Abstract
Background:
Nonfatal opioid overdose (OD) is an opportunity to identify patients who may benefit from interventions to reduce repeated overdose (rOD). In this study, we sought to determine risk and protective factors associated with rOD.
Methods:
In this retrospective cohort study of 4,155 patients aged 18–64 who presented to one of 16 emergency departments in a single Western Pennsylvania health system between July 2015 and January 2018 for index opioid overdose (iOD) and survived to discharge, we identified demographic and clinical factors association with rOD within one-year. Relative risk of repeated opioid overdose was estimated using adjusted Cox proportional hazard ratios (aHRs).
Results:
14.9% of patients (95% CI 13.9 to 16.1) had a rOD, with 29% occurring within 30 days from iOD. The adjusted hazard of opioid overdose was increased for male patients (aHR=1.19; 95%CI 1.01, 1.41), those with pre-iOD diagnoses of anxiety (aHR=1.41; 95%CI1.13, 1.77), depression (aHR=1.44; 95%CI 1.17, 1.78), substance use disorders (aHR=1.30; 95%CI 1.09, 1.55), and alcohol use disorder (aHR=1.52; 95%CI 1.02, 2.25). The hazard was lower for individuals prescribed an opioid in the 90 days prior to iOD (aHR=0.59; 95%CI 0.37, 0.97) and those admitted to the hospital for iOD (aHR=0.56; 95%CI 0.37, 0.86).
Conclusion:
We found that, among ED patients who survive an initial OD, mental health and substance use diagnoses are associated with a higher hazard of repeated overdoses whereas opioids prescriptions and admission are associated with lower hazards.
Keywords: opioid overdose, risk factors, emergency department
1. Introduction
The emergency department (ED) is a common site for treating patients who experience an opioid overdose (OD). From July 2016 to September 2017, the CDC reported that there were more than 140,000 suspected opioid-involved ODs that resulted in ED visits (Vivolo-Kantor et al.,2018). Individuals who survive an OD are at particularly high-risk for repeated overdose (Coffin et al., 2007; Stoové et al., 2009; Caudarella et al., 2016; Olfson et al., 2018), with rates ranging from 7% to 17% within a year of initial overdose. Repeat overdoses are often deadly. For example, among ED patients treated for opioid OD, 5.5% died within a year, with approximately a quarter of deaths occurring within a month of ED discharge (Weiner et al., 2019).
The time period after non-fatal OD provides an opportunity to identify high-risk individuals and engage them in treatment and harm reduction strategies (including take home naloxone, initiation of opioid substitution treatment (OST) and linkage with addiction treatment) (Bagley et al., 2019). Still, little is known about which patient and care-related factors are useful to identify individuals at risk for a rOD. Therefore, among adults who received care in the ED and survived an initial opioid OD, we sought to understand (1) prevalence and timing of rOD; and (2) patient and care factors associated with rOD.
Because prior studies have shown associations between mental health and substance use diagnoses and overdoses (Smolina et al., 2019), we hypothesized that individuals with mental health and substance use diagnoses would have a greater hazard of rOD. Given prior studies have supported the association between opioid prescriptions and rOD (Olfson et al., 2018), we hypothesized that opioid prescriptions in the 3 months prior to iOD would be associated with greater hazard of rOD. We examined both the presence of any opioid prescription as well as dosage given prior studies showing higher doses are especially hazardous (Bonhert et al., 2011). Because benzodiazepines (Park et al., 2015) and gabapentinoids (Gomes et al., 2017) have been shown to be associated with overdoses, we explored the association of these and other sedative-hypnotic prescriptions prior to iOD with rOD. Finally, we were interested in understanding whether addiction treatment was protective against rOD. We hypothesized that OST either prior to or immediately after an initial overdose would be associated with reduced hazard for rOD. We explored the role of hospitalization after iOD as a protective factor given that it could allow for initiation of specialty care for substance use disorders.
2. Materials and methods
2.1. Study Design and Data Source
We conducted a retrospective cohort study of persons presenting to an ED for a nonfatal opioid overdose. We used the electronic health record (EHR) data from a single health system in western Pennsylvania. Participating EDs serve different patient populations, including 8 located in large central metropolitan areas, 6 in non-central metro areas and 2 in rural areas. We drew our cohort from patients between July 2015 and January 2018. We obtained study approval through the University of Pittsburgh Institutional Review Board.
2.2. Patient Selection
We identified 6,559 patients aged 18 to 64 years who had an index opioid overdose (iOD), defined as an emergency department care encounter with discharge diagnosis codes (International Classification of Diseases [ICD], 9th&10th Revision) related to opioids and overdose. This method for identifying opioid overdoses has been previously developed (Green et al., 2017) and validated (Green et al., 2019), with a reported sensitivity = 97.2%, specificity = 84.6%. For ICD-9 codes, this included presence of any diagnosis codes related to heroin or non-heroin opioid overdose (956.0) and accidental poisonings by opioids (E850). For ICD-10 codes, this included both codes related to poisonings and adverse effects of opioids (T40.0) and opioid poisonings (X42,X62, Y12). Data on iOD and rOD were obtained from ED visits alone. Data on prescriptions and mental health diagnosis were based on data from the health system. We excluded 1,260 patients with less than 180 days of EHR data before the iOD date to ensure sufficient data to identify trends in pre-iOD opioid prescriptions. We then excluded 1,140 patients with less than 180 days of data after iOD and 4 who died in the ED to identify similar post-OD trends, yielding a final cohort of 4,155 patients. As medication data was obtained over 180 days, any iOD was defined as the first OD within a 180 day period. We did not include data on OD prior to the study period. (see Figure 1 for flow diagram).
Figure 1.
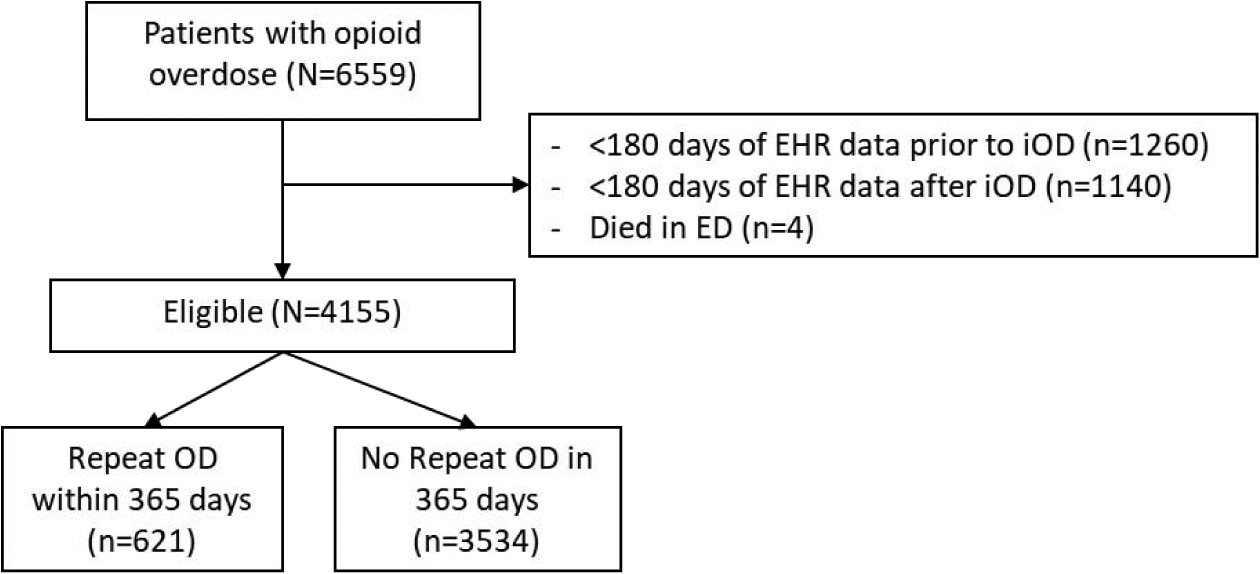
Abbreviations: ED=emergency department; iOD=initial opioid overdose.
2.3. Demographic and patient characteristics
Patients were classified by sex, age in years (18–34, 35–44, 45–64), and race (white, black, Asian, other). We used ICD diagnoses codes to identify mental health and substance use diagnoses made within 180 days prior to iOD included depression, anxiety, bipolar disorder, stress disorder, schizophrenia, as well as drug use disorder and alcohol use disorder (see Supplemental Material for ICD codes). We coded any hospital length of stay for iOD as an inpatient admission if it exceeded 23 hours.
2.4. Opioid prescriptions
We identified all opioids prescribed from 90 days prior to iOD to the day of iOD. Only prescriptions ordered by a medical provider in the health system were recorded. Opioids included codeine, fentanyl, hydrocodone, morphine, hydromorphone, oxycodone, oxymorphone, tramadol, methadone, and buprenorphine. We first calculated average morphine-equivalent (MME) per day (d) using established methods: Strength per Unit * (Number of Units/ Days Supply) * MME conversion factor = MME/d (US Department of Health and Human Services, 2018) where conversion factors were: codeine (0.15), fentanyl (7.2) hydrocodone (1.0), morphine (1.0), hydromorphone (4.0), oxycodone (1.5), oxymorphone (3.0), tramadol (0.10), methadone (4.0/8.0/10.0/12.0), and buprenorphine (30.0). Overlapping prescriptions were counted toward average daily dosage. MME average daily dose (MME/d) was converted into a categorical variable with the values of 0 mg, 1 mg to less than 50 mg, 50 mg to less than 100 mg, and 100 mg or more (Dunn et al., 2010; Gomes et al., 2011).
2.5. Mental health and other potentially sedating medications
We identified mental health-related medications prescribed within 90 days prior to iOD, including antidepressants, antipsychotics, and mood stabilizers. We also identified potentially sedating medications including benzodiazepines, gabapentinoids, and muscle relaxers. Medications from each class were identified (Clinical Handbook of Psychotropic Drugs, 2015) using techniques in Smolina et al. (2019). We categorized presence of each prescription category as a dichotomous variable. Medication names are included in Supplemental Material.
2.6. Outcomes
The primary outcome of interest was rOD, defined as any unique hospital ED visit after iOD that meets diagnostic criteria for opioid-related overdose within 365 days of iOD. Identical to our criteria for iOD, we used discharge diagnosis codes (ICD 9th&10th Revision). This included any patient who died in the ED or hospital during that admission. We recorded the number of days between iOD and rOD.
2.7. Statistical Analyses
We first determined percentages of cohort demographics, patient characteristics, and prior prescriptions overall and by rOD status. We used Cox proportional hazard models to determine unadjusted and adjusted hazard ratios (HR) for rOD during the follow-up period. The adjusted hazard model controlled for age, sex, and race given that opioid overdose death rates in the US are higher among older age groups (Bohnert et al., 2011), for males (Paulozzi et al., 2012) and for white adults (Yang et al., 2015). We did not include more predictor variables (e.g. mental health diagoses) in the adjusted models due to issues of collinearity (. We then generated Kaplan-Meier curve analyses to compute the time to rOD by sex, presence of any mental health diagnosis, hospital admission on iOD, and prior opioid prescriptions. Exponential test for hazard ratios indicated that 954 patients (477 per group) would need to detect a HR of 1.2 at 80% power and alpha=.05. We conducted a sensitivity analysis including all-cause mortality. All statistical analyses were performed with Stata 15.0 (Stata Corp).
3. RESULTS
3.1. Patient characteristics
The cohort consisted of 4,155 patients with iOD who presented to the ED and survived to discharge. Although iOD care occurred at 16 different hospitals, the number of iOD patients at any site ranged from 0.10% to 39.3%. The distribution of iOD by site is included in the Supplemental Material. Overall mean age of the cohort was 34 years (SD, 11), 64.8% were men, 85.3% were white, 16.0% had a prior diagnoses of mental health disorders, and 22.2% had prior diagnoses of substance use disorders. In the 12 months prior to iOD, 18% of patients had at least one prior ED visit, with a range of between 1 and 32 visits. 93.7% of iOD patients were discharged from the ED. Among those admitted, mean length of stay was 2.2 (SD 2.1) days.
3.2. Prescriptions
Opioid prescriptions were rare among this cohort of patients. In the 6 months prior to iOD, 4% of patients had an opioid prescription; in the month prior to iOD, 1.7% of patients had an opioid prescription; in the month following iOD, 1.3% of patients had an opioid prescription. Only 0.8% of patients were prescribed buprenorphine or methadone in the 3 months prior to or month following iOD. Among patients not prescribed buprenorphine or methadone in the 3 months prior to iOD, 0.9% were started on buprenorphine or methadone in the month post-iOD. Among patients prescribed buprenorphine or methadone in the 3 months prior to iOD, 17.7% did not have buprenorphine or methadone prescribed in the month post-iOD. There were also low prescription rates of antidepressants (5.8%), antipsychotics (1.5%), and mood stabilizers (1.8%) as well as muscle relaxers (1.4%) and gabapentinoids (5.0%), benzodiazepines (3.0%) prior to iOD.
3.3. rOD Characteristics
14.9% of patients (95% CI 13.9 to 16.1) had a rOD. 29.3% occurred within 30 days of iOD; 23.8% occurred between 30 and 90 days of iOD; 46.9% occurred between 90 and 365 days of iOD. 28.3% of rOD care took place at a different hospital from where care occurred for iOD.
3.4. Predictors of rOD
The adjusted hazard of repeated opioid overdose was increased for male patients (HR=1.19; 95%CI 1.01, 1.41), those with pre-iOD diagnoses of anxiety (HR=1.41; 95%CI1.13, 1.77), depression (HR=1.44; 95%CI 1.17, 1.78), substance use disorders (HR=1.30; 95%CI 1.09, 1.55), and alcohol use disorder (HR=1.52; 95%CI 1.02, 2.25). The hazard was lower for individuals admitted to the hospital for iOD (HR=0.56; 95%CI 0.37, 0.86) and for individuals who had any opioid in the 3 months prior to iOD (HR=0.59; 95% CI 0.37, 0.97) . The cumulative proportion of patients with rOD over time stratified by sex, presence of any mental health diagnosis, hospital admission on iOD, and prior opioid prescriptions are shown in Figure 2. In a model including all covariates, only male sex (aHR=1.20; 95% CI 0.91, 1.10) and any opioid (aHR=0.545; 95% 0.34, 0.89).
Figure 2.
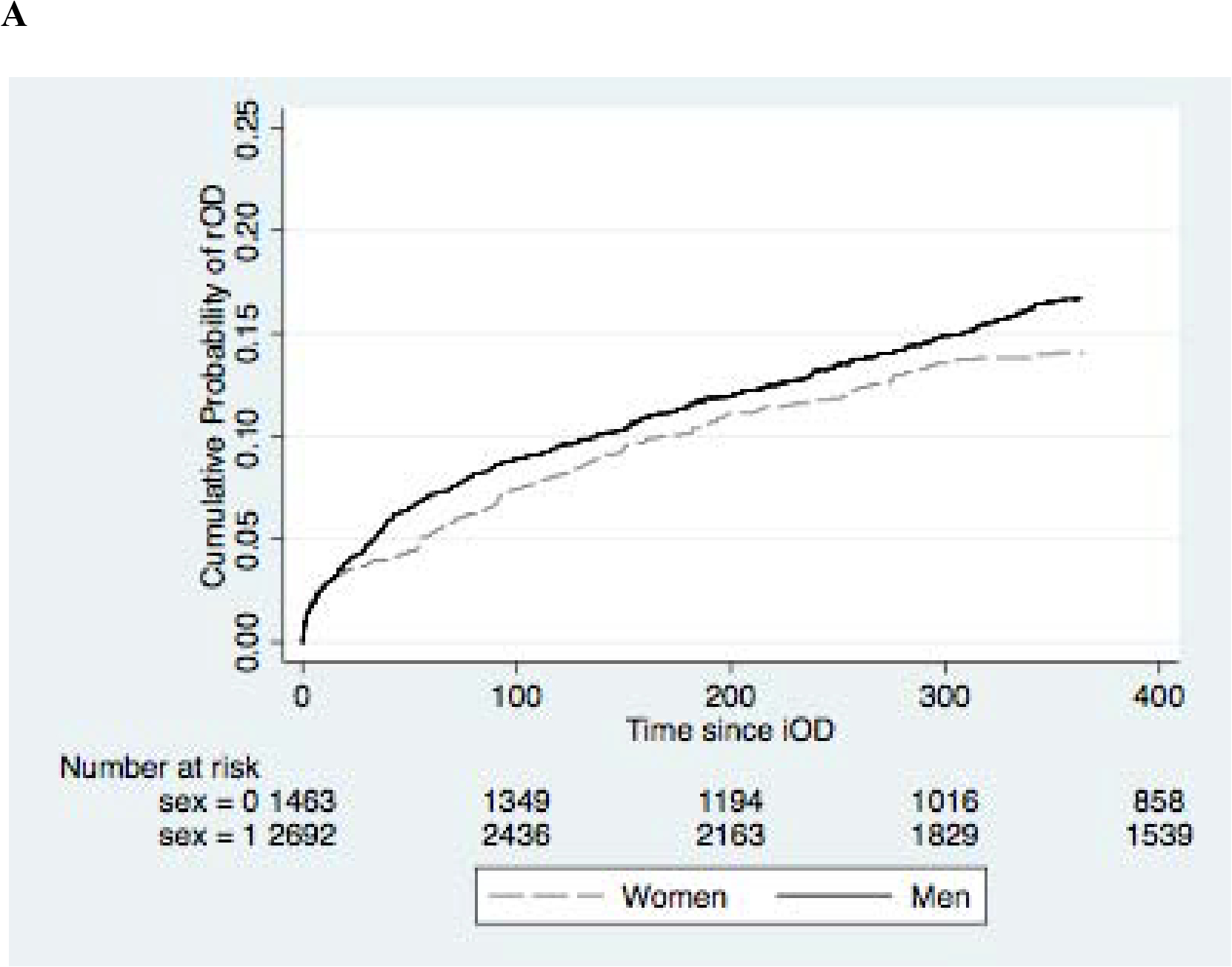
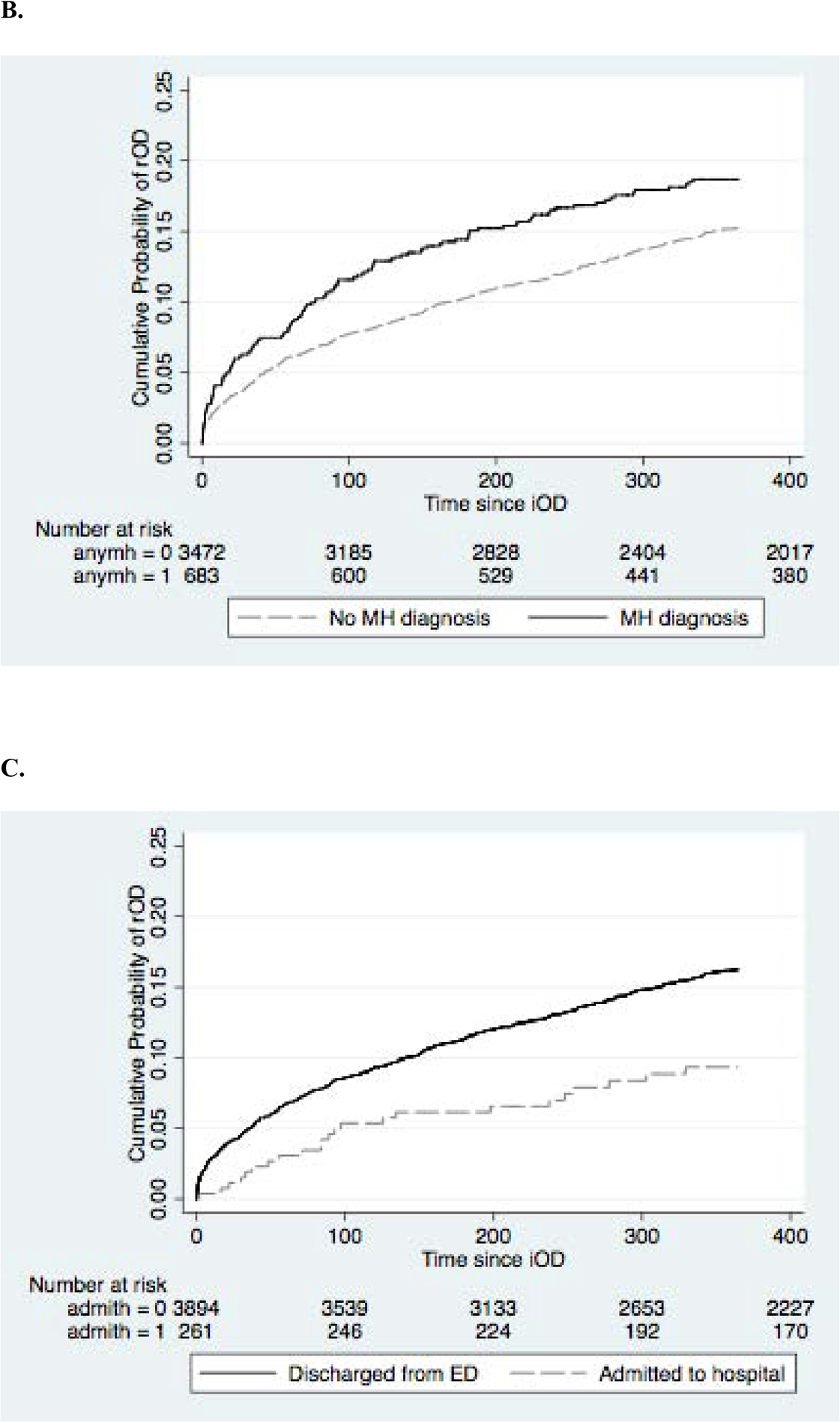
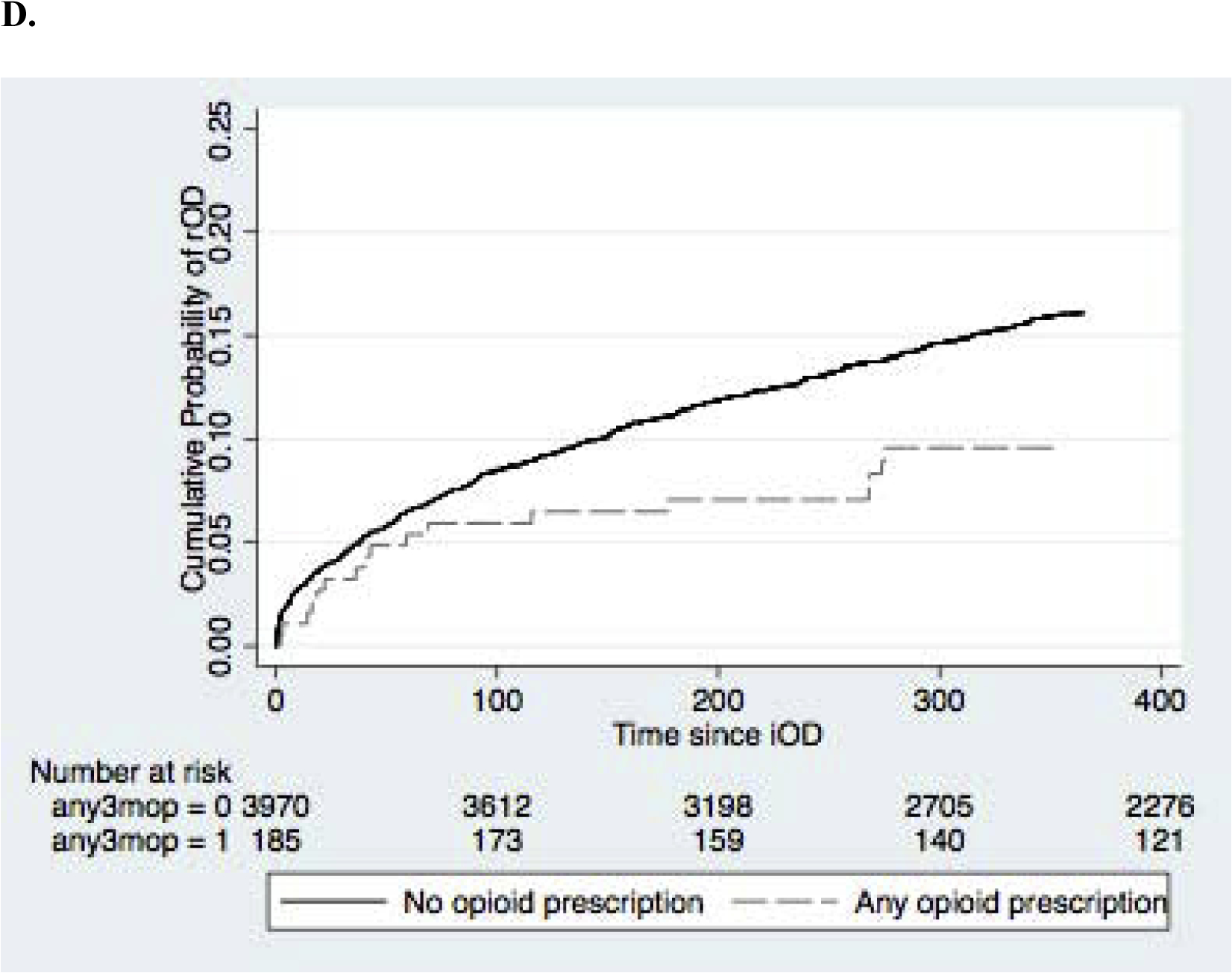
Kaplan-Meier curves of cumulative probability of rOD. (A) sex, (B) presence of any mental health diagnosis, (C) hospital admission on iOD (D) any prior opioid prescription.
3.5. Death and Sensitivity Analyses
Overall there were a total of 156 deaths within a year after iOD for an incidence of 3.7%. Of these deaths, 9% occurred within 10 days of iOD; 10.3% from 10 to 30 days after iOD; 18.6% between 30 and 90 days after iOD; and 62.2% between 90 and 365 days after iOD. When we analyzed all-cause mortality in Cox hazard models, there were no differences in strength of hazards between predictors and outcome.
4. DISCUSSION
4.1. Main findings
In this study of 4,155 individuals who received care in one of 16 hospital EDs and survived an initial opioid overdose, 15% had a repeated opioid overdose within a year. This is higher than the 7% repeated overdose rate reported among ED patients from Florida and California in 2010 (Hasegawa et al., 2014) and the 7% repeated overdose rate reported in a study of commercially insured patients who had prescription for long-term opioid from 2000 to 2011(LaRochelle et al., 2016). It is similar to the 18% rate reported among Medicaid beneficiaries from 2001 to 2007 (Olfson et al.; 2018) and fits with studies using more recent datasets, including a study from West Virginia which showed a substantial increase in the rate of OD during this time period, with rates of rOD increasing from 10.2% in 2010 to 28% in 2016 (Warfield, et al., 2019).
We found that a quarter of patients who had a repeated opioid overdose had it in first 30 days of initial overdose and 3.7% of patients died subsequently in the year following iOD. This death rate is higher than the 1.1% death rate after iOD survival reported by Weiner et al. (2019) using Massachusetts data from 2000 to 2015, and the 1% death rate in the year following opioid overdose reported by Olfson et al (2018). Our findings do however mirror national trends in increased opioid overdoses and opioid-related deaths reported over the past 10 years (Hedegaard et al., 2018).
We found overall low rates of opioid prescribing in the 3-months prior to initial opioid overdose (i.e. 4%) with less than 2% of patients prescribed an opioid in any month prior to initial overdose. This is much lower than the 35% reported among Medicaid beneficiaries from 2001 to 2007 (Olfson et al., 2018) and may represent regional or temporal differences or the markedly older population in their study (e.g. 26% vs. 42% of cohort 45 years to 65 years of age). Similar to our findings, Smolina et al. (2019) found that at the time of overdose 8% of men and 14% of women had an opioid prescription and approximately half had not filled one in the past five years. Separately, Walley et al., (2019) found that only 1.3% of overdose decedents had an active prescription for each opioid detected in toxicology reports on the date of death.
We found evidence indicating that opioid prescriptions in the 3 months prior to initial overdose was associated with lower hazard of repeated overdose. This differs from findings of Olfson et al. (2018) who reported an adjusted hazard ratio of 1.14 for any opioid prescription in the 6 months prior to iOD on repeated overdose and from Bonhert et al., (2011) who found increase overdose death rates among Veteran populations prescribed opioids. A review by Compton et al. (2016) found that those with nonmedical use of prescription opioids are more likely to transition to heroin; this was attributed to several factors including easy accessibility and purity, in addition to the inability to obtain opioid prescriptions. Another potential interpretation is that those at greater hazard for rOD seek and obtain prescription opioids outside of our health system, which creates the false impression that they are receiving lower rates of opioids in our system.
We found that patients with mental health diagnoses, especially depression and anxiety disorders, have higher hazard of repeated overdoses. This is consistent with prior research showing an associated risk of overdose among individuals with mental health diagnoses or prior psychiatric care (Smolina et al., 2019, Silva et al., 2013). Finally, although we found low rates of hospital admission after overdose, when it occurred there was lower hazard of rOD. The low rate of admission may represent patient disinterest, which may be exacerbated by physiological withdrawal symptoms precipitated by opioid reversal. It may also reflect lack of perceived need among ED providers to admit these patients. We speculate that the lower hazard of repeated overdose when admitted may represent addiction treatment initiation. Interestingly, we did not see a relative increase in either methadone or buprenorphine prescriptions among those admitted to the hospital versus those not admitted, indicating that there may be other protective factors related to a hospital admission besides the pharmacotherapy aspect of medication-assisted treatment.
Related, we found that methadone and/or buprenorphine being initiated in the month after initial overdose was <1%, which is less than previous findings by Larochelle et al (2018), who reported that in the 12 months after a nonfatal overdose form 2012 to 2014 in Massachusetts, 17% received buprenorphine and 6% received naltrexone. It is not clear from our data if patients may have been treated outside the system with more frequency at dedicated OST clinics. Regardless, this indicates an ongoing treatment gap and opportunity to improve care and outcomes for this high-risk population. Initiation of OST with buprenorphine in the ED can improve linkage with addiction treatment and long-term outcomes (D’Onofrio et al., 2015). Therefore, for individuals not admitted to the hospital after overdose, referral to peer-based services and prescription for OST from the ED should be increasingly emphasized as standard intervention.
4.2. Limitations
Our dataset was limited to emergency department patient encounters for opioid overdoses. Thus, we cannot comment on opioid overdoses that were not transported or were directly admitted to the hospital. This also limits our ability to examine whether inpatient medications were given after iOD. Our dataset also is limited to hospitals in a single health system in western Pennsylvania and may not represent other geographic regions of the US. We were only able to examine prescriptions written in this health system, therefore patients may have received prescriptions (e.g. opioids) written by medical providers in other health systems. This could have resulted in an underestimate of opioid prescribing rates. We also cannot comment on prescription fill rates, or actual rates of individual prescription opioid use. We did not evaluate which services or treatments were initiated in the hospital, therefore cannot comment on whether an admission equated with addiction treatment. We were unable to assess whether deaths recorded in the medical record were related to overdoses. Additionally, it is possible that deaths were underreported given that this included data only from a single health system. Finally, we only examined up to 6 months of prescribing prior to initial overdose, limiting our ability to assess the relationship between longer trends opioid prescribing and repeated overdose.
4.3. Conclusions
We identified several risk and protective factors associated with rOD. We found that, among ED patients who survive an initial OD, mental health diagnoses are associated with a higher hazard of repeated overdoses whereas opioids prescriptions and admission are protective. Additionally, it is notable that few patients received prescription for OST either before or after iOD, marking the gap in care from OD in the ED to receiving addiction treatment. These findings suggest that ED providers have a unique opportunity to identify patients at high risk for repeated overdoses and intervene to reduce future risk.
Supplementary Material
Table 1.
All prescriptions (i.e. opioids, mental health treatment, other potenially sedating medications) refer to presence of a prescription order in the electronic medical record in the 3 months prior to initial opioid overdose. MME/d refers only to the maximum exposure when a opioid was prescribed. Abbreviations: ED=emergency department; iOD=initial opioid overdose; OST=opioid substitution therapy (i.e. methadone, buprenorphine); MME/d=morphine milligram equivalents per day.
| Total opioid overdoses % (n) (N=4,155) | No repeat opioid overdose % (n) (N=3,534) | Repeat opioid overdose % (n) (N=621) | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) | |
|---|---|---|---|---|---|
| Age, years | |||||
| 18–34 | 47.48 (1973) | 47.45 (1677) | 47.67(296) | 1.00 | 1.00 |
| 35–44 | 25.68(1067) | 25.55 (903) | 26.41 (164) | 1.01 (0.83, 1.22) | 1.02 (0.81, 1.18) |
| 45–64 | 26.84 (1115) | 26.99 (954) | 25.93 (161) | 0.97 (0.79, 1.16) | 0.98 (0.81, 1.18) |
| Sex | |||||
| Male | 35.21 (1463) | 35.82 (1266) | 31.72 (197) | 1.19 (1.01, 1.41) | 1.19 (1.01, 1.41) |
| Female | 64.79 (2692) | 64.18 (2268) | 68.28 (424) | 1.00 | 1.00 |
| Race | |||||
| White | 85.29 (3544) | 85.09 (3007) | 86.47 (537) | 1.00 | 1.00 |
| Black | 11.82 (491) | 11.88 (420) | 11.43 (71) | 0.95 (0.74, 1.23) | 0.95 (0.74, 1.23) |
| Asian | 0.26 (11) | 0.23 (8) | 0.48 (3) | 1.97 (0.63, 6.13) | 1.99 (0.63, 6.20) |
| Other | 2.62 (109) | 2.8 (99) | 1.61 (10) | 0.59 (0.31, 1.02) | 0.57 (0.31, 1.07) |
| Any ED visit in prior 12 mo. | 6.40 (266) | 6.59 (233) | 5.31 (33) | 0.78 (0.56, 1.12) | 0.79 (0.56, 1.12) |
| Hospital admission for iOD | 6.30 (238) | 6.73 (238) | 3.70 (23) | 0.55 (0.36, 0.83) | 0.56 (0.37, 0.86) |
| Mental health diagnoses | |||||
| Depression disorder | 11.17 (464) | 10.64 (376) | 14.17 (88) | 1.35 (1.08, 1.69) | 1.38 (1.02, 1.73) |
| Anxiety disorder | 11.46 (476) | 10.89 (385) | 14.65 (91) | 1.38 (1.11, 1.73) | 1.41 (1.13, 1.77) |
| Bipolar disorder | 5.29 (220) | 5.07(179) | 6.60 (41) | 1.28 (0.94, 1.76) | 1.32 (0.96, 1.82) |
| Stress disorder | 2.00 (83) | 1.90 (67) | 2.58 (16) | 1.33 (0.81, 2.19) | 1.38 (0.84, 2.27) |
| Schizophrenia | 1.18 (49) | 1.16 (41) | 1.29 (8) | 1.10 (0.55, 2.21) | 1.14 (0.57, 2.29) |
| Any mental health disorder | 16.44 (683) | 15.85 (560) | 19.81 (123) | 1.30 (1.07, 1.58) | 1.32 (1.08, 1.61) |
| No mental health disorder | 83.56 (3472) | 84.15 (2983) | 80.19 (498) | 0.77 (0.63, 0.94) | 0.76 (0.62, 0.92) |
| Drug and alcohol diagnoses | |||||
| Substance use disorder | 22.19 (922) | 21.45 (758) | 26.41 (164) | 1.31 (1.09, 1.56) | 1.30 (1.09, 1.56) |
| Alcohol use disorder | 2.94 (122) | 2.72 (96) | 4.19 (26) | 1.53 (1.04, 2.67) | 1.52 (1.02, 2.25) |
| Opioid prescriptions | |||||
| Any prescribed opioid | 4.45 (185) | 4.84 (165) | 2.68 (20) | 0.58 (0.36, 0.94) | 0.59 (0.37, 0.97) |
| Any OST | 0.82 (34) | 0.76 (27) | 1.13 (7) | 1.43 (0.68, 3.01) | 1.45 (0.69, 3.05) |
| MME/d | |||||
| 1 to 50 | 30.28 (33) | 30.21 (29) | 30.77 (4) | 1.00 | 1.00 |
| 50 to <100 | 31.19 (34) | 29.17 (28) | 46.15 (6) | 1.47 (0.42, 5.21) | 1.65 (0.46, 5.88) |
| >=100 | 38.53 (42) | 40.62 (39) | 23.08 (3) | 0.58 (0.13, 2.60) | 0.62 (0.14, 2.79) |
| Mental health prescriptions | |||||
| Any antidepressant | 5.78 (240) | 5.75 (196) | 5.89 (44) | 0.97 (0.69, 1.36) | 0.99 (0.70, 1.39) |
| Any antipsychotic | 1.54 (64) | 1.53 (52) | 1.61 (12) | 0.95 (0.49, 1.83) | 0.98 (0.51, 1.89) |
| Any mood stabilizer | 1.18 (49) | 1.17 (40) | 1.20 (9) | 0.64 (0.27, 1.54) | 0.66 (0.27, 1.60) |
| Other potentially sedating prescriptions | |||||
| Any benzodiazepine | 3.03 (126) | 2.97 (105) | 3.38 (21) | 1.11 (0.72, 1.71) | 1.13 (0.73, 1.75) |
| Any gabapentinoid | 5.17 (215) | 5.11 (174) | 5.49 (41) | 1.06 (0.75, 1.50) | 1.08 (0.76, 1.52) |
| Any muscle relaxer | 1.44 (60) | 1.50 (51) | 1.20 (9) | 0.77 (0.37, 1.63) | 0.79 (0.37, 1.66) |
Highlights.
Mental health and substance use diagnoses increase hazard of repeated overdose.
Admission to the hospital after overdose reduce hazard of repeated overdose.
Opioid prescriptions prior to initial overdose reduce hazard of repeated overdose.
Sources of Support:
This work was supported by the National Institutes of Health [grant numbers K23 AA023284 and R01 AA023650.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest No conflict declared
References
- Bagley SM, Schoenberger SF, Waye KM, Walley AY, 2019. A scoping review of post-opioid overdose interventions. Prev Med. 19,105813. doi: 10.1016/j.ypmed.2019.105813. [DOI] [PubMed] [Google Scholar]
- Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, Blow FC, 2011. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA, 305, 1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- Caudarella A, Dong H, Milloy MJ, Kerr T, Wood E, Hayashi K, 2016. Non-fatal overdose as a risk factor for subsequent fatal overdose among people who inject drugs. Drug Alcohol Depend. 162, 51–55. doi: 10.1016/j.drugalcdep.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Handbook of Psychotropic Drugs. (21st edition ed.), Hogrefe Publishing, Toronto, Canada: (2015) [Google Scholar]
- Coffin PO, Tracy M, Bucciarelli A, Ompad D, Vlahov D, Galea S, 2007. Identifying injection drug users at risk of nonfatal overdose. Acad Emerg Med. 14, 616–623. doi: 10.1197/j.aem.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT, 2016. Relationship between nonmedical prescription-opioid use and heroin use. NEJM. 374, 154–163. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio G, O’Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH, Bernstein SL, Fiellin DA, 2015. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 313(16):1636–44. doi: 10.1001/jama.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M, 2010Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 152(2):85–92. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CA, Perrin NA, Janoff SL, Campbell CI, Chilcoat HD, Coplan PM, 2017. Assessing the accuracy of opioid overdose and poisoning codes in diagnostic information from electronic health records, claims data, and death records. Pharmacoepidemiol Drug Saf. 26, 509–517. doi: 10.1002/pds.4157. [DOI] [PubMed] [Google Scholar]
- Green CA, Perrin NA, Hazlehurst B, Janoff SL, DeVeaugh-Geiss A, Carrell DS, Grijalva CG, Liang C, Enger CL, Coplan PM, 2019. Identifying and classifying opioid-related overdoses: A validation study. Pharmacoepidemiol Drug Saf. 28(8):1127–1137. doi: 10.1002/pds.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN, 2011. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 171(7):686–91. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W, 2017. Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case-control study. PLoS Med. 14(10):e1002396. doi: 10.1371/journal.pmed.1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Brown DF, Tsugawa Y, Camargom CA Jr., 2014. Epidemiology of emergency department visits for opioid overdose: a population-based study. Mayo Clin Proc. 89, 462–71. doi: 10.1016/j.mayocp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Miniño AM, Warner M, 2018. Drug overdose deaths in the United States, 1999–2017 NCHS Data Brief, no 329 Hyattsville, MD: National Center for Health Statistics. [Google Scholar]
- Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, Wharam JF, 2016. Opioid Prescribing After Nonfatal Overdose and Association With Repeated Overdose: A Cohort Study. Ann Intern Med. 164, 1–9. doi: 10.7326/M15-0038 [DOI] [PubMed] [Google Scholar]
- Mars SG, Bourgois P, Karandinos G, Montero F, Ciccarone D, 2014. “Every ‘never’ I ever said came true”: transitions from opioid pills to heroin injecting. Int J Drug Policy. 25, 257–266. doi: 10.1016/j.drugpo.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizio KM, Baum RA, Dugan A, Martin JE, & Bailey AM, 2017. Characterization and management of patients with heroin versus nonheroin opioid overdoses: experience at an academic medical center. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 37(7), 781–790. [DOI] [PubMed] [Google Scholar]
- Olfson M, Crystal S, Wall M, Wang S, Liu SM, Blanco C, 2018. Causes of Death After Nonfatal Opioid Overdose. JAMA Psychiatry. 75(8):820–827. doi: 10.1001/jamapsychiatry.2018.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Wall M, Wang S, Crystal S, Blanco C, 2018. Risks of fatal opioid overdose during the first year following nonfatal overdose. Drug Alcohol Depend. 190, 112–119. doi: 10.1016/j.drugalcdep.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS, 2015. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 350:h2698. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi LJ, Kilbourne EM, Shah NG, Nolte KB, Desai HA, Landen MG, Harvey W, Loring LD, 2012. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 13(1):87–95. doi: 10.1111/j.1526-4637.2011.01260.x [DOI] [PubMed] [Google Scholar]
- Silva K, Schrager SM, Kecojevic A, Lankenau SE 2013. Factors associated with history of non-fatal overdose among young nonmedical users of prescription drugs. Drug Alcohol Depend. 128, 104–110. doi: 10.1016/j.drugalcdep.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolina K, Crabtree A, Chong M, Zhao B, Park M, Mill C, Schütz CG, 2019. Patterns and history of prescription drug use among opioid-related drug overdose cases in British Columbia, Canada, 2015–2016. Drug Alcohol Depend. 194, 151–158. doi: 10.1016/j.drugalcdep.2018.09.019. [DOI] [PubMed] [Google Scholar]
- Stoové MA, Dietze PM, Jolley D,2009. Overdose deaths following previous non- fatal heroin overdose: record linkage of ambulance attendance and death registry data. Drug Alcohol Rev. 28, 347–352. doi: 10.1111/j.1465-3362.2009.00057.x. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services, Centers for Disease Control, 2018. Analyzing Prescription Data and Morphine Milligram Equivalents (MME). https://www.cdc.gov/drugoverdose/resources/data.html. Page last updated July 16, 2019. [Google Scholar]
- Vivolo-Kantor AM, Seth P, Gladden RM, Mattson CL, Baldwin GT, Kite-Powell A, Coletta MA, 2018. Vital Signs: Trends in Emergency Department Visits for Suspected Opioid Overdoses — United States, July 2016–September 2017. MMWR Morb Mortal Wkly Rep. 67, 279–285. doi: 10.15585/mmwr.mm6709e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley AY, Bernson D, Larochelle MR, Green TC, Young L, & Land T (2019). The contribution of prescribed and illicit opioids to fatal overdoses in Massachusetts, 2013–2015. Public Health Reports, 0033354919878429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield S, Pollini R, Stokes CM, & Bossarte R, 2019. Opioid-Related Outcomes in West Virginia, 2008–2016. AJPH. 109(2), 303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner SG, Baker O, Bernson D, Schuur JD, 2019. One-Year Mortality of Patients After Emergency Department Treatment for Nonfatal Opioid Overdose. Ann Emerg Med. doi: 10.1016/j.annemergmed.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Wilsey B, Bohm M, Weyrich M, Roy K, Ritley D, Jones C, Melnikow J, 2015Defining risk of prescription opioid overdose: pharmacy shopping and overlapping prescriptions among long-term opioid users in medicaid. J Pain. 16(5):445–53. doi: 10.1016/j.jpain.2015.01.475. [DOI] [PubMed] [Google Scholar]
- Youssef E, Gao HT, Russell C, Hassan S, Ardolic B, Hahn B, 2018. Characteristics of prior emergency departments visits associated with subsequent opioid overdose. J Opioid Manag. 14, 327–333. doi: 10.5055/jom.2018.0465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


