Abstract
Quitting smoking is challenging in part because environmental smoking cues can trigger the desire to smoke. Neurobiological responses to smoking cues are often observed in reward-related brain regions such as the caudate and nucleus accumbens (NAc). While reward plays a well-established role in the formation of cue reactivity, whether general reward responsiveness contributes to individual differences in cue-reactivity among chronic smokers is unclear; establishing such link could provide insight into the mechanisms maintaining cue reactivity. The current study explored this relationship by assessing smoking cue reactivity during functional magnetic imaging followed by an out-of-scanner probabilistic reward task (PRT) in 24 nicotine-dependent smokers (14 women). In addition, owing to sex differences in cue reactivity and reward function, this same relationship was examined as a function of sex. Following recent smoking, greater reward responsiveness on the PRT was associated with enhanced left caudate reactivity to smoking cues. No relationship was found in any other striatal subregion. The positive relationship between reward responsiveness and caudate smoking cue reactivity was significant only in male smokers, fitting with the idea that males and females respond to the reinforcing elements of smoking cues differently. These findings are clinically relevant as they show that, following recent smoking, nicotine-dependent individuals who are more cue reactive are also more likely to be responsive to non-drug rewards, which may be useful for making individualized treatment decisions that involve behavioral reward contingencies.
Keywords: smoking, nicotine, cue-reactivity, reward responsivity, caudate, nucleus accumbens, addiction, sex differences
1. Introduction
Quitting tobacco smoking continues to be a challenge for the majority of smokers (Chaiton et al., 2016; Piasecki, 2006), in part because environmental cues associated with smoking can evoke behavioral, emotional, and neurobiological responses (i.e., cue reactivity), which drive the desire to smoke (Carpenter et al., 2009; Carter & Tiffany, 1999, 2001; Engelmann et al., 2012; Shiffman et al., 2013). Interestingly, not all smokers show the same patterns of brain reactivity to smoking cues (Janes et al., 2010, 2017; Kang et al., 2012; McClernon et al., 2007; Tang et al., 2012; McClernon et al., 2008), supporting the notion that individual variance may influence how cues motivate smoking behavior (Janes et al., 2010, 2017). Biological (e.g., sex; Doran, 2014; Dumais et al., 2017; McClernon et al., 2007; Wetherill et al., 2013) and smoking-related factors (e.g., severity of dependence; Vollstadt-Klein et al., 2011) have been linked with heightened cue reactivity, providing insights into which populations may be more prone to cue-induced relapse. It is critical to specify the cognitive mechanisms contributing to such variance in cue reactivity as this may enhance the ability to develop therapies targeted at addressing such underlying factors.
It is plausible that cue reactivity is directly influenced by one’s level of reward responsivity, which can be operationalized as the tendency to adapt behavior based on the availability of rewards (Pizzagalli et al., 2005). The idea that reward plays a role in addiction and cue reactivity is not new as it has long been shown that reward-related brain regions such as the dorsal and ventral striatum (Delgado, 2007) also respond to smoking-related cues (David et al., 2005; Franklin et al., 2007; Janes et al., 2009; Yuan et al., 2017). Furthermore, our prior work showed that following acute nicotine administration there is a positive association between brain reactivity to reward-predictive stimuli and behavioral reward responsivity (Moran et al., 2017). Building on this finding, it is plausible that brain reactivity to smoking cues may also be related to behavioral reward responsivity. Additionally, the relationship between cue reactivity and reward responsivity may be mediated by other factors known to impact nicotine dependence such as biological sex (see Benowitz & Hatsukami, 1998 for review). Specifically, nicotine-related reinforcement appears to play a larger role in motivating smoking in males relative to females (Perkins et al., 1992; see Perkins, 1996 for review), which may be related to the finding that smoking induces a larger dopamine release in males (Cosgrove et al., 2014; Weinstein et al., 2016). Whether sex influences the relationship between cue reactivity and reward sensitivity is unclear and would help explain the noted sex differences in nicotine dependence.
To determine whether there is a link between striatal reactivity to smoking cues and reward responsivity, 24 nicotine-dependent smokers performed a smoking cue-reactivity task during concurrent functional magnetic resonance imaging (fMRI) ~ 1h after smoking. After the scan, participants completed a probabilistic reward task (PRT), which quantifies behavioral responsivity to monetary rewards (Pizzagalli et al., 2005). Specifically, the relationship between striatal reactivity to smoking cues and PRT performance was assessed. The striatal regions of interest (ROIs) included the nucleus accumbens (NAc) and the caudate as these regions play a role in the establishment and expression of reward-related conditioned behavior (Knutson et al., 2001; Tricomi et al., 2004; Haruno et al., 2004). While dopamine release in the NAc underlies the reinforcing properties of abused substances such as nicotine (Brody et al., 2004; Pontieri et al., 1996; Di Chiara et al., 1988), dorsal striatal regions such as the caudate play a larger role in cue-induced craving in chronic drug users (Volkow et al., 2006). Thus, cue-induced reactivity in the caudate may better reflect the relationship between reward responsivity and cue reactivity in established long-term smokers. Finally, we investigated whether the relationship between these measures differed between males and females.
2. Methods
2.1. Study Sample
Twenty-four nicotine-dependent tobacco smokers (14 female) completed study procedures at McLean Hospital. Participants met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV-TR) criteria for current nicotine dependence and was verified by the Fagerstrom Test for Nicotine Dependence (FTND; Fagerström, 1978) with an average score of 5.95 ± 1.20 (± SD). Participants reported smoking an average of 13.72 ± 3.85 cigarettes per day over the past 6 months and had an average expired air carbon monoxide (CO) of 22.29 ppm ± 12.34 at screening. The average age of smoking onset for participants was 17.44 ± 2.01 with an average pack-year (cigarettes per day X years of smoking) of 6.57 ± 3.74. All study procedures were approved by the Partners Human Research Committee. Prior to study procedures, participants provided written informed consent and were compensated for their participation. For full demographics, see Table 1.
Table 1. Demographic characteristics.
A. Demographic characteristics for all participants. B. Demographic characteristics breakdown by sex. No significant differences in demographic variables were observed between male and female participants p > .05.
| A. | ||
|---|---|---|
| Variable | M [n] | SD |
| Age | 27.63 | 6.05 |
| Sex | ||
| Female | 14 | – |
| Male | 10 | – |
| Education | 15.00 | 2.15 |
| FTND | 5.95 | 2.03 |
| Pack-year | 6.57 | 3.74 |
| B. | |||||
|---|---|---|---|---|---|
| Males | Females | ||||
| Variable | M [n] | SD | M [n] | SD | Statistics |
| Age | 26.90 | 5.97 | 28.14 | 6.27 | p = 0.630 |
| Education | 15.65 | 2.20 | 14.54 | 2.06 | p = 0.217 |
| CO | 20.60 | 12.77 | 23.50 | 12.36 | p = 0.582 |
| FTND | 5.80 | 1.22 | 6.07 | 1.21 | p = 0.595 |
| Pack-year | 6.05 | 3.14 | 6.94 | 4.20 | p = 0.575 |
| Avg cigs/day | 14.90 | 3.92 | 12.89 | 3.72 | p = 0.216 |
Exclusionary criteria were evaluated using an amended version of the Structured Clinical Interview for DSM-IV (SCID-I; First, Gibbon, & Williams, 2002) and included current medical illness, pregnancy, recent drug/alcohol use (confirmed by a QuickTox11 Panel Drug Test Card, Branan Medical Corporation, Irvine California; Alco-Sensor IV, Intoximeters Inc., St. Louis, MO), current drug or alcohol dependence other than nicotine, current or lifetime major depressive episode (current verified with The Beck Depression Inventory-II; Beck et al., 1996), and current or lifetime diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, or psychotic disorders not otherwise specified.
Smoking time was standardized by instructing participants to smoke one of their own cigarettes in the laboratory 1 hour before the functional magnetic resonance imaging (fMRI) procedure. Following fMRI, participants were administered the PRT approximately 3 hours after scanning.
2.2. Functional neuroimaging acquisition
Scanning procedures were consistent with our previous work in an overlapping sample where only imaging metrics were evaluated and the PRT was not assessed (Janes et al., 2016). Scans were acquired on a Siemens 3T Trio scanner (Erlangen, Germany) with a 32-channel head coil. Multi-planar rapidly acquired gradient echo-structural images were acquired with the following parameters (TR = 2.1 s, TE = 3.3 ms, slices = 128, matrix = 256 × 256, flip angle = 7°, resolution = 1.0 mm × 1.0 mm × 1.33 mm) and gradient echo-planar images were acquired during cue reactivity using the following parameters (TR = 2 s, TE = 30 ms, flip angle = 75°, slices = 37, distance factor = 10%, voxel size = 3.5 mm isotropic, and GRAPPA acceleration factor = 2). Slice acquisition was aligned to the anterior and posterior commissures, and the phase encode direction was set to acquire from the posterior to anterior direction to prevent prefrontal signal loss.
2.3. Cue reactivity paradigm
Participants performed a cue reactivity task during fMRI. Across 5 blocks, participants were shown 50 smoking images that included smoking-related content (e.g. hand holding cigarette) and 50 neutral images without smoking stimuli, but otherwise matched for content (e.g. hand holding pencil). Smoking images were validated for their ability to elicit subjective craving in our previous work (Janes et al., 2015); as in our prior work, smoking cues in the current study were rated as inducing more craving than neutral cues (t(23)= 5.89, p < .001). Ten target images of animals were presented to verify that participants were paying attention. Participants were instructed to press a button every time they saw a target image. Consistent with our prior work (Janes et al., 2010, 2016), smoking, neutral, and target images were presented for 4 seconds in a pseudorandom order (with no more than two of the same picture-type occurring in a row) evenly across each 5-minute block. Images were separated by a jittered inter-trial-interval (ITI), in which participants were shown a fixation cross on a black screen. The ITI times ranged from 6 to 14 seconds in intervals of 2 seconds with a 10 second average across block.
2.4. fMRI preprocessing
The procedure for fMRI analysis was consistent with our previous work (Janes et al., 2016). All analyses were conducted using tools from the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl) and FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/). Using FSL, the first 5 volumes of each run were removed to allow for signal stabilization. Functional data pre-processing included motion correction, brain extraction, slice timing correction, spatial smoothing with a Gaussian kernel of 6 mm full-width half-maximum, and high-pass temporal filtering with Gaussian-weighted least-squares straight-line fitting with σ = 100s. Motion artifacts and intensity spiking were analyzed and adjusted for using an in-house program (https://github.com/bbfirederick/spikefix). Subject specific data were registered to standard space using the MNI152 2mm3 template (Montreal Neurological Institute, Montreal, QC, Canada).
2.5. Cue-reactivity neuroimaging analysis
Cue-reactivity analyses were also consistent with our prior work (Janes et al., 2016). First-level analysis was carried out separately for each of the participant’s cue-reactivity runs. The first-level general linear model included 3 regressors corresponding to smoking, neutral, and target image presentation, which were convolved using the standard gamma hemodynamic response function. Confound regressors modeling motion effects (x, y, z translation and rotation motion) were also included. Consistent with our prior work (Janes et al., 2015), we included a regressor representing motion/intensity artifacts identified and removed prior to preprocessing using an in-house program (https://github.com/bbfirederick/spikefix). For each participant, contrasts maps of smoking versus neutral images were created and first-level results were combined (across the 5 blocks) using a second-level fixed-effects analysis to create average brain reactivity for each participant.
FreeSurfer’s image analysis suite was used to identify and create subject-specific region of interest (ROI) masks for the left and right NAc and caudate, respectively. Output from the automated segmentation was converted to MNI space and used to create these masks. After each ROI was visually inspected for location accuracy, beta weights for each region were extracted from the second-level (subject specific) smoking > neutral contrast.
2.6. Probabilistic reward task
Similar to prior work (Pizzagalli et al., 2005), we used a computerized probabilistic reward task (PRT) to assess reward responsivity by measuring an individual’s propensity to modify behavior based on reward feedback. Briefly, the PRT consisted of 2 blocks of 100 trials each, where participants were shown one of two faces with slightly different mouth lengths (short versus long) and asked to identify which mouth-type was presented. Correct responses were financially rewarded using an asymmetrical reinforcement schedule, where the correct identification of one mouth triggered the response “Correct!! You won 5 Cents” three times more frequently (rich) than the other mouth (lean), eliciting a responses bias. Participants completed the PRT outside of the scanner ~4 hours following smoking and ~2.5 hours after completing a cue reactivity paradigm.
Following Pizzagalli et al.’s (2005) procedures, response bias was calculated for each block to measure the behavioral bias towards the rich stimulus, where higher values suggest greater responsivity to the stimulus more frequently paired with the reward. Discriminability was also calculated for each block, which captures the individual’s ability to perceptually distinguish between mouth types. Discriminability values were used as a control to ensure that any noted association was due to a response bias and not variance in perception. PRT measures were calculated using the following formulas:
Using procedures outlined in Pizzagalli et al.’s (2005), the validity of the PRT data was evaluated using four a priori criteria. Values for response bias and discriminability were calculated for each block using published formulas (Pizzagalli et al., 2005) and the change scores from blocks 1 to 2 were used in subsequent analyses as a way to capture reward learning that occurred from the first to the second block.
2.7. Data analysis
To assess for a relationship between cue reactivity and non-drug reward responsivity, we conducted multivariate regression analyses using ΔRB [=RB(Block 2) – RB(Block 1)] as the predictor, and the smoking > neutral beta weights extracted from the left and right NAc and caudate ROIs as dependent variables. To determine whether the overall ability to distinguish mouth types was driving any relationship between ΔRB and cue reactivity, we conducted the same multivariate regression again using Δd [=discriminability(Block 2) – discriminability (Block 1)] as the predictor.
To explore whether the relationship between ΔRB and striatal activation differed between males and females, the same multivariate regression was run for males (n = 10) and females (n = 14), separately. Pearson’s correlation coefficients were calculated between ΔRB and striatal regions that were significant in the multivariate regression. To compare the association between striatal activation and ΔRB between men and women, correlation coefficients were converted to a Fisher’s Z and statistically compared using Fisher’s test for independent correlation (two-tailed; Fisher, 1915;1921).
3. Results
3.1. Relationship between striatal ROIs and Response Bias
Response bias.
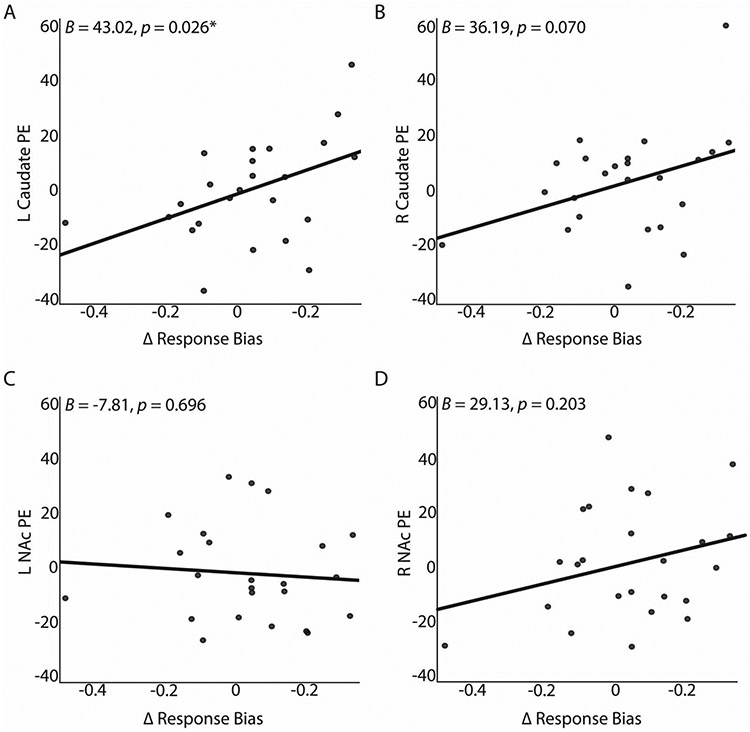
The multivariate regression model included the left and right NAc and caudate as dependent variables with ΔRB as the predictor. The overall model was significant (F(4. 19) = 3.64, p = .023, partial eta2 = .43). When probing individual striatal brain regions, ΔRB significantly predicted left caudate smoking > neutral cue activation (Fig. 1a; unstandardized B = 43.02, Standard Error = 18.05, p = .026, partial eta2 = .21). Specifically, 16.9% (adjusted R2) of the variance in left caudate activation was accounted for by ΔRB. Change in RB approached significance for predicting right caudate activation (Fig. 1b;unstandardized B = 36.19, Standard Error = 19.03, p = .070, partial eta2 = .14). Using Steiger’s Z-test for dependent correlations (Steiger, 1980), a direct comparison of the correlation coefficients of left (r = 0,42, p = .039) and right (r = 0,27, p = .201) caudate respectively with ΔRB did not reveal significant hemispheric differences (z = 0.54, p = .59, two-tailed).
Figure 1. Multivariate regression results for striatal ROIs and response bias for overall sample.
A, B. Plots of left and right caudate beta weights from smoking > neutral contrast from cue reactivity paradigm against change in response bias in probabilistic reward task. ΔRB significantly predicted the left caudate in a positive direction. ΔRB approached significance for the right caudate. C, D. Plots of left and right NAc beta weights from smoking > neutral contrast from cue reactivity paradigm against change in response bias in probabilistic reward task. ΔRB did not significantly predict left or right NAc.
For the NAc ROIs, ΔRB did not significantly predict left (Fig. 1c;unstandardized B = −7.81, Standard Error = 19.73, p = .70) or right (Fig. 1d; unstandardized B = 29.13, Standard Error = 22.21, p = .20) NAc activation.
Discriminability.
There was no significant relationship (all p-values > .05) between discriminability and striatal regions, indicating that the relationship between response bias and caudate activation was not due to overall task perceptual difficulty.
3.2. Relationship between striatal ROIs and Response Bias based on Sex
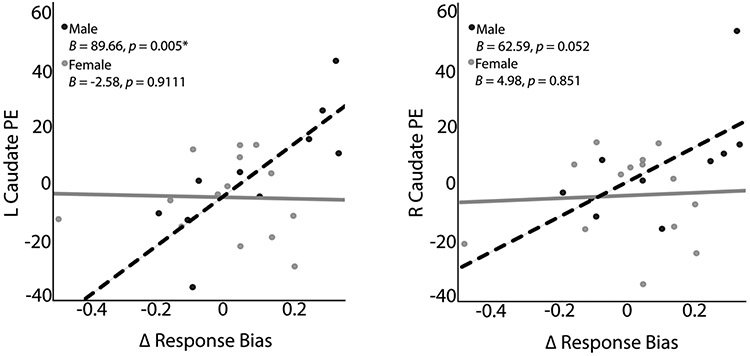
Multivariate regression models were run in males and females separately to evaluate whether the relationship between striatal reactivity to smoking cues and response bias differed based on sex. When considering male participants, the overall multivariate regression model was significant (F(4.5) = 8.18, p = .020, partial eta2 = .87). For males, ΔRB significantly predicted left caudate smoking > neutral cue activation (Figure 2; unstandardized B = 89.66, Standard Error = 23.11, p = .005, partial eta2 = .65) and approached significance for right caudate cue activation (unstandardized B = 62.59, Standard Error = 27.50, p = .052, partial eta2 = .39). Specifically, 61.0% (adjusted R2) of the variance in left caudate activation was accounted for by ΔRB in males. No significant results were observed for left (unstandardized B = −6.19, Standard Error = 26.67, p = .82) or right (unstandardized B = 36.33, Standard Error = 25.88, p = .20) NAc cue activation. For females, the overall multivariate regression was not significant (F(4.9) = 1.13, p = .40).
Figure 2. Multivariate regression results for striatal ROIs and response bias based on Sex.
Plots of smoking > neutral beta weights extracted from fMRI cue reactivity paradigm for left and right caudate for male and female subsamples. For male participants, ΔRB showed a significant association with left caudate smoking > neutral cue activation and approached significance for right caudate cue activation.
To evaluate whether the ΔRB and left caudate relationship was significantly different between males and females, separate two-tailed Pearson’s correlation analyses were run for males and females using ΔRB and left caudate. The resultant r-values were z-transformed and statistically compared using a Fisher’s test for independent correlation (two-tailed; Fisher, 1915;1921). This test confirmed that men had a significantly stronger association between left caudate cue activation and ΔRB than females (Z = 2.50, p = 0.024).
4. Discussion
The current work shows that among smokers, those with greater caudate reactivity to smoking cues are also the most reward responsive. To our knowledge, this is the first study to directly link caudate cue reactivity with behavioral responsivity to monetary reward in smokers. This relationship suggests that cue reactivity towards primary drug-related rewards does not necessarily occur at the expense of responsivity to secondary conditioned reinforcers (e.g., money), which is in contrast to theories suggesting that addiction is characterized by a hypersensitivity to drug rewards and hyposensitivity to non-drug rewards (Goldstein & Volkow, 2002; Volkow et al., 2010). However, the current work focused only on individuals who recently smoked and it is unclear how this association may be impacted following periods of extended abstinence or in those abusing other substances. Our caudate-specific finding fits with prior work demonstrating the caudate’s role in habitual responding, which is expected given that our study sample consisted of long-term established smokers (Porrino et al., 2004; see Everitt & Robbins, 2005 for review). Likewise, the lack of finding between NAc cue reactivity and reward responsivity fits with our hypothesis and the purported role of the NAc in the initial phases of learning (e.g., Parkinson et al., 2002). Specifically, the NAc plays an important role in action-outcome learning of goal-directed behaviors, while the caudate’s role is associated with facilitating cue-induced habitual behaviors (see Everitt & Robbins, 2005 for review; Tricomi et al., 2004) following established learning.
A follow-up analysis on the caudate finding showed that this positive association between caudate cue reactivity and reward responsivity was present only in males. This finding fits with the notion that there are sex-specific mechanisms contributing to nicotine dependence (Becker, 1999; Munro et al., 2006). For instance, previous work has reported that men show more smoking-induced dopamine release relative to females in areas of the striatum (Cosgrove et al., 2014; Weinstein et al., 2016), such as the caudate (Weinstein et al., 2016). While findings are mixed on sex differences in brain reactivity to smoking cues (Dumais et al., 2017; McClernon et al., 2008; Wetherill et al., 2013; Zanchi et al., 2016), the present study focused on the relationship between brain reactivity to smoking cues and behavioral reward responsivity. Our findings suggest that behavioral reward responsivity may influence smoking cue-reactivity more so in males relative to females, lending support to prior work showing that in comparison to females, males are characterized by more smoking cue reactivity in reward-related brain regions (Dumais et al., 2017; Wetherill et al., 2013). Additionally, preclinical research has demonstrated that repeated nicotine exposure enhances reward-association learning in males but not females (Quick et al., 2014), which fits with the notion that nicotine use impacts male and female brains differently (Beltz, Berenbaum, & Wilson, 2015; Cosgrove et al., 2014; Fallon et al., 2005). While we did not administer nicotine in the current study, our sample reported long-term nicotine use and it is therefore plausible that our results suggest sex differences in the relationship between reward learning and cue reactivity possibly resulting from extended nicotine use. While this specific conjecture requires further testing, our results contribute to the extant literature on sex differences in reward function and cue reactivity by highlighting a stronger relationship between these two concepts for males relative to females.
This study contributes to the field by shedding light on behavioral reward-related characteristics that underlie cue reactivity, however the study has limitations worth noting. First, the current work focused on individuals who recently smoked and therefore it is plausible the association between caudate and reward responsivity may change if participants were evaluated following extended abstinence. Second, while reward responsivity to monetary rewards was used as a predictor in the linear regression, our study design precludes us from drawing directional conclusions about whether variance in non-drug reward responsivity prior to nicotine exposure directly influenced the establishment of cue reactivity. Third, the sample size for sex-specific analyses was relatively small but we were reassured by the sizable effect size for the finding in males (partial eta2 = 0.65). However, future studies should try to replicate our findings in a larger sample. Fourth, although in this initial evaluation we chose to focus on clear a priori ROIs that have been strongly linked to reward and cue-reactivity, larger subsequent studies should be powered to conduct a whole-brain analysis, which may reveal a relationship between areas mediating executive functions, cue reactivity, and reward responsivity (Bi et al., 2017). Finally, it is plausible that hormonal variations in women may have influenced our findings (Franklin et al., 2015; Diekhof & Ratnayake, 2016), as certain hormonal phases have been shown to increase smoking cue reactivity (Franklin et al., 2015) and reward sensitivity (Diekhof & Ratnayake, 2016) in females, which should be taken into consideration by future studies.
Based on our results, we conclude that individuals who show more caudate cue reactivity are also more reward responsive to non-drug reinforcers. Extrapolating from these results, it is plausible that therapies targeting reinforcement, such as contingency management, may be useful in such reward-responsive individuals. The fact that the relationship between reward and cue reactivity was noted only in males fits with the growing literature highlighting the need to consider sex differences when trying to understand and ultimately treat nicotine dependence.
Highlights.
Caudate reactivity to smoking cues versus neutral cues is associated with behavioral responding to monetary rewards
The relationship between caudate reactivity to smoking cues and behavioral responding to monetary rewards is stronger in males relative to females
Acknowledgments
Role of Funding Source
This research was supported by National Institute on Drug Abuse [grant numbers R01 DA039135, K02 DA042987]. Dr. Pizzagalli was partially supported by Grant No. R37 MH068376 and R01 MH101521. No funding sources played a role in the study design, collection, analyses and interpretation of the data, in the writing of the report or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Pizzagalli has received consulting fees from Blackthorn Therapeutics, Boehringer Ingelheim, Compass, Takeda and an honorarium from Alkermes for activities unrelated to the current research. Dr. Pizzagalli has a financial interest in BlackThorn Therapeutics, which has licensed the copyright to the Probabilistic Reward Task through Harvard University. Dr. Pizzagalli’s interests were reviewed and are managed by McLean Hospital and Partners Healthcare in accordance with their conflict of interest policies. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- Beck AT, Steer RA, Ball R, Ranieri WF, 1996. Comparison of beck depression inventories – IA and – II in psychiatric outpatients. J. Pers. Assess 67, 588–597. [DOI] [PubMed] [Google Scholar]
- Becker JB, 1999. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol. Biochem. Behav 64, 803–812. 10.1016/S0091-3057(99)00168-9 [DOI] [PubMed] [Google Scholar]
- Beltz AM, Berenbaum SA, Wilson SJ, 2015. Sex differences in resting state brain function of cigarette smokers and links to nicotine dependence. Exp. Clin. Psychopharmacol 23, 247–254. 10.1037/pha0000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hatsukami D, 1998. Gender differences in the pharmacology of nicotine addiction. Addict. Biol 3, 383–404. [DOI] [PubMed] [Google Scholar]
- Bi Y, Yuan K, Yu D, Wang R, Li M, Li Y, Zhai J, Lin W, Tian J, 2017. White matter integrity of central executive network correlates with enhanced brain reactivity to smoking cues. Hum. Brain Mapp 38, 6239–6249. 10.1002/hbm.23830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkem MA, 2004. Smoking-induced ventral striatum dopamine release. Am. J. Psychiatry 161, 1211–1218. 10.1176/appi.ajp.161.7.1211 [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Saladin ME, DeSantis S, Gray KM, LaRowe SD, Upadhyaya HP, 2009. Laboratory-based, cue-elicited craving and cue reactivity as predictors of naturally occurring smoking behavior. Addict. Behav 34, 536–541. 10.1016/j.addbeh.2009.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST, 1999. Meta-analysis of cue-reactivity in addiction research. Addiction 94, 327–340. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST, 2001. The Cue-Availability Paradigm : The Effects of Cigarette Availability on Cue Reactivity in Smokers. Exp. Clin. Psychopharmacol 9, 183–190. 10.1037//1064-1297.9.2.183 [DOI] [PubMed] [Google Scholar]
- Chaiton M, Diemert L, Cohen JE, Bondy SJ, Selby P, Philipneri A, Schwartz R, 2016. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. Br. Med. J. Open 6, eO11045 10.1136/bmjopen-2016-011045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Wang S, Kim SJ, McGovern E, Nabulsi N, Gao H, Labaree D, Tagare HD, Sullivan JM, Morris ED, 2014. Sex differences in the brain’s dopamine signature of cigarette smoking. J. Neurosci 34, 16851–16855. 10.1523/JNEUROSCI.3661-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafò MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT, 2005. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: A functional magnetic resonance imaging study. Biol. Psychiatry 58, 488–494. 10.1016/j.biopsych.2005.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, 2007. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci 1104, 70–88. 10.1196/annals.1390.002 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A, 1988. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U. S. A 85, 5274–5278. 10.1073/pnas.85.14.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Ratnayake M, 2016. Menstrual cycle phase modulates reward sensitivity and performance monitoring in young women: Preliminary fMRI evidence. Neuropsychologia 84, 70–80. 10.1016/j.neuropsychologia.2015.10.016 [DOI] [PubMed] [Google Scholar]
- Doran N, 2014. Sex differences in smoking cue reactivity: Craving, negative affect, and preference for immediate smoking. Am. J. Addict 23, 211–217. 10.1111/j.1521-0391.2014.12094.x [DOI] [PubMed] [Google Scholar]
- Dumais KM, Franklin TR, Jagannathan K, Hager N, Gawrysiak M, Betts J, Farmer S, Guthier E, Pater H, Janes AC, Wetherill RR, 2017. Multi-site exploration of sex differences in brain reactivity to smoking cues: Consensus across sites and methodologies. Drug Alcohol Depend. 178, 469–476. 10.1016/j.drugalcdep.2017.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM, 2012. Neural substrates of smoking cue reactivity: A meta-analysis of fMRI studies. Neuroimage 60, 252–262. 10.1016/j.neuroimage.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2005. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat. Neurosci 8, 1481–1489. 10.1038/nnl579 [DOI] [PubMed] [Google Scholar]
- Fagerström KO, 1978. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict. Behav 3, 235–241. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Keator DB, Mbogori J, Taylor D, Potkin SG, 2005. Gender: A major determinant of brain response to nicotine. Int. J. Neuropsychopharmacol 8, 17–26. 10.1017/S1461145704004730 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition Biometrics Research. New York State Psychiatric Institute, New York. [Google Scholar]
- Franklin TR, Jagannathan K, Wetherill RR, Johnson B, Kelly S, Langguth J, Mumma J, Childress AR, 2015. Influence of menstrual cycle phase on neural and craving responses to appetitive smoking cues in naturally cycling females. Nicotine Tob. Res 17, 390–397. 10.1093/ntr/ntu183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen, Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR, 2007. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: A perfusion fMRI study. Neuropsychopharmacology 32, 2301–2309. 10.1038/sj.npp.1301371 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2002. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 159, 1642–1652. 10.1176/appi.ajp.159.10.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M, 2004. A neural correlate of reward-based behavioral learning in caudate nucleus: A functional magnetic resonance imaging study of a stochastic decision task. J. Neurosci 24, 1660–1665. 10.1523/JNEUROSCI.3417-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Betts J, Jensen JE, Lukas SE, 2016. Dorsal anterior cingulate glutamate is associated with engagement of the default mode network during exposure to smoking cues. Drug Alcohol Depend. 167, 75–81. 10.1016/j.drugalcdep.2016.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Frederick B. deB, Richardt S, Burbridge C, Merlo-Pich E, Renshaw PF, Evins AE, Fava M, Kaufman MJ, 2009. Brain fMRI reactivity to smoking-related images before and during extended smoking abstinence. Exp. Clin. Psychopharmacol 17, 365–373. 10.1037/a0017797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Gilman JM, Radoman M, Pachas G, Fava M, Evins AE, 2017. Revisiting the role of the insula and smoking cue-reactivity in relapse: A replication and extension of neuroimaging findings. Drug Alcohol Depend. 179, 8–12. 10.1016/j.drugalcdep.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick BDB, Holmes AJ, Sousa J, Fava M, Evins AE, Kaufman MJ, 2010. Neural substrates of attentional bias for smoking-related Cues: An fMRI study. Neuropsychopharmacology 35, 2339–2345. 10.1038/npp.2010.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Ross RS, Farmer S, Frederick BB, Nickerson LD, Lukas SE, Stem CE, 2015. Memory retrieval of smoking-related images induce greater insula activation as revealed by an fMRI-based delayed matching to sample task. Addict. Biol 20, 349–356. 10.1111/adb.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang OS, Chang DS, Jahng GH, Kim SY, Kim H, Kim JW, Chung SY, Yang SI, Park HJ, Lee H, Chae Y, 2012. Individual differences in smoking-related cue reactivity in smokers: An eye-tracking and fMRI study. Prog. Neuro-Psychopharmacology Biol. Psychiatry 38, 285–293. 10.1016/j.pnpbp.2012.04.013 [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D, 2001. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12, 3683–3687. 10.1097/00001756-200112040-00016 [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hutchison KE, Rose JE, Kozink RV, 2007. DRD4 VNTR polymorphism is associated with transient fMRI-BOLD responses to smoking cues. Psychopharmacology (Berl). 194, 433–441. 10.1007/s00213-007-0860-6 [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE, 2008. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology 33, 2148–2157. 10.1038/sj.npp.1301618 [DOI] [PubMed] [Google Scholar]
- McEwen A, West R, McRobbie H, 2008. Motives for smoking and their correlates in clients attending Stop Smoking treatment services. Nicotine Tob. Res 10, 843–850. 10.1080/14622200802027248 [DOI] [PubMed] [Google Scholar]
- Moran LV, Stoeckel LE, Wang K, Caine CE, Villafuerte R, Calderon V, Baker JT, Ongur D, Janes AC, Pizzagalli DA, Evins AE, 2017. Nicotine increases activation to anticipatory valence cues in anterior insula and striatum. Nicotine Tob. Res 0, 1–8. 10.1093/ntr/ntx217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS, 2006. Sex Differences in Striatal Dopamine Release in Healthy Adults. Biol. Psychiatry 59, 966–974. 10.1016/j.biopsych.2006.01.008 [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Dailey JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ, 2002. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: Implications for mesoaccumbens dopamine function. Behav. Brain Res 137, 149–163. 10.1016/S0166-4328(02)00291-7 [DOI] [PubMed] [Google Scholar]
- Peechatka AL, Whitton AE, Farmer SL, Pizzagalli DA, Janes AC, 2015. Cigarette craving is associated with blunted reward processing in nicotine-dependent smokers. Drug Alcohol Depend. 155, 202–207. 10.1016/j.drugalcdep.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, 1996. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp. Clin. Psychopharmacol 4, 166–177. 10.1037/1064-1297.4.2.166 [DOI] [Google Scholar]
- Perkins KA, Grobe JE, Stiller RL, Fonte C, Goettler JE, 1992. Nasal spray nicotine replacement suppresses cigarette smoking desire and behavior. Clin. Pharmacol. Ther 52, 627–634. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M, Caggiula AR, 2002. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl). 163, 194–201. 10.1007/s00213-002-1168-1 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA, 2013. Negative mood effects on craving to smoke in women versus men. Addict. Behav 38, 1527–1531. 10.1016/j.addbeh.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, 2006. Relapse to smoking. Clin. Psychol. Rev 26, 196–215. 10.1016/j.cpr.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP, 2005. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biol. Psychiatry 57, 319–327. 10.1016/j.biopsych.2004.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G, 1996. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 382, 255–257. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA, 2004. Cocaine Self-Administration Produces a Progressive Involvement of Limbic, Association, and Sensorimotor Striatal Domains. J. Neurosci. 24, 3554–3562. 10.1523/JNEUROSCI.5578-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick SL, Olausson P, Addy NA, Taylor JR, 2014. Repeated nicotine exposure during adolescence alters reward-related learning in male and female rats. Behav. Brain Res 261, 171–176. 10.1016/j.bbr.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar M, Kirchner T, Li X, Tindle H, Anderson S, Scholl S, 2013. Smoker Reactivity to Cues: Effects on Craving and on Smoking Behavior. J. Abnorm. Psychol. 122, 264–280. 10.1037/a0028339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH (1980). Tests for comparing elements of a correlation matrix. Psychol. Bull, 87, 245–251. [Google Scholar]
- Tang DW, Hello B, Mroziewicz M, Fellows LK, Tyndale RF, Dagher A, 2012. Genetic variation in CYP2A6 predicts neural reactivity to smoking cues as measured using fMRI. Neuroimage 60, 2136–2143. 10.1016/j.neuroimage.2012.01.119 [DOI] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA, 2004. Modulation of Caudate Activity by Action Contingency. Neuron 41, 281–292. 10.1016/S0896-6273(03)00848-1 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R, 2010. Addiction: Decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. BioEssays 32, 748–755. 10.1002/bies.201000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, Jayne M, Ma Y, Wong C, 2006. Cocaine Cues and Dopamine in Dorsal Striatum: Mechanism of Craving in Cocaine Addiction. J. Neurosci 26, 6583–6588. 10.1523/JNEUROSCI.1544-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Kobiella A, Bühler M, Graf C, Fehr C, Mann K, Smolka MN, 2011. Severity of dependence modulates smokers’ neuronal cue reactivity and cigarette craving elicited by tobacco advertisement. Addict. Biol 16, 166–175. 10.1111/j.1369-1600.2010.00207.x [DOI] [PubMed] [Google Scholar]
- Weinstein AM, Freedman N, Greif J, Yemini Z, Mishani E, London E, Chisin R, Bocher M, 2016. Negative association of pretreatment cigarette use with smoking-induced striatal dopamine release in smokers receiving bupropion treatment. Am. J. Addict 25, 486–492. 10.1111/ajad.12419 [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Young KA, Jagannathan K, Shin J, O’Brien CP, Childress AR, Franklin TR, 2013. The impact of sex on brain responses to smoking cues: a perfusion fMRI study. Biol. Sex Differ 4, 9 10.1186/2042-6410-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Yu D, Bi Y, Wang R, Li M, Zhang Y, Dong M, Zhai J, Li Y, Lu X, Tian J, 2017. The left dorsolateral prefrontal cortex and caudate pathway: New evidence for cue-induced craving of smokers. Hum. Brain Mapp 38, 4644–4656. 10.1002/hbm.23690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchi D, Brody A, Borgwardt S, Haller S, 2016. Sex effects on smoking cue perception in non-smokers, smokers, and ex-smokers: A pilot study. Front. Psychiatry 7, 1–7. 10.3389/fpsyt.2016.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]