Graphical abstract
Keywords: Lycorine, Anti-cancer mechanism, Structure-activity relationship
Abstract
Nature is the most abundant source for novel drug discovery. Lycorine is a natural alkaloid with immense therapeutic potential. Lycorine is active in a very low concentration and with high specificity against a number of cancers both in vivo and in vitro and against various drug-resistant cancer cells. This review summarized the therapeutic effect and the anticancer mechanisms of lycorine. At the same time, we have discussed the pharmacology and comparative structure-activity relationship for the anticancer activity of this compound. The researches outlined in this paper serve as a foundation to explain lycorine as an important lead compound for new generation anticancer drug design and provide the principle for the development of biological strategies to utilize lycorine in the treatment of cancers.
1. Introduction
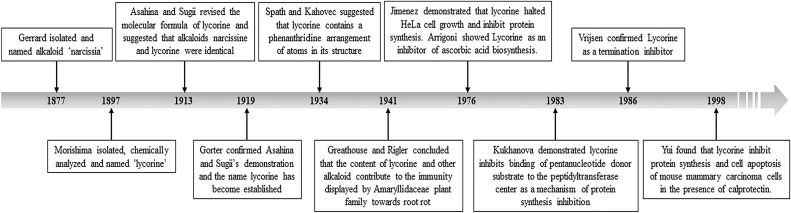
Throughout the ages, organic compounds from terrestrial and marine organisms have been applied for the treatment of a wide spectrum of diseases. Particularly plants are the richest source and the basis of these traditional medicines. The history of using plants and plant-derived substances dates back to 2600 BCE [1]. Over the past fifty years, development of combinatorial chemistries and high-throughput screening methods has made these natural products and related structures extremely important elements of pharmacopeias [2,3]. As studied by Chin et al. [4], over 20 new drugs have been launched between 2000 and 2005, which were originated from natural sources. The search for anti-cancer agents from natural sources dated back to the 1950s with the discovery and development of the vinca alkaloids vinblastine and vincristine, and the isolation of the cytotoxic podophyllotoxins which took almost 30 years for these drugs to came into clinical usage during 1990 [5]. Among 121 drugs prescribed currently for cancer treatment, 90 were derived from plants. These include vinca alkaloids (vinblastine, vincristine, vindesine, vinorelbine), taxanes (paclitaxel, docetaxel), podophyllotoxin and its derivations (topotecan, irinotecan) and anthracyclines (doxorubicin, daunorubicin, epirubicin, idarubicin) [6,7]. Plants from traditional Chinese medicine are the rich host for novel drug discovery and these traditional medicines are being used for the treatment of ailments ranging from coughs and colds to parasitic infections and inflammation [8]. Amaryllidaceae family plant Lycoris radiate is an ornamental and Chinese medicinal plant [9]. Amaryllidaceae family plants are well known as an extensive source of pharmacologically active alkaloids [[10], [11], [12], [13]], and lycorine was the first among these alkaloids to be isolated in 1877 from the plant Narcissus pseudonarcissus [14]. From then onwards, lycorine and its derivatives are drawing interest in the medicinal field due to their divergent chemical structures and strong biological effects [ Fig. 1 ].
Fig. 1.
Timeline for the history of lycorine. First isolated as narcissia from Narcissus pseudo-narcissus L., the alkaloid was later named as lycorine. Further research was performed to establish the molecular formula and general properties of this compound. After confirmation of the chemical properties by several studies, lycorine was investigated for it's in vitro properties which formed the foundation of our current knowledge of this pharmacologically potent alkaloid.
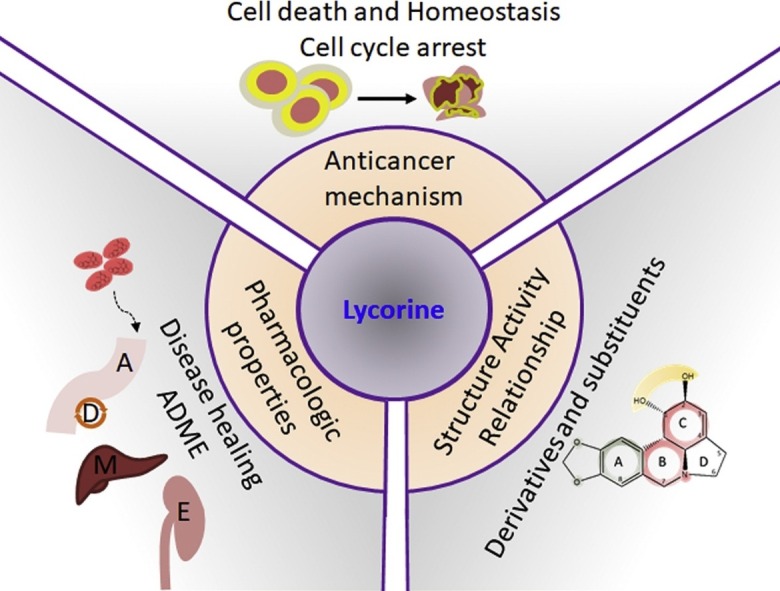
This review will focus on the diverse pharmacological function and anticancer mechanism of lycorine and the associated pharmaco-chemical characteristics.
2. Sources and chemistry of lycorine
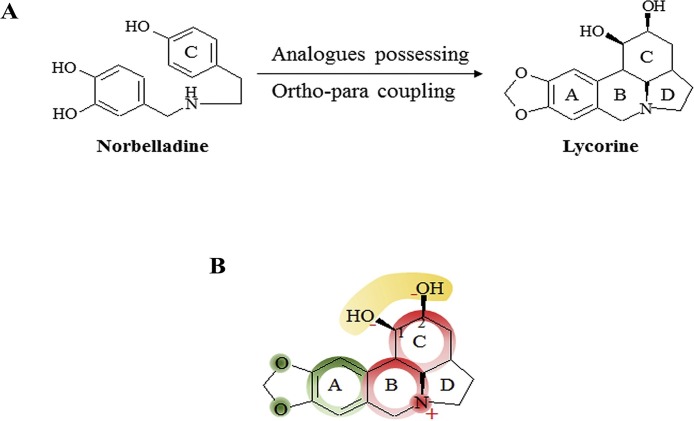
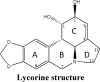
The pyrrolophenanthridine alkaloid lycorine is found in various Amaryllidaceae species. The Amaryllidaceae is a family of herbaceous, perennial and bulbous flowering plants. Compounds isolated from Amaryllidaceae plants are known for their medicinal properties over millenaries. Most frequently reported Amaryllidaceae species to contain lycorine include Lycoris radiate, Leucojum aestivum, Hymenocallis littoralis, Hippeastrum equestre [15], flowers of Clivia nobilis [16], Ammocharis coranica, Brunsvigia radulosa, Crinum macowanii, and Leucojum aestivum [17]. Lycorine can be obtained by asymmetric chemical synthesis or total synthesis strategies that are also applicable for generating other derivatives of lycorine [18,19]. Amaryllidaceae provides a great diversity of biologically potential alkaloids that have been shown to arise biosynthetically from a common intermediate, norbelladine. Norbelladine undergoes different cyclizations, rearrangements, elimination and/or recyclization to provide a variety of skeletons [9,20,21]. Lycorine comprises analogs possessing and ortho-para coupling of a double bond in the C-ring [ Fig. 2 A]. The full chemical name of lycorine is 2,4,5,7,12b,12c-hexahydro-1H-(1,3) dioxole(4,5-j)-pyrrole(3,2,1de) phenanthridine-1-diol, the molecular formula C16H17NO4 and the relative molecular mass 287.31. Lycorine is a colorless crystal with the melting point of 260–262 °C and it is immiscible to the wastewater and insoluble in ether and alcohol [22]. The biological activity of lycorine is firmly associated with its structure. For example, the anti-tumorigenic effect of lycorine is extensively attributed by its structure (discussed later in this article) and slight modification at certain group renders this compound either less active or inactive [ Fig. 2 B]. The structural parameters that provide a significant contribution to its activity include the presence of planarity of the molecule, olefin or dioxole ring, the function of hydroxyl groups and the presence of a positive charge on the nitrogen and the amine group [9,23,24].
Fig. 2.
Chemistry of lycorine. A. Synthesis of lycorine. Lycorine and lycorine like Amaryllidaceae alkaloids are generated from a common precursor norbelladine. Norbelladine undergoes analogs possessing of the −OH group of A-ring and ortho-para coupling of A and C-ring to produce lycorine. B. Structure-activity relationship of lycorine. The anticancer property of lycorine is largely depended on its structure. The red shades indicate the parts that lycorine requires absolutely to exert its activity. The green shades mean that these part can be changed with chemical modification while the yellow shades represent the part that can be replaced with only a few suitable groups.
3. Pharmacological functions of lycorine
3.1. Antiviral activity
The first reported activity of lycorine as an inhibitor of termination of protein synthesis was found in poliovirus-infected HeLa cells [25]. In subsequent studies lycorine showed moderate to potent antiviral activity and reduced viral titers of herpes simplex virus [26], retrovirus HIV-1 [27], severe acute respiratory syndrome associated coronavirus [28], poliovirus [29], West Nile Virus (WNV), dengue and yellow fever viruses [30], enterovirus 71 [31], influenza virus [32], hepatitis C virus [33] and adult zika virus vector Aedes aegypti [34,35]. Lycorine could not exert antiviral activity against alphavirus, Western equine encephalitis virus, rhabdovirus and vesicular stomatitis virus, suggesting a selective antiviral spectrum of this compound [30]. The antiviral effect of lycorine is due to the multiplication inhibition by blocking of viral polymerase activity or elongation of the viral polyprotein during protein synthesis [26,31]. Structure-activity analysis revealed that the free hydroxyl groups at C-1 and C-2, intact benzodioxole group at A-ring, the basic nitrogen, and the C3-C4 double bond are crucial for the anti-virus activity of lycorine [36].
3.2. Antibacterial effects
The main metabolite of lycorine degradation, ungeremine, and carbamate substitution at C-1 and C-2 of lycorine had stronger antibacterial activity toward fish bacterial pathogen Flavobacterium columnare isolates than lycorine itself [37,38]. Lycorine though shows almost no inhibitory activity against Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus [39], a recent study by Bendaif and colleagues demonstrated the anti-bactericidal effect of lycorine in several bacterial strains, suggesting a preferential antibiotic property of lycorine [40].
3.3. Anti-parasitic properties
Lycorine was found to be the most potent alkaloids against Plasmodium falciparum, Tribolium castaneumand, and Aphis gossypii [14,41]. This compound abolishes the nucleoside triphosphate diphosphohydrolase (NTPDase) and ecto-5′-nucleotidase activities of Trichomonas vaginalisin [42], and also causes cell cycle arrest of this parasite [43]. Lycorine shows significant inhibition of DNA topoisomerase-I activity that is required for cell growth in parasites [44,45].
The lycorine-type alkaloid acetylcaranine and dehydrolycorine has potent inhibitory activity against the malaria parasite. However, another lycorane-compound, hippadine, with slight structural differences of conjugation and substitution in the tetrahydro phenanthridine moiety was inactive [46,47]. Therefore, the structural character of these compounds seems to affect the plasmodium inhibitory activity greatly.
The promising and varied degree of anti-yeast properties of lycorine were detected in several strains of Saccharomyces cerevisiae [48], Cryptococcus laurentii [49] and Candida albicans [39]. The anti-parasitic effects of lycorine depend on the mitochondrial genes, and strains that lack the mitochondrial genome were found to be resistant to high concentrations of this compound [[50], [51], [52], [53], [54]].
3.4. Anti-inflammatory effects
Lycorine precursor norbelladine acts as an anti-inflammatory compound by inhibiting NF-κB activation and the expression of inflammation-related cyclooxygenase at relatively lower concentrations [21]. Inhibition of NF-κB signaling by lycorine suppresses endplate-chondrocyte degeneration and prevents intervertebral disc degeneration pathway [55]. Lycorine itself can block several pro-inflammatory molecules. For example, it can diminish calprotectin-influenced inflammation by targeting calprotectin or blocks lipopolysaccharide (LPS)-induced production of pro-inflammatory mediators such as nitric oxide (NO) and prostaglandin E2 (PGE2) through suppressing p38 and STATs activation [[56], [57], [58], [59], [60]]. Lycorine possess significant anti-inflammatory and hepatoprotective effect on mice at doses ranging from 1 to 2.0 mg/kg [61]. Lycorine-induced inhibition of TNF-α and HMGB1 in hematological malignancies may also contribute to its anti-inflammatory activity.
Degradation of acetylcholine (ACh) by Acetylcholinesterase (AChE) leads to brain cholinergic dysfunction in Alzheimer’s disease (AD) patients and therefore AChE provides a novel therapeutic target for the AD. Assoanine, a lycorine type alkaloid has been demonstrated as an AChE inhibitor with much lower IC50 than an anti-AD drug galanthamine [22,62]. Lycorine also has analgesic, choleretic and body-temperature lowering activity [44].
4. Anti-tumor effect of lycorine
In 1976, Jimenez and colleagues first found the anti-tumor activity of lycorine [63]. Since then numerous researches have been being conducted to discover the anti-neoplastic activity of lycorine that found it as a potent anti-tumor compound against various kind of cancer cells. Lycorine is also effective against tumor xenograft and effectively inhibited tumor growth in B16F10 melanoma-bearing mice [23], HL-60 xenografted SCID mice [64], ovarian cancer Hey1B bearing nude mice [65], and multiple myeloma (MM) cell xenografted NOD/SCID mice [66].
Several criteria of lycorine-induced anti-tumor activity can make this compound an interesting research tool for the anticancer drug design. Specificity: The selectivity of lycorine toward cancer cells is an important criterion among them. The concentration that significantly reduces the viability of cancer cells has trivial toxicity against B-lymphocytes [66,67], normal peripheral mononuclear cells, fibroblastic cells [68], normal breast [69], prostate [70], and urothelial cells [71], mammary epithelial cells [72], and even plasma cells from healthy donor [66]. Low concentration: Lycorine is effective in a very low, single-digit micromolar concentration with the reported studies suggesting the IC50 value usually not exceeded 7.5 μM (Table 1 ). Less toxicity: Lycorine is well tolerable with minimal toxicity as well. Studies with tumor xenografted mouse models revealed that 5 to 15 mg/kg/day of lycorine in tumor-bearing mice did not induce any significant change of body weight indicating the exhibition of no evident signs of toxicity [64,66,70,71]. High potency: Lycorine can enhance the effect of systemically used anti-cancer drugs and in some instance has lesser toxicity than first-line chemotherapeutic drugs. Recently Ying and colleague showed that in mouse breast-tumor cell line 4T1 orthotopic xenograft model lycorine is more potent than toxoids (paclitaxel) and while treatment of paclitaxel led to a significant loss of body weight, lycorine displayed negligible change, indicating the occurrence of less toxicity by this compound [69]. Chronic lymphocytic leukemia (CLL) cells in the lymph node engage with CD40 ligand (CD40 L) expressed by T-cells to stimulate proliferation and protection against apoptotic signals. In a study, it was showed that while in the continuous presence of CD40 L, either dasatinib or combination of dasatinib and fludarabine or bezafibrate and medroxyprogesterone acetate (MPA) fail to induce apoptosis in CLL, addition of lycorine as a third agent is able to overcome the CD40 L protective effects against bezafibrate plus MPA with the desired induction of apoptosis [73]. This compound can synergize the effect of antibodies targeting cytotoxic T-lymphocyte associated protein 4 (CTLA-4) in renal cell carcinoma [74]. Sensitivity against resistance: Lycorine and its synthetic intermediates are sensitive to Adriamycin (Doxorubicin) resistant cells [75]. Furthermore, in a recent study, we have found that lycorine is active against both dexamethasone sensitive and resistant myeloma cells. This compound can enhances bortezomib activity and re-sensitize resistant MM cells to bortezomib as well as inhibits bortezomib induced autophagy, a cellular process that provokes acquired resistance to the proteasome inhibitors [66]. Lycorine also exhibits significant anti-tumor activity against apoptosis-resistant cancer cells [23,76]. All these findings taken together provide an intense potential of lycorine itself as a single agent or in combination with other agents in counteracting cancer.
Table 1.
Anticancer effect of lycorine.
| Cancer Type | Cell lines tested | Time (hours) | IC50 (μM) | Anti-tumor ffecet | Ref. |
|---|---|---|---|---|---|
| Leukemia | HL-60 | 24 | ∼1 | Apoptosis, cell cycle arrest | [68] |
| U937 | 48 | <5 | [77] | ||
| 6T-CEM | 48 | <2 | [77] | ||
| K562 | 48 | <2 | [78] | ||
| Multiple myeloma | U266 | 48 | 0.82 | HDAC and JAK/STAT signaling inhibition, apoptosis, cell cycle arrest, programmed necrosis, autophagy inhibition | [20] |
| KM3 | 48 | 1.25 | [79] | ||
| RPMI-8226 | 48 | 0.70 | |||
| ARH-77 | 24 | 3.33 | |||
| MM.1S | 24 | 4.50 | |||
| H929 | 24 | 4.10 | |||
| Prostate cancer | PC-3M | 24 | 2∼5 | Inhibition of growth and metastasis | [70] |
| DU145 | |||||
| LNCaP | |||||
| 22RV1 | |||||
| Human breast cancer | T47D | 48 | >2 | Inhibition of invasion and metastasis | [69] |
| MDAMB231 | 48 | ∼5 | [72] | ||
| MCF-7 | 72 | 3.9 | [80] | ||
| Human bladder cancer | T24 | 48 | 7.5 | Apoptosis | [71] |
| Ovarian cancer | Hey1B | 24 | 1.2 | Anti-neo vascularization | [65] |
| SK-OV-3 | 72 | 3.3 | [80] | ||
| Non-small cell lung cancer | A549 | 72 | 6.5 | [80] | |
| Large cell lung cancer | NCI-H460 | 72 | 3.3 | [80] | |
| Colon carcinoma | HCT116 | 72 | 3.0 | [80] | |
| Human | HepG2, | 48 | 34.1 | Apoptosis, repression of Akt signaling and autophagy | [81] |
| Hepatocellular | SMMC-7721 | 36.60 | |||
| Carcinoma | HuH-7 | 38.34 | |||
5. Mechanism of anti-tumor activity of lycorine
5.1. Apoptosis induction
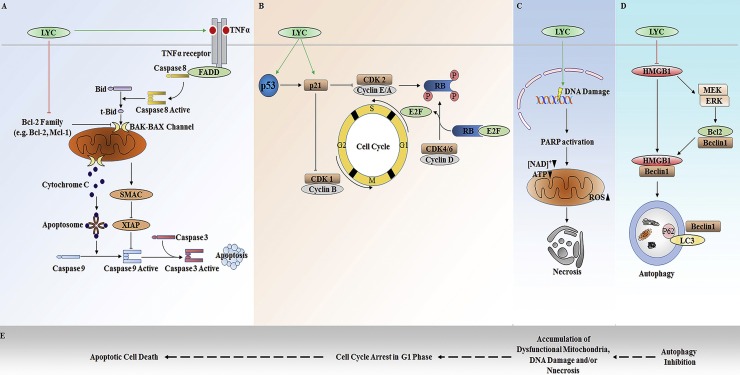
Apoptosis is the most common anti-tumor mechanism of natural compounds that operated by mitochondria-dependent intrinsic or death receptor-dependent extrinsic pathway. The mitochondrial apoptosis pathway is associated with Bcl-2 family members of pro-survival Bcl-2, Bcl-xl, Mcl-1 and pro-apoptotic BAX, BAK, Bid proteins that regulate the disruption of the mitochondrial outer membrane and cytochrome C release. The cytochrome C released from the disrupt mitochondria initiates caspase-9 activation on the scaffold protein APAF1 (apoptotic protease-activating factor 1), whereas the released protein SMAC (second mitochondria-derived activator of caspases) blocks the caspase inhibitor XIAP (X-linked inhibitor of apoptosis protein) thereby promoting apoptosis. The death receptor-mediated (or extrinsic) pathway of apoptosis is activated when certain death receptor ligands of the tumor necrosis factor (TNF) family (such as FAS ligand and TNF) engage their cognate death receptors (FAS and TNFR1, respectively) on the plasma membrane, leading to caspase-8 activation [[77], [78], [79]].
Lycorine is a strong apoptosis inducer, and it can induce both mitochondrial and death receptor-mediated apoptosis in cancer cells. The effect of lycorine on cell apoptosis has been analyzed for breast cancer, bladder cancer and hematological malignancies including leukemia and myeloma. It was found that as an inducer of mitochondrial or intrinsic apoptotic pathway, lycorine downregulates the expression of anti-apoptotic Bcl-2 family proteins and increases the expression of pro-apoptotic BAX [68,80]. Bcl-2 family protein Mcl-1 is also suggested to be a potential molecular target for lycorine-induced apoptosis [ Fig. 3 A]. Down-regulation of Mcl-1 by lycorine at translational level induces apoptosis through cytochrome C release and caspase activation in leukemia cells lacking Bcl-2 without any discernible changes in the levels of BAX, BAK, Bik, Bid, XIAP, c-IAP1 and c-IAP2 and in breast cancer [72,81]. In bladder cancer cells, lycorine suppresses the PI3K-Akt pathway with a corresponding increased expression of the negative regulator of p-Akt, PTEN protein level to activate the intrinsic apoptosis cascade [71]. As the expressional change of PI3K/Akt/mTOR pathway is associated with more than 40% of urinary bladder cancer, there is a promising therapeutic potential of lycorine against this disease [82]. Lycorine also induces apoptosis in lung cancer cells through mTOR pathway [83]. For the induction of extrinsic or death receptor pathway of apoptosis, lycorine was reported to activate death receptor ligands of the tumor necrosis factor (TNF) family TNF-α leading to caspase 8 activation [68,80,84]. It was also found that lycorine decreases the caspase-8 substrate Bid and significantly increases the expression level of truncated form of Bid protein (tBid) with a concomitant release of cytochrome C and activation of caspase-9 and caspase-3, indicating the Bid mediated crosstalk between mitochondria and death receptor apoptotic pathways upon lycorine treatment [84,85]. Lycorine also increases the portion of apoptotic cells in vivo xenografted tumor models. While lycorine has been suggested as an effective compound to induce apoptosis in a number of cancers, induction of apoptosis probably is not the principal mechanism of action by which lycorine exerts its anti-tumor effects in solid tumors. Lycorine inhibited both proliferation and migration in a panel of glioma and melanoma cell lines, which were resistant to apoptosis [86]. In addition, lycorine did not induce apoptosis in glioma and non-small cell lung cancer cells that display resistance to various proapoptotic stimuli rather it exhibited cytostatic effects through impairing the actin cytoskeleton organization leading to inhibition of cell migration and proliferation [23]. This mode of action of lycorine accounts for the inhibition of proliferation and migration of cells displaying resistance to apoptosis and suggests this compound as an excellent lead for combatting cancers which are naturally resistant to pro-apoptotic stimuli.
Fig. 3.
The anticancer mechanism of lycorine. A. Apoptosis induction by lycorine. Lycorine targets Bcl-2 pro-apoptotic family protein and downregulates them to induce intrinsic apoptosis in cells. It can also promote extrinsic apoptosis cascade by upregulating death legands. B. Cell cycle inhibition. Lycorine is suggested to target cell cycle inhibitor p21 to induce cell cycle arrest in cancer cells. C. Lycorine induced program necrosis is associated with increased ROS generation, DNA damage and ATP depletion. D. Lycorine inhibits autophagy in cancer cells. It can promote proteasomal degradation of important autophagy regulator HMGB1 and thereby sequestrates Beclin1 with Bcl-2 resulting in autophagy inhibition. E. The proposed combinatory anticancer mechanism of lycorine. Lycorine inhibits autophagy, causing accumulation of damaged mitochondria that lead to increased generation of ROS. These ROS may induce DNA damage and at the same time, cells with damaged-DNA may arrest their cell cycle at a specific checkpoint. The populations of cells with arrested cell cycle then commit death in a programmed manner either by apoptosis or by necrosis. Experimental detail is required to verify this postulation (LYC; Lycorine).
5.2. Cell cycle inhibition
Lycorine induces cell cycle arrest at G0/G1 or G2/M phase depending on the cancer type [ Fig. 3 B]. During the cell cycle, phase-specific cyclins, cyclin-dependent kinases (CDKs) and Cip/Kip or INK family CDK inhibitors (CKI) orchestrate the progression of cells through G1, S, G2 and M phases. Lycorine induces G1 phase cell cycle arrest in multiple myeloma and chronic myelocytic leukemia cell lines with downregulation of cyclin D and CDK4 [80,87,88]. Whereas, in ovarian cancer cell line HeyB1 and acute promyelocytic leukemia cell line HL-60 this compound induces cell cycle arrest at G2/M phase [65,85]. In response to growth factors, CDK4 and CDK6 combined with one of several D-type cyclins in early G1 phase. In the later phase, cyclin E and cyclin A is synthesized and form a conjugate with CDK2 that phosphorylates retinoblastoma (RB) protein allowing dissociation of RB from E2f transcription factors and E2f target gene expression. Mitogenic signal promotes the cell cycle progression from G2 phase to M phase mediated by the activity of cyclin B-associated CDK1 [89]. The key member of the Cip/Kip cyclin-dependent kinase inhibitor family, p21 can bind to and directly inhibit the activity of cyclin E-Cdk2 and cyclin B-Cdc2 [[90], [91], [92]]. Regardless of cancer cell type, lycorine increases the expression of p21 and hence it is suggested that p21 mediates lycorine-induced cell cycle arrest, either in G1 or G2 phase. The tumor suppressor p53 regulates the expression and activity of p21. Although lycorine can induce p21 expression to inhibit cell cycle progression, the upstream effector for this upregulation of p21 by lycorine is not clear yet. Lycorine was found to upregulate the expression of p53 as an upstream inducer of p21. However, it can also up-regulate p21 in p53-deficient leukemia cell lines [84,88]. Therefore, it is implied that the up-regulation of p21 mediated by p53-independent pathways, and that p21 is a possible direct target of lycorine for exerting the cell cycle inhibitory activity of this compound.
5.3. Necrotic cell death
Necrosis was formally regarded as an accidental cell death; however, upon DNA damage cells undergo necrosis in a programmed and regulated way as a consequent of the normal physiological process [93,94]. Although controlled by a manner similar to apoptosis, necrosis is a caspase expendable process involving signaling pathways distinct from apoptotic cell death where receptor-interacting protein 1 (RIP1, RIPK1) and receptor-interacting protein-3 (RIP3, RIPK3) act as initiators or effectors and necrostatin-1 (Nec-1) acts as an inhibitor of necrosis [[95], [96], [97]]. Study found that lycorine can lead to programmed necrosis with upregulation of RIP1 and RIP3 expression. The necrosis-inducing effect of lycorine was also associated with mitochondrial dysfunction, reactive oxygen species (ROS) generation, ATP depletion, and DNA damage [ Fig. 3 C] [87].
5.4. Autophagy inhibition
Autophagy is a component of stress management system of the cells that is utilized by the cell for removal of defective organelles. Autophagy acts as a survival strategy in established and advanced tumors by maintaining cellular energy levels and enhances chemo-sensitivity of a number of anticancer agents to induce cell death [[98], [99], [100], [101], [102]]. It is not clear yet, how autophagy mediates the pro-survival effects in cancerous cells, the mechanism may involve limiting DNA-damage response-mediated apoptosis or, activation of the high-mobility group box 1 (HMGB1)/ receptor for advanced glycation end products (RAGE) signaling axis [[103], [104], [105]]. Human high-mobility group box 1 protein (HMGB1), a 215 amino acid residues molecule, was named for its electrophoretic mobility on polyacrylamide gels and has been implicated in several human diseases including significant involvement in oncogenesis [106]. The action of HMGB1 depends on its subcellular localization, and this molecule is important in stress signaling as well as in autophagy activation playing extracellular, cytoplasmic and nuclear function [[107], [108], [109], [110]]. Lycorine inhibits autophagy by downregulating the expression of HMGB1 [ Fig. 3 D]. Lycorine-induced proteasomal degradation of HMGB1 inhibits the activation of MEK-ERK signaling pathway thereby decreases Bcl-2 phosphorylation leading to the constitutive association of Bcl-2 with Beclin-1 which eventually results in autophagy inhibition [66].
Combining the above studies, a possible link between the anticancer effects of lycorine can be postulated [ Fig. 3 E]. Autophagy is the key cellular process for the turnover of damaged mitochondria in cell and mitochondria are the main sources of cellular ROS [[111], [112], [113]]. Inhibition of autophagy by lycorine increases the accumulation of damaged mitochondria in cell. Although not confirmed, there is a probability that these damaged mitochondria are the sources of increased ROS after lycorine treatment, that leads to ATP depletion and DNA damage resulting in necrotic cell death. On the other hand, cell cycle arrest following DNA damage is a critical characteristic that prohibits cells from going them through mitosis [114,115]. Hence, it is possible that the cell cycle arrest observed after lycorine treatment is the result of DNA damage caused by this compound. Therefore, lycorine-induced DNA damage may cause activation of p21 for the inhibition of the cell cycle and ultimately lead to the apoptotic death of cells. However, the link between these cellular effect induced by lycorine is not experimentally proved, and further research is in demand to clarify the mechanism.
5.5. Inhibition of invasion and metastasis
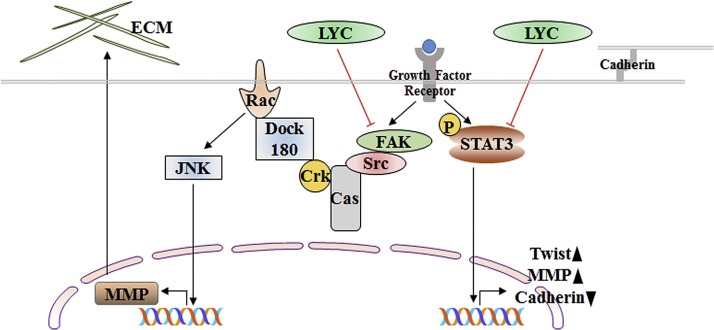
In addition to the effect of lycorine on cell death and cell cycle arrest, it can also inhibit invasion and metastasis in solid tumors [ Fig. 4 ]. Mechanistically, by modulating either Src/FAK (focal adhesion kinase) or STAT3/Twist signaling cascade, lycorine blocks the matrix-degrading metalloproteinases (MMP) mediated degradation of extracellular matrix. Lycorine inhibits breast cancer cell invasion and migration by blocking the Src/FAK-involved pathway [69]. FAK signaling in cell invasion involves proteolytic degradation of the extracellular matrix by MMP. FAK coordinates an Src-Cas complex which in interaction with Crk initiates the signaling from Rac1 to Jun N-terminal kinase (JNK) mediated transcriptional activation of MMP [[116], [117], [118]]. Treatment with lycorine significantly decreases p-FAK, p-Src, p-c-Jun, p-JNK and MMP2 in breast cancer cell lines, demonstrating the effect of lycorine in blocking matrix degradation through the Src/FAK signaling pathway to inhibit migration and invasion. Furthermore, lycorine has inhibitory effects on epidermal growth factor-induced epithelial-mesenchymal transition (EMT). In a number of studies, STAT3 was revealed to bind to the promoter of EMT-related transcription factor Twist to modulate its expression and thereby promotes the EMT process and increases the cells invasion and migration [[119], [120], [121]]. Prostate cancer cells exposed to lycorine shows significant epithelial characteristics and reduced mesenchymal features with decrease expression of N-cadherin, vimentin and fibronectin and a corresponding increase expression of E-cadherin. The reversal of growth factor-induced EMT by lycorine was associated with the inhibition of the expression of Twist and suppression of the expression of migration markers, MMP2 and MMP9. The twist is a target gene of STAT3 and lycorine-induced downregulation of Twist and thereby suppression of EMT is directly mediated by inhibition of STAT3. STAT3 is also suggested a potential target of lycorine-induced anticancer effect as no significant decrease in cell viability was observed in the STAT3 knockdown prostate cancer cell [70]. In non-small cell lung cancer, lycorine effectively suppresses the Wnt/β-catenin signaling for EMT reversal [122].
Fig. 4.
Mechanism of lycorine for inhibition of invasion and migration. Growth factors promote the FAK-JNK or STAT3 signaling that can induce the expression of certain genes like MMP. These genes can destroy the ECM or can down-regulate the expression of other genes like cadherin that maintains cell-cell connections. Lycorine downregulates the expression of FAK and inhibits FAK-JNK signaling resulting in MMP down-regulation. Lycorine can also inhibit STAT3 signaling, decreased the expression of TWIST and MMP and upregulates the expression of cadherin to maintain the cell junction (LYC; Lycorine).
Apart from the above mention effects of lycorine, it can also modulate several signaling pathways to regulate growth and proliferation of cancer cells. These include the JNK pathway [69], the phosphor-Akt pathway [71,123], the MEK/ERK pathway [66] and the JAK/STAT signaling pathway [67].
6. Structural features required for anti-tumor activity of lycorine
The structure-activity relationship (SAR) enables the determination of the chemical or 3D structure responsible for a target biological activity of a molecule. This allows the changes of chemical groups to modify the effect, potency, and bioavailability of the given molecule. Therefore, it is important to understand the structural features of a bioactive compound for further research and development of that compound into a clinically available drug.
The requirement of structural chemistry of lycorine greatly varies depending on its pharmacological activity. For example, the opening of the dioxolane ring results in loss of activity against dengue and hepatitis C virus (HCV), but substituents with suitable groups at this position can facilitate specific binding and activate Wnt signaling [[124], [125], [126]]. Wnt/β-catenin signaling pathway is very important for the development and is highly conserved. Lycorine derivatives that can act as an agonist of this pathway need the C—C single bond (C-11, C-12) of the thiazole ring-D [126]. The carbon double bond at ring-C (C-3, C-4) is vital for the anti−HCV or anti plasmodial activity and derivatives that possess free hydroxyl groups at C-1 and C-2 or esterified as acetates are active [125,14]. Although di-substitution on the free hydroxyl groups at C-1 and C-2 clearly reduces the cytotoxicity, an unsaturated ketone at either of these positions would improve the HCV inhibitory activity of lycorine [125,127]. The ketone substitution at C-2 is also effective for the anti-dengue activity of lycorine. At the same time, substitution with linear aliphatic on C-1 would enhance the activity, while the increase in carbon chain with bulky substituents or any aromatic substitution result in loss of activity against dengue virus [124,128]. The heme-containing mono-oxygenase enzymes Cytochromes P450 (CYP450) are involved in the detoxification of a wide variety of xenobiotics including environmental toxins and drugs. Preliminary structure-activity relationship points that either C-1 or C-2 substitution with bulky substances in the lycorine results in potent CYP450 inhibitory activity while di-substitution at these positions only moderately retrieve the activity [129]. The unprotected −OH groups at C-1 and C-2 position are also not necessary to the antiparasitic activity of lycorine derivatives [130]. Lycorine series of Amaryllidaceae alkaloids require a hydrogen-bond acceptor at the C-1 for acetylcholinesterase and butyrylcholinesterase inhibitory activity and the lipophilic substituent at C-2 increases the activity. However, oxidation at C2 is supposed to deactivate the compound of its inhibitory effect [131,132]. The common structural characteristics need for the pharmacologic activity of lycorine derivatives include, the planarity of the molecule, basic nitrogen, and free hydroxyl group.
Numerous studies that were aimed to determine the SAR of lycorine, found several structural features required for its anti-tumor activity [ Table 2 and Fig. 2 B]. The presence of the unaltered hydroxyl groups at C-1 and C-2 position in its original form is essential for the anti-tumor property of lycorine [23]. However, analogs incorporating changes of the diol in the C-ring by sterically bulky substituents was well tolerated. The activity is likely attributed by intracellular nucleophilic substitution of these derivatives to lycorine with water. Although the hydrophobic elements in C-ring are not part of the pharmacophore, they assist the molecule in cell penetration and any substitution at these positions that lacking the ability to intracellular hydrolysis is either inactive or moderately active [133]. The presence of nucleophilic sites in C-ring at positions C-1 and C-2 is also a structural feature required for the inhibition of amino acid biosynthesis by lycorine [23].
Table 2.
Synthetic or natural derivatives of lycorine and their anticancer activity compare to lycorine.
 | |||
|---|---|---|---|
| Position | Substitution or derivate | Activity | Ref |
| Ring-A | Open dioxole | Moderately active | [23] |
| Open dioxole with absence of -OH at C2 | Inactive | [23] | |
| Ring-B | C7-oxidation, aromatic C-ring | Inactive | [23,133] |
| C7-oxidation | Decrease | [133] | |
| C7-thiol | Inactive | [133] | |
| Quaternization of N | Inactive | [23] | |
| Ring-C | 1-acetyl | Decrease | [23,134,135] |
| 1-acetyl, 2-silyl | Inactive | [134] | |
| 1-alkyl, 2-silyl ether | Moderately active | [86,135] | |
| 1-silyl, 2-alkyl ether | Inactive | [86,134] | |
| 2-amines | Decrease | [136] | |
| 2-β acetyl | Inactive | [23] | |
| 2-epoxide | Increase | [136] | |
| 2-esters | Decrease | [136] | |
| 2-methoxy | Equipotent | [23,134] | |
| 1 or 2 methyl ether | Inactive | [86] | |
| 1 or 2 benzoate | Equipotent | [133] | |
| 1, 2-diacetyl | Inactive | [23,134,135] | |
| 1, 2-dicarbonate | Equipotent | [133] | |
| 1, 2-diether | Decrease | [86] | |
| 1,2-dipropionate | Equipotent | [133] | |
| 1,2-disilyl | Inactive | [134] | |
| 1,2,3,4-tetraol | Inactive | [133] | |
| 2.3-diallyl | Equipotent | [133] | |
| Aromatic C ring | Selective | [23] | |
| Oxiadation of C3-C4 double bond | Inactive | [23,136] | |
| Steriochemistry change | Inactive | [23] | |
| Ring-D (narciclasine type) | Narciclasine | Equipotent | [135,137] |
| Narciclasine tetraacetate | Equipotent | [135] | |
| C10b-R-hydroxypancratistatin | Moderately active | [135] | |
| Cis-dihydronarciclasine | Moderately active | [135] | |
| Trans-dihydronarciclasine | Decrease | [135] | |
| Ring-D (crinine type) | Haemanthamine | Equipotent | [135] |
| Buphanamine | Inactive | [135] | |
| 11-hydroxyvittatine | Inactive | [135,138,139] | |
| Natural derivatives | Pseudolycorine | Moderately active | [23,135] |
| Amarbellisine | Moderately active | [135] | |
| Ungeremine | Inactive | [23,135] | |
| Norpluiine | Inactive | [23,135] | |
| Lycorene | Inactive | [23] | |
There is an absolute requirement for the presence of a basic nitrogen at position N-6 of the B-ring. Quaternization by incorporation of methyl iodide or amide to this nitrogen that provides the non-basic character of the nitrogen atom resulted in a loss of activity. Exceptions to this rule are ring-C aromatized lycorine analogs, however, they are active against some but not all of the cancer cell lines [23,133].
Another structural feature required for the anti-tumor activity of lycorine is the stereochemistry and conformational freedom of the C-ring. The intact B and C-rings and β-conformation of D-ring are required for both anti-tumor effects and inhibition of ascorbic acid synthesis function of lycorine. Any di-hydro derivative at C-ring shows the absence of anti-tumor activity. However, transposition of the double bonds in C-ring proved to be equipotent to lycorine. In addition, derivatives with the open conformation of the A-ring retained similar activity as lycorine, suggests that this ring constitute no structural feature that is essential for the anti-tumor activity [9,23].
Other features that might provide useful guidelines for future analog design are lipophilicity and conjugation of long-chain fatty acid. Although the derivatives with fatty acid conjugate did not show elevated potencies, they are proved to be chemically stable in a pH range of 5-7.4. Furthermore, the difficulty of penetrating the cell for lycorine derivatives with highly hydrophilic substituent renders them inactive [133].
7. Pharmacokinetic aspects of lycorine
Despite extensive researches in the pharmacological activities of lycorine, little is known about its pharmacokinetics due to the lack of reliable methods for the pharmacokinetic study of lycorine. Preclinical studies have shown that there is no significant difference in the pharmacokinetic parameters of lycorine after either intravenous (IV) or intraperitoneal (IP) administration. However, these parameters vary with the methods applied for the pharmacokinetic studies. The maximum plasma concentration (Cmax) and area under the curve (AUC) of lycorine increase with the dose, although not proportionally. In general, plasma half-life (t1/2) of lycorine at a dose of 10 mg/kg is 3–5 h while the maximum concentration (Tmax) reaches at around 10 to 15 min [140,141]. Lycorine and galanthamine are the most abundant alkaloids isolated from Amaryllidaceae plants and the US food and drug administration (FDA) have already approved galanthamine as a prescription drug [142]. Although both of these compounds possess very similar chemical structures, their pharmacokinetic parameters following oral administration are found to be different. The AUC of lycorine is higher than that of galanthamine. However, galanthamine was found to be absorbed more quickly and eliminate more slowly from the body than lycorine probably due to their metabolic status differences in the intestine and/or liver [143]. Lycorine is extensively distributed in all tissues of the animal body such as liver, spleen, heart, brain, lung, kidney and stomach, and to be undetectable within 2 h after administration. Regardless of the route, 15 min post-administration concentration of lycorine was higher in kidney and lower in the liver. This implies that large quantity of lycorine is eliminated through the kidney and that it may metabolize in the liver [140]. In vivo experiments in beagle dog analyzing plasma (BP) and in vitro incubations with rat liver microsomes (RLM) have also found phase-I metabolites confirming the metabolic site of lycorine to be liver [144,145]. Furthermore, electrochemical (EC) metabolism simulation suggested that lycorine undergoes loss of water to form the phase-I metabolite ungeremine [145]. However, no phase-II conjugation products were found in BP, RLM or EC study.
8. Future perspectives
The phenotypic screening of natural compounds is followed by the identification of new or potential therapeutic targets that are indispensable for tumor development. No specific target for lycorine-induced anticancer effect have been identified so far. However, studies provided several potential targets for lycorine action including Bcl-2 family proteins Bcl-2 and Mcl-1, HDAC, TNF-α, STAT and HMGB1. Given that lycorine induces multiple anti-neoplastic effects, the existence of diverse molecular target of lycorine is probable. Nonetheless, specific molecular targets for lycorine such as enzymes, receptors, DNA or RNA materials should be investigated. Studies should be directed to the pharmacokinetic, safety and toxicological features to achieve a better understanding of bioavailability, metabolism, and tissue distribution behavior of this compound. High capability of lycorine for combating cancers offers motivation to medicinal and organic chemists to investigate the chemistry and active moiety associated with this alkaloid to make this compound more bioavailable and lower toxic. It is worth mentioning at this point that formulation of mannosylated lycorine-oleic acid nano-emulsion showed improved lipophilicity of and cellular uptake of lycorine with preferable growth inhibition activity [146]. Considering the activity of lycorine in resistant cells and potential in combination with other drugs, further exploration of the mechanism of lycorine in the context of resistant as a single agent or in combination with other drugs is needed. Furthermore, for the better understanding the mechanisms and metabolism of lycorine, basic molecular biological and biochemical investigations should be continued. Concerning the high morbidity of cancer worldwide with fewer effective therapies, this is the right time to work with this natural compound for improving the present cancer treatment in clinical settings.
9. Conclusion
The Amaryllidaceae alkaloid lycorine is proved to be a potent anticancer molecule that works in a low concentration and evident to be well tolerated to the animal. In this article we have outlined the anticancer mechanism of lycorine, discussed the structural characteristics related to the activity of this molecule and pharmacological aspects associated. The studies summarized in this review provides the biologist and chemist rational for further exploration of lycorine for bringing this natural compound from bench to bedside.
Author’s contribution
Mridul Roy designed the review, reviewed literature and drafted the manuscript. Long Liang, Xiaojuan Xiao, and Peifu Feng reviewed literature. Mao Ye and Jing Liu developed the idea and designed the review, analyzed, interpreted the data and revised the manuscript.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgement
This work was supported by grants 81600184, 81770107 and 81270576 from National Natural Science Foundation of China.
References
- 1.Borchardt J.K. The beginnings of drug therapy: ancient mesopotamian medicine. Drug News Perspect. 2002;15(3):187–192. doi: 10.1358/dnp.2002.15.3.840015. [DOI] [PubMed] [Google Scholar]
- 2.Ngo L.T., Okogun J.I., Folk W.R. 21st century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013;30(4):584–592. doi: 10.1039/c3np20120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cragg G.M., Newman D.J. Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta. 2013;1830(6):3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin Y.W., Balunas M.J., Chai H.B., Kinghorn A.D. Drug discovery from natural sources. AAPS J. 2006;8(2):E239–53. doi: 10.1007/BF02854894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cragg G.M., Newman D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005;100(1-2):72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee A.K., Basu S., Sarkar N., Ghosh A.C. Advances in cancer therapy with plant based natural products. Curr. Med. Chem. 2001;8(12):1467–1486. doi: 10.2174/0929867013372094. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z., Wang N., Chen J., Shen J. Emerging glycolysis targeting and drug discovery from chinese medicine in cancer therapy. Evid. Based Complem. Altern. Med.: eCAM. 2012;(2012) doi: 10.1155/2012/873175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Q., Ma D. Recent progress on the total synthesis of natural products in China. Nat. Prod. Rep. 2006;23(5):772–788. doi: 10.1039/b609469b. [DOI] [PubMed] [Google Scholar]
- 9.Lamoral-Theys D., Decaestecker C., Mathieu V., Dubois J., Kornienko A., Kiss R., Evidente A., Pottier L. Lycorine and its derivatives for anticancer drug design. Mini Rev. Med. Chem. 2010;10(1):41–50. doi: 10.2174/138955710791112604. [DOI] [PubMed] [Google Scholar]
- 10.Nair J.J., Wilhelm A., Bonnet S.L., van Staden J. Antibacterial constituents of the plant family Amaryllidaceae. Bioorg. Med. Chem. Lett. 2017;27(22):4943–4951. doi: 10.1016/j.bmcl.2017.09.052. [DOI] [PubMed] [Google Scholar]
- 11.Cimmino A., Masi M., Evidente M., Superchi S., Evidente A. Amaryllidaceae alkaloids: absolute configuration and biological activity. Chirality. 2017;29(9):486–499. doi: 10.1002/chir.22719. [DOI] [PubMed] [Google Scholar]
- 12.Nair J.J., van Staden J. Antifungal constituents of the plant family Amaryllidaceae. Phytother. Res. 2018 doi: 10.1002/ptr.6049. [DOI] [PubMed] [Google Scholar]
- 13.Hulcova D., Breiterova K., Siatka T., Klimova K., Davani L., Safratova M., Hostalkova A., De Simone A., Andrisano V., Cahlikova L. Amaryllidaceae alkaloids as potential glycogen synthase Kinase-3beta inhibitors. Molecules. 2018;23(4) doi: 10.3390/molecules23040719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cedron J.C., Gutierrez D., Flores N., Ravelo A.G., Estevez-Braun A. Synthesis and antiplasmodial activity of lycorine derivatives. Bioorg. Med. Chem. 2010;18(13):4694–4701. doi: 10.1016/j.bmc.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Lin L.Z., Hu S.F., Chai H.B., Pengsuparp T., Pezzuto J.M., Cordell G.A., Ruangrungsi N. Lycorine alkaloids from Hymenocallis littoralis. Phytochemistry. 1995;40(4):1295–1298. doi: 10.1016/0031-9422(95)00372-e. [DOI] [PubMed] [Google Scholar]
- 16.Shawky E. Phytochemical and biological investigation of Clivia nobilis flowers cultivated in Egypt. Iran. J. Pharm. Res. 2016;15(3):531–535. [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Z.F., Yang P., Zhou Q.S. Multiple biological functions and pharmacological effects of lycorine. Sci. China Chem. 2013;56(10):1382–1391. doi: 10.1007/s11426-013-4967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ptak A., El Tahchy A., Dupire F., Boisbrun M., Henry M., Chapleur Y., Mos M., Laurain-Mattar D. LCMS and GCMS for the screening of alkaloids in natural and in vitro extracts of Leucojum aestivum. J. Nat. Prod. 2009;72(1):142–147. doi: 10.1021/np800585c. [DOI] [PubMed] [Google Scholar]
- 19.Yamada K., Yamashita M., Sumiyoshi T., Nishimura K., Tomioka K. Total synthesis of (-)-lycorine and (-)-2-epi-lycorine by asymmetric conjugate addition cascade. Org. Lett. 2009;11(7):1631–1633. doi: 10.1021/ol9003564. [DOI] [PubMed] [Google Scholar]
- 20.Jin Z. Amaryllidaceae and sceletium alkaloids. Nat. Prod. Rep. 2016;33(11):1318–1343. doi: 10.1039/c6np00068a. [DOI] [PubMed] [Google Scholar]
- 21.Park J.B. Synthesis and characterization of norbelladine, a precursor of Amaryllidaceae alkaloid, as an anti-inflammatory/anti-COX compound. Bioorg. Med. Chem. Lett. 2014;24(23):5381–5384. doi: 10.1016/j.bmcl.2014.10.051. [DOI] [PubMed] [Google Scholar]
- 22.Elgorashi E.E., Stafford G.I., Van Staden J. Acetylcholinesterase enzyme inhibitory effects of amaryllidaceae alkaloids. Planta Med. 2004;70(3):260–262. doi: 10.1055/s-2004-818919. [DOI] [PubMed] [Google Scholar]
- 23.Lamoral-Theys D., Andolfi A., Van Goietsenoven G., Cimmino A., Le Calve B., Wauthoz N., Megalizzi V., Gras T., Bruyere C., Dubois J., Mathieu V., Kornienko A., Kiss R., Evidente A. Lycorine, the main phenanthridine Amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: an investigation of structure-activity relationship and mechanistic insight. J. Med. Chem. 2009;52(20):6244–6256. doi: 10.1021/jm901031h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair J.J., van Staden J. Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae. Nat. Prod. Commun. 2014;9(8):1193–1210. [PubMed] [Google Scholar]
- 25.Vrijsen R., Vanden Berghe D.A., Vlietinck A.J., Boeye A. Lycorine: a eukaryotic termination inhibitor? J. Biol. Chem. 1986;261(2):505–507. [PubMed] [Google Scholar]
- 26.Renard-Nozaki J., Kim T., Imakura Y., Kihara M., Kobayashi S. Effect of alkaloids isolated from Amaryllidaceae on herpes simplex virus. Res. Virol. 1989;140(2):115–128. doi: 10.1016/s0923-2516(89)80089-5. [DOI] [PubMed] [Google Scholar]
- 27.Szlavik L., Gyuris A., Minarovits J., Forgo P., Molnar J., Hohmann J. Alkaloids from Leucojum vernum and antiretroviral activity of Amaryllidaceae alkaloids. Planta Med. 2004;70(9):871–873. doi: 10.1055/s-2004-827239. [DOI] [PubMed] [Google Scholar]
- 28.Li S.Y., Chen C., Zhang H.Q., Guo H.Y., Wang H., Wang L., Zhang X., Hua S.N., Yu J., Xiao P.G., Li R.S., Tan X. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang Y.C., Chu J.J., Yang P.L., Chen W., Yates M.V. Rapid identification of inhibitors that interfere with poliovirus replication using a cell-based assay. Antiviral Res. 2008;77(3):232–236. doi: 10.1016/j.antiviral.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou G., Puig-Basagoiti F., Zhang B., Qing M., Chen L., Pankiewicz K.W., Felczak K., Yuan Z., Shi P.Y. A single-amino acid substitution in West Nile virus 2K peptide between NS4A and NS4B confers resistance to lycorine, a flavivirus inhibitor. Virology. 2009;384(1):242–252. doi: 10.1016/j.virol.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J., Yang Y., Xu Y., Ma C., Qin C., Zhang L. Lycorine reduces mortality of human enterovirus 71-infected mice by inhibiting virus replication. Virol. J. 2011;8(483):8–483. doi: 10.1186/1743-422X-8-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J., Qi W.B., Wang L., Tian J., Jiao P.R., Liu G.Q., Ye W.C., Liao M. Amaryllidaceae alkaloids inhibit nuclear-to-cytoplasmic export of ribonucleoprotein (RNP) complex of highly pathogenic avian influenza virus H5N1. Influenza Other Respir. Viruses. 2013;7(6):922–931. doi: 10.1111/irv.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen D., Cai J., Yin J., Jiang J., Jing C., Zhu Y., Cheng J., Di Y., Zhang Y., Cao M., Li S., Peng Z., Hao X. Lycorine-derived phenanthridine downregulators of host Hsc70 as potential hepatitis C virus inhibitors. Future Med. Chem. 2015;7(5):561–570. doi: 10.4155/fmc.15.14. [DOI] [PubMed] [Google Scholar]
- 34.Masi M., van der Westhuyzen A.E., Tabanca N., Evidente M., Cimmino A., Green I.R., Bernier U.R., Becnel J.J., Bloomquist J.R., van Otterlo W.A., Evidente A. Sarniensine, a mesembrine-type alkaloid isolated from Nerine sarniensis, an indigenous South African Amaryllidaceae, with larvicidal and adulticidal activities against Aedes aegypti. Fitoterapia. 2017;116:34–38. doi: 10.1016/j.fitote.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Masi M., Cala A., Tabanca N., Cimmino A., Green I.R., Bloomquist J.R., van Otterlo W.A., Macias F.A., Evidente A. Alkaloids with activity against the zika virus vector Aedes aegypti (L.)-Crinsarnine and Sarniensinol, two new crinine and mesembrine type alkaloids isolated from the South African Plant Nerine sarniensis. Molecules. 2016;21(11) doi: 10.3390/molecules21111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen D., Cai J., Cheng J., Jing C., Yin J., Jiang J., Peng Z., Hao X. Design, Synthesis and Structure-Activity Relationship Optimization of Lycorine Derivatives for HCV Inhibition. Sci. Rep. 2015;5(14972) doi: 10.1038/srep14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casu L., Cottiglia F., Leonti M., De Logu A., Agus E., Tse-Dinh Y.C., Lombardo V., Sissi C. Ungeremine effectively targets mammalian as well as bacterial type I and type II topoisomerases. Bioorg. Med. Chem. Lett. 2011;21(23):7041–7044. doi: 10.1016/j.bmcl.2011.09.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan C.X., Schrader K.K., Mizuno C.S., Rimando A.M. Activity of lycorine analogues against the fish bacterial pathogen Flavobacterium columnare. J. Agric. Food Chem. 2011;59(11):5977–5985. doi: 10.1021/jf200452z. [DOI] [PubMed] [Google Scholar]
- 39.Locarek M., Novakova J., Kloucek P., Host’alkovia A., Kokoska L., Lucie G., Safratova M., Opletal L., Cahlikova L. Antifungal and antibacterial activity of extracts and alkaloids of selected Amaryllidaceae species. Nat. Prod. Commun. 2015;10(9):1537–1540. [PubMed] [Google Scholar]
- 40.Bendaif H., Melhaoui A., Ramdani M., Elmsellem H., Douez C., El Ouadi Y. Antibacterial activity and virtual screening by molecular docking of lycorine from Pancratium foetidum Pom (Moroccan endemic Amaryllidaceae) Microb. Pathog. 2018;115:138–145. doi: 10.1016/j.micpath.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 41.Abbassy M.A., el-Gougary O.A., el-Hamady S., Sholo M.A. Insecticidal, acaricidal and synergistic effects of soosan, Pancratium maritimum extracts and constituents. J. Egypt. Soc. Parasitol. 1998;28(1):197–205. [PubMed] [Google Scholar]
- 42.Giordani R.B., Weizenmann M., Rosemberg D.B., De Carli G.A., Bogo M.R., Zuanazzi J.A., Tasca T. Trichomonas vaginalis nucleoside triphosphate diphosphohydrolase and ecto-5’-nucleotidase activities are inhibited by lycorine and candimine. Parasitol. Int. 2010;59(2):226–231. doi: 10.1016/j.parint.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Giordani R.B., Vieira Pde B., Weizenmann M., Rosemberg D.B., Souza A.P., Bonorino C., De Carli G.A., Bogo M.R., Zuanazzi J.A., Tasca T. Lycorine induces cell death in the amitochondriate parasite, Trichomonas vaginalis, via an alternative non-apoptotic death pathway. Phytochemistry. 2011;72(7):645–650. doi: 10.1016/j.phytochem.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 44.Cortese I., Renna G., Siro-Brigiani G., Poli G., Cagiano R. Pharmacology of lycorine. 1) Effect on biliary secretion in the rat. Boll. Soc. Ital. Biol. Sper. 1983;59(9):1261–1264. [PubMed] [Google Scholar]
- 45.Nino J., Hincapie G.M., Correa Y.M., Mosquera O.M. Alkaloids of Crinum x powellii "Album" (Amaryllidaceae) and their topoisomerase inhibitory activity. Z. Naturforsch. C. 2007;62(3-4):223–226. doi: 10.1515/znc-2007-3-411. [DOI] [PubMed] [Google Scholar]
- 46.Cho N., Du Y., Valenciano A.L., Fernandez-Murga M.L., Goetz M., Clement J., Cassera M.B., Kingston D.G.I. Antiplasmodial alkaloids from bulbs of Amaryllis belladonna Steud. Bioorg. Med. Chem. Lett. 2018;28(1):40–42. doi: 10.1016/j.bmcl.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao B., Shen S.F., Zhao Q.J. Cytotoxic and antimalarial amaryllidaceae alkaloids from the bulbs of Lycoris radiata. Molecules. 2013;18(3):2458–2468. doi: 10.3390/molecules18032458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Giudice L., Massardo D.R., Manna F., Evidente A., Randazzo G., Wolf K. Differential effect of the alkaloid lycorine on rho (+), mit (-), rho (-), and rho (o) strains of Saccharomyces cerevisiae. Curr. Genet. 1984;8(7):493–498. doi: 10.1007/BF00410435. [DOI] [PubMed] [Google Scholar]
- 49.Onofri S., Barreca D., Garuccio I. Effects of lycorine on growth and effects of L-galactonic acid-gamma-lactone on ascorbic acid biosynthesis in strains of Cryptococcus laurentii isolated from Narcissus pseudonarcissus roots and bulbs. Antonie Van Leeuwenhoek. 2003;83(1):57–61. doi: 10.1023/a:1022903504795. [DOI] [PubMed] [Google Scholar]
- 50.Del Giudice A., Massardo D.R., Manna F., Koltovaya N., Hartings H., Del Giudice L., Wolf K. Correlation of resistance to the alkaloid lycorine with the degree of suppressiveness in petite mutants of Saccharomyces cerevisiae. Curr. Microbiol. 1997;34(6):382–384. doi: 10.1007/s002849900200. [DOI] [PubMed] [Google Scholar]
- 51.Massardo D.R., Manna F., Del Giudice L., Wolf K. Interactions between the yeast mitochondrial and nuclear genomes: isogenic suppressive and hypersuppressive petites differ in their resistance to the alkaloid lycorine. Curr. Genet. 1990;17(5):455–457. doi: 10.1007/BF00334527. [DOI] [PubMed] [Google Scholar]
- 52.Del Giudice L., Massardo D.R., Pontieri P., Wolf K. Interaction between yeast mitochondrial and nuclear genomes: null alleles of RTG genes affect resistance to the alkaloid lycorine in rho0 petites of Saccharomyces cerevisiae. Gene. 2005;354:9–14. doi: 10.1016/j.gene.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 53.Massardo D.R., Manna F., Schafer B., Wolf K., Del Giudice L. Complete absence of mitochondrial DNA in the petite-negative yeast Schizosaccharomyces pombe leads to resistance towards the alkaloid lycorine. Curr. Genet. 1994;25(1):80–83. doi: 10.1007/BF00712972. [DOI] [PubMed] [Google Scholar]
- 54.Massardo D.R., Zweifel S.G., Gunge N., Miyakawa I., Sando N., Del Giudice A., Wolf K., Del Giudice L. Use of lycorine and DAPI staining in Saccharomyces cerevisiae to differentiate between rho0 and rho- cells in a cce1/delta cce1 nuclear background. Can. J. Microbiol. 2000;46(11):1058–1065. doi: 10.1139/w00-096. [DOI] [PubMed] [Google Scholar]
- 55.Wang G., Huang K., Dong Y., Chen S., Zhang J., Wang J., Xie Z., Lin X., Fang X., Fan S. Lycorine suppresses endplate-chondrocyte degeneration and prevents intervertebral disc degeneration by inhibiting NF-kappaB signalling pathway. Cell. Physiol. Biochem. 2018;45(3):1252–1269. doi: 10.1159/000487457. [DOI] [PubMed] [Google Scholar]
- 56.Kang J., Zhang Y., Cao X., Fan J., Li G., Wang Q., Diao Y., Zhao Z., Luo L., Yin Z. Lycorine inhibits lipopolysaccharide-induced iNOS and COX-2 up-regulation in RAW264.7 cells through suppressing P38 and STATs activation and increases the survival rate of mice after LPS challenge. Int. Immunopharmacol. 2012;12(1):249–256. doi: 10.1016/j.intimp.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 57.Mikami M., Kitahara M., Kitano M., Ariki Y., Mimaki Y., Sashida Y., Yamazaki M., Yui S. Suppressive activity of lycoricidinol (narciclasine) against cytotoxicity of neutrophil-derived calprotectin, and its suppressive effect on rat adjuvant arthritis model. Biol. Pharm. Bull. 1999;22(7):674–678. doi: 10.1248/bpb.22.674. [DOI] [PubMed] [Google Scholar]
- 58.Sun D., Aikawa N. The natural history of the systemic inflammatory response syndrome and the evaluation of SIRS criteria as a predictor of severity in patients hospitalized through emergency services. Keio J. Med. 1999;48(1):28–37. doi: 10.2302/kjm.48.28. [DOI] [PubMed] [Google Scholar]
- 59.Turini M.E., DuBois R.N. Cyclooxygenase-2: a therapeutic target. Annu. Rev. Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 60.Yamazaki Y., Kawano Y. Inhibitory effects of herbal alkaloids on the tumor necrosis factor-alpha and nitric oxide production in lipopolysaccharide-stimulated RAW264 macrophages. Chem. Pharm. Bull. 2011;59(3):388–391. doi: 10.1248/cpb.59.388. [DOI] [PubMed] [Google Scholar]
- 61.Citoglu G.S., Acikara O.B., Yilmaz B.S., Ozbek H. Evaluation of analgesic, anti-inflammatory and hepatoprotective effects of lycorine from Sternbergia fisheriana (Herbert) Rupr. Fitoterapia. 2012;83(1):81–87. doi: 10.1016/j.fitote.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 62.Nair J.J., van Staden J. Acetylcholinesterase inhibition within the lycorine series of Amaryllidaceae alkaloids. Nat. Prod. Commun. 2012;7(7):959–962. [PubMed] [Google Scholar]
- 63.Jimenez A., Santos A., Alonso G., Vazquez D. Inhibitors of protein synthesis in eukarytic cells. Comparative effects of some amaryllidaceae alkaloids. Biochim. Biophys. Acta. 1976;425(3):342–348. doi: 10.1016/0005-2787(76)90261-6. [DOI] [PubMed] [Google Scholar]
- 64.Liu J., Li Y., Tang L.J., Zhang G.P., Hu W.X. Treatment of lycorine on SCID mice model with human APL cells. Biomed. Pharmacother. 2007;61(4):229–234. doi: 10.1016/j.biopha.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao Z., Yu D., Fu S., Zhang G., Pan Y., Bao M., Tu J., Shang B., Guo P., Yang P., Zhou Q. Lycorine hydrochloride selectively inhibits human ovarian cancer cell proliferation and tumor neovascularization with very low toxicity. Toxicol. Lett. 2013;218(2):174–185. doi: 10.1016/j.toxlet.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 66.Roy M., Liang L., Xiao X., Peng Y., Luo Y., Zhou W., Zhang J., Qiu L., Zhang S., Liu F., Ye M., Liu J. Lycorine downregulates HMGB1 to inhibit autophagy and enhances bortezomib activity in multiple myeloma. Theranostics. 2016;6(12):2209–2224. doi: 10.7150/thno.15584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin Z., Zhou S., Zhang Y., Ye H., Jiang S., Yu K., Ma Y. Lycorine induces cell death in MM by suppressing Janus Kinase/signal transducer and activator of transcription via inducing the expression of SOCS1. Biomed. Pharmacother. 2016;84:1645–1653. doi: 10.1016/j.biopha.2016.10.069. [DOI] [PubMed] [Google Scholar]
- 68.Liu J., Hu W.X., He L.F., Ye M., Li Y. Effects of lycorine on HL-60 cells via arresting cell cycle and inducing apoptosis. FEBS Lett. 2004;578(3):245–250. doi: 10.1016/j.febslet.2004.10.095. [DOI] [PubMed] [Google Scholar]
- 69.Ying X., Huang A., Xing Y., Lan L., Yi Z., He P. Lycorine inhibits breast cancer growth and metastasis via inducing apoptosis and blocking Src/FAK-involved pathway. Sci. China Life Sci. 2017;27(10):016–0368. doi: 10.1007/s11427-016-0368-y. [DOI] [PubMed] [Google Scholar]
- 70.Hu M., Peng S., He Y., Qin M., Cong X., Xing Y., Liu M., Yi Z. Lycorine is a novel inhibitor of the growth and metastasis of hormone-refractory prostate cancer. Oncotarget. 2015;6(17):15348–15361. doi: 10.18632/oncotarget.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang C., Wang Q., Li X., Jin Z., Xu P., Xu N., Xu A., Xu Y., Zheng S., Zheng J., Liu C., Huang P. Lycorine induces apoptosis of bladder cancer T24 cells by inhibiting phospho-Akt and activating the intrinsic apoptotic cascade. Biochem. Biophys. Res. Commun. 2017;483(1):197–202. doi: 10.1016/j.bbrc.2016.12.168. [DOI] [PubMed] [Google Scholar]
- 72.Wang J., Xu J., Xing G. Lycorine inhibits the growth and metastasis of breast cancer through the blockage of STAT3 signaling pathway. Acta Biochim. Biophys. Sin. (Shanghai) 2017;49(9):771–779. doi: 10.1093/abbs/gmx076. [DOI] [PubMed] [Google Scholar]
- 73.Hayden R.E., Pratt G., Drayson M.T., Bunce C.M. Lycorine sensitizes CD40 ligand-protected chronic lymphocytic leukemia cells to bezafibrate- and medroxyprogesterone acetate-induced apoptosis but dasatanib does not overcome reported CD40-mediated drug resistance. Haematologica. 2010;95(11):1889–1896. doi: 10.3324/haematol.2010.027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X., Xu P., Wang C., Xu N., Xu A., Xu Y., Sadahira T., Araki M., Wada K., Matsuura E., Watanabe M., Zheng J., Sun P., Huang P., Nasu Y., Liu C. Synergistic effects of the immune checkpoint inhibitor CTLA-4 combined with the growth inhibitor lycorine in a mouse model of renal cell carcinoma. Oncotarget. 2017;8(13):21177–21186. doi: 10.18632/oncotarget.15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hua D.H., Saha S., Takemoto D.J. Anticancer activities of 2,5,8,9-substituted 6-oxo-1,2,3,4,5,6-hexahydrophenanthridines on multi-drug-resistant phenotype cells. Anticancer Res. 1997;17(4A):2435–2441. [PubMed] [Google Scholar]
- 76.Van Goietsenoven G., Andolfi A., Lallemand B., Cimmino A., Lamoral-Theys D., Gras T., Abou-Donia A., Dubois J., Lefranc F., Mathieu V., Kornienko A., Kiss R., Evidente A. Amaryllidaceae alkaloids belonging to different structural subgroups display activity against apoptosis-resistant cancer cells. J. Nat. Prod. 2010;73(7):1223–1227. doi: 10.1021/np9008255. [DOI] [PubMed] [Google Scholar]
- 77.Czabotar P.E., Lessene G., Strasser A., Adams J.M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014;15(1):49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 78.Marino G., Niso-Santano M., Baehrecke E.H., Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014;15(2):81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riedl S.J., Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004;5(11):897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 80.Li Y., Liu J., Tang L.J., Shi Y.W., Ren W., Hu W.X. Apoptosis induced by lycorine in KM3 cells is associated with the G0/G1 cell cycle arrest. Oncol. Rep. 2007;17(2):377–384. [PubMed] [Google Scholar]
- 81.Liu X.S., Jiang J., Jiao X.Y., Wu Y.E., Lin J.H., Cai Y.M. Lycorine induces apoptosis and down-regulation of Mcl-1 in human leukemia cells. Cancer Lett. 2009;274(1):16–24. doi: 10.1016/j.canlet.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 82.Carneiro B.A., Meeks J.J., Kuzel T.M., Scaranti M., Abdulkadir S.A., Giles F.J. Emerging therapeutic targets in bladder cancer. Cancer Treat. Rev. 2015;41(2):170–178. doi: 10.1016/j.ctrv.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 83.Zeng H., Fu R., Yan L., Huang J. Lycorine induces apoptosis of A549 cells via AMPK-Mammalian target of rapamycin (mTOR)-S6K signaling pathway. Med. Sci. Monit. 2017;23:2035–2041. doi: 10.12659/MSM.900742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J., Hu J.L., Shi B.W., He Y., Hu W.X. Up-regulation of p21 and TNF-alpha is mediated in lycorine-induced death of HL-60 cells. Cancer Cell Int. 2010;10(25):1475–2867. doi: 10.1186/1475-2867-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ji Y., Yu M., Qi Z., Cui D., Xin G., Wang B., Jia W., Chang L. Study on apoptosis effect of human breast cancer cell MCF-7 induced by lycorine hydrochloride via death receptor pathway. Saudi Pharm. J. 2017;25(4):633–637. doi: 10.1016/j.jsps.2017.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dasari R., Banuls L.M., Masi M., Pelly S.C., Mathieu V., Green I.R., van Otterlo W.A., Evidente A., Kiss R., Kornienko A. C1,C2-ether derivatives of the Amaryllidaceae alkaloid lycorine: retention of activity of highly lipophilic analogues against cancer cells. Bioorg. Med. Chem. Lett. 2014;24(3):923–927. doi: 10.1016/j.bmcl.2013.12.073. [DOI] [PubMed] [Google Scholar]
- 87.Luo Y., Roy M., Xiao X., Sun S., Liang L., Chen H., Fu Y., Sun Y., Zhu M., Ye M., Liu J. Lycorine induces programmed necrosis in the multiple myeloma cell line ARH-77. Tumour Biol. 2015;36(4):2937–2945. doi: 10.1007/s13277-014-2924-7. [DOI] [PubMed] [Google Scholar]
- 88.Li L., Dai H.J., Ye M., Wang S.L., Xiao X.J., Zheng J., Chen H.Y., Luo Y.H., Liu J. Lycorine induces cell-cycle arrest in the G0/G1 phase in K562 cells via HDAC inhibition. Cancer Cell Int. 2012;12(1):1475–2867. doi: 10.1186/1475-2867-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bertoli C., Skotheim J.M., de Bruin R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013;14(8):518–528. doi: 10.1038/nrm3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brugarolas J., Moberg K., Boyd S.D., Taya Y., Jacks T., Lees J.A. Inhibition of cyclin-dependent kinase 2 by p21 is necessary for retinoblastoma protein-mediated G1 arrest after gamma-irradiation. Proc. Natl. Acad. Sci. U. S. A. 1999;96(3):1002–1007. doi: 10.1073/pnas.96.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bartek J., Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3(5):421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 92.Rokudai S., Aikawa Y., Tagata Y., Tsuchida N., Taya Y., Kitabayashi I. Monocytic leukemia zinc finger (MOZ) interacts with p53 to induce p21 expression and cell-cycle arrest. J. Biol. Chem. 2009;284(1):237–244. doi: 10.1074/jbc.M805101200. [DOI] [PubMed] [Google Scholar]
- 93.Zong W.X., Ditsworth D., Bauer D.E., Wang Z.Q., Thompson C.B. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18(11):1272–1282. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Artal-Sanz M., Tavernarakis N. Proteolytic mechanisms in necrotic cell death and neurodegeneration. FEBS Lett. 2005;579(15):3287–3296. doi: 10.1016/j.febslet.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 95.Degterev A., Hitomi J., Germscheid M., Ch’en I.L., Korkina O., Teng X., Abbott D., Cuny G.D., Yuan C., Wagner G., Hedrick S.M., Gerber S.A., Lugovskoy A., Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008;4(5):313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He S., Wang L., Miao L., Wang T., Du F., Zhao L., Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 97.Zhang D.W., Shao J., Lin J., Zhang N., Lu B.J., Lin S.C., Dong M.Q., Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 98.Klionsky D.J. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 2007;8(11):931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 99.Bertout J.A., Patel S.A., Simon M.C. The impact of O2 availability on human cancer. Nat. Rev. Cancer. 2008;8(12):967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao S., Ma C.M., Liu C.X., Wei W., Sun Y., Yan H., Wu Y.L. Autophagy inhibition enhances isobavachalcone-induced cell death in multiple myeloma cells. Int. J. Mol. Med. 2012;30(4):939–944. doi: 10.3892/ijmm.2012.1066. [DOI] [PubMed] [Google Scholar]
- 101.Zhang H., Chen J., Zeng Z., Que W., Zhou L. Knockdown of DEPTOR induces apoptosis, increases chemosensitivity to doxorubicin and suppresses autophagy in RPMI-8226 human multiple myeloma cells in vitro. Int. J. Mol. Med. 2013;31(5):1127–1134. doi: 10.3892/ijmm.2013.1299. [DOI] [PubMed] [Google Scholar]
- 102.Mishima Y., Santo L., Eda H., Cirstea D., Nemani N., Yee A.J., O’Donnell E., Selig M.K., Quayle S.N., Arastu-Kapur S., Kirk C., Boise L.H., Jones S.S., Raje N. Ricolinostat (ACY-1215) induced inhibition of aggresome formation accelerates carfilzomib-induced multiple myeloma cell death. Br. J. Haematol. 2015;169(3):423–434. doi: 10.1111/bjh.13315. [DOI] [PubMed] [Google Scholar]
- 103.Livesey K.M., Kang R., Vernon P., Buchser W., Loughran P., Watkins S.C., Zhang L., Manfredi J.J., Zeh H.J., 3rd, Li L., Lotze M.T., Tang D. p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Res. 2012;72(8):1996–2005. doi: 10.1158/0008-5472.CAN-11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morselli E., Shen S., Ruckenstuhl C., Bauer M.A., Marino G., Galluzzi L., Criollo A., Michaud M., Maiuri M.C., Chano T., Madeo F., Kroemer G. p53 inhibits autophagy by interacting with the human ortholog of yeast Atg17, RB1CC1/FIP200. Cell Cycle. 2011;10(16):2763–2769. doi: 10.4161/cc.10.16.16868. [DOI] [PubMed] [Google Scholar]
- 105.Kang R., Loux T., Tang D., Schapiro N.E., Vernon P., Livesey K.M., Krasinskas A., Lotze M.T., Zeh H.J., 3rd The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proc. Natl. Acad. Sci. U. S. A. 2012;109(18):7031–7036. doi: 10.1073/pnas.1113865109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kang R., Chen R., Zhang Q., Hou W., Wu S., Cao L., Huang J., Yu Y., Fan X.G., Yan Z., Sun X., Wang H., Wang Q., Tsung A., Billiar T.R., Zeh H.J., 3rd, Lotze M.T., Tang D. HMGB1 in health and disease. Mol. Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kang R., Zhang Q., Zeh H.J., 3rd, Lotze M.T., Tang D. HMGB1 in cancer: good, bad, or both? Clin. Cancer Res. 2013;19(15):4046–4057. doi: 10.1158/1078-0432.CCR-13-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang D., Kang R., Cheh C.W., Livesey K.M., Liang X., Schapiro N.E., Benschop R., Sparvero L.J., Amoscato A.A., Tracey K.J., Zeh H.J., Lotze M.T. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29(38):5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dong Xda E., Ito N., Lotze M.T., Demarco R.A., Popovic P., Shand S.H., Watkins S., Winikoff S., Brown C.K., Bartlett D.L., Zeh H.J., 3rd High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J. Immunother. 2007;30(6):596–606. doi: 10.1097/CJI.0b013e31804efc76. [DOI] [PubMed] [Google Scholar]
- 110.Tang D., Kang R., Livesey K.M., Kroemer G., Billiar T.R., Van Houten B., Zeh H.J., 3rd, Lotze M.T. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011;13(6):701–711. doi: 10.1016/j.cmet.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Okamoto K., Kondo-Okamoto N. Mitochondria and autophagy: critical interplay between the two homeostats. Biochim. Biophys. Acta. 2012;1820(5):595–600. doi: 10.1016/j.bbagen.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 112.Priault M., Salin B., Schaeffer J., Vallette F.M., di Rago J.P., Martinou J.C. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ. 2005;12(12):1613–1621. doi: 10.1038/sj.cdd.4401697. [DOI] [PubMed] [Google Scholar]
- 113.Zhang D.X., Gutterman D.D. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007;292(5):H2023–31. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 114.Mombach J.C., Bugs C.A., Chaouiya C. Modelling the onset of senescence at the G1/S cell cycle checkpoint. BMC Genomics. 2014;15(Suppl 7):S7. doi: 10.1186/1471-2164-15-S7-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gomez V., Gundogdu R., Gomez M., Hoa L., Panchal N., O’Driscoll M., Hergovich A. Regulation of DNA damage responses and cell cycle progression by hMOB2. Cell. Signal. 2015;27(2):326–339. doi: 10.1016/j.cellsig.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Siesser P.M., Hanks S.K. The signaling and biological implications of FAK overexpression in cancer. Clin. Cancer Res. 2006;12(11 Pt 1):3233–3237. doi: 10.1158/1078-0432.CCR-06-0456. [DOI] [PubMed] [Google Scholar]
- 117.Hsia D.A., Mitra S.K., Hauck C.R., Streblow D.N., Nelson J.A., Ilic D., Huang S., Li E., Nemerow G.R., Leng J., Spencer K.S., Cheresh D.A., Schlaepfer D.D. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 2003;160(5):753–767. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mitra S.K., Hanson D.A., Schlaepfer D.D. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 119.Lo H.W., Hsu S.C., Xia W., Cao X., Shih J.Y., Wei Y., Abbruzzese J.L., Hortobagyi G.N., Hung M.C. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67(19):9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang J., Mani S.A., Donaher J.L., Ramaswamy S., Itzykson R.A., Come C., Savagner P., Gitelman I., Richardson A., Weinberg R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 121.Yuan J., Zhang F., Niu R. Multiple regulation pathways and pivotal biological functions of STAT3 in cancer. Sci. Rep. 2015;5(17663) doi: 10.1038/srep17663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun Y., Wu P., Sun Y., Sharopov F.S., Yang Q., Chen F., Wang P., Liang Z. Lycorine possesses notable anticancer potentials in on-small cell lung carcinoma cells via blocking Wnt/beta-catenin signaling and epithelial-mesenchymal transition (EMT) Biochem. Biophys. Res. Commun. 2018;495(1):911–921. doi: 10.1016/j.bbrc.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 123.Yu H., Qiu Y., Pang X., Li J., Wu S., Yin S., Han L., Zhang Y., Jin C., Gao X., Hu W., Wang T. Lycorine Promotes Autophagy and Apoptosis via TCRP1/Akt/mTOR Axis Inactivation in Human Hepatocellular Carcinoma. Mol. Cancer Ther. 2017;16(12):2711–2723. doi: 10.1158/1535-7163.MCT-17-0498. [DOI] [PubMed] [Google Scholar]
- 124.Wang P., Li L.F., Wang Q.Y., Shang L.Q., Shi P.Y., Yin Z. Anti-dengue-virus activity and structure-activity relationship studies of lycorine derivatives. ChemMedChem. 2014;9(7):1522–1533. doi: 10.1002/cmdc.201300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen D., Cai J., Cheng J., Jing C., Yin J., Jiang J., Peng Z., Hao X. Design, Synthesis and Structure-Activity Relationship Optimization of Lycorine Derivatives for HCV Inhibition. Sci. Rep. 2015;5:14972. doi: 10.1038/srep14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen D.Z., Jing C.X., Cai J.Y., Wu J.B., Wang S., Yin J.L., Li X.N., Li L., Hao X.J. Design, synthesis, and structural optimization of lycorine-derived phenanthridine derivatives as Wnt/beta-Catenin signaling pathway agonists. J. Nat. Prod. 2016;79(1):180–188. doi: 10.1021/acs.jnatprod.5b00825. [DOI] [PubMed] [Google Scholar]
- 127.Guo Y., Wang Y., Cao L., Wang P., Qing J., Zheng Q., Shang L., Yin Z., Sun Y. A conserved inhibitory mechanism of a lycorine derivative against enterovirus and hepatitis C virus. Antimicrob. Agents Chemother. 2016;60(2):913–924. doi: 10.1128/AAC.02274-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Awasthi M., Amin S.A., Shukla V., Jain S., Patil U.K., Gayen S. Structural requirements of some derivatives based on natural alkaloid lycorine for their dengue inhibitory activity to accelerate dengue drug discovery efforts. Indian J. Nat. Prod. Resour. 2016;7(3):221–228. [Google Scholar]
- 129.McNulty J., Nair J.J., Singh M., Crankshaw D.J., Holloway A.C., Bastida J. Cytochrome P450 3A4 inhibitory activity studies within the lycorine series of alkaloids. Nat. Prod. Commun. 2010;5(8):1195–1200. [PubMed] [Google Scholar]
- 130.Giordani R.B., Junior C.O., de Andrade J.P., Bastida J., Zuanazzi J.A., Tasca T., de Almeida M.V. Lycorine derivatives against Trichomonas vaginalis. Chem. Biol. Drug Des. 2012;80(1):129–133. doi: 10.1111/j.1747-0285.2012.01333.x. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y.H., Wan Q.L., Gu C.D., Luo H.R., Long C.L. Synthesis and biological evaluation of lycorine derivatives as dual inhibitors of human acetylcholinesterase and butyrylcholinesterase. Chem. Cent. J. 2012;6(1):96. doi: 10.1186/1752-153X-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McNulty J., Nair J.J., Little J.R., Brennan J.D., Bastida J. Structure-activity studies on acetylcholinesterase inhibition in the lycorine series of Amaryllidaceae alkaloids. Bioorg. Med. Chem. Lett. 2010;20(17):5290–5294. doi: 10.1016/j.bmcl.2010.06.130. [DOI] [PubMed] [Google Scholar]
- 133.Evdokimov N.M., Lamoral-Theys D., Mathieu V., Andolfi A., Frolova L.V., Pelly S.C., van Otterlo W.A., Magedov I.V., Kiss R., Evidente A., Kornienko A. In search of a cytostatic agent derived from the alkaloid lycorine: synthesis and growth inhibitory properties of lycorine derivatives. Bioorg. Med. Chem. 2011;19(23):7252–7261. doi: 10.1016/j.bmc.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McNulty J., Nair J.J., Bastida J., Pandey S., Griffin C. Structure-activity studies on the lycorine pharmacophore: a potent inducer of apoptosis in human leukemia cells. Phytochemistry. 2009;70(7):913–919. doi: 10.1016/j.phytochem.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Evidente A., Kireev A.S., Jenkins A.R., Romero A.E., Steelant W.F., Van Slambrouck S., Kornienko A. Biological evaluation of structurally diverse amaryllidaceae alkaloids and their synthetic derivatives: discovery of novel leads for anticancer drug design. Planta Med. 2009;75(5):501–507. doi: 10.1055/s-0029-1185340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang P., Yuan H.H., Zhang X., Li Y.P., Shang L.Q., Yin Z. Novel lycorine derivatives as anticancer agents: synthesis and in vitro biological evaluation. Molecules. 2014;19(2):2469–2480. doi: 10.3390/molecules19022469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dumont P., Ingrassia L., Rouzeau S., Ribaucour F., Thomas S., Roland I., Darro F., Lefranc F., Kiss R. The Amaryllidaceae isocarbostyril narciclasine induces apoptosis by activation of the death receptor and/or mitochondrial pathways in cancer cells but not in normal fibroblasts. Neoplasia. 2007;9(9):766–776. doi: 10.1593/neo.07535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hohmann J., Forgo P., Molnar J., Wolfard K., Molnar A., Thalhammer T., Mathe I., Sharples D. Antiproliferative amaryllidaceae alkaloids isolated from the bulbs of Sprekelia formosissima and Hymenocallis x festalis. Planta Med. 2002;68(5):454–457. doi: 10.1055/s-2002-32068. [DOI] [PubMed] [Google Scholar]
- 139.Weniger B., Italiano L., Beck J.P., Bastida J., Bergonon S., Codina C., Lobstein A., Anton R. Cytotoxic activity of Amaryllidaceae alkaloids. Planta Med. 1995;61(1):77–79. doi: 10.1055/s-2006-958007. [DOI] [PubMed] [Google Scholar]
- 140.Ren L., Zhao H., Chen Z. Study on pharmacokinetic and tissue distribution of lycorine in mice plasma and tissues by liquid chromatography-mass spectrometry. Talanta. 2014;119:401–406. doi: 10.1016/j.talanta.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 141.Liu X., Hong Y., He Q., Huang K. Rapid and sensitive HPLC-MS/MS method for quantitative determination of lycorine from the plasma of rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015;974:96–100. doi: 10.1016/j.jchromb.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 142.Azevedo Marques L., Giera M., Lingeman H., Niessen W.M. Analysis of acetylcholinesterase inhibitors: bioanalysis, degradation and metabolism. Biomed. Chromatogr. 2011;25(1-2):278–299. doi: 10.1002/bmc.1573. [DOI] [PubMed] [Google Scholar]
- 143.Zhou X., Liu Y.B., Huang S., Liu Y. An LC-MS/MS method for the simultaneous determination of lycorine and galanthamine in rat plasma and its application to pharmacokinetic study of Lycoris radiata extract in rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014;34(6):861–868. doi: 10.1007/s11596-014-1365-2. [DOI] [PubMed] [Google Scholar]
- 144.Kretzing S., Abraham G., Seiwert B., Ungemach F.R., Krugel U., Regenthal R. Dose-dependent emetic effects of the Amaryllidaceous alkaloid lycorine in beagle dogs. Toxicon. 2011;57(1):117–124. doi: 10.1016/j.toxicon.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 145.Jahn S., Seiwert B., Kretzing S., Abraham G., Regenthal R., Karst U. Metabolic studies of the Amaryllidaceous alkaloids galantamine and lycorine based on electrochemical simulation in addition to in vivo and in vitro models. Anal. Chim. Acta. 2012;756:60–72. doi: 10.1016/j.aca.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 146.Guo Y., Liu X., Sun X., Zhang Q., Gong T., Zhang Z. Mannosylated lipid nano-emulsions loaded with lycorine-oleic acid ionic complex for tumor cell-specific delivery. Theranostics. 2012;2(11):1104–1114. doi: 10.7150/thno.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]