Abstract
During evolution, certain endoparasitoid wasps have developed mechanisms to suppress the defence systems of their hosts. For this purpose, these species, all of which belong to the families Ichneumonidae and Braconidae, inject various kinds of virus-like particles. The most studied of these particles are classified as polydnaviruses (family Polydnaviridae) which are symbiotic viruses. Over the past decade, it has also been shown that several wasp species harbour reoviruses (family Reoviridae), and that two of these suppress host defence, allowing the development of the parasitoid eggs. In this paper, we summarize the key features of these viruses and their relationships with their wasp hosts. Five reoviruses are known that appear to be non-pathogenic for the wasps. Three of these, McRVLP, HeRV, OpRVLP, use their wasp hosts as vectors, and do not appear to be involved in host defence suppression. The fourth, DpRV-1, is a commensal reovirus detected in most field populations of the wasp, Diadromus pulchellus. This reovirus is always found associated with an ascovirus, DpAV-4a, which is indispensable for host immune suppression. Although DpRV-1 has not been shown to directly increase D. pulchellus parasitic success, it may contribute to this success by retarding DpAV-4a replication in the wasp. The fifth reovirus, DpRV-2, occurs in a specific population of D. pulchellus in which DpRV-1 and DpAV-4 are absent. This virus has a mutualistic relationship with its wasp host, as its injection by females during oviposition is essential for host immunosuppression. Interestingly, these viruses belong to several different reovirus genera.
Keywords: Reoviridae, Parasitoid wasp, Mutualism
1. Introduction
Parasitic success in many endoparasitoid wasps requires the injection of co-factors when they lay eggs in their insect hosts. In the absence of these factors, eggs are recognized as foreign and are destroyed by host defence mechanisms. During evolution, several mechanisms have evolved which enable endoparasitoids to escape or suppress host immune responses, and these can vary depending on the parasitoid species. Among the most interesting of these, are the virus-like particles (VLPs) co-injected with the eggs by many species of Braconid and Ichneumonid wasps. The most studied VLPs are the polydnaviruses, which have been investigated for more than 30 years (Kroemer and Webb, 2004). True viruses such as certain ascoviruses (Barratt et al., 1999; Bigot et al., 1997b; Federici et al., 2000; Renault et al., 2002; Stasiak et al., 2004), entomopoxviruses (Lawrence, 2002), have also been shown to be capable of suppressing host defence mechanisms in some species of braconid and ichneumonid wasps. These viruses have been discussed previously as well as in other papers in this volume (Federici and Bigot, 2003; Stasiak et al., 2004). The above viruses are all DNA viruses. In the present paper, we focus on the occurrence and relationships of several RNA viruses belonging to the family Reoviridae with the parasitoid wasp species that they infect. Evidence suggests that at least two of these viruses are involved in assisting endoparasitoid wasps suppress the defense systems of their hosts and increase parasitic success.
Members of the Reoviridae family are characterized by icosahedral virions that range from 60 to 80 nm in diameter and lack an envelope (see the web site: http://www.ncbi.nlm.nih.gov/ICTVdb/IctV/fs_reovi.htm). The capsid is composed of two or three layers, but the outer layers are often lost during particle purification (Fig. 1A ). All virions contain a full genome consisting of linear segments of double-stranded RNA. Depending on the genus, the genome consists of 10–12 segments, with the total length that varies from 18200 to 30500 nucleotides depending on the species. The 5′ end of each segment has a cap (m7G5ppp5′GmpNp) on the positive strand of each duplex, and negative strands have a phosphorylated terminus.
Fig. 1.
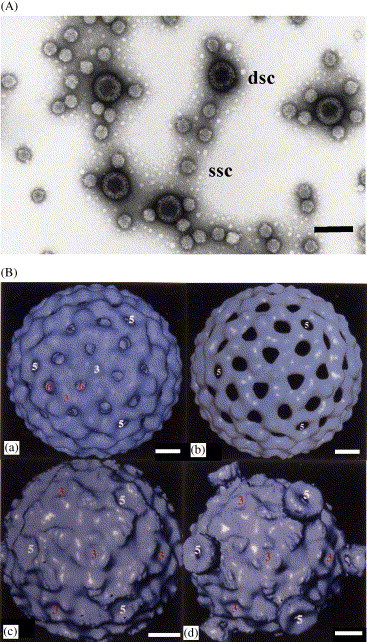
Morphology of DpRV-1 virions. (A) Negatively stained preparation of virions. Bar=100 nm. (B) Three-dimensional reconstruction of the DpRV-1 capsid: (a) DpRV-1 virion, (b) External capsid, (c) Internal capsid, and (d) Internal capsid with turrets. Bar=10 nm. Dsc: double shell capsid, ssc: single shell capsid.
The family Reoviridae consists of nine genera differentiated from one another by differences among their virions, genomes, and host ranges. Viruses belonging to the genera Fijivirus, Phytoreovirus and Oryzaviruses infect various plant species. These viruses are often transmitted by insect vectors. Four genera, Orthoreovirus, Orbivirus, Rotavirus and Coltivirus, infect warm-blood vertebrates, whereas members of the genus Aquareovirus appear only to infect cold-blooded marine vertebrates. A few Orthoreovirus, Orbivirus, Rotavirus and Coltivirus species have also been described from arthropods such as mosquitoes and ticks that feed on the blood of mammals. Although these viruses have been described from invertebrate hosts, some may have vertebrate hosts as well, the insect host serving primarily as a vector. Besides these viruses found in invertebrates, most reoviruses that exclusively infect invertebrates are classified in the genus Cypovirus, of which there are 30. In addition to these, about 14 other reoviruses infecting insects, crustaceans and arachnids remain unclassified. The variation in virion properties and host range that occurs among these different genera make it clear that the family Reoviridae contains a high degree of biodiversity.
Generally, cypoviruses are transmitted horizontally by ingestion of infected faeces from phytophagous insects that contaminate soil and plants. These viruses can also be vertically transmitted through eggs (Belloncik, 1996). Cypoviruses have several properties that are common to all the members of this genus. First, most of these viruses infect the midgut epithelium of lepidopterous or dipterous insects where the virus produces polyhedral occlusion bodies in the cytoplasm composed of polyhedrin matrix, which occludes virions. Second, virion capsid consists of only a single shell, which is structurally equivalent to the virion core of other species of the family. Cypovirus virions have icosahedric symmetry, but are smaller (55–69 nm in diameter) than the virions of other types of reoviruses. Finally, cypoviruses are the only reoviruses to display visible spikes on their surface; others have spikes which are obscured by the presence of the outer capsid. Cypoviruses can be classified using electrophoretic patterns of their 10 genomic fragments (Mertens et al., 1999). More recent assessments of their relatedness are based on molecular data. For example, sequence data from Bombyx mori cypovirus 1, Dendrolimus punctatus CPV and Antherarae mylitta CPV show that these consist of at least 14 different cypovirus species (Ikeda et al., 2001; Zhao et al., 2003; Qanungo et al., 2002). Among these, nucleotide sequences for the various cypovirus RNA-dependant RNA polymerases (RdRp) show identities ranging from 32% to 94%. This range in degree of identity is similar to that observed between RdRps of rotaviruses and Orbiviruses (25–96%; Rao et al., 2003).
So far, six reoviruses have been reported infecting hymenopteran species. Three were found in ichneumonid parasitoid wasps: DpRV-1 and DpRV-2 in Diadromus pulchellus (Rabouille et al., 1994a; Renault et al., 2003) and HeRV in one strain of Hyposoter exiguae (Stoltz and Makkay, 2000). Two correspond to reovirus-like particles described in braconid parasitoid wasps: McRVLP in Microplitis croceipes (Hamm et al., 1994) and OpRVLP in Opius concolor (Jacas et al., 1997). The last is a cypovirus found in the nest walls of the predatory wasp, Polistes hebraeus (Fouillaud and Morel, 1994). This cypovirus does not infect the wasp itself. Its source in the nest is probably lepidopteran prey of these insects, and therefore it is not considered further here. The five other reoviruses can be separated into three types depending on their relationship to and occurrence within their wasp vectors and host insect that the wasp parasitises. Thus, here we divide and examine the above reoviruses into different types that we refer to as commensal and/or mutualistic with respect to the type of relationships they share with their hymenopteran hosts. Finally, we offer a perspective on the various roles reoviruses play in host/parasitoid relationships.
2. Material and methods
2.1. Insects
The parasitoid D. pulchellus was reared in the laboratory in a lepidopteran host, Acrolepiopsis assectella. Rearing conditions used in these experiments have been described (Bigot et al., 1997a, Bigot et al., 1997b).
2.2. Detection of viral particles by immunocytochemistry with fluoresceine (FITC)
The abdomen and venom glands of D. pulchellus were fixed in paraformaldehyde/glutaraldehyde mix and dehydrated in ethanol. They were embedded at 4 °C in resin and treated with anti-DpRV-1 serum as described (Rabouille et al., 1994b).
2.3. Sequence alignment and phylogenetic analysis
The Infobiogen facilities were used for databank searches, sequence alignments and determinations of relatedness. Phylogenetic analyses of the aligned sequences were developed using the Parsimony and Neighbor-Joining programs in the PHYLIP package, version 3.5c. (Felsenstein, 1993).
2.4. Transmission electron microscopy
Sections of D. pulchellus adult midgut were placed in modified Karnovsky's fixative at 4 °C (MacDowell and Trump, 1976). Fixed tissues were stored in 1-butanol for several days, dehydrated in ethanol and embedded in Epon resin. Ultrathin sections (80 nm) were stained with uranyl acetate and lead citrate.
2.5. Cryoelectron microscopy of DpRV-1
DpRV-1 virions were isolated from 2 g of frozen parasitized D. pulchellus (Rabouille et al., 1994a) and purified by the Freon procedure (Chen and Ramig, 1992). Frozen-hydrated virus particles were analyzed as described by Hewat et al. (1992) and analysis of micrographs was performed as described previously (Schoehn, 1994).
2.6. Reverse transcription of ds RNA
RNA was isolated from samples of ten insects by guanidium isothiocyanate extraction (Chirgwin et al., 1979; Laulier et al., 1995). RNA was also isolated from purified virus particles (Rabouille et al., 1994a). Approximately 15 μg dsRNA was reverse-transcribed with 50 pmole of an oligonucleotide specific for the 1.6 kb segment: 5′ TCGTTTTGT TGAACCTGAT 3′ (O .6 R) and 16 U avian myeloblastoid virus (AMV) reverse transcriptase for 1 h at 42 °C using conditions specified by the manufacturer (Promega). Aliquots of 5 μg reverse transcription product plus 100 pmole of O1.6R⧹ and O1.6F (5′ ATGAATAATGATCCTGAC 3′) primers in 200 μm dACGT, 10 mm Tris pH 9.0, 25 mm MgCl2, 50 mm KCl and 0.5 U Taq polymerase (Appligene) were amplified by polymerase chain reaction (PCR). The PCR program used was 1 min at 95 °C, 30 s at 50 °C, 1 min at 72 °C for 30 cycles. The amplified fragment was 1.3 kb.
3. Results and discussion
Here we define three types of viral relationships between different cypoviruses and their hypenopteran hosts. These are:
-
(1)
Non-pathogenic, commensal virus: a reovirus that occurs and replicates in a wasp population and may be found in association with others types of viruses.
-
(2)
Putative mutualistic virus: a reovirus present routinely in a wasp population in association with others viruses, but which does not appear to play a major role in the host/parasitoid relationship.
-
(3)
Mutualistic virus: a reovirus present routinely in the wasp population that is not associated with other viruses, and contributes markedly to parasitic success. They are not symbiotic viruses like PDV because they can be replaced by other types of viruses.
3.1. Non-pathogenic commensal reoviruses
Three reoviruses of this type have been reported from three different parasitoid wasp species. They are the reovirus-like particles, McRVLP, reported in adult M. croceipe (Hamm et al., 1994), OpRVLP in Opius concolor (Jacas et al., 1997), and the HeRV reovirus reported in H. exiguae wasps (Stoltz and Makkay, 2000). McRVLP was detected in 9 of 11 colonies of M. croceipes surveyed at the USDA Insects Research Laboratory (Columbia, MO). HeRV was detected in a single H. exiguae strain recently established, whereas colonies of this species reared from 1978 to 1980 were free of reoviruses (Stoltz and Makkay, 2000). Nothing is known about the occurrence of OpRVLP in O. concolor populations (Jacas et al., 1997). Virion features of these three viruses indicate that they do not belong to a single genus. McRVLP, structurally, resemble members of the genus Rotavirus, OpRVLP, to members of Cypovirus whereas HeRV more closely resemble to members of Orthoreovirus. Without molecular data, these assignments were based on morphological and biological data by the authors who discovered them (Hamm et al., 1994; Jacas et al., 1997; Stoltz and Makkay, 2000).
McRVLP and HeRV are non-pathogenic commensal reoviruses, as they replicate in several tissues of the wasps and are present in both sexes (Hamm et al., 1994; Stoltz and Makkay, 2000). For OpRVLP, the authors do not indicate if it replicates in the poison gland of females where it was detected and if it could be detected in other tissues or in males (Jacas et al., 1997). The three viruses occur in the genital apparatus of their wasp hosts, suggesting that they are transmitted to hosts parasitized by the wasps at oviposition. The observation that the adults contained more virions than do larvae or pupae provides an additional evidence for this mechanism of transmission (Hamm et al., 1994; Stoltz and Makkay, 2000). McRVLP, OpRVLP and HeRV do not appear to have fitness costs for their wasp hosts. However, without studies to determine whether these viruses interfere with host defence mechanisms, their impact on the host/parasitoid system in which they occur remains unclear. In fact, delineating any effects they have may be quite difficult because these viruses occur along with other types of viruses and VLPs: a picornavirus, nudivirus or polydnavirus in M. croceipes, a coronavirus in O. concolor and a polydnavirus in H. exiguae (Hamm et al., 1994; Stoltz and Makkay, 2000).
3.2. Commensal reoviruses suspected of mutualism with the wasp
The only reovirus of this type is DpRV-1, which occurs in the ichneumonid wasp D. pulchellus. DpRV-1 was found in every D. pulchellus adult recovered from several populations that occur in the two regions of France that are 800 km apart, and over the period from 1991 to 1999 (Rabouille et al., 1994a, and unpublished data). DpRV-1 is considered a commensal virus as, in every wasp, it is always found in association with the ascovirus, DpAV-4 (Bigot et al., 1997a). DpAV-4 has been shown to be essential in the host/parasitoid relationship, as it is able to inhibit melanization, and to interfere with the cytolysis of host tissues in lepidopteran pupae parasitized by the wasp (Bigot et al., 1997b; Renault et al., 2002). Several properties of DpRV-1 virions and its biological relationship with the wasp and lepidopteran pupal host show that this virus is very unusual and requires further study.
3.2.1. Classification of DpRV-1 among the Reoviridae
First, although DpRV1 is a virus infecting insects, its particle does not show characteristic cypovirus features, such as those observed for McRVLP and HeRV. For example, intact virions have a double-shelled capsid with no spikes (Fig. 1A). This was further investigated with cryo-microscopic analyses in an attempt to reconstruct the three-dimensionnal structure of purified DpRV-1 virions (Schoehn, 1994). Data showed that the diameter of the complete particle is about 70 nm (Fig. 1B, a). The radius was from 30.3 to 36.9 nm for the outer capsid and 24.3 to 30 nm for the inner capsid (Fig. 1B, b, c). Except for cypoviruses, these dimensions are slightly less than for other reoviruses (those described to date are about 80 nm for the outer diameter). The different views virions also show that the outer and inner capsids have different symmetries (Fig. 1, b, c). The inner capsid has turrets located at the 5-fold symmetry axes. This is a cardinal feature of several genera of Reoviridae (Fig 1B, d), although the turret structure is clearly different from that of cypovirus CPV-5 from Orgya pseudotsugata (Hill et al., 1999). In terms of particle structure, our calculations showed that the closest relative of DpRV-1 would be a mammalian orthoreovirus (Schoehn, 1994)
In an attempt to verify the assignment of DpRV-1 to the genus Orthoreovirus, seven genomic segments were sequenced and analyzed (Rabouille et al., 1994a; Bigot et al., 1995). The 5′ and 3′ extremities of each DpRV-1 genomic segment showed similarities to those of a few orthoreoviruses, indicating it may belong to this group (Bigot et al., 1995). However, all the other results, presented below, do not confirm this grouping. Comparison of the nucleic acid sequences of seven segments (0.98, 1.24, 1.318, 1.652, 3.330, 3.81 and 4.23 kb) with those in the Genbank and EMBL databases resulted in no significant matches, as described previously (Bigot et al., 1995). In contrast, psi-BLAST searches done on the NCBI web site between the protein databanks and proteins encoded by the ORFs contained in the seven DpRV-1 segments allowed us to detect similarities for proteins encoded by the 4.23, 3.81 and 3.33 kb segments with proteins coded by certain reoviruses, as described below. The first significant match was between a protein domain encoded by the 4.23 kb segment (located within the amino acids 1063–3517) and the major core structural protein of several members of the genera Fijivirus, Oryzavirus and Phytovirus (p-values ranging from 4e−5 to 1e−4 after three search rounds of psi-BLAST; Fig. 2A ). However, the phylogenetic analysis of this conserved region clearly showed that DpRV-1 is different from these viruses (Distéfano et al., 2002). Alternatively, a domain between the amino acids 132–664 of the 4.23 kb protein showed similarities with a domain found in proteins of various organisms such as Saccharomyces cerevisiae, Plasmodium falciparum, Staphylococcus caprae, and a virus, the Phthorimaea perculla granulovirus (Fig. 2B). The role of this domain was identified for protein in S. cerevisiae as a weak suppressor of DNA dependant-RNA polymerase I and III (Lalo et al., 1993). The occurrence of this domain in the protein coded by the DpRV-1 4.3 kb genomic fragment might be correlated with a possible role of this virus in its interaction with the ascovirus DpAV-4 in its lepidopteran and wasp hosts. We have shown that when DpAV-4 is injected alone into host pupae, all the host cells are lysed very rapidly, which would interfere with the development of wasp larvae. In contrast, when DpAV-4 is co-injected with DpRV-1, the replication of DpAV-4 is retarded, allowing longer survival of host cells, which enables normal development of wasp larvae. Thus, it is possible that the protein encoded by the 4.23 kb DpRV-1 genomic fragment might inhibit the expression of cellular RNA needed for expression of DpAV-4 at a high level, thereby synchronising replication of DpAV-4 and the development of D. pulchellus. However, the interpretation of these results remains mainly speculative until this protein can be tested to understand its physiological role in the host-parasitoid relationships.
Fig. 2.

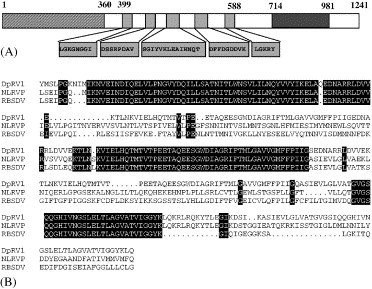
Homologies of the proteins encoded by the 4.23 kb segment of DpRV-1. (A) Alignment of the domain encoded by the 4.23 kb showing homologies with the major core protein of two Fijiviruses, Rice black streaked dwarf virus (RBSDV)(Q8UZ06) and Mal de Rio Cuarto virus (MRCV)(Q809E8). (B) Alignment of the domain encoded by the 4.23 kb segment showing homologies with a suppressor of RNApol I and III: yeast gene SRP40 (P32583), PoGV, Phtorimaea operculella granulovirus (phopgv06 gene, Q8JS13), S.c., Staphylococcus caprae gene atlc (Q9AIS0), and Plasmodium falciparum gene malp1.23 (Q8ICZ4). The slashes correspond to insertions or sequences with no homologies among the 5 sequences.
A match was also found with the 1241 aa protein encoded by the ORF contained in the 3.81 kb genomic segment. The region located between amino acids 730 and 909 showed a 20% similarity and a 45% identity with the B spike proteins found in the inner shell of several fijiviruses (Distéfano et al., 2002; Fig. 3B ). But phylogenetic analysis of this region showed that DpRV-1 is not a fijiviruses, even if the spikes protein of fijiviruses present the greatest homology to the part of this ORF of DpRV-1. Further analysis of the 1241 aa DpRV-1 protein yielded complementary information. We found that another region of this protein corresponded with that of RNA dependent RNA polymerase (RdRps) motifs, especially between amino acids 399 and 588 (Fig. 3A, Fig. 4A ). Phylogenetic analyses with parsimony or neighbor-joining programs were therefore preformed using alignment of the most conserved domains found between reoviral RdRps, as described by Distéfano et al. (2002). Both analyses revealed that the DpRV-1 RdRp belonged to a lineage comprising the RdRp's of rotaviruses, orbiviruses, and phytoreoviruses, and that the most distantly related viruses belonged to the genus Fijivirus. (Fig. 4B). In the absence of data confirming that the DpRV-1 1241 aa protein was a RdRp, we concluded that the 3.81 kb segment contained a chimeric ORF, constituted of a RdRp catalytic domain in its central part and a domain involved in the B spikes at its C-terminal end. In conclusion, these molecular data suggest that DpRV-1 cannot be assigned to one of the present genera of the family Reoviridae. It appears to be a chimeric virus consisting of segments encoding proteins originating from different reoviruses. We note that DpRV-1 occurs in a field of a tritrophic system involving a plant (leek), the leek-moth (A. assectella) that feeds on Allium species, and the parasitoid wasp (Diadromus pulchellus) that parasitizes A. assectella pupae. Each member of this system comes into contact with various reoviruses that may have contributed during evolution to the genomic and structural properties of DpRV-1 as it exists today.
Fig. 3.
Structure and homologies for the protein encoded by the DpRV-1 genomic segment. (A) Structure of the protein coded for by the 3.81 segment. The first motif of 360 amino-acids (striped box) shows high homologies with a the protein encoded bt the 3.33 kb segment of DpRV-1. The amino acids of the five motifs of the RNA-dependent-RNA polymerase (RdRp) are in light grey. The portion of sequence encoded by the 3.81 kb DpRV-1 showing homology with the B spike region of Fijiviruses is schematically represented by a dark grey box. The first and last position of each motif is noted by the amino acids. (B) Sequence homologies between the domain of the protein encoded by the 3.81 kb segment and the B “spike” of two Fijiviruses: Nilaparvata lugens reovirus (NLRV; Q8B4I4) and Rice black streaked dwarf virus (RBSDV; Q8UZ05).
Fig. 4.
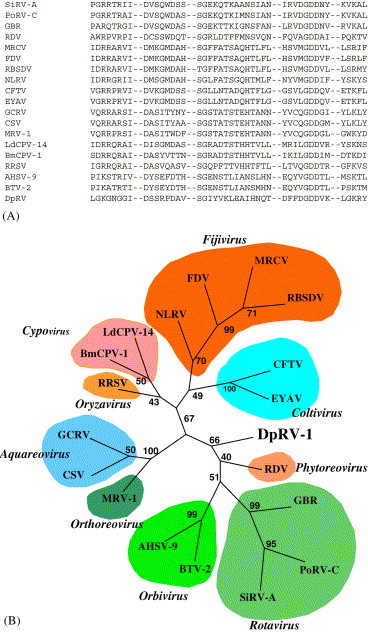
Phylogenetic analysis of the RNA-dependent-RNA polymerase (RdRp) of DpRV-1. (A) Alignment of the conserved motifs of the RdRp of reoviruses. The sequences used were from the following viruses: from of the genus Rotavirus; Simian rotavirus SA11 (SirV-A; P22678), Porcine rotavirus (PoRV-C; M74216), Group B rotavirus (GBR; P35942); from the genus Phytoreovirus; Rice dwarf virus (RDV; U73201); From the genus Fijivirus; Mal de Rio Cuarto virus (MRCV; AF49925), Fiji disease virus (FDV; AY029520), Rice black streaked dwarf virus (RBSDV; AJ294757), Nilaparvata lugens reovirus (NLRV; D49693); from the genus Coltivirus; Colorado tick fever virus (CFTV; AF133428), Eyach virus (EYAV; AF282467); from the genus Aquareovirus; Grass carp reovirus (GCRV; AF260511S2), Chulm salmon reovirus (CSV; AF418295); from the genus Orthoreovirus; Mammalian orthoreovirus subgroup 1 (MRV-1; M24734); from the genus Cypovirus; Bombyx mori cypovirus (BmCPV-1; AF323782), Lymantria dispar cypovirus (LdCPV-14; AF389452); from the genus Oryzavirus; Rice ragged stunt virus (RRSV; U66714), from the genus Orbivirus; African horse sickness virus (ASFV-9; U944887), Blue tongue virus (BTV-2; L20508) and from the unassigned reovirus, DpRV-1. (B) Phylogenetic unrooted tree of RdRp obtained by the Parsimony method. The same topology was obtained using the Neighbor-joining method.
Recombination of genome segments in DpRV-1 is illustrated by a unique property of its genome not known for any another double-stranded virus. The 1072 aa protein encoded by the 3.33 kb DpRV-1 segment resulted from recombination events that generated a well-conserved trimer of the first 360 amino acids of the 1241 aa DpRV-1 protein encoded by the 3.81 kb segment (96% of similarity; Rabouille et al., 1994a). Three rounds of psi-BLAST searches of protein databanks revealed that this 360 aa domain had a domain located between positions 25–250 within the N-terminal domain of the subunit of the DNA-directed RNA polymerases of procaryotic origin (p-values ranging from e−30 to e−50). This domain is identified as a single-strand binding domain, indicating that the N-terminal domain of the DpRV-1 RdRp probably contained a nucleic acid binding domain. It also suggests that this recombinant segment might encode an inhibition protein that could interfere with DpRV-1 replication by competing with nucleic acids binding RdRp. However, the degree of inhibition depends on biological conditions. Indeed, DpRV-1 appears to replicate without any inhibition in the midgut of males and females of the wasp D. pulchellus, and a priori with no deleterious effects on wasp viability (Rabouille et al., 1994a). Thus, the inhibitory role of the 1072 aa protein should be investigated in the wasp's lepidopteran host. This difference of activity was also reinforced by the fact that the segment encoding the 1072 aa protein is not always present in virions. In fact, we previously observed that its presence in virions depended on the ploidy of the wasp in which the virus replicated (Rabouille et al., 1994a). Briefly, sex in hymenopteran wasps is determined by the degree of ploidy; parthenogenetic eggs develop into haploid males, whereas fertilized eggs produce diploid females. We observed that the DpRV-1 genomic segment coding for the 1072 aa protein was only present in diploid wasps. Thus, its role must be related to the biology of the female (Rabouille et al., 1994a). From this perspective, our investigations were oriented to determine whether DpRV-1 was transmitted to the host parasitized by the female wasps as a co-factor that participated in parasitism. As a result, the location of DpRV-1 virions in wasp tissues was investigated first, and then its transmission at oviposition was analyzed. Finally, its putative effects in the host parasitized by the wasp were evaluated.
3.2.2. Transmission of DpRV-1
An antiserum directed against the proteins of the DpRV-1 virions was used with a FITC amplication system to locate its presence in sections of female wasp tissues (Fig. 5A , Rabouille et al., 1994b). Our results confirmed that DpRV-1 was mainly recovered in the lumen and epithelial cells of the gut (Fig. 5A, c) (Rabouille et al., 1994b), but they also revealed trace amounts of DpRV-1 in the venom gland lumen (Fig. 5A, d). Electron microscopy of the midgut showed that virions were present in the cytoplasm of epithelial cells (Fig. 5B, a) and revealed viral structures (Fig. 5B, b) arranged in pseudocrystalline arrays (Fig. 5B, c). Virions were also located near microvilli in the gut lumen (Fig. 5B, d; Rabouille et al., 1994a). Low numbers of DpRV-1 virions in the venom gland, however, did not enable their presence to be verified by electronic microscopy. Therefore, overall our data indicate that DpRV-1 might be transmitted to the host in three ways that are not mutually exclusive. The first is by the injection of DpRV-1 virions present in the venom glands during oviposition. The second is through transmission of DpRV-1 virions within wasp eggs. The third might result from oviposition behavior of the wasps. Specifically, before injection of eggs, D. pulchellus females make feeding stings and palpate host pupae with the anal extremity of their abdomen (Bénédet et al., 1999). As traces of faeces contain DpRV-1 virions, these could contaminate the end of the abdomen wasp. Lastly, it is also possible that host pupae are also contaminated by DpRV-1 during the pre-oviposition steps.
Fig. 5.
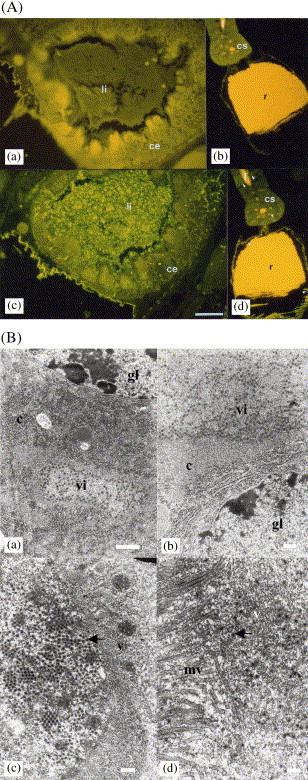
Localization of DpRV-1 virions in tissues of the wasp D. pulchellus. (A) Immunolocalization of DpRV-1 virions in the gut (a, c) and the venom gland (b, d) of a D. pulchellus female. Sections were hybridized with non-specific serum (a, b) and with anti-DpRV-1 serum (c, d), and visualized using FITC coupled anti-serum. Insect tissues are auto-fluorescent (a, b). The bar represents 10 μm. li: gut lumen, ce: epithelial cells, cs: secretory cells, r: venom gland reservoir, l: venom gland lumen. (B) Ultrastructural localization of DpRV-1 virions in the midgut of a D. pulchellus female. Bar represents 200 nm. Localization of DpRV-1 virions in epithelial cells (a, b), in viroplasm (c) and gut lumen (d). c, cytoplasm; vi , viroplasm; gl , gut lumen; mv, microvilli; v, virions.
The transmission of DpRV-1 at oviposition was examined by RT-PCR with ds RNA samples extracted from unparasitized and newly parasitized pupae, either pupae cleaned thoroughly with 5-min washings done with a 0.5% bleach solution and sterile water (twice for each), or those not surface-sterilized. RT-PCR was performed with primers specific for the 1.652 kb DpRV-1 segment. First, we observed that no RT-PCR product was obtained with RNA extracted from purified from healthy pupae. This indicated that the rearing conditions for the production of A. assectella pupae were free of DpRV-1 contamination (Fig. 6 , lane 5). Second, we observed that the amounts of RT-PCR products obtained with the extracts purified from unwashed parasitized pupae (Fig. 6, lane 2) were always much higher than those obtained with parasitized washed pupae (Fig. 6, lane 1). Similar results were obtained in three independent experiments with PCR conditions that must be considered as semi-quantitative because similar amounts of RNA were used for RNA RT-PCR amplification, and because the pupae were parasitized using the same experimental conditions. Our results, therefore indicated that contamination of the host pupa cuticle with DpRV-1 virions was the origin of these differences, which most likely resulted from the multiple contacts that occurred with the female wasps before they laid eggs in the pupae. In an attempt to solve the problem of cuticle contamination, we verified the efficiency of washing conditions to remove the DpRV-1 contamination from the surface of the pupae. This was done using A. assectella pupae in which the cuticle was artificially contaminated by immersion in freshly ground wasp guts containing DpRV-1 virions. Under these conditions, we observed that the quantity of fragments obtained after RT-PCR with RNA extracted from unwashed (Fig. 6, lane 3) and washed contaminated pupae were different (Fig. 6, lane 4). The strength of the signal obtained with extracts from unwashed pupae indicated that they were highly contaminated by DpRV-1. Their absence in extracts from the washed pupae demonstrated that the wash conditions removed all DpRV-1 virions to a non-detectable level. Similar results were obtained from two other independent experiments. As the efficiency of our washing procedure was verified, experiments were finally performed with RNA extracted from parasitized pupae thoroughly washed, in an attempt to define if DpRV-1 virions were or were not injected in pupae by wasps. RT-PCR products were detected in parasitized pupae (Fig. 6, lanes 1, 6, 8) indicating that DpRV-1 virions were recovered within the pupae after wasp oviposition. In conclusion, our results show that DpRV-1 virions were injected into A. assectella pupae parasitized by the wasp. This most likely results from co-injection of virions and wasp venom at oviposition and/or from contamination of the ovipositor by DpRV-1 virions present on the pupal cuticle surface and at the terminus of the female wasp abdomen.
Fig. 6.
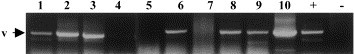
Transmission of DpRV-1 by D. pulchellus to A. assectella. RT-PCR of the 1.6 kb was performed on ds RNA extracted from washed (1) or unwashed (2) parasitized pupae, DpRV-1 artificially contaminated pupae, unwashed (3) or washed (4), unparasitized pupae (5), D. pulchellus eggs (7), and parasitized pupae at 1-day (6), 5-days (8) and 14-days (10) after parasitization, and D. pulchellus larvae 5 days after eggs were laid in the host (9). The+and – lanes correspond to positive and negative PCR controls.
The results of the last set of experiments indicated that it is highly unlikely that DpRV-1 is introduced into the host in wasp eggs. Specifically, we were unable to obtain a RT-PCR product using three different samples of RNA extracted from wasp eggs that were washed thoroughly after being dissected from parasitized pupae (Fig. 6, lane 7). Owing to the apparent absence of DpAV-1 virions or RNA within the egg, the transmission of DpRV-1 to the wasp larva during their development in host pupae was investigated. RT-PCR experiments were carried out using RNA extracted from wasp larvae that were dissected 5-days after oviposition and washed carefully. A specific fragment was observed at the larvae stage. (Fig. 6, lane 9). Seven days after oviposition, the D. pulchellus larva pupates. RT-PCR experiments performed with RNA extracted and purified from wasp nymphs 13-days post-oviposition (Fig. 6, lane 10), also confirmed that DpRV-1 is maintained and replicated in wasp pupae just prior to adult emergence. The same results were obtained with RT-PCR performed on the 3.33 and 3.81 kb segments (data not shown).
With respect to the parasitized lepidopteran host, virions of DpRV-1 were not observed in pupal tissues when examined by electronic microscopy (Bigot et al., 1997a). When pupae were injected with purified virions, the genome of DpRV-1 could not be visualized on BET agarose gels (data not shown). Together, these results provide strong evidence that the DpRV-1 genome is not replicated and expressed in the host pupae.
3.2.3. Role of DpRV-1
These results suggest the following role for DpRV-1 in the pupae parasitized by the wasp. The weak or null activities of the DpRV-1 RdRp in the lepidopteran host may be due to expression of the supernumerary 3.33 kb segment only contained in virions produced by the female wasp. As previously indicated, this segment encoded a protein that putatively showed features of a potential DpRV-1 RdRp inhibitor, possibly acting by competing with its ability to bind to nucleic acids.
The weak or null activity of DpRV-1 RdRp does not mean that this virus is unable to interfere with the host regulation without being transcribed. It has been shown in several studies that some reovirus capsid proteins are able to interfere with important factors of the immune systems, for example, NFκB, and to induce cell apoptosis (Labrada et al., 2002; Forrest and Dermody, 2003). Such properties could complement the effect of DpAV-4 on capsule melanization (Renault et al., 2002) by partially preventing capsule formation, which is observed in pupae infected by both viruses. Such interference could also inhibit DpAV-4 replication, thereby inadvertently coordinating amplification of DpAV-4 and the resulting lysis of host tissues with development of the wasp larva. In fact, we observed that when DpAV-4 is artificially injected without DpRV-1, the ascovirus amplified very quickly in the host pupae, which died in 2–3 days. In contrast, when DpAV-4 is co-injected with DpRV-1, DpAV-4 replicates slower and inhibits the melanization reaction of the pupa, allowing for the development of the parasitoid larvae (5–7 days) (Bigot et al., 1997a; Renault et al., 2002).
3.3. Mutualist reoviruses: DpRV-2
DpRV-2's relationship with D. pulchellus is unusual because it is the only known case of reovirus that occurs alone in a hymenopteran species. For example, although DpAV-4 and DpRV-1 have occured in many D. pulchellus populations, DpRV-2 has never been found in the same populations (Renault et al., 2003). The structural features of the DpRV-2 virion indicate that this virus belongs to the genus Cypovirus. However, assignment to this genus cannot be made until molecular data are available.
Electron microscopy failed to detect DpRV-2 virions in the genital tract, genitalia, midgut and adipocytes of adult D. pulchellus females. Nevertheless, injection of crude extracts of female genitalia with a glass pin into host pupae resulted in development of DpRV-2 (Renault et al., 2003). Thus, infectious virions are present in D. pulchellus females and are probably transmitted to A. assectella pupae during wasp oviposition. In A. assectella, DpRV-2 replicates primarily in the midgut epithelium, as described in cypoviruses. Virions are detected in the cytoplasm, often arranged in a pseudo-crystalline structure (Renault et al., 2003).
The most unique feature of DpRV-2 is its interference with the immune response of A. assectella by inhibition of melanization (Renault et al., 2003). This was determined through a series of experiments in which nylon filaments were inserted into healthy and parasitized moth pupae. The majority of non-parasitized pupae reacted by forming a melanotic capsule around the filaments. The same reaction occurs to wasp eggs inserted into A. assectella pupae in the absence of DpRV-2. After pupae are parasitized, many of them encapsulate the nylon filaments. Infection with the DpRV-2 virus alone had no effect on encapsulation, but reduced the degree of melanization substantially.
4. Conclusion
Most insect reoviruses described to date belong to the genus Cypovirus. These viruses have been reported as pathogens of the midgut epithelium in numerous lepidopteran and dipteran species, because these insects are, respectively, of economic or medical importance (Mertens, 2004). The typical insect cypovirus is a cytoplasmic polyhedrosis virus, so-named because they produce large polyhedral occlusion bodies that occlude virions in the cell cytoplasm. Other cypoviruses frequently are non-pathogenic and appear to be commensals, having no obvious deleterious effects on overall host viability (Maazouz, 1991). Comparatively, very few reoviruses have been described infecting hymenopteran species and none has been found that is pathogenic for its wasp host. Despite the low number, there are some distinguishing features among the five hymenopteran reoviruses described so far. First, only two apparently are cypoviruses, OpRVLP and DpRV-2. Of the others, although virions of DpRV-1 are similar to those of orthoreoviruses, our analysis of DpRV-1 genome segments indicate that this virus does not belong to any of the known reovirus genera. Moreover, in the absence of molecular data, the assignments of McRVLP and HeRV, based on virion particles, respectively, to the genera Rotavirus and Orthoreovirus are premature. Indeed, the budding of virions that occurs with HeRV is more specific of Orbivirus (Stoltz and Makkay, 2000). Nevertheless, even if the complete sequence of these hymenopteran reoviruses were known, their classification would remain difficult, as we have shown in the case of DpRV-1. The genome composition of this virus is apparently the result of different recombination events during evolution, including recombination between segments from different reovirus genera, recombination inside a segment resulting in formation of a new coding segment, and horizontal transfer of eukaryotic genes.
A second feature of these hymenopteran reoviruses is—that four have never been found alone in their wasp host—they are always associated with another virus type. HeRV is associated with a polydnavirus in H. exiguae, DpRV-1 with an ascovirus (DpAV-4) in D. pulchellus, McRVLP with baculovirus (or nudivirus) or polydnavirus, OpRVLP with a coronavirus (Stoltz and Makkay, 2000; Bigot et al., 1997a; Hamm et al., 1994; Jacas et al., 1997). The complexity of these virus associations makes it difficult to assign the reoviruses individual roles in these host/parasitoid wasp systems. Designing experimental procedures, with appropriate controls to test different hypotheses, is quite difficult. Performing such studies are costly and time-consuming, as illustrated by the 12 years and numerous individuals required just to develop an initial understanding of the interactions among three viruses, DpRV-1, DpRV-2 and DpAV-4, in the A. assectella/D.pulchellus system. Moreover, this difficulty is probably underestimated because parasitoid wasps likely depend on factors other than the viruses discussed in this paper.
Our studies of the D. pulchellus/A. assectella system have enabled us to begin to understand the complex interactions of different viruses with an insect parasitoid and its host. We found populations in which either only one virus, DpRV-2 occurred, or two viruses, DpAV-4 and DpRV-1 occurred. When only DpRV-2 occurred, the virus was maintained from generation to generation using the wasp as a vector. The benefit to the wasp is that DpRV-2 assists development of wasp larvae by down regulating the immune response of A. assectella.
When DpAV-1 and DpAV-4 occurred in the same host/parasitoid system, the relationships between the different partners were more complex. Neither of these viruses is pathogenic for the wasp. In the host, DpAV-4 has the primary role of inhibiting the immune response of the wasp's lepidopteran host, permitting development of the wasp larva (Fig. 7 ). Although, DpRV-1 replicates at only a very low level, if at all, it has an important but likely more indirect role than DpAV-4 is that it interferes with capsule formation (Fig. 7). This implies a role similar to NFκB in the host. Indirectly, the replication of DpRV-4 can be limited by the action of two DpRV-2 proteins. One is a single-strand binding protein which can play a role in the replication of the genome of DpAV-4. The other is a suppressor of RNA pol I and III that acts by down regulating overall expression of host cells, and as a consequence, expression and replication of DpAV-4. The exact role of these proteins have to be now tested at the physiological level to confirm the assigned role obtained by sequence analysis. Under these hypotheses, the two viruses are indispensable and strongly interact for the parasitism success.
Fig. 7.
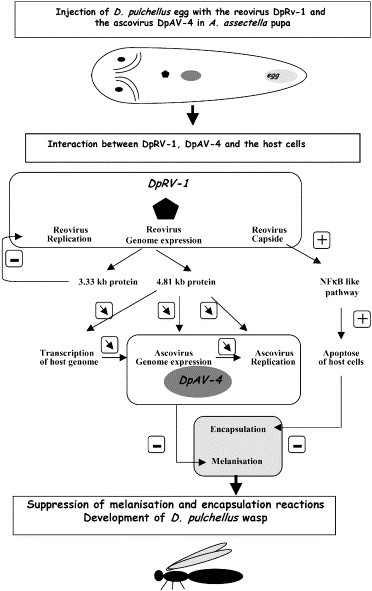
Interactions between the reovirus DpRV-1, the ascovirus DpAV-4 and the host pupa.
Understanding the associations between parasitoid wasps and true viruses such as reoviruses, as well as other viruses and micro-organisms presents a major challenge to understand how these viruses affect insect predator/prey relationships in agroecosystems. Studies of these are important and could influence decisions regarding whether the parasitoid wasps can be used in pest control programs with no significant ecological effects. So far, investments in understanding these relationships have been biased and studies in this field have focused on polydnaviruses of a few related brachonid and ichneumonid species. In terms of an ecological understanding and pest management, this was perhaps not the best model because the species without polydnaviruses represent most of the parasitoid wasps occurring in nature (Dowton and Austin, 1994).
Acknowledgments
This research was supported by grants from the Centre National de Recherche Scientifique and le Ministère de l’Education Nationale, de la Recherche et de la Technologie. We thank S. Bigot, F. Bonnin- Rouleux, M.H. Hamelin, F. Lutcher, M. Lemesle, P.Y. Sizaret, G. Doury, J.M. Drezen, H. Hewatt, A. Rabouille, G. Schoehn and E. Thibout for their technical assistance and participation during the 12 year period required for the evolution of our research on DpRV-1 and DpRV-2.
References
- Barratt B.I., Evans A.A., Stoltz D.B., Vinson S.B., Easingwood R. Virus-like particles in the ovaries of Microctonus aethiopoides loan (Hymenoptera: Braconidae), a parasitoid of adult weevils (Coleoptera: Curculionidae) Journal of Invertebrate Pathology. 1999;73:182–188. doi: 10.1006/jipa.1998.4826. [DOI] [PubMed] [Google Scholar]
- Belloncik S. Interactions of cytoplasmic polyhedrosis viruses with insects. Advances in Insect Physiology. 1996;26:233–296. [Google Scholar]
- Bénédet F., Bigot Y., Renault S., Pouzat J., Thibout E. Polypeptides of Acrolepiopsis assectella cocoon (Lepidoptera: Yponomeutoidea): an external host-acceptance kairomone for the parasitoid Diadromus pulchellus (Hymenoptera: Ichneumonidae) Journal of Insect Physiology. 1999;45:375–384. doi: 10.1016/s0022-1910(98)00136-x. [DOI] [PubMed] [Google Scholar]
- Bigot Y., Drezen J.M., Sizaret P.Y., Rabouille A., Hamelin M.H., Periquet G. The genomic segments of DpRV, a commensal reovirus of the wasp: Diadromus pulchellus (Hymenoptera) Virology. 1995;210:109–119. doi: 10.1006/viro.1995.1322. [DOI] [PubMed] [Google Scholar]
- Bigot Y., Rabouille A., Sizaret P.Y., Hamelin M.H., Periquet G. Particle and genomic characterisation of a new member of the Ascoviridae, Diadromus pulchellus ascovirus. Journal of General Virology. 1997;78:1139–1147. doi: 10.1099/0022-1317-78-5-1139. [DOI] [PubMed] [Google Scholar]
- Bigot Y., Rabouille A., Doury G., Sizaret P.Y., Delbost F., Hamelin M.H., Periquet G. Biological and molecular features of the relationships between Diadromus pulchellus ascovirus, a parasitoid hymenopteran wasp (Diadromus pulchellus) and its lepidopteran host, Acrolepiopsis assectella. Journal of General Virology. 1997;78:1149–1163. doi: 10.1099/0022-1317-78-5-1149. [DOI] [PubMed] [Google Scholar]
- Chen D., Ramig R.F. Determinants of rotavirus stability and density during CsCl purification. Virology. 1992;186:228–237. doi: 10.1016/0042-6822(92)90077-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J.M., Przybyla A.E., MacDonald R.J., Rutter W.J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Distéfano A.J., Conci L.R., Munoz Hidalgo M., Guzman F.A., Hopp H.E., del Vas M. Sequence and phylogenetic analysis of genome segments S1, S2, S3 and S6 of Mal de Rio Cuarto virus, a newly accepted Fijivirus species. Virus Research. 2002;92:113–121. doi: 10.1016/s0168-1702(02)00325-8. [DOI] [PubMed] [Google Scholar]
- Dowton M., Austin A.D. Molecular phylogeny of the insect order Hymenoptera: apocritan relationships. Proceedings of the National Academic Science USA. 1994;91:9911–9915. doi: 10.1073/pnas.91.21.9911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici B.A., Bigot Y., Hamm J.J., Granados R.R., Vlak J.M., Miller L.K. Family Ascoviridae. In: van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens E.B., Estes M.K., Lemon S.M., Maniloff J., Mayo M.A., McGeoch D.J., Pringle C.R., Wickner R.B., editors. Taxonomy of Viruses. VII Report of the International Committee on Virus Taxonomy. Academic Press; London: 2000. pp. 261–265. [Google Scholar]
- Felsenstein, J., 1993. PHILIPS (Phylogeny Inference Package) version 3.5.c University of Washington, Seattle.
- Forrest J.C., Dermody T.S. Reovirus receptors and pathogenesis. Journal of Virology. 2003;77:9109–9115. doi: 10.1128/JVI.77.17.9109-9115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouillaud M., Morel G. Characterization of cytoplasmic and nuclear polydrehosis viruses recovered from the nest of Polistes hebraeus F. (Hymenoptera: Vespidae) Journal of Invertebrate Pathology. 1994;64:89–95. [Google Scholar]
- Hamm J.J., Styer E.L., Steiner W.W. Reovirus-like particle in the parasitoid Microplitis croceipes (Hymenoptera: Braconidae) Journal of Invertebrate Pathology. 1994;63:304–306. [Google Scholar]
- Hewat E.A., Booth T.F., Roy P. Structure of bluetongue virus particles by cryoelectron microscopy. Journal of Structural Biology. 1992;109:61–69. doi: 10.1016/1047-8477(92)90068-l. [DOI] [PubMed] [Google Scholar]
- Hill C.L., Booth T.F., Prasad B.V.V., Grimes J.M., Mertens P.P.C., Sutton G.C., Stuart D.I. The structure of a cypovirus and the functional organization of dsRNA viruses. Nature Structural Biology. 1999;6:565–568. doi: 10.1038/9347. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Nagaoka S., Winkler S., Kotani K., Yagi H., Nakanishi K., Miyajima S., Kobayashi J., Mori H. Molecular characterization of Bombyx mori cytoplamic polyhedrosis genome segment 4. Journal of Virology. 2001;75:988–995. doi: 10.1128/JVI.75.2.988-995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacas J.Y., Budia F., Rodriguez-Cerezo E., Vinuela E. Virus-like particles in the poison gland of the parasitic wasp Opius concolor. Annual Applied Biology. 1997;130:587–592. doi: 10.1111/j.1744-7348.1997.tb07685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer J.A., Webb B.A. Polydnavirus genes and genomes: emerging gene families and new insights into polydnavirus replication. Annual Review Entomology. 2004;49:431–456. doi: 10.1146/annurev.ento.49.072103.120132. [DOI] [PubMed] [Google Scholar]
- Labrada L., Bodelon G., Vinuela J., Benavente J. Avian reoviruses cause apoptosis in cultured cells: viral uncoating, but not viral gene expression, is required for apoptosis induction. Journal of Virology. 2002;76:7932–7941. doi: 10.1128/JVI.76.16.7932-7941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo D., Carles C., Sentenac A., Thuriaux P. Interactions between three common subunits of yeast RNA polymerases I and III. Proceedings of the National Academic Science USA. 1993;90:5524–5528. doi: 10.1073/pnas.90.12.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulier M., Pradier E., Bigot Y., Periquet G. An easy method for preserving nucleic acids in field samples for later molecular and genetic studies without refrigerating. Journal of Evolutionary Biology. 1995;8:657–663. [Google Scholar]
- Lawrence P.O. Purification and partial characterization of an Entomopoxvirus (DIEPV) from a parasitic wasp of tephritid fruit flies. Journal of Insect Science. 2002;2:1–12. doi: 10.1093/jis/2.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maazouz, S., 1991. Etude des interactions entre l’hyménoptère Brachonide Cotesia sesamiae (Cameron 1906), le virus de la polyhèdrose nucléaire de Sesamiae calamistis (Hampton 1910, Lepidopetra, noctuidae, le virus de la polyhèdrose nucléaire de Mamestra brassicae (Linnaeus, 1758), Lepidoptera, noctuidae et deux virus de polyhédrose cytoplasmiques de Sesamiae calamistis chez les chenilles de Sesamiae calamistis. Thèse de Doctorat, Montpellier II.
- MacDowell E.M., Trump B.F. Histologic fixatives suitable for diagnostic light and electron microscopy. Archives of Pathology Laboratory Medicine. 1976;17:1–199. [PubMed] [Google Scholar]
- Mertens P. The dsRNA viruses. Virus Research. 2004;101:3–13. doi: 10.1016/j.virusres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Mertens P.P.C., Pedley S., Crook N.E., Rubinstein R., Payne C.C. A comparison of six cypovirus isolates by cross-hybridization of their dsRNA genome segments. Archives of Virology. 1999;144:561–566. doi: 10.1007/s007050050525. [DOI] [PubMed] [Google Scholar]
- Qanungo K.R., Kundu S.C., Mullins J.I., Ghosh A.K. Molecular cloning and characterization of Antharaea mylitta cytoplamic polyhedrosis genome segment 9. Journal of General Virology. 2002;83:1483–1491. doi: 10.1099/0022-1317-83-6-1483. [DOI] [PubMed] [Google Scholar]
- Rabouille A., Bigot Y., Drezen J.M., Sizaret P.Y., Hamelin M.H., Periquet G. A member of the Reoviridae (DpRV) has a ploidy-specific genomic segment in the wasp Diadromus pulchellus (Hymenoptera) Virology. 1994;205:228–237. doi: 10.1006/viro.1994.1638. [DOI] [PubMed] [Google Scholar]
- Rabouille A., Bigot Y., Periquet G. Immuno-detection en fluorescence sur des coups d’insectes entiers inclus en résine acrylique. Le brin complémentaire. 1994;10:3–4. [Google Scholar]
- Rao S., Carner G.R., Scott S.W., Omura T., Hagiwara K. Comparison of the amino acid sequences of RNA-dependent RNA polymerases of cypoviruses in the family Reoviridae. Archives of Virology. 2003;148:209–219. doi: 10.1007/s00705-002-0923-2. [DOI] [PubMed] [Google Scholar]
- Renault S., Petit A., Bénédet F., Bigot S., Bigot Y. Effects of the Diadromus pulchellus ascovirus, DpAV-4, on the hemocytic encapsulation response and capsule-melanization of the leek-moth pupa, Acrolepiopsis assectella. Journal of Insect Physiology. 2002;48:297–302. doi: 10.1016/s0022-1910(01)00174-3. [DOI] [PubMed] [Google Scholar]
- Renault S., Bigot S., Lemesle M., Sizaret P.-Y., Bigot Y. The cypovirus Diadromus pulchellus DpRV-2 is sporadically associated with the endoparasitoid wasp D. pulchellus and modulates the defence mechanisms of pupae of the parasitized leek-moth Acrolepiopsis assectella. Journal of General Virology. 2003;84:1799–1807. doi: 10.1099/vir.0.19038-0. [DOI] [PubMed] [Google Scholar]
- Schoehn, G., 1994. Etude structurale d’un virus de guêpe par cryomicroscopie électronique et reconstruction tridimensionnelle. DEA, Université Joseph Fourier, 32pp.
- Stasiak, K., Renault, S., Federici, B.A., Bigot, Y., 2004. Characteristics of the pathogenic, latent and mutualistic associations of some ascoviruses in the field populations of several parasitoid wasp species. Journal of Insect Physiology, in press. [DOI] [PubMed]
- Stoltz D., Makkay A. Co-replication of a reovirus and a polydnavirus in the ichneumonid parasitoid Hyposoter exiguae. Virology. 2000;278:266–275. doi: 10.1006/viro.2000.0652. [DOI] [PubMed] [Google Scholar]
- Zhao S.L., Liang C.Y., Hong J.J., Xu H.G., Peng H.Y. Molecular characterization of segments 7-10 of Dendrolimus punctatus cytoplamic polyhedrosis virus provides the complete genome. Virus Research. 2003;94:17–23. doi: 10.1016/s0168-1702(03)00118-7. [DOI] [PubMed] [Google Scholar]


