Abstract
Background and Purpose:
Kratom is a coffee-like plant containing compounds that cause opioid and stimulant effects. The most prevalent bioactive alkaloid of kratom is mitragynine (MG). Opioid effects of MG are apparent (e.g. antinociception and nanomolar affinity for μ, κ and δ opioid receptors), but effects encompassing interactions with additional systems, such as adrenergic and dopaminergic, remain undefined. Given that enhanced adrenergic transmission is a mechanism common to most first-line neuropathic pain medications, we tested the hypothesis that MG reduces chemotherapy-induced neuropathic pain through a mechanism involving α-adrenoceptor activation.
Methods:
Rats were injected once with oxaliplatin (6 mg/kg IP) to induce allodynia and then treated with MG (0, 1, 5, 10 mg/kg IP) for 5-7 days. To investigate receptor mechanisms, a fixed dose of MG (5 mg/kg IP) was injected with yohimbine (5 mg/kg IP, α2-adrenoceptor antagonist), prazosin (5 mg/kg IP, α1-adrenoceptor antagonist), or naltrexone (5 mg/kg IP, opioid antagonist).
Key Results:
MG (5, 10 mg/kg) dose-dependently reduced mechanical sensitivity in oxaliplatin-injected rats. Anti-allodynic effects of MG were completely inhibited by yohimbine, and significantly reduced by prazosin and naltrexone. MG produced modest hyperlocomotion but only at a dose (30 mg/kg) higher than those required to reduce allodynia.
Conclusion and Implication:
The finding that MG reduced neuropathic pain through a mechanism requiring active α-adrenoceptors indicates that the pharmacological profile of MG includes activation of adrenergic, as well as opioid, systems.
Keywords: mitragynine, kratom, neuropathic pain, oxaliplatin, adrenoceptor, adrenergic, opioid
1. Introduction
Mitragyna speciosa (i.e., kratom) contains at least 20 alkaloids, several of which are biologically active, with mitragynine (MG) being the major one (Jansen, 1988). Kratom has emerged as a popular herbal alternative to medical treatments in attempts to reduce opioid and alcohol dependence (Kruegel and Grundmann, 2018). The best defined pharmacodynamic feature of MG is μ-opioid receptor activation (Adkins et al., 2011). The affinity of MG is 7.2 nM at μ sites, 60 nM at δ sites, and >1000 nM at κ sites whereas morphine displays about 100-fold greater selectivity for μ versus δ and κ sites (Hassan et al., 2017). Compared to morphine, MG displays affinity at μ-opioid receptors that is 200-fold less, shows similar efficacy in the electrically-stimulated guinea pig ileum assay, and is about ¼ as potent (Yue et al., 2018). In preclinical pain studies, MG produces opioid-dependent antinociception in the mouse hot-plate and tail-pinch assays (Thongpradichote et al., 1998).
Despite containing alkaloids that activate μ-opioid receptors, kratom is not in the same plant family as the opium poppy. Rather, kratom is in the same plant family as coffee, the principal source of caffeine, which is the most the widely consumed psychostimulant in the world. In fact, kratom consumption elicits stimulant, as well as opioid effects, with stimulant effects presenting at low-to-moderate doses and opioid effects manifesting at higher doses (Kruegel and Grundmann, 2018). These reports suggest that kratom contains alkaloids, such as MG, that interact with non-opioid systems. To begin identifying and characterizing effects of MG on non-opioid targets, we tested the hypothesis that MG displays efficacy in a rat model of chemotherapy-induced neuropathic pain (CINP) through a mechanism requiring active α-adrenoceptors. Neuropathic pain was selected as the endpoint because enhanced adrenergic transmission at spinal and supraspinal sites inhibits neuropathic pain (Obata, 2017; Abed et al., 2017).
2. Materials and Methods
2.1. Animals and Chemicals
Male Sprague-Dawley rats (275-300 g) were pair-housed on a 12-h light/dark cycle. All animal use procedures were conducted in accordance with the National Research Council and the National Academies Press publication for the Care and Use of Laboratory Animals (adopted for use by the National Institutes of Health) and approved by the Temple University Institutional Animal Care and Use Committee. Oxaliplatin was purchased from Temple Hospital Pharmacy (Philadelphia, PA) and dissolved in sterile water. MG was purchased from Cayman Chemical Company (Ann Arbor, MI) with molecular weight and purity (>97.3%) confirmed by Dr. Allen Reitz (Fox Chase Chemical Diversity Center, Doylestown, PA). MG was dissolved in a vehicle of 20% Tween 80/sterile water. Yohimbine hydrochloride, prazosin hydrochloride, and naltrexone hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in sterile water. All drugs were administered intraperitoneally (IP) at a volume of 1 ml/kg.
2.2. Neuropathic pain experiments
Mechanical allodynia was assessed using Von Frey filaments (0.16-60 g bending force) with 5 min elapsing between consecutive filament tests on the same animal. Rats were placed on a wire mesh surface in individual boxes and allowed to habituate in the test room for 30 min. To determine paw withdrawal threshold, the 10.0 g filament was used to start, at which point if a withdrawal behavior was observed, further testing proceeded in a descending order of filament bending force until withdrawal behavior ceased. Conversely, if a withdrawal behavior was not observed using the 10.0 g filament, further testing proceeded in an ascending order of filament bending force until a withdrawal behavior was observed. The smallest bending force that elicited a response was used as the final score. Following baseline assessment, rats were injected with oxaliplatin (6 mg/kg) and returned to home cages for 24 h. Rats were then treated with MG (0, 1, 5, 10 mg/kg) for 5 days with mechanical sensitivity tested on days 2 and 5. For dose-effect experiments with MG, there were N=8 rats/group.
For antagonist experiments, rats were injected for 6 days with combinations of MG (5 mg/kg) plus vehicle, yohimbine (5 mg/kg), prazosin (5 mg/kg) or naltrexone (5 mg/kg). Mechanical sensitivity was assessed on days 2 and 5 of treatment and the day after cessation of treatment (day 7). For antagonist experiments with MG, there were N=12-16 rats/group. Doses and pretreatment paradigms for the adrenoceptor antagonists and naltrexone are based on prior work (Di Cesare Mannelli et al., 2017; Barbosa-Méndez et al., 2017; Cowan et al., 2014).
2.3. Locomotor experiments
Locomotor activity was assessed using a Digiscan DMicro system. Following a 60-min habituation in activity chambers, rats were treated with MG (0, 1, 5, 10 mg/kg) and activity was measured for 30 min. The experiment was repeated with a higher dose of MG (30 mg/kg) that included a paired vehicle (control) group. For locomotor experiments with MG, there were N=7 rats/group.
2.4. Statistical Analysis
Percentage baseline sensitivity was calculated for each rat as the sensitivity threshold value on test day divided by sensitivity threshold value at baseline multiplied by 100. Therefore, 100% baseline sensitivity represented no development of mechanical sensitivity following treatment, with lower % values equating to increasing sensitivity to mechanical stimulation. Mean percentage baseline withdrawal (±S.E.M.) was determined for each treatment group and analyzed by two-ANOVA (treatment x day) followed by Bonferonni analysis.
Locomotor dose-effect data (1-10 mg/kg) were analyzed by one-way ANOVA. For the higher MG dose (30 mg/kg), a two-way ANOVA (treatment x time) followed by Dunnett’s post-hoc test was used. A value of p<0.05 was considered statistically significant.
3. Results
3.1. MG reduced chemotherapy-induced neuropathic pain
Two-way ANOVA showed an effect of MG treatment [F(3,28)=3.42, p<0.05] (Fig. 1A). Compared to vehicle [MG (0)], 10 mg/kg of MG reduced oxaliplatin-induced allodynia on days 2 and 5 (p<0.05). On day 5, a lower dose (5 mg/kg) of MG also reduced oxaliplatin-induced allodynia (p<0.05).
Fig. 1. Mitragynine (MG) inhibits oxaliplatin-induced allodynia and enhances locomotor activation at high dose.

(A) Rats injected once with oxaliplatin (6 mg/kg) were then treated with MG (0, 1, 5 or 10 mg/kg) for 5 days and tested for mechanical sensitivity on days 2 and 5. *p<0.05, MG (10) compared to MG (0) and +p<0.05, MG (5) compared to MG (0). N=8 rats/group. (B) Locomotor activity was recorded for 30 min following treatment with MG (0, 1, 5 or 10 mg/kg). Data are expressed as the percentage of ambulatory counts in the vehicle [MG(0)] group. N=7 rats/group. (B, Inset) Locomotor activity was recorded for 30 min following treatment with MG (0, 30 mg/kg) and locomotor activity was recorded for 30 min. N=7 rats/group. For the 30-min pre-drug habituation interval preceding injection, cumulative locomotor counts for the VEH [MG(0)] (2143 ± 91) and MG (30) (2521 ± 295) groups were not significantly different (p>0.05, Student’s t-test). *p<0.05 or ***p<0.001 compared to MG (0).
3.2. MG (at a higher dose) produced a modest increase in locomotor activity
Within the a dose range of 1-10 mg/kg, MG did not affect locomotor activity (one-way ANOVA, [F(3,24)=0.3749, p>0.05]) (Fig. 1B). A higher dose of 30 mg/kg induced a modest, but significant, increase in locomotor activity as two-way ANOVA indicated a treatment effect [F(1,84)=13.43, p<0.001] and significant interaction [F(6,84)=2.653, p<0.05] (Fig 1B, inset), with enhanced activity detected 10 min (p<0.05) and 15 min (p<0.001) post-injection.
3.3. α-adrenoceptor and opioid antagonists reduced anti-allodynic efficacy of MG
Two-way ANOVA indicated a significant treatment effect [F(4,70)=14.22, p<0.001] (Fig. 2). Oxaliplatin exposure (VEH + VEH) produced allodynia that was reduced by treatment with MG (VEH + MG) on days 2 (p<0.05), 5 (p<0.001) and 7 (p<0.01). Suppression of oxaliplatin-induced allodynia by MG (VEH + MG) was reduced by treatment with (a) yohimbine and MG (YOH + MG) on days 2 (p<0.01), 5 (p<0.001), and 7 (p<0.05); (b) prazosin and MG (PRZ + MG) on days 5 (p<0.05) and 7 (p<0.05); or naltrexone and MG (NTX + MG) on days 2 (p<0.05) and 5 (p<0.05).
Fig. 2. α-adrenoceptor and opioid antagonists reduce anti-allodynic efficacy of mitragynine (MG).
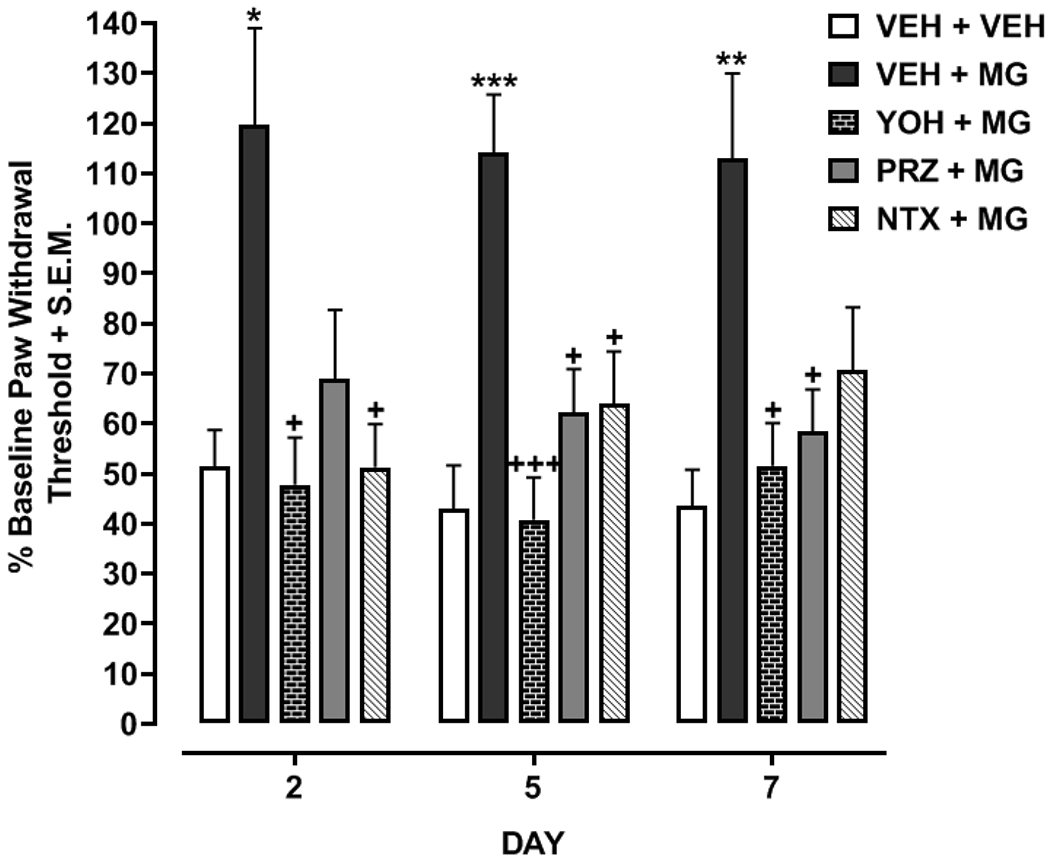
Rats injected once with oxaliplatin (6 mg/kg) were then treated for 6 days with MG (5 mg/kg) in combination with vehicle (VEH + MG), yohimbine (5 mg/kg) (YOH + MG), prazosin (5 mg/kg) (PRZ + MG), or naltrexone (5 mg/kg) (NTX + MG). A MG-naïve group that received just oxaliplatin was also included (VEH + VEH). Mechanical sensitivity was tested on days 2, 5 and 7. N=12-16 rats/group. *p<0.05, **p<0.01 or ***p<0.001 compared to VEH +VEH and +p<0.05 or +++p<0.001 compared to VEH + MG.
4. Discussion
MG displayed efficacy against chemotherapy-induced neuropathic pain that was inhibited by an α2-adrenoceptor antagonist and reduced by an α1-adrenoceptor antagonist. Mechanisms underlying the interaction between MG and α-adrenoceptors in the context of neuropathic pain are unclear, but approaches that enhance α-adrenoceptor transmission at spinal and supraspinal sites are efficacious in neuropathic pain models (Obata, 2017; Abed et al., 2017). Spinal administration of the α-adrenoceptor agonists phenylephrine (α1) and clonidine (α2), but not a β-adrenoceptor agonist (isoprenaline), decreases oxaliplatin-induced allodynia (Kim et al., 2017). Inhibition of norepinephrine reuptake in the spinal cord inhibits allodynia and hyperalgesia associated with neuropathic pain through α2-adrenoceptor activation (Kimura et al., 2013). For effects observed here with MG, multiple mechanisms are possible, including direct activation of α-adrenoceptors, as well as indirect activation, through processes such as inhibition of norepinephrine reuptake or catabolism (e.g. blockade of monoamine oxidase or catechol-O-methyltransferase). MG binding at α2-adrenoceptors has been reported (Boyer et al., 2008), but it did not act as a direct agonist in a cell line expressing α2-adrenoceptors (Tohda et al., 1997). Previous work using mice has also shown that the antinociceptive efficacy of MG in mouse tail-flick and hot-plate assays and suppression by MG of psychedelic-induced head-twitch responses is dependent on α2-adrenoceptor activity (Matsumoto et al., 1996, 1997).
Naltrexone also reduced the anti-allodynic effect of MG. In mouse hot-plate and tail-flick assays, MG produces antinociception that is antagonized by naloxone (Thongpradichote et al., 1998). To circumvent interpretational confounds between antinociceptive and anti-allodynic effects in our paradigm, daily injections of MG were administered 1 h following mechanical sensitivity testing. Thus, our results suggest that the anti-allodynic efficacy of MG is partially dependent on opioid receptor activity and are consistent with the orally-active MG derivative (MGM-16) displaying anti-allodynic efficacy in partial sciatic nerve-ligated mice that is antagonized by μ- and δ-opioid antagonists (Matsumoto et al., 2014).
Locomotor activity was modestly enhanced by MG but only at 30 mg/kg. A previous report showed that a dose range of 5-15 mg/kg of MG produced a modest, but significant, reduction in locomotor activity in the open-field test (Apryani et al., 2010). In contrast, a lower dose of MG (1 mg/kg), which entirely lacked efficacy in our experiments, enhanced locomotor activity within an hour after MG injection but not within the first 20 min (Yusoff et al., 2014). The different outcomes may be related to the fact that locomotor activity in our experiments was recorded for only 30 min after MG exposure.
In summary, a kratom alkaloid (MG) reduced neuropathic pain through adrenergic and opioid mechanisms. Future work will identify spinal and/or supraspinal sites of action and obtain dose-effect data with the different receptor antagonists using mice, which require less quantities of MG. In addition, future studies will determine if crosstalk between α-adrenoceptors and opioid receptors contributes to the anti-allodynic efficacy of MG. Indeed, combinations of opioid and α2-adrenoceptor agonists produce synergistic analgesia, and α2-adrenoceptor activation enhances the antinociceptive efficacy of μ-opioid receptors (Bahari and Meftahi, 2018).
Research Highlights.
Adrenergic profile of kratom alkaloid mitragynine was characterized.
Mitragynine reduced chemotherapy-induced neuropathic pain.
Yohimbine inhibited anti-allodynic efficacy of mitragynine.
Prazosin and naltrexone reduced anti-allodynic efficacy of mitragynine.
Mitragynine anti-allodynic efficacy depended on α-adrenoceptor activity.
Acknowledgements
The authors would like to thank the National Institute of Health and National Institute on Drug Abuse for funding through grants R21 AT010404, P30 DA013429, and T32 DA007237.
Role of Funding Source
The present work was supported by the National Center for Complementary and Integrative Health through grant R21 AT010404 and National Institute on Drug Abuse through grants P30 DA013429, and T32 DA007237.
Funding Sources: National Institutes of Health grants R21 AT010404, P30 DA013429, and T32 DA007237
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- Abed A, Khoshnoud MJ, Taghian M, Aliasgharzadeh M, Mesdaghinia A, 2017. Quetiapine reverses paclitaxel-induced neuropathic pain in mice: Role of alpha2-adrenergic receptors. Iran. J. Basic. Med. Sci 20(11), 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins JE, Boyer EW, McCurdy CR, 2011. Mitragyna speciosa, a psychoactive tree from Southeast Asia with opioid activity. Curr. Top. Med. Chem 11(9), 1165–1175. [DOI] [PubMed] [Google Scholar]
- Apryani E, Hidayat MT, Moklas MA, Fakurazi S, Idayu NF, 2010. Effects of mitragynine from Mitragyna speciosa Korth leaves on working memory. J Ethnopharmacol 129(3), 357–60. [DOI] [PubMed] [Google Scholar]
- Bahari Z, Meftahi GH, 2019. Spinal α2 -adrenoceptors and neuropathic pain modulation; therapeutic target. Br. J. Pharmacol 176(14), 2366–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Méndez S, Matus-Ortega M, Salazar-Juárez A, 2017. Synergistic interactions between mirtazapine and prazosin prevent the induction and expression of behavioral sensitization to cocaine in rats. Physiol. Behav 180, 137–145. [DOI] [PubMed] [Google Scholar]
- Cowan A, Raffa RB, Tallarida CS, Tallarida RJ, Christoph T, Schröder W, Tzschentke TM, 2014. Lack of synergistic interaction between the two mechanisms of action of tapentadol in gastrointestinal transit. Eur. J. Pain 18(8), 1148–1156. [DOI] [PubMed] [Google Scholar]
- Di Cesare Mannelli L, Ghelardini C, Micheli L, Del Bello F, Giannella M, Piergentili A, Pigini M, Quaglia W, 2017. Synergic stimulation of serotonin 5-HT1A receptor and α2-adrenoceptors for neuropathic pain relief: Preclinical effects of 2-substituted imidazoline derivatives. Eur. J. Pharmacol 810, 128–133. [DOI] [PubMed] [Google Scholar]
- Jansen KL, Prast CJ, 1988. Psychoactive properties of mitragynine (kratom). J. Psychoactive Drugs 20(4), 455–457. [DOI] [PubMed] [Google Scholar]
- Kimura M, Hayashida K, Eisenach JC, Saito S, Obata H, 2013. Relief of hypersensitivity after nerve injury from systemic donepezil involves spinal cholinergic and γ-aminobutyric acid mechanisms. Anesthesiology 118(1), 173–180. [DOI] [PubMed] [Google Scholar]
- Kruegel AC, Grundmann O, 2018. The medicinal chemistry and neuropharmacology of kratom: A preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacology 2018. 134(Pt A), 108–120. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Mizowaki M, Suchitra T, Murakami Y, Takayama H, Sakai S, Aimi N, Watanabe H, 1996. Central antinociceptive effects of mitragynine in mice: contribution of descending noradrenergic and serotonergic systems. Eur. J. Pharmacol 317(1), 75–81. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Mizowaki M, Takayama H, Sakai S, Aimi N, Watanabe H, 1997. Suppressive effect of mitragynine on the 5-methoxy-N,N-dimethyltryptamine-induced head-twitch response in mice. Pharmacol. Biochem. Behav 57(1-2), 319–323. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Narita M, Muramatsu N, Nakayama T, Misawa K, Kitajima M, Devi LA, Suzuki T, Takayama H, Horie S, 2014. Orally active opioid μ/δ dual agonist MGM-16, a derivative of the indole alkaloid mitragynine, exhibits potent antiallodynic effect on neuropathic pain in mice. J. Pharmacol. Exp. Ther 348(3), 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata H, 2017. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int. J. Mol. Sci 18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongpradichote S, Matsumoto K, Tohda M, Takayama H, Aimi N, Sakai S, Watanabe H, 1998. Identification of opioid receptor subtypes in antinociceptive actions of supraspinally-administered mitragynine in mice. Life Sci 62(16), 1371–1378. [DOI] [PubMed] [Google Scholar]
- Tohda M, Thongpraditchote S, Matsumoto K, Murakami Y, Sakai S, Aimi N, Takayama H, Tongroach P, Watanabe H, 1997. Effects of mitragynine on cAMP formation mediated by delta-opiate receptors in NG108-15 cells. Biol. Pharm. Bull 20(4), 338–340. [DOI] [PubMed] [Google Scholar]
- Yue K, Kopajtic TA, Katz JL, 2018. Abuse liability of mitragynine assessed with a self-administration procedure in rats. Psychopharmacology 235(10), 2823–2829. [DOI] [PubMed] [Google Scholar]
- Yusoff NH, Suhaimi FW, Vadivelu RK, Hassan Z, Rümler A, Rotter A, Amato D, Dringenberg HC, Mansor SM, Navaratnam V, Müller CP, 2016. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict. Biol 21(1), 98–110. [DOI] [PubMed] [Google Scholar]


