Abstract
In this study, a quantitative 1H NMR method (1H-qNMR) for determining the contents of imperatorin, byakangelicin, and oxypeucedanin in A. dahurica in traditional Chinese medicine (TCM) has been established. Dried plant material was extracted exhaustively with methanol by an ultrasonication-assisted extraction method. The 1H-qNMR measurements were performed on a 600 -MHz spectrometer with hydroquinone as the internal standard reference in deuterated dimethyl sulfoxide (DMSO-d6) solvent. Quantification was carried out using the 1H resonance signals at 6.55 ppm for hydroquinone and 7.68, 7.38-7.39, and 6.38-6.39 ppm for imperatorin, byakangelicin, and oxypeucedanin, respectively. The linearity, limit of detection (LOD), limit of quantitation (LOQ), precision, reproducibility, stability, and recovery of the methodology were evaluated, and results were good. The newly developed method has been applied to determine the three coumarins in A. dahurica.
1. Introduction
A. dahurica is a perennial medicinal plant belonging to the dry root of the umbelliferous plant A. dahurica or A. dahurica var. formosana [1]. It was first published in Shennong's Herbal classics and listed as a middle product in China and has a long history of dual use of medicine and food [2]. A. dahurica is warm, fragrant, spicy, and slightly bitter, which has the effect of relieving stuffy nose, dissipating cold, expelling wind and acesodyne, removing dampness, clearing swelling and excluding pus, spasmolysis, analgesia, relieving asthma, anti-inflammatory immunomodulation, and skin whitening. In clinical practice, it is widely applied in the treatment of common cold, headache, nasal obstruction, rhinorrhea, toothache, leucorrhea, acne and carbuncles, rheumatism, and especially for the headache caused by wind-cold invading Yang and Yin with obvious curative effect [3–9]. Except for medicinal use, it is also widely used in food, health-care products, spices, skin care and beauty, daily chemical industry, and other aspects. In particular, its dry root can be used as an important condiment and spice to increase fragrance and taste, deodorize or remove odors, and increase appetite as well [10–12].
A. dahurica mainly contains coumarins, volatile oils, polysaccharides, and trace elements as the bioactive components [13]. Coumarins consist of imperatorin, isoimperatorin, bergapten, oxypeucedanin, byakangelicin, oxypeucedanin hydrate, and cnidilin [14–16]. These compounds possess multiple biological properties [17]. As imperatorin has been documented to have versatile pharmacological effects, for example anti-inflammatory, antineoplastic, hepatoprotective, photosensitive activity, and anticonvulsant [18, 19]. Isoimperatorin is a secondary plant metabolite that possesses multiple pharmacological properties, including fighting cancer-inducing substances, analgesic, and antiviral [20, 21]. Bergapten presents in the plants of Umbelliferae family and is widely used for its medicinal values such as anticoagulant, anti-inflammatory, and antiproliferative [22–24]. Byakangelicin is considered to be a natural potent inhibitor for aldose reductase and may be applied to the development of treatment for diabetic cataract [25]. Oxypeucedanin has been reported to have antimutagenic effects, cause uterus contraction, increase blood pressure, and have anticancer effects [26]. Therefore, the content of imperatorin, byakangelicin, oxypeucedanin, isoimperatorin, and bergapten in A. dahurica was determined in this study.
Until now, the HPLC method is often adopted for the determination of coumarins, which has disadvantages such as time consumption, complex sample pretreatment, and expensive standard substance needed to obtain. Therefore, it is practically significant to establish a rapid and reliable method for the determination of coumarins in A. dahurica [27–32]. As known to us, the quantitative 1H NMR method (1H-qNMR) can effectively characterize compound mixtures and quantify its constituents [33]. It has been widely and successfully applied for the quantitative analysis of chemical drugs, traditional Chinese medicine and plant extracts, body fluid samples, isomers, food, etc. [34, 35]. This method has the advantages of short measuring times, not requiring a high-purity reference standard for accurate quantification of the compounds of interest, the simplicity of the method, the ease of sample preparation, lower solvent usage, and being rapid [36–41].
In this study, a new method for the determination of imperatorin, byakangelicin, and oxypeucedanin in A. dahurica was established by using 1H-qNMR. Furthermore, the A. dahurica samples were analyzed, and it provides a theoretical and scientific basis for the quality control and evaluation of A. dahurica.
2. Experimental
2.1. General
The reference standard (RS) of imperatorin, oxypeucedanin, and isoimperatorin was purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). The reference standard (RS) of bergapten and byakangelicin was purchased from Chengdu Pufei De Biotechnology Co. Ltd. and Chengdu Ruifensi Biotechnology Co., Ltd. (Chengdu, China). The purities of all standard substances were greater than 98%. The internal standard (IS) hydroquinone was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China), and its purity was greater than 99%. Methanol was purchased from Tianjin Fuyu Fine Chemical Co. Ltd. (Tianjin, China). DMSO-d6 was purchased from Cambridge Isotope Laboratories, Inc.
The name and grade of the balance the authors used for weighing materials are electronic balance and II.
2.2. Plant Material
The plant material in the current study was bought from different origins of A. dahurica (China) from 20 to 30th October 2018 and identified by Professor Yuan Chen from the department of cultivation and identification of Chinese herbal medicine, Gansu Agricultural University. Prior to the analyses, the plant material was powdered using a blender and was sieved (40 order).
2.3. Preparation of Solution
2.3.1. Preparation of Inner Standard Solution
The internal standard was dissolved in DMSO-d6.
2.3.2. Preparation of Standard Solution
A mixed stock solution containing reference standards (imperatorin, byakangelicin, oxypeucedanin, isoimperatorin, and bergapten) was dissolved in the inner standard solution.
2.4. Sample Preparation for 1H-qNMR Analysis
Dried plant material (25 mg) was extracted exhaustively with methanol (2 × 125 mL) by using an ultrasonic extractor (40 min, 40°C), and the combined extracts were evaporated in a water bath and dried in a desiccator and then resolved in the inner standard solution (60 mg × 0.5 mL). The extraction was performed in triplicate for every plant material, and the NMR analysis was run in triplicate for every extract.
2.5. 1H NMR Spectroscopy
1H NMR spectra were acquired with a 600-MHz NMR spectrometer with a 5-mm probe. All data were processed using MestReNova software, unless otherwise stated. The following parameters were used for acquisition of spectra of a spectral width, 11904 Hz; acquisition time, 2.8 s; relaxation delay, 50 s; pulse width, 10 s; 16 scans; and temperature, 293.6 K. In addition, the influence of different relaxation delays 1 s, 5 s, 10 s, 15 s, 20 s, 50 s, and 100 s on the integral area was verified (Table 1). From the results of the integral area, there were almost no difference and no influence on the quantitative value. In this work, 50 s was chosen as the relaxation delay.
Table 1.
Effect of relaxation delay on the integral area.
| Relaxation delay (s) | 1 | 5 | 10 | 20 | 50 | 100 |
| Integral area | 0.71 | 0.7 | 0.68 | 0.71 | 0.69 | 0.69 |
2.6. Validation
The analytical method was validated by the determination of the selectivity, linearity, limit of detection, limit of quantitation, precision, repeatability, stability, and recovery.
The selectivity was assessed by visual comparison between 1H NMR spectra of A. dahurica sample with the internal standard and reference standard.
The precision tests were performed by six replicate measurements of the reference standards with relative standard deviation (RSD) values considered as a measure of precision.
The repeatability was determined by six sample solutions (Suining sample, nonsulfur) with RSD values considered as a measure of repeatability.
To test the linearity, solutions with different concentrations (between 0.4 and 4 mg/mL) of the reference standards were prepared with the inner standard solution. The linearity was confirmed using the integral area ratio (y) and the mass ratio (x) of the standard and internal standard.
The limit of detection and quantitation can be determined by LOD = 3.3σ/s, LOQ = 10σ/s. σ shows deviation of the y-intercept of the nonzero intercept linear regression curve, and s shows the slope of the nonzero intercept linear regression curve.
The stability was determined by the same sample (Suining sample, nonsulfur) within 24 h with (RSD) values considered as a measure of stability.
Six samples of the tested content were added into the control solution of imperatorin, byakangelicin, and oxypeucedanin. The content of three coumarins in A. dahurica was determined using the developed method.
3. Results and Discussion
3.1. Selection of Solvent and Internal Standard
The suitable deuterium-substituted solvent should have good solubility for the samples and the internal standard. The internal standard should have a sharp single peak, which is easy to recognize, and the spectrum peak should not overlap with the peak to be tested. Through the preliminary experiment, DMSO-d6 was selected as the solvent and hydroquinone was selected as the internal standard. Quantification was carried out using the signals at 6.55, 7.68, 7.38-7.39, 6.38-6.39, 4.98-4.99, 4.26, 3.33, and 2.5 ppm for hydroquinone, imperatorin, byakangelicin, oxypeucedanin, isoimperatorin bergapten, H2O, and DMSO-d6 (standard reference), respectively (Figures 1 and 2).
Figure 1.
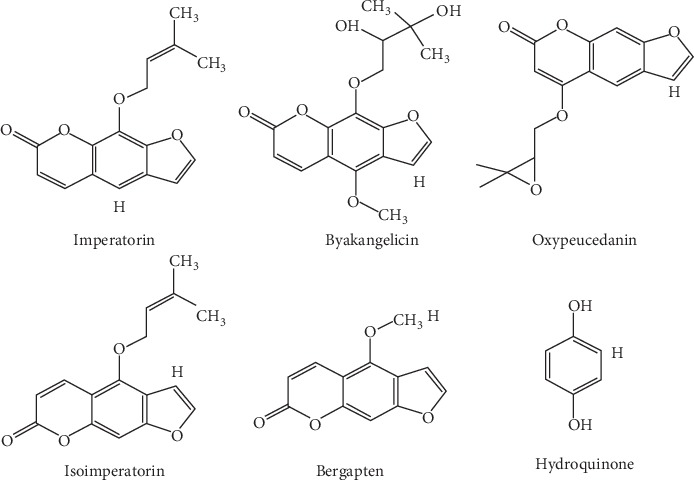
Structures of the internal standard and the reference standard.
Figure 2.
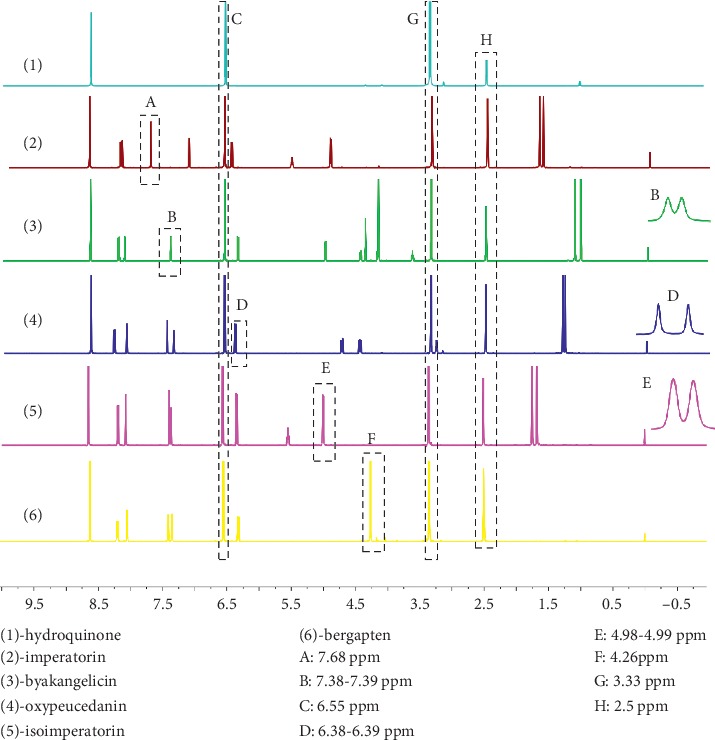
1H NMR spectrum of the internal standard and the reference standard in the DMSO-d6 solvent: (1) hydroquinone, (2) imperatorin, (3) byakangelicin, (4) oxypeucedanin, (5) isoimperatorin, and (6) bergapten; (A) 7.68 ppm, (B) 7.38-7.39 ppm, (C) 6.55 ppm, (D) 6.38-6.39 ppm, (E) 4.98-4.99 ppm, (F) 4.26 ppm, (G) 3.33 ppm, and (H) 2.5 ppm.
3.2. Validation Studies
Validation of the developed procedure was performed in terms of selectivity, linearity, precision, repeatability, stability, and recovery.
Assignments were verified by comparison with the spectra of the reference standards. Signals of the quantified compounds selected for integration did not overlap with the signals from the same molecule-related constituents, solvents, or the internal standard.
The coefficient of determination (R2) obtained from the calibration curve construction (the integral area ratio and the mass ratio of the standard and internal standard) was 0.9994, 0.9992, and 0.9992 for imperatorin, byakangelicin, and oxypeucedanin, respectively. Therefore, the constructed analytical curves presented a satisfactory linearity (Table 2).
Table 2.
Standard curves of three components in A. dahurica.
| Compound | Regression equation | r | Linearity (mg) |
|---|---|---|---|
| Imperatorin | Y = 0.1081X − 0.014 | 0.9995 | 0.2∼2 |
| Byakangelicin | Y = 0.08X − 0.0065 | 0.9992 | 0.2∼2 |
| Oxypeucedanin | Y = 0.1216X − 0.0147 | 0.9991 | 0.2∼2 |
The calculation shows that the detection and quantitation limit were 0.173 mg/mL and 0.524 mg/mL for imperatorin, 0.124 mg/mL and 0.376 mg/mL for byakangelicin, and 0.149 mg/mL and 0.452 mg/mL for oxypeucedanin, respectively.
In this study, the precision and repeatability of method are good, and the sample solutions were stable within 24 h. The results are shown in Table 3.
Table 3.
Precision, repeatability, and stability of three coumarins in A. dahurica by using the 1H-qNMR method (unit: %).
| Component | Precision | Repeatability | Stability |
|---|---|---|---|
| Imperatorin | 0.848 | 1.258 | 1.639 |
| Byakangelicin | 0.751 | 1.892 | 1.692 |
| Oxypeucedanin | 0.675 | 1.202 | 1.263 |
The recovery of the three coumarins was good in this work, which is shown in Table 4.
Table 4.
Recovery (%) of the coumarins in A. dahurica by the using the 1H-qNMR method.
| Component | Content for the sample (mg) | Standard addition (mg) | Experimental value (mg) | Recovery (%) | Average recover (%) | RSD (%) |
|---|---|---|---|---|---|---|
| Imperatorin | 1.214 | 1.217 | 2.422 | 99.297 | 97.504 | 1.300 |
| 1.214 | 1.217 | 2.406 | 97.952 | |||
| 1.214 | 1.217 | 2.406 | 97.952 | |||
| 1.214 | 1.217 | 2.373 | 95.263 | |||
| 1.214 | 1.217 | 2.373 | 95.263 | |||
| 1.214 | 1.217 | 2.406 | 97.952 | |||
|
| ||||||
| Byakangelicin | 0.675 | 0.700 | 1.377 | 100.263 | 98.817 | 1.463 |
| 0.675 | 0.700 | 1.356 | 97.371 | |||
| 0.675 | 0.700 | 1.356 | 97.371 | |||
| 0.675 | 0.700 | 1.377 | 100.263 | |||
| 0.675 | 0.700 | 1.356 | 97.371 | |||
| 0.675 | 0.700 | 1.356 | 97.371 | |||
|
| ||||||
| Oxypeucedanin | 0.968 | 1.000 | 1.907 | 93.904 | 93.038 | 0.932 |
| 0.968 | 1.000 | 1.889 | 92.171 | |||
| 0.968 | 1.000 | 1.907 | 93.904 | |||
| 0.968 | 1.000 | 1.889 | 92.171 | |||
| 0.968 | 1.000 | 1.889 | 92.171 | |||
| 0.968 | 1.000 | 1.924 | 95.638 | |||
3.3. Quantitative Results
Using the developed method, the content of three coumarins in A. dahurica was determined by using 1H-qNMR for the first time (Table 5 and Figure 3).
Table 5.
Content (%) of three coumarins in A. dahurica by using the 1H-qNMR (unit: %).
| Sample | Job operation | Component | ||
|---|---|---|---|---|
| Imperatorin | Byakangelicin | Oxypeucedanin | ||
| Suining Sichuan | — | 0.239 | 0.133 | 0.191 |
| Suining Sichuan | Stove drying | 0.132 | 0.175 | 0.219 |
| Xinqiao Sichuan | — | 0.195 | 0.120 | 0.192 |
| Daying Sichuan | — | 0.221 | 0.117 | 0.173 |
| Xinsheng Sichuan | — | 0.207 | 0.125 | 0.186 |
| Anhui | — | 0.208 | 0.257 | 0.234 |
| Bozhou Anhui | Stove drying | 0.191 | 0.287 | 0.353 |
| Xiaoying Hebei | Sun drying | 0.363 | 0.236 | 0.337 |
| Yangjiaying Hebei | Sun drying | 0.392 | 0.315 | 0.335 |
| Mengzhou Henan | Stove drying | 0.093 | 0.219 | 0.190 |
| Heze Shandong | Stove drying | 0.132 | 0.187 | 0.217 |
“—” denotes unknown.
Figure 3.
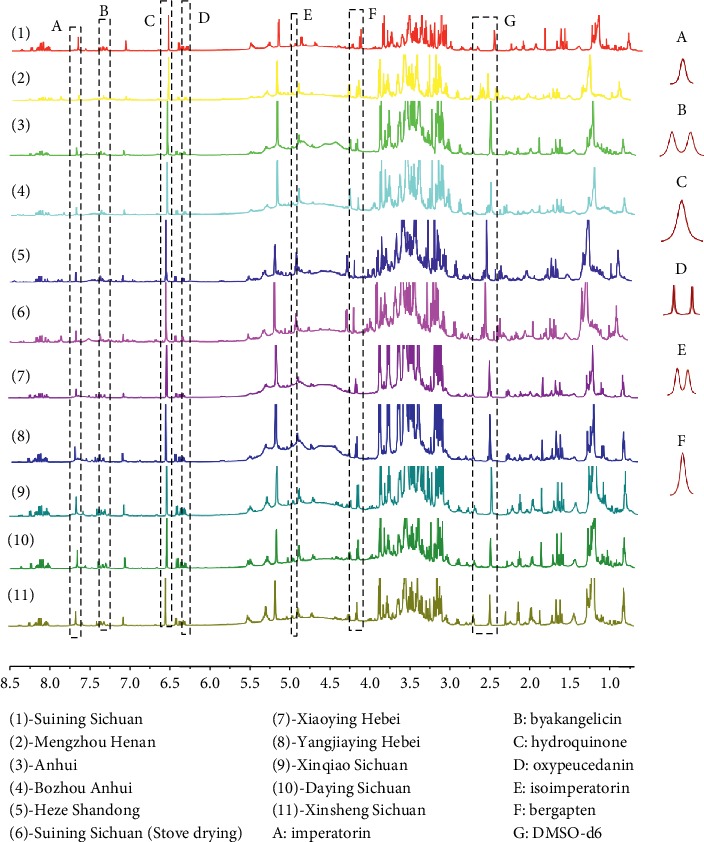
1H NMR spectrum of A. dahurica in the DMSO-d6 solvent: (1) Suining Sichuan, (2) Mengzhou Henan, (3) Anhui, (4) Bozhou Anhui, (5) Heze Shandong, (6) Suining Sichuan (stove drying), (7) Xiaoying Hebei, (8) Yangjiaying Hebei, (9) Xinqiao Sichuan, (10)- Daying Sichuan, and (11) Xinsheng Sichuan; (A) imperatorin, (B) byakangelicin, (C) hydroquinone, (D) oxypeucedanin, (E) isoimperatorin, (F) bergapten, and (G) DMSO-d6.
As can be seen from Figure 3, the base lines around the signals of isoimperatorin (E) and bergapten (F) were not flat, so we did not use the signals at 4.98-4.99 and 4.26 ppm for qNMR measurement. But it can be determined that they are the signal peaks of isoimperatorin and bergapten.
4. Conclusion
In this study, the 1H-qNMR methodology was developed for determining the content of three coumarins in A. dahurica. This work provided a fast, simple, and validated method for the quality control of A. dahurica.
Acknowledgments
This work was supported by the Discipline Construction Fund Project of Gansu Agricultural University (GSAU-XKJS-2018-086), the National Natural Science Foundation of China (31860102), the Natural Science Foundation of Gansu Province (18JR3RA185), and Research Program Sponsored by Gansu Provincial Key Laboratory of Aridland Crop Science, Gansu Agricultural University (No. GSCS- 2018-3).
Contributor Information
Qian Li, Email: liqian1984@gsau.edu.cn.
Daiyu Qiu, Email: qiudy@gsau.edu.cn.
Data Availability
The original 1HNMR spectral data and the analysis method of the data used to support the findings of this study are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Zhao L., Cao H. Optimization of extraction process of active ingredients from Chinese medicine Angelica dahurica and its quality research. Progress in Modern Biomedicine. 2011;11(14):2759–2766. in Chinese. [Google Scholar]
- 2.Cui Q. B., Zhang Y., Lan S. Basic research on the analgesic effects of Angelica dahurica. Chinese Journal of Experimental Traditional Medical Formulae. 2010;16(12):102–104. in Chinese. [Google Scholar]
- 3.Zhou S. M. Study on extraction of coumarin from Angelica dahurica and antimicrobial activities. The Food Industry. 2014;35(3):141–144. [Google Scholar]
- 4.Wang F., Wang C. Study on the relationship between the anti-aging function and antioxidation of ethanol extract from Angelica dahurica radix. China Pharmacy. 2012;23(7):599–602. [Google Scholar]
- 5.Wei W., Xu W., Yang X. W., He Z. Chemical constituents from ethyl acetate soluble parts in roots of Angelica dahurica var. formosana. Chinese Traditional and Herbal Drugs. 2016;47(15):2606–2613. [Google Scholar]
- 6.Liang W.-H., Chang T.-W., Charng Y.-C. Effects of drying methods on contents of bioactive compounds and antioxidant activities of Angelica dahurica. Food Science and Biotechnology. 2018;27(4):1085–1092. doi: 10.1007/s10068-018-0359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Y., Lu X., Peng W., Deng W., Ma Y. Study on the influence of sulfur fumigation on chemical constituents of. Tropical Journal of Pharmaceutical Research. 2015;14(5):p. 815. doi: 10.4314/tjpr.v14i5.11. [DOI] [Google Scholar]
- 8.Kang J., Zhou L., Sun J., Han J., Guo D. A. Chromatographic fingerprint analysis and characterization of furocoumarins in the roots of Angelica dahurica by HPLC/DAD/ESI-MSn technique. Journal of Pharmaceutical and Biomedical Analysis. 2008;47(4-5):778–785. doi: 10.1016/j.jpba.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Wang J. Y., Wang H. L., Zhang H. L., Liu Z. H., Ma C. Y., Kang W. Y. Immunomodulation of ADPs-1a and ADPs-3a on RAW264.7 cells through NF-κB/MAPK signaling pathway. International Journal of Biological Macromolecules. 2019;132:1024–1030. doi: 10.1016/j.ijbiomac.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 10.Deng G. G., Cui Z. J., Yang X. W. Chemical constituents from polarity part in roots of Angelica dahurica var. formosana cv. chuanbaizhi. Zhongguo Zhong yao za Zhi. 2015;40(19):3805–3810. doi: 10.4268/cjcmm20151920. [DOI] [PubMed] [Google Scholar]
- 11.Zheng L. H., Wang P. J., Li W., Feng D. Chemical composition of essential oil from Angelica dahurica roots and its DPPH radical scavenging effect. Food Science. 2014;35(14):180–183. [Google Scholar]
- 12.Zhou S. D., Xu X., Lin Y. F., Xia H. Y., Huang L., Dong M. S. On-line screening and identification of free radical scavenging compounds in Angelica dahurica fermented with Eurotium cristatum using an HPLC-PDA-Triple-TOF-MS/MS-ABTS system. Food Chemistry. 2019;272:670–678. doi: 10.1016/j.foodchem.2018.07.173. [DOI] [PubMed] [Google Scholar]
- 13.Wang J., Lian P., Yu Q., Wei J., Kang W. Purification, characterization and procoagulant activity of polysaccharides from Angelica dahurica roots. Chemistry Central Journal. 2017;11(1):p. 17. doi: 10.1186/s13065-017-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan G., Deng R., Zhou L., et al. Development of a rapid resolution liquid chromatographic method combined with chemometrics for quality control of Angelica dahurica radix. Phytochemical Analysis. 2012;23(4):299–307. doi: 10.1002/pca.1358. [DOI] [PubMed] [Google Scholar]
- 15.Li F., Song Y., Wu J., et al. Hollow fibre cell fishing and hollow fibre liquid phase microextraction research on the anticancer coumarins of Radix Angelicae dahuricae in vitro and in vivo. Journal of Liquid Chromatography & Related Technologies. 2019;42(3-4):79–88. doi: 10.1080/10826076.2019.1576141. [DOI] [Google Scholar]
- 16.Ma J., Cao Y., Chen L., et al. Determination of four coumarins in Angelica dahurica with different grades by UPLC and QAMS. Journal of Chinese Medicinal Materials. 2018;41(10):2372–2376. in Chinese. [Google Scholar]
- 17.Zhang Y. B., Deng G. G., Wang T. X., Liu L., Yang X. W. Tissue distribution study of Angelica dahurica cv. yubaizhi in rat by ultra–performance liquid chromatography with tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2019;174:43–49. doi: 10.1016/j.jpba.2019.05.046. [DOI] [PubMed] [Google Scholar]
- 18.Kaur A., Singh L., Singh N., Bhatti M. S., Bhatti R. Ameliorative effect of imperatorin in chemically induced fibromyalgia: role of NMDA/NFkB mediated downstream signaling. Biochemical Pharmacology. 2019;166:56–69. doi: 10.1016/j.bcp.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Xian Z. M., Jin G. Y., Li H. M., et al. Imperatorin suppresses anaphylactic reaction and IgE-mediated allergic responses by inhibiting multiple steps of FceRI signaling in mast cells: IMP alleviates allergic responses in PCA. BioMed Research International. 2019;2019:12. doi: 10.1155/2019/7823761.7823761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H. B., Gao H. R., Ren Y. J., et al. Effects of isoimperatorin on proliferation and apoptosis of human gastric carcinoma cells. Oncology Letters. 2018;15(5):7993–7998. doi: 10.3892/ol.2018.8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okuyama T., Takata M., Nishino H., Nishino A., Takayasu J., Iwashima A. Studies on theantitumor-promoting activity of naturally occurring substances II. Inhibition of tumor-promoter-enhanced phospholipidmetabolism by umbelliferous materials. Chemical & Pharmaceutical Bulletin. 1990;38(4):1084–1086. doi: 10.1248/cpb.38.1084. [DOI] [PubMed] [Google Scholar]
- 22.Singh G., Kaur A., Kaur J., Bhatti M. S., Singh P., Bhatti R. Bergapten inhibits chemically induced nociceptive behavior and inflammation in mice by decreasing the expression of spinal PARP, iNOS, COX-2 and inflammatory cytokines. Inflammopharmacology. 2019;27(4):749–760. doi: 10.1007/s10787-019-00585-6. [DOI] [PubMed] [Google Scholar]
- 23.Aidoo D. B., Obiri D. D., Osafo N., et al. Allergic airway-induced hypersensitivity is attenuated by bergapten in murine models of inflammation. Advances in Pharmacological Sciences. 2019;2019:12. doi: 10.1155/2019/6097349.6097349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ham J., Choi R. Y., Lee H. I., Lee M. K. Methoxsalen and bergapten prevent diabetes-induced osteoporosis by the suppression of osteoclastogenic gene expression in mice. International Journal of Molecular Sciences. 2019;20(6):p. 1298. doi: 10.3390/ijms20061298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon O. S., Song Y. S., Shin K. H., Ryu J. C. Identification of new urinary metabolites of byakangelicin, a component of Angelicae dahuricae radix, in rats. Archives of Pharmacal Research. 2003;26(8):606–611. doi: 10.1007/bf02976709. [DOI] [PubMed] [Google Scholar]
- 26.Kang T. J., Lee S. Y., Singh R. P., Agarwal R., Yim D. S. Anti-tumor activity of oxypeucedanin from Ostericum koreanum against human prostate carcinoma DU145 cells. Acta Oncologica. 2009;48(6):895–900. doi: 10.1080/02841860902824925. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z. W., Yan H. J., Wang X., et al. Quantitative determination of mollugin and purpurin from traditional Chinese medicine Rubiae Radix et Rhizoma by ∼1H NMR. Shandong Science. 2017;30(1):1–7. in Chinese. [Google Scholar]
- 28.Lin S., Su J., Ye Q., Cao B. J., Zhang W. D. Application progress in quantitative NMR in analysis of traditional Chinese medicine. Journal of Pharmaceutical Practice. 2014;32(2):92–95. in Chinese. [Google Scholar]
- 29.Chen X., Guo Y., Hu Y., Yu B., Qi J. Quantitative analysis of highly similar salvianolic acids with 1H qNMR for quality control of traditional Chinese medicinal preparation Salvianolate Lyophilized Injection. Journal of Pharmaceutical and Biomedical Analysis. 2016;124:281–287. doi: 10.1016/j.jpba.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Wang S. H., Hao H. J., Wang G. D., Wang Y. C. Determination of the absolute content of palmatine by quantitative proton nuclear magnetic resonance. Chinese Journal of Pharmaceutical Analysis. 2017;37(4):654–658. in Chinese. [Google Scholar]
- 31.Huang H. W., He L., Yue H. K., Wang T., Tao Q. F., Liu Y. Determination of entecavir by quantitative nuclear magnetic resonance (qNMR) Drug Evaluation Research. 2015;38(5):520–522. in Chinese. [Google Scholar]
- 32.Wang J. M., Peng L. N., Shi M. J., Li C. Q., Zhang Y., Kang W. Y. Spectrum effect relationship and component nock out in Angelica dahurica radix by high performance liquid chromatography-Q exactive hybrid quadrupole-orbitrap mass spectrometer. Molecules. 2017;22(7):p. 1231. doi: 10.3390/molecules22071231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma H., Pedersen C. M., Zhao Q., et al. NMR analysis of the Fischer-Tropsch process water: combination of 1D selective gradient TOCSY, 2D DOSY and qNMR. Analytica Chimica Acta. 2019;1066:21–27. doi: 10.1016/j.aca.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Owczarek A., Klys A., Olszewska M. A. A validated 1H-qNMR method for direct and simultaneous quantification of esculin, fraxin and (−)-epicatechin in Hippocastani cortex. Talanta. 2019;192:263–269. doi: 10.1016/j.talanta.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 35.Yu S., Guo Q. S., Wang H. L., Gao J. P., Xu X. Simultaneous determination of resveratrol and polydatin in Polygonum cuspidatum by quantitative nuclear magnetic resonance spectroscopy. Chinese Journal of Analytical Chemistry. 2015;43(1):69–74. in Chinese. [Google Scholar]
- 36.Cavalcante R. A. F., Silva F. L., Favero F., Resck I. S., Pereira A. L., Machado A. H. L. Quantitative 1H NMR spectroscopy (qNMR) in the early process development of a new quorum sensing inhibitor. Magnetic Resonance in Chemistry. 2019;58(1):31–40. doi: 10.1002/mrc.4906. [DOI] [PubMed] [Google Scholar]
- 37.Duangdee N., Chamboonchu N., Kongkiatpaiboon S., Prateeptongkum S. Quantitative 1H NMR spectroscopy for the determination of oxyresveratrol in Artocarpus lacucha heartwood. Phytochemical Analysis. 2019:1–6. doi: 10.1002/pca.2834. [DOI] [PubMed] [Google Scholar]
- 38.Roulard R., Fontaine J. X., Jamali A., et al. Use of qNMR for speciation of flaxseeds (Linum usitatissimum) and quantification of cyanogenic glycosides. Analytical and Bioanalytical Chemistry. 2017;409(30):7011–7026. doi: 10.1007/s00216-017-0637-7. [DOI] [PubMed] [Google Scholar]
- 39.Bertelli D., Brighenti V., Marchetti L., Reik A., Pellati F. Nuclear magnetic resonance and high-performance liquid chromatography techniques for the characterization of bioactive compounds from Humulus lupulus L. (hop) Analytical and Bioanalytical Chemistry. 2018;410(15):3521–3531. doi: 10.1007/s00216-018-0851-y. [DOI] [PubMed] [Google Scholar]
- 40.Cao R., Nonaka A., Komura F., Matsui T. Application of diffusion ordered-1H-nuclear magnetic resonance spectroscopy to quantify sucrose in beverages. Food Chemistry. 2015;171:8–12. doi: 10.1016/j.foodchem.2014.08.105. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka R., Inagaki R., Sugimoto N., Akiyama H., Nagatsu A. Application of a quantitative 1H-NMR (1H-qNMR) method for the determination of geniposidic acid and acteoside in Plantaginis semen. Journal of Natural Medicines. 2017;71(1):315–320. doi: 10.1007/s11418-016-1040-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original 1HNMR spectral data and the analysis method of the data used to support the findings of this study are available from the corresponding authors upon request.


