virus* or bacter*) N2 rhinit*) 30 S3 TI (common cold* or colds or coryza) OR AB (common cold* or colds or coryza) 888 S2 TI (respiratory N2 (infect* or illness or symptom* or acute or virus*)) OR AB (respiratory N2 (infect* or illness or symptom* or acute or virus*)) 8,217 S1 (MH "Respiratory Tract Infections") OR (MH "Bronchitis") OR (MH "Bronchitis, Acute") OR (MH "Common Cold") OR (MH "Influenza") OR (MH "Influenza, Human") OR (MH "Influenza, Seasonal") OR (MH "Laryngitis") OR (MH "Pharyngitis") OR (MH "Pneumonia+") OR (MH "Sinusitis+") OR (MH "Tonsillitis") 23,907
Appendix 4. LILACS (Bireme) search strategy
INGLÊS
MH:"Respiratory Tract Infections" OR "Upper Respiratory Tract Infections" OR MH:C01.539.739$ OR MH:C08.730$ OR MH:Bronchitis OR MH:C08.127.446$ OR MH:C08.381.495.146 OR MH:C08.730.099 OR MH:"Common Cold" OR MH:C02.782.687.207$ OR MH:C08.730.162$ OR "Cold, Common" OR "Coryza, Acute" OR MH:"Influenza, Human" OR MH:C02.782.620.365$ OR MH:C08.730.310$ OR Grippe OR "Human Flu" OR "Human Influenza" OR "Influenza in Humans" OR MH:Laryngitis OR MH:C08.360.535 OR MH:C08.730.368 OR MH:C09.400.535 OR MH:Pharyngitis OR MH:C07.550.781$ OR MH:C08.730.561$ OR MH:C09.775.649$ OR "Sore Throat" OR MH:C08.381.677$ OR MH:08.730.610$ OR "Experimental Lung Inflammation" OR "Lobar Pneumonia" OR "Lung Inflammation" OR "Pulmonary Inflammation" OR MH:Sinusitis OR MH:C08.460.692.752$ OR MH:C08.730.749$ OR MH:C09.603.692.752$ OR MH:Nasopharyngitis OR MH:C07.550.350.700$ OR MH:C07.550.781.500$ OR MH:C08.730.561.500$ OR MH:C09.775.350.700$ OR MH:C09.775.649.500$ OR MH:Cough OR MH:Tos OR MH:Tosse OR MH:C08.618.248$ MH:C23.888.852.293$ OR MH:Bronchitis OR MH:Bronquitis OR MH:Bronquite OR MH:C08.127.446$ OR MH:C08.381.495.146$ OR MH:C08.730.099$ OR MH:Bronconeumonía OR MH:C08.127.509$ OR MH:C08.381.677.127$ OR MH:C08.730.610.127$ OR MH:Pleuropneumonia OR MH:C08.381.677.473$ OR MH:C08.528.735.473$ OR MH:C08.730.582.473$ OR MH:C08.730.610.473$ AND MH:Exercise OR "Aerobic Exercise" OR "Exercise, Aerobic" OR "Exercise, Isometric" OR "Exercise, Physical" OR "Isometric Exercise" OR MH:G11.427.590.530.698.277$ OR MH:I03.350$ OR MH:"Exercise Movement Techniques" OR MH:"Exercise Therapy" OR OR MH:E02.779.483$ OR MH:E02.831.387$ OR MH:"Physical Fitness" OR "Physical Conditioning, Human" OR MH:I03.621$ OR MH:N01.400.545$ OR MH:"Physical Endurance" OR MH:G11.427.680$ OR MH:I03.450.642.845.054.600$ OR MH:"Exercise Tolerance" OR MH:G11.427.680.270$ OR MH: "Physical Exertion" OR "Physical Effort" OR MH:G11.427.590.780$ OR MH:Sports OR Athletics OR MH:I03.450.642.845$ OR MH:Dancing OR MH:I03.450.642.287$ OR MH:Gymnastics OR Calisthenics OR MH:I02.233.543.454$ OR MH:I03.450.642.845.417$ OR MH:"Weight Lifting" OR MH:I03.450.642.845.950$ OR MH:"Muscle Strength" OR MH:E01.370.600.425$ OR MH:G11.427.560$ OR MH: "Physical Education and Training" OR MH:I02.233.543$ OR MH:Running OR MH:G11.427.590.530.568.610$ OR MH:G11.427.590.530.698.277.750$ OR MH:I03.450.642.845.610$ OR MH:Jogging OR MH:G11.427.590.530.568.610.320$ OR MH:G11.427.590.530.698.277.750.320$ OR MH:I03.450.642.845.610.320$ OR MH: "Exercise Test" OR Bicycle OR "Ergometry Test" OR "Arm Ergometry Test" OR "Step Test" OR "Stress Test" OR "Treadmill Test" OR MH:E01.370.370.380.250$ OR MH:E01.370.386.700.250$ OR MH:E05.333.250$ OR "Prueba Ergométrica de Bicicleta" OR "Test Ergométrico de Bicicleta" OR MH:Swimming OR Natación OR Natação OR MH:G11.427.590.530.568.800$ OR MH:G11.427.590.530.698.277.875$ OR MH:I03.450.642.845.869$ OR MH:Bicycling OR MH:I03.450.642.845.140$ OR MH:Yoga OR MH:E02.190.525.937$ OR MH:E02.190.901.984$ OR MH:E02.779.474.937$ OR MH:K01.844.799.867$ OR MH:"Tai Ji" OR MH:E02.190.525.890$ OR MH:E02.779.474.913$ OR MH:I03.450.642.845.560.500$ OR MH:"Breathing Exercises" OR "Ch'i Kung" OR "Qi Gong" OR Qigong OR "Respiratory Muscle Training" OR MH:E02.190.525.186$ OR MH:E02.779.474.124$ OR "Ch'i Kung" OR "Qi Gong" OR Qigong
PORTUGUES
MH:"Infecções Respiratórias" OR "Infecções das Vias Respiratórias" OR "Infecções do Trato Respiratório Superior" OR "Infecções do Aparelho Respiratório" OR "Infecções das Vias Respiratórias Superiores" OR "Infecções das Vias Aéreas Superiores" OR "Infecções do Sistema Respiratório" OR "Infecções do Sistema Respiratório Superior" OR "Infecções do Trato Respiratório" OR MH:Bronquitis OR MH: "Resfriado Comum" OR "Coriza Aguda" OR Catarro OR Resfriado OR Constipação OR MH: "Gripe Humana" OR MH:"Influenza Humana" OR Gripe OR "Gripe Humana" OR "Influenza em Humanos" OR MH:Laringite OR MH:Faringite OR Inflamação OR "Experimental dos Pulmões" OR "Inflamação do Pulmão" OR "Pneumonia Lobar" OR Pneumonite OR "Inflamação Pulmonar" OR Pulmonia OR MH:Sinusite OR MH:Nasofaringite OR MH:Bronchopneumonia OR MH:Pleuroneumonía AND MH:Exercício OR "Exercício Aeróbico" OR "Exercício Isométrico" OR "Exercício Físico" OR MH:"Terapia por Exercício" OR MH:"Aptidão Física" OR "Estado Físico Humano" OR "Condicionamento Físico Humano" OR MH:"Resistência Física" OR MH:"Tolerância ao Exercício" OR MH:"Esforço Físico" OR MH:Esportes OR Atletismo OR Desportes OR MH:Dança OR MH:Ginástica OR MH:"Levantamento de Peso" OR MH:"Força Muscular" OR "Educação Física e Treinamento" OR "Educação Física" OR "Educação e Treinamento Físico" OR MH:"Corrida Moderada" OR MH:Corrida OR MH:Trote OR MH:"Teste de Esforço" OR "Teste Ergométrico de Bicicleta" OR "Teste Ergométrico com os Braços" OR "Teste de Degrau" OR "Teste de Estresse" OR "Teste de Stress" OR "Teste de Esteira Rolante" OR "Teste Ergométrico de Esteira" OR MH:Ciclismo OR MH:Yoga OR "T'ai Chi" OR "Tai Chi" OR "Tai Ji Quan" OR "Tai‐ji" OR Taiji OR Taijiquan OR MH:"Tai Ji" OR MH:"Ejercicios Respiratorios" OR "Qi Gong" OR Qigong OR "Exercícios para os Músculos Respiratórios" OR "Exercício Respiratório"
ESPANOL
MH:"Infecciones del Sistema Respiratorio" OR "Infecciones de las Vías Respiratorias" OR "Infecciones del Tracto Respiratorio Superior" OR "Infecciones del Aparato Respiratorio" OR "Infecciones de las Vías Respiratorias Superiores" OR "Infecciones del Tracto Respiratorio" OR "Infecciones Respiratorias" OR MH:"Resfriado Común" OR "Coriza Aguda" OR Catarro OR Gripe "Influenza Humana" OR "Influenza en Humanos" OR MH:Laringitis OR MH:Faringitis OR "Dolor de Garganta" OR "Dor de Garganta" OR MH:Pneumonia OR MH:Neumonía OR MH:Pneumonia OR "Pneumonia, Lobar" OR Pneumonitis OR "Inflamación Experimental del Pulmón" OR "Inflamación del Pulmón" OR "Neumonía Lobar" OR Neumonitis OR "Inflamación Pulmonar" OR Pneumonía OR Pulmonía OR MH:Sinusitis OR MH:Nasofaringitis OR MH:Broncopneumonia OR MH:Pleuropneumonia AND MH:Ejercicio OR "Ejercicio Aeróbico" OR "Ejercicio Isométrico" OR "Ejercicio Físico" OR MH:"Técnicas de Ejercicio con Movimientos" OR MH:"Técnicas de Exercício e de Movimento" OR MH:E02.779.474$ OR "Técnicas de Ejercicios con Movimiento" OR "Técnicas por Movimiento de Ejercicio" OR "Técnicas de Movimentos do Exercício" OR MH:"Terapia por Ejercicio" OR MH: "Acondicionamiento Físico" OR MH:"Resistencia Física" OR MH:"Tolerancia al Ejercicio" OR MH:"Esfuerzo Físico" OR MH:Deportes OR Atletismo OR Desportos OR MH:Baile OR MH:Gimnasia OR Calistenia OR MH:"Levantamiento de Peso" OR MH:"Fuerza Muscular" OR MH:"Educación y Entrenamiento Físico" OR "Educación Física" OR MH:Carrera OR MH:"Prueba de Esfuerzo" OR "Test de Esfuerzo" OR "Prueba Ergométrica del Brazo" OR "Test Ergométrico del Brazo" OR "Prueba del Escalón" OR "Test del Escalón" OR "Prueba de Estrés" OR "Test de Estrés" OR "Prueba de Esfuerzo en Cinta sin Fin" OR MH:Ciclismo OR MH:Ioga OR MH:"Tai Ji" OR "T'ai Chi" OR "Tai Chi" OR "Tai Ji Quan" OR Tai‐I OR Taiji OR Taijiquan OR MH:"Exercícios Respiratórios" OR "Entrenamiento del Musculo Respiratorio" OR "Ch'i Kung"
Appendix 5. SPORTDiscus (EBSCO) search strategy
S40 S33 AND S39 101 S39 S34 OR S35 OR S36 OR S37 OR S38 OR S39 35,613 S38 TI ( crossover* or cross over* ) OR AB ( crossover* or cross over* ) 2,991 S37 TI ( (singl* or doubl*) W1 (blind* or mask*) ) OR AB ( (singl* or doubl*) W1 (blind* or mask*) ) 4,406 S36 TI trial 5,644 S35 TI clinic* W1 trial* OR AB clinic* W1 trial* 4,508 S34 TI placebo* OR AB placebo* 6,609 S33 TI random* OR AB random* 26,226 S32 S19 AND S31 3,150 S31 S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 713,457 S30 TI ( run* or walk* or jog* or sprint* or treadmill* or row* or swim* or bicycl* or cycl* or danc* or yoga or tai chi or tai ji or qigong or qi gong ) OR AB ( run* or walk* or jog* or sprint* or treadmill* or row* or swim* or bicycl* or cycl* or danc* or yoga or tai chi or tai ji or qigong or qi gong ) 216,308 S39 TI activ* N2 life* OR AB activ* N2 life* 2,393 S28 TI ( physical* N5 (fit* or activ* or movement* or train* or condition* or program*) ) OR AB ( physical* N5 (fit* or activ* or movement* or train* or condition* or program*) ) 49,909 S27 TI ( (weight* or strength* or enduranc* or circuit*) N5 (program* or train* or session*) ) OR AB ( (weight* or strength* or enduranc* or circuit*) N5 (program* or train* or session*) ) 22,558 S26 TI ( exercis* or sport* or fitness* or gym* or aerobic* ) OR AB ( exercis* or sport* or fitness* or gym* or aerobic* ) 370,918 S25 DE "DANCE" OR DE "AERIAL dance" OR DE "AEROBIC dancing" OR DE "AFRICAN American dance" OR DE "BALLET" OR DE "BALLROOM dancing" OR DE "BELLY dance" OR DE "BREAK dancing" OR DE "CHA‐cha (Dance)" OR DE "COUNTRY dancing" OR DE "DANCE for people with disabilities" OR DE "FLAMENCO" OR DE "FOLK dancing" OR DE "FREE skating" OR DE "HIP‐hop dance" OR DE "ICE dancing" OR DE "JAZZ dance" OR DE "LINE dancing" OR DE "LION dance" OR DE "MODERN dance" OR DE "MOVEMENT notation" OR DE "ORIGINAL set pattern dance (Skating)" OR DE "POLE dancing" OR DE "ROUND dancing" OR DE "SALSA (Dance)" OR DE "SHISHIMAI (Dance)" OR DE "SQUARE dancing" OR DE "STEP dancing" OR DE "TANGO (Dance)" OR DE "TAP dancing" 7,883 S24 DE "PHYSICAL training & conditioning" OR DE "ACROBATICS ‐‐ Training" OR DE "ALTITUDE training" OR DE "ANAEROBIC training" OR DE "ARCHERY ‐‐ Training" OR DE "BADMINTON (Game) ‐‐ Training" OR DE "BASE training (Exercise)" OR DE "BASEBALL ‐‐ Training" OR DE "BASKETBALL ‐‐ Training" OR DE "BICYCLE racing ‐‐ Training" OR DE "BODYBUILDING ‐‐ Training" OR DE "BOWLING ‐‐ Training" OR DE "BOXING ‐‐ Training" OR DE "BULLFIGHT training & conditioning" OR DE "BUNGEE jumping training & conditioning" OR DE "CANOES & canoeing ‐‐ Training" OR DE "CAVING training & conditioning" OR DE "COMPOUND exercises" OR DE "CONTRAST training (Physical training & conditioning)" OR DE "COXSWAINING ‐‐ Training" OR DE "CRICKET training & conditioning" OR DE "CROSS‐training (Sports)" OR DE "CYCLING ‐‐ Training" OR DE "DANCE training & conditioning" OR DE "DEEP diving training & conditioning" OR DE "DIVING ‐‐ Training" OR DE "DOGSLEDDING training & conditioning" OR DE "ENDURANCE sports ‐‐ Training" OR DE "FENCING ‐‐ Training" OR DE "FIELD hockey training & conditioning" OR DE "FOOTBALL ‐‐ Training" OR DE "FUNCTIONAL training" OR DE "GLIDING & soaring training & conditioning" OR DE "GOLF ‐‐ Training" OR DE "GYMNASTICS ‐‐ Training" OR DE "HANDBALL training & conditioning" OR DE "HIKING training & conditioning" OR DE "HOCKEY ‐‐ Training" OR DE "HUNTING training & conditioning" OR DE "INTERVAL training" OR DE "ISOLATION exercises" OR DE "KAYAKING ‐‐ Training" OR DE "KNIFE fighting ‐‐ Training" OR DE "KORFBALL ‐‐ Training" OR DE "LACROSSE training & conditioning" OR DE "LONG slow distance training" OR DE "MARTIAL arts ‐‐ Training" OR DE "MOTORSPORTS training & conditioning" OR DE "MOUNTAINEERING ‐‐ Training" OR DE "NUNCHAKU ‐‐ Training" OR DE "ORIENTEERING ‐‐ Training" OR DE "OVERTRAINING" OR DE "PACE training" OR DE "PARACHUTING training & conditioning" OR DE "PARAKITING training & conditioning" OR DE "PERIODIZATION training" OR DE "PERSONAL training" OR DE "POLO training & conditioning" OR DE "PRACTICE (Sports)" OR DE "PRESEASON (Sports)" OR DE "RACQUETBALL ‐‐ Training" OR DE "RECOVERY training" OR DE "RELAY racing ‐‐ Training" OR DE "REPETITION training" OR DE "RESISTANCE training (Physical training & conditioning)" OR DE "ROCK climbing ‐‐ Training" OR DE "RODEO training & conditioning" OR DE "ROLLER skating training & conditioning" OR DE "ROWING ‐‐ Training" OR DE "RUGBY football ‐‐ Training" OR DE "RUNNING ‐‐ Training" OR DE "SHOT putting ‐‐ Training" OR DE "SKATING ‐‐ Training" OR DE "SKIS & skiing ‐‐ Training" OR DE "SKYDIVING training & conditioning" OR DE "SOCCER ‐‐ Training" OR DE "SOFTBALL ‐‐ Training" OR DE "SPEED endurance training" OR DE "SQUASH (Game) ‐‐ Training" OR DE "STRENGTH training" OR DE "SURFING ‐‐ Training" OR DE "SWIMMING ‐‐ Training" OR DE "TABLE tennis training & conditioning" OR DE "TEAM handball ‐‐ Training" OR DE "TENNIS ‐‐ Training" OR DE "TRACK & field ‐‐ Training" OR DE "TRIATHLON ‐‐ Training" OR DE "TUG of war (Game) ‐‐ Training" OR DE "VAULTING (Horsemanship) ‐‐ Training" OR DE "VOLLEYBALL ‐‐ Training" OR DE "WATER polo ‐‐ Training" OR DE "WEIGHT training" OR DE "WHEELCHAIR sports ‐‐ Training" OR DE "WINTER sports training & conditioning" OR DE "WRESTLING ‐‐ Training" OR DE "YOGA training & conditioning" 38,599 S23 DE "BALL games" OR DE "ANETSO" OR DE "BALL hockey" OR DE "BALLE au tamis (Game)" OR DE "BASEBALL" OR DE "BASKETBALL" OR DE "BATTLE ball" OR DE "BICYCLE polo" OR DE "BILLIARDS" OR DE "BOWLING games" OR DE "BROOMBALL" OR DE "CAMOGIE (Game)" OR DE "CRICKET (Sport)" OR DE "CROQUET" OR DE "DODGEBALL" OR DE "FIELD hockey" OR DE "FLICKERBALL" OR DE "FOOTBALL" OR DE "GOAL ball" OR DE "GOLF" OR DE "GOLF croquet" OR DE "HANDBALL" OR DE "HURLING (Game)" OR DE "INDOOR hockey" OR DE "JAPANESE polo" OR DE "JIAN zi (Game)" OR DE "KANG (Game)" OR DE "KICKBALL" OR DE "LACROSSE" OR DE "LAPTA (Game)" OR DE "LAWN tempest (Game)" OR DE "MINTON (Game)" OR DE "PARLOR football" OR DE "PARLOR tennis" OR DE "PICKLE ball" OR DE "PICKLEBALL (Game)" OR DE "PIZE‐ball" OR DE "POLO" OR DE "POLOCROSSE" OR DE "PUSH ball" OR DE "QUIDDITCH (Game)" OR DE "RACQUETBALL" OR DE "RAGA (Game)" OR DE "ROLL ball" OR DE "ROUNDERS" OR DE "RUGBALL" OR DE "SCHLAGBALL" OR DE "SHINTY (Game)" OR DE "SOCCER" OR DE "SOFTBALL" OR DE "SPEED‐a‐way (Game)" OR DE "SPEEDBALL" OR DE "STICKBALL (Game)" OR DE "STOOLBALL" OR DE "TABLE tennis" OR DE "TCHOUKBALL" OR DE "TENNIS" OR DE "TETHERBALL" OR DE "TRAPBALL" OR DE "VOLLEYBALL" OR DE "WALLYBALL" OR DE "WATER polo" OR DE "WICKET" OR DE "WIFFLE ball" 108,135 S22 DE "EXERCISE tolerance" 12 S21 DE "PHYSICAL fitness" OR DE "ANAEROBIC exercises" OR DE "ASTROLOGY & physical fitness" OR DE "BODYBUILDING" OR DE "CARDIOVASCULAR fitness" OR DE "CIRCUIT training" OR DE "COMPOUND exercises" OR DE "ISOLATION exercises" OR DE "LIANGONG" OR DE "MUSCLE strength" OR DE "PERIODIZATION training" OR DE "PHYSICAL fitness ‐‐ Genetic aspects" OR DE "PHYSICAL fitness for children" OR DE "PHYSICAL fitness for girls" OR DE "PHYSICAL fitness for men" OR DE "PHYSICAL fitness for older people" OR DE "PHYSICAL fitness for people with disabilities" OR DE "PHYSICAL fitness for women" OR DE "PHYSICAL fitness for youth" OR DE "SPORT for All" 96,632 S20 DE "EXERCISE" OR DE "ABDOMINAL exercises" OR DE "AEROBIC exercises" OR DE "ANAEROBIC exercises" OR DE "AQUATIC exercises" OR DE "ARM exercises" OR DE "BACK exercises" OR DE "BREATHING exercises" OR DE "BREEMA" OR DE "BUTTOCKS exercises" OR DE "CALISTHENICS" OR DE "CHAIR exercises" OR DE "CHEST exercises" OR DE "CIRCUIT training" OR DE "COMPOUND exercises" OR DE "DO‐in" OR DE "EXERCISE ‐‐ Immunological aspects" OR DE "EXERCISE adherence" OR DE "EXERCISE for children" OR DE "EXERCISE for girls" OR DE "EXERCISE for men" OR DE "EXERCISE for middle‐aged persons" OR DE "EXERCISE for older people" OR DE "EXERCISE for people with disabilities" OR DE "EXERCISE for women" OR DE "EXERCISE for youth" OR DE "EXERCISE therapy" OR DE "EXERCISE video games" OR DE "FACIAL exercises" OR DE "FALUN gong exercises" OR DE "FOOT exercises" OR DE "GYMNASTICS" OR DE "HAND exercises" OR DE "HATHA yoga" OR DE "HIP exercises" OR DE "ISOKINETIC exercise" OR DE "ISOLATION exercises" OR DE "ISOMETRIC exercise" OR DE "ISOTONIC exercise" OR DE "KNEE exercises" OR DE "LEG exercises" OR DE "LIANGONG" OR DE "METABOLIC equivalent" OR DE "MULAN quan" OR DE "MUSCLE strength" OR DE "PILATES method" OR DE "PLYOMETRICS" OR DE "QI gong" OR DE "REDUCING exercises" OR DE "RUNNING" OR DE "RUNNING ‐‐ Social aspects" OR DE "SCHOOLS ‐‐ Exercises & recreations" OR DE "SEXUAL exercises" OR DE "SHOULDER exercises" OR DE "STRENGTH training" OR DE "STRESS management exercises" OR DE "STRETCHING exercises" OR DE "TAI chi" OR DE "TREADMILL exercise" OR DE "WHEELCHAIR workouts" OR DE "YOGA" 136,880 S19 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 9,462 S18 TI ( ari or urti or lrti ) OR AB ( ari or urti or lrti ) 246 S17 TI ( bronchit* or pneumon* or bronchopneumon* or pleuropneumon* ) OR AB ( bronchit* or pneumon* or bronchopneumon* or pleuropneumon* ) 685 S16 TI ( sinusit* or rhinosinusit* or nasosinusit* ) OR AB ( sinusit* or rhinosinusit* or nasosinusit* ) 129 S15 TI cough* OR AB cough* 839 S14 TI ( nasopharyngit* or rhinopharyngit* ) OR AB ( nasopharyngit* or rhinopharyngit* ) 10 S13 TI ( throat* N3 (infect* or inflam*) ) OR AB ( throat* N3 (infect* or inflam*) ) 18 S12 TI ( pharyngit* or laryngit* or tonsillit* or sore throat* ) OR AB ( pharyngit* or laryngit* or tonsillit* or sore thoat* ) 48 S11 TI ( influenza* or flu or ili ) OR AB ( influenza* or flu or ili )1,179 S10 TI ( (acute or viral or virus* or bacter*) N2 rhinit* ) OR AB ( (acute or viral or virus* or bacter*) N2 rhinit* ) 3 S9 TI ( common cold* or colds or coryza ) OR AB ( common cold* or colds or coryza ) 5,846 S8 TI ( respiratory N2 (infect* or illness or symptom* or acute or virus*) ) OR AB ( respiratory N2 (infect* or illness or symptom* or acute or virus*) ) 936 S7 DE "BRONCHITIS" 83 S6 DE "COUGH" 148 S5 DE "PNEUMONIA" 176 S4 DE "SINUSITIS" 114 S3 DE "INFLUENZA" 378 S2 DE "COLD (Disease)" 419 S1 DE "RESPIRATORY infections" 457
Appendix 6. PEDro
exercise AND respiratory infection
Appendix 7. OTseeker
exercise AND respiratory infection
Data and analyses
Comparison 1. Exercise versus no exercise for acute respiratory infections (total).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of ARI episodes per person/year | 4 | 514 | Rate Ratio (Random, 95% CI) | 1.00 [0.77, 1.30] |
| 2 Proportion of participants who experienced at least 1 ARI over study period | 5 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.72, 1.08] |
| 3 Severity of ARI symptoms | 2 | 373 | Mean Difference (IV, Random, 95% CI) | ‐103.57 [‐198.28, ‐8.87] |
| 4 Number of symptom days during follow‐up period (12 weeks) | 4 | 483 | Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐3.50, ‐0.98] |
| 5 Number of symptom days per episode | 6 | 557 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐2.33, 1.46] |
| 6 Laboratory parameters ‐ lymphocytes | 3 | 157 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.10, 0.31] |
| 7 Laboratory parameters ‐ IgA | 4 | 166 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.37, 0.52] |
| 8 Laboratory parameters ‐ neutrophils | 4 | 489 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.54, 0.06] |
| 9 Quality of life | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Physical health | 2 | 373 | Mean Difference (IV, Random, 95% CI) | 1.37 [‐2.31, 5.05] |
| 9.2 Mental health | 2 | 373 | Mean Difference (IV, Random, 95% CI) | 3.37 [‐0.59, 7.34] |
| 10 Cost to the patient (USD) | 2 | 373 | Mean Difference (IV, Random, 95% CI) | ‐53.46 [‐128.17, 21.24] |
| 11 Exercise‐related injuries | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12 Adherence to the group intervention | 11 | 828 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.02] |
| 13 Number of symptom days per episode ‐ menopausal women | 3 | 158 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.74, ‐0.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrett 2012.
| Methods | Study design: randomised controlled trial Setting: outpatient clinic adults from University of Wisconsin Department of Family Medicine Country: USA Study period: 8‐week training in mindfulness meditation, matched; 8‐week training in moderate‐intensity sustained exercise, or observational control. For the analysis we used the exercise group and the control group. Methods of analysis: "ARI illness episode, severity was assessed once daily using a 24‐item version of the Wisconsin Upper Respiratory, Symptom Survey (WURSS). The WURSS‐24 adds items assessing headache, body aches and fever to the well‐validated WURSS‐21. With each ARI illness episode, a nasal wash was collected within 3 days of symptom onset and analysed for interleukin‐8 (IL‐8), neutrophil count and viral nucleic acid. Elevated neutrophil count and IL‐8 levels are indicators of inflammation and correlate with symptom severity and viral shedding. Multiplex polymerase chain reaction (PCR) methods developed and validated at UW were used to identify respiratory viruses. Several validated self report questionnaires were used to explore potential explanatory pathways linking behavioural interventions to ARI outcomes SF‐12; PSS‐10; Positive and Negative Affect Schedule (PANAS); State Trait Anxiety Inventory (STAI); Life Orientation Test (LOT); Positive Relationships with Others (PR) scale. The Pittsburgh Sleep Quality Index (PSQI); The International Physical Activity Questionnaire (IPAQ); Mindful Attention Awareness Scale (MAAS). Health Care Utilization and Days of Work or School Missed" Statistical analysis: "the sample size of 150 was based on power estimates contrasting (1) meditation versus control and (2) exercise versus control. To control for multiple testing, P ≤ 0.025 cutoff for null hypothesis rejection was chosen. 1‐sided testing was justified by previously published research, all in the direction of positive results" "Unadjusted between‐group contrasts were calculated using 1‐sided t tests for continuous variables and proportional difference testing for binomials. Most participants did not experience ARI illness, therefore zero inflated regression models were used to control for potential confounders. These models take into account both logistic (incidence) and linear (days of illness or global severity) data. Covariates used in these models were age, sex, education, smoking status, body mass index, baseline physical and mental health (SF‐12) and cohort. Global severity was skewed, therefore Box‐Cox transformation was used for this outcome in these models. To explore potential causal pathways, we assessed the relationship of secondary outcomes measured just after interventions to the main outcomes" |
|
| Participants | Recruitment means: community‐targeted recruitment methods included advertising in local media Target participants: adults aged 50 years or over N randomised: 154 adults N completed: 149 adults completed: 47 (exercise), 51 (meditation), 51 (control) Gender M = 27: exercise 8, meditation 9, control 10 F = 122: exercise 39, meditation 42, control 41 Age: exercise (59.0 ± 6.6 years), meditation (60.0 ± 6.5 years), control (58.8 ± 6.8 years) Baseline details: age, gender, smoking, race, BMI, education, income and every questionnaire applied |
|
| Interventions | Description of intervention: weekly group sessions were divided into didactic instruction (cognitive, logistic, and behavioural) and practice (moderately intensive exercise using stationary bicycles, treadmills, and other equipment). For most participants, home exercise consisted of brisk walking or jogging. Mindfulness meditation: the standardised 8‐week MBSR course includes weekly 2½‐hour group sessions and 45 minutes of daily at‐home practice Exercise: (8 weeks), contact time (weekly 2½‐hour group sessions), home practice (45 minutes per day), and location Delivered by: exercise was applied by 3 qualified exercise instructors in clinical exercise physiology. Meditation was applied by instructors with advanced degrees, and all were trained in Massachusetts by the Kabat‐Zinn group. Intervention period: 8 weeks Follow‐up period: 9 months Co‐interventions: didactic instruction (cognitive, logistic, and behavioural) |
|
| Outcomes |
Data collected (in 2 cohorts) over 7 or 9 months, but reported at 3 months. |
|
| Notes | Study funding: "this study was supported by a grant from the National Institutes of Health (NIH), National Center for Complementary and Alternative Medicine (1R01AT004313); and by a grant UL1RR025011 from the Clinical and Translational Science Award (CTSA) Program of the National Center for Research Resources, National Institutes of Health. Aleksandra Zgierska was supported by grant K23 AA017508 from National Institute on Alcohol Abuse and Alcoholism at NIH" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "software... was used to generate 165 unique identification numbers in balanced blocks of 3." |
| Allocation concealment (selection bias) | Unclear risk | "Codes were concealed in consecutively numbered sealed envelopes, which were opened after consent to indicate allocation." No mention of envelopes being opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants could not be blinded due to the characteristics of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome measurement is by participant self‐report, therefore cannot be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For main outcomes data were missing for only 2 people. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and the prespecified outcomes were published. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Barrett 2018.
| Methods | Study design: randomised controlled trial Location, number of centres: adults from Wisconsin Country: USA Study period: 8‐week training in mindfulness meditation, matched; 8‐week training in moderate‐intensity sustained exercise, or observational control. For the analysis we used the exercise group and the control group. Methods of analysis: "The primary outcome was global severity of ARI illness, defined as area‐under‐the‐time severity‐curve. Daily self‐reports on the Wisconsin Upper Respiratory Symptom Survey (WURSS‐24) assessed symptom severity and quality‐of‐life impact, ARI‐related absenteeism and health care utilization, virus identification, and inflammatory biomarker levels during ARI illness. Secondary outcomes also included several psychosocial domains assessed by validated self‐report instruments at baseline, then 3 or 4 times after intervention. These assessed: general mental and physical health (SF‐12), perceived stress (PSS‐10), sleep quality (PSQI), self‐efficacy (MSES,ESES), mindful awareness (MAAS), positive and negative emotion (PANAS), perceived social support (SPS), and the sense of feeling loved (www.fammed.wisc.edu/feeling‐loved; validation paper under review). Five important personality traits (BFI), the social network (SNI), and co‐morbidities (Seattle Index) were also assessed, but were not expected to be influenced by interventions. The Global Physical Activity Questionnaire (GPAQ) was used to assess self‐reported physical activity in all 3 groups. Blood and nasal wash samples were collected at baseline, 1 and 4 months after the 8‐week interventions, and approximately 24±72 hours into each ARI episode. Biomarkers included: interleukin‐6 (IL‐6), interleukin‐8 (IL‐8), high sensitivity C‐reactive protein (CRP), procalcitonin (PCT), and interferon‐gamma‐induced protein 10 (IP‐10)" Statistical analysis: "The primary efficacy analysis was done using the same zero‐inflated multivariate regression model employed in the first MEPARI trial, which incorporates a logistic sub‐model for people who do not experience ARI illness (zeroes), and a linear sub‐model accounting for variability in the continuous outcome measures (global severity, duration‐of‐illness). Prespecified covariates were: age, gender, body mass index, smoking status, highest level of education achieved, comorbidity, neuroticism, conscientiousness, general physical health, and general mental health. Primary comparisons are between: 1) EX and control, and 2) MBSR and control, with the level of statistical testing set a priori at 0.025 one‐sided for null hypothesis rejection. Secondary analyses are considered statistically significant at alpha <0.01 for evidence‐of‐effect, and at <0.05 for hypothesis‐generation or cautious support of effect based on two‐sided tests. The target sample size of N = 396 for this phase two trial was informed by data from the first MEPARI trial, using alpha = 0.025 and beta = 0.80, and one‐sided testing, for ARI illness primary outcomes" |
|
| Participants | Recruitment means: community advertising techniques, screened by telephone interview, and then met twice in person for baseline assessment, written informed consent, and enrolment Target participants: adults aged 30 to 69 years N randomised: 413 adults N completed: 390 adults completed: 146 (exercise), 111 (meditation), 133 (control) Gender M = 100: exercise 30, meditation 33, control 37 F = 313: exercise 107, meditation 105, control 101 Age: exercise (49.1 ± 11.4), meditation (49.2 ± 11.2), control (50.7 ± 12.1) Baseline details: age, gender, smoking, race, BMI, education, income and every questionnaire applied |
|
| Interventions | Setting of intervention: interventions were conducted at UW Research Park, a multipurpose outpatient clinic with exercise facilities and space suitable for meditation training. Description of intervention:
Training classes in MBSR or EX were matched in terms of location, class time (2.5 hours per week), homework. The exercise intervention lasted for 20 to 45 minutes a day, with a group of 14 to 16 people. The frequency of the exercise intervention was for at least 5 of the 9 training opportunities. Delivered by: exercise classes were taught by experienced exercise instructors. In addition to the 8 weekly classes, a 5‐hour weekend retreat was held for both EX and MBSR participants. Intervention period: 8 weeks Follow‐up period: screening occurred in the summer, with enrolment and randomisation in August, followed by MBSR or EX training in September and October. Participants were followed through May of the following year using computerised weekly self‐report, periodic in‐person visits, and close surveillance during ARI illness. Weekly self‐reports included daily minutes of MBSR or EX practice. |
|
| Outcomes | 1. ARI illness burden: severity and duration (number of days), cold severity, symptoms, based on each ARI illness episode, global severity score (area under the curve (AUC)) for all ARI illness days; symptom severity and impact on function and quality of life. 2. Laboratory‐assessed immune parameters included hsCRP neutrophils, IL‐6 (serum and nasal), IL‐8 and IP‐10 3. WURSS‐24, general mental and physical health (SF‐12), perceived stress (PSS‐10), sleep quality (PSQI), self‐efficacy (MSES, ESES), mindful awareness (MAAS), positive and negative emotion (PANAS), perceived social support (SPS), and the sense of feeling loved (www.fammed.wisc.edu/feeling‐loved). 5 important personality traits (BFI), the social network (SNI), and comorbidities (Seattle Index). Global Physical Activity Questionnaire (GPAQ) data collected (in 4 annual cohorts) at baseline, 1 and 4 months after the 8‐week interventions. |
|
| Notes | Study funding: "National Center for Complementary and Integrative Health (NCCIH) at the U.S. National Institutes of Health (R01AT006970). During the trial and writing of this paper Bruce Barrett was supported by a mid‐career research and mentoring grant from NCCIH (K24AT006543); Supriya Hayer received support from a research training grant from NCCIH (T32AT006956) directed by Dr. Barrett. MEPARI‐2 received some support from a Clinical and Translational Science Award (CTSA) through the National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427, which also provided research career development support to Elisa Torres (KL2TR000428)" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization codes generated by an independent statistician using variable block sizes" |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could not be blinded due to the characteristics of exercise and meditation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "investigators remained masked to group assignment until after the last participant exited" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was low loss to follow‐up, and intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All the outcomes (primary and secondary) described in the protocol were reported. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Chubak 2006.
| Methods | Study design: randomised controlled trial Location, number of centres: Fred Hutchinson Cancer Research Center and the University of Washington Country: USA Study period: 12 months Methods of analysis: "at baseline and 3, 6, 9 and 12 months, participants completed self administered questionnaires, modified from established, validated instruments on the number of episodes of allergies, upper respiratory tract infections (colds and flu) and other infections over the past 3 months" Statistical analysis: "Poisson regression allowed for use of data from all available time points without eliminating individuals with some missing data. We assumed an unstructured working correlation matrix, computed robust standard errors and performed an intention‐to‐treat analysis, with P < 0.05 being considered statistically significant. Results were identical or stronger when restricted to women who had assessments at all 4 time points. We also evaluated whether the exercise effect differed by age (< 60 versus ≥ 60 years) or regular multivitamin use, assessed by abstraction of vitamin bottles brought into the clinic at baseline (see Shade et al for details). All analyses were performed using SAS 8.0 (SAS Institute, Cary, NC) and Stata 8 (StataCorp, College Station, Tex) statistical software. All P values are 2‐sided" |
|
| Participants | Recruitment means: mass mailings and media placements Target participants: postmenopausal women, overweight/obese, non‐smoking, sedentary N randomised: 115 adult postmenopausal women N completed: 115 postmenopausal women: 53 (exercise) and 62 (control) Gender F = 115: exercise 53, control 62 Age: exercise 60.5 (7), control 60.9 (6.8) Inclusion criteria: "post‐menopausal; age, 50 to 75 years; in good health; non‐smoking; sedentary (< 60 minutes/week of moderate and vigorous‐intensity recreational activity and maximal oxygen consumption < 25.0 mL/kg per minute during a VO₂ test); not taking hormone replacement therapy in the past 6 months; alcohol consumption of fewer than 2 drinks per day; body mass index (BMI) between 25 and 40 or BMI 24.0 to 24.9 if body fat > 33%; no history of invasive cancer, diabetes, cardiovascular disease, asthma; no current serious allergies; no regular (≥ 2 times/week) use of aspirin or other nonsteroidal anti‐inflammatory medications; not using corticosteroids or other medications known to affect immune function. Women were ineligible if they were volunteering for the study to lose weight, had a history of surgery for weight loss, or were currently attempting, or planning to attempt, weight loss by taking diet pills or entering a structured weight loss programme. Participants had been weight stable for at least 3 months" Baseline details: demographic information, medical history, health habits, reproductive history, physical activity, diet, and anthropometric variables |
|
| Interventions | Setting of intervention: Fred Hutchinson Cancer Research Center and the University of Washington Description of intervention: "the exercise intervention consisted of at least 45 minutes of moderate‐intensity exercise 5 days/week for 12 months. During months 1 through 3, participants were required to attend 3 sessions per week at 1 of the study facilities and to exercise 2 days/week at home. For months 4 through 12, participants were required to attend at least 1 session per week at the facility and to exercise the remaining days on their own for a total of 5 days/week (participants were allowed to exercise additional days at the facility if they chose). The training programme began with a target of 40% of maximal heart rate for 16 minutes per session and gradually increased to 60% to 75% of maximal heart rate for 45 minutes per session by week 8, at which point it was maintained for the duration of the study. Participants wore heart rate monitors (Polar Electro Inc, Woodbury, NY) during their exercise sessions. Facility sessions consisted of treadmill walking and stationary bicycling. Strength training, consisting of 2 sets of 10 repetitions of leg extension, leg curls, leg press, chest press and seated dumbbell row, was recommended but not required to decrease risk of injury and maintain joint stability. A variety of home exercises were suggested and encouraged, including walking, aerobics and bicycling. Participants were encouraged to wear their heart rate monitors when exercising at home. Women randomly assigned to the control group attended weekly 45‐minute stretching sessions for 1 year and were asked not to change other exercise habits during the study. Exercise and control participants were asked to maintain their usual diet" Delivered by: not stated Follow‐up period: 12 months Co‐interventions: none |
|
| Outcomes | Multivitamin, number of colds before baseline, number of URTIs, allergy episodes, influenza immunisation Prespecified: 3 months before baseline Follow‐up period: 12 months |
|
| Notes | Study funding: "National Cancer Institute (NCI) (R01 CA 69334). Ms. Chubak was supported by grant T32 CA09168 from the NCI. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NCI or National Institutes of Health. Dr. Wener was supported in part by the University of Washington Clinical Nutrition Research Grant (DK35816)" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were enrolled in a randomised trial...". Insufficient information provided to assess this domain. |
| Allocation concealment (selection bias) | Unclear risk | No information regarding allocation concealment was provided in the text. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants could not be blinded due to the characteristics of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes are by participant self‐report. Information was obtained by telephone for outcomes ARI episodes and URI. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors reported missing data on colds or other URTI episodes at 6 and 9 months (result: < 10%). |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, although all outcomes relevant to this review were reported. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Ciloğlu 2005.
| Methods | Study design: parallel‐group randomised controlled trial Location, number of centres: Genlab Medical Diagnostics and Research Laboratory Country: Turkey Study period: September to November Methods of analysis: "the participants had anthropometrical measurements in the fasting state. Body weight and height were measured on standard scale with an attached ruler wearing light clothes and no shoes. Body mass index (BMI) was calculated as weight in kilograms (kg) divided by the square of the height in metres (m). Waist circumference was measured using a flexible measuring tape at the umbilicus level with the participants standing straight, arms at their sides and feet together. Body fat mass and fat free mass were determined by bioelectric impedance. During the supervised sessions, participants were noted for and asked about upper respiratory infection symptoms of runny stuffy nose, sore throat, coughing, sneezing coloured discharge and fever. Those who were in the non‐exercise group were phoned weekly for the same data collection. Number of URTI episodes and the number of URTI days per episode were recorded for each participant. An episode of URTI was defined as having the symptoms for more than 2 days and separated by at least 5 days from the previous episode. The saliva samples were collected prior to starting the study and at the end of the 12 weeks each time after the mouth had been rinsed thoroughly with distilled water. The saliva samples were frozen at ‐20°C and stored until the end of the study period. Salivary IgA concentrations were measured by enzyme linked immunosorbent assay (ELISA) method (Immulon II; Dynex Technologies, Chantilly, Virginia, USA)" Statistical analysis: not stated |
|
| Participants | Recruitment means: volunteers from the routine check‐up from the laboratory Target participants: postmenopausal women N screened: 90 postmenopausal women N completed: 90 postmenopausal women Gender F = 90: indoor exercise 30, outdoor exercise 30, control 30 Age: indoor exercise 55.0 ± 3.5, outdoor exercise 54.6 ± 2.1, control 54.9 ± 3.8 Exclusion criteria: "excluded for chronic disease, any medications including vitamins, having received the flu shot and having smoked cigarettes within the last 2 years" Baseline details: age, weight, BMI, waist circumference, fat mass, fat‐free mass, number of URTI episodes, number of URTI days per episode |
|
| Interventions | Setting of intervention: Genlab Medical Diagnostics and Research Laboratory Description of intervention: "both the indoor and outdoor exercise groups underwent supervised exercise sessions 5 days a week for 30 minutes each time walking on a treadmill or an outdoor tract respectively at 60% of their maximal heart rate as determined by the simple formula of Maximal Heart Rate = 220 ‐ age. Heart rate measurements were done with a Polar Heart Rate Monitor" Delivered by: supervised sessions Follow‐up period: 12 weeks Co‐interventions: none |
|
| Outcomes | Salivary IgA levels and the incidence of URTIs Follow‐up period: 12 weeks |
|
| Notes | Study funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not explain how the random sequence was generated. "They were divided into three groups ... with each group having similar characteristics and randomly assigned." |
| Allocation concealment (selection bias) | Unclear risk | No information regarding allocation concealment was provided in the text. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants could not be blinded due to the characteristics of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes measured by participant self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss of data in the outcomes analysed. |
| Selective reporting (reporting bias) | Low risk | The outcomes prespecified in the methods were reported in the results. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Dias 2014.
| Methods | Study design: randomised controlled trial Location, number of centres: gymnasium Country: Brazil Study period: 9 weeks Methods of analysis: each week the number of symptoms of acute respiratory infection was reported Statistical analysis: for frequency and intensity of symptoms of ARI, ANOVA of Friedman was applied for the intra‐group analysis and, when a difference was indicated, the post hoc model of Wilcoxon was employed with Bonferroni adjustments. In the verification of inter‐group differences at moments S1, S2, S3, S4, S5, S6, S7, and S8, the Mann‐Whitney test was adopted using P ≤ 0.05, significant. In addition, Spearman correlation was used to associate ARI symptoms to exercise. |
|
| Participants | Recruitment means: not reported Target participants: postmenopausal and sedentary women N randomised: 16 adult postmenopausal women N completed: 16 postmenopausal women: 8 (exercise) and 8 (control) Gender F = 16: exercise 8, control 8 Age: exercise 58.7 ± 6.1, control 60.9 ± 6.8 Inclusion criteria:
The exclusion criteria of the study were: patients with symptoms of IVAS classified as extreme (muscular and joint pain, pain in the back of the eyes and the nape of the neck, swelling or pain in the throat lymph nodes or fever) previously or at any point of the intervention. Baseline details: age, BMI, waist circumference, waist/hip ratio |
|
| Interventions | Setting of intervention: not reported Description of intervention: strength training alternating all body parts in all sessions. 3 sessions per week (Monday, Wednesday, Friday). The resistance exercise was conducted using free weights, and no load volume test was performed. Delivered by: researcher Follow‐up period: 9 weeks Co‐interventions: none |
|
| Outcomes | Number of symptoms of ARI collected every week using a subjective scale from 1 to 4. Follow‐up period: 9 weeks |
|
| Notes | Study funding: not reported No data could be extracted because the study authors reported only mean values and P values in the results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Just "random" was reported. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment mentioned or described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants could not be blinded due to the characteristics of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes measured by participant self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss of data in the outcomes analysed. |
| Selective reporting (reporting bias) | High risk | The study protocol is not available, the results are poorly described, and not all outcomes proposed were published in the results. |
| Other bias | High risk | The study is underpowered and is lacking much important information regarding recruitment process and where the study took place, as well as participant sociodemographic data. |
Klentrou 2002.
| Methods | Study design: parallel‐group randomised controlled trial Location, number of centres: Brock University Country: Canada Study period: February to May 2000 Methods of analysis: "to monitor the effectiveness of the exercise programme, both groups were administered a continuous incremental exercise test on an electrically braked cycle ergometer (Ergociser EC‐1600, Cateyer Co. Ltd., Japan) for the determination of maximal aerobic power (VO₂ max) at 3 separate times during the course of the study: T1 – at the onset of the study (before any activity by the exercise group began), T2 – at the mid‐point of training (6 weeks) and T3 – at the conclusion of training (12 weeks). A volume of 1 ml of unstimulated whole mixed saliva was collected from each participant at T1 and T3 using cylinder‐shaped swabs placed in the mouth for 1 minute. Each participant was provided with a Health and Sickness Logbook to record symptoms, as exhibited, daily" "Participants were asked not to take any over‐the‐counter or prescription medication that might mask their symptoms since the daily sickness log was based on symptoms that the participant experienced or felt" Statistical analysis: "all statistical analyses were performed using SPSS (SPSS Inc., Chicago Ill.). Comparison of inter‐group differences was done using an ANOVA. Changes in maximal [IgAs], [Albs] and [IgAs]:[Albs] with training were analysed using a repeated measurements ANOVA. The accepted level of significance was set at P < 0.05. The experimental power was more than 99%. Pearson correlation analysis was used to examine the strength of the relationship which existed between URTI and the salivary variables" |
|
| Participants | Recruitment means: not stated Target participants: healthy men and women N screened: 20 healthy men and women N completed: 19 completed: 9 (exercise) and 10 (control) Gender: not stated Age: 25 to 50 years Inclusion criteria: "adult men or women (aged 25 to 50 years) having a sedentary lifestyle, non‐smokers, free of asthma, no recent influenza immunisation, free from URTI at entry to the study and the women not being pregnant or planning on becoming so. Furthermore, the majority of participants were only indirectly exposed to young children and they all resided in the same area" Baseline details: age, VO₂ max, IgA |
|
| Interventions | Setting of intervention: Exercise Assessment and Research Centre Description of intervention: "the exercise programme consisted of 3 exercise sessions a week. Each exercise session was 45 minutes long. During the exercise period, the participants performed a 30‐minute aerobic protocol at 75% of heart rate reserve using stationary bicycles, treadmills, stair climbers or combined/cross‐training using more than 1 device. At the end of the aerobic activities, participants spent an additional 15 minutes doing stretching exercises involving the lower body, trunk and arms. More specifically, each participant performed approximately 10 muscle stretches including: quadriceps, calves, gluteal, lower back, triceps, biceps, shoulder, trapezius and pectoralis. During the aerobic protocol, each participant's heart rate was recorded at 3 different points: prior to starting exercise, mid‐point of exercise and completion of exercise (before cool‐down) using a Polar heart rate monitor. All exercise sessions were conducted in a group format in the Exercise Assessment and Research Center to ensure that participants exercised at the prescribed duration and intensity" Delivered by: not stated Follow‐up period: 12 weeks Co‐interventions: none |
|
| Outcomes | Influenza symptoms, cold symptoms, total sickness days, VO₂ max, symptom record, IgA concentrations, salivary albumin concentration (Albs) and minimum concentration ratio ([IgAs]:[Albs]) Follow‐up period: 12 weeks |
|
| Notes | Study funding: Faculty of Health Sciences, Brock University | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors do not explain how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | "After signing the informed consent, participants were randomly assigned to either the control group or the exercise group." Insufficient information provided to permit an assessment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants could not be blinded due to the characteristics of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes measured by participant self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant of 9 in the exercise group was not included, with no reasons given. |
| Selective reporting (reporting bias) | Low risk | The outcomes prespecified in the methods were reported in the results. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Manzaneque 2004.
| Methods | Study design: parallel‐group randomised controlled trial Location, number of centres: psychology students at the University of Malaga Country: Spain Study period: 1‐month training period, Monday to Friday for 30 minutes Methods of analysis: "the day before the study commenced, blood samples were taken from all participants, in both the control and experimental group, at 9:30 in the morning and again 1 month later, at the end of the study, when qigong training was concluded for the experimental participants. The immunological parameters investigated included the number of leukocytes (total leukocytes, monocytes, neutrophils, eosinophils, basophils, lymphocytes, T lymphocytes and T helper lymphocytes), the percentages of leukocytes (monocytes, neutrophils, eosinophils, basophils, lymphocytes and T helper lymphocytes), as well as the concentrations of immunoglobulins (IgA, IgG and IgM) and complement (C3 and C4). Total blood count ‐ Pentra 120 ABX analyser. Serum immunoglobulins and complement Immage, Immunochemistry System (Beckman Coulter)" Statistical analysis: "a between‐group analysis of covariance (ANCOVA) was performed on several dependent variables: the numbers of total leukocytes, monocytes, neutrophils, eosinophils, basophils, lymphocytes, T lymphocytes and T helper lymphocytes; the percentages of lymphocytes, T helper lymphocytes, monocytes, neutrophils, eosinophils and basophils; as well as the concentrations of IgG, IgA, IgM and the complements C3 and C4. The qigong training was considered as an independent variable with 2 levels (absence or control group and presence or experimental group) and the respective pretest scores of each dependent variable as covariates. Thus, the differences between groups were estimated with the differences in pretest scores removed. P < 0.05 was considered to be significant, while P < 0.1 was considered a trend towards significance. Lymphocytes subsets: FACScan (Becton Dickinson)" |
|
| Participants | Recruitment means: psychology student volunteers Target participants: adults N screened: 29 adults N randomised: 29 adults randomised N completed: 26 adults completed: 13 (Qigong) and 13 (control) Gender: M = 12; F = 14 Age: 18 to 21 years old Baseline details: age, gender |
|
| Interventions | Setting of intervention: a room adjoining the laboratory where the practice sessions were conducted Description of intervention: "the form of qigong taught is known as the 'eight pieces of brocade' (Ba Duan Jin in Chinese pinyin transliteration). It is a simple qigong method that contains 8 distinct movements and integrates them with breathing and a relaxed state of the mind. The whole physical sequence contains 8 discrete movements each, making a total of 64 physical movements to complete the entire set. Throughout the practice, natural, relaxed and rhythmic breathing is required. This method of qigong reportedly dates back hundreds of years and a number of physical and psychological benefits ts has traditionally been attributed to it. More recently, 2 reports published in important international journals focused on this qigong style and its health‐promoting features. 30 minutes per session, 5 days per week for the month‐long intervention with instructor. Encouraged to do extra on weekends. No data reported on this extra practice" Delivered by: qualified Qigong instructor of this discipline Intervention period: 1 month Follow‐up period: 1 month Co‐interventions: medication was kept constant during the study period |
|
| Outcomes | Leucocytes (× 103 cells/μL and %); monocytes (× 103 cells/μL and %); neutrophils (× 103 cells/μL and %); eosinophils (× 103 cells/μL and %); basophils (× 103 cells/μL and %); lymphocytes (× 103 cells/μL and %); T lymphocytes (cells/μL and %); T helper lymphocytes (cells/μL and %); IgA (mg/dL); IgG (mg/dL); IgM (mg/dL); C3 (mg/dL); C4 (mg/dL) Follow‐up period: blood sample at 9:30 a.m. the day before and again 1 month later, at the end of the study |
|
| Notes | Study funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors do not explain how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | "16 subjects were randomly allocated to the experimental group and 13 to the control group, balancing the number of males and females in each case". Insufficient information provided to permit an assessment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants could not be blinded due to the characteristics of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes measured by participant self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "One experimental subject (male) decided to abandon the experiment without reasons given within the first few days of onset and a further two (one male and one female) were excluded from the sample for non‐attendance at the qigong sessions on more than two occasions" |
| Selective reporting (reporting bias) | Low risk | The outcomes prespecified in the methods were reported in the results. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Nieman 1990.
| Methods | Study design: parallel‐group randomised controlled trial Location, number of centres: Loma Linda University Country: USA Study period: last weekend of January to mid‐May 1989 Methods of analysis: "all participants reported to the Loma Linda University Human performance Lab for testing at 0700 hours following 12 hours of fasting. After resting for at least 5 minutes, blood samples were collected. Participants returned throughout the day for assessment of the following: height and weight, body composition (hydrostatic weighing and 7‐site skinfold tests), resting 12‐lead EKG and 12‐lead EKG graded exercise testing with metabolic measurements. If a participant exhibited overt symptoms of URI, the appointment was rescheduled. Maximal graded exercise testing was conducted using the Bruce treadmill protocol on the Quinton 44000 stress test system and Q55 treadmill (Quinton Instrument Co., Seattle, WA). Metabolic measurements were taken with the Sensor Medics MMC Horizon System 4400 metabolic cart (Sensor Medics, Anaheim, CA). Log books for daily recording of health problems and exercise patterns were given to each subject at baseline. Heparinised whole blood was used for NK cell number and activity assays and EDTA whole blood for complete blood counts (CBC). CBC were performed on Coulter S‐Plus IV instrumentation with visual cell differentials in our clinical hematology laboratory" Statistical analysis: "results are expressed as mean ± SE. A 2 x 3 repeated measures ANOVA with 1 between‐participants factor (EX versus NEX) and 1 within‐participant factor (time of testing) was used to analyse the data. When Box's M suggested that the assumptions necessary for the univariate approach were not tenable, the multivariate approach to repeated measures ANOVA was used. In the latter case, Pillais trace statistic was used as the test statistic. With regard to comparison among specific means, only 7 comparisons were of interest to us. These were the contrast of the baseline measures with the 6th and 15th week measurements within the EX and NEX groups and the contrast between the EX and NEX groups at each of the 3 measurement points. The Dunn‐Sidak procedure was used to test these comparisons. Pearson correlations were used to determine the association between change in cardiorespiratory fitness, NK cell activity and URI symptomatology. Comparison between groups for age, BMI and URI were evaluated by simple univariate t‐tests" |
|
| Participants | Recruitment means: not stated Target participants: premenopausal woman N screened: 50 mildly obese premenopausal woman N completed: 36 completed: 18 (exercise) and 18 (control) Gender F = 36: placebo 18, exercise 18 Age: exercise 36.0 (1.6); control 32.8 (1.4) Inclusion criteria: "25 to 45 years of age, mildly obese (10% to 40% overweight), premenopausal, 155 cm to 170 cm in height, not presently on an exercise programme or a reducing diet, a non‐smoker without a history of alcohol or drug abuse, no current use of medications (except oral contraceptives), absence of hypertension and diabetes and no family history of heart disease" Baseline details: age, BMI, weight, compliance, NK cell response, metabolic parameters including HR, VE, and VO₂ |
|
| Interventions | Setting of intervention: Loma Linda University Human Performance Lab Description of intervention: the EX group followed a closely supervised walking programme on a measured course consisting of 5, 45‐minute sessions each week for 15 weeks at an intensity of 60% of heart rate reserve. To ensure that participants exercised at a proper intensity, heart rates were monitored by checking pulse rates every 0.8 km. At the completion of 45 minutes the supervisor recorded participant's walking distance to the nearest 0.16 km. During the 15‐week study, the NEX group was instructed not to participate in any exercise outside of normal daily activity. Delivered by: supervised exercises Follow‐up period: 15 weeks Co‐interventions: the medication was kept constant during the study period |
|
| Outcomes | Number of days with ARI; symptoms days per URI; NK cell response, metabolic parameters Follow‐up period: 15 weeks |
|
| Notes | Study funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors do not explain how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | There is no description of how allocation was concealed. "Those who qualified for the study were instructed that they would be randomly assigned to an exercise (EX) or non‐exercise (NEX) group" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blinded to the study objectives, but lacked explanation on how they did it. Personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes measured by participant self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial loss to follow‐up: 28% dropouts, 8 at the beginning of the study and 6 during the study |
| Selective reporting (reporting bias) | Low risk | The outcomes prespecified in the methods were reported in the results. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Nieman 1993.
| Methods | Study design: parallel‐group randomised controlled trial Location, number of centres: Appalachian State University Country: USA Study period: 12 weeks Methods of analysis: "measurement of immune system variables and cardiorespiratory fitness was conducted at baseline in both sedentary and highly conditioned elderly women. Following baseline testing, the 32 sedentary participants were randomly assigned to either the experimental or control group and retested after both 5 and 12 weeks of exercise training. The 5‐week testing was conducted to help determine if cardiorespiratory and immune system changes occur early in response to a moderate exercise programme. Maximal oxygen uptake (VO₂ max), weight and skin folds at the biceps, triceps, subscapular and supra iliac sites were measured at baseline in highly conditioned and sedentary participants and after 5 and 12 weeks of exercise training. Maximal graded treadmill testing using automated cardiorespiratory monitoring techniques (MMC Horizon System Exercise Evaluation Cart, Sensormedics, Yorba Linda, CA) was performed on all participants using a protocol developed in previous research with elderly participants. Blood specimens were collected from all participants in the seated position at 0700 hours after resting for a minimum of 10 minutes and abstaining from all food, beverages (except water) and vigorous physical activity for at least 12 hours. Samples were taken from the 32 sedentary participants at baseline and then again after 5 and 12 weeks of training. Samples from the 12 highly conditioned elderly participants were taken only at baseline and from the 13 young women only at the 12‐week testing. Routine complete blood counts (CBC) were performed using a Coulter STKS instrument (Coulter Electronics, Inc., Hialeah, FL). Heparinised whole blood was used for immune cell phenotyping for analysis of lymphocyte subset profiles" Statistical analysis: "results are expressed as means ± SE. Baseline comparisons between the elderly and young females were made using simple univariate t‐tests. A 2 x 3 repeated measures ANOVA with 1 between‐participants factor (walking versus calisthenic groups) and 1 within‐subject factor (baseline, 5 and 12 week times for testing) was used to analyse the training data. When Box's M suggested that the assumptions necessary for the univariate approach to repeated measures ANOVA was used. In the latter case Pillais trace statistic was used as the test statistic. With regard to comparison among specific means, only 2 comparisons were of interest to us. These were the contrast of the change in baseline measures with the 5th and 12th week measurements between the walking and calisthenic groups. The Dunn‐Sidak procedure was used to test the comparisons. The chi‐square test of association was used to test the relationship between incidence of URTI and varying levels of cardiorespiratory exercise according to group status (highly conditioned, walking and calisthenic groups) during the 12‐week study. The Pearson correlation coefficient was used to measure the linear correlationship between immune function and physical fitness (aerobic power and sum of 4 skin‐folds) in all elderly women at baseline" |
|
| Participants | Recruitment means: newspaper advertisements and direct mailings to local senior citizen groups Target participants: sedentary healthy elderly women N randomised: 32 women N completed: 30 completed: 14 (experimental) and 16 (control) Gender F = 30 Age: experimental 73.4 (1.1), control 73.5 (1.2) Inclusion criteria: "between the ages of 67 and 85; did not smoke or abuse alcohol; had not been on a reducing diet or exercise programme (≤ 3 moderate‐to‐vigorous aerobic sessions of > 20‐minute duration per week) for the previous 6 months; were non‐diseased (no current symptoms or signs suggestive of heart disease or cancer; did not use medications known to affect immune function)" Baseline details: age, sex, height, weight, BMI, sum of 4 skin‐folds, VO₂ max |
|
| Interventions | Setting of intervention: university activity centre Description of intervention: "participants in both groups met at the university activity centre and exercised 5 days/week, 30 to 40 minutes per session, under supervision. Participants in the experimental group engaged in 5 sessions of 30‐minute to 40‐minute brisk walking sessions per week at 60% of their heart rate reserve on either an outdoor or indoor (during bad weather) track. Participants warmed up for 5 minutes before each walking session with range‐of‐motion callisthenics. Total walking distances were recorded by the supervisor and heart rates monitored every 10 minutes through use of Polar pacer heart rate monitors (Polar USA, Inc.). Walking duration started at 30 minutes and was increased 2 minutes each week until participants were walking for 40 minutes by the mid‐point of the study. Training heart rates were recalculated after the 5‐week testing to adjust for improvement in cardio‐respiratory fitness and ensure that subjects maintained the 60% intensity level. To control for subject expectations and attention, the control group met in the same facility as the experimental group, and engaged in mild flexibility and musculoskeletal callisthenics under the direction of a second supervisor. Emphasis was placed on range‐of‐motion and stretching movements" Delivered by: supervised exercise Follow‐up period: 12 weeks Co‐interventions: none |
|
| Outcomes | Leukocyte and lymphocyte subsets; NK cell activity; VO₂ max; weight, sum of 4 skin‐folds; incidence of URTI Follow‐up period: 12 weeks |
|
| Notes | Study funding: this research was supported by a grant from the Cybex Corporation through the American College of Sports Medicine Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors do not explain how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No description of how allocation was concealed: "randomised to either the experimental or control groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants could not be blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes measured by participant self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were 2 participants with missing data. |
| Selective reporting (reporting bias) | Low risk | The outcomes prespecified in the methods were reported in the results. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Nieman 1997.
| Methods | Study design: factorial randomised controlled trial Location, number of centres: Loma Linda University Center Country: USA Study period: late January to April Methods of analysis: "log books for daily recording of health problems were given to each participant at baseline. Careful verbal and written instructions were given during a pre‐study orientation session. Participants recorded health problems each day of the 12‐week study using codes used in previous studies. The coded health problems included: 1. No health problems today; 2. Cold symptoms (runny, stuffy nose, sore throat, coughing, sneezing, coloured discharge; 3. Flu symptoms (fever, headache, general aches and pains, fatigue and weakness, chest discomfort, cough); 4. Nausea, vomiting and/or diarrhoea; 5. Muscle, joint or bone problems/injury; 6. Other health problems (describe). The severity of the symptoms was rated by participants as mild, moderate or severe. The number of days with URTI symptoms was calculated for each subject, with days counted only if 2 or more consecutive days of cold or flu symptoms were reported with a mild to severe rating. Body mass and composition were determined for all participants by means of underwater weighing. Residual volume was measured by the nitrogen washout procedure using the System 2100 Computerized Pulmonary Function Laboratory (Sensor Medics Corp, Yorba Linda, Calif). Maximal aerobic power (Vo₂ max) was determined using the Bruce graded maximal treadmill protocol (20). Oxygen uptake was measured using the MedGraphics CPX metabolic system (MedGraphics Corporation, St Paul, Minn). Immune assay measurement blood samples were drawn at 7 am from an antecubital vein with participants in the seated position (after 10 to 15 minutes of rest). Routine complete blood counts were performed by clinical haematology laboratory staff using a Coulter STKS instrument (Coulter Electronics, Hialeah, Fla)" Statistical analysis: "statistical significance was set at the P < 0.05 level and values are expressed as mean ± standard deviation. Data analysed using a 4 (control, exercise, diet, exercise+diet groups) x 2 (pre‐ and post‐study) repeated measures ANOVA. Duncan multiple comparison test. Pearson product‐moment correlations for changes in body mass, body mass index, body fat mass and VO₂ max" |
|
| Participants | Recruitment means: participants were recruited from the surrounding community through advertisements Target participants: obese females N screened: 102 obese females N completed: 91 completed: 22 (control); 21 (exercise); diet (26); exercise + diet (22) Gender F = 91 Age: 45.6 ± 1.1 Inclusion criteria: "between the ages of 25 and 75 years; in good health with no known diseases, including diabetes, cancer and heart disease; body mass index (BMI, calculated as kg/m²) between 25 and 65 for obese participants and fewer than 25 for non‐obese participants; not currently following a reducing diet or exercise programme not using medications known to affect immune function; not using supplements in excess of 100% of the Recommended Dietary Allowance on a regular basis; not experiencing chronic pain, marked sleep disturbance, serious allergies, salient emotional or mood problems; no recent history of systemic infection, bone fracture, or surgery; and not smoking cigarettes or abusing alcohol" Baseline details: compliance, body composition and immune function, blood cholesterol, triglycerides and glucose |
|
| Interventions | Setting of the intervention: indoor track Description of intervention: participants in 2 exercise groups (E and ED) were required to walk 5 times a week, 45 minutes per session, at 60% to 80% of maximum heart rate (MHR) for 12 weeks (60 total exercise sessions). Supervised sessions were held 4 days per week at an indoor track with duration, heart rate, and distance walked measured and recorded. Participants walked 1 session per week without supervision. Duration and intensity of exercise was gradually increased over a 3‐week period from 25 to 30 minutes/session at 60% to 65% MHR during the first week to 45 minutes at 70% to 80% MHR from weeks 4 to 12. Participants in the 2 non‐walking groups (C and D) reported to the exercise facility 4 days week for a 45‐minute session of stretching and mild range‐of‐motion calisthenic exercises. Delivered by: supervised sessions Follow‐up period: 12 weeks Co‐interventions: none |
|
| Outcomes | Body composition, aerobic power and immune function, blood cholesterol, triglycerides and glucose, days of URTI Follow‐up period: 12 weeks |
|
| Notes | Study funding: this work was funded by The Cybex Grant from the American College of Sports Medicine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors do not explain how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No description of how allocation was concealed. "Before being included in the study, participants had to agree to be randomised to any 1 of the 4 groups (control, exercise, diet, diet and exercise)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants could not be blinded due to the characteristics of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes measured by participant self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was loss of 10.8% (dropouts), and no intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | The outcomes prespecified in the methods were reported in the results. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Silva 2018.
| Methods | Study design: controlled clinical trial Location, number of centres: northern Portugal Country: Portugal Study period: November to July Methods of analysis: "For respiratory infection assessment, a symptom diary was assembled using previously developed questionnaires and indexes (Barrett et al., 2009; Jackson, Dowling, Spiesman, & Boand, 1958; Powell et al., 2008). The questionnaire was subdivided into five main symptom domains: general (shivering, muscle pain, tiredness, chest pain, dyspnoea), nasal (runny nose, obstructed nose, sneezing, throat (sore throat, itching throat, cough and hoarseness)) and ear (ear pain). Body temperature was registered if the absolute value was above 37.5°C. Allergic symptoms were also included (nose and eye itching). To assess the suitability of the questionnaire, a pilot application was administered to a population of older adults in an exercise programme. Symptoms were easily understood; however, the grading scale was simplified from a Likert scale of 0–7 to a scale grading the symptoms as mild, moderate or severe due to comprehensibility issues. All participants were given a symptom diary with a written and verbal individual explanation of how to document symptoms. Participants were contacted monthly to assure compliance and asked whether they had been sick and whether medical care had been administered. In the EG, 17 participants completed the exercise programme and RIs diary. Three individuals dropped out due to the inability to continue training, 1 at the beginning and 2 after 3 months. In the CG, 9 participants were included, 3 were not compliant with the respiratory symptoms diary and 6 were inappropriate in their answers and could not be used." Statistical analysis: "Analysis was conducted per‐protocol. The results are presented as the mean values [95% confidence interval (CI)], medians (interquartile range) or counts (n, %). The Mann–Whitney U‐test was used for comparison of continuous data and Fisher’s exact test for categorical data. Univariate and multiple logistic regression models were developed using independent variables as risk factors for having an RI; results are presented as odds ratio (OR) with 95% CI. Variables used in the model included gender, age, body mass index, history of respiratory tract disease, diabetes, smoking habits, influenza immunization and history of performing exercise training. The model was performed by data driven and adjusted by considering its goodness‐of‐fit (Hosmer–Lemeshow test) and predictive power (ROC curve analysis). Analyses were performed using SPSS 23.0 software (Chicago, IL)." |
|
| Participants | Recruitment means: public advertisement from senior universities and nursing homes in northern Portugal Target participants: physically independent, community‐dwelling individuals over 60 years of age N screened: 80 participants (38 included) N completed: 26 completed: 9 (control); 17 (exercise) Gender F = 19; M = 7 Age: 70 (65 to 79) IQR Inclusion criteria: individuals were included if they had a medical report describing that they were physically able for exercise and had not been involved in regular exercise training in the previous 6 months. Exclusion criteria: volunteers were excluded if they had: acute or terminal illnesses; severe or uncontrolled cardiovascular or respiratory disorders; neurological or musculoskeletal disorders that compromised their compliance; or had undergone treatments that could affect exercise training or increase susceptibility to RI. Baseline details: body composition, other diseases (hypertension, diabetes, etc.), practice of exercise, smoking. Comorbidities: many comorbidities were described here from respiratory disease to osteoarticular disease, with only osteoporosis significantly different between groups. |
|
| Interventions | Setting of intervention: not reported Description of intervention: the exercise programme was performed 3 times per week (non‐consecutive days) for 36 weeks in sports facility of University of Porto. Each session lasted 90 min (10 min of warm‐up, 30 min of aerobic training, and 45 min of resistance training, ending with a 5‐minute cool‐down). Aerobic training consisted of 30 min of walking at moderate intensity reaching 60% to 80% of the maximum heart rate. Heart rate monitors (Polar Team System, Finland) were used during the exercise sessions. Resistance training was performed on variable‐resistance machines (Nautilus Sports/Medical Industries, Independence, USA) aimed at 9 different muscular groups (leg press, chest press, leg extension, seated row, seated leg curl, abdominal flexion, low‐back extension, biceps curl, and triceps extension), and participants completed 2 sets of 10 to 12 repetitions at 60% to 80% of repetition maximum (1RM calculated based on Epley formula). Every month, the 1RM was measured to keep the training stimulus consistently at 80% of the 1RM. Training was standardised but adapted to each participant according to their limitations. Delivered by: physical education instructor specialised in training older adults Follow‐up period: 36 weeks Co‐interventions: none |
|
| Outcomes | Acute respiratory infection, duration and severity of ARI, serum metabolomic profiling Follow‐up period: 36 weeks |
|
| Notes | Study funding: this work was supported by the European Regional Development Fund (FEDER) through the Competitive Factors Thematic Operational Programme (COMPETE) and Foundation for Science and Technology (FCT), Portugal, under the projects PEst‐C/QUI/UI0062/2013 (Research Unit 62/94 QOPNA) and PTDC/QUI‐QUI/117803/2010 (Future asthma management helped by non‐invasive sampling: contributes for the definition of a rapid and non‐invasive diagnostic tool). Project NORTE‐01‐0145‐FEDER‐000010 – Health, Comfort and Energy in the Built Environment (HEBE), co‐financed by Programa Operacional Regional do Norte (NORTE2020) through FEDER. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Participants were assigned to the exercise group (EG) and the control group (CG) according to the availability to attend the sessions" |
| Allocation concealment (selection bias) | High risk | Researchers did not conceal the allocation of each participant. They chose according to the availability to attend the sessions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants could not be blinded due to the characteristics of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors do not report who assessed the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High losses to follow‐up and no proper statistical adjustments. A total of 20 participants were enrolled in the exercise group, with data provided for only 17. Of the 18 original control participants, complete data were provided for only 9. |
| Selective reporting (reporting bias) | Low risk | All the outcomes proposed are reported in the study and additional tables. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Sloan 2013.
| Methods | Study design: prospective randomised controlled trial Location, number of centres: information not provided Country: USA Study period: information not provided Methods of analysis: "each participant visited the exercise physiology laboratory before the first experimental test session for screening purposes and to familiarise themselves with the laboratory testing procedures. At this session, participants also provided written informed consent. During this preliminary visit participants underwent the same test procedures that were used during subsequent graded maximal exercise testing except that the graded exercise test protocol was stopped once a participant reached an exercise intensity level corresponding to 75% of her age‐predicted maximal heart rate (HR max = 220 – age) and successfully demonstrated the ability to maintain this level of exercise intensity for 30 minutes without becoming unduly fatigued" Inclusion criteria for participant selection were: "(1) female; (2) 1 to 5 years since cessation of menses; (3) FSH levels > 40 IU/L; (4) not on oestrogen replacement therapy; (5) sedentary, defined as no participation in a regular exercise programme for 2 or more times per week for at least 20 minutes per session or in a participative sport at least twice per week during the preceding 6 months; (6) written clearance from personal primary health care provider to participate in the study; and (7) willingness to accept random assignment" Statistical analysis: "using a 2 sample t test, the differences in the mean EG and CG at baseline on key demographic variables of age, height, weight, body mass index (BMI), FSH and VO₂ max between the 2 groups were evaluated. The distributions of all obtained measures were plotted graphically for visual inspection regarding deviation from normality. The result of the Shapiro‐Wilk Test for Normality indicated that the null hypothesis for normality assumptions of mucosal immune measures could not be rejected. The mucosal immune measures data were analysed using multivariate repeated measures analysis of variance (ANOVA). To compare the difference in outcome variables from the baseline and subsequent measurements, the contrast and profile transformations in repeated‐measures ANOVA were employed. P < 0.05 was considered statistically significant. For simultaneous testing of hypotheses, the Bonferroni method for controlling the overall error rate was used. All statistical analysis was performed using Statistical Analysis System (SAS, version 9.2) software. Values have been shown as means ± standard deviations" |
|
| Participants | Recruitment means: information not provided Target participants: healthy postmenopausal women N screened: 32 participants N completed: 32 participants Gender F = 32: intervention 16, control 16 Age: 54.1 ± 5.3 years old Baseline details: age, gender, physical activity profile, symptom checklist, health history, immune deficiency, medications |
|
| Interventions | Setting of intervention: home‐based walking programme Description of intervention: 5 days/week of 30‐minute brisk walking at a prescribed moderate aerobic exercise intensity corresponding to 75% of individual HRmax Delivered by: self‐delivered Intervention period: 16 weeks Co‐interventions: none described |
|
| Outcomes | Height, weight, BMI, FSH, VO₂ max, VE max, RER max, HR max, SIgA measures, incidence and duration of URTI Follow‐up period: 16 weeks |
|
| Notes | Study funding: supported by NIH/NINR R01 NR 008024 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors mention randomisation, but do not describe the methods used. |
| Allocation concealment (selection bias) | Unclear risk | The authors do not describe how participants were allocated. "Following random assignment to the EG or CG ..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants could not be blinded due to the characteristics of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes measured by participant self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The outcomes prespecified in the methods were reported in the results. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Weidner 1998.
| Methods | Study design: randomised controlled trial Location, number of centres: School of Physical Education at Ball State University Country: USA Study period: 6 exercise sessions in a 10‐day period Methods of analysis: "all participants reported to the laboratory every 12 hours for 10 consecutive days. Beginning on day 2 (the day of the second inoculation), all participants completed a 13‐item symptom severity checklist for each reporting period for virus detection and quantification. Just before HRV 16 inoculation, a pre‐inoculation nasal wash was taken from all participants. This nasopharyngeal sample, designed to detect most subclinical or incubating respiratory viruses, allowed us to eliminate previously infected participants from the experiment. The cultures were examined by microscope approximately every other day; other standard techniques were used for detection and identification of viruses (e.g. hemadsorption for myxo and paramyxoviruses, acid lability for rhinoviruses, etc.). These cell cultures could not detect all possible viruses (e.g. most coronavirus infections and many coxsackie A viruses). Beginning the day after inoculation (day 2), nasal washings were obtained and virus specimens were quantitated for HRV 16. Instruments included cycling on either the Air‐Dyne bicycle (Schwinn Bicycle Co., Chicago, IL) or Cybex MET 100 cycle (Cybex Metabolic Systems, Ronkonkoma, NY); walking or jogging on a treadmill (Trotter, Millis, MA) or at an indoor track; or stair climbing on the Stepmill (Stair Master Sports and Medical Products, Kirkland, WA). All participants performed the same mode for each training session. HR was monitored continuously via Polar HR telemetry units; rating of perceived exertion via the Borg 6‐20 RPE scale was recorded twice per training session" Statistical analysis: "symptom severity scores from the cold symptom checklist were summed. 3 statistical analysis were performed. A 2 group by 9 measure (2 × 9) repeated measures ANOVA procedure was used to compare the symptom questionnaire mean z‐value scores and the mucous weights for days 2 to 10. A participant's values obtained during the a.m. and p.m. testing were averaged to arrive at a participant's value for a day. The statistical power for comparing the differences between the EX and NEX groups over the 9 days (P value < 0.05) was 0.96 for Cohen's medium‐sized effect (Eta = 0.25) and 0.99 for his large effect (Eta = 0.37). Preliminary analyses of the questionnaire and mucous data suggested an alternative to the usual ANOVA procedure was desirable. The alternative procedure employed for these data was the assignment of ranks to the data values, normalising the ranks (obtaining normal distribution z‐values for percentiles of the ranks) and evaluating the data via conventional ANOVA procedures and F‐tests. The other 2 statistical procedures were a 2 by 5 (2 × 5) repeated measures ANOVA for differences between the EX pre‐ and post‐exercise cold symptom scores and a one‐way ANOVA for differences between the quantity of recreational physical activity performed by the EX and NEX groups. The statistical power for the EX group pre post differences (P value < 0.05) was 0.67 for Cohen's medium‐sized effect (Eta = 0.25) and 0.97 for his large effect (Eta = 0.37). The SPSS MANOVA program (SPSS, Inc., Chicago, IL) was used for these analyses" |
|
| Participants | Recruitment means: student volunteers solicited from classes Target participants: healthy adults N screened: 50 adult students N randomised: 50 adults randomised N completed: 50 adults completed the study: 34 (intervention group) and 16 (control group) Gender M = 24: intervention 17, control 7 F = 14: intervention 17, control 9 Age: 19 to 29 years old Baseline details: age, gender, physical activity profile, symptom checklist, health history, immune deficiency, medications |
|
| Interventions | Setting of intervention: Ball State University, School of Physical Education laboratory Description of intervention: "2 standardised incremental treadmill protocols, 1 for men and 1 for women, were used in this study. Both protocols consisted of 1‐minute stages (1‐MET increments) and began with 5 to 6 minutes of graded walking and then progressed to running speeds. All participants were encouraged to give a maximal effort and were provided with strong verbal prompts throughout the testing sessions. HR and RPE were recorded during the last 10 seconds of each stage. Exercise training. Within 18 hours of the first inoculation, EX participants began the supervised exercise training programme previously described. Participants were scheduled for 1 of 2 possible exercise times, either morning or evening. Participants who were assigned to exercise in the morning were expected to exercise at the same time for the entire 6 days of training; likewise, participants assigned to exercise in the evening did so regularly. Exercise consisted of training at 70% of HR reserve for 40 minutes, with the mode of exercise designed to match each participant's regular form of workout. Choices included cycling on either the Air‐Dyne bicycle (Schwinn Bicycle Co., Chicago, IL) or Cybex MET 100 cycle (Cybex Metabolic Systems, Ronkonkoma, NY); walking or jogging on a treadmill (Trotter, Millis, MA) or at an indoor track; or stair climbing on the Stepmill (StairMaster Sports and Medical Products, Kirkland, WA). All participants performed the same mode for each training session. HR was monitored continuously via Polar HR telemetry units; rating of perceived exertion via the Borg 6‐20 RPE scale was recorded twice per training session" Delivered by: supervised by the researchers Intervention period: 10 days Co‐interventions: rhinovirus‐induced disease |
|
| Outcomes | Cold symptom, upper respiratory infection and severity of disease measured by questionnaire and facial tissues Follow‐up period: 10 days |
|
| Notes | Study funding: this research was supported by NIH HL 50123 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors do not explain how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No description of how allocation was concealed. "Fifty subjects who tested negative to the HRV 16 antibody were randomly assigned to the exercise (EX) group or the non‐exercise (NEX) group" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants could not be blinded due to the characteristics of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes measured by participant self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The outcomes prespecified in the methods were reported in the results. |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Weidner 2003.
| Methods | Study design: quasi‐randomised controlled trial Location, number of centres: School of Physical Education at Ball State University Country: USA Study period: 7 days Methods of analysis: "volunteers were interviewed about their physical activity levels and completed a 13‐item symptom severity checklist as part of the initial screening process. A physical examination by a doctor and screening included a health history questionnaire about acute and chronic diseases, asthma, bronchitis, chronic colds, allergies, pregnancy, immune deficiency, medications, smoking and physical activity level. Volunteers were sedentary (2 or fewer days a week of recreational exercise for fewer than 30 minutes a day for the preceding 3 months). Participants had no symptoms of lower respiratory tract illness, were afebrile (< 100°F) and apparently healthy according to the criteria of the American College of Sports Medicine. All participants agreed to refrain from self treating their colds with over the counter medicines. Each participant signed an informed consent form approved by the institutional review board. Participants who completed the study received some remuneration. All participants reported to the laboratory every 12 hours (0700 and 1900) for 7 consecutive days, beginning on the evening of the day on which they were selected into the study. All completed the 13‐item symptom severity checklist at each reporting period. They all also completed an activity log during each evening reporting period to monitor their physical activity levels. After the seventh day of the study, participants reported to the laboratory once a day until they were asymptomatic" Statistical analysis: "symptom severity scores from the cold symptom checklist were summed. 2 statistical analyses were performed. A 2 group (EX and NEX) by 2 factor repeated measures analysis of variance was used to compare the mean symptom questionnaire values of study participants for mornings and evenings (AM/PM) of 6 time periods (DAY) after collection of the baseline symptom data (2 x 2 x 6). The analyses included data obtained for only days 2 to 7 of the study because some study participants were unable to participate on the first day after collection of the baseline data. An independent groups t test was used to compare the number of days from baseline until the study participants were symptom free. The analysis of variance was performed on scores obtained by: (a) subtracting baseline symptom values from values obtained during the study; (b) ranking the resulting difference values; and (c) obtaining normalised z scores for the ranks. A set of polynomial contrasts was specified in the SPSS MANOVA program (SPSS, Inc, Chicago, Illinois, USA) for the day factor. Statistical tests were conducted for the linear relation component of elapsed time from baseline scores and for the relations of the other components combined with the scores. The latter statistical test identified if systematic variation among the score means existed beyond that identified by the linear component ‐ that is, deviation from linearity. The statistical power for evaluating the relation of the day factor with the scores and for the difference between the EX and NEX groups over the days (P value < 0.05) was 0.89 for Cohen's large effect size and 0.45 for his medium effect size (x). A 1 way analysis of variance for differences between the measures on the physical activity logs for the EX and NEX groups was also completed. A P < 0.05 was considered significant in this investigation" |
|
| Participants | Recruitment means: newspaper advertisements Target participants: students that had acquired a URTI within the preceding 3 to 4 days (typical peak of illness) N randomised: 22 adult students N completed: 22 adults completed the study: 11 (intervention group) and 11 (control group) Gender M = 7: intervention 4, control 3 F = 15: intervention 7, control 8 Age: 19 to 29 years old Baseline details: age, gender, physical activity profile, symptom checklist, health history, immune deficiency, medications |
|
| Interventions | Setting of intervention: Ball State University School of Physical Education laboratory Description of intervention: "by the second day of the study, participants in the EX group began the supervised exercise training sessions. They were scheduled for either a morning or an evening exercise session and were expected to exercise at the same time for all 5 days of the study. Exercise sessions lasted 30 minutes at 70% of target heart rate with the mode of exercise chosen by the participant from the following list of choices: the Air‐Dyne bicycle (Schwinn Bicycle Co, Chicago, Illinois, USA); the Cybex MET 100 cycle (Cybex Metabolic Systems, Ronkonkoma, New York, USA); walking or jogging on a treadmill (Trotter, Millis, Massachusetts) or on an indoor track; or stair climbing on the Stepmill (StairMaster Sports and Medical Products, Kirkland, Washington, USA). All participants performed the same mode for each training session. Heart rate was monitored continuously via Polar heart rate telemetry units" Delivered by: supervised by the researchers Intervention period: 7 days Co‐interventions: none |
|
| Outcomes | Symptom severity/duration Follow‐up period: until the end of symptoms |
|
| Notes | Study funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The participants were alternately assigned to either group. |
| Allocation concealment (selection bias) | High risk | The allocation to group was predictable. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants could not be blinded due to the characteristics of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes measured by participant self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow‐up. |
| Selective reporting (reporting bias) | High risk | The study protocol is not available. The outcomes reported in the methods were reported incompletely in the results; no data were provided for each outcome, just a sentence: "no differences between the groups for...". |
| Other bias | Low risk | The study seems to be free of other sources of bias. |
Albs: salivary albumin ANCOVA: analysis of covariance ANOVA: analysis of variance ARI: acute respiratory infection BFI: big five inventory BMI: body mass index C3: complement 3 C4: complement 4 CBC: complete blood counts CG: control group CTSA: Clinical and Translational Science Award EDTA: Ethylenediamine Tetraacetic Acid EG: exercise group EKG: electrocardiogram ELISA: enzyme‐linked immunosorbent assay ESES: Enriched Soul Empowering Space Eta: eta‐squared values from multifactor ANOVA EX: exercise FSH: follicle‐stimulating hormone hsCRP: high sensitivity C‐reactive protein HR: heart rate HRV: Human Rhinovirus IP‐10: Inducible Protein 10 IgA: immunoglobulin A IgG: immunoglobulin G IgM: immunoglobulin M IL‐6: interleukin‐6 IL‐8: interleukin‐8 IPAQ: International Physical Activity Questionnaire IQR: Interquartile range IVAS: Upper airway tract infections LOT: Life Orientation Test MAAS: Mindful Attention Awareness Scale MBSR: mindfulness‐based stress reduction MET: metabolic equivalent of task MSES: Mindfulness‐Based Self Efficacy Scale NEX: non‐exercise NIH: National Institutes of Health NK: natural killer PANAS: Positive and Negative Affect Schedule PR: positive relationships PSQI: Pittsburgh Sleep Quality Index PSS‐10: perceived stress scale RER: respiratory exchange ratio ROC: Receiver Operating Characteristic RPE: Rated Perceived Exertion RI: Respiratory Infection SD: standard deviation SE: standard error SF‐12: 12‐item Short Form Health Survey SIgA: salivary immunoglobulin A SNI: server name indication SPS: Social perceived support SPSS: Statistical Package for the Social Sciences STAI: State‐Trait Anxiety Inventory URI: upper respiratory infection URTI: upper respiratory tract infection VE: pulmonary ventilation VO₂: oxygen uptake
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boukelia 2017 | Did not investigate acute respiratory infection; authors investigated diurnal physiological and immunological responses to exercise |
| Colburn 2018 | Investigated adjuvants to seasonal influenza vaccine |
| Couto 2014 | Investigated swimming on airway inflammation in a longitudinal study |
| Crabtree 2015 | Investigated effects of exercise in the cold on ghrelin, PYY, and food intake, and not ARI |
| Dubnov‐Raz 2015 | Investigated effects of vitamin D supplementation on upper respiratory tract infections in adolescent swimmers, focusing on supplementation and not exercise |
| Edwards 2015 | Investigated effects of exercise prior to influenza vaccination |
| Fujimaki 2017 | Investigated effects of exercise for glottal incompetence to improve vocal problems to prevent aspiration pneumonia in the elderly |
| Johansson 2017 | Investigated the effects of physical therapy treatment on impaired chest mobility and respiratory movement in patients with airway environmental sensitivity, not ARI |
| Killer 2015 | Investigated the effects of hydration status during prolonged endurance exercise on salivary antimicrobial proteins, not ARI |
| Komano 2018 | Investigated the effects of heat‐killed Lactococcus lactis JCM 5805 on high‐intensity exercise, focus was the supplementation |
| Kulnik 2014 | Investigated respiratory muscle training to improve cough and reduce the incidence of pneumonia in acute stroke, not ARI |
| Kunz 2015 | Investigated the effect of fitness level on salivary antimicrobial protein responses to a single bout of cycling exercise, not RCT |
| Kurowski 2014 | Investigated serum Clara cell protein CC16 with respiratory infections and immune response to respiratory pathogens in elite athletes; not an RCT |
| Langeskov‐Christensen 2015 | Investigated aerobic exercise to alleviate flu‐like symptoms following interferon beta‐1a injections in patients with multiple sclerosis, not ARI |
| Lee 2015 | Investigated the effects on immunoglobulin and changes of physiological stress and physical fitness level induced by increased cold stress, not an RCT |
| Morgado 2018 | Investigated if long‐term swimming training modifies acute immune cell response compared to a high‐intensity session, not an RCT |
| Morris 2016 | Investigated standardised rehabilitation and hospital length of stay amongst patients with acute respiratory failure, not ARI |
| Nieman 2014 | Investigated the effects on immune and inflammation responses of a 3‐day period of intensified running versus cycling, there was no non‐exercise control group |
| Oliveira 2016 | Investigated respiratory physiotherapy for patients who had lower respiratory tract infections |
| Qieqeshlaq 2016 | Investigated high‐intensity intermittent training and consumption of probiotic supplement on immune cells; study focused on supplementation |
| Rocco 2018 | Systematic review; not an RCT |
| Shing 2007 | Investigated the effect of dairy‐based beverages after exercise; study focused on supplementation |
| Strasser 2016 | Investigated probiotic supplementation in trained athletes, RCT focusing on probiotics and not exercise |
| van Middendorp 2016 | Investigated healthy men after a training programme consisting of meditation, breathing techniques, and exposure to cold; RCT focusing on multimodal interventions and not ARI |
| Vaz Fragoso 2016 | Investigated the effect of structured physical activity on respiratory outcomes in community‐dwelling elderly adults with mobility limitations, RCT focusing on breathing capacity and not ARI |
| Witard 2014 | Investigated high‐protein diet and exercise, RCT focusing on supplementation |
ARI: acute respiratory infection HRV: human rhinovirus type 16 PYY: peptide YY RCT: randomised controlled trial
Differences between protocol and review
We found one quasi‐randomised controlled trial (not specifically excluded in our protocol) in the search (Weidner 2003). We included this study in the review, although it contributed no data to the meta‐analysis.
We changed the title of the review from 'Exercise for acute respiratory infections' to 'Exercise versus no exercise for the occurrence, severity, and duration of acute respiratory infections' to more accurately describe the focus of the review.
We included the proportion of people who experienced at least one acute respiratory infection (ARI) over the study period as a primary outcome, even though it was not described as such in our protocol, because we judged this outcome to be important to the understanding of ARI episodes. Similarly, we separated the outcome mean number of ARI symptoms days (data not extractable from most trials) into two new primary outcomes: number of symptom days in the follow‐up period and number of symptom days per episode.
We had planned to compare exercise versus no exercise, and exercise versus usual care, but data were available to compare exercise to no exercise only.
We had also planned to calculate number needed to treat for an additional beneficial outcome, but there were no significant dichotomous outcomes (only one continuous outcome was significant).
Had we included cross‐over randomised controlled trials in the review, we would have included only the phase before crossing over interventions. Similarly, had we included cluster‐randomised controlled trials, we would have made statistical adjustments for clustering of participants.
We planned the following subgroup analyses: participant age; setting for the exercise; whether the exercise was supervised or not; any associated chronic conditions (asthma, diabetes, hypertension, chronic obstructive pulmonary disease); types of exercise (resistance, endurance, stretching); frequency of exercise (how many sessions per week); and intensity of exercise: light (1.6 to 2.9 metabolic equivalents), moderate (3 to 5.9 metabolic equivalents), or vigorous (≥ 6 metabolic equivalents). However, we identified data for length of the intervention only, which we had not planned.
In this 2020 update, we added an explanation to the Methods section considering heterogeneity classification according to the Cochrane Handbook for Systematic Reviews of Interventions: heterogeneity I² = 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% considerable heterogeneity. Additionally, one of the possible comparisons was whether participants were regular exercisers or had a sedentary lifestyle; we excluded this comparison, since the concept of sedentary compared to no exercise was not easily differentiated across studies.
We did not prespecify the subgroup analysis of menopausal women in the protocol. However, because sufficient data were available, on the suggestion of a peer reviewer we added a post hoc analysis of the number of symptom days per episode of illness in menopausal women (Analysis 1.13).
Contributions of authors
AJG co‐ordinated the retrieval of papers and wrote the Background, Methods, Results, Discussion, and Authors' conclusions. JK participated in the retrieval of papers and co‐wrote the Background, Methods, Results, Discussion, and Authors' conclusions. VS resolved any disagreements and appraised the review. AMS: analysed data and critically appraised the review.
Authors' contributions for previous versions of the review
Tammy Hoffmann co‐wrote the Background, Methods, Results, Discussion, and Authors' conclusions. Elaine M Beller co‐wrote the Background, Methods, Results, Discussion, and Authors' conclusions. Chris B Del Mar participated in the development of the review and co‐wrote the Background, Methods, Results, Discussion, and Authors' conclusions.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Antonio Jose Grande: none known. Justin Keogh: none known. Valter Silva: none known. Anna Scott: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Barrett 2012 {published data only}
- Barrett B, Hayney MS, Muller D, Rakel D, Ward A, Obasi CN, et al. Meditation or exercise for preventing acute respiratory infection: a randomized controlled trial. Annals of Family Medicine 2012;10(4):337‐46. [DOI: 10.1370/afm.1376] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obasi CN, Brown R, Ewers T, Barlow S, Gassman M, Zgierska A, et al. Advantage of meditation over exercise in reducing cold and flu illness is related to improved function and quality of life. Influenza and Other Respiratory Viruses 2013;7(6):938‐44. [DOI: 10.1111/irv.12053] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakel D, Mundt M, Ewers T, Fortney L, Zgierska A, Gassman M, et al. Value associated with mindfulness meditation and moderate exercise intervention in acute respiratory infection: the MEPARI Study. Family Practice 2013;30(4):390‐7. [DOI: 10.1093/fampra/cmt008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgierska A, Obasi CN, Brown R, Ewers T, Muller D, Gassman M, et al. Randomized controlled trial of mindfulness meditation and exercise for the prevention of acute respiratory infection: possible mechanisms of action. Evidence‐Based Complementary and Alternative Medicine 2013;1:1‐14. [DOI: 10.1155/2013/952716] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrett 2018 {published data only}
- Barrett B, Hayney MS, Muller D, Rakel D, Brown R, Zgierska AE, et al. Meditation or exercise for preventing acute respiratory infection (MEPARI‐2): a randomized controlled trial. PLoS ONE 2018;13(6):e0197778. [DOI: 10.1371/journal.pone.0197778] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah E, Kaden VN, Kelley KM, Liska DJ. Beverage containing dispersible yeast beta‐glucan decreases cold/flu symptomatic days after intense exercise: a randomized controlled trial. Journal of Dietary Supplements 2018 Oct 31 [Epub ahead of print]. [DOI: 10.1080/19390211.2018.1495676] [DOI] [PubMed]
Chubak 2006 {published data only}
- Chubak J, McTiernan A, Sorensen B, Wener MH, Yasui Y, Velasquez M, et al. Moderate‐intensity exercise reduces the incidence of colds among postmenopausal women. American Journal of Medicine 2006;119(11):937‐42. [DOI: 10.1016/j.amjmed.2006.06.033] [DOI] [PubMed] [Google Scholar]
Ciloğlu 2005 {published data only}
- Ciloğlu F. The effect of exercise on salivary IgA levels and the incidence of upper respiratory tract infections in postmenopausal women. Kulak Burun Bogaz Ihtis Derg 2005;15(5‐6):112‐6. [PUBMED: 16444091] [PubMed] [Google Scholar]
Dias 2014 {published data only}
- Dias R, Terciotti de Oliveira A, Cieslak F, Krinski K, Júlio Baganha R, Alexandre Pezolato V, et al. Resistance training and symptoms of respiratory infections in postmenopausal women [Treinamento de força e sintomas de infecções respiratórias em mulheres pós‐menopausadas]. ConScientiae Saude 2014;13(4):586‐94. [DOI: 10.5585/conssaude.v13n4.5053] [DOI] [Google Scholar]
Klentrou 2002 {published data only}
- Klentrou P, Cieslak T, MacNeil M, Vintinner A, Plyley M. Effect of moderate exercise on salivary immunoglobulin A and infection risk in humans. European Journal of Applied Physiology 2002;87(2):153‐8. [DOI: 10.1007/s00421-002-0609-1] [DOI] [PubMed] [Google Scholar]
Manzaneque 2004 {published data only}
- Manzaneque JM, Vera FM, Maldonado EF, Carranque G, Cubero VM, Morell M, et al. Assessment of immunological parameters following a qigong training program. Medical Science Monitor 2004;10(6):264‐70. [PUBMED: 15173671] [PubMed] [Google Scholar]
Nieman 1990 {published data only}
- Nieman DC, Nehlsen‐Cannarella SL, Markoff PA, Balk‐Lamberton AJ, Yang H, Chritton DB, et al. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. International Journal of Sports Medicine 1990;11(6):467‐73. [DOI: 10.1055/s-2007-1024839] [DOI] [PubMed] [Google Scholar]
Nieman 1993 {published data only}
- Nieman DC, Henson DA, Gusewitch G, Warren BJ, Dotson RC, Butterworth DE, et al. Physical activity and immune function in elderly women. Medicine & Science in Sports & Exercise 1993;25(7):823‐31. [PUBMED: 8350705] [DOI] [PubMed] [Google Scholar]
Nieman 1997 {published data only}
- Nieman DC, Nehlsen‐Cannarella SL, Henson DA, Koch AJ, Butterworth DE, Fagoaga OR, et al. Immune response to exercise training and/or energy restriction in obese women. Medicine & Science in Sports & Exercise 1998;30(5):679‐86. [PUBMED: 9588608] [DOI] [PubMed] [Google Scholar]
Silva 2018 {published data only}
- Silva D, Arend E, Rocha SM, Rudnitskaya A, Delgado L, Moreira A, et al. The impact of exercise training on the lipid peroxidation metabolomic profile and respiratory infection risk in older adults. European Journal of Sport Science (EJSS) 2018;19(3):384‐93. [DOI: 10.1080/17461391.2018.1499809] [DOI] [PubMed] [Google Scholar]
Sloan 2013 {published data only}
- Sloan CA, Engels HJ, Fahlman MM, Yarandi HE, Davis JE. Effects of exercise on S‐IGA and URS in postmenopausal women. International Journal of Sports Medicine 2013;34(1):81‐6. [DOI: 10.1055/s-0032-1314817] [DOI] [PubMed] [Google Scholar]
Weidner 1998 {published data only}
- Weidner TG, Cranston T, Schurr T, Kaminsky LA. The effect of exercise training on the severity and duration of a viral upper respiratory illness. Medicine & Science in Sports & Exercise 1998;30(11):1578‐83. [PUBMED: 9813869] [DOI] [PubMed] [Google Scholar]
Weidner 2003 {published data only}
- Weidner T, Schurr T. Effect of exercise on upper respiratory tract infection in sedentary subjects. British Journal of Sports Medicine 2003;37(4):304‐6. [DOI: 10.1136/bjsm.37.4.304] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Boukelia 2017 {published data only}
- Boukelia B, Fogarty M, Davison R, Florida‐James G, Fogarty MC, Davison RCR, et al. Diurnal physiological and immunological responses to a 10‐km run in highly trained athletes in an environmentally controlled condition of 6 °C. European Journal of Applied Physiology 2017;117(1):1‐6. [DOI: 10.1007/s00421-016-3489-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colburn 2018 {published data only}
- Colburn A, Wright S, Lopez V, Giersch G, Belval L, Hosokawa Y, et al. Aerobic exercise and environmental heat stress as adjuvants to seasonal influenza vaccine. FASEB Journal 2018;32(Suppl 1):lb255. [Google Scholar]
Couto 2014 {published data only}
- Couto M, Andrade P, Pereira M, Araujo J, Moreira P, Delgado L, et al. Effect of competitive swimming on airway inflammation: a 3‐yr longitudinal study. Pediatric Allergy and Immunology 2014;25(2):193‐5. [DOI: 10.1111/pai.12172] [DOI] [PubMed] [Google Scholar]
Crabtree 2015 {published data only}
- Crabtree DR, Blannin AK. Effects of exercise in the cold on ghrelin, PYY, and food intake in overweight adults. Medicine & Science in Sports & Exercise 2015;47(1):49‐57. [DOI: 10.1249/MSS.0000000000000391] [DOI] [PubMed] [Google Scholar]
Dubnov‐Raz 2015 {published data only}
- Dubnov‐Raz G, Rinat B, Hemila H, Choleva L, Cohen AH, Constantini NW. Vitamin D supplementation and upper respiratory tract infections in adolescent swimmers: a randomized controlled trial. Pediatric Exercise Science 2015;27(1):113‐9. [DOI: 10.1123/pes.2014-0030] [DOI] [PubMed] [Google Scholar]
Edwards 2015 {published data only}
- Edwards KM, Pascoe AR, Fiatarone‐Singh MA, Singh NA, Kok J, Booy R. A randomised controlled trial of resistance exercise prior to administration of influenza vaccination in older adults. Brain, Behavior, and Immunity 2015;49:e24‐5. [DOI: 10.1016/j.bbi.2015.06.102] [DOI] [Google Scholar]
Fujimaki 2017 {published data only}
- Fujimaki Y, Tsunoda K, Kobayashi R, Tonghyo C, Tanaka F, Kuroda H, et al. Independent exercise for glottal incompetence to improve vocal problems and prevent aspiration pneumonia in the elderly: a randomized controlled trial. Clinical Rehabilitation 2017;31(8):1049‐56. [DOI: 10.1177/0269215516673208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johansson 2017 {published data only}
- Johansson EL, Ternestén‐Hasseus E, Fagevik Olsén M, Millqvist E. Physical therapy treatment of impaired chest mobility and respiratory movement in patients with airway environmental sensitivity. Physiotherapy Research International 2017;22(2):e1658. [DOI: 10.1002/pri.1658] [DOI] [PubMed] [Google Scholar]
Killer 2015 {published data only}
- Killer SC, Svendsen IS, Gleeson M. The influence of hydration status during prolonged endurance exercise on salivary antimicrobial proteins. European Journal of Applied Physiology 2015;115(9):1887‐95. [DOI: 10.1007/s00421-015-3173-1] [DOI] [PubMed] [Google Scholar]
Komano 2018 {published data only}
- Komano Y, Shimada K, Naito H, Fukao K, Ishihara Y, Fujii T, et al. Efficacy of heat‐killed Lactococcus lactis JCM 5805 on immunity and fatigue during consecutive high intensity exercise in male athletes: a randomized, placebo‐controlled, double‐blinded trial. Journal of the International Society of Sports Nutrition 2018;15(1):39. [DOI: 10.1186/s12970-018-0244-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kulnik 2014 {published data only}
- Kulnik S, Rafferty G, Birring S, Moxham J, Kalra L. A pilot study of respiratory muscle training to improve cough effectiveness and reduce the incidence of pneumonia in acute stroke: study protocol for a randomized controlled trial. Trials 2014;15:123. [DOI: 10.1186/1745-6215-15-123] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunz 2015 {published data only}
- Kunz H, Bishop NC, Spielmann G, Pistillo M, Reed J, Ograjsek T, et al. Fitness level impacts salivary antimicrobial protein responses to a single bout of cycling exercise. European Journal of Applied Physiology 2015;115(5):1015‐27. [DOI: 10.1007/s00421-014-3082-8] [DOI] [PubMed] [Google Scholar]
Kurowski 2014 {published data only}
- Kurowski M, Jurczyk J, Jarzebska M, Moskwa S, Makowska JS, Krysztofiak H, et al. Association of serum Clara cell protein CC16 with respiratory infections and immune response to respiratory pathogens in elite athletes. Respiratory Research 2014;15:45. [DOI: 10.1186/1465-9921-15-45] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langeskov‐Christensen 2015 {published data only}
- Langeskov‐Christensen M, Dalgas U, Stenager E, Boye Jensen H. Can aerobic exercise alleviate flu‐like symptoms following interferon beta‐1a injections in patients with multiple sclerosis? Preliminary results. Multiple Sclerosis 2015;21(4):525. [DOI] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee N, Kim J, Hyung GA, Park JH, Kim SJ, Kim HB, et al. Training effects on immune function in judoists. Asian Journal of Sports Medicine 2015;6(3):1‐8. [DOI: 10.5812/asjsm.24050] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgado 2018 {published data only}
- Morgado JP, Monteiro CP, Matias CN, Reis JF, Teles J, Laires MJ, et al. Long‐term swimming training modifies acute immune cell response to a high‐intensity session. European Journal of Applied Physiology 2018;118(3):573‐83. [DOI: 10.1007/s00421-017-3777-8] [DOI] [PubMed] [Google Scholar]
Morris 2016 {published data only}
- Morris PE, Berry MJ, Files DC, Thompson JC, Hauser J, Flores L, et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: a randomized clinical trial. JAMA 2016;315(24):2694‐702. [DOI: 10.1001/jama.2016.7201] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nieman 2014 {published data only}
- Nieman DC, Luo B, Dreau D, Henson DA, Shanely RA, Dew D, et al. Immune and inflammation responses to a 3‐day period of intensified running versus cycling. Brain, Behavior, and Immunity 2014;39:180‐5. [DOI: 10.1016/j.bbi.2013.09.004] [DOI] [PubMed] [Google Scholar]
Oliveira 2016 {published data only}
- Oliveira A, Marques A. Exploratory mixed methods study of respiratory physiotherapy for patients with lower respiratory tract infections. Physiotherapy 2016;102(1):111‐8. [DOI: 10.1016/j.physio.2015.03.3723] [DOI] [PubMed] [Google Scholar]
Qieqeshlaq 2016 {published data only}
- Qieqeshlaq G, Reza J, Abkar AR, Heidari H. The effect of high‐intensity intermittent training (HIIT) and consumption of probiotic supplement on immune cells, C‐reactive protein, and IgA in young football player. Qom University of Medical Sciences Journal 2016;10(8):14‐6. [journal.muq.ac.ir/article‐1‐758‐en.html] [Google Scholar]
Rocco 2018 {published data only}
- Rocco M, Bravo‐Soto G, Ortigoza A. Is the exercise effective for the prevention of upper respiratory tract infections?. Medwave 2018;18(4):e7226. [DOI: 10.5867/medwave.2018.04.7225] [DOI] [PubMed] [Google Scholar]
Shing 2007 {published data only}
- Shing CM, Peake J, Suzuki K, Okutsu M, Pereira R, Stevenson L, et al. Effects of bovine colostrum supplementation on immune variables in highly trained cyclists. Journal of Applied Physiology 2007;102(3):1113‐22. [DOI: 10.1152/japplphysiol.00553.2006] [DOI] [PubMed] [Google Scholar]
Strasser 2016 {published data only}
- Strasser B, Geiger D, Schauer M, Gostner JM, Gatterer H, Burtscher M, et al. Probiotic supplements beneficially affect tryptophan‐kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: a randomized, double‐blinded, placebo‐controlled trial. Nutrients 2016;8(11):23. [DOI: 10.3390/nu8110752] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Middendorp 2016 {published data only}
- Middendorp H, Kox M, Pickkers P, Evers AW. The role of outcome expectancies for a training program consisting of meditation, breathing exercises, and cold exposure on the response to endotoxin administration: a proof‐of‐principle study. Clinical Rheumatology 2016;35(4):1081‐5. [DOI: 10.1007/s10067-015-3009-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaz Fragoso 2016 {published data only}
- Vaz Fragoso CA, Beavers DP, Anton SD, Liu CK, McDermott MM, Newman AB, et al. Effect of structured physical activity on respiratory outcomes in sedentary elderly adults with mobility limitations. Journal of the American Geriatrics Society 2016;64(3):501‐9. [DOI: 10.1111/jgs.14013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Witard 2014 {published data only}
- Witard OC, Turner JE, Jackman SR, Kies AK, Jeukendrup AE, Bosch JA, et al. High dietary protein restores overreaching induced impairments in leukocyte trafficking and reduces the incidence of upper respiratory tract infection in elite cyclists. Brain, Behavior, and Immunity 2014;39:211‐9. [DOI: 10.1016/j.bbi.2013.10.002] [DOI] [PubMed] [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellos 2010
- Bellos A, Mulholland K, O'Brien KL, Qazi SA, Gayer M, Checchi F. The burden of acute respiratory infections in crisis‐affected populations: a systematic review. Conflict and Health 2010;11(4):3. [DOI: 10.1186/1752-1505-4-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2007
- Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;334(7591):455‐9. [DOI: 10.1136/bmj.39108.379965.BE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpersen 1985
- Carpersen JC, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health‐related research. Public Health Reports 1985;100(2):126‐31. [PUBMED: 3920711 ] [PMC free article] [PubMed] [Google Scholar]
Cohen 1993
- Cohen S, Tyrrell DA, Russell MAH, Jarvis MJ, Smith AP. Smoking, alcohol consumption, and susceptibility to the common cold. American Journal of Public Health 1993;83(9):1277‐83. [DOI: 10.2105/ajph.83.9.1277] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dasaraju 1996
- Dasaraju PV, Liu C. Chapter 93: Infections of the respiratory system. In: Baron S editor(s). Medical Microbiology. 4th Edition. Galveston (TX): University of Texas Medical Branch at Galveston, 1996. [PubMed] [Google Scholar]
Del Mar 2000
- Mar C. Understanding the global burden of acute respiratory infections. Annales Nestle 2000;58(2):41‐8. [trove.nla.gov.au/version/49479161] [Google Scholar]
Engels 2004
- Engels H, Fahlman MM, Morgan AL, Formolo LR. Mucosal IgA response to intense intermittent exercise in healthy male and female adults. Journal of Exercise Physiology 2004;7(5):21‐6. [Google Scholar]
Fendrick 2003
- Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non‐influenza‐related viral respiratory tract infection in the United States. Archives of Internal Medicine 2003;163(4):487–94. [PUBMED: 12588210] [DOI] [PubMed] [Google Scholar]
Ghosh 2014
- Ghosh M, Rodriguez‐Garcia M, Wira CR. The immune system in menopause: pros and cons of hormone therapy. Journal of Steroid Biochemistry and Molecular Biology 2014;142:171‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 14 May 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2011a
- Higgins JPT, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5(13):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferson 2011
- Jefferson T, Mar CB, Dooley L, Ferroni E, Al‐Ansary LA, Bawazeer GA, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD006207.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2014
- Lee HK, Hwang IH, Kim SY, Pyo SY. The effect of exercise on prevention of the common cold: a meta‐analysis of randomized controlled trial studies. Korean Journal of Family Medicine 2014;35(3):119‐26. [DOI: 10.4082/kjfm.2014.35.3.119] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lim 2012
- Lim WS. Acute Respiratory Infections. 1st Edition. Oxford: Oxford University Press, 2012. [Google Scholar]
Marciniuk 2014
- Marciniuk D, Ferkol T, Nana A, Oca MM, Rabe K, Billo N, et al. Respiratory diseases in the world. Realities of today – opportunities for tomorrow. African Journal of Respiratory Medicine 2014;9(1):4‐13. [Google Scholar]
Moreira 2009
- Moreira A, Delgado L, Moreira P, Haahtela T. Does exercise increase the risk of upper respiratory tract infections?. BMJ 2009;90:111‐31. [DOI: 10.1093/bmb/ldp010] [DOI] [PubMed] [Google Scholar]
Nieman 2010
- Nieman DC, Henson DA, Austin MD, Sha W. Upper respiratory tract infection is reduced in physically fit and active adults. British Journal of Sports Medicine 2010;45(12):987‐92. [DOI: 10.1136/bjsm.2010.077875] [DOI] [PubMed] [Google Scholar]
Nieman 2019
- Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. Journal of Sport and Health Science 2019;8(3):201‐17. [DOI: 10.1016/j.jshs.2018.09.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Obasi 2013
- Obasi CN, Brown R, Ewers T, Barlow S, Gassman M, Zgierska A, et al. Advantage of meditation over exercise in reducing cold and flu illness is related to improved function and quality of life. Influenza and Other Respiratory Viruses 2013; Vol. 7, issue 6:938‐44. [DOI: 10.1111/irv.12053] [DOI] [PMC free article] [PubMed]
Pugh 2015
- Pugh R, Grant C, Cooke RP, Dempsey G. Short‐course versus prolonged‐course antibiotic therapy for hospital‐acquired pneumonia in critically ill adults. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD007577.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Putri 2018
- Putri WCWS, Muscatello DJ, Stockwell MS, Newall AT. Economic burden of seasonal influenza in the United States. Vaccine 2018;36(27):3960‐6. [DOI] [PubMed] [Google Scholar]
Renati 2016
- Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Family Practice 2016;33(3):312‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roth 2008
- Roth DE, Caulfield LE, Ezzati M, Black RE. Acute lower respiratory infections in childhood: opportunities for reducing the global burden through nutritional interventions. Bulletin of the World Health Organization 2008;86:356–64. [DOI: 10.2471/blt.07.049114] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2019
- Shi T, Arnott A, Semogas I, Falsey AR, Openshaw P, Wedzicha JA, et al. The etiological role of common respiratory viruses in acute respiratory infections in older adults: a systematic review and meta‐analysis. Journal of Infectious Diseases 2019 March 8 [Epub ahead of print]. [DOI: 10.1093/infdis/jiy662] [DOI] [PMC free article] [PubMed]
Thomas 2000
- Thomas M. The management of acute lower respiratory tract infection in adults in primary care. Primary Care Respiratory Journal 2000;9(1):4‐7. [www.nature.com/articles/pcrj20008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warburton 2006
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. Canadian Medical Association Journal 2006;174(6):801‐9. [DOI: 10.1503/cmaj.051351] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1990
- World Health Organization. Programme of Acute Respiratory Infections. Acute respiratory infections in children : case management in small hospitals in developing countries, a manual for doctors and other senior health workers. A Manual for Doctors and Senior Health Workers 1990:https://apps.who.int/iris/handle/10665/61873.
WHO 2002
- World Health Organization. Acute respiratory infection. www.who.int/whr/2002/whr2002_annex2.pdf (accessed 4 January 2013).
WLF 2010
- World Lung Foundation. The acute respiratory infection ATLAS. www.ariatlas.org/tools/downloads/files/ARIA.pdf (accessed 4 January 2013).
References to other published versions of this review
Grande 2013
- Grande AJ, Keogh J, Hoffmann T, Mar CB, Peccin MS. Exercise for acute respiratory infections. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010596] [DOI] [Google Scholar]
Grande 2015
- Grande AJ, Keogh J, Hoffmann TC, Beller EM, Mar CB. Exercise versus no exercise for the occurrence, severity and duration of acute respiratory infections. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD010596.pub2] [DOI] [PubMed] [Google Scholar]


