Abstract
Porcine reproductive and respiratory syndrome (PRRS) is an immunosuppressive chronic respiratory viral disease of pigs that is responsible for major economic losses to the swine industry worldwide. The efficacy of parenteral administration of widely used modified live virus PRRS vaccine (PRRS-MLV) against genetically divergent PRRSV strains remains questionable. Therefore, we evaluated an alternate and proven mucosal immunization approach by intranasal delivery of PRRS-MLV (strain VR2332) with a potent adjuvant to elicit cross-protective immunity against a heterologous PRRSV (strain MN184). Mycobacterium tuberculosis whole cell lysate (Mtb WCL) was chosen as a potent mucosal adjuvant due to its Th1 biased immune response to PRRS-MLV. Unvaccinated pigs challenged with MN184 had clinical PRRS with severe lung pathology; however, vaccinated (PRRS-MLV+ Mtb WCL) pigs challenged with MN184 were apparently healthy. There was a significant increase in the body weight gain in vaccinated compared to unvaccinated PRRSV challenged pigs. Vaccinated compared to unvaccinated, virus-challenged pigs had reduced lung pathology associated with enhanced PRRSV neutralizing antibody titers and reduced viremia. Immunologically, an increased frequency of Th cells, Th/memory cells, γδ T cells, dendritic cells, and activated Th cells and a reduced frequency of T-regulatory cells were detected at both mucosal and systemic sites. Further, reduced secretion of immunosuppressive cytokines (IL-10 and TGF-β) and upregulation of the Th1 cytokine IFN-γ in blood and lungs were detected in mucosally vaccinated, PRRSV-challenged pigs. In conclusion, intranasal immunization of pigs with PRRS-MLV administered with Mtb WCL generated effective cross-protective immunity against PRRSV.
Keywords: Porcine reproductive and respiratory syndrome virus, Mucosal vaccination, M. tuberculosis whole cell lysate, Cytokines, Immune cells, Cross-protection
1. Introduction
Porcine reproductive and respiratory syndrome (PRRS) is a chronic immunosuppressive disease of pigs responsible for huge economic loss to the swine industry worldwide. The economic impact of PRRS alone to the US swine producers has been estimated to be approximately $560 million annually [1]. According to the Animal and Plant Health Inspection Service (APHIS) report of January 2009, overall, 49.8% of unvaccinated grower/finisher pigs were positive for PRRSV antibodies in the US (http://www.aphis.usda.gov/animal_health/nahms/swine/downloads/swine2006/Swine2006_is_PRRS.pdf). As a member of the Arteriviridae family, PRRSV contains a positive-sense, single-stranded RNA genome [2]. PRRSV causes respiratory distress in pigs of all ages and reproductive failure in sows [3], [4]. Infection with this virus results in suppression of innate immune response (reduced IFN-α production and NK cell cytotoxicity) and a delay in the onset of adaptive immune response [5], [6], [7].
Different field isolates of PRRSV have been found to carry variable levels of genetic diversity ranging between 84 and 100% and one of the virulent strains, MN184 is antigenically highly divergent from the vaccine strain, VR2332 [8]. Currently, modified live and inactivated PRRSV vaccines have been licensed for use; however, these vaccines are not always efficacious in preventing PRRSV reinfections and transmission. There continue to be questions regarding vaccine safety and efficacy against existing as well as emerging antigenically heterologous PRRSV strains [9], [10]. Therefore, development of a broadly protective PRRSV vaccine has been a challenge to PRRSV researchers. Recently, PRRSV experts and researchers collectively agreed that only replicating PRRSV vaccines have the most promise in the field and successful protection can be achieved by improving the efficacy of live PRRSV vaccines (http://vetmed.illinois.edu/news/PRRSwhitepaper.pdf).
Mucosal surfaces cover the largest surface area in the body and almost 80% of total immune cells in the body are localized in the mucosa-associated lymphoid tissues (MALT) and at mucosal sites. Nasopharyngeal MALT contains the entire repertoire of immune cells which are strategically located to orchestrate regional immune functions against airborne infections [11]. A majority of pathogens are transmitted through mucosal surfaces, but some of them cause disease primarily at these mucosal sites (e.g. Influenza, PRRSV, HIV, Rotavirus, etc.). It has been demonstrated that systemic stimulation of the immune system mainly results in systemic protection with low mucosal immune responses. Conversely, optimal stimulation of the mucosal immune system generates both mucosal and systemic immunity [12]. The mucosal immune system possesses strong immunoregulatory mechanisms to dampen inflammation-induced pathology; therefore, mucosal immunization generally results in tolerance in the absence of suitable adjuvants or delivery systems [13], [14]. Intranasal delivery of live attenuated vaccines against bovine herpes virus-1, influenza, and parainfluenza-3 when administered with adjuvants has proven effective [15], [16]. Intranasal delivery of influenza vaccine with a potent adjuvant resulted in effective cross protection due to immune responses generated against conserved internal viral proteins [17]. Thus, mucosal immunization may be an attractive method to induce PRRSV specific cross-protective immunity.
Design of an effective mucosal vaccine is not easy because the adjuvant/delivery system used must not elicit any toxicity and should overcome the host- and/or vaccine antigens-induced immunosuppression. Heat-killed Mycobacterium tuberculosis (Mtb) in an oil emulsion has been used extensively for experimental purposes as complete Freund's adjuvant [18]. Unfortunately, adverse effects mediated by major cell wall components of Mtb such as mycolic acids, peptidoglycan, and wax D preclude use of complete Freund's adjuvant in humans and food animals [19], [20]. Adjuvanticity of various purified individual components of Mtb have been investigated [21], [22], including water soluble whole cell lysate (WCL) of Mtb [23]. Interestingly, a few of the Mtb WCL fractions, such as heat shock protein-70 (HSP70) [21], [24] and PE (Pro-Glu)/PPE (Pro-Pro-Glu) have been identified to elicit potent adjuvant activity [25], [26]. However, the knowledge related to mucosal adjuvanticity of Mtb WCL to mucosal viral vaccines is limited.
Initially, we performed studies to choose a suitable bacterial candidate mucosal adjuvant to use with PRRS-MLV. Pigs were inoculated intranasally with nine different bacterial preparations belonging to Mycobacterium, Vibrio, and Streptococcus, and selected Mtb WCL due to its ability to induce increased Th1 and reduced immunosuppressive responses; the detailed results of which will be published elsewhere. Subsequently, PRRSV specific immune responses to PRRS-MLV+/− Mtb WCL in pigs inoculated intranasally was evaluated in detail in a pre-challenge study, which resulted in satisfactory immune correlates of protection mediated through Mtb WCL [27]. The purpose of the current study was to confirm the mucosal adjuvanticity of Mtb WCL to PRRS-MLV in inducing effective cross-protection to challenge with a genetically divergent and virulent PRRSV strain.
2. Materials and methods
2.1. Cells, PRRSV, and adjuvant
Stable Mycoplasma-free MARC-145 cells which support the growth of PRRSV [28] were used to prepare PRRSV stocks and to perform immunological assays. Cells were maintained in DMEM with 10% FBS (Atlanta Biologicals, Lawrenceville, GA) at 37 °C with 5% CO2. For virus infection and titration of viral stock, DMEM supplemented with 2% horse serum was used. Modified live virus PRRS vaccine (PRRS-MLV) (Ingelvac® PRRS) was a kind gift from Dr. Mike Roof (Bio-R&D, Boehringer Ingelheim Vetmedica Inc., St. Joseph, MO). For some experiments, M. tuberculosis whole cell lysate (Mtb WCL) was provided by Drs. Dobos and Belisle under NIH/NIAID funded contract HHSN266200400091c “TB Vaccine Testing and Research Materials” (Colorado State University, Fort Collins, CO).
2.2. Pigs and inoculations
Conventional large White-Duroc crossbred weaned specific-pathogen-free piglets at 3–4 wks of age were transported to animal facilities of the Food Animal Health Research Program at the Ohio Agricultural Research and Development Center, Wooster, OH. The swine herd was confirmed seronegative for antibodies to PRRSV, porcine respiratory corona virus, transmissible gastroenteritis virus, and porcine circo virus 2. Piglets were bled on arrival, and the sera were tested to confirm the absence of PRRSV antibodies. Pigs were allowed to acclimate for an additional week before initiation of the experiment. Animals were maintained in the large animal BSL-2 facility under the supervision of a veterinarian. Throughout the duration of the study, all animals received food and water ad libitum. All inoculations such as adjuvant (Mtb WCL, 3 mg/pig), vaccine (PRRS-MLV, 2 × 106 TCID50 per pig) and challenge (PRRSV, 1 × 106 TCID50 per pig) were administered intranasally. Adjuvant and vaccine were inoculated separately into each nostril. All pigs were maintained, samples collected, and euthanized as per the protocol approved by the Institutional Animal Care and Use Committee (IACUC), Wooster, and Institutional Biosafety Committee (IBC), Columbus, The Ohio State University, Ohio.
For the primary study, 22 pigs were randomly allocated to one of three groups: group 1, mock pigs (n = 4) received DMEM and they were unvaccinated and unchallenged; group 2, unvaccinated pigs (n = 9); group 3, vaccinated (PRRS-MLV+ Mtb WCL) pigs (n = 9). Groups 2 and 3 were challenged with PRRSV MN184 on day post-vaccination (DPV) 21. Three pigs each from groups 2 and 3 were euthanized on 15, 30, and 60 day post-challenge (DPC). Mock inoculated pigs (n = 4) were euthanized separately prior to killing of any infected animals.
In another study, a total of nine pigs were split into three groups and housed in three separate isolation rooms (n = 3 per group). Pigs were inoculated intranasally as follows: group 1, mock (DMEM); group 2, vaccine (PRRS-MLV); group 3, vaccine with adjuvant (PRRS-MLV+ Mtb WCL). At DPI-21, groups 2 and 3 were challenged with PRRSV MN184 on DPV 21. Infection was allowed to proceed for 30 days at which time all pigs were euthanized. Mock inoculated pigs were euthanized prior to the euthanasia of any PRRSV challenged animals.
2.3. Collection of blood samples for analysis
Blood (3–5 ml) was collected on DPI 0 and DPC 0, 4, 7, 14, 21, 28, 31, 35, 42, 49, 56, and 60, serum was separated from the clotted blood, and aliquots of serum were preserved at −20 °C until used in assays. Serum was used for evaluation of viremia, viral titer, PRRSV serum neutralizing antibody titers, and cytokine production. Pigs were monitored daily for respiratory disease, and rectal temperature and body weight were recorded twice a week.
2.4. Isolation of cells
Blood was collected in acid citrate dextrose solution and processed for isolation of peripheral blood mononuclear cells (PBMC) as previously described [29]. Lung mononuclear cells (lung MNC) from individual pigs were isolated at necropsy as described previously [6], [30], [31]. Tracheobronchial lymph nodes (TBLN) draining the lungs were collected in DMEM, cut into small pieces, and homogenized in stainless steel cellectors. Homogenates were washed and the pellet was dissolved in RPMI containing 43% Percoll and centrifuged for 25 min at 2800 × g at 4 °C, with no brake. Red blood cells in the cell pellet were lysed and the mononuclear cells were washed and resuspended in enriched RPMI [RPMI-1640, 10% fetal bovine serum, gentamicin (100 μg/ml), ampicillin (20 μg/ml), 20 mM HEPES, 2 mM l-glutamine, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, and 50 nM of 2-ME].
2.5. Gross lung lesion analysis
Necropsy was performed and lungs, tonsils, and TBLN were examined for gross lesions. Grossly evident pulmonary changes were assigned a score based upon the percent of virus-affected lesions (purple-red colored consolidation) in each lung lobe, and a total score for the entire lung was calculated as described previously [32].
2.6. Analyses of PRRSV load, viral titer, and virus neutralizing antibody titer
All the analyses were performed using a standard indirect immunofluorescence assay (IFA) as described previously [4], [28], [33], [34]. For virus titration, a confluent monolayer of MARC-145 cells in a 96-well microtiter plate was incubated with a 10-fold dilution of serum for 24 h. For determining the virus neutralization (VN) titers, serum was heat treated for complement inactivation, diluted two-fold in DMEM, and incubated with an equal volume of PRRSV MN184 containing 500 TCID50 per well for 2 h at 37 °C. One hundred microliter of that suspension was transferred into a 96-well microtiter plate containing a confluent monolayer of MARC-145 cells and incubated for 24 h at 37 °C in a CO2 incubator. Cytopathic effects were examined following fixation with acetone water and addition of anti-PPRSV Nucleoprotein mAb (clone, SDOW17; Rural Technologies, Inc., Brookings, SD) and Alexa-488 conjugated anti-mouse IgG(H+L) secondary antibody, and observed under a fluorescent microscope after mounting with glycerol–PBS (6:4 ratio).
2.7. PRRSV specific recall/memory immune response
Five million PBMC, TBLN MNC, and lung MNC were subjected to in vitro restimulation in a 24-well tissue culture plate in the presence of killed crude PRRSV MN184 antigens (Ags) (50 μg/ml), or recombinant PRRSV carboxy terminal 88 amino acid fragment of matrix protein (M3′) (2 μg/ml) [35], [36] in enriched RPMI for 48 h at 37 °C. The harvested culture supernatant was analyzed for cytokines by ELISA. Cells cultured in the absence of any antigens were included as a control, and the amount of cytokines secreted by these cells were subtracted from the respective restimulated experimental well values.
2.8. ELISPOT assay to determine PRRSV specific IFN-γ-secreting cells
The frequency of IFN-γ-secreting cells in PBMC was determined by an ELISPOT assay as described previously [9], [37]. Briefly, PBMC were plated (5 × 105 cells/well) in enriched RPMI in a 96-well MultiScreen plate (Millipore, Billerica, MA) pre-coated overnight with a mouse anti-pig IFN-γ mAb (BD PharMingen, San Diego, CA) at 4 °C. Cells were restimulated with killed crude PRRSV MN184 Ags (50 μg/ml) for 24 h at 37 °C in a CO2 incubator. Plates were incubated with biotinylated anti-pig IFN-γ detection antibody, subsequently with streptavidin-HRP conjugate and developed using an insoluble substrate tetramethylbenzidine with H2O2 peroxidase substrate system (KPL Inc., Gaithersburg, Maryland). The frequency of PRRSV specific IFN-γ-secreting cells was counted using an AID® ELISpot Reader System. The background values were subtracted from the respective counts of the unstimulated cells and the immune responses were expressed as the number of IFN-γ-secreting cells per million PBMC. Cells stimulated with phytohemaglutinin-P were included as a positive control on every plate.
2.9. Analysis of cytokine response
Pig sera collected at indicated DPC and culture supernatants harvested after in vitro restimulation of PBMC, TBLN and lung MNC were analyzed by ELISA for secretion of different interleukin (IL) classes, such as Th1 (IFN-γ and IL-12), Th2 (IL-4), pro-inflammatory (IL-6), and immunosuppressive (IL-10 and TGF-β) cytokines as described previously [6], [37].
2.10. Flow cytometric study of immune cell populations
Flow cytometric analysis was performed to determine the phenotype and frequency of different immune cells in a multicolor immunoassay as described previously [6], [31]. Briefly, previously isolated cell types (PBMC, lung MNC, and TBLN MNC) were treated with 2% pig serum to block Fc receptors. Cells were then stained with an appropriate mAb which was either directly conjugated to a specific fluorochrome or biotinylated, or with a purified antibody to pig specific immune cell surface markers [CD3ɛ, CD172 (SouthernBiotech, Birmingham, Alabama), CD4α, CD8α/β, CD11c (BD PharMingen), CD25, SLA class II (Serotec, Raleigh, NC), TcR1N4 (VMRD, Pullman, WA), Foxp3 (eBioscience, San Diego, CA)] or with their respective isotype control mAb and labeled cells were treated with streptavidin-conjugated fluorochrome or respective anti-species isotype specific secondary antibody conjugated with fluorochrome. Finally, cells were fixed with 1% paraformaldehyde. For intracellular Foxp3 staining, cells were surface stained for CD4 and CD25 as described above and overnight incubated at 4 °C in permeabilization buffer, and stained with fluorochrome-conjugated pig Foxp3 cross-reactive anti-rat Foxp3 mAb [38], [39].Immunostained cells were acquired using a FACS AriaII (BD Biosciences, San Jose, CA) flow cytometer. Analysis was performed to determine different immune cell populations based on the cell surface marker phenotypes: natural killer (NK) cells (CD3−CD4−CD8α+) [40]; T-helper cells (CD3+CD4+CD8−); cytotoxic T lymphocytes (CTLs) (CD3+CD4−CD8+); CD4CD8 double positive T cells (CD3+CD4+CD8+) also called as T-helper/memory cells [41], [42]; γδ T cells (CD8α+TcR1N4+); T-regulatory cells (CD4+CD25+Foxp3+); CD172+ (myeloid cells); CD4+CD25+Foxp3− (activated T-helper cells); dendritic cells rich fraction (CD172+CD11c+SLAII+) using FlowJo software (Tree Star, Inc., OR, USA). Frequencies of individual lymphocyte and myeloid cell subsets were analyzed from a total 50,000 to 100,000 events.
2.11. Statistical analysis
All data were expressed as the mean value of three, six, or nine pigs ± SEM. Statistical analyses were performed using a nonparametric “Wilcoxon t-test” where functionality was compared between two study groups (unvaccinated vs. vaccinated, and in Fig. 5 and Table 2, PRRS-MLV vs. PRRS-MLV+ Mtb WCL), or nonparametric “Kruskal–Wallis test” followed by a “Dunn's test” for multiple comparisons when comparing three or more groups for VN titers and body weight gain using SAS software (SAS Institute Inc., Cary, NC). Statistical significance was assessed as P < 0.05.
Fig. 5.
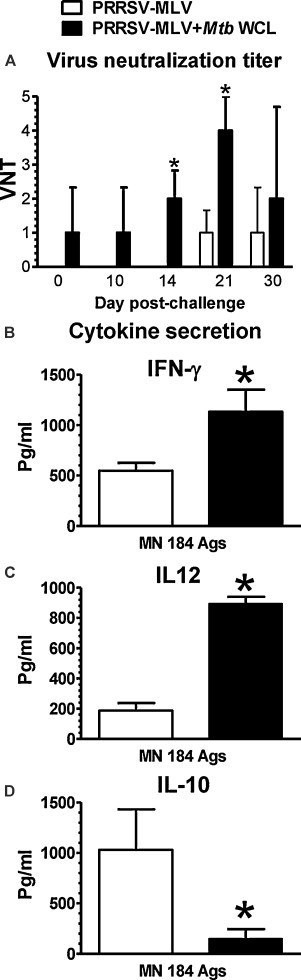
Protective anti-PRRSV specific humoral and cell-mediated immune responses to PRRS-MLV were mediated by Mtb WCL. Pigs (three each) were inoculated intranasally with mock, PRRS-MLV, or PRRS-MLV+ Mtb WCL and then vaccinated pigs were challenged with MN184 at DPI 21 and euthanized at DPC 30. (A) Serum collected at indicated DPC were analyzed for PRRSV specific VN titers by a standard immunofluorescence assay. Supernatants harvested from lung MNC and PBMC cultures restimulated using killed MN184 Ags were analyzed for cytokines: (B) IFN-γ; (C) IL-12; (D) IL-10 by ELISA. Each bar represents the average values from three pigs ± SEM. Asterisk denotes a statistically significant difference (P < 0.05) between PRRS-MLV vs. PRRS-MLV+ Mtb WCL received and PRRSV challenged pigs.
Table 2.
Frequency of immune cells in pigs inoculated intranasally with mock (no vaccination and no challenge), PRRS-MLV, or PRRS-MLV+ Mtb WCL and then challenged with PRRSV MN184.
| Immune cells | Mocka | PRRS-MLVb | PRRS-MLV+ Mtb WCLb |
|---|---|---|---|
| A. Lung MNC | |||
| Th cellsc | 18.3 ± 4.2 | 4.3 ± 0.3* | 17.5 ± 1.4* |
| CTLsc | 17.5 ± 3.8 | 47.9 ± 6.2 | 50.9 ± 3.7 |
| Activated Th cells | 3.4 ± 1.4 | 19.9 ± 6.9* | 43.0 ± 2.4* |
| NK cellsc | 9.6 ± 1.9 | 12.7 ± 4.6* | 33.6 ± 4.9* |
| γδ T cells | 7.6 ± 0.2 | 6.8 ± 3.5 | 14.6 ± 2.4 |
| T-regulatory cellsd | 2.3 ± 0.2 | 6.0 ± 2.4 | 3.5 ± 1.0 |
| B. PBMC | |||
| CTLsc | 12.2 ± 2.5 | 19.2 ± 2.3 | 22.3 ± 1.7 |
| Activated Th cells | 5.2 ± 1.9 | 8.4 ± 4.2 | 11.8 ± 2.0 |
| NK cellsc | 16.6 ± 2.8 | 13.9 ± 9.3 | 20.3 ± 3.6 |
| γδ T cells | 21.2 ± 2.3 | 12.3 ± 4.0* | 27.1 ± 0.8* |
| Dendritic cellsd | 6.7 ± 3.6 | 10.9 ± 3.7 | 19.1 ± 4.6 |
| T-regulatory cellse | 6.3 ± 3.7 | 8.5 ± 4.2* | 2.76 ± 0.9* |
| C. TBLN MNC | |||
| Activated Th cellsc | 13.6 ± 4.9 | 13.2 ± 2.0 | 20.3 ± 1.5 |
| T-regulatory cellse | 5.7 ± 1.6 | 8.0 ± 2.3 | 1.6 ± 1.2* |
Each number is an average percent of immune cells from three pigs ± SEM. Asterisk indicates a statistically significant difference (P < 0.05) between PRRS-MLV vs. PRRS-MLV+ Mtb WCL and PRRSV challenged pigs.
Three mock (5–6 wks of age) pigs were euthanized separately.
Three each of indicated vaccine inoculated and virus challenged pigs were euthanized at DPC 30. Different immune cell subsets present in lung MNC, PBMC, and TBLN MNC were enumerated by flow cytometry.
CD3+ and CD3− cells were gated to enumerate CD4 and CD8α expression.
CD172+ cells were gated to enumerate CD11c and SLAII expression and the percent of triple positive (CD172+CD11c+SLAII+) cells are shown.
CD25+ cells were gated to enumerate CD4 and Foxp3 expression and the percent of triple positive (CD4+CD25+Foxp3+) cells are shown.
3. Results
3.1. Mucosal immunization to PRRSV protected pigs against virulent viral challenge
In this study, pigs were either unvaccinated or vaccinated with PRRS-MLV+ Mtb WCL and challenged using a virulent heterologous PRRSV MN184. Clinically, unvaccinated pigs developed typical PRRS symptoms with fever, mild cough, reduced food intake, and lethargy during first 2 wks post-challenge. Vaccinated, MN184 challenged pigs did not suffer from clinical PRRS. The mean body temperature of unvaccinated, PRRSV challenged pigs (n = 6) until DPC 30 was 0.3 °F higher compared to vaccinated pigs.
Pigs vaccinated and challenged with MN184 had significant increase in the net body weight gain compared to control challenged pigs (Fig. 1A). Unvaccinated, MN184 challenged pigs had severe gross lung lesions on both ventral and dorsal surfaces (Fig. 1B), and the lung lesion scores were significantly higher at DPC 15 and DPC 30 compared to vaccinated challenged pigs (Fig. 1C). Protection in mucosally vaccinated pigs against PRRSV challenge was associated with a significantly reduced viral load (Fig. 2A) and viral titer (Fig. 2B) at DPC 15. The circulating PRRSV in the blood was less but not statistically significant at DPC 30 in vaccinated pigs, and it was almost cleared by DPC 60 in both the pig groups (Fig. 2A).
Fig. 1.
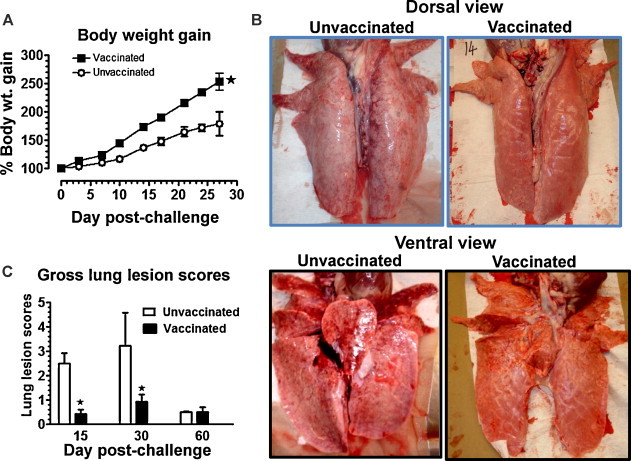
Mucosal vaccination with adjuvant rescued pigs from clinical PRRS with reduced gross lung pathology to a virulent PRRSV challenge. Pigs were unvaccinated (n = 9) or vaccinated (n = 9) with PRRS-MLV+ Mtb WCL and challenged with PRRSV MN184 on day post-immunization (DPI) 21 and then three pigs from each group were euthanized on day post-challenge (DPC) 15, 30, and 60. (A) Body weight of pigs was monitored on every third day post-challenge for 4 wks. Percentage body weight gain of individual pig was calculated by considering the weight of the pig at DPC 0 as 100%. (B) A representative lung picture of an unvaccinated or vaccinated and PRRSV challenged and then euthanized at DPC 15 is shown. (C) Gross lung lesion scores present in all the pig lung lobes at DPC 15, 30 and 60 were scored using a standard procedure. Each data point represents the average body weight gain from nine and six pigs ± SEM at DPC 15 and 30, respectively, and each bar represents the average lung lesion score from three pigs ± SEM. Asterisk denotes a statistically significant difference (P < 0.05) between unvaccinated vs. vaccinated and PRRSV challenged pigs.
Fig. 2.
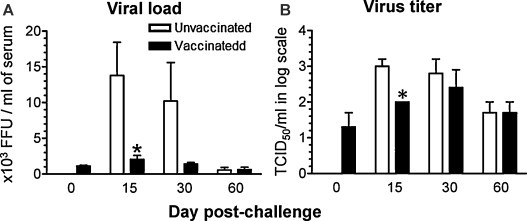
Reduced viremia and PRRSV titer in mucosally vaccinated and MN184 challenged pigs. Experimental details were as described in legend to Fig. 1. Serum collected at indicated DPC were analyzed for (A) total PRRS viral load; (B) PRRS viral titer; by a standard immunofluorescence assay. Each bar represents the average of six pigs ± SEM at DPC 15 and 30, and three pigs ± SEM at DPC 60. Asterisk denotes a statistically significant difference (P < 0.05) between unvaccinated vs. vaccinated and PRRSV challenged pigs.
3.2. Enhanced neutralizing antibody and reduced immunosuppressive cytokine response in the blood of mucosally vaccinated pigs
PRRSV neutralizing antibody titers in serum at different time points until DPC 60 were analyzed. A significant increase in the VN titers in vaccinated and MN184 challenged pigs was detected (Fig. 3A). We found a significant difference in the VN titers in vaccinated pigs at DPC 7, 21, and 35 when the titer was compared at each DPC. In mucosally vaccinated, PRRSV-challenged pigs, there was a significant reduction in serum IL-10 levels at DPC 14, 21, and 28 (Fig. 3B). Similarly, TGF-β serum levels were also significantly reduced but at a later stage of challenge (DPC 42 and 60) (Fig. 3C). Overall, both the immunosuppressive cytokines IL-10 and TGF-β were detected at reduced levels in vaccinated pigs (Fig. 3B and C).
Fig. 3.
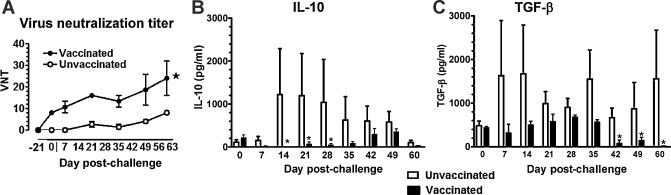
Enhanced PRRSV specific neutralizing antibody titers and reduced immunosuppressive cytokines in serum of mucosally vaccinated and MN184 challenged pigs. Experimental details were as described in legend to Fig. 1. Serum samples collected at indicated DPC were analyzed for (A) anti-PRRSV specific neutralizing antibody titers by standard immunofluorescence assay, (B) IL-10, and (C) TGF-β by ELISA. Each bar or data point in the graph represents the average VN titer or amount of cytokines from three pigs ± SEM. Asterisk denotes a statistically significant difference (P < 0.05) between unvaccinated vs. vaccinated and PRRSV challenged pigs.
3.3. Increased PRRSV-specific Th1 cytokines and reduced immunosuppressive cytokines were secreted by immune cells of vaccinated and virus challenged pigs
Information regarding secretion of cytokines by pig MNC after PRRSV restimulation ex vivo is important to understand virus specific memory immune responses. Consistent with the reduced lung lesions and viremia, a significantly increased frequency of IFN-γ-secreting cells in PBMC of vaccinated, PRRSV challenged pigs at DPC 15 was detected (Fig. 4A). Such a trend was noted for IFN-γ-secreting cells in the lungs at DPC 15 (data not shown) with a concomitant reduction in IL-6-secretion by lung MNC (Fig. 4C). The extended clinical protection in vaccinated PRRSV challenged pigs was indicated by an increased trend in the secretion of IFN-γ with a concomitant reduction in IL-10 and TGF-β secretion by lung MNC and PBMC at both DPC 30 and 60 (data not shown).
Fig. 4.
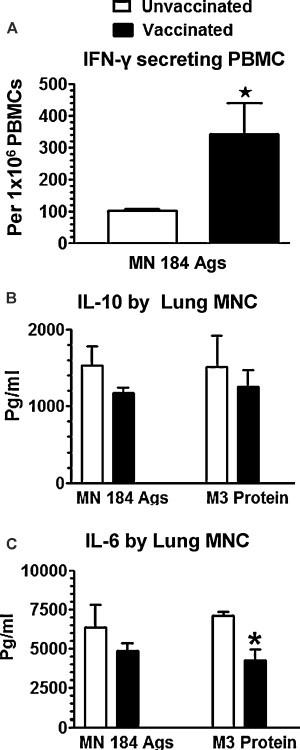
Mucosally vaccinated and PRRSV challenged pig immune cells secreted increased recall Th1 and suppressed immunosuppressive cytokines. Experimental details were as described in legend to Fig. 1, and the data from only DPC 15 pigs is shown. (A) PBMC was restimulated in the presence of killed MN184 antigens and the IFN-γ-secreting cells were analyzed by the ELISPOT assay. Supernatants harvested from lung MNC and PBMC cultures restimulated using killed MN184 antigens or PRRSV recombinant matrix (M3′) protein were analyzed for cytokines: (B) IL-10; (C) IL-6 by ELISA. Each bar represents the average number of IFN-γ-secreting cell spots or amount of cytokines from three pigs ± SEM. Asterisk denotes a statistically significant difference (P < 0.05) between unvaccinated vs. vaccinated and PRRSV challenged pigs.
3.4. Frequencies of lymphoid and myeloid cells in mucosally vaccinated and PRRSV challenged pigs correlate with the cytokine responses
Evaluation of the frequency of various immune cells at both mucosal (lung and TBLN MNC) and systemic sites (PBMC) in vaccinated and virulent PRRSV challenged pigs is important for associating cytokine responses. At DPC 15, a significant increase in the frequency of dendritic cells (DCs) and an increased frequency of Th cells, Th/memory cells, and γδ T cells were detected in the lungs of vaccinated, virus challenged pigs (Table 1A). In addition, in both PBMC and TBLN MNC, a significant increase in the frequency of activated Th cells was detected at DPC 15 (Table 1B and C).
Table 1.
Frequency of immune cells in pigs inoculated intranasally with mock (no vaccination and no challenge), unvaccinated (n = 9) or vaccinated with PRRS-MLV+ Mtb WCL (n = 9) and then challenged with PRRSV MN184.
| Immune cells | Mocka | Unvaccinatedb |
Vaccinatedb |
||||
|---|---|---|---|---|---|---|---|
| Days post-challenge |
|||||||
| 15 | 30 | 60 | 15 | 30 | 60 | ||
| A. Lung MNC | |||||||
| T lymphocytes | 29 ± 0.3 | 21.9 ± 5.0 | 19.5 ± 4.0 | 6.9 ± 1.6 | 26.4 ± 3.7 | 22.9 ± 0.4 | 10.1 ± 2.3 |
| Th cellsc | 25.9 ± 0.7 | 6.4 ± 4.7 | 13.3 ± 1.3 | ND | 11.5 ± 2.0 | 17.5 ± 1.4 | ND |
| CTLsc | 25.0 ± 2.9 | ND | 40.5 ± 3.9 | 2.4 ± 1.8 | ND | 44.3 ± 2.1 | 6.2 ± 1.7 |
| Th/memory cellsc | 12.10 ± 1.8 | 15.4 ± 1.5 | ND | 25.9 ± 0.6 | 24.1 ± 4.6 | ND | 36.5 ± 1.2 |
| Dendritic cellsd | 10.6 ± 5.4 | 5.2 ± 1.7* | ND | ND | 7.9 ± 0.8* | ND | ND |
| Myeloid cells | 71.4 ± 5.3 | 30.2 ± 2.3 | ND | 24.2 ± 2.8 | 37.6 ± 5.3 | ND | 30.8 ± 3.8 |
| γδ T cells | 6.9 ± 0.6 | 16.2 ± 2.2 | ND | 5.9 ± 2.9 | 22.5 ± 5.2 | ND | 8.5 ± 2.3 |
| T-regulatory cellse | 12.5 ± 2.8 | ND | 9.10 ± 0.9 | 8.2 ± 2.2* | ND | 5.73 ± 2.0 | 1.2 ± 0.4* |
| B. PBMC | |||||||
| Th cellsc | 18.8 ± 0.9 | ND | 9.3 ± 4.7* | ND | ND | 22.3 ± 1.8* | ND |
| Activated Th cells | 5.2 ± 1.9 | 5.9 ± 1.2* | ND | ND | 11.1 ± 2.7* | ND | ND |
| Th/memory cellsc | 5.2 ± 0.1 | 7.8 ± 0.4 | ND | ND | 23.6 ± 17.3 | ND | ND |
| NK cellsc | 2.5 ± 2.1 | ND | 14.8 ± 2.6 | ND | ND | 26.9 ± 3.3 | ND |
| Dendritic cellsd | 4.4 ± 3.1 | ND | ND | 3.5 ± 1.5* | ND | ND | 12.6 ± 0.7* |
| γδ T cells | 21.2 ± 2.3 | ND | 14.8 ± 3.9 | 15.7 ± 8.1 | ND | 27.1 ± 2.6 | 25.4 ± 1.5 |
| T-regulatory cellse | 9.5 ± 1.6 | ND | ND | 17.4 ± 0.9* | ND | ND | 10.7 ± 3.5* |
| C. TBLN MNC | |||||||
| T lymphocytes | 34.7 ± 11.7 | 23.2 ± 2.3 | 16.2 ± 3.1 | 30.5 ± 6.4 | 28.4 ± 7.4 | 28.0 ± 1.8 | 34.5 ± 3.6 |
| Th cellsc | 37.8 ± 4.7 | ND | 17.7 ± 1.3* | ND | ND | 46.2 ± 5.6* | ND |
| Activated Th cells | 13.6 ± 4.9 | 15.9 ± 1.0 | ND | ND | 20.1 ± 0.6 | ND | ND |
| γδ T cells | 5.1 ± 0.2 | 16.4 ± 3.9 | ND | ND | 21.2 ± 5.0 | ND | ND |
| T-regulatory cellse | 6.8 ± 3.7 | ND | 6.9 ± 1.6 | 29.6 ± 12.7 | ND | 3.8 ± 1.4 | 7.4 ± 2.5 |
Each number is an average percent of immune cells from three or four pigs ± SEM. Asterisk indicates a statistically significant difference (P < 0.05) between unvaccinated vs. vaccinated pig groups. ND – no difference in the immune cell frequency between unvaccinated vs. vaccinated pigs.
Four control mock pigs (5–6 wks of age) were euthanized separately.
Three each of unvaccinated or vaccinated and then challenged were euthanized at DPC 15, 30, and 60. Different immune cell subsets present in lung MNC, PBMC, and TBLN MNC were enumerated by flow cytometry.
CD3+ and CD3− cells were gated to enumerate CD4 and CD8α expression.
CD172+ cells were gated to enumerate CD11c and SLAII expression and the percent of triple positive (CD172+CD11c+SLAII+) cells are shown.
CD25+ cells were gated to enumerate CD4 and Foxp3 expression and the percent of triple positive (CD4+CD25+Foxp3+) cells are shown.
At DPC 30, a 50% reduction in the frequency of T-regulatory cells (Tregs) in the lungs and TBLN in vaccinated, virus challenged pigs was detected (Table 1A and C). In blood and TBLN, the frequency of Th cells was significantly increased in vaccinated pigs (Table 1B and C). At DPC 60, a significant decrease in the frequency of Tregs in both lungs and blood was detected (Table 1A and B). In addition, a significantly increased frequency of DCs in the blood was detected in vaccinated and PRRSV MN184 challenged pigs at DPC 60 (Table 1B).
3.5. Protective anti-PRRSV specific humoral and cell-mediated immune responses to PRRS-MLV were mediated by Mtb WCL
To strengthen our data on the superior adjuvanticity of Mtb WCL to PRRS-MLV, we performed challenge studies in pigs vaccinated intranasally with PRRS-MLV in the presence or absence of Mtb WCL. PRRSV specific VN titers were detected at significantly higher levels at DPC 14 and 21 in pigs vaccinated with PRRS-MLV+ Mtb WCL compared to PRRS-MLV alone (Fig. 5A). Virus neutralizing antibody titers were present at low levels in pigs vaccinated with PRRS-MLV+ Mtb WCL from DPI 21 (DPC 0), but not in pig groups receiving PRRS-MLV alone (Fig. 5A).
In support of adjuvant Mtb WCL mediated enhanced cell mediated immune (CMI) responses to PRRSV, lung MNC of pigs vaccinated with Mtb WCL secreted significantly higher amounts of IFN-γ following restimulation using MN184 Ags (Fig. 5B). Another Th1 response inducing cytokine IL-12 was also secreted at significantly higher levels in a Mtb WCL dependent manner in vaccinated pigs (Fig. 5C). As expected, the immunosuppressive cytokine, IL-10 was secreted at significantly reduced levels in pigs vaccinated with Mtb WCL compared those pigs vaccinated without Mtb WCL following challenge with PRRSV MN184 (Fig. 5D).
Frequency of immune cell populations was also evaluated at both mucosal and systemic sites between these two pig groups to determine the Mtb WCL mediated adjuvant effects. In lung MNC, a significant increase in the frequency of Th cells, activated Th cells, and NK cells was detected in challenged pigs vaccinated with PRRS-MLV+ Mtb WCL compared to pigs vaccinated with PRRS-MLV alone (Table 2A). In PBMC, a significant increase in the frequency of γδ T cells was detected in PRRS-MLV+ Mtb WCL inoculated pigs (Table 2B). As expected, the frequency of Tregs was significantly reduced in both PBMC and TBLN MNC of pigs inoculated with vaccine with Mtb WCL compared to vaccine alone (Table 2B and C). Overall, data from these particular pig groups combination study demonstrated the superior adjuvanticity of the mucosal adjuvant Mtb WCL to PRRS-MLV.
4. Discussion
Until now, as per our knowledge, no successful attempts have been made to elicit effective anti-PRRSV immunity by intranasal delivery of live PRRSV vaccine. Viruses evade the host immunity by promoting secretion of IL-10 and TGF-β which antagonize the protective Th1 immune response [43]. PRRSV induces a strong immunosuppressive response resulting in the delayed onset of cell mediated immune responses [6], [43], [44], [45]. Both live and inactivated PRRSV significantly increase IL-10 gene expression [45]. An increased concentration of IL-10 in pig lungs was detected from PRRSV-infected pigs for long periods of time [6], [43]. Nonetheless, expression of both IL-10 and TGF-β genes was also increased in pigs vaccinated against PRRSV by a systemic route [46]. In our pre-challenge study, increased secretion of both IL-10 and TGF-β in both the lungs and blood of pigs vaccinated intranasally with PRRS-MLV without Mtb WCL was detected [27]. In contrast, in both pre- and post-challenge studies when PRRS-MLV was inoculated intranasally with Mtb WCL, secretion of both IL-10 and TGF-β was suppressed.
Infiltration of Tregs into the infected pig lungs contributes to the secretion of high levels of IL-10 and TGF-β [47]. The role of Tregs in establishment of chronic persistent HIV, hepatitis C and B viruses, cytomegalovirus, and Epstein–Barr virus infections has been reported [48], [49], [50]. Like in mice and humans, Foxp3-expressing CD4+CD25+ cells with comparable immunosuppressive properties have been identified in pigs [38]. Studies have been reported on the PRRSV mediated proliferation of Tregs in infected pigs, indicating their involvement in disease progression [51], [52], [53]. In our study, a consistently reduced frequency of Tregs in the lungs, blood, and TBLN of pigs vaccinated intranasally with PRRS-MLV+ Mtb WCL was detected which was associated with reduced secretion of both the immunosuppressive cytokines, IL-10 and TGF-β. Co-ordinated immunosuppressive functions of IL-10, TGF-β, and Tregs have been reported [54], [55]. PRRSV increases the expression of TGF-β from myeloid cells, and TGF-β is essential for the de novo induction of Foxp3 and for the regulation of differentiation and function of Tregs in mice, humans, and pigs [52], [56].
All the mucosal sites are interconnected by a common mucosal immune system, and immunization at one primary site will stimulate proliferation and migration of antigen-specific lymphocytes, resulting in both mucosal and systemic immunity [57]. Virus neutralizing antibodies play an important role in the clearance of PRRS viremia [58], [59]. Like in natural PRRSV infection, the PRRSV vaccine also induced delayed neutralizing antibody and CMI responses in pigs [60], [61]. However, an increase in the PRRSV-VN titers with a concomitant reduction in PRRSV load in pigs vaccinated with PRRS-MLV+ Mtb WCL and challenged with MN184 was detected in our study. This suggests that reduced viremia detected from vaccinated pigs correlates with increased PRRSV specific VN titers.
Apart from an effective humoral response, a potent CMI response is essential for complete viral clearance. The key cytokine associated with a host CMI response is IFN-γ produced by NK cells, γδ T cells, Th cells, CTLs, and Th/memory cells [62]. In our study, reduced clinical PRRS and viremia in pigs vaccinated with PRRS-MLV+ Mtb WCL were associated with increased frequency of IFN-γ-secreting cells and increased levels of activated Th cells, memory/Th cells, and NK cells in both the lungs and blood. CD4CD8 double positive T cells possess memory, T-helper, and cytolytic properties. They are generally called Th/memory cells, and they also secrete IFN-γ [41], [42]. This important T cell subset was associated with protection in pigs vaccinated against pseudorabies virus [41], [42].
Recently, recombinant BCG based respiratory syncitial virus vaccine induced enhanced recruitment of CD4+ and CD8+ T cells into the lungs resulting in increased Th1 cytokine responses and protection [63]. The in vivo adjuvant effects of the water soluble fraction of Mtb WCL containing HSP70 resulted in rapid and prolonged activation of antigen-specific CD8+ T cells [21]. Consistent with that in our study, Mtb WCL-mediated, increased frequency of CD4+ and CD8+ T cells with reduced frequency of Tregs was detected. Importantly, this was associated with enhanced secretion of Th1 cytokines (IFN-γ) and downregulated secretion of immunosuppressive (IL-10 and TGF-β) cytokines.
The pro-inflammatory cytokine IL-6 is critical for induction of specific adaptive immunity [37], [64], but excess secretion of IL-6 results in inflammation-induced pathology [6]. In our pre-challenge study, increased secretion of IL-6 in the lungs and blood of PRRS-MLV+ Mtb WCL inoculated pigs at DPV 7 and 15 was associated with enhanced PRRSV specific CMI responses [27]. In contrast, in the current study, reduced gross lung lesions observed in vaccinated, MN184 challenged pigs were associated with reduced secretion of IL-6. These data suggest that adjuvant Mtb WCL mediated regulated secretion of IL-6 might play a role in inducing a PRRSV-specific CMI response.
Pigs possess a higher frequency of γδ T cells and these cells are considered to be an important innate immune cell at mucosal sites. In addition, pigs have non-MHC class I cytolytic activity against PRRSV infection [65]. In mucosally vaccinated and PRRSV challenged pigs, increased frequency of γδ T cells in blood, lungs, and TBLN that is mediated by Mtb WCL was detected, suggesting a possible protective role played by γδ T cells to PRRSV MN184 in these pigs.
In summary, mucosal immunization of pigs using a live PRRSV vaccine along with a potent adjuvant has the potential to induce heightened innate and adaptive immune responses, with a concomitant reduction in immunosuppressive responses. Thus, based on immune correlates of protection detected against virulent heterologous PRRSV MN184 in pigs intranasally vaccinated with PRRS-MLV+ Mtb WCL, it may be possible to achieve an effective cross-protective immunity against PPRS. Our future aim will be to conduct field trials to evaluate the efficacy of our mucosal immunization approach to control PRRS.
Acknowledgements
This work was supported by a National Pork Board award (NPB #08-187) and a USDA-NIFA PRRS CAP2 award (2008-55620-19132) to RJG. Salaries and research support were provided by the state and federal funds appropriated to Ohio Agricultural Research and Development Center, The Ohio State University. We would like to thank Drs. Juliette Hanson, Mahesh Khatri, and Hadi Yassine, and Todd Root and Matthew Weeman for their help in animal studies. We would like to thank Drs. Eric Nelson, Mike Roof, Dobos, and Belisle for providing reagents. We also thank Bert Bishop for statistics, and Dr. Michele Williams for editing the manuscript.
Role of the funding source: Sponsors have no role in study design, in the writing of the report,or in the decision to submit the paper for publication.
References
- 1.Neumann E.J., Kliebenstein J.B., Johnson C.D., Mabry J.W., Bush E.J., Seitzinger A.H. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc. 2005;227(August (3)):385–392. doi: 10.2460/javma.2005.227.385. [DOI] [PubMed] [Google Scholar]
- 2.Meulenberg J.J., Hulst M.M., de Meijer E.J., Moonen P.L., den Besten A., de Kluyver E.P. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993;192(January (1)):62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albina E. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet Microbiol. 1997;55(April (1–4)):309–316. doi: 10.1016/s0378-1135(96)01322-3. [DOI] [PubMed] [Google Scholar]
- 4.Benfield D.A., Nelson E., Collins J.E., Harris L., Goyal S.M., Robison D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J Vet Diagn Invest. 1992;4(April (2)):127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- 5.Albina E., Carrat C., Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J Interferon Cytokine Res. 1998;18(July (7)):485–490. doi: 10.1089/jir.1998.18.485. [DOI] [PubMed] [Google Scholar]
- 6.Renukaradhya G.J., Alekseev K., Jung K., Fang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus-induced immunosuppression exacerbates the inflammatory response to porcine respiratory coronavirus in pigs. Viral Immunol. 2010;23(October (5)):457–466. doi: 10.1089/vim.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao Z., Batista L., Dee S., Halbur P., Murtaugh M.P. The level of virus-specific T-cell and macrophage recruitment in porcine reproductive and respiratory syndrome virus infection in pigs is independent of virus load. J Virol. 2004;78(June (11)):5923–5933. doi: 10.1128/JVI.78.11.5923-5933.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim W.I., Lee D.S., Johnson W., Roof M., Cha S.H., Yoon K.J. Effect of genotypic and biotypic differences among PRRS viruses on the serologic assessment of pigs for virus infection. Vet Microbiol. 2007;123(July (1–3)):1–14. doi: 10.1016/j.vetmic.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Martelli P., Gozio S., Ferrari L., Rosina S., De Angelis E., Quintavalla C. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: clinical protection and cell-mediated immunity. Vaccine. 2009;27(June (28)):3788–3799. doi: 10.1016/j.vaccine.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Kimman T.G., Cornelissen L.A., Moormann R.J., Rebel J.M., Stockhofe-Zurwieden N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine. 2009;27(June (28)):3704–3718. doi: 10.1016/j.vaccine.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Mann J.F., Acevedo R., Campo J.D., Perez O., Ferro V.A. Delivery systems: a vaccine strategy for overcoming mucosal tolerance? Expert Rev Vaccine. 2009;8(January (1)):103–112. doi: 10.1586/14760584.8.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Etchart N., Wild F., Kaiserlian D. Mucosal and systemic immune responses to measles virus haemagglutinin in mice immunized with a recombinant vaccinia virus. J Gen Virol. 1996;77(October (Pt 10)):2471–2478. doi: 10.1099/0022-1317-77-10-2471. [DOI] [PubMed] [Google Scholar]
- 13.McMenamin C., Holt P.G. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993;178(September (3)):889–899. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian J., Atkinson M.A., Clare-Salzler M., Herschenfeld A., Forsthuber T., Lehmann P.V. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med. 1996;183(April (4)):1561–1567. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Poel W.H., Kramps J.A., Quak J., Brand A., Van Oirschot J.T. Persistence of bovine herpesvirus-1-specific antibodies in cattle after intranasal vaccination with a live virus vaccine. Vet Rec. 1995;137(September (14)):347–348. doi: 10.1136/vr.137.14.347. [DOI] [PubMed] [Google Scholar]
- 16.Karron R.A., Wright P.F., Hall S.L., Makhene M., Thompson J., Burns B.A. A live attenuated bovine parainfluenza virus type 3 vaccine is safe, infectious, immunogenic, and phenotypically stable in infants and children. J Infect Dis. 1995;171(May (5)):1107–1114. doi: 10.1093/infdis/171.5.1107. [DOI] [PubMed] [Google Scholar]
- 17.Guillonneau C., Mintern J.D., Hubert F.X., Hurt A.C., Besra G.S., Porcelli S. Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc Natl Acad Sci USA. 2009;106(March (9)):3330–3335. doi: 10.1073/pnas.0813309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts P., Jeffery P.K., Mitchell T.J., Andrew P.W., Boulnois G.J., Feldman C. Effect of immunization with Freund's adjuvant and pneumolysin on histologic features of pneumococcal infection in the rat lung in vivo. Infect Immun. 1992;60(November (11)):4969–4972. doi: 10.1128/iai.60.11.4969-4972.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White R.G., Coons A.H., Connolly J.M. Studies on antibody production. IV. The role of a wax fraction of Mycobacterium tuberculosis in adjuvant emulsions on the production of antibody to egg albumin. J Exp Med. 1955;102(July (1)):83–104. doi: 10.1084/jem.102.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bekierkunst A. Acute granulomatous response produced in mice by trehalose-6,6-dimycolate. J Bacteriol. 1968;96(October (4)):958–961. doi: 10.1128/jb.96.4.958-961.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmala L.A., Ingulli E.G., Curtsinger J.M., Lucido M.M., Schmidt C.S., Weigel B.J. The adjuvant effects of Mycobacterium tuberculosis heat shock protein 70 result from the rapid and prolonged activation of antigen-specific CD8+ T cells in vivo. J Immunol. 2002;169(November (10)):5622–5629. doi: 10.4049/jimmunol.169.10.5622. [DOI] [PubMed] [Google Scholar]
- 22.Bekierkunst A., Yarkoni E., Flechner I., Morecki S., Vilkas E., Lederer E. Immune response to sheep red blood cells in mice pretreated with mycobacterial fractions. Infect Immun. 1971;4(September (3)):256–263. doi: 10.1128/iai.4.3.256-263.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner G.H., Maral R., Floch F., Migliore-Samour D., Jolles P. Adjuvant and immunostimulating activities of water-soluble substances extracted from Mycobacterium tuberculosis (var. hominis) Biomedicine. 1975;22(September (5)):440–452. [PubMed] [Google Scholar]
- 24.Srivastava P.K., Udono H., Blachere N.E., Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39(2):93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 25.Bansal K., Elluru S.R., Narayana Y., Chaturvedi R., Patil S.A., Kaveri S.V. PE_PGRS antigens of Mycobacterium tuberculosis induce maturation and activation of human dendritic cells. J Immunol. 2010;184(April (7)):3495–3504. doi: 10.4049/jimmunol.0903299. [DOI] [PubMed] [Google Scholar]
- 26.Choudhary R.K., Mukhopadhyay S., Chakhaiyar P., Sharma N., Murthy K.J., Katoch V.M. PPE antigen Rv2430c of Mycobacterium tuberculosis induces a strong B-cell response. Infect Immun. 2003;71(November (11)):6338–6343. doi: 10.1128/IAI.71.11.6338-6343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dwivedi V., Manickam C., Patterson R., Dodson K., Weeman M., Renukaradhya G.J. Intranasal delivery of whole cell lysate of Mycobacterium tuberculosis induces protective immune responses to a modified live porcine reproductive and respiratory syndrome virus vaccine in pigs. Vaccine. 2011;29(23):4067–4076. doi: 10.1016/j.vaccine.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christopher-Hennings J., Holler L.D., Benfield D.A., Nelson E.A. Detection and duration of porcine reproductive and respiratory syndrome virus in semen, serum, peripheral blood mononuclear cells, and tissues from Yorkshire, Hampshire, and Landrace boars. J Vet Diagn Invest. 2001;13(March (2)):133–142. doi: 10.1177/104063870101300207. [DOI] [PubMed] [Google Scholar]
- 29.VanCott J.L., Brim T.A., Simkins R.A., Saif L.J. Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of suckling pigs. J Immunol. 1993;150(May (9)):3990–4000. [PubMed] [Google Scholar]
- 30.Loving C.L., Brockmeier S.L., Sacco R.E. Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology. 2007;120(February (2)):217–229. doi: 10.1111/j.1365-2567.2006.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khatri M., Dwivedi V., Krakowka S., Manickam C., Ali A., Wang L. Swine influenza H1N1 virus induces acute inflammatory immune responses in pig lungs: a potential animal model for human H1N1 influenza virus. J Virol. 2010;84(November (21)):11210–11218. doi: 10.1128/JVI.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung K., Renukaradhya G.J., Alekseev K.P., Fang Y., Tang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J Gen Virol. 2009;90(November (Pt 11)):2713–2723. doi: 10.1099/vir.0.014001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed L.J., Muench L. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27(3):493–497. [Google Scholar]
- 34.Thanawongnuwech R., Young T.F., Thacker B.J., Thacker E.L. Differential production of proinflammatory cytokines: in vitro PRRSV and Mycoplasma hyopneumoniae co-infection model. Vet Immunol Immunopathol. 2001;79(May (1–2)):115–127. doi: 10.1016/s0165-2427(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 35.Johnson C.R., Yu W., Murtaugh M.P. Cross-reactive antibody responses to nsp1 and nsp2 of Porcine reproductive and respiratory syndrome virus. J Gen Virol. 2007;88(April (Pt 4)):1184–1195. doi: 10.1099/vir.0.82587-0. [DOI] [PubMed] [Google Scholar]
- 36.Baker R.B., Yu W., Fuentes M., Johnson C.R., Peterson L., Rossow K. Prairie dog (Cynomys ludovicianus) is not a host for porcine reproductive and respiratory syndrome virus. J Swine Health Prod. 2007;15:22–29. [Google Scholar]
- 37.Azevedo M.S., Yuan L., Pouly S., Gonzales A.M., Jeong K.I., Nguyen T.V. Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. J Virol. 2006;80(January (1)):372–382. doi: 10.1128/JVI.80.1.372-382.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaser T., Gerner W., Hammer S.E., Patzl M., Saalmuller A. Phenotypic and functional characterisation of porcine CD4(+)CD25(high) regulatory T cells. Vet Immunol Immunopathol. 2008;122(March (1–2)):153–158. doi: 10.1016/j.vetimm.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Kaser T., Gerner W., Hammer S.E., Patzl M., Saalmuller A. Detection of Foxp3 protein expression in porcine T lymphocytes. Vet Immunol Immunopathol. 2008;125(September (1–2)):92–101. doi: 10.1016/j.vetimm.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Gerner W., Kaser T., Saalmuller A. Porcine T lymphocytes and NK cells – an update. Dev Comp Immunol. 2009;33(3):310–320. doi: 10.1016/j.dci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Ober B.T., Summerfield A., Mattlinger C., Wiesmuller K.H., Jung G., Pfaff E. Vaccine-induced, pseudorabies virus-specific, extrathymic CD4+CD8+ memory T-helper cells in swine. J Virol. 1998;72(June (6)):4866–4873. doi: 10.1128/jvi.72.6.4866-4873.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Bruin T.G., Van Rooij E.M., De Visser Y.E., Bianchi A.T. Cytolytic function for pseudorabies virus-stimulated porcine CD4+ CD8dull+ lymphocytes. Viral Immunol. 2000;13(4):511–520. doi: 10.1089/vim.2000.13.511. [DOI] [PubMed] [Google Scholar]
- 43.Johnsen C.K., Botner A., Kamstrup S., Lind P., Nielsen J. Cytokine mRNA profiles in bronchoalveolar cells of piglets experimentally infected in utero with porcine reproductive and respiratory syndrome virus: association of sustained expression of IFN-gamma and IL-10 after viral clearance. Viral Immunol. 2002;15(4):549–556. doi: 10.1089/088282402320914494. [DOI] [PubMed] [Google Scholar]
- 44.Diaz I., Darwich L., Pappaterra G., Pujols J., Mateu E. Immune responses of pigs after experimental infection with a European strain of Porcine reproductive and respiratory syndrome virus. J Gen Virol. 2005;86(July (Pt 7)):1943–1951. doi: 10.1099/vir.0.80959-0. [DOI] [PubMed] [Google Scholar]
- 45.Suradhat S., Thanawongnuwech R., Poovorawan Y. Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J Gen Virol. 2003;84(February (Pt 2)):453–459. doi: 10.1099/vir.0.18698-0. [DOI] [PubMed] [Google Scholar]
- 46.Royaee A.R., Husmann R.J., Dawson H.D., Calzada-Nova G., Schnitzlein W.M., Zuckermann F.A. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet Immunol Immunopathol. 2004;102(December (3)):199–216. doi: 10.1016/j.vetimm.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Didierlaurent A., Goulding J., Hussell T. The impact of successive infections on the lung microenvironment. Immunology. 2007;122(December (4)):457–465. doi: 10.1111/j.1365-2567.2007.02729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinter A.L., Hennessey M., Bell A., Kern S., Lin Y., Daucher M. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200(August (3)):331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng G., Li S., Wu W., Sun Z., Chen Y., Chen Z. Circulating CD4+ CD25+ regulatory T cells correlate with chronic hepatitis B infection. Immunology. 2008;123(January (1)):57–65. doi: 10.1111/j.1365-2567.2007.02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vahlenkamp T.W., Tompkins M.B., Tompkins W.A. The role of CD4+CD25+ regulatory T cells in viral infections. Vet Immunol Immunopathol. 2005;108(October (1–2)):219–225. doi: 10.1016/j.vetimm.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Silva-Campa E., Cordoba L., Fraile L., Flores-Mendoza L., Montoya M., Hernandez J. European genotype of porcine reproductive and respiratory syndrome (PRRSV) infects monocyte-derived dendritic cells but does not induce Treg cells. Virology. 2009;396(January (2)):264–271. doi: 10.1016/j.virol.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 52.Silva-Campa E., Flores-Mendoza L., Resendiz M., Pinelli-Saavedra A., Mata-Haro V., Mwangi W. Induction of T helper 3 regulatory cells by dendritic cells infected with porcine reproductive and respiratory syndrome virus. Virology. 2009;387(May (2)):373–379. doi: 10.1016/j.virol.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 53.Wongyanin P., Buranapraditkun S., Chokeshai-Usaha K., Thanawonguwech R., Suradhat S. Induction of inducible CD4+CD25+Foxp3+ regulatory T lymphocytes by porcine reproductive and respiratory syndrome virus (PRRSV) Vet Immunol Immunopathol. 2010;133(February (2–4)):170–182. doi: 10.1016/j.vetimm.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Read S., Malmstrom V., Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(July (2)):295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakaguchi S., Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21(October (10)):1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 56.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7(November (11)):875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 57.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7(July (4)):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 58.Batista L., Pijoan C., Dee S., Olin M., Molitor T., Joo H.S. Virological and immunological responses to porcine reproductive and respiratory syndrome virus in a large population of gilts. Can J Vet Res. 2004;68(October (4)):267–273. [PMC free article] [PubMed] [Google Scholar]
- 59.Lowe J.E., Husmann R., Firkins L.D., Zuckermann F.A., Goldberg T.L. Correlation of cell-mediated immunity against porcine reproductive and respiratory syndrome virus with protection against reproductive failure in sows during outbreaks of porcine reproductive and respiratory syndrome in commercial herds. J Am Vet Med Assoc. 2005;226(May (10)):1707–1711. doi: 10.2460/javma.2005.226.1707. [DOI] [PubMed] [Google Scholar]
- 60.Charerntantanakul W., Platt R., Johnson W., Roof M., Vaughn E., Roth J.A. Immune responses and protection by vaccine and various vaccine adjuvant candidates to virulent porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2006;109(January (1–2)):99–115. doi: 10.1016/j.vetimm.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 61.Foss D.L., Zilliox M.J., Meier W., Zuckermann F., Murtaugh M.P. Adjuvant danger signals increase the immune response to porcine reproductive and respiratory syndrome virus. Viral Immunol. 2002;15(4):557–566. doi: 10.1089/088282402320914502. [DOI] [PubMed] [Google Scholar]
- 62.Costers S., Lefebvre D.J., Goddeeris B., Delputte P.L., Nauwynck H.J. Functional impairment of PRRSV-specific peripheral CD3+CD8high cells. Vet Res. 2009;40(September–October (5)):46. doi: 10.1051/vetres/2009029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cautivo K.M., Bueno S.M., Cortes C.M., Wozniak A., Riedel C.A., Kalergis A.M. Efficient lung recruitment of respiratory syncytial virus-specific Th1 cells induced by recombinant bacillus Calmette-Guerin promotes virus clearance and protects from infection. J Immunol. 2010;185(December (12)):7633–7645. doi: 10.4049/jimmunol.0903452. [DOI] [PubMed] [Google Scholar]
- 64.Barbe F., Atanasova K., Van Reeth K. Cytokines and acute phase proteins associated with acute swine influenza infection in pigs. Vet J. 2011;187(January (1)):48–53. doi: 10.1016/j.tvjl.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olin M.R., Batista L., Xiao Z., Dee S.A., Murtaugh M.P., Pijoan C.C. Gammadelta lymphocyte response to porcine reproductive and respiratory syndrome virus. Viral Immunol. 2005;18(3):490–499. doi: 10.1089/vim.2005.18.490. [DOI] [PubMed] [Google Scholar]


