Abstract
Purpose
This retrospective audit aimed to evaluate the impact of combined iStent® Inject (iSI) and phacoemulsification on medication number in Australians with open-angle glaucoma. Secondary outcomes included intraocular pressure (IOP), best-corrected visual acuity, refraction and visual fields.
Patients and Methods
Patients with glaucoma that received combined iSI and phacoemulsification by the same surgeon between 1 February 2016 and 1 February 2018 were audited for postoperative medication number, pressures after 1 day, 1 week, 4 weeks and 6, 12, 18 and 24 months, visual acuity, refraction and visual fields. These parameters were compared to baseline levels and with those from a separate cohort of patients without glaucoma that received standalone phacoemulsification.
Results
Forty-one patients (63 eyes) received the combined procedure. Thirty-four patients (59 eyes) received standalone phacoemulsification. Four weeks after receiving combined iSI and phacoemulsification the mean medication number was significantly reduced by 1.3 (p < 0.001) for those on medication at baseline and by 0.5 (p = 0.002) overall. Mean IOP was significantly reduced from baseline after 6 months (–16%; p = 0.012; n = 35) and 12 months (–29%; p = 0.004; n = 16). Patients receiving standalone phacoemulsification had short-term reductions in IOP at 4 weeks (–8%; p < 0.001; n = 57) and 6 months (–16%; p < 0.001; n = 32). These patients without glaucoma had lower pressures overall compared to those with glaucoma that received the combined procedure (p = 0.019). There were no differences in final visual acuity or refractive outcomes between groups.
Conclusion
This audit suggests that iSI and phacoemulsification are at least as effective in controlling IOP as medical therapy. It may have an important role in reducing the medication burden in Australians with cataract and glaucoma. This study is one of the first to confirm refractive stability in concomitant iSI and phacoemulsification.
Keywords: glaucoma, iStent Inject, micro-invasive glaucoma surgery, trabecular micro-bypass, phacoemulsification
Plain Language Summary
The medical (drop) therapies that have historically been used to manage intraocular pressure (IOP) in primary open-angle glaucoma (POAG) have important drawbacks. For some patients, these may become expensive, associated with unpleasant ocular symptoms and prove less effective over time. The iStent® Inject (iSI) trabecular micro-bypass is a type of minimally invasive surgery that may effectively manage IOP and reduce dependence on medication. It can be performed at the time of cataract surgery. This study evaluated the outcomes of Australians receiving iSI and cataract surgery along with those receiving cataract surgery alone. Patients that received iSI with cataract surgery were found to be less dependent on medications after 4 weeks and had lower IOPs at 6 and 12 months. This study is also one of the first to demonstrate that iSI does not change the refractive outcomes of cataract surgery. It may therefore have a role to play in reducing medication burden for Australians with POAG at the time of cataract removal.
Introduction
Glaucoma is the second leading cause of irreversible blindness and affects over 60 million people worldwide.1,2 The most common glaucoma subtype is primary open-angle glaucoma (POAG). It affects 2% of those over 40 years and 10% of those over 80 years.4,5 POAG pathogenesis is multifactorial6–9 but is typically associated with an increased resistance to outflow of aqueous humour through damaged trabecular meshwork (TM).
Medical (drop) therapies are commonly used as first-line treatments for glaucoma but have important drawbacks inherent with long-term use. Preservatives inherent in these medications are known to cause corneal erosion, conjunctival injection and superficial punctate keratitis in some patients.10,11 As therapeutic intervals increase over months to years the efficacy of anti-glaucoma drops may wane and their cost can become prohibitive to treatment.12 Selective laser trabeculoplasty (SLT) is capable of producing the same 20–30% reduction in intraocular pressure (IOP) as medication.13,14 However, its efficacy also wanes after months to years and success rates may drop with retreatment.13,15,16 External filtration surgery is invasive and typically performed in cases where the maximum-tolerated levels of medication and SLT reoperations have failed to control IOP. Although effective, it is associated with a risk of potentially sight-threatening complications such as damage to anterior chamber structures, postoperative hypotony, bleb leak and endophthalmitis.17,18
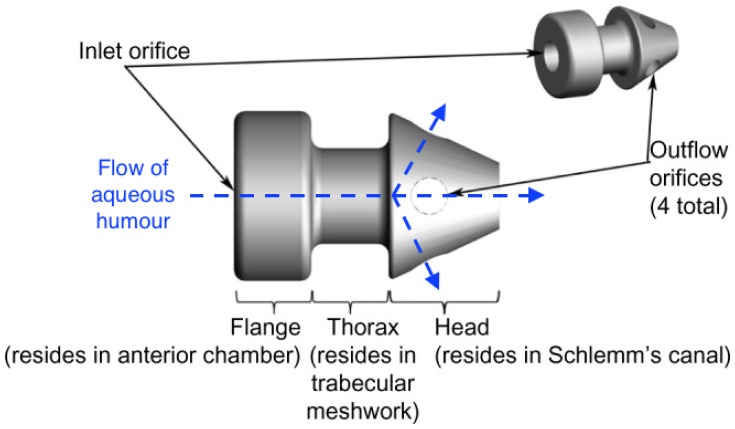
Micro-invasive glaucoma surgery (MIGS) refers to an increasingly popular category of surgical procedures defined by minimal tissue disruption, generally sustained efficacy in lowering IOP, a high safety profile and a rapid recovery phase with minimal impact on quality of life.19 The Glaukos iStent® Inject (iSI) Trabecular Micro-Bypass (Glaukos Corporation, Laguna Hills, CA, USA; Figures 1 and 2) consists of two non-ferromagnetic, heparin-coated, l-shaped stents (360 μm x 230 μm) that can be inserted individually into the TM through a stainless steel insertion tube with a handheld injector. These can easily be implanted alongside phacoemulsification. The iSI reduces IOP via bypass of the damaged TM and restoration of the physiologic mechanism of outflow.
Figure 1.
The iSI micro-bypass system (Adapted from Voskanyan L, Garcia-Feijoo J, Belda JI, et al. Prospective, unmasked evaluation of the iStent(R) inject system for open-angle glaucoma: synergy trial. Adv Ther. 2014;31(2):189–201. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode21).
Figure 2.
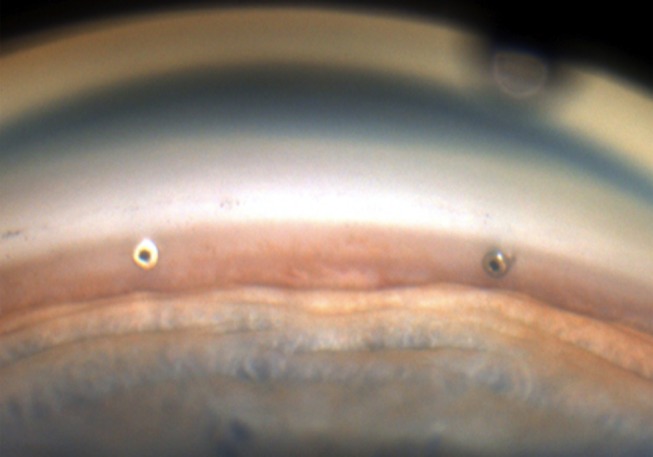
Gonioscopic view of two iSIs within the trabecular meshwork.
Several studies published over the past 5 years in overseas cohorts suggest that iSI is capable of reducing IOP in the long-term, has a favourable safety profile and, importantly, may reduce reliance on anti-glaucoma medications.20–24 It may therefore be a valuable therapeutic tool in minimising medication burden and ultimately optimising quality of life also in Australians with POAG and cataract.
Presently, no published study is known to have investigated the impact of combined iSI and phacoemulsification on medication dependence, IOP, final visual acuity, field indices and refractive outcomes in Australian patients. This study aimed to compare these parameters before and after combined iSI and phacoemulsification in Australians with coexistent POAG and cataract.
Materials and Methods
Participants and Settings
This was a retrospective audit of cases operated by a single surgeon (S.E.S.) in his private practice over a 24-month period (1 February 2016–1 February 2018). In accordance with the Health Records Act 2001 (State of Victoria) and the Privacy Act 1988 (Commonwealth of Australia) only cases from the practitioner’s private practice and none from his public hospital practice were included. Outcomes were collected by intervention type: either iSI and phacoemulsification (combined procedure) or phacoemulsification alone (single procedure). In this surgeon’s practice, glaucoma patients requiring cataract surgery were treated with combined iSI and phacoemulsification when IOP was relatively well controlled on medication in order to reduce topical drop dependence or when the therapeutic effect of SLT was likely to wear off. All patients included in this audit provided signed consent for their de-identified data to be collected and published for scientific review and also their informed consent for surgery to be performed at a subsequent visit.
Inclusion criteria for patients in the combined procedure group were: (a) a diagnosis of mild-to-moderate POAG as per the Hodapp classification;24 (b) concurrent iSI and phacoemulsification performed by a single surgeon between 1 February 2016 and 1 February 2018; (c) high-fidelity of follow-up (defined as having attended at least 3 follow-up appointments during this time). Inclusion criteria for patients in the single procedure group were: phacoemulsification performed by the same surgeon between 1 February 2016 and 1 February 2018 and high-fidelity of follow-up. Patients in the single procedure group did not have glaucoma. A small minority had received SLT or topical glaucoma medications pre-operatively for ocular hypertension.
Exclusion criteria for patients in either group were: previous glaucoma filtration surgery, advanced POAG not suitable for MIGS and lack of follow-up within 1 month postoperatively.
Surgical Technique
The technique used for iSI adhered to the recommendations provided by Glaukos Corporation25 and was similar to that described in similar studies.20 All procedures were performed by the same surgeon (S.E.S).
In all patients, standard clear corneal phacoemulsification with the implant of a foldable acrylic intraocular lens was conducted under local anaesthesia. After the intraocular lens was implanted, two GTS-400 iStent® were inserted through the same corneal incision (~2.6 mm) placed for phacoemulsification. A viscoelastic agent (Healon, Abbott Medical Optics, Santa Ana, California, USA) was inserted into the anterior chamber to facilitate clear visualisation of the TM through a Swan-Jacob gonioscope. The head of the patient was repositioned at 45° to the non-operated eye so that the surgical microscope could be tilted to view a nasal 35° arc on the operated eye. Once inserted through the corneal incision, the insertion sleeve was retracted to expose the insertion tube and trocar. The trocar tip was carefully inserted into the TM and, once in position, the injector trigger was pressed to gently release the stent through the TM and into the Canal of Schlemm. The injector tip was then carefully relocated within the eye to the second implant location and the second iStent® inserted in the same manner (Figure 2). The injector was then removed from the eye and the anterior chamber irrigated with balanced salt solution to remove all viscoelastic. The anterior chamber was left inflated with saline solution to achieve physiologic IOP. Postoperative management consisted of a 4-week course of topical steroid (Tobradex, Alcon Cusi) and antibiotic (tobramycin 0.3%) medications; topical glaucoma medications were generally stopped day 1 post-operatively, unless the measured IOP was high.
Data Collection
Age and sex were collected as demographic factors. The number of topical anti-glaucoma medications required to control IOP was recorded at the first session prior to surgery and 4 weeks post-operatively. Combination drops containing two or more anti-glaucoma drugs were considered as multiple individual medications. Mean preoperative IOP was calculated from the three measurements closest to the date of surgery. There was no preoperative medication washout. Postoperative IOP was collected at 1 day, 1 week, 4 weeks, 6 months, 12 months, 18 months and 24 months follow-up. Mean pre-operative and post-operative best-corrected LogMAR visual acuities were calculated from the three independent measurements closest to the date of surgery. Spherical equivalent was calculated based on the cylinder and sphere powers assessed directly prior to surgery and at 4 weeks follow-up. Mean deviation was collected at the first consultation before and after the combined procedure.
Approximately 54% of eyes in the combined iSI and phacoemulsification cohort were not on topical therapy at baseline. This was because many of these patients were intolerant to drops. Others had received SLT in the past and IOPs were unlikely to remain therapeutic in future months to years. Furthermore, since IOP control can change over time and in Australia iSI can only be implanted concurrently with phacoemulsification, it is important to offer glaucoma patients the procedure at the time of cataract surgery. Since the time of this study Australian regulations have changed such that iSI can only be considered in patients with an intolerance to topical therapy.
Statistical Analyses
Statistical analyses and tests for normality were performed using JASP® for Mac (version 0.8.6; University of Amsterdam, Netherlands). Baseline characteristics were assessed using the Mann–Whitney U-test for continuous variables and Chi-squared test for categorical variables. Univariate analysis of outcomes relative to baseline within cohorts was performed using the Wilcoxon signed-rank test. Univariate analysis of outcomes between cohorts was performed using the Mann–Whitney U-test and repeated measures ANOVA. P-values ≤0.05 were considered significant.
Ethics
This audit was performed with approval from the Royal Australian and New Zealand College of Ophthalmologists Human Research Ethics Committee, granted on 13 December 2017 (reference: 81.17).
Results
A total of 122 eyes from 75 patients were included in the study. Of these, 63 eyes were included from 41 patients in the group receiving the combined intervention while 59 eyes from 34 patients were included in the single intervention group. Of the 63 eyes included in the iStent® group, 36 eyes from 24 patients had received previous SLT. Seven eyes from 4 patients in the phacoemulsification group had received previous SLT (for ocular hypertension without glaucoma). The mean duration of follow-up was 6.5 ± 5.2 months in the combined procedure group and 6.8 ± 6.9 months in the single procedure group. No eyes were excluded due to insufficient follow-up.
Table 1 outlines the baseline patient characteristics. The mean age at the time of surgery was 71 and 72 years for the combined and single procedure groups, respectively. There were significantly more females (74%) in the group receiving phacoemulsification than in the group receiving the combined procedure (56%; p = 0.009). The mean number of medications for the combined procedure group was 0.8 as a whole and 1.9 for the 29 patients medicated at baseline. In the standalone phacoemulsification group at baseline, there was a single patient that received a topical medication for ocular hypertension without glaucoma. Mean baseline IOPs were significantly higher in the combined procedure group (16.0 mmHg) relative to the single procedure group ((13.5 mmHg); p < 0.001).
Table 1.
Baseline Characteristics of Patients Receiving Combined iSI and Phacoemulsification versus Phacoemulsification Alone
| Comparison of Baseline Characteristics Between Intervention Groups† | |||
|---|---|---|---|
| iStent® Inject and Phacoemulsification | Phacoemulsification | p | |
| Number of eyes | 63 | 59 | − |
| Number of patients | 41 | 34 | − |
| Demographics | |||
| Age (years) | 71.2 ± 8.3 | 72.4 ± 9.0 | 0.533 |
| Sex (female) [n%] | 23 (56) | 25 (74) | 0.009* |
| Number of Medications^ | |||
| Overall | 0.8 ± 1.1 | 0.0 ± 0.1 | − |
| For patients on medication at baseline | 1.9 ± 0.9 | 1.0 ± 0.0 | − |
| Number of patients on medication at baseline (n%) | 29 (46) | 1 (1.6) | − |
| Baseline Ocular Measurements | |||
| Intraocular pressure in mmHg | 16.0 ± 3.9 | 13.5 ± 3.3 | <0.001* |
| Best corrected visual acuity in logMAR | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.515 |
| Spherical equivalent in D | −1.2 ± 2.4 | 1.0 ± 2.2 | <0.001* |
| Mean deviation in dB | −3.6 ± 3.9 | −3.2 ± 5.1 | 0.121 |
| Previous selective laser trabeculoplasty (n%) | 36 (57) | 7(12) | <0.001* |
Notes: †Presented as [mean ± SD] unless otherwise specified. –Not applicable. ^Anti-glaucoma medication. *Denotes significance at p < 0.05. Number (n); p-value (p); standard deviation (SD)
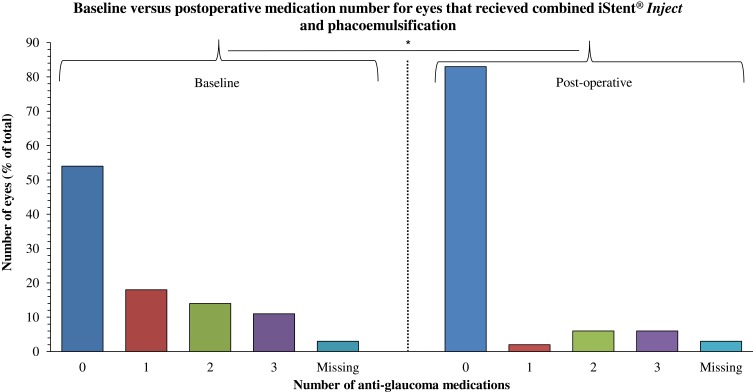
The number of medications required to maintain target IOP following combined iSI and phacoemulsification was significantly reduced by 0.5 in the combined group overall (p = 0.002) and by 1.3 for the 29 patients medicated at baseline (p < 0.001, Table 2); only 11 patients required glaucoma drops post-operatively (Figure 3).
Table 2.
Changes Relative to Baseline in Postoperative Treatment Outcomes Between Patients Receiving Combined iSI and Phacoemulsification versus Phacoemulsification Alone. Postoperative Visual Field Indices Were Not Evaluated in Patients Receiving Phacoemulsification Alone
| Univariate Analysis of Outcomes Relative to Baseline by Intervention Group† | ||||
|---|---|---|---|---|
| iStent® Inject and Phacoemulsification | p | Phacoemulsification | p | |
| Number of eyes | 63 | − | 59 | − |
| Number of Medications^ ~ (Absolute Change from Baseline) | ||||
| Overall | 0.3 ± 0.9 (−0.5) | 0.002* | 0.0 ± 0.1 (−0.0) | − |
| For patients on medication at baseline | 0.56 ± 1.0 (−1.3) | <0.001* | 1.0 ± 0.0 (−0.0) | − |
| Intraocular Pressure in mmHg (% Change from Baseline) | ||||
| 1 day | 17.1 ± 7.6 (+6.9) | 0.793 | 13.4 ± 4.8 (−0.7) | 0.808 |
| 1 week | 15.7 ± 5.4 (−1.9) | 0.208 | 13.3 ± 6.0 (−1.5) | 0.158 |
| 4 weeks | 15.2 ± 4.0 (−5.0) | 0.052 | 12.4 ± 3.5 (−8.2) | <0.001* |
| 6 months | 13.5 ± 3.2 (−15.6) | 0.012* | 11.4 ± 2.5 (−15.6) | <0.001* |
| 12 months | 11.4 ± 2.6 (−28.8) | 0.004* | 13.3 ± 3.4 (−1.5) | 0.553 |
| 18 months | 14.6 ± 2.5 (−8.8) | 0.498 | 9.8 ± 1.3 (−27.4) | 0.713 |
| 24 months | 12.7 ± 3.8 (−20.6) | − | 12.6 ± 5.1 (−6.7) | >0.99 |
| Best corrected visual acuity in logMAR | 0.1 ± 0.2 (−0.1) | <0.001* | 0.0 ± 0.1 (−0.1) | <0.001* |
| (absolute change from baseline) | ||||
| Final spherical equivalent in D~ | −0.3 ± 0.5 (+0.9) | 0.04* | −0.5 ± 0.5 (−1.5) | 0.04* |
| (absolute change from baseline) | ||||
| Final mean deviation in dB | −2.8 ± 4.3 (+0.8) | >0.99 | − | − |
| (absolute change from baseline) | ||||
Notes: †Presented as [mean ± SD] unless otherwise specified. –Not applicable. ^Anti-glaucoma medication. ~After 4 weeks. *Denotes significance at p < 0.05. Number (n); p-value (p); standard deviation (SD)
Figure 3.
Number of anti-glaucoma medications required to achieve therapeutic IOP at baseline versus 4 weeks after combined iSI and phacoemulsification. Number of eyes (percent of cohort total) is represented on the left axis and medication number is represented on the bottom axis. A significant (p = 0.002) difference postoperatively is denoted by *.
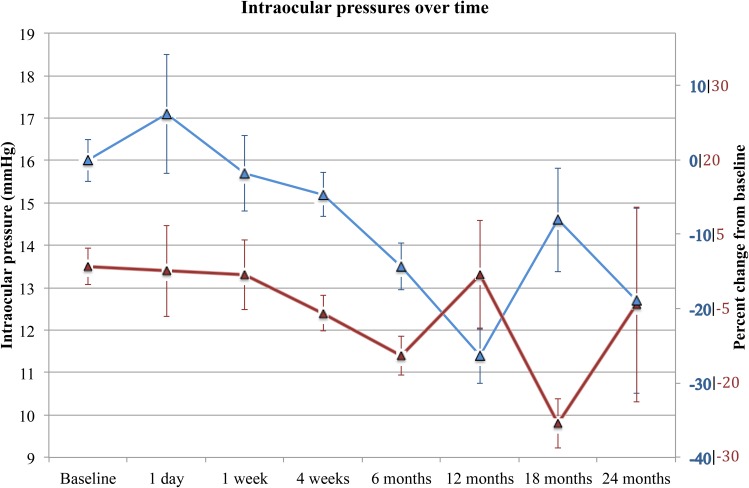
Overall, the IOP of patients in the single procedure group was lower over 24 months (p = 0.019; Table 3). As demonstrated in Figure 4, patients in the combined intervention group had a greater reduction in IOP after 12 months (–28%, n = 16) and 24 months (–20%, n = 3) compared to those receiving the single intervention (–3%, n = 9; –8%, n = 5, respectively). In the combined procedure group, IOP was significantly reduced from baseline at both 6 months (–16%; p = 0.012; n = 35) and 12 months (–29%; p = 0.004; n = 16). In the standalone phacoemulsification group IOP was reduced after 4 weeks (–8%; p < 0.001; n = 57) and 6 months (–16%; p < 0.001; n = 32).
Table 3.
Comparison of Treatment Outcomes Between Patients Receiving Combined iSI and Phacoemulsification versus Phacoemulsification Alone
| Univariate Analysis of Outcomes Between Intervention Groups† | |||
|---|---|---|---|
| iStent® Inject and Phacoemulsification | Phacoemulsification | p | |
| Number of eyes | 63 | 59 | − |
| Number of Medications^ ~ | |||
| Overall | 0.3 ± 0.9 | 0.1 ± 0.6 | − |
| For patients on medication at baseline | 0.6 ± 1.0 | − | − |
| Intraocular Pressure in mmHg (% Change from Baseline) | |||
| 1 day | 17.1 ± 7.6 (+8.2) | 13.4 ± 4.8 (−2.2) | 0.15 |
| 1 week | 15.7 ± 5.4 (−0.6) | 13.3 ± 6.0 (−2.9) | 0.01* |
| 4 weeks | 15.2 ± 4.0 (−3.8) | 12.4 ± 3.5 (−9.5) | <0.001* |
| 6 months | 13.5 ± 3.2 (−14.6) | 11.4 ± 2.5 (−16.8) | 0.002* |
| 12 months | 11.4 ± 2.6 (−27.8) | 13.3 ± 3.4 (−2.9) | 0.35 |
| 18 months | 14.6 ± 2.5 (−7.6) | 9.8 ± 1.3 (−28.5) | 0.02* |
| 24 months | 12.7 ± 3.8 (−19.6) | 12.6 ± 5.1 (−8.0) | 0.65 |
| Best corrected visual acuity in logMAR (Change from baseline) | 0.1 ± 0.2 (−0.1) | 0.0 ± 0.1 (−0.1) | 0.608 |
| Spherical equivalent in D~ (Change from baseline) | −0.3 ± 0.5 (+0.9) | −0.5 ± 0.5 (−1.5) | >0.99 |
Notes: †Presented as [mean ± SD] unless otherwise specified. –Not applicable. ^Anti-glaucoma medication. ~After 4 weeks. *Denotes significance at p < 0.05. Number (n); p-value (p); standard deviation (SD)
Figure 4.
IOPs 24 months after iSI and phacoemulsification (blue) and phacoemulsification alone (red). Absolute IOP (in mmHg) is represented on the left axis. Percent change from baseline IOP is represented on the right axis in the colour corresponding to each cohort. Error bars represent standard error of the mean.
There were no between-group differences in final BCVA or, interestingly, final refractive outcome (Table 3).
Discussion
This study demonstrated that combined iSI and phacoemulsification were capable of reducing dependence on anti-glaucoma medications in a cohort of Australian patients with POAG and cataract. This finding is largely consistent with the results of one of the only other published papers known to have evaluated the impact of the combined procedure on medication dependence.20 In that study, Arriola-Villalobos et al demonstrated that patients receiving combined iSI and phacoemulsification were on average one medication less dependent while maintaining target IOP. The present study demonstrated a significant reduction of 0.5 medications in the cohort overall and a reduction of 1.3 medications in those medicated at baseline – greater than that reported by Arriola-Villalobos et al. The medication outcomes demonstrated in Australians herein are consistent also with those at 623 and 12 months22 post standalone iSI in overseas cohorts. The present findings support a role for combined iSI and phacoemulsification in reducing medication dependence in Australians also.
IOP was significantly reduced relative to baseline levels after 6 and 12 months following combined iSI and phacoemulsification. At no point during follow-up did pressure significantly exceed baseline levels. This suggests that, in parallel with the findings of Fea et al,21 the procedure is at least as capable of achieving therapeutic IOP as medical therapy alone. This is in keeping with the findings of Arriola-Villalobos et al20 who report reduced IOP at each stage of follow-up between 1 day and 12 months.
The overall trend of reduced IOP relative to medicated baseline described in the present study is consistent with most of the current published findings. However, there is some discrepancy in the degree of postoperative IOP change at each time point. In this study, IOP was reduced significantly at only 6 and 12 months however similar cohorts receiving standalone iSI demonstrated significant IOP reductions throughout this period. Fea et al21 reported a 12% reduction in IOP after 1 and 12 months. IOP outcomes from this study are lower than those reported by Voskanyan et al22 after 1, 6 and 12 months. Relative to Klamann et al,23 this study demonstrates a higher mean IOP after 24 hrs but a lower mean IOP after 6 months. This variability likely reflects the fact that pre-treatment IOPs from the present combined procedure group were noticeably lower than those from other cohorts. In this cohort of private patients, medication washout was not incorporated into the study design and iSI was offered only to patients with well-controlled mild-to-moderate glaucoma. It is therefore likely that higher pre-treatment IOPs would have yielded more exaggerated reductions in pressure comparable to those published elsewhere. Final IOP outcomes reported in this combined-procedure cohort are, in general, lower than those from standalone iSI cohorts. This might be further evidence of a synergistic relationship between iSI and phacoemulsification as suggested by several emerging studies.20,26,27,28,29
IOP outcomes were lower for patients receiving the single procedure at 1 week, 4 weeks, 6 months, and 18 months relative to those receiving iSI. This is best explained by the fact that no patients receiving phacoemulsification alone had POAG and therefore had lower IOPs at baseline. Since there was a significant difference in IOP prior to surgery these absolute IOP values should be compared with discretion. It is important to note, however, that the percent IOP drop is greater in the combined procedure group. In both cohorts IOP at 24 months was approximately 12.5 mmHg. This likely reflects an attrition bias common to most retrospective studies, ie, fewer eyes to be evaluated at late stages of follow-up thus reducing statistical power at those time points. It may also indicate loss of efficacy of iSI over time.
Refractive error was reduced in both groups relative to baseline with no difference in final refractive outcome between groups. This was likely because IOL selection was aimed for emmetropia as is conventional in modern practice. This study is one of the first to confirm the refractive stability of iSI in that it does not interfere with refractive outcomes of cataract surgery. Expectedly, visual field indices remained unchanged after the combined procedure.
All retrospective studies share a potential for systematic bias relating to patient selection and loss to follow-up. Cases included in this audit were identified through a comprehensive review of electronic medical records designed to provide a complete cohort of patients reviewed by the same surgeon for either procedure. Despite this, the interval between 2016 and 2018 from which cases were selected limited the opportunity for some patients to receive the full duration of follow-up after 24 months. However, it is unlikely that this would have significantly skewed results from within the first 12 months of follow-up. A washout period was not included in the study design for ethical and safety reasons. The IOPs included in this audit were therefore likely to be better controlled at baseline when compared to those described in other studies with medication washout. This would almost certainly have masked a more exaggerated drop in IOP during the early stages of follow-up. Finally, only few POAG patients of the practice received phacoemulsification alone and in order to prevent crossover between intervention groups those in the single intervention group did not have POAG. Although this does not impact the validity of analyses performed within-groups it should be acknowledged as a limitation of between-groups analyses.
Future studies should aim to evaluate long-term medication outcomes in Australian patients following combined iSI and phacoemulsification. Secondary endpoints might be expanded to include retinal nerve fibre layer volume over time. A cross-sectional, comparative analysis directly quantifying the impact of reduced medication dependence on patient satisfaction using QoL metrics would also encourage a better understanding of the role of iSI in the landscape of glaucoma treatment in Australia.
Conclusions
Combined iSI and phacoemulsification, as discussed in this study, was capable of reducing medication dependence in Australians with coexistent cataract and POAG. The procedure seemed to be at least as effective in controlling IOP as medical therapy and was not associated with interference in postoperative refractive outcomes. It may therefore be a valuable therapeutic tool in relieving medication burden at the time of cataract surgery.
Summary
In a cohort of Australians with open-angle glaucoma combined iStent® Inject and phacoemulsification reduced dependence on topical therapy after 4 weeks. Pressures were reduced over 24 months relative to baseline without interference in refractive outcomes.
Acknowledgments
Thank you to the Department of Ophthalmology at the Royal Melbourne Hospital and to the staff at Eye Surgery Associates (East Melbourne and Vermont South). The abstract of this paper was presented as a poster with interim findings at the Royal Australian and New Zealand College of Ophthalmologists Congress in 2018 and the 8th World Glaucoma Congress in 2019. The poster’s abstract was published in “Poster Abstracts” in Clinical & Experimental Ophthalmology: https://doi.org/10.1111/ceo.13405.
Abbreviations
BCVA, best-corrected visual acuity; IOP, intraocular pressure; POAG, open-angle glaucoma; SLT, selective laser trabeculoplasty; TM, Trabecular meshwork.
Disclosure
Dr Simon E Skalicky received travel fees for local interstate conferences held by Glaukos Corp and also honoraria from Glaukos as an invited speaker. The authors report no other conflicts of interest in this work.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1(6):e339–e49. doi: 10.1016/S2214-109X(13)70113-X [DOI] [PubMed] [Google Scholar]
- 3.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360(11):1113–1124. doi: 10.1056/NEJMra0804630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapetanakis VV, Chan MP, Foster PJ, Cook DG, Owen CG, Rudnicka AR. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br J Ophthalmol. 2016;100(1):86–93. doi: 10.1136/bjophthalmol-2015-307223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resch H, Garhofer G, Fuchsjager-Mayrl G, Hommer A, Schmetterer L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009;87(1):4–12. doi: 10.1111/aos.2009.87.issue-1 [DOI] [PubMed] [Google Scholar]
- 6.Su WW, Cheng ST, Ho WJ, Tsay PK, Wu SC, Chang SH. Glaucoma is associated with peripheral vascular endothelial dysfunction. Ophthalmology. 2008;115(7):1173–8 e1. doi: 10.1016/j.ophtha.2007.10.026 [DOI] [PubMed] [Google Scholar]
- 7.Kong GY, Van Bergen NJ, Trounce IA, Crowston JG. Mitochondrial dysfunction and glaucoma. J Glaucoma. 2009;18(2):93–100. doi: 10.1097/IJG.0b013e318181284f [DOI] [PubMed] [Google Scholar]
- 8.Chrysostomou V, Rezania F, Trounce IA, Crowston JG. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr Opin Pharmacol. 2013;13(1):12–15. doi: 10.1016/j.coph.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 9.Noecker R, Miller KV. Benzalkonium chloride in glaucoma medications. Ocul Surf. 2011;9(3):159–162. doi: 10.1016/S1542-0124(11)70025-8 [DOI] [PubMed] [Google Scholar]
- 10.Inoue K. Managing adverse effects of glaucoma medications. Clin Ophthalmol. 2014;8:903–913. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ting NS, Li Yim JF, Ng JY. Different strategies and cost-effectiveness in the treatment of primary open angle glaucoma. Clinicoecon Outcomes Res. 2014;6:523–530. doi: 10.2147/CEOR.S30697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Realini T. Selective laser trabeculoplasty: a review. J Glaucoma. 2008;17(6):497–502. doi: 10.1097/IJG.0b013e31817d2386 [DOI] [PubMed] [Google Scholar]
- 13.Nagar M, Luhishi E, Shah N. Intraocular pressure control and fluctuation: the effect of treatment with selective laser trabeculoplasty. Br J Ophthalmol. 2008;93(4):497–501. doi: 10.1136/bjo.2008.148510 [DOI] [PubMed] [Google Scholar]
- 14.Song J, Lee PP, Epstein DL, et al. High failure rate associated with 180 degrees selective laser trabeculoplasty. J Glaucoma. 2005;14(5):400–408. doi: 10.1097/01.ijg.0000176939.43681.c2 [DOI] [PubMed] [Google Scholar]
- 15.Ayala M, Chen E. Long-term outcomes of selective laser trabeculoplasty (SLT) treatment. Open Ophthalmol J. 2011;5:32–34. doi: 10.2174/1874364101105010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razeghinejad MR, Fudemberg SJ, Spaeth GL. The changing conceptual basis of trabeculectomy: a review of past and current surgical techniques. Surv Ophthalmol. 2012;57(1):1–25. doi: 10.1016/j.survophthal.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 17.Watson PG, Jakeman C, Ozturk M, Barnett MF, Barnett F, Khaw KT. The complications of trabeculectomy (a 20-year follow-up). Eye (Lond). 1990;4(Pt 3):425–438. doi: 10.1038/eye.1990.54 [DOI] [PubMed] [Google Scholar]
- 18.Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96–104. doi: 10.1097/ICU.0b013e32834ff1e7 [DOI] [PubMed] [Google Scholar]
- 19.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, et al. Mid-term evaluation of the new Glaukos iStent with phacoemulsification in coexistent open-angle glaucoma or ocular hypertension and cataract. Br J Ophthalmol. 2013;97(10):1250–1255. doi: 10.1136/bjophthalmol-2012-302394 [DOI] [PubMed] [Google Scholar]
- 20.Fea AM, Belda JI, Rekas M, et al. Prospective unmasked randomised evaluation of the iStent inject versus two ocular hypotensive agents in patients with primary glaucoma. Clin Ophthalmol. 2014;8:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voskanyan L, Garcia-Feijoo J, Belda JI, et al. Prospective, unmasked evaluation of the iStent(R) inject system for open-angle glaucoma: synergy trial. Adv Ther. 2014;31(2):189–201. doi: 10.1007/s12325-014-0095-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klamann MK, Gonnermann J, Pahlitzsch M, et al. iStent inject in phakic open angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2015;253(6):941–947. doi: 10.1007/s00417-015-3014-2 [DOI] [PubMed] [Google Scholar]
- 23.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, Morales-Fernandez L, Fernandez-Perez C, Garcia-Feijoo J. Glaukos iStent inject trabecular micro-bypass implantation associated with cataract surgery in patients with coexisting cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmol. 2016;2016:1056573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodapp E, Parish R, Anderson D. Initial treatment of primary open-angle glaucoma. Clin Decisions Glaucoma. 1993;52(9). [Google Scholar]
- 25.Glaukos Corporation. iStent Inject® Trabecular Micro-Bypass System Instructions for Use. 2 ed. 2017; 1–2. [Google Scholar]
- 26.Poley BJ, Lindstrom RL, Samuelson TW, Schulze R Jr. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg. 2009;35(11):1946–1955. doi: 10.1016/j.jcrs.2009.05.061 [DOI] [PubMed] [Google Scholar]
- 27.Shrivastava A, Singh K. The effect of cataract extraction on intraocular pressure. Curr Opin Ophthalmol. 2010;21(2):118–122. doi: 10.1097/ICU.0b013e3283360ac3 [DOI] [PubMed] [Google Scholar]
- 28.Alnawaiseh M, Muller V, Lahme L, Merte RL, Eter N. Changes in flow density measured using optical coherence tomography angiography after iStent insertion in combination with phacoemulsification in patients with open-angle glaucoma. J Ophthalmol. 2018;2018:2890357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz LJ, Erb C, Carceller Guillamet A, et al. Long-term titrated IOP control with one, two, or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clin Ophthalmol. 2018;12:255–262. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]