Summary
The aim of this study was to compare experimentally the pathogenicity and tissue distribution of the recently emerged QX-like strain of infectious bronchitis virus (IBV) with the widespread M41 and 793/B serotypes of the virus. Histopathological and immunohistochemical methods were employed to define the main sites of virus replication. One-day-old specific pathogen free chickens were inoculated with five different QX-like strains, or with the M41 and 793/B IBV strains and monitored for 42 days post-infection. Tracheal lesions developed in all infected birds, confirming the ability of all of the tested strains to induce respiratory disease. Replication of the isolates in the alimentary tract was detected, but the infection did not cause significant gut lesions. Four of the five QX-like IBV strains induced severe kidney lesions. Dilation of the oviduct with accumulation of serum-like fluid in the lumen of this structure, reported previously from field cases of QX-like IBV infection, was observed following experimental infection with all of the five QX-like strains. Microscopical and immunohistochemical examination of the affected oviducts did not help to elucidate the pathogenesis of this lesion.
Keywords: chicken, histopathology, immunohistochemistry, infectious bronchitis virus
Introduction
Infectious bronchitis virus (IBV), the coronavirus of the domestic chicken (Gallus gallus), is primarily known as a respiratory pathogen. The disease attributed to this virus was first recognized in 1941 in North Dakota, where outbreaks of severe respiratory infection occurred in flocks of young chickens (Fabricant, 1998). Some years later the infection reached layer flocks, causing a considerable decline in egg production (Fabricant, 1998). Kidney lesions due to IBV infection were reported in affected birds in the USA (Winterfield and Hitchner, 1962) and Australia (Cumming, 1963). Further clinical manifestations associated with IBV infection were then reported (Ambali and Jones, 1990, YuDong et al., 1998, Boltz et al., 2004, Landman et al., 2005, Villarreal et al., 2007). Different parts of the intestinal tract are suspected to be target tissues for the virus. IBV has also been suggested to play a role in the aetiology of proventriculitis (YuDong et al., 1998) and from time to time it has been isolated from birds with a range of other enteric signs (Ambali and Jones, 1990, Villarreal et al., 2007). Age-dependent adverse effects of IBV on the female reproductive tract have also been recognized (Broadfoot and Smith, 1954, Broadfoot et al., 1956), although the means by which the virus damages the oviduct of young birds has not yet been elucidated. Recent publications suggest that IBV may also have a role in male (Boltz et al., 2004, Boltz et al., 2006, Villarreal et al., 2007).
It is now recognized that IBV comprises more than one serotype. The original serotype (Massachusetts [Mass]) has now spread worldwide (Fabricant, 1998) and is included in most of the commercially available vaccines. In Europe, the second most frequent serotype is 793/B. This serotype has been present since the mid 1980s, but the majority of current isolates are suspected to be re-isolates of vaccine virus (Worthington et al., 2008).
The continuous emergence of new antigenic variants makes prevention of infectious bronchitis (IB) more challenging. The majority of the serotypes that have emerged over the past two decades appear to be endemic (Bochkov et al., 2006). The QX serotype was first isolated in China in 1996 from birds with proventriculitis (YuDong et al., 1998), but spread rapidly so that within a few years genetically related strains were isolated from outbreaks with various clinical manifestations from several provinces in China (Yu et al., 2001, Liu and Kong, 2004, Liu et al., 2006) and from the far eastern and European region of Russia (Bochkov et al., 2006). In 2005 the QX serotype was reported from the European continent (Beato et al., 2005, Landman et al., 2005, Zanella et al., 2006) and rapidly spread to become the most widespread serotype of non-vaccine origin (Worthington et al., 2008).
In addition to respiratory lesions, the QX-like serotype has been associated with proventriculitis (YuDong et al., 1998), severe kidney damage (Liu and Kong, 2004, Beato et al., 2005, Zanella et al., 2006, Terregino et al., 2008, Worthington et al., 2008) and false-layer syndrome (Landman et al., 2005). The field cases reported until now suggest that the QX-like strains have wide tissue tropism and high pathogenicity, although the effect of individual isolates varies greatly. Therefore, a comprehensive experiment was performed to investigate the tissue distribution and tropism of QX-like strains originating from different countries and to compare them to each other and to the M41 and 793/B reference strains (Benyeda et al., 2009). The aim of the present study was to extend that investigation to the immunohistochemical examination of different organs in order to localize the viral antigen and determine its relationship to the observed tissue lesions.
Materials and Methods
Chickens
One-day-old specific pathogen free (SPF) Spafas, Line 22 chickens were randomly divided into eight groups and kept in isolated animal houses with feed and water provided ad libitum.
Viruses
Five different QX-like strains, isolated from different pathological conditions and from different countries (China, France, Slovakia, Greece and Hungary) were used in parallel with the reference M41 and 793/B strains. The Chinese strain was isolated in 2005 from a 3–4-week-old broiler flock with severe renal disease. The French strain was isolated from a layer flock showing respiratory signs at young age. Later in the laying period, ‘false-layer syndrome’ developed in this flock. The Slovakian strain originated from a 2-week-old broiler flock with respiratory signs and renal disease. The Greek strain, which was isolated from 5-week-old broilers, was only associated with respiratory signs. The Hungarian isolate induced respiratory and kidney lesions in 4–5-week-old broilers. Each virus was propagated in 9–11-day-old SPF chicken embryos. The French strain was passaged once, the Chinese, Slovakian and Hungarian strains were passaged twice and the Greek strain was passaged three times before being used for infection. The reference M41 and 793/B strains were obtained from the Veterinary Laboratories Agency, Avian Virology Department (Weybridge, Surrey, UK). The M41 strain was in the third passage and the 793/B strain was in the second passage when received. Both strains were passaged one further time before being used in this experiment.
Experimental Infection
Chickens in each group were infected by the intranasal and intraocular routes with 40 μl of inoculum containing 1 × 105 embryo infectious dose (EID50) of the relevant virus strain (one of the seven different strains). One group served as non-inoculated controls.
Experimental Design
Four groups (A, B, C and D), each comprising 50 1-day-old female chicks were inoculated with one of the following QX-like IBV strains (Chinese, French, Slovakian or Greek). Three groups (E, F and G), each comprising 50 1-day-old female and 50 1-day-old male chicks, were each inoculated with the Hungarian QX-like, the M41 or the 793/B IBV strains. Twenty female and 10 male chicks were kept as non-inoculated controls (group K). The birds were monitored daily by visual observation for clinical signs. Five birds from each of groups A, B, C and D, five female and five male chicks from groups E, F and G, and two female and one male chicks from group K were killed at 4, 7, 11, 14, 21, 28, 35 and 42 days post-infection (dpi).
Histopathology
During necropsy examination, tissue samples were taken from the trachea, lung, ileum, kidney, ovary, oviduct and testis (testis was taken only from male birds in the Hungarian QX-like, M41, 793/B and control groups). Glandular stomach was collected only between 7 and 21 dpi. Samples were fixed in 4% buffered formaldehyde solution for 1 day, embedded in paraffin wax, sectioned (4 μm) and stained with haematoxylin and eosin (HE). The slides were examined by light microscopy and the lesions, induced presumptively by IBV (Table 1 ), were scored as: no change (0), mild (1), moderate (2) or severe (3), as described by Nakamura et al. (1991) and Chen et al. (1996). Selected samples were stained with periodic acid–Schiff (PAS) and alcian blue in order to detect mucus, or by von Kossa’s method to demonstrate calcium deposition. Data were analyzed by one-way analysis of variance (ANOVA) to determine the main effects of the different IBV challenge strains, compared with the control group. All statistical analyses were conducted using ‘R’ (R Development Core Team, 2008).
Table 1.
Lesions evaluated and scored by histology
| Trachea (upper and lower part) | Kidney |
| Loss of epithelial cells | Focal lymphoid cell infiltration |
| Degeneration of epithelial cells | Diffuse lymphoid cell infiltration |
| Lymphoid cell infiltration of the lamina propria | Degeneration of tubular epithelial cells |
| Depletion of mucus secreting cells | Lymphoid infiltration in the collecting duct or ureter |
| Heterophil exudate in the lumen | |
| Hyperplasia of mucus-secreting cells | Gastrointestinal tract |
| Metaplasia of epithelial cells | Lymphoid infiltration in the glandular stomach |
| Hyperplasia of epithelial cells | Lymphoid infiltration in the small intestine |
| Lung | Reproductive tract |
| Bronchitis, mesobronchitis | Oophoritis |
| Focal lymphoid cell infiltration | Calcium deposition in the oocytes |
| Diffuse lymphoid cell infiltration | Lymphoid infiltration of the wall of the oviduct |
| Peribronchitis | Dilation of the oviduct |
| Orchitis, epididymitis | |
Immunohistochemistry
Immunohistochemistry (IHC) was performed on serial sections prepared from the tissues collected at 4, 7, 11 and 14 dpi. After de-waxing, the tissue sections were incubated in citrate buffer (pH 6.0) in a microwave oven at 700–800 W for 10 min and then at 200–300 W for 30 min. Endogenous peroxidase activity was blocked with 3% H2O2 solution for 10 min and then sections were treated with 2% low-fat milk powder solution for 10 min at room temperature. IBV was detected with N-protein-specific mouse monoclonal antibody (dilution 1 in 100; clone Ch/IBV 48.4; Prionics, Lelystad, The Netherlands) by incubating the tissue sections at 37°C overnight. Antibody binding was detected by a horseradish peroxidase-labelled polymer kit (EnVision+ System-HRP; Dako, Glostrup, Denmark). Slides were counterstained with Mayer’s Haematoxylin and the presence of viral antigen was assessed by light microscopy.
Results
Clinical Findings
Respiratory signs (sneezing, nasal discharge and tracheal rales) developed at 3–4 dpi and lasted until 7 dpi in all groups. At 11 dpi birds in group A showed minor respiratory signs and watery diarrhoea, while birds in the other groups were clinically normal. No birds died during the experiment and no clinical signs were observed in the control group.
Gross Pathology
In all infected birds, slight hyperaemia of the tracheal mucosa with minor catarrhal exudation was observed at 4–7 dpi. Air sac lesions were present in all groups from 11 dpi until 21 dpi, after which these lesions disappeared. Kidney lesions were detected from 11 dpi for the remainder of the experiment in every group except F and G. The affected kidneys were slightly enlarged and pale, and in later stages of the experiment, urate deposition in the tubules and ureters was seen occasionally. Characteristic dilatation of the oviduct developed in a few birds of all QX-like strain-infected groups (Fig. 1 ), but no such lesions were observed in the birds infected with the M41 or 793/B strains. The dilatation and serous fluid accumulation in the oviduct became obvious from 14 dpi and was detected sporadically for the remainder of the experiment. This change was recorded most often (five times) in group A and was seen only once in group B (Table 2 ). No gross lesions were observed in birds of the control group.
Fig. 1.
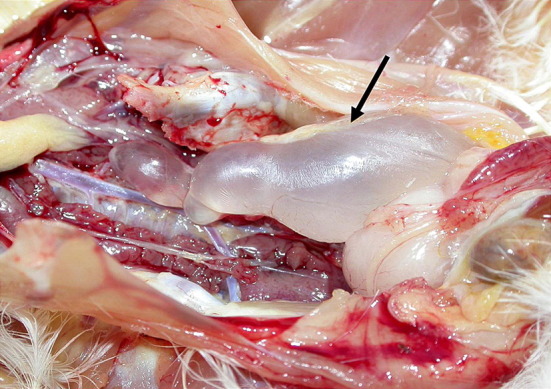
Dilatation and fluid accumulation in the oviduct (arrowed) of a chick infected with the Hungarian QX-like strain of IBV at 28 dpi.
Table 2.
Occurrence of oviduct dilation and serum-like fluid accumulation
| 4 dpi | 7 dpi | 11 dpi | 14 dpi | 21 dpi | 28 dpi | 35 dpi | 42 dpi | |
|---|---|---|---|---|---|---|---|---|
| Chinese | 0/5 | 0/5 | 0/5 | 2/5 | 0/5 | 2/5 | 0/5 | 1/5 |
| French | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 |
| Slovakian | 0/5 | 0/5 | 0/5 | 1/5 | 1/5 | 1/5 | 0/5 | 0/5 |
| Greek | 0/5 | 0/5 | 0/5 | 1/5 | 1/5 | 0/5 | 0/5 | 0/5 |
| Hungarian | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 1/5 | 0/5 |
| M41 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 793/B | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| K- | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
Table presents number of birds with lesion/number of sampled birds.
Histopathology and Immunohistochemistry
Trachea
The nature of the microscopical findings in the infected groups was similar, but the severity and persistence of the lesions differed between the strains. Lesions had already developed by the time of first sampling (4 dpi). The progression of the lesions with time could be divided into degenerative, hyperplastic and recovery stages.
Extensive degeneration and desquamation of the ciliated epithelial cells dominated the microscopical picture in the early phase of infection, although degenerative processes also affected the mucus-secreting and goblet cells. Mononuclear cells infiltrated the affected lamina propria and in serious cases, inflammatory exudate, together with detached epithelial cells and mucus, partly obstructed the tracheal lumen. Pronounced lymphohistiocytic infiltration of the lamina propria, accompanied by epithelial metaplasia, became predominant during the hyperplastic stage and by the end of the third phase the inflammatory process had diminished and the epithelial layer had completely recovered. The mucosal epithelium was normal by 21 dpi.
The most severe lesions were caused by the Chinese and M41 strains at 4 dpi. The severity of lesions induced by the other strains peaked at 7dpi. The destructive effects of the Chinese, French, Slovakian, Hungarian and M41 strains were similar, while the Greek and 793/B strains caused milder changes. By 14 dpi, regeneration of the epithelium had started in all groups. Apart from the Greek isolate, all strains had significant adverse effect (P < 0.05) on the tracheal mucosa compared with the control group (Table 3 ).
Table 3.
Significance in lesion severity for different strains of IBV
| Trachea | Lung | Ileum | Kidney | Ovary | Oviduct | Testis | |
|---|---|---|---|---|---|---|---|
| Chinese | 0.000634∗ | 0.44387 | 1.0000 | 0.00307∗ | 0.3581 | 0.017∗ | NT |
| French | 0.004773∗ | 0.04924 | 0.3032 | 0.00839∗ | 1.0000 | 0.689 | NT |
| Slovakian | 0.000213∗ | 0.18765 | 0.5219 | 2.55 × 10−5∗ | 0.2874 | 0.120 | NT |
| Greek | 0.070627 | 0.04924 | 0.3032 | 0.07421 | 0.0810 | 0.689 | NT |
| Hungarian | 0.003986∗ | 0.15844 | 0.6579 | 0.00450∗ | 0.1587 | 0.410 | 0.616 |
| M41 | 0.002204∗ | 0.59348 | 0.0243 | 0.74020 | 0.4232 | 1.000 | 0.377 |
| 793/B | 0.040321∗ | 0.95504 | 0.3032 | 0.37414 | 0.0351 | 1.000 | 0.319 |
P values are presented for the investigated organs infected with the different strains (one-way ANOVA). The differences in the results were not significant for the lung, ileum, ovary and kidney.
NT, not tested.
Statistically significant difference.
Viral antigen was detected at 4 dpi in the epithelial cells of the tracheal mucosa (Fig. 2 ), except for in the group infected with the Greek strain. In severe cases the cytoplasm of the desquamated epithelial and inflammatory cells also contained viral antigen. At 7 dpi only the chicks infected with the Hungarian strain were positive for virus antigen (Table 4 ). No viral antigen was detected in samples collected after 7 dpi.
Fig. 2.

Desquamated epithelial and inflammatory cells in the lumen of the trachea of a chick infected with the Chinese strain of IBV at 4 dpi. IHC. ×100.
Table 4.
Expression of IBV antigen in affected tissues
| Strains | Trachea |
Kidney |
||
|---|---|---|---|---|
| 4 dpi | 7 dpi | 4 dpi | 7 dpi | |
| Chinese | 2/5 | 0/5 | 1/5 | 3/5 |
| French | 3/5 | 0/5 | 1/5 | 4/5 |
| Slovakian | 0/5 | 0/5 | 1/5 | 0/5 |
| Greek | 0/5 | 0/5 | 0/5 | 0/5 |
| Hungarian | 3/5 | 1/5 | 2/5 | 1/5 |
| M41 | 2/5 | 0/5 | 0/5 | 0/5 |
| 793/B | 3/5 | 0/5 | 0/5 | 0/5 |
| K- | 0/5 | 0/5 | 0/5 | 0/5 |
Immunohistochemical examination of the trachea and kidney at 4 and 7 dpi. Data presented as number of birds with positive labelling/number of sampled birds.
Kidney
Renal lesions developed in all infected groups from 4 dpi onwards and included all segments of tubules and ducts. Mild interstitial oedema and slight dilation of the collecting tubules were observed initially and then the interstitium became infiltrated by lymphocytes and histiocytes. The severity of the inflammation ranged from mild to severe and the extent of this change was focal or diffuse. Severe infiltration was detected in the lamina propria of the mucous membrane of the collecting ducts and ureter in several samples. Sporadic vacuolar degeneration, necrosis and desquamation of epithelial cells were observed.
At 4 dpi, lesions appeared in four out of the five birds infected with the Chinese strain, while birds from the other groups had lesser pathology. From 7 dpi the incidence and severity of lesions increased in all groups and persisted until the time of last sampling, when a slight decrease in incidence was found. Interstitial nephritis due to the QX-like strains was more frequent and more severe than that induced by M41 and 793/B. Microscopical changes in the group infected with the Greek strain were milder than those seen in the other groups infected with the same serotype, which was also supported by the statistical analysis (Table 3).
Viral antigen was detected at 4 and 7 dpi (Table 4) and in one case at 14 dpi in the group infected with the Slovakian isolate. Apart from the Greek strain, severe lesions and positive immunohistochemical reactions developed only in the groups infected with the QX-like strains. Antigen was found in the cytoplasm of the tubular epithelial cells and in the mucous membrane of the ureters and collecting ducts (Fig. 3 ).
Fig. 3.

(a) The expression of viral antigen in the tubular epithelial cells of the kidney, and (b) in the mucous membrane of the ureter, in a chick infected with the Chinese strain of IBV at 4 dpi. IHC, ×100.
Oviduct
Characteristic dilation and serum-like fluid accumulation developed in the oviduct in all groups infected with QX-like strains. Contrary to the gross findings, very few changes were observed microscopically in this organ and no obvious alteration was detected that might explain the pathogenesis of this lesion. In the dilated oviducts, flattening of the developing folds and thinning of the walls could be seen. Focal mononuclear cell infiltration was also found beneath the epithelial layer or the serosal lining. No signs of epithelial damage, excessive secretion or extensive inflammation were found in the oviduct samples. Control birds and birds infected with M41 or 793/B did not show any changes in this organ.
The lesions were detected mainly in those samples in which gross alteration had been described. No viral antigen was detected in the oviduct.
Lung
Microscopical lesions developed in the primary and secondary bronchi and in the interstitium. Lesions were not consistent and were not specific in character. No significant difference among the strains was found regarding the extent and the severity of the lesions (Table 3). Viral antigen was only in the lung of chicks infected with the French, Chinese and Hungarian QX-like isolates.
Glandular Stomach
No characteristic lesions were found in the stomach. Viral antigen was detected in only one case in the epithelial cells of the mucous membrane of a bird infected with the French strain.
Ileum
Mononuclear cell infiltration developed occasionally in this organ and viral antigen was found in two samples from birds infected with the Chinese and Hungarian strains. No significant difference among the strains was found regarding the extent and the severity of the lesions (Table 3).
Ovary
No significant microscopical lesions or viral antigen were detected in the ovary of any bird.
Testis
Lymphohistiocytic infiltration was seen occasionally in the interstitium of the testis or the epididymis of the infected chicks. No significant difference among the strains was found regarding the extent and the severity of the lesions (Table 3). No viral antigen was detected in any testis sample.
Discussion
The present study has shown that all seven strains of IBV studied have affinity for the respiratory tract of chicks. The characteristics and the chronological development of the lesions observed were in agreement with the findings of previous experiments (Purcell and McFerran, 1972, Nakamura et al., 1991, Owen et al., 1991, Chen et al., 1996, Kotani et al., 2000, Chousalkar et al., 2007). Despite the severity of the lesions in the trachea mucosa, expression of viral antigen was not high compared with other studies (Nakamura et al., 1991, Owen et al., 1991, Chen et al., 1996, Kotani et al., 2000). This might reflect the relatively late timing of collection of the first samples, since in previous studies antigen was first detected between 1–3 dpi and peaked at 3 dpi. The absence of viral antigen in the samples from birds infected with the Greek strain was not surprising, as the lesions induced in these chicks were mild. In the case of the Slovakian strain, the negative immunohistochemical findings might be explained by rapid clearance of the virus despite the presence of severe microscopical lesions.
Renal disease is often associated with IBV infection and nephropathogenic strains have been identified worldwide (Fabricant, 1998). Several strains of the Mass serotype have been shown to produce kidney lesions in young (Butcher et al., 1990, Owen et al., 1991; Chen et al., 1996) and older birds (Jones, 1974). In our study the reference M41 strain of this serotype was used, but only mild histopathological lesions were recorded in the absence of virus antigen. Infection with the 793/B strain induced similar findings, while the nephropathogenic effect of the QX-like strains was greater and confirmed the high affinity of these strains (apart from the Greek strain) for the kidney. Viral antigen in the kidney peaked at 7 dpi and then became undetectable immunohistochemically. However, it is likely that a low concentration of virus persisted until the end of the experiment, associated with the observed chronic renal pathology.
Microscopical lesions and virus antigen were not found in the ovary, consistent with previous results (Crinion et al., 1971a, Crinion et al., 1971b, Jones and Jordan, 1972). It would appear that the ovary remains intact and functional after infection and it is only the impairment of the oviduct that results in the ‘false-layer syndrome’ during the laying period. The pathogenesis of this syndrome has not been determined. Previous studies of the aetiology of cystic ovarian changes induced by infection with Mass and other serotypes of 1-day-old and older birds suggested the role of hormonal responses (Jones and Jordan, 1972) or the obliteration of the lumen due to the persistent epithelial damage (Crinion and Hofstad, 1972). In those studies, gross cyst formation developed from 20 dpi, while in the present experiment lesions were detected as early as 14 dpi in groups infected with the QX-like strains. Contrary to the findings of Crinion and Hofstad (1972), we did not observe microscopical lesions or viral antigen in the oviduct. The absence of lesions might be due to inappropriate sampling time or site of sampling.
Grossly, the distal part of the dilated cystic oviduct always ended in a blind sac, close to the cloacovaginal entrance. The opening of the infantile oviduct is the final stage in the development of the Müller duct, which ensures the connection between the cloaca and the vagina and which occurs in the post-embryonal period of development and is normally achieved by the seventh day of development. Any disturbance during this short period may block the physiological process and inhibit the opening of the duct. Impairment of the normal fluid movement within the blocked oviduct might result in the development of the characteristic lesion and explain its occurrence only following early infection.
The severity of histopathological lesions and the amount of viral antigen expression in the lung were mild and occasional. This finding may relate to the route of experimental infection, which differs from the natural aerosol route of infection.
Proventriculitis, reported in the original infections with the QX isolate (YuDong et al., 1998), was not observed in the present experiment. The lesions detected in the proventriculus were non-specific in character and were present also in the control birds.
The virus strains investigated had no significant effect on the remainder of the organs of the birds. This may reflect the route of inoculation, the dose of inoculum, the inappropriate timing of sampling and the lower susceptibility of these tissues for the virus. Viral antigen was not detected in most of these samples, but positivity, occasionally found in the samples infected with the QX-like strains, indicates a wider tissue distribution.
Acknowledgment
The authors thank T. Adley for his grammatical suggestions and R. Pop and Á. Mészáros for assistance with histopathology and immunohistochemistry.
References
- Ambali A.G., Jones R.C. Early pathogenesis in chicks of infection with an enterotropic strain of infectious bronchitis virus. Avian Diseases. 1990;34:809–817. [PubMed] [Google Scholar]
- Beato M.S., De Battisti C., Terregino C., Drago A., Capua I. Evidence of circulation of a Chinese strain of infectious bronchitis virus (QXIBV) in Italy. Veterinary Record. 2005;156:720. doi: 10.1136/vr.156.22.720. [DOI] [PubMed] [Google Scholar]
- Benyeda Z., Mató T., Süveges T., Szabó É, Kardi V. Comparison of the pathogenicity of QX-like, M41 and 793/B infectious bronchitis strains from different pathological conditions. Avian Pathology. 2009;38:449–456. doi: 10.1080/03079450903349196. [DOI] [PubMed] [Google Scholar]
- Bochkov Y.A., Batchenko G.V., Shcherbakova L.O., Borisov A.V., Drygin V.V. Molecular epizootiology of avian infectious bronchitis virus in Russia. Avian Pathology. 2006;35:379–393. doi: 10.1080/03079450600921008. [DOI] [PubMed] [Google Scholar]
- Boltz D.A., Nakai M., Bahr J.M. Avian infectious bronchitis virus: a possible cause of reduced fertility in the rooster. Avian Diseases. 2004;48:909–915. doi: 10.1637/7192-040808R1. [DOI] [PubMed] [Google Scholar]
- Boltz D.A., Zimmerman C.R., Nakai M., Bunick D., Scherba G. Epididymal stone formation and decreased sperm production in roosters vaccinated with a killed strain of avian infectious bronchitis virus. Avian Diseases. 2006;50:594–598. doi: 10.1637/7654-052506R.1. [DOI] [PubMed] [Google Scholar]
- Broadfoot D.I., Pomeroy B.S., Smith W.M., Jr. Effects of infectious bronchitis in baby chicks. Poultry Science. 1956;35:757–762. [Google Scholar]
- Broadfoot D.I., Smith W.M., Jr. Effects of infectious bronchitis in laying hens on egg production, percent unsettable eggs and hatchability. Poultry Science. 1954;33:653–655. [Google Scholar]
- Butcher G.D., Winterfield R.W., Shapiro D.P. Pathogenesis of H13 nephropathogenic infectious bronchitis virus. Avian Diseases. 1990;34:916–921. [PubMed] [Google Scholar]
- Chen B.Y., Hosi S., Nunoya T., Itkura C. Histopathology and immunohistochemistry of renal lesions due to infectious bronchitis virus in chicks. Avian Pathology. 1996;25:269–283. doi: 10.1080/03079459608419141. [DOI] [PubMed] [Google Scholar]
- Chousalkar K.K., Roberts J.R., Reece R. Comparative histopathology of two serotypes of infectious bronchitis virus (T and N1/88) in laying hens and cockerels. Poultry Science. 2007;86:50–58. doi: 10.1093/ps/86.1.50. [DOI] [PubMed] [Google Scholar]
- Crinion R.A.P., Ball R.A., Hofstad M.S. Pathogenesis of oviduct lesions in immature chickens following exposure to infectious bronchitis virus at one day old. Avian Diseases. 1971;15:32–41. [PubMed] [Google Scholar]
- Crinion R.A.P., Ball R.A., Hofstad M.S. Abnormalities in laying chickens following exposure to infectious bronchitis virus at one day old. Avian Diseases. 1971;15:42–48. [PubMed] [Google Scholar]
- Crinion R.A.P., Hofstad M.S. Pathogenicity of four serotypes of avian infectious bronchitis virus for the oviduct of young chickens of various ages. Avian Diseases. 1972;16:351–363. [PubMed] [Google Scholar]
- Cumming R.B. Infectious avian nephrosis (uraemia) in Australia. Australian Veterinary Journal. 1963;39:145–147. [Google Scholar]
- Fabricant J. The early history of infectious bronchitis. Avian Diseases. 1998;42:648–650. [PubMed] [Google Scholar]
- Jones R.C., Jordan F.T.W. Persistence of virus in the tissues and development of the oviduct in the fowl following infection at day old with infectious bronchitis virus. Research in Veterinary Science. 1972;13:52–60. [PubMed] [Google Scholar]
- Jones R.C. Nephrosis in laying chickens caused by Massachusetts-type infectious bronchitis virus. Veterinary Record. 1974;95:319. doi: 10.1136/vr.95.14.319-a. [DOI] [PubMed] [Google Scholar]
- Kotani T., Shiraishi Y., Tsukamoto Y., Kuwamura M., Yamate Epithelial cell kinetics in the inflammatory process of chicken trachea infected with infectious bronchitis virus. Journal of Veterinary Medicine Science. 2000;62:129–134. doi: 10.1292/jvms.62.129. [DOI] [PubMed] [Google Scholar]
- Landman WJM, Dwars RM, de Wit JJ (2005) High incidence of false layers in (re)production hens supposedly attributed to a juvenile infectious bronchitis virus infection. Proceedings of the 14th World Veterinary Poultry Congress, Istanbul, Turkey, pp. 369.
- Liu S., Kong X. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China. Avian Pathology. 2004;33:321–327. doi: 10.1080/0307945042000220697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.W., Zhang Q.X., Chen J.D., Han Z.X., Liu X. Genetic diversity of avian infectious bronchitis coronavirus strains isolated in China between 1995 and 2004. Archives of Virology. 2006;151:1133–1148. doi: 10.1007/s00705-005-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Cook J.K.A., Otsuki K., Huggins M.B., Frazier J. Comparative study of respiratory lesions in two chicken lines of different susceptibility infected with infectious bronchitis virus: histology, ultrastructure and immunohistochemistry. Avian Pathology. 1991;20:241–257. doi: 10.1080/03079459108418761. [DOI] [PubMed] [Google Scholar]
- Owen R.L., Cowen B.S., Hattel A.L., Naqi S.A., Wilson R.A. Detection of viral antigen following exposure of one-day old chickens to the Holland-52 strain of infectious bronchitis virus. Avian Pathology. 1991;20:663–673. doi: 10.1080/03079459108418805. [DOI] [PubMed] [Google Scholar]
- Purcell D.A., McFerran J.B. The histopathology of infectious bronchitis in the domestic fowl. Journal of Veterinary Science. 1972;13:116–122. [PubMed] [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2008. R: a Language and Environment for Statistical Computing.http://www.R-project.org [Google Scholar]
- Terregino C., Toffan A., Beato M.S., De Nardi R., Vascellari M. Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathology. 2008;37:487–493. doi: 10.1080/03079450802356938. [DOI] [PubMed] [Google Scholar]
- Villarreal L.Y., Brandão P.E., Chacón J.L., Assayag M.S., Maiorka P.C. Orchitis in roosters with reduced fertility associated with avian infectious bronchitis virus and avian metapneumovirus infections. Avian Diseases. 2007;51:900–904. doi: 10.1637/7815-121306-REGR4.1. [DOI] [PubMed] [Google Scholar]
- Winterfield R.W., Hitchner S.B. Etiology of an infectious nephritis–nephrosis syndrome of chickens. American Journal of Veterinary Research. 1962;23:1273–1279. [PubMed] [Google Scholar]
- Worthington K.J., Currie R.J.W., Jones R.C. A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathology. 2008;37:247–257. doi: 10.1080/03079450801986529. [DOI] [PubMed] [Google Scholar]
- Yu L., Jian Y., Low S., Wang Z., Nam S.J. Characterisation of three infectious bronchitis virus isolates from China associated with proventriculitis in vaccinated chickens. Avian Diseases. 2001;45:416–424. [PubMed] [Google Scholar]
- YuDong W., YongLin W., ZiChun Z., GenChe F., YiHai J. Isolation and identification of glandular stomach type IBV (QX IBV) in chickens. Chinese Journal of Animal Quarantine. 1998;15:1–3. [Google Scholar]
- Zanella A, Barbieri I, Gavazzi L, Tosi G (2006) Avian infectious bronchitis: isolation of an umpteenth new serotype of virus in Italy. Proceedings of the Vth International Symposium on Avian Corona- and Pneumoviruses, Rauischholzhausen, Germany, pp. 161–168.


