Abstract
During 2013, a novel avian-origin H7N9 influenza A virus (IAV) emerged in China and subsequently caused large economic and public health burdens. We experimentally infected three common peridomestic wild mammals with H7N9 (A/Anhui/1/2013) IAV. Striped skunks exhibited the highest burden of disease followed by raccoons and cottontail rabbits. Striped skunks also produced the highest levels of viral shedding (up to 106.4 PFU/mL nasal flush) followed by cottontail rabbits (up to 105.8 PFU/mL nasal flush) and raccoons (up to 105.2 PFU/mL nasal flush). Thus, various mammalian species, especially those that are peridomestic, could play a role in the epidemiology of emergent H7N9 IAV. Mammals should be accounted for in biosecurity plans associated with H7N9 and their presence in wet markets, dependent on species, could lead to increased transmission among interspecific species aggregations and may also pose an elevated zoonotic disease risk to visitors and workers of such markets.
Keywords: Cottontail rabbit, Experimental infection, Influenza A virus, H7N9, Mammals, Mephitis, Pathology, Procyon, Raccoon, Shedding, Skunk, Sylvilagus
Highlights
-
•
Common peridomestic mammal species shed H7N9 influenza A virus (IAV).
-
•
Certain mammalian species shed H7N9 IAV for long periods.
-
•
Mammals should be taken into account in biosecurity plans for H7N9 IAV.
Introduction
Human infections with a novel avian-origin influenza A virus (IAV; H7N9) were first reported from Shanghai and Anhui Province, China during 2013 (Gao et al., 2013b). As of February 2015, over 575 cases of human infection with this virus have been reported in China (ProMED-mail, 2015c). Notably, several human exposures of H7N9 are thought to have been associated with visitations to live poultry markets, but not necessarily with direct contact with poultry (Liu et al., 2014a). For obvious reasons, this virus has caused economic losses and is a public health concern (Bi et al., 2015). For example, soon after the emergence of H7N9, economic losses to the poultry industry were estimated to be >1 billion U.S. dollars, while losses incurred from medical expenses and disability-adjusted life years were smaller but also quite significant (Liu et al., 2014b). Unfortunately, there appears to have been a surge of human cases associated with H7N9 IAV during the winter of 2014–2015 and imported cases have been recently observed in Hong Kong, British Columbia, Canada, and possibly in the Philippines (ProMED-mail, 2015a, ProMED-mail, 2015b, ProMED-mail, 2015c).
Although exposure to the H7N9 virus has resulted in infection of birds in experimental and live-animal market settings (Nicoll and Danielsson, 2013, Pantin-Jackwood et al., 2014), clinical signs of disease in birds are largely lacking, even though certain species are known to shed the virus (Pantin-Jackwood et al., 2014), which is the general pattern of a low pathogenic IAV in certain bird species. However, this virus has shown a much different pattern in humans, as high case fatality rates were noted in admitted patients during the first few months following the discovery of this virus (Gao et al., 2013a). Varying degrees of pathogenicity of H7N9 in other mammalian species have been noted. In one study of domestic ferrets, experimental infections with the Anhui strain of the virus by the nasal route caused respiratory symptoms and lethargy in some animals, but was not lethal during the experimental period (Belser et al., 2013). However, a second study indicated the virus can cause high morbidity and mortality rates in ferrets inoculated intratracheally, especially at high doses (Kreijtz et al., 2013). Guinea pigs were found to be productively infected with this virus and showed a very low minimum infectious dose (Gabbard et al., 2014). A recent study contrasted infections of ferrets, chickens, and pigeons with emergent H7N9 (A/Anhui/1/13) and a LP avian-origin H7N7 (A/turkey/Germany/AR534/2013) (Kalthoff et al., 2014). Results suggested that the mammals tested in this study typically shed ten-fold or greater quantities of the H7N9 virus than the tested birds and also often shed ten-fold or greater quantities when H7N9 was compared to H7N7 (Kalthoff et al., 2014).
Several North American mammalian species, such as striped skunks (Mephitis mephitis), raccoons (Procyon lotor), and cottontail rabbits (Sylvilagus sp.), have recently been shown to shed moderate to relatively high quantities of IAV by select routes (Hall et al., 2008, Root et al., 2014a, Root et al., 2014b, Root et al., 2014c). Due to the peridomestic tendencies of these animals, along with their shedding potentials, some of them have been proposed to be a potential threat to biosecurity at poultry operation facilities (Hall et al., 2008, Root et al., 2014b, Root et al., 2014c).
In preparation for potential introductions of foreign viruses, it is important to identify key common species that may facilitate trafficking to and among agricultural operations or pose a public health threat due to their peridomestic behavioral tendencies. For these reasons, the objective of this study was to assess the capacity of select North American mammals to shed a novel H7N9 virus (A/Anhui/1/2013) recently identified in China during 2013.
Results
Striped skunks
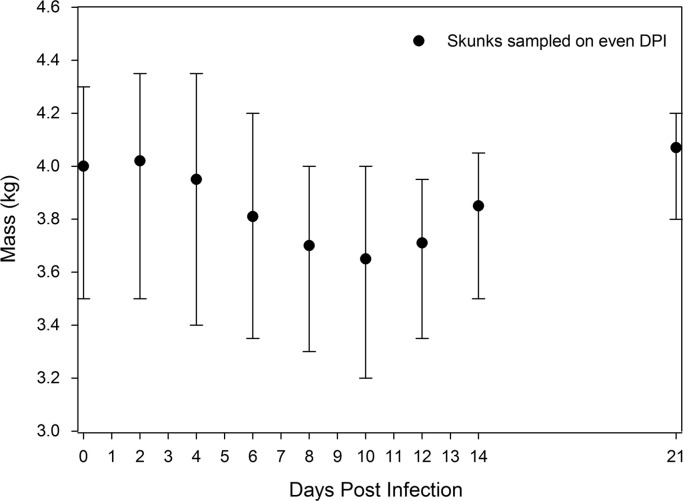
All skunks showed evidence of H7N9 IAV infection on multiple DPI of the experiment. The highest titer nasal flush was collected on 2 DPI yielding 106.4 PFU/mL, while the highest titer oral swab was collected on 1 DPI (104.2 PFU/mL; Table 1). All skunks displayed nasal shedding through 7–8 DPI, while one skunk continued to shed nasally through 12 DPI. Of interest, all tested skunks yielded at least one nasal flush of >105.0 PFU/mL. Observed clinical signs included nasal and ocular discharge, cough, sneezes, and loss of appetite in most animals. Moderate fevers were noted in these animals, as temperatures peaked at 6 DPI and began to drop thereafter ( Fig. 1). At 21 DPI, temperatures were lower than baseline conditions, likely the result of acclimatization. In addition, most animals underwent moderate weight loss through 10 DPI of the experimental infection ( Fig. 2), but those sampled on 21 DPI had largely regained their lost weight to pre-test conditions (average of 4.0 kg versus 4.06 kg for animals sampled at 0 and 21 DPI, respectively). None of the skunks sampled for the duration of the study lost more than 10% of their body mass. Serological assessments were conducted on the three animals that were maintained through 21 DPI. Two animals had titers of >1280 and 320, while the third did not yield a detectable HI antibody response. The reason for the lack of an antibody response in this animal is unclear, especially considering that it shed virus. Histopathology of three skunks euthanized on 7 DPI showed marked to severe inflammation and/or pneumocyte hypertrophy affecting greater than 50% of the lobe in at least one lung lobe for all three animals. Two of those same skunks had minimal to mild inflammatory changes in the tracheal epithelium. No lesions were detected in the nasal turbinates.
Table 1.
Nasal and oral shedding of striped skunks (Mephitis mephitis) experimentally infected with emergent H7N9 (A/Anhui/1/2013) influenza A virus. Values represent log10 PFU/mL.
| Skunk | Samplea |
Days Post Infection (DPI) |
Serology | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7b | 8 | 10 | 12 | 14 | HI titerc | |||
| 1 | Oral | 4.2 | 3.0 | 3.3 | 2.0 | N/A | ||||||||
| Nasal | 5.7 | 6.0 | 5.7 | 5.6 | ||||||||||
| 2 | Oral | 4.1 | 2.2 | 2.3 | <1 | N/A | ||||||||
| Nasal | 4.8 | 5.8 | 4.3 | 4.4 | ||||||||||
| 3 | Oral | 3.6 | 2.5 | 2.5 | <1 | N/A | ||||||||
| Nasal | 5.8 | 5.9 | 5.0 | 4.0 | ||||||||||
| 4 | Oral | 2.8 | 1.6 | 1.6 | <1 | <1 | <1 | <1 | Neg | |||||
| Nasal | 5.7 | 6.1 | 5.8 | 4.0 | <1.7 | <1.7 | <1.7 | |||||||
| 5 | Oral | 3.6 | 3.6 | 2.8 | <1 | <1 | <1 | <1 | >1280 | |||||
| Nasal | 6.4 | 5.5 | 5.7 | 3.6 | 2.3 | <1.7 | <1.7 | |||||||
| 6 | Oral | 3.4 | 2.1 | 2.0 | <1 | <1 | <1 | <1 | 320 | |||||
| Nasal | 6.2 | 6.0 | 5.7 | 3.8 | 4.9 | 2.9 | <1.7 | |||||||
Oral=oral swab; Nasal=nasal flush.
Striped skunks 1, 2, and 3 were euthanized on 7 DPI for pathological examination.
HI titers were assessed from sera collected at 21 DPI.
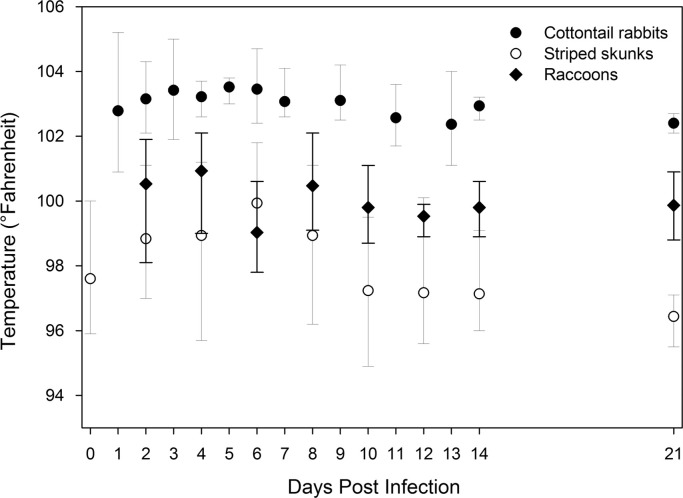
Fig. 1.
Average body temperatures of cottontail rabbits (Sylvilagus sp.), striped skunks (Mephitis mephitis), and raccoons (Procyon lotor) during select days postinfection (DPI) following experimental infection with emergent H7N9 (A/Anhui/1/2013) influenza A virus. Vertical bars represent the maximum and minimum temperatures of individual animals during a given DPI. All cottontail rabbits (n=6) are shown through 7 DPI and the remaining three are shown through subsequent DPI. Skunks (n=3) and raccoons (n=3) sampled through 21 DPI are presented.
Fig. 2.
Average striped skunk (Mephitis mephitis) mass sampled during even days (n=3) postinfection (DPI) following experimental infection with emergent H7N9 (A/Anhui/1/2013) influenza A virus. Vertical bars represent the maximum and minimum mass of individual skunks on a given DPI.
Raccoons
Infected raccoons displayed a delayed, less prominent, and shorter-duration virus shedding pattern as compared to striped skunks ( Table 1, Table 2). For example, only one of three individuals sampled yielded evidence of shedding on 1 DPI and three animals did not begin shedding until 3, 4, or 5 DPI (Table 2). The highest titers from nasal flush samples were collected on 2 and 6 DPI, both yielding 105.2 PFU/mL. The highest titer from oral swab samples occurred on 2 DPI yielding 103.7 PFU/mL. Of interest and in contrast to other species tested, oral shedding was observed in two of three animals that were shedding on 1 or 2 DPI in the absence of detectable nasal shedding. Clinical signs of disease in raccoons were more moderate as compared to striped skunks. These signs commonly included various degrees of nasal discharge (i.e., moist noses, moderate nasal discharge, or more prominent nasal discharge) and loss of appetite. No clinical signs of disease were noted until 3 DPI and were largely absent in most animals by 7–8 DPI, although one animal showed nasal discharge through 14 DPI. Body temperatures were quite variable among raccoons over time and a discernable pattern could not be readily obtained from these data; however, a mild fever (Fig. 1) and moderate mean weight loss (average of 8 kg on 2 DPI versus 7.86 kg on 4 DPI) were noted on 4 DPI. These animals regained their lost weight at 6 DPI (average of 8.1 kg). The three raccoons maintained until 21 DPI all developed HI titers of 1:80 (Table 2). Histopathologic evaluation of the three raccoons euthanized on 7 DPI showed moderate to marked inflammation and pneumocyte hypertrophy in at least one lung lobe for two of the raccoons.
Table 2.
Nasal and oral shedding of raccoons (Procyon lotor) experimentally infected with emergent H7N9 (A/Anhui/1/2013) influenza A virus. Values represent log10 PFU/mL.
| Raccoon | Samplea |
Days Post Infection (DPI) |
Serology | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8b | 10 | 12 | 14 | HI titerc | ||
| 1 | Oral | 1.4 | <1 | 1.7 | <1 | N/A | |||||||
| Nasal | <1 | 3.7 | 4.8 | <1 | |||||||||
| 2 | Oral | <1 | <1 | <1 | <1 | N/A | |||||||
| Nasal | <1 | <1 | 2.1 | <1 | |||||||||
| 3 | Oral | <1 | <1 | <1 | 3.2 | N/A | |||||||
| Nasal | <1 | 3.6 | 4.3 | 1 | |||||||||
| 4 | Oral | 3.7 | 2.7 | 1.9 | <1 | <1 | <1 | <1 | 80 | ||||
| Nasal | 5.2 | 4.9 | 5.2 | 1.7 | <1 | <1 | <1 | ||||||
| 5 | Oral | 1.2 | <1 | <1 | <1 | <1 | <1 | <1 | 80 | ||||
| Nasal | <1 | <1 | 2.0 | <1 | <1 | <1 | <1 | ||||||
| 6 | Oral | <1 | 1.6 | 2.1 | <1 | <1 | <1 | <1 | 80 | ||||
| Nasal | <1 | 3.6 | 5.0 | 4.8 | <1 | <1 | <1 | ||||||
Oral=oral swab; Nasal=nasal flush.
Raccoons 1, 2, and 3 were euthanized on 8 DPI for pathological examination.
HI titers were assessed from sera collected at 21 DPI.
Cottontail rabbits
All cottontail rabbits showed evidence of viral shedding by at least one route at 1 DPI. The highest titer from nasal flush samples was 105.8 PFU/mL and the highest titer from oral swab samples was 102.5 PFU/mL ( Table 3). Each of the cottontail rabbits produced nasal shedding of ≥104.0 PFU/mL on 1 DPI. Only mild clinical signs of disease were noted for cottontails, which included mild nasal discharge, loss of appetite, and apparent lethargy in some individuals. These symptoms were not observed after 6 DPI. The weight of cottontail rabbits was relatively stable during the experimental period. For example, although weights varied by day, the average mass of cottontail rabbits at 3 DPI (0.936 kg during the infection period) and 7 DPI (0.935 kg when the infection had largely cleared) were quite similar. However, daily temperatures suggested these animals have had mild to moderate fevers during the infection period, peaking at 5–6 DPI and decreasing during most DPI thereafter (Fig. 1). The three cottontails that were maintained until 21 DPI developed detectable antibody responses at the end of the experiment with titers of 1:80, 1:80, and 1:160 (Table 3). Histopathology revealed marked inflammation in one lung lobe of one rabbit, but no other lesions were observed in that rabbit or the other two animals.
Table 3.
Nasal and oral shedding of cottontail rabbits (Sylvilagus sp.) experimentally infected with emergent H7N9 (A/Anhui/1/2013) influenza A virus. Values represent log10 PFU/mL.
| Rabbit | Samplea |
Days Post Infection (DPI) |
Serology | |||
|---|---|---|---|---|---|---|
| 1 DPI | 3 DPI | 5 DPI | 7 DPIb | HI titerc | ||
| 1 | Oral | <1 | <1 | <1 | <1 | N/A |
| Nasal | 5.2 | 4.5 | 3.0 | <1.7 | ||
| 2 | Oral | <1 | <1 | 1.0 | <1 | N/A |
| Nasal | 4.0 | 1.7 | 4.0 | <1.7 | ||
| 3 | Oral | 2.0 | <1 | 1.3 | <1 | 80 |
| Nasal | 5.3 | 3.7 | 5.1 | <1.7 | ||
| 4 | Oral | 2.5 | <1 | <1 | <1 | 160 |
| Nasal | 5.8 | 3.6 | 2.6 | 2.3 | ||
| 5 | Oral | <1 | 1 | <1 | <1 | N/A |
| Nasal | 4.1 | 3.9 | <1.7 | <1.7 | ||
| 6 | Oral | <1 | 1.0 | <1 | <1 | 80 |
| Nasal | 4.2 | 2.7 | 2.8 | <1.7 | ||
Oral=oral swab; Nasal=nasal flush.
Cottontail rabbits 1, 2, and 5 were euthanized on 7 DPI for pathological examination.
HI titers were assessed from sera collected at 21 DPI.
Discussion
Striped skunks were clearly the species most affected clinically by virus infection, as they showed several signs of illness, including sneezing. This feature of their infection is of obvious epidemiological importance. For example, if an infected peridomestic skunk were to feed upon unprotected poultry feed or use unprotected watering devices, sneezes or oral secretions could be sufficient to contaminate poultry feed or water with virus-laden secretions. This scenario may be enhanced by the nasal shedding profiles of these animals. Every skunk tested shed a minimum of 105.8 PFU/mL on at least one DPI (Table 1). Furthermore, one of the animals tested was still shedding IAV at 12 DPI, thereby suggesting these animals could pose a transmission threat long after their initial infection. This is consistent with a previous report that suggested that striped skunks can shed IAV for extended time periods (Root et al., 2014b).
Raccoons did not have the same viral shedding potential as striped skunks, as shedding in this species was largely delayed and short-lived. For example, one animal ceased shedding as early as 5–7 DPI and no animals yielded evidence of shedding after 8 DPI (Table 2). Overall, viral shedding in raccoons was reduced when compared to striped skunks. However, there was individual heterogeneity in shedding profiles of the raccoons, with some individuals clearly shedding at relatively high rates during few DPI. In general, the shedding of A/Anhui/1/2013 in raccoons was greater than what has been previously reported for other IAVs tested in this species (Hall et al., 2008, Root et al., 2014a). Although raccoons typically do not shed the viral loads seen in certain other mammals such as striped skunks and cottontail rabbits (Root et al., 2014b, Root et al., 2014c), they have been shown to be naturally exposed to IAV in North America and in introduced populations elsewhere (Hall et al., 2008, Horimoto et al., 2011, Yamaguchi et al., 2014), suggesting that the behavioral tendencies of this species facilitates events where transmission can occur.
Consistent with a previous report, infection in cottontail rabbits peaked quickly and was largely cleared within one week (Root et al., 2014c). The highest titers from nasal flush samples were generally observed at 1 DPI and typically decreased on subsequent DPI. Although very mild clinical signs were noted during early DPI, these were not observed after 6 DPI. Various IAVs have been reported in naturally infected lagomorphs. For example, antibodies reactive with an H9N2 subtype as well as an H5N1 subtype have been documented in plateau pika (Ochotona curzoniae) sampled near Qinghai Lake, China (Yu et al., 2014, Zhou et al., 2009). It was suggested that infections in pika were possibly caused by environmental contamination of common foraging sites with wild birds (Zhou et al., 2009). This indicates that North American lagomorphs may also be susceptible to natural infections with IAVs when found in environments conducive to resources shared with birds, such as similar environments and species compositions as found at Qinghai Lake.
The body temperature data collected during this study suggest that some wildlife species develop fever following infection with H7N9 (A/Anhui/1/2013) IAV. In the current study, this condition appeared to be most prominent in striped skunks and possibly in cottontail rabbits (Fig. 1). As with other non-data-logging devices, these data were likely influenced by anesthesia and/or handling stress. As such, these temperature readings, even during pre-experiment or early or late DPI, should not be assumed to represent baseline temperatures of these animals. However, the temperature trend of apparent fevers was clear from these data.
Results from gross and histopathological examinations indicated that striped skunks and raccoons developed lesions consistent with bronchopneumonia. Histopathological examination indicated the presence of lesions associated with the bronchioles and alveoli of the lungs ( Fig. 3) and occasionally mild changes in the trachea.
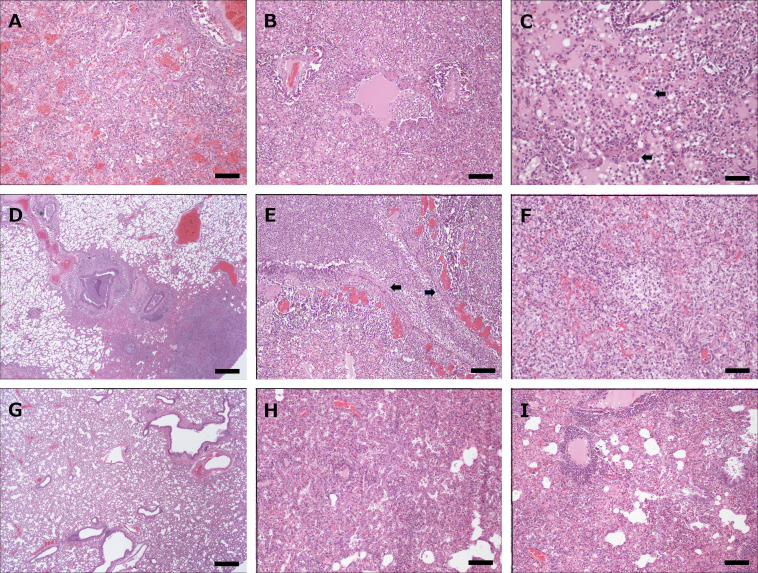
Fig. 3.
Variable severity of bronchopneumonia as a result of experimental infection with H7N9 in raccoons (A,B,C), skunks (D,E,F) and cottontail rabbits (G,H,I). (A) Severe fibrinous and necrotizing pneumonia with alveolar edema and hemorrhage in a raccoon. (B) Epithelial loss and attenuation in terminal bronchioles and severe alveolar edema and infiltration of leukocytes, mainly neutrophils and monocyte/macrophages in alveolar septae and alveoli in lung of a raccoon. (C) Higher magnification of lung of an infected raccoon showing severe alveolar neutrophilic and monocyte/macrophage infiltration and edema. Scattered type 2 pneumocytes (arrows) are hypertrophic. (D) Focally very severe bronchopneumonia in an infected skunk with marked intraluminal accumulation of cellular debris in bronchioles and severe parenchyma consolidation due to inflammatory cell infiltration and alveolar edema and fibrin deposition. (E) Severe necrotizing bronchopneumonia in a skunk with destruction of bronchiolar epithelium (arrows) and luminal plugs composed of cellular debris. The adjacent alveolar tissue is severely infiltrated with neutrophils and monocytes/macrophages. (F) Complete destruction of a terminal bronchiole and necrosis of associated alveoli in the lung of an infected skunk accompanied by severe inflammatory cell infiltration. (G) Lung lobe from an H7N9-infected cottontail rabbit showing complete absence of lesions. (H) Focally restricted pneumonia with marked type 2 pneumocyte hypertrophy and proliferation in a lung lobe of a virus-challenged cottontail rabbit. The pneumocyte reaction reflects regeneration and recovery from mild pneumonia. (I) Focally restricted pneumonia with marked perivascular lymphocyte cuffing and type 2 pneumocyte proliferation in the lung of a cottontail rabbit. Both features reflect recovery and regeneration of the parenchyma. Scale bars indicate 1500 um (D, G), 200 um (A, B, E, H, and I) and 150 um (C, F).
These studies have clearly shown that three common peridomestic mammals can replicate H7N9 IAV and some species can shed high viral loads through the nasal route. Because Asian H7N9 is not naturally present in North America, the potential role of these species in the epidemiology of this virus is unknown at this time. However, the three species tested developed productive infections and all have peridomestic tendencies, which are behavioral attributes that could put them into close contact with humans or farms. An additional risk factor includes the existence of wet markets that contain both mammals and birds. For example, stacked pens of rabbits, pigeons, chickens, and ducks have been described as a representative wet market in China (Guan et al., 2013) and rabbits can be present in live bird markets in the U.S. (Cardona et al., 2009). Although less common, some small carnivores, such as Chinese ferret-badgers (Melogale moschata), hog badgers (Arctonyx collaris), Himalayan palm civets (Paguna larvata), and raccoon dogs (Nyctereutes procyonoides), have also been sold in some wet markets in Asia (Guan et al., 2003). Two of these species, Himalayan palm civets and raccoon dogs, showed evidence of infection with a SARS-like coronavirus (Guan et al., 2003). Following the identification of this virus in palm civets, this species was barred from Chinese wet markets for a time period but were again made available soon thereafter (Webster, 2004). Notably, some close relatives to some of the species mentioned above, such as Owston’s civet (Chrotogale owstoni) and stone marten (Martes foina), have been shown to be susceptible to IAV infection (Klopfleisch et al., 2007, Roberton et al., 2006). Furthermore, raccoon dogs, which have been sold in wet markets (Guan et al., 2003), are known to be susceptible to infection with highly pathogenic H5N1 IAV (Qi et al., 2009). Thus, considering the virus shedding demonstrated in mesocarnivores (i.e., skunk and raccoon) of the current study, it is reasonable to assume that some of the ecologically similar species from Asian wet markets mentioned above may pose a risk for transmission of IAV in these types of artificial high density interspecific aggregations. These findings suggest that certain mammal species should be considered in biosecurity plans for live animal markets and poultry production facilities located in the endemic region of H7N9 IAV.
Materials and methods
Study animals
Six raccoons, six striped skunks, and six cottontail rabbits were tested in three independent experimental infection studies. Raccoons and cottontails were wild-caught near the front range of northern Colorado, USA, while striped skunks were purchased from a commercial vendor (Ruby Fur Farm, New Sharon, IA, USA). Following brief quarantine periods, animals were transferred to an animal biosafety level-three (ABSL-3) facility on the Foothills Research Campus of Colorado State University. For experimental infection purposes, raccoons and striped skunks were housed in 1.08l×0.76w×0.692h m3 metal dog crates equipped with rubber livestock mat floors. These animals were maintained with omnivore diet (omnivore diet; Mazuri, Purina Mills, LLC, St. Louis, MO) and supplemented with hardboiled eggs. Both food and water were replenished each day. Cottontail rabbits were housed in standard rabbit racks that included a PVC nest box and floor grates lined with cardboard. Food (MannaPro®, Pro Formula, St. Louis, MO, and alfalfa supplemented with fruits and vegetables) and water were replenished daily. All animals were bled and assessed to be antibody negative by hemagglutination-inhibition (HI) assay with homologous virus and chicken erythrocytes (cottontail rabbits) or ELISA (striped skunks and raccoons; FlockCheck® Avian Influenza MultiS-Screen Antibody Test Kit, IDEXX Laboratories, Inc, Westbrook, ME) prior to the initiation of the study. This ELISA test has been used successfully in raccoon and striped skunks in earlier studies (Root et al., 2014a, Root et al., 2014b). In addition, each animal was outfitted with a Bio Medic Data Systems (BMDS) subcutaneous transponder (IPPT-300 transponders) that transmitted an individual identification number for each animal as well as individual temperature readings. Scans were conducted using a BMDS IPPT 7009 reader (Bio Medic Data Systems, Seaford, Delaware, USA). Animal care and use protocols were approved by the National Wildlife Research Center (protocol number 2209) and Colorado State University (protocol number 13-4666A).
Experimental infections
The skunks and raccoons were anesthetized (intramuscular injection of a 5:1 ratio of ketamine/xylazine at approximately 100 mg/kg and 20 mg/kg, respectively), and nasally inoculated with approximately 104.5 PFU of H7N9 (A/Anhui/1/2013) delivered in 1 mL of BA-1 viral transport media. Each nostril received approximately 0.5 mL. Cottontails were anesthetized using the methods listed above and were inoculated with approximately 104.5 PFU of the virus delivered in a 100 µl vehicle of BA-1; each nostril received approximately 50 µl.
Following inoculation, three of six striped skunks were sampled on even days while the other three were sampled on odd days. One-half of this cohort was euthanized on 7 days post infection (DPI) to assess gross and histopathological lesions. The remaining animals were sampled every other day through 14 DPI and on 21 DPI when they were bled and euthanized. Daily sampling consisted of an oral swab, a nasal flush, a temperature reading and general health observations while animals were under anesthesia. Nasal flushes were conducted by dripping BA-1 medium into the nares and collecting material that was expelled by sneezing. This procedure was used for the three species tested. Raccoon sampling mirrored that of striped skunks with one exception: the three animals sacrificed early in the infection were euthanized on 8 DPI. The sampling regime was modified for cottontail rabbits, as this species has been previously shown to have peak shedding of IAV and clear their infections more quickly than the other species tested in this study (Root et al., 2014c). As such, all of the rabbits were sampled on odd days from 1–7 DPI. Three animals were euthanized on 7 DPI and the remaining animals were euthanized on 21 DPI at which time a blood sample was collected for serologic analyses.
Laboratory assays
Nasal flush fluids and oral swab samples were tested by plaque assay as described previously (Achenbach and Bowen, 2011). The limit of detection for oral swabs was 101.0 PFU/mL while the limit of detection for nasal flushes was 101.7 PFU/mL. Post-experiment serology was conducted with standard HI assays using the inoculation virus on sera collected at 21 DPI. Serology was not attempted on animals euthanized on 7 or 8 DPI.
Necropsy
Following euthanasia on 7 or 8 DPI, one-half of each cohort of each species was necropsied to observe gross lesions and to collect tissues for subsequent histopathological analyses. The following tissues were routinely collected: lung, trachea, nasal turbinates, and liver. Tissues were preserved in 10% neutral-buffered formalin, embedded in paraffin wax and 5 µm sections were cut on a microtome and stained with hemotoxylin and eosin using standard methods.
Acknowledgments
We thank the National Wildlife Research Center (NWRC) animal care staff for excellent animal care, NWRC rabies project personnel for assistance with obtaining and processing skunks, and NWRC staff for assistance with animal processing and with ELISAs. The opinions and conclusions of this article are those of the authors and do necessarily represent those of the U.S. Department of Agriculture (USDA) or Colorado State University. The mention of commercial products herein is for identification purposes only and does not constitute endorsement or censure. Funding was provided by the USDA.
References
- Achenbach J.E., Bowen R.A. Transmission of avian influenza A viruses among species in an artificial barnyard. PLoS One. 2011;6:e17643. doi: 10.1371/journal.pone.0017643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser J.A., Gustin K.M., Pearce M.B., Maines T.R., Zeng H., Pappas C., Sun X., Carney P.J., Villanueva J.M., Stevens J., Katz J.M., Tumpey T.M. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature. 2013;501:556–559. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y., Xie Q., Zhang S., Li Y., Xiao H., Jin T., Zheng W., Li J., Jia X., Sun L., Liu J., Qin C., Gao G.F., Liu W. Assessment of the internal genes of influenza A (H7N9) virus contributing to high pathogenicity in mice. J. Virol. 2015;89:2–13. doi: 10.1128/JVI.02390-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona C., Yee K., Carpenter T. Are live bird markets reservoirs of avian influenza? Poult. Sci. 2009;88:856–859. doi: 10.3382/ps.2008-00338. [DOI] [PubMed] [Google Scholar]
- Gabbard J.D., Dlugolenski D., Van Riel D., Marshall N., Galloway S.E., Howerth E.W., Campbell P.J., Jones C., Johnson S., Byrd-Leotis L., Steinhauer D.A., Kuiken T., Tompkins S.M., Tripp R., Lowen A.C., Steel J. Novel H7N9 influenza virus shows low infectious dose, high growth rate, and efficient contact transmission in the Guinea pig model. J. Virol. 2014;88:1502–1512. doi: 10.1128/JVI.02959-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H.N., Lu H.Z., Cao B., Du B., Shang H., Gan J.H., Lu S.H., Yang Y.D., Fang Q., Shen Y.Z., Xi X.M., Gu Q., Zhou X.M., Qu H.P., Yan Z., Li F.M., Zhao W., Gao Z.C., Wang G.F., Ruan L.X., Wang W.H., Ye J., Cao H.F., Li X.W., Zhang W.H., Fang X.C., He J., Liang W.F., Xie J., Zeng M., Wu X.Z., Li J., Xia Q., Jin Z.C., Chen Q., Tang C., Zhang Z.Y., Hou B.M., Feng Z.X., Sheng J.F., Zhong N.S., Li L.J. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N. Engl. J. Med. 2013;368:2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- Gao R., Cao B., Hu Y., Feng Z., Wang D., Hu W., Chen J., Jie Z., Qiu H., Xu K., Xu X., Lu H., Zhu W., Gao Z., Xiang N., Shen Y., He Z., Gu Y., Zhang Z., Yang Y., Zhao X., Zhou L., Li X., Zou S., Zhang Y., Li X., Yang L., Guo J., Dong J., Li Q., Dong L., Zhu Y., Bai T., Wang S., Hao P., Yang W., Zhang Y., Han J., Yu H., Li D., Gao G.F., Wu G., Wang Y., Yuan Z., Shu Y. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- Guan Y., Farooqui A., Zhu H., Dong W., Wang J., Kelvin D.J. H7N9 incident, immune status, the elderly and a warning of an influenza pandemic. J. Infect. Dev. Ctries. 2013;7:302–307. doi: 10.3855/jidc.3675. [DOI] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J., Butt K.M., Wong K.L., Chan K.W., Lim W., Shortridge K.F., Yuen K.Y., Peiris J.S.M., Poon L.L.M. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Hall J.S., Bentler K.T., Landolt G., Elmore S.A., Minnis R.B., Campbell T.A., Barras S.C., Root J.J., Pilon J., Pabilonia K., Driscoll C., Slate D., Sullivan H., McLean R.G. Influenza infection in wild raccoons. Emerg. Infect. Dis. 2008;14:1842–1848. doi: 10.3201/eid1412.071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horimoto T., Maeda K., Murakami S., Kiso M., Iwatsuki-Horimoto K., Sashika M., Ito T., Suzuki K., Yokoyama M., Kawaoka Y. Highly pathogenic avian influenza virus infection in feral raccoons, Japan. Emerg. Infect. Dis. 2011;17:714–717. doi: 10.3201/eid1704.101604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalthoff D., Bogs J., Grund C., Tauscher K., Teifke J.P., Starick E., Harder T., Beer M. Avian influenza H7N9/13 and H7N7/13: a comparative virulence study in chickens, pigeons, and ferrets. J. Virol. 2014;88:9153–9165. doi: 10.1128/JVI.01241-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfleisch R., Wolf P.U., Wolf C., Harder T., Starick E., Niebuhr M., Mettenleiter T.C., Teifke J.P. Encephalitis in a stone marten (Martes foina) after natural infection with highly pathogenic avian influenza virus subtype H5N1. J. Comp. Pathol. 2007;137:155–159. doi: 10.1016/j.jcpa.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Kreijtz J.H.C.M., Kroeze E.J.B.V., Stittelaar K.J., de Waal L., van Amerongen G., van Trierum S., van Run P., Bestebroer T., Kuiken T., Fouchier R.A.M., Rimmelzwaan G.F., Osterhaus A.D.M.E. Low pathogenic avian influenza A (H7N9) virus causes high mortality in ferrets upon intratracheal challenge: a model to study intervention strategies. Vaccine. 2013;31:4995–4999. doi: 10.1016/j.vaccine.2013.06.071. [DOI] [PubMed] [Google Scholar]
- Liu B., Havers F., Chen E., Yuan Z., Yuan H., Ou J., Shang M., Kang K., Liao K., Liu F., Li D., Ding H., Zhou L., Zhu W., Ding F., Zhang P., Wang X., Yao J., Xiang N., Zhou S., Liu X., Song Y., Su H., Wang R., Cai J., Cao Y., Wang X., Bai T., Wang J., Feng Z., Zhang Y., Widdowson M.A., Li Q. Risk factors for influenza A (H7N9) disease – China, 2013. Clin. Infect. Dis. 2014;59:787–794. doi: 10.1093/cid/ciu423. [DOI] [PubMed] [Google Scholar]
- Liu J., Xiao H., Wu Y., Liu D., Qi X., Shi Y., Gao G.F. H7N9: a low pathogenic avian influenza A virus infecting humans. Curr. Opin. Virol. 2014;5:91–97. doi: 10.1016/j.coviro.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll A., Danielsson N. A novel reassortant avian influenza A (H7N9) virus in China – what are the implications for Europe. Eurosurveillance. 2013;18:20452. [PubMed] [Google Scholar]
- Pantin-Jackwood M.J., Miller P.J., Spackman E., Swayne D.E., Susta L., Costa-Hurtado M., Suarez D.L. Role of poultry in the spread of novel H7N9 influenza virus in China. J. Virol. 2014;88:5381–5390. doi: 10.1128/JVI.03689-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ProMED-mail, 2015a. Avian Influenza, Human (15): WHO Update, China (Guandong), H7N9. 20150119.3104096.
- ProMED-mail, 2015b. Avian Influenza, Human (24): Update Week 4, China (Guandong), Case Update H7N9 20150127.3123681.
- ProMED-mail, 2015c. Avian Influenza, Human (54): China (Guangdong), H7N9, Philippines ex China. 20150224.3189776.
- Qi X., Li X., Rider P., Fan W., Gu H., Xu L., Yang Y., Lu S., Wang H., Liu F. Molecular characterization of highly pathogenic H5N1 avian influenza A viruses isolated from raccoon dogs in China. PLoS One. 2009;4:e4682. doi: 10.1371/journal.pone.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton S.I., Bell D.J., Smith G.J.D., Nicholls J.M., Chan K.H., Nguyen D.T., Tran P.Q., Streicher U., Poon L.L.M., Chen H., Horby P., Guardo M., Guan Y., Peiris J.S.M. Avian influenza H5N1 in viverrids: Implications for wildlife health and conservation. Proc. R. Soc. B: Biol. Sci. 2006;273:1729–1732. doi: 10.1098/rspb.2006.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root J.J., Bentler K.T., Shriner S.A., Mooers N.L., VanDalen K.K., Sullivan H.J., Franklin A.B. Ecological routes of avian influenza virus transmission to a common mesopredator: an experimental evaluation of alternatives. PLoS One. 2014;9:e102964. doi: 10.1371/journal.pone.0102964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root J.J., Shriner S.A., Bentler K.T., Gidlewski T., Mooers N.L., Ellis J.W., Spraker T.R., VanDalen K.K., Sullivan H.J., Franklin A.B. Extended viral shedding of a low pathogenic avian influenza virus by striped skunks (Mephitis mephitis) PLoS One. 2014;9:e70639. doi: 10.1371/journal.pone.0070639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root J.J., Shriner S.A., Bentler K.T., Gidlewski T., Mooers N.L., Spraker T.R., VanDalen K.K., Sullivan H.J., Franklin A.B. Shedding of a low pathogenic avian influenza virus in a common synanthropic mammal-the cottontail rabbit. PLoS One. 2014;9:e103513. doi: 10.1371/journal.pone.0102513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R.G. Wet markets – a continuing source of severe acute respiratory syndrome and influenza? Lancet. 2004;363:234–236. doi: 10.1016/S0140-6736(03)15329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi E., Sashika M., Fujii K., Kobayashi K., Bui V.N., Ogawa H., Imai K. Prevalence of multiple subtypes of influenza A virus in Japanese wild raccoons. Virus Res. 2014;189:8–13. doi: 10.1016/j.virusres.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Yu Z., Cheng K., Sun W., Xin Y., Cai J., Ma R., Zhao Q., Li L., Huang J., Sang X., Li X., Zhang K., Wang T., Qin C., Qian J., Gao Y., Xia X. Lowly pathogenic avian influenza (H9N2) infection in Plateau pika (Ochotona curzoniae), Qinghai Lake, China. Vet. Microbiol. 2014;173:132–135. doi: 10.1016/j.vetmic.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Zhou J., Sun W., Wang J., Guo J., Yin W., Wu N., Li L., Yan Y., Liao M., Huang Y., Luo K., Jiang X., Chen H. Characterization of the H5N1 highly pathogenic avian influenza virus derived from wild pikas in China. J. Virol. 2009;83:8957–8964. doi: 10.1128/JVI.00793-09. [DOI] [PMC free article] [PubMed] [Google Scholar]