Abstract
We describe our design, synthesis, and chemical study of a set of functional epidithiodiketopiperazines (ETPs) and evaluation of their activity against five human cancer cell lines. Our structure–activity relationship-guided substitution of ETP alkaloids offers versatile derivatization while maintaining potent anticancer activity, offering exciting opportunity for their use as there are no examples of complex and potently anticancer (nM) ETPs being directly used as conjugatable probes or warheads. Our synthetic solutions to strategically designed ETPs with functional linkers required advances in stereoselective late-stage oxidation and thiolation chemistry in complex settings, including the application of novel reagents for dihydroxylation and cis-sulfidation of diketopiperazines. We demonstrate that complex ETPs equipped with a strategically substituted azide functional group are readily derivatized to the corresponding ETP-triazoles without compromising anticancer activity. Our chemical stability studies of ETPs along with cytotoxic evaluation of our designed ETPs against A549, DU 145, HeLa, HCT 116, and MCF7 human cancer cell lines provide insights into the impact of structural features on potency and chemical stability, informing future utility of ETPs in chemical and biological studies.
Introduction
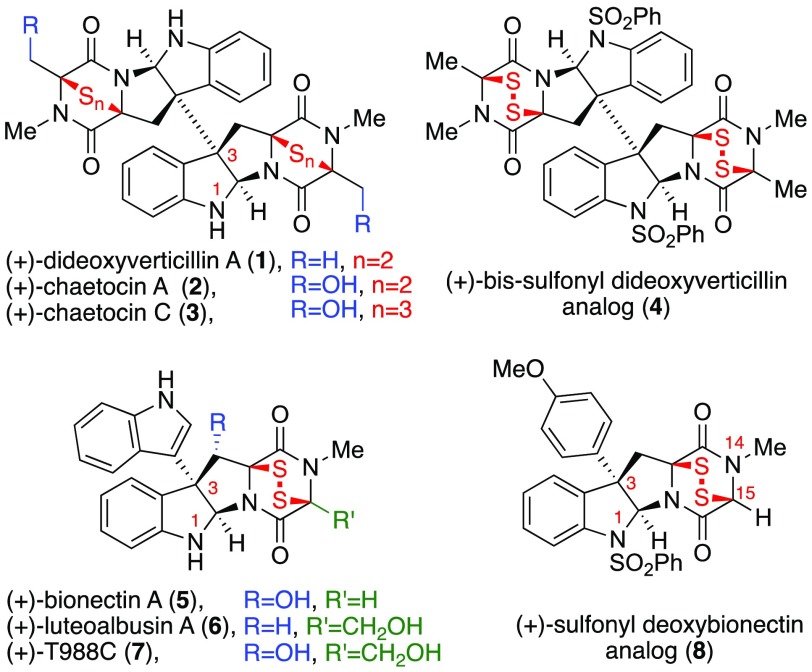
Epipolythiodiketopiperazine alkaloids comprise a structurally diverse and biologically active family of fungal metabolites characterized by a polysulfide bridged 2,5-diketopiperazine substructure (Figure 1).1−4 These natural products possess myriad biological activities, including anticancer,4,5 antifungal,6 antibacterial,6,7 and antiviral properties,8 and thus have prompted considerable interest in chemistry and allied sciences.9,10 While the mechanism of action of these compounds is not precisely understood, the pivotal role of the polysulfide bridge for bioactivity is well appreciated.1,4−8 At least three pathways of toxicity have been proposed in the literature1,4−8 for ETP-containing compounds: (1) redox cycling generating deleterious reactive oxygen species (ROS) (e.g., superoxide radical anion, hydroxyl radical, hydrogen peroxide) and causing oxidative stress, DNA strand cleavage, and apoptosis;5b (2) disruption of the global tertiary structures of proteins and/or inhibition of protein function due to thiol–disulfide exchange;5a−5e and (3) disruption of zinc-binding proteins by promoting intramolecular protein disulfide formation concomitant with zinc ion (or zinc ETP complex) ejection.5c,6,8 Our previous structure–activity relationship (SAR) study of a diverse set of cyclotryptophan-containing epipolythiodiketopiperazines for cytotoxic activity against several human cancer cell lines4 inspired our pursuit of synthetic epidithiodiketopiperazines (ETPs) with strategic substitution that enhances their translational potential as chemical probes11 and anticancer payloads.12 Herein, we describe our design and synthesis of complex ETPs (Figure 2), their chemical study and derivatization, and their cytotoxicity against A549, DU 145, HeLa, HCT 116, and MCF7 human cancer cell lines.
Figure 1.
Representative natural and unnatural ETPs.
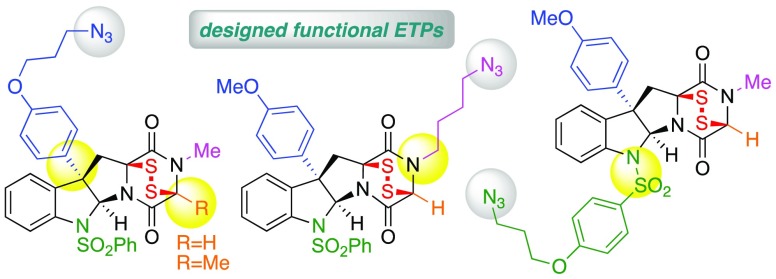
Figure 2.
Design of structurally diverse complex ETP-azides (+)-9a–d.
Our SAR study4 identified the dimeric ETP (+)-4 (Figure 1), a bis-sulfonyl derivative9a of the natural product (+)-dideoxyverticillin A (1),13 as a highly potent anticancer compound against five human cancer cell lines. Additionally, we recognized minimal required structural features in complex cyclotryptophan-ETPs for optimal cytotoxicity as well as strategic positions of the common substructure that allow substitution with minimal impact on anticancer activity.4 For example, the activity of N1-benzenesulfonyl substituted derivative (+)-4 was increased by up to 2 orders of magnitude compared to the natural product (+)-dideoxyverticillin A (1) {(+)-4 vs (+)-1; IC50 (U-937, histiocytic lymphoma): 0.18 nM vs 15.5 nM; IC50 (HeLa, cervical carcinoma): 0.09 nM vs 7.2 nM; IC50 (NCl-H460, lung carcinoma): 1.53 nM vs 42 nM; IC50 (786-O, renal carcinoma): 1.55 nM vs 33.5 nM; IC50 (MCF7, breast carcinoma): 1.65 nM vs 28.4 nM}.14 Likewise, the unnatural C3-aryl ETP (+)-8, a truncated analogue of dimeric alkaloid (+)-1, maintained ample cytotoxicity against the same panel of cell lines {IC50 (U-937): 5.0 nM; IC50 (HeLa): 26.8 nM; IC50 (NCl-H460): 46.7 nM; IC50 (786-O): 83 nM; IC50 (MCF7): 63 nM}. The readily accessible ETP (+)-8 offers an opportunity for the design and development of functional ETPs for use in detailed chemical and biological investigations.
Results and Discussion
Design and Synthesis of Complex ETP-Azides (+)-9a–c
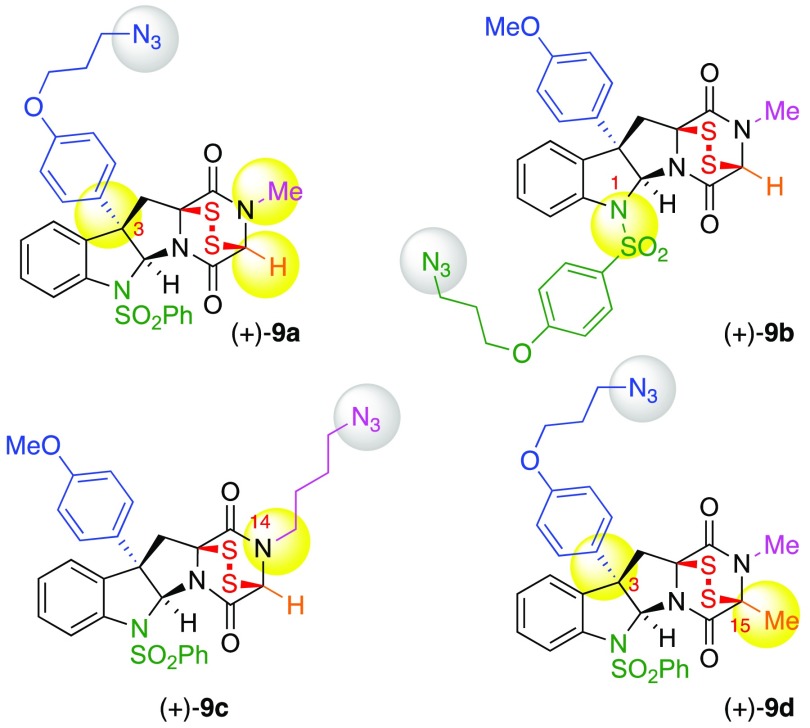
We targeted compounds (+)-9a–c (Figure 2) guided by the insights gained in our prior SAR study.4 We envisioned C3, N1, and N14 (for positional numbering system, see Figure S1) as optimal positions for introduction of a functional handle for further chemical modification while maintaining the cytotoxicity of ETP (+)-8. Herein, we report the synthesis of functional ETPs (+)-9a–c. Lacking full C15-substitution, these ETPs avoid the often seen challenges concerning C15-epimerization or elimination of alanine- or serine-derived diketopiperazine (DKP) precursors.2,9 The synthesis of ETPs (+)-9a and (+)-9b from sarcosine streamlined their preparation,15 whereas ETP (+)-9c required the development of a DKP N-alkylation strategy. Additionally, informed by mechanistic studies on the potent yet chemically sensitive C15–H substituted ETPs (vide infra), we designed C15-Me substituted ETP (+)-9d as a variant of ETP (+)-9a with improved chemical stability. Our syntheses of ETPs (+)-9a–d and related derivatives leverage advances in late-stage oxidation and thiolation strategies, including the application of a novel thiolating reagent for stereoselective introduction of two C–S bonds onto a DKP. Additionally, we provide a platform for rapid derivatization and conjugation of complex ETPs. The presence of the alkyl azide and the compatibility of the sensitive epidisulfide bridge with the planned copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction16 enabled the facile conjugation of these designed ETPs as potent anticancer payloads (vide infra). Bioactive small molecules have been structurally modified17 and used in various contexts, including antibody–drug conjugates for targeted drug delivery,12 activity-based protein profiling,18 photoaffinity labels for target identification,19 small molecule imaging probes,20 and polymer–drug conjugates for improved pharmacokinetics.21 While advances in synthesis of ETPs continue to enable informative biochemical studies,22 there are no examples of complex and potently anticancer (nM) ETPs being directly used as conjugatable probes or warheads as described here. We anticipate that the functional alkyl azide handle on our designed ETPs provides a versatile strategy for ligation of complex ETPs using CuAAC, providing exciting new opportunities for chemical and biological studies.
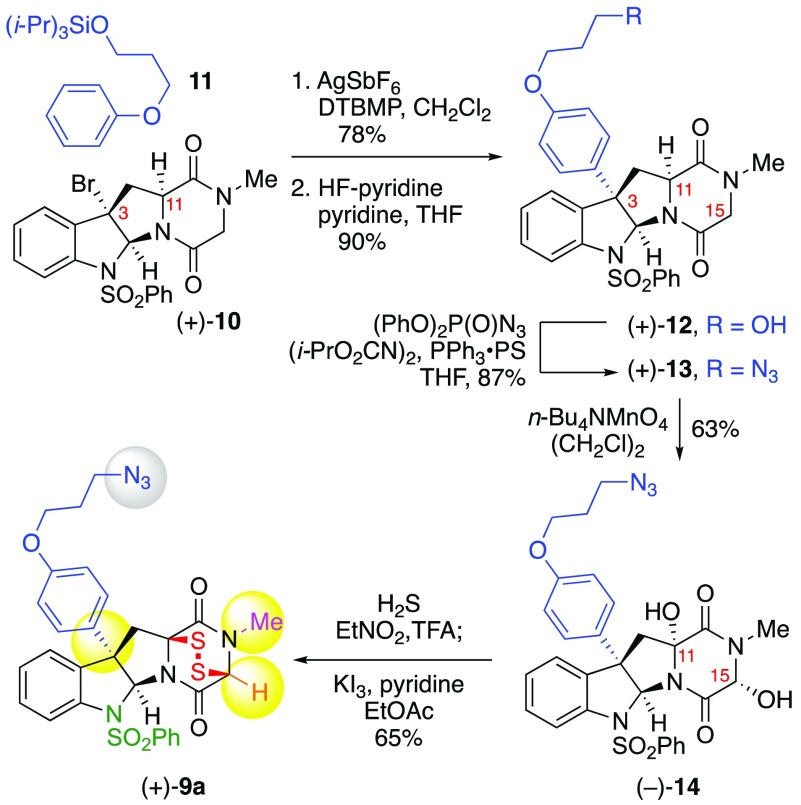
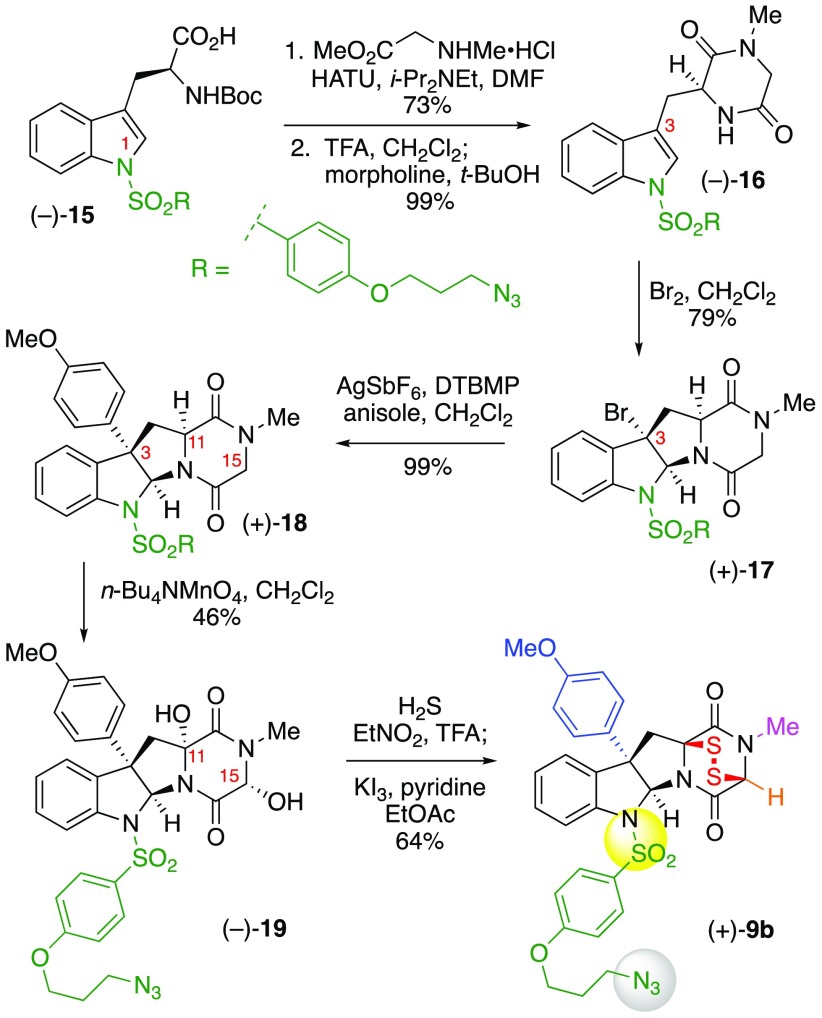
We designed ETP (+)-9a (Scheme 1) with an alkyl azide incorporated via C3-substitution based on our observations that aryl substituents at C3 of the cyclotryptophan substructure of ETPs led to an increase in potency relative to short chain alkyl substituents.4 Our synthesis of ETP (+)-9a commenced with treatment of endo-tetracyclic bromide (+)-104 with aryl ether 11(23) under our silver-promoted C3-arylation of cyclotryptophan-DKPs24 to provide the desired Friedel–Crafts product in 78% yield. Epimerization of the base-sensitive C11 stereocenter during desilylation was completely suppressed by employing hydrogen fluoride in a mixture of pyridine and THF to furnish alcohol (+)-12 in 90% yield as a single diastereomer.25 Conversion of alcohol (+)-12 into the corresponding azide (+)-13, via the Bose–Mitsunobu protocol with polymer-supported triphenylphosphine (PPh3·PS),26 set the stage for the planned stereoselective DKP dihydroxylation2,27 and sulfidation.2−4,9 Exposure of azide (+)-13 to tetra-n-butylammonium permanganate28 in 1,2-dichloroethane gave diol (−)-14 in 63% yield as a single diastereomer.2 Introduction of the critical epidisulfide bridge was achieved by treatment of diol (−)-14 with trifluoroacetic acid in a saturated solution of hydrogen sulfide in nitroethane, followed by oxidative disulfide formation upon exposure to potassium triiodide9a to afford C3-functionalized ETP-azide (+)-9a in 65% yield.
Scheme 1. Synthesis of Designed ETP-azide (+)-9a.
As illustrated in Scheme 2, the synthesis of N1-substituted ETP-azide (+)-9b necessitated early introduction of the azide functional group via N1-sulfonylation. The condensation of the readily prepared carboxylic acid (−)-1523 with sarcosine methyl ester hydrogen chloride promoted by N-[(dimethylamino)-1H-1,2,3-triazolo-[4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide (HATU) afforded the corresponding dipeptide in 73% yield. Subsequent deprotection of the tert-butoxycarbonyl group with trifluoroacetic acid in dichloromethane followed by treatment with morpholine in tert-butanol resulted in cyclization to DKP (−)-16 in 99% yield.9a,29 Exposure of DKP (−)-16 to bromine29 in dichloromethane afforded endo-tetracyclic bromide (+)-17 in 79% yield and >18:1 dr. Application of our methodologies2 for C3-arylation (99%),24 permanganate-promoted DKP dihydroxylation (46%),27 and sulfidation4,9 of diol (−)-19 provided N1-functionalized ETP-azide (+)-9b in 64% yield.
Scheme 2. Synthesis of Designed ETP-azide (+)-9b.
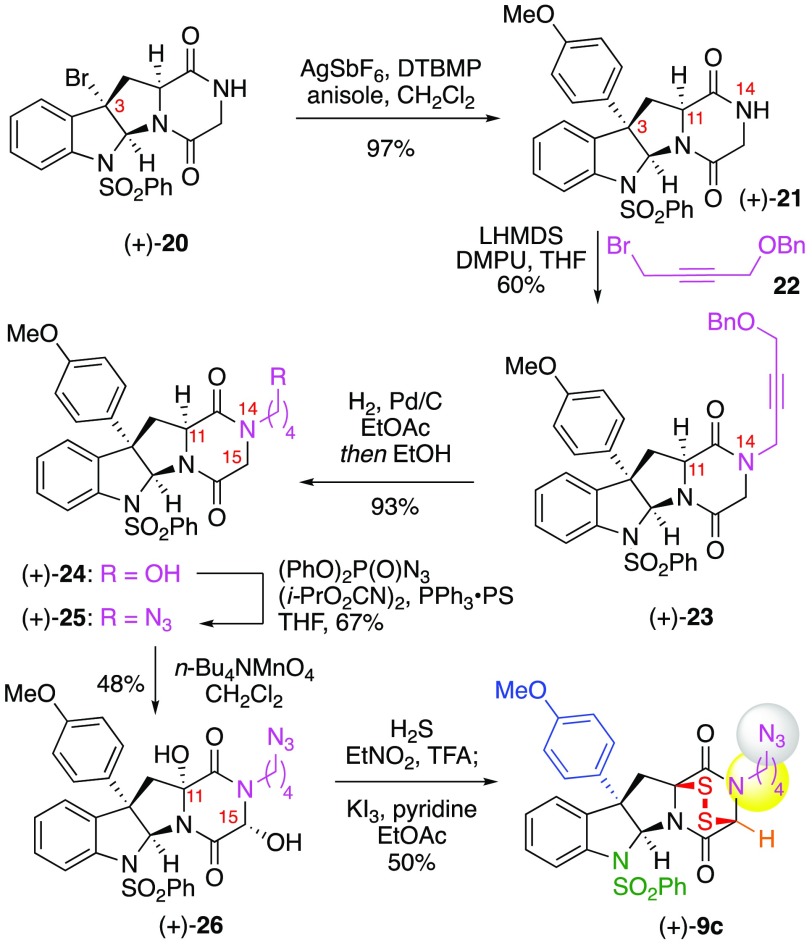
Considering the presence of N14-substitution in the vast majority of ETP natural products,1,5−8 we designed ETP (+)-9c (Scheme 3) consistent with parameters described above to incorporate the alkyl azide via N14-substitution. Our synthesis of ETP-azide (+)-9c began with N-alkylation of diketopiperazine (+)-20.23 However, direct N-alkylation of (+)-20 with alkyl iodide or allyl bromide derivatives resulted in no reactivity or low conversions, respectively, with significant C11 epimerization. We hypothesized that converting the electron withdrawing C3-bromide to the desired aryl substitution may enhance nucleophilicity of N14 and suppress C11 epimerization. Furthermore, we postulated the use of a propargylic electrophile might provide superior N-alkylation. Under optimal conditions, treatment of C3-aryl diketopiperazine (+)-21 with lithium hexamethyldisilylamide (LHMDS) in a mixture of N,N′-dimethylpropyleneurea–tetrahydrofuran (DMPU–THF, 1:4) at −30 °C followed by addition of propargyl bromide 22 afforded alkyne (+)-23 in 60% yield.23 Hydrogenation of benzyl ether (+)-23 proved challenging due to competitive reduction of a putative allylic alcohol/ether intermediate that gave rise to an undesired N14-n-butyl derivative of alcohol (+)-24.30 Temporal control of the reduction events was achieved by means of a solvent change, wherein alkyne (+)-23 was subjected to palladium on carbon (Pd/C, 5 wt %) in ethyl acetate under an atmosphere of dihydrogen to fully reduce the alkyne functional group, followed by dilution of the reaction mixture with ethanol to hydrogenolyze the benzyl protective group and to afford alcohol (+)-24 in 93% yield. The remaining steps to ETP-azide (+)-9c follow our general synthetic strategy described in the synthesis of ETP-azide (+)-9a, involving the application of the Bose–Mitsunobu26 azidation chemistry to afford DKP-azide (+)-25 in 67% yield, permanganate-mediated hydroxylation27 to give DKP-diol (+)-26 in 48% yield, nucleophilic DKP-sulfidation,4,9 and subsequent triiodide-promoted disulfide formation9a to give N14-functionalized ETP-azide (+)-9c in 50% yield.
Scheme 3. Synthesis of Designed ETP-azide (+)-9c.
Synthesis of Derivatized ETP-Triazoles (+)-28a–c
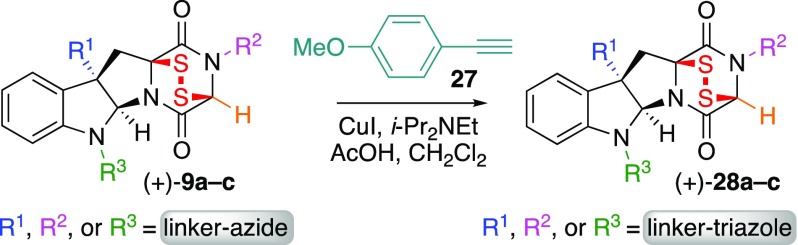
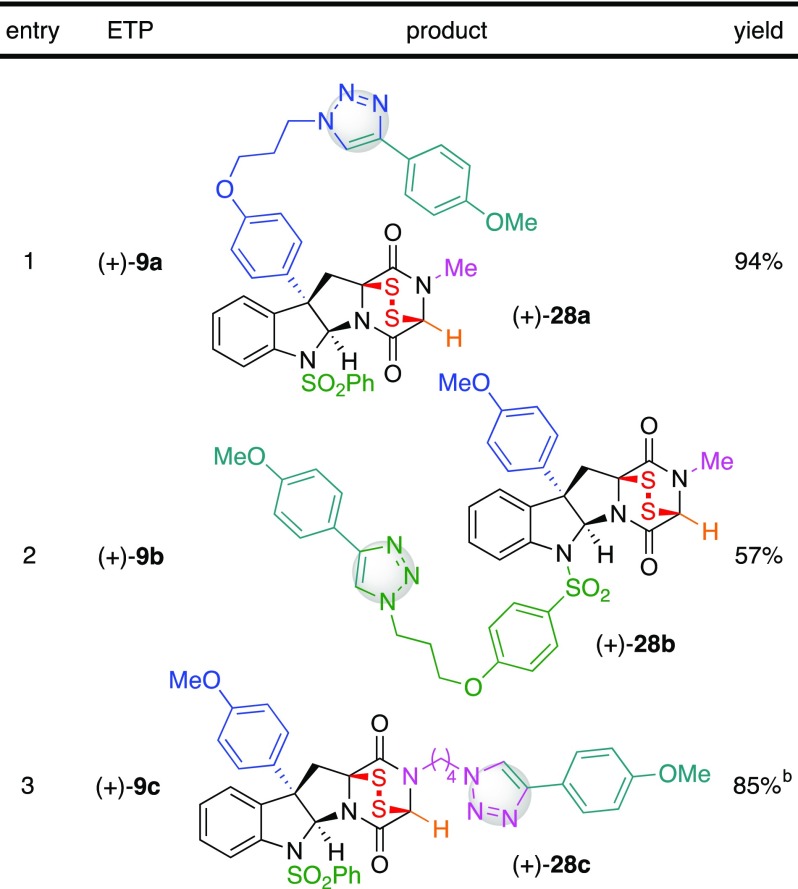
With ETP-azides (+)-9a–c in hand, we next evaluated the compatibility of the sensitive epidisulfide-based warhead with the planned CuAAC reaction.16 We employed 4-ethynylanisole (27) as a model substrate for CuAAC-based conjugation of complex ETPs with alkyne tethered partners (Table 1). Importantly, treatment of a solution of ETP-azides (+)-9a–c with alkyne 27 and copper iodide31 at 23 °C proceeded smoothly to provide the corresponding triazoles (+)-28a–c in 94, 57, and 85%32 yield, respectively. We next obtained promising IC50 values (vida infra) for both ETP-azides and the corresponding triazoles, as discussed below in greater detail (Table 2), and determined that conjugated ETP-triazoles (+)-28a–c retain the potent anticancer activity of their ETP-azide precursors. This observation highlights the outstanding potential for use of complex and potently anticancer ETP-azides as ready-to-conjugate payloads for synthesis of probes and future use in targeted delivery.11,12,17−21
Table 1. Derivatization of ETP-azides (+)-9a–c with Alkyne 27a.
Conditions:23 ETP-azide (1 equiv), 4-ethynylanisole 27 (5.0 equiv), CuI (0.50–1.5 equiv), acetic acid (1.0–3.0 equiv), N,N-diisopropylethylamine (1.0–3.0 equiv), dichloromethane, 23 °C.
Toluene was used as the solvent.
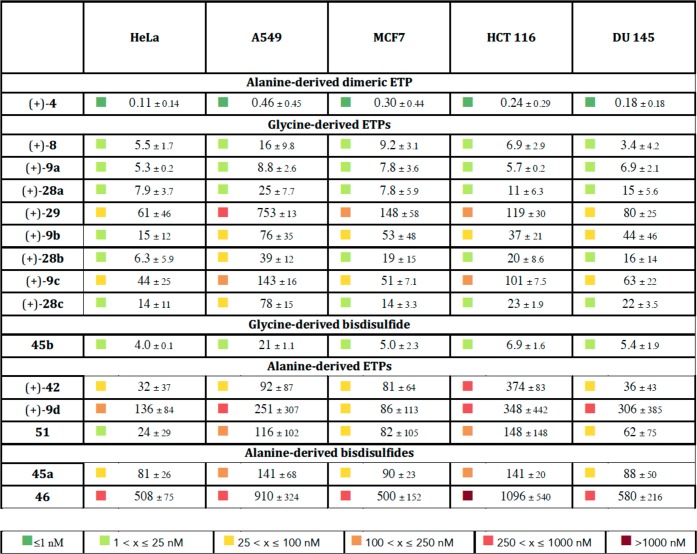
Table 2. Assessment of Designed ETPs for Cytotoxicity in Five Human Cancer Cell Linesa.
HeLa (cervical carcinoma), A549 (alveolar adenocarcinoma), MCF7 (breast adenocarcinoma), HCT 116 (colorectal carcinoma), and DU 145 (prostate carcinoma). 72-h IC50 values (in nM) as determined by Cell Titer-Glo (Promega), measuring ATP levels as a surrogate for cell viability. Error is standard deviation of the mean, n ≥ 2; IC50 = half maximal inhibitory concentration.
Derivatization of Functional Linker
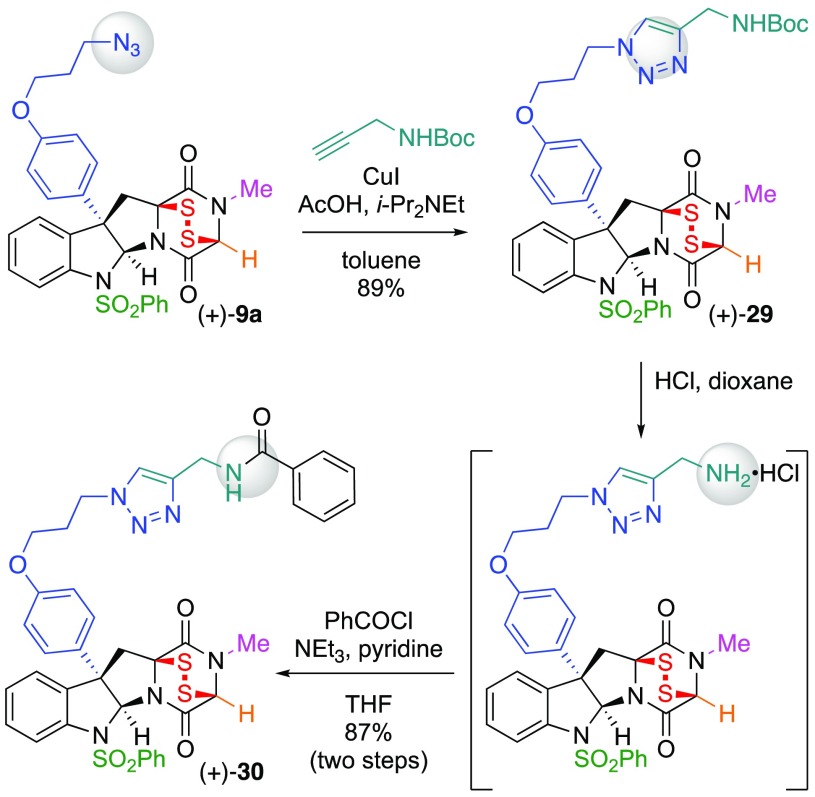
The ability to couple our ETP azides with bifunctional alkynes also provides an expedient opportunity for final stage diversification of the functional linker. For example, where introduction of a primary amine may be of interest for ligation and further derivatization,33 such as bioconjugation11,12,18−20 or synthesis of a focused library using acyl donors,34 the conjugation of N-Boc-propargylamine with ETP-azide (+)-9a directly affords the protected ETP-amine (+)-29 in 89% yield (Scheme 4). Unraveling of the primary amine under acidic conditions followed by direct acylation with benzoyl chloride as a model acyl donor34 affords ETP-amide (+)-30 in 87% yield, highlighting the versatility of our ETP-azide derivatives for rapid diversification.
Scheme 4. Representative Derivatization of ETP-azide (+)-9a.
Chemical Stability Studies and Design Enhancement Based on C15-Substitution
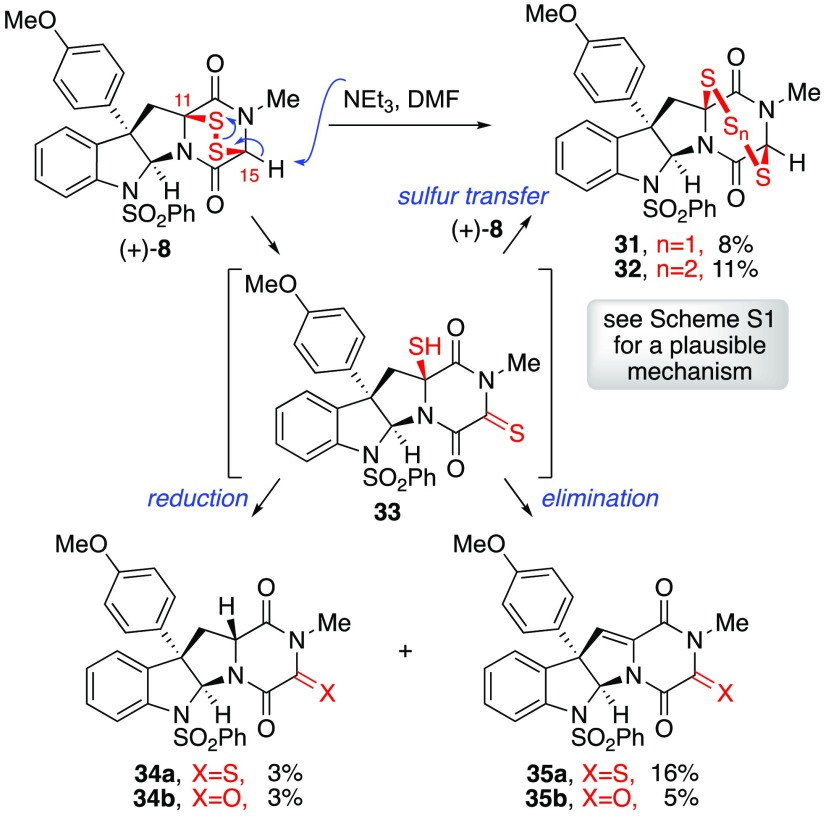
We next aimed to better understand the chemical stability of our synthetic ETPs to inform their future use. To this end, we chose to study the stability of ETP (+)-8 (Scheme 5) under conditions relevant to common conjugation reactions. Importantly, ETP (+)-8 can be recovered intact and with excellent mass balance from critical control experiments involving exposure to bioconjugation conditions35 such as those used in amidation of an activated ester36 or incubation in cellular lysing buffer (pH 7.4) for 24 h.37 However, the observed formation of minor side products in our studies, including sulfur-congeners of ETP (+)-8, prompted a deeper investigation. To facilitate these mechanistic studies, we independently prepared possible decomposition products including the corresponding epitrisulfide 31 (Scheme 5) and epitetrasulfide 32 to confirm their detection.23 While ETP (+)-8 is stable in deuterochloroform [20 mM] at 23 °C over 20 h as monitored by 1H NMR analysis, introduction of triethylamine (2 equiv) led to gradual consumption of ETP (+)-8 (∼15%) and concomitant formation of epitrisulfide 31 (∼5%) over 20 h. Similar observations were made using Hünig’s base and DABCO as the base additive, and the rate of decomposition was greater using higher dielectric constant media including acetonitrile, dimethyl sulfoxide, and N,N-dimethylformamide. For example, treatment of ETP (+)-8 with triethylamine (2 equiv) in DMF [20 mM] for 2 h (Scheme 5) led to isolation of epitrisulfide 31 (8%) and epitetrasulfide 32 (11%) as well as diketopiperazinethione 34a (3%) and corresponding hydrolysis product triketopiperazine 34b (3%), diketopiperazinethione 35a (16%), and triketopiperazine 35b (5%).38 As highlighted in Scheme 5, our hypothesis for formation of these side products, under the basic conditions described above, involves C15−H deprotonation of ETP (+)-8 resulting in S–S bond scission and formation of intermediate thiol 33, a reactive species that leads to consumption of the starting disulfide and ultimately give rise to the higher order polysulfanes via electrophilic sulfur transfer.2,9b A plausible mechanism for the degradation of ETP (+)-8 and formation of its congeners 31–32 is depicted in Scheme S1.23
Scheme 5. Base Sensitivity of ETP (+)-8 and Formation of Its Congeners 31 and 32.
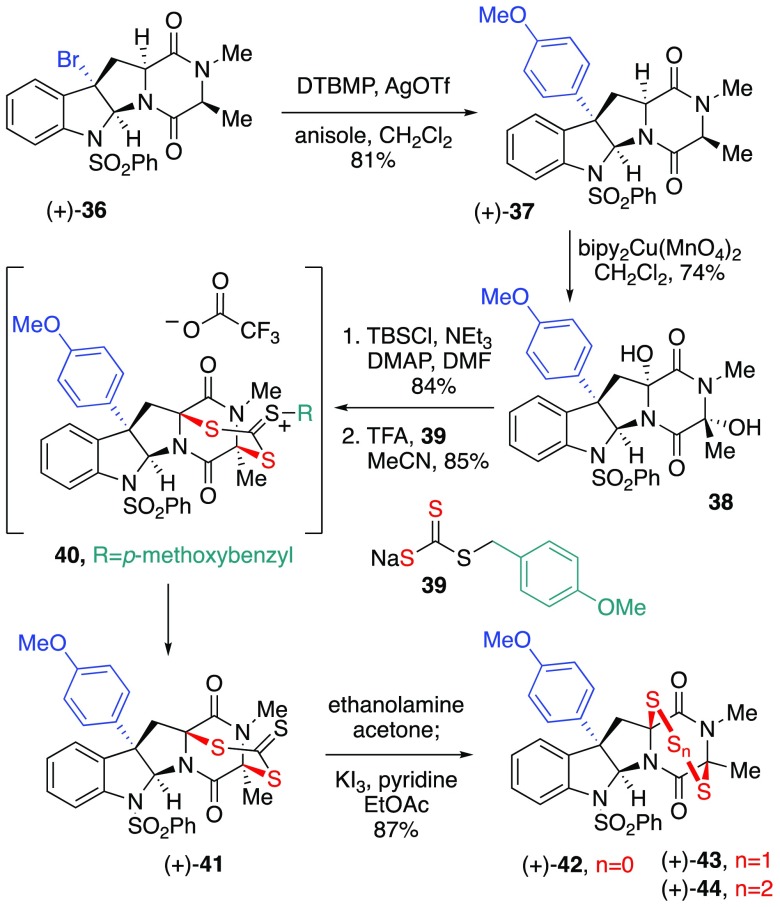
We hypothesized the C15-Me-substitution of alanine-derived ETP (+)-42 (Scheme 6) could avoid the decomposition pathway described above and provide a more stable ETP. While our earlier SAR studies suggested that alanine-based ETPs were approximately an order of magnitude less active than their sarcosine analogues,4 we envisioned the additional C15-substitution would translate to enhanced stability and a superior mechanistic probe. Due to the expected altered reactivity profile of C15-substitution,2−4,9 the synthesis of epidisulfide (+)-42 also offered opportunities to refine our hydroxylation and sulfidation strategies en route to ETPs. As illustrated in Scheme 6, silver-mediated activation24 of bromide (+)-362,24 gave C3-adduct (+)-37 in 81% yield. We found that permanganate-mediated dihydroxylation using bis(2,2′-bipyridyl)-copper(II) permanganate {bipy2Cu(MnO4)2}39 proved particularly effective in furnishing DKP-diol 38 as a single diastereomer in 74% yield.40 This represents the first application of this mild oxidant for DKP dihydroxylation. The impact of the counter-cation on the outcome of the permanganate-promoted oxidation of diketopiperazines is consistent with our prior observations and mechanistic studies.2,27 The tactical conversion of diol 38 to an alcohol by monosilylation (84%) resulted in a mixture of regioisomeric (1.1:1) monosilylether alcohols with improved stability and solubility characteristics,9a setting the stage for stereoselective introduction of the epidisulfide bridge.
Scheme 6. Synthesis of C15-Me ETP (+)-42.
As part of an ongoing effort to advance the selectivity and efficiency of our methodology in accessing ETPs,2 we discovered a practical reagent that can be used to give cis-sulfidation en route to epidisulfide (+)-42 and its sulfur-congeners. After establishing that independent exposure of either monosilyl regioisomer derivative of diol 38 (Scheme 6) to potassium trithiocarbonate9a and trifluoroacetic acid in dichloromethane led to formation of dithiepanethione (+)-41 in moderate yield (66–73%), we hypothesized if a suitably designed alkyl trithiocarbonate derivative could enhance the overall efficiency through superior reagent solubility and stability. As illustrated in Scheme 6, we found that monosodium trithiocarbonate 39, conveniently prepared from commercially available p-methoxybenzyl thiol23 without the use of dihydrogen sulfide, gave dithiepanethione (+)-41 in 85% yield, likely via efficient formation of sulfonium ion 40. The versatile dithiepanethione (+)-41 was efficiently converted to the ETP (+)-42 in 87% yield upon aminolysis to a bisthiol intermediate followed by oxidative disulfide formation. Additionally, exposure of the same bisthiol intermediate to sulfur dichloride and disulfur dichloride provided the epitrisulfide 43 (Scheme 6) and epitetrasulfide 44 in 22 and 66% yield, respectively.23
Synthesis of Bisdisulfides via Thiol–Disulfide Exchange
In comparison to C15-desmethyl ETP (+)-8, the enhanced chemical stability of C15-substituted ETP (+)-42 provided an excellent opportunity to investigate the reactivity of the epidisulfide bridge in thiol–disulfide exchange reactions. Consistent with the stability studies described above (Scheme 5), exposure of a solution of C15-desmethyl ETP (+)-8 in deuteroacetonitrile [20 mM] to triethylamine (2.2 equiv) at 23 °C led to complete consumption of epidisulfide (+)-8 over 2 h as observed by in situ 1H NMR monitoring experiments, followed by the isolation of epitrisulfide 31 (16%) and epitetrasulfide 32 (24%). Conversely, exposure of C15-substituted ETP (+)-42 to identical conditions led to no decomposition and allowed quantitative recovery of ETP (+)-42. Given the importance of ETP’s reactivity with cellular thiols for its biological activity,1,5−8,41,42 we set out to study disulfide exchange reactions using ETPs (+)-8, (+)-42, and the corresponding mixed bisdisulfides. Our SAR profile of ETPs demonstrated bisdisulfides also served as competent anticancer agents.4 We hypothesized that these species might serve as prodrugs, being converted to their corresponding epidisulfide pharmacophores under biological conditions, which are then concentrated within the cell via thiol-mediated uptake.42
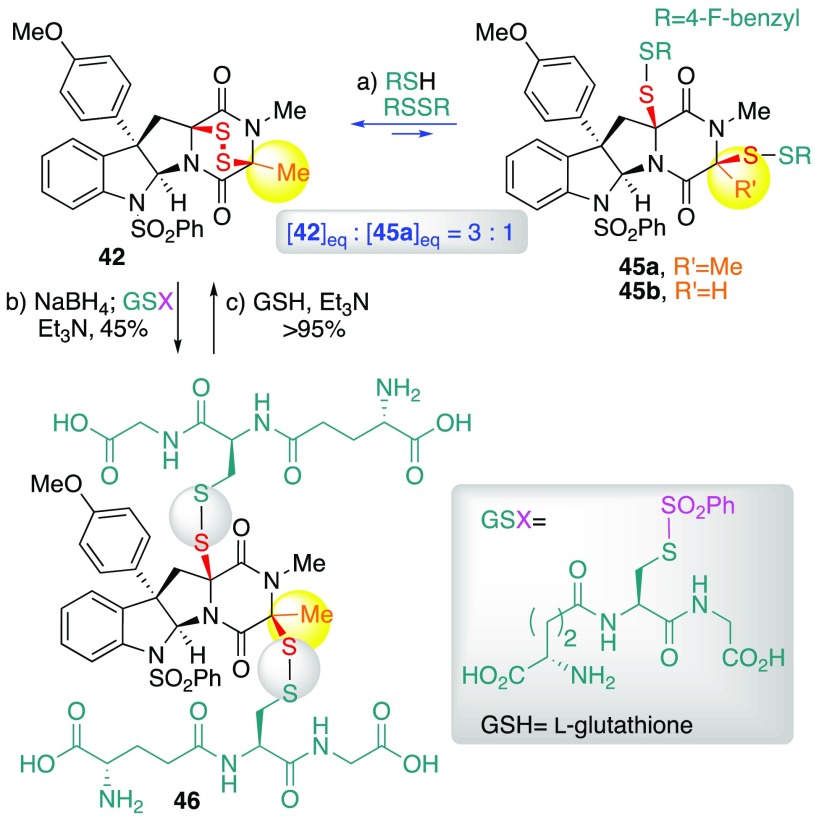
As illustrated in Scheme 7, treatment of ETP (+)-42 with excess (para-fluorobenzyl)disulfane (10 equiv) in the presence of para-fluorobenzylthiol (1.0 equiv) and triethylamine (2.5 equiv) in THF [0.1 M] at 23 °C for 65 h resulted in isolation of bis(para-fluorobenzyl)disulfide 45a in 22% yield, alongside the recovery of ETP (+)-42 in 66% yield.23 This exchange reaction could be monitored with 1H NMR experiments by diluting aliquots of the reaction mixture into deuterochloroform; we found that the 3:1 equilibrium ratio favoring ETP (+)-42, consistent with isolated yields, could be established from either direction by treating either (+)-42 or 45a under identical conditions (see Scheme S2).23 In parallel studies, we found that C15-desmethyl ETP (+)-8 could be converted to the corresponding bisdisulfide 45b (Scheme 7), but that analogous disulfide-exchange equilibration experiments resulted in the appearance of undesired sulfur-congeners trisulfide 31 and tetrasulfide 32 (Scheme 5). Specifically, treatment of epidisulfide (+)-8 with excess (para-fluorobenzyl)disulfane (3.0 equiv) in the presence of para-fluorobenzylthiol (0.5 equiv) and triethylamine (2.0 equiv) in THF [20 mM] at 23 °C for 30 min afforded bisdisulfide 45b (69%), epitrisulfide 31 (2%), and recovered epidisulfide (+)-8 (17%).43 Notably, exposure of bisdisulfide 45b to identical conditions resulted in a mixture of di-, tri-, and tetra-sulfides consistent with our earlier observations (Scheme 5).
Scheme 7. Thiol–Disulfide Exchange Studies of ETP 42.
Conditions: (a) para-fluorobenzylthiol, (para-fluorobenzyl)disulfane, NEt3, THF, 23 °C. (b) NaBH4, MeOH, THF; S-(phenylsulfonyl)-l-glutathione hydrogen chloride, NEt3, MeOH, THF, 23 °C. (c) l-Glutathione, NEt3, D2O, CD3CN, 23 °C.
We next aimed to evaluate the feasibility of bisdisulfides undergoing reversion to epidisulfides under biologically relevant, aqueous conditions. We prepared water-soluble glutathione bisdisulfide 46 (Scheme 7) in 45% isolated yield by stepwise reduction with sodium borohydride and subsequent exposure of the crude bisthiol to electrophilic S-(phenylsulfonyl)-l-glutathione44 (5 equiv) and triethylamine (11 equiv) in a mixture of methanol and tetrahydrofuran.23 Monitoring by 1H NMR spectroscopy, we found that exposure of bisdisulfide 46 to l-glutathione (1.0 equiv) and triethylamine (1.0 equiv) in deuterium oxide–deuteroacetonitrile [2:3, 2 mM] led to formation of epidisulfide (+)-42 in 15 min (Scheme 7, 42:46, >50:1).45 As mixed bisdisulfides 45a, 45b, and 46 readily revert to their respective ETP derivative, our thiol–disulfide exchange studies highlight the remarkable thermodynamic stability of the ETP substructure but also present a potential strategy to modulate ETP cytotoxicity in prodrug form.
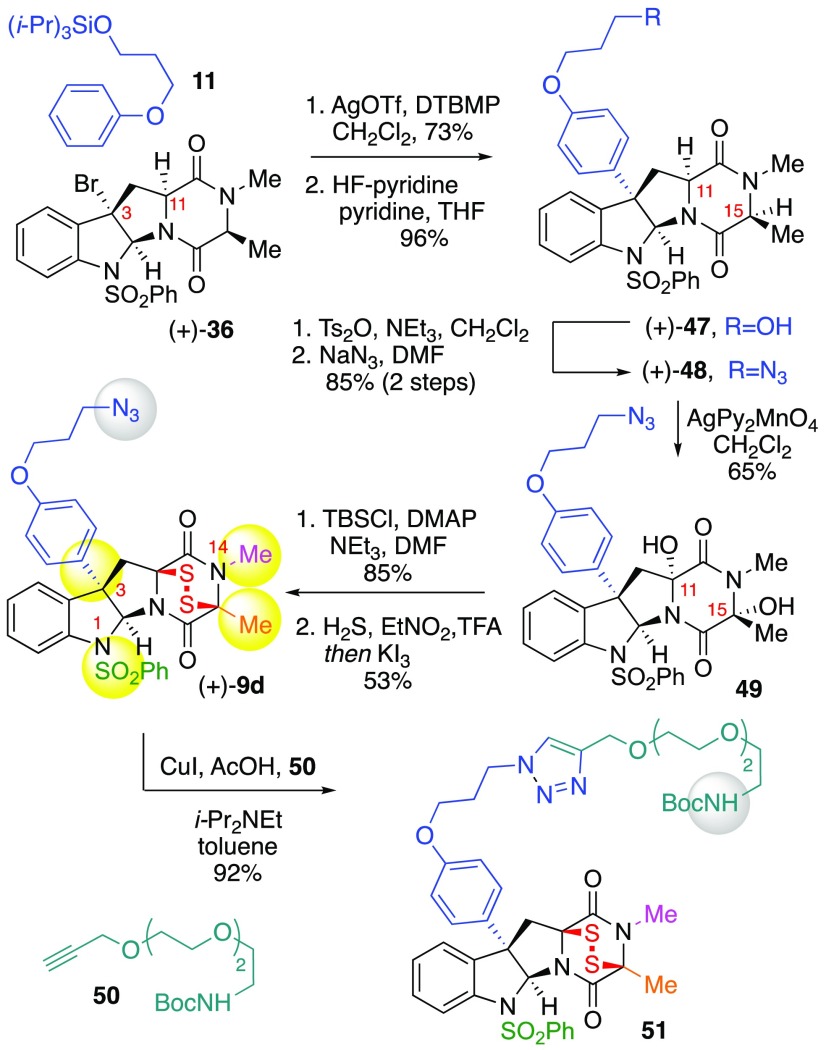
Design and Synthesis of Complex ETP-Azide (+)-9d
Having demonstrated the superior stability of alanine-derived ETPs, we chose to prepare C15-Me substituted functional ETP (+)-9d (Scheme 8). We were guided by comparisons in the activities (vida infra) of ETPs (+)-9a–c to prepare C3-functionalized ETP (+)-9d, keeping the N1- and N14-substitutions for added stability and potency as described above. The synthesis of ETP (+)-9d parallels our strategy described above for synthesis of related ETPs, beginning with C3-arylation24 of bromide (+)-369a with aryl silyl ether 11 (73%). Removal of the silyl ether to give alcohol (+)-47 (96%) followed by conversion to the corresponding azide (85%)23 afforded the DKP (+)-48. Application of our DKP sulfidation strategy involved oxidation of DKP (+)-48 to give diol 49 in 65% yield, which upon exposure to t-butyldimethylsilyl chloride gave a regioisomeric mixture of monosilyl monoalcohols (1.1:1) in 85% yield. Consistent with our observation in synthesis of ETP (+)-42 and use of monosilyl ether intermediates, exposure of the regioisomeric monosilyl ethers to nitroethane saturated with hydrogen sulfide gas followed by oxidation with triiodide gave the desired C15-substituted C3-functionalized ETP azide (+)-9d in 53% yield. Efforts to apply potassium trithiocarbonate or monosodium trithiocarbonate 39 toward the cis-sulfidation of diol 49 or its corresponding monosilyl ether intermediates were unsuccessful due to the competitive reduction of the alkyl azide. To further highlight the diversity of linkers that we may couple with our functional ETPs without compromising anticancer activity, we conjugated epidisulfide probe (+)-9d with ethylene glycol-derived alkyne 50 through application of the CuAAC coupling strategy, affording triazole 51 in 92% yield. The protected amine in ETP 51 can be derivatized as demonstrated in Scheme 4. We anticipate the superior stability of ETP (+)-9d may result in clearer biochemical readouts when used in mechanistic studies.
Scheme 8. Synthesis and Utility of Designed ETP-azide (+)-9d.
Anticancer Activity of Designed ETP Derivatives
The evaluation of our model and functionalized ETP probes as anticancer agents against a panel of five human cancer cell lines is illustrated in Table 2. A range of complex derivatives, including ETPs (+)-4, (+)-8, and (+)-42, ETP-azides (+)-9a–d, and ETP-triazole conjugates (+)-28a–c, (+)-29, and 51, in addition to bisdisulfides 45a, 45b, and 46, were evaluated for cytotoxicity against cervical carcinoma (HeLa), alveolar adenocarcinoma (A549), breast adenocarcinoma (MCF7), colorectal carcinoma (HCT 116), and prostate carcinoma (DU 145) cell lines. Importantly, our designed ETPs displayed similar patterns of potency in the form of low nanomolar cytotoxicity across all cell lines examined in this study.
Comparisons between ETPs (+)-8 and (+)-42, along with their respective functionalized ETP-azide derivatives (+)-9a–c and (+)-9d, indicate that ETPs possessing conjugatable chemical handles about either the C3, N1, or N14 positions are well tolerated (Table 2). While we demonstrated that C15-substituted ETP (+)-42 is chemically more stable than glycine-derived ETP (+)-8 (Scheme 5), glycine-derived ETPs were more active against the same cell lines {(+)-42 vs (+)-8: IC50 (HeLa): 32 vs 5.5 nM; IC50 (A549): 92 vs 16 nM; IC50 (MCF7): 81 vs 9.2 nM; IC50 (HCT 116): 374 vs 6.9 nM; IC50 (DU 145): 36 vs 3.4 nM}. The degree to which the sarcosine-derived ETPs (+)-9a–c maintained cytotoxicity compared to dimeric ETP (+)-4, despite only having a single epidisulfide bridge, may in part be due to greater access to the epidisulfane bridge lacking substitution at C15. In comparing model ETP (+)-8 to its functionalized derivatives ETP-azides (+)-9a–c, we found the activity of ETP (+)-9a to be unaffected across all five cell lines (<2-fold difference), (+)-9b to be slightly reduced {(+)-9b vs (+)-8: IC50 (HeLa): 3-fold decrease; IC50 (A549): 5-fold decrease; IC50 (MCF7): 6-fold decrease; IC50 (HCT 116): 5-fold decrease; IC50 (DU 145): 13-fold decrease}, and (+)-9c to be most impaired {(+)-9c vs (+)-8: IC50 (HeLa): 8-fold decrease; IC50 (A549): 9-fold decrease; IC50 (MCF7): 6-fold decrease; IC50 (HCT 116): 15-fold decrease; IC50 (DU 145): 19-fold decrease}. Similarly, functionalization of C15-Me substituted ETP (+)-42 as ETP-azide (+)-9d did not impact the activity against MCF7 or HCT 116 cell lines but resulted in slightly reduced activities against HeLa, A549, and DU 145 cell lines {(+)-9d vs (+)-42: IC50 (HeLa): 136 vs 32 nM; IC50 (A549): 251 vs 92 nM; IC50 (DU 145): 306 vs 36 nM}.
As illustrated in Table 2, triazole conjugates of ETP-azides (+)-9a–d prepared using CuAAC chemistry largely retain the anticancer potency. The conversion of azide (+)-9a to triazole (+)-28a resulted in a minimal (<3-fold) loss of activity across all five cell lines, whereas an analogous comparison between azide (+)-9b and triazole (+)-28b resulted in a minimal (2- to 3-fold) increase in activity upon conjugation. Compared to azide (+)-9c, triazole (+)-28c was slightly more active (4-fold) against MCF7 and HCT 116 cell lines and (2- to 3-fold) against HeLa, A549, and DU 145 cell lines. Interestingly, the derivatization of ETP-azide (+)-9d as PEG-triazole 51 increased the activity for HeLa and DU 145 cell lines (5-fold), resulting in activities comparable to parent ETP (+)-42 {51 vs (+)-42; IC50 (HeLa): 24 vs 32 nM; IC50 (A549): 116 vs 92 nM; IC50 (MCF7): 82 vs 81 nM; IC50 (HCT 116): 148 vs 374 nM; IC50 (DU 145): 62 vs 36 nM} as well as the C15-desmethyl triazole (+)-29. The anticancer potency of both our ETP-azides and their corresponding conjugated ETP-triazoles highlights the exciting opportunity for their use as biochemical probes and in targeted delivery.
Expanding on our prior observations4 that bisdisulfides derived from ETPs retain anticancer activity, we found that the composition of the mixed disulfide impacts anticancer activity (Table 2). For example, whereas bis(para-fluorobenzyl)-disulfides 45a and 45b have similar activities to their parent ETPs (+)-42 and (+)-8, respectively, the larger bis(l-glutathione)disulfide 46 derived from ETP (+)-42 was significantly less active {46 vs (+)-42; IC50 (HeLa): 508 vs 32 nM; IC50 (A549): 910 vs 92 nM; IC50 (MCF7): 500 vs 81 nM; IC50 (HCT 116): 1096 vs 374 nM; IC50 (DU 145): 580 vs 36 nM}. The reduced activity of bis(l-glutathione)disulfide 46 compared to bis(para-fluorobenzyl)disulfides 45a and 45b is likely due to a combination of factors including cellular permeability, pharmacodynamic properties, steric crowding at the sulfur atoms, and variation in the reduction potentials.46 The application of these bisdisulfides as ETP prodrugs may find utility in the treatment of cancers with higher glutathione (GSH) to glutathione disulfide (GSSG) ratios. For example, several studies have found that invasive and metastatic colon and prostate tumors have higher extracellular thiol concentrations than healthy tissue.47 Our observations suggest possible modulation of ETP toxicity in prodrug form as the corresponding bisdisulfides for a more controlled ETP formation at the local tumor environment with higher GSH concentration.
Conclusions
In summary, we have described the design, synthesis, chemical stability studies, and evaluation of functional ETPs as potent anticancer compounds. Our SAR informed strategic substitution of designed ETPs (+)-9a–c with an alkyl azide at the C3, N1, and N14 positions, respectively, enabled versatile derivatization while maintaining potency. Employing sarcosine as starting material streamlined the synthesis of ETP-azides (+)-9a and (+)-9b, whereas ETP-azide (+)-9c required the development of a mild diketopiperazine N-alkylation strategy. Mechanistic studies and cytotoxic evaluation of the potent C15–H substituted ETPs (+)-8 and (+)-9a–c led us to design C15-Me substituted ETP-azide (+)-9d to reduce the rate of base-promoted decomposition of the ETP warhead. Our synthetic solutions to these complex ETPs required advances in stereoselective late-stage dihydroxylation and sulfidation strategies, including the application of novel reagents for dihydroxylation and cis-sulfidation of diketopiperazines. While C15–H substituted ETP-azides (+)-9a–c offer outstanding anticancer activity, the C15-Me substituted ETP-azide (+)-9d with its enhanced chemical stability may provide more clear readouts when used in biochemical studies. The results of our thiol–disulfide exchange studies revealed that mixed bisdisulfides 45a, 45b, and 46 readily revert to their respective ETPs, demonstrating the remarkable thermodynamic stability of the ETP substructure as well as revealing a potential strategy to modulate ETP cytotoxicity and pharmacodynamics in prodrug form. The facile conjugation of ETP-azides (+)-9a–d using CuAAC chemistry provides a flexible approach for further functionalization of complex ETPs, affording access to corresponding ETP-triazoles without compromising anticancer activity. Our findings highlight the outstanding potential for diversification of functional ETP-azides to enhance their translational potential as chemical probes or anticancer warheads.
Experimental Section
General Methods
All reactions were performed in oven-dried or flame-dried round-bottom flasks, modified Schlenk (Kjeldahl shape) flasks, or glass pressure vessels. The flasks were fitted with rubber septa, and reactions were conducted under a positive pressure of argon. Cannulae or gastight syringes with stainless steel needles were used to transfer air- or moisture-sensitive liquids. Flash column chromatography was performed as described by Still48 using granular silica gel (60-Å pore size, 40–63 μm, 4–6% H2O content) or C18-reversed-phase silica gel (90-Å pore size, 40–63 μm). Analytical thin layer chromatography (TLC) was performed using glass plates precoated with 0.25 mm 230–400 mesh silica gel impregnated with a fluorescent indicator (254 nm) or basic alumina impregnated with a fluorescent indicator (254 nm). Thin layer chromatography plates were visualized by exposure to short wave ultraviolet light (254 nm) and/or irreversibly stained by treatment with an aqueous solution of ceric ammonium molybdate (CAM), an ethanolic solution of phosphomolybdic acid (PMA), an aqueous solution of silver nitrate (AgNO3), Ellman’s reagent (5,5′-dithiobis(2-nitrobenzoic acid), DTNB) in N,N-dimethylformamide,49 or an aqueous solution of potassium permanganate (KMnO4), followed by heating (∼1 min) on a hot plate (∼250 °C). Organic solutions were concentrated at 30 °C on rotary evaporators capable of achieving a minimum pressure of ∼2 Torr. Proton (1H) and carbon (13C) nuclear magnetic resonance spectra were recorded with 600, 500, or 400 MHz spectrometers. Proton nuclear magnetic resonance (1H NMR) spectra are reported in parts per million on the δ scale and are referenced from the residual protium in the NMR solvent (CHCl3: δ 7.26, CD2HCN: 1.94, CD2HOD: 3.31, CD3SOCD2H: 2.50, H2O: 4.79).50 Data are reported as follows: chemical shift [multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, p = pentet, m = multiplet, br = broad), coupling constant(s) in Hertz, integration, assignment]. Broadband proton-decoupled carbon-13 nuclear magnetic resonance (13C{1H} NMR) spectra are reported in parts per million on the δ scale and are referenced from the carbon resonances of the solvent (CDCl3: δ 77.16, CD3CN: 118.26, CD3OD: 49.00, DMSO-d6: 39.52). Structural assignments were made with additional information from gCOSY, gHSQC, and gHMBC experiments. Infrared data (IR) were obtained with a FTIR or an ATR and are reported as follows: [frequency of absorption (cm–1), intensity of absorption (s = strong, m = medium, w = weak, br = broad)]. Optical rotations were recorded on a polarimeter, and specific rotations are reported as follows: [wavelength of light, temperature (°C), specific rotation, concentration in grams/100 mL of solution, solvent]. High-resolution mass spectra (HRMS) were recorded on a FT-ICR-MS using electrospray (ESI) (m/z) ionization source or direct analysis in real time (DART), a Q-TOF LC/MS using ESI, or an AccuTOF LC/MS using DART.
Representative Procedure for C3-Derivatization: Synthesis of (+)-(5aS,10bS,11aS)-2-Methyl-6-(phenylsulfonyl)-10b-(4-(3-((triisopropylsilyl)oxy)propoxy)phenyl)-2,3,6,10b,11,11a-hexahydro-4H-pyrazino[1′,2′:1,5]pyrrolo[2,3-b]indole-1,4(5aH)-dione (S2);51 C3-Adduct (+)-S2
endo-Tetracyclic bromide (+)-10 (1.67 g, 3.50 mmol, 1 equiv), 2,6-di-tert-butyl-4-methylpyridine (DTBMP, 1.81 g, 8.80 mmol, 2.51 equiv), and triisopropyl(3-phenoxypropoxy)silane (11, 2.16 g, 6.99 mmol, 2.00 equiv) were azeotropically dried by concentration from anhydrous benzene (30 mL) under reduced pressure. Dichloromethane (35 mL) was added via syringe, and silver hexafluoroantimonate (2.40 g, 6.99 mmol, 2.00 equiv) was added as a solid in one portion to the solution at 23 °C. After 1 h, the reaction mixture was diluted with dichloromethane (100 mL) and was filtered through a pad of diatomaceous earth. The filter cake was washed with dichloromethane (3 × 50 mL), and the filtrate was concentrated under reduced pressure. The resulting residue was purified by flash column chromatography on silica gel (eluent: 0 → 20% acetone in dichloromethane) to afford C3-adduct (+)-S2 (1.93 g, 78%) as a white solid. 1H NMR (400 MHz, CDCl3, 25 °C): δ 7.58 (d, J = 8.1 Hz, 1H), 7.46 (app-d, J = 8.5 Hz, 2H, SO2Ph), 7.30 (app-t, J = 7.5 Hz, 1H), 7.28–7.24 (m, 1H), 7.10 (m, 4H), 6.68–6.61 (m, 4H), 6.13 (s, 1H), 4.39 (app-t, J = 8.3 Hz, 1H), 4.10 (d, J = 17.4 Hz, 1H), 4.04 (t, J = 6.3 Hz, 2H), 3.86 (t, J = 6.1 Hz, 2H), 3.82 (d, J = 17.4 Hz, 1H), 3.06 (dd, J = 7.0, 14.1 Hz, 1H), 2.89–2.83 (m, 4H), 1.98 (p, J = 6.1 Hz, 2H), 1.11–1.03 (m, 21H). 13C{1H} NMR (100 MHz, CDCl3, 25 °C): δ 167.1, 165.2, 158.4, 139.9, 138.2, 135.8, 133.0, 132.5, 129.2, 128.7, 128.1, 128.0, 126.0, 125.4, 117.2, 115.0, 87.2, 64.9, 59.8, 59.4, 58.6, 54.5, 39.1, 33.7, 32.7, 18.2, 12.1. FTIR (thin film) cm–1: 3065 (m), 2943 (s), 2868 (s), 1684 (s), 1610 (m), 1512 (m), 1253 (m), 1171 (m), 883 (m), 686 (w). HRMS (DART) m/z: [M + H]+ calcd for C38H50N3O6SSi 704.3184; Found 704.3195. [α]D23: +19 (c = 0.24, CHCl3). TLC (30% acetone in dichloromethane), Rf: 0.63 (UV, CAM).
Representative Synthesis of a Base-Sensitive Azide Precursor: Synthesis of (+)-(5aS,10bS,11aS)-10b-(4-(3-Hydroxypropoxy)phenyl)-2-methyl-6-(phenylsulfonyl)-2,3,6,10b,11,11a-hexahydro-4H-pyrazino[1′,2′:1,5]pyrrolo[2,3-b]indole-1,4(5aH)-dione (12);51 Alcohol (+)-12
A freshly prepared solution of hydrogen fluoride–pyridine (70% HF, 9 mL), pyridine (18 mL), and tetrahydrofuran (72 mL) at 0 °C was poured into a solution of C3-adduct (+)-S2 (1.89 g, 2.69 mmol, 1 equiv) in tetrahydrofuran (90 mL) at 0 °C contained in a 1 L polypropylene vessel. After 5 min, the ice–water bath was removed, and the solution was allowed to stir and warm to 23 °C. After 20 h, the reaction mixture was cooled to 0 °C and was diluted with a saturated aqueous sodium bicarbonate solution (500 mL) in portions (50 mL) over 15 min. The resulting mixture was extracted with ethyl acetate (300 mL), the layers were separated, and the aqueous layer was extracted with ethyl acetate (2 × 75 mL). The combined organic extracts were washed sequentially with a saturated aqueous copper(II) sulfate solution (3 × 100 mL), with a saturated aqueous ammonium chloride solution (3 × 100 mL), and with a saturated aqueous sodium chloride solution (75 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The resulting residue was purified by flash column chromatography on silica gel (eluent: 0 → 60% acetone in dichloromethane) to afford alcohol (+)-12 (1.33 g, 90%) as a white solid. 1H NMR (400 MHz, CDCl3, 25 °C): δ 7.57 (d, J = 8.1 Hz, 1H), 7.45 (app-d, J = 9.7 Hz, 2H), 7.33 (app-t, J = 7.5 Hz, 1H), 7.28–7.23 (m, 1H), 7.12–7.08 (m, 4H), 6.65 (app-d, J = 9.0 Hz, 2H) 6.60 (app-d, J = 9.0 Hz, 2H), 6.13 (s, 1H), 4.41 (app-t, J = 8.3 Hz, 1H), 4.10 (d, J = 17.3 Hz, 1H), 4.05 (t, J = 6.0 Hz, 2H), 3.84 (t, J = 6.0 Hz, 2H), 3.81 (d, J = 17.7 Hz, 1H), 3.06 (dd, J = 7.0, 14.1 Hz, 1H), 2.88–2.82 (m, 4H), 2.02 (p, J = 5.9 Hz, 2H), 1.88 (br-s, 1H). 13C{1H} NMR (100 MHz, CDCl3, 25 °C): δ 167.1, 165.3, 158.1, 139.9, 138.2, 135.9, 133.1, 132.9, 129.3, 128.8, 128.2, 127.6, 126.0, 125.5, 117.2, 115.0, 87.2, 65.8, 60.2, 59.4, 58.6, 54.4, 39.0, 33.7, 32.1. FTIR (thin film) cm–1: 2954 (w), 1700 (s), 1684 (s), 1507 (m), 1362 (m), 1169 (m), 832 (w), 668 (m). HRMS (DART) m/z: [M + H]+ calcd for C29H30N3O6S 548.1850; Found 548.1872. [α]D23: +26 (c = 0.12, CHCl3). TLC (30% acetone in dichloromethane), Rf: 0.21 (UV, CAM).
Representative Azide Synthesis: Synthesis of (+)-(5aS,10bS,11aS)-10b-(4-(3-Azidopropoxy)phenyl)-2-methyl-6-(phenylsulfonyl)-2,3,6,10b,11,11a-hexahydro-4H-pyrazino[1′,2′:1,5]pyrrolo[2,3-b]indole-1,4(5aH)-dione (13);51 Azide (+)-13
Diisopropyl azodicarboxylate (DIAD, 256 μL, 1.28 mmol, 1.50 equiv) and diphenylphosphoryl azide (DPPA, 276 μL, 1.28 mmol, 1.50 equiv) were added dropwise via syringe to a suspension of alcohol (+)-12 (466 mg, 851 μmol, 1 equiv) and resin-bound triphenylphosphine (1.31 mmol/g on 100–200 mesh polystyrene cross-linked with 1% divinylbenzene, 973 mg, 1.28 mmol, 1.50 equiv) in tetrahydrofuran (20 mL) at 0 °C. After 5 min, the ice–water bath was removed, and the reaction mixture was allowed to stir and warm to 23 °C. After 16 h, the reaction mixture was filtered through a 1 cm pad of diatomaceous earth in a 60 mL medium-porosity fritted-glass funnel. The filter cake was washed with dichloromethane (100 mL), and the filtrate was concentrated under reduced pressure. The resulting residue was purified by flash column chromatography on silica gel (eluent: 30% acetone in dichloromethane) to afford azide (+)-13 (425 mg, 87%) as a white solid. 1H NMR (400 MHz, CDCl3, 25 °C): δ 7.58 (d, J = 8.1 Hz, 1H), 7.49 (app-d, J = 8.4 Hz, 2H), 7.34 (app-t, J = 7.5 Hz, 1H), 7.28–7.23 (m, 1H), 7.14–7.09 (m, 4H), 6.68 (app-d, J = 9.0 Hz, 2H) 6.62 (app-d, J = 9.0 Hz, 2H), 6.13 (s, 1H), 4.39 (app-t, J = 8.2 Hz, 1H), 4.10 (d, J = 17.4 Hz, 1H), 3.99 (t, J = 5.9 Hz, 2H), 3.82 (d, J = 17.4 Hz, 1H), 3.51 (t, J = 6.5 Hz, 2H), 3.06 (dd, J = 7.1, 14.2 Hz, 1H), 2.89–2.83 (m, 4H), 2.04 (p, J = 6.2 Hz, 2H). 13C{1H} NMR (100 MHz, CDCl3, 25 °C): δ 167.1, 165.3, 157.9, 139.9, 138.2, 135.8, 133.1, 133.0, 129.3, 128.7, 128.2, 127.7, 126.0, 125.4, 117.2, 115.0, 87.1, 64.7, 59.4, 58.6, 54.4, 48.3, 39.0, 33.7, 28.9. FTIR (thin film) cm–1: 2929 (w), 2099 (s), 1700 (s), 1684 (s), 1512 (m), 1362 (m), 1252 (m), 1169 (m), 1091 (w), 832 (w), 668 (m). HRMS (DART) m/z: [M + H]+ calcd for C29H29N6O5S 573.1915; Found 573.1921. [α]D23: +21.8 (c = 0.22, CHCl3). TLC (30% acetone in dichloromethane), Rf: 0.55 (UV, CAM).
Representative DKP-Dihydroxylation: Synthesis of (−)-(3R,5aS,10bS,11aR)-10b-(4-(3-Azidopropoxy) phenyl)-3,11a-dihydroxy-2-methyl-6-(phenylsulfonyl)-2,3,6,10b,11,11a-hexahydro-4H-pyrazino[1′,2′:1,5] pyrrolo[2,3-b]indole-1,4(5aH)-dione (14);51 Diol (−)-14
Tetra-n-butylammonium permanganate28 (807 mg, 2.23 mmol, 5.05 equiv) was added as a solid to a solution of azide (+)-13 (253 mg, 442 μmol, 1 equiv) in 1,2-dichloroethane (16 mL) at 23 °C. After 1 h, the reaction mixture was diluted with a saturated aqueous sodium sulfite solution (50 mL) and with ethyl acetate–hexanes (9:1, 200 mL). The resulting mixture was washed with a saturated aqueous sodium bicarbonate solution (50 mL); the layers were separated, and the organic layer was washed sequentially with a saturated aqueous sodium bicarbonate solution (50 mL), deionized water (50 mL), and a saturated aqueous sodium chloride solution (25 mL). The combined aqueous layers were extracted with ethyl acetate–hexanes (9:1, 2 × 50 mL), and the combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The resulting residue was purified by flash column chromatography on silica gel (eluent: 0 → 40% acetone in dichloromethane) to afford diol (−)-14 (169 mg, 63%) as a white solid. 1H NMR (400 MHz, DMSO-d6, 25 °C): δ 7.43 (app-t, J = 7.4 Hz, 1H), 7.39–7.32 (m, 4H), 7.26–7.19 (m, 3H), 7.13 (app-t, J = 7.5 Hz, 2H), 7.01 (d, J = 7.2 Hz, 1H), 6.75 (app-d, J = 8.9 Hz), 6.66 (app-d, J = 8.9 Hz, 2H), 6.21 (s, 1H), 5.00 (d, J = 6.8 Hz, 1H), 4.02 (t, J = 6.0 Hz, 2H), 3.54 (t, J = 6.7 Hz, 2H), 3.19 (d, J = 14.9 Hz, 1H), 2.77 (s, 3H), 2.66 (d, J = 14.9 Hz, 1H), 1.99 (p, J = 6.3 Hz, 2H). 13C{1H} NMR (100 MHz, DMSO-d6, 25 °C): δ 166.6, 165.8, 157.1, 139.3, 138.0, 137.7, 133.6, 133.2, 128.9, 128.7, 128.0, 126.7, 126.6, 125.7, 117.0, 114.5, 87.3, 86.0, 80.9, 64.6, 57.4, 49.7, 47.7, 30.5, 28.1. FTIR (thin film) cm–1: 2095 (m), 1844 (m), 1734 (m), 1700 (s), 1685 (s), 1653 (s), 1559 (s), 1540 (m), 1507 (m), 1457 (m), 1055 (w), 668 (m). HRMS (DART) m/z: [M + H]+ calcd for C29H29N6O7S 605.1813; Found 605.1814. [α]D23: – 6 (c = 0.16, DMSO). TLC (30% acetone in dichloromethane), Rf: 0.40 (UV, CAM).
Representative DKP-Sulfidation: Synthesis of (+)-(3S,5aS,10bS,11aS)-10b-(4-(3-Azidopropoxy)phenyl)-2-methyl-6-(phenylsulfonyl)-2,3,5a,6,10b,11-hexahydro-3,11a-epidithiopyrazino[1′,2′:1,5]pyrrolo[2,3-b]indole-1,4-dione;51 Epidithiodiketopiperazine Azide (+)-9a
A solution of diol (−)-14 (190 mg, 314 μmol, 1 equiv) in anhydrous nitroethane (13 mL) at 0 °C was sparged with hydrogen sulfide gas for 20 min by discharge of a balloon equipped with a needle extending into the reaction mixture, providing a saturated hydrogen sulfide solution. Trifluoroacetic acid (TFA, 9.8 mL) was added via syringe over 20 s, and the sparging with hydrogen sulfide gas was maintained for another 20 min. The ice–water bath was removed and the solution was allowed to stir and warm to 23 °C under an atmosphere of hydrogen sulfide. After 2 h, the reaction mixture was diluted with ethyl acetate (125 mL) and slowly poured into a stirring saturated aqueous sodium bicarbonate solution (50 mL), and the organic layer was washed with a saturated aqueous sodium chloride solution (35 mL). A stock solution of potassium triiodide in pyridine9a was added dropwise into the organic layer containing crude bisthiol until a persistent yellow color was observed. The resulting mixture was washed with an aqueous hydrogen chloride solution (1 M, 2 × 35 mL), washed with a saturated aqueous sodium chloride solution (35 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The resulting residue was purified by flash column chromatography on silica gel (eluent: 10 → 20% ethyl acetate in dichloromethane) to afford epidithiodiketopiperazine azide (+)-9a (129 mg, 65%) as a beige solid. 1H NMR (400 MHz, CDCl3, 25 °C): δ 7.59 (d, J = 8.0 Hz, 1H), 7.40–7.34 (m, 3H), 7.29 (app-t, J = 7.5 Hz, 1H), 7.25–7.21 (m, 2H), 7.03 (t, J = 7.9 Hz, 2H), 6.75 (app-d, J = 8.9 Hz, 2H), 6.61 (app-d, J = 8.9 Hz, 2H), 6.38 (s, 1H), 5.24 (s, 1H), 3.99 (t, J = 6.0 Hz, 2H), 3.62 (d, J = 15.5 Hz, 1H), 3.51 (t, J = 6.5 Hz, 2H), 3.11 (s, 3H), 2.84 (d, J = 15.5 Hz, 1H), 2.03 (p, J = 6.1 Hz, 2H). 13C{1H} NMR (100 MHz, CDCl3, 25 °C): δ 165.2, 160.2, 158.1, 141.3, 138.5, 135.9, 133.1, 131.6, 129.9, 128.7, 128.1, 127.3, 126.2, 125.7, 119.0, 115.1, 87.7, 74.6, 68.5, 64.7, 59.6, 48.3, 45.5, 32.2, 28.9. FTIR (thin film) cm–1: 2926 (w), 2098 (m), 1717 (s), 1700 (s), 1685 (s), 1559 (m), 1507 (m), 1473 (w), 972 (w), 668 (m). HRMS (DART) m/z: [M + NH4]+ calcd for C29H30N7O5S3 652.1465; Found 652.1454. [α]D23: +236 (c = 0.10, CHCl3). TLC (20% ethyl acetate in dichloromethane), Rf: 0.32 (UV, CAM, AgNO3).
Representative Procedure of CuAAC Ligation: Synthesis of (+)-(3S,5aS,10bS,11aS)-10b-(4-(3-(4-(4-Methoxyphenyl)-1H-1,2,3-triazol-1-yl)propoxy)phenyl)-2-methyl-6-(phenylsulfonyl)-2,3,5a,6,10b,11-hexahydro-3,11a-epidithiopyrazino[1′,2′:1,5]pyrrolo[2,3-b]indole-1,4-dione (28a);51 Triazole (+)-28a
Copper(I) iodide (45.7 mg, 0.240 mmol, 1.50 equiv) was added as a solid to a solution of epidithiodiketopiperazine azide (+)-9a (102 mg, 0.160 mmol, 1 equiv), 4-ethynylanisole 27 (104 μL, 0.800 mmol, 5.00 equiv), acetic acid (28 μL, 0.48 mmol, 3.0 equiv), and N,N-diisopropylethylamine (84 μL, 0.48 mmol, 3.0 equiv) in dichloromethane (1.6 mL) at 23 °C. After 11 h, the reaction mixture was directly purified by flash column chromatography on silica gel (eluent: 20% ethyl acetate in dichloromethane → 100% ethyl acetate) to afford triazole (+)-28a (116 mg, 94%) as a yellow solid. 1H NMR (400 MHz, CDCl3, 25 °C): δ 7.70 (app-d, J = 8.8 Hz, 2H), 7.68 (s, 1H), 7.57 (d, J = 8.0 Hz, 1H), 7.39–7.35 (m, 3H), 7.30–7.19 (m, 3H), 7.03 (t, J = 7.9 Hz, 2H), 6.92 (app-d, J = 8.8 Hz, 2H), 6.76 (app-d, J = 8.8 Hz, 2H), 6.60 (app-d, J = 8.8 Hz, 2H), 6.38 (s, 1H), 5.21 (s, 1H), 4.61 (t, J = 6.7 Hz, 2H), 3.94–3.91 (m, 2H), 3.81 (s, 3H,), 3.62 (d, J = 15.5 Hz, 1H), 3.10 (s, 3H), 2.83 (d, J = 15.5 Hz, 1H), 2.42 (p, J = 6.3 Hz, 2H). 13C{1H} NMR (100 MHz, CDCl3, 25 °C): δ 165.1, 160.1, 159.7, 157.8, 147.8, 141.3, 138.3, 135.8, 133.1, 131.7, 129.8, 128.6, 128.0, 127.2, 127.1, 126.2, 125.6, 123.3, 119.3, 118.9, 115.0, 114.4, 87.6, 74.5, 68.4, 64.3, 59.5, 55.4, 47.1, 45.4, 32.1, 30.0. FTIR (thin film) cm–1: 3058 (m), 2958 (w), 1700 (s), 1646 (s), 1559 (m), 1512 (s), 1458 (m), 1250 (w), 1171 (s), 1032 (w), 836 (m). HRMS (ESI) m/z: [M + H]+ calcd for C38H35N6O6S3 767.1775; Found 767.1796. [α]D23: +315 (c = 0.10, CHCl3). TLC (100% ethyl acetate), Rf: 0.38 (UV, CAM, AgNO3).
Representative Synthesis of ETP-Amine Acylation: Synthesis of (+)-N-((1-(3-(4-((3S,5aS,10bS,11aS)-2-Methyl-1,4-dioxo-6-(phenylsulfonyl)-1,2,3,4,5a,6-hexahydro-3,11a-epidithiopyrazino[1′,2′:1,5]pyrrolo[2,3-b]indol-10b(11H)-yl)phenoxy)propyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (30);51 Benzamide (+)-30
A solution of hydrogen chloride in 1,4-dioxane (4.0 M, 1.0 mL) was added via syringe to a solution of triazole (+)-29 (15.0 mg, 19.0 μmol, 1 equiv) in 1,4-dioxane (0.5 mL) at 23 °C. After 20 min, the reaction mixture was concentrated under reduced pressure, and the resulting yellow solid was dissolved in pyridine (240 μL). A solution of benzoyl chloride (48 mM, 0.60 mL, 29 μmol, 1.5 equiv) in tetrahydrofuran was added via syringe, followed by the addition of triethylamine (40 μL, 290 μmol, 15 equiv) via syringe. After 30 min, the reaction mixture was diluted with ethyl acetate (30 mL) and was slowly poured into an aqueous hydrogen chloride solution (1 M, 5 mL). The organic layer was washed sequentially with an aqueous hydrogen chloride solution (1 M, 5 mL), a saturated aqueous sodium bicarbonate solution (5 mL), and a saturated aqueous sodium chloride solution (5 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The resulting residue was purified by flash column chromatography on silica gel (eluent: 1 → 2% methanol in dichloromethane) to afford benzamide (+)-30 (13.1 mg, 87%) as a beige solid. 1H NMR (400 MHz, CDCl3, 25 °C): δ 7.77 (app-d, J = 7.3 Hz, 2H), 7.71 (br-s, 1H), 7.57 (d, J = 8.0 Hz, 1H), 7.46 (app-t, J = 7.4 Hz, 1H), 7.40–7.32 (m, 5H), 7.28–7.20 (m, 3H), 7.16 (br-s, 1H), 7.01 (app-t, J = 7.8 Hz, 2H), 6.71 (app-d, J = 8.8 Hz, 2H), 6.56 (app-d, J = 8.8 Hz, 2H), 6.36 (s, 1H), 5.27 (s, 1H), 4.68 (br-s, 2H), 4.55 (t, J = 6.3 Hz, 2H), 3.95–3.84 (m, 2H), 3.60 (d, J = 15.5 Hz, 1H), 3.10 (s, 3H), 2.83 (d, J = 15.5 Hz, 1H), 2.37 (p, J = 5.9 Hz, 2H). 13C{1H} NMR (150 MHz, CDCl3, 25 °C): δ 167.6, 165.2, 160.2, 157.8, 145.0, 141.3, 138.4, 135.9, 134.0, 133.2, 131.9, 131.8, 129.9, 128.8, 128.7, 128.1, 127.3, 127.2, 126.3, 125.7, 123.3, 119.0, 115.1, 87.7, 74.6, 68.5, 64.3, 59.5, 47.4, 45.5, 35.5, 32.2, 30.0. FTIR (thin film) cm–1: 3345 (w), 3001 (w), 1695 (s), 1512 (m), 1461 (m), 1169 (m), 755 (m). HRMS (ESI) m/z: [M + H]+ calcd for C39H36N7O6S3 794.1884; Found 794.1890. [α]D23: +175 (c = 0.11, CHCl3). TLC (10% methanol in dichloromethane), Rf: 0.52 (UV, CAM, AgNO3).
Synthesis of (3R,5aS,10bS,11aR)-3,11a-Dihydroxy-10b-(4-methoxyphenyl)-2,3-dimethyl-6-(phenylsulfonyl)-2,3,6,10b,11,11a-hexahydro-4H-pyrazino[1′,2′:1,5]pyrrolo[2,3-b]indole-1,4(5aH)-dione (38);51 Diol 38
Bis(2,2′-bipyridyl)copper(II) permanganate39 (1.61 g, 2.62 mmol, 2.70 equiv) was added as a solid to solution of anisole adduct (+)-37 (502 mg, 0.970 mmol, 1 equiv) in dichloromethane (10 mL) at 23 °C. After 50 min, the reaction mixture was diluted with dichloromethane (100 mL) and poured into an aqueous sodium bisulfite solution (1 M, 200 mL). The layers were separated, and the organic layer was washed sequentially with an aqueous sodium bisulfite solution (1 M, 75 mL), a mixture of a saturated aqueous copper(II) sulfate solution and deionized water (1:1, 100 mL), a saturated aqueous ammonium chloride solution (100 mL), and a saturated aqueous sodium chloride solution (100 mL). The aqueous layers were separately extracted with dichloromethane (2 × 75 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The resulting light blue foam was purified by flash column chromatography on silica gel (eluent: 0 → 30% acetone in dichloromethane) to afford diol 38 (393 mg, 74%) as a white foam. 1H NMR (400 MHz, CDCl3, 25 °C): δ 7.61 (d, J = 8.1 Hz, 1H), 7.34–7.26 (m, 4H), 7.22–7.15 (m, 2H), 7.02 (app-t, J = 7.9 Hz, 2H), 6.78 (app-d, J = 8.9 Hz, 2H), 6.55 (app-d, J = 8.9 Hz, 2H), 6.35, (s, 1H), 5.62 (br-s, 1H), 5.24 (br-s, 1H), 3.76 (s, 3H), 3.38 (d, J = 15.1 Hz, 1H), 2.99 (s, 3H), 2.92 (d, J = 15.1 Hz, 1H), 1.81 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3, 25 °C): δ 168.2, 166.8, 158.4, 140.0, 138.2, 137.7, 133.9, 132.9, 129.1, 128.6, 128.5, 127.5, 126.5, 126.1, 118.0, 114.3, 88.7, 87.4, 85.7, 58.1, 55.4, 49.6, 28.1, 22.8. FTIR (thin film) cm–1: 3375 (br), 3067 (w), 1687 (m), 1512 (m), 1361 (m), 1252 (m), 1169 (s), 832 (w), 737 (w), 600 (m). HRMS (ESI) m/z: [M + H]+ calcd for C28H28N3O7S 550.1642; Found 550.1640. TLC (20% acetone in dichloromethane), Rf: 0.22 (UV, CAM).
Synthesis of Sodium 4-Methoxybenzyl Carbonotrithioate (39); Monosodium Trithiocarbonate 39
A suspension of sodium hydride (60% dispersion, 1.03 g, 25.8 mmol, 1 equiv) in diethyl ether (125 mL) at 0 °C was sparged with argon for 20 min by discharge of a balloon equipped with a needle extending into the reaction mixture. p-Methoxybenzyl thiol (4.5 mL, 33 mmol, 1.3 equiv) was added dropwise via syringe over 2 min. The solution was stirred for 5 min; the ice–water bath was removed, and the reaction mixture was allowed to stir and warm to 23 °C. After 1 h, the light-gray suspension was cooled to 0 °C, and carbon disulfide (2.0 mL, 33 mmol, 1.3 equiv) was added dropwise via syringe over 3.5 min. The ice–water bath was removed, and the reaction mixture was allowed to stir and warm to 23 °C. After 2 h, a yellow precipitate was collected by filtration of the yellow suspension through a 350 mL medium-porosity fritted-glass funnel. The yellow precipitate was washed with hexanes (2 × 50 mL) and dried under reduced pressure to afford monosodium trithiocarbonate 39 (5.76 g, 88%) as a yellow solid. 1H NMR (500 MHz, DMSO-d6, 25 °C): δ 7.20 (d, J = 8.6 Hz, 2H), 6.81 (d, J = 8.6 Hz, 2H), 4.29 (s, 2H), 3.71 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6, 25 °C): δ 239.0, 157.8, 130.9, 129.8, 113.5, 55.0, 44.6. FTIR (thin film) cm–1: 1507 (w), 1248 (w), 1229 (w), 1177 (w), 1003 (s), 833 (m), 539 (m). HRMS (DART-TOF) m/z: [M – Na]− calcd for C9H9OS3 228.9821; Found 228.9813.
Synthesis of (+)-(4S,6aS,11bS,12aS)-11b-(4-Methoxyphenyl)-4,14-dimethyl-7-(phenylsulfonyl)-2-thioxo-6a,7,11b,12-tetrahydro-4,12a-(epiminomethano)[1,3,5]dithiazepino[5′,4′:1,5]pyrrolo[2,3-b]indole-5,13(4H)-dione (41);51 Dithiepanethione (+)-41
A mixture of regioisomeric silyl ethers23S15 and S16 (1.1:1, 956 mg, 1.44 mmol, 1 equiv) was azeotropically dried by concentration from dichloromethane (5 mL) and anhydrous benzene (50 mL) under reduced pressure. The resulting white foam was dissolved in acetonitrile (100 mL) via cannula, and monosodium trithiocarbonate 39 (1.82 g, 7.21 mmol, 5.01 equiv) was added as a solid. Trifluoroacetic acid (TFA, 50 mL) was poured rapidly into the reaction mixture over 15 s, resulting in a homogeneous yellow solution. After 1 h, the dark orange solution was diluted with ethyl acetate–hexanes (9:1, 100 mL) and slowly poured into a saturated aqueous sodium bicarbonate solution (650 mL), and the biphasic mixture was stirred vigorously for 30 min. The aqueous layer was extracted with ethyl acetate–hexanes (9:1, 2 × 100 mL), and the combined organic extracts were washed sequentially with deionized water (200 mL) and a saturated aqueous sodium chloride solution (150 mL). The combined aqueous layers were extracted with a single portion of ethyl acetate–hexanes (4:1, 100 mL), and the combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The resulting residue was purified by flash column chromatography on silica gel (eluent: 0 → 7.5% diethyl ether in dichloromethane) to afford dithiepanethione (+)-41 (766 mg, 85%) as a yellow foam. 1H NMR (400 MHz, CDCl3, 25 °C): δ 7.55 (d, J = 8.1 Hz, 1H), 7.43 (app-d, J = 7.6 Hz, 2H), 7.30–7.21 (m, 2H), 7.30–7.21 (m, 2H), 7.13 (app-t, 2H), 6.87 (app-d, J = 8.8 Hz, 2H), 6.68 (app-d, J = 8.8 Hz, 2H), 6.59 (s, 1H), 3.78 (s, 3H), 3.53 (d, J = 15.3 Hz, 1H), 3.06 (s, 3H), 3.05 (d, J = 15.2 Hz, 1H), 1.92 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3, 25 °C): δ 215.7, 164.7, 160.5, 159.0, 141.5, 138.9, 134.9, 133.1, 131.4, 130.1, 128.7, 127.5, 126.8, 126.4, 125.5, 118.7, 114.6, 87.8, 75.0, 73.5, 57.8, 55.5, 48.7, 28.4, 19.8. FTIR (thin film) cm–1: 3002 (w), 1713 (s), 1685 (s), 1476 (w), 1362 (s), 1169 (s), 1034 (m), 999 (m), 895 (w), 737 (m), 599 (m). HRMS (ESI) m/z: [M + H]+ calcd for C29H26N3O5S4 624.0750; Found 624.0747. [α]D23: +148 (c = 0.61, CHCl3). TLC (5% diethyl ether in dichloromethane), Rf: 0.31 (UV, CAM, AgNO3, DTNB).
Synthesis of (+)-(3S,5aS,10bS,11aS)-10b-(4-Methoxyphenyl)-2,3-dimethyl-6-(phenylsulfonyl)-2,3,5a,6,10b,11-hexahydro-3,11a-epidithiopyrazino[1′,2′:1,5]pyrrolo[2,3-b]indole-1,4-dione (42);51 Epidithiodiketopiperazine (+)-42
A yellow solution of dithiepanethione (+)-41 (374 mg, 0.600 mmol, 1 equiv) in acetone (15 mL) at 23 °C was sparged with argon for 10 min by discharge of a balloon equipped with a needle extending into the reaction mixture. Ethanolamine (3.75 mL) was added via syringe over 30 s, resulting in a nearly colorless solution. After 1 h, the reaction mixture was diluted with ethyl acetate–hexanes (9:1, 100 mL) and was washed with an aqueous hydrogen chloride solution (1 M, 150 mL). The aqueous layer was extracted with ethyl acetate–hexanes (9:1, 2 × 50 mL), and the combined organic extracts were washed with a saturated aqueous sodium chloride solution (100 mL). A stock solution of potassium triiodide in pyridine was added dropwise into the organic layer containing crude bisthiol until a persistent yellow color was observed. The resulting mixture was washed sequentially with an aqueous hydrogen chloride solution (1 M, 2 × 75 mL), a mixture of deionized water and a saturated aqueous sodium thiosulfate solution (3:1, 100 mL), deionized water (100 mL), and a saturated aqueous sodium chloride solution (100 mL). The aqueous layers were separately extracted with a single portion of ethyl acetate–hexanes (9:1, 100 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The resulting residue was purified by flash column chromatography on silica gel (eluent: 15% dichloromethane, 0 → 7.5% isopropanol in hexanes) to afford epidithiodiketopiperazine (+)-42 (304 mg, 87%) as a white solid. 1H NMR (500 MHz, CDCl3, 25 °C): δ 7.65 (d, J = 8.0 Hz, 1H), 7.40 (app-t, d, J = 7.1, 1.5 Hz, 1H), 7.34 (dd, J = 8.5, 1.2 Hz, 2H), 7.31–7.22 (m, 3H), 7.02 (app-t, J = 7.5 Hz, 2H), 6.74 (app-d, J = 8.8 Hz, 2H), 6.62 (app-d, J = 8.7 Hz, 2H), 6.42 (s, 1H), 3.79 (s, 3H), 3.67 (d, J = 15.6 Hz, 1H), 3.05 (s, 3H), 2.88 (d, J = 15.5 Hz, 1H), 1.97 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3, 25 °C): δ 165.8, 161.4, 158.8, 141.2, 138.3, 135.8, 132.9, 131.4, 129.7, 128.5, 127.9, 127.2, 126.1, 125.6, 119.0, 114.5, 88.0, 73.9, 73.5, 59.1, 55.5, 46.1, 27.6, 18.2. FTIR (thin film) cm–1: 2951 (br), 2359 (w), 1679 (s), 1514 (s), 1457 (m), 1341 (s), 1249 (s), 1163 (s), 1028 (m), 905 (m), 730 (s). HRMS (ESI) m/z: [M + H]+ calcd for C28H26N3O5S3 580.1029; Found 580.1032. [α]D23: +293 (c = 0.57, CHCl3). TLC (15% dichloromethane and 15% isopropanol in hexanes), Rf: 0.42 (UV, CAM, AgNO3).
Synthesis of (3S,5aS,10bS,11aS)-3,11a-Bis((4-fluorobenzyl)disulfaneyl)-10b-(4-methoxyphenyl)-2,3-dimethyl-6-(phenylsulfonyl)-2,3,6,10b,11,11a-hexahydro-4H-pyrazino[1′,2′:1,5]pyrrolo[2,3-b]indole-1,4(5aH)-dione (45a);51 Bis(p-fluorobenzyl)disulfide 45a
Triethylamine (70 μL, 0.50 mmol, 2.5 equiv) and (p-fluorophenyl)methanethiol (PFB-SH, 25 μL, 0.20 mmol, 1.0 equiv) were added via syringe to a solution of epidithiodiketopiperazine (+)-42 (116 mg, 0.200 mmol, 1 equiv) and 1,2-bis(p-fluorobenzyl)disulfane (PFB-SS-PFB, 552 mg, 1.95 mmol, 9.75 equiv) in tetrahydrofuran (0.5 mL) at 23 °C. After 15 h, additional tetrahydrofuran (1.1 mL) was added via syringe to dissolve a white precipitate. After an additional 50 h, the reaction mixture was concentrated under reduced pressure, and the resulting residue was purified by flash column chromatography on silica gel (eluent: 0 → 15% ethyl acetate in dichloromethane) to afford bisdisulfide 45a (38.7 mg, 22.4%) as a white solid and recovered epidithiodiketopiperazine (+)-42 (76.6 mg, 66%) as a white solid. 1H NMR (400 MHz, CDCl3, 25 °C): δ 7.67 (d, J = 8.1 Hz, 1H), 7.48 (app-d, J = 7.6 Hz, 2H), 7.38–7.33 (m, 3H), 7.30 (app-t, J = 7.7 Hz, 1H), 7.22–7.15 (m, 2H), 7.14–7.09 (m, 4H), 6.95 (app-t, J = 8.7 Hz, 2H), 6.90 (app-t, J = 8.6 Hz), 6.67 (app-d, J = 8.8 Hz, 2H), 6.59 (s, 1H), 6.58 (app-d, J = 9.1 Hz, 2H), 4.09 (d, J = 12.9 Hz, 1H), 3.99 (d, J = 12.9 Hz, 1H), 3.84 (d, J = 14.7 Hz, 1H), 3.83 (s, 2H), 3.76 (s, 3H), 3.10 (s, 3H), 2.99 (d, J = 14.8 Hz, 1H), 2.09 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3, 25 °C): δ 167.4, 164.2, 162.3 (d, J = 245.6 Hz), 162.3 (d, J = 246.3 Hz), 158.6, 142.2, 137.9, 135.5, 133.2, 133.1, 132.9 (d, J = 3.2 Hz), 132.4 (d, J = 3.3 Hz), 131.7 (d, J = 8.2 Hz), 131.3 (d, J = 8.2 Hz), 129.4, 128.7, 127.5, 127.5, 125.9, 125.7, 118.5, 115.5 (d, J = 21.5 Hz), 115.4 (d, J = 21.5 Hz), 114.3, 88.3, 73.7, 71.1, 57.1, 55.5, 46.9, 42.2, 41.7, 29.5, 22.8. FTIR (thin film) cm–1: 3485 (br), 2927 (br), 2106 (w), 1663 (m), 1600 (w), 1509 (s), 1362 (s), 833 (m), 687 (w), 599 (m). HRMS (ESI) m/z: [M + H]+ calcd for C42H38F2N3O5S5 862.1378; Found 862.1371. TLC (5% ethyl acetate in dichloromethane), Rf: 0.35 (UV, CAM, AgNO3).
Synthesis of Triethylammonium S-(((3S,5aS,10bS,11aS)-3-(((R)-2-((S)-4-Amino-4-carboxybutanamido)-3-((carboxymethyl)amino)-3-oxopropyl)disulfaneyl)-10b-(4-methoxyphenyl)-2,3-dimethyl-1,4-dioxo-6-(phenylsulfonyl)-1,2,3,4,5a,6,10b,11-octahydro-11aH-pyrazino[1′,2′:1,5]pyrrolo[2,3-b]indol-11a-yl)thio)-N-((S)-4-amino-4-carboxybutanoyl)-l-cysteinylglycinate (46);51 Bis(l-glutathione)disulfide 46
Sodium borohydride (4.9 mg, 0.13 mmol, 4.3 equiv) was added as a solid in one portion to a solution of epidithiodiketopiperazine (+)-42 (17.3 mg, 29.8 μmol, 1 equiv) in tetrahydrofuran (4.0 mL) and methanol (30 μL). After 35 min, the reaction mixture was diluted with ethyl acetate–hexanes (9:1, 40 mL) and was washed sequentially with a saturated aqueous ammonium chloride solution (40 mL), deionized water (30 mL), and a saturated aqueous sodium chloride solution (20 mL). The aqueous layers were separately extracted with a single portion of ethyl acetate–hexanes (9:1, 25 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and sparged with argon for 15 min by discharge of a balloon equipped with a needle extending into the stirring reaction mixture. The reaction mixture was then concentrated under reduced pressure, and the resulting residue containing bisthiol was dissolved in tetrahydrofuran (0.25 mL) and added dropwise via syringe to a solution of S-(phenylsulfonyl)-l-glutathione hydrogen chloride44 (72.9 mg, 163 μmol, 5.45 equiv) and triethylamine (45 μL, 320 μmol, 11 equiv) in tetrahydrofuran (1.1 mL) and methanol (1.1 mL). The transfer was quantitated with additional tetrahydrofuran (2 × 0.25 mL). After 19 h, the reaction mixture was diluted with methanol and adsorbed onto diatomaceous earth (0.4 g) by concentration under reduced pressure until a free-flowing powder was obtained. The diatomaceous earth-absorbed crude mixture was purified by flash column chromatography on C18-reversed phase silica gel (eluent: 10 → 80% acetonitrile in water) to afford the bisdisulfide 46 (17.2 mg, 45%) as a white solid and recovered epidithiodiketopiperazine (+)-42 (6.0 mg, 21%). 1H NMR (500 MHz, 5:1 D2O:CD3CN, 25 °C): δ 7.45 (d, J = 8.2 Hz, 1H), 7.42–7.34 (m, 3H), 7.32 (app-t, J = 7.7 Hz, 1H), 7.26 (d, J = 7.6 Hz, 1H), 7.16 (app-t, J = 7.5 Hz, 1H), 7.10 (app-t, J = 7.8 Hz, 2H), 6.70 (app-d, J = 8.4 Hz, 2H), 6.59 (app-d, J = 8.4 Hz, 2H), 6.40 (s, 1H), 4.66 (dd, J = 8.6, 5.1 Hz, 1H), 4.42 (dd, J = 10.0, 4.0 Hz, 1H), 3.80–3.57 (m, 9H), 3.54 (d, J = 14.6 Hz), 3.26–3.03 (m, 9H), 3.03–2.93 (m, 4H), 2.65–2.54 (m, 1H), 2.42 (app-t, J = 7.6 Hz, 2H), 2.34 (app-t, J = 7.7 Hz, 2H), 2.04 (app-q, J = 7.2 Hz, 2H), 1.98 (app-q, J = 7.5 Hz, 2H), 1.89 (s, 3H), 1.17 (t, J = 7.3 Hz, 9H). 13C{1H} NMR (125 MHz, 5:1 D2O:CD3CN, 25 °C): δ 174.5 (br, 2C), 173.7, 173.6, 172.8, 170.5, 170.0, 166.6, 163.9, 157.0, 140.3, 135.6, 134.7, 133.5, 132.3, 128.8, 128.4, 126.7, 125.9, 125.6, 125.3, 117.3, 113.7, 87.1, 73.1, 71.5, 56.1, 54.6, 53.3, 53.2, 52.1, 51.7, 45.8, 44.4, 42.3, 42.2, 40.4, 37.5, 30.8, 30.7, 29.2, 25.5, 25.4, 20.8, 7.4. FTIR (thin film) cm–1: 3273 (br), 1645 (s), 1513 (s), 1253 (m), 1167 (m), 1109 (w), 1028 (w), 832 (w), 686 (m). HRMS (ESI) m/z: [M + Na]+ calcd for C48H57N9NaO17S5 1214.2368; Found 1214.2359. TLC (30% acetonitrile in water, C18-reversed phase), Rf: 0.25 (UV, CAM, AgNO3).
Synthesis of tert-Butyl (2-(2-(2-((1-(3-(4-((3S,5aS,10bS,11aS)-2,3-Dimethyl-1,4-dioxo-6-(phenylsulfonyl)-1,2,3,4,5a,6-hexahydro-3,11a-epidithiopyrazino[1′,2′:1,5]pyrrolo[2,3-b]indol-10b(11H)-yl)phenoxy)propyl)-1H-1,2,3-triazol-4-yl)methoxy)ethoxy)ethoxy)ethyl)carbamate;51 Triazole 51
A solution of N,N-diisopropylethylamine (DIPEA, 2.7 μL, 16 μmol, 1.5 equiv) and acetic acid (AcOH, 0.90 μL, 16 μmol, 1.5 equiv) in toluene (0.2 mL) was added to a flask containing azide (+)-9d (6.8 mg, 11 μmol, 1 equiv) and alkyne2350 (11.6 mg, 40.4 μmol, 3.67 equiv). Copper(I) iodide (0.9 mg, 5 μmol, 0.5 equiv) was added as a solid, and the suspension was sparged with argon for 2 min by discharge of balloon equipped with a needle extending into the reaction mixture. After 17 h, the reaction mixture was diluted with dichloromethane (0.5 mL) and purified by flash chromatography on silica gel (eluent: 5 → 40% acetone in dichloromethane) to afford triazole 51 as a yellow solid. The mixture was further purified by flash column chromatography on silica gel (eluent: 0 → 4% methanol in dichloromethane) to afford triazole 51 (9.0 mg, 92%) as a white solid. 1H NMR (500 MHz, CDCl3, 25 °C): δ 7.68–7.60 (m, 2H), 7.40 (app-t, J = 7.6 Hz, 1H), 7.36 (app-d, J = 7.9 Hz, 2H), 7.31 (app-t, J = 7.5 Hz, 1H), 7.29–7.22 (m, 2H), 7.05 (app-t, J = 7.7 Hz, 2H), 6.74 (app-d, J = 8.2 Hz, 2H), 6.59 (br-s, 2H), 6.42 (s, 1H), 5.04 (br-s, 1H), 4.70 (s, 2H), 4.60 (t, J = 5.7 Hz, 2H), 3.97–3.88 (m, 2H), 3.73–3.57 (m, 9H), 3.53 (t, J = 5.0 Hz, 2H), 3.30 (app-q, J = 5.5 Hz, 2H), 3.05 (s, 3H), 2.88 (d, J = 15.5 Hz, 1H), 2.40 (p, J = 6.4 Hz, 2H), 1.96 (s, 3H), 1.43 (s, 9H). 13C{1H} NMR (125 MHz, CDCl3, 25 °C): δ 165.8, 161.4, 157.7, 156.1, 145.5, 141.3, 138.5, 135.8, 133.1, 132.0, 129.8, 128.6, 128.0, 127.2, 126.1, 125.5, 123.0, 119.0, 115.0, 87.9, 79.3, 73.9, 73.5, 70.7 (3C), 70.4, 69.9, 64.8, 64.2, 59.1, 47.1, 46.0, 40.5, 30.0, 28.6 (3C), 27.7, 18.2. FTIR (thin film) cm–1: 3360 (br-m), 2921 (s), 2851 (m), 1659 (m), 1632 (m), 1468 (w), 1411 (w), 1024 (w), 801 (w). HRMS (ESI) m/z: [M + Na]+ calcd for C44H53N7NaO10S3 958.2908; Found 958.2902. TLC (5% methanol in dichloromethane), Rf: 0.26 (UV, CAM, AgNO3).
Cell Culture Information
Cells were grown in media supplemented with fetal bovine serum (FBS) and antibiotics (100 μg/mL penicillin and 100 U/mL streptomycin). Specifically, experiments were performed using the following cell lines and media compositions: HeLa (cervical adenocarcinoma) and A549 (lung carcinoma) were grown in RPMI-1640 + 10% FBS; HCT 116 (colorectal carcinoma) was grown in DMEM + 10% FBS; MCF7 (breast adenocarcinoma) and DU 145 (prostate carcinoma) were grown in EMEM + 10% FBS. Cells were incubated at 37 °C in a 5% CO2, 95% humidity atmosphere.
Cell Viability Assays
Cells were plated at 250 cells/well into duplicate assay plates in 50 μL of media into 384-well white, opaque, tissue-culture treated plates and allowed to adhere overnight at 37 °C/5% CO2. Compounds were solubilized in DMSO as 1000× stocks, and 100 nL was pin-transferred to cells. Compounds were tested in 10-pt, 2-fold dilution with concentrations tested between 1 nM and 20 μM for most compounds except where indicated. DMSO (32 wells of 384-wells) was used as vehicle control. After 72 h of incubation at 37 °C/5% CO2, 10 μL of Cell Titer-Glo was added to each well, and plates were incubated at room temperature for 10 min before the luminescence was read on a plate reader. Cell Titer-Glo measures ATP levels of cells as a surrogate for cell viability. All compound-treated wells were normalized to the DMSO control averages and expressed as a % of DMSO viability. IC50 values were determined from the dose curves using Spotfire.
Acknowledgments
We are grateful for financial support from NIH-NIGMS (GM089732). This work was supported in part by the Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.9b03371.
Detailed experimental procedures, complete characterization data for new compounds, and copies of 1H and 13C{1H} NMR spectra of new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- For reviews on epipolythiodiketopiperazines, see:; a Hino T.; Nakagawa M. In The Alkaloids: Chemistry and Pharmacology; Brossi A., Ed.; Academic Press: New York, 1989, Vol. 34, Chapter 1, pp 1–75. [Google Scholar]; b Gardiner D. M.; Waring P.; Howlett B. J. The Epipolythiodioxopiperazine (ETP) Class of Fungal Toxins: Distribution, Mode of Action, Functions and Biosynthesis. Microbiology 2005, 151, 1021–1032. 10.1099/mic.0.27847-0. [DOI] [PubMed] [Google Scholar]; c Jiang C.-S.; Guo Y.-W. Epipolythiodioxopiperazines from Fungi: Chemistry and Bioactivities. Mini-Rev. Med. Chem. 2011, 11, 728–745. 10.2174/138955711796355276. [DOI] [PubMed] [Google Scholar]
- Kim J.; Movassaghi M. Biogenetically-Inspired Total Synthesis of Epidithiodiketopiperazines and Related Alkaloids. Acc. Chem. Res. 2015, 48, 1159–1171. 10.1021/ar500454v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Movassaghi M. Biogenetically Inspired Syntheses of Alkaloid Natural Products. Chem. Soc. Rev. 2009, 38, 3035–3050. 10.1039/b819925f. [DOI] [PubMed] [Google Scholar]
- Boyer N.; Morrison K. C.; Kim J.; Hergenrother P. J.; Movassaghi M. Synthesis and Anticancer Activity of Epipolythiodiketopiperazine Alkaloids. Chem. Sci. 2013, 4, 1646–1657. 10.1039/c3sc50174d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For representative anticancer activity of epipolythiodiketopiperazines, see:; a Vigushin D. M.; Mirsaidi N.; Brooke G.; Sun C.; Pace P.; Inman L.; Moody C. J.; Coombes R. C. Gliotoxin is a Dual Inhibitor of Farnesyltransferase and Geranylgeranylteransferase I with Antitumor Activity Against Breast Cancer In Vivo. Med. Oncol. 2004, 21, 21–30. 10.1385/MO:21:1:21. [DOI] [PubMed] [Google Scholar]; b Isham C. R.; Tibodeau J. D.; Jin W.; Xu R.; Timm M. M.; Bible K. C. Chaeotocin: A Promising New Antimyeloma Agent with in vitro and in vivo Activity Mediated via Imposition of Oxidative Stress. Blood 2007, 109, 2579–2588. 10.1182/blood-2006-07-027326. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Cook K. M.; Hilton S. T.; Mecinović J.; Motherwell W. B.; Figg W. D.; Schofield C. J. Epidithiodiketopiperazines Block the Interaction between Hypoxia-Inducible-Factor-1α(HIF-1α) and p300 by a Zinc Ejection Mechanism. J. Biol. Chem. 2009, 39, 26831–26838. 10.1074/jbc.M109.009498. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Liu F.; Liu Q.; Yang D.; Bollag W. B.; Robertson K.; Wu P.; Liu K. Verticillin A Overcomes Apoptosis Resistance in Human Colon Carcinoma Through DNA Methylation-Dependent Upregulation of BNIP3. Cancer Res. 2011, 71, 6807–6816. 10.1158/0008-5472.CAN-11-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Dubey R.; Levin M. D.; Szabo L. Z.; Laszlo C. F.; Kushal S.; Singh J. B.; Oh P.; Schnitzer J. E.; Olenyuk B. Z. Suppression of Tumor Growth by Designed Dimeric Epidithiodiketopiperazine Targeting Hypoxia-Inducible Transcription Factor Complex. J. Am. Chem. Soc. 2013, 135, 4537–4549. 10.1021/ja400805b. [DOI] [PubMed] [Google Scholar]; f Dewangan J.; Srivastava S.; Mishra S.; Pandey P. K.; Divakar A.; Rath S. K. Chetomin Induces Apoptosis in Human Triple-Negative Breast Cancer Cells by Promoting Calcium Overload and Mitochondrial Dysfunction. Biochem. Biophys. Res. Commun. 2018, 495, 1915–1921. 10.1016/j.bbrc.2017.11.199. [DOI] [PubMed] [Google Scholar]; and references cited therein.
- For antifungal activity of epipolythiodiketopiperazines, see:Saleh A. A.; Jones G. W.; Tinley F. C.; Delaney S. F.; Alabbadi S. H.; Fenlon K.; Doyle S.; Owens R. A. Systems Impact of Zinc Chelation by the Epipolythiodioxopiperazine Dithiol Gliotoxin in Aspergillus fumigatus: A New Direction in Natural Product Functionality. Metallomics 2018, 10, 854–866. 10.1039/C8MT00052B. [DOI] [PubMed] [Google Scholar]
- For antibacterial activity of epipolythiodiketopiperazines, see:Zheng C.-J.; Kim C.-J.; Bae K. S.; Kim Y.-H.; Kim W.-G. Bionectins A–C, Epidithiodioxopiperazines with Anti-MRSA Activity, from Bionectra byssicola F120. J. Nat. Prod. 2006, 69, 1816–1819. 10.1021/np060348t. [DOI] [PubMed] [Google Scholar]
- For antiviral activity of epipolythiodiketopiperazines, see:Asquith C. R. M.; Sil B. C.; Laitinen T.; Tizzard G. J.; Coles S. J.; Poso A.; Hofmann-Lehmann R.; Hilton S. T. Novel Epidithiodiketopiperazines as Anti-Viral Zinc Ejectors of the Feline Immunodeficiency Virus (FIV) Nucleocapsid Protein as a Model for HIV Infection. Bioorg. Med. Chem. 2019, 27, 4174–4184. 10.1016/j.bmc.2019.07.047. [DOI] [PubMed] [Google Scholar]
- For representative syntheses of epipolythiodiketopiperazines from our laboratory, see:; a Kim J.; Ashenhurst J. A.; Movassaghi M. Total Synthesis of (+)-11,11′-Dideoxyverticillin A. Science 2009, 324, 238–241. 10.1126/science.1170777. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kim J.; Movassaghi M. General Approach to Epipolythiodiketopiperazine Alkaloids: Total Synthesis of (+)-Chaetocins A and C and (+)-12,12′-Dideoxychetracin A. J. Am. Chem. Soc. 2010, 132, 14376–14378. 10.1021/ja106869s. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Coste A.; Kim J.; Adams T. C.; Movassaghi M. Concise Total Synthesis of (+)-Bionectins A and C. Chem. Sci. 2013, 4, 3191–3197. 10.1039/c3sc51150b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For representative syntheses of epipolythiodiketopiperazines, see:; a Fukuyama T.; Nakatsuka S.-I.; Kishi Y. Total Synthesis of Gliotoxin, Dehydrogliotoxin, and Hyalodendrin. Tetrahedron 1981, 37, 2045–2078. 10.1016/S0040-4020(01)97960-8. [DOI] [Google Scholar]; b Overman L. E.; Sato T. Construction of Epidithiodioxopiperazines by Directed Oxidation of Hydroxyproline-Derived Dioxopiperazines. Org. Lett. 2007, 9, 5267–5270. 10.1021/ol702518t. [DOI] [PubMed] [Google Scholar]; c Iwasa E.; Hamashima Y.; Fujishiro S.; Higuchi E.; Ito A.; Yoshida M.; Sodeoka M. Total Synthesis of (+)-Chaetocin and its Analogues: Their Histone Methyltransferase G9a Inhibitory Activity. J. Am. Chem. Soc. 2010, 132, 4078–4079. 10.1021/ja101280p. [DOI] [PubMed] [Google Scholar]; d Codelli J. A.; Puchlopek A. L. A.; Reisman S. E. Enantioselective Total Synthesis of (−)-Acetylaranotin, a Dihydrooxepine Epidithiodiketopiperazine. J. Am. Chem. Soc. 2012, 134, 1930–1933. 10.1021/ja209354e. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Takeuchi R.; Shimokawa J.; Fukuyama T. Development of a Route to Chiral Epidithiodioxopiperazine Moieties and Application to the Asymmetric Synthesis of (+)-Hyalodendrin. Chem. Sci. 2014, 5, 2003–2006. 10.1039/C3SC53222D. [DOI] [Google Scholar]; f Baumann M.; Dieskau A. P.; Loertscher B. M.; Walton M. C.; Nam S.; Xie J.; Horne D.; Overman L. E. Tricyclic Analogues of Epidithiodioxopiperazine Alkaloids with Promising in vitro and in vivo Antitumor Activity. Chem. Sci. 2015, 6, 4451–4457. 10.1039/C5SC01536G. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Snaddon T. N.; Scaggs T. D.; Pearson C. M.; Fyfe J. W. B. A Modular Construction of Epidithiodiketopiperazines. Org. Lett. 2019, 21, 4873–4877. 10.1021/acs.orglett.9b01770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bunnage M. E.; Chekler E. L. P.; Jones L. H. Target Validation Using Chemical Probes. Nat. Chem. Biol. 2013, 9, 195–199. 10.1038/nchembio.1197. [DOI] [PubMed] [Google Scholar]; b Wright M. H.; Sieber S. A. Chemical Proteomics Approaches for Identifying the Cellular Targets of Natural Products. Nat. Prod. Rep. 2016, 33, 681–708. 10.1039/C6NP00001K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Flygare J. A.; Pillow T. H.; Aristoff P. Antibody-Drug Conjugates for the Treatment of Cancer. Chem. Biol. Drug Des. 2013, 81, 113–121. 10.1111/cbdd.12085. [DOI] [PubMed] [Google Scholar]; b Chari R. V. J.; Miller M. L.; Widdison W. C. Antibody-Drug Conjugates: An Emerging Concept in Cancer Therapy. Angew. Chem., Int. Ed. 2014, 53, 3796–3827. 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]; c Beck A.; Goetsch L.; Dumontet C.; Corvaïa N. Strategies and Challenges for the Next Generation of Antibody–Drug Conjugates. Nat. Rev. Drug Discovery 2017, 16, 315–337. 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]; d Birrer M. J.; Moore K. N.; Betella I.; Bates R. C. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J. Natl. Cancer Inst. 2019, 111, 538–549. 10.1093/jnci/djz035. [DOI] [PubMed] [Google Scholar]
- Son B. W.; Jensen P. R.; Kauffman C. A.; Fenical W. New Cytotoxic Epidithiodioxopiperazines Related to Verticillin A from a Marine Isolate of the Fungus Penicillium. Nat. Prod. Lett. 1999, 13, 213–222. 10.1080/10575639908048788. [DOI] [Google Scholar]
- The increased potency of N1-sulfonyl derivatives may be due to enhanced stability of the disulfide bridge or reduced metabolic degradation.
- The advantages include bypassing N-methylation of a complex DKP and more favorable reactivity differences at C11 vs C15 hemiaminals, facilitating regio- and stereoselective DKP-sulfidation.
- a Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A Stepwise Hüisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. . [DOI] [PubMed] [Google Scholar]; b Hein J. E.; Fokin V. V. Copper-catalyzed Azide-Alkyne Cycloaddition (CuAAC) and Beyond: New Reactivity of Copper(I) Acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpilman A. M.; Carreira E. M. Probing the Biology of Natural Products: Molecular Editing by Diverting Total Synthesis. Angew. Chem., Int. Ed. 2010, 49, 9592–9628. 10.1002/anie.200904761. [DOI] [PubMed] [Google Scholar]
- a Cravatt B. F.; Wright A. T.; Kozarich J. W. Activity-Based Protein Profiling: From Enzyme Chemistry to Proteomic Chemistry. Annu. Rev. Biochem. 2008, 77, 383–414. 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]; b Fonović M.; Bogyo M. Activity-based Probes as a Tool for Functional Proteomic Analysis of Proteases. Expert Rev. Proteomics 2008, 5, 721–730. 10.1586/14789450.5.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Smith E.; Collins I. Photoaffinity Labeling in Target- and Binding-site Identification. Future Med. Chem. 2015, 7, 159–183. 10.4155/fmc.14.152. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lapinsky D. J.; Johnson D. S. Recent Developments and Applications of Clickable Photoprobes in Medicinal Chemistry and Chemical Biology. Future Med. Chem. 2015, 7, 2143–2171. 10.4155/fmc.15.136. [DOI] [PubMed] [Google Scholar]
- Ghosh B.; Jones L. H. Target Validation Using In-Cell Small Molecular Clickable Imaging Probes. MedChemComm 2014, 5, 247–254. 10.1039/C3MD00277B. [DOI] [Google Scholar]
- Larson N.; Ghandehari H. Polymeric Conjugates for Drug Delivery. Chem. Mater. 2012, 24, 840–853. 10.1021/cm2031569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a study employing a nontoxic ETP with an activated ester, see:Zong L.; Bartolami E.; Abegg D.; Adibekian A.; Sakai N.; Matile S. Epidithiodiketopiperazines: Strain-Promoted Thiol-Mediated Cellular Uptake at the Highest Tension. ACS Cent. Sci. 2017, 3, 449–453. 10.1021/acscentsci.7b00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See the Supporting Information for further details.
- Kim J.; Movassaghi M. Concise Total Synthesis and Stereochemical Revision of (+)-Naseseazines A and B: Regioselective Arylative Dimerization of Diketopiperazine Alkaloids. J. Am. Chem. Soc. 2011, 133, 14940–14943. 10.1021/ja206743v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unveiling of the primary alcohol using tetra-n-butylammonium fluoride in THF at 0 °C afforded an inseparable mixture of alcohol (+)-12 and its C11-epimer (3:1, respectively) in 88% yield.
- Lal B.; Pramanik B. N.; Manhas M. S.; Bose A. K. Diphenylphosphoryl Azide: A Novel Reagent for the Stereospecific Synthesis of Azides from Alcohols. Tetrahedron Lett. 1977, 18, 1977–1980. 10.1016/S0040-4039(01)83657-1. [DOI] [Google Scholar]
- For our mechanistic studies of permanganate-promoted DKP-dihydroxylation, see:; a Bischoff A. J.; Nelson B. M.; Niemeyer Z. L.; Sigman M. S.; Movassaghi M. Quantitative Modeling of Bis(pyridine)silver(I) Permanganate Oxidation of Hydantoin Derivatives: Guidelines for Predicting the Site of Oxidation in Complex Substrates. J. Am. Chem. Soc. 2017, 139, 15539–15547. 10.1021/jacs.7b09541. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Haines B. E.; Nelson B. M.; Grandner J. M.; Kim J.; Houk K. N.; Movassaghi M.; Musaev D. G. Mechanism of Permanganate-Promoted Dihydroxylation of Complex Diketopiperazines: Critical Roles of Counter-Cation and Ion-Pairing. J. Am. Chem. Soc. 2018, 140, 13375–13386. 10.1021/jacs.8b08371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman H.; Barton R. J.; Robertson B. E.; Lee D. G. Preparation and Properties of Quaternary Ammonium and Phosphonium Permanganates. J. Org. Chem. 1984, 49, 4509–4516. 10.1021/jo00197a037. [DOI] [Google Scholar]
- Movassaghi M.; Schmidt M. A.; Ashenhurst J. A. Concise Total Synthesis of (+)-WIN 64821 and (−)-Ditryptophenaline. Angew. Chem., Int. Ed. 2008, 47, 1485–1487. 10.1002/anie.200704960. [DOI] [PubMed] [Google Scholar]
- Exposure of alkyne (+)-23 to palladium on carbon (Pd/C, 5 wt %) and dihydrogen in ethyl acetate at 23 °C for 24 h gave an equimolar mixture of alcohol (+)-24 and the hydrogenated side product possessing the benzyloxy group, whereas otherwise identical treatment in ethanol provided alcohol (+)-24 (67%) alongside a deoxygenated N14-n-butyl side product (18%).
- Shao C.; Wang X.; Zhang Q.; Luo S.; Zhao J.; Hu Y. Acid–Base Jointly Promoted Copper(I)-Catalyzed Azide-Alkyne Cycloaddition. J. Org. Chem. 2011, 76, 6832–6836. 10.1021/jo200869a. [DOI] [PubMed] [Google Scholar]
- Use of dichloromethane and toluene as solvent in the CuAAC reaction of ETP (+)-9c and alkyne 27 afforded ETP-triazole (+)-28c in 73 and 85% yield, respectively.
- Reduction of the azide linker to the corresponding amine or early introduction of a N-Boc amine linker is not optimal given the sensitivity of the epidisulfide functional group and the conditions needed for accessing it.
- Thompson L.; Ellman J. A. Synthesis and Applications of Small Molecule Libraries. Chem. Rev. 1996, 96, 555–600. 10.1021/cr9402081. [DOI] [PubMed] [Google Scholar]
- Stephanopoulos N.; Francis M. B. Choosing an Effective Protein Bioconjugation Strategy. Nat. Chem. Biol. 2011, 7, 876–884. 10.1038/nchembio.720. [DOI] [PubMed] [Google Scholar]
- Barré A.; Tîntas M.-L.; Levacher V.; Papamicaël C.; Gembus V. An Overview of the Synthesis of Highly Versatile N-Hydroxysuccinimide Esters. Synthesis 2017, 49, 472–483. 10.1055/s-0036-1588607. [DOI] [Google Scholar]
- Janes K. A. An Analysis of Critical Factors for Quantitative Immunoblotting. Sci. Signaling 2015, 8, rs2. 10.1126/scisignal.2005966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The structures of triketopiperazines 34b and 35b were confirmed through independent synthesis.
- Firouzabadi H.; Sardarian A. R.; Naderi M.; Vessal B. Bis(2,2′-bipyridyl)-copper(II) Permanganate (BBCP): A Mild and Versatile Oxidant in Organic Synthesis. Tetrahedron 1984, 40, 5001–5004. 10.1016/S0040-4020(01)91340-7. [DOI] [Google Scholar]
- The oxidation of C3-adduct (+)-37 with bis(pyridine)silver(I) permanganate gave diol 38 in 50–65% yield as a mixture of diastereomers (10:1 to 5:1), whereas the oxidation with tetra-n-butylammonium permanganate gave a complex mixture.
- a Chai C. L. L.; Waring P. Redox Sensitive Epidithiodioxopiperazines in Biological Mechanisms of Toxicity. Redox Rep. 2000, 5, 257–264. 10.1179/135100000101535799. [DOI] [PubMed] [Google Scholar]; b Srinivasan U.; Bala A.; Jao S.-C.; Starke D. W.; Jordan T. W.; Mieyal J. J. Selective Inactivation of Glutaredoxin by Sporidesmin and Other Epidithiopiperazinediones. Biochemistry 2006, 45, 8978–8987. 10.1021/bi060440o. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Bertling A.; Niemann S.; Uekötter A.; Fegeler W.; Lass-Flörl C.; von Eiff C.; Kehrel B. E. Candida albicans and its Metabolite Gliotoxin Inhibit Platelet Function via Interaction with Thiols. Thromb. Haemostasis 2010, 104, 270–278. 10.1160/TH09-11-0769. [DOI] [PubMed] [Google Scholar]
- a Bernardo P. H.; Chai C. L. L.; Deeble G. J.; Liu X.-M.; Waring P. Evidence for Gliotoxin–Glutathione Conjugate Adducts. Bioorg. Med. Chem. Lett. 2001, 11, 483–485. 10.1016/S0960-894X(00)00696-X. [DOI] [PubMed] [Google Scholar]; b Bernardo P. H.; Brasch N.; Chai C. L. L.; Waring P. A Novel Redox Mechanism for the Glutathione-dependent Reversible Uptake of a Fungal Toxin In Cells. J. Biol. Chem. 2003, 278, 46549–46555. 10.1074/jbc.M304825200. [DOI] [PubMed] [Google Scholar]
- The isolation of bisdisulfide 45b was capricious due to its high reactivity in the presence of base and poor stability.
- Hart T. W. Some Observations Concerning the S-Nitroso and S-phenylsulphonyl Derivatives of L-Cysteine and Glutathione. Tetrahedron Lett. 1985, 26, 2013–2016. 10.1016/S0040-4039(00)98368-0. [DOI] [Google Scholar]
- As ETP (+)-42 is insoluble in deuterium oxide, it was necessary to include deuteroacetonitrile to monitor the reversion of bisdisulfide 46 to ETP (+)-42.
- Szajewski R. P.; Whitesides G. M. Rate Constants and Equilibrium Constants for Thiol-Disulfide Interchange Reactions Involving Oxidized Glutathione. J. Am. Chem. Soc. 1980, 102, 2011–2026. 10.1021/ja00526a042. [DOI] [Google Scholar]
- a Ramirez A.; Ramadan B.; Ritzenthaler J. D.; Rivera H. N.; Jones D. P.; Roman J. Extracellular Cysteine/Cystine Redox Potential Controls Lung Fibroblast Proliferation and Matrix Expression through Upregulation of Transforming Growth Factor-Beta. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2007, 293, 972–981. 10.1152/ajplung.00010.2007. [DOI] [PubMed] [Google Scholar]; b Chaiswing L.; Zhong W.; Cullen J. J.; Oberley L. W.; Oberley T. D. Extracellular Redox State Regulates Features Associated with Prostate Cancer Cell Invasion. Cancer Res. 2008, 68, 5820–5826. 10.1158/0008-5472.CAN-08-0162. [DOI] [PubMed] [Google Scholar]
- Still W. C.; Kahn M.; Mitra A. Rapid Chromatographic Technique for Preparative Separations with Moderate Resolution. J. Org. Chem. 1978, 43, 2923–2925. 10.1021/jo00408a041. [DOI] [Google Scholar]
- Murdock K. C. Antiviral Agents. Chemical Modifications of a Disulfide Antibiotic, Acetylaranotin. J. Med. Chem. 1974, 17, 827–835. 10.1021/jm00254a010. [DOI] [PubMed] [Google Scholar]
- Fulmer G. R.; Miller A. J. M.; Sherden N. H.; Gottlieb H. E.; Nudelman A.; Stoltz B. M.; Bercaw J. E.; Goldberg K. I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. 10.1021/om100106e. [DOI] [Google Scholar]
- Pursuant to The Journal of Organic Chemistry requirements, we have provided the systematic compound names following the International Union of Pure and Applied Chemistry (IUPAC) convention. For a complementary unified numbering system used throughout this report for comparison with related compounds, see Figure S1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.