Abstract
Noroviruses have emerged as the leading worldwide cause of acute non-bacterial gastroenteritis in humans. The presence of noroviruses in diarrheic stool samples from calves on Michigan and Wisconsin dairy farms was investigated by RT-PCR. Norovirus-positive samples were found on all eight farms studied in Michigan and on 2 out of 14 farms in Wisconsin. Phylogenetic analyses of partial polymerase and capsid sequences, derived for a subset of these bovine noroviruses, showed that these strains formed a group which is genetically distinct from the human noroviruses, but more closely related to genogroup I than to genogroup II human noroviruses. Examination of 2 full and 10 additional partial capsid (ORF2) sequences of these bovine strains revealed the presence of two genetic subgroups or clusters of bovine noroviruses circulating on Michigan and Wisconsin farms. One subgroup is “Jena-like”, the other “Newbury agent-2-like”.
Keywords: Norovirus, NLV, Jena, Newbury agent-2, Bovine enteric calicivirus, RT-PCR
1. Introduction
Noroviruses, formerly known as Norwalk-like viruses (NLVs), have been recognized as the most common pathogens involved in outbreaks of acute non-bacterial gastroenteritis in humans (Fankhauser et al., 1998, Mead et al., 1999, Glass et al., 2000, Monroe et al., 2000, Parashar and Monroe, 2001, Bresee et al., 2002, Fankhauser et al., 2002). These agents belong to the Norovirus genus, one of the four genera in the Caliciviridae family, the other three being Sapovirus (formerly, Sapporo-like viruses), Vesivirus and Lagovirus (Green et al., 2000a). Human noroviruses are a genetically heterogeneous group of viruses currently classified into two genogroups: genogroup I (GGI) and genogroup II (GGII) (Ando et al., 1994, Green et al., 1994, Wang et al., 1994). In the late 1970s, and throughout the 1980s, calici-like viruses with norovirus morphology were observed in diarrheic feces of newborn calves in Germany and the United Kingdom (Bridger et al., 1984, Wood and Bridger, 1978, Gunther and Otto, 1987). Sequencing showed that the bovine noroviruses Jena and Newbury agent-2 are closely related to GGI human noroviruses (Liu et al., 1999, Dastjerdi et al., 1999) and that porcine noroviruses are closely related to GGII human noroviruses (Sugieda et al., 1998, Sugieda and Nakajima, 2002). Studies in the Netherlands further confirmed this genetic relationship between animal and human noroviruses (van der Poel et al., 2000). These findings raised the possibility that animals may be a reservoir for human noroviruses.
The assignment of bovine noroviruses to a third genogroup, GGIII, of the Norovirus genus was first suggested by Ando et al. (2000), based on comparison of the Jena and Newbury agent-2 sequences to corresponding sequences of human norovirus strains. Oliver et al. (2003) recently reported on the characterization of a number of bovine enteric caliciviruses from the UK and found that these strains definitely comprise a third genogroup of noroviruses distinct from the two human genogroups. Using three sets of primer pairs routinely used for the detection of noroviruses in human diarrheic feces, the authors did not identify any human strains from cattle specimens. This finding made them suggest that the bovine noroviruses circulating in the UK are unlikely to be pathogenic to humans. Most recently, Smiley et al. (2003) reported the identification of bovine noroviruses in Ohio veal calf herds. The Ohio strains were found to be genetically more related to Jena and Newbury agent-2 than to human noroviruses, supporting previous findings that bovine noroviruses fall into a third genogroup within the norovirus genus. In addition to the bovine norovirus strains, these authors also found NB-like bovine enteric caliciviruses in some of the calf specimens examined. The NB strain, originally identified in diarrheic feces from a dairy calf in Nebraska, was recently characterized and shown to be a novel enteropathogenic bovine calicivirus, genetically most similar to lagoviruses and sapoviruses (Smiley et al., 2002).
The aim of this study was to determine the presence of noroviruses in diarrheic feces from calves on Michigan and Wisconsin dairy farms initially by using a modified RT-PCR assay for detecting human norovirus strains. A subset of these strains were molecularly characterized by sequencing partial or entire genes, followed by phylogenetic analyses with corresponding sequences of human and bovine norovirus strains from the GenBank database.
2. Materials and methods
2.1. Origin of the samples
Fifty-seven individual fecal samples were randomly collected from 5- to 10-day-old neonatal calves with diarrhea on six dairy farms (designated as farms A–F) in Michigan. Twelve of these samples originated from farm A, 1 from farm B, 3 from farm C, 3 from farm D, 26 from farm E and 12 from farm F. All farms, except B and C, were experiencing high rates of neonatal diarrhea. Comprehensive disease investigations were not conducted, except on farm E. Other findings in calves from farm E included septicemia and the presence of Salmonella DT104, Cryptosporidium parvum, rotavirus and coronavirus. Clinical signs were variable. There was mild to severe diarrhea, generally between 5 and 14 days of age, and mortality was low (less than 0.5%). Diarrhea was normally off-white in color, with a consistency ranging from watery to semi-liquid. Blood was generally not observed in the fecal samples. The calves typically responded to electrolyte therapy.
Three more samples originating from two different dairy farms in Michigan (designated as farms G and H, with two samples from farm G and one from farm H) were diagnostic submissions to the Diagnostic Center for Population and Animal Health at Michigan State University (MSU). The samples were from 5- to 10-day-old calves with diarrhea that was not responsive to antimicrobial therapy. Enterovirus was found in the two samples from farm G, and small round virus-like particles were identified in the single sample from farm H, both by electron microscopy.
Fourteen individual fecal samples from Wisconsin neonatal calves were diagnostic submissions to the Wisconsin Veterinary Diagnostic Laboratory at the University of Wisconsin. These samples originated from 14 different dairy farms (one sample per farm). The specific age range and clinical signs observed in the calves from which the diagnostic specimens were collected were not specified. Laboratory findings included the presence of C. parvum (seven farms), coronavirus (two farms) and rotavirus (four farms).
2.2. Detection of noroviruses by RT-PCR
RNA from fecal samples was extracted using the QIAquick Gel Extraction Kit (QIAGEN) RNeasy Mini Kit (QIAGEN Inc., Valencia, CA). Total RNA was eluted from the columns with 30 μl of RNase-free water. A modified RT-PCR protocol for detecting human noroviruses, obtained from the Centers for Disease Control and Prevention (Dr. Stephan Monroe, CDC, Atlanta, GA, personal communication), was used for the initial detection of norovirus RNA. The assay makes use of a degenerate primer pair (MON 432: 5′-TGGACICGYGGICCYAAYCA-3′; MON 477: 5′-AAAICGCATCCAIGCAAACAT-3′) targeting a 213-nucleotide region at the 3′ end of the norovirus RNA polymerase gene (Region B) (Anderson et al., 2003). This region corresponds to nucleotides 5093–5305 in the Norwalk virus genome (GenBank accession number M87661), and nucleotides 4786–4998 in the Jena virus genome (GenBank accession number AJ011099). The RT-PCR assay was performed in a 50 μl reaction volume, using 25 μl MasterAmp 2X PCR Premix G (Epicentre Technologies, Madison, WI), 1 mM dithiothreitol, 0.2% Triton X-100, 2 mM 2-mercaptoethanol, 0.6 μM MON 432, 0.6 μM MON 477, 20 U of RNase Inhibitor (Roche Molecular Biochemicals, Indianapolis, IN), 2.7 U of avian myeloblastosis virus super reverse transcriptase (Molecular Genetic Resources, Tampa, FL), and 1.25 U of AmpliTaq DNA polymerase (Perkin Elmer Biosystems, Inc., Foster City, CA). The cycling conditions were as follows: 1 cycle of reverse transcription at 42 °C for 10 min, 1 cycle of pre-denaturation at 94 °C for 3 min, 40 amplification cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 1 min 30 s, and extension at 60 °C for 30 s, followed by a final cycle incubation at 72 °C for 7 min.
The 213 bp amplicons corresponding to the first eight norovirus-positive samples (six from Michigan, two from Wisconsin) were cloned in the sequencing plasmid, pCR4-TOPO, of the TOPO TA Cloning Kit for Sequencing (Invitrogen, Carlsbad, CA), according to procedures outlined by the manufacturer. The plasmids were submitted to the Genomic Technology Support Facility (GTSF) at Michigan State University for automated sequencing. Based on the partial RNA polymerase sequence alignment data generated for these eight strains (designated as: B-1SVD, A-1SVD, 2086MR, 3470DD, 11MSU-MW, 803466WI, 803484WI), a pair of primers was selected from conserved regions: forward primer, 5′-GGGCCTAATCACGATGACC-3′; and reverse primer, 5′-AAGCAAACATGGAGCGRTGTG-3′. These primers target a 192 bp region of the norovirus RNA polymerase gene. With this new set of non-degenerate (except for one “R” in the reverse primer) diagnostic primers, a one-tube RT-PCR assay was developed, based upon the QIAGEN One-Step RT-PCR Kit. Five microliters of RNA template was used. The optimal primer concentration for both the forward and reverse primer was 0.6 μM. Optimized cycling conditions were as follows: cDNA synthesis at 50 °C for 30 min, followed by pre-denaturation at 95 °C for 15 min, then 40 cycles of PCR at 94 °C for 30 s, 54 °C for 45 s, and 72 °C for 1 min. The last cycle was followed by a final extension at 72 °C for 7 min. PCR products were analyzed by electrophoresis in 2% agarose gels stained with ethidium bromide.
Forty of the 60 Michigan fecal samples and all 14 Wisconsin samples were tested with the CDC primer set. Not all Michigan samples were tested with the CDC protocol, due to the limited quantity of reagents available at the time of the study. All 60 Michigan and 12 of the Wisconsin samples were tested using the newly designed diagnostic primer set. Two of the 14 Wisconsin samples were no longer available during rescreening with the diagnostic primer set.
Partial RNA polymerase sequences derived for the set of eight Michigan/Wisconsin strains (above) were then compared with corresponding sequences of the Jena strain, two bovine calicivirus strains from Ohio, three bovine noroviruses from the UK, three GGI and two GGII human noroviruses.
2.3. Acquisition of extended sequence data for bovine norovirus strains B-1SVD and 11MSU-MW
After obtaining sequence data from a portion of the 3′ end of the polymerase gene, extended sequence data of approximately 2.5 kb from the 3′ end of the norovirus genome were derived by a 3′ RACE System for Rapid Amplification of cDNA Ends (GIBCO BRL, Life Technologies, Rockville, MD). The determination of extended sequence data was done to determine the genome organization of the strains. Strain B-1SVD was the first bovine norovirus identified in this study. A second strain, 11MSU-MW, was chosen for further sequencing, based on its maximum divergence from B-1SVD within the previously analyzed partial polymerase region. Complementary DNA was synthesized from 3 μl of fecal RNA template with the oligo(dT)-containing adapter primer provided in the kit, according to the manufacturer’s recommended protocol. The gene-specific primer (GSP) used for PCR amplification of the target cDNA was the diagnostic forward primer, 5′-GGGCCTAATCACGATGACC-3′, mentioned above. The abridged universal amplification primer (AUAP) provided in the kit was used as reverse primer. PCR was carried out with the QIAGEN HotStar Taq DNA Polymerase Kit, using 2 μl of cDNA template, 0.5 μM of the GSP, 2 μl of AUAP and the following cycling conditions: pre-denaturation at 95 °C for 15 min, followed by 45 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 4 min, with a final extension of 72 °C for 10 min. PCR products of the expected size were extracted and purified from agarose gels with the QIAquick Gel Extraction Kit and submitted to GTSF-MSU for automated sequencing. Sequence data for the 2.5 kb product were obtained by initially using the diagnostic forward primer (GSP) and a poly-T sequencing primer, followed by primer-walking with newly designed 5′ and 3′ primers derived with the OLIGO 6 software (Molecular Biology Insights, Cascade, CO).
2.4. Amplification of bovine norovirus partial capsid sequences
The marked divergence observed between strains B-1SVD and 11MSU-MW within the ORF2 region prompted further investigation of more strains to assess sequence diversity within the capsid gene. Capsid sequence data obtained for Michigan bovine norovirus strains B-1SVD and 11MSU-MW were aligned with corresponding sequences of the German strain, Jena, and the Dutch strain, Bo/NLV/CH126/1998/NL, from the GenBank database. A primer pair from generally conserved sequences, spanning a 784 nucleotide central variable region of the capsid gene, was identified: forward primer 5′-CCCAGGCTACWCAGTTCCC-3′ and reverse primer, 5′-AGGRGCAAACTCAGAACCAAG-3′. This region is located at nucleotides 5457–6238 in the Jena virus genome (GenBank accession number AJ011099). A one-tube RT-PCR using the One-Step RT-PCR Kit (QIAGEN) was carried out with 0.6 μM primer concentrations. Cycling conditions were as follows: cDNA synthesis at 50 °C for 30 min, followed by pre-denaturation at 95 °C for 15 min, and 40 cycles of 94 °C for 30 s, 56 °C for 1 min and 72 °C for 1 min and 30 s, with a final extension of 72 °C for 7 min after the last cycle. Purified products were submitted to GTSF-MSU with both forward and reverse capsid-specific primers for automated sequencing.
Partial capsid sequences were derived for 10 additional strains: A-1SVD, 2086MR, 2167MR, 3505DD, 1GM, 17–19MSU-MW, 21MSU-MW, 32MSU-MW, 2387108MI, and 803484WI. Nine of these strains were from Michigan and one (803484WI) was from Wisconsin. Selection of these strains was based upon the fact that they were the first 10 yielding a sufficient amount of PCR product. Deduced amino acid sequences of the partial capsid region of these strains were then compared to corresponding GenBank sequences of Jena, bovine noroviruses from the UK and the Netherlands, and to human GGI and GGII noroviruses.
2.5. Sequence and phylogenetic analyses
BLAST (Altschul et al., 1990) searches against the GenBank database were performed to confirm that the sequence data obtained were authentic calicivirus sequences. Sequence assembly and analyses, including multiple alignments of nucleotide and predicted amino acid sequences using the ClustalV method (Higgins and Sharp, 1989), were done with the Lasergene biocomputing software (DNASTAR, Inc., Madison, WI). Phylogenetic trees were constructed with the Treecon software package (Van de Peer and De Wachter, 1994), using the neighbor-joining method (Saitou and Nei, 1987) and 500 bootstrap analyses (Efron and Gong, 1983, Felsenstein, 1985). Sequence alignment data generated with Lasergene were converted to the Treecon format using the ForCon software (Raes and Van de Peer, 1999).
3. Results
3.1. Detection of noroviruses by RT-PCR
Out of 40 Michigan samples screened initially using the CDC primer set, 19 (∼48%) were found to be positive for norovirus. Screening the same 40 samples with our diagnostic primer set, 30 (75%) were found to be positive. All 19 samples determined positive with the CDC protocol were also positive with the diagnostic primer set. The remaining 20 fecal samples from Michigan were assayed only with the diagnostic primer set, due to the limited amount of reagents available for the CDC protocol. Eighteen more positive samples out of the 20 were found, leading to a total of 48 (80%) positive samples from Michigan farms. Positive samples were found on all eight dairy farms in Michigan. The numbers of positive samples per farm were as follows: 12 from farm A (n=12), 1 from farm B (n=1), 1 from farm C (n=3), 1 from farm D (n=3), 19 from farm E (n=26), 11 from farm F (n=12), 2 from farm G (n=2) and 1 from farm H (n=1).
Two of the 14 samples from Wisconsin (from 14 different farms) were found to be positive for norovirus with the CDC degenerate primer set. These same 2 positive samples and 2 additional ones were found to be positive with the diagnostic primer set, for a total of 4 positive samples out of the 12 that were rescreened using the diagnostic primer set.
3.2. Partial polymerase sequence and phylogenetic analyses
Partial RNA polymerase sequences, corresponding to the first eight norovirus-positive samples (six from Michigan, two from Wisconsin) detected with the CDC protocol, were derived and analyzed to obtain an initial impression of potential strain variation. BLAST analysis confirmed the homology of the sequences to those of previously reported bovine and human calicivirus strains deposited in GenBank. Table 1 shows the sequence similarities, based upon alignments of partial RNA polymerase sequences (172-bp segment, excluding primer sequences) of the eight Michigan/Wisconsin bovine norovirus strains with corresponding sequences of the Jena strain, two bovine calicivirus strains from Ohio, three bovine noroviruses from the UK, three GGI and two GGII human noroviruses. The GenBank accession numbers for these strains are given in Fig. 1 . Nucleotide and amino acid sequence identities between the eight Michigan/Wisconsin bovine norovirus strains ranged from 81.4 to 100 and 94.7 to 100%, respectively. The Michigan/Wisconsin strains shared amino acid sequence identities ranging from 82.5 to 87.2% with Jena, 93–100% with the Ohio strains, and 91.2–100% with the UK strains. Sequence identities between the Michigan/Wisconsin bovine noroviruses and GGI human noroviruses ranged from 46.5 to 54.1% at the nucleotide and 61.4–68.4%, at the amino acid level. Phylogenetic analyses, based on the deduced amino acid sequence of the 172-nucleotide region of the RNA polymerase gene (Fig. 1), showed a clustering of the Michigan/Wisconsin bovine norovirus strains with their counterparts from Europe and Ohio, in a third group, genogroup III (GGIII), distinct from GGI human noroviruses. However, the bovine norovirus group still appeared to be more closely related to GGI than to GGII human norovirus strains.
Table 1.
Percent aa and nt sequence identities between NLVs based on partial sequence of the RNA polymerase gene
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | ||
| – | 100.0 | 100.0 | 96.5 | 98.2 | 96.5 | 98.2 | 96.5 | 84.2 | 96.5 | 93.0 | 94.7 | 96.5 | 94.7 | 68.4 | 64.3 | 63.2 | 57.9 | 52.6 | 1 | A-1SVD |
| 100.0 | – | 100.0 | 96.5 | 98.2 | 96.5 | 98.2 | 96.5 | 84.2 | 96.5 | 93.0 | 94.7 | 96.5 | 94.7 | 68.4 | 64.3 | 63.2 | 57.9 | 52.6 | 2 | B-1SVD |
| 98.3 | 98.3 | – | 96.5 | 98.2 | 96.5 | 98.2 | 96.5 | 84.2 | 96.5 | 93.0 | 94.7 | 96.5 | 94.7 | 68.4 | 64.3 | 63.2 | 57.9 | 52.6 | 3 | 2086MR |
| 87.2 | 87.2 | 86.6 | – | 94.7 | 100.0 | 98.2 | 100.0 | 87.2 | 100.0 | 96.5 | 98.2 | 100.0 | 98.2 | 66.7 | 62.5 | 61.4 | 56.1 | 52.6 | 4 | 3470DD |
| 97.7 | 97.7 | 99.4 | 86.0 | – | 94.7 | 96.5 | 94.7 | 82.5 | 94.7 | 91.2 | 93.0 | 94.7 | 93.0 | 68.4 | 62.5 | 61.4 | 56.1 | 50.9 | 5 | 3503DD |
| 83.7 | 83.7 | 82.0 | 94.2 | 81.4 | – | 98.2 | 100.0 | 84.2 | 100.0 | 96.5 | 98.2 | 100.0 | 98.2 | 66.7 | 62.5 | 61.4 | 56.1 | 52.6 | 6 | 11MSU-MW |
| 85.5 | 85.5 | 84.9 | 89.5 | 84.3 | 86.6 | – | 98.2 | 82.5 | 98.2 | 94.7 | 96.5 | 98.2 | 96.5 | 66.7 | 62.5 | 61.4 | 56.1 | 52.6 | 7 | 803466WI |
| 84.9 | 84.9 | 84.3 | 95.9 | 83.7 | 96.5 | 90.1 | – | 84.2 | 100.0 | 96.5 | 98.2 | 100.0 | 98.2 | 66.7 | 62.5 | 61.4 | 56.1 | 52.6 | 8 | 803484WI |
| 69.8 | 69.8 | 70.9 | 70.9 | 70.3 | 66.9 | 72.1 | 69.8 | – | 84.2 | 80.7 | 82.5 | 84.2 | 84.2 | 57.9 | 62.5 | 57.9 | 56.1 | 50.9 | 9 | Jena |
| 84.3 | 84.3 | 83.7 | 95.3 | 83.1 | 95.9 | 89.5 | 99.4 | 69.8 | – | 96.5 | 98.2 | 100.0 | 98.2 | 66.7 | 62.5 | 61.4 | 56.1 | 52.6 | 10 | Bo/Penrith55/00/UK |
| 81.4 | 81.4 | 80.2 | 84.3 | 79.7 | 84.9 | 84.3 | 85.5 | 71.5 | 84.9 | – | 98.2 | 96.5 | 94.7 | 66.7 | 62.5 | 63.2 | 56.1 | 52.6 | 11 | Bo/Dumfries/94/UK |
| 80.8 | 80.8 | 79.7 | 85.5 | 79.1 | 87.2 | 83.1 | 86.6 | 69.8 | 87.2 | 87.8 | – | 98.2 | 96.5 | 66.7 | 64.3 | 63.2 | 57.9 | 54.4 | 12 | Newbury agent-2 |
| 87.2 | 87.2 | 86.6 | 100.0 | 86.0 | 84.2 | 89.5 | 95.9 | 70.9 | 95.3 | 84.3 | 85.5 | – | 98.2 | 66.7 | 62.5 | 61.4 | 56.1 | 52.6 | 13 | CV500-OH |
| 86.6 | 86.6 | 86.0 | 99.4 | 85.5 | 93.6 | 88.4 | 95.3 | 70.9 | 94.8 | 83.7 | 84.9 | 99.4 | – | 64.9 | 62.5 | 61.4 | 54.4 | 50.9 | 14 | CV514-OH |
| 52.9 | 52.9 | 52.9 | 54.1 | 52.9 | 52.9 | 51.7 | 54.1 | 45.9 | 54.1 | 51.2 | 51.7 | 54.1 | 52.9 | – | 80.4 | 84.2 | 57.9 | 49.1 | 15 | Desert Shield |
| 50.0 | 50.0 | 48.8 | 46.5 | 48.3 | 48.8 | 50.0 | 47.7 | 49.4 | 47.7 | 50.6 | 52.3 | 46.5 | 46.5 | 69.2 | – | 92.9 | 62.5 | 53.6 | 16 | Norwalk |
| 47.7 | 47.7 | 47.7 | 50.0 | 46.5 | 48.3 | 48.8 | 48.3 | 43.6 | 48.3 | 45.3 | 46.5 | 50.0 | 50.0 | 71.5 | 71.5 | – | 57.9 | 52.6 | 17 | Southampton |
| 46.5 | 46.5 | 47.7 | 50.0 | 45.9 | 47.1 | 48.3 | 48.3 | 50.0 | 47.7 | 47.7 | 47.1 | 50.0 | 49.4 | 55.8 | 55.2 | 56.4 | – | 80.7 | 18 | Lordsdale |
| 50.0 | 50.0 | 49.4 | 49.4 | 48.8 | 47.7 | 48.3 | 48.3 | 47.1 | 47.7 | 51.2 | 50.0 | 49.4 | 48.8 | 51.2 | 51.2 | 50.0 | 69.8 | – | 19 | MX |
Percent identities based on deduced amino acid (aa) sequences, in upper triangle; Percent identities based on nucleotide (nt) sequences, in lower triangle.
Fig. 1.
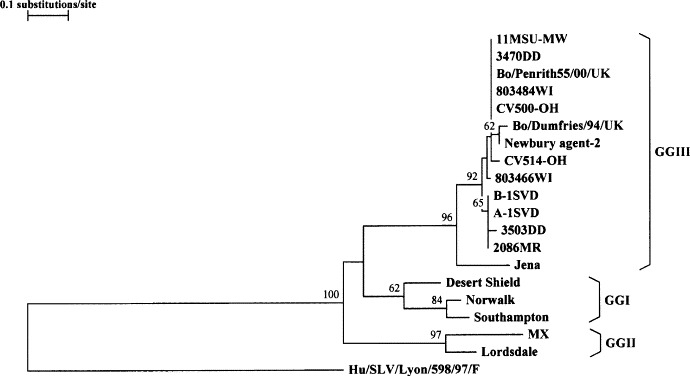
Phylogenetic analysis based on deduced amino acid sequences of a 172-nucleotide region of the RNA polymerase gene of bovine norovirus strains from Michigan,11MSU-MW, 3470DD, A-1SVD, B-1SVD, 3503DD and 2086MR (this study); from Wisconsin, 803484WI and 803466WI (this study); from Germany, Jena (AJ011099); from the UK, Bo/Penrith55/00/UK (AY126476), Bo/Dumfries/94/UK (AY126474), and Newbury agent-2 (AF097917); of bovine caliciviruses from Ohio, CV500-OH (AY151258) and CV514-OH (AY151259); and of human norovirus strains Desert Shield (U04469), Norwalk (M87661), Southampton (L07418), MX (U22498) and Lordsdale (X86557). Human sapovirus strain, Hu/SLV/Lyon/598/97/F (AJ271056), was included as the outgroup sequence. GenBank accession numbers are in parentheses. Bootstrap values at nodes are given if >50%.
3.3. Analysis of extended 3′ genomic sequence data for Michigan bovine norovirus strains B-1SVD and 11MSU-MW
Extended genomic sequence data were derived by the 3′ RACE method for two Michigan strains, B-1SVD and 11MSU-MW. Approximately 2.5 kb of sequence data were obtained for each strain, spanning the 3′ end of the polymerase gene [3′ end of open reading frame (ORF)1] to the start of the 3′ untranslated region of the genome. BLAST analyses showed that the entire ORF2 and ORF3 sequences obtained from these two strains corresponded to calicivirus capsid and small protein sequences. For both strains, the start codon of ORF2 overlaps the 3′ end of ORF1 by 14 nucleotides, the sequence of which is 100% conserved between the two strains (5′-AUGAAGAUGACUGA-3′). This sequence overlap between ORF1 and ORF2 is also conserved in Jena and in the UK bovine norovirus strains reported in the GenBank database. Genome organizations observed for B-1SVD and 11MSU-MW were consistent with those of strains classified under the genus norovirus.
ORF2 is 1560 and 1569 nucleotides long for B-1SVD and 11MSU-MW, respectively. The sequences translate into 519 amino acids for the former, and 522 amino acids for the latter. Results of pairwise sequence analyses showed these two Michigan bovine noroviruses sharing only 61.2% nucleotide and 67.4% amino acid sequence identities. Based upon a protein BLAST search, strain B-1SVD was found to be most closely related to the Jena strain, sharing with it an amino acid sequence identity of 94.2%. In contrast, strain 11MSU-MW was most closely related to bovine norovirus strains Bo/Dumfries/94/UK from the UK and Bo/NLV/CH126/1998/NL from the Netherlands, with amino acid sequence similarities of 97.1 and 96.2%, respectively. The Newbury agent-2 sequence was among those producing significant alignments with 11MSU-MW, with an amino acid sequence similarity of 95.8%. An alignment of the entire amino acid capsid sequences of B-1SVD, 11MSU-MW, Jena and Newbury agent-2 is shown in Fig. 2 . B-1SVD and Jena shared three amino acid deletions at positions 292, 417 and 423, in comparison to both 11MSU-MW and Newbury agent-2 sequences.
Fig. 2.
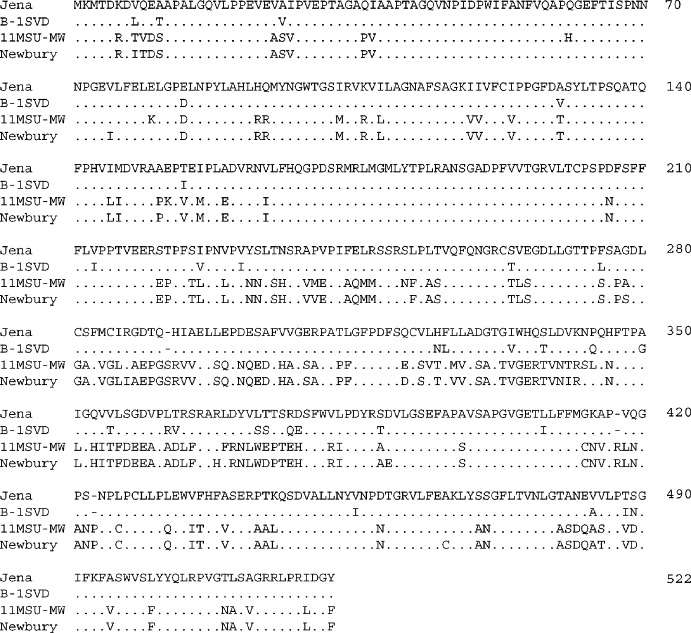
Alignment of the deduced amino acid sequences of the entire capsid gene (ORF2) of bovine norovirus strains: Michigan strains, B-1SVD and 11MSU-MW (this study); Jena (AJ011099) and Newbury (Newbury agent-2, AF097917). Full name of strain and/or GenBank accession numbers are in parentheses.
ORF3 of strain B-1SVD is 672 nucleotides long and encodes a small protein of 223 amino acids. ORF3 of strain 11MSU-MW is much longer, with 849 nucleotides translating into a 282-amino acid protein. The predicted ORF3 protein sequences of these two Michigan strains shared a sequence identity of only 60.5%. Protein BLAST analysis for B-1SVD showed Jena to have the highest sequence match at 87.4%. As is the case for the Jena strain, the ORF3 of B-1SVD also codes for a 223-amino acid protein. The 11 nucleotide ORF2–ORF3 sequence overlap (5′-AUGGCUACUGA-3′) is conserved between B-1SVD and Jena. For 11MSU-MW, its 282-amino acid ORF3 protein is identical in length to that of the UK strain, Bo/Aberystwth24/00/UK (GenBank accession no. AY126475), which showed the highest sequence match at 91.1%. Newbury agent-2 and Bo/Dumfries/94/UK also showed high sequence identities with 11MSU-MW, at 90.8 and 90.4%, respectively. The much longer ORF3 found in 11MSU-MW and in the UK strains, compared to B-1SVD and Jena, also presents a much longer ORF2–ORF3 sequence overlap of 209 nucleotides. However, there is a second potential AUG start codon for the ORF3 of 11MSU-MW, which reduces the overlap with ORF2 to 11 nucleotides, identical to the overlap seen in B-1SVD and Jena, except for one nucleotide change from an “A” to an “U” at the 7th nucleotide position within the overlap. This shorter overlap is 100% conserved between 11MSU-MW and the UK strains examined.
3.4. Partial capsid sequence and phylogenetic analyses
The high capsid sequence divergence observed between Michigan strains B-1SVD and 11MSU-MW led us to further investigate the extent of genetic diversity among the Michigan/Wisconsin bovine norovirus strains. Deduced amino acid sequences corresponding to a 744-nucleotide (excluding primer sequences) central variable region of the capsid gene were derived for 10 more Michigan/Wisconsin strains and compared to corresponding GenBank sequences of Jena, bovine noroviruses from the UK and the Netherlands and also to human GGI and GGII noroviruses (GenBank accession numbers are given in Fig. 4). Amino acid identities among the 12 US bovine norovirus partial capsid sequences (Table 2 ) showed the presence of two genetic groups. The first group, composed of five Michigan strains (B-1SVD, A-1SVD, 3505DD, 2086MR and 2167MR), shared an amino acid sequence identity of 100%. Strains B-1SVD and A-1SVD, originating from the same dairy farm, had identical sequences at the nucleotide level as well. The other three strains shared nucleotide identities of 99.6–100% with each other, but were only 97.6–97.7% similar to strains B-1SVD and A-1SVD. Strains 2086MR and 3505DD, originating from different farms, were identical at the nucleotide level. This group of five Michigan strains shared an amino acid sequence identity of 91.9% with the German strain, Jena. The second group includes six Michigan strains (11MSU-MW, 1GM, 21MSU-MW, 17–19MSU-MW, 32MSU-MW and 2387108MI) and one Wisconsin strain (803484WI). Strains with initials, “MSU-MW”, were from the same dairy farm. This group of seven strains shared amino acid identities with one another of 92.7–99.2% and average identities of 94.5% with the Dutch strains and 95.5% with the UK strains. Amino acid identities between the two groups range from only 56.3 to 57.1%. An alignment of the partial capsid sequences (deduced amino acid sequences) of the Michigan/Wisconsin strains and their counterparts from Germany, the UK and the Netherlands, is shown in Fig. 3 . Each of the sequences derived for the first group (five Michigan strains), had a serine residue missing at position number 150, as is the case for Jena. The sequence alignment in Fig. 3 clearly distinguishes “Jena-like” sequences from “Newbury agent-2-like” sequences, as exemplified in the alignment of sequences of the entire capsid of Michigan strains B-1SVD and 11MSU-MW with Jena and Newbury agent-2 in Fig. 2.
Fig. 4.
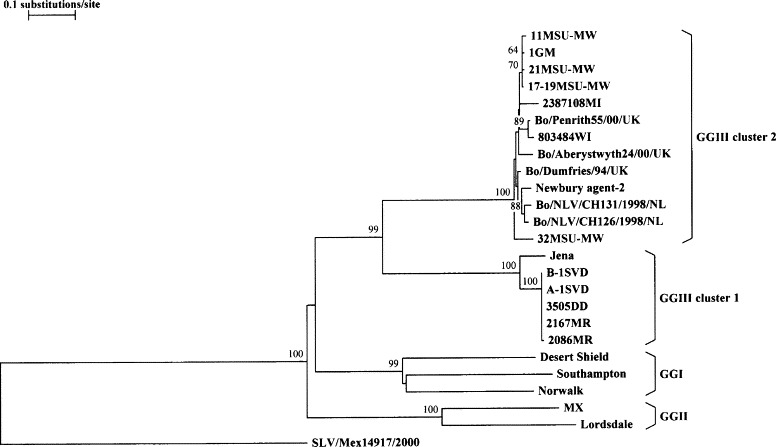
Phylogenetic analysis based on deduced amino acid sequences corresponding to a 744-nucleotide central variable region of the capsid gene of bovine noroviruses 11MSU-MW, 1GM, 21MSU-MW, 17–19MSU-MW, 2387108MI, 803484WI, 32MSU-MW, B-1SVD, A-1SVD, 3505DD, 2167MR, and 2086MR (this study); Jena (AJ011099); Bo/Penrith55/00/UK (AY126476); Bo/Aberystwyth24/00/UK (AY126475); Bo/Dumfries/94/UK (AY126474); Newbury agent-2 (AF097917); Bo/NLV/CH131/1998/NL (AF320113); Bo/NLV/CH126/1998/NL (AF320625); and of human noroviruses Desert Shield (U04469), Southampton (L07418), Norwalk (M87661), MX (U22498) and Lordsdale (X86557). Human sapovirus strain, SLV/Mex14917/2000 (AF435813), was included as the outgroup sequence. GenBank accession numbers are in parentheses. Bootstrap values at nodes are given if >60%.
Table 2.
Percent amino acid sequence identities between NLVs based on partial sequence of the capsid gene
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | ||
| 99.2 | 56.7 | 56.7 | 95.6 | 56.7 | 96.4 | 56.7 | 56.7 | 98.8 | 99.2 | 94.8 | 97.6 | 97.2 | 96.8 | 95.2 | 95.6 | 95.2 | 56.7 | 40.7 | 37.9 | 37.5 | 33.5 | 35.1 | 1 | 17–19MSU-MW | |
| 56.3 | 56.3 | 95.6 | 56.3 | 95.6 | 56.3 | 56.3 | 98.8 | 99.2 | 94.0 | 97.6 | 96.4 | 96.0 | 94.4 | 95.2 | 94.8 | 56.3 | 40.7 | 37.9 | 37.5 | 33.1 | 35.1 | 2 | 1GM | ||
| 100.0 | 56.3 | 100.0 | 57.1 | 100.0 | 100.0 | 55.9 | 56.3 | 56.7 | 56.7 | 56.3 | 56.3 | 57.1 | 56.7 | 56.7 | 91.9 | 40.5 | 39.3 | 37.2 | 37.2 | 35.6 | 3 | 2086MR | |||
| 56.3 | 100.0 | 57.1 | 100.0 | 100.0 | 55.9 | 56.3 | 56.7 | 56.7 | 56.3 | 56.3 | 57.1 | 56.7 | 56.7 | 91.9 | 40.5 | 39.3 | 37.2 | 37.2 | 35.6 | 4 | 2167MR | ||||
| 56.3 | 94.0 | 56.3 | 56.3 | 95.2 | 95.6 | 92.7 | 94.8 | 94.8 | 93.1 | 92.7 | 94.4 | 93.5 | 56.3 | 40.3 | 37.1 | 37.1 | 32.7 | 35.1 | 5 | 2387108MI | |||||
| 57.1 | 100.0 | 100.0 | 55.9 | 56.3 | 56.7 | 56.7 | 56.3 | 56.3 | 57.1 | 56.7 | 56.7 | 91.9 | 40.5 | 39.3 | 37.2 | 37.2 | 35.6 | 6 | 3505DD | ||||||
| 57.1 | 57.1 | 95.2 | 96.0 | 92.7 | 94.8 | 98.4 | 94.4 | 95.6 | 93.5 | 93.1 | 57.5 | 41.5 | 37.5 | 37.9 | 33.1 | 35.5 | 7 | 803484WI | |||||||
| 100.0 | 55.9 | 56.3 | 56.7 | 56.7 | 56.3 | 56.3 | 57.1 | 56.7 | 56.7 | 91.9 | 40.5 | 39.3 | 37.2 | 37.2 | 35.6 | 8 | A-1SVD | ||||||||
| 55.9 | 56.3 | 56.7 | 56.7 | 56.3 | 56.3 | 57.1 | 56.7 | 56.7 | 91.9 | 40.5 | 39.3 | 37.2 | 37.2 | 35.6 | 9 | B-1SVD | |||||||||
| 98.8 | 93.5 | 97.2 | 96.0 | 95.6 | 94.0 | 94.8 | 94.4 | 55.9 | 40.3 | 37.9 | 37.1 | 32.7 | 34.7 | 10 | 11MSU-MW | ||||||||||
| 94.0 | 97.6 | 96.8 | 96.0 | 94.4 | 95.2 | 94.8 | 56.3 | 40.7 | 37.9 | 37.5 | 33.1 | 35.1 | 11 | 21MSU-MW | |||||||||||
| 95.6 | 94.4 | 92.7 | 94.0 | 94.8 | 94.0 | 56.7 | 40.3 | 37.5 | 56.7 | 33.1 | 33.9 | 12 | 32MSU-MW | ||||||||||||
| 95.6 | 95.6 | 96.8 | 97.6 | 97.2 | 56.7 | 40.7 | 37.5 | 37.9 | 33.1 | 35.1 | 13 | Bo/Dumfries/94/UK | |||||||||||||
| 94.8 | 95.6 | 94.4 | 94.0 | 56.3 | 41.1 | 38.3 | 38.3 | 33.1 | 35.5 | 14 | Bo/Penrith55/00/UK | ||||||||||||||
| 94.8 | 94.4 | 94.8 | 55.9 | 41.1 | 37.9 | 38.3 | 32.7 | 35.1 | 15 | Bo/Aberywtwth24/00/UK | |||||||||||||||
| 96.8 | 96.4 | 57.5 | 41.9 | 37.9 | 38.7 | 33.1 | 35.9 | 16 | Newbury agent-2 | ||||||||||||||||
| 98.0 | 56.7 | 41.1 | 37.5 | 37.9 | 32.7 | 36.7 | 17 | Bo/NLV/CH126/1998/NL | |||||||||||||||||
| 56.7 | 41.1 | 37.5 | 37.5 | 32.7 | 36.7 | 18 | Bo/NLV/CH131/1998/NL | ||||||||||||||||||
| 40.9 | 38.5 | 37.2 | 36.4 | 35.6 | 19 | Jena | |||||||||||||||||||
| 57.4 | 56.9 | 33.6 | 33.2 | 20 | Desert Shield | ||||||||||||||||||||
| 55.8 | 36.3 | 40.6 | 21 | Norwalk | |||||||||||||||||||||
| 32.8 | 32.1 | 22 | Southampton | ||||||||||||||||||||||
| 58.2 | 23 | Lordsdale | |||||||||||||||||||||||
| 24 | MX | ||||||||||||||||||||||||
Fig. 3.
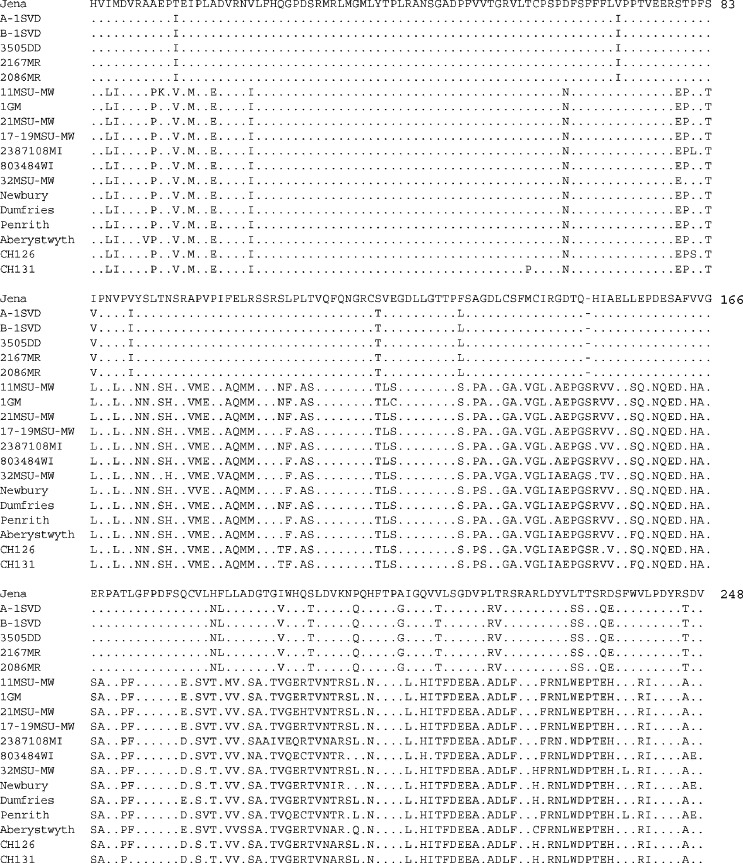
Alignment of deduced amino acid sequences corresponding to a 744-nucleotide central variable region of the capsid gene of bovine norovirus strains from Michigan and Wisconsin, A-1SVD, B-1SVD, 3505DD, 2167MR, 2086MR, 11MSU-MW, 1GM, 21MSU-MW, 17–19MSU-MW, 2387108MI, 803484WI, 32MSU-MW (this study); from Germany, Jena (AJ011099); from the UK, Newbury (Newbury agent-2, AF097917), Dumfries (Bo/Dumfries/94/UK, AY126474), Penrith (Bo/Penrith55/00/UK, AY126476), and Aberystwyth (Bo/Aberystwyth24/00/UK, AY126475); and from the Netherlands, CH126 (Bo/NLV/CH126/1998/NL, AF320625) and CH131 (Bo/NLV/CH131/1998/NL, AF320113). Full name of strain and/or GenBank accession numbers are in parentheses.
A phylogenetic tree, based on partial amino acid sequences of the capsid protein (Fig. 4 ), shows that the Michigan/Wisconsin bovine norovirus strains characterized thus far fall into one group (GGIII) with their European counterparts, genetically distinct from the human noroviruses, but more related to GGI than to GGII human strains. The bovine group is further subdivided into two distinct clusters or subgroups. Cluster 1 is composed of Jena and the five “Jena-like” Michigan strains. Cluster 2 consists of Newbury agent-2 and the “Newbury agent-2-like” strains from Michigan, Wisconsin, the UK and the Netherlands.
3.5. Nucleotide sequence accession numbers
Partial polymerase sequences of strains A-1SVD, B-1SVD,3470DD, 3503DD, 2086MR, 11MSU-MW, 803466WI and 803484WI, have been assigned GenBank accession numbers, AY266459–AY266466, respectively. Partial capsid sequences of strains 1GM, 21MSU-MW, 17–19MSU-MW, 2387108MI, 803484WI, 32MSU-MW, A-1SVD, 3505DD, 2167MR and 2086MR, have been assigned GenBank accession numbers, AY274261–AY274270, respectively. Partial polymerase sequences and complete capsid and ORF3 (small basic protein) sequences of strains B-1SVD and 11MSU-MW have been assigned GenBank accession numbers AY274819 and AY274820, respectively.
4. Discussion
In this study, we have used RT-PCR to show that diarrheic fecal samples from neonatal dairy calves on Michigan and Wisconsin farms contained norovirus-specific RNA. The samples were initially screened with a modified RT-PCR protocol from CDC, which makes use of a degenerate primer pair for detecting human norovirus RNA polymerase gene sequences. Once bovine norovirus polymerase sequences were derived for eight positive samples, a specific set of primers was designed to screen all available samples. The prevalence of bovine noroviruses in Michigan was high, with all eight farms and 80% of fecal samples examined testing positive. A high prevalence of bovine noroviruses in the field was similarly observed by researchers in the neighboring state of Ohio (Smiley et al., 2003). In contrast, only 4 positive samples (from 4 farms) were found among the 14 Wisconsin samples (from 14 farms) assayed with either of the RT-PCR protocols. Based on this very limited data, it is not possible to reach a conclusion about the true prevalence rate of noroviruses in Wisconsin. It is very apparent though that more positive samples were detected using our diagnostic set of bovine norovirus-specific primers. However, the lack of an internal control to monitor potential inhibition of the PCR reaction does not rule out the possibility that the number of positives may have been underestimated.
Results of phylogenetic analyses of partial RNA polymerase and capsid sequences derived for a subset of these Michigan/Wisconsin bovine norovirus strains are consistent with the suggestion of Ando et al. (2000) and the recent findings of Oliver et al. (2003) in the UK and of Smiley et al. (2003), that bovine noroviruses form a third genogroup, GGIII, distinct from the two human norovirus genogroups.
Noroviruses are known to be a genetically diverse group of viruses (Green et al., 1994, Wang et al., 1994, Lew et al., 1994). Within the two established human genogroups, GGI and GGII, strains can further be assigned to subgroups or clusters, depending upon the classification scheme used. The CDC has outlined a classification based on ORF2 sequences, in which GGI and GGII consisted of 5 and 10 genetic clusters, respectively (Ando et al., 2000). Seven phylogenetic groups in GGI and at least five phylogenetic groups in GGII were identified by Vinje et al. (2000), based on greater than 80% nucleotide identity in the N-terminus of the capsid protein. Green et al. (2000b) suggested seven genomic subgroups within GGI and eight in GGII when sequences of either the N-terminus or the central variable region of the capsid were analyzed. To date, little is known about the sequence diversity of bovine noroviruses. Ando et al. (2000) predicted two putative clusters within GGIII, represented by Jena and Newbury agent-2, respectively, based on the analysis of a 349 nucleotide region at the 5′ end of ORF2. Since then, more bovine norovirus capsid gene sequences have been submitted to the database. This allowed us to broaden the analysis of sequence diversity within the capsid region. Our findings demonstrate the existence of two genetic clusters/subgroups within the group we examined. One is more “Jena-like”, the other, more “Newbury agent-2-like”, as predicted by Ando et al. (2000). The Michigan strains are represented in either cluster, while the Wisconsin strain, along with the Dutch and other UK strains, all fall within the “Newbury agent-2-like” cluster.
The capsid primers designed in our study are from generally conserved regions which flank a central hypervariable region of the capsid gene. This region is useful for genetic diversity studies, as pointed out by Green et al. (2000b) and Vinje et al. (2000). Moreover, it has been shown that clustering of norovirus strains based on capsid sequences correlates well with their antigenic grouping based on antigen and antibody ELISAs using expressed norovirus capsid proteins (Jiang et al., 1992; Kobayashi et al., 2000a, Kobayashi et al., 2000b; Katayama et al., 2002).
Smiley et al. (2003) observed the formation of two distinct clusters of Ohio bovine norovirus strains based on the analysis of the 3′ terminal RNA polymerase gene segment (∼155 aa). One cluster was comprised of Jena and one Ohio strain. Another cluster was formed by four Newbury agent-2-like Ohio strains, two of which were previously characterized as being Newbury agent-2-like, based also on a partial segment of the RNA polymerase gene. In our study, the clustering of the Michigan/Wisconsin bovine norovirus strains into two distinct subgroups was not apparent when partial RNA polymerase-specific sequences were analyzed. Michigan strains B-1SVD, A-1SVD and 2086MR, which were determined “Jena-like” based on the analysis of capsid sequences, shared 84.2 and 94.7% amino acid similarities with Jena and Newbury agent-2, respectively, in the analyzed region of the RNA polymerase gene. The phylogenetic tree based on partial RNA polymerase sequences in Fig. 1 shows the Jena strain in a branch by itself, separated from the rest of the bovine strains in the GGIII cluster. In a phylogenetic study of 18 Norwalk-like viruses, Katayama et al. (2002) similarly observed that an RNA polymerase gene-based classification did not separate the strains into internal clusters within the genogroup. Although the RNA polymerase regions of noroviruses have been widely used for diagnostic purposes, results of genotyping within a genogroup based on sequences from these regions often do not correlate with those based upon capsid sequence data (Katayama et al., 2002). It has also been suggested that strains with genotypes that differ between the polymerase and capsid regions may be naturally occurring genetic recombinants (Vinje et al., 2000, Katayama et al., 2002, Jiang et al., 1999). A potential recombination site at the polymerase/capsid junction had already been identified in human norovirus strains (Jiang et al., 1999). Sequencing the entire polymerase gene of the Jena-like Michigan strains observed in this study will determine whether any of the strains are actually genetic recombinants between Jena-like and Newbury agent-2-like caliciviruses.
The divergence observed between the ORF3 sequences derived for the two Michigan strains, B-1SVD and 11MSU-MW, is further evidence for the assignment of these two strains in the two separate genetic clusters. ORF3 sequence-specific BLAST analyses showed the B-1SVD strain to be more “Jena-like” and the 11MSU-MW strain to be, more “Newbury agent-2-like”. It will be of interest to determine whether a consistent pattern exists among the ORF3 sequences of the other 10 Michigan/Wisconsin strains.
Our main focus for this study was to detect the presence of noroviruses in Michigan and Wisconsin dairy calves. In an ongoing study, we are examining the presence of other enteric viruses, including the novel enteropathogenic NB and NB-like bovine caliciviruses (Smiley et al., 2002, Smiley et al., 2003), in our collection of diarrheic calf fecal samples.
It is clear that, like their human-associated counterparts, bovine norovirus strains form a genetically heterogeneous group. It is anticipated that more genetic clusters will be identified in the future, as the bank of molecularly characterized bovine norovirus strains further expands. New genogroups of both human and animal noroviruses may emerge and the natural occurrence of genetic recombination between genomes of two very different norovirus strains (Jiang et al., 1999) may even result in host-range shifts, possibly turning animals into reservoirs for human infection and vice versa.
Acknowledgments
We thank Jacqueline Noel of the Centers for Disease Control and Prevention, Atlanta, GA, for sending primers and reagents used in our initial RT-PCR tests. We also thank Craig Radi of the Wisconsin Veterinary Diagnostic Laboratories, Madison, WI, for sending the fecal samples from Wisconsin. This study was supported in part by a grant from the National Food Safety and Toxicology Center, Michigan State University, East Lansing, MI.
Contributor Information
Annabel G Wise, Email: wisea@msu.edu.
Stephan S Monroe, Email: stm2@cdc.gov.
Lora E Hanson, Email: hansonlo@cvm.msu.edu.
Daniel L Grooms, Email: groomsd@cvm.msu.edu.
Donald Sockett, Email: donald.sockett@wvdl.wisc.edu.
Roger K Maes, Email: maes@dcpah.msu.edu.
References
- Anderson A.D, Heryford A.G, Sarisky J.P, Higgins C, Monroe S.S, Beard R.S, Newport C.M, Cashdollar J.L, Fout G.S, Robbins D.E, Seys S.A, Musgrave K.J, Medus C, Vinje J, Bresee J.S, Mainzer H.M, Glass R.I. A waterborne outbreak of Norwalk-like virus among snowmobilers—Wyoming, 2001. J. Infect. Dis. 2003;187:303–306. doi: 10.1086/346239. [DOI] [PubMed] [Google Scholar]
- Ando T, Mulders M.N, Lewis D.C, Estes M.K, Monroe S.S, Glass R.I. Comparison of the polymerase region of small round structured virus strains previously classified in three antigenic types by solid-phase immune electron microscopy. Arch. Virol. 1994;135:217–226. doi: 10.1007/BF01309781. [DOI] [PubMed] [Google Scholar]
- Ando T, Noel J.S, Fankhauser R.L. Genetic classification of “Norwalk-like viruses”. J. Infect. Dis. 2000;181:S336–S348. doi: 10.1086/315589. [DOI] [PubMed] [Google Scholar]
- Altschul S.F, Gish W, Miller W, Myers E.W, Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bresee J.S, Widdowson M.-A, Monroe S.S, Glass R.I. Foodborne viral gastroenteritis: challenges and opportunities. Clin. Infect. Dis. 2002;35:748–753. doi: 10.1086/342386. [DOI] [PubMed] [Google Scholar]
- Bridger J.C, Hall G.A, Brown J.F. Characterization of a calici-like virus (Newbury agent) found in association with astrovirus in bovine diarrhea. Infect. Immun. 1984;43:133–138. doi: 10.1128/iai.43.1.133-138.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastjerdi A.M, Green J, Gallimore C.I, Brown D.W.G, Bridger J.C. The bovine Newbury Agent-2 is genetically more closely related to human SRSVs than to animal caliciviruses. Virology. 1999;254:1–5. doi: 10.1006/viro.1998.9514. [DOI] [PubMed] [Google Scholar]
- Efron B, Gong G. A leisurely look at the bootstrap, the jackknife, and cross-validation. Am. Stat. 1983;37:36–48. [Google Scholar]
- Fankhauser R.L, Monroe S.S, Noel J.S, Humphrey C.D, Bresee J.S, Parashar U.D, Ando T, Glass R.I. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 2002;186:1–7. doi: 10.1086/341085. [DOI] [PubMed] [Google Scholar]
- Fankhauser R.L, Noel J.S, Monroe S.S, Ando T.A, Glass R.I. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 1998;178:1571–1578. doi: 10.1086/314525. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Glass R.I, Noel J, Ando T, Fankhauser R, Belliot G, Mounts A, Parashar U.D, Bresee J.S, Monroe S.S. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J. Infect. Dis. 2000;181:S254–S261. doi: 10.1086/315588. [DOI] [PubMed] [Google Scholar]
- Green K.Y, Ando T, Balayan M.S, Berke T, Clarke I.N, Estes M.K, Matson D.O, Nakata S, Neill J.D, Studdert M.J, Thiel H.-J. Taxonomy of Caliciviruses. J. Infect. Dis. 2000;181:S322–S330. doi: 10.1086/315591. [DOI] [PubMed] [Google Scholar]
- Green S.M, Dingle K.E, Lambden P.R, Caul E.O, Ashley C.R, Clarke I.N. Human enteric Caliciviridae: a new prevalent small round-structured group defined by RNA-dependent RNA polymerase and capsid diversity. J. Gen. Virol. 1994;5:1883–1888. doi: 10.1099/0022-1317-75-8-1883. [DOI] [PubMed] [Google Scholar]
- Green J, Vinje J, Gallimore C.I, Koopmans M, Hale A, Brown D.W.G. Capsid protein diversity among Norwalk-like viruses. Virus Genes. 2000;20:227–236. doi: 10.1023/a:1008140611929. [DOI] [PubMed] [Google Scholar]
- Günther H, Otto P. Studies into diarrhea of young calves. Seventh communication: “Zackenvirus” (Jena-Agens 117/80)—a new diarrhea pathogen to calf. Arch. Exp. Vet. Med. Leipzig. 1987;41:934–938. [PubMed] [Google Scholar]
- Higgins D.G, Sharp P.M. Fast and sensitive multiple sequence alignments on a microcomputer. CABIOS. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Jiang X, Espul C, Zhong W.M, Cuello H, Matson D.O. Characterization of a novel human calicivirus that may be a naturally occurring recombinant. Arch. Virol. 1999;144:2377–2387. doi: 10.1007/s007050050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wang M, Graham D.Y, Estes M.K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 1992;66:6525–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Shirato-Horikoshi H, Kojima S, Kageyama T, Oka T, Hoshino F, Fukushi S, Shinohara M, Uchida K, Suzuki Y, Gojobori T, Takeda N. Phylogenetic analysis of the complete genome of 18 Norwalk-like viruses. Virology. 2002;299:225–239. doi: 10.1006/viro.2002.1568. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Sakae K, Suzuki Y, Ishiko H, Kamada K, Suzuki K, Natori K, Miyamura T, Takeda N. Expression of recombinant capsid proteins of chitta virus, a genogroup II Norwalk virus, and development of an ELISA to detect the viral antigen. Microbiol. Immunol. 2000;44:687–693. doi: 10.1111/j.1348-0421.2000.tb02550.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Sakae K, Suzuki Y, Shinozaki K, Okada M, Ishiko H, Kamata K, Suzuki K, Natori K, Miyamura T, Takeda N. Molecular cloning, expression, and antigenicity of seto virus belonging to genogroup I Norwalk-like viruses. J. Clin. Microbiol. 2000;38:3492–3494. doi: 10.1128/jcm.38.9.3492-3494.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew J.F, Kapikian A.Z, Valdesuso J, Green K.Y. Molecular characterization of Hawaii virus and other Norwalk-like viruses: evidence for genetic polymorphism among human caliciviruses. J. Infect. Dis. 1994;170:535–542. doi: 10.1093/infdis/170.3.535. [DOI] [PubMed] [Google Scholar]
- Liu B.L, Lambden P.R, Günther H, Otto P, Elschner M, Clarke I.N. Molecular characterization of a bovine enteric calicivirus: relationship to the Norwalk-like viruses. J. Virol. 1999;73:819–825. doi: 10.1128/jvi.73.1.819-825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead P.S, Slutsker L, Dietz V, McCaig L.F, Bresee J.S, Shapiro C, Griffin P.M, Tauxe R.V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S.S, Ando T, Glass R.I. Introduction: Human enteric caliciviruses—an emerging pathogen whose time has come. J. Infect. Dis. 2000;181:S249–S251. doi: 10.1086/315594. [DOI] [PubMed] [Google Scholar]
- Oliver S.L, Dastjerdi A.M, Wong S, El-Attar L, Gallimore C, Brown D.W.G, Green J, Bridger J.C. Molecular characterization of bovine enteric caliciviruses: a distinct third genogroup of noroviruses (Norwalk-like viruses) unlikely to be of risk to humans. J. Virol. 2003;77:2789–2798. doi: 10.1128/JVI.77.4.2789-2798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar U.D, Monroe S.S. ‘Norwalk-like viruses’ as a cause of foodborne disease outbreaks. Rev. Med. Virol. 2001;11:243–252. doi: 10.1002/rmv.321. [DOI] [PubMed] [Google Scholar]
- Raes, J., Van de Peer, Y., 1999. ForCon: a software tool for the conversion of sequence alignments. EMBnet.news 6(1). Available at: http://www.hgmp.mrc.ac.uk/embnet.news/vol6_1/.
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. E. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Smiley J.R, Chang K.O, Hayes J, Vinje J, Saif L.J. Characterization of an enteropathogenic bovine calicivirus representing a potential new genus. J. Virol. 2002;76:10089–10098. doi: 10.1128/JVI.76.20.10089-10098.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J.R, Hoet A.E, Traven M, Tsunemitsu H, Saif L.J. Reverse transcription-PCR assays for detection of bovine enteric caliciviruses (BEC) and analysis of the genetic relationships among BEC and human caliciviruses. J. Clin. Microbiol. 2003;41:3089–3099. doi: 10.1128/JCM.41.7.3089-3099.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugieda M, Nagaoka H, Kakishima Y, Ohshita T, Nakamura S, Nakajima S. Detection of Norwalk-like virus genes in the caecum contents of pigs. Arch. Virol. 1998;143:1215–1221. doi: 10.1007/s007050050369. [DOI] [PubMed] [Google Scholar]
- Sugieda M, Nakajima S. Viruses detected in the caecum contents of healthy pigs representing a new genetic cluster in genogroup II of the genus ‘Norwalk-like viruses’. Virus Res. 2002;87:165–172. doi: 10.1016/s0168-1702(02)00107-7. [DOI] [PubMed] [Google Scholar]
- Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- van der Poel W.H.M, Vinje J, van der Heide R, Herrera M.-I, Vivo A, Koopmans M.P.G. Norwalk-like calicivirus genes in farm animals. Emerg. Infect. Dis. 2000;6:36–41. doi: 10.3201/eid0601.000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinje J, Green J, Lewis D.C, Gallimore C.I, Brown D.W.G, Koopmans M.P.G. Genetic polymorphism across regions of the three open reading frames of “Norwalk-like viruses”. Arch. Virol. 2000;145:223–241. doi: 10.1007/s007050050020. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang X, Madore H.P, Gray J, Desselberger U, Ando T, Seto Y, Oishi I, Lew J.F, Green K.Y, Estes M.K. Sequence diversity of small* round-structured viruses in the Norwalk virus group. J. Virol. 1994;68:5982–5990. doi: 10.1128/jvi.68.9.5982-5990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G.N, Bridger J.C. Isolation of small viruses resembling astroviruses and caliciviruses from acute enteritis of calves. J. Med. Microbiol. 1978;11:411–452. doi: 10.1099/00222615-11-4-441. [DOI] [PubMed] [Google Scholar]


