Abstract
Gastrointestinal infectious diseases are very common worldwide and an important cause of morbidity and mortality, particularly in infants in developing countries. Diarrhea and other intestinal infections are caused by a wide range of bacteria, viruses, protozoa, and parasites. Conventional diagnosis of these infections is performed by culture, microscopy, and antigen detection immunoassays. The traditional culture and microscopy procedures are time-consuming, lack sensitivity, and require special laboratory setup and well-trained staff. However, based on the advancement in the molecular diagnostics and with the introduction of commercially available tests, traditional diagnostic techniques have been continuously replaced by these newer rapid antigen detection and molecular-based methods. This review summarizes and discusses the availability, advantages, and disadvantages of molecular methods in the detection and identification of human gastrointestinal pathogens.
1. Introduction
Gastrointestinal infections are among the most common infectious diseases worldwide, being exceeded only by respiratory tract infections [1]. Gastrointestinal infections are caused by a variety of bacterial, viral, and parasitic pathogens. These infections are mostly transmitted in poor hygienic conditions and by consuming contaminated food or water. Infections can also be transmitted from person to person by direct contact or through fomites. The most common symptom is diarrhea, which is usually a self-limiting disease, and most of the healthy individuals recover within few days. However, in very young patients, and with poor hygiene, diarrheal disease may progress, leading to severe dehydration, malnourishment, bacteremia, and other complications that may lead to death [2]. According to the report of the World Health Organization (WHO), there are over 1.7 billion cases of diarrheal disease worldwide every year [3]. Furthermore, diarrheal diseases are the second leading cause of death in children under five years of age [3].
The incidence rate and mortality from gastrointestinal infections and diarrhea are relatively low in developed countries including the United States. However, these illnesses remain a major public health burden. In the US, 211–375 million cases of diarrheal disease are estimated each year, including 1.8 million hospitalizations, and up to 6,000 deaths [4, 5]. Furthermore, in the United States, the cost of hospitalization due to gastrointestinal infections exceeds 6 billion dollars annually. Early diagnosis of enteric diseases and identification of etiological agents are helpful in patient management and in making appropriate treatment decisions. Furthermore, it is also very helpful for infection control from a public health point of view.
The important causative agents of bacterial gastroenteritis and diarrhea are Campylobacter, Salmonella, Shigella, Plesiomonas, Vibrio, Yersinia enterocolitica, Clostridioides difficile (formerly Clostridium difficile), and pathogenic strains of Escherichia coli [4, 6]. Important causative agents of viral gastroenteritis are Adenovirus, Rotavirus, Astrovirus, and Norovirus [4, 6]. Parasitic infections are caused by a variety of helminths, protozoa, ciliates, and coccidian organisms. Acute gastrointestinal infections with diarrhea are mostly caused by Giardia lamblia, Entamoeba histolytica, Cyclospora cayetanensis, and Cryptosporidium species [7].
Recent development in the field of Molecular Diagnostics and the availability of commercial Nucleic Acid Amplification Techniques (NAATs) based assays have changed the way we used to perform laboratory diagnosis of enteric infections. This review summarizes the currently available Food and Drug Administration- (FDA-) approved and some commonly available European In Vitro Diagnostic Devices (CE-IVD) marked molecular methods for the diagnosis of gastrointestinal infectious diseases. Advantages and disadvantages are discussed to see if we are ready to move from traditional and immunological based assays to molecular methods.
2. Traditional Culture, Microscopy, and Immunological Techniques
Traditional laboratory diagnoses of gastrointestinal infections and enteric pathogens detection are performed by (1) culture and antibiotic susceptibility testing, (2) ova and parasite microscopy examination, and (3) antigen detection via immunoassays.
In the clinical and diagnostic microbiology, assay sensitivity and specificity are important parameters and are used in the evaluation of a newly developed test after comparison with a reference gold standard method. A test or a newly developed test validation sensitivity is the ability of a test to correctly identify those with the disease (true positive), whereas test specificity is the ability of the test to correctly identify those without the disease (true negative). When a newly developed test is evaluated by comparison with a nonreference method, the terms sensitivity and specificity are not used. Rather numerical calculations are called as positive percent agreement (PPA) and negative percent agreement (NPA) instead of sensitivity and specificity. While sensitivity and specificity are characteristics of a test, two other parameters, positive predictive value (PPV) and negative predictive value (NPV), are used to determine clinical relevance and effectiveness of a test in determining a specific disease in a specific patient population. PPV is the probability that following a positive test result, that individual will truly have that specific disease, and NPV is the probability that following a negative test result, that individual will truly not have that specific disease.
Conventional culture remains a gold standard for the diagnosis of bacterial enteropathogens with several advantages and disadvantages. The major advantage of the culture method is its specificity. Specificity of culture is 100% if the pathogenic organism is not found in healthy subjects. However, the sensitivity of culture varies and is usually low and more difficult to determine. Another advantage of the culture method is the availability of isolate, which may be used for further testing including antibiotic susceptibility testing. When a traditional culture method is performed, the isolate can be referred to state public health laboratories for further identification, outbreak investigations, and epidemiological studies. The disadvantage of the culture method is poor sensitivity and the fact that it requires 3–5 days for pathogen detection and finalizing reports. For traditional stool culture, virus or parasite detection, patient history, and request for specific testing may be required for the proper selection of media and method. Laboratories may lack resources, trained staff, and equipment to detect some of the pathogens in the clinical specimens. Furthermore, in case of traditional bacterial culture, time and experience are required to screen all the normal flora, look for the possible pathogen and subculture, and setup further identification procedures [8]. Ova and parasite microscopic examination of stool sample is useful in the direct detection of intestinal parasites. However, microscopic examination of the direct smear or stained smear of the stool for the intestinal parasite has low sensitivity, is technically challenging, and requires highly trained and experienced personnel [9]. Ova and parasite detection can be complicated due to low organism burden and/or intermittent shedding. Antigen detection by commercially available immunoassays is popular and easy to perform procedure to detect certain intestinal parasites and viruses. However, these antigen-based assay doses generally have low sensitivity and do not detect all the pathogens involved in gastrointestinal infections.
3. Nucleic Acid-Based Amplification Techniques (NAATs)
In the last decade, commercially available nucleic acid-based methods have focused on the detection of either a single pathogen or multiple pathogens in a multiplex assay format. Several molecular assays are available for the detection of a single gastrointestinal pathogen. These assays are especially designed to target specific patient population and to meet medical coding and billing requirements. Furthermore, these single NAAT assays allow for particular testing that a physician may order. Common single molecular assays are used for the detection of toxigenic C. difficile and Norovirus infections [1, 10–12].
Current molecular techniques include (1) polymerase chain reaction (PCR) in a real-time format, (2) endpoint PCR with microfluidics and array technologies, and (3) integrated platforms in which nucleic acid extraction, amplification, and analysis are performed in a single step. Recently, isothermal amplification, in which thermal cycling is not required, has been gaining popularity. These isothermal helicase-dependent amplification techniques do not require expensive thermal cycling equipment and are more suitable for the detection of a single pathogen.
3.1. Molecular Tests for the Detection of Toxigenic Clostridioides difficile
Clostridioides difficile is a major causative agent of nosocomial and antibiotic-associated diarrhea and pseudomembranous colitis [13, 14]. C. difficile can normally colonize the gastrointestinal tract of up to 90% of healthy newborns and infants and up to 15% healthy adult population. Risk factors for C. difficile-associated disease are older age, hospitalization, or stay in long-term care facilities, and typically diarrhea symptoms occur after antibiotic treatment. The pathogenicity of C. difficile is associated with the production of a binary toxin, and a large clostridial toxin comprising of the toxin A (TcdA) and toxin B (TcdB), and non-toxin-producing strains are considered as nonpathogenic [15]. C. difficile toxin A, encoded by the tcdA gene, is an enterotoxin that causes diarrhea. Toxin B, encoded by the tcdB gene, causes cellular destruction leading to pseudomembranous colitis, which may progress to the complications of the development of toxic megacolon, perforation of the colon, and sepsis. The tcdC gene regulates toxin A and B production. Genes encoding for toxins A and B are present in the pathogenicity locus (PaLoc) together with three additional genes that have been implicated in regulation (tcdR and tcdC) and secretion (tcdE) [16]. In recent years, several outbreaks have been reported with increased morbidity and mortality by a hypervirulent ribotype 027 strain of C. difficile (NAP1 strain) [17, 18]. This strain shows high virulence due to a base pair frameshift mutation in the regulatory tcdC gene, which leads to the increased toxin production and pathogenicity [19]. Based on the fact that C. difficile can normally colonize, multiplex assays for the detection of enteric pathogens discussed in this article are not recommended for the diagnosis of C. difficile-associated disease. According to the most recent Infectious Diseases Society of America/Society for Healthcare Epidemiology of America (IDSA/SHEA), C. difficile testing is recommended in high-risk adults and children of ≥2 years of age with onset of ≥3 unformed stools in 24 hours, following antimicrobial treatment, healthcare-associated diarrhea, and in patients with persistent chronic diarrhea without any etiology [20].
Laboratory diagnosis of C. difficile colonization and disease is performed by the demonstration of the presence of the pathogen and their toxin production in the stool samples. Initial screening of C. difficile colonization is performed by the antigen-based immunoassays, e.g., toxin A and B detection using chromatographic/lateral flow membrane cartridge devices or enzyme immunoassay (EIA). Initial screening can also be performed by detection of the presence of glutamate dehydrogenase (GDH) enzyme in the patient stool sample in a solid-phase microtiter plate format or in a chromatographic/lateral flow membrane cartridge device as a single test, or as a combined test with the detection of toxins A and B. GDH is a constitutive enzyme produced by all strains of C. difficile independent of toxigenicity, and its presence indicates colonization and not necessarily active C. difficile-associated disease. All positive screen results need to be further confirmed by either molecular NAAT-based assay, including PCR for the detection of the toxin-producing genes, or culture and cytotoxin production assays. Toxigenic culture involves isolation of C. difficile from the stool sample on cycloserine-cefoxitin-fructose agar (CCFA) and then demonstration of toxin production of the isolates by cell culture cytotoxicity assay. Cytotoxin production assay can also be used directly to detect the presence and activity of the toxin in the stool filtrates. Exposure of cell lines with cell culture supernatant or stool filtrates can typically show the cytopathic effect of the cell rounding and is due to the presence of the toxin B than toxin A. Since C. difficile can be normally present in stool samples, most of the institutes use a two-step reflex algorithm to determine active C. difficile infection (Figure 1). This two-step approach uses a combination of screen test by toxin A and B EIA/GHD antigen and confirmatory NAAT testing for the detection of toxin gene or demonstration of toxin production by toxigenic culture/cytotoxicity assay [21, 22]. The diagnosis can be performed by EIA/GDH antigen screen first and, if positive, demonstration of the presence of the toxin-producing genes by NAAT testing or toxigenic culture/cytotoxicity assay. Alternately, NAAT can be performed first, and, if positive, it usually indicates infection. However, a positive NAAT test may be because of asymptomatic colonization of toxigenic C. difficile, which can be further confirmed by the use of highly specific toxin A and B EIA or demonstration of toxin production by toxigenic culture/cytotoxicity assays (Figure 1).
Figure 1.
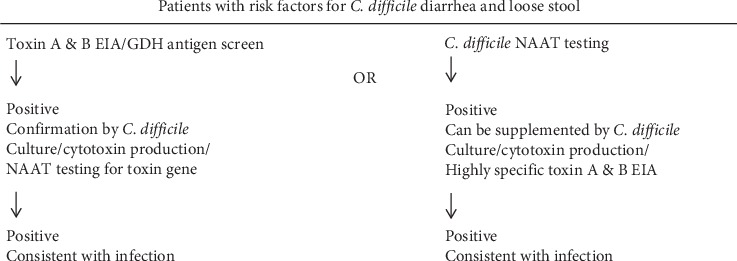
Clostridium difficile testing algorithm.
Several FDA-approved methods are available for the diagnosis of C. difficile infection (Table 1). AmpliVue C. difficile (Quidel Diagnostics, San Diego, CA, USA) is a semiautomated assay which uses isothermal helicase-dependent amplification and hybridization in a cartridge/chip-based format. A comparative study of AmpliVue C. difficile with illumigene C. difficile and reference toxigenic culture method showed this assay sensitivity and specificity to be 96% and 100%, respectively [23]. Illumigene C. difficile assay (Meridian Bioscience, Cincinnati, OH, USA) is also an isothermal amplification system and uses loop-mediated isothermal amplification (LAMP) technology. In an independent study, the sensitivity and specificity of this assay were reported as 88.1% and 96.7%, respectively, as compared with the reference toxigenic culture method [24]. These integrated isothermal amplification-based assays provide an alternative to more expensive PCR-based tests and equipment and are easy to perform.
Table 1.
Molecular tests for detection of toxigenic Clostridioides difficile.
| Assay | Manufacturer | Devise/analyzer | Targets/methodology | |
|---|---|---|---|---|
| AmpliVue®C. difficile | FDA/CE | Quidel Diagnostics, San Diego, CA, USA | Hand-held Disposable cassette | tcdA, isothermal helicase-dependent |
| Illumigene™ (Alethia) C. difficile | FDA/CE | Meridian Bioscience, Cincinnati, OH, USA | Incubator/reader | tcdA, loop-mediated isothermal amplification (LAMP) |
| ProGastro™ Cd test | FDA/CE | Hologic, San Diego, CA, USA | SmartCycler | tcdB, real-time PCR |
| Lyra®C. difficile | FDA/CE | Quidel, San Diego, CA, USA | SmartCycler, ABI 7500, QuantStudio | tcdA and tcdB, real-time PCR |
| Simplexa™C. difficile | FDA/CE | Focus Dx, Cypress, CA, USA | 3M Integrated Cycler | tcdB, real-time PCR |
| BD Max™C. difficile | FDA/CE | Becton Dickinson & Co., Sparks, MD, USA | BD Max System | tcdB, real-time PCR |
| Cobas® Liat Cdiff | FDA/CE | Roche Diagnostics, Indianapolis, IN, USA | Cobas Liat System | tcdB, real-time PCR |
| GenePOC™ CDiff assay | FDA/CE | Meridian Bioscience, Cincinnati, OH, USA | Revogene integrated analyzer | tcdB, real-time PCR |
| ARIES®C. difficile | FDA/CE | Luminex, Austin, TX, USA | ARIES system | tcdA, tcdB, real-time PCR |
| GeneXpert®C. difficile/Epi | FDA/CE | Cepheid, Sunnyvale, CA, USA | GeneXpert system | tcdB, Δ117tcdC (BI/NAP1/027, real-time PCR |
| Verigene®C. difficile | FDA/CE | Luminex, Austin, TX, USA | Verigene processor and reader | tcdA, tcdB, Δ117tcdC (BI/NAP1/027), multiplex PCR/nanoparticle array hybridization |
| Qiagen® artus C. difficile QS-RGQ | FDA/CE | Qiagen, Hilden, Germany | QIAsymphony rotor-gene Q instruments | tcdA, tcdB, real-time PCR |
| EntericBio real-time®C. difficile | CE-IVD | Serosep, Limerick, Ireland | ABI 7500, LightCycler 480 | tcdB, real-time PCR |
Other FDA-approved real-time PCR-based assays that detect tcdB gene include ProGastro Cd Test (Hologic, San Diego, CA, USA) using SmartCycler (Cepheid, Sunnyvale, CA, USA), Lyra C. difficile (Quidel, San Diego, CA, USA) using SmartCycler/ABI 7500/QuantStudio, and Simplexa C. difficile based on 3M Integrated Cycler (Focus Dx, Cypress, CA, USA) (Table 1). These assays require separate nucleic acid extraction step, and amplification and detection are performed on respective real-time PCR analyzers. Other assays that detect tcdB gene using integrated dedicated real-time PCR systems include BD Max C. difficile (Becton Dickinson, Sparks, MD, USA), Cobas Liat Cdiff (Roche Diagnostics, Indianapolis, IN, USA), and GenePOC CDiff assay (Meridian Bioscience, Cincinnati, OH, USA). These assays are based on integrated systems and do not require the separate nucleic acid extraction step. Several comparative studies with culture and other molecular methods showed these assays to have good correlation and sensitivities and specificities [25–28]. The remaining three assays, ARIES C. difficile (Luminex, Austin, TX, USA), GeneXpert C. difficile/Epi (Cepheid, Sunnyvale, CA, USA), and Verigene C. difficile (Luminex, Austin, TX, USA), use real-time PCR-based ARIES system, GeneXpert multiplex PCR system, and nanoparticle array hybridization-based Verigene equipment, respectively (Table 1). All of these assays use closed cartridge-based system in which nucleic acid extraction, amplification, and detection are performed simultaneously without separate processing, thus minimizing the chances of contamination and false-positive results. Several independent studies have been performed on the evaluation of these assays and have sensitivities and specificities in the range of upper 90s [25, 26, 28]. All of these assays target C. difficile tcdB gene, while GeneXpert C. difficile and Verigene C. difficile assays can also detect hypervirulent ribotype 027 (BI/NAP1/027) strain of C. difficile by targeting the regulatory tcdC gene in which there is a deletion of nucleotide at position number 117 (Δ117tcdC) [25, 26, 28, 29]. Besides, there are several CE-IVD and in-house assays available for the detection of C. difficile genes (Table 1). Performance characteristics of these assays are mostly performed by manufacturers, and there are limited data available from the independent studies.
3.2. Commercially Available Multiplex Assays for the Detection of Enteric Pathogens
There are several multiplex commercial assays available that can detect most of the common pathogens in an open system, in which separate nucleic acid extraction step is required, or closed assays and systems, in which simultaneous nucleic acid extraction, amplification, and product analysis are performed. These assays can detect pathogens that may or may not be prevalent in a setting, and local epidemiology as well as institutional need should be considered before acquisition. Some assays offer separate bacterial, viral, and parasite panels, making them flexible in situations where specific testing may have been requested by a physician. Furthermore, these separate bacterial, viral, and parasite panels can be used to resolve patient billing issues. This review article discusses current FDA-approved (Table 2) and commonly available CE-IVD marked approved (Table 3) multiplex NAAT commercial assays for the identification of enteric pathogens. For the identification of pathogens, these assays utilize multiplex PCR followed by either hybridization to microarray, hybridization probes, or melting curve analysis.
Table 2.
Comparative summary of commercial enteropathogen multiplex PCR assays.
| Product name | BioFire® FilmArray® Gastrointestinal (GI) Panel FDA/CE-IVD | xTAG® Gastrointestinal Pathogen Panel FDA/CE-IVD | Verigene® Enteric Pathogens TestFDA/CE-IVD | Prodesse® ProGastro™ SSCS assay FDA/CE-IVD | BD MAX™ Enteric Bacterial, Ext Bacterial, Parasite, and Viral Panels FDA/CE-IVD | Stool Bacterial Pathogens Panel FDA/CE-IVD |
|---|---|---|---|---|---|---|
| Manufacturer | BioFire, Salt Lake City, UT, USA | Luminex, Austin, TX, USA | Luminex, Austin, TX, USA | Hologic, San Diego, CA, USA | BD, Sparks, MD, USA | Great Basin Scientific, Salt Lake City, UT, USA |
| Bacteria | ||||||
| Campylobacter spp | √C. jejuni, C. coli, C. upsaliensis | √ | √C. jejuni, C. coli, and C. lari | √C. jejuni, C. coli | √ | √C. jejuni, C. coli |
| Clostridioides difficile | √Toxin A/B | √Toxin A/B | ||||
| Plesiomonas shigelloides | √ | |||||
| Salmonella spp | √ | √ | √ | √ | √ | √ |
| Yersinia enterocolitica | √ | √∗ | √ | √ | ||
| Vibrio spp | √V. cholera, V. parahaemolyticus, V. vulnificus | √V. cholera | √V. cholera, V. parahaemolyticus | √V. cholera, V. parahaemolyticus, V. vulnificus | ||
| Enteroaggregative E. coli (EAEC) | √ | |||||
| Enteropathogenic E. coli (EPEC) | √ | |||||
| Enterotoxigenic E. coli (ETEC) lt/st | √ | √ | √ | |||
| Shiga-like toxin-producing E. coli (STEC) stx1/stx2 | √ | √ | √ | √ | √ | |
| E. coli O157 | √ | √ | √ | |||
| Enteroinvasive E. coli (EIEC)/Shigella spp | √ | √ | √S. dysenteriae, S. boydii, S. sonnei, and S. flexneri | √ | √ | √Shigella spp |
| Viruses | ||||||
| Adenovirus | √F40/41 | √F40/41 | √F40/41 | |||
| Astrovirus | √ | √ | ||||
| Norovirus | √GI/GII | √GI/GII | √GI/GII | √ | ||
| Rotavirus | √A | √A | √A | √A | ||
| Sapovirus | √I, II, IV, and V | √ | ||||
| Parasites | √F40/41 | |||||
| Cryptosporidium spp | √ | √ | √C. parvum, C. hominis | |||
| Cyclospora cayetanensis | √ | |||||
| Entamoeba histolytica | √ | √ | √ | |||
| Giardia lamblia | √ | √ | √ |
∗Not available in the United States.
Table 3.
Comparative summary of commercial enteropathogen multiplex PCR testing kit.
| Product name | Allplex™ Gastrointestinal Panel (virus, bacteria 1, bacterial 2, and parasite) CE-IVD | Seeplex® Diarrhea ACE Detection (virus, bacteria 1, and bacterial 2) CE-IVD | QIAstat-Dx® Gastrointestinal Panel CE-IVD | RIDA® GENE real-time PCR kits (bacterial, parasite, and viral stool) panel CE-IVD | EntericBio real-time® Gastro Panel I, II and III. Virus panel CE-IVD | CLART® EnteroBac CE-IVD | GastroFinder® 2SMART CE-IVD |
|---|---|---|---|---|---|---|---|
| Manufacturer | Seegene, Seoul, South Korea | Seegene, Seoul, South Korea | Qiagen, Hilden, Germany | R-Biopharm AG, Darmstadt, Germany | Serosep, Limerick, Ireland | Genomica, Madrid, Spain | PathoFinder, the Netherlands |
| Bacteria | |||||||
| Aeromonas spp. | √ | √A. media, A. veronii, A. salmonicida, A. sobria, A. bivalvium, A. hydrophila |
√ | ||||
| Campylobacter spp. | √ | √C. jejuni, C. coli | √C. jejuni, C. coli, C. upsaliensis | √ | √ | √C. jejuni, C. coli, C. lari, C. laridis, C. upsaliensis | √ |
| Clostridium difficile | √Toxin B and hypervirulent stain | √Toxin B | √Toxin A/B | √ | √Toxin B | √Toxins A and B | |
| Clostridium perfringens | √ | ||||||
| Plesiomonas shigelloides | √ | ||||||
| Salmonella spp. | √ | √S. bongori, S. enterica | √ | √ | √ | √ | √ |
| Yersinia enterocolitica | √ | √ | √ | √ | √Y. enterocolitica, Y. pestis, Y. pseudotuberculosis | √ | |
| Vibrio cholerae and other Vibrio spp. | √ | √V. cholerae, V. parahaemolyticus, V. vulnificus | √V. cholerae, V parahaemolyticus, V. vulnificus | √ | |||
| Enteroaggregative E. coli (EAEC) | √ | √ | |||||
| Enteropathogenic E. coli (EPEC) | √ | √ | √ | √ | |||
| Enterotoxigenic E. coli (ETEC) lt/st | √ | √ | √ | √ | |||
| Shiga-like toxin-producing E. coli (STEC) stx1/stx2 | √ | √ | √ | √ | √ | ||
| E. coli O157 | √ | √O157 : H7 | √ | √ | √ | ||
| Enteroinvasive E. coli (EIEC)/Shigella spp. | √ | √S. flexneri, S. boydii, S. sonnei, S. dysenteriae | √ | √ | √ | √S. flexneri, S. boydii, S. sonnei, S. dysenteriae | √ |
| Viruses | |||||||
| Adenovirus | √ | √ | √F40/41 | √ | √ | √F40/41 | |
| Astrovirus | √ | √ | √ | √ | √ | ||
| Norovirus | √GI/GII | √GII | √GI/GII | √ | √GI/GII | √GI/GII/IV | |
| Rotavirus | √A | √A | √A | √ | √A | √A | |
| Sapovirus | √ | √ (I, II, IV, V) | √ | √ (I, II, IV, V) | |||
| Parasites | |||||||
| Blastocystis hominis | √ | ||||||
| Cryptosporidium spp. | √ | √ | √ | √ | √ | ||
| Cyclospora cayetanensis | √ | √ | |||||
| Dientamoeba fragilis | √ | √ | √ | ||||
| Entamoeba histolytica | √ | √ | √ | √ | √ | ||
| Giardia lamblia | √ | √ | √ | √ | √ |
3.2.1. BioFire Gastrointestinal (GI) Panel
The BioFire Gastrointestinal (GI) Panel (BioFire, Salt Lake City, UT, USA) is a fully integrated system that allows for the simultaneous detection of a greater number of bacteria (13 pathogens), viruses (5 pathogens), and parasites (4 pathogens) than other assays (Table 2). This system simultaneously performs nucleic acid extraction, reverse transcription, amplification, and analysis within one hour. The technology is based on multiplex PCR amplification followed by endpoint melting curve data analysis. The main advantage of BioFire FilmArray is its comprehensive coverage of most the pathogen and low hands-on and turnaround time. The disadvantage of filmarray is low throughput and inability to separate bacterial, viral, or parasitic testing if needed from patients need, or billing point of view. A multicenter evaluation of BioFire GI Panel with conventional stool culture and molecular methods showed the FilmArray GI Panel sensitivity to be 100% for 12 of the 22 and >94.5% for an additional 7 of the 22 target pathogens tested. For the remaining 3 targets, sensitivity could not be calculated due to the low prevalence of the pathogens in the study [30].
3.2.2. Luminex Gastrointestinal Pathogen Panel (xTAG GPP)
The Luminex xTAG GPP (Luminex, Austin, TX, USA) is an FDA-approved assay which allows for the detection of 14 broad range of pathogens in a single test (9 bacterial, 3 viral, and 3 parasitic) (Table 2). Luminex xTAG GPP is not an integrated system and requires a separate nucleic acid extraction step, followed by multiplex PCR and reverse transcriptase PCR, hybridization to bead array, and detection by Luminex equipment. Luminex xTAG GPP test sensitivity is in between 90 and 100%, depending on pathogen present, and specificity in the range of 91 to 99% [31–33]. The main advantage of Luminex xTAG GPP is its high sample throughput and the ability to detect multiple pathogens. However, the major disadvantage is that it is not an integrated platform and requires separate nucleic acid extraction and post-PCR handling, which increases the potential of cross-contamination and false-positive results [31, 34].
3.2.3. Verigene Enteric Pathogen (EP) Test
The Verigene Enteric Pathogen (EP) (Luminex, Austin, TX, USA) is an integrated FDA-approved system for the simultaneous detection of common stool pathogens (Table 2). This system detects up to 5 bacterial (Campylobacter, Salmonella, Shigella, Vibrio, and Yersenia enterocolitica), Shiga Toxin 1 (stx1), Shiga Toxin 2 (stx2), and 2 viral pathogens (Norovirus and Rotavirus) and does not cover any of the parasitic pathogens. The Verigene platform uses a processor and reader which can simultaneously perform nucleic acid extraction, amplification, and hybridization to probes on a glass slide in a microarray format. The manufacturer reported sensitivities and specificities of the test are in the range of >91% and >99%, respectively, for the target organisms. A comparative study of Verigene EP test with BioFire FilmArray GI panel and Luminex xTAG GI panel showed this assay to be less sensitive and specific as compared to BioFire Array GI panel [35].
3.2.4. ProGastro SSCS Assay
The Prodesse ProGastro SSCS (Hologic, San Diego, CA, USA) is another commercially available FDA-approved assay that is used for the simultaneous detection of 4 bacterial pathogens (Campylobacter, Salmonella, Shigella, and Shiga toxin-producing E. coli (STEC) stx1 and stx2 genes) (Table 2). ProGastro SSCS is not an integrated system and requires a separate nucleic acid extraction step, followed by PCR amplification in SmartCycler (Cepheid, Sunnyvale, CA, USA) and data analysis. The overall sensitivity of this assay is 98.5% and specificity is in the range of 98.9% to 99.4%, depending on the target pathogen [1, 36].
3.2.5. BD Max Enteric and Extended Enteric Panels
The BD Max (Becton Dickinson, Sparks, MD, USA) is an integrated system that incorporates simultaneous sample preparation, nucleic acid extraction, amplification, and detection. BD Max microfluidic real-time PCR-based system batches up to 24 samples within 3 hours and required 2 minutes of hands-on time per sample. BD Max Enteric panel is an FDA-approved assay that can be used to detect 5 bacterial pathogens, i.e., Campylobacter, Salmonella, Shigella, Enteroinvasive E. coli (EIEC), and Shiga toxin-producing E. coli (STEC) stx1 and stx2 genes (Table 2). A comparison of BD Max enteric panel testing with the conventional culture method showed increased sensitivity and specificity in the detection of Campylobacter, Salmonella, Shigella, and STEC [37, 38]. The other BD Max panels include the extended enteric bacterial panel that can detect (Yersinia enterocolitica, V. cholera, V. parahaemolyticus, and V. vulnificus), viral panel (Adenovirus, Astrovirus, Norovirus, and Sapovirus), and parasite panel (C. parvum, C. hominis, Entamoeba histolytica, and Giardia lamblia) [39].
3.2.6. Allplex Gastrointestinal Panel
The Allplex Gastrointestinal Assays (Seegene, Seoul, South Korea) is a new CE-IVD marked multiplex real-time PCR assay that detects 13 bacteria, 5 viruses, and 6 parasites in 4 multiplex PCRs (Table 3). This assay uses the novel analytical multiple detection temperature (MuDT) technique which is able to detect multiple targets in a single fluorescence channel without melting curve analysis. The procedure involves separate nucleic acid extraction from stool samples, followed by multiplex real-time PCR using the CFX96TM real-time PCR system (Bio-Rad Laboratories, Richmond, CA, USA) and detection by data analysis. Two comparative studies with routine methods showed Allplex Gastrointestinal multiplex PCR assay to be more sensitive and specific as compared to traditional methods [40, 41]. A comparative evaluation and laboratory performance of Seegene Allplex Gastrointestinal with the conventional procedure and two other NAT methods showed this assay to have an overall 94% positive percent agreement for the detection of gastrointestinal pathogens [42].
3.2.7. Seeplex Diarrhea ACE Detection
The Seeplex Diarrhea ACE Detection kits (Seegene, Seoul, Korea) for Bacteria 1, Bacterial 2, and Virus are CE-IVD-approved panels, and these multiplex PCR-based kits allow the detection of common bacterial and viral pathogens [43]. This multiplex PCR assay enables simultaneous multipathogen detection of 9 bacteria, 4 viruses, and a C. difficile toxin-producing gene using three multiplex assays (Table 3). The test procedure includes separate nucleic acid extraction, reverse transcription followed by PCR, and product separation by capillary electrophoresis. The major disadvantages of these kits are that separate nucleic acid extraction is required and none of the parasitic pathogens can be detected. The sensitivity of these assays is in the range of 40–100%, and specificity is in the range of 96–100% depending on the pathogen present in the sample [43–45].
3.2.8. RIDA GENE Real-Time PCR Kits
The RIDA GENE gastrointestinal kits (R-Biopharm AG, Darmstadt, Germany) offers real-time PCR-based separate bacterial, viral, and parasitic panel that can detect a range of common pathogens (Table 3). These CE-IVD diagnostic tests require separate nucleic acid isolation procedure and can be performed on most commonly available real-time PCR equipment. A comparative study of RIDA GENE Bacterial Stool and two other molecular methods, the Fast Track Diagnostics (FTD) Bacterial Gastroenteritis Panel and the BD MAX Enteric Bacterial Panel, indicates RIDA GENE gastrointestinal to be more sensitive than culture methods for the detection of Campylobacter and Shigella species [46]. However, the sensitivity of RIDA GENE GI Kit for the detection of Salmonella spp. was found to be low at 25% as compared to the culture method [1, 46].
3.2.9. FTD Bacterial Gastroenteritis Panel
The Fast Track Bacterial GI panel (Fast Track Diagnostics, Junglinster, Luxembourg) is a CE-IVD marked two-tube multiplex real-time PCR test for the detection of pathogen genes by TaqMan technology using the ABI 7500 Fast instrument (Applied Biosystems, Foster City, CA, USA). This system requires separate nucleic acid extraction step. The first tube performs multiplex detection of Campylobacter coli/jejuni/lari and Enterohemorrhagic E. coli. Second tube performs multiplex PCR detection of Salmonella spp., Shigella/Enteroinvasive E. coli, Yersinia enterocolitica, and C. difficile. Validation and performance characteristics of these assays are determined by the manufacturer. One comparative study of FTD Bacterial GI panel, RIDA GENE GI, and BD Max showed FTD GI panel to be more sensitive than culture methods for the detection of Campylobacter and Shigella species. However, for Salmonella spp., FTD Bacterial GI panel showed a low sensitivity of 50% as compared to the culture method [46].
3.2.10. EntericBio Gastro Panels
The EntericBio Gastro panels (Serosep, Limerick, Ireland) are CE-IVD assays that offer several bacterial, viral, parasite, and combo panels that cover most of the enteropathogens (Table 3). Compared with the previous version and culture methods, the sensitivity and specificity of these assays are reported to be in the range of 100% and 97.8%, respectively [47, 48]. These assays require separate nucleic acid extraction, followed by real-time PCR amplification by LightCycler 480 II (Roche Diagnostics, Indianapolis, IN, USA) instrument and data analysis.
3.2.11. QIAstat-Dx Gastrointestinal Panel
The QIAstat GIP (Qiagen, Hilden, Germany) is a new multiplex PCR assay that can simultaneously detect and identify 24 gastroenteritis pathogens from stool samples in Cary-Blair transport medium (Table 3). QIAstat GIP is an integrated system and uses cartridge and QIAstat-Dx analyzer, in which nucleic acid extraction, real-time PCR amplification, and fluorescent amplicon detection are performed in a closed system. Manufacturer reports the overall assay sensitivity and specificity to be 97.9% and 97.8%, respectively. A multicenter comparative study of QIAstat GIP with BioFire FilmArray GIP and Seegene Allplex GIP indicates a good correlation and positive percent agreement of 98.2% with these other assays [49].
3.2.12. CLART EnteroBac
The CLART EnteroBac (Genomica, Madrid, Spain) is a PCR array-based system that simultaneously allows detection and identification of 8 bacterial pathogens (Table 3). The test procedure includes nucleic acid extraction, multiplex PCR amplification, microarray hybridization, and automated data analysis. The advantage of this assay is high throughput and disadvantage is that it does not detect any viral and parasitic pathogens. Validation and performance characteristics of this assay are performed by manufacturer, and there are limited independent studies available.
3.2.13. GastroFinder 2SMART
The GastroFinder 2SMART assay (PathoFinder, the Netherlands) is a CE-IVD real-time PCR-based assay which is able to detect 9 bacterial, 5 viral, and 4 parasitic pathogens causing gastrointestinal infection in one multiplex assay (Table 3). This is not an integrated assay and requires separate nucleic acid extraction followed by real-time PCR amplification and identification of organisms on the basis of melting curve analysis. Performance of this assay is evaluated by the manufacturer with limited independent studies.
The other non-FDA and non-CE-IVD assays are EasyScreen Enteric assay (Genetic Signature's, Sydney, Australia) and Faecal Pathogens M detection assay (AusDiagnostics, Mascot, Australia). The Genetic Signature EasyScreen Enteric assay uses company's 3base technology to convert all cytosine bases (C) in the starting nucleic acid samples to thymidine (T). The resulting reduction in sequence variation allows for a higher number of multiplex targets to be run under similar conditions. Separate panels are available to detect common bacterial, viral, parasitic pathogens, and C. difficile including hypervirulent 027 and 078 strains. Several studies have been performed on the detection and identification of Blastocystis spp., Cryptosporidium spp., Dientamoeba fragilis, Entamoeba spp., and Giardia lamblia in human clinical samples [50–52]. The EasyScreen Enteric Parasite Detection Kit exhibited 92–100% sensitivity and 100% specificity [53]. AusDiagnostics Faecal Pathogens M detection assay can detect 14 common bacterial, viral, and parasitic pathogens. Faecal Pathogens M detection assay uses multiplexed tandem PCR (MT-PCR) technique comprising of two amplification steps. In the first step, extracted nucleic acid is preamplified as a single well multiplex reaction. The amplified product in the first step is diluted, and second step multiplex real-time PCR is performed using SYBR green dye, and identification of organisms is performed by melting curve analysis.
3.2.14. Advantages of Molecular Testing
The main advantages of molecular testing are improved workflow and faster turnaround time with high sensitivity and specificity as compared to traditional methods. An additional advantage is the capability of multiplexing, which allows for the simultaneous detection of multiple enteric pathogens.
Multiplex assays can be particularly helpful for severely ill patients and in certain patient population where rapid diagnosis, treatment, and management decision are required. Multiplex molecular assays are helpful from the therapeutic point of view to avoid inappropriate and unnecessary antimicrobial treatment, for example, in case of Shiga toxin-producing E. coli (STEC) infection where antimicrobial exposure may increase the risk of patient developing hemolytic uremic syndrome (HUS). Multiplex NAAT assays can detect a variety of enteric pathogens, thus eliminating the need to stock special media and perform separate parasitology and virology testing. From public health and infection control point of view, rapid detection can be helpful in the infection control measure and prevention and spread of infections.
Because of the increased sensitivity and specificity and multiplex detection, several studies have reported increased rate in the detection of enteric pathogens as compared to traditional methods. Furthermore, there are reports of increased detection of multiple pathogens (more than one) from single specimens and in identifying coinfections [30, 49]. This increased detection of multiple pathogens may be beneficial and indicates coinfection. Multiplex gastrointestinal pathogen detection has been particularly found to be useful in one infection control study in which 22.2% of patients with negative conventional tests for C. difficile and/or rotavirus had the unsuspected gastrointestinal pathogen detected leading to more rational patient isolation and prevention of the nosocomial transmission [54].
3.2.15. Disadvantages of Molecular Testing
The main disadvantage of NAAT is the initial setup and cost, but in the longer run replacing conventional culture methods with molecular methods is mostly beneficial. Another disadvantage is that NAAT cannot differentiate between dead and living organisms and results need to be interpreted carefully depending on the patient condition [55]. Furthermore, depending on the patient, the physician's need, and public health department requirements, a custom culture may be necessary to identify the pathogen and perform antibiotic susceptibility. The availability of antibiotic susceptibility profile is very helpful especially in critical conditions to determine if antibiotic treatment is necessary and which antibiotic to be used. Submission of selected bacterial isolates to state public health laboratories is a requirement and plays an important in the public health surveillance, outbreak investigations, and monitoring the antibiotic susceptibilities. In order to fulfil regulations, it may be necessary for labs to communicate with state labs to get approved protocol for reporting in case of NAAT testing on stool samples. Based on these complexities and individual hospital/laboratory needs, a custom multiplex NAAT algorithm can be used to determine if further testing by conventional culture method is required (Figure 2). A custom culture for identification and susceptibility testing may be required for bacterial isolates, and further identification of pathogenic E. coli can be performed [56]. In general, multiplex NAAT procedure should not be used for C. difficile. However, if the patient is found to be positive for C. difficile using multiplex NAAT method, separate C. difficile algorithm should be followed. No further testing is required if a patient is positive for viral and parasitic pathogens by NAAT test, as usually it confirms infection.
Figure 2.

Multiplex NAAT algorithm for enteric pathogens.
In conclusion, NAAT-based technologies provide better options in the diagnosis of infectious gastroenteritis caused by a wide range of pathogens and overcoming some of the challenges faced in the traditional microbiological and culture methods. High throughput, sensitivity, and specificity of molecular-based testing allow rapid diagnosis, treatment, and management of gastrointestinal infections. With the improvement in the technology and availability of commercially available methods, traditional laboratory diagnostic techniques for the diagnosis of gastrointestinal infectious diseases have rapidly been replaced by these newer molecular methods.
Conflicts of Interest
The author has no financial relationships with any of the manufacturer or their products mentioned in this article and has not received any financial assistance. This article reviewed commonly available commercial products on which independent studies had been performed and it does to necessarily include all the available products. Furthermore, this article overviews mostly independent studies and does not reflect or endorse manufacturers' data and views.
References
- 1.Zboromyrska Y., Vila J. Advanced PCR-based molecular diagnosis of gastrointestinal infections: challenges and opportunities. Expert Review of Molecular Diagnostics. 2016;16(6):631–640. doi: 10.1586/14737159.2016.1167599. [DOI] [PubMed] [Google Scholar]
- 2.Fischer Walker C. L., Sack D., Black R. E. Etiology of diarrhea in older children, adolescents and adults: a systematic review. PLoS Neglected Tropical Diseases. 2010;4(8):p. e768. doi: 10.1371/journal.pntd.0000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diarrhoeal Disease. Key Facts. Geneva, Switzerland: World Health Orgaization Website; 2017. [Google Scholar]
- 4.Thielman N. M., Guerrant R. L. Acute infectious diarrhea. New England Journal of Medicine. 2004;350(1):38–47. doi: 10.1056/nejmcp031534. [DOI] [PubMed] [Google Scholar]
- 5.Guerrant R. L., Van Gilder T., Steiner T. S, et al. Practice guidelines for the management of infectious diarrhea. Clinical Infectious Diseases : 2001;32(3):331–351. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 6.Stockmann C., Pavia A. T., Graham B., et al. Detection of 23 gastrointestinal pathogens among children who present with diarrhea. Journal of the Pediatric Infectious Diseases Society. 2017;6(3):231–238. doi: 10.1093/jpids/piw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz D. E., Taylor D. N. Parasitic infections of the gastrointestinal tract. Gastroenterology Clinics of North America. 2001;30(3):797–815. doi: 10.1016/s0889-8553(05)70211-9. [DOI] [PubMed] [Google Scholar]
- 8.Bennett W. E., Jr., Tarr P. I. Enteric infections and diagnostic testing. Current Opinion in Gastroenterology. 2009;25(1):1–7. doi: 10.1097/mog.0b013e32831ba094. [DOI] [PubMed] [Google Scholar]
- 9.Elsafi S. H., Al-Maqati T. N., Hussein M. I., Adam A. A., Hassan M. M. A., Al Zahrani E. M. Comparison of microscopy, rapid immunoassay, and molecular techniques for the detection of Giardia lamblia and Cryptosporidium parvum. Parasitology Research. 2013;112(4):1641–1646. doi: 10.1007/s00436-013-3319-1. [DOI] [PubMed] [Google Scholar]
- 10.Daniel-Wayman S., Fahle G., Palmore T., Green K. Y., Prevots D. R. Norovirus, astrovirus, and sapovirus among immunocompromised patients at a tertiary care research hospital. Diagnostic Microbiology and Infectious Disease. 2018;92(2):143–146. doi: 10.1016/j.diagmicrobio.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramanan P., Espy M. J., Khare R., Binnicker M. J. Detection and differentiation of norovirus genogroups I and II from clinical stool specimens using real-time PCR. Diagnostic Microbiology and Infectious Disease. 2017;87(4):325–327. doi: 10.1016/j.diagmicrobio.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Morris K. A., Davies K., Wilcox M. H. Impact of Clostridium difficile toxin gene PCR result on decisions to de-isolate patients: do the ends justify the means? Journal of Infection Prevention. 2018;19(3):138–140. doi: 10.1177/1757177418755309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanzas C., Dubberke E. R., Lu Z., Reske K. A., Gröhn Y. T. Epidemiological model for Clostridium difficile transmission in healthcare settings. Infection Control & Hospital Epidemiology. 2011;32(6):553–561. doi: 10.1086/660013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akbari M., Vodonos A., Silva G., et al. The impact of PCR on Clostridium difficile detection and clinical outcomes. Journal of Medical Microbiology. 2015;64(9):1082–1086. doi: 10.1099/jmm.0.000126. [DOI] [PubMed] [Google Scholar]
- 15.Furuya-Kanamori L., Ascenzi P., Siarakas S., Petrosillo N., Masi A. D. Asymptomatic Clostridium difficile colonization: epidemiology and clinical implications. BMC Infectious Diseases. 2015;15:p. 516. doi: 10.1186/s12879-015-1258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Bella S., Ascenzi P., Siarakas S., Petrosillo N., Masi A. D. Clostridium difficile toxins A and B: insights into pathogenic properties and extraintestinal effects. Toxins. 2016;8(5) doi: 10.3390/toxins8050134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies K. A., Helen A., Christopher M. L., David A., Georgina L. D., Mark H. W. Diversity of Clostridium difficile PCR ribotypes in Europe: results from the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID), 2012 and 2013. Eurosurveillance. 2016;21(29) doi: 10.2807/1560-7917.es.2016.21.29.30294. [DOI] [PubMed] [Google Scholar]
- 18.Valiente E., Cairns M. D., Wren B. W. The Clostridium difficile PCR ribotype 027 lineage: a pathogen on the move. Clinical Microbiology and Infection. 2014;20(5):396–404. doi: 10.1111/1469-0691.12619. [DOI] [PubMed] [Google Scholar]
- 19.Fatima R., Aziz M. The hypervirulent strain of Clostridium difficile: NAP1/B1/027-a brief overview. Cureus. 2019;11(1):p. e3977. doi: 10.7759/cureus.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald L. C., Gerding D. N., Johnson S., et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and society for healthcare epidemiology of America (SHEA) Clinical Infectious Diseases. 2018;66(7):987–994. doi: 10.1093/cid/ciy149. [DOI] [PubMed] [Google Scholar]
- 21.Vasoo S., Stevens J., Portillo L., et al. Cost-effectiveness of a modified two-step algorithm using a combined glutamate dehydrogenase/toxin enzyme immunoassay and real-time PCR for the diagnosis of Clostridium difficile infection. Journal of Microbiology, Immunology and Infection. 2014;47(1):75–78. doi: 10.1016/j.jmii.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Schultz K., Bennett E. S., Marx A., et al. Preventable patient harm: a multidisciplinary, bundled approach to reducing Clostridium difficile infections while using a glutamate dehydrogenase/toxin immunochromatographic assay/nucleic acid amplification test diagnostic algorithm. Journal of Clinical Microbiology. 2018;56(9) doi: 10.1128/jcm.00625-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deak E., Miller S. A., Humphries R. M. Comparison of illumigene, simplexa, and amplivue Clostridium difficile molecular assays for diagnosis of C. difficile infection. Journal of Clinical Microbiology. 2014;52(3):960–963. doi: 10.1128/jcm.02354-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong G., Park K. S., Ki C.-S., Lee N. Y. Evaluation of the illumigene C. difficile assay for toxigenic Clostridium difficile detection: a prospective study of 302 consecutive clinical fecal samples. Diagnostic Microbiology and Infectious Disease. 2014;80(3):177–180. doi: 10.1016/j.diagmicrobio.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Gilbreath J. J., Verma P., Abbott A. N., Butler-Wu S. M. Comparison of the verigene Clostridium difficile, Simplexa C. difficile universal direct, BD MAX Cdiff, and xpert C. difficile assays for the detection of toxigenic C. difficile. Diagnostic Microbiology and Infectious Disease. 2014;80(1):13–18. doi: 10.1016/j.diagmicrobio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Paitan Y., Miller-Roll T., Adler A. Comparative performance study of six commercial molecular assays for rapid detection of toxigenic Clostridium difficile. Clinical Microbiology and Infection. 2017;23(8):567–572. doi: 10.1016/j.cmi.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Peterson L. R., Young S. A., Davis T. E., et al. Evaluation of the cobas Cdiff test for detection of toxigenic Clostridium difficile in stool samples. Journal of Clinical Microbiology. 2017;55(12):3426–3436. doi: 10.1128/jcm.01135-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granato P. A., Hansen G., Herding E., et al. Performance comparison of the cobas Liat and Cepheid GeneXpert systems for Clostridium difficile detection. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0200498.e0200498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosai K., Iwanaga Y., Akamatsu N., et al. Performance evaluation of the Verigene ® Clostridium difficile nucleic acid test, an automated multiplex molecular testing system for detection of C. difficile toxin. Journal of Infection and Chemotherapy. 2017;23(10):674–677. doi: 10.1016/j.jiac.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Buss S. N., Leber A., Chapin K., et al. Multicenter evaluation of the biofire filmarray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. Journal of Clinical Microbiology. 2015;53(3):915–925. doi: 10.1128/jcm.02674-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wessels E., Rusman L. G., van Bussel M. J. A. W. M., Claas E. C. J. Added value of multiplex luminex gastrointestinal pathogen panel (xTAG GPP) testing in the diagnosis of infectious gastroenteritis. Clinical Microbiology and Infection. 2014;20(3):O182–O187. doi: 10.1111/1469-0691.12364. [DOI] [PubMed] [Google Scholar]
- 32.Claas E. C. Performance of the xTAG gastrointestinal pathogen panel, a multiplex molecular assay for simultaneous detection of bacterial, viral, and parasitic causes of infectious gastroenteritis. Journal of Microbiology and Biotechnology. 2013;23(7):1041–1045. doi: 10.4014/jmb.1212.12042. [DOI] [PubMed] [Google Scholar]
- 33.Navidad J. F., Griswold D. J., Gradus M. S., Bhattacharyya S. Evaluation of Luminex xTAG gastrointestinal pathogen analyte-specific reagents for high-throughput, simultaneous detection of bacteria, viruses, and parasites of clinical and public health importance. Journal of Clinical Microbiology. 2013;51(9):3018–3024. doi: 10.1128/jcm.00896-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddington K., Tuite N., Minogue E., Barry T. A current overview of commercially available nucleic acid diagnostics approaches to detect and identify human gastroenteritis pathogens. Biomolecular Detection and Quantification. 2014;1(1):3–7. doi: 10.1016/j.bdq.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang R. S. P., Johnson C. L., Pritchard L., Hepler R., Ton T. T., Dunn J. J. Performance of the Verigene ® enteric pathogens test, Biofire FilmArray™ gastrointestinal panel and Luminex xTAG ® gastrointestinal pathogen panel for detection of common enteric pathogens. Diagnostic Microbiology and Infectious Disease. 2016;86(4):336–339. doi: 10.1016/j.diagmicrobio.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Buchan B. W., Olson W. J., Pezewski M., et al. Clinical evaluation of a real-time PCR assay for identification of Salmonella, Shigella, Campylobacter (Campylobacter jejuni and C. coli), and shiga toxin-producing Escherichia coli isolates in stool specimens. Journal of Clinical Microbiology. 2013;51(12):4001–4007. doi: 10.1128/jcm.02056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knabl L., Grutsch I., Orth-Höller D. Comparison of the BD MAX ® enteric bacterial panel assay with conventional diagnostic procedures in diarrheal stool samples. European Journal of Clinical Microbiology & Infectious Diseases. 2016;35(1):131–136. doi: 10.1007/s10096-015-2517-4. [DOI] [PubMed] [Google Scholar]
- 38.DeBurger B., Sarah H., Eleanor A. P., Cindi V., Joel E. M. Utilizing BD MAX enteric bacterial panel to detect stool pathogens from rectal swabs. BMC Clinical Pathology. 2017;17:p. 7. doi: 10.1186/s12907-017-0043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeBurger B. A., Hanna S., Mortensen J. E. Evaluation of alternate parasite transport systems for the BD MAX enteric parasite panel. Diagnostic Microbiology and Infectious Disease. 2018;92(3):204–205. doi: 10.1016/j.diagmicrobio.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Martín A., Pérez-Ayala A., Chaves F., Lora D., Orellana M. Á. Evaluation of the multiplex PCR Allplex-GI assay in the detection of bacterial pathogens in diarrheic stool samples. Journal of Microbiological Methods. 2018;144:33–36. doi: 10.1016/j.mimet.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Amrud K., Slinger R., Sant N., Desjardins M., Toye B. A comparison of the Allplex ™ bacterial and viral assays to conventional methods for detection of gastroenteritis agents. BMC Research Notes. 2018;11(1):p. 514. doi: 10.1186/s13104-018-3645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoo J., Park J., Lee H. K., et al. Comparative evaluation of Seegene allplex gastrointestinal, luminex xTAG gastrointestinal pathogen panel, and BD MAX enteric assays for detection of gastrointestinal pathogens in clinical stool specimens. Archives of Pathology & Laboratory Medicine. 2019;143(8):999–1005. doi: 10.5858/arpa.2018-0002-oa. [DOI] [PubMed] [Google Scholar]
- 43.Coupland L. J., Mcelarney I., Meader E., et al. Simultaneous detection of viral and bacterial enteric pathogens using the Seeplex ® Diarrhea ACE detection system. Epidemiology and Infection. 2013;141(10):2111–2121. doi: 10.1017/s0950268812002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higgins R. R., Beniprashad M., Cardona M., Masney S., Low D. E., Gubbay J. B. Evaluation and verification of the Seeplex Diarrhea-V ACE assay for simultaneous detection of adenovirus, rotavirus, and norovirus genogroups I and II in clinical stool specimens. Journal of Clinical Microbiology. 2011;49(9):3154–3162. doi: 10.1128/jcm.00599-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onori M., Coltella L., Mancinelli L., et al. Evaluation of a multiplex PCR assay for simultaneous detection of bacterial and viral enteropathogens in stool samples of paediatric patients. Diagnostic Microbiology and Infectious Disease. 2014;79(2):149–154. doi: 10.1016/j.diagmicrobio.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Biswas J. S., Al-Ali A., Rajput P., Smith D., Goldenberg S. D. A parallel diagnostic accuracy study of three molecular panels for the detection of bacterial gastroenteritis. European Journal of Clinical Microbiology & Infectious Diseases. 2014;33(11):2075–2081. doi: 10.1007/s10096-014-2177-9. [DOI] [PubMed] [Google Scholar]
- 47.Koziel M., Kiely R., Blake L., et al. Improved detection of bacterial pathogens in patients presenting with gastroenteritis by use of the entericbio real-time gastro panel I assay. Journal of Clinical Microbiology. 2013;51(8):2679–2685. doi: 10.1128/jcm.00809-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koziel M., Corcoran D., O’Callaghan I., Sleator R. D., Lucey B. Validation of the EntericBio Panel II multiplex polymerase chain reaction system for detection of Campylobacter spp., Salmonella spp., Shigella spp., and verotoxigenic E. coli for use in a clinical diagnostic setting. Diagnostic Microbiology and Infectious Disease. 2013;75(1):46–49. doi: 10.1016/j.diagmicrobio.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Hannet I., Anne L. E., Josep P., et al. Multicenter evaluation of the new QIAstat gastrointestinal panel for the rapid syndromic testing of acute gastroenteritis. European Journal of Clinical Microbiology & Infectious Diseases. 2019;38 doi: 10.1007/s10096-019-03646-4. [DOI] [PubMed] [Google Scholar]
- 50.Gough R., Ellis J., Stark D. Comparison and recommendations for use of Dientamoeba fragilis real-time PCR assays. Journal of Clinical Microbiology. 2019;57(5) doi: 10.1128/jcm.01466-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dirani G., Zannoli S., Paesini E., et al. Easyscreen enteric Protozoa assay for the detection of intestinal parasites: a retrospective bi-center study. Journal of Parasitology. 2019;105(1):58–63. doi: 10.1645/18-52. [DOI] [PubMed] [Google Scholar]
- 52.Cao M., Ellis J. T., Marriott D., Harkness J., Stark D. Evaluation of the easy screen Protozoan detection kit for the diagnosis of Entamoeba histolytica. Pathology. 2019;51(4):426–428. doi: 10.1016/j.pathol.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Stark D., Roberts T., Ellis J. T., Marriott D., Harkness J. Evaluation of the EasyScreen ™ enteric parasite detection kit for the detection of Blastocystis spp., Cryptosporidium spp., Dientamoeba fragilis, Entamoeba complex, and Giardia intestinalis from clinical stool samples. Diagnostic Microbiology and Infectious Disease. 2014;78(2):149–152. doi: 10.1016/j.diagmicrobio.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Rand K. H., Tremblay E. E., Hoidal M., Fisher L. B., Grau K. R., Karst S. M. Multiplex gastrointestinal pathogen panels: implications for infection control. Diagnostic Microbiology and Infectious Disease. 2015;82(2):154–157. doi: 10.1016/j.diagmicrobio.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Momcilovic S., Cantacessi C., Arsić-Arsenijević V., Otranto D., Otašević S. T. Rapid diagnosis of parasitic diseases: current scenario and future needs. Clinical Microbiology and Infection. 2019;25(3):290–309. doi: 10.1016/j.cmi.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 56.Wild D. Next Step? The switch from stool culture to PCR. CAP Today. 2018;52:p. 54. [Google Scholar]


