Abstract
Background:
Chronic non-cancer pain (CNCP) among patients with substance use disorder (SUD) poses a risk for worse treatment outcomes. Understanding the association of CNCP with SUD is important for informing the need and potential benefits of pain assessment/management among those with SUDs.
Methods:
We analyzed electronic health record data from 2013–2018 among adults aged ≥18 years (N=951,533; mean age: 48.4 years; 57.4% female) in a large academic healthcare system. Adjusted logistic regression models were conducted to estimate the association of CNCP conditions with opioid overdose, emergency department utilization, and inpatient hospitalization stratified by different SUD diagnoses and by gender.
Results:
Among the total sample, the prevalence of CNCP was 46.6% and any SUD was 11.2%. The majority of patients with a SUD had CNCP (opioid: 74.7%; sedative: 72.3%; cannabis: 64.3%; alcohol: 58.7%; tobacco: 59.5%). The prevalence of CNCP was greater in females vs. males for most SUD diagnoses. The presence of CNCP was associated with more mental health disorders and chronic medical conditions among each SUD group. CNCP was associated with significantly decreased odds of overdose among those with opioid use disorder but increased odds of overdose and healthcare utilization among other SUDs. CNCP was positively associated with overdose in females, but not males, with alcohol or non-opioid drug use disorders.
Conclusions:
The direction and magnitude of the association between CNCP and negative health indicators differed as a function of SUD type and gender, respectively. Greater awareness of potential unmet pain treatment need may have implications for improving SUD outcomes.
Keywords: Chronic pain, substance use disorder, opioid, drug overdose, healthcare utilization
1. Introduction
Chronic non-cancer pain (CNCP) affects approximately 50 million adults in the U.S. and contributes to high health care costs and lost productivity (Dahlhamer et al., 2018; Simon, 2012). CNCP is particularly overrepresented among individuals with substance use disorder (SUD) (Manhapra and Becker, 2018; Rosenblum et al., 2003). For example, nationally representative survey data show that the lifetime prevalence of chronic pain was reported by 52.8% of individuals reporting a lifetime SUD (Ilgen et al., 2010). Samples of patients in treatment for SUD indicate that the prevalence of chronic pain is even higher, with prevalence estimates ranging up to 75% (Manhapra and Becker, 2018; Witkiewitz and Vowles, 2018). Research suggests that the frequent co-occurrence of CNCP and SUD may be driven by several different reasons including substance use as a coping response to pain-related symptoms, substance use-related injury leading to chronic pain conditions, or the diathesis-stress model (Alford et al., 2016; Dersh et al., 2002; Ilgen et al., 2010).
Research also suggests that SUD treatment is complicated by co-occurring CNCP. Due to a lack of established guidelines for treating co-occurring SUD and CNCP, research suggests that treatment for SUD may often be prioritized (Berg et al., 2009), which could result in leaving CNCP symptoms untreated and potential prognostic risk factors. Indeed, studies have shown that the presence of CNCP among patients with SUD is associated with increased craving and withdrawal (Ditre et al., 2016; Tsui et al., 2016), relapse during treatment or following treatment discharge (Caldeiro et al., 2008; Jakubczyk et al., 2016; Witkiewitz et al., 2015; Worley et al., 2017), continued substance use following detoxification (Larson et al., 2007; Potter et al., 2010), or lower treatment retention (Caldeiro et al., 2008). Inadequately managed CNCP also often results in disability, which in turn may negatively affect aspects of psychosocial functioning and quality of life (e.g., employment, interpersonal relationships, attending healthcare visits) essential to SUD recovery (Speed et al., 2018). Moreover, it has been shown that CNCP is associated with higher prevalence of medical and psychiatric comorbidities among patients with SUDs, which may further contribute to treatment complications (Caldeiro et al., 2008). Among patients with opioid use disorder (OUD), CNCP has been associated with greater costly healthcare utilization than those without CNCP (Alford et al., 2016; Caldeiro et al., 2008; Dunn et al., 2015; Trafton et al., 2004). Furthermore, research suggests that CNCP may be a barrier to SUD treatment initiation due to perceived fear of uncontrolled pain relief (Stumbo et al., 2017).
Given these findings, it is important to understand the extent of CNCP among those with SUDs in order to inform the need for integrated pain management strategies as a way to potentially improve SUD treatment access and outcomes. In particular, emerging evidence supports the efficacy of some psychosocial pain management approaches (e.g., cognitive behavioral therapy with acceptance-based content) to simultaneously reduce substance use and improve pain-related function (Ilgen et al., 2016; Morasco et al., 2016). It is also important to better understand the association between CNCP and costly healthcare utilization (e.g., emergency department (ED) utilization and hospitalization) among patients with SUD for indicating potential benefits of integrated CNCP treatment. Moreover, studies have shown that alcohol or drug use disorder is associated with substantially increased odds of opioid overdose among patients prescribed opioids for chronic pain (Liang et al., 2016). However, information is needed on the association of CNCP and overdose in relation to specific types of SUDs, which may further inform clinical decision making on whether to initiate opioid therapy or how to monitor certain individuals receiving opioid therapy. Prior research also suggests that SUD and pain treatment needs may differ between males and females given reported gender differences in pain manifestation, coping, and aberrant behaviors (Bartley and Fillingim, 2013; Manubay et al., 2015; Mogil, 2012). Thus, understanding gender differences with respect to comorbid CNCP and SUD has the potential to further inform tailored intervention efforts to improve outcomes.
The use of electronic health record (EHR) data provides an opportunity to investigate these gaps in the literature and inform treatment. Other data sources such as national surveys or clinical research samples are often limited by study inclusion/exclusion criteria, self-report bias, or small sample sizes that constrain subgroup analysis. The aims of this study were to leverage an EHR database from a large academic healthcare system to 1) examine the prevalence of CNCP by SUD diagnoses, 2) to examine demographic and clinical characteristics among patients with SUD diagnoses by CNCP status, and 3) to examine the association between CNCP and opioid overdose, ED utilization, and inpatient hospitalization among patients with SUD diagnoses. Gender differences in the prevalence of CNCP and its association with healthcare utilization were also examined.
2. Methods
2.1. Study sample
We analyzed EHR data from patients receiving healthcare within the Duke University Health System (DUHS) between January 1, 2013 and December 31, 2018. The DUHS includes 3 hospitals and over 300 ambulatory clinics. All EHR data generated within the DUHS was stored within the Duke Medicine Enterprise Data Warehouse, which employs a formal extract, transform, and load procedure to integrate data from source systems on a nightly basis to ensure consistency and quality and to minimize redundancy (Horvath et al., 2014).
The analytic sample included 951,533 unique adult patients who were aged 18–90 years old as of January 1, 2013, had information about gender and race/ethnicity, and had at least two healthcare encounters during the study period. Healthcare encounters included ambulatory visits, ED visits, hospitalizations, and other visits (e.g., visits for procedures). The use of these data for analysis was approved by the Duke University Health System Institutional Review board. All aspects of this study were conducted in accordance with principles set forth in the Declaration of Helsinki.
2.2. Study variables
Demographic variables included age (as of January 1, 2013), gender, and patient-identified race and ethnicity (i.e., Caucasian/white, Black/African-American, Hispanic, Asian, American Indian, Alaska Native, native Hawaiian or Pacific Islander, multiracial, or other). Diagnostic variables were based on the International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) and ICD-10-CM codes listed in the discharge or final diagnoses codes for outpatient, ED, or inpatient encounters. Grouping of the ICD-9/10-CM codes for each diagnostic variable was based on the codes listed for each condition in the DSM-5 and the Centers for Medicare and Medicaid Services (CMS) code lists (CMS, 2019). The list of codes utilized for this study can be found in the supplementary material (Table S1).
CNCP was defined by ICD-9/10-CM codes for conditions that may represent chronic pain provided by the Centers for Disease Control and Prevention (CDC, 2018), and by not having a code for cancer-related pain (338.3, G89.3) or any cancer diagnosis (excluding non-melanoma skin cancer). SUD diagnosis variables included tobacco, alcohol, cannabis, opioid, cocaine/amphetamine, sedative/hypnotic/anxiolytic, and other drug (hallucinogen, inhalant, other/unspecified drug) use disorder. Mental health disorder diagnosis variables included depressive, bipolar, anxiety, psychotic, sleep, and other mental health disorders (i.e., adjustment, personality, attention-deficit/impulse/conduct, somatoform, or eating disorders). Common non-cancer chronic health condition diagnosis variables included diabetes (type 1 or 2), asthma, chronic obstructive pulmonary disease (COPD), ischemic heart disease, hepatitis B or C, chronic kidney disease, and hypertension (Heron, 2016). Healthcare utilization variables included any ED visit or inpatient hospitalization during the study period of January 1, 2013-December 31, 2018. Opioid overdose was defined by ICD-9/10-CM diagnostic codes and included unintentional, undetermined, and suicidal overdoses (CDC, 2013; CDC, 2017).
2.3. Data analysis
Descriptive statistics were used to examine the frequency of demographic variables, CNCP, SUDs, mental health disorder, chronic medical conditions, opioid overdose, and healthcare utilization among the overall sample and stratified by sex. Differences in the demographic and clinical characteristics between males and females were assessed using chi-square and t-tests for categorical and continuous variables, respectively. The prevalence of CNCP was examined by SUD diagnoses among the overall sample and stratified by gender. We examined the demographic and clinical characteristics of four SUD groups, stratified by CNCP status, including tobacco use disorder, alcohol use disorder, opioid use disorder, and other drug (non-opioid) use disorder. Finally, logistic regression analyses, stratified by SUD group, were conducted to examine the association between having CNCP and opioid overdose, ED utilization, and hospitalization, respectively, while adjusting for age, gender, race/ethnicity, any mental health disorder diagnosis (not including SUD diagnoses), any chronic medical condition diagnosis, and any cancer diagnosis. Models were conducted among the overall sample and stratified by gender. We also conducted models that included an interaction term between CNCP and gender to explore gender differences in the association of CNCP and outcomes. All analyses were conducted using SAS, version 9.4.
3. Results
3.1. Demographic and clinical characteristics of the sample (Table 1)
Table 1.
Demographic and clinical characteristics of adult patients aged 18 or older.
| Overall | Males | Females | |||
|---|---|---|---|---|---|
| (N= 951,533) | (n= 405,366) | (n= 546,167) | p-value | ||
| Column % | n | % (SE) | % (SE) | % (SE) | |
| Sex | |||||
| Male | 405,366 | 42.6 (0.05) | ----- | ----- | |
| Female | 546,167 | 57.4 (0.05) | ----- | ----- | |
| Age in years on Jan. 1, 2013 | |||||
| 18–25 | 118,044 | 12.4 (0.03) | 11.2 (0.05) | 13.3 (0.05) | <0.0001 |
| 26–34 | 135,524 | 14.2 (0.04) | 13.2 (0.05) | 15.0 (0.05) | |
| 35–49 | 235,999 | 24.8 (0.04) | 25.0 (0.07) | 24.6 (0.06) | |
| 50–64 | 260,808 | 27.4 (0.05) | 28.7 (0.07) | 26.4 (0.06) | |
| 65+ | 201,158 | 21.1 (0.04) | 21.8 (0.06) | 20.6 (0.05) | |
| Race/ethnicity | |||||
| White, non-Hispanic | 626,983 | 65.9 (0.05) | 67.9 (0.07) | 64.4 (0.06) | <0.0001 |
| Black, non-Hispanic | 226,556 | 23.8 (0.04) | 21.8 (0.06) | 25.3 (0.06) | |
| Hispanic | 38,895 | 4.09 (0.02) | 4.14 (0.03) | 4.05 (0.03) | |
| Other, non-Hispanic | 59,099 | 6.21 (0.02) | 6.13 (0.04) | 6.27 (0.03) | |
| SUD diagnoses | |||||
| Tobacco | 85,406 | 8.98 (0.03) | 11.0 (0.05) | 7.46 (0.04) | <0.0001 |
| Alcohol | 19,195 | 2.02 (0.01) | 3.25 (0.03) | 1.10 (0.01) | <0.0001 |
| Cannabis | 6,978 | 0.73 (0.01) | 0.99 (0.02) | 0.54 (0.01) | <0.0001 |
| Opioid | 12,142 | 1.28 (0.01) | 1.34 (0.02) | 1.23 (0.01) | <0.0001 |
| Cocaine/amphetamine | 6,185 | 0.65 (0.01) | 0.96 (0.02) | 0.42 (0.01) | <0.0001 |
| Sedative/hypnotic/anxiolytic | 1,544 | 0.16 (0.00) | 0.17 (0.01) | 0.15 (0.01) | 0.04 |
| Other druga | 7,266 | 0.76 (0.01) | 0.97 (0.02) | 0.61 (0.01) | <0.0001 |
| Mental health disorder diagnoses | |||||
| Depressive disorder | 107,762 | 11.3 (0.03) | 8.46 (0.04) | 13.4 (0.05) | <0.0001 |
| Bipolar disorder | 11,728 | 1.23 (0.01) | 1.01 (0.02) | 1.40 (0.02) | <0.0001 |
| Mood disorder (any) | 117,546 | 12.4 (0.03) | 9.36 (0.05) | 14.6 (0.05) | <0.0001 |
| Anxiety disorder | 131,654 | 13.8 (0.04) | 10.6 (0.05) | 16.3 (0.05) | <0.0001 |
| Psychotic disorder | 10,014 | 1.05 (0.01) | 1.20 (0.02) | 0.94 (0.01) | <0.0001 |
| Sleep disorder | 128,970 | 13.6 (0.04) | 14.7 (0.06) | 12.7 (0.05) | <0.0001 |
| Otherb | 45,693 | 4.80 (0.02) | 4.13 (0.03) | 5.30 (0.03) | <0.0001 |
| Any | 268,064 | 28.2 (0.05) | 26.1 (0.07) | 29.7 (0.06) | <0.0001 |
| Chronic medical conditions | |||||
| Diabetes, type 1 or 2 | 128,291 | 13.5 (0.04) | 15.3 (0.06) | 12.2 (0.04) | <0.0001 |
| Asthma | 67,371 | 7.08 (0.03) | 5.15 (0.03) | 8.51 (0.04) | <0.0001 |
| COPD | 80,210 | 8.43 (0.03) | 8.70 (0.04) | 8.23 (0.04) | <0.0001 |
| Ischemic heart disease | 81,242 | 8.54 (0.03) | 12.2 (0.05) | 5.83 (0.03) | <0.0001 |
| Hepatitis B or C | 8,479 | 0.89 (0.01) | 1.27 (0.02) | 0.61 (0.01) | <0.0001 |
| Chronic kidney disease | 87,956 | 9.24 (0.03) | 11.3 (0.05) | 7.69 (0.04) | <0.0001 |
| Hypertension | 303,089 | 31.9 (0.05) | 35.3 (0.08) | 29.3 (0.06) | <0.0001 |
| Any | 411,396 | 43.2 (0.05) | 46.7 (0.08) | 40.7 (0.07) | <0.0001 |
| Cancer | |||||
| No | 843,357 | 88.6 (0.03) | 87.2 (0.05) | 89.7 (0.04) | <0.0001 |
| Yes | 108,176 | 11.4 (0.03) | 12.8 (0.05) | 10.3 (0.04) | |
| Chronic non-cancer pain | |||||
| No | 508,040 | 53.4 (0.05) | 55.0 (0.08) | 52.2 (0.07) | <0.0001 |
| Yes | 443,493 | 46.6 (0.05) | 45.0 (0.08) | 47.8 (0.07) | |
| Opioid overdose, yes | 1,289 | 0.14 (0.00) | 0.16 (0.01) | 0.11 (0.00) | <0.0001 |
| ED admission, yes | 251,681 | 26.5 (0.05) | 26.9 (0.07) | 26.1 (0.06) | <0.0001 |
| Inpatient hospitalization, yes | 178,711 | 18.8 (0.04) | 18.6 (0.06) | 18.9 (0.05) | <0.001 |
| Total encounters, mean (SD) | 22.2 (35.8) | 20.5 (34.4) | 23.5 (36.6) | <0.0001 | |
Note: the analysis (N=951,533) was based on EHR data from January 1, 2013 to December 31, 2018. SE: standard error; SUD: substance use disorder; COPD: chronic obstructive pulmonary disease; ED: emergency department.
Other drug use disorder diagnoses included hallucinogen, inhalant, or other/unspecified substance use disorders.
Other mental health disorders included adjustment, personality, attention-deficit/impulse/conduct, somatoform, or eating disorders.
Among the total sample (N=951,533), 57.4% were female and the mean (SD) age of the sample on January 1, 2013 was 48.4 (17.7) years old. Approximately two-thirds (65.9%) of the sample were white, 23.8% were Black/African-American, and 4.1% were Hispanic.
The prevalence of CNCP in the sample during the 5-year study period was 46.6% (n=443,493). The prevalence of any SUD diagnosis in the sample was 11.2% (n=106,732), including 9.0% for tobacco (n=85,406), 2.0% for alcohol (n=19,195), 0.73% for cannabis (n=6,978), 1.28% for opioid (n=12,142), 0.65% for cocaine/amphetamine (n=6,185), 0.16% for sedative/hypnotic/anxiolytic (n=1,544), and 0.76% for other/unspecified drug use disorder (n=7,266). More than one-fourth of the sample (28.2%; n=268,064) had a mental health disorder diagnosis (not including SUD). More than 2 out of 5 patients in the sample (43.2%; n=411,396) had a comorbid, chronic non-cancer medical condition diagnosis and 11.4% (n=108,176) had a cancer diagnosis.
Higher proportions of females in the sample compared to males were younger, Black/African-American, and had CNCP. A higher proportion of males had SUD diagnoses for each substance category than females. The prevalence of each mental health disorder diagnosis was higher in females, except psychotic and sleep disorders, which were higher in males. Males also had a higher prevalence of each chronic medical condition, except asthma, which was higher in females.
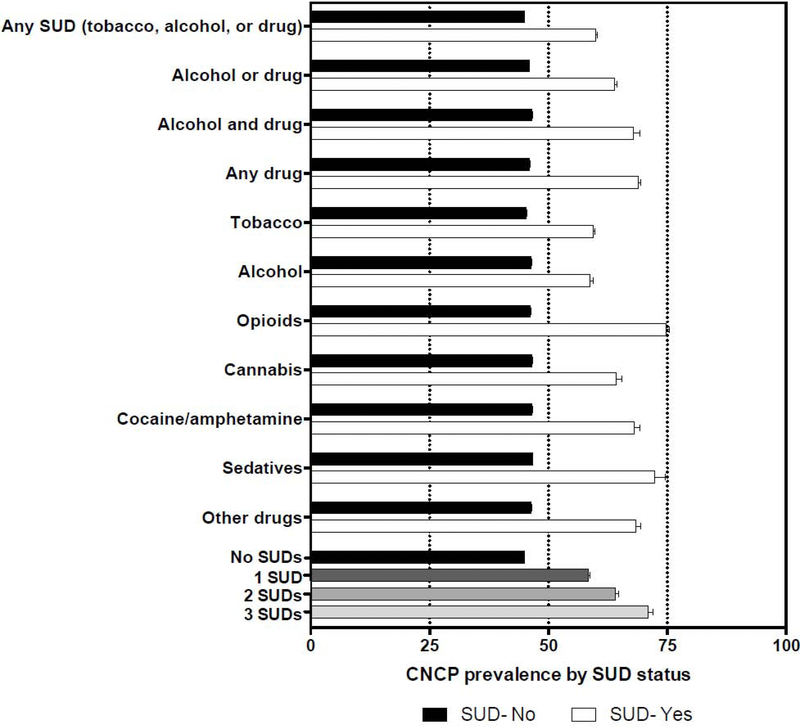
3.2. Prevalence of CNCP by SUD diagnosis and gender (Figure 1; Table 2)
Figure 1.
Prevalence of chronic non-cancer pain (CNCP) by substance use disorder (SUD) status.
Table 2.
Prevalence of chronic non-cancer pain by substance use disorder (SUD) diagnoses among adult patients aged 18 or older.
| Prevalence of chronic non-cancer pain | |||
|---|---|---|---|
| Overall | Males | Females | |
| (N= 951,533) | (n= 405,366) | (n= 546,167) | |
| SUD diagnosis | Row % (95% CI) | Row % (95% CI) | Row % (95% CI) |
| Any SUD (tobacco, alcohol, or drug) | |||
| No | 44.9 (44.8–45.0) | 43.1 (42.9–43.3) | 46.2 (46.1–46.3) |
| Yes | 59.9 (59.6–60.2) | 56.4 (56.0–56.8) | 63.9 (63.5–64.3) |
| Alcohol or drug | |||
| No | 45.9 (45.8–46.0) | 44.0 (43.9–44.2) | 47.2 (47.1–47.3) |
| Yes | 63.9 (63.5–64.4) | 60.7 (60.0–61.3) | 68.4 (67.7–69.1) |
| Alcohol and drug | |||
| No | 46.5 (46.4–46.6) | 44.8 (44.6–44.9) | 47.8 (47.6–47.9) |
| Yes | 67.9 (66.6–69.2) | 65.6 (64.1–67.2) | 73.1 (70.8–75.4) |
| Any druga | |||
| No | 46.0 (45.9–46.1) | 44.3 (44.1–44.4) | 47.3 (47.2–47.4) |
| Yes | 68.9 (68.3–69.4) | 66.0 (65.1–66.8) | 71.9 (71.1–72.7) |
| Tobacco | |||
| No | 45.3 (45.2–45.4) | 43.6 (43.4–43.7) | 46.6 (46.4–46.7) |
| Yes | 59.5 (59.2–59.8) | 56.0 (55.5–56.4) | 63.4 (62.9–63.8) |
| Alcohol | |||
| No | 46.4 (46.3–46.5) | 44.6 (44.4–44.7) | 47.7 (47.5–47.8) |
| Yes | 58.7 (58.0–59.4) | 56.9 (56.0–57.7) | 62.6 (61.4–63.8) |
| Opioid | |||
| No | 46.2 (46.1–46.3) | 44.6 (44.4–44.7) | 47.5 (47.4–47.6) |
| Yes | 74.7 (73.9–75.5) | 72.8 (71.6–74.0) | 76.3 (75.3–77.3) |
| Cannabis | |||
| No | 46.5 (46.4–46.6) | 44.8 (44.6–44.9) | 47.7 (47.6–47.9) |
| Yes | 64.3 (63.2–65.4) | 62.0 (60.5–63.5) | 67.4 (65.7–69.1) |
| Cocaine/amphetamine | |||
| No | 46.5 (46.4–46.6) | 44.8 (44.6–44.9) | 47.7 (47.6–47.9) |
| Yes | 68.1 (67.0–69.3) | 65.5 (64.0–67.0) | 72.5 (70.6–74.3) |
| Sedative/hypnotic/anxiolytic | |||
| No | 46.6 (46.5–46.7) | 44.9 (44.8–45.1) | 47.8 (47.7–47.9) |
| Yes | 72.3 (70.0–74.5) | 72.5 (69.2–75.8) | 72.1 (69.0–75.1) |
| Other drugb | |||
| No | 46.4 (46.3–46.5) | 44.7 (44.6–44.9) | 47.7 (47.6–47.8) |
| Yes | 68.3 (67.3–69.4) | 66.8 (65.3–68.3) | 70.1 (68.6–71.7) |
| Total number of SUD diagnoses | |||
| None | 44.9 (44.8–45.0) | 43.1 (42.9–43.3) | 46.2 (46.1–46.3) |
| 1 | 58.4 (58.1–58.7) | 54.5 (54.0–54.9) | 62.5 (62.1–63.0) |
| 2 | 64.0 (63.1–64.8) | 60.5 (59.5–61.6) | 69.0 (67.8–70.2) |
| 3 or more | 71.0 (69.9–72.0) | 68.5 (67.1–69.9) | 74.7 (73.1–76.4) |
Note: the analysis (N=951,533) was based on the EHR data from January 1, 2013 to December 31, 2018. CI: confidence interval; SUD: substance use disorder.
Any drug use disorder included diagnoses for opioid, cannabis, cocaine/amphetamine, sedative/hypnotic/anxiolytic, hallucinogen, inhalant, or other/unspecified drug use disorder.
Other drug use disorders included hallucinogen, inhalant, or other/unspecified drug use disorder. Boldface: estimate for females significantly different from estimate for males (p<0.05).
The prevalence of CNCP during the study period among patients with any SUD diagnosis was 59.9% compared to 44.9% among patients without a SUD diagnosis. Over two-thirds of patients (68.9%) with any drug use disorder had CNCP. Across individual SUD categories, the prevalence of CNCP was highest among patients with OUD (74.7%), followed by those with sedative use disorder (72.3%). Moreover, the prevalence of CNCP increased with the number of comorbid SUD diagnoses (none: 44.9%, 1 SUD: 58.4%, 2 SUDs: 64.0%, and ≥3 SUDs: 71.0%). The prevalence of CNCP was higher among females than males for all SUD diagnosis categories except for sedative use disorder. The prevalence of CNCP within one year of SUD diagnoses logged in the EHRs was similar for all subgroups (Table S2).
3.3. Demographic and clinical characteristics among patients with SUDs by CNCP status (Table 3)
Table 3.
Clinical characteristics among patients with substance use disorder (SUD) diagnoses by chronic non-cancer pain (CNCP) status among adult patients aged 18 or older.
| Tobacco use disorder | Alcohol use disorder | Opioid use disorder | Other drug use disordera | |||||
|---|---|---|---|---|---|---|---|---|
| No CNCP | CNCP | No CNCP | CNCP | No CNCP | CNCP | No CNCP | CNCP | |
| n=34,583 | n=50,823 | n=7,932 | n=11,263 | n=3,070 | n=9,072 | n=5,505 | n=10,498 | |
| Column % | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) |
| Comorbid SUD diagnosis | ||||||||
| Tobacco | ----- | ----- | 41.0 (39.9–42.0) | 49.2 (48.3–50.1) | 39.5 (37.7–41.2) | 41.5 (40.5–42.5) | 54.2 (52.9–55.5) | 65.2 (64.3–66.1) |
| Alcohol | 9.39 (9.09–9.70) | 10.9 (10.6–11.2) | ----- | ----- | 12.7 (11.5–13.9) | 11.8 (11.1–12.4) | 26.1 (25.0–27.3) | 28.7 (27.8–29.5) |
| Cannabis | 4.11 (3.90–4.32) | 6.26 (6.04–6.47) | 8.11 (7.51–8.71) | 12.1 (11.5–12.7) | 10.0 (8.94–11.1) | 10.0 (9.40–10.6) | 45.3 (44.0–46.6) | 42.7 (41.8–43.7) |
| Opioid | 3.50 (3.31–3.70) | 7.41 (7.19–7.64) | 4.92 (4.44–5.39) | 9.48 (8.94–10.0) | ----- | ----- | 16.5 (15.5–17.5) | 25.0 (24.2–25.9) |
| Cocaine/amphetamine | 3.91 (3.71–4.12) | 6.49 (6.27–6.70) | 9.78 (9.13–10.4) | 15.7 (15.1–16.4) | 13.7 (12.5–14.9) | 13.6 (12.9–14.3) | 35.8 (34.6–37.1) | 40.1 (39.2–41.1) |
| Sedative | 0.64 (0.55–0.72) | 1.31 (1.21–1.41) | 1.49 (1.22–1.75) | 3.33 (3.00–3.66) | 5.37 (4.58–6.17) | 5.73 (5.25–6.21) | 7.77 (7.07–8.48) | 10.6 (10.0–11.2) |
| Other drugb | 3.51 (3.32–3.71) | 6.42 (6.21–6.64) | 7.74 (7.15–8.33) | 13.6 (12.9–14.2) | 19.1 (17.7–20.4) | 20.1 (19.3–20.9) | 41.8 (40.5–43.1) | 47.3 (46.3–48.2) |
| Mental health disorders | ||||||||
| Depressive disorder | 17.4 (17.0–17.8) | 27.1 (26.7–27.5) | 28.4 (27.4–29.4) | 38.3 (37.4–39.2) | 46.4 (44.6–48.1) | 52.6 (51.6–53.6) | 35.3 (34.1–36.6) | 47.2 (46.2–48.1) |
| Bipolar disorder | 3.33 (3.14–3.52) | 6.13 (5.92–6.33) | 5.66 (5.15–6.17) | 9.32 (8.79–9.86) | 8.34 (7.36–9.32) | 10.6 (9.95–11.2) | 12.6 (11.7–13.5) | 16.6 (15.9–17.4) |
| Mood disorder (any) | 20.0 (19.6–20.4) | 30.9 (30.5–31.3) | 32.2 (31.2–33.2) | 42.9 (42.0–43.8) | 50.7 (48.9–52.4) | 58.1 (57.1–59.1) | 43.9 (42.6–45.2) | 55.8 (54.8–56.7) |
| Anxiety disorder | 20.7 (20.2–21.1) | 29.2 (28.8–29.6) | 30.5 (29.5–31.5) | 39.5 (38.6–40.4) | 51.4 (49.6–53.1) | 52.5 (51.5–53.6) | 39.0 (37.7–40.3) | 49.9 (48.9–50.8) |
| Psychotic disorder | 3.52 (3.33–3.72) | 4.20 (4.03–4.38) | 5.70 (5.19–6.21) | 7.57 (7.08–8.06) | 5.93 (5.09–6.76) | 6.10 (5.60–6.59) | 12.2 (11.3–13.1) | 12.6 (12.0–13.2) |
| Sleep disorder | 15.9 (15.5–16.3) | 23.2 (22.8–23.5) | 19.9 (19.0–20.7) | 28.9 (28.1–29.8) | 36.6 (34.9–38.3) | 42.1 (41.1–43.1) | 21.9 (20.8–23.0) | 30.1 (29.2–31.0) |
| Otherc | 6.03 (5.78–6.28) | 9.95 (9.69–10.2) | 11.3 (10.6–12.0) | 15.7 (15.0–16.4) | 19.5 (18.1–20.9) | 21.5 (20.6–22.3) | 17.1 (16.1–18.1) | 22.3 (21.5–23.1) |
| Any | 38.5 (38.0–39.0) | 50.6 (50.2–51.1) | 53.4 (52.3–54.5) | 64.2 (63.3–65.1) | 74.0 (72.4–75.5) | 78.1 (77.3–79.0) | 63.9 (62.7–65.2) | 73.9 (73.1–74.7) |
| Chronic medical conditions | ||||||||
| Diabetes, type 1 or 2 | 17.8 (17.4–18.2) | 20.1 (19.8–20.5) | 15.7 (14.9–16.5) | 19.6 (18.9–20.4) | 24.0 (22.5–25.5) | 25.6 (24.7–26.5) | 15.1 (14.1–16.0) | 20.7 (19.9–21.4) |
| Asthma | 8.49 (8.20–8.79) | 14.4 (14.1–14.7) | 7.44 (6.86–8.02) | 13.7 (13.1–14.3) | 14.4 (13.1–15.6) | 19.1 (18.3–20.0) | 11.2 (10.4–12.0) | 19.3 (18.5–20.1) |
| COPD | 24.0 (23.6–24.5) | 22.1 (21.7–22.4) | 18.1 (17.3–19.0) | 19.3 (18.6–20.0) | 25.3 (23.8–26.8) | 22.7 (21.8–23.6) | 16.1 (15.1–17.0) | 20.0 (19.2–20.8) |
| Ischemic heart disease | 18.6 (18.2–19.0) | 15.3 (15.0–15.6) | 16.4 (15.6–17.2) | 16.3 (15.6–17.0) | 20.8 (19.3–22.2) | 17.4 (16.6–18.2) | 13.9 (12.9–14.8) | 15.9 (15.2–16.6) |
| Hepatitis B or C | 3.16 (2.98–3.34) | 3.91 (3.74–4.08) | 6.15 (5.62–6.68) | 7.32 (6.84–7.80) | 9.12 (8.10–10.1) | 8.19 (7.63–8.75) | 8.59 (7.85–9.33) | 10.4 (9.77–10.9) |
| Chronic kidney disease | 17.8 (17.4–18.2) | 15.7 (15.4–16.0) | 22.6 (21.7–23.5) | 21.9 (21.2–22.7) | 33.8 (32.2–35.5) | 25.1 (24.2–26.0) | 22.1 (21.0–23.2) | 24.5 (23.6–25.3) |
| Hypertension | 45.9 (45.4–46.4) | 48.9 (48.5–49.3) | 51.9 (50.8–53.0) | 58.2 (57.3–59.1) | 53.4 (51.6–55.2) | 55.0 (53.9–56.0) | 40.9 (39.6–42.2) | 50.4 (49.4–51.3) |
| Any | 63.1 (62.6–63.7) | 65.1 (64.7–65.5) | 65.2 (64.2–66.3) | 72.1 (71.2–72.9) | 72.6 (71.1–74.2) | 71.8 (70.8–72.7) | 57.8 (56.5–59.1) | 69.7 (68.8–70.5) |
Note: the analysis was based on the EHR data from January 1, 2013 to December 31, 2018. CI: confidence interval; CNCP: chronic non-cancer pain; SUD: substance use disorder; COPD: chronic obstructive pulmonary disease.
Other (non-opioid) drug use disorders included cannabis, cocaine/amphetamine, sedative/hypnotic/anxiolytic, hallucinogen, inhalant, or other/unspecified drug use disorder.
Other drug use disorders included hallucinogen, inhalant, or other/unspecified drug use disorder.
Other mental health disorders included adjustment, personality, attention-deficit/impulse/conduct, somatoform, or eating disorders.
Boldface: the number in the CNCP group differed significantly from the number in the no CNCP group.
Among each SUD group, a higher proportion of those with CNCP than those without CNCP were female, of older age, and Black/African-American (Table S3). Among patients with tobacco or alcohol use disorder, the prevalence of each comorbid SUD diagnosis was higher among those with CNCP than those without CNCP. Among those with OUD, there was no significant difference in the prevalence of comorbid SUD diagnosis as a function of CNCP status. Among patients with non-opioid drug use disorders, the prevalence of each comorbid SUD was higher among those with CNCP than those without CNCP, except for cannabis use disorder, which was higher among those without CNCP. Moreover, the prevalence of most mental health disorder diagnoses was highest among each SUD group with CNCP than without CNCP. The prevalence of each mental health disorder diagnosis was highest among those with CNCP and OUD, except for bipolar and psychotic disorders, which were higher among those with CNCP and other drug use disorders. The overall prevalence of any chronic medical condition diagnoses was higher in each SUD group with CNCP than without CNCP, except among those with OUD, in which there was no difference.
3.4. Association of CNCP with opioid overdose and healthcare utilization (Table 4)
Table 4.
Adjusted odds ratio (AOR) of opioid overdose, emergency department (ED) admission, and inpatient hospitalization in relation to having chronic non-cancer pain (CNCP) vs. not having CNCP: stratified by substance use disorder diagnosis and by gender.
| Patients with substance use disorder | Overall | Males | Females |
|---|---|---|---|
| AOR (95% CI) of opioid overdose | |||
| Tobacco | 1.50 (1.25–1.81) | 1.42 (1.13–1.80) | 1.62 (1.18–2.22) |
| Alcohol | 1.47 (1.10–1.96) | 1.23 (0.89–1.70) | 2.48 (1.30–4.73) |
| Opioid | 0.49 (0.39–0.63) | 0.42 (0.31–0.56) | 0.70 (0.46–1.07) |
| Other druga | 1.32 (1.06–1.63) | 1.08 (0.83–1.40) | 1.94 (1.29–2.90) |
| AOR (95% CI) of ED admission | |||
| Tobacco | 2.07 (1.99–2.14) | 1.95 (1.85–2.04) | 2.22 (2.11–2.35) |
| Alcohol | 1.73 (1.60–1.87) | 1.80 (1.64–1.97) | 1.57 (1.36–1.81) |
| Opioids | 1.09 (0.94–1.28) | 1.10 (0.89–1.36) | 1.10 (0.88–1.39) |
| Other druga | 2.29 (2.08–2.53) | 2.03 (1.78–2.31) | 2.71 (2.33–3.16) |
| AOR (95% CI) of inpatient hospitalization | |||
| Tobacco | 1.28 (1.23–1.32) | 1.26 (1.20–1.32) | 1.31 (1.24–1.38) |
| Alcohol | 1.26 (1.17–1.36) | 1.28 (1.17–1.39) | 1.22 (1.06–1.39) |
| Opioids | 1.05 (0.91–1.22) | 1.30 (1.06–1.59) | 0.81 (0.65–1.02) |
| Other druga | 1.21 (1.11–1.31) | 1.28 (1.15–1.42) | 1.14 (1.00–1.29) |
Note: Separate models were conducted among patients with tobacco, alcohol, opioid, or other (non-opioid) drug use disorder, respectively, and for each dependent variable including opioid overdose, ED admission, and inpatient hospitalization, respectively. The overall model was controlled for gender, age group, race/ethnicity, any mental health disorder, chronic medical condition, and any cancer diagnosis. Each gender-specific model was controlled for age group, race/ethnicity, any mental health disorder, and any chronic medical condition. CI: confidence interval; CNCP: chronic non-cancer pain; AOR: adjusted logistic regression; ED: emergency department.
Other (non-opioid) drug use disorders included cannabis, cocaine/amphetamine, sedative/hypnotic/anxiolytic, hallucinogen, inhalant, or other/unspecified drug use disorder.
Boldface: estimate significantly differed from the estimate without CNCP (p<0.05)
The prevalence of opioid overdose was 5.09% among those with CNCP and OUD, followed by 4.22% among those with CNCP and other drug use disorders, and 2.03% and 1.13% among those with CNCP and alcohol and tobacco use disorder, respectively. The prevalence of opioid overdose was highest, however, among those with OUD and no CNCP (6.12%). More than two-thirds of each SUD diagnosis group with CNCP had an ED visit, including 85.9% of those with CNCP and non-opioid drug use disorder (Table S4). The prevalence of inpatient hospitalization was between 38.7%−63.1% among those with CNCP and a SUD diagnosis (Table S4).
Adjusted logistic regression models indicated that the presence of CNCP was associated with increased odds of opioid overdose, ED utilization, and inpatient hospitalization among those with tobacco, alcohol, or non-opioid drug use disorders. In contrast, CNCP was associated with decreased odds of overdose among those with OUD.
There was a significant CNCP and gender interaction for opioid overdose among those with alcohol (p<0.01) and other drug use disorder (p<0.01); for ED utilization among those with tobacco (p<0.001) or other drug use disorder (p<0.01); and for inpatient hospitalization among those with tobacco use disorder (p<0.001). Notably, having CNCP was associated with increased odds of opioid overdose among females, but not males, with alcohol or non-opioid drug use disorder.
4. Discussion
This study leveraged EHR data from a large healthcare system to examine the prevalence and associations of CNCP among patients with SUDs. Such data are important for informing the need and potential benefits of pain assessment/management among those with SUDs. Key features of this study extending prior research included the stratification of analyses by various SUD diagnoses and by gender. In this regard, several major findings emerged. First, we found that the majority of patients with each SUD diagnosis (58.7%−74.7%) had a co-occurring CNCP condition diagnosis, while the prevalence of CNCP was higher among females than males for most SUDs. Second, we found that CNCP was associated with distinct demographic and clinical characteristics, which differed further as a function of the type of SUD diagnosis. Finally, we found that CNCP among patients with SUDs was significantly associated with opioid overdose, ED admission, and hospitalization; however, the direction and magnitude of those associations differed by type of SUD diagnosis and gender, respectively.
The findings from this study underscore the importance of considering the impact of CNCP on the course and prognosis of not only OUD, but also the broader spectrum of non-opioid SUDs. That is, the close association between OUD and CNCP has been well-documented in the literature given the use of prescription opioids for pain as a common pathway to OUD (Stumbo et al., 2017). However, we found that a high proportion of patients with SUDs other than OUD also had CNCP, including nearly three-fourths of those with sedative use disorder (72.3%), more than half of those with tobacco (59.5%), alcohol (58.7%), and approximately two-thirds of those with cannabis (64.3%) or cocaine/amphetamine use disorder (68.1%).
Moreover, our study suggests that CNCP among patients with SUD may be an especially prominent factor within the general medical setting, given that the prevalence among our sample was within the upper range of what has been reported among samples from other settings (Alford et al., 2016; Caldeiro et al., 2008; Hser et al., 2017; Larson et al., 2007; Zale et al., 2015). While comparisons across studies are limited by various definitions of CNCP and differing sample characteristics, these findings likely reflect the common utilization of general medical settings for managing conditions causing CNCP or associated symptoms. Nonetheless, general medical physicians often report disinterest in or feeling unprepared to treat chronic pain, especially in the context of addiction (Barry et al., 2010), which highlights the importance of enhanced physician training around pain management principles of assessment and treatment.
Our findings also revealed that the prevalence of CNCP was greater among females than males for nearly all SUD diagnosis groups, the exception being sedative use disorder. Among those without each SUD diagnosis, the prevalence of CNCP was also higher among females than males, which is consistent with data from samples of the general population (Fullerton et al., 2018). Together, these findings suggest that females may be at a relatively increased risk for CNCP conditions independent of SUD status. Potential mechanisms may include biological sex differences in pain transmission, psychological factors, and/or sociocultural (e.g., gender role expectations) factors (Bartley and Fillingim, 2013; Mogil, 2012). Some previous research suggests that CNCP may be relatively under-treated among females compared to men (Calderone, 1990; LeResche, 2011), which could be reflected from our data as well. Moreover, the greater prevalence of CNCP among females with SUD in our sample may reflect gender differences in CNCP as a risk factor for developing SUD or vice versa. For instance, CNCP is commonly associated with mood-related problems (e.g., depression and anxiety), which has been shown to be more frequently associated with aberrant drug-related behavior in women than men (Jamison et al., 2010). It is also possible that women with SUDs in our sample were more likely to attribute their problematic substance use to self-medication of pain symptoms. A better understanding of gender-specific factors underlying the association between SUD and CNCP has implications for developing improved prevention and intervention strategies.
Other characteristics associated with CNCP among each SUD group included a higher proportion of patients who were of older age, Black/African-American, and had comorbid mental health disorder and chronic medical condition diagnoses. These findings indicate patient subgroups that may have increased vulnerability to CNCP or distinct factors that may influence treatment outcome, in which increased monitoring may be warranted. Differences, however, were found across SUD groups with regard to the prevalence of comorbid SUDs. That is, the prevalence of any comorbid SUD among those with OUD was not significantly different as a function of CNCP status. In contrast, it was found that CNCP was associated with a higher prevalence of comorbid SUDs among the other SUD groups. These findings may be explained in part by the relatively higher proportion of females, in which polysubstance use disorders are less common (John et al., 2018), among those with CNCP and OUD relative to the other SUD groups. Together, these data emphasize the issue of multicomorbidity of chronic conditions with SUDs, which is of concern given its particularly robust association with increased disease burden and costly healthcare utilization (Wu et al., 2018). Hence, chronic care treatment models with behavioral healthcare integration are important considerations for most effectively managing the care of patients with SUD and CNCP (Laderman, 2015; McLellan et al., 2013).
Among patients with tobacco, alcohol, and drug use disorders other than OUD, CNCP was associated with increased odds of ED utilization, hospitalization, and opioid overdose while controlling for demographic and clinical variables. These findings are consistent with prior studies and suggest that pain management strategies could potentially improve outcomes and reduce costly healthcare utilization (Alford et al., 2016; Caldeiro et al., 2008; Heimer et al., 2015). Interestingly, the opposite result for opioid overdose was found among those with OUD such that CNCP was associated with lower odds of opioid overdose, and no association was found between CNCP and ED utilization or hospitalization. One possible explanation for the differences in the association of CNCP with negative health outcomes as a function of SUD type may be related to motives of substance use among those with CNCP. For instance, prior research shows that individuals who reported nonmedical opioid use for non-pain relief purposes (e.g., to improve mood, sleep, or to relax) were more likely to have greater substance use severity and to have had an overdose compared to those who used for pain relief purposes (Bohnert et al., 2013). Thus, it is possible that substance use motives among patients with CNCP and OUD, compared to those with CNCP and other SUDs, were more directly related to physical pain relief, which may have constituted relatively less severity of use. Another hypothesis for the differences in the association of CNCP with overdose/healthcare utilization by SUD type is that CNCP may be relatively undertreated in patients with SUDs other than OUD. While more research is needed to explore these potential underlying factors, awareness of the frequent co-occurrence of CNCP with SUD as well as pain assessment/management should be key components of SUD treatment.
Gender differences were found in the association of CNCP with overdose and healthcare utilization in relation to type of SUD diagnosis. Notably, among those with alcohol or non-opioid drug use disorders, CNCP was associated with overdose only among females. On the other hand, CNCP among those with OUD was associated with decreased odds of overdose only among males. CNCP was also associated with hospitalization only among males with opioid or other drug use disorders. These findings extend prior work to not only suggest that CNCP may manifest in different ways as a function of gender (Bartley and Fillingim, 2013; Liang et al., 2016; Manubay et al., 2015), but also as a function of type of SUD diagnosis. In particular, these findings may suggest disparities in pain management, in which opioid overdose or healthcare utilization was a consequence. A better understanding of these relationships is needed, which has implications for maximizing the effectiveness of gender-specific prevention and intervention strategies.
There are some limitations to our study, which should be taken into consideration. Foremost, our findings are correlational in nature, which precludes cause and effect determinations from being made. The present results, however, provide a basis for which future studies can be conducted to better understand underlying mechanisms. It should also be noted that our data were derived from a single healthcare system in North Carolina, which may limit the generalizability of our results to other settings or regions. In particular, findings from this sample may contain an age-related bias considering the greater prevalence of chronic pain as a function of age and almost half of our sample was 50+ years of age (Cicero et al., 2012). Our data were also not able to capture the severity of pain intensity or degree of functional interference. These factors may have been especially important in the observed gender differences, given prior research showing greater pain severity in females than males (Fillingim et al., 2003; Keefe et al., 2000; Ruau et al., 2012). It was also not possible to infer whether nonmedical substance use was directly related to pain, which prior research suggests could be an important determinant of outcomes among those with chronic pain (Bohnert et al., 2013). Finally, it should be noted that EHR data were collected as part of routine care that may be influenced by biases including misclassification, condition severity, or provider specialty. However, to mitigate these biases, comorbidity was controlled for in logistic regression analyses.
4.1. Conclusions
In summary, the present study revealed four key findings. First, the majority of patients with various SUD diagnoses in our sample had a CNCP condition, which underscores the need for pain assessment and management as an integral component of SUD treatment in the general medical setting. Second, CNCP was associated with overdose and costly healthcare utilization in patients with tobacco, alcohol, and drug use disorders, but not those with OUD, which may indicate unmet treatment needs for pain. While emerging data from clinical trials support integrated treatment strategies (e.g., cognitive behavioral therapy, mindfulness) targeting both CNCP and SUD (Barry et al., 2019; Barry et al., 2014; Garland et al., 2014; Ilgen et al., 2016), the majority of these studies have been conducted only among individuals with CNCP and OUD. Thus, more research is greatly needed to inform treatment strategies for individuals with CNCP comorbid with non-opioid SUDs, in which distinct barriers and treatment needs may be present. Third, this study highlights the multicomorbidity of patients with SUDs and CNCP. These findings emphasize the need for integrated services and multidisciplinary care models to most effectively meet the complex treatment demands of patients with SUDs and CNCP and potentially improve outcomes. Finally, our findings suggest that CNCP may manifest differently among males and females in relation to the type of SUD diagnosis. In particular, CNCP may play a greater role in opioid overdoses among females with alcohol or drug use disorders than their male counterparts. More research is needed to inform the optimization of pain management strategies among those with SUDs, in which gender and SUD-specific strategies may be necessary for achieving maximum benefits.
Supplementary Material
Highlights.
Most patients with substance use disorders (SUDs) had chronic pain (CP) conditions.
The prevalence of CP was greater among females than males for most SUDs.
CP was associated with significant multicomorbidity among patients with SUDs.
Results suggest CP may be relatively untreated in patients with non-opioid SUDs.
CP may play a greater role in opioid overdoses among females than males with SUDs.
Acknowledgments
Role of the funding source: This study was made possible by the U.S. National Institutes of Health (grant numbers UG1DA040317 and R01MD007658; Principal Investigator: Li-Tzy Wu). The sponsoring agency had no further role in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the article for publication.
Footnotes
Conflict of interest: William S. John has received research funding from Patient-Centered Outcomes Research Institute. Li-Tzy Wu has also received research funding from Patient-Centered Outcomes Research Institute, as well as from the Centers for Disease Control and Prevention, Duke Endowment, and Alkermes Inc.
Conflict of interest statement: Li-Tzy Wu also has received research funding from Patient-Centered Outcomes Research Institute, Centers for Disease Control and Prevention, Duke Endowment, and Alkermes Inc. William S. John also has received research funding from Patient-Centered Outcomes Research Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alford DP, German JS, Samet JH, Cheng DM, Lloyd-Travaglini CA, Saitz R, 2016. Primary care patients with drug use report chronic pain and self-medicate with alcohol and other drugs. J. Gen. Intern. Med 31, 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Beitel M, Cutter CJ, Fiellin DA, Kerns RD, Moore BA, Oberleitner L, Madden LM, Liong C, Ginn J, Schottenfeld RS, 2019. An evaluation of the feasibility, acceptability, and preliminary efficacy of cognitive-behavioral therapy for opioid use disorder and chronic pain. Drug Alcohol Depend. 194, 460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Irwin KS, Jones ES, Becker WC, Tetrault JM, Sullivan LE, Hansen H, O’Connor PG, Schottenfeld RS, Fiellin DA, 2010. Opioids, chronic pain, and addiction in primary care. The Journal of Pain 11, 1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Savant JD, Beitel M, Cutter CJ, Schottenfeld RS, Kerns RD, Moore BA, Oberleitner L, Joy MT, Keneally N, 2014. The feasibility and acceptability of groups for pain management in methadone maintenance treatment. J. Addict. Med 8, 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley EJ, Fillingim RB, 2013. Sex differences in pain: a brief review of clinical and experimental findings. Br. J. Anaesth 111, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Arnsten JH, Sacajiu G, Karasz A, 2009. Providers’ experiences treating chronic pain among opioid-dependent drug users. J. Gen. Intern. Med 24, 482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert AS, Eisenberg A, Whiteside L, Price A, McCabe SE, Ilgen MA, 2013. Prescription opioid use among addictions treatment patients: Nonmedical use for pain relief vs. other forms of nonmedical use. Addict. Behav 38, 1776–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeiro RM, Malte CA, Calsyn DA, Baer JS, Nichol P, Kivlahan DR, Saxon AJ, 2008. The association of persistent pain with out-patient addiction treatment outcomes and service utilization. Addiction 103, 1996–2005. [DOI] [PubMed] [Google Scholar]
- Calderone KL, 1990. The influence of gender on the frequency of pain and sedative medication administered to postoperative patients. Sex Roles 23, 713–725. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Prescription drug overdose data & statistics: Guide to ICD-9-CM and ICD-10 codes related to poisoning and pain. Verson 1.3. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, State Health Department Training and Technical Assitance meeting 2013; https://www.cdc.gov/drugoverdose/pdf/pdo_guide_to_icd-9-cm_and_icd-10_codes-a.pdf. [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2018. Quality Improvement and Care Coordination: Implementing the CDC Guideline for Prescribing Opioids for Chronic Pain. National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention, Atlanta, GA. [Google Scholar]
- Centers for Disease Control Prevention (CDC), 2017. Annual surveillance report of drug-related risks and outcomes—United States, 2017. Surveillance Special Report 1. [Google Scholar]
- Centers for Medicare and Medicaid Services (CMS). Chronic Conditions. 2019; https://www.ccwdata.org/web/guest/condition-categories. Accessed June 10, 2019.
- Cicero TJ, Surratt HL, Kurtz S, Ellis M, Inciardi JA, 2012. Patterns of prescription opioid abuse and comorbidity in an aging treatment population. J. Subst. Abuse Treat 42, 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C, 2018. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morbidity and Mortality Weekly Report 67, 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersh J, Polatin PB, Gatchel RJ, 2002. Chronic pain and psychopathology: research findings and theoretical considerations. Psychosom. Med 64, 773–786. [DOI] [PubMed] [Google Scholar]
- Ditre JW, Kosiba JD, Zale EL, Zvolensky MJ, Maisto SA, 2016. Chronic pain status, nicotine withdrawal, and expectancies for smoking cessation among lighter smokers. Ann. Behav. Med 50, 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Finan PH, Tompkins DA, Fingerhood M, Strain EC, 2015. Characterizing pain and associated coping strategies in methadone and buprenorphine-maintained patients. Drug Alcohol Depend. 157, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Doleys DM, Edwards RR, Lowery D, 2003. Clinical characteristics of chronic back pain as a function of gender and oral opioid use. Spine (Phila Pa 1976) 28, 143–150. [DOI] [PubMed] [Google Scholar]
- Fullerton EF, Doyle HH, Murphy AZ, 2018. Impact of sex on pain and opioid analgesia: a review. Current opinion in behavioral sciences 23, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO, 2014. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: Results from an early-stage randomized controlled trial. J. Consult. Clin. Psychol 82, 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer R, Zhan W, Grau LE, 2015. Prevalence and experience of chronic pain in suburban drug injectors. Drug Alcohol Depend. 151, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M, 2016. Deaths: Leading Causes for 2013. Natl. Vital Stat. Rep 65, 1–95. [PubMed] [Google Scholar]
- Horvath MM, Rusincovitch SA, Brinson S, Shang HC, Evans S, Ferranti JM, 2014. Modular design, application architecture, and usage of a self-service model for enterprise data delivery: the Duke Enterprise Data Unified Content Explorer (DEDUCE). J Biomed Inform 52, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Mooney LJ, Saxon AJ, Miotto K, Bell DS, Huang D, 2017. Chronic pain among patients with opioid use disorder: Results from electronic health records data. J. Subst. Abuse Treat 77, 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgen MA, Bohnert AS, Chermack S, Conran C, Jannausch M, Trafton J, Blow FC, 2016. A randomized trial of a pain management intervention for adults receiving substance use disorder treatment. Addiction 111, 1385–1393. [DOI] [PubMed] [Google Scholar]
- Ilgen MA, Perron B, Czyz EK, McCammon RJ, Trafton J, 2010. The timing of onset of pain and substance use disorders. The American journal on addictions 19, 409–415. [DOI] [PubMed] [Google Scholar]
- Jakubczyk A, Ilgen M, Kopera M, Krasowska A, Klimkiewicz A, Bohnert A, Blow F, Brower K, Wojnar M, 2016. Reductions in physical pain predict lower risk of relapse following alcohol treatment. Drug Alcohol Depend. 158, 167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison RN, Butler SF, Budman SH, Edwards RR, Wasan AD, 2010. Gender differences in risk factors for aberrant prescription opioid use. The Journal of Pain 11, 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Zhu H, Mannelli P, Schwartz RP, Subramaniam GA, Wu L-T, 2018. Prevalence, patterns, and correlates of multiple substance use disorders among adult primary care patients. Drug Alcohol Depend. 187, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS, 2000. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain 87, 325–334. [DOI] [PubMed] [Google Scholar]
- Laderman M, 2015. Behavioral health integration: a key component of the triple aim. Population health management 18, 320–322. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Paasche-Orlow M, Cheng DM, Lloyd-Travaglini C, Saitz R, Samet JH, 2007. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction 102, 752–760. [DOI] [PubMed] [Google Scholar]
- LeResche L, 2011. Defining gender disparities in pain management. Clinical Orthopaedics and Related Research® 469, 1871–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Goros MW, Turner BJ, 2016. Drug overdose: differing risk models for women and men among opioid users with non-cancer pain. Pain Med. 17, 2268–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhapra A, Becker WC, 2018. Pain and addiction: an integrative therapeutic approach. Med. Clin. North Am 102, 745–763. [DOI] [PubMed] [Google Scholar]
- Manubay J, Davidson J, Vosburg S, Jones J, Comer S, Sullivan M, 2015. Sex differences among opioid-abusing chronic pain patients in a clinical trial. J. Addict. Med 9, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Starrels JL, Tai B, Gordon AJ, Brown R, Ghitza U, Gourevitch M, Stein J, Oros M, Horton T, 2013. Can substance use disorders be managed using the chronic care model? Review and recommendations from a NIDA consensus group. Public Health Rev. 35, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, 2012. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nature Reviews Neuroscience 13, 859. [DOI] [PubMed] [Google Scholar]
- Morasco BJ, Greaves DW, Lovejoy TI, Turk DC, Dobscha SK, Hauser P, 2016. Development and preliminary evaluation of an integrated cognitive-behavior treatment for chronic pain and substance use disorder in patients with the hepatitis C virus. Pain Med. 17, 2280–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JS, Chakrabarti A, Domier CP, Hillhouse MP, Weiss RD, Ling W, 2010. Pain and continued opioid use in individuals receiving buprenorphine-naloxone for opioid detoxification: secondary analyses from the Clinical Trials Network. J. Subst. Abuse Treat 38 Suppl 1, S80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK, 2003. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA 289, 2370–2378. [DOI] [PubMed] [Google Scholar]
- Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ, 2012. Sex differences in reported pain across 11,000 patients captured in electronic medical records. The Journal of Pain 13, 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon LS, 2012. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. J. Pain Palliat. Care Pharmacother. 26, 197–198. [Google Scholar]
- Speed TJ, Parekh V, Coe W, Antoine D, 2018. Comorbid chronic pain and opioid use disorder: literature review and potential treatment innovations. Int. Rev. Psychiatry 30, 136–146. [DOI] [PubMed] [Google Scholar]
- Stumbo SP, Yarborough BJ, McCarty D, Weisner C, Green CA, 2017. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J. Subst. Abuse Treat 73, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Oliva EM, Horst DA, Minkel JD, Humphreys K, 2004. Treatment needs associated with pain in substance use disorder patients: implications for concurrent treatment. Drug Alcohol Depend. 73, 23–31. [DOI] [PubMed] [Google Scholar]
- Tsui JI, Lira MC, Cheng DM, Winter MR, Alford DP, Liebschutz JM, Edwards RR, Samet JH, 2016. Chronic pain, craving, and illicit opioid use among patients receiving opioid agonist therapy. Drug Alcohol Depend. 166, 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, McCallion E, Vowles KE, Kirouac M, Frohe T, Maisto SA, Hodgson R, Heather N, 2015. Association between physical pain and alcohol treatment outcomes: The mediating role of negative affect. J. Consult. Clin. Psychol 83, 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Vowles KE, 2018. Alcohol and opioid use, co- use, and chronic pain in the context of the opioid epidemic: a critical review. Alcoholism: clinical and experimental research 42, 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley MJ, Heinzerling KG, Shoptaw S, Ling W, 2017. Volatility and change in chronic pain severity predict outcomes of treatment for prescription opioid addiction. Addiction 112, 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-T, Zhu H, Ghitza UE, 2018. Multicomorbidity of chronic diseases and substance use disorders and their association with hospitalization: Results from electronic health records data. Drug Alcohol Depend. 192, 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, Maisto SA, Ditre JW, 2015. Interrelations between pain and alcohol: An integrative review. Clin. Psychol. Rev 37, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.