Abstract
Thirty colostrum-deprived piglets aged 1 day were inoculated with a Korean strain of porcine epidemic diarrhoea virus (PEDV). The purpose was to elucidate the pathogenicity and viral distribution in PEDV-infected piglets over a period of 60 h, by morphometric analysis, in-situ hybridization and immunohistochemistry. At 24–60 h post-inoculation (hpi), the villous height/crypt depth (VH/CD) ratio of infected pigs was significantly less than that of control pigs. Positive cells typically exhibited a dark black reaction product (in-situ hybridization) or brown reaction product (immunohistochemistry) in the cytoplasm, without background staining. PEDV nucleic acid and antigen were detected in the duodenum, jejunum and ileum of experimentally infected pigs. The results suggested that the Korean strain was virulent and caused severe villous atrophy in the small intestine.
Keywords: coronavirus, PEDV, pig, poraine epidemic diarrhoea virus, viral infection
1. Introduction
Porcine epidemic diarrhoea (PED) virus (PEDV) is an enveloped RNA virus with a single-stranded positive-sense genome. It belongs to the family Coronaviridae which, together with the Arteriviridae family, constitutes the order Nidovirales (Cavanagh, 1997). PEDV mainly affects unweaned piglets but occasionally older animals. Piglets up to 7 days old often vomit and develop a severe watery fetid diarrhoea that leads to dehydration, rapid weight loss, and death in 2 to 4 days. Clinical signs last 3 to 5 weeks in farrowing units (Chasey and Cartwright, 1978, Pensaert and Debouck, 1978). Two forms of PED have been described, namely, (1) epidemic diarrhoea type I, clinically characterized by diarrhoea in swine of all ages except unweaned piglets (Anonymous, 1972), and (2) epidemic diarrhoea type II, characterized by acute diarrhoea in swine of all ages (Wood, 1977). Both forms of PED are recognized in Korea (Chae et al., 2000).
PED was first reported in Belgium and the United Kingdom in 1978 (Wood, 1977, Pensaert and Debouck, 1978). Since then, outbreaks of the disease have been reported in many swine-raising countries, notably in Europe and Korea (Debouck et al., 1982, Jimenez et al., 1986, Chae et al., 2000). The pathogenicity of PEDV isolated in Europe was studied by Debouck et al., 1981, Pospischil et al., 1981. The pathogenicity of Korean strains of PEDV, however, has not been studied, despite their high prevalence (Chae et al., 2000). The objective of the present study was to elucidate the pathogenicity and viral distribution of a Korean strain in piglets over a period of 60 h after oral infection.
2. Materials and Methods
2.1. PEDV Strain
Strain SNUVR971496 at the third passage in tissue culture was used, having been isolated from the small intestine of a 3-day-old pig with severe diarrhoea in Korea. The virus was titrated by growth of serial 10-fold dilutions in Vero cells, followed by immunofluorescent labelling for viral antigen (Ellis et al., 1998, Ellis et al., 1999) with PEDV monoclonal antibody. The monoclonal antibody (C9-9-9) reacted with the 30 kD membrane protein of PEDV in Western blotting analysis (Kim et al., 1999).
2.2. Experimental Design
Thirty colostrum-deprived Large White–Duroc cross-bred pigs aged 1 day were randomly allocated to two equal groups. Fifteen piglets were each dosed orally with 2 ml of virus stock (106.5 tissue culture infective doses 50% [TCID50]/ml) of the strain SNUVR971496. Fifteen control piglets were similarly treated with uninfected cell culture supernat. All the piglets were maintained in sterile stainless steel isolators (three per isolator) and fed a commercial sterile milk substitute. All piglets were examined three times daily for clinical signs. Subgroups of three piglets from the infected and control groups were killed by electrocution and subjected to necropsy at 12, 24, 36, 48 and 60 h post-inoculation (hpi). Tissue specimens were collected as previously described (Kim and Chae, 2002a).
2.3. In-situ Hybridization (ISH)
A 412 base pair cDNA probe for the viral RNA encoding the membrane protein of PEDV was used as a probe. The forward and reverse primers were 5′-GGGCGCCTGTATAGAGTTTA-3′ (nucleotides 927-946) and 5′-AGACCACCAAGAATGTGTCC-3′ (nucleotides 1319-1338), respectively. The polymerase chain reaction (PCR) was carried out as previously described (Kim et al., 2000, Kim and Chae, 2002b). The PCR product was purified with Wizard PCR Preps (Promega Biotech, Madison, WI, USA) and labelled by random priming with digoxigenin-dUTP with a commercial kit (Boehringer Mannheim, Indianapolis, IN, USA), according to the manufacturer's instructions. In-situ hybridization was carried out as previously described (Kim and Chae, 2002a). The digoxigenin-labelled cDNA probe of transmissible gastroenteritis virus (TGEV) was used as negative probe (Kim and Chae, 2001). Negative tissue (jejunum and ileum) controls were collected from three 1-day-old colostrum-deprived piglets that had been experimentally infected with TGEV (Kim and Chae, 2002a).
2.4. Immunohistochemistry (IHC)
Tissues were dewaxed with xylene, rehydrated through graded alcohols, and air dried. Endogenous peroxidase was quenched with absolute methanol containing hydrogen peroxide 1% for 1 h. All slides were then treated with 0.01% protease XXIV (Sigma Chemical Company, St Louis, MO, USA) in phosphate-buffered saline (PBS; 0.1 M, pH 7.4) for 2 min at room temperature. To saturate nonspecific protein-binding sites, all slides were then treated for 1 h at room temperature with a mixture of 10% normal horse serum (NHS) (Vector Laboratories, Burlingame, CA, USA) and 3% bovine serum albumin in a ratio of 1:20, the diluent used being PBS. The slides were next treated overnight at 4 °C in a humidity chamber with PEDV monoclonal antibody (C9-9-9) diluted 1 in 100 with PBS containing NHS 2% and Triton X100 (Sigma Chemical Company) 3%. After three washes with PBS, sections were flooded and incubated for 1–2 h at room temperature with biotinylated horse anti-mouse IgG (Vector Laboratories) diluted 1 in 400 in PBS containing NHS 2% and Triton X100 0.3%. The slides were washed in PBS, followed by incubation for 30 min in avidin-biotin complex (ABC) solution (Vector Laboratories), prepared according to the manufacturer's instructions. After washing in PBS, the final reaction product was developed by immersing the sections in a solution of hydrogen peroxide 0.01% and 3,3′-diaminobenzidine tetrahydrochloride (Vector Laboratories) 0.05% in PBS for 10 min. The sections were lightly counterstained with Mayer's haematoxylin, dehydrated through graded concentrations of ethanol and xylene, and mounted.
2.5. Morphometric Analysis
Three pieces of formalin-fixed jejunum were taken from each virus-infected and control piglet for morphometric analysis, as previously described (Kim and Chae, 2002a).
2.6. Statistical Analysis
The Wilcoxon matched pairs test was used to determine the significance of differences between infected and control piglets in terms of villous height/crypt depth(VH/CD) ratio.
3. Results
3.1. Clinical Signs
The signs observed in infected piglets ranged from diarrhoea to vomiting and dehydration. Diarrhoea was seen in some piglets at 12 hpi but by 36 hpi all infected animals had diarrhoea. Anorexia was seen in some piglets at the onset of diarrhoea, but others continued to eat until they became too weak to do so. Vomiting was seen in some piglets at 24 hpi. In addition, they appeared dehydrated. No clinical signs were seen in the control piglets.
3.2. VH/CD Ratio
The results are summarized in Table 1 . At 12 hpi, the mean VH/CD ratio in pigs dosed with PEDV was not significantly different from that of the control pigs. At 24, 36, 48 and 60 hpi, however, the mean value in infected piglets was significantly different from that of the controls.
Table 1.
Mean jejunal villous height and crypt depth (VH/CD) ratio in piglets infected orally with the Korean strain of porcine epidemic diarrhoea virus (PEDV)
| Hours post-inoculation | Mean VH/CD ratio (±SD) in |
|
|---|---|---|
| Uninfected control piglets | PEDV-infected piglets | |
| 12 | 7.70±0.73 | 7.60±0.65 |
| 24 | 7.01±0.70 | 3.36±0.38* |
| 36 | 7.32±0.73 | 2.58±0.23* |
| 48 | 7.58±0.64 | 1.36±0.27* |
| 60 | 7.38±0.78 |
1.11±0.34* |
*Significantly different from control (P<0.05), SD, standard deviation.
3.3. Viral Distribution in Infected Piglets as Demonstrated by ISH and IHC
Table 2 summarizes the number of positive results (PEDV nucleic acid and antigen) in four specific tissues from the subgroups of three infected piglets killed at the five chosen intervals after inoculation. The sensitivity and specificity of PEDV in-situ hybridization and immunohistochemistry were calculated from the data in Table 2. The sensitivity of each of the two methods, determined by dividing the number of PEDV nucleic acid- and antigen-positive jejuna by the number of PEDV-inoculated piglets, was 80% (12/15) and 66.7% (10/15), respectively. In fact, the observed difference in sensitivity between the two methods relates to samples collected at 24 hpi (Table 2). The specificity of the two methods, determined by dividing the number of control samples negative for PEDV nucleic acid or antigen by the number of control piglets, was 100% (0/15) in each case. Positive cells typically exhibited a dark black reaction product (in-situ hybridization) or brown reaction product (immunohistochemistry) in the cytoplasm, without background staining (Fig. 1, Fig. 2) .
Table 2.
In-situ hybridization (ISH) and immunohistochemistry (IHC) results in piglets infected orally with the Korean strain of porcine epidemic diarrhoea virus
| Positive results (in subgroups of 3 piglets) by ISH/IHC at hours post-inoculation |
Total ISH/IHC results (out of 15) | |||||
|---|---|---|---|---|---|---|
| Tissues | 12 | 24 | 36 | 48 | 60 | |
| Duodenum | 0*/0* | 2/1 | 2/2 | 3/3 | 3/3 | 10/9 |
| Jejunum | 0/0 | 3/1 | 3/3 | 3/3 | 3/3 | 12/10 |
| Ileum | 0/0 | 2/1 | 2/2 | 3/3 | 3/3 | 10/9 |
| Colon | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
A section was considered positive for ISH and IHC when at least one enterocyte was positive. The 15 uninfected control piglets gave consistently negative results.
Fig. 1.
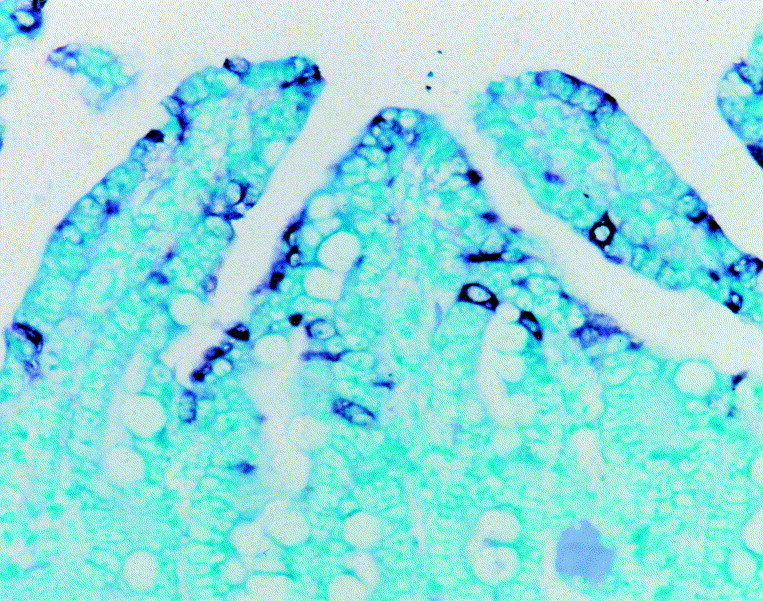
Jejunum from a piglet dosed orally with PEDV; 36 h post-inoculation (hpi). PEDV nucleic acid (dark black reaction product) was detected in the villous enterocytes. In-situ hybridization. ×200.
Fig. 2.
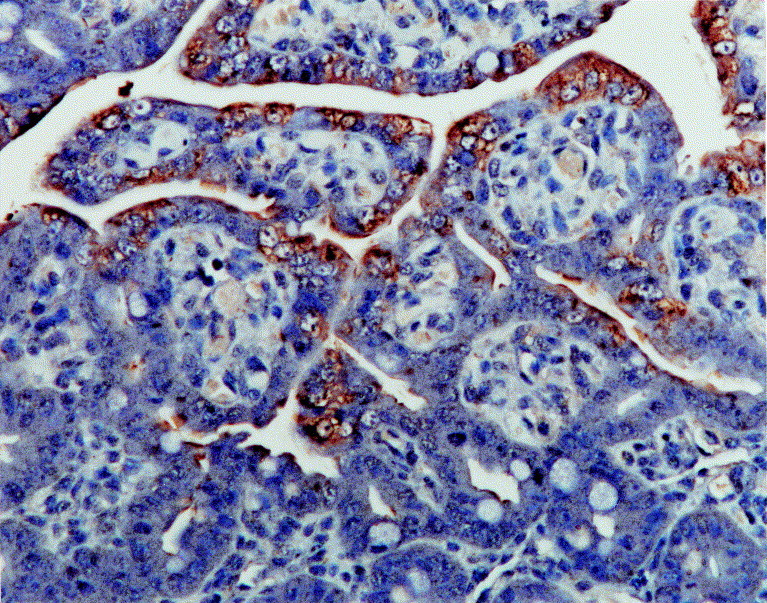
Jejunum from a piglet dosed orally with PEDV; 48 hpi. PEDV antigen (brown reaction product) was detected in the villous enterocytes. Immunohistochemistry. ×200.
PEDV nucleic acid and antigen were not detected in the duodenum, jejunum or ileum of any inoculated animal at 12 hpi but were detected at 24, 36, 48 and 60 hpi, being seen almost exclusively in the cytoplasm of duodenal, jejunal, and ileal villous enterocytes. Intense and specific hybridization and immunohistochemical signals were most often seen within jejunal villous enterocytes (Fig. 1, Fig. 2). Strong hybridization signals and immunolabelling were seen in areas of moderate to severe villous atrophy or vacuolation; positive cells were arranged continuously over the villi. In the lumen, exfoliated enterocytes were strongly positive. Hybridization signals and immunolabelling were also seen in the cytoplasm of ileal villous enterocytes. Hybridization signals were not detected in epithelial cells of the colon or rectum, or in lung specimens.
No hybridization signal was consistently seen in tissue sections pretreated with RNase A. The cDNA probes for TGEV were consistently negative in tissues tested from both infected and uninfected pigs. Neither PEDV nucleic acid nor antigen was detected in the intestinal tissues from uninfected control pigs.
4. Discussion
The SNUVR971496 strain of PEDV, which caused severe diarrhoea in the experimental piglets, appeared to be particularly virulent. This conclusion was consistent with the devastating diarrhoea observed on the farm from which the strain originated. PEDV nucleic acid and antigen were detected in intestinal tissues from infected piglets, the most consistent labelling for PEDV occurring in the jejunal villous enterocytes. Simultaneous detection of viral protein and nucleic acid provided molecular evidence of viral replication in these cells.
The large amounts of viral protein expressed in villous enterocytes, together with the presence of high copy numbers of virus-specific RNA, strongly suggest that villous enterocytes support PEDV replication and represent a main target of the virus. There was a direct relation between viral replication and the development of histopathological lesions in the small intestine. A first peak in the number of virus- infected cells, observed shortly after the onset of diarrhoea, coincided with a shortening of the villi. Infection of villous enterocytes by PEDV apparently led to destruction or sloughing of the cells in the intestinal lumen, resulting in a degree of villous atrophy. Ultrastructural studies have revealed considerable damage to the villous enterocytes by PEDV infection (Ducatelle et al., 1981, Ducatelle et al., 1982, Pospischil et al., 1981). Destruction of, or damage to, these cells by PEDV may induce villous atrophy and fusion, which result in a decreased intestinal surface area; this, in turn, results in intestinal maldigestion and malabsorption, severe diarrhoea, and dehydration (Coussement et al., 1982, Pensaert, 1989).
In-situ hybridization and immunohistochemistry detect PEDV nucleic acid and proteins and enable comparisons to be made of transcriptional and translational events in cells infected with PEDV. The slightly greater sensitivity of ISH has been attributed to higher quantities of PEDV nucleic acid than of proteins. Early in the viral cycle, quantities of viral nucleic acid may predominate over protein. The current understanding of the replication cycle of coronavirus is that large quantities of mRNA begin forming within 12–24 h of cellular infection; this is then followed by protein synthesis (Spaan et al., 1988, Lai, 1990). It is conceivable, therefore, that ISH is superior to immunohistochemistry for the detection of PEDV during early infection. This explanation is supported by our observation that in-situ hybridization succeeded in detecting PEDV nucleic acid in jejunal tissue sections in the apparent absence of viral antigen in two PEDV-inoculated piglets at 24 hpi. Viral nucleic acid and antigen were detected equally, however, in the jejunum thereafter.
The clinical signs of PEDV and TGEV infections are virtually indistinguishable. Moreover, both infections cause destruction of villous enterocytes and villous atrophy in the jejunum and ileum (Debouck and Pensaert, 1980, Debouck et al., 1981, Ducatelle et al., 1981, Pospischil et al., 1981, Kim and Chae, 2002a). Despite these similarities, the two viruses are distinct. The progression of enterocyte infection by PEDV appears to be slower than that by TGEV. In TGEV infection, viral nucleic acid could be detected as early as 12 h after inoculation (Kim and Chae, 2002a), whereas in PEDV infection in the present study, viral nucleic acid was absent at 12 h after inoculation, becoming distinct only at 24 h. The longer incubation period observed in PEDV infection is a result of this slower infection rate. Moreover, replication of PEDV is confined to the epithelial cells covering the villi of the intestinal tract, as previously reported (Kim et al., 1999, Kim and Chae, 2000). This differs from TGEV infections, in which hybridization signal is also seen in crypt enterocytes (Sirinarumitr et al., 1996, Kim and Chae, 2001, Kim and Chae, 2002a). Since the viral distribution corresponded to the distribution of viral receptor (Weingartl and Derbyshire, 1993), crypt enterocytes may not possess the PEDV receptor.
Korean and European strains of PEDV both lead to severe villous atrophy in the jejunum; however, in this study, replication of a Korean strain could not be demonstrated in colonic enterocytes, whereas positive fluorescence for a European strain was observed in the colon of pigs with diarrhoea by Debouck and Pensaert, 1980, Debouck et al., 1981. This difference in site of viral replication may be due to differences in tissue tropism among PEDV strains. The Korean strain appeared to have a tropism for villous enterocytes in the jejunum and ileum. The difference in replication sites could also have been due to other factors, such as differences in the age or breed of the piglet, and further studies are needed to resolve this matter.
Acknowledgments
This research was supported by the Ministry of Agriculture, Forestry and Fisheries—Special Grants Research Program (MAFF—SGRP), and the Brain Korea 21 Project, Republic of Korea.
References
- Anonymous Information supplement. Veterinary Record. 1972;95:49. [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Archives of Virology. 1997;142:629–633. [PubMed] [Google Scholar]
- Chae C., Kim O., Choi C., Min K., Cho W.-S., Kim J., Tai J.H. Prevalence of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus infection in Korean pigs. Veterinary Record. 2000;147:606–608. doi: 10.1136/vr.147.21.606. [DOI] [PubMed] [Google Scholar]
- Chasey D., Cartwright S.F. Virus-like particles associated with porcine epidemic diarrhoea. Research in Veterinary Science. 1978;25:255–256. doi: 10.1016/S0034-5288(18)32994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussement W., Ducatelle R., Debouck P., Hoorens J. Pathology of experimental CV777 coronavirus enteritis in piglets. I. Histological and histochemical study. Veterinary Pathology. 1982;19:46–56. doi: 10.1177/030098588201900108. [DOI] [PubMed] [Google Scholar]
- Debouck P., Callebaut P., Pensaert M. Prevalence of the porcine epidemic diarrhea (PED) virus in the pig population of different countries. Proceedings of the International Pig Veterinary Society Congress. 1982;7:53. [Google Scholar]
- Debouck P., Pensaert M. Experimental infection of pigs with a new porcine enteric coronavirus, CV777. American Journal of Veterinary Research. 1980;41:219–223. [PubMed] [Google Scholar]
- Debouck P., Pensaert M., Coussement W. The pathogenesis of an enteric infection in pigs, experimentally induced by the coronavirus-like agent, CV777. Veterinary Microbiology. 1981;6:157–165. [Google Scholar]
- Ducatelle R., Coussement W., Charlier G., Debouck P., Hoorens J. Three-dimensional sequential study of the intestinal surface in experimental porcine CV777 coronavirus enteritis. Journal of Veterinary Medicine B. 1981;28:483–493. doi: 10.1111/j.1439-0450.1981.tb01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatelle R., Coussement W., Debouck P., Hoorens J. Pathology of experimental CV777 coronavirus enteritis in piglets. II. Electron microscopic study. Veterinary Pathology. 1982;19:57–66. doi: 10.1177/030098588201900109. [DOI] [PubMed] [Google Scholar]
- Ellis J., Hassard L., Clark E., Harding J., Allan G., Willson P., Strokappe J., Martin K., McNeilly F., Meehan B., Todd D., Haines D. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Canadian Veterinary Journal. 1998;39:44–51. [PMC free article] [PubMed] [Google Scholar]
- Ellis J., Krakowka S., Laimore M., Haines D., Brantanich A., Clark E., Allan G., Konoby C., Hassard L., Meehan B., Martin K., Harding J., Kennedy S., McNeilly F. Reproduction of lesions of postweaning multisystemic wasting syndrome in gnotobiotic piglets. Journal of Veterinary Diagnostic Investigation. 1999;11:3–14. doi: 10.1177/104063879901100101. [DOI] [PubMed] [Google Scholar]
- Jimenez G., Castro J.M., del Pozzo M., Correa I., de la Torre J., Enjuanes L. Identification of a coronavirus inducing porcine gastroenteritis in Spain. Proceedings of the International Pig Veterinary Society Congress. 1986;9:186. [Google Scholar]
- Kim O., Chae C. In situ hybridization for the detection and localization of porcine epidemic diarrhea virus in the intestinal tissues from naturally infected piglets. Veterinary Pathology. 2000;37:62–67. doi: 10.1354/vp.37-1-62. [DOI] [PubMed] [Google Scholar]
- Kim B., Chae C. In-situ hybridization for the detection of transmissible gastroenteritis virus in pigs and comparison with other methods. Canadian Journal of Veterinary Research. 2001;65:33–37. [PMC free article] [PubMed] [Google Scholar]
- Kim B., Chae C. Experimental infection of piglets with transmissible gastroenteritis virus: a comparison of three strains (Korean Purdue and Miller) Journal of Comparative Pathology. 2002;126:30–37. doi: 10.1053/jcpa.2001.0517. [DOI] [PubMed] [Google Scholar]
- Kim O., Chae C. Comparison of reverse transcription polymerase chain reaction, immunohistochemistry, and in situ hybridization for the detection of porcine epidemic diarrhea virus in pigs. Canadian Journal of Veterinary Research. 2002;66:112–116. [PMC free article] [PubMed] [Google Scholar]
- Kim O., Chae C., Kweon C.-H. Monoclonal antibody-based immunohistochemical detection of porcine epidemic diarrhea virus antigen in formalin-fixed, paraffin-embedded intestinal tissues. Journal of Veterinary Diagnostic Investigation. 1999;11:458–462. doi: 10.1177/104063879901100512. [DOI] [PubMed] [Google Scholar]
- Kim O., Choi C., Kim B., Chae C. Detection and differentiation of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus in clinical samples by multiplex RT-PCR. Veterinary Record. 2000;146:637–640. doi: 10.1136/vr.146.22.637. [DOI] [PubMed] [Google Scholar]
- Lai M.M.C. Coronavirus: organization, replication and expression of genome. Annual Review of Microbiology. 1990;44:303–333. doi: 10.1146/annurev.mi.44.100190.001511. [DOI] [PubMed] [Google Scholar]
- Pensaert M.B. Virus Infections of Porcines. Elsevier; Amsterdam: 1989. (Virus Infections of Porcines). pp. 167–176. [Google Scholar]
- Pensaert M.B., Debouck P. A new coronavirus-like particle associated with diarrhea in swine. Archives of Virology. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospischil A., Hess R.G., Bachmann P.A. Light microscopy and ultrahistology of intestinal changes in pigs infected with epizootic diarrhoea virus (EDV): comparison with transmissible gastroenteritis (TGE) virus and porcine rotavirus infections. Journal of Veterinary Medicine B. 1981;28:564–577. doi: 10.1111/j.1439-0450.1981.tb01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirinarumitr T., Paul P.S., Kluge J.P., Halbur P.G. In-situ hybridization technique for the detection of swine enteric and respiratory coronaviruses, transmissible gastroenteritis virus (TGEV) and porcine respiratory coronavirus (PRCV), in formalin-fixed paraffin-embedded tissues. Journal of Virological Methods. 1996;56:149–160. doi: 10.1016/0166-0934(95)01901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan W., Cavanagh D., Horzinek M.C. Coronaviruses: structure and genome expression. Journal of General Virology. 1988;69:2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- Weingartl H.M., Derbyshire J.B. Binding of porcine transmissible gastroenteritis virus by enterocytes from newborn and weaned piglets. Veterinary Microbiology. 1993;35:23–32. doi: 10.1016/0378-1135(93)90113-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E.N. An apparently new syndrome of porcine epidemic diarrhoea. Veterinary Record. 1977;100:243–244. doi: 10.1136/vr.100.12.243. [DOI] [PubMed] [Google Scholar]


