Abstract
Aim
To investigate the association between intravitreal ranibizumab therapy and serum cytokine concentrations in patients with diabetic macular edema (DME).
Methods
Twenty-five patients with center-involved DME were recruited prospectively. Serum samples were collected from the patients before and 4 weeks after two ranibizumab injections. The levels of 32 cytokines at these two time points were assessed using a multiplex array assay.
Results
Following two ranibizumab injections, there was a statistically significant decrease in the median [interquartile range] levels of Interleukin 1-1beta (IL-1β) from 5.56 [3.6, 8.75] to 2.33 [1.51, 2.89], Interleukin 13 (IL-13) from 4.30 [1.84, 18.55] to 0.38 [0.38, 0.78], granulocyte-colony stimulating factor (G-CSF) from 64.65 [42.9, 108] to 37.8 [27.3, 46.37], Interferon gamma (IFN-γ) from 241 [103.33, 753.4] to 94.4626 [42.04, 118.58], Interferon gamma-induced protein 10 (IP-10) from 234.68 [144.16, 285.98] to 158.73 [94.71, 198.64], Macrophage Inflammatory Protein-1 alpha (MIP-1α) from 3.65 [2.62, 11.02] to 1.41 [0.94, 1.88], and Tumor necrosis factor- alpha (TNF-α) from 131.09 [100.68,28 240.27] to 45.19 [24.04, 68.55]. There was a statistically significant increase in the levels of Interleukin 9 (IL-9) from 0.76 [0.76, 7.03] to 19.67 [5.36 27.76], Macrophage Inflammatory Protein-1 beta (MIP-1β) from 0.28 [0.28, 30 0.28] to 6.79 [I3.74, 14.16], Vascular endothelial growth factor (VEGF) from 2.55 [2.55, 2.55] to 25.24 [14.51, 41.73], and soluble vascular endothelial growth factor -1 (sVEGFR-1) from 333.92 [204.99, 440.43] to 500.12 [38.7, 786.91]. A Bonferroni-corrected p value of 0.00156 was considered statistically significant.
Conclusions
In patients with DME, intravitreal ranibizumab therapy appears to influence the serum levels of a range of cytokines. After two injections, intravitreal ranibizumab therapy appears to be associated with a significant decrease in inflammatory mediators and a rise in VEGF and sVEGFR1.
Introduction
Diabetic retinopathy (DR) is a microvascular complication of diabetes mellitus. DME is the major cause of central vision loss in DR and results from leakage of intravascular fluid from retinal microaneurysms and capillaries into the macula. The breakdown of the blood–retinal barrier (BRB) leads to the accumulation of plasma and lipids in the intraretinal and subretinal space [1]. DME results from retinal microvascular changes secondary to endothelial cell dysfunction, thickening of the retinal capillary basement membrane, and reduction in the number of the surrounding pericytes leading to increased permeability and incompetence of the retinal vasculature. Although the mechanism for these retinal changes remains poorly understood, there is increasing evidence of a possible role for inflammation in the pathogenesis of diabetic retinopathy [2-4]. Although vascular endothelial growth factor (VEGF) upregulation is important in increasing intraretinal vascular permeability, it has increasingly been shown that non-VEGF-dependent inflammatory pathways also play a crucial role. VEGF and inflammatory pathways are also intimately connected with increased expression of VEGF leading to upregulation of inflammatory mediators, such as TNF-α, IL-6, and IL-1β. Similarly, upregulation of proinflammatory cytokines in the diabetic environment may lead to leukostasis and subsequent hypoxia promoting expression of VEGF [5,6].
In keeping with this, several groups have found elevation in several inflammation-associated cytokines (IL-6, IL-8, interferon-induced protein [IP]-10, monocyte chemoattractant protein-1 (MCP-1), and platelet-derived growth factor [PDGF]-AA) in addition to VEGF in the plasma and aqueous of eyes with proliferative and severe non-proliferative diabetic retinopathy compared to control eyes without diabetes [3,7].
Ranibizumab is a monoclonal antibody that inhibits human vascular endothelial growth factor A (VEGF-A) through binding, thus preventing activation of vascular endothelial growth factor receptors (VEGFR-1 and VEGFR-2). In several studies, intravitreal injections of anti-VEGF agents have been shown to reduce DME [8-11]. The exact mechanisms for this response to treatment remain unknown. Although VEGF inhibition is expected to reduce intraocular VEGF levels, whether this is the only mechanism for DME reduction or whether there are more widespread changes in the intraocular as well as systemic cytokine milieu is not clear.
Additionally, although the plasma half-life of ranibizumab is approximately 2 h [12] there have been concerns regarding the increased risk of arterial thromboembolic events with all anti-VEGF drugs that include ranibizumab treatment, and some have attributed this to the potential systemic effects of anti-VEGF therapy [13]. To further elucidate the changes in VEGF, as well as inflammation-associated cytokines in the serum, in this study, we investigated the serum cytokine profiles of patients with DME following two doses of intravitreal ranibizumab.
Methods
Patients with center-involved DME were recruited from the Royal Victorian Eye and Ear Hospital (Melbourne, Australia) between September 2015 and May 2016. This study was approved by the Human Research and Ethics Committee of the Royal Victorian Eye and Ear Hospital (RVEEH) (approval number 13/ 1123H) as part of the DIabetic macular edema: aqueous and Serum Cytokine profiling to determine the Efficacy of RaNibizumab (DISCERN) Study. Research adhered to the tenets of the Declaration of Helsinki and adhered to the Association of Research and Vision in Ophthalmology (ARVO) statement on human subjects.
Patients
Patients with center-involved DME requiring anti-VEGF therapy were recruited prospectively. A full list of inclusion and exclusion criteria can be found in Appendix 1. At baseline and each visit thereafter, all patients underwent a complete ophthalmic examination that included best-corrected visual acuity (BCVA) letters using an Early Treatment Diabetic Retinography Study (ETDRS) chart, intraocular pressure (IOP) measurements, slit-lamp examination, and optical coherence tomography (OCT). Fast macular thickness scans and 6-mm cross-hair scans were obtained using Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany). Central macular thickness (CMT) was determined automatically and analyzed with OCT software. In all OCT maps, automated macular thickness detection was performed by the instrument’s software analysis without manual operator adjustment.
Visits and treatment schedule
All study participants received an intravitreal ranibizumab (Novartis, Novartis Pharmaceuticals, Sydney, Australia) 0.5 mg/0.05 ml injection 3.5–4 mm from the corneal limbus at baseline, 1 month (+/− 7 days), and 2 months (+/− 7 days). Injections did not occur fewer than 21 days apart. An ophthalmologist performed all injections under sterile conditions using a 30-gauge needle and a prefilled syringe.
Serum sampling
Serum samples were taken at baseline and at 8 weeks (immediately before the third injection of ranibizumab) to assess response to treatment. Samples were collected from a peripheral vein in two 8-ml serum tubes. These tubes were gently inverted for good mixing and sat upright for 30 min before centrifuging. The centrifuge was run at 1000 ×g for 10 min at 12 °C. One-thousand-microliter tips were used to transfer 750 μl serum samples into Axygen (San Francisco, CA) tubes that were then stored at −80 °C until assayed.
Serum processing and analysis
The levels of 32 analytes were measured using commercially available Luminex systems from Bio-Rad Laboratories Ltd. (Hercules, CA) and Millipore (Billerica, MA) on the Bio-Plex 200 System. Initial data analysis was undertaken using Bio-Plex Manager 5.0 software to determine concentrations. Serially diluted standards (50 μl) and test serum, diluted 1 in 4 in sample diluent (50 μl), were added to a plate containing magnetic antibody-coupled beads for each of the 32 analytes. The samples were incubated in replicates of four at room temperature on a plate shaker at 300 rpm for 30 min. Following washing with the Bio-Plex Washing buffer, the secondary detection antibodies (25 μl) were added to the plate and incubated as previously. The plate was washed again, streptavidin-phycoerythrin (streptavidin-PE; 50 μl) was added, and the plate incubated at room temperature on a plate shaker at 300 rpm for 10 min. Assay buffer (125 μl) was added to each well of the plate before analysis on the Bio-Plex 200 machine. Fluorescent intensities obtained for the test samples were read from the standard curve to give picograms per milliliter (pg/ml) values for each of the 32 analytes. Values below the reference range were assigned the value of the lowest standard.
Statistical analysis
At the first stage of the analysis, changes in serum cytokine levels from baseline to month 2 were summarized as medians with interquartile ranges (IQRs), and the hypothesis of no change was tested using the Wilcoxon signed-rank test. Due to the multiplicity of testing where 32 individual analytes were screened, a Bonferroni-corrected threshold value for Type I error of 0.00156=0.05/32 was used as indicative of statistical significance.
At the second stage of the analysis, a correlation between the change in the VEGF concentration and changes in the other analytes identified as exhibiting statistically significant change from baseline to month 2 at the first stage of the analysis was estimated using Spearman’s rank correlation coefficient. Due to the multiplicity of testing where ten individual analytes were investigated, a Bonferroni-corrected threshold value for Type I error of 0.005=0.05/10 was used as indicative of statistical significance. Statistical analysis was conducted using Stata v14IC (StataCorp, College Station, TX) statistical software.
Results
As part of the DISCERN study, 25 patients were recruited with center-involved DME. In this sub-analysis looking at the changes in cytokine concentrations following ranibizumab treatment, to eliminate any potential confounding effect on the serum concentrations of VEGF and other cytokines by both eyes being treated, only the first recruited eye was considered eligible. Therefore, a total of 25 eyes from 25 patients were included. Baseline characteristics of the study participants are shown in Table 1. The mean (standard deviation) patient age was 63.4 (9.0) years, with a mean BCVA of 59.3 (10.3) letters (range 28 to 70 letters). In the 25 study eyes, 23 (92.0%) had non-proliferative diabetic retinopathy (NPDR), and two (8.00%) had PDR, both of which regressed after pan-retinal photocoagulation. Seven eyes (28.0%) had a previous intravitreal anti-VEGF injection at a mean of 18 (range 4–60) months before entry into the study, and one had previously received intravitreal corticosteroids 6 years before entry into the study. At baseline, none of the cytokines measured in the serum were associated with baseline BCVA, central macular thickness, or Hemoglobin A1c (HbA1c; p>0.10).
Table 1. Baseline clinical characteristics of the patients with diabetic macular edema.
| Characteristic | Values |
|---|---|
| Number of patients |
25 |
| Gender, male/ female (%) |
18/7 (72.0/ 28.0) |
| Mean age of patients, years, mean (±SD) |
63.8 (9.5) |
| Glycated hemoglobin, %, mean (±SD) |
7.54 (1.19) |
| Treatment |
|
| Oral hypoglycaemic agent, n (%) |
10 (40.0) |
| Insulin, n (%) |
1 (4.0) |
| Oral hypoglycaemic agent plus insulin, n (%) |
14 (56.0) |
| Hypertension, n (%) |
19 (76.0) |
| Dyslipidaemia, n (%) |
23 (92.0) |
| Duration of diabetes |
|
| <5 years, n (%) |
4 (13.3) |
| 5 to 10 years, n (%) |
4 (13.3) |
| 10 to 15 years, n (%) |
5 (16.7) |
| >15 years, n (%) |
17 (56.7) |
| BCVA (logMAR), median (IQR) |
61.5 (55 – 67) |
| CMT, µm (mean ± SD) |
484.5 (134.3) |
| Phakic lens status, n (%) |
21 (84.0) |
| Stage of diabetic retinopathy, n (%) |
|
| Mild NPDR |
0 (0.0) |
| Moderate NPDR |
13 (52.0) |
| Severe NPDR |
10 (40.0) |
| Proliferative DR |
2 (8.0) |
| History of focal photocoagulation, n (%) |
8 (32.0) |
| History of panretinal photocoagulation, n (%) |
6 (24.0) |
| History of intravitreal anti-VEGF, n (%) |
7 (28.0) |
| History of intravitreal corticosteroids, n (%) | 1 (4) |
BCVA=best corrected visual acuity, CMT=central macular thickness, NPDR=non-proliferative diabetic retinopathy, DR=diabetic retinopathy, anti-VEGF=anti-vascular endothelial growth factor, n=number, SD=standard deviation
A statistically significant improvement in the number of letters read on the logarithm of the minimal angle of resolution (LogMAR) chart following two intravitreal ranibizumab injections of +7.4 letters was noted (95% confidence interval [CI] 4.9 to 9.9, paired t test, p<0.0001; range, −4 to +21 letters, SD +6.67 letters. Similarly, there was a statistically significant reduction in the CMT of −93.5 μm (95% CI 47.6 to 139.5, paired t test, p=0.0003) over the same period.
Changes in serum cytokine levels from baseline to month 2
Baseline cytokine concentrations were compared to cytokine concentrations after two doses of intravitreal ranibizumab. Only cytokines with measurable concentrations were included in the analysis. Thus, Interleukin 2 (IL-2), Interleukin 15 (IL-15), Interleukin 17 (IL-17), and Granulocyte-macrophage colony-stimulating factor (GM-CSF) were excluded due to undetectable levels with the serum at baseline and at month 2. No statistically significant changes following intravitreal ranibizumab treatment were identified in the following cytokines (using Bonferroni-corrected threshold for Type 1 error p=0.00156); Interleukin 1-ra (IL-1ra), Interleukin 4 (IL-4), Interleukin 5 (IL-5), Interleukin 6 (IL-6), Interleukin 7 (IL-7), Interleukin 8 (IL-8), Interleukin 10 (IL-10), Interleukin 12 (IL-12), eotaxin, fibroblastic growth factor-beta (FGFb), macrophage inflammatory protein-1 alpha (MCP-1α), platelet derived growth factor- beta (PDGFB), Regulated on Activation Normal T Cell Expressed and Secreted (RANTES), erythropoietin (EPO), soluble vascular endothelial growth factor receptor 2 (sVEGR-2), epidermal growth factor (EGF), and angiopoietin 2 (ANG-2). (Table 2). Of the remaining cytokines, seven showed a statistically significant decrease, and four showed a statistically significant increase in the serum concentration following ranibizumab treatment.
Table 2. Changes in serum concentrations (pg/ml) of inflammatory and angiogenic cytokines after intravitreal ranibizumab in diabetic macular edema.
| Biomarkers | Pre-injection, median (IQR) | Post-injection, median (IQR) | Change, median (IQR) | Match-pairs signed rank test, p value |
|---|---|---|---|---|
| IL-1β |
5.56 (3.6, 8.75) |
2.33 (1.51, 2.89) |
−3.6 (−6.67, −1.05) |
<0.0001* |
| IL-1ra |
42.49 (5.44, 154.44) |
66.9 (29.42, 81.42) |
0 (−134.33, 46.4) |
0.451 |
| IL-2 |
0.91 (0.91, 0.91) |
0.91 (0.91, 0.91) |
0 (0, 0) |
undefined |
| IL-4 |
4.09 (3.29, 5.42) |
4.29 (1.83, 4.98) |
−0.68 (−3.46, 1.41) |
0.093 |
| IL-5 |
2.81 (0.82, 24.43) |
2.87 (0.82, 5.91) |
0 (−21.56, 0.52) |
0.278 |
| IL-6 |
1.58 (0.88, 5.36) |
2.61 (1.75, 3.94) |
0.76 (−0.78, 2.36) |
0.389 |
| IL-7 |
4.12 (0.64, 12.34) |
1.65 (0.64, 6.6) |
0 (−7.38, 1.21) |
0.202 |
| IL-8 |
13.91 (7.27, 32.45) |
15.39 (10.43, 17.93) |
2.54 (−16.54, 5.04) |
0.696 |
| IL-9 |
0.76 (0.76, 7.03) |
19.67 (5.36, 27.76) |
18.76 (2.33, 24.33) |
0.0003* |
| IL-10 |
2.62 (2.62, 10.21) |
6.9 (4.06, 8.17) |
3.04 (−0.1, 4.57) |
0.242 |
| IL-12 |
2.22 (2.22, 2.22) |
2.33 (2.1, 3.34) |
0 (−0.49, 1.02) |
0.58 |
| IL-13 |
4.30(1.84, 18.55) |
0.38 (0.38, 0.78) |
−2.16 (−18.17, −1.32) |
<0.0001* |
| IL-15 |
1.91 (1.91, 1.91) |
1.91 (1.91, 1.91) |
0 (0, 0) |
undefined |
| IL-17 |
2.27 (2.27, 2.27) |
2.27 (2.27, 2.27) |
0 (0, 0) |
undefined |
| Eotaxin |
187.06 (114.66, 342. 6) |
119.68 (70.47, 207.41) |
−19.24 (−96.47, 4.2) |
0.0058 |
| FGFb |
0.6 (0.6, 0.6) |
0.6 (0.6, 0.6) |
0 (0, 0) |
0.157 |
| G-CSF |
64.65 (42.9, 108) |
37.8 (27.3, 46.37) |
−41.17 (−72.56, −10.54) |
0.0001* |
| GM-CSF |
0.67 (0.67, 0.67) |
0.67 (0.67, 0.67) |
0 (0, 0) |
undefined |
| IFNγ |
241 (103.33, 753.4) |
94.46 (42.04, 118.58) |
−192.06 (−668.51, −29.83) |
0.0001* |
| IP-10 |
234.68 (144.16, 285.98) |
158.73 (94.71, 198.64) |
−39.68 (−138.39, −16.81) |
0.0003* |
| MCP-1 |
1.62 (1.62, 1.62) |
15.23 (4.55, 23.84) |
12.54 (−1.62, 22.22) |
0.563 |
| MIP-1α |
3.65 (2.62, 11.02) |
1.41 (0.94, 1.88) |
−2.54 (−10.55, −1.09) |
<0.0001* |
| MIP-1β |
0.28 (0.28, 0.28) |
6.79 (3.74, 14.16) |
6.08 (2.51, 13.1) |
0.0003* |
| PDGFB |
276.25 (161.28, 375.51) |
296.35 (105.09, 465.09) |
29.07 (−76.18, 148.58) |
0.242 |
| RANTES |
303.62 (228.61, 355.61) |
352 (148.03, 512.27) |
99.73 (−140.96, 181.95) |
0.276 |
| TNF-α |
131.09 (100.68, 240.27) |
45.19 (24.04, 68.55) |
−73.44 (−175.77, −49.17) |
<0.0001* |
| VEGF |
2.55 (2.55, 2.55) |
25.24 (14.51, 41.73) |
22.56 (10.93, 38.66) |
0.0001* |
| EPO |
0 (0, 0) |
0 (0, 2687.73) |
0 (0, 0) |
0.699 |
| SVEGFR-1 |
333.92 (204.99, 440.43) |
500.12 (338.7, 786.91) |
214.76 (53.59, 371.86) |
0.0016* |
| SVEGFR-2 |
6695 (6060.22, 8495.57) |
8674.84 (7364.98, 10,228.7) |
1690.28 (−129.55, 2437.81) |
0.156 |
| EGF |
79.59 (47.38, 125.12) |
64.57 (36.78, 132.55) |
12.04 (−52.42, 43.06) |
0.599 |
| ANG-2 | 0 (0, 0) | 0 (0, 0) | 0 (0,0) | 0.597 |
Decreased and increased concentrations
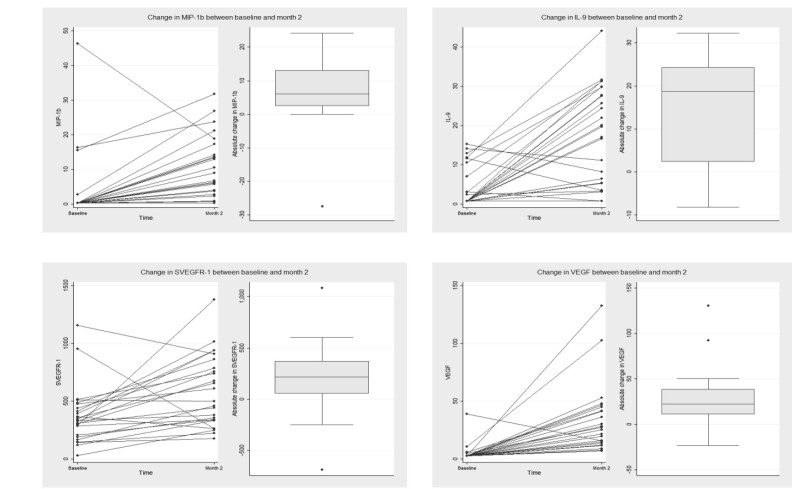
Levels of IL-1β (−3.60 [IQR −6.67, −1.05], p<0.0001), IL-13 (−2.16 [IQR −18.17, −1.32], p<0.0001), G-CSF (−41.17 [IQR −72.56.68, −10.54], p=0.0001), IFN-γ (−192.06 [IQR −668.51, −29.83], p=0.0001), IP-10 (−39.68 [IQR −138.39, −16.81], p=0.0003), MIP-1α (−2.54 [IQR −10.55, −1.09], p<0.0001), and TNF-α (−73.44 [IQR –175.77, −49.17], p<0.0001) were statistically significantly reduced (Figure 1). Levels of IL-9 (+18.76 [IQR +2.33, +24.33] p=0.0003), MIP-1β (+6.08 [IQR +2.51, +13.1], p=0.0003), VEGF (+22.56 [IQR +10.93, +38.66], p=0.0001), and sVEGFR-1 (+214.76 [IQR +53.59, +371.86], p=0.0016) were statistically significantly increased (Figure 2). Of the serum cytokines observed to change statistically significantly from baseline to month 2, only changes in the serum concentrations of IL-9 (rho=0.6141, p=0.0011) were statistically significantly correlated with the change in the VEGF concentration (Table 3).
Figure 1.
Serum cytokine concentrations inflammatory and angiogenic cytokines before and after two intravitreal ranibizumab injections in eyes with diabetic macular edema.
Figure 2.
Serum cytokine concentrations of inflammatory and angiogenic cytokines before and after two intravitreal ranibizumab injections in eyes with diabetic macular edema.
Table 3. Correlation between changes in serum cytokines with change in serum vascular endothelial growth factor following two ranibizumab injections.
| Cytokines | VEGF Correlation (Spearman rho) | P value |
|---|---|---|
| IL-1β |
0.2916 |
0.1572 |
| IL-9 |
0.6141 |
0.0011 |
| IL-13 |
0.1625 |
0.4376 |
| G-CSF |
0.306 |
0.1368 |
| IFNγ |
0.2441 |
0.2397 |
| IP-10 |
0.0248 |
0.9065 |
| MIP-1α |
0.0467 |
0.8247 |
| MIP-1β |
0.1479 |
0.4804 |
| TNF-α |
0.2351 |
0.2579 |
| SVEGFR-1 | −0.2897 | 0.1602 |
Discussion
This is the first study to show an association between intravitreal ranibizumab therapy for DME and serum cytokine levels. After two intravitreal ranibizumab injections, reductions in the levels of circulating cytokines IL-1β, IL-13, G-CSF, IFN-γ, IP-10, MIP-1α, and TNF-α were measured, with increased levels recorded for IL-9, MIP-1b, VEGF, and sVEGFR-1, after correcting for the multiple comparisons.
The findings suggest that there may be diffusion of ranibizumab across the neural retina into the choroidal and systemic circulation after injection [14]. The vitreous half-life of ranibizumab is estimated to be 9 days, but the systemic clearance of ranibizumab is only 2 h [12] as its absence of an fragment crystallizable (Fc) domain allows it to avoid neonatal Fc receptor (FcRN) receptors on endothelial cells, and thus, Fc recycling [12]. The present results suggest that despite this short systemic half-life, there are longer-lasting effects triggered by ranibizumab therapy that result in longer-lasting changes in systemic cytokine profiles.
Inflammation has long been considered to be central to type 2 diabetes mellitus [15], and inflammatory cytokines are elevated in the vitreous, aqueous, and plasma of patients with DR and DME [7,16,17]. IL-1β, IFN-γ, IP-10, MIP-1α, and TNF-α are potent inflammatory meditators important for cell recruitment and inflammation [18-20]. These cytokines were statistically significantly reduced in the serum following two, monthly intravitreal ranibizumab injections. Similarly, G-CSF and IL-13, also statistically significantly decreased following intravitreal ranibizumab injections, play key roles in the inflammatory processes involved in rheumatoid arthritis and allergic asthma, respectively [21,22].
In addition to the reduced concentrations of certain cytokines, we found that at 28 days after two consecutive monthly intravitreal ranibizumab treatments IL-9, MIP-1b, VEGF, and sVEGFR-1 were elevated in the serum. There is evidence to support an important role for IL-9 in inflammation resolution [23], and MIP-1b is a known potent chemoattractant [24]. IL-9 has also previously been shown to promote VEGF secretion from mast cells [25], and may explain the strong correlation between IL-9 and VEGF that we observed in this study.
In contrast to these results, previous studies that looked at plasma or serum VEGF levels following intravitreal ranibizumab found no statistically significant change at 7 days and 1 month follow-up [26-28]. Most studies looked at patients with age-related macular degeneration, although one study had multiple arms, one of which looked at patients with DME [29]. In ten patients with DME, no statistically significant change in VEGF levels was found at 1 week and 1 month follow-up [29]. In another larger trial assessing plasma VEGF in patients with treated with anti-VEGF therapy (bevacizumab, ranibizumab, and aflibercept) for DME found that the median plasma VEGF concentration increased by +0.20 pg/ml (IQR −5.00 to +4.90) 4 weeks after the first injection of ranibizumab [30]. Likewise at 52 weeks, following a pro re nata injection strategy, a similar increase (+0.20 pg/ml, IQR −5.20, +3.80) in plasma VEGF was observed, and at 104 weeks, plasma VEGF increased by +0.14 pg/ml (IQR −9.54, +5.45). Conversely, the mean plasma VEGF levels decreased at 4 weeks in the patients treated with ranibizumab (−7.12 pg/ml) but subsequently increased by +1.43 pg/ml and +6.51 pg/ml at 52 and 104 weeks, respectively. In contrast, the mean and median plasma VEGF concentrations were reduced in patients treated with bevacizumab and aflibercept at all time periods. This difference was greater for bevacizumab compared to aflibercept.
The patients in the present study received two injections, and the time point for serum analysis was 28 days following the second ranibizumab injection. Thus, it is possible that the difference between the present results and previous work may reflect the possibility that a single treatment may not alter systemic VEGF levels, while multiple treatments may trigger signaling cascades that lead to cytokine changes [31].
Interestingly, we previously reported in the same cohort of 25 patients that following ranibizumab treatment, there was a statistically significant reduction in inflammation-associated cytokines as well as VEGF within the aqueous. The rise in serum VEGF is thus counterintuitive; however, increases in serum and plasma VEGF with anti-VEGF therapy have previously been reported [32-34]. Shao and associates reported multiphasic changes in total and free plasma VEGF in a child who received intravitreal ranibizumab injections for choroidal neovascularization [32]. They found a 30% increase in circulating VEGF following one injection, a return to baseline, and then a rebound 67% increase following a further two injections [32]. Similarly, in patients with metastatic renal cancer, Yang and coworkers found that intravenous administration of bevacizumab led to an increase in plasma VEGF and hypothesized that this may be the result of decreased clearance of VEGF following anti-VEGF binding of VEGF [34]. In the cancer literature, other groups have suggested that the increase in serum VEGF in patients with advanced cancer may be due to increased synthesis of VEGF in response to anti-VEGF therapy [35]. It has also been suggested that in response to anti-VEGF therapy there may be a redistribution of VEGF from tissue into the circulation [33,36,37]. Through binding VEGF, sVEGFR1 acts as a negative regulator of VEGF-induced angiogenesis and edema [38]. The upregulation of serum sVEGFR may be a compensatory response to the elevation in VEGF.
We also found that there was a strong correlation between the rise in serum VEGF and the rise in serum IL-9 levels. IL-9 has previously been shown to promote VEGF secretion from mast cells, and may represent one possible explanation for this correlation [25]. The multiplex serum analysis technique used in the present study measured only free serum VEGF, and thus, we postulate a feedback mechanism or signaling cascade in response to effective VEGF blockade is increasing VEGF production or redistributing VEGF into the systemic circulation from tissue. In a pharmacokinetic model, Stefanini et al. suggested that the anti-VEGF/VEGF complex intravasates and subsequently, dissociates leading to increases in the systemic unbound VEGF [32].
The finding of increased serum VEGF following intravitreal anti-VEGF therapy has important implications. Anti-VEGF therapy has been postulated to increase the risk of systemic arterial thromboembolic events (ATEs), and this has been hypothesized to be related to systemic inhibition of VEGF following redistribution of drug from the vitreous cavity into the choroidal vasculature and thus, the systemic circulation [39]. The present results suggest that although it is likely that ranibizumab enters the circulation and reduces concentrations of inflammatory cytokines within the serum, it does not reduce serum VEGF levels, and thus, the potentially increased risk of ATEs is unlikely to relate to systemic VEGF inhibition, at least in the short term. The longer-term effects of intravitreal ranibizumab therapy are uncertain, however.
This study has several limitations. First, as we did not have a control group we cannot claim the causality of the observed association and comment on the comparisons to serum cytokine changes or variations in patients with DME not receiving intravitreal ranibizumab. Second, we measured serum cytokine levels only before and 28 days after two intravitreal injections. There is some evidence that ranibizumab has varying effects on circulating VEGF [32]; thus, the timing and frequency of injections may be important. Third, there is some diurnal variation in the levels of circulating cytokines, particularly plasma concentrations of cytokines influenced by circulating cortisol [40,41]. In this study, we collected serum samples at the same time of day, the afternoon. This reduced the impact of diurnal effects on the results [41]. Serum cytokine concentrations are also likely to be influenced by several systemic factors, including HbA1c and the severity of diabetic retinopathy [3,42]. Given our small sample size, a statistical adjustment through a regression model that accounts for these influences was not possible. Finally, we used multiplex array assays to measure the serum levels of various cytokines. Where levels were below the detectable reference range, the sample was assigned the value of the lowest standard.
In conclusion, intravitreal injections of ranibizumab appear to be associated with changes in a range of serum cytokines in patients with DME, several inflammation-associated cytokines, and VEGF. The VEGF results are intriguing but consistent with previous results [32-34]. The effects on serum cytokines associated with long-term ranibizumab treatment are uncertain, and ultimately, larger controlled studies are needed to establish causality and to investigate whether the serum changes seen in this study are maintained.
Acknowledgments
Funding sources: This study was supported by a grant from Novartis Pharma AG. SW (GNT 1128343) and LLL (GNT 1109330) are supported by a National Health and Medical Research Council (NHMRC) Early Career Fellowships. The Centre for Eye Research Australia receives Operational Infrastructure Support from the Victorian Government.
Appendix 1. DISCERN Study inclusion and exclusion criteria.
To access the data, click or select the words “Appendix 1.”
References
- 1.Das A, McGuire PG, Rangasamy S. Diabetic Macular Edema: Pathophysiology and Novel Therapeutic Targets. Ophthalmology. 2015;122:1375–94. doi: 10.1016/j.ophtha.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–2. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 3.Meleth AD, Agron E, Chan CC, Reed GF, Arora K, Byrnes G, Csaky KG, Ferris FL, 3rd, Chew EY. Serum inflammatory markers in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46:4295–301. doi: 10.1167/iovs.04-1057. [DOI] [PubMed] [Google Scholar]
- 4.van Hecke MV, Dekker JM, Nijpels G, Moll AC, Heine RJ, Bouter LM, Polak BC, Stehouwer CD. Inflammation and endothelial dysfunction are associated with retinopathy: the Hoorn Study. Diabetologia. 2005;48:1300–6. doi: 10.1007/s00125-005-1799-y. [DOI] [PubMed] [Google Scholar]
- 5.dell’Omo R, Semeraro F, Bamonte G, Cifariello F, Romano MR, Costagliola C. Vitreous mediators in retinal hypoxic diseases. Mediators Inflamm. 2013;2013:935301. doi: 10.1155/2013/935301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costagliola C, Romano V, De Tollis M, Aceto F, dell’Omo R, Romano MR, Pedicino C, Semeraro F. TNF-alpha levels in tears: a novel biomarker to assess the degree of diabetic retinopathy. Mediators Inflamm. 2013;2013:935301. doi: 10.1155/2013/629529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maier R, Weger M, Haller-Schober EM, El-Shabrawi Y, Wedrich A, Theisl A, Aigner R, Barth A, Haas A. Multiplex bead analysis of vitreous and serum concentrations of inflammatory and proangiogenic factors in diabetic patients. Mol Vis. 2008;14:637–43. [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS, RISE and RIDE Research Group Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A, RESTORE study group The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Do DV, Nguyen QD, Vitti R, Berliner AJ, Gibson A, Saroj N, Soo Y, Boyer DS. Intravitreal Aflibercept Injection in Diabetic Macular Edema Patients with and without Prior Anti-Vascular Endothelial Growth Factor Treatment: Outcomes from the Phase 3 Program. Ophthalmology. 2016;123:850–7. doi: 10.1016/j.ophtha.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, Brucker AJ, Ferris FL, Hampton GR, Jhaveri C, Melia M, Beck RW. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. 2016;123:1351–9. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Lu T, Tuomi L, Jumbe N, Lu J, Eppler S, Kuebler P, Damico-Beyer LA, Joshi A. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci. 2013;54:1616–24. doi: 10.1167/iovs.12-10260. [DOI] [PubMed] [Google Scholar]
- 13.Rogers CA, Scott LJ, Reeves BC, Downes S, Lotery AJ, Dick AD, Chakravarthy U. Serum Vascular Endothelial Growth Factor Levels in the IVAN Trial; Relationships with Drug, Dosing, and Systemic Serious Adverse Events. Ophthalmology Retina. 2018;2:118–27. doi: 10.1016/j.oret.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mordenti J, Cuthbertson RA, Ferrara N, Thomsen K, Berleau L, Licko V, Allen PC, Valverde CR, Meng YG, Fei DT, Fourre KM, Ryan AM. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999;27:536–44. doi: 10.1177/019262339902700507. [DOI] [PubMed] [Google Scholar]
- 15.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas JB, Jonas RA, Neumaier M, Findeisen P. Cytokine concentration in aqueous humor of eyes with diabetic macular edema. Retina. 2012;32:2150–7. doi: 10.1097/IAE.0b013e3182576d07. [DOI] [PubMed] [Google Scholar]
- 17.Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86:363–5. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–6. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 20.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 21.Eyles JL, Hickey MJ, Norman MU, Croker BA, Roberts AW, Drake SF, James WG, Metcalf D, Campbell IK, Wicks IP. A key role for G-CSF–induced neutrophil production and trafficking during inflammatory arthritis. Blood. 2008;112:5193–201. doi: 10.1182/blood-2008-02-139535. [DOI] [PubMed] [Google Scholar]
- 22.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. •••;98:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 23.Rauber S, Luber M, Weber S, Maul L, Soare A, Wohlfahrt T, Lin NY, Dietel K, Bozec A, Herrmann M, Kaplan MH, Weigmann B, Zaiss MM, Fearon U, Veale DJ. [5]., Cañete JD, Distler O, Rivellese F, Pitzalis C, Neurath MF, McKenzie ANJ, Wirtz S, Schett G, Distler JHW, Ramming A. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med. 2017;23:938–44. doi: 10.1038/nm.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–81. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 25.Sismanopoulos N, Delivanis DA, Alysandratos KD, Angelidou A, Vasiadi M, Therianou A, Theoharides TC. IL-9 Induces VEGF Secretion from Human Mast Cells and IL-9/IL-9 Receptor Genes Are Overexpressed in Atopic Dermatitis. PLoS One. 2012;7:e33271. doi: 10.1371/journal.pone.0033271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zehetner C, Kralinger MT, Modi YS, Waltl I, Ulmer H, Kirchmair R, Bechrakis NE, Kieselbach GF. Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: a randomised, prospective trial. Acta Ophthalmol. 2015;93:e154–9. doi: 10.1111/aos.12604. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Sawada T, Sawada O, Saishin Y, Liu P, Ohji M. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol. 2014;158:738–44.e1. doi: 10.1016/j.ajo.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Jin E, Bai Y, Luo L, Huang L, Zhu X, Ding X, Qi H, Zhao M. Serum levels of vascular endothelial growth factor before and after intravitreal injection of ranibizumab or conbercept for neovascular age-related macular degeneration. Retina. 2017;37:971–7. doi: 10.1097/IAE.0000000000001274. [DOI] [PubMed] [Google Scholar]
- 29.Zehetner C, Kirchmair R, Huber S, Kralinger MT, Kieselbach GF. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol. 2013;97:454–9. doi: 10.1136/bjophthalmol-2012-302451. [DOI] [PubMed] [Google Scholar]
- 30.Jampol LM, Glassman AR, Liu D, Aiello LP, Bressler NM, Duh EJ, Quaggin S, Wells JA, Wykoff CC. Plasma Vascular Endothelial Growth Factor Concentrations after Intravitreous Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema. Ophthalmology. 2018;125:1054–63. doi: 10.1016/j.ophtha.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillier RJ, Ojaimi E, Wong DT, Mak MYK, Berger AR, Kohly RP, Kertes PJ, Forooghian F, Boyd SR, Eng K, Altomare F, Giavedoni LR, Nisenbaum R, Muni RH. Aqueous Humor Cytokine Levels and Anatomic Response to Intravitreal Ranibizumab in Diabetic Macular Edema. JAMA Ophthalmol. 2018;136:382–8. doi: 10.1001/jamaophthalmol.2018.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao EH, Sivagnanavel V, Dabbagh A, Dave R, Tempest-Roe S, Tam FW, Taylor SR. Multiphasic changes in systemic VEGF following intravitreal injections of ranibizumab in a child. Eye (Lond) 2015;29:569–73. doi: 10.1038/eye.2014.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefanini MO, Wu FT, Mac Gabhann F, Popel AS. Increase of plasma VEGF after intravenous administration of bevacizumab is predicted by a pharmacokinetic model. Cancer Res. 2010;70:9886–94. doi: 10.1158/0008-5472.CAN-10-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon MS, Margolin K, Talpaz M, Sledge GW, Jr, Holmgren E, Benjamin R, Stalter S, Shak S, Adelman D. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–50. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 36.Bocci G, Man S, Green SK, Francia G, Ebos JM, du Manoir JM, Weinerman A, Emmenegger U, Ma L, Thorpe P, Davidoff A, Huber J, Hicklin DJ, Kerbel RS. Increased Plasma Vascular Endothelial Growth Factor (VEGF) as a Surrogate Marker for Optimal Therapeutic Dosing of VEGF Receptor-2 Monoclonal Antibodies. Cancer Res. 2004;64:6616. doi: 10.1158/0008-5472.CAN-04-0401. [DOI] [PubMed] [Google Scholar]
- 37.Yang JC. Bevacizumab for Patients with Metastatic Renal Cancer. An Update. Clin Cancer Res. 2004;10:6367S–70S. doi: 10.1158/1078-0432.CCR-050006. [DOI] [PubMed] [Google Scholar]
- 38.Olsson A-K, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 39.Ueta T, Noda Y, Toyama T, Yamaguchi T, Amano S. Systemic vascular safety of ranibizumab for age-related macular degeneration: systematic review and meta-analysis of randomized trials. Ophthalmology. 2014;121:2193–203. doi: 10.1016/j.ophtha.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 40.Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–12. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- 41.Altara R, Manca M, Hermans KCM, Daskalopoulos EP, Brunner-La Rocca HP, Hermans RJ, Struijker-Boudier HA, Blankesteijn MW. Diurnal rhythms of serum and plasma cytokine profiles in healthy elderly individuals assessed using membrane based multiplexed immunoassay. J Transl Med. 2015;13:129. doi: 10.1186/s12967-015-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavusoglu AC, Bilgili S, Alaluf A, Doğan A, Yilmaz F, Aslanca D, Karaca B, Yüksel B, Topaloglu E. Vascular endothelial growth factor level in the serum of diabetic patients with retinopathy. Ann Ophthalmol. 2007;39:205–8. doi: 10.1007/s12009-007-0037-2. [DOI] [PubMed] [Google Scholar]