Highlights
-
•
Emergence of 2019 novel coronavirus (2019-nCoV) in China has caused a large global outbreak and major public health issue.
-
•
At 9 February 2020, data from the WHO has shown >37 000 confirmed cases in 28 countries (>99% of cases detected in China).
-
•
2019-nCoV is spread by human-to-human transmission via droplets or direct contact.
-
•
Infection estimated to have an incubation period of 2–14 days and a basic reproduction number of 2.24–3.58.
-
•
Controlling infection to prevent spread of the 2019-nCoV is the primary intervention being used.
Keywords: 2019-nCoV, SARS-CoV-2, COVID-19, China, Epidemic, Remdesivir
Abstract
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; previously provisionally named 2019 novel coronavirus or 2019-nCoV) disease (COVID-19) in China at the end of 2019 has caused a large global outbreak and is a major public health issue. As of 11 February 2020, data from the World Health Organization (WHO) have shown that more than 43 000 confirmed cases have been identified in 28 countries/regions, with >99% of cases being detected in China. On 30 January 2020, the WHO declared COVID-19 as the sixth public health emergency of international concern. SARS-CoV-2 is closely related to two bat-derived severe acute respiratory syndrome-like coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZXC21. It is spread by human-to-human transmission via droplets or direct contact, and infection has been estimated to have mean incubation period of 6.4 days and a basic reproduction number of 2.24–3.58. Among patients with pneumonia caused by SARS-CoV-2 (novel coronavirus pneumonia or Wuhan pneumonia), fever was the most common symptom, followed by cough. Bilateral lung involvement with ground-glass opacity was the most common finding from computed tomography images of the chest. The one case of SARS-CoV-2 pneumonia in the USA is responding well to remdesivir, which is now undergoing a clinical trial in China. Currently, controlling infection to prevent the spread of SARS-CoV-2 is the primary intervention being used. However, public health authorities should keep monitoring the situation closely, as the more we can learn about this novel virus and its associated outbreak, the better we can respond.
1. Introduction
Since the emergence of the 2019 novel coronavirus (2019-nCoV) infection in Wuhan, China, in December 2019 [1], it has rapidly spread across China and many other countries [2], [3], [4], [5], [6], [7], [8]. So far, 2019-nCoV has affected more than 43 000 patients in 28 countries/regions and has became a major global health concern (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=6f80d1b9_4). On 11 February 2020, the World Health Organization (WHO) announced a new name for the epidemic disease caused by 2019-nCoV: coronavirus disease (COVID-19). Regarding the virus itself, the International Committee on Taxonomy of Viruses has renamed the previously provisionally named 2019-nCoV as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [3].
Although early studies reported a link between a single local fish and wild animal market and most cases of infection, indicating possible animal-to-human transmission, studies have increasingly demonstrated human-to-human transmission of SARS-CoV-2 through droplets or direct contact [2,[8], [9], [10]. Moreover, according to one study, presumed hospital-related transmission of SARS-CoV-2 was suspected in 41% of patients [8]. Based on the evidence of a rapidly increasing incidence of infections [11] and the possibility of transmission by asymptomatic carriers [12], SARS-CoV-2 can be transmitted effectively among humans and exhibits high potential for a pandemic [5,10,13]. In addition to the high transmission efficiency of SARS-CoV-2, the advancement and convenience of global travel could further enhance its worldwide spread [12]. On 30 January 2020, the WHO declared the COVID-19 outbreak as the sixth public health emergency of international concern, following H1N1 (2009), polio (2014), Ebola in West Africa (2014), Zika (2016) and Ebola in the Democratic Republic of Congo (2019). Therefore, health workers, governments and the public need to co-operate globally to prevent its spread [14].
2. SARS-CoV-2 and COVID-19
In addition to seasonal influenza, reported pathogens of pneumonia include adenovirus, coronavirus 229E/NL63/OC43, human bocavirus, human metapneumovirus, parainfluenza virus 1/2/3, rhinovirus and respiratory syncytial virus A/B [15], [16], [17], [18]. Moreover, these viruses can cause co-infection in the setting of community-acquired bacterial pneumonia [16], [17], [18]. Using molecular methods, knowledge about the role of these viruses in the setting of pneumonia has achieved significant advancements [19], [20], [21]. SARS-CoV-2 was found to be a positive-sense, single-stranded RNA virus belonging to the genus Betacoronavirus [22], [23], [24]. Phylogenetic analysis revealed that SARS-CoV-2 is closely related (88–89% similarity) to two bat-derived SARS-like coronaviruses, namely bat-SL-CoVZC45 (GenBank accession no. MG772933.1) and bat-SL-CoVZXC21 (GenBank accession no. MG772934.1), but it is more distant from SARS-CoV (~79% similarity) and Middle East respiratory syndrome coronavirus (MERS-CoV) (~50% similarity) [23,25,26]. Chen et al. applied an RNA-based metagenomic next-generation sequencing approach to identify a human coronavirus from two pneumonia cases during the Wuhan outbreak in 2019 [27]. Its entire genome was 29 881 bp in length [27]. Phylogenetic analysis indicates that SARS-CoV-2 is similar to the coronavirus circulating in Rhinolophus (horseshoe bats), with 98.7% nucleotide similarity to the partial RNA-dependent RNA polymerase (RdRp) gene of the bat coronavirus strain BtCoV/4991 (GenBank KP876546, 370 bp sequence of RdRp) and 87.9% nucleotide similarity to bat coronavirus strain bat-SL-CoVZC45 and bat-SL-CoVZXC21. Evolutionary analysis based on ORF1a/1b, S and N genes suggests that SARS-CoV-2 is more likely a novel coronavirus that was independently introduced from animals to humans [27]. Based on the findings of genomic investigations and the presence of some bats and live animals in the seafood market in Wuhan, SARS-CoV-2 may have originated from bats or bat droppings associated with contaminated materials in the market or surrounding region [25,28].
3. Epidemiology
Based on observations of data from the early outbreak in mainland China from 10–24 January 2020, the trend of an increasing incidence largely follows exponential growth, and the mean basic reproduction number (R 0) was estimated to range from 2.24 [95% confidence interval (CI) 1.96–2.55] to 3.58 (95% CI 2.89–4.39), associated with two- to eight-fold increases in the reporting rate [11]. Another estimation based on data from 31 December 2019 to 28 January 2020 suggested similar findings, with the R 0 for COVID-19 being 2.68 [95% credible interval (CrI) 2.47–2.86] and the epidemic doubling time being 6.4 days (95% CrI 5.8–7.1 days) [29]. The current estimate of the mean incubation period for COVID-19 is 6.4 days, ranging from 2.1 days to 11.1 days (2.5th to 97.5th percentile) [30], with potential asymptomatic transmission. Although the situation is evolving and further updated data are required to confirm these estimations, there is great potential for a large outbreak of COVID-19 soon.
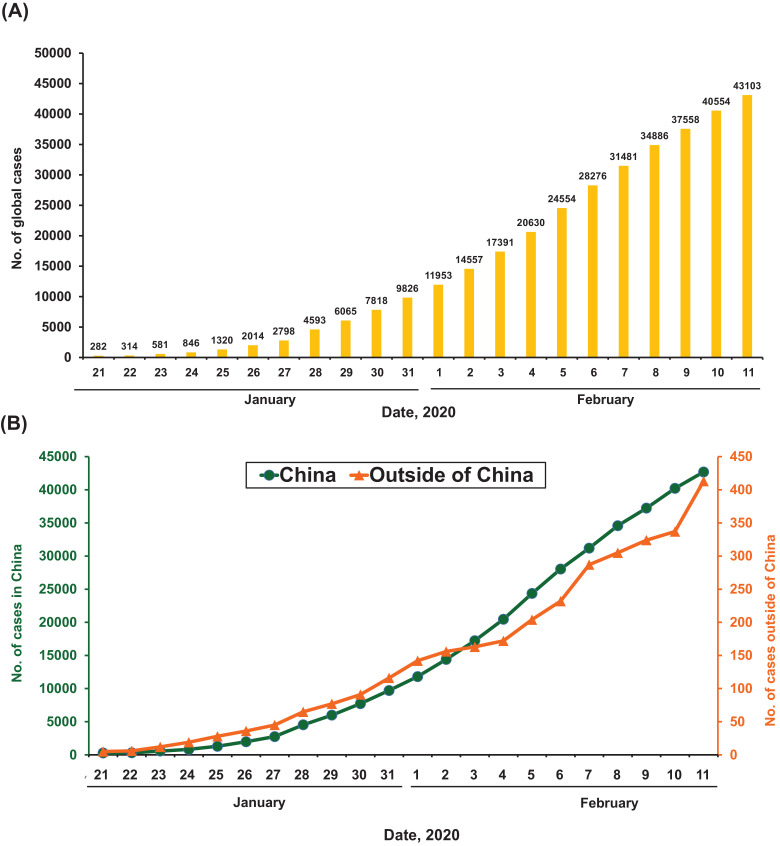
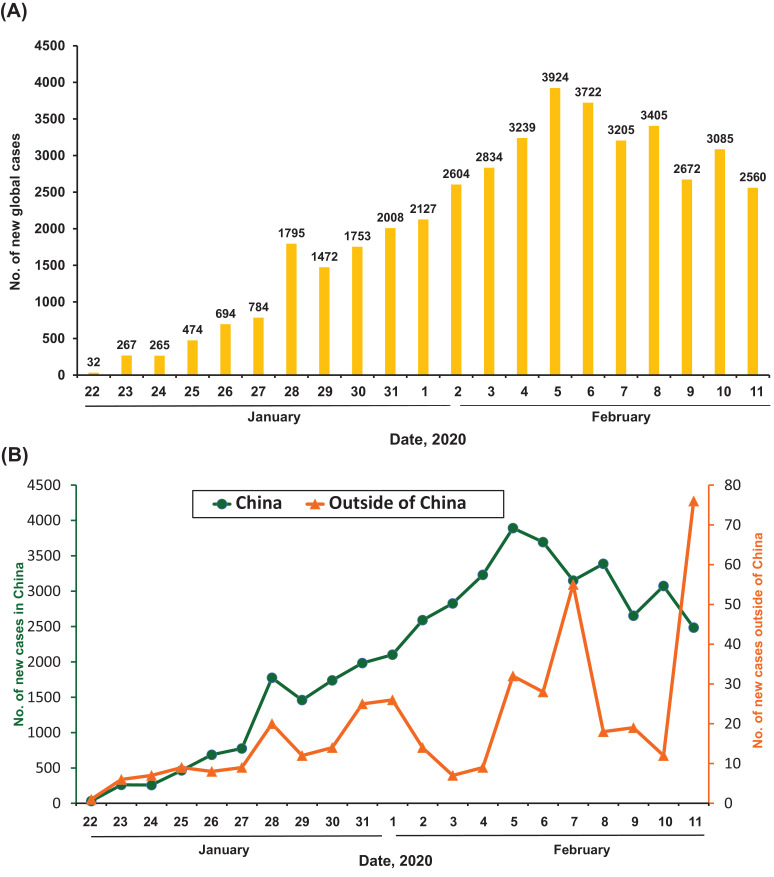
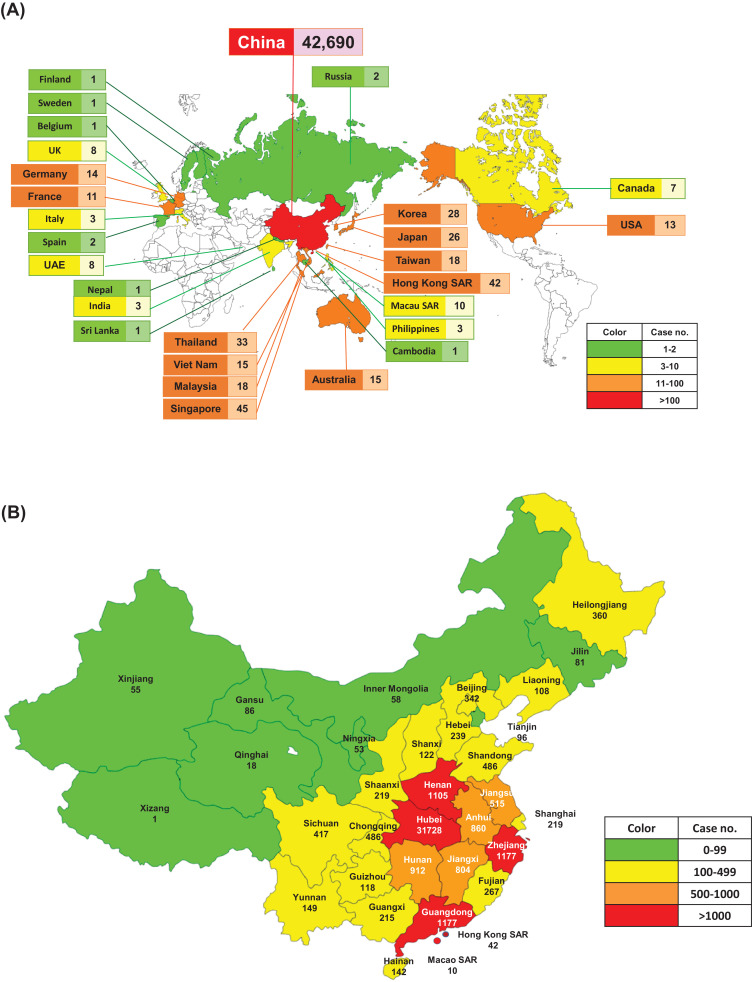
As of 11 February 2020, data from the WHO showed that there were a total of 43 103 cases of COVID-19 (Fig. 1, Fig. 2 ) (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=6f80d1b9_4). There has been a steady rise in the daily total number of COVID-19 cases globally, both within and outside China (Fig. 1). Regarding new cases of COVID-19, a declining trend was found globally (Fig. 2A), in China but not outside China (Fig. 2B), mainly in international conveyance (Japan) on 11 February 2020. Twenty-eight countries/regions have reported confirmed cases, including mainland China, Japan, Singapore, Hong Kong Special Administrative Region (SAR), Thailand, South Korea, Taiwan, Australia, Malaysia, Germany, Vietnam, the USA, Macao SAR, the United Arab Emirates, Canada, France, the Philippines, the UK, Italy, India, Russia, Finland, Sweden, Sri Lanka, Cambodia, Nepal, Spain and Belgium. China has had the largest number of patients with COVID-19 (n = 42 690), followed by Singapore (n = 45) (Fig. 3 A). Asia has had most of the reported cases, followed by Europe, North America and Australia, but no cases have been reported in Africa. Within China, Hubei has endured the largest number of infected patients (n = 31 728), followed by Guangdong (n = 1177), Zhejiang (n = 1117) and Henan (n = 1105) (Fig. 3B). A total of 1017 mortalities have been reported globally, with only 2 mortalities occurring outside of mainland China, one each in Hong Kong SAR and the Philippines. According to the Taiwan Centers for Disease Control (https://www.cdc.gov.tw/En), as of 12 February 2020 there were 45 167 cases of COVID-19 reported from 28 countries/region and 1115 (2.5%) of patients had died. Among the 45 167 cases, most were found in mainland China (n = 44 653) and the reported mortality was 2.5% (n = 1113).
Fig. 1.
Daily accumulative cases of laboratory-confirmed cases of 2019 coronavirus disease (COVID-19) as of 11 February 2020: (A) daily numbers of global cases; and (B) daily numbers of cases from China [including Hong Kong Special Administrative Region (SAR) and Macau SAR] and outside of China.
Fig. 2.
New daily cases of laboratory-confirmed 2019 coronavirus disease (COVID-19) as of 11 February 2020: (A) daily numbers of new cases globally; and (B) daily numbers of new cases from China [including Hong Kong Special Administrative Region (SAR) and Macau SAR] and outside of China.
Fig. 3.
Distribution of laboratory-confirmed cases of 2019 coronavirus disease (COVID-19) (A) globally by country and (B) in China by province/region as of 11 February 2020.
4. Clinical manifestations
As of 10 February 2020, only three relatively large-scale case studies have thoroughly demonstrated the clinical features of patients with pneumonia caused by SARS-CoV-2 (SARS-CoV-2 pneumonia) in Wuhan [4,5,8]. Herein, we summarise the clinical manifestations of the 278 pooled patients with SARS-CoV-2 pneumonia, which is also referred to as novel coronavirus pneumonia or Wuhan pneumonia (Table 1 ). All of the patients were adults older than 18 years of age, and males comprised 61.9% of the patients (n = 172). A recent study in Beijing reported that 2 of the 13 patients with SARS-CoV-2 pneumonia were children aged between 2–15 years [9]. As of 10 February 2020, more than 20 paediatric cases have been reported in China, 10 of whom were identified in Zhejiang Province and were in the age range of 112 days to 17 years [31]. Among adult patients, cardiovascular disease and hypertension were the most common underlying diseases, followed by diabetes mellitus. Fever was the most common symptom (92.8%; n = 258), followed by cough (69.8%; n = 194), dyspnoea (34.5%; n = 96), myalgia (27.7%; n = 77), headache (7.2%; n = 20) and diarrhoea (6.1%; n = 17). Rhinorrhoea was noted in only 4.0% [4], a sore throat in 5.1% [4] and pharyngalgia in 17.4% [8] of patients with relevant clinical information. Most patients had a normal white blood cell count, but 56.8% (n = 158) of patients had leukopenia. In one study, patients requiring intensive care were significantly older and more likely to have underlying diseases [8], but another study showed different findings [5]. According to two studies, patients admitted to the intensive care unit (ICU) were more likely to have dyspnoea than non-ICU patients [5,8]. Among the 13 patients with SARS-CoV-2 pneumonia reported in Beijing, 12 (92.3%) had fever with a mean duration of 1.6 days before hospitalisation [9]. Other symptoms included cough (46.3%), upper airway congestion (61.5%), myalgia (23.1%) and headache (23.1%) [9]. Although some of the epidemiological characteristics were identified, considerable uncertainties are still present and additional studies are needed with detailed information from confirmed cases [32].
Table 1.
Demographic data, underlying medical conditions, clinical manifestations and laboratory findings from three studies of 278 patients with SARS-CoV-2 pneumonia in Wuhan, China [4,5,8]a.
| Huang et al. [5] (n = 41) | Chen et al. [4] (n = 99) | Wang et al. [8] (n = 138) | |
|---|---|---|---|
| Study site | Wuhan local health authority | Wuhan Jinyintan Hospital | Zhongnan Hospital of Wuhan University |
| Age (years) | 49 (41–58) | 55.5 (13.1) | 56 (42–68) |
| ≥65 years | 6 (14.6) | NA | NA |
| Sex | |||
| Male | 30 (73.2) | 67 (67.7) | 75 (54.3) |
| Female | 11 (26.8) | 32 (32.3) | 63 (45.7) |
| Presumed hospital-related infection | NA | NA | 57 (41.3) |
| Healthcare worker | NA | NA | 40 (29.0) |
| Any co-morbidity | 13 (31.7) | 50 (51.5) | 64 (46.4) |
| Co-morbidities | |||
| Cardiovascular disease | 6 (14.6) | 40 (40.4) | 20 (14.5) |
| Hypertension | 6 (14.6) | NA | 43 (31.2) |
| Diabetes | 8 (19.5) | 12 (12.1) | 14 (10.1) |
| Respiratory disease | 1 (2.4) | 1 (1.0) | 4 (2.9) |
| Malignancy | 1 (2.4) | 1 (1.0) | 10 (7.2) |
| Chronic kidney disease | NA | NA | 4 (2.9) |
| Chronic liver disease | 1 (2.4) | NA | 4 (2.9) |
| Symptoms and signs | |||
| Fever | 40 (97.6) | 82 (82.8) | 136 (98.6) |
| Cough | 31 (75.6) | 81 (81.8) | 82 (59.4) |
| Dyspnoea | 22/40 (55.0) | 31 (31.3) | 43 (31.2) |
| Sputum production | 11/38 (28.9) | 37 (26.8) | |
| Myalgia | 18 (43.9) | 11 (11.1) | 48 (34.8) |
| Headache | 3/38 (7.9) | 8 (8.1) | 9 (6.5) |
| Diarrhoea | 1/38 (2.6) | 2 (2.0) | 14 (10.1) |
| Rhinorrhoea | NA | 4 (4.0) | NA |
| Sore throat or pharyngalgia | NA | 5 (5.1) | 24 (17.4) |
| Duration of onset to dyspnoea | 8 (5–13) | NA | 5 (1–10) |
| Duration of onset to hospital admission | 7 (4–8) | NA | 7 (4–8) |
| Duration of onset to ARDS | 9 (8–14) | NA | 8 (6–12) |
| Laboratory findings | |||
| White blood cell count (× 109/L) | 6.2 (4.1–10.5) | 7.5 (3.6) | 4.5 (3.3–6.2) |
| Neutrophil count (× 109/L) | 5.0 (3.3–8.9) | 5.0 (3.3–8.1) | 3.0 (2.0–4.9) |
| Lymphocyte count (× 109/L) | 0.8 (0.6–1.1) | 0.9 (0.5) | 0.8 (0.6–1.1) |
| Platelet count (× 109/L) | 164.5 (131.5–263.0) | 213.5 (79.1) | 163 (123–191) |
| aPTT (s) (range) | 27.0 (24.2–34.1) | 27.3 (10.2) | 31.4 (29.4–33.5) |
| PT (s) (range) | 11.1 (10.1–12.4) | 11.3 (1.9) | 13.0 (12.3–13.7) |
| Creatine kinase (U/L) | 132.5 (62.0–219.0) | 850 (51–184) | 92 (56–130) |
| ALT (U/L) | 32.0 (21.0–50.0) | 39 (22–53) | 24 (16–40) |
| AST (U/L) | 34 (26–48) | 34 (26–48) | 31 (24–51) |
| Total bilirubin (mmol/L) | 11.7 (9.5–13.9) | 15.1 (17.3) | 9.8 (8.4–11.1) |
| Creatinine (μmol/L) | 74.2 (57.5–85.7) | 75.6 (25.0) | 72 (60–87) |
| Lactate dehydrogenase (U/L) | 286 (242–408) | 336 (260–447) | 261 (182–403) |
NA, not available; ARDS, acute respiratory distress syndrome; aPTT, activated partial thromboplastin time; PT, prothrombin time; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Data are n (%), n/N (%), mean (standard deviation) or median (interquartile range).
5. Imaging
Radiological findings of SARS-CoV-2 pneumonia are variable. More than 75% of patients presented with bilateral lung involvement [4,5,8,32], and multilobe involvement was also common (71%) [33]. Ground-glass opacity (GGO) was the most common finding from chest computed tomography (CT) [8,34], and in a series of 21 patients, 86% had GGO on chest CT and 29% showed consolidation [33]. Approximately one-third of patients showed a peripheral distribution of GGO. In contrast, no discrete nodules, cavitation, pleural effusion or lymphadenopathy were observed on the chest CT images [33,34]. Another study including 51 cases showed similar findings [35]: most CT images showed pure GGO (77%), followed by GGO with reticular and/or interlobular septal thickening (75%), GGO with consolidation (59%) and pure consolidation (55%). Of the 51 cases, 86% showed bilateral lung involvement, and the above findings were peripherally distributed in 86% of cases [35].
6. Potential treatment options
According to recent reports [4,5,8], >85% of patients received antiviral agents, including oseltamivir (75 mg every 12 h orally), ganciclovir (0.25 g every 12 h intravenously) and lopinavir/ritonavir tablets (400/100 mg twice daily orally). Empirical antibiotics were prescribed for 90% of patients in three reports [4,5,8], and according to one study 15 patients (15%) received antifungal agents [4]. Five cases (5.1%) of bacterial (n = 1) or Candida (n = 4) co-infections were reported among 99 patients in one study [4], and 4 cases (9.8%) of secondary bacterial infections were reported in another study of 41 patients [5] (Table 2 ). Although intravenous immunoglobulin and systemic steroids have been used in several reports [4,5,8], their efficacy and associated adverse effects remain unclear.
Table 2.
| Huang et al. [5] (n = 41) | Chen et al. [4] (n = 99) | Wang et al. [8] (n = 138) | |
|---|---|---|---|
| Treatment | |||
| Antiviral treatment | 38 (92.7) | 75 (75.8) | 124 (89.9) |
| Antibiotic treatment | 41 (100) | 70 (70.7) | 138 (100) |
| Antifungal treatment | NA | 15 (15.2) | NA |
| Corticosteroid treatment | 9 (22.0) | 19 (19.2) | 62 (44.9) |
| CRRT | 3 (7.3) | 9 (9.1) | 2 (1.4) |
| IVIg therapy | NA | 27 (27.3) | NA |
| Invasive mechanical ventilation | 2 (4.9) | 4 (4.0) | 17 (12.3) |
| ECMO | 2 (4.9) | 3 (3.0) | 4 (2.9) |
| Complications | |||
| ARDS | 12 (29.3) | 17 (17.2) | 27 (19.6) |
| Acute kidney injury | 3 (7.3) | 3 (3.0) | 5 (3.6) |
| Acute cardiac injury | 5 (12.2) | NA | 10 (7.2) |
| Co- or secondary infection | 4 (9.8) | 5 (5.1) | NA |
| Shock | 3 (7.3) | 4 (4.0) | 12 (8.7) |
| ICU unit admission | 13 (31.7) | 23 (23.2) | 36 (26.1) |
| Mortality | 6 (14.6) | 11 (11.1) | 6 (4.3) |
CRRT, continuous renal replacement therapy; IVIg, intravenous immunoglobulin; ECMO, extracorporeal membrane oxygenation; ARDS, acute respiratory distress syndrome; NA, not available; ICU, intensive care unit.
aData are number (%) of confirmed patients.
So far, there has been no effective treatment of COVID-19. Several potential drug candidates, including lopinavir/ritonavir (KaletraⓇ), nucleoside analogues, neuraminidase inhibitors, remdesivir, umifenovir (ArbidolⓇ), DNA synthesis inhibitors (such as tenofovir disoproxil and lamivudine), chloroquine and Chinese traditional medicines (such as ShuFeng JieDu or Lianhua Qingwen capsules), have been proposed [36,37]. In addition, an angiotensin-converting enzyme 2 (ACE2)-based peptide, 3CLpro inhibitor (3CLpro-1) and a novel vinylsulfone protease inhibitor, theoretically, appear to show potential for antiviral activity against SARS-CoV-2 [38]. Chloroquine has been well described with in vitro effects on inhibition of uncoating and/or alteration of post-translational modifications of newly synthesised proteins, especially inhibition of glycosylation in many viruses, including human immunodeficiency virus (HIV) [39]. Preliminary in vivo clinical studies suggest that chloroquine alone or in combination with antiretroviral agents might play an interesting role in treating HIV infection [39]. A recent study by Wang et al. revealed that remdesivir and chloroquine were highly effective in the control of 2019-nCoV in vitro [37]. In addition to the one case of SARS-CoV-2 pneumonia with a promising clinical response to remdesivir [7] and two clinical trials in China, further case-controlled clinical studies of remdesivir therapy are warranted to verify its therapeutic efficacy.
7. Outcomes
In the three pooled studies of 278 patients [4,5,8], 72 patients (25.9%) with SARS-CoV-2 pneumonia required ICU admission, 56 (20.1%) developed acute respiratory distress syndrome, and 23 (8.3%) and 9 (3.2%) required invasive mechanical ventilation and extracorporeal membrane oxygenation for refractory hypoxemia, respectively (Table 2). Shock was observed in 19 patients (6.8%), acute kidney injury in 11 patients (4.0%) and continuous renal replacement therapy was required in 14 patients (5.0%). Acute cardiac injury was reported in 5 patients (12.2%) in one study [5] and 10 patients (7.2%) in another study [8]. Although two earlier studies demonstrated that SARS-CoV-2 pneumonia was associated with high mortality rates of 11.1% (n = 11) [4] and 14.6% (n = 6) [5], one recent study showed a mortality rate of 4.3% (n = 6) [8] (Table 2). Among 13 patients with SARS-CoV-2 pneumonia outside of Wuhan, as of 4 February 2020 all of the patients recovered but 12 were still being quarantined in hospital in Beijing [9]. It might be suggested that the real-world mortality rate may be lower than that reported in a few published clinical series, when clinical data from more systematic testing would be available, and as the ratio between fatality cases and total reported cases of COVID-19 on 12 February 2020 was currently 0.025 (mortality rate 2.5%). However, most deaths developed in male and elderly patients [4,40]. The median number of days from the appearance of the first symptom to death was 14 days, and it was significantly shorter among patients aged ≥70 years (11.5 days) compared with those aged <70 years (20 days) (P = 0.033) [40].
8. Infection control and prevention
To decrease the damage associated with COVID-19, public health and infection control measures are urgently required to limit the global spread of the virus [35]. Experience from the early phase of SARS-CoV-2 pneumonia strongly highlighted that travel history, rather than chest radiography, is of paramount importance for early detection and isolation of SARS-CoV-2 pneumonia cases [41]. It is essential to limit human-to-human transmission in order to reduce secondary infections among close contacts and healthcare workers and to prevent transmission amplification events and further international spread from China. Based on previous experience of management of MERS and SARS infections, the WHO recommend infection control interventions to reduce the general risk of transmission of acute respiratory infections, including avoiding close contact with people suffering from acute respiratory infections, frequent hand-washing especially after direct contact with ill people or their environment, and avoiding unprotected contact with farm or wild animals. Moreover, people with symptoms of acute respiratory infection should practice cough etiquette, which is to maintain distance, cover coughs and sneezes with disposable tissues or clothing, and wash hands, and within healthcare facilities enhanced standard infection prevention and control practices are recommended in hospitals, especially in emergency departments [42]. The US Centers for Disease Control and Prevention (CDC) has established interim clinical guidance for the COVID-19 outbreak to implement aggressive measures to slow the transmission of SARS-CoV-2 in the USA [43]. These measures include identification of cases and their contacts in the USA as well as appropriate assessment and care of travellers arriving from mainland China to the USA [43]. All efforts are being made to slow the spread of the illness in order to provide time to better prepare healthcare systems and the general public, to better characterise COVID-19 to guide public-health recommendations, and to develop timely diagnostics, therapeutics and vaccines [43]. Finally, although the improvement of internet communication largely enhances the availability and dissemination of knowledge, the internet also has the potential for the development and spread of misinformation or fake news. Governments should be responsible for providing accurate knowledge and clarifying misinformation to help the public face this novel infection.
9. Unresolved issues
Despite the whole world's efforts to understand COVID-19, many issues remain unclear. First, one report has demonstrated the presence of SARS-CoV-2 in patient stools [7]. However, whether SARS-CoV-2 can be transmitted through the faecal–oral route remains unclear. Second, previous studies showed that SARS-CoV and other coronaviruses could survive on environmental surfaces and inanimate objects [44,45]; however, the presence of SARS-CoV-2 in the environment has not been reported. Previous studies have shown that coronaviruses could be efficiently inactivated using surface disinfectants with 62–71% ethanol, 0.5% hydrogen peroxide or 0.1% sodium hypochlorite within 1 min, but other biocidal agents such as 0.05–0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective [45]. However, current investigation of the efficacy of commonly used disinfection agents against SARS-CoV-2 is lacking. Third, although travel restriction was exerted in many countries, whether this intervention was effective is unclear. Fourth, although one case responded well to remdesivir [7] and one in vitro study [37] showed that remdesivir and chloroquine were promising for the treatment of COVID-19, further clinical trials on the effectiveness of remdesivir and chloroquine for treating SARS-CoV-2 pneumonia should be conducted. Fifth, although several studies have reported the clinical features of COVID-19 [4,5,8,9], all of the patients had pneumonia and were treated in Wuhan and Beijing. Most recently, an article has described 1099 patients with acute respiratory disease (ARD) caused by SARS-CoV-2 treated at 552 hospitals across 31 provinces/provincial municipalities in China [46]. The article reported that only 43.8% of patients had a initial presentation of fever, and severe pneumonia occurred in 15.7% of cases. The study indicated the median incubation period to be 3.0 days (range, 0–24.0 days) and the fatality rate to be only 1.36% [46]. However, further evaluation of the content of the above report is warranted to clarify the epidemiological and clinical characteristics of asymptomatic carriers and of ARD and pneumonia caused by SARS-CoV-2. Finally, although 32.4% (n = 90) of the reported 278 cases with SARS-CoV-2 pneumonia received systemic steroid therapy [4,5,8], a study on the temporal features of the SARS-CoV-2‐induced inflammatory response in relation to the timing of therapeutic interventions is lacking. Previous experiences of systemic steroids in the treatment of coronavirus-related infections, such as SARS and MERS, showed disappointing results. In the interim, clinical use of glucocorticoids to control SARS-CoV-2 pneumonia with the intention of regulating cytokine production and the inflammatory response and avoiding lung injury should be avoided [47,48].
10. Conclusions
The outbreak of COVID-19 has become a clinical threat to the general population and healthcare workers worldwide. However, knowledge about this novel virus remains limited. The effective option of antiviral therapy and vaccination are currently under evaluation and development. What we can do now is aggressively implement infection control measures to prevent the spread of SARS-CoV-2 via human-to-human transmission. Public health authorities should keep monitoring the situation, as the more we learn about this novel virus and its associated outbreaks, the better we can respond.
Acknowledgments
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
Editor: Jean-Marc Rolain
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020 Jan 16 doi: 10.1002/jmv.25678. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 Jan 29 doi: 10.1056/NEJMoa2001316. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A. Severe acute respiratory syndrome-related coronavirus: the species and its viruses—a statement of the Coronavirus Study Group. bioRxiv. 2020 Feb 11 doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 Jan 31 doi: 10.1056/NEJMoa2001191. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7 doi: 10.1001/jama.2020.1585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang D., Lin M., Wei L., Xie L., Zhu G., Dela Cruz C.S. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020 Feb 7 doi: 10.1001/jama.2020.1623. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlos W.G., Dela Cruz C.S., Cao B., Pasnick S., Jamil S. Novel Wuhan (2019-nCoV) coronavirus. Am J Respir Crit Care Med. 2020;201:P7–P8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 11.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biscayart C., Angeleri P., Lloveras S., Chaves T., Schlagenhauf P., Rodriguez-Morales A.J. The next big threat to global health? 2019 novel coronavirus (2019-nCoV): What advice can we give to travellers? — Interim recommendations January 2020, from the Latin-American Society for Travel Medicine (SLAMVI) Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E. A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med. 2020 Jan 24 doi: 10.1056/NEJMp2000929. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Yoo J.H. The fight against the 2019-nCoV outbreak: an arduous march has just begun. J Korean Med Sci. 2020;35:e56. doi: 10.3346/jkms.2020.35.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee K.H., Yoo S.G., Cho Y., Kwon D.E., La Y., Han S.H. Characteristics of community-acquired respiratory viruses infections except seasonal influenza in transplant recipients and non-transplant critically ill patients. J Microbiol Immunol Infect. 2019 Jun 19 doi: 10.1016/j.jmii.2019.05.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou C.C., Shen C.F., Chen S.J., Chen H.M., Wang Y.C., Chang W.S. Recommendations and guidelines for the treatment of pneumonia in Taiwan. J Microbiol Immunol Infect. 2019;52:172–199. doi: 10.1016/j.jmii.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Lee J.Y., Yang P.C., Chang C., Lin I.T., Ko W.C., Cia C.T. Community-acquired adenoviral and pneumococcal pneumonia complicated by pulmonary aspergillosis in an immunocompetent adult. J Microbiol Immunol Infect. 2019;52:838–839. doi: 10.1016/j.jmii.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Su I.C., Lee K.L., Liu H.Y., Chuang H.C., Chen L.Y., Lee Y.J. Severe community-acquired pneumonia due to Pseudomonas aeruginosa coinfection in an influenza A(H1N1)pdm09 patient. J Microbiol Immunol Infect. 2019;52:365–366. doi: 10.1016/j.jmii.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Lee S.H., Ruan S.Y., Pan S.C., Lee T.F., Chien J.Y., Hsueh P.R. Performance of a multiplex PCR pneumonia panel for the identification of respiratory pathogens and the main determinants of resistance from the lower respiratory tract specimens of adult patients in intensive care units. J Microbiol Immunol Infect. 2019;52:920–928. doi: 10.1016/j.jmii.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung H.M., Yang S.L., Chen C.J., Chiu C.H., Kuo C.Y., Huang K.A. Molecular epidemiology and clinical features of rhinovirus infections among hospitalized patients in a medical center in Taiwan. J Microbiol Immunol Infect. 2019;52:233–241. doi: 10.1016/j.jmii.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Lin G.L., Lu C.Y., Chen J.M., Lee P.I., Ho S.Y., Weng K.C. Molecular epidemiology and clinical features of adenovirus infection in Taiwanese children, 2014. J Microbiol Immunol Infect. 2019;52:215–224. doi: 10.1016/j.jmii.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 Jan 30 doi: 10.1016/S0140-6736(20)30251-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Jan 24 doi: 10.1056/NEJMoa2001017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang S., Du L., Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerg Microbes Infect. 2020;9:275–277. doi: 10.1080/22221751.2020.1723441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren L.L., Wang Y.M., Wu Z.Q., Xiang Z.C., Guo L., Xu T. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020 Jan 30 doi: 10.1097/CM9.0000000000000722. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L., Liu W., Zhang Q., Xu K., Ye G., Wu W. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect. 2020;9:313–319. doi: 10.1080/22221751.2020.1725399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Feb 3 doi: 10.1038/s41586-020-2012-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020 Jan 31 doi: 10.1016/S0140-6736(20)30260-9. [Epub ahead of print] [DOI] [Google Scholar]
- 30.Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travelers from Wuhan, China, 20–28 January 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z.M., Fu J.F., Shu Q., Chen Y.H., Hua C.Z., Li F.B. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020 Feb 5 doi: 10.1007/s12519-020-00345-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryu S., Chun B.C. Epidemiological characteristics of 2019 novel coronavirus: an interim review. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020 Feb 4 doi: 10.1148/radiol.2020200230. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanne J.P. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020 Feb 4 doi: 10.1148/radiol.2020200241. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song F., Shi N., Shan F., Zhang Z., Shen J., Lu H. Emerging coronavirus 2019-nCoV pneumonia. Radiology. 2020 Feb 6 doi: 10.1148/radiol.2020200274. [Epub ahead of print] [DOI] [Google Scholar]
- 36.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020 Jan 28 doi: 10.5582/bst.2020.01020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 Feb 4 doi: 10.1038/s41422-020-0282-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W., Morse J.S., Lalonde T., Xu S. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020 Feb 5 doi: 10.1002/cbic.202000047. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rolain J.M., Colson P., Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30:297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020 Jan 29 doi: 10.1002/jmv.25689. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J.Y., Choe P.G., Oh Y., Oh K.J., Kim J., Park S.J. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020;35:e61. doi: 10.3346/jkms.2020.35.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization (WHO). Novel coronavirus (2019-nCoV). Situation report. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200207-sitrep-18-ncov.pdf?sfvrsn=fa644293_2[accessed 9 February 2020].
- 43.Patel A., Jernigan D.B., 2019-nCoV CDC Response Team Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak—United States, December 31, 2019–February 4, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:140–146. doi: 10.15585/mmwr.mm6905e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casanova L.M., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl Environ Microbiol. 2010;76:2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kampf G., Todt D., Pfaeder S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and its inactivation with biocidal agents. J Hosp Infect. 2020 Feb 6 doi: 10.1016/j.jhin.2020.01.022. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guan W.J., Ni Z.Y., Hu Y., Laing W.H., Ou C.Q., He J.X. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020 Feb 9 doi: 10.1101/2020.02.06.20020974. [DOI] [Google Scholar]
- 47.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. Coronavirus infections and immune responses. J Med Virol. 2020 Jan 25 doi: 10.1002/jmv.25685. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]