Abstract
Rapid emergence of aggressive, multidrug-resistant Mycobacteria strain represents the main cause of the current antimycobacterial-drug crisis and status of tuberculosis (TB) as a major global health problem. The relatively low-output of newly approved antibiotics contributes to the current orientation of research towards alternative antibacterial molecules such as advanced materials. Nanotechnology and nanoparticle research offers several exciting new-concepts and strategies which may prove to be valuable tools in improving the TB therapy. A new paradigm in antituberculous therapy using silver nanoparticles has the potential to overcome the medical limitations imposed in TB treatment by the drug resistance which is commonly reported for most of the current organic antibiotics. There is no doubt that AgNPs are promising future therapeutics for the medication of mycobacterial-induced diseases but the viability of this complementary strategy depends on overcoming several critical therapeutic issues as, poor delivery, variable intramacrophagic antimycobacterial efficiency, and residual toxicity. In this paper, we provide an overview of the pathology of mycobacterial-induced diseases, andhighlight the advantages and limitations of silver nanoparticles (AgNPs) in TB treatment.
Keywords: nanoparticles, antimycobacterial, Mycobacterium, tuberculosis, macrophage, granuloma
Introduction
The emergence of multidrug-resistance, the intercurrent immunosuppressive diseases, the relatively low-output and high costs of newly-approved antituberculous antibiotics, and the partially protective vaccines, represents the main cause of the current status of tuberculosis as a regionally re-emerging and global health problem1–4 slowing in the same time the progress towards TB eradication. Tuberculosis infects globally more than one-third of human population,5 and despite the lastest progress, it remains according to the latest WHO report the world’s leading infectious-bacterial cause of deaths among adults, accounting only in 2018 more than 1.5 million deaths and 10 million new cases.6,7 Moreover, in some endemic areas, TB was the first cause of hospital death.8
Tuberculosis (TB) is a zoonotic and anthropozoonotic disease with a complex pathogenesis, produced by bacteria from Mycobacterium tuberculosis complex (MtbC), mainly M. tuberculosis, and in a lesser amount by the infections with other mycobacteria such as M. bovis, M. africanum, M. caprae, M. canetti, and occasionally Mycobacterium pinnipedii or M. microti.9–11 For some newly included members of MtbC as M. mungi,12 the exact role in human tuberculosis is currently poorly understood. MtbC bacteria are nonmotile and non-sporulated bacilli with a distinctively thick and lipid-rich cell wall included in the Actinomycetales order. The emergence of drug-resistant strains of MtbC observed in the last decades gives rise to additional challenges to the anti-TB prevention and control efforts.8
Nanotechnology and nanoparticle science are emerging disciplines connecting interdisciplinary areas of research such as chemistry, physics, and medicine providing innovative approaches and new-practical solutions for several critical-issues, including bacterial-induced infectious diseases.13,14 Metallic silver has a long history in medical applications, but its popularity markedly declined following the introduction and broad-usage of antibiotics.15 Nowadays, in the context of continuous rise in the rate of antibiotics consumption and “antibioresistance crisis”, silver in the form of AgNPs or in combination with classical antibiotics has made a remarkable comeback as a potential antibacterial molecule in the medicine and health care industry.16,17 Intracellular survival represents peculiar pathogenic factors of Mycobacteria, and this combined with the thick, hydrophobic (waxy) bacterial cell wall rich in mycolic acid and arabinogalactan contributes to the “phagocyte sabotage”, failure of the immune system to clear the septic focus, ensures the long-term persistence and furthermore, the local to systemic dissemination of infection.18 Recent reports have shown that AgNPs have a high antimycobacterial effect in both bacterial cultures and within macrophages,19,20 thus, the exploration of this new-concept of antimycobacterial-nanoparticles could change the current optics regarding TB-therapy.
This review explores in detail the main pathological features of mycobacteria and TB-pathogenesis, the AgNPs antibacterial mechanism of action per se and in combination with antibiotics, and not least the advantages and the limitation on using AgNP in TB therapy. Also, we up-to-date review of the main in vitro, in vivo and clinical studies assessing the antimycobacterial potential of AgNPs.
The Emergence of Drug Resistance Tuberculosis
Drug-resistant (DR) (defined as resistant to one or more antituberculosis drugs) and finally Multi-Drug-resistant tuberculosis (MDR) (defined as antibioresistace to at least rifampicin and isoniazid, the two most powerful antituberculosis drugs)7 is the most urgent and difficult provocation in TB treatment, a major public health concern, and an important cause or global TB reemergence noticed in the last three decades.21,22 New cases of both DR and MDR are typically expected to appear following the amplification of TB-resistance patterns through inadequate usage of antituberculosis chemotherapy, mainly the therapeutic use of ineffective-antibiotics formulations as first-line treatment and the premature stoppage of treatment and not last the inter-patient transmission of DR/MDR/XDR (Drug-/Multidrug-/Extensively drug-resistant tuberculosis) strains of TB, especially observed in areas with a high prevalence of DR/MDR-TB infections of following nosocomial transmission.7,23,24 Infection with MDR-TB strains is associated with a high mortality rate (up to 55%, compared to 4.5−17% mortality in infections with nonresistant TB-strains). A low treatment success despite the usage of appropriate second-line treatment, and typically spans a relatively short clinical course from diagnosis to death, especially in cases with concurrent infections like HIV or reduced body mass index.7,25-27
The current antituberculous therapy involves the first-line treatment during a 6 to 9 months, involving four antibiotics in sequential combination (isoniazid, rifampin, pyrazinamide, and ethambutol). In case of relapse or antibioresistace, the second-line therapy-treatment (during 18–24 months) of combination therapy with second-line drugs as aminosalicylic acid, fluoroquinolones, aminoglycosides, cycloserine, linezolid, and clofazimine, which are typically more toxic, more expensive and less efficient.28–30 In addition to poor efficiency in the case of MDR and XDR-strains of M tuberculosis, major adverse reactions (mainly hepatitis, gastrointestinal events) are present in more than 30% of cases following first-line therapy31 and in 83% following the second-line antituberculous therapy.32 Following the second-line antituberculous therapy, the adverse reactions are more severe and include mainly gastrointestinal and hepatic reactions, CNS adverse effects (including reactions raging from insomnia to psychosis and delirium), arthropathies, nephrotoxicity and electrolyte abnormalities, ototoxicity, hypothyroidism and hematological toxicity.30,33
The prolonged antituberculous therapy, limited antibacterial activity and intercurrent diseases are the main reason for patient noncompliance and finally, the induction of DR/MDR/XDR strains MtbC. The DR reaches approx. 20% among the previously treated TB patients, while the MDR tuberculosis appears in 4–10% of the same group population.34
The development of new antimycobacterial drugs and identification of new drug targets must take into account firstly the peculiarity of MtbC pathogeneses35 and the high adaptability of this classically known as an intracellular bacterial pathogen. The most intriguing property of MtbC assured by over 150 virulence factors5,36 is the capacity of MtbC to survive and multiply in certain conditions inside the macrophages, monocytes and dendritic cells.8
Mycobacterial Infection Pathology
Mycobacteria are classified according to their pathogenesis and role in human tuberculous as Mycobacterium tuberculosis complex (detailed above) and non-tuberculous mycobacteria (NTM, previously named “atypical mycobacteria”) (e.g. M. avium, M. kansasii, M. terrae, M. abscessus, etc).37,38
Non-tuberculous mycobacteria are ubiquitous, free-living, acid-fast bacteria, generally with reduced human pathogenicity (most of them are saprophytic) compared with M. tuberculosis complex. Even so, infections with both types of mycobacteria have several common characteristics and some NTM are used as infectious agents in experimental models of tuberculosis (e.g. Mycobacterium marinum in the zebrafish model of tuberculosis).39 This material is mainly intended to review the pathogenesis of bacteria included in the M. tuberculosis complex with few examples of NTM when adequate.
Although a dual intracellular and extracellular-type of infectivity is described for MtbC, the essential mechanism of disease in TB is based on the ability of mycobacteria to inhibit within the cells of the monocyte-macrophage system (MMS) the fusion of the phagosomes (containing microbes) with.18 Modulation of macrophage intracellular organelle compartment is essential not only for MtbC survival but also for its intracellular multiplication. Replication within the MMS-cells leads not only to the destruction of these cells but also of all cell populations surrounding the inflammatory focus. Within the affected organ and regional lymph nodes, this process will result is massive caseous necrosis and formation of a granulomatous reaction (caseating granuloma/tubercle)18,40 with a typical morphology.
Virulence and Pathogenesis Factors of Mycobacterium Tuberculosis
The complex pathogenicity of MtbC is determined by a plethora of virulence factors and literature dedicated to these factors is vast.5,41,42 This is particularly important in the disease process and gives TB a peculiar progression of biological events and interaction with the immune cells.
In a comprehensive review by Forrellad et al5 the MtbC virulence factors were classified in nine groups based on their activity, chemical structure and bacterial location: (1) virulence factors involved in the metabolism of lipids and fatty acids, (2) bacterial-wall proteins and lipoproteins (including secretion systems cell wall), (3) proteins suppressing the antimicrobial effectors of macrophage, (4) proteases (5) protein kinases (6) proteins involved in metal transport, (7) regulator gene, (8) proteins of unknown function and (9) other virulence proteins.5
The main virulence factors and the mechanisms by which they enhance MtbC infectious capability and resistance are summarized in Table 1.
Table 1.
A Synopsis of the m Tuberculosis Main Virulence Factor and Their Pathogenic Mechanism
| Virulence Factor | Mechanism of Bacterial Virulence |
|---|---|
| ● Lipoarabinomannan (LAM) and Mannose-capped-LAM | Bacterial Adherence and phagocytosis by macrophages43 |
| Inhibits phagosome maturation44 and phagolysosomal fusion45 | |
| Block transcription of IFN-g, antioxidative defense and inhibition of protein kinase C activity46 | |
| DownregulateTh1 cytokine expression47 | |
| Induction of IL-10 production and Inhibition of dendritic-cell maturation48 | |
| ● Lipomannan | Induction of IL-12 production and apoptosis in macrophages49,50 |
| ● Cord factor (Trehalose-6,6´-dimycolate) | Inhibits acidification of phagolysosome, delayed maturation of phagosomes, phagosome-lysosome fusion51,52 |
| TB-granuloma development and maintenance(dependent mainly on TNF-α and IL6 increased production) and cachexia53,54 | |
| Damage to mitochondria membranes and oxidative phosphorylation impairment55,56 | |
| Induction of apoptosis and thymus atrophy57 | |
| ● Phosphatidylinositol mannosides | Granuloma development and maintenance58 |
| Inhibition of TNF, IL-12p40 production within macrophages59 | |
| ● Phthiocerol dimycocerosate and phenolic glycolipids | Evade recruitment of MyD88-dependent macrophage populations60 |
| Intracellular bacterial survival (bacterial protection against nitrogen intermediates species)61 | |
| Bacterial Adherence and phagocytosis by macrophages62 | |
| Phagosome membrane rupture followed by apoptosis63 | |
| ● Twin-arginine transporter | Cell wall biogenesis and resistance to beta-lactam antibiotics64,65 |
| ● Exported repetitive protein (Erp) | Intracellular MTb growth66 |
| ● ESAT-6 family | T cell stimulation (gamma interferon release)67 |
| Delayed-type hypersensitivity68 | |
| Downregulate ROS production and LPS‐induced nuclear factor‐κB activity in macrophage69 | |
| Inhibit TLR2-mediated signaling in macrophage70 | |
| Apoptosis of macrophage71 | |
| Cytolysis of macrophages, red blood cells,72 and pneumocytes73 by pore formation | |
| Bacterial translocation from the phagolysosomes to the cytoplasm74 | |
| ● Phenolic glycolipids | Immunosuppression (release of this pro-inflammatory mediators)75 |
Entry into Macrophages, Monocytes, and Dendritic Cells
The route of entry into the organism of MtbC is most often by inhalator route; the digestive pathway and other nonrespiratory route are less important for the TB transmission and are often used by other Mycobacteria of MtbC group (e.g. M. mungi is transmitted by an environmental pathway mainly through anal gland secretions and infected urine).76
Following the initial mechanical entrapment in the bilaminar protective mucus covering the respiratory or digestive system, mycobacteria enter in contact with the local macrophages (occasionally suspended in the respiratory mucous blanket) or, rarely, with intestinal M cells. Following mainly a specific ligand–receptor interaction with the membrane receptors (pattern recognition receptors-PPR) of macrophages, mycobacteria are engulfed by phagocytosis (Figure 1). Although macrophages are the main cells responsible for MtbC engulfment, all cells or the MMS, including monocytes and dendritic cells are capable of MtbC phagocytosis.39,77 Other professional-phagocytic cells as neutrophils, although are capable to phagocytose and destroy MtbC,78 have a less-known of the role in TB infection.
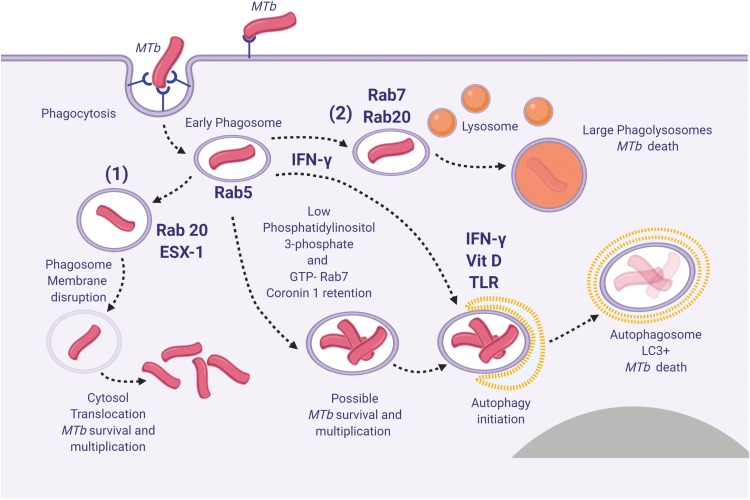
Figure 1.
Spatiotemporal dynamic model of the possible fates of Mycobacterium tuberculosis (MTb) following macrophage phagocytosis (1) MTb can prevent early phagosome maturation and by the action of Rab20-trafficking, the ESX-1 will destabilize and disrupt the phagosome membrane allowing MTb direct access into the macrophage cytosol, followed in certain conditions by MTb survival and multiplication; (2) Some early phagosomes will undergo normal maturation, will fuse with the lysosomes and MTb will be killed (by reactive nitrogen intermediates, low pH, ROS, antimicrobial peptides and Fe deprivation mediated by iron scavengers, as lactoferrin, and NRAMP1);80 occasionally MTb can survive within the mature phagolysosome; (3) Blocking of the early phagosome maturation (mainly by inhibiting PI3P generation) followed by intravesicular MTb replication; (4) Delivery of the early endosomes or early-endosomes-to autolysosomes, where typically the activity of Mtb will be suppressed. Inspired from Philips et al81 and Schnettger et al.82 Figure 1 was created using BioRender.
Abbreviations: NRAMP1, natural resistance-associated macrophage protein 1.
There are several phagocytic receptors (surface-expressed PPRs) that assures MtbC recognition and phagocytosis by macrophages/newly-recruited monocytes, such as those for: complement (CR1, CR3, and CR4), macrophage mannose receptors, CD14, surfactant protein receptors (surfactant protein A) (Sp-A), Fc (FcR) and macrophage scavenger receptors.18,36 These receptors recognize different components of MtbC: lipoarabinomannan (LAM) from the bacterial cell wall is recognized by CD14 and macrophage scavenger receptors, mannose, and mannose-capped-LAM by the macrophage mannose receptor, polyanionic macromolecules by the scavenger receptors and mycolyl-arabinogalactan by the intracellular NOD2 receptors.
Typically, the recognition and MMC-internalization of MtbC is mediated through the interaction of several of the PPRs listed above. The active types of PPRs influence the downstream inflammatory events and the fate of MtbC infection. Also, some intracellular PPRs as NOD2 (nucleotide oligomerization domain protein) are able to recognize the MtbC and further regulate the inflammatory process79 (mainly mediated through the NF-kB pathway). The involvement of these receptors could also be sequential, dominating different stages of the MtbC infection (engulfment in the early infections vs phagocytosis in systemically disseminated TB).
Replication in Macrophages
Once internalized in macrophages (or other MMC), MtbC resides in a phagocytic vacuole where they are capable to delay or block the fusion of primary/early phagosomes with lysosomes (Figure 1) and thus to prevent the maturation, acidification of lysosomes, MtbC destruction, and activation of other antimycobacterial mechanisms.18,83 This process is actively mediated by MtbC and implies a reduction of proton ATPase amount within the phagosome and inhibition of Ca2 signals84,85 although the exact events that lead to this effect are still controversial. Several MtbC pathogenic factors as sulfolipids, trehalose dimycolate, lipoarabinomannan/mannose-capped-lipoarabinomannan (MC-LAM), tryptophan aspartate coat protein (TACO) and SapM are involved in this process.86–89 Finally, the mycobacterial phagosomes have the biochemical features of the early endosomes90 and are a favorable milieu for MtbC replication and systemic (lymphatic and/or sanguine) dissemination.
Even if these mechanisms seem to robustly block the phagosome-lysosome activity, several acute-phase cytokines (as IL-1 and tumor necrosis factor-TNF) and IFNγ can stimulate the MtbC-infected macrophages to overcome this dysregulation of the intracellular compartments and to regain the antimycobacterial activity (essentially by changing the macrophage polarization state-discussed below).
Tuberculosis Progression: Th1 to Th2 Response Imbalance
The polarization of the immune system activity is critical in the control and evolution of the MtbC infections. The CD4+ T lymphocytes orchestrate by the types of cytokines produced the inflammatory process (including the autoimmune processes) and are responsible for the normal multi-step evolution of a typical inflammation.
In tuberculosis, initially, a TH1 response induces a “classically” activated, M1-bactericidal macrophage (which mainly by secreting IFN-γ is able in certain limits to control the initial MtbC infection). Additional to TH1, also the TH17 cells are considered to induce a protective inflammatory response during MtbC infection.91
By the TH2 response CD4+ T secrete IL-4, IL-5, and IL-13 (promoting an “alternative” M2-activated macrophage); M2-polarised macrophages are commonly responsible for a protective effect against extracellular pathogen.85 The TH2 response typically is not inducing any protective activity against MtbC infection and replication. Moreover, TH2 response is responsible for the development of delayed (Type IV/T cell-mediated) hypersensitivity to MtbC antigens (used as a diagnostic tool – intradermal reaction/tuberculin test), granuloma formation (Figure 2) and progression of clinical tuberculosis.91–93 Although with a relative opposing effect, both TH1/TH2 inflammatory “phenotypes” usually coexists in MtbC infections. The modulation of these two components during the TB evolution is under the influence of several factors, among which individual-genetic variations (“genotype”), immune-system reactivity, microbial products (MtbC strain), intercurrent infections and physiological status.
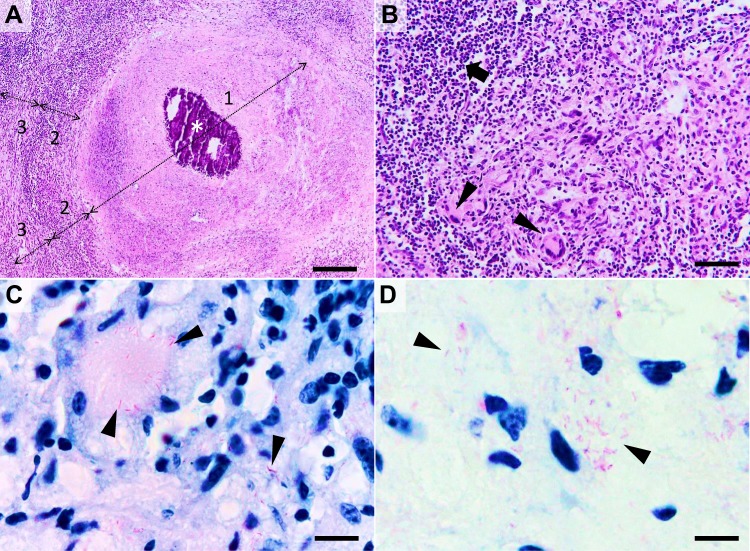
Figure 2.
Histological characteristics of a tuberculous granuloma in the late caseo-calcareous stage. Image (A) “caseating tubercule” consisting of a large central area of caseating necrosis (zone 1) with extensive calcification (asterisk), surrounded by a reactive rim (zone 2) of lymphocytes and macrophages (including macrophage-derived epithelioid and multinucleated giant cells) and bordered by a partially formed fibrous capsule (zone 3) focally infiltrated by the above-mentioned cells; Image (B) detail of the leukocyte rim (zone 2), depicting several multinucleated giant cells (Langhans type) (arrowheads) admixed with fewer histiocytes, macrophages, and lymphocytes (arrow). Image (C and D) many acid-fast bacilli located intracellularly within the Langhans type multinucleate giant cells and histiocytes (image (C), arrowheads) and extracellularly (image (D), arrowheads). Image (A and B), Hematoxylin and eosin stain; Image (C and D) Ziehl–Neelsen stain for mycobacteria; ob x 4 for image (A) (scale bar=500 µm), x20 for image (B) (scale bar=100 µm), and x100 for images (C and D) (scale bar=20 µm).
Persistence of Viable Mycobacteria in Dead Cells and Necrotic Tissue
The capacity of mycobacteria to hijack the type of macrophage calls death is well known,94 but recently a new adaptive mechanism of mycobacteria was found. Mainly, following macrophage necrosis and neutrophil necrosis, a subset of mycobacteria exploits the necrotic cell-debris as a nutrient-rich growing substrate.95 More interestingly is the fact that tissular necrosis tends to enhance the overall mycobacterial replication.96 In a dynamic representation of this pathogenicity, the macrophage and neutrophil necrosis represents the starting point for a vicious cycle which continues with the uptake of the Mtb-infected cell debris from the newly recruited monocytes and neutrophils, de novo Mtb replication, sustained infection and finally the induction of cell death.96,97 The mycobacteria can utilize this growing niche for enhanced replication and survival, contributes to the success of mycobacteria to resist host defense and antibacterial therapy.95,97
Metallic Nanoparticles as Antiinfective Agents
Due to the increasing capacity of bacterial pathogens to acquire resistance to classical anti–infectious agents, nosocomial infections become a major cause of morbidity in patients of all age groups.98 Metallic nanoparticles have unique antiviral, antibacterial, and antiparasitic properties, making them promising candidates for future applications in the treatment of infectious diseases.99 From this class of molecules, zirconium oxide (ZrO2NPs)100 and Co3O4@ZrO2 (CoZ) core/shell NP101 proved to have an antibacterial effect against both gram-negative (E. coli and Pseudomonas aeruginosa) and positive bacteria (Bacillus subtilis and Staphylococcus aureus), copper oxide nanoparticles (CuONP) have shown antifungal (Candida albicans) and antibacterial effect against gram-positive (Staphylococcus aureus and Staphylococcus epidermidis) and gram-negative (E. coli and Proteus vulgaris) bacteria13,102 and iron oxide nanoparticles (FeONP) bactericidal effect against E. coli, Klebsiella pneumoniae, and Staphylococcus aureus.103 Gold nanoparticles (AuNP) have shown broad antibacterial effect against both gram-positive (Staphylococcus epidermidis) and gram-negative (E. coli) bacteria,104 and following appropriate functionalization a selective antibacterial effect against methicillin-resistant Staphylococcus aureus.105 Also, gold nanoparticles (AuNP) synthesized from marine seaweed Gracilaria verrucosa and Gelidium pusillum shows good biocompatibility to human embryonic kidney cells even at high concentrations of 100 and 150 μgmL−1.106,107
Additionally to their antimicrobial properties, some form of nanoparticles posses also antiproliferative-antitumoral effect as was recently shown for magnesium oxide nanoparticles (MgONPs) synthesized from the brown algae Sargassum wighitii,108 for titanium dioxide (TiO2) nanoparticles109,110 and for AgNPs synthesized from Enteromorpha compressa.111 Also, TiO2 nanoparticles show immunomodulatory effects,112 having a hypothetical application in infectious diseases with a hypersensitive component (including some phases of TB).
Moreover, some nanoparticles as copper nanoparticles (CuNPs) show catalytic degradation of organic dyes with application in wastewater treatment113 and interestingly, some nanoparticles as CuO and CuO/Cu(OH)2 show multimodal effects including in addition to antibacterial effects against E. coli and S. aureus also photocatalytic activity with potential application in wastewater management114 and a dose-dependent anticancer activity against tumor rat C6 cell line.115 A similar photocatalytic activity was shown also for zinc oxide nanoparticles synthesized from Cyanometra ramiflora.116
From the metallic nanoparticles, AgNPs are the most popular choice as anti–infectious nanoparticle-adjuvants.17 In conjunction with appropriate-drug delivery systems as chitosan117 AgNP per se or in combination with proanthocyanidin shown also a good in vitro antitumoral effect, against HT 29 human adenocarcinoma cells.118,119 The antibacterial properties of AgNP, their mechanism of action and especially their antimycobacterial effects will be further detailed.
Silver Nanoparticles as an Emerging Therapeutic Approach in Mycobacterial Infections
Silver per sei or incorporated in different compounds has long been used empirically as antimicrobial agents and tested since the XIX century as a natural antibiotic.15,120
In the quest for more efficient antimycobacterial drugs that are able to overcome the “classical” issues discussed above and partially responsible for the global TB status, the antibacterial peptides and nanoparticles gained recently special attention.19,121 Several classes of nanoparticles with intrinsic antibacterial and antibiofilm effects are proven,122 including metallic nanoparticles (e.g copper,123 iron,124 gold125 or silver-based126), carbon nanotubes,127 polysaccharides as chitosan128 and chitosan in conjunction with polycationic polymer129 or combinations of the above-mentioned antibacterial molecules as chitosan-gold NP.130 Among these antibacterial nanoparticles, due to their strong antibacterial activity and long-history of using silver as antiseptic, AgNPs have received most of the attention.131,132 This new paradigm in antituberculous therapy is based on the fact that the efficiency of Ag was already proven for many classes of bacteria and their microorganisms, the long tradition in using Ag salts as disinfectants,15 and due to the fact that unlike antibiotic drugs, most of the currently known pathogenic bacteria rarely develop resistance to metallic nanoparticles. The conditions under which this phenomenon can appear will be discussed in a separate section.
Antibacterial Effect and Mechanism of Silver Nanoparticles
Antibacterial action of AgNPs is mediated by several, generally, accepted-mechanisms (depicted in Figure 3) which in a biological context have a complementary action: 1) Direct contact with the bacteria components (biofilm and bacterial cell wall); 2) Release of bioactive ions (ex. Ag+ ions); 3) Disruption of several metabolic pathways; 4) Generation of reactive oxygen species (ROS); 5) Genotoxicity; 6) Alteration of cell wall and cytoplasm; 7) Inhibition of bacterial DNA replication; 8) Alteration of bacterial membrane permeability and ionic change.133–140
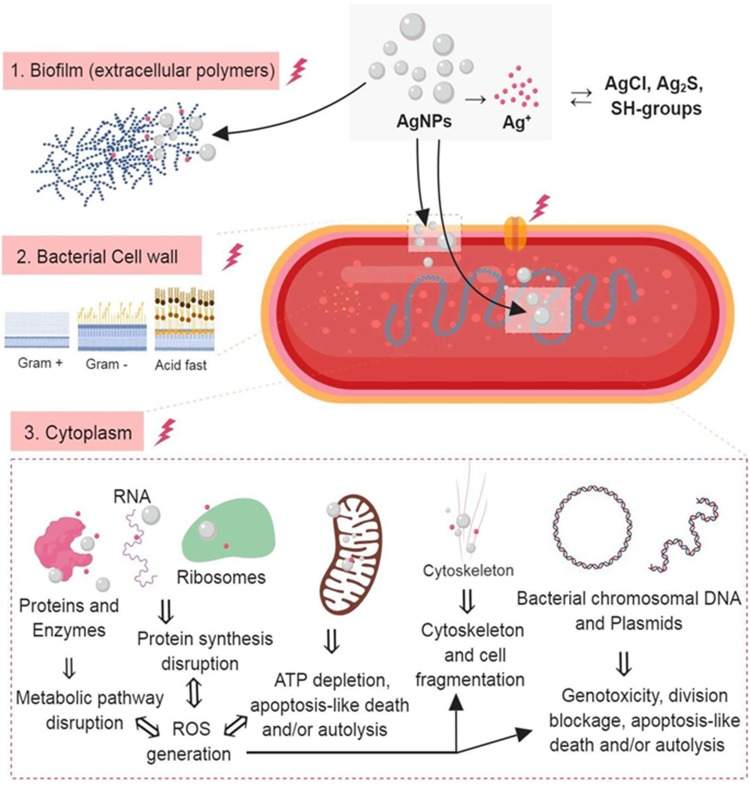
Figure 3.
The three most important routes of antimicrobial action of AgNPs. 1. Accumulation and disruption of the extracellular polymers of the bacterial biofilm; silver ions (Ag+) could also biochemically alter the biofilm overall adherence, structure, and porosity.2. AgNPs adhere to bacterial cell surface (documented for Gram-positive, negative and also for the acid-fast bacteria) resulting in microbial membrane disruption, altered transmembranar transport, cellular content leakage (mainly electrolytes dysregulation) and bacterial death (apoptosis/lysis); as for the biofilm, Ag+ generated extracellularly contribute to the microbial cell wall disruption by biochemical alteration of the SH– groups. 3. AgNPs penetrate bacterial cell wall and access microbial cytoplasm where can interact with the organelles, cytosolic molecules (as free amino acids, peptides, and enzymes) and bacterial cytoskeleton; By direct action of AgNPs and Ag+ results the alteration of several metabolic pathways, bacterial organelles dysfunction (mainly mitochondria), ROS generation and bacterial DNA alteration ultimately causing cell death apoptosis/lysis). Figure 3 was created using BioRender.
Abbreviations: AgNPs, silver nanoparticle; ROS, reactive oxygen species: Ag+, silver ions.
These effects are mediated mainly by the primary action of the AgNP, or by the release of Ag+ species and ROS will further disrupt the metabolic pathways and DNA. Although the main antibacterial effect of AgNPs is believed mediated by the release of bio-active Ag+ ions,141 more exactly, the AgNPs antibacterial mechanisms employ targeting multiple components in the bacterial cell,142 including bacterial wall (disruption and/or increasing the membrane permeability), tRNA (transfer ribonucleic acid), inactivating the respiratory chain (ATP depletion), enzyme and protein synthesis and DNA-binding (resulting cleavage, inhibition of replication).138–140
The overall bactericidal effect of AgNPs depends, in addition to the rate of Ag+ production, also on the AgNPs size and shape, overall NP surface area, type of coating/corona, and rate of Ag+ generation.133–140 The difference in the efficiency of AgNPs against Gram-positive, Gram-negative or acid-fast bacteria is believed to be mainly dependent on the structural and thickness differences of their cell walls. Usually, acid-fast bacteria due to the presence of a thicker-waxy cell wall have a stronger defense-system against Ag-NPs. As in the case of the Gram-positive bacteria, this structural particularity prevents the action of Ag-NPs rendering acid-fast and gram-positive bacteria more resistance to the antimicrobial activity of Ag-NPs comparatively with Gram-positive bacteria.143,144 For example, the Gram-positive Bacillus subtilis, have a cell wall of 55.4 nm, the acid-fast M tuberculosis a 20.2 nm145 while the Gram-negative Pseudomonas aeruginosa, has a cell wall of only a 2.4 nm.146 Interestingly, although there are important functional differences between the mycobacteria cell wall and gram-positive bacteria, the DNA-based molecular taxonomy of bacteria based on the high similarity to genes, groups the classical acid fast-mycobacteria as gram-positive bacteria.147 But this overall generalization regarding the susceptibility towards AgNPs has many exceptions, thus, AgNPS synthesized from Bacillus brevis has a maximum antibacterial effect against the Gram-positive, multi-drug resistant for Staphylococcus aureus and moderate for the Gram-negative Salmonella typhi.148
Role of Ag in Particle State (Ag0) and Ag+ Species in Mediating the Bactericidal Effect of Silver Nanoparticles
Ag in Particle State (Ag0)
This is the first (direct or “primary”), of antibacterial effect of AgNPs and is considered to be due to: (1) nanoparticles damage the bacterial wall and on (2) entrance of particles into the bacterial cytosol and directly interact with the intrabacterial environment.149–151 The adherence of the AgNPs on the bacterial surface and formation of particle agglomerates is followed by disruption of bacterial membrane integrity by induction of cell-wall pits and gaps, and alteration in membrane selectivity and permeability, including ionic transport.151–154 This first, step is dominated by the wall changes is followed by bacterial-cytosol leakage, lost the intracellular contents and finally the collapse of the cell or apoptotic-like bacterial cell death and formation of an amorphous mass of cell debris.149,150,155 Due to the massive loss of the bacterial content the “ghost cells” morphology is used to describe lysed bacteria following this process.150,156
Nanoparticles have the property to be adsorbed at the bacterial membrane mainly by electrostatic adhesion, a process mediated by surface charge of the particle- the zeta (ζ)-potential – and the outer layers of the bacterial cell wall.151 Thus, a study designed to explore this surface-interaction between AgNP and bacteria (Bacillus spp), El Badawy et al found that positively charged BPEI-caped AgNPs were the most bacteriotoxic NPs, mainly due to the local agglomeration. The negatively charged citrate-caped AgNPs were the least bacteriotoxic.151 The outer layer of bacteria (G+) is negatively charged due to the presence of carboxyl, phosphate and amino groups,157 thus influencing the electro repulsion between bacteria and negatively charged AgNP. The highly-negatively charged bacterial wall is believed to be an important fact in explaining the superior activity of AgNP against G- compared with G+ bacteria which is frequently reported.158
In a similar study, positively charged AgNP by functionalization with PHMB functionalized exhibited superior antibacterial effects against E. coli. Also, the bactericidal activity of PHMB was enhanced by the combination with AgNPs.159 Indeed, this hypothesis was further confirmed by Ivask et al,160 which observed that the pathways involved in G- bacterial responses to AgNP are highly dependent on the surface characteristics of the Ag composite, including zeta (ζ)-potential.
At least partially the enhancement of the antibacterial effect of observed in AgNPs with surface-modified by surfactants (SDS) and polymers (PVP 360),161 in addition to stabilization of particles against aggregation, can be attributed to this facilitated-adhesion to the bacterial wall.
Additionally to the wall thickness and structure, the difference in resistance of different classes of bacteria can be explained by the fact that due to the high-presence of LPS the cell wall, Gram-negative bacteria has a higher negative charge, which promotes local adhesion and membrane-clustering of particles and finally enhances the antibacterial effect of Ag-NPs.144,162,163 Therefore, electrostatic interaction between bacterial cells (charged negatively) and AgNPs (charged positively) is critical for the antibacterial activity of NPs.144,161,164
Moreover, to the above-mentioned action against the bacterial wall, AgNPs have the ability to enter inside bacteria's cytosol, to form cytoplasmic precipitates and to disrupt several bacterial-physiological processes. The type of bacterial- metabolic pathways disrupted directly by the Ag in particle state is largely unknown.
Role of Ag+ Species
The antibacterial effect of AgNP is complementary enhanced by the local elimination of Ag+ species which have high affinity especially for thiols, selenols, organic amines and phosphates and forms strong covalent bonds.141 The formation of this covalent bonds (e.g. silver thiolate) in which Ag act as a bridging agent linking several thiols-groups for different molecules can irreversibly alter their tridimensional structure and function141,165 and finally will disrupt simultaneously several enzymatic pathways and constitutive cell-structure elements (DNA, cytoskeleton, plasmatic and organelle membrane, etc.). This multi-molecule disruption mediated by a broad chemical affinity and not by a targeted-element is the main cause of the complex antibacterial mechanism in comparison with classical antibiotics which typically target a narrow groups of molecules, as for example, restricted to cell membrane (beta-lactamides) or interfere with molecules synthesis and also broad spectrum of micro-organisms sensible to Ag.15 Regarding the involvement of different metabolic pathways following the above-described mechanism, probably one of the most important is the disruption of the ROS-regulation system (by interfering with reductase enzymes and other cofactors) and thus increasing their intracellular oxidative stress and triggering cell senescence or death. The ROS-generation as a mechanism of AgNP/Ag+ action will be separately discussed.
In the AgNP/Ag+ model of action, AgNPs acts as a nanoparticulate reservoir for the continuous release of Ag+ species. The rate of release of Ag+ is dependent on many factors including NP size, surface, porosity, O2 amount in the environment, and is mediated by release (“desorption”) of chemisorbed ions from the particulate surface, oxidative dissolution (which is the main way to release Ag+ in the aqueous environment).166
In a dynamic presentation of the plausible effect, the AgNP adherent on the bacterial-cell wall or entrapped inside the bacterial cytoplasm (“Trojan horse effect”) will release in the adjacent environment large amounts of Ag+ species generating a locally high concentration of antibacterial ions.160,167
Generation of Reactive Oxygen Species (ROS)
The generation of ROS is considered a second mechanism by which AgNPs can induce bactericidal or bacteriostatic effects. The ROS generation is due to (1) particle–cell interactions (alteration of local cell activity, e.g. inflammation-driven enhancement of oxygen respiration and oxidative/antioxidative imbalance) or due to (2) in situ production of hydroxyl radicals due to an Ag-mediated Fenton-like reaction168,169 (acellular induction of ROS). The presence of transition metals including Fe, Cu, or Cr as synthesis contaminants enhances ROS generation via direct catalytic Haber–Weiss and Fenton-type reactions.170 This in situ production of free radicals by AgNPs is usually enhanced by exposure to light-sources of variable wavelengths, this feature is currently explored also for photocatalytic degradation of pigments.171,172
The ROS generation within the activated cells is mediated by enhancement of: 1. cytoplasmic ROS (cytoROS) production by NADPH oxidase family of enzymes (e.g. endothelial, neuronal and inducible nitric oxide synthases)(eNOS, nNOS, iNOS) during inflammation; 2. Peroxisome ROS generation as a by-product of enzymatic activity (as hypoxanthine and β-oxidation, polyamine synthesis and amino acid deamination); 3. mitochondrial ROS (mitoROS) as a byproduct of metabolic-enzyme activity and mitochondrial respiration, activity upregulated, for example, by complex I NADH reductase and dehydrogenase via RET (reverse electron transfer) during inflammation. 4. lysosomal and phagolysosomal ROS mediated mainly by NADPH oxidase and myeloperoxidase produced mainly within the professional phagocytic cells (neutrophils, monocytes, and macrophages) during the intracellular destruction of microbes and removal of cell debris.85,173 AgNPs were shown to interact with all of the above systems, including increased expression of iNOS and generation of NO,174 impairment of mitochondrial function and ROS generation,175,176 upregulation of peroxisome oxidative stress-related genes, such as catalase,177 enhancement of phagolysosomal activity (reduction of lysosomes pH)178 and macrophage and neutrophil activation and stimulation of ROS generation.179
Another clear advantage of using AgNPs is based on the well-known fact that NP persists much longer in the body (even years) compared with the small molecule used currently in antibacterial therapy. This would increase the long term releasing of active compounds and thus the sustained therapeutic effects.142,180 Although AgNPs can have a direct effect on the microorganisms, the main effect is considered to be mediated through the biochemical interactions of Ag+.181,182
Presence of Antibacterial – Active Products in Biosynthesized AgNPs, a Possible Source of Antibacterial Synergy?
The antibacterial effect of AgNP, especially in the green-synthesis context (plant, viral, bacterial, fungic, and algal extracts or biomimetic compounds as reducing agents),183,184 can be, at least partially enhanced by the extra bio-active component introduced in the particle synthesis.185,186 AgNP can be prepared using elements that possess per se an antibacterial activity. The synergistic effect between AgNP and other and bioactive phytocompounds can be expected in the green-synthesis, leading to antibacterial effects via different mechanisms as those described above.185 Also, the concentrations of antibacterial-active compounds can be observed below the minimal dose of individual compounds. Therefore, the enhanced antimicrobial effect of NP synthesized by green-extraction which can be occasionally observed can also be determined by the presence of the bioactive molecules of the synthesis attached on the surface of nanoparticles as stabilizing agent.186,187
In the green-synthesis of AgNP, the biological extracts are mixed with the metal salt solutions, the bio-extract (containing starch, steroids, sapogenins, flavonoids, terpenoids, amino cellulose, etc.)188 acts in situ as reducing agents of the silver salts (Ag+) to form metallic silver Ag0, and also as capping agents to provide stability of silver nanoparticles in solution186,189 and partially can be further be found in the structure of the AgNP as surface-stabilizing ligands.190
Indeed, in a recent study, Shaik et al190 tested the efficiency of AgNP synthesized from Origanum vulgar against various bacteria (Escherichia coli, Shigella sonnei, Micrococcus luteus), and fungi (Aspergillus flavus, Alternaria alternate, Paecilomyces variotii, Phialophora alba). The bactericidal and antifungal efficiency was proportional to the amount of plant extract employed for the preparation of AgNPs. Similar findings of obtaining biogenic NP with broad antibacterial effect were reported by Pugazhendhi et al from AgNP synthesized from red algae Gelidium amansii.191
Also, a study which compares the antibacterial effect of AgNP produced by green synthesis (from S. persica) versus chemical against Gram-negative (E. coli and P. aeruginosa) and Gram-positive (M. luteus and S. aureus) bacteria shows that the green synthesized Ag-NPs exhibited slightly higher antimicrobial activity in comparison to the chemically synthesized Ag-NP.158 The conclusion of the study was that, although S. persica root has antibacterial properties per sei, due to the small amount of active compound included in the synthesis process, the increased activity of the green-synthetized Ag-NPs was mainly due to the improved solubility of the Ag-NPs rather than the microbicidal potential of plant-derived compounds used for the synthesis of NPs.
In a study designed to assess the antibacterial effect of AgNPs from Asparagusspp. against 4 mycobacterium species (M.tuberculosis, M.pheli, M.avim, and M. smegmatis), Kote et al185 found a direct connection between the green approach of AgNPs synthesis (mainly due to the enhanced stability) and the antimycobacterial effect. Also, some forms of biosynthesized AgNPs have multimodal action, proving simultaneous antibacterial, antimycotic and antitumoral effects as was recently shown for AgNPs produced from Phoenix dactylifera.192
In the above-mentioned studies, the enhancement of the antibacterial efficiency of AgNP produced by green synthesis is more likely mediated by the uniformity of the dispersion and a better stabilizing of molecules in aqueous solution compared with chemical synthesis.
In a study exploring comparatively the antimycobacterial effects of green-synthetized vs chemically produced AgNPs found that chemically AgNP exhibited greater efficiency in terms of mycobacterial inhibition, specificity and selectivity compared with bio-AgNPs193
Thus, although the presence of co-synthesis products in the green-synthesis of AgNP definitely have a role in determining and fine-tuning the biological activity of the obtained nanoparticles,194 the exact mechanisms and the possible synergism with Ag in mediating antibacterial activity should be further explored.
Enhancement of Antibacterial Efficiency Antibiotics by AgNP
An emerging practice in antituberculous experimental therapy is to combine a metallic nanoparticle (TiNP, CuNP, AuNP, AgNP, ZnNP, etc.) with antibiotics (“nano-antimicrobials”) to enhance their antimycobacterial efficiency, especially in the context of bacterial antibioresistance.195,196 Also, antibacterial-AgNP synthesis using tetracycline as co-reducing and a stabilizing agent was described by Djafari et al.197
It is postulated that combining AgNPs and an antibiotic can synergistically inhibit both Gram + and Gram - multidrug-resistant bacteria.198–200 But this synergism is observed only for certain types of antibiotics, thus Deng et al198 showed AgNP/antibiotic synergistic growth inhibition against the multidrug-resistant bacterium Salmonella typhimurium for enoxacin, kanamycin, neomycin, and tetracycline, while ampicillin and penicillin did not show any enhancement of the antibacterial activity. Regarding the mechanisms of synergy (depicted in Figure 4), the presence of tetracycline enhances the bacterial binding of Ag, followed by an enhancement in Ag+ release which finally leads to a high local-concentration of Ag+ near the bacteria cell wall which leads to bacterial-growth inhibition and death.198 Enhanced positive synergistic response against S. aureus and E. coli was observed also for AgNPs synthesized from Argyreia nervosa associated with seven commercial antibiotics (streptomycin, vancomycin, tetracycline, amoxicillin, gentamicin, erythromycin and ciprofloxacin).201 Similarly, enhanced antibacterial efficiency of ceftriaxone against ceftriaxone-resistant human pathogens was reported following conjugation with biogenic AgNP.202
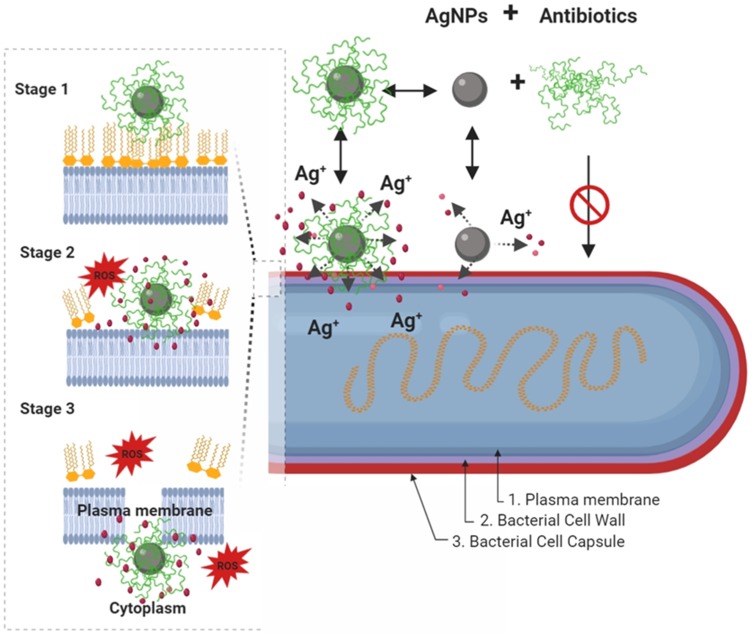
Figure 4.
Schematic diagram showing, in a step by step fashion, the synergistic pathways and mechanisms of AgNP and antibiotics against multidrug-resistant bacteria (depicted in G- bacteria). Enhancement of the accumulation of the AgNPs conjugates with antibiotics within the bacterial cell membrane is associated with potentiation of Ag+ release and damage of the bacterial capsule, cell wall, and plasma membrane components. In this paradigm, the pathway mediated by AgNPs is a minor antibacterial mechanism, and the activity mediated by antibiotics-only is not effective due to antibacterial resistance. In a step by step diagram of the bacterial membrane destabilization (depicted for AgNPs/nisin conjugates), the interaction between AgNP/antibiotic complexes with bacterial cell membrane (stage 1) will results in enhancement Ag+ release, in situ ROS generation, membrane-insertion of nisin (methyl)-lanthionine rings, followed by local dissolution of lipidic molecules, membrane-pore formation, and internalization of AgNPs/nisin complexes within the bacterial cytoplasm. Inspired from Deng et al198 and Arakha et al206 schematic concepts of AgNP/nisin and AgNPs/tetracycline complexes-mediated antibacterial activity. Figure 4 was created using BioRender.
Abbreviations: AgNPs= silver nanoparticle; ROS, reactive oxy gen species: Ag+=silver ions.
Another mechanism of AgNPs/antibiotics synergy was described by Hwang et al.203 and is the anti-biofilm effect. This was observed following a combination of AgNPs with ampicillin, chloramphenicol, and kanamycin against various pathogenic bacteria (Enterococcus faecium, Staphylococcus aureus, Streptococcus mutans, E. coli, and P. aeruginosa) inhibits the formation of biofilm which is a major resistance mechanism for several types of bacteria. This antibacterial effect can be related to the high surface to volume ratio of NPs which can permit their deep infiltration into mature biofilms.17 Recently by Farooq et al.204 showed an enhancement of antibiofilm efficiency of rifampicin following conjugation with silver (Rif-AgNPs) in methicillin-resistant K pneumoniae and S aureus.
Other mechanistic studies exploring the antibacterial effect of NPs, shown that AgNPs and amoxicillin, in addition to their intrinsic antibacterial activity, can form a new complex in which amoxicillin-molecules surround the AgNPs metallic core.205
Antimycobacterial Effect of Silver Nanoparticles
Nanotechnology brings a novel and promising therapeutic approach to improve the current antimycobacterial treatments. This include improvement of the efficiency of the currently used first-or second-line antibiotics following generation of different formulations (e.g. liposomes, solid lipid nanoparticles, alginate nanoparticles, niosomes, dendrimers)207 or by adding new antituberculous compounds which can synergies the classical therapy, as metallic metal-based nanoparticles (mainly silver, iron oxide, gold, copper oxide, aluminum oxide, zinc oxide, titanium dioxide, etc.).99,187 Several of the therapeutical advantages of such nanoparticle-based therapy of tuberculosis are, among others: a) prolonged time of action, b) a high carrier ability; c) flexibility of various routes of administration, d) possibility of multiple drugs-encapsulation in the matrix, e) fewer side effects and improved compliance (especially important in prolonged anti-TB therapy).207
In vitro Studies
Multiple experiments carried recently determined the antimycobacterial effect of AgNP.133,208 For example, a good activity against mycobacteria and low cytotoxicity (10 times the dose established as MIC for Mtb) on infected macrophages was recently reported by Singh et al (2016)180 for phytogenic AgNPs.
One of the earliest reports on the antimycobacterial effect of AgNP came from Song et al.209 who tested in vitro small, non-biogenic AgNP measuring <10 nm to several bacteria species, including beside M. tuberculosis, also E. coli, S. aureus, and Salmonella typhi. The antimycobacterial effect was observed at 10 ppm, and the proposed mechanism is based on the presence of AgNPs in the cytoplasm of mycobacteria and the following bacterial-metabolic disturbances.209
A good in vitro antimycobacterial effect, observed mainly by inhibition of the mycobacterial growth, was reported also by studies employing biogenic AgNP produced from Plumbago auriculata,210 Coriandrum sativum,211 Catharanthus roseus,212 Asparagus race,185 Psidium guajava,213 Ipomoea carnea,214 Rhizopus stolonifer,215 and Cucumis sativus.216
In vitro inhibition of MDR and XDR strains of M. tuberculosis was found for physicochemically (“non-green”) synthesized AgNP in doses as low 1 µg/mL.217 Overall, no bactericidal effect was found, and although the AgNP are internalized within THP-1 macrophages, the intramacrophagic antimycobacterial effect was modest. A similar effect of multimetallic nanoparticles (MMN) including AgNP for intramacrophagic mycobacteria was reported by Ellis et al.218 Although internalized within the phagolysosomal apparatus, the AgNP have a limited antitubercular effect for the intracellular bacteria, but increase the antitubercular effect of rifampicin. The co-administration of rifampicin led to a reduction of 68% of M. tuberculosis colony-forming units. Using spherical AgNP measuring 13 nm, Jafari et al.219 observed no antibacterial effect for intramacrophagic M. tuberculosis, but the addition of Zn in the molecule is inducing an anti-tubercular effect. Additionally, the 5Ag:5ZnO report was found to have both an intracellular antibacterial effect and also no significant toxicity to normal lung (MRC-5) cell lines. By contrary, a good antitubercular effect against intramacrophagic M. marinum and M. smegmatis was observed by Mohanty et al.19 for spherical, biogenic AgNP combined with antimicrobial peptides in doses of 0.1 and 0.5 ppm. The tested particles measured 50–100 nm and were synthesized from Alstonia macrophylla and Trichoderma sp. The enhanced antitubercular effect was not correlated with high levels of NO, thus the proposed antibacterial mechanism was associated with superoxide radicals formation and the activation of macrophages by cytokines. In the same study, an increased antibacterial effect against M. smegmatis was observed following the combination of NPs with the classical-antituberculosis drug rifampin.19 Intramacrophagic killing of M. smegmatis internalized in RAW264.7 macrophages (in both pre/and postexposure treatments) was reported for spherical chitosan-coated AgNP (CS-AgNPs) in 3 ppm dose.220 The bactericidal effect was time and concentration-dependent and most of the antibacterial effect was observed in the first hour of incubation. The hypothesized antibacterial mechanism was cell membrane disruption or chemical inactivation of thiol-containing molecules. Also, CS-AgNPs were noncytotoxic on RAW264.7 macrophages at the bactericidal concentration. An increased antitubercular activity was observed following the addition of gentamicin. In the same study, in addition to antimycobacterial effect, CS-AgNPs were found to be also active against other bacteria like Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella typhi.220
In addition to the direct antitubercular activity in doses beginning with 5 mg/l, AgNPs measuring 10–150 nm were shown to potentiation of the antibacterial effect of isoniazid, rifampicin, ethionamide, levofloxacin, ofloxacin and kanamycin against clinical isolates of M. tuberculosis by Kreytsberg et al.133
In another study employing the antimycobacterial effect of physicochemically synthesized, tetrahedral and spherical AgNP measuring 50 nm, an antibacterial effect against field isolates and standard strains of M. tuberculosis and M. bovis were reported. The minimal inhibitory concentration (MIC) was found to be 1 and 4 µg/mL for standard cultures of M. tuberculosis and M. bovis. Higher doses were needed to inhibit the clinical isolates, being in the range of 4–32 µg/mL for M. bovis and 1–16 µg/mL for M. tuberculosis.221
Promising antimycobacterial results against both M. tuberculosis and M. smegmatis are also observed for AgCl-NP produced from commercial yeast extract in doses of 37 µg/mL concentration.222 In addition to the Ag effect, in this study, the antibacterial effect could be also mediated by the activity of Cl, a potent and broad antiseptic.223
Using biogenic spherical (20–56 nm) AgNP synthesized from Sesbania grandiflora, Patel et al showed that MIC for standard cultures of M. tuberculosis is 12.5 µg/mL. This dose was half of the MIC observed for silver nitrate and approximately 30% of the MIC of Rifampicin. Also, the Sesbania grandiflora extract shown an antimycobacterial effect, but much higher compared with the AgNPs (100 µg/mL).224 A similar MIC was obtained for M.tuberculosis by Punjabi et al.
An interesting strategy is combining AgNP with peptides or chitosan for antibacterial/antitumoral effect. Thus in a recent study, Abdel-Aziz et al150 shown that spherical N,N,N-trimethyl chitosan chloride (TMC)/AgNP in a 0.98 to 125 mg/mL dose have an antibacterial effect on M. tuberculosis mainly by disrupting the bacterial cell wall. Also, the same nanocomposite was found to have a cytotoxic effect against A-549-lung adenocarcinoma cells in 12.3 µg/mL dose and to have reduced toxicity against normal lung cells.
Also, spherical PVP and BSA-caped AgNP measuring 5–9 nm (BSA-AgNP) and 6–45 nm (PVP-AgNP) were shown to have antibacterial effects against clinically isolated and standard M. tuberculosis cultures. The antibacterial effect was mediated by mycobacterial cell membrane injury, followed by bacterial lysis.225
Although differences regarding the sensitivity towards AgNPs were reported between different species of Mycobacteria, most of the tested materials have a simultaneous antibacterial effect against multiple species, including M. tuberculosis, M. pheli, M. avium and M. smegmatis.185
A synopsis of the in vitro studies using AgNPs in the treatment of mycobacteria-induced diseases, including the species and strain of mycobacteria tested, the experimental model, the type of AgNP and the main results are presented in Table 2.
Table 2.
A Synopsis on Studies Using AgNPs in the Treatment of Mycobacteria-Induced Diseases
| Mycobacterium Species/Strain | Experimental Model | Nanoformulation | Tested Doses | AgNP Shape and Size Distribution | Effect on Bacteria | References | |
|---|---|---|---|---|---|---|---|
| 1 | ● M. tuberculosis (ATCC 25177) | Bacterial culture | TMC/AgNP* | 0.98 to 125 mg/mL | Spherical 11 to17.5 nm | Inhibition of growth. Disruption of the bacterial cell wall. |
Abdel-Aziz et al 2019150 |
| 2 | ● M. tuberculosis (H37Ra, and MDR/XDR strains) | Bacterial culture and within THP-1 macrophages | AgNP* * | 1–128 μg/mL | Spherical 5.4±2.6 nm | Inhibition of growth (not bactericidal). In vitro following macrophage internalization: poor antibacterial activities. |
Heidary et al 2019217 |
| 3 | ● M. tuberculosis (H37Rv) ● M. smegmatis (MC2 155) |
Bacterial culture | AgCl NP* (from commercial yeast) | 37 µg/mL | Spherical 9 to 51 nm | Inhibition of growth. | Sivaraj et al 2019222 |
| 4 | ● M. tuberculosis (H37Rv) ● M. tuberculosis (clinical isolate MDR strain) ● M. bovis (reference strain and clinical isolate) |
Bacterial culture | AgNP* (suspended in sodium citrate) | 0,25 to 256μg/mL | Tetrahedral and spherical 50 nm | Inhibition of growth. | Selim et al 2018221 |
| 5 | ● M. tuberculosis (H37Rv) | Bacterial culture | AgNP (from Sesbania grandiflora) | 100 μg/mL and 25 g/mL (based on MIC) |
Spherical 20 to 56 nm. | Inhibition of growth. | Patel et al 2018224 |
| 6 | ● M. tuberculosis (H37Rv) | Bacterial culture | AgNP (from Pseudomonas hibiscicola) | 1.25–10 mg/mL | Spherical and polygonal 10–70 nm (average 39 nm) |
Inhibition of growth. | Punjabi et al 2018226 |
| 7 | ● M. tuberculosis | THP-1 macrophages | AgNP*, AgNP+ZnNP* and ZnNP* (embedded in PLGA polymer) |
60 µg mL-1 | Spherical 20 nm MMP-AgNp-1,5µm |
Limited antitubercular effect (reduction with 4.5% of CFU). Increase rifampicin antitubercular potency. Global disruption to the bacterial membrane. |
Ellis et al, 2018218 |
| 8 | ● M. tuberculosis (H37RvMTB) | THP-1 macrophages | AgNP* | 1.562 ppm, 0.781 ppm, 0.390 ppm, 0.195 ppm | Spherical 13 nm | No antibacterial activities following TB phagocytosis, only after combination with ZnONP. | Jafari et al 2017219 |
| 9 | ● M. smegmatis | Bacterial culture | AgNP* and AgNP/VAM (conjugated with vancomycin) |
Not detailed for AgNP; for AgNP-VAM inhibitory concentration was 54 µg/mL, | Spherical AgNP: 17 ± 3 nm AgNPVAM: 30 ± 3 nm |
Internalization within bacteria (without specific binding of interaction). Reduction of viability and Inhibition of growth (Mild); AgNPs potentiate the effect of VAM |
Sun et al 2017227 |
| 10 | ● M. tuberculosis | Bacterial culture | AgNP (from Plumbago auriculata) | 0.2 to 100 μg/mL. | Spherical 15–45 nm | Inhibition of growth | Jaryal et al 2017210 |
| 11 | ● M. tuberculosis (H73Rv) | Bacterial culture | AgNP (from Coriandrum sativum) | 0.2μg/mL to 100 | Spherical and polygonal 50–200 nm228 |
Inhibition of growth | Paarakh et al 2017211 |
| 12 | ● M. avium subsp. paratuberculosis (K10/GFP) | Bacterial culture | AgN* (in distilled water containing 2% fetal calf serum) | 0 to 100 μg/mL | Spherical <50 nm | Inhibition of growth. | Donnellan et al 2016229 |
| 13 | ● M. tuberculosis | Bacterial culture | AgNP* | 20 ppm and 60 ppm | Shape not specified 30–80 nm |
No anti-Mtb effects | Jafari et al 2016230 |
| 14 | ● M. tuberculosis (MTTC300) ● M. smegmatis |
Bacterial culture | AgNP (from Catharanthus roseus) | 5 μg/disc | Not provided (possible spherical) 38–52 nm |
Inhibition of growth. | Raja et al 2016212 |
| 15 | ● M. tuberculosis (H37Ra) ● M. bovis (BCG) |
Bacterial culture and within THP-1 macrophages | AgNP (from Barleria prionitis, Plumbago zeylanica and Syzygium cumini) | 0.1, 0.3, 1, 3, 10, 30, and 100 μg/mL. | Spherical and polydisperse 10–120 nm (from B prionitis) 60 nm (extracted from P. zeylanica) 9–35 nm (extracted from S. cumini) |
Inhibition of active and dormant mycobacteria in both culture and following internalization in THP-1 macrophages | Singh et al 2016180 |
| 16 | ● M. tuberculosis (MTCC-300), ● M. pheli (MTCC-1723) ● M.avium (MTCC-1724) ● M. smegmatis (MTCC-994) |
Bacterial culture | AgNP (from Asparagus race) | 176 mg/100 mL | Spherical and rectangular | Inhibition of growth | Kote et al 2016185 |
| 17 | ● M. tuberculosis (H37Ra) ● M. bovis (BCG) |
Bacterial culture | AgNp (sol A: from Acinetobacter sp and sol. B: from reduction of 1% trisodium citrate) | 0.02–2.56 μg/mL. | Spherical (Sol A:) and Spherical-oval (Sol B) 8–12 nm (Sol A:) 1–5 nm (Sol B) |
Inhibition of growth | Singh et al 2015193 |
| 18 | ● M. tuberculosis ● M. smegmatis ● M. pheli |
Bacterial culture | AgNP (from Psidium guajava) | 100–500 µL/disc | Unknown | Inhibition of growth | Kote et al 2014213 |
| 19 | ● M. smegmatis | Bacterial culture | AgNP (from Ipomoea carnea) | 5 mg/mL (impregnated) | Spherical and oval 30 to 130 nm |
Inhibition of growth | Daniel et al 2014214 |
| 20 | ● M. smegmatis (MC2155 ATCC 700084) ● M. marinum (ATCC 927) |
Bacterial culture and within RAW264.7 macrophages | AgCl NPs (sol A: from Alstonia macrophylla and sol B: from Trichoderma sp) | 0.1 and 0.5 ppm | Spherical, A: 50 nm and B: 100 nm | Inhibition of growth Enhancing the destruction of Mycobacteria within macrophages (0.5pppm) |
Mohanty et al 201319 |
| 21 | ● M. avium ● M. smegmatis ● M. marinum |
Bacterial culture | AgNP* | 6.25, 12.5, 25, 50, and 100 μM. | Spherical 12.6 ± 5.7 nm | Inhibition of growth | Islam et al 2013231 |
| 22 | ● M. tuberculosis (clinical isolate) | Bacterial culture | AgNP (from Rhizopus stolonifer) | 8 to 64 μg/mL. |
Spherical 3 to 20 nm. | Inhibition of growth. | Banu et al 2013215 |
| 23 | ● M. tuberculosis (H37Rv and clinical isolates, including MDR and XDR strains) ● Mycobacterium spp other than tuberculosis (no data further specified) |
Bacterial culture | AgNP (from Cucumis sativus) | 50, 31.2, 25, 15.6, 12.5, 7.8 and 6.2 g/mL | Spherical 10–20 nm | Inhibition of growth | Agarwal et al 2013216 |
| 24 | ● M. smegmatis | Bacterial culture and RAW264.7 macrophage culture | AgNP* (chitosan-coated: CS-AgNPs) | 1,2 and 3ppm CS-AgNPs | Spherical Two size-population 55 and 278 nm | Disruption of bacterial cell wall Intramacrophagic killing of M. smegmatis (in both pre/and postexposure treatment) |
Jena et al 2012220 |
| 25 | ● M. smegmatis | Bacterial culture | AgNP* (starch-stabilized) | 0.1, 1, 2, 5, 10 μM | Spherical 20 nm | Inhibition of growth | Mohanty et al 2012232 |
| 26 | ● M. bovis (BCG) | Bacterial culture | AgNP* (suspended in sodium citrate) | 1, 5, 10, 20 μg/mL | Spherical 20 and 30 nm | Bactericidal Activity (cell lysis) |
Zhou et al 2012233 |
| 27 | ● M. tuberculosis (clinical isolates, including DR strains) | Bacterial culture | AgNP* (in distilled water and in combination with antibiotics) | 5, 25 and 50 μg/mL | Shape not specified 10–150 nm |
Inhibition of growth Potentate the effect of isoniazid, rifampicin, ethionamide, levofloxacin, ofloxacin and kanamycin |
Kreytsberg et al 2011133 |
| 28 | ● M. tuberculosis (H37Rv and clinical isolates) ● M. xenopi |
Bacterial culture | AgNP* (BSA and PVP-capped) | 1.6, 4 and 8 μg/mL | Spherical 5–9 nm (BSA nano-Ag) 6–45 nm (PVP nano-Ag) |
Inhibition of growth. TB cell membrane injury; bacterial lysis |
Seth et al 2011225 |
| 29 | ● M. smegmatis (ATCC 700084) ● M. bovis (BCG, ATCC 35374) |
Bacterial culture | AgNP* | 0.22 to 25 μg/mL | Spherical and polygonal A: 20–25 nm B: 80–90 nm |
Inhibition of growth | Martinez-Gutierrez et al 2010234 |
| 30 | ● M; tuberculosis (H37Rv) | Bacterial culture | AgNP* and AgNp/Cys (cysteine –caped AgNp) |
Tested interval not provided, 6 and 10 ppm | Shape not provided AgNp 10.45 ± 0.546 AgNp/Cys 45.67 ± 0.951 |
Inhibition of growth | Varghese et al 2009235 |
| 31 | ● M. tuberculosis | Bacterial culture | AgNPs* | 0.5, 1, 5, 10 and 30 ppm | Spherical (?) <10 nm | Inhibition of growth | Song at al., 2006209 |
Note: *AgNPS produced by physicochemical synthesis (non-green synthesis)
Abbreviations: DR/MDR/XDR, drug-/multi drug-/extensively drug-resistant; TMC-N, N,N-trimethyl chitosan chloride; BCG, bacillus Calmette-Guérin
In vivo Preclinical and Clinical Studies
Compared with the large number of in vitro studies exploring the potential used of AgNp as antimycobacterial drugs only a few in vivo studies were up to date carried.
In a clinical trial carried on 50 human patients with ages from 26 to 55 years suffering from with laryngeal tuberculosis, including cases with DR-tuberculosis, an AgNP aqueous suspension (Argovit-C, 10 mg/mL silver, in a concentration of 3.3%) was tested for 2 months as local therapy.236 The AgNP group (n=30) received the treatment topically by inhalation for 2 times a day for 10 mins. The control group (n=20) received classical TB therapy. The suspension was previously characterized as containing spherical AgNP with bimodal size distribution (14.1 ± 9.9 and 50.1 ± 40.3 nm).237
After 60 days of therapy, the sputum was negative for M. tuberculosis in 93.3% of patients enrolled in the AgNP group compared with 70% of patients who received the standard anti-TB treatment. Also, the patients enrolled in the AgNP group – showed faster healing of the laryngeal TB-lesion, including ulcerations and voice function compared to standard tuberculosis drugs.236
An experiment designed to investigate the effect of isoniazid combined with AgNPs on MDR strains of M. tuberculosis was carried by Zakharov et al in 68 BALB/c inbred mice. Spherical AgNP measuring 3–60 nm were tested initially in vitro in ascending concentrations (5;25;50 μg/mL) in 651 MDR strains of M. tuberculosis. In the rodent study, based on the survival rates and histopathology of the lung, the combination of isoniazid and silver nanoparticles was preferable compared to the single-use of the above components.238
In a recently published study carried out by the same author, the effect of isoniazid combined with AgNPs was tested in 3 experimental groups of MDR-TB-infected mice: group 1 received only isoniazid (50 mg/kg); group 2 received intramuscularly AgNPs in doses of 12.5 to 125 µg/kg; group 3 received a combination of the treatments detailed for groups 1 and 2. Based on the histopathologic grading of lesions, the use of AgNPs in the treatment of TB induced by MDR strains enhances the efficiency of isoniazid.239
In an in vivo study carried out in 65 mice experimentally infected with MDR strains of Mycobacterium tuberculosis isolated from human patients, the efficiency of AgNP as a single therapeutic molecule or in combination with isoniazid was tested. The AgNP measured 10–150 nm. The survival rate of TB-infected animals following the combined treatment with isoniazid and AgNP was 95% and 35% in the group receiving AgNP only, compared with 100% mortality in the TB-infected control group.133
The Main Limitation of the Usage of AgNP in the Treatment of Tuberculosis
Potential Toxicity of AgNPs
One of the potential drawbacks of AgNPs, as four most of the inorganic nanoparticles, is their toxicity which may limit their usage in a biological context,240–242 but despite the extension of use in the last decades, the evidence for the toxicity of AgNPs is still unclear.243 However, an in depth discussion regarding the toxicity of AgNP is something that goes beyond the purpose of this manuscript.
The increased production of ROS which is presented above as one of the antibacterial mechanisms of AgNP can be harmful to the normal cells if the cellular protective antioxidative mechanisms are overcome, which will trigger several detrimental biological effects like inflammation, autophagia, apoptosis, necrosis or irreversible DNA-damage followed by mutations and possible oncogenesis.244 There are several studies in which a good antibacterial efficiency and a low-toxicity for the explored doses were observed. Thus, for AgNPs produced from Phenerochaete chrysosporium, a good antibacterial effect against Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, and Staphylococcus epidermidis was observed but no in vitro toxic effect on mouse embryo fibroblasts for doses up to 12.5 μg/mL AgNPS.245
Moreover, biogenic AgNPs measuring 50–100 nm synthesized from Alstonia macrophylla and Trichoderma sp showed no cytotoxic effects on macrophages at the mycobactericidal dose (0.1 and 0.5 ppm), but the exposure to higher doses of AgNPs induced cytotoxicity and DNA-damage.19
Low Penetrability in Tuberculous Granulomas
A clinical limitation of the usage of AgNP in TB therapy is based on the low tissue penetrability of large molecules following a non-intravenous route of administration.246 Also in a lack of proper functionalization following an intravenous route of administration, a low intra-lesional accumulation is predictable considering the poorly vascularization of tuberculose-lung cavities present in chronic cases of tuberculosis.247 Additionally, the persistence of mycobacteria in the nonvascularized necrotic material within the granuloma center can assure the persistence of a reservoir of viable mycobacteria in a biological environment largely inaccessible for large molecules and immune cells.18
The Immunomodulatory Effect of AgNP
Especially important in the clinical context of MtbC infection and macrophage polarization, Sarkar et al.248 observed an upregulation of macrophage Hsp72 by AgNP, which is possible to be linked with further suppression of NF-κB pathway and reduction of the macrophage antimycobacterial effect. In vivo following 28 days repeated dose toxicity study in rats, AgNP in high doses induce a marked suppression of natural killer cell (NK) activities and decreased interferon-γ and interleukin (IL)-10 release in response to Concanavalin A (ConA)- mediated activation of the spleen cells.249 Interestingly, in the same study, the lipopolysaccharide (LPS) stimulation was associated with increased IL-1β and decreased IL-6, IL-10 and TNF-α production in the spleen, proving a complex immunomodulatory effect of AgNP. The exposure of human NK cells to AgNPs also resulted in reduced viability and altered function enhancement of expression of the inhibitory receptor CD159a.250
The conclusion drawn by their observation brings new perspectives regarding the drug-designs which intend using AgNP in obligate intra-macrophage pathogens. But this immunomodulatory effect seems to be more complex and interferes with specific immune cell activities. Thus, although exposure of neutrophils to AgNP (50 µg/mL) was associated with reduced neutrophilic degranulation (elastase release) and oxidative burst, the overall phagocytic activity was enhanced.251 In the same study, pre-exposure of macrophages (RAW 264.7) to AgNP, stimulates the release of inflammatory cytokine interleukin-6 as well as enhancement of phagocytic ability in response to lipopolysaccharide stimulation.251 Also, the exposure of J774 A1 murine macrophage to AgNPs resulted in early activation of the inflammatory response by up-regulation of IL-1, IL-6, and TNF-a genes, but in a lesser amount compared with AuNPs.252 Therefore, taking into account the divergent data regarding the immune impact of AgNPs, a better understanding of activation or suppression of immune cell pathways and functions following AgNPs needs to carry before a clinical-functional significance in the context of the complex inflammatory environment associated with mycobacterial infections to be drawn. As a solution to the above-mentioned issues in AgNP usage the utilization of a gold-silver alloy NP promise to be a viable solution in overcoming the macrophage function suppression due to the significant improvement of AgNP biocompatibility following the introduction of gold NP (AuNP) as alloy.253 Additionally, AuNP produced from Terminalia arjuna show important antioxidant and anticholinesterase effects254 which could antagonize the AsNP toxicity.
Development of Bacterial Resistance Towards Silver Nanoparticles
Although rarer and less studied compared with the classical antibioresistance, the mutation in bacteria to resist Ag is similar in certain limits to the pathway that led to chemoresistant bacterial strains.255–258 The widespread use of Ag- and Ag-ions containing nanomaterials and nanocomposites is considered to be the main determinant in a possible bacterial selection and evolution towards a biological resistance to Ag NP and/of Ag ions.258,259 The resistance to antibacterial Ag is reported among nosocomial infections,260 in bacteria present within wounds, including burns,261–264 diabetic foot ulcers,265 dental bacteria,266,267 or exterior natural-environments containing high amounts of Ag.268 Occasionally, the resistance towards Ag is developed in parallel with the multidrug-resistance, as shown in Staphylococcus aureus, klebsiella pneumoniae, Acinetobacter baumannii, and Enterococcus faecium.269 This cross-resistance to antibiotic and metal resistance are typically mediated when genes for different resistant phenotypes (metal/chemioresistant) are located on the same mobile genetic elements as plasmids and conserved regions of integrons.270
Generally observed in fast-growing bacteria, Ag-resistance was described also in Mycobacteria, as Mycobacterium smegmatis,255 M avium, M fortuitum, and M mucogenicum.271 Resistance to both silver nanoparticle and AgNO3 was observed for saprophytic bacteria (as Mycobacterium smegmatis)255 and could be proven also for the other pathogenic classes of bacteria.
As mechanism, the Ag resistance can be associated with elimination and neutralization of ionic forms of silver, as active efflux of Ag+ from bacteria (e.g by P-type ATP/SilP, membrane potential-dependent three-polypeptide cation/proton antiporter or multidrug resistance/MDR efflux pumps), increased capacity for reducing Ag+ to a neutral-oxidation state which are typically less bacteriotoxic.272–274 Recently, in gram-negative bacteria, the resistance to AgNP was induced by overexpression of bacterial flagellum protein-flagellin, which induced aggregation of particles at the surface of bacteria and reduction of AgNPp antibacterial effect.192
The difficulty in gaining such a resistance against AgNPs s is due to the fact that the antibacterial effect of nanoparticles is more complex (illustrated in Figures 2 and 3) compared with classical antibiotics.
Conclusion
Tuberculosis is still a major public health issue, but currently, nanotechnology and nanoparticle research offers several exciting concepts which may prove to be valuable tools in improving the TB-therapy, especially in the context of broad-antibioresistant stains of MtbC.
There is no doubt that AgNPs per sei or in conjunction with different biomolecules as peptides and chitosan have good antimycobacterial effects, but this effect is limited following macrophagic internalization of mycobacteria. A promising strategy is combining AgNPs with classical anti-TB therapeutics which synergically enhance the antimycotic activity both extra and intracellularly. Despite the encouraging in the in vitro-stage of research, still, there are few in vivo studies exploring the anti-TB potential of AgNPs. As a result of the peculiar structure and visceral distribution and of TB-induced lesions, the on-going research efforts for the synthesis of novel anti-TB nanoparticles should be focused on strategies for enhancing the local availability of antibacterial nanoparticles. Increased local availability, associated with good intra-macrophagic disponibility, a potent antimycobacterial effect, and a low-immunosuppressive and toxic effect should be the cumulative characteristics of a good nanoparticle candidate for future therapy of tuberculosis.
Acknowledgments
This work was supported by a grant of Ministery of Research and Innovation, CNCS – UEFISCDI, project numbers PN-III-P1-1.1-PD-2016-1840, PN-III-P1-1.1-PD-2016-1831 and PN-III-P1-1.1-TE2016-2161within PNCDI III.
All figures presented in this work were created with BioRender.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Thoen CO, LoBue PA, Enarson DA, Kaneene JB, de Kantor IN. Tuberculosis: a re-emerging disease in animals and humans. Vet Ital. 2009;45(1):135–181. [PubMed] [Google Scholar]
- 2.De Lorenzo S, Tiberi S. Tuberculosis a re-emerging disease. Intern Emerg Med. 2012;7(S3):185–187. doi: 10.1007/s11739-012-0822-9 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Tuberculosis Report 2015, 20th Ed. World Health Organization; 2015. [Google Scholar]
- 4.Schneider E, Moore M, Castro KG. Epidemiology of Tuberculosis in the United States. Clin Chest Med. 2005;26(2):183–195. doi: 10.1016/j.ccm.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 5.Forrellad MA, Klepp LI, Gioffré A, et al. Virulence factors of the Mycobacterium tuberculosis complex. Virulence. 2013;4(1):3–66. doi: 10.4161/viru.22329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.who.tb.99.260.pdf. Available from: https://www.who.int/docstore/gtb/publications/mdrtb/PDF/who.tb.99.260.pdf. Accessed November21, 2019.
- 7.9789241565714-eng.pdf. Available from: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf. Accessed November24, 2019..
- 8.Saravanan M, Niguse S, Abdulkader M, et al. Review on emergence of drug-resistant tuberculosis (MDR & XDR-TB) and its molecular diagnosis in Ethiopia. Microb Pathog. 2018;117:237–242. doi: 10.1016/j.micpath.2018.02.047 [DOI] [PubMed] [Google Scholar]
- 9.Richter E, Weizenegger M, Rüsch-gerdes S, Niemann S. Evaluation of genotype MTBC assay for differentiation of clinical Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2003;41(6):2672–2675. doi: 10.1128/JCM.41.6.2672-2675.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thoen CO, Steele JH, Kaneene JB. Zoonotic Tuberculosis: Mycobacterium Bovis and Other Pathogenic Mycobacteria. John Wiley & Sons; 2014. [Google Scholar]
- 11.Kiers A, Klarenbeek A, Mendelts B, Van Soolingen D, Koëter G. Transmission of Mycobacterium pinnipedii to humans in a zoo with marine mammals. Int J Tuberc Lung Dis off J Int Union Tuberc Lung Dis. 2008;12(12):1469–1473. [PubMed] [Google Scholar]
- 12.Alexander KA, Laver PN, Michel AL, et al. Novel Mycobacterium tuberculosis complex pathogen, M. mungi. Emerg Infect Dis. 2010;16(8):1296–1299. doi: 10.3201/eid1608.100314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathiyavimal S, Vasantharaj S, Bharathi D, et al. Biogenesis of copper oxide nanoparticles (CuONPs) using Sida acuta and their incorporation over cotton fabrics to prevent the pathogenicity of Gram negative and Gram positive bacteria. J Photochem Photobiol B. 2018;188:126–134. doi: 10.1016/j.jphotobiol.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 14.Shankar PD, Shobana S, Karuppusamy I, et al. A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: formation mechanism and applications. Enzyme Microb Technol. 2016;95:28–44. doi: 10.1016/j.enzmictec.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 15.Silvestry-Rodriguez N, Sicairos-Ruelas EE, Gerba CP, Bright KR. Silver as a Disinfectant In: Ware GW, editor. Reviews of Environmental Contamination and Toxicology. Vol. 191 New York: Springer New York; 2007:23–45. doi: 10.1007/978-0-387-69163-3_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paladini F, Pollini M. Antimicrobial silver nanoparticles for wound healing application: progress and future trends. Materials. 2019;12(16):2540. doi: 10.3390/ma12162540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shanmuganathan R, Karuppusamy I, Saravanan M, Muthukumar H, Ponnuchamy K, Pugazhendhi VSR. Synthesis of silver nanoparticles and their biomedical applications - a comprehensive review. Current Pharmaceut Design 2019. doi: 10.2174/1381612825666190708185506 [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto K. The pathology of Mycobacterium tuberculosis infection. Vet Pathol. 2012;49(3):423–439. doi: 10.1177/0300985811429313 [DOI] [PubMed] [Google Scholar]
- 19.Mohanty S, Jena P, Mehta R, et al. Cationic antimicrobial peptides and biogenic silver nanoparticles kill mycobacteria without eliciting DNA damage and cytotoxicity in mouse macrophages. Antimicrob Agents Chemother. 2013;57(8):3688–3698. doi: 10.1128/AAC.02475-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montelongo-Peralta LZ, León-Buitimea A, Palma-Nicolás JP, Gonzalez-Christen J, Morones-Ramírez JR. Antibacterial activity of combinatorial treatments composed of transition-metal/antibiotics against Mycobacterium tuberculosis. Sci Rep. 2019;9(1):1–6. doi: 10.1038/s41598-019-42049-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maher D, Raviglione M. Global epidemiology of tuberculosis. Clin Chest Med. 2005;26(2):167–182. doi: 10.1016/j.ccm.2005.02.009 [DOI] [PubMed] [Google Scholar]
- 22.Seung et al. 2015. Multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis.pdf. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4561400/pdf/cshperspectmed-TUB-a017863.pdf. Accessed November24, 2019. [DOI] [PMC free article] [PubMed]
- 23.Seung KJ, Keshavjee S, Rich ML. Multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis. Cold Spring Harb Perspect Med. 2015;5:9. doi: 10.1101/cshperspect.a017863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization 2007. - Global tuberculosis control surveillance, plannin.pdf. Available from: https://apps.who.int/iris/bitstream/handle/10665/144567/9241563141_eng.pdf?sequence=1. Accessed November24, 2019.
- 25.Chung-Delgado K, Guillen-Bravo S, Revilla-Montag A, Bernabe-Ortiz A. Mortality among MDR-TB cases: comparison with drug-susceptible tuberculosis and associated factors. PLoS One. 2015;10:3. doi:10/f7b7v6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin SS, Furin JJ, Alcántara F, Bayona J, Sánchez E, Mitnick CD. Long-term follow-up for multidrug-resistant tuberculosis. Emerg Infect Dis. 2006;12(4):687–688. doi: 10.3201/eid1204.041256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernabé-Ortiz A. Factores asociados a supervivencia en pacientes con tuberculosis en Lima, Perú [Factors associated with survival of patients with tuberculosis in Lima, Peru]. Rev Chil Infectologia Organo of Soc Chil Infectologia. 2008;25(2):104–107. Spanish. [PubMed] [Google Scholar]
- 28.Ginsberg AM, Spigelman M. Challenges in tuberculosis drug research and development. Nat Med. 2007;13(3):290–294. doi: 10.1038/nm0307-290 [DOI] [PubMed] [Google Scholar]
- 29.Takiff H, Guerrero E. Current prospects for the fluoroquinolones as first-line tuberculosis therapy. Antimicrob Agents Chemother. 2011;55(12):5421–5429. doi: 10.1128/AAC.00695-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramachandran G, Swaminathan S. Safety and tolerability profile of second-line anti-tuberculosis medications. Drug Saf. 2015;38(3):253–269. doi: 10.1007/s40264-015-0267-y [DOI] [PubMed] [Google Scholar]
- 31.Marra F, Marra CA, Bruchet N, et al. Adverse drug reactions associated with first-line anti-tuberculosis drug regimens. Available from: https://www.ingentaconnect.com/content/iuatld/ijtld/2007/00000011/00000008/art00007. August 2007. Accessed December2, 2019. [PubMed]
- 32.Natarajan S, Subramanian P. Adverse drug reactions to second line anti tuberculosis drugs: a prospective study in Mumbai, India. Eur Respir J. 2013;42(Suppl57). [Google Scholar]
- 33.Sotgiu G, Centis R, D’ambrosio L, Migliori GB. Tuberculosis treatment and drug regimens. Cold Spring Harb Perspect Med. 2015;5(5):a017822–a017822. doi: 10.1101/cshperspect.a017822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crofton SJ, Chaulet P, Maher D, et al. Guidelines for the Management of Drug-Resistant Tuberculosis. Vol No. WHO/TB/96.210 (Rev. 1). Geneva: World Health Organization; 1997. [Google Scholar]
- 35.O’Brien RJ, Spigelman M. New drugs for tuberculosis: current status and future prospects. Clin Chest Med. 2005;26(2):327–340. doi: 10.1016/j.ccm.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 36.Ernst JD. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun. 1998;66(4):1277–1281. doi: 10.1128/IAI.66.4.1277-1281.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porvaznik I, Solovič I, Mokrý J. Non-tuberculous mycobacteria: classification, diagnostics, and therapy. Adv Exp Med Biol. 2017;944:19–25. doi: 10.1007/978-3-319-44488-8_45 [DOI] [PubMed] [Google Scholar]
- 38.Azadi D, Motallebirad T, Ghaffari K, Shojaei H. Mycobacteriosis and tuberculosis: laboratory diagnosis. Open Microbiol J. 2018;12:41–58. doi: 10.2174/1874285801812010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesley R, Ramakrishnan L. Insights into early mycobacterial pathogenesis from the zebrafish. Curr Opin Microbiol. 2008;11(3):277–283. doi: 10.1016/j.mib.2008.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zachary JF, McGavin MD. Pathologic Basis of Veterinary Disease Expert Consult. Elsevier Health Sciences; 2016. [Google Scholar]
- 41.Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev. 2003;16(3):463–496. doi: 10.1128/CMR.16.3.463-496.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Echeverria-Valencia G, Flores-Villalva S, Espitia CI. Virulence factors and pathogenicity of Mycobacterium In: Ribón W editor. Mycobacterium - Research and Development. InTech; 2018:231–255. doi: 10.5772/intechopen.72027 [DOI] [Google Scholar]
- 43.Torrelles JB, Sieling PA, Zhang N, et al. Isolation of a distinct Mycobacterium tuberculosis mannose-capped lipoarabinomannan isoform responsible for recognition by CD1b-restricted T cells. Glycobiology. 2012;22(8):1118–1127. doi: 10.1093/glycob/cws078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc Natl Acad Sci. 2003;100(9):5437–5442. doi: 10.1073/pnas.0737613100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hmama Z. Quantitative analysis of phagolysosome fusion in intact cells: inhibition by mycobacterial lipoarabinomannan and rescue by an 1,25-dihydroxyvitamin D3-phosphoinositide 3-kinase pathway. J Cell Sci. 2004;117(10):2131–2140. doi: 10.1242/jcs.01072 [DOI] [PubMed] [Google Scholar]
- 46.Chan J, Xuedong F, Shirley WH, Patrick B, Bloom B. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59(5):1755–1761. doi: 10.1128/IAI.59.5.1755-1761.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shabaana AK, Kulangara K, Semac I, et al. Mycobacterial lipoarabinomannans modulate cytokine production in human T helper cells by interfering with raft/microdomain signalling. CMLS Cell Mol Life Sci. 2005;62(2):179–187. doi: 10.1007/s00018-004-4404-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geijtenbeek TBH, van Vliet SJ, Koppel EA, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197(1):7–17. doi: 10.1084/jem.20021229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dao et al. 2004. Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12.pdf. Available from: https://iai.asm.org/content/72/4/2067.full.pdf. Accessed November22, 2019. [DOI] [PMC free article] [PubMed]
- 50.Quesniaux VJ, Nicolle DM, Torres D, et al. Toll-Like Receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol. 2004;172(7):4425–4434. doi: 10.4049/jimmunol.172.7.4425 [DOI] [PubMed] [Google Scholar]
- 51.Indrigo J. Cord factor trehalose 6,6ʹ-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology. 2003;149(8):2049–2059. doi: 10.1099/mic.0.26226-0 [DOI] [PubMed] [Google Scholar]
- 52.Axelrod S, Oschkinat H, Enders J, et al. Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell Microbiol. 2008;10(7):1530–1545. doi: 10.1111/j.1462-5822.2008.01147.x [DOI] [PubMed] [Google Scholar]
- 53.Welsh KJ, Abbott AN, Hwang S-A, et al. A role for tumour necrosis factor-, complement C5 and interleukin-6 in the initiation and development of the mycobacterial cord factor trehalose 6,6ʹ-dimycolate induced granulomatous response. Microbiology. 2008;154(6):1813–1824. doi: 10.1099/mic.0.2008/016923-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez RL, Roman J, Roser S, et al. Cytokine message and protein expression during lung granuloma formation and resolution induced by the mycobacterial cord factor trehalose-6,6′-dimycolate. J Interferon Cytokine Res. 2000;20(9):795–804. doi: 10.1089/10799900050151067 [DOI] [PubMed] [Google Scholar]
- 55.Laneelle G, Tocanne J-F. Evidence for penetration in liposomes and in mitochondrial membranes of a fluorescent analogue of cord factor. Eur J Biochem. 1980;109(1):177–182. doi: 10.1111/j.1432-1033.1980.tb04782.x [DOI] [PubMed] [Google Scholar]
- 56.Kato M. Site II-specific inhibition of mitochondrial oxidative phosphorylation by trehalose-6,6′-dimycolate (cord factor) of Mycobacterium tuberculosis. Arch Biochem Biophys. 1970;140(2):379–390. doi: 10.1016/0003-9861(70)90079-2 [DOI] [PubMed] [Google Scholar]
- 57.Ozeki Y, Kaneda K, Fujiwara N, Morimoto M, Oka S, Yano I. In vivo induction of apoptosis in the thymus by administration of mycobacterial cord factor (trehalose 6,6’-dimycolate).. Infect Immun. 1997;65(5):1793–1799. doi: 10.1128/IAI.65.5.1793-1799.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilleron M, Ronet C, Mempel M, Monsarrat B, Gachelin G, Puzo G. Acylation state of the phosphatidylinositol mannosides from mycobacterium bovis bacillus calmette guérin and ability to induce granuloma and recruit natural killer T cells. J Biol Chem. 2001;276(37):34896–34904. doi: 10.1074/jbc.M103908200 [DOI] [PubMed] [Google Scholar]
- 59.Court N, Rose S, Bourigault M-L, et al. Mycobacterial PIMs inhibit host inflammatory responses through CD14-Dependent and CD14-Independent mechanisms. PLoS One. 2011;6(9):e24631. doi: 10.1371/journal.pone.0024631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cambier CJ, Takaki KK, Larson RP, et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505(7482):218–222. doi: 10.1038/nature12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rousseau C, Winter N, Pivert E, et al. Production of phthiocerol dimycocerosates protects Mycobacterium tuberculosis from the cidal activity of reactive nitrogen intermediates produced by macrophages and modulates the early immune response to infection. Cell Microbiol. 2004;6(3):277–287. doi: 10.1046/j.1462-5822.2004.00368.x [DOI] [PubMed] [Google Scholar]
- 62.Astarie-Dequeker C, Le Guyader L, Malaga W, et al. Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog. 2009;5(2):e1000289. doi: 10.1371/journal.ppat.1000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Augenstreich J, Arbues A, Simeone R, et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell Microbiol. 2017;19(7):e12726. doi: 10.1111/cmi.12726 [DOI] [PubMed] [Google Scholar]
- 64.Saint-Joanis B, Demangel C, Jackson M, et al. Inactivation of Rv2525c, a substrate of the Twin Arginine Translocation (Tat) System of Mycobacterium tuberculosis, increases -lactam susceptibility and virulence. J Bacteriol. 2006;188(18):6669–6679. doi: 10.1128/JB.00631-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhuwan M, Arora N, Sharma A, et al. Interaction of Mycobacterium tuberculosis virulence factor RipA with chaperone MoxR1 is required for transport through the TAT secretion system. mBio. 2016;7(2):e02259–15. doi: 10.1128/mBio.02259-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berthet F. Attenuation of virulence by disruption of the Mycobacterium tuberculosis erp Gene. Science. 1998;282(5389):759–762. doi: 10.1126/science.282.5389.759 [DOI] [PubMed] [Google Scholar]
- 67.Skjot RLV, Oettinger T, Rosenkrands I, et al. Comparative evaluation of low-molecular-mass proteins from mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect Immun. 2000;68(1):214–220. doi: 10.1128/IAI.68.1.214-220.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elhay MJ, Oettinger T, Andersen P. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the Guinea Pig.:3. Infect Immun. 1998;66:3454–3456. doi: 10.1128/IAI.66.7.3454-3456.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ganguly N, Giang PH, Gupta C, et al. Mycobacterium tuberculosis secretory proteins CFP-10, ESAT-6 and the CFP10: ESAT6complex inhibit lipopolysaccharide-induced NF-κB transactivation by downregulation of reactive oxidative species (ROS) production. Immunol Cell Biol. 2008;86(1):98–106. doi: 10.1038/sj.icb.7100117 [DOI] [PubMed] [Google Scholar]
- 70.Pathak SK, Basu S, Basu KK, et al. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol. 2007;8(6):610–618. doi: 10.1038/ni1468 [DOI] [PubMed] [Google Scholar]
- 71.Derrick SC, Morris SL. The ESAT6 protein of Mycobacterium tuberculosis induces apoptosis of macrophages by activating caspase expression. Cell Microbiol. 2007;9(6):1547–1555. doi: 10.1111/j.1462-5822.2007.00892.x [DOI] [PubMed] [Google Scholar]
- 72.Smith J, Manoranjan J, Pan M, et al. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect Immun. 2008;76(12):5478–5487. doi: 10.1128/IAI.00614-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kinhikar AG, Verma I, Chandra D, et al. Potential role for ESAT6 in dissemination of Maf tuberculosis via human lung epithelial cells. Mol Microbiol. 2010;75(1):92–106. doi: 10.1111/j.1365-2958.2009.06959.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Wel N, Hava D, Houben D, et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129(7):1287–1298. doi: 10.1016/j.cell.2007.05.059 [DOI] [PubMed] [Google Scholar]
- 75.Reed MB, Domenech P, Manca C, et al. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature. 2004;431(7004):84–87. doi: 10.1038/nature02837 [DOI] [PubMed] [Google Scholar]
- 76.Alexander KA, Sanderson CE, Larsen MH, Robbe-austerman S, Williams MC, Palmer MV. Emerging tuberculosis pathogen hijacks social communication behavior in the group-living banded mongoose (Mungos mungo). mBio. 2016;7:3. doi: 10.1128/mBio.00281-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henderson RA, Watkins SC, Flynn JL. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159(2):635–643. [PubMed] [Google Scholar]
- 78.Ganbat D, Seehase S, Richter E, et al. Mycobacteria infect different cell types in the human lung and cause species dependent cellular changes in infected cells. BMC Pulm Med. 2016:16. doi: 10.1186/s12890-016-0185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Divangahi M, Mostowy S, Coulombe F, et al. NOD2-deficient mice have impaired resistance to mycobacterium tuberculosis infection through defective innate and adaptive immunity. J Immunol. 2008;181(10):7157–7165. doi: 10.4049/jimmunol.181.10.7157 [DOI] [PubMed] [Google Scholar]
- 80.Flannagan RS, Cosío G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7(5):355–366. doi: 10.1038/nrmicro2128 [DOI] [PubMed] [Google Scholar]
- 81.Philips JA, Ernst JD. Tuberculosis pathogenesis and Immunity. Annu Rev Pathol Mech Dis. 2012;7(1):353–384. doi: 10.1146/annurev-pathol-011811-132458 [DOI] [PubMed] [Google Scholar]
- 82.Schnettger L, Rodgers A, Repnik U, et al. A Rab20-dependent membrane trafficking pathway controls M. tuberculosis replication by regulating phagosome spaciousness and integrity. Cell Host Microbe. 2017;21(5):619–628.e5. doi: 10.1021/es1034188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tăbăran A-F, Cornel C. Macrophages targeted drug delivery as a key therapy in infectious disease. Biotechnol Mol Biol Nanomedicine. 2014;2:1. [Google Scholar]
- 84.Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263(5147):678–681. doi: 10.1126/science.8303277 [DOI] [PubMed] [Google Scholar]
- 85.Stanley LR, Vinay K, Abul K, Ramzi S, Nelson F. Robbins & Cotran Pathologic Basis of Disease. Saunders/Elsevier; 2010. [Google Scholar]
- 86.Ferrari G, Langen H, Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97(4):435–447. doi: 10.1016/S0092-8674(00)80754-0 [DOI] [PubMed] [Google Scholar]
- 87.Hmama Z, Sendide K, Talal A, Garcia R, Dobos K, Reiner NE. Quantitative analysis of phagolysosome fusion in intact cells: inhibition by mycobacterial lipoarabinomannan and rescue by an 1α, 25-dihydroxyvitamin D3–phosphoinositide 3-kinase pathway. J Cell Sci. 2004;117(10):2131–2140. doi: 10.1242/jcs.01072 [DOI] [PubMed] [Google Scholar]
- 88.Pabst M, Gross J, Brozna J, Goren M. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J Immunol. 1988;140(2):634–640. [PubMed] [Google Scholar]
- 89.Vergne I, Chua J, Deretic V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J Exp Med. 2003;198(4):653–659. doi: 10.1084/jem.20030527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clemens DL, Horwitz MA. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J Exp Med. 1996;184(4):1349–1355. doi: 10.1084/jem.184.4.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lyadova IV, Panteleev AV. Th1 and Th17 cells in tuberculosis: protection, pathology, and biomarkers. Mediators Inflamm. 2015;2015:1–13. doi: 10.1155/2015/854507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ashenafi S, Aderaye G, Bekele A, et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clin Immunol. 2014;151(2):84–99. doi: 10.1016/j.clim.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 93.Infante-Duarto C, Kamradt T Thl/Th2 balance in infection.:22.
- 94.Mohareer K, Asalla S, Banerjee S. Cell death at the cross roads of host-pathogen interaction in Mycobacterium tuberculosis infection. Tuberculosis. 2018;113:99–121. doi: 10.1016/j.tube.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 95.Lerner TR, Borel S, Greenwood DJ, et al. Mycobacterium tuberculosis replicates within necrotic human macrophages. J Cell Biol. 2017;216(3):583–594. doi: 10.1161/CIRCRESAHA.117.311401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dallenga T, Repnik U, Corleis B, et al. M. tuberculosis-induced necrosis of infected neutrophils promotes bacterial growth following phagocytosis by macrophages. Cell Host Microbe. 2017;22(4):519–530.e3. doi: 10.1016/j.chom.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 97.Queval CJ, Brosch R, Simeone R. The macrophage: a disputed fortress in the battle against mycobacterium tuberculosis. Front Microbiol. 2017;8. doi: 10.3389/fmicb.2017.02284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shanmugasundaram T, Radhakrishnan M, Gopikrishnan V, Pazhanimurugan R, Balagurunathan R. A study of the bactericidal, anti-biofouling, cytotoxic and antioxidant properties of actinobacterially synthesised silver nanoparticles. Colloids Surf B Biointerfaces. 2013;111:680–687. doi: 10.1016/j.colsurfb.2013.06.045 [DOI] [PubMed] [Google Scholar]
- 99.Aderibigbe BA. Metal-based nanoparticles for the treatment of infectious diseases. Mol Basel Switz. 2017;22:8. doi: 10.3390/molecules22081370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fathima JB, Pugazhendhi A, Venis R. Synthesis and characterization of ZrO2 nanoparticles-antimicrobial activity and their prospective role in dental care. Microb Pathog. 2017;110:245–251. doi: 10.1016/j.micpath.2017.06.039 [DOI] [PubMed] [Google Scholar]
- 101.Shanmuganathan R, LewisOscar F, Shanmugam S, et al. Core/shell nanoparticles: synthesis, investigation of antimicrobial potential and photocatalytic degradation of Rhodamine B. J Photochem Photobiol B. 2020;202:111729. doi: 10.1016/j.jphotobiol.2019.111729 [DOI] [PubMed] [Google Scholar]
- 102.Pugazhendhi A, Kumar SS, Manikandan M, Saravanan M. Photocatalytic properties and antimicrobial efficacy of Fe doped CuO nanoparticles against the pathogenic bacteria and fungi. Microb Pathog. 2018;122:84–89. doi: 10.1016/j.micpath.2018.06.016 [DOI] [PubMed] [Google Scholar]
- 103.Vasantharaj S, Sathiyavimal S, Senthilkumar P, LewisOscar F, Pugazhendhi A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: antimicrobial properties and their applications in photocatalytic degradation. J Photochem Photobiol B. 2019;192:74–82. doi: 10.1016/j.jphotobiol.2018.12.025 [DOI] [PubMed] [Google Scholar]
- 104.Reznickova A, Slavikova N, Kolska Z, et al. PEGylated gold nanoparticles: stability, cytotoxicity and antibacterial activity. Colloids Surf Physicochem Eng Asp. 2019;560:26–34. doi: 10.1016/j.colsurfa.2018.09.083 [DOI] [Google Scholar]
- 105.Mocan L, Matea C, Tabaran FA, et al. Selective in vitro photothermal nano-therapy of MRSA infections mediated by IgG conjugated gold nanoparticles. Sci Rep. 2016:6. doi: 10.1038/srep39466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jeyarani S, Vinita NM, Puja P, et al. Biomimetic gold nanoparticles for its cytotoxicity and biocompatibility evidenced by fluorescence-based assays in cancer (MDA-MB-231) and non-cancerous (HEK-293) cells. J Photochem Photobiol B. 2020;202:111715. doi: 10.1016/j.jphotobiol.2019.111715 [DOI] [PubMed] [Google Scholar]
- 107.Chellapandian C, Ramkumar B, Puja P, Shanmuganathan R, Pugazhendhi A, Kumar P. Gold nanoparticles using red seaweed Gracilaria verrucosa: green synthesis, characterization and biocompatibility studies. Process Biochem. 2019;80:58–63. doi: 10.1016/j.procbio.2019.02.009 [DOI] [Google Scholar]
- 108.Pugazhendhi A, Prabhu R, Muruganantham K, Shanmuganathan R, Natarajan S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J Photochem Photobiol B. 2019;190:86–97. doi: 10.1016/j.jphotobiol.2018.11.014 [DOI] [PubMed] [Google Scholar]
- 109.Hariharan D, Thangamuniyandi P, Jegatha Christy A, et al. Enhanced photocatalysis and anticancer activity of green hydrothermal synthesized Ag@TiO2 nanoparticles. J Photochem Photobiol B. 2020;202:111636. doi: 10.1016/j.jphotobiol.2019.111636 [DOI] [PubMed] [Google Scholar]
- 110.Hariharan D, Thangamuniyandi P, Selvakumar P, et al. Green approach synthesis of Pd@TiO2 nanoparticles: characterization, visible light active picric acid degradation and anticancer activity. Process Biochem. 2019;87:83–88. doi: 10.1016/j.procbio.2019.09.024 [DOI] [Google Scholar]
- 111.Ramkumar VS, Pugazhendhi A, Gopalakrishnan K, et al. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol Rep. 2017;14:1–7. doi: 10.1016/j.btre.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Madhubala V, Pugazhendhi A, Thirunavukarasu K. Cytotoxic and immunomodulatory effects of the low concentration of titanium dioxide nanoparticles (TiO2 NPs) on human cell lines - An in vitro study. Process Biochem. 2019;86:186–195. doi: 10.1016/j.procbio.2019.08.004 [DOI] [Google Scholar]
- 113.Fathima JB, Pugazhendhi A, Oves M, Venis R. Synthesis of eco-friendly copper nanoparticles for augmentation of catalytic degradation of organic dyes. J Mol Liq. 2018;260:1–8. doi: 10.1016/j.molliq.2018.03.033 [DOI] [Google Scholar]
- 114.Vasantharaj S, Sathiyavimal S, Saravanan M, et al. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: characterization of antibacterial activity and dye degradation potential. J Photochem Photobiol B. 2019;191:143–149. doi: 10.1016/j.jphotobiol.2018.12.026 [DOI] [PubMed] [Google Scholar]
- 115.Saratale RG, Ghodake GS, Shinde SK, et al. Photocatalytic activity of CuO/Cu(OH)2 nanostructures in the degradation of Reactive Green 19A and textile effluent, phytotoxicity studies and their biogenic properties (antibacterial and anticancer). J Environ Manage. 2018;223:1086–1097. doi: 10.1016/j.jenvman.2018.04.072 [DOI] [PubMed] [Google Scholar]
- 116.Varadavenkatesan T, Lyubchik E, Pai S, Pugazhendhi A, Vinayagam R, Selvaraj R. Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J Photochem Photobiol B. 2019;199:111621. doi: 10.1016/j.jphotobiol.2019.111621 [DOI] [PubMed] [Google Scholar]
- 117.Shanmuganathan R, Edison TNJI, LewisOscar F, Kumar P, Shanmugam S, Pugazhendhi A. Chitosan nanopolymers: an overview of drug delivery against cancer. Int J Biol Macromol. 2019;130:727–736. doi: 10.1016/j.ijbiomac.2019.02.060 [DOI] [PubMed] [Google Scholar]
- 118.Sanpui P, Chattopadhyay A, Ghosh SS. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl Mater Interfaces. 2011;3(2):218–228. doi: 10.1021/am100840c [DOI] [PubMed] [Google Scholar]
- 119.Suganya M, Gnanamangai BM, Govindasamy C, et al. Mitochondrial dysfunction mediated apoptosis of HT-29 cells through CS-PAC-AgNPs and investigation of genotoxic effects in zebra (Danio rerio) fish model for drug delivery. Saudi J Biol Sci. 2019;26(4):767–776. doi: 10.1016/j.sjbs.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Russell AD, Hugo WB. 7 Antimicrobial activity and action of silver In: Progress in Medicinal Chemistry. Vol. 31 Elsevier;1994::351–370. doi: 10.1016/S0079-6468(08)70024-9 [DOI] [PubMed] [Google Scholar]
- 121.Silva JP, Appelberg R, Gama FM. Antimicrobial peptides as novel anti-tuberculosis therapeutics. Biotechnol Adv. 2016;34(5):924–940. doi: 10.1016/j.biotechadv.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 122.Khan F, Khan MM, Kim Y-M. Recent progress and future perspectives of antibiofilm drugs immobilized on nanomaterials. Curr Pharm Biotechnol. 2018;19(8):631–643. doi: 10.2174/1389201019666180828090052 [DOI] [PubMed] [Google Scholar]
- 123.Raffi M, Mehrwan S, Bhatti TM, et al. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann Microbiol. 2010;60(1):75–80. doi: 10.1007/s13213-010-0015-6 [DOI] [Google Scholar]
- 124.Pham DTN, Khan F, Phan TTV, et al. Biofilm inhibition, modulation of virulence and motility properties by FeOOH nanoparticle in Pseudomonas aeruginosa. Braz J Microbiol. 2019;50(3):791–805. doi: 10.1007/s42770-019-00108-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khan F, Manivasagan P, Lee J-W, Pham DTN, Oh J, Kim Y-M. Fucoidan-stabilized gold nanoparticle-mediated biofilm inhibition, attenuation of virulence and motility properties in Pseudomonas aeruginosa PAO1. Mar Drugs. 2019;17:4. doi: 10.3390/md17040208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Javaid A, Oloketuyi SF, Khan MM, Khan F. Diversity of bacterial synthesis of silver nanoparticles. BioNanoScience. 2018;8(1):43–59. doi: 10.1007/s12668-017-0496-x [DOI] [Google Scholar]
- 127.Mocan T, Matea CT, Pop T, et al. Carbon nanotubes as anti-bacterial agents. Cell Mol Life Sci CMLS. 2017;74(19):3467–3479. doi: 10.1007/s00018-017-2532-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khan F, Pham DTN, Oloketuyi SF, Manivasagan P, Oh J, Kim Y-M. Chitosan and their derivatives: antibiofilm drugs against pathogenic bacteria. Colloids Surf B Biointerfaces. 2020;185:110627. doi: 10.1016/j.colsurfb.2019.110627 [DOI] [PubMed] [Google Scholar]
- 129.Khan F, Manivasagan P, Pham DTN, Oh J, Kim S-K, Kim Y-M. Antibiofilm and antivirulence properties of chitosan-polypyrrole nanocomposites to Pseudomonas aeruginosa. Microb Pathog. 2019;128:363–373. doi: 10.1016/j.micpath.2019.01.033 [DOI] [PubMed] [Google Scholar]
- 130.Manivasagan P, Khan F, Hoang G, et al. Thiol chitosan-wrapped gold nanoshells for near-infrared laser-induced photothermal destruction of antibiotic-resistant bacteria. Carbohydr Polym. 2019;225:115228. doi: 10.1016/j.carbpol.2019.115228 [DOI] [PubMed] [Google Scholar]
- 131.Zazo H, Colino CI, Lanao JM. Current applications of nanoparticles in infectious diseases. J Control Release off J Control Release Soc. 2016;224:86–102. doi: 10.1016/j.jconrel.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 132.Costa-gouveia J, Aínsa JA, Brodin P, Lucía A. How can nanoparticles contribute to antituberculosis therapy? Drug Discov Today. 2017;22(3):600–607. doi: 10.1016/j.drudis.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 133.Kreytsberg GN, Gracheva IE, Kibrik BS, Golikov IV. Antituberculous effect of silver nanoparticles. J Phys Conf Ser. 2011;291:012030. doi: 10.1088/1742-6596/291/1/012030 [DOI] [Google Scholar]
- 134.Song B, Zhang C, Zeng G, Gong J, Chang Y, Jiang Y. Antibacterial properties and mechanism of graphene oxide-silver nanocomposites as bactericidal agents for water disinfection. Arch Biochem Biophys. 2016;604:167–176. doi: 10.1016/j.abb.2016.04.018 [DOI] [PubMed] [Google Scholar]
- 135.Liu C, Guo J, Yan X, et al. Antimicrobial nanomaterials against biofilms: an alternative strategy. Environ Rev. 2016;25(2):225–244. doi: 10.1139/er-2016-0046 [DOI] [Google Scholar]
- 136.Franci G, Falanga A, Galdiero S, et al. Silver nanoparticles as potential antibacterial agents. Mol Basel Switz. 2015;20(5):8856–8874. doi: 10.3390/molecules20058856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. 2016;7:1831. doi: 10.3389/fmicb.2016.01831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346. doi: 10.1088/0957-4484/16/10/059 [DOI] [PubMed] [Google Scholar]
- 139.Kumar DA, Palanichamy V, Roopan SM. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2014;127:168–171. doi: 10.1016/j.saa.2014.02.058 [DOI] [PubMed] [Google Scholar]
- 140.Jain J, Arora S, Rajwade JM, Omray P, Khandelwal S, Paknikar KM. Silver nanoparticles in therapeutics: development of an antimicrobial gel formulation for topical use. Mol Pharm. 2009;6(5):1388–1401. doi: 10.1021/mp900056g [DOI] [PubMed] [Google Scholar]
- 141.Le Ouay B, Stellacci F. Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today. 2015;10(3):339–354. doi: 10.1016/j.nantod.2015.04.002 [DOI] [Google Scholar]
- 142.Huh AJ, Kwon YJ. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release off J Control Release Soc. 2011;156(2):128–145. doi: 10.1016/j.jconrel.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 143.Kim JS, Kuk E, Yu KN, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine Nanotechnol Biol Med. 2007;3(1):95–101. doi: 10.1016/j.nano.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 144.Al-Sharqi A, Apun K, Vincent M, Kanakaraju D, Bilung LM. Enhancement of the antibacterial efficiency of silver nanoparticles against gram-positive and gram-negative bacteria using blue laser light. Int J Photoenergy. doi: 10.1155/2019/2528490 [DOI] [Google Scholar]
- 145.Velayati AA, Farnia P, Ibrahim TA, et al. Differences in cell wall thickness between resistant and nonresistant strains of Mycobacterium tuberculosis: using transmission electron microscopy. Chemotherapy. 2009;55(5):303–307. doi: 10.1159/000226425 [DOI] [PubMed] [Google Scholar]
- 146.Mai-Prochnow A, Clauson M, Hong J, Murphy AB. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci Rep. 2016;6. doi: 10.1038/srep38610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hett EC, Rubin EJ. Bacterial growth and cell division: a mycobacterial perspective. Microbiol Mol Biol Rev MMBR. 2008;72(1):126–156. doi: 10.1128/MMBR.00028-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saravanan M, Barik SK, MubarakAli D, Prakash P, Pugazhendhi A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb Pathog. 2018;116:221–226. doi: 10.1016/j.micpath.2018.01.038 [DOI] [PubMed] [Google Scholar]
- 149.Seth D, Sarkar A, Mitra D. Nanomedicine to counter syndemic tuberculosis and HIV infection: current knowledge and state of art. Nanosci Nanoeng. 2014;9. [Google Scholar]
- 150.Abdel-Aziz MM, Elella MHA, Mohamed RR. Green synthesis of quaternized chitosan/silver nanocomposites for targeting mycobacterium tuberculosis and lung carcinoma cells (A-549). Int J Biol Macromol. 2019. doi:10/ggdd6n [DOI] [PubMed] [Google Scholar]
- 151.El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM. Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol. 2011;45(1):283–287. doi:10/cc29h5 [DOI] [PubMed] [Google Scholar]
- 152.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 153.Cho K-H, Park J-E, Osaka T, Park S-G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim Acta. 2005;51(5):956–960. doi: 10.1016/j.electacta.2005.04.071 [DOI] [Google Scholar]
- 154.McQuillan JS, Groenaga Infante H, Stokes E, Shaw AM. Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12. Nanotoxicology. 2012;6(8):857–866. doi: 10.3109/17435390.2011.626532 [DOI] [PubMed] [Google Scholar]
- 155.Orlov IA, Sankova TP, Babich PS, et al. New silver nanoparticles induce apoptosis-like process in E. coli and interfere with mammalian copper metabolism. Int J Nanomedicine. doi: 10.2147/IJN.S117745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rodgers FG, Tzianabos AO, Elliott TSJ. The effect of antibiotics that inhibit cell-wall, protein, and DNA synthesis on the growth and morphology of Legionella pneumophila. J Med Microbiol. 1990;31(1):37–44. doi: 10.1099/00222615-31-1-37 [DOI] [PubMed] [Google Scholar]
- 157.van der Wal A, Norde W, Zehnder AJB, Lyklema J. Determination of the total charge in the cell walls of Gram-positive bacteria. Colloids Surf B Biointerfaces. 1997;9(1–2):81–100. doi: 10.1016/S0927-7765(96)01340-9 [DOI] [Google Scholar]
- 158.Shaik M, Albalawi G, Khan S, et al. “Miswak” based green synthesis of silver nanoparticles: evaluation and comparison of their microbicidal activities with the chemical synthesis. Molecules. 2016;21(11):1478. doi: 10.3390/molecules21111478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ashraf S, Akhtar N, Ghauri M, Rajoka M, Khalid ZM, Hussain I. Polyhexamethylene biguanide functionalized cationic silver nanoparticles for enhanced antimicrobial activity. Nanoscale Res Lett. 2012;7(1):267. doi: 10.1186/1556-276X-7-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ivask A, ElBadawy A, Kaweeteerawat C, et al. Toxicity mechanisms in escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano. 2014;8(1):374–386. doi:10/f5qqhz [DOI] [PubMed] [Google Scholar]
- 161.Kvítek L, Panáček A, Soukupová J, et al. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J Phys Chem C. 2008;112(15):5825–5834. doi: 10.1021/jp711616v [DOI] [Google Scholar]
- 162.Yin R, Agrawal T, Khan U, et al. Antimicrobial photodynamic inactivation in nanomedicine: small light strides against bad bugs. Nanomed. 2015;10(15):2379–2404. doi: 10.2217/nnm.15.67 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 163.Yun’an Qing LC, Li R, Liu G, et al. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int J Nanomedicine. 2018;13:3311. doi: 10.2147/IJN.S165125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bonnet M, Massard C, Veisseire P, Camares O, Awitor KO. Environmental toxicity and antimicrobial efficiency of titanium dioxide nanoparticles in suspension. J Biomater Nanobiotech. 2015;6(3):213–224. doi: 10.4236/jbnb.2015.63020 [DOI] [Google Scholar]
- 165.Parikh AN, Gillmor SD, Beers JD, Beardmore KM, Cutts RW, Swanson BI. Characterization of chain molecular assemblies in long-chain, layered silver thiolates: a joint infrared spectroscopy and X-ray diffraction study. J Phys Chem B. 1999;103(15):2850–2861. doi: 10.1021/jp983938b [DOI] [Google Scholar]
- 166.Dobias J, Bernier-Latmani R. Silver release from silver nanoparticles in natural waters. Environ Sci Technol. 2013;47(9):4140–4146. doi: 10.1021/es304023p [DOI] [PubMed] [Google Scholar]
- 167.Park E-J, Yi J, Kim Y, Choi K, Park K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol in Vitro. 2010;24(3):872–878. doi: 10.1016/j.tiv.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 168.He D, Miller CJ, Waite TD. Fenton-like zero-valent silver nanoparticle-mediated hydroxyl radical production. J Catal. 2014;317:198–205. doi: 10.1016/j.jcat.2014.06.016 [DOI] [Google Scholar]
- 169.Sisubalan N, Ramkumar VS, Pugazhendhi A, et al. ROS-mediated cytotoxic activity of ZnO and CeO2 nanoparticles synthesized using the Rubia cordifolia L. leaf extract on MG-63 human osteosarcoma cell lines. Environ Sci Pollut Res. 2018;25(11):10482–10492. doi: 10.1007/s11356-017-0003-5 [DOI] [PubMed] [Google Scholar]
- 170.Knaapen AM, Borm PJA, Albrecht C, Schins RPF. Inhaled particles and lung cancer. Part A: mechanisms. Int J Cancer. 2004;109(6):799–809. doi: 10.1002/ijc.11708 [DOI] [PubMed] [Google Scholar]
- 171.Samuel MS, Jose S, Selvarajan E, Mathimani T, Pugazhendhi A. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J Photochem Photobiol B. 2020;202:111642. doi: 10.1016/j.jphotobiol.2019.111642 [DOI] [PubMed] [Google Scholar]
- 172.Speight JG, editor. Redox Transformations In: Reaction Mechanisms in Environmental Engineering. Elsevier; 2018:231–267. doi: 10.1016/B978-0-12-804422-3.00007-9 [DOI] [Google Scholar]
- 173.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902. doi: 10.1161/CIRCRESAHA.117.311401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zielinska E, Tukaj C, Radomski MW, Inkielewicz-stepniak I. Molecular mechanism of silver nanoparticles-induced human osteoblast cell death: protective effect of inducible nitric oxide synthase inhibitor. PLoS One. 2016;11(10):e0164137. doi:10/f9rvdx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bressan E, Ferroni L, Gardin C, et al. Silver nanoparticles and mitochondrial interaction. Int J Dent. 2013;2013:1–8. doi: 10.1155/2013/312747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Maurer LL, Meyer JN. A systematic review of evidence for silver nanoparticle-induced mitochondrial toxicity. Environ Sci Nano. 2016;3(2):311–322. doi: 10.1039/C5EN00187K [DOI] [Google Scholar]
- 177.Kim S, Choi JE, Choi J, et al. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol in Vitro. 2009;23(6):1076–1084. doi: 10.1016/j.tiv.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 178.Miyayama T, Matsuoka M. Involvement of lysosomal dysfunction in silver nanoparticle-induced cellular damage in A549 human lung alveolar epithelial cells. J Occup Med Toxicol. 2016;11(1):1. doi: 10.1186/s12995-016-0090-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Park MVDZ, Neigh AM, Vermeulen JP, et al. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. 2011;32(36):9810–9817. doi: 10.1016/j.biomaterials.2011.08.085 [DOI] [PubMed] [Google Scholar]
- 180.Singh R, Nawale L, Arkile M, et al. Phytogenic silver, gold, and bimetallic nanoparticles as novel antitubercular agents. Int J Nanomedicine. 2016;11:1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xiu Z, Zhang Q, Puppala HL, Colvin VL, Alvarez PJJ. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012;12(8):4271–4275. doi: 10.1021/nl301934w [DOI] [PubMed] [Google Scholar]
- 182.Behra R, Sigg L, Clift MJD, et al. Bioavailability of silver nanoparticles and ions: from a chemical and biochemical perspective. J R Soc Interface. 2013;10:87. doi: 10.1098/rsif.2013.0396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Saratale RG, Karuppusamy I, Saratale GD, et al. A comprehensive review on green nanomaterials using biological systems: recent perception and their future applications. Colloids Surf B Biointerfaces. 2018;170:20–35. doi: 10.1016/j.colsurfb.2018.05.045 [DOI] [PubMed] [Google Scholar]
- 184.Saratale RG, Saratale GD, Shin HS, et al. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: current knowledge, their agricultural and environmental applications. Environ Sci Pollut Res. 2018;25(11):10164–10183. doi: 10.1007/s11356-017-9912-6 [DOI] [PubMed] [Google Scholar]
- 185.Kote JR, Kadam AS, Patil SS, Mane RS. Green functionalized silver nanoparticles with significantly enhanced antimycobactericidal and cytotoxicity performances of asparagus racemosus Linn. Int J New Technol Sci. 2016;3(2):15. [Google Scholar]
- 186.Roy A, Bulut O, Some S, Mandal AK, Yilmaz MD. Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9(5):2673–2702. doi: 10.1039/C8RA08982E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kerry RG, Gouda S, Sil B, et al. Cure of tuberculosis using nanotechnology: an overview. J Microbiol. 2018;56(5):287–299. doi: 10.1007/s12275-018-7414-y [DOI] [PubMed] [Google Scholar]
- 188.Arokiyaraj S, Arasu MV, Vincent S, et al. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L and its antibacterial and cytotoxic effects: an in vitro study. Int J Nanomedicine. doi: 10.2147/IJN.S53546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17–28. doi: 10.1016/j.jare.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shaik M, Khan M, Kuniyil M, et al. Plant-extract-assisted green synthesis of silver nanoparticles using origanum vulgare L. Extract and their microbicidal activities. Sustainability. 2018;10(4):913. doi: 10.3390/su10040913 [DOI] [Google Scholar]
- 191.Pugazhendhi A, Prabakar D, Jacob JM, Karuppusamy I, Saratale RG. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb Pathog. 2018;114:41–45. doi: 10.1016/j.micpath.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 192.Oves M, Aslam M, Rauf MA, et al. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater Sci Eng C. 2018;89:429–443. doi: 10.1016/j.msec.2018.03.035 [DOI] [PubMed] [Google Scholar]
- 193.Singh R, Nawale LU, Arkile M, et al. Chemical and biological metal nanoparticles as antimycobacterial agents: a comparative study. Int J Antimicrob Agents. 2015;46(2):183–188. doi: 10.1016/j.ijantimicag.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 194.Rónavári A, Kovács D, Igaz N, et al. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: a comprehensive study. Int J Nanomedicine. 2017;12:871–883. doi: 10.2147/IJN.S122842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rai M, Ingle AP, Pandit R, et al. Broadening the spectrum of small-molecule antibacterials by metallic nanoparticles to overcome microbial resistance. Int J Pharm. 2017;532(1):139–148. doi: 10.1016/j.ijpharm.2017.08.127 [DOI] [PubMed] [Google Scholar]
- 196.Baptista PV, McCusker MP, Carvalho A, et al. Nano-strategies to fight multidrug resistant bacteria—“a battle of the titans. Front Microbiol. 2018;9:1441. doi: 10.3389/fmicb.2018.01441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Djafari J, Marinho C, Santos T, et al. New synthesis of gold- and silver-based nano-tetracycline composites. ChemistryOpen. 2016;5(3):206–212. doi: 10.1002/open.201600016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Deng H, McShan D, Zhang Y, et al. Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environ Sci Technol. 2016;50(16):8840–8848. doi: 10.1021/acs.est.6b00998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Birla SS, Tiwari VV, Gade AK, Ingle AP, Yadav AP, Rai MK. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol. 2009;48(2):173–179. doi: 10.1111/j.1472-765X.2008.02510.x [DOI] [PubMed] [Google Scholar]
- 200.Brown AN, Smith K, Samuels TA, Lu J, Obare SO, Scott ME. Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of pseudomonas aeruginosa and enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl Environ Microbiol. 2012;78(8):2768–2774. doi: 10.1128/AEM.06513-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Saratale GD, Saratale RG, Benelli G, et al. Anti-diabetic potential of silver nanoparticles synthesized with argyreia nervosa leaf extract high synergistic antibacterial activity with standard antibiotics against foodborne bacteria. J Clust Sci. 2017;28(3):1709–1727. doi: 10.1007/s10876-017-1179-z [DOI] [Google Scholar]
- 202.Shanmuganathan R, MubarakAli D, Prabakar D, et al. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environ Sci Pollut Res. 2018;25(11):10362–10370. doi: 10.1007/s11356-017-9367-9 [DOI] [PubMed] [Google Scholar]
- 203.Hwang I-S, Hwang JH, Choi H, Kim K-J, Lee DG. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J Med Microbiol. 2012;61(Pt_12):1719–1726. doi: 10.1099/jmm.0.047100-0 [DOI] [PubMed] [Google Scholar]
- 204.Farooq U, Ahmad T, Khan A, et al. Rifampicin conjugated silver nanoparticles: a new arena for development of antibiofilm potential against methicillin resistant Staphylococcus aureus and Klebsiella pneumoniae. Int J Nanomedicine. 2019;14:3983–3993. doi: 10.2147/IJN.S198194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Li P, Li J, Wu C, Wu Q, Li J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology. 2005;16(9):1912–1917. doi: 10.1088/0957-4484/16/9/082 [DOI] [Google Scholar]
- 206.Arakha M, Jha S. Effects of photocatalytic nanoparticle interfaces on biological membranes and biomacromolecules. 2017. doi:10/ggdp7k
- 207.Nasiruddin M, Neyaz M, Das S. Nanotechnology-based approach in tuberculosis treatment. Tuberc Res Treat. 2017;2017. doi: 10.1155/2017/4920209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Singh R, Nawale L, Arkile M, et al. Phytogenic silver, gold, and bimetallic nanoparticles as novel antitubercular agents. Int J Nanomedicine. 2016;11:1889–1897. doi: 10.2147/IJN.S102488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Song HY, Ko KK, Oh IH, Lee BT. Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur Cell Mater. 2006;11(suppl 1):58. [Google Scholar]
- 210.Jaryal N, Kaur H. Plumbago auriculata leaf extract-mediated AgNPs and its activities as antioxidant, anti-TB and dye degrading agents. J Biomater Sci Polym Ed. 2017;28(16):1847–1858. doi: 10.1080/09205063.2017.1354673 [DOI] [PubMed] [Google Scholar]
- 211.Paarakh PM. Anti-tubercular activity of silver nanoparticle synthesized from the fruits of coriandrum sativum linn. World J Pharm Pharm Sci. 2017;1720–1727. doi:10/ggdkzz [Google Scholar]
- 212.Raja A, Salique SM, Gajalakshmi P, James A. Antibacterial and hemolytic activity of green silver nanoparticles from Catharanthus roseus. Int J Pharm Sci Nanotechnol. 2016;9(1):7. [Google Scholar]
- 213.Kote JR, Mulani RM, Kadam AS, Solankar BM. Anti-Mycobacterial Activity of Nanoparticles from Psidium Guajava L. 2014:5. [Google Scholar]
- 214.Daniel SCGK, Banu BN, Harshiny M, et al. Ipomea carnea -based silver nanoparticle synthesis for antibacterial activity against selected human pathogens. J Exp Nanosci. 2014;9(2):197–209. doi: 10.1080/17458080.2011.654274 [DOI] [Google Scholar]
- 215.Banu A. Biosynthesis of monodispersed silver nanoparticles and their activity against Mycobacterium tuberculosis. J Nanomedicine Biotherapeutic Discov. 2013;03:01. doi: 10.4172/2155-983X.1000110 [DOI] [Google Scholar]
- 216.Agarwal P, Mehta A, Kachhwaha S, Kothari SL. Green synthesis of silver nanoparticles and their activity against Mycobacterium tuberculosis. Adv Sci Eng Med. 2013;5(7):709–714. doi: 10.1166/asem.2013.1307 [DOI] [Google Scholar]
- 217.Heidary M, Zaker Bostanabad S, Amini SM, et al. The anti-mycobacterial activity of Ag, ZnO, And Ag- ZnO nanoparticles against MDR- and XDR-Mycobacterium tuberculosis. Infect Drug Resist. 2019;12:3425–3435. doi: 10.2147/IDR.S221408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ellis T, Chiappi M, García-trenco A, et al. Multimetallic microparticles increase the potency of rifampicin against intracellular Mycobacterium tuberculosis. ACS Nano. 2018;12(6):5228–5240. doi: 10.1021/acsnano.7b08264 [DOI] [PubMed] [Google Scholar]
- 219.Jafari A, Mosavari N, Movahedzadeh F, et al. Bactericidal impact of Ag, ZnO and mixed AgZnO colloidal nanoparticles on HRv Mycobacterium tuberculosis phagocytized by THP-1 cell lines. Microb Pathog. 2017;110:335–344. doi: 10.1016/j.micpath.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 220.Jena P, Mohanty S, Mallick R, Jacob B, Sonawane A. Toxicity and antibacterial assessment of chitosancoated silver nanoparticles on human pathogens and macrophage cells. Int J Nanomedicine. 2012;7:1805. doi: 10.2147/IJN.S30631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Selim A, Elhaig MM, Taha SA, Nasr EA. Antibacterial activity of silver nanoparticles against field and reference strains of Mycobacterium tuberculosis, Mycobacterium bovis and multiple-drug-resistant tuberculosis strains. Rev Sci Tech OIE. 2018;37(3):823–830. doi: 10.20506/rst.37.3.2888 [DOI] [PubMed] [Google Scholar]
- 222.Sivaraj A, Kumar V, Sunder R, Parthasarathy K, Kasivelu G. Commercial yeast extracts mediated green synthesis of silver chloride nanoparticles and their anti-mycobacterial activity. J Clust Sci. 2019. doi:10/ggdgtw [Google Scholar]
- 223.Kim J, Pitts B, Stewart PS, Camper A, Yoon J. Comparison of the antimicrobial effects of chlorine, silver ion, and tobramycin on biofilm. Antimicrob Agents Chemother. 2008;52(4):1446–1453. doi: 10.1128/AAC.00054-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Patel S. Biogenic silver nanoparticles as potential agent against mycobacterium tuberculosis. Int J Res Appl Sci Eng Technol. 2018;6(1):505–511. doi: 10.22214/ijraset.2018.1075 [DOI] [Google Scholar]
- 225.Seth D, Choudhury SR, Pradhan S, et al. Nature-inspired novel drug design paradigm using nanosilver: efficacy on multi-drug-resistant clinical isolates of tuberculosis. Curr Microbiol. 2011;62(3):715–726. doi: 10.1007/s00284-010-9770-7 [DOI] [PubMed] [Google Scholar]
- 226.Punjabi K, Mehta S, Chavan R, Chitalia V, Deogharkar D, Deshpande S. Efficiency of biosynthesized silver and zinc nanoparticles against multi-drug resistant pathogens. Front Microbiol. 2018;9. doi:10/gfdvdk [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sun F, Oh S, Kim J, et al. Enhanced internalization of macromolecular drugs into mycobacterium smegmatis with the assistance of silver nanoparticles. J Microbiol Biotechnol. 2017;27(8):1483–1490. doi: 10.4014/jmb.1612.12041 [DOI] [PubMed] [Google Scholar]
- 228.Padmaa MP. Green synthesis of silver nanoparticles using fruits of coriandrum sativum linn and its antioxidant activity. J Nat Prod Resour. 2015;1(1):19–22. [Google Scholar]
- 229.Donnellan S, Tran L, Johnston H, McLuckie J, Stevenson K, Stone V. A rapid screening assay for identifying mycobacteria targeted nanoparticle antibiotics. Nanotoxicology. 2016;10(6):761–769. doi: 10.3109/17435390.2015.1124468 [DOI] [PubMed] [Google Scholar]
- 230.Jafari AR, Mosavi T, Mosavari N, et al. Mixed metal oxide nanoparticles inhibit growth of Mycobacterium tuberculosis into THP-1 cells. Int J Mycobacteriology. 2016;5:S181–S183. doi: 10.1016/j.ijmyco.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 231.Islam MS, Larimer C, Ojha A, Nettleship I. Antimycobacterial efficacy of silver nanoparticles as deposited on porous membrane filters. Mater Sci Eng C. 2013;33(8):4575–4581. doi: 10.1016/j.msec.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 232.Mohanty S, Mishra S, Jena P, Jacob B, Sarkar B, Sonawane A. An investigation on the antibacterial, cytotoxic, and antibiofilm efficacy of starch-stabilized silver nanoparticles. Nanomedicine Nanotechnol Biol Med. 2012;8(6):916–924. doi: 10.1016/j.nano.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 233.Zhou Y, Kong Y, Kundu S, Cirillo JD, Liang H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J Nanobiotechnology. 2012;10(1):19. doi: 10.1186/1477-3155-10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Martinez-Gutierrez F, Olive PL, Banuelos A, et al. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomedicine Nanotechnol Biol Med. 2010;6(5):681–688. doi: 10.1016/j.nano.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 235.Varghese MV, Dhumal RS, Patil SS, Paradkar AR, Khanna PK. Synthesis and in-vitro antimycobacterial studies of cysteine capped silver nano-particles. Synth React Inorg Met-Org Nano-Met Chem. 2009;39(9):554–558. doi: 10.1080/15533170903327869 [DOI] [Google Scholar]
- 236.Uraskulova BB, Gyusan AO. The clinical and bacteriological study of the effectiveness of the application of silver nanoparticle for the treatment of tuberculosis. Vestn Otorinolaringol. 2017;82(3):54–57. doi: 10.17116/otorino201782354-57 [DOI] [PubMed] [Google Scholar]
- 237.Gmoshinski IV, Shumakova AA, Shipelin VA, Maltsev G, Khotimchenko SA. Influence of orally introduced silver nanoparticles on content of essential and toxic trace elements in organism. Nanotechnologies Russ. 2016;11(9–10):646–652. doi: 10.1134/S1995078016050074 [DOI] [Google Scholar]
- 238.Zakharov AV, Khokhlov AL, Kibrik BS. Effectiveness of combination of isoniazid and silver nanoparticles in the treatment of experimental tuberculosis. Tuberc Lung Dis. 2017;95(6):51–58. doi: 10.21292/2075-1230-2017-95-6-51-58 [DOI] [Google Scholar]
- 239.Zakharov AV, Khokhlov A. The results of experimental studies of the use of silver nanoparticles in tuberculosis drug-resistant pathogen. Med News North Cauc. 2019;14:1. doi:10/ggdjnk [Google Scholar]
- 240.Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;539(1):104–111. doi: 10.1016/j.ijpharm.2018.01.034 [DOI] [PubMed] [Google Scholar]
- 241.Srinivasan M, Venkatesan M, Arumugam V, et al. Green synthesis and characterization of titanium dioxide nanoparticles (TiO2 NPs) using Sesbania grandiflora and evaluation of toxicity in zebrafish embryos. Process Biochem. 2019;80:197–202. doi: 10.1016/j.procbio.2019.02.010 [DOI] [Google Scholar]
- 242.Vazquez-Muñoz R, Borrego B, Juárez-moreno K, et al. Toxicity of silver nanoparticles in biological systems: does the complexity of biological systems matter? Toxicol Lett. 2017;276:11–20. doi: 10.1016/j.toxlet.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 243.Tarannum N, Divya K, Gautam Y. Facile green synthesis and applications of silver nanoparticles: a state-of-the-art review. RSC Adv. 2019;9(60):34926–34948. doi: 10.1039/C9RA04164H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dey Bhowmik A, Bandyopadhyay A, Chattopadhyay A. Cytotoxic and mutagenic effects of green silver nanoparticles in cancer and normal cells: a brief review. The Nucleus. 2019;62(3):277–285. doi: 10.1007/s13237-019-00293-0 [DOI] [Google Scholar]
- 245.Saravanan M, Arokiyaraj S, Lakshmi T, Pugazhendhi A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb Pathog. 2018;117:68–72. doi: 10.1016/j.micpath.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 246.Yuan D, He H, Wu Y, Fan J, Cao Y. Physiologically based pharmacokinetic modeling of nanoparticles. J Pharm Sci. 2019;108(1):58–72. doi: 10.1016/j.xphs.2018.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ehlers S, Schaible UE. The granuloma in tuberculosis: dynamics of a host–pathogen collusion. Front Immunol. 2013;3. doi: 10.3389/fimmu.2012.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sarkar S, Leo BF, Carranza C, et al. Modulation of human macrophage responses to mycobacterium tuberculosis by silver nanoparticles of different size and surface modification. PLoS One. 2015;10(11):e0143077. doi: 10.1371/journal.pone.0143077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.De Jong WH, Van Der Ven LTM, Sleijffers A, et al. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials. 2013;34(33):8333–8343. doi: 10.1016/j.biomaterials.2013.06.048 [DOI] [PubMed] [Google Scholar]
- 250.Müller L, Steiner SK, Rodriguez-lorenzo L, Petri-fink A, Rothen-rutishauser B, Latzin P. Exposure to silver nanoparticles affects viability and function of natural killer cells, mostly via the release of ions. Cell Biol Toxicol. 2018;34(3):167–176. doi: 10.1007/s10565-017-9403-z [DOI] [PubMed] [Google Scholar]
- 251.Alsaleh NB, Minarchick VC, Mendoza RP, Sharma B, Podila R, Brown JM. Silver nanoparticle immunomodulatory potential in absence of direct cytotoxicity in RAW 264.7 macrophages and MPRO 2.1 neutrophils. J Immunotoxicol. 2019;16(1):63–73. doi: 10.1080/1547691X.2019.1588928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yen H, Hsu S, Tsai C. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small. 2009;5(13):1553–1561. doi: 10.1002/smll.200900126 [DOI] [PubMed] [Google Scholar]
- 253.Li T, Albee B, Alemayehu M, et al. Comparative toxicity study of Ag, Au, and Ag–Au bimetallic nanoparticles on Daphnia magna. Anal Bioanal Chem. 2010;398(2):689–700. doi: 10.1007/s00216-010-3915-1 [DOI] [PubMed] [Google Scholar]
- 254.Suganthy N, Sri Ramkumar V, Pugazhendhi A, Benelli G, Archunan G. Biogenic synthesis of gold nanoparticles from Terminalia arjuna bark extract: assessment of safety aspects and neuroprotective potential via antioxidant, anticholinesterase, and antiamyloidogenic effects. Environ Sci Pollut Res. 2018;25(11):10418–10433. doi: 10.1007/s11356-017-9789-4 [DOI] [PubMed] [Google Scholar]
- 255.Larimer C, Islam MS, Ojha A, Nettleship I. Mutation of environmental mycobacteria to resist silver nanoparticles also confers resistance to a common antibiotic. BioMetals. 2014;27(4):695–702. doi: 10.1007/s10534-014-9761-4 [DOI] [PubMed] [Google Scholar]
- 256.Panáček A, Kvítek L, Smékalová M, et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat Nanotechnol. 2018;13(1):65–71. doi: 10.1038/s41565-017-0013-y [DOI] [PubMed] [Google Scholar]
- 257.Muller M. Bacterial silver resistance gained by cooperative interspecies redox behavior. Antimicrob Agents Chemother. 2018;62:8. doi: 10.1128/AAC.00672-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev. 2003;27(2–3):341–353. doi: 10.1016/S0168-6445(03)00047-0 [DOI] [PubMed] [Google Scholar]
- 259.Mijnendonckx K, Ali MM, Provoost A, et al. Spontaneous mutation in the AgrRS two-component regulatory system of Cupriavidus metallidurans results in enhanced silver resistance. Metallomics. 2019;11(11):1912–1924. doi: 10.1039/C9MT00123A [DOI] [PubMed] [Google Scholar]
- 260.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147–179. doi: 10.1128/CMR.12.1.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. J Hosp Infect. 2005;60(1):1–7. doi: 10.1016/j.jhin.2004.11.014 [DOI] [PubMed] [Google Scholar]
- 262.Woods EJ, Cochrane CA, Percival SL. Prevalence of silver resistance genes in bacteria isolated from human and horse wounds. Vet Microbiol. 2009;138(3–4):325–329. doi: 10.1016/j.vetmic.2009.03.023 [DOI] [PubMed] [Google Scholar]
- 263.Loh JV, Percival SL, Woods EJ, Williams NJ, Cochrane CA. Silver resistance in MRSA isolated from wound and nasal sources in humans and animals. Int Wound J. 2009;6(1):32–38. doi: 10.1111/j.1742-481X.2008.00563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Finley PJ, Norton R, Austin C, Mitchell A, Zank S, Durham P. Unprecedented silver resistance in clinically isolated enterobacteriaceae: major implications for burn and wound management. Antimicrob Agents Chemother. 2015;59(8):4734–4741. doi: 10.1128/AAC.00026-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Percival SL, Woods E, Nutekpor M, Bowler P, Radford A, Cochrane C. Prevalence of silver resistance in bacteria isolated from diabetic foot ulcers and efficacy of silver-containing wound dressings. Ostomy Wound Manage. 2008;54(3):30–40. [PubMed] [Google Scholar]
- 266.Davis IJ, Richards H, Mullany P. Isolation of silver- and antibiotic-resistant Enterobacter cloacae from teeth. Oral Microbiol Immunol. 2005;20(3):191–194. doi: 10.1111/j.1399-302X.2005.00218.x [DOI] [PubMed] [Google Scholar]
- 267.Summers AO, Wireman J, Vimy MJ, et al. Mercury released from dental “silver” fillings provokes an increase in mercury- and antibiotic-resistant bacteria in oral and intestinal floras of primates. Antimicrob Agents Chemother. 1993;37(4):825–834. doi: 10.1128/AAC.37.4.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Haefeli C, Franklin C, Hardy K. Plasmid-determined silver resistance in Pseudomonas stutzeri isolated from a silver mine. J Bacteriol. 1984;158(1):389–392. doi: 10.1128/JB.158.1.389-392.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hanczvikkel A, Víg A, Tóth Á. Survival capability of healthcare-associated, multidrug-resistant bacteria on untreated and on antimicrobial textiles. J Ind Text. 2019;48(7):1113–1135. doi: 10.1177/1528083718754901 [DOI] [Google Scholar]
- 270.Chapman JS. Disinfectant resistance mechanisms, cross-resistance, and co-resistance. Int Biodeterior Biodegrad. 2003;51(4):271–276. doi: 10.1016/S0964-8305(03)00044-1 [DOI] [Google Scholar]
- 271.Rodgers MR, Blackstone BJ, Reyes AL, Covert TC. Colonisation of point of use water filters by silver resistant non-tuberculous mycobacteria. J Clin Pathol. 1999;52(8):629. doi: 10.1136/jcp.52.8.629a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Li XZ, Nikaido H, Williams KE. Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J Bacteriol. 1997;179(19):6127–6132. doi: 10.1128/JB.179.19.6127-6132.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gupta A, Matsui K, Lo J-F, Silver S. Molecular basis for resistance to silver cations in Salmonella. Nat Med. 1999;5(2):183–188. doi: 10.1038/5545 [DOI] [PubMed] [Google Scholar]
- 274.Li W, Zhang H, Assaraf YG, et al. Overcoming ABC transporter-mediated multidrug resistance: molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updat Rev Comment Antimicrob Anticancer Chemother. 2016;27:14–29. doi: 10.1016/j.drup.2016.05.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- who.tb.99.260.pdf. Available from: https://www.who.int/docstore/gtb/publications/mdrtb/PDF/who.tb.99.260.pdf. Accessed November21, 2019.