Abstract
Apoptosis in the cystic epithelium is observed in most rodent models of polycystic kidney disease (PKD) and in human autosomal dominant PKD (ADPKD). Apoptosis inhibition decreases cyst growth, whereas induction of apoptosis in the kidney of Bcl-2 deficient mice increases proliferation of the tubular epithelium and subsequent cyst formation. However, alternative evidence indicates that both induction of apoptosis as well as increased overall rates of apoptosis are associated with decreased cyst growth. Autophagic flux is suppressed in cell, zebra fish and mouse models of PKD and suppressed autophagy is known to be associated with increased apoptosis. There may be a link between apoptosis and autophagy in PKD. The mammalian target of rapamycin (mTOR), B-cell lymphoma 2 (Bcl-2) and caspase pathways that are known to be dysregulated in PKD, are also known to regulate both autophagy and apoptosis. Induction of autophagy in cell and zebrafish models of PKD results in suppression of apoptosis and reduced cyst growth supporting the hypothesis autophagy induction may have a therapeutic role in decreasing cyst growth, perhaps by decreasing apoptosis and proliferation in PKD. Future research is needed to evaluate the effects of direct autophagy inducers on apoptosis in rodent PKD models, as well as the cause and effect relationship between autophagy, apoptosis and cyst growth in PKD.
Keywords: Apoptosis, Autophagy, Polycystic kidney disease
1. Introduction
ADPKD is the commonest life threatening hereditary disease often resulting in end stage kidney disease requiring dialysis and kidney transplantation [1]. Most ADPKD is caused by a mutation in either the Pkd1 gene (85% of cases) or the Pkd2 gene [2]. There are a multitude of signaling pathways that are either increased or decreased in PKD [3] [4] [5] [6]. Abnormal cross-talk between intracellular calcium and cAMP signaling is likely one of the first effects of PKD mutations and can result in increased proliferation of the tubular cells lining the cyst [3]. Protein kinase A-induced phosphorylation of cystic fibrosis transmembrane conductance regulator (CFTR) allows chloride and fluid secretion into the cysts and increases cyst growth [3]. Mammalian target of rapamycin (mTOR), that plays a role in both autophagy and apoptosis signaling, is increased in PKD kidneys [7]. mTOR inhibitors that protect against cyst growth in animal models [7] [8] are known to affect both autophagy and apoptosis. The Bcl-2 family of proteins that is dyregulated in PKD [9] [10] [11] also plays an important role in both apoptosis and autophagy signaling. Caspases, the major mediators of apoptosis that can also affect autophagy are increased in PKD kidneys [12] [9] [13] [14]. Studies in Pkd1−/− cells [15], Pkd1−/− zebrafish [16] and mouse models of PKD [17] [18] [19] suggest that there may be a disturbance in the balance between tubular cell proliferation, apoptosis, and autophagy in PKD. This review will focus on the role of tubular cell apoptosis and autophagy in cyst growth in PKD and explore the possible interactions between apoptosis and autophagy in PKD.
2. Apoptosis
Apoptosis is a process of programmed cell death characterized by volume reduction, cell surface blebbing, chromatin condensation, internucleosomal cleavage of DNA, and formation of apoptotic bodies [20] [21] [22]. Apoptotic cells are quickly phagocytosed by macrophages to prevent the release of intracellular components and inflammatory factors, resulting in “clean” cell death. Lockshin & Williams [23] originally defined programmed cell death in the context of insect development. Subsequently, Kerr et al. noticed by ultrastructural analysis two morphologically different types of cell death in humans: apoptosis and necrosis [24]. In necrosis, the cells swell, plasma mem-branes rupture, and cellular components are released. In apoptosis, the cells shrink with intact plasma membranes, and nuclei are condensed and fragmented. As apoptosis was discovered to be mediated by gene products, it was termed as being programmed. Thus, the term programmed cell death has been used in the context of apoptosis. It should be noted that cell death with a necrotic morphology that occurs during inflammation or infection can also be programmed or regulated by gene products, and is categorized as necroptosis and pyroptosis [25] [26].
3. Apoptosis in PKD
Apoptosis in the tubular epithelial cells lining the cyst and/or noncystic tubular epithelium in PKD kidneys is observed in most rodent models of PKD, regardless of the genetic defect (Table 1).
Table 1.
Apoptosis of tubular cells lining the cyst is seen in most rodent models of PKD and in human PKD kidneys. Oak Ridge polycystic kidney (orpk), post natal (PN), Not applicable (NA)
| Model | Inheritance | Gene | Characteristics | Ref |
|---|---|---|---|---|
| Male Han:SPRD rat | ADPKD | Anks6 | Doubling kidney size and kidney failure by 8 wks of age. Apoptosis in cystic and non cystic tubules. Hypertension, anemia, ESRD | [12] [51] |
| pck rat | ADPKD | Pkhd1 | Cysts in kidney and liver at 1 yr of age. Apoptosis in cystic and non cystic tubules. | [169] |
| C57BL6 PKD1RC/RC | ADPKD | Pkd1 | Hypomorphic PKD1 gene knockout matching a human disease variant. Renal failure at 70 d of age. Apoptosis in cystic and non cystic tubules. | [37] |
| Pkdlnl/nl mice | ADPKD | Pkd1 | Apoptosis induced in cystic epithelium by smac-mimetic | [90] |
| PKD2WS25/− mice | ADPKD | Pkd2 | PKD and renal failure at 16 wks of age. Apoptosis in cystic epithelium and caspase-3 activity not affected by sirolimus. | [33] |
| orpk mouse | ARPKD | Ift88Tg737Rpw | Ciliary defect model of ARPKD. Sirolimus increases apoptosis in cystic epithelial cells. | [8] |
| C57BL6 jck mouse | ARPKD | Nphp9 | Mutated gene product in primary cilia affects normal expression of PC1/2. High rate of epithelial cell proliferation and apoptosis in cystic epithelium | [170] |
| cpk mouse | ARPKD | Cys1 | Lifespan 4–6 weeks. Apoptosis of tubular and interstitial cells. Deletion of caspase-3 gene increases lifespan. | [14] |
| pcy mouse | ADPKD | Nphp3 | Orthologous to adolescent nephronophthisis. Apoptotic DNA fragmentation evident in preuremic kidneys. | [27] |
| SBM mice | ADPKD | c-myc | Over expression of c-myc. Tubular apoptosis increased 3–9 -fold over controls. | [95] [104] [96] |
| AP-2 β −/− knockout mice | NA | AP-2 β | Mice lack transcription factor AP-2 β die at PN day 1–2 from PKD. Enhanced apoptotic cell death of renal epithelial cells. | [171] |
| Bcl-2−/− mice | NA | Bcl-2 | Knockout of anti-apoptotic Bcl-2. Fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Apoptosis in kidney interstitium. | [50] |
| Dysplastic human renal disease | NA | NA | Cell death prominent in undifferentiated cells around dysplastic tubules and occasionally cystic epithelia | [172] |
| Human kidneys | ADPKD | Pkd1/2 | Apoptotic cells in glomeruli, cyst walls, cystic and noncystic tubules regardless of renal function | [27] |
| Human kidneys | ARPKD | Pkhd1 | Apoptosis more prevalent in human ARPKD kidneys than in age-matched normal kidneys | [91] [173] |
Apoptosis has also been detected in human ADPKD kidneys compared to normal kidneys, regardless of renal function [27] (Table 1). Specifically, apoptosis is evident in normal non cystic tubular epithelium in human polycystic kidneys from non-uremic ADPKD patients [27].
As apoptosis in renal tubular epithelium is detected in most models of PKD resulting from differing gene defects, it is unlikely that the Pkd1 or Pkd2 gene defect directly causes apoptosis.
mTOR, which exists in association with two different complexes, mTORC1 and mTORC2, may provide the link between cyst growth and apoptosis in diverse models of PKD [28] [29]. mTORC1 consists of mTOR and regulatory associated protein of mTOR (Raptor), while mTORC2 consists of mTOR and rapamycin-independent companion of mTOR (Rictor) [29]. mTOR is known to regulate apoptosis and proliferation [30]. Increased mTOR signaling is an almost universal phenomenon in PKD kidneys from different gene defects. Increased mTORC1 signaling has been observed in many rodent models of PKD [12] [31] [32] [8] [33] and in human ARPKD kidneys [34] [35]. Both mTORC1 and 2 signaling are increased in mouse models of PKD [36] [37]. The effects of mTORC½ inhibition on apoptosis in PKD models will be discussed later in this review.
4. Pathways of caspase-mediated apoptosis in PKD
A family of cysteine proteases, known as caspases, are the major mediators of apoptosis. The caspase pathways that are centrally important in apoptosis involve the “initiator” caspases-8 and −9 and the “executioner” caspases-3 and −7 [38] [39] [40]. Caspase-3 plays a crucial and extensively studied role in the promotion of apoptosis [41] [42] [43]. Caspase-3 terminates contacts with surrounding cells, re-organizes the cytoskeleton, shuts down DNA replication, interrupts splicing, destroys DNA, disrupts the nuclear structure, marks the cell for phagocytosis, and disintegrates the cell into apoptotic bodies [44] [45].
There are two major pathways of caspase-mediated apoptosis [39]20. In the mitochondrial or “intrinsic” pathway, stress-induced signals act via Bcl-2 proteins to promote cytochrome c release from mitochondria [46] [47]. Cytochrome c binds to the cytosolic protein Apaf-1 to facilitate the formation of an apoptosome. Once formed, the apoptosome can then recruit and activate caspase-9, that can in turn activate caspase-3. In support of a role of the Bcl-2 protein family in PKD, Bcl-2 deficient mice have increased apoptosis and die from severe PKD [48] [49] [50]. Activation of caspase-3 and dysregulation of the balance between pro- and anti-apoptotic Bcl-2 family members, specifically a downregulation of anti-apoptotic Bcl-XL, correlates with increased apoptosis in polycystic Han:SPRD rat kidneys [9]. In Han:SPRD rat kidneys, the pro form of caspase-9, cytochrome c release into the cytosol, and caspase-2 protein and activity are increased, demonstrating involvement of the intrinsic pathway [51]. In the “ extrinsic” pathway, the binding of a ligand to its death receptor recruits an adaptor protein that in turn recruits and activates procaspase-8, which subequently activates caspase-3 [52]. The proform of caspase-8 is also increased in PKD, demonstrating involvement of the extrinsic pathway [51]. No differences in Fas ligand (FasL) mRNA are observed, suggesting that the extrinsic pathway is independent of the death receptor ligand, FasL [51].
5. Relationship between apoptosis and cyst growth in PKD
Apoptosis may be causally linked to the development of renal cystic disease. For example, apoptosis is essential for Madin-Darby canine kidney (MDCK) cell cyst cavitation in collagen type 1 matrix. Cystogenesis in this system is inhibited by overexpression of the anti-apoptotic gene, Bcl-2 [53]. In a novel cell culture system for studying how PKD1 regulates apoptosis, proliferation and cyst formation, expression of human PKD1 in MDCK cells slows their growth and protects them from apoptosis [54]. MDCK cells expressing PKD1 also spontaneously form branching tubules, while control cells form simple cysts. Thus, PKD1 may function to regulate both apoptosis and proliferation pathways, allowing cells to enter a differentiation pathway that results in tubule formation. Collectively, this provides evidence that poly-cystin-1 (PC-1) may inhibit apoptosis, and confirms that increased apoptosis is associated with cyst formation in MDCK cells. Similarly, inhibition of fibrocystin (the gene product of PKHD1 that is responsible for autosomal recessive PKD) by short hairpin RNA inhibition in inner medullary collecting duct cells disrupts normal tubulomorphogenesis and results in increased apoptosis and proliferation [55].
Y528C and R1942H are two missense mutations that have been identified from a PKD1-affected individual. MDCK cells expressing the Y528C variant form cysts in culture and display increased rates of growth and apoptosis compared to MDCK cells stably expressing the wild-type [56]. The protein product of PKD1 (PC-1) is a large transmembrane protein with a short intracellular C terminus that interacts with numerous signaling molecules, including Galpha(12). G12/G13 alpha subunits are alpha subunits of heterotrimeric G proteins. MDCK cells can increase apoptosis via Galpha(12) stimulation of JNK and degradation of the anti-apoptotic protein Bcl-2, with PC1 expression levels determining the activity of the JNK/Bcl-2 apoptosis pathway [57]. Thus, there appears to be a causal relationship between apoptosis, proliferation and cyst formation. In summary, most (but not all) in vitro studies in PKD cells support that increased levels of apoptosis are associated with formation and growth of cysts. Next, the effect of therapies that decrease cyst growth in vivo on apoptosis will be discussed.
6. The effect of therapies that decrease cyst growth on apoptosis in vivo (Table 2)
Table 2.
Effects of treatments on apoptosis/autophagy and PKD.
| Treatment | In vivo model | Effect on apoptosis | Effect on autophagy | Effect on PKD | Ref |
|---|---|---|---|---|---|
| Cell cycle inhibitor, roscovitine | jck, cpk mice | Decreased | ND | Long-lasting arrest of cystogenesis | [58] [59]. |
| Hydroxyestradiol (2-OHE) | Han: SPRD rat | Decreased | ND | Decreased cyst growth | [61]. |
| GlcCer synthase inhibitor, Genz-123346 | jck and pcy mice | Decreased | ND. | Decreased cyst growth | [62] |
| Immunodepletion of CD8 + T cells | C57BL/6 PkdlRC/RC mice | Decreased | ND | Worsening | [63] |
| Caspase inhibitor, IDN-8050 | Han:SPRD rat | Decreased apoptosis and proliferation | ND | Decreased cyst growth | [12] |
| MIF inhibitor ISO-1 | Pkdl−/− mice | Increased | ND | Delays cyst growth | [67] |
| High dose sirolimus | Pkdl−/−, orpk mice | Increased | ND | Decreased cyst growth | [8] [69] [70] |
| Low dose sirolimus | Pkd2WS25/− mice | No effect | ND | Decreased cyst growth | [33] |
| mTOR kinase inhibitor, torin-2 | PkdlRC/RC mice | Increased | ND | Decreased cyst growth | [37] |
| mTOR kinase inhibitor, PP242 | Han:SPRD rat | No effect | ND | Decreased cyst growth | [71] |
| mTOR ASO | Pkd2 WS25/− mice | Decreased | ND | Decreased cyst growth | [36]. |
| Smac-mimetic | Pkdl−/− mice | Increased TNF-α-dependent apoptosis | ND | Delays cyst growth | [90] |
| Bafilomycin | cpk mice | ND | Decreased | ND | [17] |
| Autophagy inducer: Trehalose | Hypomorphic Pkdl−/− mice | ND | No effect on suppressed autophagy | No effect | [18] |
| Caloric restriction mimetic, 2DG | Pkdl−/− mice | ND | ND | Delays cyst growth | [160] [161] [15] |
| Saikosaponin-d (SSd), a SERCA inhibitor | UCL93, 0X161 ADPKD cells. MDCK cells | ND | Increased | Decreased | [164] |
| Glucose deprivation | Pkdl−/− cells | Increased | Decreased. Rescued by sirolimus | ND | [15] |
| Autophagy activators: Beclin-1, sirolimus, carbamazepine and minoxidil | Zebrafish pkdla mutants | Decreased | Increased | Decreased cysts | [16] |
| Metformin | Zebrafish pkd2 mutants | ND | Increased | Decreased cysts | [118] |
| HDACi, Trichostatin A | Pkdl−/− cells | ND | Increased | Prevents cyst formation | [165] |
Not determined (ND), glucosylceramine (GlcCer), antisense oligonucleotide (ASO), macrophage migration inhibitory factor (MIF-1), 2-deoxy-glucose (2DG), second mitochondria-derived activator of caspase (Smac), Madin-Darby canine kidney cells (MDCK), Sarcoplasmic/endoplasmic reticulum Ca2 + ATPase pump (SERCA).
Therapies that decrease cyst growth in PKD have variable effects on apoptosis. Some therapies that decrease cyst growth also decrease apoptosis. The cell cycle inhibitor roscovitine results in long-lasting arrest of cystogenesis, along with decreased apoptosis [58] [59]. Erb-b2 receptor tyrosine kinase 4 (ErbB4) is a receptor tyrosine kinase and member of the epidermal growth factor receptor family that is highly expressed in cystic kidneys. In cpk mice, ErbB4 deletion results in accelerated cyst progression, renal function deterioration, increased cell proliferation in the cyst-lining epithelial cells, and significantly more apoptotic cells. This is associated with decreased levels of cyclin D1, increased levels of p21, p27, and cleaved caspase 3, suggesting that decreased cell cycle progression may contribute to apoptosis [60]. Similarly, in Han:SPRD rats treated with 2-hydroxyestradiol (2-OHE), the resultant decrease in cyst growth and preservation of kidney function is associated with suppression of proliferation, apoptosis, and markers of angiogenesis [61]. Sphingolipids and glycosphingolipids are emerging as major regulators of proliferation, apoptosis, and activation of growth regulatory pathways. Blockade of glucosylceramide (GlcCer; a glyco-sphingolipid) accumulation with the GlcCer synthase inhibitor Genz-123346 effectively inhibits cystogenesis in Pkd1 conditional knockout mice and jck and pcy mouse models of nephronophthisis [62]. Mechanism-of-action studies suggest that GlcCer synthase inhibition results in effective cell cycle arrest and inhibition of the Akt-mTOR pathway, ultimately leading to decreased apoptosis and mitogenic signaling [62]. Of note, enrollment in a phase II/III trial in adults with ADPKD evaluating the efficacy of Venglustat, which reduces accumulation of GlcCer, is ongoing (NCT03523728).
In contrast, some therapies that decrease cyst growth can increase apoptosis; likewise, worsening ADPKD may associate with reduced apoptosis. Immunodepletion of CD8+ T cells from one to three months in C57Bl/6 Pkd1RC/RC mice results in worsening of ADPKD pathology, decreased apoptosis, and increased proliferation compared to IgG-control, consistent with a reno-protective role of CD8+ T cells [63]. microRNAs (miRNAs) are short noncoding RNAs that function as sequence-specific inhibitors of gene expression [64]. The miR-17 family and miR-21 are both upregulated in kidney cysts and promote ADPKD progression in mouse models. miR-21 represses proapoptotic genes and thus inhibits cyst apoptosis and promotes PKD progression [65]. Overexpression of exogenous kidney-specific neutrophil gelatinase-associated lipocalin (Ngal) attenuates progressive cyst development and prolongs lifespan in a murine model of PKD, which is associated with reductions in interstitial fibrosis and proliferation and augmented apoptosis [66]. Macrophage migration inhibitory factor (MIF) is another important regulator of cyst growth in ADPKD. MIF is upregulated in cyst-lining epithelial cells in PC-1-deficient murine kidneys and accumulates in cyst fluid of human ADPKD kidneys [67]. MIF regulates cystic renal epithelial cell apoptosis through p53-dependent signaling. MIF deletion or pharmacologic inhibition of MIF delays cyst growth and increases p53-dependent apoptosis in multiple murine ADPKD models [67].
Sirolimus is an autophagy inducer, but its effects on apoptosis in PKD are complex [68]. The effect of sirolimus on apoptosis in PKD may be determined by the dose. High-dose sirolimus increases apoptosis of cyst lining epithelium in PKD [8] [69] [70]. However, low-dose sirolimus may also result in effective blockade of PKD without an effect on apoptosis [33]. Thus, an appropriate therapeutic dose of sirolimus may not have an effect on apoptosis in PKD. Furthermore, the effect of mTOR inhibitors on apoptosis may depend on whether there is dual blockade of both mTORC1 and 2. The mTOR kinase inhibitor, torin-2, decreases PKD severity and improves kidney function in the Pkd1RC/RC mouse model of PKD [37]. Torin-2 decreases proliferation and increases apoptosis in cells lining the cysts in these mice [37]. However, in Han:SPRD rats with PKD, the mTOR kinase inhibitor PP242, which inhibits both mTORC1 and 2, has no effect on caspase-3 activity, TUNEL positive or active caspase-3-positive tubular cells, despite significantly reducing kidney enlargement, cyst density, blood urea nitrogen, and proliferation in cells lining the cysts and non-cystic tubules [71]. Finally, an mTOR anti-sense oligonucleotide (ASO) that blocks both mTORC1 and 2, decreases PKD severity in mice with a targeted mutation in Pkd2, which is associated with a significant decrease in proliferation and apoptosis of tubular epithelial cells [36]. Thus, blockade of both mTORC1 and 2 does not have a consistent effect on apoptosis in the cells lining the cysts in the PKD kidney.
Differences in apoptosis observeed in in vivo studies may be due to different methods used to detect apoptosis in vivo. Numerous studies simply count TUNEL-positive cells in an automated fashion. However, the gold-standard is to count cells that have morphologic criteria of apoptosis that include cellular rounding and shrinkage, nuclear chromatin compaction, and formation of apoptotic bodies [72]. Morphology is the gold-standard for detection of apoptosis and TUNEL staining fails to discriminate between proximal tubule apoptosis and necrosis, particularly in vivo in the kidney, and it grossly overestimates proximal tubule apoptosis in the kidney [73] [72] [74] [75] [76]. This is especially true in cystic tubular epithelial cells that may have injury that can cause necrosis [77]. For these reasons, the number of TUNEL–stained nuclei that also demonstrate the morphological features of apoptosis should be also be quantitated.
With this background, studies have been performed to determine the effect of direct apoptosis inhibition or apoptosis induction on tubular cell proliferation, cyst formation, kidney size, and kidney function in PKD.
7. Caspase inhibition in PKD
Caspases are attractive potential targets for treatment of diseases because of their central role in apoptosis and the appealing prospect of small-molecule inhibitor therapy [78]. In animal models, caspase inhibitors decrease ischemia-perfusion injury in heart [79] [80], liver [81] and kidney [82] [83]. The pan-caspase inhibitor IDN-1965 improves cardiac function and decreases mortality in mouse cardiomyopathy [84] and reduces apoptosis of sinusoidal endothelial cells during liver preservation injury [85]. The pan-caspase inhibitor IDN-6556 decreases liver enzyme elevations in patients with hepatic dysfunction [86]. Of note, the orally active caspase inhibitor, Emricasan, is currently being tested in multiple clinical trials conducted in patients with liver disease [87] (see clinicaltrials.gov).
Early evidence in rodents suggests that caspase inhibitors may also be an attractive therapy to slow PKD progression. Three-week old heterozygous and littermate control Han:SPRD male rats treated with the caspase inhibitor, IDN-8050 (10 mg/kg/d) via a minipump for 5 weeks, have a 44% reduction in kidney enlargement, a 29% reduction in cyst volume density, and attenuated increase in BUN, as compared to vehicle [12]. IDN-8050-treatment additionally reduces the number of proliferating cell nuclear antigen (PCNA) positive tubular cells and apoptotic tubular cells in non-cystic and cystic tubules. Collectively, this indicates that in a rat model of PKD, caspase-inhibition with IDN-8050: 1) decreases apoptosis and proliferation in cystic and non-cystic tubules, 2) inhibits renal enlargement and cystogenesis, and 3) attenuates the loss of kidney function. These findings will require confirmation in other animal models of PKD prior to translation to clinical trials in humans with ADPKD.
Mice with caspase-3 gene deletion have been crossed with mice harboring the congenital polycystic kidney (cpk) mutation to generate double-mutant mice [14]. Homozygous caspase-3 (cpk;casp3−/− mice) live nearly four-times longer than littermate control cpk mice, and heterozygous (cpk;casp3+/− mice) live significantly longer than controls. In addition, kidney weight, relative to body weight, is significantly lower in the cpk;casp3−/− mice than in the cpk and cpk;casp3+/− mice. However, despite deletion of caspase-3, apoptosis occurs and cysts form; therefore, alternative pathways of apoptosis in cystic kidneys have been investigated. Caspase-7 is up-regulated and the anti-apoptotic protein Bcl-2 is down-regulated in cpk, cpk;casp3+/−, and cpk;casp3−/− mice compared with wild-type controls. In summary, homozygous deletion of caspase-3 markedly prolongs survival of cpk mice, but a caspase-7-mediated pathway may compensate for the deficiency of functional caspase-3 [14]. These findings suggest that pan-caspase inhibition may have a greater therapeutic effect than selective caspase inhibition in PKD.
8. Apoptosis induction in PKD
The mitochondrial protein Smac promotes caspase activation in the cytochrome c/Apaf-1/caspase-9 pathway by binding to inhibitor of apoptosis proteins and removing their inhibitory activity [88]. While Smac is normally a mitochondrial protein, it is released into the cytosol when cells undergo apoptosis [89]. Overexpression of Smac increases cells’ sensitivity to apoptotic stimuli. In vitro, a Smac-mimetic and co-treatment with tumor necrosis factor-α (TNF-α) augments the formation and activation of the receptor-interacting serine/threonine-protein kinase 1-dependent death complex and the degradation and cleavage of the caspase-8 inhibitor FLIP. This results in death in Pkd1 mutant epithelial cells, with no effect on normal renal epithelial cells [90]. In vitro, a Smac-mimetic selectively induces TNF-α-dependent cystic renal epithelial cell death and delays cyst formation [90]. Similarly, treatment with a Smac-mimetic slows cyst growth and kidney enlargement, as well as preserves renal function in two genetic strains of mice with Pkd1 mutations, without affecting proliferation.
How do we reconcile the seeming contradiction that both apoptosis inhibition and apoptosis induction can lessen cyst growth? Whether apoptosis promotes or retards cyst growth is confounded by factors such as differing rodent models of PKD with differing mutations, early-versus late-disease, and apoptosis in cysts versus apoptosis limited to non-cystic tubules and interstitial cells [91]. It is possible that apoptosis inhibition and apoptosis induction decrease cyst growth by very different mechanisms that can act independently. Under certain conditions, enhanced apoptosis may preserve renal structure by eliminating mural cells from cysts that otherwise would expand endlessly without having an effect on proliferation [90]. Likewise, apoptosis inhibition can decrease proliferation of cystic epithelial cells that is crucial for growth of the cyst [12].
9. Apoptosis may be causally linked to proliferation and the development of cysts
Abnormal proliferation in tubular epithelial cells plays a crucial role in cyst development and/or growth in PKD [92] [2,93] and apoptosis may be causally linked to these processes. Convincing evidence indicates that increased tubular cell proliferation is accompanied by tubular apoptosis in PKD, and apoptosis and proliferation are directly related [94] [95] [50] [96] [97]. A massive increase in both apoptosis and proliferation is observed in both SBM mice that overexpress c-myc and Bcl-2 deficient mouse models of cystic disease [95] [50]. In fact, increased apoptosis and proliferation occur early in the course of the disease and precede cystogenesis in SBM mice [95]. In Han:SPRD rats fed soy protein, the improved renal function and decreased cyst formation is accompanied by decreases in both tubular cell proliferation and apoptosis [97]34. Also, mice deficient in the pro-apoptotic Bcl-2 gene have hyperproliferation as well as apoptosis that accompanies renal cysts [50,98] [48]. Kidneys from patients with ADPKD have high levels of apoptosis as well as cellular proliferation [27] [99]. Thus, epithelial cell apoptosis and proliferation are dysregulated in ADPKD and may represent a general mechanism for cyst growth and tissue remodeling [94] [2] [100].
The precise pathways that link apoptosis and proliferation in PKD remain to be determined. However, a common pathway of apoptosis and proliferation may involve adhesion-dependent control of apoptosis and overexpression of the proto-oncogene c-myc. Changes in cell shape and loss of cell to cell adhesion during apoptosis may stimulate surrounding cells to proliferate [101] [102]. Theoretically, in PKD loss of tubular cells by apoptosis may initiate proliferation of neighboring tubular cells. In this case, both apoptosis and proliferation would be observed in the same cyst and caspase inhibition would attenuate both of these processes. Overexpression of c-myc is thought to play a role in the dysregulation of both proliferation and apoptosis in ADPKD. Mice overexpressing c-myc (also known as SBM mice) develop PKD concomitant with massively increased apoptosis and proliferation [103,104]. Renal c-myc expression is also increased in the Han:SPRD rat model of ADPKD [105]. The pathway of c-myc-induced apoptosis is thought to be mediated by the “initiator” caspase-9 [106] and the “executioner” caspase-3 [107] [108]. A novel Ste20-related kinase (SLK) is cleaved and activated by caspase-3 during c-myc-induced apoptosis [109]. In human ADPKD, a large increase in c-myc expression is associated with both tubular cell proliferation and apoptosis [96] [110] [111] [94]. Thus, caspase inhibition may have therapeutic relevance by impeding a common pathway for both apoptosis and proliferation, resulting in reduced cyst formation.
With caspase inhibition, it is important to consider that apoptosis in ADPKD may be a double-edged sword [112]. While increased apoptosis may result in increased proliferation, cyst growth, and deterioration of renal function in PKD, apoptosis may also be a defense against oxidative or other forms of DNA damage and thus reduce the risk of neoplastic transformation. In this regard, the simultaneous induction of cell proliferation and apoptosis has been regarded as a safeguard against neoplastic transformation. However, cancer-prone mice treated with the caspase inhibitor IDN-8050 (Emricasan) from a young age have no detectable deliterious effects, including no evidence of treatment-related tumor formation or carcionogenicity [113]. Emricasan does not affect apoptosis in normal healthy cells, and as noted previously, it is currently being tested in multiple Phase 3 clinical trials, without reports of carcinogenicity (see clinicaltrials.gov). So it is unlikely that caspase inhibition would result in tumor development in PKD kidneys.
10. Summary on apoptosis
There is abundant evidence that apoptosis plays a causal role in cyst formation: 1) induction of apoptosis in tubular cells in culture results in cyst formation; 2) tubular epithelial cell apoptosis occurs in most animal models of PKD and in kidneys from humans with ADPKD; 3) both apoptosis and proliferation occur in non-cystic as well as cystic epithelial cells early in the course of PKD; 4) caspase inhibition results in less apoptosis and proliferation in the tubular epithelium, with attenuation of cyst formation and kidney failure.
11. Autophagy
Autophagy is a process that occurs in all eukaryotic cells to keeps cells alive under stressful conditions [114]. In autophagy, damaged organelles are sequestered into double-membraned autophagosomes that subsequently fuse with lysosomes to deliver their cargoes for degradation and recycling. Autophagy received prominence in 2016 when the Nobel Prize in Physiology or Medicine was awarded to Dr. Yoshinori Ohsumi for “discoveries of the mechanisms for autophagy.” It is now known that there are 3 major forms of autophagy: macroautophagy, microautophagy and chaperone—mediated autophagy (CMA) [26]: 1) Macroautophagy is the best characterized variant of autophagy, that occurs in all eukaryotic cells and involves the sequestration of cytoplasmic components in double-membraned autophagosomes that subsequently fuse with lysosomes, where the cargo e.g. damaged organelles is delivered for degradation and recycling; 2) Microautophagy, or endosomal microautophagy, is a form of autophagy where cytoplasmic cargo destined for degradation is taken up by the vacuole via direct membrane invagination; 3) Chaperone-mediated autophagy (CMA) involves the direct delivery of cytosolic proteins targeted for degradation to the lysosome. The characteristic feature of CMA is that neither vesicles nor membrane invaginations are required for substrate delivery to lysosomes, and substrates reach the lysosomal lumen via a proteintranslocation complex at the lysosomal membrane. Other forms of autophagy target mitochondria (mitophagy), peroxisomes (pexophagy), endoplasmic reticulum (reticulophagy), protein aggregates (aggrephagy), lipid droplets (lipophagy), and inactive proteasomes (proteaphagy).
Autophagic flux is the most well accepted measure of the state of autophagy. For example, increased autophagosomes may be used to characterize systems of either increased autophagosome production or decreased autophagosome clearance by the lysosome, two dissimilar cellular responses [115,116]. According to the 2016 Guidelines for the use and interpretation of assays for monitoring autophagy, if the basal increase in microtubule-associate protein 1A/1B-light chain 3-II (LC3-II; a central protein in the autophagy pathway) is due to increased autophagosome production, then it is expected that lysosomal inhibition will further increase LC3-II [116]. Alternatively, if the increase in LC3-II is due to a block in autophagosome-lysosome fusion or a defect in lysosome function, then lysosomal inhibition would not affect LC3-II expression. The measurement of autophagic flux requires the measurement of autophagosomes (e.g., LC3-II, a marker of autophagosomes), in the presence and absence of lysosomal inhibition. Autophagic flux, measured as described above, is decreased in the cpk mouse model of ARPKD [17].
Autophagy was originally characterized as a hormonal and starvation response. It is now known that autophagy has a broad role in biology, including organelle remodeling, protein and organelle quality control, prevention of genotoxic stress, tumor suppression, pathogen elimination, regulation of immunity and inflammation, maternal DNA inheritance, metabolism, and cellular survival [117]. While autophagy is usually a degradative pathway, it also plays a role in biosynthetic and secretory processes. As autophagy is critical to many essential cellular functions, it is not surprising that defects in autophagy have been implicated in a variety of diseases [16,17,19,118–121]. Autophagy is crucial in maintaining cell and organ homeostasis, protecting against disease, and promoting recovery after injury [122], particularly in the kidneys. There is accumulating evidence that autophagy may be dysregulated in kidney disease and injury. Acute kidney injury, induced by either cisplatin or ischemia, has been hypothesized to upregulate autophagy as a recovery mechanism [123–126]. Autophagy enhancement benefits mice with cisplatin-induced acute kidney injury, and autophagy inhibition exacerbates injury in this model [124]. Further, autophagy is important for maintaining podocyte proteostasis in aging mice [127]. Autophagy also has implications as a stress response during kidney transplant injury, with opposing effects resulting from immunosuppressive drugs or ischemic stresses [119].
12. Autophagy in PKD
Autophagy research in PKD is in its infancy, but there are reasons to believe that autophagy may be dysregulated in the PKD kidney and specifically that autophagy may be supressed in PKD. Autophagic flux is suppressed in ADPKD as evidenced by studies in PKD1−/− cells, Pkd1−/− zebrafish and rodent models of PKD.
12.1. In vitro models
In human Pkd1−/− cells, there is an attenuated increase in LC3-II expression after bafilomycin A1 (BafA1) treatment demonstrating decreased autophagic flux [16]. Additionally, by immunofluorescence, the number of both autophagosomes and autolysosomes in PKD1−/− cells is significantly lower, and the number of autophagosomes fails to be up-regulated in response to BafA1 treatment, suggesting inadequate autophagosome formation and diminished autophagosome-lysosome fusion. Autophagy impairment accounts for a weakened ability to remove protein aggregates, as PKD1−/− cells accumulate more aggregates in the absence of any stress and also break down MG132-induced protein aggregation less efficiently. Mouse Pkd1−/− cells show a similar deficiency in the clearance of protein aggregates [16]. Zebrafish mutants for pkd1a develop mTOR activation, impaired autophagic flux and cystic kidneys [16].
12.2. In vivo models
Dysregulated autophagy has also been described in rodent models of PKD. Autophagic vacuoles in the cells surrounding cysts have been described in polycystic kidneys in the Han:SPRD rat [17]. By electron microscopy, features suggestive of autophagy-like autophagosomes, mitophagy, and autolysosomes are observed in both wild-type and PKD kidneys [17]. Specific to the Han:SPRD rat, autophagosomes are found by electron microscopy in the tubular cells lining the cysts. LC3 staining by immunofluorescence is also present in the tubular epithelial cells lining the cysts. Autophagic flux is also dysregulated in the cpk mouse model of ARPKD [17]. LC3-II expression is increased in PKD kidneys of cpk mice as compared to wild-type kidneys. Additionally, in vivo treatment with the lysosomal inhibitor BafA1 increases LC3-II expression in the kidneys of wild-type mice. In contrast, BafA1 has no effect on LC3-II in the polycystic kidneys of cpk mice, suggesting a defect in autophagic flux in PKD resulting from impaired autophagosome-lysosme fusion and degradation. In a Pkd1-hypomorphic mouse model (Pkdd1 miRNA transgenic mice) there is a significantly lower renal mRNA expression of autophagy-related genes, including Atg12, Atg3, beclin1, and p62, compared with wild-type control mice [18]. Collectively, these results suggest a modifying effect of ADPKD on autophagy and establish autophagy activation as a potential novel therapy for ADPKD [128].
13. Interactions between apoptosis and autophagy in PKD
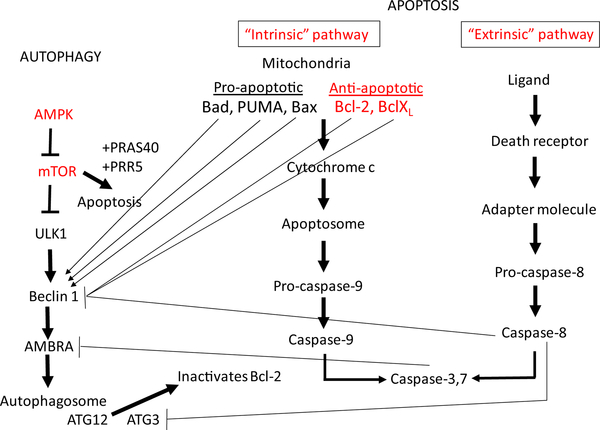
There is cross talk between mTOR pathway of autophagy and the apoptosis pathways [129] (Figure 1). The mTOR pathway that inhibits autophagy is activated in PKD [28]. The intrinsic and extrinsic pathways of apoptosis are activated in PKD [51]. Many of the signals that regulate apoptosis in PKD also regulate autophagy, for example, the Bcl-2 family of proteins and caspases [130] [131] [132] [133] [134]. The relationship between apoptosis and autophagy is complex, depending on the type of cell and the nature and timing of the injury [133]. For example, increased autophagy may result in a delay of apoptosis [135] [136] [137] and genetic deletion of crucial autophagy proteins increases apoptotic cell death [138]. Autophagy is closely related to apoptosis, a process that is dysregulated in PKD. PKD kidneys have a significantly lower renal expression of autophagy-related genes, including Atg12, Atg3, beclin1, and p62, compared with wild-type control mice [166]. Inhibition of autophagy by knocking down the essential autophagy protein Atg5 promotes cystogenesis and specific induction of autophagy inhibits apoptosis [16]. Thus, it could be hypothesized that suppressed autophagy in PKD may be associated with increased apoptosis and cyst growth.
Figure 1.
Molecular pathways involved in PKD, autophagy and apoptosis. Autophagy and apoptosis are intimately related. Pathways known to be activated in PKD are shown in red. The mTOR pathway that inhibits autophagy is activated in PKD [28]. There is cross talk between mTOR pathway of autophagy and the apoptosis pathways [129]. Bcl-2 is a central regulator of autophagy and apoptosis and functions by interacting with Beclin-1 and inhibiting autophagy. The pro-apoptotic mitochondrial protein, Bad, disrupts the interaction between Bcl-2 and Beclin-1 to induce autophagy. The pro-apoptotic protein, PUMA, a p53-inducible BH3-only protein, triggers mitochondrial-specific autophagy. Activation of caspase-3 and dysregulation of the balance between pro- and anti-apoptotic Bcl-2 family members, specifically a down-regulation of anti-apoptotic Bcl-xL, has been shown in PKD [9] and Bcl-2 downregulation worsens PKD [10]. Caspases activated during apoptosis can cleave and inactivate Beclin-1. Ambra-1, a key molecule that promotes the initial steps of autophagy, is irreversibly cleaved by both calpains and caspases. During apoptosis, Atg3 is cleaved by caspase 8 and cleaved Atg3 inhibits autophagy. Autophagy is inhibited by caspase-8-mediated cleavage of Beclin-1 [174]. The intrinsic and extrinsic pathways of caspase activation in apoptosis are activated in PKD [51]. In addition to the role that apoptosis-related proteins play in modulating autophagy, many autophagic proteins can induce apoptosis. mTOR forms a complex with PRAS40 and PRR5-like proteins to induce apoptosis. Activation of AMPK is known to inhibit mTOR and induce autophagy. AMPK activation with metformin is protective in PKD [152] but the effect of metformin on autophagy and apoptosis in PKD is not known. Atg12 increases mitochondrial apoptosis by directly binding to and inactivating Bcl-2. PKD mice have a significantly lower renal mRNA expression of Atg12 and other autophagy-related genes, Atg3, beclin1 and p62, [18].
Abbreviations: B-cell lymphoma 2 (Bcl-2), B-cell lymphoma-extra large (Bcl-xL), p53 upregulated modulator of apoptosis (PUMA), proline-rich Akt substrate of 40 kDa (PRAS40), PRoline-Rich protein 5 (PRR5), activating molecule in Beclin1-regulated autophagy protein-1 (Ambra-1), 5’ AMP-activated protein kinase (AMPK).
The hypothesis that autophagy suppression may contribute to cyst formation and growth is based on studies that many of the agents that protect against PKD, such as mTOR inhibitors, cyclin-dependent kinase inhibitors, caspase inhibitors, tyrosine kinase inhibitors, metformin, curcumin, and triptolide, affect both autophagy and apoptosis. Discussion of the effect of these agents on autophagy and apoptosis will be divided into agents that have been used in human studies, agents that have shown positive effects on PKD in animals and represent potential new therapies for PKD and in vitro studies.
13.1. Human studies
The mTOR inhibitors sirolimus and everolimus have been used in patients with ADPKD [139] [140]. The effect of mTOR inhibitors on PKD in humans was disappointing most likely related factors like dosage, side effects and penetration of mTOR inhibitors into the polycystic kidney [141]. mTOR is the major regulator of autophagy. mTOR activation inhibits autophagy, while mTOR inhibitors induce autophagy. With present technology, the effect of mTOR inhibitors on autophagy in human kidneys would be near impossible to determine.
13.2. Rodent studies
The effects of treatments on apoptosis/autophagy and PKD in in vivo models of PKD is shown in Table 2. There are autophagy inducers that have shown positive effects on PKD in animals and represent potential new therapies for PKD. mTOR inhibitors that induce autophagy can protect against PKD in rat and mouse models with variable effects to either increase or decrease apoptosis depending on the dose of mTOR inhibitor and PKD model [7] [69]. The traditional Chinese medicine, triptolide, which affects Ca2+ signaling as well as autophagy and apoptosis, protects against PKD [142] [143]. Curcumin, a hydrophobic polyphenol compound extracted from the spice turmeric, is an autophagy inducer [144]. Curcumin inhibits cystogenesis in Pkd-1 knockout mice by inhibiting signal transducers and activator of transcription 3 (STAT3), which play a major role in the regulation of autophagy [145] [146] [147] [148] [149]. Of note, an ongoing randomized controlled trial is evaluating the efficacy of curcumin supplementation in children and young adults with ADPKD (NCT02494141). The cyclin-dependent kinase inhibitor, Roscovitine [59], a dual Src and tyrosine kinase inhibitor, and epidermal growth factor receptor (EGFR) tyrosine kinase inhibition, all known autophagy inducers, also ameliorates PKD in rodent models [150] [151]. While roscovitine decreased apoptosis in PKD, the effect on autophagy was not determined [59]. The adenosine monophosphate-activated protein kinase (AMPK) inhibitor, metformin, a known autophagy inducer, also slows cyst growth in mouse models of PKD [152] [153,154]. However, the effects of metformin on autophagic flux, mitophagy, or apoptosis in PKD were not described.
Trehalose is a natural, nonreducing disaccharide that has been shown to enhance autophagy independent of mTOR [155]. In a Pkd1-hypomorphic mouse model, renal mRNA expression of autophagy-related genes, including atg5, atg12, ulk1, beclin1, and p62 are reduced and there is positive staining for the p62 protein in cystic lining cells, indicating impaired degradation by the autophagy-lysosome pathway [18]. However, trehalose treatment does not affect autophagy signaling, nor does it reduce kidney cysts or improve kidney function in this model, suggesting that treholose supplementation is not a candidate to slow PKD progression.
Lifestyle interventions may also modulate autophagy in PKD. Fasting inhibits the mTOR pathway and stimulates autophagy to remove damaged molecules and organelles, including in the kidney [156]. Thirty-percent caloric restriction for two weeks prior to ischemia reperfusion injury in a rodent model improves renal function, which may be mediated by increased autophagy [157]. More recently, the effects of caloric restriction and agents targeting metabolic pathways on autophagy have been evaluated in rodent of models of PKD. Mild-to-moderate caloric restriction resulted in a decrease in cleaved casapse-3, a marker of apoptosis, but autophagy was not measured [158]. Mild food restricition decreased PKD associated with no change in LC3-II, a marker of autophagosomes, but not a marker of true autophagic flux [159]. In this study, apoptosis was not determined. The caloric restriction mimetic, 2-deoxy-glucose (2DG) [160] [161] [15] also protects against PKD, however the effect of 2DG on apoptosis or autophagy was not determined in the PKD kidney.
Notably, overweight, and particularly obesity, are strong independent predictors of more rapid kidney growth, as well as kidney function decline in adults with early-stage ADPKD [162]. Of relevance to this obervation, mouse models of deficient autophagic competence exhibit significantly greater weight gain in response to metabolic challenge, suggesting an intimate link between autophagy and obesity [163]. An ongoing clinical trial (NCT03342742) is evaluating the feasibility of two weight loss interventions in adults with ADPKD who are oveweight or obese (daily caloric restriction and intermittent fasting); these diets may influence disease progression via weight loss and/or periods of fasting, both of which are tied to autophagy.
In summary, while many potential autophagy inducers are potential therapies for PKD, the role of autophagy induction in the protection against PKD is not known. mTOR inhibitors that protect against PKD have variable effects on apoptosis, but autophagy was not determined in mTOR inhibition studies (Table 2). The effect of potential autophagy inducers on both apoptosis and autophagy has not been systematically studied in preclinical studies of PKD.
13.3. In vitro studies
The effects of treatments on apoptosis/autophagy and PKD in in vitro models of PKD is shown in Table 2. Saikosaponin-d (SSd), a sarcoplasmic/endoplasmic reticulum Ca2+ ATPase pump (SERCA) inhibitor, may suppress proliferation in ADPKD cells by up-regulating autophagy [164]. SSd results in the accumulation of intracellular calcium, activation of the calcium/calmodulin-dependent protein kinase β (CaMKKβ)-AMPK signaling cascade, inhibition of mTOR signaling, and induction of autophagy. Notably, treatment with an autophagy inhibitor (3-methyladenine), AMPK inhibitor (Compound C), CaMKKβ inhibitor (STO-609), or an intracellular calcium chelator (BAPTA/AM) reduces autophagy puncta formation mediated by SSd. Thus, SERCA may represent a new autophagy target in ADPKD. Likewise, histone deacetylase inhibitors (HDACi) have therapeutic effects in in vitro models of ADPKD. Treatment with trichostatin A, a specific HDACi, prevents cyst formation in Pkd1−/− cells and also stimulates autophagy, suggesting a role for autophagy in slowing cyst growth with HDACi [165]. However, in these two studies, the effects of SSd or trichostatin on apoptosis was not determined.
Studies in Pkd1−/− zebrafish and Pkd1−/− cell models have shown consistent relationship between apoptosis and autophagy. Evidence from a zebrafish model of PKD implicates autophagy in cystogenesis [16]. Zebrafish mutants for pkd1a develop cystic kidneys and mTOR activation, suggesting a conserved ADPKD model. Further assessment of the pkd1a mutants reveals impaired autophagic flux and increased apoptosis. Inhibition of autophagy by knocking down the core autophagy protein Atg5 promotes cystogenesis. Activation of autophagy using a specific inducer Beclin-1 peptide results in a decrease in apoptosis and ameliorates cysts in this model. Additionally, treatment with both mTOR-dependent (sirolimus) and mTOR-independent (carbamazepine and minoxidil) autophagy activators, markedly attenuate cyst formation.
There appears to be an important connection between the Pkd1 gene, apoptosis, and autophagy. Pkd1+/+ cells deprived of glucose activated cell autophagy to survive; however, two different Pkd1−/− cell lines fail to activate autophagy, but instead increase apoptotic rates [15]. The effect is in part dependent on mTORC1, as treatment with sirolimus partially restores autophagy and decreases apoptosis in Pkd1−/− cells. This is the first direct evidence of a connection between the Pkd1 gene, apoptosis, and autophagy.
Metformin also reduces cyst formation in a zebrafish model of polycystin-2 deficiency, in part via modulation of autophagy [118]. In this model, metformin inhibits pronephric cyst formation by 42–61% compared to untreated controls. Metformin also reduces the number of proliferating cells in the pronephric ducts, increases the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK), and enhances autophagy in the pronephros. In this study, the effect of autophagy induction with metformin on apoptosis was not determined.
In summary, the lack of autophagy and increased apoptosis in Pkd1−/− zebrafish and Pkd1−/− cells and the effect of autophagy inducers to increase autophagy and decrease apoptosis in these cells supports the hypothesis that a lack of autophagy and increased apoptosis are central features of PKD and may explain why drugs or lifestyle interventions that induce autophagy and inhibit apoptosis may have therapeutic value in PKD.
14. Autophagy inhibition in the polycystic liver
Contrary to the hypothesis that increasing autophagy will slow PKD, autophagy appears to be increased in polycystic liver disease (PLD) cholangiocytes and contribute to hepatic cystogensis [166]. PLD cholangiocytes have increased number and size of autophagosomes, lysosomes, and autolysosomes both in vitro and in vivo, overexpress autophagy-related proteins (Atg5, Beclin1, Atg7, and LC3), and have enhanced autophagic flux. Molecular and pharmacologic interventions to inhibit autophagy with ATG7 small interfering RNA, BafA1, or hydroxychloroquine, reduce proliferation of PLD cholangiocytes and growth of hepatic cysts. Hydroxychloroquine also efficiently inhibits hepatic cystogenesis in the pck rat. In contrast, autophagy inhibition using siRNA against LC3 and the inhibitor 3-methyladenine significantly increase the cell proliferative activity of pck rat cholangiocytes treated with NVP-BEZ235, a combined PI3K, mTORC½ inhibitor [167]. In vivo, NVP-BEZ235 treatment attenuates cystic dilatation of the intrahepatic bile ducts, without affecting renal cyst development. Finally, knockdown of hepatocystin, a gene implicated in autosomal dominant PLD, results in an autophagy a defect that can be rescued by ectopic expression of wild-type hepatocystin [168]. The pathogenesis of PLD is different from PKD [4]; for example, growth of liver compared to kidney cysts is very estrogen dependent [4], and this may influence the role of autophagy in the polycystic liver versus the polycystic kidney. However, in pursuing autophagy inducers as a potential therapeutic option to treat PKD, it will be important to also determine whether there is an adverse effect on PLD.
15. Summary
Apoptosis is present in the cells lining cysts in most rodent models of PKD and in human PKD kidneys. Inhibition of apoptosis using caspase inhibitors, knockout of apoptosis in PKD mice, as well as apoptosis induction using a SMAC-mimetic can all protect against PKD, highlighting the complex nature of the role of apoptosis in cyst growth. Increased proliferation of the cells lining the cyst is a major factor in cyst growth. Highlighting the connection between apoptosis and proliferation in PKD, both proliferation and apoptosis are hugely increased in the SBM mouse, a unique transgenic model of PKD induced by the dysregulated expression of c-myc in renal tissue. Apoptosis is likely closely related to dysregulated autophagy in PKD. Pkd1−/− zebrafish, Pkd1−/− cells, and some PKD mouse models demonstrate both increased apoptosis and suppressed autophagy in the kidney. Autophagy induction directly leads to decreased apoptosis and protection against PKD in zebrafish models. Autophagy induction directly leads to decreased apoptosis in Pkd1−/− cells suggesting a direct connection between apoptosis and autophagy in PKD. However, mTOR inhibitors, that are known autophagy inducers, have a variable effect on apoptosis depending on dose and PKD model. In conclusion, autophagy is suppressed in in vitro and in vivo PKD models and autophagy induction may have a therapeutic role in decreasing cyst growth, perhaps by decreasing apoptosis and proliferation. In the future, direct autophagy inducers should be evaluated in rodent PKD models, and the cause and effect relationship between autophagy, apoptosis and cyst growth in PKD should be tested via autophagy knockout in PKD kidneys.
Acknowledgments
Funding
This work was supported by the National Institutes of Health, USA [R03DK118215; K01DK103678] and the Department of Veterans Affairs, USA [1I01BX003803001A1].
Credit Author Statement
Charles Edelstein: Conceptualization, Writing-original draft preparation, Writing-review and editing, supervision, Funding acquisition. Kristen Nowak: Writing-review and editing.
Abbreviations:
- ADPKD
autosomal dominant polycystic kidney disease
- AMPK
adenosine monophosphate-activated protein kinase
- ARPKD
autosomal recessive polycystic kidney disease
- BafA1
bafilomycin A1
- ErbB4
Erb-b2 receptor tyrosine kinase 4
- HDACi
histone deacetylase inhibitors
- LC3-II
microtubule-associate protein 1A/1B-light chain 3-II
- MDCK
Madin-Darby canine kidney
- mTOR
mammalian target of rapamycin
- Ngal
neutrophil gelatinase-associated lipocalin
- PKD
polycystic kidney disease
- PC-1
polycystin-1
- PC-2
polycystin-2; TNF-α, tumor necrosis factor-α
References
- [1].Fick GM, Gabow PA, Natural history of autosomal dominant polycystic kidney disease, Annu. Rev. Med 45 (1994) 23–29. [DOI] [PubMed] [Google Scholar]
- [2].Wilson PD, Polycystic kidney disease N. Engl. J. Med 350 (2) (2004) 151–164. [DOI] [PubMed] [Google Scholar]
- [3].Antignac C, Calvet JP, Germino GG, Grantham JJ, Guay-Woodford LM, Harris PC, Hildebrandt F, Peters DJ, Somlo S, Torres VE, et al. , The future of polycystic kidney disease research-as seen by the 12 Kaplan Awardees, J. Am. Soc. Nephrol 26 (9) (2015. September) 2081–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Belibi FA, Edelstein CL, Novel targets for the treatment of autosomal dominant polycystic kidney disease, Expert Opin. Investig. Drugs 19 (3) (2010) 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Torres VE, Harris PC, Autosomal dominant polycystic kidney disease: the last 3 years, Kidney Int. 76 (2) (2009) 149–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wu G, Somlo S, Molecular genetics and mechanism of autosomal dominant polycystic kidney disease, Mol. Genet. Metab 69 (1) (2000) 1–15. [DOI] [PubMed] [Google Scholar]
- [7].Tao Y, Kim J, Schrier RW, Edelstein CL, Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease(PKD), J. Am. Soc. Nephrol 16 (2005) 46–51. [DOI] [PubMed] [Google Scholar]
- [8].Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, et al. , The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease, Proc. Natl. Acad. Sci. U. S. A 103 (14) (2006) 5466–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ecder T, Melnikov VY, Stanley M, Korular D, Lucia MS, Schrier RW, Edelstein CL, Caspases, Bcl-2 proteins and apoptosis in autosomal-dominant polycystic kidney disease, Kidney Int. 61 (4) (2002) 1220–1230. [DOI] [PubMed] [Google Scholar]
- [10].Duplomb L, Droin N, Bouchot O, Thauvin-Robinet C, Bruel AL, Thevenon J, Callier P, Meurice G, Pata-Merci N, Loffroy R, et al. , A constitutive BCL2 down-regulation aggravates the phenotype of PKD1-mutant-induced polycystic kidney disease, Hum. Mol. Genet 26 (23) (2017) 4680–4688. [DOI] [PubMed] [Google Scholar]
- [11].Holditch SJBC, Atwood D, Brown SE, Lombardi AM, Nguyen KN, Hill RC, Lanaspa M, Hopp K, Weiser-Evans M, Edelstein CL, The consequences of increased 4E-BP1 in polycystic kidney disease, Hum. Mol. Genet (2019) In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tao Y, Kim J, Faubel S, Wu JC, Falk SA, Schrier RW, Edelstein CL, Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease(PKD), Proc. Natl. Acad. Sci. USA 102 (19) (2005) 6954–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ali SM, Wong VY, Kikly K, Fredrickson TA, Keller PM, DeWolf WE Jr., Lee D, Brooks DP, Apoptosis in polycystic kidney disease: involvement of caspases, Am. J. Physiol. Regul. Integr. Comp. Physiol 278 (3) (2000) R763–R769. [DOI] [PubMed] [Google Scholar]
- [14].Tao Y, Zafar I, Kim J, Schrier RW, Edelstein CL, Deletion of the caspase-3 gene markedly prolongs survival in the cpk mouse model of polycystic kidney disease (PKD), J. Am. Soc. Nephrol 19 (2007) 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F, et al. , Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy, Nat. Med 19 (4) (2013) 488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhu P, Sieben CJ, Xu X, Harris PC, Lin X, Autophagy activators suppress cystogenesis in an autosomal dominant polycystic kidney disease model, Hum. Mol. Genet 26 (1) (2017) 158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Belibi F, Zafar I, Ravichandran K, Segvic AB, Jani A, Ljubanovic DG, Edelstein CL, Hypoxia-inducible factor-1alpha (HIF-1alpha) and autophagy in polycystic kidney disease (PKD), Am. J. Physiol. Renal Physiol 300 (5) (2011) F1235–F1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chou LF, Cheng YL, Hsieh CY, Lin CY, Yang HY, Chen YC, Hung CC, Tian YC, Yang CW, Chang MY, Effect of trehalose supplementation on autophagy and cystogenesis in a mouse model of polycystic kidney disease, Nutrients 11 (1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ravichandran K, Edelstein CL, Polycystic kidney disease: a case of suppressed autophagy? Semin. Nephrol 34 (1) (2014) 27–33. [DOI] [PubMed] [Google Scholar]
- [20].Sorenson CM, Life, death and kidneys: regulation of renal programmed cell death, Curr. Opin. Nephrol. Hypertens 7 (1) (1998) 5–12. [DOI] [PubMed] [Google Scholar]
- [21].Savill J, Apoptosis and the kidney [editorial], J. Am. Soc. Nephrol 5 (1) (1994) 12–21. [DOI] [PubMed] [Google Scholar]
- [22].Hammerman MR, Renal programmed cell death and the treatment of renal disease [editorial], Curr. Opin. Nephrol. Hypertens 7 (1) (1998) 1–3. [DOI] [PubMed] [Google Scholar]
- [23].Lockshin RA, Early work on apoptosis, an interview with Richard Lockshin, Cell Death Differ. 15 (7) (2008) 1091–1095. [DOI] [PubMed] [Google Scholar]
- [24].Kerr JF, Wyllie AH, Currie AR, Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics, Br. J. Cancer 26 (4) (1972) 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. , Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018, Cell Death Differ. 25 (3) (2018) 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, et al. , Molecular definitions of autophagy and related processes, EMBO J. 36 (13) (2017) 1811–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Woo D, Apoptosis and loss of renal tissue in polycystic kidney diseases, N. Engl. J. Med 333 (1) (1995) 18–25. [DOI] [PubMed] [Google Scholar]
- [28].Edelstein CL, Mammalian target of rapamycin and caspase inhibitors in polycystic kidney disease, Clin. J. Am. Soc. Nephrol 3 (4) (2008) 1219–1226. [DOI] [PubMed] [Google Scholar]
- [29].Saxton RA, Sabatini DM, mTOR signaling in growth, metabolism, and disease, Cell 168 (6) (2017) 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ravikumar B, Berger Z, Vacher C, O’Kane CJ, Rubinsztein DC, Rapamycin pretreatment protects against apoptosis, Hum. Mol. Genet 15 (7) (2006) 1209–1216. [DOI] [PubMed] [Google Scholar]
- [31].Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP, Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD), Nephrol. Dial. Transplant 21 (3) (2006) 598–604. [DOI] [PubMed] [Google Scholar]
- [32].Wu M, Wahl PR, Le Hir M, Wackerle-Men Y, Wuthrich RP, Serra AL, Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease, Kidney Blood Press. Res 30 (4) (2007) 253–259. [DOI] [PubMed] [Google Scholar]
- [33].Zafar I, Ravichandran K, Belibi FA, Doctor RB, Edelstein CL, Sirolimus attenuates disease progression in an orthologous mouse model of human autosomal dominant polycystic kidney disease, Kidney Int. 78 (8) (2010) 754–761. [DOI] [PubMed] [Google Scholar]
- [34].Becker JU, Saez AO, Zerres K, Witzke O, Hoyer PF, Schmid KW, Kribben A, Bergmann C, Nurnberger J, The mTOR pathway is activated in human autosomal-recessive polycystic kidney disease, Kidney Blood Press. Res 33 (2) (2010) 129–138. [DOI] [PubMed] [Google Scholar]
- [35].Fischer DC, Jacoby U, Pape L, Ward CJ, Kuwertz-Broeking E, Renken C, Nizze H, Querfeld U, Rudolph B, Mueller-Wiefel DE, et al. , Activation of the AKT/mTOR pathway in autosomal recessive polycystic kidney disease (ARPKD), Nephrol. Dial. Transplant 24 (6) (2009) 1819–1827. [DOI] [PubMed] [Google Scholar]
- [36].Ravichandran K, Zafar I, He Z, Doctor RB, Moldovan R, Mullick AE, Edelstein CL, An mTOR anti-sense oligonucleotide decreases polycystic kidney disease in mice with a targeted mutation in Pkd2, Hum. Mol. Genet 23 (18) (2014) 4919–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Holditch SJ, Brown CN, Atwood DJ, Lombardi AM, Nguyen KN, Toll HW, Hopp K, Edelstein CL, A study of sirolimus and an mTOR kinase inhibitor (TORKi) in a hypomorphic Pkd1 mouse model of autosomal dominant polycystic kidney disease (ADPKD), Am. J. Physiol. Renal Physiol 317 (1) (2019. July 1) F187–F196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Green DR, Apoptotic pathways: the roads to ruin, Cell 94 (1998) 695–698. [DOI] [PubMed] [Google Scholar]
- [39].Green DR, Apoptotic pathways: paper wraps stone blunts scissors, Cell 102 (2000) 1–4. [DOI] [PubMed] [Google Scholar]
- [40].Green DR, Reed JC, Mitochondria and apoptosis, Science 281 (5381) (1998) 1309–1312. [DOI] [PubMed] [Google Scholar]
- [41].Barinaga M, Cell suicide:by ICE, not fire, Science 263 (1994) 754–756. [DOI] [PubMed] [Google Scholar]
- [42].Barinaga M, Death by dozens of cuts, Science 280 (1998) 32–34. [DOI] [PubMed] [Google Scholar]
- [43].Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, Identification and inhibition of the ICE/CED-3 protease neccessary for mammalian apoptosis, Nature 376 (1995) 37–43. [DOI] [PubMed] [Google Scholar]
- [44].Salvesen GS, Dixit VM, Caspases: intracellular signaling by proteolysis, Cell 91 (4) (1997) 443–446. [DOI] [PubMed] [Google Scholar]
- [45].Fraser A, Evan G, A license to kill, Cell 85 (1996) 781–784. [DOI] [PubMed] [Google Scholar]
- [46].Adams JM, Cory S, The Bcl-2 protein family: arbiters of cell survival, Science 281 (5381) (1998) 1322–1326. [DOI] [PubMed] [Google Scholar]
- [47].Korsmeyer SJ, BCL-2 gene family and the regulation of programmed cell death, Cancer Res. 59 (1999) 1693s–1700s. [PubMed] [Google Scholar]
- 48.[] Nakayama K, Nakayama K, Negishi I, Kuida K, Sawa H, Loh DY, Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia, Proc. Natl. Acad. Sci. U. S. A 91 (9) (1994) 3700–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kamada S, Shimono A, Shinto Y, Tsujimura T, Takahashi T, Noda T, Kitamura Y, Kondoh H, Tsujimoto Y, bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine, Cancer Res. 55 (2) (1995) 354–359. [PubMed] [Google Scholar]
- [50].Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ, Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair, Cell 75 (2) (1993) 229–240. [DOI] [PubMed] [Google Scholar]
- [51].Tao Y, Kim J, He SM,Z, Faubel SG, Schrier RW, Edelstein CL, Pathways of caspase-mediated apoptosis in autosomal dominant polycystic kidney disease (ADPKD), Kidney Int. 67 (3) (2004) 909–919. [DOI] [PubMed] [Google Scholar]
- [52].Ashkenazi A, Dixit VM, Death receptors: signaling and modulation, Science 281 (1998) 1305–1308. [DOI] [PubMed] [Google Scholar]
- [53].Lin HH, Yang TP, Jiang ST, Yang HY, Tang MJ, Bcl-2 overexpression prevents apoptosis-induced Madin-Darby canine kidney simple epithelial cyst formation, Kidney Int. 55 (1) (1999) 168–178. [DOI] [PubMed] [Google Scholar]
- [54].Boletta A, Qian F, Onuchic LF, Bhunia AK, Phakdeekitcharoen B, Hanaoka K, Guggino W, Monaco L, Germino GG, Polycystin-1, the gene product of PKD1, induces resistance to apoptosis and spontaneous tubulogenesis in MDCK cells, Mol. Cell 6 (5) (2000) 1267–1273. [DOI] [PubMed] [Google Scholar]
- [55].Mai W, Chen D, Ding T, Kim I, Park S, Cho SY, Chu JS, Liang D, Wang N, Wu D, et al. , Inhibition of Pkhd1 impairs tubulomorphogenesis of cultured IMCD cells, Mol. Biol. Cell 16 (9) (2005) 4398–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pei Y, Lan Z, Wang K, Garcia-Gonzalez M, He N, Dicks E, Parfrey P, Germino G, Watnick T, A missense mutation in PKD1 attenuates the severity of renal disease, Kidney Int. 81 (4) (2012) 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yu W, Kong T, Beaudry S, Tran M, Negoro H, Yanamadala V, Denker BM, Polycystin-1 protein level determines activity of the Galpha12/JNK apoptosis pathway, J. Biol. Chem 285 (14) (2010) 10243–10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bukanov NO, Moreno SE, Natoli TA, Rogers KA, Smith LA, Ledbetter SR, Oumata N, Galons H, Meijer L, Ibraghimov-Beskrovnaya O, CDK inhibitors R-roscovitine and S-CR8 effectively block renal and hepatic cystogenesis in an orthologous model of ADPKD, Cell Cycle 11 (21) (2012) 4040–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O, Long lasting arrest of murine polycystic kidney disease with CDK inhibitor rosocovitine, Nature 444 (7121) (2006) 949–952. [DOI] [PubMed] [Google Scholar]
- [60].Zeng F, Miyazawa T, Kloepfer LA, Harris RC, Deletion of ErbB4 accelerates polycystic kidney disease progression in cpk mice, Kidney Int. 86 (3) (2014) 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Anderson S, Oyama TT, Lindsley JN, Schutzer WE, Beard DR, Gattone VH, Komers R, 2-Hydroxyestradiol slows progression of experimental polycystic kidney disease, Am. J. Physiol. Renal Physiol 302 (5) (2012) F636–F645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Natoli TA, Smith LA, Rogers KA, Wang B, Komarnitsky S, Budman Y, Belenky A, Bukanov NO, Dackowski WR, Husson H, et al. , Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models, Nat. Med 16 (7) (2010) 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kleczko EK, Marsh KH, Tyler LC, Furgeson SB, Bullock BL, Altmann CJ, Miyazaki M, Gitomer BY, Harris PC, Weiser-Evans MCM, et al. , CD8( + ) T cells modulate autosomal dominant polycystic kidney disease progression, Kidney Int. 94 (6) (2018) 1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lee CG, Kim JG, Kim HJ, Kwon HK, Cho IJ, Choi DW, Lee WH, Kim WD, Hwang SJ, Choi S, et al. , Discovery of an integrative network of microRNAs and transcriptomics changes for acute kidney injury, Kidney Int. 86 (5) (2014) 943–953. [DOI] [PubMed] [Google Scholar]
- [65].Yheskel M, Patel V, Therapeutic microRNAs in polycystic kidney disease, Curr. Opin. Nephrol. Hypertens 26 (4) (2017) 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wang E, Chiou YY, Jeng WY, Lin HK, Lin HH, Chin HJ, Leo Wang CK, Yu SS, Tsai SC, Chiang CY, et al. , Overexpression of exogenous kidney-specific Ngal attenuates progressive cyst development and prolongs lifespan in a murine model of polycystic kidney disease, Kidney Int. 91 (2) (2017) 412–422. [DOI] [PubMed] [Google Scholar]
- [67].Chen L, Zhou X, Fan LX, Yao Y, Swenson-Fields KI, Gadjeva M, Wallace DP, Peters DJ, Yu A, Grantham JJ, et al. , Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease, J. Clin. Invest 125 (6) (2015) 2399–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Carayol N, Vakana E, Sassano A, Kaur S, Goussetis DJ, Glaser H, Druker BJ, Donato NJ, Altman JK, Barr S, et al. , Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells, Proc. Natl. Acad. Sci. U. S. A 107 (28) (2010) 12469–12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shillingford JM, Piontek KB, Germino GG, Weimbs T, Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1, J. Am. Soc. Nephrol 21 (3) (2010) 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shillingford JM, Leamon CP, Vlahov IR, Weimbs T, Folate-conjugated rapamycin slows progression of polycystic kidney disease, J. Am. Soc. Nephrol 23 (10) (2012) 1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ravichandran K, Zafar I, Ozkok A, Edelstein CL, An mTOR kinase inhibitor slows disease progression in a rat model of polycystic kidney disease (PKD), Nephrol. Dial. Transplant 30 (1) (2014) 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gobe G, Willgoss D, Hogg N, Schoch E, Endre Z, Cell survival or death in renal tubular epithelium after ischemia-reperfusion injury, Kidney Int. 56 (4) (1999) 1299–1304. [DOI] [PubMed] [Google Scholar]
- [73].Gobe G, Zhang XJ, Cuttle L, Pat B, Willgoss D, Hancock J, Barnard R, Endre RB, Bcl-2 genes and growth factors in the pathology of ischaemic acute renal failure, Immunol. Cell Biol 77 (3) (1999) 279–286. [DOI] [PubMed] [Google Scholar]
- [74].A. L, V. JM, S. R, How cells die counts, Am. J. Kidney Dis 36 (2000) 662–668. [DOI] [PubMed] [Google Scholar]
- [75].Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R, In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note, Hepatology 21 (5) (1995) 1465–1468. [DOI] [PubMed] [Google Scholar]
- [76].Gobe G, Zhang XJ, Willgoss DA, Schoch E, Hogg NA, Endre ZH, Relationship between expression of Bcl-2 genes and growth factors in ischemic acute renal failure in the rat, J. Am. Soc. Nephrol 11 (3) (2000) 454–467. [DOI] [PubMed] [Google Scholar]
- [77].Weimbs T, Regulation of mTOR by polycystin-1: is polycystic kidney disease a case of futile repair? Cell Cycle 5 (21) (2006) 2425–2429. [DOI] [PubMed] [Google Scholar]
- [78].Boxer MB, Quinn AM, Shen M, Jadhav A, Leister W, Simeonov A, Auld DS, Thomas CJ, A highly potent and selective caspase 1 inhibitor that utilizes a key 3-cyanopropanoic acid moiety, ChemMedChem 5 (5) (2010) 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chapman JG, Magee WP, Stukenbrok HA, Beckius GE, Milici AJ, Tracey WR, A novel nonpeptidic caspase-3/7 inhibitor, (S)-( + )-5-[1-(2-meth-oxymethylpyrrolidinyl)sulfonyl]isatin reduces myocardial ischemic injury, Eur. J. Pharmacol 456 (1–3) (2002) 59–68. [DOI] [PubMed] [Google Scholar]
- [80].Abbate A, Biondi-Zoccai GG, Baldi A, Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling, J. Cell. Physiol 193 (2) (2002) 145–153. [DOI] [PubMed] [Google Scholar]
- [81].Cursio R, Gugenheim J, Ricci JE, Crenesse D, Rostagno P, Maulon L, Saint-Paul MC, Ferrua B, Auberger AP, A caspase inhibitor fully protects rats against lethal normothermic liver ischemia by inhibition of liver apoptosis, FASEB J. 13 (2) (1999) 253–261. [DOI] [PubMed] [Google Scholar]
- [82].Melnikov VY, Faubel SG, Lucia SB,MS, Ljubanovic D, Edelstein CL, Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice, J. Clin. Invest 110 (2002) 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Faubel S, Edelstein CL, Caspases as drug targets in ischemic organ injury, Curr. Drug Targets Immune Endocr. Metabol. Disord 5 (3) (2005) 269–287. [DOI] [PubMed] [Google Scholar]
- [84].Hayakawa Y, Chandra M, Miao W, Shirani J, Brown JH, Dorn GW, Armstrong RC, Kitsis RN, Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice, Circulation 108 (24) (2003) 3036–3041. [DOI] [PubMed] [Google Scholar]
- [85].Natori S, Higuchi H, Contreras P, Gores GJ, The caspase inhibitor IDN-6556 prevents caspase activation and apoptosis in sinusoidal endothelial cells during liver preservation injury, Liver Transpl. 9 (3) (2003) 278–284. [DOI] [PubMed] [Google Scholar]
- [86].Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ, The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse, J. Pharmacol. Exp. Therap 308 (3) (2004) 1191–1196. [DOI] [PubMed] [Google Scholar]
- [87].Valentino KL, Gutierrez M, Sanchez R, Winship MJ, Shapiro DA, First clinical trial of a novel caspase inhibitor: anti-apoptotic caspase inhibitor, IDN-6556, improves liver enzymes, Int. J. Clin. Pharmacol. Ther 41 (10) (2003) 441–449. [DOI] [PubMed] [Google Scholar]
- [88].Du C, Fang M, Li Y, Li L, Wang X, Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition, Cell 102 (2000) 33–42. [DOI] [PubMed] [Google Scholar]
- [89].Srinivasula SM, Datta P, Fan XJ, Fernandes-Alnemri T, Huang Z, Alnemri ES, Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway, J. Biol. Chem 275 (46) (2000) 36152–36157. [DOI] [PubMed] [Google Scholar]
- [90].Fan LX, Zhou X, Sweeney WE Jr., Wallace DP, Avner ED, Grantham JJ, Li X, Smac-mimetic-induced epithelial cell death reduces the growth of renal cysts, J. Am. Soc. Nephrol 24 (12) (2013) 2010–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Goilav B, Apoptosis in polycystic kidney disease, Biochim. Biophys. Acta 1812 (10) (2011) 1272–1280. [DOI] [PubMed] [Google Scholar]
- [92].Murcia NS, Sweeney WE Jr., Avner ED, New insights into the molecular pathophysiology of polycystic kidney disease, Kidney Int. 55 (4) (1999) 1187–1197. [DOI] [PubMed] [Google Scholar]
- [93].Ibrahim S, Increased apoptosis and proliferative capacity are early events in cyst formation in autosomal-dominant, polycystic kidney disease, Sci. World J 7 (2007) 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lanoix J, D’Agati V, Szabolcs M, Trudel M, Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD), Oncogene 13 (6) (1996) 1153–1160. [PubMed] [Google Scholar]
- [95].Trudel M, Barisoni L, Lanoix J, D’Agati V, Polycystic kidney disease in SBM transgenic mice: role of c-myc in disease induction and progression, Am. J. Pathol 152 (1) (1998) 219–229. [PMC free article] [PubMed] [Google Scholar]
- [96].Trudel M, Lanoix J, Barisoni L, Blouin MJ, Desforges M, L’Italien C, D’Agati V, C-myc-induced apoptosis in polycystic kidney disease is Bcl-2 and p53 independent, J. Exp. Med 186 (11) (1997) 1873–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ogborn MR, Bankovic-Calic N, Shoesmith C, Buist R, Peeling J, Soy protein modification of rat polycystic kidney disease, Am. J. Physiol 274 (3 Pt 2) (1998) F541–F549. [DOI] [PubMed] [Google Scholar]
- [98].Sorenson CM, Padanilam BJ, Hammerman MR, Abnormal postpartum renal development and cystogenesis in the bcl-2 (−/−) mouse, Am. J. Physiol 271 (1 Pt 2) (1996) F184–F193. [DOI] [PubMed] [Google Scholar]
- [99].Woo D, Loss of renal function in polycystic kidney diseases is a result of apoptosis, J. Am. Soc. Nephrol 4 (1993) 268. [Google Scholar]
- [100].Wilson MR, Apoptosis: unmasking the executioner, Cell Death Differ. 5 (8) (1998) 646–652. [DOI] [PubMed] [Google Scholar]
- [101].Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE, Geometric control of cell life and death, Science 276 (1997) 1425–1429. [DOI] [PubMed] [Google Scholar]
- [102].Tsuboi A, Ohsawa S, Umetsu D, Sando Y, Kuranaga E, Igaki T, Fujimoto K, Competition for space is controlled by apoptosis-induced change of local epithelial topology, Curr. Biol 28 (13) (2018) 2115–2128 e2115. [DOI] [PubMed] [Google Scholar]
- [103].Couillard M, Guillaume R, Tanji N, D’Agati V, Trudel M, c-myc-induced apoptosis in polycystic kidney disease is independent of FasL/Fas interaction, Cancer Res. 62 (8) (2002) 2210–2214. [PubMed] [Google Scholar]
- [104].Trudel M, D’Agati V, Costantini F, C-myc as an inducer of polycystic kidney disease in transgenic mice, Kidney Int. 39 (4) (1991) 665–671. [DOI] [PubMed] [Google Scholar]
- [105].Cowley BD Jr., Gudapaty S, Kraybill AL, Barash BD, Harding MA, Calvet JP, Gattone VH 2d, Autosomal-dominant polycystic kidney disease in the rat, Kidney Int. 43 (3) (1993) 522–534. [DOI] [PubMed] [Google Scholar]
- [106].Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW, Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition, Science 284 (5411) (1999) 156–159. [DOI] [PubMed] [Google Scholar]
- [107].Kangas A, Nicholson DW, Hottla E, Involvement of CPP32/caspase-3 in c-myc-induced apoptosis, Oncogene 16 (1998) 387–398. [DOI] [PubMed] [Google Scholar]
- [108].Mochizuki T, Kitanaka C, Noguchi K, Sugiyama A, Kagaya S, Chi S, Asai A, Kuchino Y, Pim-1 kinase stimulates c-myc-mediated death signaling upstream of caspase-3 (CPP32)-like protease activation, Oncogene 15 (1997) 1471–1480. [DOI] [PubMed] [Google Scholar]
- [109].Sabourin LA, Seale P, Wagner J, Rudnicki MA, Caspase-3 cleavage of the Ste20-related kinase SLk releases and activates an apoptosis-inducing kinase domain and an actin-disassembling region, Mol. Cell. Biol 20 (2000) 684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Husson H, Manavalan P, Akmaev VR, Russo RJ, Cook B, Richards B, Barberio D, Liu D, Cao X, Landes GM, et al. , New insights into ADPKD molecular pathways using combination of SAGE and microarray technologies, Genomics 84 (3) (2004) 497–510. [DOI] [PubMed] [Google Scholar]
- [111].Song X, Di GV, He N, Wang K, Ingram A, Rosenblum ND, Pei Y, Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks, Hum. Mol. Genet 18 (13) (2009) 2328–2343. [DOI] [PubMed] [Google Scholar]
- [112].Torres VE, Apoptosis in cystogenesis: hands on or hands off? Kidney Int. 55 (1) (1999) 334–335. [DOI] [PubMed] [Google Scholar]
- [113].Elbekai RH, Paranjpe MG, Contreras PC, Spada A, Carcinogenicity assessment of the pan-caspase inhibitor, emricasan, in Tg.rasH2 mice, Regul. Toxicol. Pharmacol 72 (2) (2015) 169–178. [DOI] [PubMed] [Google Scholar]
- [114].Levine B, Kroemer G, Autophagy in the pathogenesis of disease, Cell 132 (1) (2008) 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Loos B, du Toit A, Hofmeyr JH, Defining and measuring autophagosome fluxconcept and reality, Autophagy 10 (11) (2014) 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, et al. , Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition), Autophagy 12 (1) (2016) 1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Levine B, Klionsky DJ, Autophagy wins the 2016 Nobel Prize in Physiology or Medicine: breakthroughs in baker’s yeast fuel advances in biomedical research, Proc. Natl. Acad. Sci. U. S. A 114 (2) (2017) 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Chang MY, Ma TL, Hung CC, Tian YC, Chen YC, Yang CW, Cheng YC, Metformin inhibits cyst formation in a zebrafish model of polycystin-2 deficiency, Sci. Rep 7 (1) (2017) 7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Huber TB, Edelstein CL, Hartleben B, Inoki K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, et al. , Emerging role of autophagy in kidney function, diseases and aging, Autophagy 8 (7) (2012) 1009–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Bustamante HA, Gonzalez AE, Cerda-Troncoso C, Shaughnessy R, Otth C, Soza A, Burgos PV, Interplay between the autophagy-lysosomal pathway and the ubiquitin-proteasome system: a target for therapeutic development in Alzheimer’s disease, Front. Cell. Neurosci 12 (2018) 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, Xie Y, Carlson D, Rothermel BA, Sun Y, et al. , Beclin-1-dependent autophagy protects the heart during sepsis, Circulation 138 (20) (2018. November 13) 2247–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Sureshbabu A, Ryter SW, Choi ME, Oxidative stress and autophagy: crucial modulators of kidney injury, Redox Biol. 4 (2015) 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N, Walz G, Huber TB, Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury, Autophagy 8 (5) (2012) 826–837. [DOI] [PubMed] [Google Scholar]
- [124].Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z, Autophagy in proximal tubules protects against acute kidney injury, Kidney Int. 82 (12) (2012) 1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chien CT, Shyue SK, Lai MK, Bcl-xL augmentation potentially reduces ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy, Transplantation 84 (9) (2007) 1183–1190. [DOI] [PubMed] [Google Scholar]
- [126].Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z, Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells, Kidney Int. 74 (5) (2008) 631–640. [DOI] [PubMed] [Google Scholar]
- [127].Hartleben B, Godel M, Meyer-Schwesinger C, Liu S, Ulrich T, Kobler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, et al. , Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice, J. Clin. Invest 120 (4) (2010) 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Aguilar A, Polycystic kidney disease: autophagy boost to treat ADPKD? Nat. Rev. Nephrol 13 (3) (2017) 134. [DOI] [PubMed] [Google Scholar]
- [129].Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK, Autophagy and apoptosis: where do they meet? Apoptosis 19 (4) (2014) 555–566. [DOI] [PubMed] [Google Scholar]
- [130].Zhou F, Yang Y, Xing D, Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis, FEBS J. 278 (3) (2011) 403–413. [DOI] [PubMed] [Google Scholar]
- [131].Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y, Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes, Nat. Cell Biol 6 (12) (2004) 1221–1228. [DOI] [PubMed] [Google Scholar]
- [132].Song J, Zhao X, Feng Y, Xu S, Zhang Y, Wei L, Involvement of proapoptotic genes in autophagic cell death induced by irradiation, Cell Death Dis. 3 (2017) 17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Giansanti V, Torriglia A, Scovassi AI, Conversation between apoptosis and autophagy: “Is it your turn or mine?”, Apoptosis 16 (4) (2011) 321–333. [DOI] [PubMed] [Google Scholar]
- [134].Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ, Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8, Science 304 (5676) (2004) 1500–1502. [DOI] [PubMed] [Google Scholar]
- [135].Kaushal GP, Kaushal V, Herzog C, Yang C, Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity, Autophagy 4 (5) (2008) 710–712. [DOI] [PubMed] [Google Scholar]
- [136].Kroemer G, Levine B, Autophagic cell death: the story of a misnomer, Nat. Rev. Mol. Cell Biol 9 (12) (2008) 1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Maiuri MC, Zalckvar E, Kimchi A, Kroemer G, Self-eating and self-killing: crosstalk between autophagy and apoptosis, Nat. Rev. Mol. Cell Biol 8 (9) (2007) 741–752. [DOI] [PubMed] [Google Scholar]
- [138].Hotchkiss RS, Strasser A, McDunn JE, Swanson PE, Cell death, N. Engl. J. Med 361 (16) (2009) 1570–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N, et al. , Everolimus in patients with autosomal dominant polycystic kidney disease, New Engl. J. Med 363 (9) (2010) 830–840. [DOI] [PubMed] [Google Scholar]
- [140].Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, et al. , Sirolimus and kidney growth in autosomal dominant polycystic kidney disease, New Engl. J. Med 363 (9) (2010) 820–829. [DOI] [PubMed] [Google Scholar]
- [141].Watnick T, Germino GG, mTOR inhibitors in polycystic kidney disease, New Engl. J. Med 363 (9) (2010) 879–881. [DOI] [PubMed] [Google Scholar]
- [142].Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, Crews CM, Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease, Proc. Natl. Acad. Sci. U. S. A 104 (11) (2007) 4389–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Leuenroth SJ, Bencivenga N, Igarashi P, Somlo S, Crews CM, Triptolide reduces cystogenesis in a model of ADPKD, J. Am. Soc. Nephrol 19 (9) (2008) 1659–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Shakeri A, Cicero AFG, Panahi Y, Mohajeri M, Sahebkar A, Curcumin: a naturally occurring autophagy modulator, J. Cell. Physiol 234 (5) (2019) 5643–5654. [DOI] [PubMed] [Google Scholar]
- [145].Leonhard WN, van der WA, Novalic Z, Kunnen SJ, Gansevoort RT, Breuning MH, de Heer E, Peters DJ, Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: in vivo evidence from a Pkd1-deletion model, Am. J. Physiol. Renal Physiol 300 (5) (2011. May) F1193–F1202. [DOI] [PubMed] [Google Scholar]
- [146].Han J, Pan XY, Xu Y, Xiao Y, An Y, Tie L, Pan Y, Li XJ, Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage, Autophagy 8 (5) (2012) 812–825. [DOI] [PubMed] [Google Scholar]
- [147].Cheng Y, Ren X, Zhang Y, Shan Y, Huber-Keener KJ, Zhang L, Kimball SR, Harvey H, Jefferson LS, Yang JM, Integrated regulation of autophagy and apoptosis by EEF2K controls cellular fate and modulates the efficacy of curcumin and velcade against tumor cells, Autophagy 9 (2) (2013) 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Talbot JJ, Shillingford JM, Vasanth S, Doerr N, Mukherjee S, Kinter MT, Watnick T, Weimbs T, Polycystin-1 regulates STAT activity by a dual mechanism, Proc. Natl. Acad. Sci. U. S. A 108 (19) (2011) 7985–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Pietrocola F, Izzo V, Niso-Santano M, Vacchelli E, Galluzi L, Maiuri MC, Kroemer G, Regulation of autophagy by stress-responsive transcription factors, Semin. Cancer Biol 23 (5) (2013. October) 310–322 E pub ahead of print. [DOI] [PubMed] [Google Scholar]
- [150].Chandrasekar B, Smith JB, Freeman GL, Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine, Circulation 103 (18) (2001) 2296–2302. [DOI] [PubMed] [Google Scholar]
- [151].Sweeney WE Jr., von Vigier RO, Frost P, Avner ED, Src inhibition ameliorates polycystic kidney disease, J. Am. Soc. Nephrol 19 (7) (2008) 1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Takiar V, Nishio S, Seo-Mayer P, King JD Jr., Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ, Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis, Proc. Natl. Acad. Sci. U. S. A 108 (6) (2011) 2462–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Hoyer-Hansen M, Jaattela M, AMP-activated protein kinase: a universal regulator of autophagy? Autophagy 3 (4) (2007) 381–383. August. [DOI] [PubMed] [Google Scholar]
- [154].Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, et al. , Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice, Diabetes 60 (6) (2011) 1770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Seshia SS, Yager JY, Johnston B, Haese P, Inter-observer agreement in assessing comatose children, Can. J. Neurol. Sci 18 (4) (1991) 472–475. [DOI] [PubMed] [Google Scholar]
- [156].Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D, Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney, J. Clin. Invest 120 (4) (2010) 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Lempiainen J, Finckenberg P, Mervaala EE, Sankari S, Levijoki J, Mervaala EM, Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1alpha-eNOS pathway and enhanced autophagy, Acta Physiol (Oxf.) 208 (4) (2013) 410–421. [DOI] [PubMed] [Google Scholar]
- [158].Warner G, Hein KZ, Nin V, Edwards M, Chini CC, Hopp K, Harris PC, Torres VE, Chini EN, Food restriction ameliorates the development of polycystic kidney disease, J. Am. Soc. Nephrol 27 (5) (2016. May) 1437–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Kipp KR, Rezaei M, Lin L, Dewey EC, Weimbs T, A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease, Am. J. Physiol. Renal Physiol 310 (8) (2016. April 15) F726–F731 ajprenal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Chiaravalli M, Rowe I, Mannella V, Quilici G, Canu T, Bianchi V, Gurgone A, Antunes S, D’Adamo P, Esposito A, et al. , 2-Deoxy-d-glucose ameliorates PKD progression, J. Am. Soc. Nephrol 27 (7) (2016. July) 1958–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Kraus A, Schley G, Kunzelmann K, Schreiber R, Peters DJ, Stadler R, Eckardt KU, Buchholz B, Glucose promotes secretion-dependent renal cyst growth, J. Mol. Med. (Berl) 94 (1) (2016. January) 107–117. [DOI] [PubMed] [Google Scholar]
- [162].Nowak KL, You Z, Gitomer B, Brosnahan G, Torres VE, Chapman AB, Perrone RD, Steinman TI, Abebe KZ, Rahbari-Oskoui FF, et al. , Overweight and obesity are predictors of progression in early autosomal dominant polycystic kidney disease, J. Am. Soc. Nephrol 29 (2) (2018) 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Fernandez AF, Barcena C, Martinez-Garcia GG, Tamargo-Gomez I, Suarez MF, Pietrocola F, Castoldi F, Esteban L, Sierra-Filardi E, Boya P, et al. , Autophagy couteracts weight gain, lipotoxicity and pancreatic beta-cell death upon hypercaloric pro-diabetic regimens, Cell Death Dis. 8 (8) (2017) e2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Shi W, Xu D, Gu J, Xue C, Yang B, Fu L, Song S, Liu D, Zhou W, Lv J, et al. , Saikosaponin-d inhibits proliferation by up-regulating autophagy via the CaMKKbeta-AMPK-mTOR pathway in ADPKD cells, Mol. Cell. Biochem 449 (1–2) (2018) 219–226. [DOI] [PubMed] [Google Scholar]
- [165].Sun L, Hu C, Zhang X, Histone deacetylase inhibitors reduce cysts by activating autophagy in polycystic kidney disease, Kidney Dis. (Basel) 5 (3) (2019) 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Masyuk AI, Masyuk TV, Lorenzo Pisarello MJ, Ding JF, Loarca L, Huang BQ, LaRusso NF, Cholangiocyte autophagy contributes to hepatic cystogenesis in polycystic liver disease and represents a potential therapeutic target, Hepatology 67 (3) (2018) 1088–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ren XS, Sato Y, Harada K, Sasaki M, Furubo S, Song JY, Nakanuma Y, Activation of the PI3K/mTOR pathway is involved in cystic proliferation of cholangiocytes of the PCK rat, PLoS One 9 (1) (2014) e87660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Yang J, Zhao Y, Ma K, Jiang FJ, Liao W, Zhang P, Zhou J, Tu B, Wang L, Kampinga HH, et al. , Deficiency of hepatocystin induces autophagy through an mTOR-dependent pathway, Autophagy 7 (7) (2011) 748–759. [DOI] [PubMed] [Google Scholar]
- [169].Lager DJ, Qian Q, Bengal RJ, Ishibashi M, Torres VE, The pck rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease, Kidney Int. 59 (1) (2001) 126–136. [DOI] [PubMed] [Google Scholar]
- [170].Smith LA, Bukanov NO, Husson H, Russo RJ, Barry TC, Taylor AL, Beier DR, Ibraghimov-Beskrovnaya O, Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease, J. Am. Soc. Nephrol 17 (10) (2006) 2821–2831. [DOI] [PubMed] [Google Scholar]
- [171].Moser M, Pscherer A, Roth C, Becker J, Mucher G, Zerres K, Dixkens C, Weis J, Guay-Woodford L, Buettner R, et al. , Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2beta, Genes Dev. 11 (15) (1997) 1938–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Winyard PJ, Nauta J, Lirenman DS, Hardman P, Sams VR, Risdon RA, Woolf AS, Deregulation of cell survival in cystic and dysplastic renal development, Kidney Int. 49 (1) (1996) 135–146. [DOI] [PubMed] [Google Scholar]
- [173].Goilav B, Satlin LM, Wilson PD, Pathways of apoptosis in human autosomal recessive and autosomal dominant polycystic kidney diseases, Pediatr. Nephrol 23 (9) (2008) 1473–1482. [DOI] [PubMed] [Google Scholar]
- [174].Li H, Wang P, Sun Q, Ding WX, Yin XM, Sobol RW, Stolz DB, Yu J, Zhang L, Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of Beclin 1, Cancer Res. 71 (10) (2011) 3625–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]