Abstract
Background:
Rapid and reliable diagnosis of respiratory viral infections (RVI) in lung transplant recipients is essential to direct therapy of acute graft dysfunction and identify epidemic trends. Traditional techniques of serology and viral culture are limited by the lack of antibody response and delay in diagnosis.
Methods:
We examined the clinical utility of indirect fluorescent antibody (IFA) testing in adult lung transplant patients with suspected RVI, compared with serology and culture. Nasopharyngeal and throat swabs (NT) were obtained to sample epithelial cells, followed by application of monoclonal antibody to respiratory syncytial virus, adenovirus, parainfluenza 1–3 and influenza A and B. The Bartels Respiratory Viral Detection kit was used with IFA results available within 24 hours.
Results:
Nine of 18 patients tested positive for RVI with influenza A (n = 8) and influenza B (n = 1) detected. The sensitivity of IFA (67%) was higher than that of cell culture (45%). With intensive supportive therapy, infection was self-limiting in bronchiolitis obliterans syndrome (BOS) Grade 0–2 patients. However, patients with BOS Grade 3 manifested an acute exacerbation of airflow obstruction, which proved to be irreversible.
Conclusions:
Lung transplant patients with “flu-like” symptoms should proceed to IFA testing of NT swab specimens for early diagnosis. Samples collected within 7 days of symptom onset have high sensitivity as compared with serology and viral culture techniques.
Respiratory viral infections (RVI) are common in immunocompromised patients and have been associated with significant morbidity and mortality approaching 20%.1, 2 Lung transplant recipients have a unique predisposition to infection because of diminished cough reflex, abnormal lymphatic drainage, impaired mucociliary clearance and pre-existing airways damage with obliterative bronchiolitis.3 Clinical manifestations may include acute self-limiting pharyngitis, bronchiolitis, viral pneumonia and respiratory failure. Secondary bacterial infection is well recognized along with a predisposition to acute allograft rejection and bronchiolitis obliterans syndrome (BOS) through local immune upregulation. Early diagnosis is essential to direct therapy of acute graft dysfunction, identify epidemic trends in the transplant community and prevent nosocomial acquisition of infection. Historically, 3 laboratory techniques have been utilized in the diagnosis of RVI: serology; viral culture; and direct antigen detection. Serologic confirmation of infection using acute and convalescent serum has significant drawbacks in transplant recipients including delay in diagnosis, lack of antibody response and possibility of cross-reaction. Traditional viral culture remains the “gold standard” of diagnosis, although this requires 7 to 10 days of incubation to achieve maximal sensitivity. Isolation in embryonated hen eggs, A549 lung carcinoma, primary monkey kidney or Madin–Darby canine kidney (MDCK) cell lines constitutes the classic method of diagnosis of respiratory viruses.4
Detection of viral antigen within clinical samples using direct or indirect fluorescent antibody (DFA/IFA) techniques is a proposed alternative to achieve rapid diagnosis in immunocompromised patients. Palmer and colleagues in 1998 described 2 cases of RVI in lung transplant recipients diagnosed by DFA performed on bronchoalveolar lavage (BAL) fluid.5 However, exfoliated epithelial cells derived from the upper respiratory tract may be a more practical, less invasive source of diagnostic material in such patients. Successful examination for influenza virus in nasal smears with fluorescein-labeled antibody was first described over 45 years ago.6 Although nasopharyngeal aspirates are widely recognized as providing sufficient cells for fluorescent antibody screening,7, 8 they have a number of inherent problems, including inconvenience of collection and a propensity to induce trauma in patients with fragile mucosa or scant nasal secretions. A nasopharyngeal and throat (NT) swab may be the ideal specimen to provide cellular material; however, its application to viral diagnosis in transplant recipients remains poorly described.
In this study we prospectively analyzed the clinical utility of IFA testing of NT swab specimens in the diagnosis of RVI, compared with serology and cell culture in adult lung transplant recipients. We also characterized the local epidemiology, clinical manifestations and potential long-term complications of RVI in our transplant population.
Methods
Patients
During a 3-month study period commencing in July 2000, 18 adult lung transplant patients presenting with “flu-like” symptoms at St Vincent’s Hospital, Sydney, underwent NT swabs for IFA testing and viral culture using the Bartels Respiratory Viral Detection Kit (cost $15 US). “Flu-like” symptoms were defined as any combination of sore throat, nasal irritation, low-grade fever, myalgia and arthralgia with or without lower respiratory tract (LRT) symptoms of cough, dyspnea or wheeze. Following NT swabs, blood samples were sent for acute viral serology (influenza A or B and adenovirus) using the complement fixation assay, cytomegalovirus polymerase chain reaction (CMV PCR) analysis, full blood count, biochemistry and immunosuppressant levels. Detailed pulmonary function tests, a chest radiograph and measurement of oxygen saturation were also performed. Selected patients with significant LRT symptoms and/or radiographic infiltrates proceeded to early fiber-optic bronchoscopy with transbronchial lung biopsy (TBB). BAL samples were not processed for viral studies and IFA test results were available within 24 hours of collection. Convalescent serology for respiratory viruses was collected 6 weeks after clinical presentation and lung function charted for 6 months post-infection. Baseline forced expiratory volume in 1 second (FEV1) was defined as the average of 2 measurements recorded in the 4 months preceding clinical presentation separated by at least 4 weeks. Diagnosis of RVI required a positive viral culture, IFA test or demonstration of a 4-fold rise in serum antibody titer. Study participants received similar immunosuppressive therapy early post-transplantation consisting of oral cyclosporine, azathioprine and prednisolone (maintenance dose on .20 mg/kg). Acute rejection was treated with intravenous methylprednisolone 15 mg/kg per day for 3 days and generally followed by an oral taper commencing at 1 mg/kg. Patients were switched from cyclosporine to tacrolimus 0.15 mg/kg for recurrent or persistent rejection.
Procedure for NT swabs
A cotton swab moistened in physiologic saline was inserted into both nostrils and gently rotated to absorb secretions from the posterior nasopharyngeal area. A second swab was used to vigorously rub the posterior oropharynx. Each swab was placed in 2 ml of viral transport medium9 and transported immediately to an outside reference laboratory 5 km from our institution. Upon arrival, the liquid medium from each swab was mixed together and centrifuged to provide a cellular supernatant. Only specimens with at least 3 epithelial cells per 400× field under light microscopy were considered adequate for viral detection. Half of the cells were removed with a Pasteur pipette and placed onto 8-well slides. The IFA test consisted of two immunologic reagents. First, 20 μl of each anti-viral mouse monoclonal antibody to adenovirus, respiratory syncytial virus (RSV), influenza A and B and parainfluenza 1–3 were inoculated with the wells. Then 20 μl of non-immune mouse antibody was added to the final well as a negative control. Specimens were incubated in a humid chamber at 35° to 37°C for 30 minutes then washed with phosphate-buffered saline. Anti-mouse immunoglobulin conjugated with fluorescein-isothiocyanate was added and then washed to remove any unattached reagent. The antigen wells were examined at 400× magnification with the non-immune mouse well examined first to detect any non-specific background immunofluorescence. Cells infected with virus displayed apple-green fluorescence that was cytoplasmic and/or nuclear, in a uniform or punctate distribution. The remaining cells with supernatant from the NT swab were processed for culture isolation and incubated with MDCK cells at 35° to 37°C. Cells were observed daily for cytopathic changes of vacuolation and stained for respiratory virus after 4 to 7-day incubation as outlined earlier.4
Results
Viral infection was observed in 50% (9 of 18) of subjects tested, with all cases being influenza infection (8 influenza A, 1 influenza B). No cases of adenovirus, RSV or parainfluenza occurred during the evaluation period. Our presumed diagnoses in those patients with negative diagnostic tests (n = 9) were common respiratory viral pathogens not routinely tested, such as rhinovirus and coronavirus. Figure 1 outlines the distribution of infection vs time post-transplantation for the evaluation months of August and September. No cases of RVI were detected in the month of July. Infection peaked in September, which corresponds to early spring in Australia, and was observed predominately in patients >2 years post-transplantation. Table I highlights the method of diagnosis in influenza patients according to symptom duration at presentation. Seven influenza patients were tested within 1 week of clinical symptoms, with the IFA component of the diagnostic test detecting 6 cases (sensitivity 86%). The 1 case missed on IFA was subsequently detected on viral culture assay. However, 3 of the 6 IFA-positive cases were culture negative, giving a sensitivity of culture in this patient sub-group of only 56%. Two patients with delayed clinical presentation beyond 1 week had negative NT swabs and were diagnosed on the basis of serologic conversion. Therefore, the overall sensitivity of IFA testing was 67% vs 45% for viral culture and 33% for serology.
FIGURE 1.
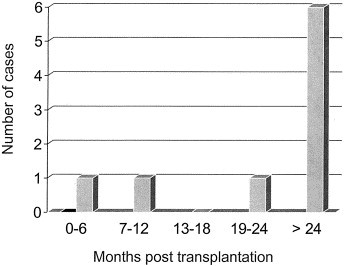
Distribution of influenza infection according to time post-transplantation.
TABLE I.
Method of diagnosis in influenza subjects according to symptom duration
| Duration (days) | IFA | Culture∗ | Serology |
|---|---|---|---|
| 0–3 | 3 /4 | 2 /4 | 1 /4 |
| 3–7 | 3 /3 | 2 /3 | 0 /3 |
| >7 | 0 /2 | 0 /2 | 2 /2 |
IFA, indirect fluorescent antibody.
Viral culture using Madin–Darby canine kidney cells.
The referral population of transplant subjects was community based with no cases of nosocomial acquisition during the study period. All NT swabs were processed for IFA and viral culture with no specimens rejected because of inadequate cell numbers. The characteristics of lung transplant recipients with influenza infection are shown in Table II. Patients ranged in age from 15 to 56 years and were diagnosed 134 to 1,795 days post-transplant (mean 988 days). Prior to influenza infection, the majority of patients had baseline lung function consistent with BOS Grade 0 (n = 5). No patient had received augmented immunosuppression or anti-rejection treatment before the diagnosis. All subjects, except 1, experienced significant morbidity from infection with LRT symptoms, acute decline in lung function and/or a requirement for hospital admission. Bacterial co-infection was documented on sputum or bronchoalveolar lavage (BAL) samples in 5 of 9 patients, with 7 receiving intravenous antibiotics. Most patients also received anti-inflammatory doses of oral prednisolone commencing at 1 mg/kg tapering by 5 mg/day to their usual maintenance dose. However, a steroid pulse was not given to the patient with symptoms limited to the upper respiratory tract with normal graft function or the patient with significant methicillin-resistant Staphylococcus aureus (MRSA) infection. Anti-viral therapy with amantadine or selective neuraminidase inhibitors was not administered to study participants. TBB was performed in 3 patients with radiologic infiltrates and revealed intense airway-centered inflammation with neutrophils in 2 cases. One procedure confirmed moderate acute allograft rejection with associated lymphocytic bronchiolitis Grade B2.10
TABLE II.
Characteristics of influenza subjects
| Patient, age (years), gender, transplant type | Diagnosis | POD | BOS grade | Radiographic changes | Concurrent infection/rejection | Decline in FEV1† | LRT symptoms | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1. 56, M, BSLT | Influenza A IFA+, VC+ | 1,353 | 3 | Bibasal patchy consolidation | Pseudomonas | >330 ml, 20% | Yes | IV antibiotics, p.o. steroids |
| 2. 30, F, BSLT | Influenza A, IFA+, VC+ | 1,325 | 3 | Interstitial infiltrates | Pseudomonas | 270 ml, 27% | Yes | IV antibiotics, p.o. steroids |
| 3. 39, M, BSLT | Influenza A IFA+, VC+ | 134 | 2 | No change | MRSA | 290 ml, 26% | Yes | Vancomycin |
| 4. 55, F∗, SLT | Influenza B IFA+, VC− | 1,199 | 1 | Left lower lobe infiltrates | Pseudomonas | 340 ml, 31% | Yes | IV antibiotics, p.o. steroids |
| 5. 49, M∗, BSLT | Influenza A IFA+, VC− | 1,175 | 0 | Interstitial infiltrates | Nil | 1330 ml, 44% | Yes | IV antibiotics, p.o. steroids |
| 6. 37, M, BSLT | Influenza A IFA+, VC− | 1,795 | 0 | No change | Nil | 560 ml, 18% | Yes | p.o. antibiotics, p.o. steroids |
| 7. 15, M, HLT | Influenza A IFA−, VC+ | 756 | 0 | No change | Nil | No change | No | p.o. antibiotics |
| 8. 55, M, BSLT | Influenza A serology | 256 | 0 | No change | Nil | 460 ml, 32% | Yes | IV antibiotics, p.o. steroids |
| 9. 45, F∗, SLT | Influenza A serology | 905 | 0 | Left lower lobe consolidation | Aspergillus, Pseudomonas, Grade A3B2 |
180 ml, 9.2% |
Yes |
IV antibiotics, IV steroid pulse |
BSLT, bilateral sequential lung transplant; SLT, single-lung transplant; HLT, heart–lung transplant; IFA, indirect fluorescent antibody; VC, viral culture; POD, post-operative day; BOS, bronchiolitis obliterans syndrome; LRT, lower respiratory tract; MRSA, methicillin-resistant Staphylococcus aureus; p.o., per oral; IV, intravenous.
Patients who underwent bronchoscopy and transbronchial lung biopsy.
Decline in FEV1 from baseline.
As shown in Table III, influenza subjects were of similar age, gender, transplant type and post-operative days to those patients in whom no respiratory virus was isolated (n = 9). All participants from both groups were on 3 maintenance immunosuppressive agents at the time of clinical infection. However, the decline in FEV1 for influenza subjects was significantly greater compared with the non-influenza group (p = 0.0469, Mann–Whitney U-test). Five of 9 influenza patients developed new chest radiograph infiltrates compared with none in the non-influenza group. Although the presence of hypoxemia and leukocytosis showed similar distribution among the groups, fever was particularly characteristic of influenza infection. Non-influenza patients experienced a less severe clinical presentation requiring hospitalization in only 3 cases and augmented oral steroids infrequently. Interestingly, all study participants except 1 had received a single-dose influenza vaccine 4 months prior to the evaluation period.
TABLE III.
Comparison of lung transplant recipients with influenza to those without
| Patients with influenza (n = 9) | Patients without influenza (n = 9) | |
|---|---|---|
| Mean age (years) | 42.3 | 42.7 |
| Gender M:F | 6:3 | 5:4 |
| Transplant type | 6 BSLT, 2 SLT, 1 HLT | 6 BSLT, 2 SLT, 1 HLT |
| Acute rejection episodes∗ | 1.22 | 1.33 |
| POD† | 988 ± 535 (134–1795) | 1,488 ± 902 (44–2890) |
| FEV1 decline† | ||
| (1) Volume | 418 ± 377 ml (0–1330) | 176 ± 187 ml (0–585) |
| (2) Percentage | 23 ± 13 (0–44) | 15 ± 14 (0–33) |
| LRT symptoms | 8/9 | 4/9 |
| Clinical signs | ||
| Hypoxia‡ | 4/9 | 2/9 |
| Leukocytosis§ | 1/9 | 0/9 |
| Fever∥ | 8/9 | 2/9 |
| Radiographic changes | 5/9 | 0/9 |
| Influenza vaccine | 8/9 | 9/9 |
| Bronchoscopy | 3/9 | 0/9 |
| Number hospitalized | 8/9 | 3/9 |
| Treatment | ||
| Steroids (p.o. or IV) | 7/9 | 2/9 |
| IV antibiotics | 7/9 | 4/9¶ |
BSLT, bilateral sequential single-lung transplant; SLT, single-lung transplant; HLT, heart–lung transplant; POD, post-operative day; LRT, lower respiratory tract; p.o., per oral; IV, intravenous.
Average number per patient post-transplant.
Values expressed as mean ± standard deviation (range).
Resting oxygen saturation <92.
White cell count >11,000 mm3.
Temperature ≥37.6°C.
Anti-microbial therapy included ambisome for 2 cases of Aspergillus infection.
Outcome was favorable for all patients with no cases of progressive respiratory failure requiring intubation or assisted ventilation. Figure 2 demonstrates lung function of study participants with BOS Grade 3 at baseline for 6 months before and after clinical presentation. BOS Grade 0–2 influenza and non-influenza subjects experienced acute reversible declines in FEV1 with return to baseline at a mean of 3 weeks after clinical presentation. However, influenza patients with BOS Grade 3 failed to return to baseline and suffered a progressive loss in lung function, thereafter, in contrast to those BOS Grade 3 subjects in the non-influenza group. This occurred despite all BOS Grade 3 subjects having received intravenous cefepime and tobramycin for concurrent Pseudomonas chest infection and an oral pulse of steroids over a 10 to 14-day inpatient stay. Although both non-influenza subjects were hypoxemic at presentation, decline in lung function was less severe and neither had new radiographic infiltrates. One non-influenza BOS Grade 3 patient had concurrent Aspergillus infection that necessitated treatment with intravenous ambisome to a total dose of 2 g.
FIGURE 2.

Outcome of BOS Grade 3 subjects according to lung function (n = 4). Filled squares: influenza subject; open squares: non-influenza subject.
Discussion
The reported incidence of influenza infection in lung transplant recipients varies in the literature, although it is generally low. Palmer and colleagues5 described 10 episodes of RVI in 122 adult lung transplant patients over a 5-year review, with not a single case of influenza identified. Holt et al11 reported 1,820 lavage samples in 137 lung or heart–lung recipients over a 7-year period with influenza isolated only twice. At the Duke University Medical Center, Matar and colleagues2 diagnosed just 2 subjects with influenza over 5 years on lavage samples taken from 176 lung transplant patients. These retrospective reviews incorporated both surveillance and diagnostic biopsy protocols, with BAL fluid the diagnostic specimen. They contrast significantly with prospective evaluations performed in immunocompromised patients with hematologic malignancies before and after bone marrow transplantation (BMT) using upper respiratory tract sampling techniques. Ljungman et al12 reported that 19% of such patients (15 of 78) had a respiratory virus detectable on nasal wash during a 3-month study period with the vast majority of patients symptomatic. A large prospective study post-BMT, comparing isolation sites for confirmed influenza A infection, showed that only 6% of lavage samples vs 94% of nasal wash/throat swab specimens were DFA- or culture-positive for virus.13 We report 9 cases of influenza infection over a 3-month period in 18 lung transplant recipients with “flu-like” symptoms, from a transplant community of some 115. Several additional factors may explain our higher prevalence of infection such as seasonal and geographic variation along with heightened efforts to diagnose infection. Nonetheless, the incidence of influenza infection in adult lung transplant recipients is likely to be considerably greater than that reported to date, with the more specific application of diagnostic tests obtained from the upper respiratory tract.
Traditional cell culture techniques have remained the gold standard in respiratory viral diagnosis. However, the sensitivity of viral culture compared with rapid diagnostic methods is not well described in lung transplant recipients. In our series, IFA testing of samples had a superior sensitivity vs cell culture (67% vs 45%). Garantziotis et al14 reported similar findings with lavage fluid in their small series of 3 cases of influenza pneumonia in lung transplant recipients. Our lower rate of positive viral culture may reflect in vitro viral instability or sub-optimal specimen handling. Although all samples were sent to an outside reference laboratory, inoculation into cell culture and IFA staining was achieved within a few hours. No sample was frozen in transport, which may otherwise promote cellular degradation and reduce viral stability. If we exclude patients with clinical presentation beyond 1 week, the sensitivity of IFA improves significantly to 86%. The possibility of viral isolation is enhanced when specimens are collected within 7 days of clinical onset.15
The high sensitivity of IFA testing in our series supports the use of the nasopharyngeal and throat swab as a diagnostic specimen. This collection method was well tolerated by all patients and no specimen was discarded or re-collected because of inadequate cell numbers. Nasopharyngeal aspirates and/or washes have been proposed as alternative methods to collect larger numbers of exfoliated epithelial cells. However, these techniques are potentially more invasive and inconvenient to collect compared with NT swabs. In addition, if nasal secretions are minimal or mucosa particularly fragile, as is common with influenza,15 NT swabs may inflict less local trauma. The stipulation on cellularity applied in our study would likely reduce any disparity in yield between the various upper airway sampling techniques.
Influenza produced significant clinical infection in our lung transplant patients, necessitating hospitalization, single bed isolation and supportive care in the majority of affected individuals. Radiographic anomalies were not predictive of more severe graft dysfunction or development of respiratory failure. No patient required ventilatory support or died as a result of their acute infection. To our knowledge, no anti-viral agents have proven efficacy against influenza infection in transplant recipients. Inhaled zanamivir and oral oseltamivir are selective inhibitors of the neuraminidase glycoprotein essential for replication of influenza A and B viruses. Studies in immunocompetent volunteers have shown that these agents reduce the duration of disease (by up to 1.5 days), severity of illness and secondary complications compared with placebo when initiated within 36 hours of symptom onset.16, 17 IFA testing may facilitate the broader application of these novel agents in transplant recipients by rapid confirmation of infection. Although we have described influenza as generally self-limiting in the acute setting, strategies to reduce morbidity may prove cost-effective. Further research is needed to define potential sub-groups of transplant recipients who may benefit from early introduction of neuraminidase inhibitors.
Serology has a low sensitivity in diagnosing recent influenza infection, confirming only 33% percent of cases in our series. This poor response is similar to that achieved after influenza vaccination in patients after thoracic organ transplantation, where protective antibody levels have been seen in 36% to 41% of recipients.18, 19 The major public health measure for the prevention of influenza infection remains the use of inactivated vaccine administered in autumn each year. Nonetheless, vaccination of lung transplant recipients with a yearly single-dose influenza vaccine may afford minimal protection against viral replication and infection. All except 1 patient was vaccinated for influenza some 4 months prior to the evaluation period. This adds further controversy to the debate regarding the need for booster vaccination and serologic confirmation of antibody response. However, it is feasible that vaccination of our patient group protected individuals from more severe clinical manifestations by inducing low serum levels of protective antibody. Until specific assays to measure antibodies to vaccine constituents become more widely available, we continue to recommend at least the single-dose influenza vaccine.
Retrospective clinical reviews support an association between RVI and the development of BOS.5 Seasonal clustering of onset of BOS suggests that infectious triggers such as respiratory viruses may play an etiologic role.20 Obliterative bronchiolitis has recently been described shortly after influenza pneumonia in both pediatric and adult lung transplant recipients.14, 21 Experimentally, the intratracheal instillation of parainfluenza 1 in rat lung allografts induces the typical lesion of obliterative bronchiolitis within 56 days on histologic section.22 Palmer5 described 4 of 8 patients developing BOS immediately following RVI or several months after infection. In our study, patients experienced a similar reversible decline in lung function with no progression to BOS or worsening BOS grade on follow-up. However, BOS Grade 3 patients with influenza failed to return to baseline and experienced ongoing loss thereafter. This suggests that patients with severe pre-existing bronchiolar epithelial damage have a limited repertoire of defense mechanisms to respiratory viruses.22 A component of the pathology in influenza is caused by recruitment of T cells to the site of infection.23 Perhaps the use of oral prednisolone in treatment regimens for lower BOS grades protected the patients from initiation or amplification of the chronic rejection process. Mechanisms of action of this steroid blockade may include downregulation of lymphocyte alloreactivity or minimization of the sub-epithelial inflammatory reaction. In addition, acute rejection was an infrequent association with influenza infection, with only 1 biopsy-proven Grade A3 rejection on Day 5 of hospitalization.
In conclusion, influenza in lung transplant recipients is probably far more prevalent than previously reported. Although our study population was relatively small, we believe IFA testing of NT swabs is a low-cost less invasive test of high sensitivity in the diagnosis of infection. Routine viral culture of NT swabs may not be necessary after an initial positive IFA, given its lower sensitivity in our experience. With early diagnosis and institution of intensive supportive treatment, infection is generally self-limiting, showing an infrequent association with acute rejection. With the exception of BOS Grade 3 patients, we found no association with the subsequent development of BOS in 6 months of follow-up. Although we recommend an annual influenza vaccination, single-dose protocols may provide only minimal protection against disease.
Acknowledgements
The authors are grateful to all patients who took part in this study. We also thank the South East Area Laboratory Services division of Prince of Wales Hospital for specimen handling and processing.
References
- 1.Apalsch A.M., Green M., Ledesma-Medina J., Nour B., Wald E.R. Parainfluenza and influenza virus infections in paediatric organ transplant recipients. Clin Infect Dis. 1995;20:394–399. doi: 10.1093/clinids/20.2.394. [DOI] [PubMed] [Google Scholar]
- 2.Matar L.D., McAdams H.P., Palmer S.M. Respiratory viral infections in lung transplant recipients: Radiologic findings with clinical correlation. Radiology. 1999;213:735–742. doi: 10.1148/radiology.213.3.r99dc25735. [DOI] [PubMed] [Google Scholar]
- 3.Wendt C.H. Community respiratory viruses: Organ transplant recipients. Am J Med. 1997;102:31–36. doi: 10.1016/s0002-9343(97)80008-3. [DOI] [PubMed] [Google Scholar]
- 4.The Diagnostics Division of Intracel Corporation . Report on the Bartels Viral Respiratory Screening and Identification Kit. Publication no. 3450-0185E. Intracel Corp; Issaquah, WA: 1995. [Google Scholar]
- 5.Palmer S.M., Nancy M.D., Henshaw G. Community respiratory viral infection in adult lung transplant recipients. Chest. 1998;113:944–951. doi: 10.1378/chest.113.4.944. [DOI] [PubMed] [Google Scholar]
- 6.Liu C. Rapid diagnosis of human influenza infection from nasalsmears by means of fluorescein-labelled antibody. Proc Soc Exp Biol Med. 1956;92:883–887. doi: 10.3181/00379727-92-22642. [DOI] [PubMed] [Google Scholar]
- 7.Mackie P.L., Joannidis P.A., Beattie J. Evaluation of an acute point of caresystem screening for respiratory syncytial virus infection. J Hosp Infect. 2001;48:66–71. doi: 10.1053/jhin.2001.0942. [DOI] [PubMed] [Google Scholar]
- 8.Rolston K., Englund J.A., Henrickson K. Symposium on respiratory viral infections/discussion. Am J Med. 1997;102:53–54. [Google Scholar]
- 9.Lennette E.H., Balows A., Hausler W.J., Shadomy H.J. Manual of clinical microbiology. 4th ed. American Society of Microbiology; Washington DC: 1995. [Google Scholar]
- 10.Yousem S.A., Berry G.J., Cagle P.T. Revision of the working formulation for the classification of pulmonary allograft rejection: Lung rejection study group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 11.Holt N.D., Gould F.K., Taylor C.E. Incidence and significance of noncytomegalovirus viral respiratory infection after adult lung transplantation. J Heart Lung Transplant. 1997;16:416–419. [PubMed] [Google Scholar]
- 12.Ljungman P., Gleaves C.A., Meyers J.D. Respiratory virus infection in immunocompromised patients. Bone Marrow Transplant. 1989;4:35–40. [PubMed] [Google Scholar]
- 13.Bowden R.A. Respiratory virus infections after marrow transplant: The Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102:27–30. doi: 10.1016/s0002-9343(97)00007-7. [DOI] [PubMed] [Google Scholar]
- 14.Garantziotis S., Howell D.N., McAdams H.P., Davis R.D., Henshaw N.G., Palmer S.M. Influenza pneumonia in lung transplant recipients: Clinical features and association with bronchiolitis obliterans syndrome. Chest. 2001;119:1277–1280. doi: 10.1378/chest.119.4.1277. [DOI] [PubMed] [Google Scholar]
- 15.Fraser R.S., Muller N.L., Colman N., Pare P.D. Diagnosis of diseases of the chest. 4th ed. W. B. Saunders; London: 1999. [Google Scholar]
- 16.Hayden F.G., Osterhaus A.D.M.E., Treanor J.J. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. GG167 Influenza Study Group. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 17.McClellan K., Perry C.M. Oseltamivir: A review of its use in influenza. Drugs. 2001;61:263–283. doi: 10.2165/00003495-200161020-00011. [DOI] [PubMed] [Google Scholar]
- 18.Kimball P., Verbeke S., Flattery M., Rhodes C., Tolman D. Influenza vaccination does not promote cellular or humoral activation among heart transplant recipients. Transplantation. 2000;69:2449–2451. doi: 10.1097/00007890-200006150-00042. [DOI] [PubMed] [Google Scholar]
- 19.Admon D., Engelhard D., Strauss N., Goldman N., Zakay-Rones Z. Antibody response to influenza immunization in patients after heart transplantation. Vaccine. 1997;15:1518–1522. doi: 10.1016/s0264-410x(97)00193-x. [DOI] [PubMed] [Google Scholar]
- 20.Hohlfeld J., Niedermeyer J., Hamm H., Schafers H.J., Wagner T.O.F., Fabel H. Seasonal onset of bronchiolitis obliterans syndrome in lung transplant recipients. J Heart Lung Transplant. 1996;15:888–894. [PubMed] [Google Scholar]
- 21.Faul J.L., Akindipe O.A., Berry G.J., Theodore J. Influenza pneumonia in a pediatric lung transplant recipient. Transplant Int. 2000;13:79–81. doi: 10.1007/s001470050013. [DOI] [PubMed] [Google Scholar]
- 22.Winter J.B., Gouw A.S.H., Groen M., Wildevuur C., Prop J. Respiratory viral infections aggravate airway damage caused by chronic rejection in rat lung allografts. Transplantation. 1994;57:418–422. doi: 10.1097/00007890-199402150-00018. [DOI] [PubMed] [Google Scholar]
- 23.Rubin E., Farber J.L. Pathology. 3rd ed. Lippincott-Raven; Philadelphia: 1998. [Google Scholar]


