Highlights
-
•
Current allergen immunotherapy approaches are limited by long treatment duration, cost and adverse reactions.
-
•
Immune adjuvants may improve efficacy, durability and cost of allergen immunotherapy.
-
•
Delta inulin (Advax™) is a well-tolerated and safe carbohydrate-based immune adjuvant.
-
•
Jack Jumper ant venom immunotherapy is compatible with Advax adjuvant.
-
•
Delta inulin enhances the immunogenicity of Jack Jumper ant venom immunotherapy.
Keywords: Adjuvants, Advax, Allergy, Delta inulin, Venom immunotherapy
Abstract
A major challenge in broader clinical application of Jack Jumper ant venom immunotherapy (JJA VIT) is the scarcity of ant venom which needs to be manually harvested from wild ants. Adjuvants are commonly used for antigen sparing in other vaccines, and thereby could potentially have major benefits to extend JJA supplies if they were to similarly enhance JJA VIT immunogenicity. The purpose of this study was to evaluate the physicochemical and microbiological stability and murine immunogenicity of low-dose JJA VIT formulated with a novel polysaccharide adjuvant referred to as delta inulin or Advax™. Jack Jumper ant venom (JJAV) protein stability was assessed by UPLC-UV, SDS-PAGE, SDS-PAGE immunoblot, and ELISA inhibition. Diffraction light scattering was used to assess particle size distribution of Advax; pH and benzyl alcohol quantification by UPLC-UV were used to assess the physicochemical stability of JJAV diluent, and endotoxin content and preservative efficacy test was used to investigate the microbiological properties of the adjuvanted VIT formulation. To assess the effect of adjuvant on JJA venom immunogenicity, mice were immunised four times with JJAV alone or formulated with Advax adjuvant. JJA VIT formulated with Advax was found to be physicochemically and microbiologically stable for at least 2 days when stored at 4 and 25 °C with a trend for an increase in allergenic potency observed beyond 2 days of storage. Low-dose JJAV formulated with Advax adjuvant induced significantly higher JJAV-specific IgG than a 5-fold higher dose of JJAV alone, consistent with a powerful allergen-sparing effect. The pharmaceutical data provides important guidance on the formulation, storage and use of JJA VIT formulated with Advax adjuvant, with the murine immunogenicity studies providing a strong rationale for a planned clinical trial to test the ability of Advax adjuvant to achieve 4-fold JJAV dose sparing in JJA-allergic human patients.
1. Introduction
Rapid onset, systemic allergic reactions to Jack Jumper ant (Myrmecia pilosula, JJA) sting represent a significant public health problem in the south-eastern and south-western part of Australia, affecting up to 3% of the population [1]. Immunotherapy for the prevention of stinging insect anaphylaxis involves the administration of increasing doses of purified insect venom to induce clinical tolerance, as reflected by a progressive reduction in venom-specific IgE and increase in IgG4 [2]. JJA venom immunotherapy (VIT) involving a large maintenance dose of 100 μg of venom has been shown to be highly effective at preventing JJA sting anaphylaxis, reducing the risk of severe systemic sting reactions by 95% [3]. Real-world experience shows efficacy comparable with yellow jacket and better than honeybee VIT [4]. The applicability of JJA VIT to the wider population, however, is limited by highly restricted JJA venom (JJAV) extract availability, production cost, and high incidence of adverse events [[3], [4], [5]].
An adjuvant that could facilitate a desirable immune response to JJAV and allow allergen dose reduction would have the potential to minimise adverse effects and reduce cost by reducing venom requirements [6]. A potential candidate adjuvant for VIT is the delta inulin-based adjuvant, Advax™. Advax is based on the plant derived fructan inulin (β-d-[2→1] poly(fructo-furanosyl) α-d-glucose) [7]. Inulin has no immunological activity when in soluble form, but once formulated into delta inulin microparticles of 1–5 μm size, it has potent immune-adjuvant activity [8]. Advax has been shown in preclinical studies to enhance immunogenicity of a broad range of vaccines including influenza, hepatitis B virus (HBV), Japanese encephalitis, SARS coronavirus, HIV, listeria, RSV, and anthrax [9,10]. The safety and efficacy of Advax have been shown in adult humans when formulated with HBV and influenza vaccines [9,11,12]. When combined with honeybee VIT and administered to individuals with honeybee-sting anaphylaxis, Advax was shown to be safe and well tolerated [13], and enhanced the immunogenicity of the honeybee venom with strongly enhanced venom-specific IgG4 responses [14], a potential marker of successful immunotherapy [15].
Understanding the stability of JJA VIT and its aggregation potential with Advax microparticles in a combined formulation is essential as aggregation or decomposition of allergen components in JJAV may affect their allergenic potency [16], which has been found to increase with storage in a previous study [17]. The objective of the current study was therefore to explore the stability and aggregation potential of JJA VIT and Advax in a combined formulation as a prelude to a clinical trial of low-dose JJA VIT with Advax. Although there are currently no animal models of JJAV allergy, it was further necessary to demonstrate using a murine immunogenicity model that co-administration of JJA VIT with Advax was safe and well tolerated and that the inclusion of the Advax adjuvant was able to enhance the immunogenicity of low-dose JJAV.
2. Materials and methods
2.1. Materials
Chemicals, reagents and consumables used in the experiments were as previously described [16]. Chemicals used in the formulation of JJA VIT products were of pharmacopoeial grade; sucrose and polysorbate 80 from Avantor (Centre Valley, PA, USA), benzyl alcohol from PCCA (Houston, TX, USA), sodium chloride 20% solution from AstraZeneca (North Ryde, NSW, Australia), sodium dihydrogen phosphate solution from Phebra (Lane Cove West, NSW, Australia), disodium hydrogen phosphate from SAFC (St. Louis, MO, USA), 0.9% sodium chloride from Pfizer (West Ryde, NSW, Australia), Water for Injection from Pfizer and Baxter (Old Toongabbie, NSW, Australia). Microbiological media and ATCC® strains microorganisms were from Oxoid (Basingstoke, Hampshire, UK). Pooled positive sera (PPS) with high JJAV-specific positive IgE levels was obtained from JJAV allergic individuals with clinically proven history of allergy, as previously described [16].
2.2. Jack Jumper ant venom immunotherapy and Advax adjuvant
JJAV Active Pharmaceutical Ingredient (API) was prepared as previously described [16] and formulated in 22% sucrose solution to produce clinical grade JJA VIT containing 1.1 mg/mL venom proteins. JJAV diluent solution containing 0.9% sodium chloride, phosphate buffer (pH 6.0; 10 mM), 0.05% polysorbate 80 and 0.9% benzyl alcohol was manufactured at the Pharmacy Department, Royal Hobart Hospital as previously described [17]. Advax adjuvant in buffered saline (pH 7.5–8.5) was supplied by Vaxine Pty Ltd (Bedford Park, Adelaide, Australia). The Advax adjuvant was prepared according to current Good Manufacturing Practice [8] and supplied as a sterile suspension containing 50 mg/mL delta inulin microparticles.
2.3. Preparation of JJA VIT/Advax formulations and stability trial conditions
For Advax-free samples, JJAV was diluted to 25 μg/mL using JJAV diluent. Where Advax was included in the formulation, JJA VIT and Advax were first diluted using JJAV diluent to 50 μg/mL and 20 mg/mL, respectively. When the two diluted components were mixed in equal quantities this gave final concentrations of 25 μg/mL and 10 mg/mL, respectively, the planned doses to be used in the future human trial. For allergen potency studies, JJAV was further diluted using JJAV diluent and Advax to obtain final concentrations of 10, 1 and 0.1 μg/mL of venom and 5 mg/mL of Advax. Samples were prepared using aseptic techniques and packaged in U-100 insulin syringes (Terumo, Elkton, Maryland, USA), protected from light and stored at 4 °C and 25 °C for up to 28 days.
2.4. Antigen extraction, efficiency of antigen extraction and control of samples pre-analysis
Baseline samples were processed within 15 min post preparation. All stability samples were collected and processed after 2, 6, 24 and 48 h, and the 25 μg/mL samples were also collected at 3, 7, 14, 21, and 28 days post storage. Except for the particle size analysis, where samples were analysed without antigen extraction, JJAV was extracted from the stability samples by centrifugation. Samples were transferred to Protein LoBind microtubes (Eppendorf AG, Hamburg, Germany) and centrifuged for 5 min at 5000 rpm (2348× g) and 5 °C (Eppendorf 5424R Microcentrifuge) to pellet the Advax particles. Supernatant was transferred to fresh microtubes and stored at −80 °C until analysis.
2.5. Analysis of JJAV allergen stability
2.5.1. ELISA inhibition
Allergenic potency in 25, 10, 1, and 0.1 μg/mL samples was analysed using JJAV IgE-specific ELISA inhibition assay, as previously described [16]. Briefly, 96-well Amino Immobilizer plates (Nunc, Roskilde, Denmark) were coated overnight at 4 °C with 100 μL of 10 μg/mL JJAV in sodium carbonate (0.05 M, pH 9.6). Inhibition mixtures were prepared by adding between 0.15 and 4 ng of JJAV to 25 μL PPS and diluting to 100 μL with phosphate buffered saline (0.01 M, pH = 7.2) containing 0.05% polysorbate 20 (PBS-T) and 0.5% bovine serum albumin (BSA). JJAV in the inhibition mixtures was either baseline or stability samples as described in Section 2.4. A positive control was prepared by diluting PPS 1:4 (v/v) in PBS-T with 0.5% BSA and negative control consisted of PBS-T with 0.5% BSA and incubated overnight at 4 °C. Subsequently, venom coating solution was discarded and the plate was washed three times with PBS-T. The venom-coated plate was incubated with 100 μL of inhibition mixtures, positive control and negative control for 60 min at room temperature (RT). The plate was washed three times with PBS-T, and 100 μL of biotinylated mouse monoclonal anti-human IgE antibody (1:2000 (v/v) dilution in PBS-T, clone HP6029, Life Technologies, Carlsbad, CA, USA) was added to each well and incubated in the dark for 60 min at RT. The plate was washed three times with PBS-T, and then 100 μL of streptavidin–horseradish peroxidase (1:1000 (v/v) dilution in PBS-T, BD Pharmingen, San Diego, CA, USA) was added to each well and the plate was incubated in the dark for 20 min at RT. The plate was washed three times with PBS-T, and then 100 μL of 1-Step Ultra TMB-ELISA (Thermo Fisher Scientific, Rockford, IL, USA) was added to each well and the plate was incubated in the dark for 30 min at RT. Colour development was stopped by adding 100 μL of 2 M sulphuric acid and absorbance was read at 450 nm utilizing an ELISA plate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). Samples were run in triplicates, and the mean and standard deviation calculated. The percentage inhibition for each sample was calculated using the following equation:
Allergenic potency of JJAV was measured as the amount of venom in solution that was required to inhibit 50% of IgE binding to the JJAV coated onto the plate. The stability of venom samples were calculated as a percentage relative to baseline samples.
2.5.2. SDS-PAGE and SDS-PAGE resolved JJAV immunoblot
The 25 μg/mL samples were concentrated to 0.5 mg/mL using a centrifugal filter device (Amicon Ultra-0.5 with 3 kDa nominal molecular weight limit, Merck Millipore, Darmstadt, Germany), after which they were mixed with SDS sample buffer and analysed by SDS-PAGE or SDS-PAGE immunoblot assay as previously described [16]. PPS was used in the SDS-PAGE immunoblot assay.
2.5.3. Ultra performance liquid chromatography
To assess Myr p allergen peptide changes, the 25 μg/mL venom samples were analysed by UPLC-UV using an Acquity H-series UPLC (Waters Corporation, Milford, Massachusetts, USA) coupled to an Acquity Photo Diode Array (PDA) detector. An Acquity UPLC BEH C18 column (2.1 x 100 mm x 1.7 μm particles) held at 40 °C was used. The mobile phase flow rate was 0.35 mL/min and the solvent system was water:acetonitrile:1% trifluoroacetic acid in water (80:12:8, v/v/v) to (24:68:8, v/v/v) in a linear gradient over 12 min before re-equilibration for 3 min to initial conditions. Triplicate injections of 40 μL were made and data for quantitative measurements of peak areas corresponding to the Myr p 1–3 allergen peptides were extracted at 220 nm. Under these conditions Myr p 2 eluted at 7.22 min, Myr p 3 at 7.52 min, and Myr p 1 at 8.39 min (Fig S1). Data were analysed using Waters MassLynx and TargetLynx software. The mean peak area for each allergen peptide was calculated and the analysis of remaining concentration of the allergen peptides was calculated as a percentage relative to baseline samples.
2.6. Analysis of JJAV diluent stability
2.6.1. Measurement of pH
Sample(s) pH was measured using a calibrated pH meter (Hanna Instrument, Woonsocket, RI, USA).
2.6.2. Benzyl alcohol determination
Benzyl alcohol concentration was determined by UPLC-UV using the instrument and column described above. The mobile phase flow rate was 0.35 mL/min and the solvent system was an isocratic mixture of 1% acetic acid in water:acetonitrile (70:30, v/v). Quadruple injections of 20 μL were made and data for quantitative measurements were extracted at 270 nm to maintain on scale signals. Benzyl alcohol eluted at 1.3 min and the peak area was determined using Waters TargetLynx software. Where benzyl alcohol-free Advax was added, the concentration of benzyl alcohol in the JJAV diluent was reduced by 19%, consistent with dilutional effect.
2.7. Analysis of advax stability
Particle size of Advax (10 mg/mL) in the presence of JJAV (25 μg/mL) in diluent solution was determined by dynamic light scattering (DLS) using a Zetasizer Nano S (Malvern Instruments Ltd., Worcestershire, UK). Samples were mixed by vortexing to form a uniform suspension and transferred to UVette plastic cuvettes (Eppendorf AG). Sample compartment temperature was adjusted to 25 °C before measurements were taken. Measurements were performed in triplicate and the average particle size (Z-Average size) and particle size distribution (via Polydispersity Index; PdI) were obtained [18].
2.8. Microbiological analysis
2.8.1. Endotoxin content
Samples were analysed for endotoxin contents using Limulus Amebocyte Lysate QCL-1000 chromogenic assay (Lonza, Walkersville, MD, USA) as previously described [16].
2.8.2. Antimicrobial efficacy test
The antimicrobial efficacy of benzyl alcohol in JJAV diluent was evaluated using the United States Pharmacopoeia Antimicrobial Effectiveness Test (AET) [19]. The following challenge organisms were used: Candida albicans (ATCC 10231), Escherichia coli (ATCC 25922), and Staphylococcus aureus (ATCC 25923). Bacterial stock cultures were grown for 20–24 hours in Tryptone Soya Broth and yeast stock culture was grown for 44–48 hours in Sabaroud Dextrose Broth. Microorganisms were harvested by centrifugation, washed, resuspended, and diluted in 0.9% normal saline to obtain a microbial count of approximately 1 × 108 colony forming units per mL (CFU/mL). Microbial suspensions were used within 30 min of harvest. For each challenge organism, AET was conducted in five replicates. Test samples consisting of 400 μL of JJAV diluent containing either JJAV (25 μg/mL) alone or with Advax (10 mg/mL), were inoculated with the microbial suspension at 1.0% (v/v) to yield a final concentration between 1 × 105 and 1 × 106 CFU/mL and incubated at 23 °C for up to 28 days. Aliquots (100 μL) were taken from each sample at days 7, 14 and 28, and the number of CFUs in each sample was determined using plate-count method. Positive control samples (400 μL of 0.9% normal saline) were inoculated with the microbial suspension at 1.0% (v/v) to yield a final concentration between 1 × 105 and 1 × 106 CFU/mL. A 100 μL aliquot was taken from each control sample at baseline and the number of CFUs was determined using plate-count method. Negative control samples were growth media (from the same batches) devoid of any manipulation.
2.9. Murine immunogenicity studies
To assess the potential of Advax to provide JJAV antigen-sparing, female BALB/c mice, 6–8 week old were immunized 4 times intramuscularly at 2-week intervals with JJAV 2 μg (Royal Hobart Hospital, Tasmania, Australia) alone or formulated with Advax 1 mg (Vaxine Pty Ltd, Adelaide, Australia) versus JJAV 10 μg alone in 50 μL total injection volume. Blood samples were collected 2 weeks after the last immunization for measurement of JJAV-specific IgG responses by ELISA.
2.9.1. JJAV-specific antibody ELISA
Mouse JJAV-specific IgG, IgG1 and IgG2a antibodies were determined by ELISA, as previously described, with minor modifications [20]. Briefly, JJAV (1 μg/mL) in coating buffer (pH 5.0; 50 mM MES + 25 mM HEPES in PBS) was absorbed to 96 well plates, blocked with 0.2% Casein, then 100 μL of 1:200 dilution of sera was incubated for 2 h at RT, followed by washing then incubation with biotinylated anti-mouse IgG, IgG1 and IgG2a antibodies (Abcam, Cambridge, MA, USA) mixed with HRP-conjugated Streptavidin (BD Biosciences, Franklin Lakes, NJ, USA) for 1 h. Washed wells were then incubated with TMB substrate (KPL, Gaithersburg, MD, USA) for 10 min before the reaction was stopped with 1 M phosphoric acid and optical density measured at 450 nm (OD450 nm) using a VersaMax plate reader and analysed using SoftMax Pro software (Molecular Devices, Sunnyvale, CA, USA). Average OD450 nm values obtained from negative control wells were subtracted.
2.10. Statistical and data analysis
Statistical analysis was performed using GraphPad Prism 5.03 and CurveExpert Basic 1.4. Statistical analyses, including descriptive statistics, t-test and ANOVA with Bonferroni’s post-hoc test were used as appropriate, and p < 0.05 was considered significant. Image analysis was performed using ImageJ 1.46 r (Wayne Rasband, National Institutes of Health). For the noninferiority analysis, we performed an independent t-test on the log transformed IgG data, and assessed whether the upper limit of the 95% confidence interval for the difference between treatments (low dose adjuvanted JJAV treatment minus standard high dose JJAV alone treatment) was below a predefined margin of non-inferiority of 0.5.
2.11. Stability criteria
Table I describes the acceptance criteria for the physicochemical and microbiological stability of JJA VIT components and Advax adjuvant.
3. Results
3.1. The effect of Advax on the allergenic potency of JJAV
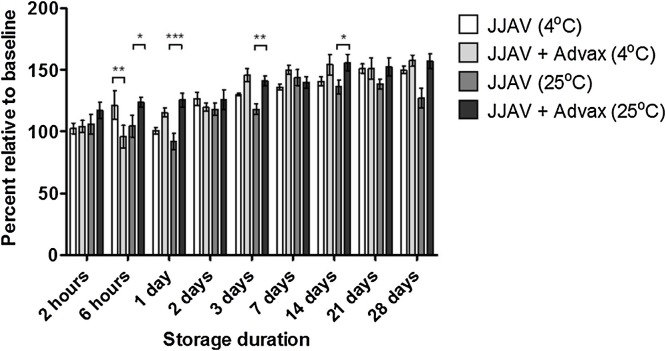
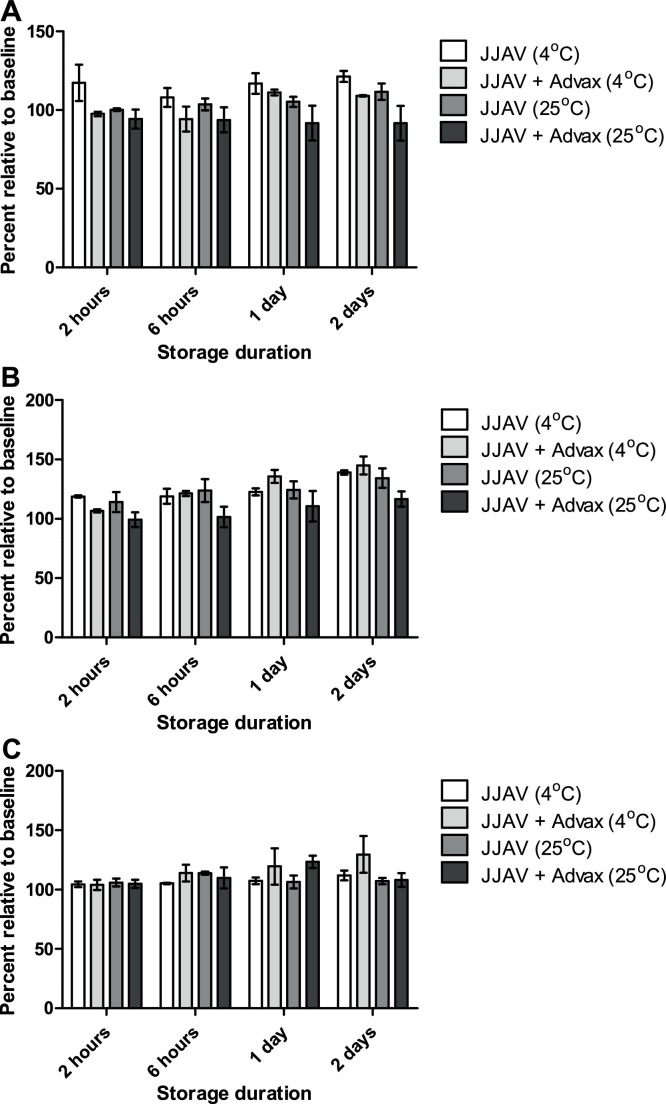
The allergenic potency of JJAV at baseline was not significantly affected by the presence of Advax in the formulation (p = 0.06‒0.99; Supplementary Table 1 ). The overall allergenic potency of JJAV with and without Advax was acceptable for at least 2 days when stored at 4 and 25 °C, with relative allergenic potencies between 102.4–139.0% and 97.5–144.9% for all stability samples stored at 4 °C with and without Advax respectively, and between 100.2–134.3% and 94.3–126.0% for all stability samples stored at 25 °C with and without Advax respectively (Fig. 1, Fig. 2 ). A trend for a progressive increase in allergenic potency was observed in the 25 μg/mL JJAV stability samples, which continued to increase beyond the acceptable limits of 50–150% after 14 days of storage at either 4 or 25 °C, particularly for the stability samples with Advax (Fig. 1).
Table 1.
Acceptance criteria for physicochemical and microbiological stability of JJA VIT, JJAV diluent and Advax adjuvant.
| Characteristic | Assay | Predetermined criteria |
|---|---|---|
| JJAV allergens stability | ||
| Allergenic potency | ELISA inhibition | 50 - 150% relative to baseline |
| Individual allergen concentration | UPLC-UV | 75 - 150% relative to baseline |
| Protein profile | SDS-PAGE | Identical to baseline |
| Allergen profile | SDS-PAGE immunoblot | Identical to baseline |
| JJAV diluent stability | ||
| Potential of hydrogen | pH | 5.9 - 6.3 |
| Benzyl alcohol concentration | UPLC-UV | > 90% relative to baseline |
| Advax adjuvant stability | ||
| Particle diameter | Dynamic Light Scattering | 1 - 7 μm |
| Microbiological purity | ||
| Endotoxin content | Limulus Amebocyte Lysate QCL-1000 |
< 50 EU/ml |
| Preservative efficacy | Antimicrobial Efficacy Test | 1 × 105 - 1 × 106 CFU/plate on baseline; ≥ 1 log reduction relative to baseline on day 7; ≥ 3 log reduction relative to baseline on day 14; no increase on day 28 relative to day 14 count |
Fig. 1.
Effects of Advax (10 mg/mL) and storage temperature (4 °C or 25 °C) on the allergenic potency of JJAV (25 μg/mL) stored for up to 28 days. Analysis of allergenic potency was calculated as a percentage relative to baseline samples. Each sample was analysed in triplicate and presented as mean and SD. Asterisks designate significant differences (* p < 0.05, ** p < 0.01, *** p < 0.001).
Fig. 2.
Effects of Advax (5 mg/mL) and storage temperature (4 °C or 25 °C) on the allergenic potency of JJAV at (A) 0.1 μg/mL, (B) 1 μg/mL, and (C) 10 μg/mL stored for up to 2 days. Analysis was calculated as a percentage relative to baseline samples. Each sample was analysed in triplicate and presented as mean and SD.
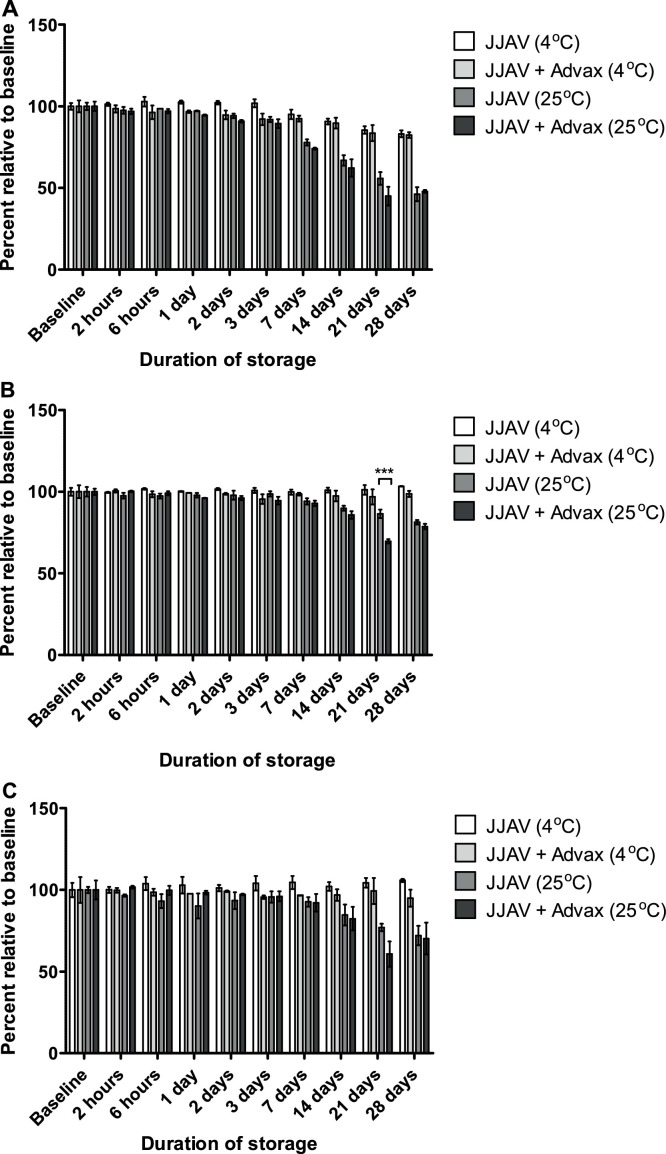
3.2. The effects of Advax on the stability of JJAV allergens
Myr p 1 was the least stable of the 3 allergens that were quantified via UPLC-UV, and as such, stability was limited by the stability of Myr p 1 (Fig S1). Therefore, in the presence of Advax, the JJAV was stable for up to 7 and 3 days when stored at 4 °C and 25 °C, respectively (Fig. 3 ). No significant differences were found between ‘with’ and ‘without’ Advax for either baseline samples (Supplementary Table 2) or for all but one of the stability pairs (Fig. 3). SDS-PAGE results showed that a protein band at approximately 23 kDa disappeared slowly throughout the storage period and appeared to be replaced with new bands at approximately 17, 19 and 21 kDa (Fig S2). The changes occurred regardless of sample treatments and were more obvious with prolonged storage. A similar pattern was also observed on the SDS-PAGE immunoblot results, whereby an IgE-binding band at 23 kDa slowly disappeared and was replaced with a new IgE-binding band at 19 kDa (Fig S3). The changes occurred regardless of Advax, and were most obvious in the stability samples stored at 25 °C for 24 h.
Fig. 3.
Effects of Advax (10 mg/mL) and storage temperature (4 °C or 25 °C) on JJAV allergen peptides (A) Myr p 1, (B) Myr p 2, and (C) Myr p 3 in formulations of JJAV (25 μg/mL) stored for up to 28 days. Analysis of remaining concentration of Myr p allergen peptides was calculated as a percentage relative to baseline samples. Each sample was analysed in triplicate and presented as mean and SD. Asterisks designate significant differences (*** p < 0.001).
3.3. The effects of Advax on JJAV diluent stability
The addition of Advax did not cause a substantial change in the pH of JJAV diluent (Fig S4), and storage temperature had a minimal effect on the stability of benzyl alcohol for the period studied (Fig S5).
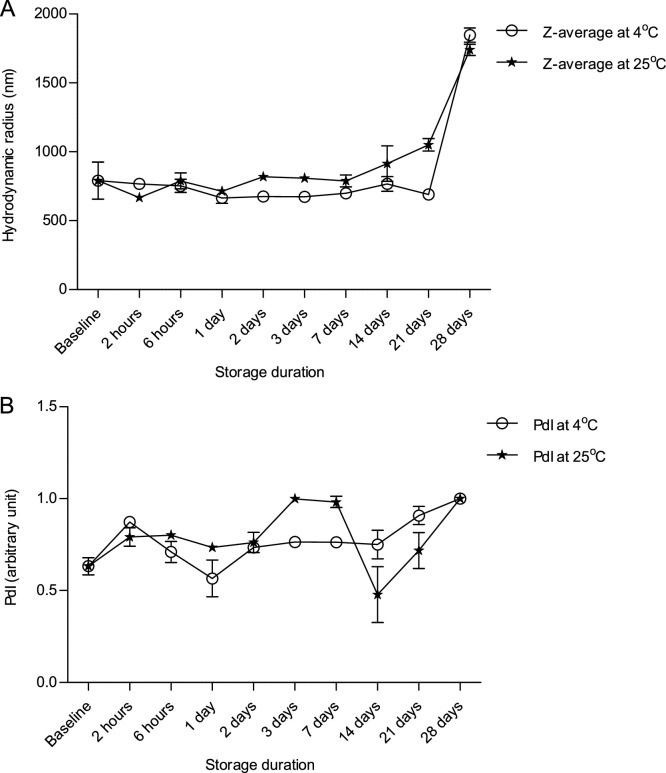
3.4. Size distribution of advax microparticles formulated with JJA VIT
Advax delta inulin microparticles had a mean hydrodynamic diameter of 1.6 μm at baseline (Fig. 4 A), and this did not change after storage at 4 and 25 °C for up to 21 and 7 days respectively, but a two-fold increase in diameter was observed at the 28 day study endpoint at both 4 and 25 °C. The polydispersity index (PdI) of Advax was approximately 0.6 at baseline and did not change substantially between day 1 and 21 of the study period, although there were noticeable fluctuations over time, particularly in the sample stored at 25 °C, and the PdI increased to 1.0 at day 28 of study period at both 4 and 25 °C (Fig. 4B).
Fig. 4.
Particle size distribution of Advax (10 mg/mL) as a function of (A) Z-average and (B) polydispersity index (PdI) when combined with JJAV (25 μg/mL) and stored for up to 28 days at either 4 °C or 25 °C. Analysis was performed using diffraction light scattering technique. Each sample was analysed in triplicate and presented as mean and SD.
3.5. Antimicrobial activity of JJA VIT formulated with Advax
The batches of JJA VIT, JJAV diluents, and Advax were virtually free of endotoxins (Supplementary Table 3), and sterility testing of JJA VIT and JJAV diluents further supported the aseptic nature of the combined formulation (data not shown). All samples including JJAV diluent containing only JJAV and samples where Advax was added met the Pharmacopoeial requirements for antimicrobial activity at each sampling time point (Supplementary Table 4).
3.6. Effect of Advax adjuvant on immunogenicity and tolerability of JJA VIT
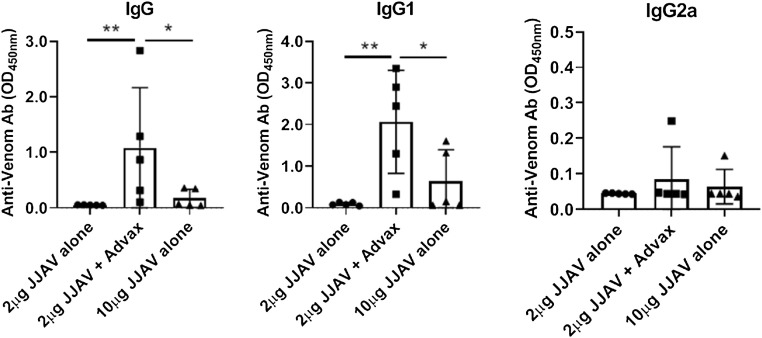
A murine immunogenicity model was used to test the hypothesis that Advax adjuvant could be used for JJAV dose sparing and to confirm that it had no negative effects on safety and tolerability. Female BALB/c mice, 6–8 week of age (n = 5/group), were immunized 4 times intramuscularly at 2-week intervals with JJAV 2 μg alone or combined with 1 mg Advax adjuvant versus JJAV 10 μg alone, with the aim to assess the non-inferiority of the JJAV specific IgG responses in the JJAV 2 μg + Advax low dose group when compared to the JJAV 10 μg high dose group. Blood samples were collected 2 weeks after the last immunization for measurement of JJAV-specific total IgG and IgG subtypes by ELISA. While only low or undetectable levels of JJAV-specific IgG were seen in animals immunised with 10 μg of JJAV alone, all animals in the low dose JJAV (2 μg) + Advax arm had detectable JJAV-specific IgG. A formal non-inferiority analysis performed using pre-specified limits, confirmed the low dose JJAV + Advax arm was statistically non-inferior to the high dose JJAV alone group (p = 0.976). A subsequent test of superiority confirmed that the JJAV-specific IgG responses in the low dose JJAV + Advax arm were superior (p = 0.048) to those in the high dose alone arm (Fig. 5 ). Hence, Advax was confirmed to provide at least 5-fold JJAV dose-sparing. Analysis of IgG isotypes confirmed that the predominant effect of Advax adjuvant was predominantly through significant enhancement of specific IgG1 rather than IgG2a. No JJAV-specific IgE was detected in the sera of the immunized mice and no local (swelling, redness, hair loss) or systemic (weight loss, fever, inactivity, loss of grooming) adverse effects were observed.
Fig. 5.
Antigen-sparing effect of Advax adjuvant on JJAV-specific IgG responses. Female BALB/c mice, 6–8 week old (n = 5/group) were immunized 4 times i.m. at 2-week intervals with JJAV 2 μg alone or with Adva x 1 mg compared to a 5-fold higher dose of JJAV 10 μg alone, all in 50 μL total volume. Blood samples were collected 2 weeks after the last immunization and JJAV-specific IgG, IgG1 and IgG2a measured by ELISA (* p < 0.05, ** p < 0.01).
4. Discussion
Preclinical studies of novel adjuvanted allergen-specific immunotherapy (AIT) formulations are generally required by regulatory authorities prior to initiation of clinical trials. Such studies should demonstrate physicochemical and microbiological compatibility of the adjuvant with the antigenic components contained within the vaccines [21], together with immunogenicity data to justify the inclusion of the adjuvant in the vaccine. Although many human trials have been conducted on Advax adjuvant in combination with various infectious disease vaccines and one study of Advax with honeybee VIT [8,13,14], no information was previously available on the formulation, stability or immunogenicity of JJAV antigens in the presence of Advax.
In the current study, we performed stability study of low-dose JJA VIT with and without Advax adjuvant, pre-packaged in plastic syringes thereby confirming that the JJA VIT and Advax components were compatible with each other, and minimal aggregation of delta inulin microparticles was observed in the period studied. Based on the data obtained, in particular the allergenic potency studies, low-dose JJA VIT formulated with Advax is both physicochemically and microbiologically stable for at least 2 days when stored at 4 °C and 25 °C in plastic syringes. This will allow low-dose JJA VIT, formulated with or without Advax, to be prepared at a central pharmacy location in advance of clinical trial use and then transported to the site of patient administration, removing the need to prepare the vaccine at the patient’s bedside and may reduce the likelihood of errors in dosing occurring [22].
The JJAV stability results obtained in the current study are consistent with a previous report [17], although the conditions employed were somewhat different. In particular, the current study used U-100 plastic insulin syringes to store the samples and a lower concentration of JJAV was used than in the previous study. Measurement of total allergenic activity as determined by ELISA inhibition assay is required by regulatory bodies for the standardization and batch control of AIT products [23,24]. The slight increase in JJAV allergenic activity with storage is entirely consistent with previous findings and this has been proposed to be due to conformational changes in the JJAV proteins in the presence of polysorbate 80 in the JJAV diluent [17]. Despite ELISA inhibition data indicating that the vast majority of allergenic activity was preserved, it does not exclude the possibility that one or more “minor” allergens might have degraded. This perhaps is better monitored using the immunoblot assay where we observed the shift down in the 22–23 kDa bands, recently found to be Phospholipase A2 enzyme [1], to a band at approximately 19 kDa (Fig S2). This pattern of allergen degradation is consistent with prior findings and it was not affected by the inclusion of Advax in the formulation. Wanandy et al. recently reported the adsorption of Myr p 1 and Myr p 3 allergens when JJAV API grade products were allowed to contact rubber stoppers for more than 24 h [16]. Even though the low-dose JJAV in the current study was stored in plastic syringes with rubber plungers, negligible adsorption was identified. This difference might be due to the inclusion of polysorbate 80 as a surface active agent in the current samples, or different adsorption capacity and surface area of the rubbers.
JJAV solutions above 25 μg/mL that are used for immunotherapy are intended for multi-dose use. The addition of benzyl alcohol as an antimicrobial preservative in the JJAV diluent is essential to reduce the risk of bacterial contamination [25,26]. The insignificant change in the pH of the JJAV diluent in the presence of Advax was important as the solubility and optimal antimicrobial activity of benzyl alcohol is considerably reduced above pH 7 [17,27]. When used as an antimicrobial preservative, benzyl alcohol is commonly added to parenteral preparations at a concentration between 0.75–5% [27,28]. A 19% reduction of benzyl alcohol concentration in JJAV diluent due to dilutional effects from the addition of Advax reduced the concentration of benzyl alcohol to below this usual range and could therefore have affected its effectiveness as a preservative. However, the AET experiments confirmed that the antimicrobial efficacy of the combined formulation against challenged organisms was maintained throughout the study period. It is possible that this was due, at least in part, to the inherent antimicrobial activities of JJAV [29], which provided additive or synergistic effects to the preservative activity of benzyl alcohol. If this was the case, since low concentrations of venom proteins are used during the induction phase of a VIT, a greater reliance on the antimicrobial efficacy of benzyl alcohol is required during this phase.
A PdI of < 0.7 suggests a monodisperse preparation [18], and the results from these studies showed that Advax had a relatively monodisperse particle size distribution at the start of the study as indicated by the PdI of 0.6. The increase in average particle size and PdI observed at the end of study period may be caused by formation of delta inulin aggregates. However, the absence of multimodal peaks in all samples tested throughout the study period suggests that aggregation, if present, is limited under the study conditions employed and importantly the size of these particles was within the accepted range for stability (Table 1). Advax has been reported to have minimal protein adsorptive capacity [30], and similarly we found no evidence that Advax adsorbs JJAV allergens as there was minimal difference in the SDS-PAGE and UPLC-UV analysis of venom components between samples formulated with and without Advax.
Notably, the murine immunogenicity data supported the hypothesis that Advax adjuvant can provide significant antigen-sparing for JJA VIT, inducing significantly higher serum JJAV-specific IgG levels in the mice that received the adjuvanted venom formulation than a five times higher dose of JJAV alone. Whilst there is currently no animal model of JJAV allergy, and hence the effects of Advax on inhibition of JJAV IgE during VIT or its ability to induce blocking IgG4 antibodies is unable to be assessed outside of human studies, the ability of Advax to enhance venom-specific IgG responses when compared to immunisation with venom alone has served as a useful predictive marker of favourable human responses in previous human studies of Advax-adjuvanted honeybee VIT, where inclusion of Advax led to an earlier and higher rise of venom-specific total IgG and IgG4 with potential blocking activity [13,14]. Hence the current results support the rationale for a planned human trial of Advax combined with a reduced dose of JJAV (25 μg in maintenance phase) as compared to the currently clinically proven 100 μg maintenance JJAV dose. Notably, no safety or reactogenicity issues were identified with the Advax-adjuvanted low dose JJAV formulation in the current study.
5. Conclusion
Advax adjuvant provided at least 5-fold JJAV antigen sparing in murine immunogenicity studies, with no observed issues of reactogenicity or safety. No detrimental effects of Advax on JJAV stability were found, supporting use of this combined formulation as a JJAV-sparing strategy in planned human trials. Plans are in place to commence a human clinical trial to assess the ability of Advax to reduce the required maintenance dose of JJAV from 100 to 25 μg per dose, which if successful would result in a 4-fold higher number of subjects able to be treated with JJA VIT with current supplies, while also significantly reducing the potential cost of therapy, which largely reflects JJAV costs.
Role of funding source
TW was supported through an Australian Government Research Training Program Scholarship and ELITE PhD Research Scholarship from the University of Tasmania to conduct part of this study. This work was funded by the Tasmanian Health Service. Development of Advax adjuvant was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and US Department of Health and Human Services, under Contracts No. HHSN272200800039C and U01AI061142. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Contributors
RJH, SGAB, NP and MDW conceived the study. TW, MDW and NP designed the study methodologies. TW, NWD, HER and YH-O performed the experiments. TW, NWD, NP, RW and MDW analysed the data. TW prepared the manuscript. All authors participated in its revisions and have approved the final form.
Conflict of interest
NP and YH-O are associates of Vaxine Pty Ltd which owns the Advax™ adjuvant platform. TW, HER, SGAB, and MDW are either employee or consultants to the Tasmanian Health Service, the organization owning the intellectual property over JJA VIT. All other authors declare no conflict of interest.
Acknowledgements
We are grateful to Sandra Ahokas, Jenny Gudden, Judith Hawker, and Rhys Gunner for their assistance in collecting the ants; Judith Hawker and Hayley Webb for their assistance in performing venom sac dissection; Judith Hawker, Amanda Bannister, Roseanne Ford, Elizabeth Jordan, and Anne Kaye of the Pharmacy Department, Royal Hobart Hospital for their technical assistance in the manufacture of JJA Venom Immunotherapy products; David Jones of the Microbiology Department, Royal Hobart Hospital for the supply of ATCC grade microorganisms, Dr David Nichols of the Central Science Laboratory, University of Tasmania for UPLC-UV analysis of stability samples, Tim Chataway of Flinders University for assisting with analysis of the JJAV-specific antibodies and Mrs. Leonie McLean for reviewing the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jpba.2019.04.017.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Wanandy T., Wilson R., Gell D., Rose H.E., Gueven N., Davies N.W., Brown S.G., Wiese M.D. Towards complete identification of allergens in Jack Jumper (Myrmecia pilosula) ant venom and their clinical relevance: an immunoproteomic approach. Clin. Exp. Allergy. 2018;48(9):1222–1234. doi: 10.1111/cea.13224. [DOI] [PubMed] [Google Scholar]
- 2.James L., Durham S. Update on mechanisms of allergen injection immunotherapy. Clin. Exp. Allergy. 2008;38(7):1074–1088. doi: 10.1111/j.1365-2222.2008.02976.x. [DOI] [PubMed] [Google Scholar]
- 3.Brown S.G.A., Wiese M.D., Blackman K.E., Heddle R.J. Ant venom immunotherapy: a double-blind, placebo-controlled, crossover trial. Lancet. 2003;361(9362):1001–1006. doi: 10.1016/S0140-6736(03)12827-9. [DOI] [PubMed] [Google Scholar]
- 4.Mullins R.J., Brown S.G.A. Ant venom immunotherapy in Australia: the unmet need. Med. J. Aust. 2014;201(1):33–34. doi: 10.5694/mja13.00035. [DOI] [PubMed] [Google Scholar]
- 5.Brown S.G.A., Wiese M.D., van Eeden P., Stone S.F., Chuter C.L., Gunner J., Wanandy T., Phillips M., Heddle R.J. Ultrarush versus semirush initiation of insect venom immunotherapy: a randomized controlled trial. J. Allergy Clin. Immunol. 2012;130(1):162–168. doi: 10.1016/j.jaci.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Chesné J., Schmidt-Weber C.B. J.E. von-Bieren, the use of adjuvants for enhancing allergen immunotherapy efficacy. Immunol. Allergy Clin. North Am. 2016;36(1):125–145. doi: 10.1016/j.iac.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Petrovsky N., Cooper P.D. Carbohydrate-based immune adjuvants. Expert Rev. Vaccines. 2011;10(4):523–537. doi: 10.1586/erv.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrovsky N., Cooper P.D. Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine. 2015;33(44):5920–5926. doi: 10.1016/j.vaccine.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon D.L., Sajkov D., Honda-Okubo Y., Wilks S.H., Aban M., Barr I.G., Petrovsky N. Human Phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax™ delta inulin adjuvant. Vaccine. 2016;34(33):3780–3786. doi: 10.1016/j.vaccine.2016.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barclay T., Petrovsky N. Vaccine adjuvant nanotechnologies. In: Skwarczynski M., Toth I., editors. Micro- and Nanotechnology in Vaccine Development. Elsevier; 2017. pp. 99–113. [Google Scholar]
- 11.Gordon D.L., Sajkov D., Woodman R.J., Honda-Okubo Y., Cox M.M.J., Heinzel S., Petrovsky N. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax™ polysaccharide adjuvant. Vaccine. 2012;30(36):5407–5416. doi: 10.1016/j.vaccine.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrovsky N. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf. 2015;38(11):1059–1074. doi: 10.1007/s40264-015-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heddle R., Russo P., Petrovsky N., Hannah R., Smith A. Immunotherapy - 2076. A controlled study of delta inulin-adjuvanted honey bee venom immunotherapy. Second WAO International Scientific Conference (WISC 2012), Hyderabad, India. World Allergy Organ. J. 2013;6(Supplement 1):P158. [Google Scholar]
- 14.Heddle R.J., Smith A., Woodman R.J., Hissaria P., Petrovsky N. A randomized controlled trial to assess the safety and benefits of immunotherapy with a delta inulin adjuvanted venom extract in honey bee allergic patients. J. Allergy Clin. Immunol. 2019 doi: 10.1016/j.jaci.2019.03.035. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akdis M., van de Veen W. Allergen immunotherapy. In: Ratcliffe M.J., editor. Encyclopedia of Immunobiology. Academic Press; Oxford: 2016. pp. 313–320. [Google Scholar]
- 16.Wanandy T., Dwyer H.E., McLean L., Davies N.W., Nichols D., Gueven N., Brown S.G., Wiese M.D. Factors influencing the quality of Myrmecia pilosula (Jack Jumper) ant venom for use in in vitro and in vivo diagnosis of allergen sensitization and in allergen immunotherapy. Clin. Exp. Allergy. 2017;47(11):1478–1490. doi: 10.1111/cea.12987. [DOI] [PubMed] [Google Scholar]
- 17.Wiese M.D., Davies N.W., Chataway T.K., Milne R.W., Brown S.G.A., Heddle R.J. Stability of Myrmecia pilosula (Jack Jumper) ant venom for use in immunotherapy. J. Pharm. Biomed. Anal. 2011;54(2):303–310. doi: 10.1016/j.jpba.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 18.2011. Malvern Instruments, Dynamic Light Scattering Common Terms Defined, Inform White Paper. [Google Scholar]
- 19.Sutton S.V., Porter D. Development of the antimicrobial effectiveness test as USP chapter <51>. PDA J. Pharm. Sci. Technol. 2002;56(6):300–311. [PubMed] [Google Scholar]
- 20.Honda-Okubo Y., Saade F., Petrovsky N. Advax, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012;30(36):5373–5381. doi: 10.1016/j.vaccine.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EMEA . 2005. Guideline on Adjuvants in Vaccines for Human Use, EMEA/CHMP/VEG/134716/2004. [Google Scholar]
- 22.Cohen M.R., Smetzer J.L., Tuohy N.R., Kilo C.M. 2nd ed. American Pharmaceutical Association; Washington (DC): 2007. High-alert Medications: Safeguarding Against Errors, Medication Errors; pp. 317–411. [Google Scholar]
- 23.Morrow K.S., Slater J.E. Regulatory aspects of allergen vaccines in the US. Clin. Rev. Allergy Immunol. 2001;21(2-3):141–152. doi: 10.1385/CRIAI:21:2-3:141. [DOI] [PubMed] [Google Scholar]
- 24.EMA . European Medicines Agency; 2008. Guideline on Allergen Products: Production and Quality Issues. [Google Scholar]
- 25.Letz A.G., Tankersley M.S., Dice J.P., England R.W. Monitoring bacteriostasis in allergen extract mixing: 10 years of culture data. J. Allergy Clin. Immunol. 2009;123(5):1175–1176. doi: 10.1016/j.jaci.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 26.Rossow K., Lowe D., Butler M., Li J. Bacteriostatic agents and sterility requirements for allergen immunotherapy. J. Allergy Clin. Immunol. 2010;125(2):AB64. doi: 10.1016/j.anai.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Meyer B.K., Ni A., Hu B., Shi L. Antimicrobial preservative use in parenteral products: past and present. J. Pharm. Sci. 2007;96(12):3155–3167. doi: 10.1002/jps.20976. [DOI] [PubMed] [Google Scholar]
- 28.Nema S., Brendel R.J. Excipients and their role in approved injectable products: current usage and future directions. PDA J. Pharm. Sci. Technol. 2011;65(3):287–332. doi: 10.5731/pdajpst.2011.00634. [DOI] [PubMed] [Google Scholar]
- 29.Wanandy T., Gueven N., Davies N.W., Brown S.G., Wiese M.D. Pilosulins: a review of the structure and mode of action of venom peptides from an Australian ant Myrmecia pilosula. Toxicon. 2015;98:54–61. doi: 10.1016/j.toxicon.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Saade F., Honda-Okubo Y., Trec S., Petrovsky N. A novel hepatitis B vaccine containing Advax, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013;31(15):1999–2007. doi: 10.1016/j.vaccine.2012.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.