Abstract
Background
Risk factors for acute wheezing among children in subtropical areas are largely unknown.
Objective
To investigate the role of viral infections, allergen sensitization, and exposure to indoor allergens as risk factors for acute wheezing in children 0 to 12 years old.
Methods
One hundred thirty-two children 0 to 12 years of age who sought emergency department care for wheezing and 65 children with no history of wheezing were enrolled in this case-control study. Detection of respiratory syncytial virus antigen, rhinovirus and coronavirus RNA, adenovirus, influenza, and parainfluenza antigens was performed in nasal washes. Total IgE and specific IgE to mites, cockroach, cat, and dog were measured with the CAP system. Major allergens from mites, cockroach, cat, and dog were quantified in dust samples by ELISA. Univariate and multivariate analyses were performed by logistic regression.
Results
In children under 2 years of age, infection with respiratory viruses and family history of allergy were independently associated with wheezing (odds ratio, 15.5 and 4.2; P = .0001 and P = .008, respectively). Among children 2 to 12 years old, sensitization to inhalant allergens was the major risk factor for wheezing (odds ratio, 2.7; P = .03). High-level allergen exposure, exposure to tobacco smoke, and lack of breast-feeding showed no association with wheezing.
Conclusions
Some risk factors for wheezing previously identified in temperate climates were present in a subtropical area, including respiratory syncytial virus infection in infants and allergy in children older than 2 years. Rhinovirus was not associated with wheezing and did not appear to be a trigger for asthma exacerbations.
Keywords: Wheezing, asthma, allergy, mites, cockroach, respiratory viruses, respiratory syncytial virus, rhinovirus, allergens
Abbreviations: ED, Emergency department; GM, Geometric mean; RSV, Respiratory syncytial virus
Asthma is the most common chronic disease of childhood. Evidence indicates that more than half of the cases of persistent asthma start before the age of 3 years, and it is now clear that airway inflammation and irreversible remodeling may be initiated early in life.1, 2 Definitive diagnosis of asthma and subsequent decision to initiate long-term anti-inflammatory treatment at an early age has been a challenge in pediatric practice.3 Although asthma in young children appears to represent a heterogeneous disease with distinct phenotypes, sensitization to common inhalant allergens including those derived from dust mites, cockroach, cat, and Alternaria remains a major risk factor for persistent asthma associated with airway hyperreactivity and decreased lung function.4, 5, 6
Respiratory viruses have been recognized as major triggers of wheezing episodes and asthma exacerbation.7 In the northern hemisphere, respiratory syncytial virus (RSV) accounts for the majority of wheezing episodes in children younger than 2 years of age, being detected in 60% to 80% of infants.7 Results of the Tucson Children's Respiratory Study8 revealed that lower respiratory tract infection with RSV early in life was associated with persistent wheezing; however, by age 13 years, lung function abnormalities were no longer present and wheezing resolved in most children. In one study, severe RSV bronchiolitis in the first year of life has been linked to asthma and allergic sensitization at age 7 years9; however, studies to determine whether RSV infections enhance allergy have provided conflicting results.7 It has been definitely established that specific IgE response to RSV is not an important element in the pathogenesis of RSV-induced wheezing10; however, the virus has been shown to induce expression of cytokines and chemokines and activation of monocytes, macrophages, and lymphocytes, which may influence the mechanisms involved in the development of asthma in children.7 In older children, rhinovirus becomes the dominant viral pathogen linked to wheezing exacerbations. This has been demonstrated in community-based and emergency department studies.11, 12 In the emergency department setting, rhinovirus infections act synergistically with allergen sensitization and/or allergen exposure to induce severe asthma symptoms in both children and adults.12, 13 In keeping with this, lower respiratory tract symptoms and markers of inflammation in young adults with mild asthma experimentally infected with rhinovirus are more pronounced in those with high total IgE.14 It is thought that a deficient TH1-type response with decreased IFN-γ production underlies the observation that rhinovirus infections in atopic individuals are more severe and longer-lasting and induce more lower respiratory symptoms as compared with nonatopic subjects.15
The purpose of the current study was to investigate the role of viral infections, allergen sensitization, and exposure to indoor allergens as risk factors for acute wheezing in children 0 to 12 years old living in a subtropical environment. The results of the current study may contribute to promote better care of children with wheezing in these areas.
Methods
Subjects
One hundred thirty-two children 0 to 12 years of age (80 boys) who sought emergency department (ED) care for wheezing either at the Clinical Hospital of the University of São Paulo School of Medicine of Ribeirão Preto or Santa Lydia community Hospital were enrolled in this case-control study. Patients were selected for the study if they presented with wheezing that required therapy with inhaled β2-agonists as judged by the attending physician. In addition, 65 children 0 to 12 years of age (39 boys) without respiratory symptoms and no previous history of wheezing who sought ED care at the same hospitals for other reasons were selected as control subjects. The most frequent complaints among the control subjects were acute gastroenteritis, fever, abdominal pain, headache, vomiting, and seizures. Children with chronic respiratory illnesses (n = 2) (bronchopulmonary dysplasia) and those who reported therapy with corticosteroids within 4 weeks before the ED visit (n = 8) were not included in the study. All children were residents in the city of Ribeirão Preto. Wheezing and control children were enrolled every month from October 1998 to June 2000, except for December 1998. Approximately 3% of all children seen in the ED over the study period were enrolled in the study, and 95% of the children approached were enrolled. Peaks of attendance to the ED for wheezing were observed in both years; 61% and 68%, respectively, of wheezing and control children under 2 years of age were enrolled from February through May and 52% and 60% of wheezing and control children older than 2 years were enrolled from March through June.
Parents or guardians completed a questionnaire that included questions on personal and family history of allergies and asthma, passive exposure to tobacco smoke, breast-feeding, and housing conditions. A positive personal history of allergy was defined as a report of physician-diagnosed asthma, rhinitis, and/or atopic dermatitis. A positive family history for allergy was characterized by the report of asthma, rhinitis, and/or atopic dermatitis diagnosed by a physician in the mother, father, and/or sibling(s). History of prior wheezing illnesses was reported for 48% of the wheezing children younger than 2 years and for 93% of the older wheezing children. However, only 16% of the wheezing infants and 51% of the wheezing children older than 2 years reported asthma diagnosed by a physician. The study protocol was approved by the ethics committee of both hospitals and the children's parents and/or guardians gave written informed consent to participate in the study.
Detection of respiratory viruses
Nasal washings were collected and processed for detection of respiratory viruses as previously described.12 RSV antigen was detected by using both a rapid enzyme immunoassay (Test-Pack, Abbott, Chicago, Ill) and an indirect immunofluorescence (Chemicon, Temecula, Calif). Human rhinovirus and coronavirus RNA was detected by RT-PCR.12, 16 Influenza and parainfluenza viruses were detected by indirect immunofluorescence.17, 18 In a subset consisting of 90 of 132 wheezing children and 48 of 65 control subjects, from whom remaining material was available, detection of adenovirus was investigated by culture of frozen nasal aspirates on A549 cells, with cytopathic effect confirmed by immunofluorescence. Typing of adenovirus isolated from cultures to the subgenus level was performed by amplification of the VA-RNA region of the viral genome by PCR, according to previously described method.19 PCR products of 520 to 522 bp and 505 bp in length corresponded to adenovirus subgenera B and C, respectively. Certain adenovirus serotypes including those of subgenus C can be identified in nasal aspirates up to several weeks after acute infection.20 To minimize the interference of latent adenovirus, only subgenus B was included in the analysis.
Total IgE, specific IgE antibodies, and peripheral blood eosinophils
Serum levels of total IgE and specific IgE to mites (Dermatophagoides pteronyssinus, D farinae, and Blomia tropicalis), cockroach (Blattella germanica), cat, dog, foods (cow's milk, egg, wheat, soy, peanut, and fish), and to Ascaris lumbricoides were measured with the use of the UniCAP system (Pharmacia, Uppsala, Sweden). Specific IgE levels ≥0.7 kU/L (CAP score ≥2) were considered positive. Automated white blood cell counts were carried out with the use of a Colter T540 system, and differential counts, including eosinophil quantification, were performed manually.
Allergen levels in house dust samples
Dust samples were collected from four sites in each subject's home: bedding, bedroom floor, TV room, and kitchen, within 3 weeks of the ED visit. Dust collection and preparation of dust extracts were performed according to standard procedures.21 Measurements of major allergens from mites (group 1 and group 2), cockroach (Bla g 1 and Bla g 2), cat (Fel d 1), and dog (Can f 1) in dust extracts were carried out with the use of monoclonal antibody–based ELISA.22 Group 1 mite allergens comprised the sum of levels of Der p 1 and Der f 1. Group 2 allergens (Der p 2 and Der f 2) were simultaneously detected in the monoclonal antibody ELISA with a cross-reactive capture antibody (1D8). High-level exposure was defined as the presence of at least one dust sample in the house containing concentrations of group 1 mite allergens (≥10 μg/g, Bla g 1 ≥8 U/g, and/or Bla g 2 ≥0.32 μg/g, Fel d 1 ≥8 μg/g, and Can f 1 ≥10 μg/g).22
Statistical analysis
Data from wheezing and control children were compared in two age groups: children younger than 2 years of age (n = 74 and n = 30, respectively) and children 2 to 12 years of age (n = 58 and n = 35, respectively). Data on total IgE levels were log-transformed and analyzed by Student t test. Peripheral blood eosinophils and allergen levels in house dust were analyzed by Mann-Whitney test. Chi-square analysis was used to compare the prevalence of elevated specific IgE, positive viral tests, frequency of passive smoke exposure, and high-level allergen exposure. Univariate and multivariate analysis were carried out by logistic regression, using the software STATA version 6.0.
Results
Detection of respiratory viruses
In the group of children under 2 years of age, respiratory viruses were detected in 60.8% (45 of 74) wheezing infants and in 13.3% (4 of 30) control infants (P < .001). RSV was detected in 39% wheezing children and none of the control infants (P < .001). Rhinovirus RNA was found in 20.2% and 10% of the wheezing and control children, respectively (P = .21). Adenovirus was cultured from 29.4% (15/51) of wheezing children and 20.8% (5/24) of control children; 4 and 1 isolates were identified as subgenus B (7.8% and 4.1%), respectively (P = .55). Corona-virus RNA was found in 3 of 73 wheezing subjects and none of 30 control subjects. Influenza A and B and parainfluenza viruses were not detected (Fig 1). Five wheezing children were positive for more than one virus.
FIG 1.
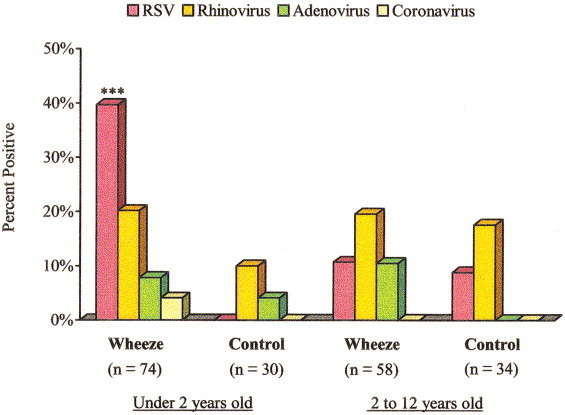
Viral identification in nasal washes by age group. Pink bars indicate nasal washes positive for RSV antigen; dark yellow bars represent nasal washes positive for rhinovirus; green bars indicate nasal washes positive for adenovirus B; and light yellow bars represent nasal washes positive for coronavirus. Other common viral pathogens (influenza A and B and parainfluenza viruses) were not detected. ∗∗∗P < .001 for wheezing versus control subjects in age group.
In the group of children 2 to 12 years of age, respiratory viruses were not significantly associated with wheezing. Rhinovirus RNA was detected in 19.3% wheezing children as compared with 17.1% of the nonwheezing control children (P = .80); RSV antigen was detected in 10.3% and 8.5%, respectively (P = .70), and adenovirus was isolated from 23% (9/39) and 17.8% (5/28), respectively; 4 and 0 (10.5% and 0%) of the adenovirus isolates were identified as subgenus B (P = .07). Coronavirus, influenza A and B, and parainfluenza viruses were not found in children older than 2 years (Fig 1).
Relation of wheezing, IgE response, and eosinophilia
In children under 2 years of age, geometric mean (GM) total IgE levels were 19.7 IU/mL (range <2 IU/mL to 1709 IU/mL) in wheezing infants and 9.4 IU/mL (range < 2 IU/mL to 620 IU/mL) in control infants (P = .09). Only four wheezing infants, all over 1 year old, presented IgE antibodies to inhalant allergens: three to mites and one to mites, dog, and egg; three of these had a reported history of allergy in both mother and father. Both wheezing and control infants presented IgE to foods: 8.9% (6 of 67) and 11.1% (3 of 27) were sensitized to at least one food allergen, respectively (P = .74). The most common allergens were egg and cow's milk, followed by wheat and soy. No sensitization to peanut or fish was found. IgE to Ascaris lumbricoides was not detectable in children under 2 years of age. No significant differences in peripheral blood eosinophil counts were observed in the wheezing and control groups (GM = 218/mm3 and 208/mm3, respectively) (P = .21).
In children older than 2 years, GM value of total IgE was 278 IU/mL (range, 7.9 to 2627 IU/mL) in wheezing children and 97.4 IU/mL (range, 2.0 to 1067 IU/mL) in control children (P = .002). Sensitization to inhalant allergens was present in 72.4% of wheezing children and 42.8% of control children (P = .005). Mites and cockroach were the sensitizing allergens associated with wheezing. Specific IgE to A lumbricoides was more frequently found in wheezing children, 33.9% (19 of 56) as compared with 14.2% (5 of 35) of the control children (P = .04). Peripheral blood eosinophil counts were higher but did not reach significance (GM = 350/mm3 and 199/mm3 in wheezing and control children, respectively) (P = .08).
Role of allergen exposure
Concentrations of mite, cockroach, cat, and dog allergens in dust samples of homes of wheezing children and control children were compared (Table I). Levels of group 2 mite allergens were significantly higher in homes of wheezing children under 2 years of age. Levels of group 1 mite allergens, Bla g 1 and Bla g 2, Fel d 1, and Can f 1, in the homes were not different between wheezing and control children for either age group (Table I). The highest levels of mite allergens were found in bedding samples.
Table I.
Median levels and range (in parenthesis) of major allergens from mites, cockroach, cat, and dog in dust samples of homes of wheezing and control children by age group
| Under 2 y | 2 to 12 y | |||||
|---|---|---|---|---|---|---|
| Allergens | Wheeze (n = 65) | Control (n = 25) | P value | Wheeze (n = 51) | Control (n = 29) | P value |
| Group 1 mite allergens (μg/g of dust) | 9.6 | 9.2 | .5 | 16.7† | 28.3‡ | .3 |
| (0.05 to 200.7) | (0.9 to 56.6) | (1.2 to 654.4) | (1.5 to 131) | |||
| Group 2 mite allergens (μg/g of dust) | 1.9 | 1.0 | .01 | 3.6 | 3.2 | .7 |
| (0.1 to 191.6) | (0.1 to 12.1) | (0.1 to 90.0) | (0.2 to 39.4) | |||
| Bla g 1 (U/g of dust) | 2.0 | 3.0 | .2 | 2.0 | 3.0 | .5 |
| (0.7 to 35.2) | (0.7 to 42.4) | (0.7 to 86.4) | (0.7 to 25.9) | |||
| Bla g 2 (μg/g of dust) | 0.048 | 0.08 | .1 | 0.048 | 0.08 | .5 |
| (0.028 to 1.11) | (0.028 to 1.38) | (0.028 to 1.58) | (0.028 to 1.82) | |||
| Fel d 1 (μg/g of dust)∗ | 0.2 | 0.1 | .2 | 0.2 | 0.3 | .4 |
| (0.06 to 23.7) | (0.06 to 2.6) | (0.06 to 23.1) | (0.06 to 3.0) | |||
| Can f 1 (μg/g of dust)∗ | 0.9 | 1.2 | .9 | 4.0§ | 2.6 | .4 |
| (0.01 to 200) | (0.1 to 76.8) | (0.04 to 194.5) | (0.07 to 267.4) | |||
Presence of cats or dogs was found in 13 of 172 (7.5%) and 87 of 172 (50.2%) homes visited, respectively. In most cases, animals were kept outside the house.
P < .05 compared with wheezing children under 2 years of age.
P < .05 compared with control children younger than 2 years of age.
P < .05 compared with wheezing children under 2 years of age.
Analysis of risk factors for wheezing
Infection with respiratory viruses, particularly with RSV, was the major risk factor for wheezing among children younger than 2 years of age, as revealed by univariate analysis. Personal and family history of allergy were both significantly associated with wheezing (Table II). In the multivariate model, viral infection and family history of allergy were each independently associated with wheezing, and sensitization to inhalant allergens played no significant role in wheezing in infants (Table III).
Table II.
Univariate analysis of risk factors for acute wheezing among children 0 to 12 years old
| Under 2 y | 2 to 12 y | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | Wheeze (n = 74) | Control (n = 30) | Odds ratio (95% CI) | P value | Wheeze (n = 58) | Control (n = 35) | Odds ratio (95% CI) | P value |
| Age in mo (median) | 6 | 4 | 1.0 (0.9 to 1.0) | .6 | 48 | 60 | 0.9 (0.9 to 1.0) | .2 |
| Male sex (%) | 62.1 | 56.6 | 0.8 (0.3 to 1.9) | .6 | 58.6 | 62.8 | 1.1 (0.5 to 2.8) | .6 |
| Personal history of allergy (%) | 33.7 | 13.3 | 3.3 (1.04 to 10.5) | .04 | 75.8 | 11.4 | 24 (7.3 to 81.0) | < .001 |
| Family history of allergy (%) | 72.9 | 46.6 | 3.0 (1.2 to 7.4) | .01 | 65.5 | 62.8 | 1.1 (0.4 to 2.6) | .79 |
| Total serum IgE ≥400 IU/mL (%) | 4.1 | 3.4 | 1.2 (0.1 to 12.0) | .8 | 44.8 | 22.8 | 2.7 (1.06 to 7.0) | .03 |
| Sensitization to inhalant allergens (%)∗ | 5.4 | 0 | — | — | 72.4 | 42.8 | 3.5 (1.4 to 8.4) | .005 |
| Mite (%) | 5.4 | 0 | — | — | 68.9 | 40 | 3.3 (1.3 to 8.0) | .007 |
| Cockroach (%) | 0 | 0 | — | — | 26.3 | 2.8 | 12.1 (1.5 to 96.6) | .018 |
| Cat (%) | 0 | 0 | — | — | 5.2 | 5.7 | 0.9 (0.1 to 5.7) | .9 |
| Dog (%) | 1.4 | 0 | — | — | 7.0 | 0 | — | — |
| Sensitization to food allergens (%)∗ | 8.9 | 11.1 | 0.7 (0.1 to 3.4) | .7 | n.d. | n.d. | n.d. | n.d. |
| Specific IgE to Ascaris lumbricoides (%)† | 0 | 0 | — | — | 33.9 | 14.2 | 3.0 (1.02 to 9.2) | .04 |
| Viral infection (%)‡ | 60.8 | 13.3 | 10.0 (2.9 to 37.9) | .0001 | 32.7 | 25.7 | 1.4 (0.5 to 3.5) | .47 |
| Passive smoke exposure (%) | 67.5 | 50 | 2.0 (0.8 to 4.9) | .09 | 61.4 | 51.4 | 1.5 (0.6 to 3.5) | .34 |
| High-level exposure§ to: | ||||||||
| Mites (%) | 47.6 | 36 | 1.6 (0.6 to 4.1) | .3 | 68.6 | 72.4 | 0.8 (0.3 to 2.2) | .72 |
| Cockroach (%) | 23 | 28 | 0.7 (0.2 to 2.1) | .6 | 25.4 | 24.1 | 1.0 (0.3 to 3.0) | .89 |
| Cat (%) | 1.5 | 0 | — | — | 2.0 | 0 | — | — |
| Dog (%) | 12.5 | 16.0 | 0.7 (0.2 to 2.7) | .6 | 34.0 | 32.1 | 1.0 (0.4 to 2.9) | .8 |
n.d., Not done. IgE to food allergens was not assayed on children ages 2 to 12 years; —, odds ratios for variables with a zero value could not be calculated with STATA 6.0.
Levels of specific IgE antibodies ≥0.7 kUA/L (CAP class ≥2) to at least one inhalant allergen (mite, cockroach, cat, or dog) or food allergen (egg, milk, soy, wheat, fish, or peanut).
Levels of IgE antibodies to A lumbricoides≥0.7 kUA/L (CAP class ≥2).
Detection of RSV antigen, rhinovirus RNA, adenovirus B, and/or coronavirus RNA in nasal washings.
High-level exposure to mites, cockroach, cat, and dog were defined as group 1 mite allergen levels ≥10 μg/g of dust; cockroach allergens Bla g 1 ≥8 U/g or Bla g 2 ≥0.32 μg/g of dust; cat allergen Fel d 1 ≥8 μg/g of dust; and dog allergen Can f 1 ≥10 μg/g of dust, respectively, at least in one site of the home.
Table III.
Multivariate analysis of risk factors for wheezing in children under 2 years of age
| Risk factor | Wheeze (%) n = 74 | Control (%) n = 30 | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Viral infection∗ | 45/74 (60.8) | 4/30 (13.3) | 15.5 (4.0 to 60.5) | .0001 |
| Family history of allergy† | 54/74 (72.9) | 14/30 (46.6) | 4.2 (1.4 to 12.4) | .008 |
| Sensitization to inhalant and/or food allergens∗ | 9/70 (12.8) | 3/29 (10.3) | 1.3 (0.2 to 6.5) | .7 |
| Sex, male | 46/74 (62.1) | 17/30 (56.6) | 0.9 (0.3 to 2.7) | .9 |
‡Sensitization defined as IgE antibody levels ≥0.7 kUA/L (CAP class ≥2) to at least one inhalant allergen (mites, cockroach, cat, dog) or food allergen (egg, milk, soy, wheat, fish, or peanut).
Detection of rhinovirus RNA, coronavirus RNA, adenovirus B, and/or RSV antigen in nasal washings.
History of asthma, rhinitis, and/or atopic dermatitis in parent(s) and/or siblings.
Among children 2 to 12 years old, allergy was the most important risk factor for wheezing, indicated by the higher frequency of positive tests for specific IgE to inhalant allergens and elevated total IgE levels (Table II). IgE to A lumbricoidesand personal history of allergy were both associated with wheezing (Table II). On the other hand, viral infection was not a significant risk factor for wheezing in this age group. Two models of multivariate analysis were generated by using levels of specific IgE antibodies ≥ 0.7 kUA/L or ≥ 3.5 kUA/L (considered as high level). In both models, sensitization to inhalant allergens was the major independent risk factor for wheezing (Table IV).
Table IV.
Multivariate analysis of risk factors for wheezing in children 2 to 12 years of age (model 1 and model 2)
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Risk factor | Wheeze (%) (n = 58) | Control (%) (n = 35) | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value |
| Sensitization to inhalant allergens (IgE ≥0.7 kUA/L)∗ | 42/58 (72.4) | 15/35 (42.8) | 2.7 (1.06 to 7.1) | .03 | — | — |
| Sensitization to inhalant allergens (IgE ≥3.5 kUA/L)∗ | 36/58 (62) | 7/35 (20) | — | — | 5.7 (1.9 to 16.7) | .001 |
| Viral infection† | 19/58 (32.7) | 9/35 (25.7) | 1.3 (0.4 to 3.6) | .5 | 1.1 (0.4 to 2.9) | .8 |
| Family history of allergy‡ | 38/58 (65.5) | 22/35 (62.8) | 1.1 (0.4 to 3.0) | .7 | 1.0 (0.4 to 2.9) | .8 |
| Specific IgE to Ascaris lumbricoides(IgE ≥0.7 kUA/L)§ | 19/56 (33.9) | 5/35 (14.2) | 2.1 (0.6 to 7.2) | .2 | 1.5 (0.4 to 5.4) | .5 |
| Sex, male | 34/58 (58.6) | 22/35 (62.8) | 1.1 (0.4 to 3.0) | .7 | 1.1 (0.4 to 2.9) | .8 |
Sensitization defined as IgE antibody levels ≥0.7 kUA/L (model 1) or IgE antibody levels ≥3.5 kUA/L (model 2) to at least 1 inhalant allergen (mites, cockroach, cat, dog).
Detection of rhinovirus RNA, coronavirus RNA, adenovirus B, and/or RSV antigen in nasal washings.
History of asthma, rhinitis, and/or atopic dermatitis in parent(s) and/or siblings.
Positive specific IgE defined as IgE antibody levels ≥0.7 kUA/L to Ascaris lumbricoides.
Current exposure to high levels of indoor allergens was not significantly associated with wheezing (Table I). Levels of group 1 mite allergens >10 μg/g of dust were found in approximately 70% of the homes of children older than 2 years and in 36% to 46% of the homes of children under 2 years, with no differences between wheezing and control groups. Approximately 25% of homes presented high levels of cockroach allergens in dust samples. In 13 of 172 (7.5%) homes, cats were present in the house. Levels of cat allergen were undetectable or very low in the majority of samples; however, Fel d 1 levels in homes with cats were higher than in those without a cat (GM = 1.06 μg/g and 0.19 μg/g, P < .0001). The presence of dogs was found in 87 of 172 (50.2%) homes visited, and Can f 1 levels were also higher in homes with dogs as compared with homes without dogs (GM = 6.4 μg/g and 0.5 μg/g, P < .0001). No synergistic effect was observed when the combination of sensitization and exposure to specific allergens was analyzed.
No association of passive smoke exposure and wheezing was found (Table II). Breast-feeding for at least 6 months was reported for 21.4% (12 of 56) of wheezing children older than 2 years of age as compared with 31.4% (11 of 35) of control children (P = .28).
Discussion
In the current study, we have identified for the first time risk factors associated with acute wheezing among children living in a subtropical area of South America. We have found that RSV infection was strongly associated with wheezing among children under 2 years of age.7, 12 The frequency of RSV was lower than that reported in temperate regions (39% versus 60% to 80%).7 However, if we consider the subgroup of infants 0 to 6 months old, 61% tested positive for RSV antigen. RSV infections were predominantly found in the months of February to May, corresponding to late summer and early to mid-fall, indicating that the virus occurs in a different seasonal pattern as compared with that of the northern hemisphere. The available data indicate that the main age group affected by RSV in developing countries is children under 6 months of age and that in tropical or subtropical climates RSV outbreaks are frequently associated with the rainy season.23 Household and day care–based prospective studies in coastal northeast Brazil have identified RSV at an unexpectedly low frequency, 0% to 4%.24, 25 A previous study in Ribeirão Preto, analyzing samples of 829 children under 5 years of age with nonspecific respiratory tract symptoms seen in the ED revealed that RSV was present in 21.6% of nasal washes. However, the vast majority of RSV infections (157/188 episodes, 83.5%) were found in children under 1 year of age.26
The lack of evidence for association of rhinovirus and wheezing in the current study is striking. It is possible that genetic factors may play a role in determining immunologic responses to rhinovirus in different populations. In addition, rhinovirus serotypes circulating in tropical areas may be different from those in temperate climates, particularly regarding their ability to replicate in the lower respiratory airways. Temperatures in the lower airways may be critical for the intensity of replication, at least for some rhinovirus serotypes.27 It has been demonstrated that inhalation of cold air results in substantial temperature decrease in the lower airways28 that may favor rhinovirus replication. In the northern hemisphere, rhinovirus peak prevalence occurs in early fall and late spring and has been associated with new school terms.29 In subtropical climates, it is possible that higher temperatures year-round may be less conducive to productive growth of rhinovirus. Reduced rhinovirus spread in these areas could, at least in part, be due to the styles of housing peculiar to warmer climates, with homes and schools being ventilated by keeping windows open.
The role of adenovirus in causing wheezing and persistent asthma in children is largely unknown. It has been suggested that latency of adenovirus in infected lung epithelial cells is associated with amplification of cigarette smoke–induced inflammation and may cause allergen-induced eosinophilic inflammation to become steroid resistant.30 Adenoviruses play an important role as a cause of lower respiratory infections among children in Argentina and Chile,31, 32 being secondary only to RSV.
Similar to previous studies,12, 33, 34, 35 sensitization to inhalant allergens was the major independent risk factor for wheezing among children 2 to 12 years of age, and home exposure to high levels of inhalant allergens was not associated with current wheezing. We have previously reported that mites and cockroach were major causes of sensitization among children with asthma and/or allergic rhinitis, seen at university-based allergy clinics in Ribeirão Preto and São Paulo, Brazil.36 In the current study, the frequency of allergic sensitization was very high, notably among control children (43% of the children). Part of the reason for finding a higher-than-expected frequency of sensitization among control subjects may be due to the fact that we have not aimed to exclude subjects with allergic rhinitis from the control group. It would be difficult to have a definitive diagnosis of allergic rhinitis among children with respiratory distress at the ED setting. It is possible that some subjects with allergic rhinitis may have been included as control subjects, particularly in the group of children older than 2 years of age.
The relation of asthma and allergy with parasitic infection is controversial. Our studies in children living in poverty in northeast Brazil have shown that current infection with A lumbricoideswas an independent risk factor for wheezing, in addition to positive skin tests to inhalant allergens.37 A study of 2164 children 8 to 18 years of age from China revealed that infection with A lumbricoides was associated with increased risk of asthma and with sensitization to common inhalant allergens.38 On the other hand, studies in rural Africa point to an inverse correlation between helminthic infections and atopy and asthma.39 Medeiros et al40 have recently shown that subjects from a Schistosoma mansoni endemic area in Brazil had lower frequency of skin test positivity to indoor allergens and a milder course of asthma than individuals with asthma not living in an area endemic for S mansoni. In the current study, we have found that IgE antibodies to A lumbricoideswere more frequently found in wheezing children than in control children; however, this association was no longer significant after multivariate analysis.
In conclusion, results of the current study have shed light on risk factors associated with acute wheezing in children living in a subtropical environment, an area where the prevalence of asthma is comparable with that in the United States and other developed countries.41 Wheezing in the prior 12 months, which has been shown to correlate better with diagnosis of asthma in Brazil,42 was reported in 22.5% and 16.7% of children 6 to 7 years old and 13 to 14 years old, respectively, evaluated by the ISAAC questionnaire.41 Some of the risk factors for acute wheezing identified in the current study, including RSV infections in the first 2 years of life, are similar to those previously reported in studies carried out in temperate developed countries. Inhalant allergens appear to be a major cause of asthma attacks in this environment, and rhinovirus does not appear to play a remarkable role in triggering asthma symptoms, as previously described in temperate regions.
Acknowledgements
The authors wish to thank Gustavo F. Pacca, MD, and the Pediatric residents of Santa Lydia hospital for help with enrolling of the patients; Heloisa Bettiol, MD, for assistance with statistical analysis; and Maria Lucia da Silva, BSc, for technical assistance.
Footnotes
Supported by FAPESP grants 95/9690-0 and 00/01875-0, CNPq-Instituto de Investigação em Imunologia (iii), and National Institutes of Health grant AI-20565. V.P.L.F., E.A., and L.K.A are recipients of CNPq scholarships. M.D.C. was supported in part by a research grant from Philip Morris Inc to Indoor Biotechnologies, Inc.
References
References
- 1.Martinez FD. Development of wheezing disorders and asthma in preschool children. Pediatrics. 2002;109:362–367. [PubMed] [Google Scholar]
- 2.Krawiec ME, Westcott JY, Chu HW, Balzar S, Trudeau JB, Schwartz LB. Persistent wheezing in very young children is associated lower respiratory inflammation. Am J Respir Crit Care Med. 2001;163:1338–1343. doi: 10.1164/ajrccm.163.6.2005116. [DOI] [PubMed] [Google Scholar]
- 3.Strunk RC. Defining asthma in the preschool-aged child. Pediatrics. 2002;109:357–361. [PubMed] [Google Scholar]
- 4.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson children's respiratory study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–675. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 5.Platts-Mills TAE, Rakes GP, Heymann PW. The relevance of allergen exposure to the development of asthma in childhood. J Allergy Clin Immunol. 2000;105:S503–S508. doi: 10.1016/S0091-6749(00)90051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahn U, von Mutius E. Childhood risk factors for atopy and the importance of early intervention. J Allergy Clin Immunol. 2001;107:567–574. doi: 10.1067/mai.2001.112943. [DOI] [PubMed] [Google Scholar]
- 7.Gern J, Busse WW. The role of viral infections in the natural history of asthma. J Allergy Clin Immunol. 2000;106:201–212. doi: 10.1067/mai.2000.108604. [DOI] [PubMed] [Google Scholar]
- 8.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 9.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 10.Alarcon A, Walsh EE, Carper HT, La Russa JB, Evans BA, Rakes GP. Detection of IgA and IgG but not IgE antibody to respiratory syncytial virus in nasal washes and sera from infants with wheezing. J Pediatr. 2001;138:311–317. doi: 10.1067/mpd.2001.111277. [DOI] [PubMed] [Google Scholar]
- 11.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 13.Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002;324:1–5. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zambrano JC, Carper HT, Rakes GP, Patrie J, Murphy DD, Platts-Mills TAE. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003;111:1008–1016. doi: 10.1067/mai.2003.1396. [DOI] [PubMed] [Google Scholar]
- 15.Corn JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 16.Pitkaranta A, Virolainen A, Jero J, Arruda E, Hayden FG. Detection of rhinovirus, respiratory syncytial virus, and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics. 1998;102:291–295. doi: 10.1542/peds.102.2.291. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler T, Cox NJ. Influenza viruses. In: Murray PR, Baron EJ, Pfaller MA, Tenover FG, Yolken RH, editors. Manual of Clinical Microbiology. 7th edition. American Society for Microbiology Press; Washington, DC: 1999. pp. 928–935. [Google Scholar]
- 18.Waner JL. Parainfluenza viruses. In: Murray PR, Baron EJ, Pfaller MA, Tenover FG, Yolken RH, editors. Manual of Clinical Microbiology. 7th edition. American Society for Microbiology Press; Washington, DC: 1999. pp. 936–941. [Google Scholar]
- 19.Kidd AH, Jonsson M, Garwicz D, Kajon AE, Wermenbol AG, Verweij MW, Jong JC. Rapid subgenus identification of human adenovirus isolates by a general PCR. J Clin Microbiol. 1996;34:622–627. doi: 10.1128/jcm.34.3.622-627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruuskanen O, Meurman O, Akusjarvi G. Adenoviruses. In: Richman DD, Whitley RJ, Hayden FG, editors. Clinical virology. 2nd edition. American Society for Microbiology Press; Washington, DC: 2002. pp. 515–535. [Google Scholar]
- 21.Arruda LK, Rizzo MC, Chapman MD, Fernandez-Caldas E, Baggio D, Platts-Mills TAE. Exposure and sensitization to dust mite allergens among asthmatic children in öo Paulo, Brazil. Clin Exp Allergy. 1991;21:433–439. doi: 10.1111/j.1365-2222.1991.tb01683.x. [DOI] [PubMed] [Google Scholar]
- 22.Platts-Mills TAE, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100:S1–24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- 23.Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Intern Health. 1998;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 24.de Arruda E, Hayden FG, McAuliffe JF, de Souza MA, Mota SB, McAuliffe MI. Acute respiratory viral infections in ambulatory children of urban northeast Brazil. J Infect Dis. 1991;164:252–258. doi: 10.1093/infdis/164.2.252. [DOI] [PubMed] [Google Scholar]
- 25.Souza LSF, Ramos EAG, Carvalho FM, Guedes VMCR, Souza LS, Rocha GM. Viral respiratory infections in young children attending day care in urban Northeast Brazil. Pediatr Pulmonol. 2003;35:184–191. doi: 10.1002/ppul.10194. [DOI] [PubMed] [Google Scholar]
- 26.Cintra OAL, Owa MA, Machado AA, Cervi MC, Figueiredo LTM, Rocha GM. Occurrence and severity of infections caused by subgroup A and B respiratory syncytial virus in children in Southeast Brazil. J Med Virol. 2001;65:408–412. doi: 10.1002/jmv.2049. [DOI] [PubMed] [Google Scholar]
- 27.Schroth MK, Grimm E, Frindt P, Galagan DM, Konno SI, Love R, Gern JE. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20:1220–1228. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]
- 28.McFadden ER, Jr, Pichurko BM, Bowman HF, Ingenito E, Burns S, Dowling N. Thermal mapping of the airways in humans. J Appl Physiol. 1985;58:564–570. doi: 10.1152/jappl.1985.58.2.564. [DOI] [PubMed] [Google Scholar]
- 29.Gwaltney JM., Jr . Rhinoviruses. In: Evans AS, Kaslow RA, editors. Viral Infections of Humans. Plenum Medical Book Company; New York/London: 1997. pp. 815–838. [Google Scholar]
- 30.Hogg JC. Role of latent viral infections in chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med. 2001;164:S71–S75. doi: 10.1164/ajrccm.164.supplement_2.2106063. [DOI] [PubMed] [Google Scholar]
- 31.Carballal G, Videla C, Misirlian A, Requeijo PV, Aguilar MC. Adeno-virus type 7 associated with severe and fatal acute lower respiratory infections in Argentine children. BMC Pediatr. 2002;2:6–12. doi: 10.1186/1471-2431-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larranaga C, Kajon A, Villagra E, Avendano LF. Adenovirus surveillance on children hospitalized for acute lower respiratory infection in Chile (1988-1996) J Med Virol. 2000;60:343–346. [PubMed] [Google Scholar]
- 33.Duff AL, Pomeranz ES, Gelber LE, Price GW, Farris H, Hayden FG. Risk factors for acute wheezing in infants and children: viruses, passive smoke and IgE antibodies to inhalants allergens. Pediatrics. 1993;92:535–540. [PubMed] [Google Scholar]
- 34.Gelber LE, Seltzer LH, Bouzoukis JK, Pollart SM, Chapman MD, Platts-Mills TAE. Sensitization and exposure to indoor allergens as risk factors for asthma among patients presenting to hospital. Am Rev Respir Dis. 1993;147:573–578. doi: 10.1164/ajrccm/147.3.573. [DOI] [PubMed] [Google Scholar]
- 35.Call RS, Smith TF, Morris E, Chapman MD, Platts-Mills TAE. Risk factors for asthma in inner city children. J Pediatr. 1992;121:862–866. doi: 10.1016/s0022-3476(05)80329-4. [DOI] [PubMed] [Google Scholar]
- 36.Santos ABR, Chapman MD, Aalberse RC, Vailes LD, Ferriani VPL, Oliver C. Cockroach allergens and asthma in Brazil: identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens. J Allergy Clin Immunol. 1999;104:329–337. doi: 10.1016/s0091-6749(99)70375-1. [DOI] [PubMed] [Google Scholar]
- 37.Sales VS, Rodrigues CE, Cavalcanti GB, Trombone APF, Lima RC, Santos ABR. Infection with. Ascaris lumbricoides in pre-school children: role in wheezing and IgE responses to inhalant allergens [Abstract] 2002;109:S27. [Google Scholar]
- 38.Palmer LJ, Celedon JC, Weiss ST, Wang B, Fang Z, Xu X. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Respir Crit Care Med. 2002;165:1489–1493. doi: 10.1164/rccm.2107020. [DOI] [PubMed] [Google Scholar]
- 39.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 40.Medeiros M, Jr, Figueiredo JP, Almeida MC, Matos MA, Araujo MI, Cruz AA. Schistosoma mansoni infection is associated with a reduced course of asthma. J Allergy Clin Immunol. 2003;111:947–951. doi: 10.1067/mai.2003.1381. [DOI] [PubMed] [Google Scholar]
- 41.Costa SRR, Ferriani VPL. Prevalence of asthma and related symptoms in children and adolescents from public and private schools: an ISAAC study [Abstract] J Allergy Clin Immunol. 2002;109:S55. [Google Scholar]
- 42.Camelo-Nunes IC, Wandalsen GF, Melo KC, Sole D, Naspitz CK. Non-specific bronchial hyperresponsiveness to methacholine (M) among probable asthmatic children identified by the International Study of Asthma and Allergies in Childhood (ISAAC) protocol [Abstract] J Allergy Clin Immunol. 2001;107:S230. [Google Scholar]


