Abstract
Chronic lung diseases are prevalent worldwide and cause significant mortality and suffering. This article discusses infections that occur in three chronic lung diseases: chronic obstructive pulmonary disease, bronchiectasis, and cystic fibrosis. Rather than discussing the role of infections as etiology of these diseases, this article focuses on infections that occur in the background of established chronic lung disease.
Chronic lung diseases are prevalent worldwide and cause significant mortality and suffering. This article discusses infections that occur in three chronic lung diseases: (1) chronic obstructive pulmonary disease (COPD), (2) bronchiectasis, and (3) cystic fibrosis (CF). Rather than discussing the role of infections as causes of these diseases, this article focuses on infections that occur in the background of established chronic lung disease.
Chronic obstructive pulmonary disease
COPD is a major health problem worldwide. Nearly 7% of the adult United States population has COPD and it is currently the fourth leading cause of death in United States [1]. COPD is projected to become the third leading cause of death worldwide by 2020 [2]. In patients with established COPD, infections of the lower respiratory tract are frequent. These present as an exacerbation of the chronic symptoms of COPD (acute exacerbation of COPD) or as pneumonia. Either of these can lead to complications including respiratory failure requiring mechanical ventilation in intensive care units.
Several mechanisms account for the increased frequency and severity of lower airway infections in COPD, including anatomic abnormalities, disordered mucus secretion, decreased ciliary clearance, and host defense defects [3]. Mucus hypersecretion by airway cells, long thought to be of no consequence, has now been shown to be associated with worsening lung function, increased frequency of acute exacerbations, and hospitalizations [4], [5]. Alveolar macrophages from patients with COPD show decreased response to nontypeable Haemophilus influenzae antigens and decreased ability to phagocytose these bacteria [6], [7]. These problems cause increased susceptibility to damage by environmental insults and microbial infections, causing an increase in baseline lower airway inflammation [8].
Acute exacerbation of chronic obstructive pulmonary disease
Patients with COPD show evidence of increased inflammation in their airway even at the baseline state. Sputum levels of interleukin (IL)-6, IL-8, neutrophil elastase, and myeloperoxidase are increased in patients with COPD compared with those without COPD [9], [10]. Chronic symptoms of cough, increased sputum production, or dyspnea are present at baseline; episodes of worsening of these symptoms occur periodically, an exacerbation. An increase in airway inflammation is observed during exacerbations [11], [12], [13]. C-reactive protein levels in blood, a marker of systemic inflammation, rise during an exacerbation and decrease more slowly in patients who do not improve [14]. Levels of sputum tumor necrosis factor-α and IL-8 rise during an exacerbation, then decline during convalescence [11]. These observations suggest that the symptoms of an acute exacerbation are caused by increased airway inflammation.
The clinical diagnosis of acute exacerbation of COPD is distinguished from pneumonia by the absence of lung infiltrate in chest radiograph in the former. Longitudinal studies have explored the incidence of acute exacerbations in patients with COPD. In general, the number of exacerbations per year increases with severity of COPD. In a 2-year study of 101 patients with moderate COPD, the median exacerbation rate was 2.4 per patient per year [15]. Exacerbations requiring hospitalization have been associated with increased mortality that persists up to 2 years after discharge from hospital [16]. A hallmark of COPD is heterogeneity in clinical course among patients.
Several etiologies account for acute exacerbations of COPD and the increase in airway inflammation. The relative proportion of these causes has been difficult to determine. Part of the difficulty stems from inconsistency in definition of acute exacerbation in various studies. There is concern that the spectrum of acute exacerbations, defined by a worsening in one or more symptoms, may represent different diseases. For example, patients with purulent sputum are more likely to have a bacterial infection as the cause of acute exacerbation than those with mucoid sputum [17]. At present, the most used definition is the one proposed by Anthonisen and colleagues [18] in their study of antibiotics in acute exacerbation of COPD. In this study of antibiotic efficacy, an exacerbation was defined as increase in dyspnea, sputum volume, and purulence (type 1 or severe); any two of these symptoms (type 2 or moderate); or any one of these symptoms with fever and symptoms of upper respiratory tract infection (type 3 or mild). Using this definition, there are various probable causes for worsening in lower airway inflammation causing acute exacerbation ( Box 1).
Box 1. Causes of acute exacerbation of COPD.
Bacterial infection
Viral infection
Coinfection (bacteria and viruses)
Environmental causes
Other diseases (heart failure, pneumothorax, pulmonary emboli)
Unknown
Another reason for the difficulty in precise definition of the proportional contribution from the various etiologies is the focus on one etiology over others in various studies. There are several studies focusing on the role of bacteria or viruses in acute exacerbations, but few have studied both in the same set of patients. In one group of 64 hospitalized patients with acute exacerbations, sputa were analyzed for bacteria by culture and for viruses by polymerase chain reaction (PCR). An infectious cause was established in 78.1% (29.7% bacterial, 23.4% viral, and 25% viral-bacterial coinfection) [13]. Various authors have estimated that 50% to 80% of exacerbations are caused by infections from bacteria and viruses, and about 10% by environmental causes [19], [20], [21]. Up to 30% of exacerbations have no identifiable etiology (see Box 1) [16].
Bacterial infections
Many patients with COPD have potentially pathogenic bacteria in their lower airway even at their baseline state. Several studies, using bronchoscopy, bronchoalveolar lavage (BAL), and protected specimen brushes to avoid contamination from upper airway flora, have recovered such bacteria in about 40% of these patients at their baseline. The literature is divided on the role of bacteria in the pathogenesis of COPD, but there is little doubt that bacteria cause at least some of the acute exacerbations of COPD. Box 2 lists the lines of evidence that show bacteria play a significant role in causing acute exacerbation.
Box 2. Evidence for role of bacteria in acute exacerbation.
-
1.
Increased presence of pathogenic bacteria in lower airway, using protected specimen brush, in acute exacerbations.
-
2.
Increased indices of airway inflammation correlating with positive cultures
-
3.
Immune responses to bacteria in exacerbations
-
4.
Acquisition of new bacterial strain associated with acute exacerbations
-
5.
Placebo-controlled trials of antibiotic therapy in acute exacerbations
Increase in pathogenic bacteria during exacerbation
The percentage of patients with pathogenic bacteria in lower airway increases from the baseline during an exacerbation [13], [22]. A pooled analysis of studies using protected specimen brush sampling showed that pathogenic bacteria were present in lower airway of 29% stable COPD patients and 54% of patients with acute exacerbation [23]. As the microbial load in lower airway increased, the rate of exacerbations rose.
Correlation of increased airway inflammation with presence of pathogenic bacteria
In patients with exacerbation, sputum markers of inflammation are higher in those with bacteria in the sputum than those without bacteria [24]. Furthermore, as the number of pathogen colony-forming units increases, sputum markers of inflammation increase [25]. These observations suggest that bacteria contribute to the increased airway inflammation in exacerbations of COPD.
Antibody response to bacteria
Patients with acute exacerbation develop antibodies to infecting strains of H influenzae and Moraxella catarrhalis, showing triggering of the humoral immune system by the bacteria [26], [27].
Molecular epidemiology
Part of the confusion regarding the role of bacteria as etiologic agents in acute exacerbation of COPD is because of isolation of the same species of bacteria in stable and exacerbation states. Factors related to differences in bacterial pathogenicity might explain this paradox. Most of the older studies of exacerbation did not pay attention to differences in strains of bacteria. In a longitudinal study of patients with COPD, acquisition of new strains of H influenzae, M catarrhalis, or Streptococcus pneumoniae was associated with a significantly increased risk of an exacerbation [28]. One might speculate that the older strains were kept in check by neutralizing antibodies and the newer strain is not subject to such limitation for a period.
Benefits of antibiotics in acute exacerbation
Two recent systematic reviews have shown that antibiotics significantly reduced mortality and treatment failure in severe acute exacerbations of COPD [29] and in exacerbations with purulent sputum [30]. Antibiotics seem less beneficial in mild and moderate exacerbations and when sputum purulence is absent. Prior studies have shown that the presence of purulent sputum during an exacerbation, as reported by the patient, correlated with a higher chance of recovering pathogenic bacteria from sputum [17], [31]. In the study by Anthonisen and coworkers [18], the benefits of antibiotic therapy in acute exacerbation were mostly in the type 1, the group with all three symptoms (increased dyspnea, sputum volume, and purulence). This emphasizes that acute exacerbation of COPD, a syndrome defined by symptoms, has subsets that are more likely caused by bacterial infection than other causes.
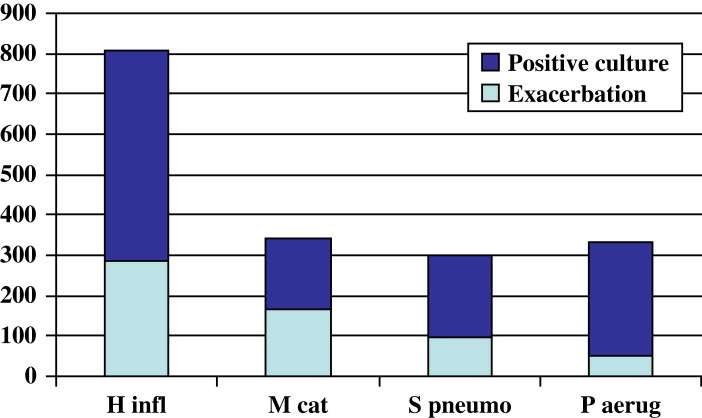
Haemophilus influenzae is the commonest bacterial pathogen in COPD. Fig. 1 shows the bacterial isolates from the COPD study clinic at VA Western New York Healthcare System (Buffalo, New York) over a 12-year period (Timothy F. Murphy, MD, unpublished data).
Fig. 1.
Bacterial infection in chronic obstructive pulmonary disease. Exacerbation defined by acquisition of a new strain associated with clinical symptoms of an exacerbation. H infl, H influenzae; M cat, M catarrhalis; P aerug, P aeruginosa, S pneumo, S pneumoniae.
Viruses
Respiratory viral infections can cause acute exacerbations and hospitalizations in COPD [32]. Rhinovirus [33], influenza virus, parainfluenza virus, coronavirus, adenovirus, respiratory syncytial virus [19], [33], and human metapneumovirus [34] have been associated with acute exacerbations in COPD. In general, 20% to 40% of acute exacerbation episodes are probably caused by viral respiratory infections [19], [21]. PCR techniques detect viruses in a higher proportion of respiratory secretions than culture or serology. A study of patients with acute exacerbations, using PCR, viral culture of nasal secretions, and serology for respiratory viruses, detected evidence of viral infection in 39.2% of acute exacerbation episodes. Rhinovirus accounted for 58.2% [33]. In another study, the same group of authors reported detecting, by PCR, rhinovirus in induced sputum in 10 (23.2%) of 43 exacerbation episodes [35]. In four of these instances, they could not find rhinovirus in nasal secretions. Another study of patients hospitalized with severe exacerbations found evidence of viral respiratory infection in 56% [36]. Human metapneumovirus has been detected, using PCR, in respiratory secretions of patients with acute exacerbation [34], [37]. These reports establish association, however, not causation. Respiratory viruses have been detected in 16% of patients with stable COPD [33]. Detection of virus by PCR need not mean infection in all cases, and further studies exploring a causal link between viruses and acute exacerbations are needed [38].
How viruses cause acute exacerbations is not yet clearly understood. Rhinovirus, an upper airway pathogen, also is capable of replication in lower airway epithelium [39]. Respiratory viruses, especially influenza virus, cause lower airway epithelial cell damage. In addition to the direct cytotoxicity, release of cytokines and chemokines leads to an inflammatory response, release of proteolytic enzymes, and further tissue damage [40]. Secondary bacterial infections following viral respiratory infections can worsen the inflammation. Influenza infections increase the odds of infection with S pneumoniae and H influenzae [41].
Atypical bacteria
Serologic evidence of Chlamydophila (Chlamydia) pneumoniae infection, without isolation of the organism, has been reported in 4% to 8% of patients with acute exacerbations [42], [43]. Similarly, serologic evidence of acute Mycoplasma infection, but without isolation of the microbe, has been reported in a small percentage of patients with acute exacerbation [44].
Environmental causes
Ozone and particulate air pollutants of diameter less than 10 μM have been shown, in epidemiologic studies, to be associated with acute exacerbations [45]. In Linden, Utah, the number of hospital admissions for exacerbations of bronchitis fell when the local steel mill closed, only to go back to prior levels when the mill opened again. The number of atmospheric particles less than 10 μM paralleled this trend [46]. Atmospheric black smoke and sulfur dioxide have been associated with increase in emergency room visits for acute exacerbation of COPD [20]. Atmospheric pollutants and tobacco smoke cause loss of ciliated cells, decreased mucociliary clearance, mucosal gland hypertrophy, and increased mucus production [47]. Mucus contains glycoproteins with carbohydrate side chains to which bacteria attach [48].
Other causes
Noncompliance with medications and incorrect use of inhalers can induce an exacerbation. Conditions like heart failure, pulmonary embolism, and pneumothorax can mimic symptoms of an acute exacerbation and have to be considered in the clinical analysis. Finally, up to 30% of acute exacerbations have no identifiable cause [16].
Pneumonia
COPD patients with pneumonia present with symptoms similar to acute exacerbation, but have lung infiltrates on chest radiographs. COPD raises the risk for community-acquired pneumonia [49]. Patients with COPD and community-acquired pneumonia have higher mortality than patients without COPD [50] and patients with nonpneumonic acute exacerbation of COPD [51].
Among patients who have influenza A respiratory illness, COPD is a risk factor for developing influenza-associated pneumonia [52]. These patients have pulmonary infiltrates and test positive for influenza A in their respiratory secretions. They often have superimposed bacterial pneumonia, caused most commonly by Staphylococcus aureus and S pneumoniae. Mortality is high, around 30%.
Opportunistic infections
Patients with COPD are often treated with oral glucocorticoids for acute exacerbations and often with inhaled corticosteroids. Stuck and colleagues [53], in a meta-analysis, found increased rates of infections in patients receiving more than 10 mg prednisone per day and in those who had received more than 700 mg prednisone cumulative dose.
Invasive aspergillosis has been most well described in patients with prolonged neutropenia and those receiving high-dose steroids, usually in the setting of organ transplants and hematologic malignancy. There have been several reports of invasive aspergillosis in patients without these problems, especially in COPD patients on long-term steroid therapy. The diagnosis is often not made until after death and the mortality is high [54], [55], [56], [57]. The possibility of Aspergillus pneumonia should be considered in COPD patients who have pulmonary infiltrates and are not responding to standard therapy [58].
An acute exacerbation in COPD is defined by patient-reported symptoms that increase from a baseline. There are many causes for acute exacerbation of COPD. Infections are involved in more than half of these, with viruses causing a third and the rest by bacteria. Patients with pneumonia have similar symptoms, with an infiltrate on chest radiograph. In patients who have been on steroids, opportunistic infections are possible, especially if the patient does not respond to standard therapy.
Bronchiectasis
Bronchiectasis is defined as permanent abnormal dilatation of bronchi. These patients often have chronic, baseline respiratory symptoms of cough, increased sputum production, and shortness of breath. These symptoms are more intense during an exacerbation. Lower respiratory tract infections (bronchitis, pneumonia, and its complications) are more frequent in patients with bronchiectasis compared with healthy volunteers [59], [60], [61]. Infections play a prominent role in these exacerbations and probably in the baseline symptoms.
The exact prevalence of bronchiectasis is unknown. In New Zealand, a survey of pediatricians produced an estimated prevalence of 1 in 3000 children for non-CF bronchiectasis [62]. A report from the United Kingdom found a prevalence of 1 in 5800 children [63]. The description of the disease is similar in different countries. These patients have decreased survival as compared with healthy subjects. Keistinen and colleagues [64] followed a cohort of patients with bronchiectasis in Finland over a 7-year period. Patients with bronchiectasis had higher mortality and lower quality of life than those with asthma, although they fared better than those with COPD.
Etiology
Numerous factors have been identified as the cause of bronchiectasis. These include postinfectious (childhood viral respiratory infections, bacterial pneumonia, tuberculosis); inherited diseases, such as ciliary dyskinesia; autoimmune disorders, such as rheumatoid arthritis; inflammatory bowel disease; and immune deficiencies [61]. A sizable number of patients, however, have idiopathic bronchiectasis with no clearly identifiable cause [65], [66]. In adults who present with bronchiectasis without an obvious cause, genetic testing has identified CF in 3% to 20% of these patients [65], [67].
Pathophysiology
The obvious reason for chronic infections and exacerbations in patients with bronchiectasis is the anatomic abnormality. The bronchial walls are damaged and dilated, with possible strictures in the varicose variety of bronchiectasis. This reduces the patient's ability to clear respiratory secretions from the segments. Clearance of bacteria by the ciliary mechanism is also affected. Recurrent infections lead to further damage to the bronchi, setting up a vicious cycle of suppuration and tissue damage [68]. Recent investigations have focused on defects in host defense against bacteria. Patients with bronchiectasis, in comparison with normal persons, have more of a Th2 immune response to nontypeable H influenzae infection [69]. It is not yet clear if this represents increased susceptibility and inability to clear bacteria in a certain group of patients who are more prone to develop bronchiectasis in response to infections, or a secondary effect of chronic infection.
Baseline symptoms
The baseline symptoms suggest chronic inflammation in the airways that worsens during an exacerbation. Sputum studies show an increase in neutrophils, markers of inflammation, and cytokines [70] including IL-8 and IL-6 [71]. Moreover, those who have pathogenic bacteria in their lower airway cultures have more sputum neutrophils, elastase, myeloperoxidase, tumor necrosis factor-α, and IL-8. Systemic markers of inflammation including total blood white count, neutrophil count, erythrocyte sedimentation rate, and C-reactive protein are increased in patients with bronchiectasis at their baseline, compared with controls [72].
Symptoms during an exacerbation respond well to appropriate antibiotics [61], [73]. These reports support the role of bacteria causing the exacerbation of symptoms. The role of microbes in the baseline state symptoms is less clear. Evidence supporting a role comes from studies that show an increased bacterial load in the lower airways of these patients. In studies using techniques that have avoided contamination by upper airway flora (using bronchoscopy, protected specimen brush, and BAL), more than 50% of patients with bronchiectasis have potentially pathogenic bacteria in their lower airway [71], [74]. The presence of pathogenic bacteria in the lower airways of these patients has often been termed “colonization.” Improvement in baseline symptoms after long-term antibiotics [73] suggests, however, that these bacteria may be causing inflammation. It is very likely that these patients have chronic lower airways inflammation, caused at least in part by pathogenic bacteria.
Exacerbation
If patients with bronchiectasis have chronic lower airway bacterial infection, what causes exacerbations? In addition to noninfective causes (eg, tobacco smoke), microbes play a role. The spectrum of pathogenic bacteria isolated in patients at baseline and during exacerbations is remarkably similar. H influenzae, Pseudomonas aeruginosa, S pneumoniae, and M catarrhalis are the more common bacteria in most studies. H influenzae has been the commonest pathogen isolated, in nearly 40% of patients [71], [75], [76]. S pneumoniae and P aeruginosa are the next most frequent.
In lesser proportions, Enterobacteriaceae, Nocardia, and S aureus are isolated. Recently nontuberculous mycobacteria (NTMB) (see later) have been recognized more frequently. What changes in the baseline microbial situation to cause an exacerbation is unclear. It has been proposed that going beyond a certain threshold in the lower airways bacterial load causes the increase in inflammation and an exacerbation [25]. Recent studies in COPD, however, point to acquisition of new strains as a cause. The acquisition and clearance of different strains of M catarrhalis in bronchiectasis has been shown to be a dynamic process [77], with intermittent acquisition and then clearing of new strains. In COPD, acquisition of a new strain of pathogenic bacteria has been associated with a significantly increased risk of an exacerbation [28]. A similar mechanism is very possible in bronchiectasis, but this remains to be established.
The isolation of P aeruginosa correlates with more severe clinical disease in bronchiectasis and occurs later in the course of disease [78], [79]. This could be the cause of the disease progression, or more likely the effect. At least one report [80] notes that the rate of decline in lung function was no worse in patients infected with P aeruginosa. This suggests that as the bronchiectasis becomes more severe, with increasing bronchial wall damage, P aeruginosa gains a foothold and serves as a marker for severe disease. S aureus is an uncommon isolate in bronchiectasis that is not caused by CF. One report found that in patients with bronchiectasis who had persistent S aureus isolation in sputum, there was a high incidence of abnormal sweat chloride test. The authors advise that persistent S aureus isolation in bronchiectasis should prompt testing for CF [81].
NTMB have been recognized as causing chronic lung disease, including bronchiectasis [82]. Although it is clear that Mycobacterium avium complex causes nodular infiltrates and bronchiectasis in elderly white women, it is not clear if NTMB infections occur more frequently in established bronchiectasis. Fowler and colleagues [83] isolated NTMB in sputum cultures of 10% of patients with bronchiectasis; however, many of these patients were later diagnosed to have CF. Others have found NTMB to be uncommon in non-CF bronchiectasis [84].
Cystic fibrosis
CF is the most common lethal inherited disorder in the white population [85]. It is inherited as an autosomal-recessive disorder. Homozygotes have clinical disease, heterozygotes are carriers. The gene involved codes for CF transmembrane conductance regulator (CFTR), a c-AMP regulated protein that functions as a chloride channel in the apical membrane of epithelial cells [86], [87], [88], [89]. Epithelial cells in the airways, submucosal glands of the airways, sweat glands, nasal epithelium, salivary glands, biliary tree, pancreas, vas deferens, and intestines express this gene. The classic triad of clinical features (increased sweat chloride, pancreatic insufficiency, and chronic pulmonary disease) is caused by mutations in the gene coding for CFTR [90]. The median survival age has gradually increased to about 30 years. At present, most of the morbidity and mortality from CF are caused by respiratory illness [89]. Patients with CF develop pulmonary infections caused by a limited group of bacteria from a very early age. Within a short time, these infections become chronic and almost impossible to eradicate. Repeated, chronic infection leads to problems with growth, quality of life, and ultimately to respiratory failure and death.
Pathophysiology
Except for mild increase in acinar diameter of submucosal glands in airways, the lungs are normal in appearance at birth in patients with CF [91]. The glands produce mucus with increased viscosity. There is decreased mucociliary clearance of particulate matter and bacteria. Soon after birth, pulmonary infections start. There is intense neutrophilic inflammation in the airways, which continues through life. Mucopurulent exudate accumulates in airways, causing plugging of the smaller airways. There is destruction of bronchial wall, including cartilage. Bronchiectasis, pneumonia, and increasing respiratory difficulty occur [85].
There are two primary issues involved in the pathophysiology of lung disease in CF. There is increased susceptibility to infection in the lung (but not in other organs) and excessive neutrophilic inflammation in CF (this is seen in other organs like pancreas and intestines) [90]. The combination of these two factors leads to suppurative pulmonary infections. Increased susceptibility to infections is probably caused by a combination of host and pathogen factors.
Increased susceptibility to infection
Host factors
The lungs in CF present an unusually fertile environment for growth of some bacteria. P aeruginosa, the most serious pathogen in CF, flourishes in CF lungs, but rarely causes infections elsewhere [92]. Mucociliary clearance of particulate matter is abnormal in CF because of a reduction in the volume of periciliary fluid [93], [94], [95]. Moreover, the mucus in CF is more viscid and of a greater depth, causing increased oxygen gradient across this layer [96].
Increased adherence of bacteria, especially P aeruginosa, to airway epithelial cells occurs in CF. CFTR has been proposed to be a receptor for P aeruginosa, causing internalization of the bacteria followed by apoptosis of the cell. In CF, a defective CFTR is not able to function in a similar manner [97]. An abnormal glycolipid, asialoGM1, functions as a receptor for P aeruginosa pilin and is increased in CF airway cells [98]. Mechanical factors (bronchiectasis, viscid pools of mucus and pus) certainly contribute to increased susceptibility to airway infection in CF. Although the function of antimicrobial peptides has not been shown to be defective in CF, there are reports of reduced nitric oxide production by CF airway epithelial cells [99], which may impair killing of bacteria.
Pathogen factors
The unusual host environment also induces phenotypic changes in P aeruginosa that favor chronic infection. Although infecting P aeruginosa strains resemble environmental strains in the early years of the disease, they change to mucoid strains later. These strains produce an exopolysaccharide, alginate, which causes the colonies to look mucoid. The onset of this change in phenotype is associated with further worsening of lung function [100]. The trigger for this change is not clearly known. It seems reasonable to assume that it is a response to some host environmental conditions and various triggers, such as neutrophil products, surfactants, and suboptimal doses of antibiotics, have been proposed [101].
Pseudomonas aeruginosa also changes its lipopolysaccharide side chain structure, which could make it more resistant to antimicrobial peptides [102]. Recent evidence suggests that P aeruginosa produces biofilms. Biofilms are sessile communities of bacteria attached to a surface and living in an extracellular matrix of their own synthesis [103]. Indirect evidence for biofilm formation in CF comes from observation of discrete bacterial colonies in CF sputum, and detection of quorum-sensing signals in these bacterial communities [104]. Bacteria in biofilms grow more slowly and are more resistant to antibiotics [105].
Increased inflammation
In addition to the increased susceptibility, patients with CF have an abnormally intense and prolonged inflammatory response to infections. CF patients have increased numbers of neutrophils and levels of IL-8 in BAL fluid, as compared with non-CF patients, irrespective of the species of pathogen [106]. Neutrophils and IL-8 are increased in BAL even when cultures are negative [107]. Infections are associated with an increase in inflammation markers in sputum [108]. The production of IL-10, an anti-inflammatory cytokine, is reduced in bronchial epithelial cells of patients with CF as compared with normal controls [109]. This evidence points to a disordered inflammatory response to infection in CF airways. The products of excessive inflammation, which include neutrophil elastase and DNA fragments from apoptotic neutrophils, contribute to anatomic damage and airway plugging [89], [90].
Exacerbations
The course of CF pulmonary disease is punctuated by recurrent exacerbations, which cause increase in symptoms and worsening of lung function [110]. The triggers for these episodes are not well known. Possible causes are viral respiratory infections [111], clonal expansion of P aeruginosa population [112], and dispersal within airways.
Pulmonary clinical features in cystic fibrosis
Most newborns with CF do not have respiratory symptoms or signs. Within the first year, however, most develop cough, wheezing, and tachypnea. The severity of these symptoms varies among patients. These symptoms are worsened by common childhood viral respiratory infections [113]. Infants with mild disease are often not diagnosed until older. As the child becomes older, cough becomes persistent, and if the child is able to expectorate, increasing sputum volume and purulence is noted. Shortness of breath leads to decreased quality of life and deficiencies in growth may be apparent. Once bronchiectasis sets in, hemoptysis, which can be mild to severe, can occur. Cough, wheezing, and purulent sputum become a daily occurrence. Bronchial cysts develop, and occasionally rupture to cause pneumothorax. Exacerbations of the baseline symptoms often necessitate hospitalization [85], [89]. Eventually, respiratory failure is the cause of death in 80% of these patients.
There are less severe phenotypes of CF, however, with patients surviving well into adulthood (adult CF) and with lesser frequency of P aeruginosa infection [114].
Microbiology
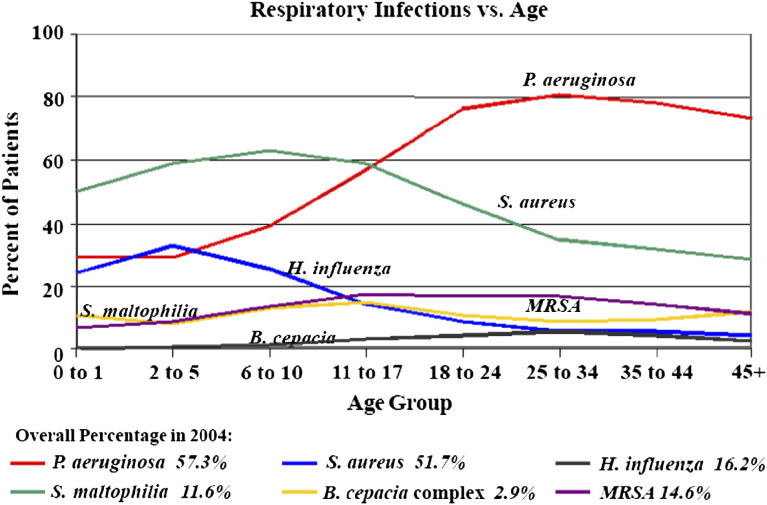
Patients with CF develop pulmonary infections caused by a limited group of bacteria, but the list of bacteria isolated from CF patients is growing ( Box 3). The acquisition of various bacteria varies by age ( Fig. 2). Of these, P aeruginosa, Burkholderia cepacia, and probably S aureus are associated with decline in clinical status and mortality. The spectrum of bacteria varies with age. S aureus was reported to be the first organism cultured by BAL within the first few months of life in CF [108]. Rosenfeld and colleagues [115], in a longitudinal study of infants with CF using bronchoscopy and BAL, reported that H influenzae was the commonest infecting microbe at age 1 year. H influenzae declined over the next 2 years of life, whereas S aureus and P aeruginosa increased. By 3 years of age, 33% had P aeruginosa in the BAL. By early adulthood 80% have chronic P aeruginosa infection. Most patients with CF have polymicrobial chronic airway infection. In an analysis of 595 patients, an average of 2.9 pathogenic species per sputum were cultured [116].
Box 3. Bacteria and fungi that colonize and cause infection in CF.
Pseudomonas aeruginosa
Staphylococcus aureus (including methicillin-resistant S aureus)
Nontypeable Haemophilus influenzae
Burkholderia cepacia complex
Stenotrophomonas maltophilia
Alcaligenes xylosoxidans
Nontuberculous mycobacteria
Pandoraea apista
Aspergillus fumigatus
Scedosporium apiospermum
Fig. 2.
Bacterial infection in cystic fibrosis. MRSA, methicillin-resistant S aureus. (From Cystic Fibrosis Foundation, Patient Registry 2004 Annual Report. Bethesda, Maryland; with permission.)
Staphylococcus aureus is one of the earliest to be cultured from CF patients. Historically, S aureus has been thought to be of significance and treated with antibiotics [85], [117]. Definitive evidence, however, for a role in causing infection in CF is lacking. A meta-analysis of antistaphylococcal antibiotic trials showed that, although sputum clearance was often achieved, improvement in lung function was not observed or reported [118]. Most authorities, however, advise treatment of S aureus in sputum. The percentage of methicillin-resistant S aureus has been increasing across the United States. In 2004, the prevalence in CF was 14.6% (see Fig. 2).
Similarly, evidence for a definite role for nontypeable H influenzae in causing lung inflammation in CF is lacking [119]. It is often the earliest bacterial pathogen cultured, however, from the lower airway in CF patients [115]. Recent work has shown evidence that H influenzae forms biofilms in CF airways [120].
Pseudomonas aeruginosa
Pseudomonas aeruginosa is the most important pathogen in CF. The onset of infection with P aeruginosa is associated with decline in lung function. As mentioned previously, Rosenfeld and coworkers [115] found that 33% of patients have P aeruginosa in BAL by age 3 years. Serologic studies show that infection probably occurs even earlier. Antibodies to P aeruginosa can be shown in blood 6 to 12 months before the bacteria can be recovered by sputum or oropharyngeal cultures [121]. The database of the Cystic Fibrosis Foundation shows that about 80% of young adults with CF have chronic P aeruginosa infection (see Fig. 2). Others have found an even higher incidence. Using BAL cultures, 47.5% had P aeruginosa by age 3 years; however, when combined with serology, 97.5% had evidence of P aeruginosa infection [121]. Female gender, homozygous ΔF508 CFTR mutation, and S aureus isolation from sputum have been identified as risk factors for early acquisition of P aeruginosa [122]. There are less severe phenotypes of CF where the burden of P aeruginosa infection is less common. In a series of patients with adult CF, the mean age was 41.8 years and P aeruginosa airway infection was present in only 48% [114].
The initial isolates of P aeruginosa in CF phenotypically resemble environmental isolates and those seen in non-CF patients. Early infections are cleared, only to be infected by a different strain and cleared again [121]. Within a few years, however, P aeruginosa becomes persistent. Once persistent, it is extremely difficult to eradicate. Chronic pseudomonas airway infection in CF has been defined as the continuous presence of P aeruginosa in lower airway secretions for greater than or equal to 6 months or the presence of two precipitating antibodies against P aeruginosa [123]. Chronic infection is more likely to be caused by the same strain of P aeruginosa over many years, albeit with continuing genetic adaptation to life in host airways [124].
Once well established, P aeruginosa changes its phenotype and produces alginate. The colonies look mucoid. P aeruginosa uses various strategies to survive on a long-term basis in CF patients' lungs. There is evidence that P aeruginosa produces biofilms. Biofilm formation is associated with the production of rhamnolipids, which facilitate dispersal of bacteria within airways [125]. In biofilm mode of growth, P aeruginosa down-regulates virulence factors and tissue invasion [124]. Transcription of the flagellin protein is down-regulated [126]. This is advantageous to the bacterium because flagellin acts as a ligand for bacterial recognition proteins [127]. This probably helps with evasion from detection by host immune system and an effective immune response [128].
The change to mucoid type is associated with further decline in lung function [100]. Once well established, it is extremely difficult to eradicate P aeruginosa in these patients [89]. Many have strains that are resistant to multiple antibiotics. Being infected by mucoid P aeruginosa increases mortality and morbidity in CF children [129], [130]. Early in the course of CF, however, P aeruginosa infections are intermittent. This offers a window of opportunity for clearing the infection with aggressive antibiotic therapy [131]. In Denmark, such a strategy has markedly reduced the proportion of CF patients who develop chronic infection during adolescence and early adulthood [123].
Burkholderia cepacia
Initially described as Pseudomonas cepacia, this bacterium was renamed Burkholderia and given its own genus. Later work showed that the prototype species, B cepacia, has at least nine genetic variants (genomovars) and many of these have been named as new species. It was renamed B cepacia complex. Most clinical disease is caused by genomovars 2, 3, and 5; severe disease is mostly by genomovar 3 (B cenocepacia) [132], [133]. Colonization and infection of airways in CF by B cepacia had been sporadically reported, but the incidence increased dramatically in 1970s and 1980s [134], [135], [136]. It frequently coexists with P aeruginosa, but occurs later in the disease [136]. B cepacia can be transmitted in an epidemic fashion between CF patients at CF clinics and outside-clinic social interaction [135]. So far, the prevalence of B cepacia infection remains under 20% in the CF population (see Fig. 1). In a Canadian cohort of CF, B cepacia colonization was associated with increased mortality at all levels of lung function [137].
Burkholderia cepacia causes three types of host response [136]: (1) type 1, colonization of airways without any change in clinical status; (2) type 2, gradual clinical decline with frequent exacerbations of respiratory symptoms and decline in lung function; and (3) type 3, abrupt, rapid deterioration and respiratory failure, necrotizing pneumonia, frequently causing death. Most of type 3 response occurs in girls. Unfortunately, B cepacia remains resistant to most commonly used antibiotics.
Other bacteria
Stenotrophomonas maltophilia [138], [139] and Alcaligenes xylosoxidans (previously called Achromobacter xylosoxidans) [140] are both gram-negative bacilli and are recovered from CF airway cultures in 8% to 10% (see Box 3). The age of acquisition is later than for P aeruginosa, usually in late adolescence. When adjusted for P aeruginosa infection status, neither of these bacteria affect mortality or severity of the course in CF [138], [140].
Pandoraea apista, a nonfermenting gram-negative bacillus, has been reported to infect lower airways in CF [141], [142]. Burkholderia pseudomallei infection (melioidosis) has been reported in CF patients from northern Australia [143]. A large variety of other gram-negative bacilli have been isolated from sputa of patients with CF. These include Ralstonia, Enterobacteriaceae, and Burkholderia gladioli [116], [144]. It is unclear whether these bacteria adversely affect the course of disease in CF.
Allergic bronchopulmonary aspergillosis
Allergic bronchopulmonary aspergillosis is a clinical syndrome of wheezing, pulmonary infiltrates, and central bronchiectasis, caused by sensitization to Aspergillus, mostly Aspergillus fumigatus (as opposed to invasive disease). These patients have immediate cutaneous hypersensitivity to A fumigatus, increased total serum IgE, eosinophilia, and serum precipitating antibodies against A fumigatus [145]. The incidence of allergic bronchopulmonary aspergillosis is increased in CF. Estimates vary because of geographic variation and differences in definitions of diagnosis. Two large United States databases estimate prevalence at around 2% in CF [145], [146], whereas a large European registry reported 7.8% [147]. Patients with CF and atopy have higher prevalence [145]. Prevalence is higher in late adolescence.
Nontuberculous mycobacteria
Olivier and colleagues [148] reported a prevalence of 13% for NTMB in patients with CF. M avium complex accounted for 72%, and 16% were caused by Mycobacterium abscessus. The prevalence was higher in coastal areas. Whether isolation of NTMB represents lung disease is a matter of much research. In an autopsy study, patients with CF who had repeatedly positive antemortem cultures for NTMB were more likely to have granulomatous lung disease on autopsy [149]. In addition to repeatedly positive cultures, changes on high-resolution CT showing bronchiectasis or lung involvement are also suggestive of the need for treatment [148], [150].
Infection control
Because patients with CF are often treated in centralized clinics, interpatient spread of virulent and multidrug-resistant pathogens has been a concern. Interpatient transmission has been well documented for B cepacia [135], [151] and P aeruginosa [152], [153]. The evidence for interpatient spread is much less robust for S maltophilia and A xylosoxidans. Cohorting of patients, by isolation of infected from noninfected, has been shown to reduce new infections for B cepacia [151] and P aeruginosa [153]. A meta-analysis advised separation of patients with B cepacia from noninfected patients, and separation of patients with multidrug-resistant P aeruginosa, S maltophilia, and A xylosoxidans from other CF patients and immunocompromised patients [154].
References
- 1.Celli B.R., MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 2.Murray C.J., Lopez A.D. Alternative projections of mortality and disability by cause 1990–2020. Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Roisin R. The airway pathophysiology of COPD: implications for treatment. COPD. 2005;2:253–262. [PubMed] [Google Scholar]
- 4.Vestbo J., Prescott E., Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med. 1996;153:1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 5.Melton L. Does mucus hypersecretion matter in airway disease? Lancet. 2002;359:1924. doi: 10.1016/S0140-6736(02)08788-3. [DOI] [PubMed] [Google Scholar]
- 6.Berenson C.S., Garlipp M.A., Grove L.J. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J Infect Dis. 2006;194:1375–1384. doi: 10.1086/508428. [DOI] [PubMed] [Google Scholar]
- 7.Berenson C.S., Wrona C.T., Grove L.J. Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;174:31–40. doi: 10.1164/rccm.200509-1461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veeramachaneni S.B., Sethi S. Pathogenesis of bacterial exacerbations of COPD. COPD. 2006;3:109–115. doi: 10.1080/15412550600651347. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto C., Yoneda T., Yoshikawa M. Airway inflammation in COPD assessed by sputum levels of interleukin-8. Chest. 1997;112:505–510. doi: 10.1378/chest.112.2.505. [DOI] [PubMed] [Google Scholar]
- 10.Hill A.T., Campbell E.J., Bayley D.L. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with alpha(1)-antitrypsin deficiency (PiZ) Am J Respir Crit Care Med. 1999;160:1968–1975. doi: 10.1164/ajrccm.160.6.9904097. [DOI] [PubMed] [Google Scholar]
- 11.Aaron S.D., Angel J.B., Lunau M. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:349–355. doi: 10.1164/ajrccm.163.2.2003122. [DOI] [PubMed] [Google Scholar]
- 12.Hurst J.R., Perera W.R., Wilkinson T.M. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:71–78. doi: 10.1164/rccm.200505-704OC. [DOI] [PubMed] [Google Scholar]
- 13.Papi A., Bellettato C.M., Braccioni F. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 14.Perera W.R., Hurst J.R., Wilkinson T.M. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J. 2007;29:527–534. doi: 10.1183/09031936.00092506. [DOI] [PubMed] [Google Scholar]
- 15.Seemungal T.A., Donaldson G.C., Bhowmik A. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 16.Connors A.F., Jr., Dawson N.V., Thomas C. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (study to understand prognoses and preferences for outcomes and risks of treatments) Am J Respir Crit Care Med. 1996;154:959–967. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 17.Stockley R.A., O'Brien C., Pye A. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638–1645. doi: 10.1378/chest.117.6.1638. [DOI] [PubMed] [Google Scholar]
- 18.Anthonisen N.R., Manfreda J., Warren C.P. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 19.Sethi S. Infectious etiology of acute exacerbations of chronic bronchitis. Chest. 2000;117:380S–385S. doi: 10.1378/chest.117.5_suppl_2.380s. [DOI] [PubMed] [Google Scholar]
- 20.Sunyer J., Saez M., Murillo C. Air pollution and emergency room admissions for chronic obstructive pulmonary disease: a 5-year study. Am J Epidemiol. 1993;137:701–705. doi: 10.1093/oxfordjournals.aje.a116730. [DOI] [PubMed] [Google Scholar]
- 21.Sapey E., Stockley R.A. COPD exacerbations. 2: Aetiology. Thorax. 2006;61:250–258. doi: 10.1136/thx.2005.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monso E., Ruiz J., Rosell A. Bacterial infection in chronic obstructive pulmonary disease: a study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med. 1995;152:1316–1320. doi: 10.1164/ajrccm.152.4.7551388. [DOI] [PubMed] [Google Scholar]
- 23.Rosell A., Monso E., Soler N. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005;165:891–897. doi: 10.1001/archinte.165.8.891. [DOI] [PubMed] [Google Scholar]
- 24.Sethi S., Muscarella K., Evans N. Airway inflammation and etiology of acute exacerbations of chronic bronchitis. Chest. 2000;118:1557–1565. doi: 10.1378/chest.118.6.1557. [DOI] [PubMed] [Google Scholar]
- 25.Hill A.T., Campbell E.J., Hill S.L. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med. 2000;109:288–295. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]
- 26.Sethi S., Wrona C., Grant B.J. Strain-specific immune response to Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169:448–453. doi: 10.1164/rccm.200308-1181OC. [DOI] [PubMed] [Google Scholar]
- 27.Murphy T.F., Brauer A.L., Grant B.J. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med. 2005;172:195–199. doi: 10.1164/rccm.200412-1747OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sethi S., Evans N., Grant B.J. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 29.Puhan M.A., Vollenweider D., Latshang T. Exacerbations of chronic obstructive pulmonary disease: when are antibiotics indicated? A systematic review. Respir Res. 2007;8:30. doi: 10.1186/1465-9921-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ram F.S., Rodriguez-Roisin R., Granados-Navarrete A. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD004403.pub2. CD004403. [DOI] [PubMed] [Google Scholar]
- 31.Soler N., Agusti C., Angrill J. Bronchoscopic validation of the significance of sputum purulence in severe exacerbations of chronic obstructive pulmonary disease. Thorax. 2007;62:29–35. doi: 10.1136/thx.2005.056374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glezen W.P., Greenberg S.B., Atmar R.L. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 33.Seemungal T., Harper-Owen R., Bhowmik A. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 34.Hamelin M.E., Cote S., Laforge J. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis. 2005;41:498–502. doi: 10.1086/431981. [DOI] [PubMed] [Google Scholar]
- 35.Seemungal T.A., Harper-Owen R., Bhowmik A. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J. 2000;16:677–683. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohde G., Wiethege A., Borg I. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58:37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinello R.A., Esper F., Weibel C. Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J Infect. 2006;53:248–254. doi: 10.1016/j.jinf.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luyt C.E. Virus diseases in ICU patients: a long time underestimated; but be aware of overestimation. Intensive Care Med. 2006;32:968–970. doi: 10.1007/s00134-006-0203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papadopoulos N.G., Bates P.J., Bardin P.G. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 40.Mallia P., Johnston S.L. How viral infections cause exacerbation of airway diseases. Chest. 2006;130:1203–1210. doi: 10.1378/chest.130.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith C.B., Golden C., Klauber M.R. Interactions between viruses and bacteria in patients with chronic bronchitis. J Infect Dis. 1976;134:552–561. doi: 10.1093/infdis/134.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blasi F., Legnani D., Lombardo V.M. Chlamydia pneumoniae infection in acute exacerbations of COPD. Eur Respir J. 1993;6:19–22. [PubMed] [Google Scholar]
- 43.Miyashita N., Niki Y., Nakajima M. Chlamydia pneumoniae infection in patients with diffuse panbronchiolitis and COPD. Chest. 1998;114:969–971. doi: 10.1378/chest.114.4.969. [DOI] [PubMed] [Google Scholar]
- 44.Buscho R.O., Saxtan D., Shultz P.S. Infections with viruses and Mycoplasma pneumoniae during exacerbations of chronic bronchitis. J Infect Dis. 1978;137:377–383. doi: 10.1093/infdis/137.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacNee W., Donaldson K. Exacerbations of COPD: environmental mechanisms. Chest. 2000;117:390S–397S. doi: 10.1378/chest.117.5_suppl_2.390s. [DOI] [PubMed] [Google Scholar]
- 46.Pope C.A., III Particulate pollution and health: a review of the Utah valley experience. J Expo Anal Environ Epidemiol. 1996;6:23–34. [PubMed] [Google Scholar]
- 47.Wilson R. Bacteria, antibiotics and COPD. Eur Respir J. 2001;17:995–1007. doi: 10.1183/09031936.01.17509950. [DOI] [PubMed] [Google Scholar]
- 48.Wilson R., Dowling R.B., Jackson A.D. The biology of bacterial colonization and invasion of the respiratory mucosa. Eur Respir J. 1996;9:1523–1530. doi: 10.1183/09031936.96.09071523. [DOI] [PubMed] [Google Scholar]
- 49.Farr B.M., Woodhead M.A., Macfarlane J.T. Risk factors for community-acquired pneumonia diagnosed by general practitioners in the community. Respir Med. 2000;94:422–427. doi: 10.1053/rmed.1999.0743. [DOI] [PubMed] [Google Scholar]
- 50.Restrepo M.I., Mortensen E.M., Pugh J.A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. 2006;28:346–351. doi: 10.1183/09031936.06.00131905. [DOI] [PubMed] [Google Scholar]
- 51.Lieberman D., Lieberman D., Gelfer Y. Pneumonic vs nonpneumonic acute exacerbations of COPD. Chest. 2002;122:1264–1270. doi: 10.1378/chest.122.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oliveira E.C., Marik P.E., Colice G. Influenza pneumonia: a descriptive study. Chest. 2001;119:1717–1723. doi: 10.1378/chest.119.6.1717. [DOI] [PubMed] [Google Scholar]
- 53.Stuck A.E., Minder C.E., Frey F.J. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11:954–963. doi: 10.1093/clinids/11.6.954. [DOI] [PubMed] [Google Scholar]
- 54.Rello J., Esandi M.E., Mariscal D. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: report of eight cases and review. Clin Infect Dis. 1998;26:1473–1475. doi: 10.1086/517672. [DOI] [PubMed] [Google Scholar]
- 55.Denning D.W. Aspergillosis in nonimmunocompromised critically ill patients. Am J Respir Crit Care Med. 2004;170:580–581. doi: 10.1164/rccm.2407004. [DOI] [PubMed] [Google Scholar]
- 56.Meersseman W., Vandecasteele S.J., Wilmer A. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170:621–625. doi: 10.1164/rccm.200401-093OC. [DOI] [PubMed] [Google Scholar]
- 57.Ader F., Nseir S., Le Berre R. Invasive pulmonary aspergillosis in chronic obstructive pulmonary disease: an emerging fungal pathogen. Clin Microbiol Infect. 2005;11:427–429. doi: 10.1111/j.1469-0691.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 58.Rodrigues J., Niederman M.S., Fein A.M. Nonresolving pneumonia in steroid-treated patients with obstructive lung disease. Am J Med. 1992;93:29–34. doi: 10.1016/0002-9343(92)90676-3. [DOI] [PubMed] [Google Scholar]
- 59.Singer J.J. Bronchiectasis. Chest. 1948;14:92–106. doi: 10.1378/chest.14.1.92. [DOI] [PubMed] [Google Scholar]
- 60.Souders C.R. Bronchiectasis and its management: a report of 277 cases. Chest. 1949;16:381–407. doi: 10.1378/chest.16.4.381. [DOI] [PubMed] [Google Scholar]
- 61.Barker A.F. Bronchiectasis. N Engl J Med. 2002;346:1383–1393. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 62.Twiss J., Metcalfe R., Edwards E. New Zealand national incidence of bronchiectasis too high for a developed country. Arch Dis Child. 2005;90:737–740. doi: 10.1136/adc.2004.066472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eastham K.M., Fall A.J., Mitchell L. The need to redefine non-cystic fibrosis bronchiectasis in childhood. Thorax. 2004;59:324–327. doi: 10.1136/thx.2003.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keistinen T., Saynajakangas O., Tuuponen T. Bronchiectasis: an orphan disease with a poorly-understood prognosis. Eur Respir J. 1997;10:2784–2787. doi: 10.1183/09031936.97.10122784. [DOI] [PubMed] [Google Scholar]
- 65.Pasteur M.C., Helliwell S.M., Houghton S.J. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162:1277–1284. doi: 10.1164/ajrccm.162.4.9906120. [DOI] [PubMed] [Google Scholar]
- 66.Shoemark A., Ozerovitch L., Wilson R. Aetiology in adult patients with bronchiectasis. Respir Med. 2007;101:1163–1170. doi: 10.1016/j.rmed.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 67.Ziedalski T.M., Kao P.N., Henig N.R. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest. 2006;130:995–1002. doi: 10.1378/chest.130.4.995. [DOI] [PubMed] [Google Scholar]
- 68.Cole P. The damaging role of bacteria in chronic lung infection. J Antimicrob Chemother. 1997;40(Suppl A):5–10. doi: 10.1093/jac/40.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 69.King P.T., Hutchinson P.E., Johnson P.D. Adaptive immunity to nontypeable Haemophilus influenzae. Am J Respir Crit Care Med. 2003;167:587–592. doi: 10.1164/rccm.200207-728OC. [DOI] [PubMed] [Google Scholar]
- 70.Gaga M., Bentley A.M., Humbert M. Increases in CD4 + T lymphocytes, macrophages, neutrophils and interleukin 8 positive cells in the airways of patients with bronchiectasis. Thorax. 1998;53:685–691. doi: 10.1136/thx.53.8.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Angrill J., Agusti C., de Celis R. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax. 2002;57:15–19. doi: 10.1136/thorax.57.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson C.B., Jones P.W., O'Leary C.J. Systemic markers of inflammation in stable bronchiectasis. Eur Respir J. 1998;12:820–824. doi: 10.1183/09031936.98.12040820. [DOI] [PubMed] [Google Scholar]
- 73.Tsang K.W., Ho P.I., Chan K.N. A pilot study of low-dose erythromycin in bronchiectasis. Eur Respir J. 1999;13:361–364. doi: 10.1183/09031936.99.13236199. [DOI] [PubMed] [Google Scholar]
- 74.Pang J.A., Cheng A., Chan H.S. The bacteriology of bronchiectasis in Hong Kong investigated by protected catheter brush and bronchoalveolar lavage. Am Rev Respir Dis. 1989;139:14–17. doi: 10.1164/ajrccm/139.1.14. [DOI] [PubMed] [Google Scholar]
- 75.Cabello H., Torres A., Celis R. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: a bronchoscopic study. Eur Respir J. 1997;10:1137–1144. doi: 10.1183/09031936.97.10051137. [DOI] [PubMed] [Google Scholar]
- 76.King P.T., Holdsworth S.R., Freezer N.J. Microbiologic follow-up study in adult bronchiectasis. Respir Med. 2007;101:1633–1638. doi: 10.1016/j.rmed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 77.Klingman K.L., Pye A., Murphy T.F. Dynamics of respiratory tract colonization by Branhamella catarrhalis in bronchiectasis. Am J Respir Crit Care Med. 1995;152:1072–1078. doi: 10.1164/ajrccm.152.3.7663786. [DOI] [PubMed] [Google Scholar]
- 78.Ho P.L., Chan K.N., Ip M.S. The effect of Pseudomonas aeruginosa infection on clinical parameters in steady-state bronchiectasis. Chest. 1998;114:1594–1598. doi: 10.1378/chest.114.6.1594. [DOI] [PubMed] [Google Scholar]
- 79.Caballero E., Drobnic M.E., Perez M.T. Anti-Pseudomonas aeruginosa antibody detection in patients with bronchiectasis without cystic fibrosis. Thorax. 2001;56:669–674. doi: 10.1136/thorax.56.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davies G., Wells A.U., Doffman S. The effect of Pseudomonas aeruginosa on pulmonary function in patients with bronchiectasis. Eur Respir J. 2006;28:974–979. doi: 10.1183/09031936.06.00074605. [DOI] [PubMed] [Google Scholar]
- 81.Shah P.L., Mawdsley S., Nash K. Determinants of chronic infection with Staphylococcus aureus in patients with bronchiectasis. Eur Respir J. 1999;14:1340–1344. doi: 10.1183/09031936.99.14613409. [DOI] [PubMed] [Google Scholar]
- 82.Kunst H., Wickremasinghe M., Wells A. Nontuberculous mycobacterial disease and Aspergillus-related lung disease in bronchiectasis. Eur Respir J. 2006;28:352–357. doi: 10.1183/09031936.06.00139005. [DOI] [PubMed] [Google Scholar]
- 83.Fowler S.J., French J., Screaton N.J. Nontuberculous mycobacteria in bronchiectasis: prevalence and patient characteristics. Eur Respir J. 2006;28:1204–1210. doi: 10.1183/09031936.06.00149805. [DOI] [PubMed] [Google Scholar]
- 84.Wickremasinghe M., Ozerovitch L.J., Davies G. Non-tuberculous mycobacteria in patients with bronchiectasis. Thorax. 2005;60:1045–1051. doi: 10.1136/thx.2005.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gibson R.L., Burns J.L., Ramsey B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 86.Rommens J.M., Zengerling S., Burns J. Identification and regional localization of DNA markers on chromosome 7 for the cloning of the cystic fibrosis gene. Am J Hum Genet. 1988;43:645–663. [PMC free article] [PubMed] [Google Scholar]
- 87.Rommens J.M., Iannuzzi M.C., Kerem B. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 88.Kerem B., Rommens J.M., Buchanan J.A. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 89.Davis P.B. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 90.Chmiel J.F., Davis P.B. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir Res. 2003;4:8. doi: 10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chow C.W., Landau L.I., Taussig L.M. Bronchial mucous glands in the newborn with cystic fibrosis. Eur J Pediatr. 1982;139:240–243. doi: 10.1007/BF00442171. [DOI] [PubMed] [Google Scholar]
- 92.Baltimore R.S., Christie C.D., Smith G.J. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis: implications for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis. 1989;140:1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- 93.Tarran R., Loewen M.E., Paradiso A.M. Regulation of murine airway surface liquid volume by CFTR and Ca2+-activated Cl- conductances. J Gen Physiol. 2002;120:407–418. doi: 10.1085/jgp.20028599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Briel M., Greger R., Kunzelmann K. Cl- transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na+ channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J Physiol. 1998;508(Pt 3):825–836. doi: 10.1111/j.1469-7793.1998.825bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang Q., Li J., Dubroff R. Epithelial sodium channels regulate cystic fibrosis transmembrane conductance regulator chloride channels in Xenopus oocytes. J Biol Chem. 2000;275:13266–13274. doi: 10.1074/jbc.275.18.13266. [DOI] [PubMed] [Google Scholar]
- 96.Worlitzsch D., Tarran R., Ulrich M. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pier G.B., Grout M., Zaidi T.S. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saiman L., Prince A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Invest. 1993;92:1875–1880. doi: 10.1172/JCI116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kelley T.J., Drumm M.L. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Invest. 1998;102:1200–1207. doi: 10.1172/JCI2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Demko C.A., Byard P.J., Davis P.B. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol. 1995;48:1041–1049. doi: 10.1016/0895-4356(94)00230-n. [DOI] [PubMed] [Google Scholar]
- 101.Donlan R.M., Costerton J.W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ernst R.K., Yi E.C., Guo L. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science. 1999;286:1561–1565. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- 103.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 104.Singh P.K., Schaefer A.L., Parsek M.R. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 105.Hoiby N., Krogh Johansen H., Moser C. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect. 2001;3:23–35. doi: 10.1016/s1286-4579(00)01349-6. [DOI] [PubMed] [Google Scholar]
- 106.Muhlebach M.S., Stewart P.W., Leigh M.W. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med. 1999;160:186–191. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- 107.Khan T.Z., Wagener J.S., Bost T. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 108.Armstrong D.S., Grimwood K., Carlin J.B. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156:1197–1204. doi: 10.1164/ajrccm.156.4.96-11058. [DOI] [PubMed] [Google Scholar]
- 109.Bonfield T.L., Konstan M.W., Burfeind P. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–261. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 110.Goss C.H., Burns J.L. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62:360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hiatt P.W., Grace S.C., Kozinetz C.A. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics. 1999;103:619–626. doi: 10.1542/peds.103.3.619. [DOI] [PubMed] [Google Scholar]
- 112.Aaron S.D., Ramotar K., Ferris W. Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2004;169:811–815. doi: 10.1164/rccm.200309-1306OC. [DOI] [PubMed] [Google Scholar]
- 113.Armstrong D., Grimwood K., Carlin J.B. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr Pulmonol. 1998;26:371–379. doi: 10.1002/(sici)1099-0496(199812)26:6<371::aid-ppul1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 114.Paschoal I.A., de Oliveira Villalba W., Bertuzzo C.S. Cystic fibrosis in adults. Lung. 2007;185:81–87. doi: 10.1007/s00408-006-2597-0. [DOI] [PubMed] [Google Scholar]
- 115.Rosenfeld M., Gibson R.L., McNamara S. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol. 2001;32:356–366. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 116.Burns J.L., Emerson J., Stapp J.R. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 117.Thomassen M.J., Demko C.A., Doershuk C.F. Cystic fibrosis: a review of pulmonary infections and interventions. Pediatr Pulmonol. 1987;3:334–351. doi: 10.1002/ppul.1950030510. [DOI] [PubMed] [Google Scholar]
- 118.McCaffery K., Olver R.E., Franklin M. Systematic review of antistaphylococcal antibiotic therapy in cystic fibrosis. Thorax. 1999;54:380–383. doi: 10.1136/thx.54.5.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lyczak J.B., Cannon C.L., Pier G.B. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Starner T.D., Zhang N., Kim G. Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis. Am J Respir Crit Care Med. 2006;174:213–220. doi: 10.1164/rccm.200509-1459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Burns J.L., Gibson R.L., McNamara S. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis. 2001;183:444–452. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- 122.Maselli J.H., Sontag M.K., Norris J.M. Risk factors for initial acquisition of Pseudomonas aeruginosa in children with cystic fibrosis identified by newborn screening. Pediatr Pulmonol. 2003;35:257–262. doi: 10.1002/ppul.10230. [DOI] [PubMed] [Google Scholar]
- 123.Hoiby N., Frederiksen B., Pressler T. Eradication of early Pseudomonas aeruginosa infection. J Cyst Fibros. 2005;4(Suppl 2):49–54. doi: 10.1016/j.jcf.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 124.Smith E.E., Buckley D.G., Wu Z. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A. 2006;103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boles B.R., Thoendel M., Singh P.K. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol Microbiol. 2005;57:1210–1223. doi: 10.1111/j.1365-2958.2005.04743.x. [DOI] [PubMed] [Google Scholar]
- 126.Palmer K.L., Mashburn L.M., Singh P.K. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol. 2005;187:5267–5277. doi: 10.1128/JB.187.15.5267-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hybiske K., Ichikawa J.K., Huang V. Cystic fibrosis airway epithelial cell polarity and bacterial flagellin determine host response to Pseudomonas aeruginosa. Cell Microbiol. 2004;6:49–63. doi: 10.1046/j.1462-5822.2003.00342.x. [DOI] [PubMed] [Google Scholar]
- 128.Murray T.S., Egan M., Kazmierczak B.I. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr Opin Pediatr. 2007;19:83–88. doi: 10.1097/MOP.0b013e3280123a5d. [DOI] [PubMed] [Google Scholar]
- 129.Nixon G.M., Armstrong D.S., Carzino R. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr. 2001;138:699–704. doi: 10.1067/mpd.2001.112897. [DOI] [PubMed] [Google Scholar]
- 130.Emerson J., Rosenfeld M., McNamara S. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 131.Starner T.D., McCray P.B., Jr. Pathogenesis of early lung disease in cystic fibrosis: a window of opportunity to eradicate bacteria. Ann Intern Med. 2005;143:816–822. doi: 10.7326/0003-4819-143-11-200512060-00010. [DOI] [PubMed] [Google Scholar]
- 132.Mahenthiralingam E., Baldwin A., Vandamme P. Burkholderia cepacia complex infection in patients with cystic fibrosis. J Med Microbiol. 2002;51:533–538. doi: 10.1099/0022-1317-51-7-533. [DOI] [PubMed] [Google Scholar]
- 133.Mahenthiralingam E., Vandamme P. Taxonomy and pathogenesis of the Burkholderia cepacia complex. Chron Respir Dis. 2005;2:209–217. doi: 10.1191/1479972305cd053ra. [DOI] [PubMed] [Google Scholar]
- 134.Isles A., Maclusky I., Corey M. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104:206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 135.Govan J.R., Brown P.H., Maddison J. Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet. 1993;342:15–19. doi: 10.1016/0140-6736(93)91881-l. [DOI] [PubMed] [Google Scholar]
- 136.Thomassen M.J., Demko C.A., Klinger J.D. Pseudomonas cepacia colonization among patients with cystic fibrosis. A new opportunist. Am Rev Respir Dis. 1985;131:791–796. doi: 10.1164/arrd.1985.131.5.791. [DOI] [PubMed] [Google Scholar]
- 137.Corey M., Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970–1989. Am J Epidemiol. 1996;143:1007–1017. doi: 10.1093/oxfordjournals.aje.a008664. [DOI] [PubMed] [Google Scholar]
- 138.Goss C.H., Otto K., Aitken M.L. Detecting Stenotrophomonas maltophilia does not reduce survival of patients with cystic fibrosis. Am J Respir Crit Care Med. 2002;166:356–361. doi: 10.1164/rccm.2109078. [DOI] [PubMed] [Google Scholar]
- 139.Demko C.A., Stern R.C., Doershuk C.F. Stenotrophomonas maltophilia in cystic fibrosis: incidence and prevalence. Pediatr Pulmonol. 1998;25:304–308. doi: 10.1002/(sici)1099-0496(199805)25:5<304::aid-ppul3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 140.Tan K., Conway S.P., Brownlee K.G. Alcaligenes infection in cystic fibrosis. Pediatr Pulmonol. 2002;34:101–104. doi: 10.1002/ppul.10143. [DOI] [PubMed] [Google Scholar]
- 141.Atkinson R.M., Lipuma J.J., Rosenbluth D.B. Chronic colonization with Pandoraea apista in cystic fibrosis patients determined by repetitive-element-sequence PCR. J Clin Microbiol. 2006;44:833–836. doi: 10.1128/JCM.44.3.833-836.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jorgensen I.M., Johansen H.K., Frederiksen B. Epidemic spread of Pandoraea apista, a new pathogen causing severe lung disease in cystic fibrosis patients. Pediatr Pulmonol. 2003;36:439–446. doi: 10.1002/ppul.10383. [DOI] [PubMed] [Google Scholar]
- 143.Holland D.J., Wesley A., Drinkovic D. Cystic fibrosis and Burkholderia pseudomallei infection: an emerging problem? Clin Infect Dis. 2002;35:e138–e140. doi: 10.1086/344447. [DOI] [PubMed] [Google Scholar]
- 144.Coenye T., Vandamme P., LiPuma J.J. Infection by Ralstonia species in cystic fibrosis patients: identification of R. pickettii and R. mannitolilytica by polymerase chain reaction. Emerg Infect Dis. 2002;8:692–696. doi: 10.3201/eid0807.010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stevens D.A., Moss R.B., Kurup V.P. Allergic bronchopulmonary aspergillosis in cystic fibrosis: state of the art. Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003;37(Suppl 3):S225–S264. doi: 10.1086/376525. [DOI] [PubMed] [Google Scholar]
- 146.Geller D.E., Kaplowitz H., Light M.J. Allergic bronchopulmonary aspergillosis in cystic fibrosis: reported prevalence, regional distribution, and patient characteristics. Scientific Advisory Group, Investigators, and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Chest. 1999;116:639–646. doi: 10.1378/chest.116.3.639. [DOI] [PubMed] [Google Scholar]
- 147.Mastella G., Rainisio M., Harms H.K. Allergic bronchopulmonary aspergillosis in cystic fibrosis: a European epidemiological study. Epidemiologic registry of cystic fibrosis. Eur Respir J. 2000;16:464–471. doi: 10.1034/j.1399-3003.2000.016003464.x. [DOI] [PubMed] [Google Scholar]
- 148.Olivier K.N., Weber D.J., Lee J.H. Nontuberculous mycobacteria. II: Nested-cohort study of impact on cystic fibrosis lung disease. Am J Respir Crit Care Med. 2003;167:835–840. doi: 10.1164/rccm.200207-679OC. [DOI] [PubMed] [Google Scholar]
- 149.Tomashefski J.F., Jr., Stern R.C., Demko C.A. Nontuberculous mycobacteria in cystic fibrosis: an autopsy study. Am J Respir Crit Care Med. 1996;154:523–528. doi: 10.1164/ajrccm.154.2.8756832. [DOI] [PubMed] [Google Scholar]
- 150.Oliver A., Maiz L., Canton R. Nontuberculous mycobacteria in patients with cystic fibrosis. Clin Infect Dis. 2001;32:1298–1303. doi: 10.1086/319987. [DOI] [PubMed] [Google Scholar]
- 151.Thomassen M.J., Demko C.A., Doershuk C.F. Pseudomonas cepacia: decrease in colonization in patients with cystic fibrosis. Am Rev Respir Dis. 1986;134:669–671. doi: 10.1164/arrd.1986.134.4.669. [DOI] [PubMed] [Google Scholar]
- 152.Pedersen S.S., Koch C., Hoiby N. An epidemic spread of multiresistant Pseudomonas aeruginosa in a cystic fibrosis centre. J Antimicrob Chemother. 1986;17:505–516. doi: 10.1093/jac/17.4.505. [DOI] [PubMed] [Google Scholar]
- 153.Frederiksen B., Koch C., Hoiby N. Changing epidemiology of Pseudomonas aeruginosa infection in Danish cystic fibrosis patients (1974-1995) Pediatr Pulmonol. 1999;28:159–166. doi: 10.1002/(sici)1099-0496(199909)28:3<159::aid-ppul1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 154.Vonberg R.P., Gastmeier P. Isolation of infectious cystic fibrosis patients: results of a systematic review. Infect Control Hosp Epidemiol. 2005;26:401–409. doi: 10.1086/502558. [DOI] [PubMed] [Google Scholar]