Abstract
Objectives:
Studies examining the effects of statins after acute myocardial infarction (AMI) excluded frail older adults, especially nursing home (NH) residents, and few examined functional outcomes. Older NH residents may benefit less from statins and be particularly susceptible to adverse drug events like myopathy-related functional decline. We evaluated the effects of statins on 1-year functional decline, rehospitalization, and death in NH residents.
Design:
We conducted a retrospective cohort study using 2007 to 2010 linked national data from Minimum Data Set (MDS) assessments; Medicare claims; and Online Survey Certification and Reporting System records.
Setting and Participants:
We included U.S. NH residents 65 years and older who were statin non-users, were hospitalized for AMI between May 2007 and March 2010, and returned to the NH.
Measures:
Outcomes were functional decline, death, and rehospitalization in the first year after post-AMI NH admission. New statin users were 1:1 propensity-score matched to non-users to adjust for 92 characteristics. We estimated hazard ratios (HRs) and restricted mean survival time differences with 95% confidence intervals (CI) comparing individuals who did versus did not initiate statin therapy after AMI hospitalization.
Results:
Propensity-score matching yielded a cohort of 5,440 residents. Mean age was 83 years and 69% were female. Statin use was associated with a reduction in mortality (HR 0.80, 95%CI 0.73–0.87), corresponding to a mean of 15.9 (95%CI 9.9–22.0) days of extended life expectancy. No overall differences in rehospitalization (HR 1.06, 95%CI 0.98–1.14) or functional decline (HR 1.00, 95%CI 0.88–1.14) were observed.
Conclusions and Implications:
Statins may reduce one-year mortality by 20% without affecting function among older NH residents who wish to live longer after AMI. During shared decision-making with these patients or their representatives, clinicians should consider communicating that the average benefit of statins is 16 days of additional survival over one year.
Keywords: hydroxymethylglutaryl-CoA reductase inhibitors, frailty, nursing homes, secondary prevention, activities of daily living
Brief Summary:
Statins confer two weeks of additional survival without affecting function or rehospitalization risk among older nursing home residents after acute myocardial infarction.
INTRODUCTION
Statins are a mainstay of guideline-recommended secondary prevention after acute myocardial infarction (AMI) for adults of all ages.1–3 Data from multiple randomized clinical trials (RCTs) support the use of statins to reduce mortality after AMI in older adults aged ≥65 years, among whom more than two-thirds of all cardiovascular disease (CVD) deaths occur.4 However, after decades of research, it remains unclear whether the mortality benefit of statin use generalizes to older adults who are multimorbid, cognitively or functionally impaired, and frail.5 Nearly all individuals with this phenotype were excluded from RCTs of statins, even those that enrolled older adults.5 The potential lack of generalizability is most pronounced for long-term nursing home (NH) residents because they are the frailest and oldest subpopulation in the U.S.6–8 NH residents have limited life expectancy and may not derive a mortality benefit from statin use after AMI.
Adults residing in the NH are at high risk of functional decline and, given their limited life expectancy, often prioritize preserving their remaining functional independence and quality of life.9,10 Therefore, the muscle pain and related symptoms that some studies have found to be associated with statin use may be of importance to frail older adults. The muscle pain may be especially important if it interferes with activities of daily living (ADLs) and ultimately results in a functional decline. If such a relationship exists, NH residents are likely at greater risk than other populations due to their frailty and physiologic susceptibility to medication adverse effects.
To evaluate the potential benefit and potential function harms of statin use in this highly vulnerable patient population, we estimated the association between statin use after AMI and functional decline, rehospitalization, and mortality outcomes among frail older adults in the NH setting. We hypothesized that statins would be associated with an increased risk of functional decline, but a decreased risk of hospitalization and mortality.
METHODS
Study Design and Data Source
This was a retrospective new-user cohort study using existing7,8,11–14 national Medicare data linked to the Minimum Data Set (MDS)15 version 2.0 and Online Survey Certification and Reporting System (OSCAR) data. The MDS is a quarterly assessment tool of resident characteristics that is required for all facilities that are certified to receive Medicare or Medicaid funding. MDS assessments occur at least every 3 months, and more often for patients with a major recent change in clinical status and those receiving care under the Medicare Skilled Nursing Facility benefit. The OSCAR data16 provides facility-level information on NH characteristics, staffing levels, and quality indicators. Medicare claims include information on inpatient care (Part A), outpatient care (Part B), and prescription drug dispensings (Part D17). Part D covers over 90% of NH residents and is the sole source of prescription drug coverage for these patients. A previously validated residential history file algorithm was used to track the timing and location of health service use.18
Study Population
The study population was a previously established7,8,11–14 national cohort of long-stay NH residents aged ≥65 years without a history of AMI who were hospitalized for AMI (ICD-9 codes 410.XX or 411.1 in principal or secondary position on inpatient claim), had not taken statins for at least 12 months before the AMI, and were readmitted to a U.S. NH directly after hospital discharge between May 1, 2007 and December 31, 2010 (Figure A1). We selected previous non-users to permit an evaluation of the decision to initiate statins after AMI, distinct from the decision to continue these agents in patients who had already been taking them before their AMI. However, we excluded patients with extremely poor functional status before the AMI hospitalization (ADL score ≥24) because they had little opportunity for further functional decline (see “Outcomes” below).11,19 Additional details of the cohort have been previously described.7,8,11
Exposures and Causal Contrast of Interest
Statin initiation after AMI was identified in Medicare Part D prescription drug claims (individual drugs enumerated in Table A1).7,8,11,17 The causal contrast of interest was defined as the effect of initiating statins versus not, regardless of subsequent treatment discontinuation or switching among treatment groups, analogous to intention-to-treat analyses in randomized controlled trials.20–22
Outcomes
The three outcomes were all-cause death, all-cause rehospitalization, and functional decline. We used data from Medicare Part A and Medicare enrollment files to identify hospital admissions and date of death. Functional decline was defined as an increase of 3 points on the validated 28-point MDS Morris scale of independence in ADLs between the prehospital baseline assessment and the first available assessment after hospitalization up to 12 months after discharge.19,23 This measure indicates the degree of dependence on staff assistance in seven areas of ADL function (bed mobility, transfer, locomotion, dressing, eating, toilet use, personal hygiene), which are summed to create a validated score that ranges from 0 (no assistance required) to 28 (total dependence in ADL functioning).19 Increases in this score over time have been validated as an important marker of functional decline, and a 3-point increase corresponds to a major loss of independence in one ADL or incremental losses in two or more ADLs.11,23
Follow-up
Prior studies suggest that the lag time to benefit from statins (i.e., the time from statin initiation to when outcomes are observably improved) is between six months and one year, so we selected a one-year follow-up period.5,24,25 We excluded individuals who died or were rehospitalized within 14 days of hospital discharge because reliable ascertainment of secondary prevention medication use is difficult in such short-stay situations. Follow-up therefore started on day 14 (index date) after hospital discharge and continued up to 1 year.11 For the rehospitalization outcome, at the end of the 1-year follow-up, participants were classified as alive without rehospitalization, having had a rehospitalization, or having died without a rehospitalization. For the functional decline outcome, at the end of the 1-year follow-up, participants were classified as alive without functional decline, having had functional decline documented on an MDS assessment in that period, or having died without evidence of functional decline on the MDS. For death, individuals were simply categorized as alive or dead at one year.
Baseline Characteristics
Variables that could potentially confound the relationship between statins and outcomes were prespecified and all measured prior to the index date. A complete list of these 92 characteristics and details about their measurement are provided in Table A2.
Statistical Analyses
We adjusted for confounding by baseline covariates using methods that rely on estimating the propensity score. We estimated the propensity scores via a flexible logistic regression model that used the aforementioned 92 baseline variables to (Table A2) to predict the use of statins. The initial model achieved good balance in measured covariates (see below) with good discrimination (c-statistic = 0.78), and was thus used for all analyses.
We matched one new user of statins to one non-user on the propensity score via a greedy 5-to-1 digit matching algorithm without replacement.26 Propensity score distributions in each treatment group were examined using histograms and descriptive statistics.27 Matching resulted in good covariate balance based on standardized mean differences (Table A3).27
We estimated hazard ratios (HRs) with 95% confidence intervals (CIs) using Cox proportional hazards regression models to compare statin new users versus non-users for all outcomes of interest.28 The robust sandwich estimator was used to account for the clustering within propensity score-matched pairs. To better understand the difference in outcomes between treatment groups, we calculated the difference in restricted mean survival time (RMST).29,30 The difference in RMST is interpretable as the average number of days that prescribing a statin will delay a negative health outcome over one year of follow-up time. As an alternative to the difference in RMST, we estimated 1-year risk differences with 95% CIs calculated using the non-parametric bootstrap, and the accompanying numbers needed to treat (NNT) or harm (NNH).
Statistical significance was defined as p-value < 0.05.
Stability and Sensitivity Analyses
We chose Cox regression models as the main analysis to facilitate the calculation of the RMST measures and thus, provision of clinically relevant information. However, we also evaluated the Fine and Gray approach to estimation in a stability analysis to determine if our results were robust to our analytic decision. The Fine and Gray approach helps to account for the competing risk of death.
To assess how robust our findings were to potential unmeasured or residual confounding, we conducted a sensitivity analysis using the E-value.31 The E-value is the minimum strength of association, on the risk ratio scale, that an unmeasured confounder would need to have with both statin use and an outcome to fully explain away the observed treatment effect estimate (i.e., if there truly was no effect).31
Subgroup Analyses
In separate analyses to evaluate whether the association between statins and outcomes varied across participant characteristics (i.e., effect measure modification32), we included interaction terms between the exposure and characteristic (i.e., multiplied the two independent variables). These baseline characteristics included levels of age (≤85 versus >85)33, sex (male versus female), cognitive function (moderate to severe impairment versus no to mild impairment), functional status (moderate to severe impairment versus no to mild impairment), ICU/CCU stay (any versus none), and polypharmacy (<11 medications versus ≥11 medications). Individuals were re-matched on the propensity score for subgroup analyses to ensure covariate balance between treatment groups within subgroups. We observed good covariate balance using standardized mean differences.
Software
Data were analyzed using Stata, version 15.0 (Stata Corp., College Station, TX), software.
Ethics Approval
The study protocol was approved by the institutional review boards of all participating institutions.
RESULTS
Study Cohort
Our initial cohort included 11,192 NH residents, of whom 3,217 (28.7%) were new statin users and 7,975 (71.3%) were nonusers after AMI. The mean (SD) age of the study cohort was 84 (8) years, approximately 50% of the cohort had moderate to severe cognitive impairment (n=5,801), and 74% required extensive or greater assistance with their ADLs (n=8,259). On average, residents were actively taking 11 medications (SD=5) before their AMI. The median pre-AMI length of NH stay was 554 (interquartile range [IQR] 144–1,277) days. Propensity-score matching yielded a cohort of 5,440 residents—2,720 statin users matched to an equal number of non-users.
The prevalence of resident characteristics by treatment group are shown in Table 1 and accompanying standardized differences in Table A3. Even before matching, the distribution of resident characteristics between statin users and non-users after AMI were quite similar. The characteristics of the NHs in which residents resided were also highly similar between statin users and non-users before and after matching (Table 2).
Table 1.
Characteristics of statin new users and non-users before and after propensity score matching
| No. (%)* | ||||
|---|---|---|---|---|
| Before Matching | After Matching | |||
| Characteristics | Statins (n=3217) | No Statins (n=7975) | Statins (n=2720) | No Statins (n=2720) |
| Age, mean (SD), years | 82.1 (8.0) | 84.8 (8.1) | 82.8 (7.8) | 82.7 (8.2) |
| Female sex | 2135 (66.4) | 5892 (73.9) | 1872 (68.8) | 1852 (68.1) |
| Race | ||||
| White | 2608 (81.1) | 6629 (83.1) | 2216 (81.4) | 2195 (80.7) |
| African American | 416 (12.9) | 909 (11.4) | 347 (12.8) | 362 (13.3) |
| Other | 193 (6.0) | 437 (5.5) | 157 (5.8) | 163 (6.0) |
| Body Mass Index, mean (SD), kg/m2 | 26.9 (7.0) | 25.7 (6.5) | 26.9 (7.0) | 26.2 (6.9) |
| Chronic Conditions | ||||
| Hyperlipidemia | 712 (22.1) | 631 (7.9) | 458 (16.8) | 407 (15.0) |
| Diabetes | 1013 (31.5) | 1990 (25.0) | 804 (29.6) | 814 (29.9) |
| Hypertension | 1967 (61.1) | 4373 (54.8) | 1612 (59.3) | 1627 (59.8) |
| Heart Failure | 1563 (48.6) | 3850 (48.3) | 1349 (49.6) | 1341 (49.3) |
| Atrial fibrillation | 705 (21.9) | 2038 (25.6) | 622 (22.9) | 622 (22.9) |
| Peripheral vascular disease | 296 (9.2) | 533 (6.7) | 240 (8.8) | 233 (8.6) |
| Depression | 426 (13.2) | 978 (12.3) | 353 (13.0) | 367 (13.5) |
| COPD | 828 (25.7) | 2096 (26.3) | 698 (25.7) | 720 (26.5) |
| Arthritis | 357 (11.1) | 984 (12.3) | 308 (11.3) | 297 (10.9) |
| Elixhauser comorbidity score, median (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) |
| ADL status before hospitalization† | ||||
| Independent to limited assistance required | 1150 (35.8) | 2541 (31.9) | 934 (34.3) | 996 (36.6) |
| Extensive assistance required | 1137 (35.3) | 2577 (32.3) | 962 (35.4) | 926 (34.0) |
| Extensive dependency | 930 (28.9) | 2857 (35.8) | 824 (30.3) | 798 (29.3) |
| Cognitive status before hospitalization‡ | ||||
| Intact or borderline intact | 1094 (34.0) | 2139 (26.8) | 871 (32.0) | 893 (32.8) |
| Mild to moderate dementia | 1679 (52.2) | 4146 (52.0) | 1434 (52.7) | 1403 (51.6) |
| Moderately severe to very severe dementia | 444 (13.8) | 1690 (21.2) | 415 (15.3) | 424 (15.6) |
| Health instability (CHESS score) before hospitalization, mean (SD)§ | 0.6 (0.8) | 0.7 (0.8) | 0.6 (0.8) | 0.6 (0.8) |
| Geriatric symptoms before hospitalization | ||||
| Falls | 620 (19.3) | 1771 (22.2) | 528 (19.4) | 546 (20.1) |
| Dyspnea | 237 (7.4) | 643 (8.1) | 204 (7.5) | 231 (8.5) |
| Number of medications before hospitalization, median (IQR) | 11 (8–15) | 11 (8–14) | 11 (8–14) | 11 (8–15) |
| Medication use before hospitalization | ||||
| Angiotensin-converting enzyme inhibitor | 817 (25.4) | 2292 (28.7) | 736 (27.1) | 779 (28.6) |
| Angiotensin receptor blocker | 263 (8.2) | 746 (9.4) | 238 (8.8) | 231 (8.5) |
| Warfarin | 279 (8.7) | 930 (11.7) | 246 (9.0) | 256 (9.4) |
| Antiplatelet agent | 320 (10.0) | 990 (12.4) | 292 (10.7) | 304 (11.2) |
| Calcium channel blocker | 505 (15.7) | 1316 (16.5) | 436 (16.0) | 436 (16.0) |
| Diuretic | 1140 (35.4) | 3670 (46.0) | 1033 (38.0) | 1042 (38.3) |
| Nitrate | 428 (13.3) | 1601 (20.1) | 385 (14.2) | 379 (13.9) |
| Length of hospital stay for AMI, median (IQR), d | 6 (4–9) | 6 (4–9) | 6 (4–9) | 6 (4–9) |
| No. of days in ICU or CCU | ||||
| None | 1156 (35.9) | 3717 (46.6) | 1034 (38.0) | 1011 (37.2) |
| 1–2 | 929 (28.9) | 1900 (23.8) | 741 (27.2) | 728 (26.8) |
| ≥3 | 1132 (35.2) | 2358 (29.6) | 945 (34.7) | 981 (36.1) |
| Nursing home length of stay before AMI, median (IQR), d | 413 (92–1,094) | 617 (175–1,336) | 450 (104–1,140) | 509 (110–1,229) |
| Nursing home care pathway after hospitalization | ||||
| Skilled nursing facility benefit | 2480 (77.1) | 5547 (69.6) | 668 (24.6) | 638 (23.5) |
| Long-term care | 737 (22.9) | 2428 (30.5) | 2052 (75.4) | 2082 (76.5) |
Abbreviations: SD, standard deviation; IQR, interquartile range; COPD; chronic obstructive pulmonary disease; CHESS, Changes in Health, End-stage Disease, Signs, and Symptoms; ADL, activities of daily living; AMI, acute myocardial infarction; ICU, intensive care unit; CCU, coronary care unit.
Percentages have been rounded and may not total 100.
Measured by the Morris 28-point scale of independence in ADLs, and categorized as 0 to 14 (independent to limited assistance required), 15 to 19 (extensive assistance required), and 20 or higher (extensive dependency).
Measured by the Cognitive Performance Scale and trichotomized as 0 to 1 (intact to borderline intact), 2 to 3 (mild to moderate dementia), and 4 to 6 (moderately severe to very severe dementia).
Scores ranging from 0 to 5, with higher scores indicating greater health instability.
Table 2.
Nursing home facility characteristics of statin new users and non-users before and after propensity score matching
| No. (%)* | ||||
|---|---|---|---|---|
| Before Matching | After Matching | |||
| Characteristics | Statins (n=3217) | No Statins (n=7975) | Statins (n=2720) | No Statins (n=2720) |
| Ownership | ||||
| For profit | 2339 (72.7) | 5758 (72.2) | 1970 (72.4) | 1982 (72.9) |
| Not for profit | 716 (22.3) | 1726 (21.6) | 609 (22.4) | 610 (22.4) |
| Government | 162 (5.0) | 491 (6.2) | 151 (5.2) | 128 (4.7) |
| Size, No. of beds, mean (SD) | 148.6 (91.4) | 139.9 (79.8) | 147.0 (88.1) | 146.2 (88.9) |
| Occupancy, % of beds occupied, median (IQR) | 90.4 (82.3–95.0) | 90.0 (81.3–95.0) | 90.3 (82.3–95.0) | 90.5 (82.0–95.0) |
| Quality indicators | ||||
| Residents restrained, median (IQR), % | 2.8 (0.4–6.5) | 3.0 (0.4–6.8) | 2.8 (0.5–6.5) | 2.8 (0–5.7) |
| No. of quality-of-life deficiencies, mean (SD) | 0.7 (1.1) | 0.7 (1.1) | 0.7 (1.0) | 0.7 (1.0) |
| Residents with pressure sores, mean (SD), % | 7.2 (4.4) | 7.1 (4.5) | 7.1 (4.4) | 7.2 (4.4) |
| Direct care per resident per day, mean (SD), h | 3.4 (0.8) | 3.3 (0.8) | 3.4 (0.8) | 3.4 (0.8) |
| Physician staffing, median (IQR), full time equivalents per 100 beds | 0.15 (0.06–0.28) | 0.13 (0.06–0.26) | 0.14 (0.06–0.28) | 0.14 (0.06–0.27) |
Abbreviations: SD, standard deviation; IQR, interquartile range.
Percentages have been rounded and may not total 100.
After matching, there were 991 (18.2%) functional decline, 2,812 (51.7%) rehospitalization, and 2,036 (37.4%) death events. Figure A2 displays the propensity score distributions before and after matching.
Outcomes of Statin Use
Prescribing statins was associated with a significant decrease in the risk of mortality compared to non-use after AMI (HR 0.80, 95%CI 0.73–0.87), but no significant difference in all-cause rehospitalization (HR 1.06, 95%CI 0.98–1.14) (Table 3; Table A4). The mortality benefit of statin use corresponded to an increase in survival time of 16 days (95%CI 10–22) and an NNT of 17 (95%CI 12–28) over one year of follow-up. Statin use was not associated with functional decline (HR 1.00, 95%CI 0.88–1.14).
Table 3.
Effect of statin use versus non-use following myocardial infarction on outcomes among frail older adults after propensity score matching
| Percent with Outcome | HR (95% CLs) | Risk Difference (95% CLs)*,† | NNT / NNH (95% CLs)‡ | Difference in RMST (95% CLs)§ | ||
|---|---|---|---|---|---|---|
| Outcome | Statin | No Statin | ||||
| Mortality | 34.3 | 40.5 | 0.80 (0.73, 0.87) | −6.2 (−8.7, −3.6) | NNT 17 (12, 28) | 16 (10, 22) |
| Rehospitalization | 52.9 | 50.5 | 1.06 (0.98, 1.14) | 2.4 (−0.3, 5.0) | NNH 43 (NNT 313 to ∞ to NNH 20) | −4 (−12, 3) |
| Functional Decline | 18.6 | 17.8 | 1.00 (0.88, 1.14) | 0.9 (−1.2, 2.9) | NNH 118 (NNT 86 to ∞ to NNH 35) | −1 (−6, 5) |
Abbreviations: PY, person-years; HR, hazard ratio; CLs, confidence limits; NNT, number needed to treat; NNH, number needed to harm; RMST, restricted mean survival time.
Reported as a percent rather than a proportion.
Confidence intervals estimated using bootstrapping with 10,000 replicates.
Confidence intervals for non-significant NNT/NNH expressed in the format recommended by “Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998 Nov 7;317(7168):1309–12”.
Restricted mean survival time is interpretable as the average gain or loss in event-free days due to statin use versus non-use during a 1-year follow-up period; for example, residents who initiated statins would increase the time they survived after myocardial infarction by 16 days on average over a 1-year follow-up period.
Results before propensity score matching in Table A4.
Stability and Sensitivity Analyses
In stability analyses accounting for the competing risk of death (Table A5), results were similar to the main analyses: statin use after AMI was not associated with a significant increase in the risk of all-cause rehospitalization (HR 1.06, 95%CI 0.99–1.15) and functional decline (HR 1.03, 95%CI 0.91–1.17).
The E-value for the matched mortality HR was 1.61. Thus, an unmeasured confounder that was associated with both statin use and mortality with a risk ratio of 1.61 each could explain the observed effect on mortality, suggesting moderate sensitivity to unmeasured confounding.
Treatment Effects in Subgroups
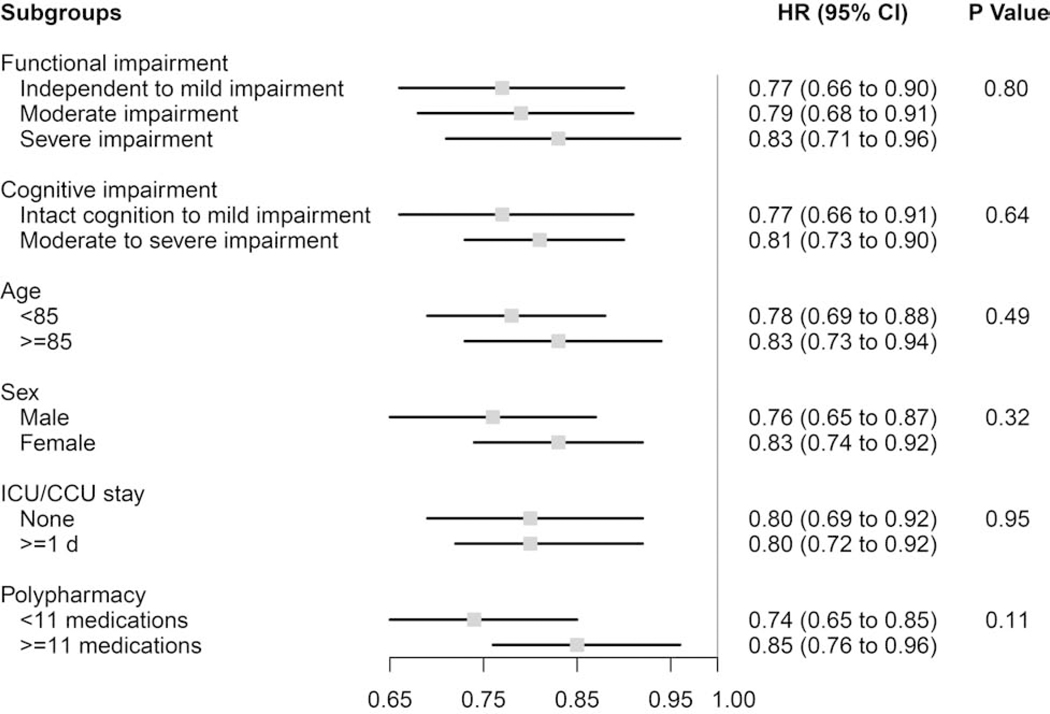
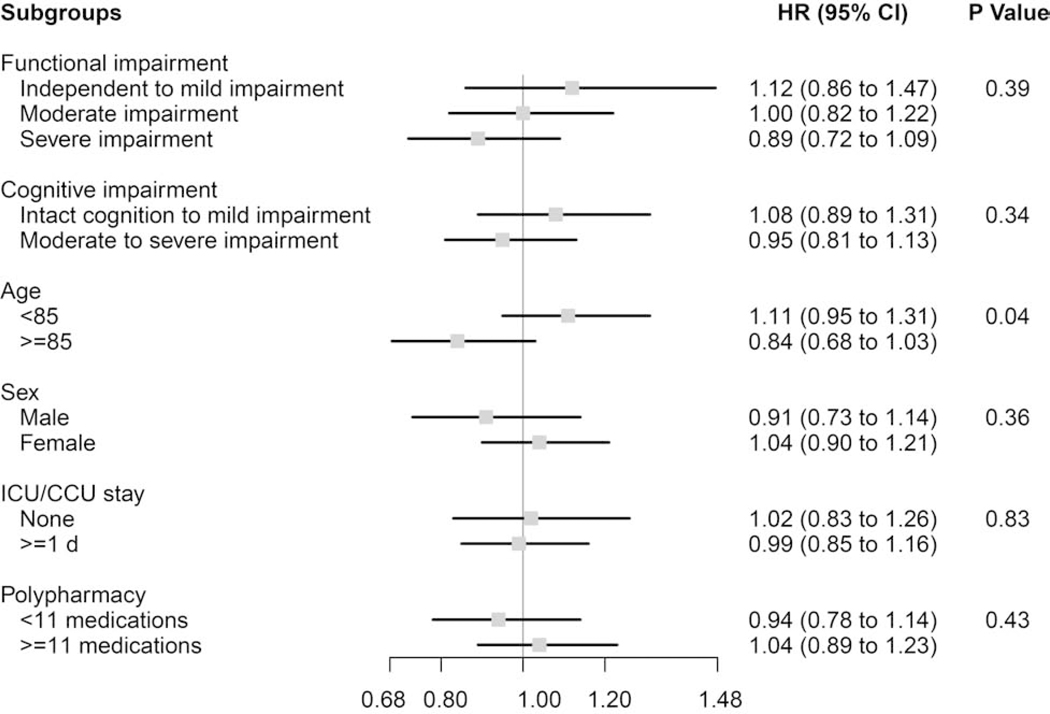
In subgroup analyses stratifying residents by functional impairment, cognitive impairment, age, sex, critical care use during the AMI, and polypharmacy, almost no notable differences were observed for the associations between statin use and mortality (Figure 1), functional decline (Figure 2), or rehospitalization (Table A6). The one exception was that statins were non-significantly associated with a slight increase in functional decline (Figure 2) among residents <85 years old (HR 1.11, 95%CI 0.95–1.31), but were non-significantly associated with a decrease among residents aged ≥85 years (HR 0.84, 95%CI 0.68–1.03), resulting in a significant difference by age subgroup (P-value for effect modification=0.04). Statins were associated with a significant mortality benefit across all subgroups examined.
Figure 1. Subgroup analyses of the effect of statin use versus non-use on mortality among frail older adults after myocardial infarction (N=5,440).
P Values for effect modification by subgroup characteristic.
Functional impairment measured using the Minimum Data Set 28-point Activities of Daily Living (ADL) Scale; Independent to mild impairment is represented by an ADL score of 0 to 14 (independent to limited assistance required with ADLs), moderate impairment is represented by an ADL score of 15 to 19 (extensive assistance required with ADLs), and severe impairment is represented by an ADL score of ≥20 (extensive dependency in ADLs).
Cognitive impairment measured using the Minimum Data Set Cognitive Performance Score (CPS); Intact to Mild Impairment is represented by a CPS score of 0 to 2, and Moderate to Severe Impairment is a score of ≥3.
Figure 2. Subgroup analyses of the effect of statin use versus non-use on functional decline among frail older adults after myocardial infarction (N=5,440).
P Values for effect modification by subgroup characteristic.
Functional impairment measured using the Minimum Data Set 28-point Activities of Daily Living (ADL) Scale; Independent to mild impairment is represented by an ADL score of 0 to 14 (independent to limited assistance required with ADLs), moderate impairment is represented by an ADL score of 15 to 19 (extensive assistance required with ADLs), and severe impairment is represented by an ADL score of ≥20 (extensive dependency in ADLs).
Cognitive impairment measured using the Minimum Data Set Cognitive Performance Score (CPS); Intact to Mild Impairment is represented by a CPS score of 0 to 2, and Moderate to Severe Impairment is a score of ≥3.
DISCUSSION
In this national retrospective cohort study, statin use post-AMI was associated with a 20% decrease in one-year mortality in frail, older adults residing in NHs. The decrease corresponded to approximately 16 additional days of survival on average, which concords with estimates ranging from −10 to 27 days in secondary prevention trials of statins.34 Use of statins did not appear to influence the risk of functional decline or rehospitalization. These results suggest that even frail older adults may derive a mortality benefit from statins, although an average gain in survival of 16 days may not be meaningful to many frail, older adults, especially those with very limited life expectancy. Providers should elicit whether a mortality benefit of that magnitude is important to a given older adult and their family before prescribing statins for secondary prevention post-AMI. The costs of statin treatment, and of monitoring for adverse effects, is also another relevant consideration.
Our study meaningfully contributes to the existing literature by: using one of the largest and most nationally representative samples of frail older adults; examining the immediate post-acute care period after AMI; assessing validated functional outcomes in addition to traditional ones; and using modern advanced methods to adjust for a wide array of potentially confounding covariates. Other data on the effects of statin use in frail, older adults are scarce, but our study is consistent with another using 1992–1997 Medicare and MDS data from four states.35 The study population was NH residents with prevalent CVD rather than incident AMI as in our study.35 Among other study design and analytic differences, statin exposure was prevalent rather than new use because the authors had no information on medication use before NH admission. The authors found that statins were associated with a 31% reduction in one-year mortality (HR=0.69, 95%CI 0.58–0.81). The larger mortality reduction estimated by the authors was likely due, at least in part, to their mixed prevalent and incident statin user cohort.35,36 For comparison, estimates from statin secondary prevention trials ranged from a 0% to 30% reduction in all-cause mortality.34 The authors observed no difference in functional decline (HR=0.95, 95%CI 0.75–1.19) or rehospitalization (HR=0.98, 95%CI 0.68–1.12).
Little other literature on the potential effects of statins on functioning is available, though many hypotheses and potential mechanisms for the relationship between statins and functioning have been introduced over time.37 Of note, although myopathy-mediated functional decline may be most plausible, studies have not examined whether a relationship between myopathy and functional decline exists. Available studies do not focus on frail, older adults or secondary prevention after AMI. One study found that statins were associated with better self-reported physical function, but not a summary performance measure of walking, chair rises, dressing, and a tandem stand in predominantly non-frail older adults.38 Two others of mainly non-frail community-dweller older adults (mostly aged<80) provided evidence of no effect: one39 found no association between statins and ability to walk one-quarter of a mile or climb 10 steps without resting over 6.5 years of follow-up, while the other40 found no association between statins and decrease in the number of activities the study participants were able to perform at follow-up. These findings are generally consistent with our observed statins-functional decline HR of 1.00.
Limitations
The findings of this study must be interpreted in light of several limitations.
First, because our study was observational, we cannot rule out the possibility of residual confounding. One plausible mechanism is that individuals with more severe AMI are more likely to receive statins after AMI. A second potential mechanism is that individuals who were more frail or had a worse prognosis were less likely to receive statins because prescribers expected such patients not to benefit from use. A third mechanism is that providers were more likely to prescribe statins to individuals with higher laboratory values of lipids, which were unmeasured in our data. However, several factors support the robustness of our findings. The various proposed mechanisms of confounding would counteract one another, at least to some extent. We also obtained good balance on over 92 measured covariates between treatment groups, including several validated measures related to frailty (e.g., baseline ADL status), even before propensity score matching. Furthermore, in prior work, we conducted a companion validation study using national data from the Department of Veterans Affairs, which contains information on vital signs, laboratory test results, and measures of cardiac function that was missing from our linked Medicare and MDS data.11 Those analyses suggested that such variables would not substantially alter the observed results.
Secondly, we could not conduct analyses of statin dose (i.e., intensity) due to the nature of our data. Myalgias may be dose-dependent in older adults. Although our results suggest that overall use of statins is not associated with functioning, it remains possible that high-dose statin use could increase the risk of myalgias and subsequent functional decline. Future studies should aim to address this question. Although guidelines recommend moderate- or high-intensity statins for secondary prevention in adults aged >75 years, until more evidence is available about frail adults specifically, providers might consider starting low-dose statins and increasing dose as tolerated.41
Thirdly, to enable robust assessment of statin exposure, we excluded patients who died or were rehospitalized within the first 14 days of hospital discharge. Thus, our results should be interpreted as providing evidence about the effect of statins on outcomes starting 14 days after hospital discharge, among people who had survived and remained in the NH until then. In addition, these exclusions could induce selection bias42, though prior work suggests this bias would be mild.11
Fourth, our study was also limited by the frequency of functional outcome assessments. Functional outcomes were measured intermittently on MDS assessments rather than continuously like mortality and rehospitalization. Declines in function would have been unmeasured if they occurred between an MDS assessment and death.
Finally, although we present HRs with 95% CIs, it is important to note that HRs tend to exaggerate treatment effects and may misinform treatment decisions.43 We therefore provide the differences in RMST alongside the HRs to improve clinical interpretability and present results on a time scale that can be intuitively understood by patients and their clinicians. However, the difference in RMST measure still has potential disadvantages. Primarily, that it provides an estimate of the gain or loss in the event-free survival time due to a treatment in a specified follow-up period, and this follow-up period must be suitably chosen.30
Conclusions and Implications
In summary, the use of statins post-AMI was associated with decreased mortality in frail, older NH residents, but no difference in functional decline or rehospitalization. The mortality benefit was consistently observed across subgroups defined by baseline age, cognition, functional status, and other characteristics. However, given the limited life expectancy of frail older NH residents, the mortality benefit conferred by statins is likely to be small in absolute terms, and should be factored into decision-making.
Supplementary Material
Acknowledgements:
Sponsor’s Role: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Meeting Presentations: Parts of this paper were presented at the International Society of Pharmacoepidemiology Midyear Meeting, April 23, 2018, Toronto, Canada and at the American Geriatrics Society Annual Meeting, May 4, 2018, Orlando, FL.
Funding: This study was supported by grants R01HL111032 and K24AG049057 from the National Institutes of Health (NIH), and by awards 5K12HS022998 from the Agency for Healthcare Research and Quality, R21AG061632 from the National Institute on Aging, and U54GM115677 from the National Institute of General Medical Sciences to Dr. Zullo. Dr. Zullo is also supported by a U.S. Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship in Health Services Research and Development.
Footnotes
CONFLICT OF INTEREST
All other authors have no relevant conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–2473. [DOI] [PubMed] [Google Scholar]
- 2.American College of Emergency P, Society for Cardiovascular A, Interventions, O’Gara PT, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;61(4):e78–140. [DOI] [PubMed] [Google Scholar]
- 3.Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):e344–426. [DOI] [PubMed] [Google Scholar]
- 4.Afilalo J, Duque G, Steele R, Jukema JW, et al. Statins for secondary prevention in elderly patients: a hierarchical bayesian meta-analysis. Journal of the American College of Cardiology. 2008;51(1):37–45. [DOI] [PubMed] [Google Scholar]
- 5.Fleg JL, Forman DE, Berra K, Bittner V, et al. Secondary prevention of atherosclerotic cardiovascular disease in older adults: a scientific statement from the American Heart Association. Circulation. 2013;128(22):2422–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy CR, Radcliff TA, Williams ET, Hutt E. Acute myocardial infarction in nursing home residents: adherence to treatment guidelines reduces mortality, but why is adherence so low? Journal of the American Medical Directors Association. 2009;10(1):56–61. [DOI] [PubMed] [Google Scholar]
- 7.Zullo AR, Sharmin S, Lee Y, Daiello LA, et al. Secondary Prevention Medication Use After Myocardial Infarction in U.S. Nursing Home Residents. Journal of the American Geriatrics Society. 2017;65(11):2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zullo AR, Lee Y, Daiello LA, Mor V, et al. Beta-Blocker Use in U.S. Nursing Home Residents After Myocardial Infarction: A National Study. Journal of the American Geriatrics Society. 2017;65(4):754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerety MB, Chiodo LK, Kanten DN, Tuley MR, et al. Medical treatment preferences of nursing home residents: relationship to function and concordance with surrogate decision-makers. Journal of the American Geriatrics Society. 1993;41(9):953–960. [DOI] [PubMed] [Google Scholar]
- 10.Harris-Kojetin L, Sengupta M, Park-Lee E, Valverde R, et al. Long-Term Care Providers and services users in the United States: data from the National Study of Long-Term Care Providers, 2013–2014. Vital Health Stat 3 2016(38):x–xii; 1–105. [PubMed] [Google Scholar]
- 11.Steinman MA, Zullo AR, Lee Y, Daiello LA, et al. Association of beta-Blockers With Functional Outcomes, Death, and Rehospitalization in Older Nursing Home Residents After Acute Myocardial Infarction. JAMA internal medicine. 2017;177(2):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zullo AR, Hersey M, Lee Y, Sharmin S, et al. Outcomes of “Diabetes‐Friendly” versus “Diabetes‐Unfriendly” Beta‐blockers in Older Nursing Home Residents with Diabetes after Acute Myocardial Infarction. Diabetes, obesity & metabolism. 2018;20(12):2724–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zullo AR, Mogul A, Corsi K, Shah NR, et al. Association Between Secondary Prevention Medication Use and Outcomes in Frail Older Adults After Acute Myocardial Infarction. Circulation Cardiovascular quality and outcomes. 2019;12(4):e004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zullo AR, Hersey M, Lee Y, Sharmin S, et al. Outcomes of “Diabetes-Friendly” versus “Diabetes-Unfriendly” Beta-blockers in Older Nursing Home Residents with Diabetes after Acute Myocardial Infarction. Diabetes, obesity & metabolism. 2018;20(12):2724–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris JN, Hawes C, Fries BE, Phillips CD, et al. Designing the national resident assessment instrument for nursing homes. The Gerontologist. 1990;30(3):293–307. [DOI] [PubMed] [Google Scholar]
- 16.Kash BA, Hawes C, Phillips CD. Comparing staffing levels in the Online Survey Certification and Reporting (OSCAR) system with the Medicaid Cost Report data: are differences systematic? The Gerontologist. 2007;47(4):480–489. [DOI] [PubMed] [Google Scholar]
- 17.Briesacher BA, Soumerai SB, Field TS, Fouayzi H, et al. Nursing home residents and enrollment in Medicare Part D. J Am Geriatr Soc. 2009;57(10):1902–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Intrator O, Hiris J, Berg K, Miller SC, et al. The residential history file: studying nursing home residents’ long-term care histories(*). Health services research. 2011;46(1 Pt 1):120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris JN, Fries BE, Morris SA Scaling ADLs within the MDS. The journals of gerontology Series A, Biological sciences and medical sciences. 1999;54(11):M546–553. [DOI] [PubMed] [Google Scholar]
- 20.Danaei G, Rodriguez LA, Cantero OF, Logan R, et al. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Statistical methods in medical research. 2013;22(1):70–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernan MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. American journal of epidemiology. 2016;183(8):758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huitfeldt A, Hernan MA, Kalager M, Robins JM. Comparative Effectiveness Research Using Observational Data: Active Comparators to Emulate Target Trials with Inactive Comparators. EGEMS (Wash DC). 2016;4(1):1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpenter GI, Hastie CL, Morris JN, Fries BE, et al. Measuring change in activities of daily living in nursing home residents with moderate to severe cognitive impairment. BMC geriatrics. 2006;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barter PJ, Waters DD. Variations in time to benefit among clinical trials of cholesterol-lowering drugs. J Clin Lipidol. 2018;12(4):857–862. [DOI] [PubMed] [Google Scholar]
- 25.Yourman LC, Lee SJ, Schonberg MA, Widera EW, et al. Prognostic indices for older adults: a systematic review. JAMA : the journal of the American Medical Association.2012;307(2):182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Statistics in medicine. 2014;33(6):1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuart EA. Matching methods for causal inference: A review and a look forward. Statistical science : a review journal of the Institute of Mathematical Statistics. 2010;25(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. Journal of the American Statistical Association. 1989;84(408):1074–1078. [Google Scholar]
- 29.Royston P, Parmar MK. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Statistics in medicine. 2011;30(19):2409–2421. [DOI] [PubMed] [Google Scholar]
- 30.Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC medical research methodology. 2013;13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Annals of internal medicine. 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 32.VanderWeele TJ. On the distinction between interaction and effect modification. Epidemiology (Cambridge, Mass). 2009;20(6):863–871. [DOI] [PubMed] [Google Scholar]
- 33.Rockwood K, Howlett SE, MacKnight C, Beattie BL, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59(12):1310–1317. [DOI] [PubMed] [Google Scholar]
- 34.Kristensen ML, Christensen PM, Hallas J. The effect of statins on average survival in randomised trials, an analysis of end point postponement. BMJ open. 2015;5(9):e007118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eaton CB, Lapane KL, Murphy JB, Hume AL. Effect of statin (HMG-Co-A-Reductase Inhibitor) use on 1-year mortality and hospitalization rates in older patients with cardiovascular disease living in nursing homes. Journal of the American Geriatrics Society. 2002;50(8):1389–1395. [DOI] [PubMed] [Google Scholar]
- 36.Schneeweiss S, Patrick AR, Sturmer T, Brookhart MA, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Medical care. 2007;45(10 Supl 2):S131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savo A, Maiorano PM, Onder G, Bernabei R. Pharmacoepidemiology and disability in older adults: can medications slow the age-related decline in physical function? Expert opinion on pharmacotherapy. 2004;5(2):407–413. [DOI] [PubMed] [Google Scholar]
- 38.van Boheemen L, Tett SE, Sohl E, Hugtenburg JG, et al. Associations Between Statin Use and Physical Function in Older Adults from The Netherlands and Australia: Longitudinal Aging Study Amsterdam and Australian Longitudinal Study on Women’s Health. Drugs & aging. 2016;33(6):437–445. [DOI] [PubMed] [Google Scholar]
- 39.Gray SL, Boudreau RM, Newman AB, Studenski SA, et al. Angiotensin-converting enzyme inhibitor and statin use and incident mobility limitation in community-dwelling older adults: the Health, Aging and Body Composition study. Journal of the American Geriatrics Society. 2011;59(12):2226–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAvay G, Allore HG, Cohen AB, Gnjidic D, et al. Guideline-Recommended Medications and Physical Function in Older Adults with Multiple Chronic Conditions. Journal of the American Geriatrics Society. 2017;65(12):2619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grundy SM, Stone NJ, Bailey AL, Beam C, et al. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2018. [Google Scholar]
- 42.Hernán MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology (Cambridge, Mass). 2004;15(5):615–625. [DOI] [PubMed] [Google Scholar]
- 43.Trinquart L, Jacot J, Conner SC, Porcher R. Comparison of Treatment Effects Measured by the Hazard Ratio and by the Ratio of Restricted Mean Survival Times in Oncology Randomized Controlled Trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(15):1813–1819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.