Abstract
The paradigm modifications in the metallic crystals from bulky to micro-size to nano-scale have resulted in excellent and amazing properties; which have been the remarkable interests in a wider range of applications. Particularly, Ag NPs have much attention owing to their distinctive optical, chemical, electrical and catalytic properties that can be tuned with surface nature, size, shapes, etc. and hence these crystals have been used in various fields such as catalysis, sensor, electronic components, antimicrobial agents in the health industry etc. Among them, Ag NPs based disinfectants have paid attention due to the practical applications in our daily life. Therefore the Ag NPs have been used in different sectors such as silver-based air/water filters, textile, animal husbandry, biomedical and food packaging etc. In this review, the Ag NPs as a disinfectant in different sectors have been included in detail.
Keywords: Disinfectant; Silver nanoparticles; Biomedical; HAIs; Food packaging, textiles; Animal husbandry
1. Introduction
In spite of the contemporary improvement of the hygiene in the biomedical (hospitals), education (school/colleges), surrounding environment (air/water), and industry (food/textile/animal husbandry); it is an increasingly important public health issue globally. In particular, the infectious diseases are the major challenges to the human being because of emerging >300 infectious diseases with a new adaptation. The microbial based infections are a key cause of the diverse infections because of which >50% people are dying in Africa due to a variety of infections [1]. To overcome the various strategies have been used to reduce infections by using different disinfectant. The disinfectants are chemical substances applied on the surface to kill or inhibit microorganisms. It is an ideal way to disinfect various surfaces in hospitals, kitchens and in clinics. They are useful in our daily life because they particularly kill microorganisms without causing health hazards to human beings. In addition to that, they are abundant in quantity, efficient, a cheaper antimicrobial agent in short periods and unable to generate toxic compounds after their use [2]. The various chemical compounds such as alcohols, quaternary ammonium cation, aldehydes, oxidizing agents such as sodium hypochlorite, hydrogen peroxides, iodine etc. have been used as disinfectant effectively. However, these compounds are suffered from various constraints such as harmfulness, corrosive nature and bacterial resistance.
To overcome those problems, the nanomaterials have created a new field in wider sectors. The International Organization for Standardization states a nanomaterial as a material with any exterior dimension in between 1 and 100 nm; which have also been in the multi-fold domain due to their remarkable properties. The various nanomaterials have been employed as efficient disinfectants by optimizing their physicochemical properties. Hence, many investigators are probing to generate multifunctional nanomaterials as a potent disinfectant. The nanomaterials have a wide range of uses like water disinfectant, hospital acquired disinfectant, food preservative, and medical devices etc. Among the various materials inorganic metals such as the copper, silver and gold are used eating utensils, plates, cups, jewellery, and coins water/food container for disinfection of water/food as well as human infections. Particularly, silver ions and silver-based compounds are the well-known antimicrobial agent for the medicinal importance from the 1000 BCE and they have been used as an efficient health additive in Chinese and Indian Ayurveda medicine [3]. The choice of silver is due to its multiple functions in the medical field. As usual silver nitrate is used for antimicrobial action a long time, but nowadays nano-based silver has efficient in antimicrobial action due to its physicochemical property in which larger surface to volume ratio resulted into higher surface exposure to the microbes which leads to furnish better antimicrobial activity. In addition, the special properties such as size, shape, phases play a crucial role of bacterial inactivation or killing of bacteria. These physicochemical properties of the silver nanomaterials and its compounds have foremost applications in the environmental, biomedical and industry sectors. Ag NPs are playing the crucial role in the air/water purifications, in biomedical fields as a therapeutic agent, textile consumer products as well as wound dressing which are shown in Table 1 . Its bactericide effects are observed on Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Bacillus cereus, Listeria innocua, Salmonella choleraesuis bacteria due to higher toxic effect to the bacterial cells [4]. Ag NPs have imparting broad scope to enhance efficiency by optimizing its physicochemical parameters, which also leads to greater binding capability with sulphur and phosphorous functionalized biomolecules of bacteria for killing the cells [5]. Our research group exhibited a detail study of nanocrystalline Ag connected to the surface of TiO2 electrostatically for the photoinactivation antibacterial studies in the presence of UV/Visible light [6]. Therefore, due to its multi-domain uses, Ag NPs would have the broad spectrum of the biomedical sector for innovative formulation to resist the bacterial growth. This review is majorly focus on the role of Ag NPs as a disinfectant for controlling the various infections observed in water, air, textile, poultry, hospital acquired infections, wound healing infection and food packaging infection.
Table 1.
The physicochemical properties of silver nanoparticles as a disinfectant.
| Sr. No. | Materials | Physicochemical properties of Ag NPs | Disinfectant activity | References |
|---|---|---|---|---|
| 1. | Ag/TiO2 | Reduction in band gap leads to visible active material | Visible active antibacterial activity | [6,76] |
| 2. | Ag@ZnO | O2−, •OH | Photo-oxidative killing of bacteria | [37,61] |
| 3. | Ag NPs | Surface functionalization | Photocatalytic, self-cleaning bacterial inactivation | [52,54,58,61] |
| 4. | Ag polyamide | Size 40–60 nm | Sustainable release of Ag + ion for antibacterial effect | [62] |
| 5. | PLA/ZnO:Cu/Ag bionanocomposites | Mechanical/structural, antibacterial and barrier property to UV light | Enhance shelf life of food | [73] |
| 6. | Ag NPs | Anti-viral | Elimination of aerosolized bacteriophage MS2 virus particles | [26] |
| 7. | Ag@Co-NPs | Magnetic and antibacterial | Water purification | [21] |
| 8. | AgNP@SiO2 | Spherical morphology | Prompt and synergic antibacterial activity of air filter | [25] |
| 9. | Ag NPs/Chitosan | Porosity, moisture retention capability, blood-clotting capability | Wound healing ability | [47] |
| 10. | Ag/BC | Porosity | Wound healing ability | [54] |
Schematic representation for potential applications of silver nanoparticles (Ag NPs) shown in the Fig. 1 .
Fig. 1.
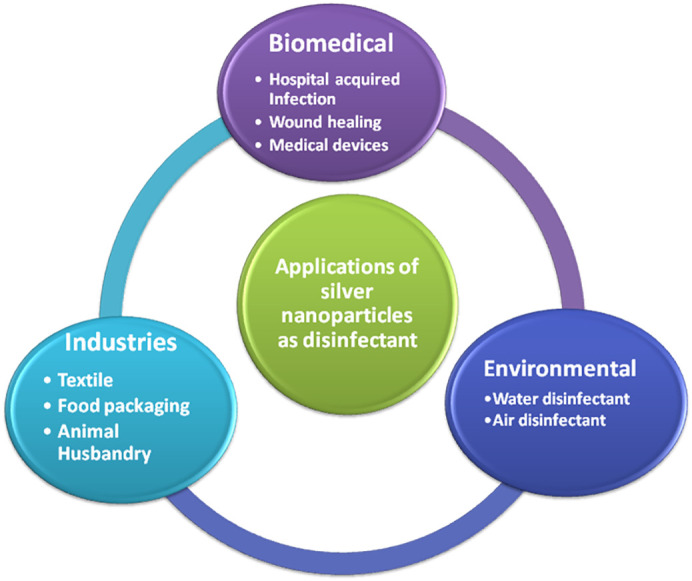
Applications of silver nanoparticles as a disinfectant.
2. Environmental sector
2.1. Water disinfections
Providing the pure water to human beings is one of the sustainable development goals to be achieved up to 2030. Presently, the various methods are available to pure the water. Filtration is commonly used everywhere; but it can remove only the suspended or micro-particles present in water. Advanced oxidation treatment is another technique used to decompose the organic matter present in water; but it is not feasible to remove all moieties. In reverse osmosis, one can get the pure water; but having more cost as well. In addition, UV-treated filters are also available to remove the microorganisms present in water. Therefore, the present water filters should be multi-functional to remove or decompose the moieties or microbes present in water and hence these are un-economical to the common peoples. However, the bare or composites of Ag NPs would have the multi-tasking ability as these nanomaterials can degrade the moieties as well as can also kill the different organisms in a single treatment [7]. Therefore, in connection to these constraints, we have highlighted the use of composites in water disinfection fields.
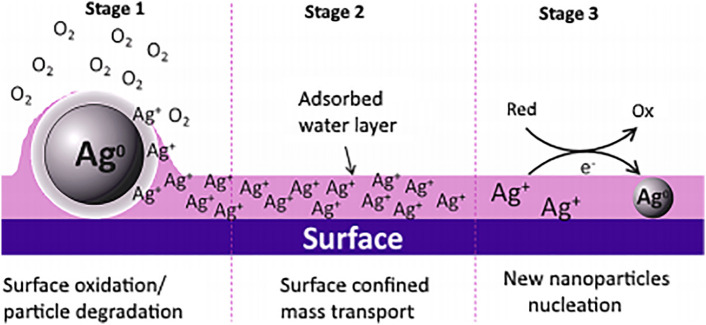
Estimated 1.87 million deaths occur worldwide due to lack of pure drinking water and sanitation problems. As per evaluated data, 780 million people did not have a safe water supply for their daily needs [8]. World Health Organization (WHO) report suggests household drinking water quality needs to be improved by various treatments at the point of use (POU) [9]. As increasing water inadequacy is an alarming rate, it is required to generate POU or advanced water treatment facilities for safe, easy, secure drinking water. In this regards, the use of nanomaterials as a disinfectant is a new approach. Drinking water purification has involved the various water treatment processes such as settlement, coagulation and filtration in addition of chemical process which covers ozonation and chlorination. Chlorination is widely used as a disinfectant method but it has some disadvantages such as bad taste and odor. In some cases, it is ineffective due to microbial resistance and generated toxic product in the water. The aim of water disinfection is to remove or inactivate microbiological contaminates from the water, without changing the physicochemical properties of water, in addition to these process, silver as a disinfectant is one of the traditional routes to kill or inactivate the bacteria [10]. Also, per the U.S. Environmental Protection Agency (EPA) and WHO, silver at a low concentration in drinking water i.e. <0.1 mg/L is safe [11]. Silver disinfection depends on various parameters such as concentration, pH, exposure time and temperature. In addition, the other content in the water such as calcium, chlorine and sulphide also play a crucial role in the disinfection process. Calcium and sulphide in water affect the bactericidal activity of silver, but chloride has less effect on its bactericidal activity [12]. Therefore nanocrystalline silver form would have better efficacy in water disinfection. The impregnation of silver nanocrystals in ceramic water filter can act as an effective disinfectant than simple filter [13]. Water filters implanted with Ag NPs proved efficient for the removal and deactivation of microbes by using two mechanisms such as metallic disinfection and physical filtration. Actual impact of silver salt and its NPs on the C. parvum pathogen and its complete removal from water by filtration has been revealed [14]. Nowadays, low-pressure membrane filtrations methods have increasing usage in water treatment due to lower processing cost. Generally, low-pressure membrane equipment involve microfiltration (MF) and ultrafiltration (UF) processes. But, they have some issues related to performance. In which, biofouling is the major problem which can created bacterial film on the membrane resulted into decreasing efficiency of membrane flux, thereby reducing the life of membranes. Therefore, it is needed to add antimicrobial agent into the membrane to reduce biofouling and increase capacity of the membrane. Thus, Ag NPs play dual role such as anti-adhesive and antimicrobial agent on the surface of membrane to inhibit bacterial adsorption, attachment and growth; resulted into the prohibition of biofilm formation on the membrane [15]. The antibiofouling performance depends on the particle morphology such as microparticle (mAg), nanoparticle (npAg), nanowire (nwAg) and leaching ability of silver which has incorporated polysulfone membrane. Here, water flux is enhanced by 7 fold due to nanowire Ag NPs and overall antibiofouling property of polysulfone membrane has been enhanced [16].Thin film composite was formed with uniform distribution of Ag NPs on reverse osmosis membranes having surface roughness, hydrophilicity and zeta potential properties. It exhibited high functional property, in which 75% active bacteria anchored on the membrane hence filtered water is free from bacteria and it also reduces biofilm formation rate. Such type of ease method for potent antibacterial ability resulted into practical approach to water filter membrane [17]. The cryogels are another efficient superabsorbent material focused as alternative to ceramic filter with efficient disinfection process. Silver impregnated polysodium acrylate (PSA) cryogels are used as efficient disinfectant in POU process. This is because of its higher porosity, better mechanical and water absorption ability. Homogeneous dispersion of silver nanocrystals on pore surface of cryogels resulted into efficient disinfection with lower release rate of silver ions [13].In another method the percolation of water through silver adsorbed blotting paper sheet has play major role in the bacterial deactivation. It's deactivation performance and leaching test confirmed that, this method is good for water purification process [18]. Polyurethane foams are homogeneously coated with Ag NPs without leaching during the washing in presence of water. This foam is used in water filtration in which flow rate of 0.5 L/min was maintained and filtered water does not have any E. coli bacteria. It confirmed that this water filtration unit is compatible with drinking water purposes [19]. To reduce hardness of water cation exchange polymer matrices being routinely used in water treatment process with little focus on the trapping of bacteria in the membrane. Glover et al. reported the dynamic behaviour of Ag NPs on the surface under ambient conditions. The humidity dependent particle generation from the host NPs explored with successive steps are involved in this process. In the first stage Ag NPs are dissolved and oxidized on the highly humid surfaces that lead to formation of the silver ions. Afterwards, in the second stage these silver ions are diffused in the water layer from the parent particles that are different from the parent particles. Later in the third stage formation of Ag NPs are observed at the surface through the photo-reduction or mild reduction process [20]. Various stages involved in the Ag NPs generation under the ambient conditions are shown in Fig. 2 .
Fig. 2.
Proposed steps involved in Ag nanoparticle generation from the parent nanoparticles.
.
For bacterial inactivation, Ag@Co NPs were embedded into polymer matrices by soft reaction condition. These nanoparticles and their functional groups are efficiently acted as antibacterial agents for water purification [21]. In the current era, self-propelled micro- and nanomotor have projected in the various fields, but in the water disinfection process micro- or nano-devices have been effectively used. The self-propelled Janus microbots incorporated Ag NPs acts as a potent bactericide for water disinfection process, anchored with magnetic iron particles for controlling its migration and recovery after use from the treated water. It has specific properties, such as better swimming capability and deactivation of bacteria within short time. Such innovative ways for water disinfection are phenomenally effective and encouraging for the development of micro- and nano-scale devices [22]. Therefore, incorporation of silver in materials like ceramic filter, cryogels, blotting papers, polyurethane foams and cation exchange polymer matrices to design nano- or micro-devices have generated new avenues for water purification than routine water disinfection methods.
2.2. Air disinfection
In the contemporary era, multiple human activities during the various development processes lead to have an adverse effect on the air quality. The current confront involve the clean environment free from toxic gases, particles, volatile organic compounds, airborne pathogenic bacteria and viruses. Among the various challenges of air infections, the removal of airborne microorganisms such as viruses, bacteria and fungi have received great attention as these microbes are responsible for the chronic communicable diseases. It increases vigilance about air purification in the health context. The air purification is needed to eradicate the generation of airborne particles from the various viruses, bacteria, fungi and all of biological living organism. They are majorly responsible for the various diseases such as anthrax, SARS (severe acute respiratory syndrome), asthma etc. These bioaerosols are deposited on the filter, air conditioning systems in excessive amount and multiply due to the higher amount of moisture condition [23]. Therefore, various engineering solutions are available for the removal of bioaerosols using ultraviolet germicide irradiations, photocatalytic oxidation and air ozonolysis methods. To control air quality in the engineering nanomaterials the Ag NPs are used as efficient antimicrobial activity. Silver aerosol nanoparticles are generated from atomizer has been studied as an antimicrobial agent against B. subtilis bioaerosols under meticulous conditions. It is observed that Ag NPs are good enough to improve the air quality using air filters [24].Young et al. reported the simple approach used to fabricate the mono-disperse Ag NPs decorated silica particles for synergic antibacterial activity with gram positive and gram negative bacteria in the air filtration unit. In addition, the solution of AgNP@SiO2 was stable up to six months and exhibited 99.99% antibacterial efficiencies of the both bacteria. Therefore, such hybrid materials are useful as coating for the air purification devices and appliances for prospect green environment applications [25]. Silver coated silica particles are designed and coated on the air filter for the measurement of filtration efficiency and anti-viral ability in the presence of aerosolized virus particles. Subsequently, mathematical model has been used for the determination of anti-viral ability of the air filters. As per calculated data the stable inlet virus concentration 500 PFU/m3; three inlet dust concentrations 0, 100, and 200 g/m3; and three outside layer areal densities of 9.34 × 108, 2.8 × 109, and 4.7 × 109 particles/cm2. These measurements show the anti-viral efficiency of the filter increased with coating areal density and diminish with dust loading. Moreover, it does not affect filtration quality of air of the air filter [26].
Fig. 3 shows the antiviral action of Ag NPs on dust particles of the air filter. Ag NPs are coated on medium air filter as an anti-viral agent and analysed its efficiency as well as anti-viral capability with aerosolized bacteriophage MS2 virus in the presence of dust particles as compared with the theoretical model [26]. Herzong et al. demonstrated the human epithelial airway barrier model at the air-liquid interface using aerosolized Ag NPs exhibited minimum cytotoxicity [27]. The utilization of nanotechnology-based products have been increased and thus the exposures of NPs to the human have also been amplified. These NPs have exposed to the consumer via inhalation, ingestion, and dermal pathways, but inhalation is the more routine way to introduce NPs into the human body. Therefore, the releases of airborne particles and silver compounds from nanotechnology-based sprays have studied in the context of model population exposure and human health effect. Thus air quality has improved by using Ag NPs incorporated in various supporting materials without affecting the air filtration efficiency [28,29]. Thus air quality has improved by using Ag NPs incorporated in various supporting materials without affecting the air filtration efficiency. Though various strategies have been employed for the air/water purification using improved devices with NPs exhibiting better performance; still there is a lot of scope for the optimization based research output with long-term impact of NPs on living organism as well as optimum concentration for increasing efficacy without toxic effect.
Fig. 3.
Antiviral action of silver nanoparticles with dust particles.
3. Biomedical sector
3.1. Hospital acquired infections (HAIs)
The current challenges in the biomedical sector are the antibiotic resistant of the organism, product development protocols and its utility in terms of toxicity, healing time period and a side effect on the human cells. In addition, the detection of infection causing non-bacterial pathogen, monitoring infection control and prevention of nosocomial infections are key confront towards the scientific community [30]. HAIs are well known as nosocomial infections that occurred in the hospital and health care facility centre. There are many factors responsible for HAIs, such as decreased immunity of patient, multistep treatment of patient resulted into increase in infections, spreading of drug resistant bacteria and less care is taken for employing bacterial infection protocols. Fig. 4 shows the various factors responsible for HAIs. A worldwide survey conducted by WHO revealed average 8.7% peoples are suffered by nosocomial infection and about 1.4 million people got affected with HAIs. Eastern Mediterranean and south-east Asia regions are more prone to such infections compared to the western world. Nosocomial infections include urinary tract infections, surgical wounds and lower respiratory tract infections [31]. Prevention of nosocomial infections requires integrated and monitoring programme in which different aspects are essential to be considered. The transfer of microorganism from patient to care taker has been reduced by personal hygiene including hand washing, hand gloves, masks, working clothes, shoes and sterilization of hospital equipment [32]. Moreover, increasing bacterial resistance has a major impact on health and enhancing economic burdens. A large number of policies have been applied for campaigning against HAIs. Particularly, the use of Ag NPs is emerging one to control the HAIs and efficient nano-weapon against multidrug resistance bacteria [33]. The metal NPs synthesized by the biological route benefits more than those prepared by the physical and chemical methods. This is because of a cheaper processing cost and eco-friendly nature of the biological method. In the biological protocol, biomass has been formed around the metal to neutralize its toxic effect. Biologically prepared Ag NPs using Bacillus marisflavi showed high antibacterial activity against bacteria responsible for HAIs [34]. The uncontrolled and excessive use of antibiotics and their bacterial resistance is a present threat in front of the medical community, which is also addressed by synergetic combinations of antibiotics with Ag NPs [35].
Fig. 4.
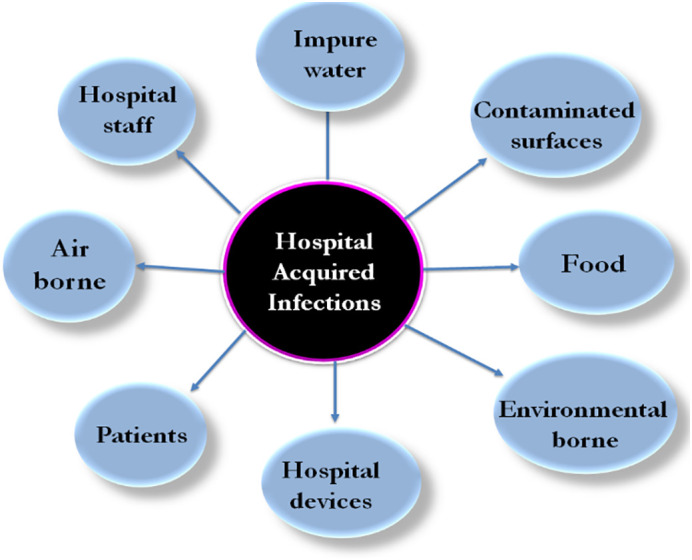
Various factors responsible for hospital acquired infections.
The allicin and Ag NPs combination have been studied for skin infection occurred due to Methicillin-Resistant Staphylococcus aureus. This study revealed that the Minimum inhibitory concentration (MIC) and Minimum bacterial concentration (MBC) values for this drug combination are lower and thus useful in skin treatment to avoid skin infections. Various medical tools used in hospitals are also potential sources of infections. These are coated with Ag NPs to avoid bacterial contamination. Ag NPs are effectively used in catheters for better antimicrobial activity and zero thrombogenicity [36]. The effect of Ag NPs due to higher surface to volume ratio and release of silver ion on the coagulation of contacting blood has been examined. Visible light assisted disinfections by using antimicrobial agent are another path to reduce infections. Antimicrobial effect of silver coated titania films was revealed under indoor light condition for photoinactivation of bacteria. A complete killing of MRSA bacteria due to synergic effect of Ag NPs as a photocatalyst and visible light has been observed [37,38]. The stents and catheters used in cardiovascular applications required to be coated with antimicrobial agents like Ag NPs to prevent thrombosis. The Ag NPs have prolonged activity, higher bactericidal and bacteriostatic property, biocompatibility and lower vivo toxicity [39]. A bone cement used in the hip and knee replacement surgery, where infection rate is lower with Ag NPs along with poly (methyl methacrylate) (PMMA) in order to reduce risk of bacterial infections. It does not display any cytotoxicity in mouse fibroblasts or human osteoblasts indicating good biocompatibility [40]. Wound dressing is another area in which nanocrystalline silver has been used as commercial product from the decade. In clinical trial, the wound healing efficiency of exiting 1% silver sulfadiazine was compared with new chitosan-nanocrystalline silver. Healing rate of chitosan-nanocrystalline silver after 13 days was higher than control by at 98.98 ± 6.09% which is compared with 1% silver sulfadiazine at 81.67 ± 6.30%. In addition, the healing period was 13.51 ± 4.56 days and 17.45 ± 6.23 days for chitosan-nanocrystalline silver dressing group and 1% silver sulfadiazine group, respectively. It has been found that antibacterial efficacy and wound healing property is significantly high for the chitosan-nanocrystalline silver [41]. The chronic infections are mainly related to biofilm formation on the surface of medical devices in which these bacteria have resistance to antibiotic agent. Such biofilms are effectively degraded by using Ag NPs. After isolation of biofilm from wounds its anti-biofilm efficacy of Ag NPs is examined. The observed lower MIC value range of 11.25–45 μg/mL and anti-biofilm efficiency of Ag NPs is higher at lower concentration of 50 μg/mL [42]. Ag NPs were incubated into polymer matrix exhibit hydrophilic properties that reduce surface attachment of microorganism, biofilm formation and proteins accumulations. Hence, the regular dispersion of activated Ag NPs on the inner and outer catheters avoids formation of biofilm and showed greater antimicrobial property into wide range of in vitro studies [43]. In addition, the human infections occur due to the C. albicans pathogenic microorganisms that correspond to the commensal fungi which are usually present on skin, oral cavity, vaginal and gastrointestinal tracts. Candida biofilms have been mainly studied on abiotic surfaces of medical devices but it is found that lower dose has limited efficacy. In order to improve its efficacy use of higher dose required that results in damage of various organs such as kidney, liver etc. To overcome these problems, the biogenic Ag NPs are used to control the biofilms formation on the surface of catheters at the lower dose [44]. External ventricular drainage catheters impregnated with Ag NPs are new path ways to avoid catheter-associated ventriculitis in neurocritical patients. It is validated with recent vitro study on the biomedical devices exhibited the segregation of silver ion in catheter is lower than accepted levels [45]. In conclusion, in vitro and animal studies exhibited Ag NPs have significant level of toxicity. In vivo studies showed long term exposure causes increased argyremia. Subsequently, Ag NPs have been used for wound dressing purpose for safe and broad spectrum. So, the Ag NPs or its composites are useful materials for the different purposes for the control of various infections occurs in the hospitals. In addition, proper precautions are needed to be taken to avoid their toxic effects on human.
3.2. Wound healing
Silver has been used as an effective antimicrobial agent from century to decrease bio-burden. Worldwide spreading of microbial resistance is the alarming concern in the clinical practice. The various antibiotic agents are effectively used from many years for the treatment, but excessive use resulted in antimicrobial resistance. So, to reduce microbial resistance it is needed to design new strategies making use of nanotechnology. Generally, infections are responsible for the postpone healing of closed wounds, traumatic, burn wounds and chronic skin ulcers. The silver based antimicrobial agent is emerging disinfectant promoted for the wound healing to combat infections without affecting human cells. But, its resistance, toxicity and analysis of product protocols are main factors of consideration before its use. [46]. Ag NPs (AgNPs)/chitosan composite as wound healing agent are prepared by simple two step route. The inclusion of Ag NPs into chitosan could improve the antibacterial action against drug responsive and drug-resistant harmful bacteria. The hydrophobic parts of dressing exhibit waterproof and anti-adhesion properties to avoid contamination of the surfaces. On the contrary, hydrophilic surface shows water penetrating ability and diminish bacterial growth [47]. In chronic wound care dressing, the hydrogels with wide functions are the new emerging technologies to avoid infections. Hydrogels, with a high amount of moisture and prepared with silver encapsulation exhibited that this low concentration (0.1 and 1.0% w/v) have promising potent antibacterial performance against S. aureus, P. aeruginosa, and E. coli which are majorly responsible for development of antibiotic resistance [48]. Green methodologies for the synthesis of nanomaterials are point of focus due to health concern and biocompatibility. Therefore, Ag NPs have been synthesized by Lansium domesticum (LD) fruit peel extract for the wound healing purposes. In vivo wound healing analysis demonstrated that it intensify wound closure time and excellent histocompatibility. Thus, it has good prospectus in the disinfection applications [49]. Typically, inflammatory skin diseases, with atopic dermatitis, psoriasis, and contact dermatitis are the majorly widespread skin conditions impact on adults and children. Therefore, immunosuppressant drugs have been used for the treatments but they have lower efficacy and with bad effects. Hence, nanocrystalline silver cream is used as substitute treatments for inflammatory skin diseases and it is found that it has efficient anti-inflammatory activity in comparison with routine steroids and immune suppressant in presence of pig model of dermatitis diseases. It reduced erythema in 1 day of treatment at variation in concentration with noteworthy reduction at silver concentrations of 0.5% and 1% (P < 0.05) and this decreases during the study stage [50]. Direct application of such antimicrobial agents on the human skin may cause toxicity. Toxicity is the major concern to humans rather than bacteria. Therefore, in vitro study of Ag NPs for toxicity assessment exhibited that antimicrobial concentrations (1.56–6.25 g/mL) are safe for its use. Lower concentration of silver selectively attacked on bacterial cell lines without harming host cells and therefore useful as well as safe to cure burn wound infections. It established its utility as a good antimicrobial agent due to less cytotoxicity, oxidative stress and apoptosis of the bacteria [51]. There is a wide range of typical silver containing antibacterial products, such as silver nitrate, silver sulphadiazine, silver sulphadiazine/chlorhexidine, silver sulphadiazine with cerium nitrate and silver sulphadiazine impregnated lipidocolloid wound dressing. On the other hand, newly improved products such as ActicoatTM and Silverlon1 have a more systematic and prolonged release of nanocrystalline silver to the wound surfaces. The modes of action like liberation of silver have changed the path of wound dressing. This is due to the reducing threat of nosocomial infection, cost of product, and without damaging tissue efficient healing would be takes place [52]. The release of silver ions depends on the size of the particles. When the size is <20 nm, it exhibited 100 times more release rate than bulk silver particles. It has been revealed from many studies that Ag NPs are efficient bactericidal in vivo conditions as compared to the bulk silver. The wound dressings are designed in such a way that, they can constantly release silver ion to the wound for the period up to seven days for bacterial death [53]. Ag NPs are functionalized with bacterial cellulose using green synthetic methods with formation of three dimensional webs like structure through the UV light irradiation. Bacterial cellulose is used as supporting material for wound dressing. A greater porosity of the cellulose is due to the nano-fibrous network which enhances water retention ability. The zone of inhibition of silver/bacterial cellulose is observed at 6.5 mm as compared to reference bacterial cellulose is 10 mm, shows efficient antimicrobial substrate against E. coli bacteria for wound healing treatment [54]. To understand antibacterial action of silver ion on infected wounds in the presence of organic materials or inorganic ions like chloride, sulphate and phosphate ions have major impact on wound healing process. Therefore, to recognize the bacterial death mechanism there is major causes such as the penetration of silver ions into peptidoglycan cell wall and interaction with plasma membrane, bacterial cytoplasmic DNA and bacterial proteins [55]. In clinical studies the wider chronic non-healing wounds of 29 patients have been quantitatively studied. The bacterial biopsies depicted same number of bacteria. But, swabs used for healing showed reduced number of bacteria on the wound surfaces and causing less pain in wound healing process [56]. In controlled clinical examinations, it is observed that Ag NPs are efficient disinfectant for the wound healing at lower dose. Despite phenomenally efficient wound healing property of silver, its production cost, clinical excellence and safety as compared with well-known antiseptic agent povidone‑iodine are also considered. At the end, wound healing using Ag NPs as the potent antimicrobial agent is an advanced process with lower toxicity to the human cell line and shorter time period of wound healing. In the future, the excessive use of Ag NPs based drugs in the biomedical sector may cause of resistance to the organism so that there is scope for designing, developing and modification of the Ag NPs based drug to avoid bacterial resistance.
4. Industrial sector
4.1. Textiles
Nowadays, there is growing interest of the use of silver based nanomaterials as antimicrobial agents in the textile sector as well. Sterile fabrics are one of the common goal defined by the scientists so there would be the bacterial free fabrics subject to the various different conditions. The different usage of nanomaterials for textile fabrics has been continuously demanded due to increasing customer costumes for different purposes. To functionalize fabrics the nano-moieties can play the major role with their specific properties. The various properties of fabrics such as stimulate stain repellent, wrinkle free, antistatic, strength enhancement; water repellent and antimicrobial are very significant to enhance fabric durability, luxuries and flexibility. Textile industries have changing and adopting new technologies not only in fabric processing but also in the use of antimicrobial agent to avoid bacterial contamination. Currently, peoples are also aware about bacterial infections occurred due to textile products. The spreading of microorganisms from fabric surfaces to the human skin is the major health concern. Therefore, fabrics can be treated to avoid bacterial infections [57]. In current scenario, textile fabrics have different properties such as antimicrobial activity, UV protection and self-cleaning. The contemporary methods in textiles have adopted nanotechnology in order to accomplish antimicrobial property. But, in this direction selection of proper antimicrobial agent is a challenging task. The choice of Ag NPs is preferable as compared with traditional antimicrobial agents such as metal salts, quaternary ammonium compounds and triclosan. This is superior due to various factors such as bacterial resistance, stability, cheaper and environmental benign [58,59]. Therefore it is more useful than the ionic silver as they cannot generate stain on the fabrics, maintaining fabric breathability and handling. The antimicrobial agent in bare or composite form on the fabric cannot leads to the decolourizations or of destruction of mechanical strength of fibre [60]. Cotton fabric exhibits self-cleaning properties under visible light by anchoring its surface with Ag@ZnO nanostructured materials. In addition, the preparation of lightweight and wearable clothes with antimicrobial activity the polyamine-mediated bioinspired approach is simple for functionalization of antimicrobial agents on the surfaces. Nanostructured materials are formed by using polyamines which is coated on the surface of fabrics for better antimicrobial activity under the sunlight. It also exhibited proficient photocatalytic, self-cleaning and stain removal property of the fabric [61]. Plasma pre-treated polyamine coated with Ag NPs are studied for antimicrobial activity and aging effect. The bacterial inhibition is observed after 30 days in the presence of Ag NPs with size <100 nm. Furthermore, a longer period bacterial inhibition is observed at lower concentration with 40–60 nm size Ag NPs [62]. Plasma treated polyester fabric developed for increasing binding property of Ag NPs and pre-treatment initiated both ionic and covalent interactions to create oxygen species on the fibres, resulting deposition of smaller size Ag NPs which promote antimicrobial property [63].The functional clothing has good scope in the market because it has designed to get better performance to the user in the extreme conditions. The various functional clothing such as protective, medical or sport clothing are available in the market. In functional clothing studies have been focused on antimicrobial effects in laboratory scale to the real life conditions [64]. Now a day's engineered nanoparticles (ENPs) are the new technology, in which functionalized silver and titania based consumer products are used as antimicrobial and photoactive agents. However, the various external exposure pathways such as the contact between fabric and skin as well as ingestion and inhalation transfer to children by oral routes are responsible for toxicity to human cell lines. Goetz et al. studied the dermal exposure to nano-object and their aggregated and agglomerates of the loaded ENPs on the commercial textile fabrics to artificial sweat using simulated wear-and-tear through physical process. These ENPs uptake rates on skin have less exposure therefore they are exhibited less toxicity than oral pathways used in dietary supplements [65]. Silver coating by various techniques on the textile fibres having longer period of release of silver ion and uniform distribution of silver on fabric is important parameter for antimicrobial fabrics. In the realistic approach leaching of silver from fabrics used for children is studied in the presence of different liquids such as water, milk, sweat and urine. Subsequently, leaching of silver in presence of sweat and urine found to be higher than the tap water. This study reveals the less connectivity of silver particles to the fabrics; which would be further optimized for decreasing the leaching rate of Ag NPs from the fabrics so that these particles would not be harmful to the human beings [66]. Fig. 5 shows the antimicrobial efficacy of Ag NPs in the textiles. Microbial efficacy of Ag NPs was determined on the textile fibres during various life cycle stages. Multiple cycles washing exhibited range of silver release but does not affect antimicrobial efficacy of Ag NPs [67].
Fig. 5.
Antimicrobial actions of silver nanoparticles in the textiles.
In supposition, the overall antimicrobial efficacy of Ag NPs depends on its concentration, surface area, size and release rate. The higher surface area and concentration of silver help to increase bacterial contact by binding with —SH group of a protein. This resulted into reduction of bacterial functions and ultimately its inhibition. It also hamper respiratory system, basal metabolism of electron transfer in the cell membrane causing bacterial death [68].Nonetheless, because of its high antimicrobial activity and stability on the fabrics next generations prefer to use various types of costumes which are free from bacterial contamination and having self-cleaning ability of the fabrics. Accordingly, the textile industries have been changing from their old fashion technology to the new nano-based technology for advanced textile products.
4.2. Food packaging
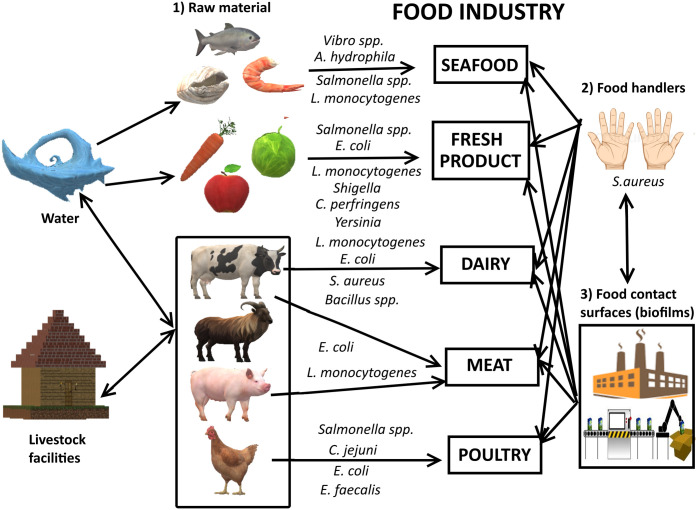
Food safety is the major concern in front of the food industry and government worldwide. A fresh, clean, hygienic and long life food without chemicals or the chemicals that are less harmful to human being globally. Market trends have also been changing to lower processed, readily prepared and ready-to-eat “fresh” food goods. On the other hand, foodborne microbial bursts, worldwide food trade and transportation of processed food to consumers are motivating for searching new ways to inhibit microbial contamination of food along with key challenges like quality, freshness and safety. The Centre for Disease Control and Prevention (CDC) of USA, evaluated that 90% of the infections are occurred due to different types of bacteria. As per CDC report almost 48 million peoples are infected out of that 128,000 are hospitalized and 3000 died in United States due to foodborne diseases. These are not only bacterial infection but also toxins released from the various microorganisms during metabolic processes [69]. Therefore, antimicrobial packaging technologies are needed to enhance shelf life of food, inhibit bacterial contamination and to prevent or delay the spoilage. Ag NPs are promising bactericidal materials in food packaging against broad range of microorganism such as bacteria, yeasts, fungi and viruses. Most of the inorganic metals and metal oxides used as antimicrobial agents are more tolerable to the drastic food processing conditions as compared to the organic materials having a less stability. Ag NPs are potent biocides against various pathogens. But, migration of silver from the packaging surfaces to the food stuffs is the potential health concern risks [70]. The European Food Safety Authority (EFSA) recommended upper limits of silver migration from packaging should not go beyond 0.05 mg/L in water and 0.05 mg/kg in food. It exhibits that determination of silver migration framework are essential to ensure potent antimicrobial activity [71]. Food packaging are classified into two categories; firstly improved packaging in which nanomaterials are embedded into the polymer to enhance its gas barrier properties and secondly active packaging in which nanomaterials interact directly with food and prevent contamination of food from microbes. In the film formation process Ag NPs were coated, absorbed, or directly incorporated by the simple chemical route [72,73]. Active packaging is the emerging technology focused on the protection of food product from microbial contamination and deteriorations. It involves the three sub-categories, such as absorbing systems, releasing systems and other specific temperature, UV light and microwave based systems [74]. Though the Ag NPs increase shelf life of food, there is need to evaluate hazards and risks of migration from packaging to food for the customer safeness. The better food quality and shelf life have been achieved by active packaging which leads to the reduction of microbial infection from field, cold storage and consumer places. The low cost and environmental friendly packets embedded with Ag NPs for vegetable storage have been investigated. After periodic determination it was observed that shelf life of vegetables increased without reducing nutritional values [75]. Cozmuta et al. reported Ag/TiO2 nanocomposites in high density polyethylene (HDP-P) film in packaging which increases shelf life and microbiological safety of bread in comparison with the routine packages [76]. Polyethylene based packages with Ag–TiO2–Fe composite kept orange juice with same colour, consistency, flavour and taste as like the freshly prepared juice, even after 10 days of storage. It was also found that, silver and iron have a better antimicrobial property on the yeast and molds than TiO2 [77]. Food packaging has various factors such as size, concentration detection limit, size resolution were determined for effective packaging. The biokinetic behaviour such as size, shape, capping agent, charge and solubility and aggregation state of the active packaging are have impact on the shelf life of food. Investigators were used SP-ICPMS and AF4-ICP-MS techniques for studying the properties of NPs for better understanding the effect of NPs on the active food packaging [78]. Ramos et al. reported the migration study of Ag NPs, from plastic baby bottle and food container revealed less agglomeration and oxidation of Ag NPs, it is depends on the nature of polymer and its storage conditions. SP-ICPMS techniques were used for the determination of ionic silver and Ag NPs in extremely diluted samples. Hence this method is better for getting precise information of NPs size and concentration in complex extracts at lower quantity with short duration of time, that avoids agglomeration and oxidation of Ag NPs [79]. Silver and copper NPs were impregnated into guar gum nanocomposites and effect of on thermo-mechanical, optical, spectral, oxygen barrier and antimicrobial properties on film have been studied. It exhibited good properties of film for active food packaging applications. While commercialization of such films require to study the effect of NPs on food and its impact on the human health [80]. Commercially available containers are used to determine migration of Ag NPs under acetic acid as stimulant at 40 °C under 10 day's observations. Migration values were found to be higher under heating in the microwave oven compared to conventional oven. These values were also depending on size and aggregation of Ag NPs [81]. PVC nanocomposite films incorporated with Ag NPs decrease the thermal with retained mechanical properties and prolonged the shelf life as well as decrease the lipid oxidation of packed chicken breast fillets [82]. Thus, active packaging with Ag NPs is breakthrough technology for food safety and its processing. Vast commercialization of this technology offers clean and fresh food in ready to eat format without losing its nutritional qualities. Fig. 6 shows the correlations between raw materials, food handlers and biofilms in the food packaging [83]. Thus, the nanoscale silver is one of the best materials for disinfection purpose with its potent antimicrobial activity. Presently, it has been profoundly used in daily life, in environment for water and air disinfection, in industries such as textile and animal husbandry, in medicine. With such widespread use of Ag NPs as disinfectant; it is required to give special emphasis on the risk of its toxicity for the welfare of human health.
Fig. 6.
Correlations between raw materials, food handlers and biofilms.
4.3. Animal husbandry
Animal husbandry is the one sector of agriculture related to animals as the source of meat, fibre, milk, eggs, or other foodstuffs. During the routine activity of the animals, there is a possibility of infections due to various pathogenic microorganisms. So there is a need to expand healthy animals with fresh and better food products. Among the disinfectants, Ag NPs is also used as a surface disinfectant, water disinfectant and therapeutic material in animal husbandry including poultry, livestock and aquatic industry. The various diseases caused by bacteria, viruses, fungi and other mono-cellular microorganisms were effectively controlled by using Ag NPs. It inhibits reproduction and growth of those bacteria and fungi responsible for the infection, bad odor, itchiness and sores. Ag NPs are found to be highly efficient, fast acting, deodorizing, nontoxic, non-stimulating, non-allergic, tolerance free, hydrophilic and thus very effective for bacterial resistance. Therefore, Ag NPs are used as a disinfectant in animal husbandry to disinfect and prevent disease [84]. All the products of poultry farming viz eggs, chicken etc. are mainly based on biological material, which is the potential source of infections and thus the poultry diseases. Various microorganisms and their endotoxins are responsible for the infectious diseases and spread in the environment through bioaerosols called organic dust. This organic dust is reaching about three kilometers away from the place of its formation and causes serious respiratory tract infections [85,86]. A lot of efforts have been made to conquer such infections by eco-friendly ways without having a negative impact on human health. Many chemical compounds such as organic acids, hydrogen peroxide, sodium bicarbonates, sodium orthophosphate etc. have been used for the destruction of microorganisms. But, all those have one or more limitations like less solubility, a possibility of direct application on the product, high cost, toxicity [86].
One of the significant examples of an organic compound used for the disinfection is formaldehyde due to its low cost and high biocidal activity. But, it is toxic as well as carcinogenic in nature. Thus, it is needed to find effective methods for destroying bacteria and fungi without being harmful to human health. Literature survey revealed that the Ag NPs with good biocidal properties, may be an outstanding alternative [87,88]. They are effective in abolishing a wide range of Gram-negative and Gram-positive bacteria. Gram-negative bacteria include the genera Acinetobacter, Escherichia, Pseudomonas, Salmonella, and Vibrio, while Gram-positive bacteria include genera such as Bacillus, Clostridium, Enterococcus, Listeria, Staphylococcus, and Streptococcus [89]. Studies proved that Ag NPs with a diameter of 22.5 nm increase the antibacterial activity of some antibiotics, such as penicillin G, amoxicillin, erythromycin, clindamycin, and vancomycin [90]. Sun et al. observed that Ag NPs are effective against many viruses and it also inhibit the replication HIV 1 virus. Ag NPs are also found effective for the inhibition of a large number of fungi Aspergillum, Candida and Saccharomyces. On the other hand, they are markedly useful against yeast isolated from infected cow udders [91]. As a disinfectant, nanosilver plays a very significant role in animal breeding where sanitation of transport chambers or the space used for the storage of animals are important factors. Ag NPs are applied for animal disinfection. In animal husbandry, strong antibacterial, fungicidal and deodorizing properties of Ag NPs are noteworthy for the disinfection and the prevention of contamination in animal breeding facilities [92]. Some workers [93] reported that a nanomaterial-supplemented diet reduces the toxic activity of aflatoxin-contaminated feeds. Sawosz et al. assessed levels of Ag NPs residues in eggshells and tissues. This study revealed that nanosilver stimulates the oxidative stress condition in chicks hatched from nanosilver disinfected eggs. Disinfection proved to be very effective in the development of embryos and makes them sensitive to even very small amounts of toxic substances [94]. The application of Ag NPs is investigated as feed additives for encouraging birds and weaned pigs' growth. In the study, it was well established that Ag NPs with size up to 100 nm showed higher antimicrobial activity than the silver salts. This is because; silver salts get deactivated by gastric acids and easily absorbed into the body through the intestinal mucosa. At the same time, Ag NPs cannot be digested through intestinal gastric juice and render less toxic effect as compared to silver salts [95]. Ag NPs as a potential dietary additive ascertained beneficial for the growth of weaned pig which might be facilitated through its antimicrobial properties by killing bacterial groups or reducing the microbial load of the small intestine of pigs [96]. In the future, there is wide scope to build up a new avenue in the industrial sector to avoid bacterial infections on silver based nanocomposites. In which, the pave the way to reduce cost, competent and low toxic nanomaterials for ease to use silver based fabrics, food packaging and clean, safe and fresh animal food products.
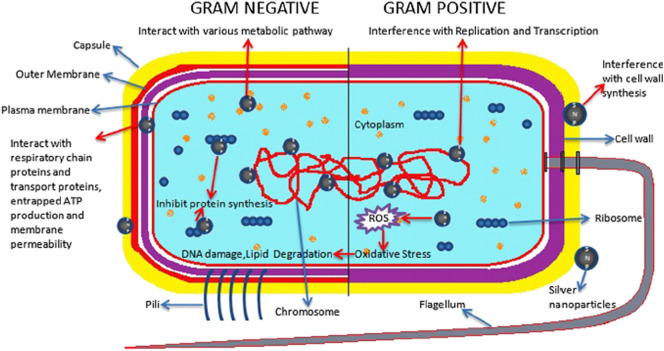
5. Antibacterial mechanism of silver nanoparticles
The antibacterial mechanisms of silver NPs are reported by the various investigators. The bacterial cell membranes contain sulphur constituting proteins and sulphur containing amino acids; inside and outside the cell membrane silver can interact with them which resulted in bacterial inactivation. In addition, silver ion released from Ag NPs interacts with phosphorus in DNA as well as with sulphur containing proteins resulted into inhibition of enzymes activities. Particle size and shape are also other parameters to determine antimicrobial activity. In the size dependent study, it can be revealed that if a size of NPs is <20 nm, it can exhibited greater attachment of sulphur containing protein of membrane resulted into maximum permeability through membrane and finally cell death of bacteria [97].
Fig. 7 shows the detail mechanism of Ag NPs. This has to be considered together with the high surface to volume ratio typically present in nanomaterials the smaller the particles, the higher the metallic surface exposed and subsequently higher micro-biocidal effect can be expected [98,99]. The shape is the other parameters of nanocrystals that are responsible for the interaction with cell wall of bacteria. Truncated triangular shaped silver nanoplates exhibited higher antibacterial activity against E. coli bacteria rather than spherical and rod-shaped NPs [100]. Recently, Ag NPs <10 nm create pores on cell wall due to these pores the cytoplasmic amount is discharged into the medium, which governs cell death without interacting the intracellular and extracellular proteins and nucleic acids of the bacteria. The interaction of Ag NPs with some cells may cause the process of programmed cell death i.e. apoptosis [101].
Fig. 7.
Antibacterial mechanism of silver nanoparticles.
In summarize, the Ag NPs as an effective disinfectant in the various commercialized products such as Acticoat™ for wound dressing, Silverline® for polyurethane ventricular catheter, SilvaSorb® as hand gels, wound dressing and cavity fillers, ON-Q SilverSoaker™ as a catheter for drug delivery [39]. In addition to that it is used in various products such as shirt, cloths and medical mask, toothpaste, hand wash, shampoo, toys, detergent as well as humidifiers. Nevertheless, the use of Ag NPs in a consumer product is safe or not is the current topic of debate.
6. Hazardous effects of Silver nanoparticles
Silver NPs have been used in various sectors due to fabulous and efficient antimicrobial nature; a lot of awareness has arisen among the researcher and policy makers because of the adverse effects of silver NPs on the environment as well as on the human health. Therefore it is needed to tackle its health hazards as well as to understand long-term associated risk which fulfils the knowledge gap of toxicity. As we aware of about silver NPs are efficient material used in different sectors as food materials, health and fitness, cleaning, electronics devices, household appliances, toys, medical devices are depicted in the Fig. 8 [102].
Fig. 8.
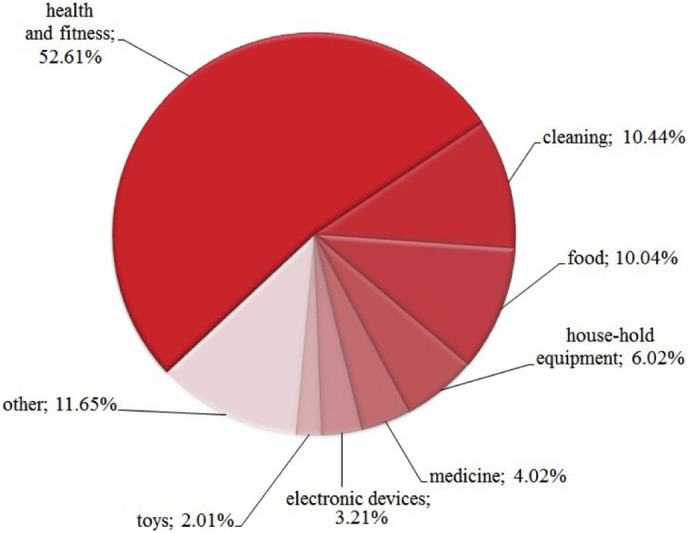
Contribution of silver nanoparticles in different sectors.
Silver NPs show signs of some vivo or virto toxicity due to their physicochemical properties. The other form of toxic effect in the environment observed due to discharge of silver NPs that are readily absorbed by the aquatic species. In addition, extensive use of silver NPs as a disinfectant may be a risk of microbial resistance that reduces its applicability. The change in bluish-gray colour of skin is reported as Argyria diseases due to the toxicity of nano‑silver. Actually, toxicity of silver is low but its consequence other than Argyria was observed at a higher concentration; the available literature data exhibits the 0.9 g is threshold limit throughout life time for the Argyria diseases [103]. Furthermore, the drinking water limit is 100 μg/L for nano‑silver constituents. Toxicity arises from the nano‑silver or dissolved silver is a lot of debate but current research reports show the toxicity arise due to a discharge of silver in the environment in the particulate type as well as in nano-size rather than dissolved silver. The sensitivity of toxicity of silver NPs is higher for the aquatic species with the concentration of 1–5 μg/L3 as compared to the human and mammals [104]. In the environment, the toxicity of silver in nanoscale is introduced in sequential forms as a release of nano‑silver from the product, emission, distribution and effect on the aquatic life. AshaRani et al. reported Ag-np has the probable reason of toxicity to human cell line as determined by cytotoxicity, genotoxicity and antiproliferative parameters [105]. A review discusses the various aspect of the transformation of silver NPs surface property as phase transformation, aggregation and sulfidation in the environment lead to toxicity to the aquatic living organism. In addition, it revealed the toxicity of silver NPs to the aquatic, terrestrial, plant, algae, fungi, vertebrate and human cells skin (keratinocytes, lung fibroblast cells, and glioblastoma cells) [106].Gliga et al. reported the detail nanotoxicology studies of silver NPs were investigated with particles agglomeration in cell medium, cellular uptake, intracellular localization and release of silver; and revealed intracellular release of silver is accountable for the toxicity to human lung cells [107]. Even though enriching knowledge about the hazardous effect of silver NPs some issues needed to be assessed and optimized the toxicity limit, dose and concentration to the aquatic living organism and human, thereafter it can be safely and efficiently used in various functions.
7. Conclusions
At present, the use of nanomaterials in a wide range of products specifically in the medical and daily life sectors have been increased. Moreover, for the safe, peaceful and paramount life there is need to avoid various infections associated with water, textile, foods and hospital environment. To avoid microbial infections, the Ag NPs have been used from last few decades. It is used in various daily life products and medical devices which give a new approach towards the microbial resistance bacteria to avoid bacterial infections. Beside the wide range of applications of silver and silver based products in various fields as antimicrobial agents, the actual impact of its toxicity on a human has to be encountered as the major risk factor. Furthermore, researcher are also seeking to understand impact of Ag NPs for long term health effect, generating bacterial resistance that are wider scope for the future study.
Acknowledgements
The authors are thankful to University Grants Commission, New Delhi for financial assistance under FDP scheme [UGC-F. No.–38-11/15 and UGC-F. No.–36-40/14 (WRO) Pune] which is gratefully acknowledged.
References
- 1.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutala W.A., Weber D.J. 2008. Guideline for Disinfection and Sterilization in Healthcare Facilities; p. 2008. [Google Scholar]
- 3.Gupta A., Silver S. Molecular genetics: silver as a biocide: will resistance become a problem? Nat. Biotechnol. 1998;16(10):888. doi: 10.1038/nbt1098-888. [DOI] [PubMed] [Google Scholar]
- 4.Pantic I. Application of silver nanoparticles in experimental physiology and clinical medicine: current status and future prospects. Rev. Adv. Mater. Sci. 2014;37 [Google Scholar]
- 5.Pandey J.K., Swarnkar R.K., Soumya K.K., Dwivedi P., Singh M.K., Sundaram S., Gopal R. Silver nanoparticles synthesized by pulsed laser ablation: as a potent antibacterial agent for human enteropathogenic gram-positive and gram-negative bacterial strains. Appl. Biochem. Biotechnol. 2014;174(3):1021–1031. doi: 10.1007/s12010-014-0934-y. [DOI] [PubMed] [Google Scholar]
- 6.Deshmukh S.P., Mullani S.B., Koli V.B., Patil S.M., Kasabe P.J., Dandge P.B., Pawar S.A., Delekar S.D. Ag nanoparticles connected to the surface of TiO2 electrostatically for antibacterial photoinactivation studies. Photochem. Photobiol. 2018 Nov;94(6):1249–1262. doi: 10.1111/php.12983. [DOI] [PubMed] [Google Scholar]
- 7.Villanueva C.M., Kogevinas M., Cordier S., Templeton M.R., Vermeulen R., Nuckols J.R., Nieuwenhuijsen M.J., Levallois P. Assessing exposure and health consequences of chemicals in drinking water: current state of knowledge and research needs. Environ. Health Perspect. 2014;122(3):213. doi: 10.1289/ehp.1206229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Supply W.U.J.W., Programme S.M. World Health Organization; 2014. Progress on Drinking Water and Sanitation: 2014 Update. [Google Scholar]
- 9.Clasen T., Roberts I., Rabie T., Schmidt W., Cairncross S. Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst. Rev. 2006;3(3) doi: 10.1002/14651858.CD004794.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Lantagne D.S., Quick R., Mintz E.D. Household water treatment and safe: storage options in developing countries. Navi. 2006;17 [Google Scholar]
- 11.W.H. Organization . World Health Organization; 2004. Guidelines for Drinking-water Quality. [Google Scholar]
- 12.Woodward R.L. Review of the bactericidal effectiveness of silver. J. Am. Water Works Assoc. 1963;55(7):881–886. [Google Scholar]
- 13.Oyanedel-Craver V.A., Smith J.A. Sustainable colloidal-silver-impregnated ceramic filter for point-of-use water treatment. Environ. Sci. Technol. 2007;42(3):927–933. doi: 10.1021/es071268u. [DOI] [PubMed] [Google Scholar]
- 14.Abebe L.S., Su Y.-H., Guerrant R.L., Swami N.S., Smith J.A. Point-of-use removal of Cryptosporidium parvum from water: independent effects of disinfection by silver nanoparticles and silver ions and by physical filtration in ceramic porous media. Environ. Sci. Technol. 2015;49(21):12958–12967. doi: 10.1021/acs.est.5b02183. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Rosenfield E., Hu M., Mi B. Direct observation of bacterial deposition on and detachment from nanocomposite membranes embedded with silver nanoparticles. Water Res. 2013;47(9):2949–2958. doi: 10.1016/j.watres.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Hu M., Zhong K., Liang Y., Ehrman S.H., Mi B. Effects of particle morphology on the antibiofouling performance of silver embedded polysulfone membranes and rate of silver leaching. Ind. Eng. Chem. Res. 2017;56(8):2240–2246. [Google Scholar]
- 17.Ben-Sasson M., Lu X., Bar-Zeev E., Zodrow K.R., Nejati S., Qi G., Giannelis E.P., Elimelech M. In situ formation of silver nanoparticles on thin-film composite reverse osmosis membranes for biofouling mitigation. Water Res. 2014;62:260–270. doi: 10.1016/j.watres.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 18.Dankovich T.A., Gray D.G. Bactericidal paper impregnated with silver nanoparticles for point-of-use water treatment. Environ. Sci. Technol. 2011;45(5):1992–1998. doi: 10.1021/es103302t. [DOI] [PubMed] [Google Scholar]
- 19.Jain P., Pradeep T. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005;90(1):59–63. doi: 10.1002/bit.20368. [DOI] [PubMed] [Google Scholar]
- 20.Glover R.D., Miller J.M., Hutchison J.E. Generation of metal nanoparticles from silver and copper objects: nanoparticle dynamics on surfaces and potential sources of nanoparticles in the environment. ACS Nano. 2011;5(11):8950–8957. doi: 10.1021/nn2031319. [DOI] [PubMed] [Google Scholar]
- 21.Alonso A., Muñoz-Berbel X., Vigués N., Rodríguez-Rodríguez R., Macanás J., Muñoz M., Mas J., Muraviev D.N. Superparamagnetic Ag@co-nanocomposites on granulated cation exchange polymeric matrices with enhanced antibacterial activity for the environmentally safe purification of water. Adv. Funct. Mater. 2013;23(19):2450–2458. [Google Scholar]
- 22.Vilela D., Stanton M.M., Parmar J., Sanchez s. Microbots decorated with silver nanoparticles kill bacteria in aqueous media. ACS Appl. Mater. Interfaces. 2017;9(27):22093–22100. doi: 10.1021/acsami.7b03006. [DOI] [PubMed] [Google Scholar]
- 23.Schleibinger H., Rüden H. Air filters from HVAC systems as possible source of volatile organic compounds (VOC)–laboratory and field assays. Atmos. Environ. 1999;33(28):4571–4577. [Google Scholar]
- 24.Yoon K.-Y., Byeon J.H., Park J.-H., Ji J.H., Bae G.N., Hwang J. Antimicrobial characteristics of silver aerosol nanoparticles against Bacillus subtilis bioaerosols. Environ. Eng. Sci. 2008;25(2):289–294. [Google Scholar]
- 25.Ko Y.-S., Joe Y.H., Seo M., Lim K., Hwang J., Woo K. Prompt and synergistic antibacterial activity of silver nanoparticle-decorated silica hybrid particles on air filtration. J. Mater. Chem. B. 2014;2(39):6714–6722. doi: 10.1039/c4tb01068j. [DOI] [PubMed] [Google Scholar]
- 26.Joe Y.H., Park D.H., Hwang J. Evaluation of Ag nanoparticle coated air filter against aerosolized virus: anti-viral efficiency with dust loading. J. Hazard. Mater. 2016;301:547–553. doi: 10.1016/j.jhazmat.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzog F., Clift M.J., Piccapietra F., Behra R., Schmid O., Petri-Fink A., Rothen-Rutishauser B. Exposure of silver-nanoparticles and silver-ions to lung cells in vitro at the air-liquid interface. Part. Fibre Toxicol. 2013;10(1):11. doi: 10.1186/1743-8977-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calderón L., Han T.T., McGilvery C.M., Yang L., Subramaniam P., Lee K.-B., Schwander S., Tetley T.D., Georgopoulos P.G., Ryan M. Release of airborne particles and Ag and Zn compounds from nanotechnology-enabled consumer sprays: implications for inhalation exposure. Atmos. Environ. 2017;155:85–96. [Google Scholar]
- 29.M Patil S., P Deshmukh S., G Dhodamani A., V More K., D Delekar S. Different strategies for modification of titanium dioxide as heterogeneous catalyst in chemical transformations. Curr. Org. Chem. 2017;21(9):821–833. [Google Scholar]
- 30.Mehta Y., Gupta A., Todi S., Myatra S., Samaddar D.P., Patil V., Bhattacharya P.K., Ramasubban S. Guidelines for prevention of hospital acquired infections. Indian J. Crit. Care Med. 2014;18(3):149–163. doi: 10.4103/0972-5229.128705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ducel G., Fabry J., Nicolle L., W.H. Organization . 2002. Prevention of Hospital-acquired Infections: A Practical Guide. [Google Scholar]
- 32.Shlaes D.M., Gerding D.N., John J.F., Craig W.A., Bornstein D.L., Duncan R.A., Eckman M.R., Farrer W.E., Greene W.H., Lorian V. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the prevention of antimicrobial resistance guidelines for the prevention of antimicrobial resistance in hospitals. Infect. Control Hosp. Epidemiol. 1997;18(4):275–291. doi: 10.1086/647610. [DOI] [PubMed] [Google Scholar]
- 33.Rai M., Deshmukh S., Ingle A., Gade A. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012;112(5):841–852. doi: 10.1111/j.1365-2672.2012.05253.x. [DOI] [PubMed] [Google Scholar]
- 34.Nadaf N., Kanase S. Aantibacterial activity of silver nanoparticles singly and in combination with third generation antibiotics against bacteria causing hospital acquired infections biosynthesized by isolated Bacillus marisflavi YCIS MN 5. Dig. J. Nanomater. Biostruct. 2015;10(4):1189–1199. [Google Scholar]
- 35.Sharifi-Rad J., Hoseini-Alfatemi S., Sharifi-Rad M., Iriti M. Antimicrobial synergic effect of Allicin and silver nanoparticles on skin infection caused by methicillin resistant Staphylococcus aureus. Ann. Med. Health Sci. Res. 2014;4(6):863–868. doi: 10.4103/2141-9248.144883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens K.N., Crespo-Biel O., van den Bosch E.E., Dias A.A., Knetsch M.L., Aldenhoff Y.B., van der Veen F.H., Maessen J.G., Stobberingh E.E., Koole L.H. The relationship between the antimicrobial effect of catheter coatings containing silver nanoparticles and the coagulation of contacting blood. Biomaterials. 2009;30(22):3682–3690. doi: 10.1016/j.biomaterials.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 37.Dunnill C.W., Page K., Aiken Z.A., Noimark S., Hyett G., Kafizas A., Pratten J., Wilson M., Parkin I.P. Nanoparticulate silver coated-titania thin films—photo-oxidative destruction of stearic acid under different light sources and antimicrobial effects under hospital lighting conditions. J. Photochem. Photobiol. 2011;220(2):113–123. [Google Scholar]
- 38.Patil S., Dhodamani A., Vanalakar S., Deshmukh S., Delekar S. Multi-applicative tetragonal TiO2/SnO2 nanocomposites for photocatalysis and gas sensing. J. Phys. Chem. Solids. 2018;115:127–136. [Google Scholar]
- 39.Chaloupka K., Malam Y., Seifalian A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28(11):580–588. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Jiranek W.A., Hanssen A.D., Greenwald A.S. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. JBJS. 2006;88(11):2487–2500. doi: 10.2106/JBJS.E.01126. [DOI] [PubMed] [Google Scholar]
- 41.Lu S., Gao W., Gu H.Y. Construction, application and biosafety of silver nanocrystalline chitosan wound dressing. Burns. 2008;34(5):623–628. doi: 10.1016/j.burns.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Ansari M., Khan H., Khan A., Cameotra S., Alzohairy M. Anti-biofilm efficacy of silver nanoparticles against MRSA and MRSE isolated from wounds in a tertiary care hospital. Indian J. Med. Microbiol. 2015;33(1):101. doi: 10.4103/0255-0857.148402. [DOI] [PubMed] [Google Scholar]
- 43.Samuel U., Guggenbichler J. Prevention of catheter-related infections: the potential of a new nano-silver impregnated catheter. Int. J. Antimicrob. Agents. 2004;23:75–78. doi: 10.1016/j.ijantimicag.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Hamid S., Zainab S., Faryal R., Ali N. Deterrence in metabolic and biofilms forming activity of Candida species by mycogenic silver nanoparticles. J. Appl. Biomed. 2017;15(4):249–255. [Google Scholar]
- 45.Lackner P., Beer R., Broessner G., Helbok R., Galiano K., Pleifer C., Pfausler B., Brenneis C., Huck C., Engelhardt K. Efficacy of silver nanoparticles-impregnated external ventricular drain catheters in patients with acute occlusive hydrocephalus. Neurocrit. Care. 2008;8(3):360–365. doi: 10.1007/s12028-008-9071-1. [DOI] [PubMed] [Google Scholar]
- 46.Edwards-Jones V. The benefits of silver in hygiene, personal care and healthcare. Lett. Appl. Microbiol. 2009;49(2):147–152. doi: 10.1111/j.1472-765X.2009.02648.x. [DOI] [PubMed] [Google Scholar]
- 47.Liang D., Lu Z., Yang H., Gao J., Chen R. Novel asymmetric wettable AgNPs/chitosan wound dressing: in vitro and in vivo evaluation. ACS Appl. Mater. Interfaces. 2016;8(6):3958–3968. doi: 10.1021/acsami.5b11160. [DOI] [PubMed] [Google Scholar]
- 48.McMahon S., Kennedy R., Duffy P., Vasquez J.M., Wall J.G., Tai H., Wang W. Poly(ethylene glycol)-based hyperbranched polymer from RAFT and its application as a silver-sulfadiazine-loaded antibacterial hydrogel in wound care. ACS Appl. Mater. Interfaces. 2016;8(40):26648–26656. doi: 10.1021/acsami.6b11371. [DOI] [PubMed] [Google Scholar]
- 49.Shankar S., Jaiswal L., Aparna R.S.L., Prasad R.G.S.V., Kumar G.P., Manohara C.M. Wound healing potential of green synthesized silver nanoparticles prepared from Lansium domesticum fruit peel extract. Mater. Express. 2015;5(2):159–164. [Google Scholar]
- 50.Bhol K., Alroy J., Schechter P. Anti-inflammatory effect of topical nanocrystalline silver cream on allergic contact dermatitis in a Guinea pig model. J. Clin. Exp. Dermatol. 2004;29(3):282–287. doi: 10.1111/j.1365-2230.2004.01515.x. [DOI] [PubMed] [Google Scholar]
- 51.Arora S., Jain J., Rajwade J., Paknikar K. Cellular responses induced by silver nanoparticles: in vitro studies. Toxicol. Lett. 2008;179(2):93–100. doi: 10.1016/j.toxlet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Atiyeh B.S., Costagliola M., Hayek S.N., Dibo S.A. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33(2):139–148. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Wilkinson L., White R., Chipman J. Silver and nanoparticles of silver in wound dressings: a review of efficacy and safety. J. Wound Care. 2011;20(11) doi: 10.12968/jowc.2011.20.11.543. [DOI] [PubMed] [Google Scholar]
- 54.Pal S., Nisi R., Stoppa M., Licciulli A. Silver-functionalized bacterial cellulose as antibacterial membrane for wound-healing applications. ACS Omega. 2017;2(7):3632–3639. doi: 10.1021/acsomega.7b00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leaper D.J. Silver dressings: their role in wound management. Int. Wound J. 2006;3(4):282–294. doi: 10.1111/j.1742-481X.2006.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sibbald R., Browne A., Coutts P., Queen D. Screening evaluation of an ionized nanocrystalline silver dressing in chronic wound care. Ostomy Wound Manage. 2001;47(10):38–43. [PubMed] [Google Scholar]
- 57.Yetisen A.K., Qu H., Manbachi A., Butt H., Dokmeci M.R., Hinestroza J.P., Skorobogatiy M., Khademhosseini A., Yun S.H. Nanotechnology in textiles. ACS Nano. 2016;10(3):3042–3068. doi: 10.1021/acsnano.5b08176. [DOI] [PubMed] [Google Scholar]
- 58.Wagener S., Dommershausen N., Jungnickel H., Laux P., Mitrano D., Nowack B., Schneider G., Luch A. Textile functionalization and its effects on the release of silver nanoparticles into artificial sweat. Environ. Sci. Technol. 2016;50(11):5927–5934. doi: 10.1021/acs.est.5b06137. [DOI] [PubMed] [Google Scholar]
- 59.Tamboli M., Kulkarni M., Deshmukh S., Kale B. Synthesis and spectroscopic characterisation of silver–polyaniline nanocomposite. Mater. Res. Innov. 2013;17(2):112–116. [Google Scholar]
- 60.Bender W., Stutte P. ACS Publications; 2001. Antimicrobials for Synthetic Fibers. [Google Scholar]
- 61.Manna J., Goswami S., Shilpa N., Sahu N., Rana R.K. Biomimetic method to assemble nanostructured Ag@ ZnO on cotton fabrics: application as self-cleaning flexible materials with visible-light photocatalysis and antibacterial activities. ACS Appl. Mater. Interfaces. 2015;7(15):8076–8082. doi: 10.1021/acsami.5b00633. [DOI] [PubMed] [Google Scholar]
- 62.Zille A., Fernandes M.M., Francesko A., Tzanov T., Fernandes M., Oliveira F.R., Almeida L., Amorim T., Carneiro N., Esteves M.F. Size and aging effects on antimicrobial efficiency of silver nanoparticles coated on polyamide fabrics activated by atmospheric DBD plasma. ACS Appl. Mater. Interfaces. 2015;7(25):13731–13744. doi: 10.1021/acsami.5b04340. [DOI] [PubMed] [Google Scholar]
- 63.Ilic V., Šaponjić Z., Vodnik V., Lazović S.A., Dimitrijevic S., Jovancic P., Nedeljkovic J.M., Radetic M. Bactericidal efficiency of silver nanoparticles deposited onto radio frequency plasma pretreated polyester fabrics. Ind. Eng. Chem. Res. 2010;49(16):7287–7293. [Google Scholar]
- 64.Pan N., Sun G. Elsevier; 2011. Functional Textiles for Improved Performance, Protection and Health. [Google Scholar]
- 65.von Goetz N., Lorenz C., Windler L., Nowack B., Heuberger M., Hungerbuhler K. Migration of Ag-and TiO2-(Nano) particles from textiles into artificial sweat under physical stress: experiments and exposure modeling. Environ. Sci. Technol. 2013;47(17):9979–9987. doi: 10.1021/es304329w. [DOI] [PubMed] [Google Scholar]
- 66.Quadros M.E., Pierson R., IV, Tulve N.S., Willis R., Rogers K., Thomas T.A., Marr L.C. Release of silver from nanotechnology-based consumer products for children. Environ. Sci. Technol. 2013;47(15):8894–8901. doi: 10.1021/es4015844. [DOI] [PubMed] [Google Scholar]
- 67.Reed R.B., Zaikova T., Barber A., Simonich M., Lankone R., Marco M., Hristovski K., Herckes P., Passantino L., Fairbrother D.H. Potential environmental impacts and antimicrobial efficacy of silver-and nanosilver-containing textiles. Environ. Sci. Technol. 2016;50(7):4018–4026. doi: 10.1021/acs.est.5b06043. [DOI] [PubMed] [Google Scholar]
- 68.Feng Q., Wu J., Chen G., Cui F., Kim T., Kim J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000;52(4):662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 69.Appendini P., Hotchkiss J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002;3(2):113–126. [Google Scholar]
- 70.Cushen M., Kerry J., Morris M., Cruz-Romero M., Cummins E. Nanotechnologies in the food industry–recent developments, risks and regulation. Trends Food Sci. Technol. 2012;24(1):30–46. [Google Scholar]
- 71.Committee E.S. Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA J. 2011;9(5) doi: 10.2903/j.efsa.2018.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duncan T.V. Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011;363(1):1–24. doi: 10.1016/j.jcis.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vasile C., Râpă M., Ştefan M., Stan M., Macavei S., Darie-Niţă R., Barbu-Tudoran L., Vodnar D., Popa E., Ştefan R. New PLA/ZnO: Cu/Ag bionanocomposites for food packaging. Express Polym Lett. 2017;11(7) [Google Scholar]
- 74.Ahvenainen R. Elsevier; 2003. Novel Food Packaging Techniques. [Google Scholar]
- 75.Singh M., Sahareen T. Investigation of cellulosic packets impregnated with silver nanoparticles for enhancing shelf-life of vegetables. LWT Food Sci. Technol. 2017;86:116–122. [Google Scholar]
- 76.Mihaly Cozmuta A., Peter A., Mihaly Cozmuta L., Nicula C., Crisan L., Baia L., Turila A. Active packaging system based on Ag/TiO2 nanocomposite used for extending the shelf life of bread. Chemical and microbiological investigations. Packag. Technol. Sci. 2015;28(4):271–284. [Google Scholar]
- 77.Peter A., Mihaly-Cozmuta L., Mihaly-Cozmuta A., Nicula C., Indrea E., Barbu-Tudoran L. Testing the preservation activity of Ag-TiO2-Fe and TiO2 composites included in the polyethylene during orange juice storage. J. Food Process Eng. 2014;37(6):596–608. [Google Scholar]
- 78.Mitrano D.M., Barber A., Bednar A., Westerhoff P., Higgins C.P., Ranville J.F. Silver nanoparticle characterization using single particle ICP-MS (SP-ICP-MS) and asymmetrical flow field flow fractionation ICP-MS (AF4-ICP-MS) J. Anal. At. Spectrom. 2012;27(7):1131–1142. [Google Scholar]
- 79.Ramos K., Gómez-Gómez M., Cámara C., Ramos L. Silver speciation and characterization of nanoparticles released from plastic food containers by single particle ICPMS. Talanta. 2016;151:83–90. doi: 10.1016/j.talanta.2015.12.071. [DOI] [PubMed] [Google Scholar]
- 80.Arfat Y.A., Ejaz M., Jacob H., Ahmed J. Deciphering the potential of guar gum/Ag-Cu nanocomposite films as an active food packaging material. Carbohydr. Polym. 2017;157:65–71. doi: 10.1016/j.carbpol.2016.09.069. [DOI] [PubMed] [Google Scholar]
- 81.Echegoyen Y., Nerín C. Nanoparticle release from nano-silver antimicrobial food containers. Food Chem. Toxicol. 2013;62:16–22. doi: 10.1016/j.fct.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 82.Azlin-Hasim S., Cruz-Romero M.C., Morris M.A., Padmanabhan S.C., Cummins E., Kerry J.P. The potential application of antimicrobial silver polyvinyl chloride nanocomposite films to extend the shelf-life of chicken breast fillets. Food Bioprocess Technol. 2016;9(10):1661–1673. [Google Scholar]
- 83.Gutiérrez D., Rodríguez-Rubio L., Martínez B., Rodríguez A., García P. Bacteriophages as weapons against bacterial biofilms in the food industry. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nia J.R. Google Patents; 2007. Using of Nanosilver in Poultry, Livestock and Aquatics Industry. [Google Scholar]
- 85.Tymczyna L., Chmielowiec-Korzeniowska A., Drabik A. The effectiveness of various biofiltration substrates in removing bacteria, endotoxins, and dust from ventilation system exhaust from a chicken hatchery. Poult. Sci. 2007;86(10):2095–2100. doi: 10.1093/ps/86.10.2095. [DOI] [PubMed] [Google Scholar]
- 86.Hegarty R., Goopy J., Herd R., McCorkell B. Cattle selected for lower residual feed intake have reduced daily methane production. J. Anim. Sci. 2007;85(6):1479–1486. doi: 10.2527/jas.2006-236. [DOI] [PubMed] [Google Scholar]
- 87.Konopka M., Kowalski Z., Wzorek Z. Disinfection of meat industry equipment and production rooms with the use of liquids containing silver nano-particles. Arch. Environ. Prot. 2009;35(1):107–115. [Google Scholar]
- 88.Metak A., Ajaal T. Investigation on polymer based nano-silver as food packaging materials. International J. Bio. Food.Veter. Agri. Eng. 2013;7(12):772–778. [Google Scholar]
- 89.Banach M., Tymczyna L., Chmielowiec-Korzeniowska A., Pulit-Prociak J. Nanosilver biocidal properties and their application in disinfection of hatchers in poultry processing plants. Bioinorg. Chem. Appl. 2016;2016 doi: 10.1155/2016/5214783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shahverdi A.R., Fakhimi A., Shahverdi H.R., Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine. 2007;3(2):168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 91.Sun R.W.-Y., Chen R., Chung N.P.-Y., Ho C.-M., Lin C.-L.S., Che C.-M. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem. Commun. (Camb.) 2005;40:5059–5061. doi: 10.1039/b510984a. [DOI] [PubMed] [Google Scholar]
- 92.Chmielowiec-Korzeniowska A., Tymczyna L., Dobrowolska M., Banach M., Nowakowicz-Dębek B., Bryl M., Drabik A., Tymczyna-Sobotka M., Kolejko M. Silver (Ag) in tissues and eggshells, biochemical parameters and oxidative stress in chickens. Open Chem. 2015;13(1) [Google Scholar]
- 93.Gholami-Ahangaran M., Zia-Jahromi N. Nanosilver effects on growth parameters in experimental aflatoxicosis in broiler chickens. Toxicol. Ind. Health. 2013;29(2):121–125. doi: 10.1177/0748233711425078. [DOI] [PubMed] [Google Scholar]
- 94.Sawosz F., Pineda L.M., Hotowy A.M., Hyttel P., Sawosz E., Szmidt M., Niemiec T., Chwalibog A. Nano-nutrition of chicken embryos. The effect of silver nanoparticles and glutamine on molecular responses, and the morphology of pectoral muscle: the effect of silver nanoparticles and glutamine on molecular responses, and the morphology of pectoral muscle, Baltic. J. Comp. Clin. Bio. 2012;2:29–45. [Google Scholar]
- 95.Fondevila M. Silver Nanoparticles. InTech; 2010. Potential use of silver nanoparticles as an additive in animal feeding. [Google Scholar]
- 96.Fondevila M., Herrer R., Casallas M., Abecia L., Ducha J. Silver nanoparticles as a potential antimicrobial additive for weaned pigs. Anim. Feed Sci. Technol. 2009;150(3):259–269. [Google Scholar]
- 97.Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramírez J.T., Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 98.Wigginton N.S., De Titta A., Piccapietra F., Dobias J., Nesatyy V., Suter M.J., Bernier-Latmani R. Binding of silver nanoparticles to bacterial proteins depends on surface modifications and inhibits enzymatic activity. Environ. Sci. Technol. 2010;44:2163–2168. doi: 10.1021/es903187s. [DOI] [PubMed] [Google Scholar]
- 99.Khanna P., Singh N., Kulkarni D., Deshmukh S., Charan S., Adhyapak P. Water based simple synthesis of re-dispersible silver nano-particles. Mater. Lett. 2007;61(16):3366–3370. [Google Scholar]
- 100.Pal S., Tak Y.K., Song J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007;73(6):1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 102.Pulit-Prociak J., Banach M. Silver nanoparticles – a material of the future…? Open Chem. 2016:76. [Google Scholar]
- 103.Height M.J. 2011. Nanosilver in Perspective, Presentation “Health Risk Assessment of Nanosilver” Workshop. [Google Scholar]
- 104.Nowack B., Krug H.F., Height M. 120 years of nanosilver history: implications for policy makers. ACS Publications. 2011;45(4):1177–1183. doi: 10.1021/es103316q. [DOI] [PubMed] [Google Scholar]
- 105.AshaRani P., Low Kah G., Mun M.P., Hande S. Valiyaveettil. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2008;3(2):279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 106.Levard C., Hotze E.M., Lowry G.V., Brown G.E. Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ. Sci. Technol. 2012;46(13):6900–6914. doi: 10.1021/es2037405. [DOI] [PubMed] [Google Scholar]
- 107.Gliga A.R., Skoglund S., Wallinder I.O., Fadeel B., Karlsson H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014;11(1):11. doi: 10.1186/1743-8977-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]