Abstract
This article reviews diagnosis and management of gastrointestinal diseases in guinea pigs and rabbits. The review includes established causes of gastrointestinal disease in these species. The authors highlight syndromes that may be considered emerging or less-recognized causes of gastrointestinal stasis, including gastric dilation and volvulus in guinea pigs and lead toxicity, colonic entrapment, and liver torsion in rabbits. Practitioners should recommend initial diagnostics, including radiographs and blood work on guinea pigs and rabbits presenting with nonspecific signs of gastrointestinal stasis, to better determine possible cause and make the best treatment recommendations.
Keywords: Gastrointestinal disease, Rabbit, Guinea pig, Stasis, Enteritis, GDV, Liver torsion
Key points
-
•
Dental disease is a commonly encountered problem in guinea pigs and rabbits.
-
•
Even minor changes in the diet or digestive process can lead to significant gastrointestinal (GI) disease in guinea pigs and rabbits.
-
•
Diarrheal disease is common and frequently results from alterations in intestinal microflora balance.
-
•
Several cases of dilatation have been documented in guinea pigs.
-
•
Rabbits secrete higher levels of gastric acid and pepsin than do rats and guinea pigs, likely contributing to their higher incidence of gastric ulceration.
-
•
Signs of lead toxicity in rabbits include neurologic presentations, such as seizures, torticollis, and blindness, but more common signs may be nonspecific and include anemia, anorexia, loss of body condition, and GI stasis.
-
•
One of the authors has documented 16 cases of liver torsion in rabbits at a single referral institution in 5 years.
Dental disease, gastrointestinal stasis, and dysbiosis in guinea pigs and rabbits
Dental disease is a commonly encountered problem in guinea pigs and rabbits. Guinea pig and rabbit teeth are elodont (continuously growing/erupting), aradicular (open rooted), and hypsodont (long-crowned) and contain incisors and cheek teeth (premolars and molars). With insufficient wear, particularly from low-fiber, low-abrasion diets, molar crowns elongate and alter the slope of the occlusal plane, eventually creating sharp points that inhibit closure, often resulting in secondary incisor elongation.1 Malocclusion also results from diets deficient in vitamin C in guinea pigs because it is critical for gingival health and anchoring of teeth. Trauma, infection, and genetics are also implicated. Dental disease can lead to oral ulcerations, infection, abscess formation, and tongue entrapment in guinea pigs. Guinea pigs are more sensitive to subtle occlusal changes than rabbits, and even mild disease can lead to anorexia and malnutrition.2 Buccal ulcerations generally form along the upper arcades and lingual ulcerations along the lower arcades in rabbits. Congenital deformities can also result in malocclusion and produce dental disease at several months of age. In rabbits with mandibular prognathism, most commonly seen in dwarf rabbits, misalignment causes overgrowth of unopposed incisors, with upper incisors curving inward and upward toward the roof of the mouth and lower incisors curving outward and upward, sometimes into the upper lip or nose.1, 3, 4
Clinical signs of primary dental disease include anorexia, dysphagia, excessive salivation and drooling, weight loss, emaciation, and changes in fecal appearance and quantity. The presence of facial masses, excessive swelling, exophthalmos, and purulent nasal discharge suggests secondary infection/abscess formation. Animals with anorexia or dysphagia from a systemic disease, or with ocular disease restricting feeding, can develop secondary dental disease.4 Diagnosis requires a routine physical examination, including thorough oral examination of the incisors, cheek teeth, periapical structures, bone, tongue, and oral mucosa. Complete dental examinations require extraoral radiographic studies from multiple projections (lateral, obliques, ventrodorsal, and rostrocaudal) using high-definition (mammography) film5 as well as a thorough examination with patients under anesthesia, assisted by oral endoscopy, when available.6 Diagnosis of periapical disease and abscess can be aided by CT, when available.7
Dental correction involves shortening of overgrown teeth, restoring the occlusal plane, extracting any diseased teeth, and treating abscessation. Dental procedures8 and anesthetic and analgesic considerations4, 8, 9 have recently been reviewed. Surgical treatment must be combined with medical therapy to manage pain, restore health (hydration, diet correction, and vitamin C supplementation for guinea pigs), and minimize infection as well as the risk of postoperative GI stasis.2 Long-term management of dental disease includes providing a high-fiber diet with Timothy grass hay (ad lib),10 adequate vitamin C for guinea pigs (10–30 mg/d), and regular rechecks with tooth trimming as needed.
Guinea pigs are less prone than rabbits to develop periapical infections and osteomyelitis, although they frequently present with more advanced disease and poor prognosis.11 Periapical infection, abscessation and/or osteomyelitis of the surrounding bones are common sequelae in rabbits with dental disease. Treatment typically requires extraction, opening and excising an entire abscess capsule, careful débridement of bone, marsupialization with secondary closure, and packing the surgical site. Antibiotic therapy should be based on culture and sensitivity results and can include combinations of oral and/or injectable agents as well as impregnated beads.11
Gastrointestinal hypomotility and stasis
GI stasis is a common problem in guinea pigs and rabbits. The GI tract is specialized for its high-fiber herbivorous diet; even minor changes in the diet or digestive process can lead to significant GI disease. GI stasis has a multifactorial etiology. In animals receiving an adequate diet, GI stasis can result from reduced intake secondary to one of several or a combination of factors causing anorexia, including dental disease, dysphagia, pain, anxiety, environmental changes, infection, dysbiosis, neoplasia, chronic disease, drug effects (anesthetics, anticholinergics, opioids, and antibiotics), obstruction/foreign bodies, and accidental or forced restriction (preoperative fasting). Restricted water intake and activity also impair adequate processing of dietary fiber and promote stasis. In addition to reducing intake, chronic stress causes increased catecholamine signaling, acting on the enteric nervous system to impair intestinal motility. Once initiated, dysmotility leads to reduced colonic transit, with decreased fecal output, increased dehydration of intestinal contents, dehydration of gastric contents with trichobezoar formation, impaired cecal fermentation, and disruption of the enteric microflora, creating a cycle of further anorexia and worsening stasis.12, 13 In severe cases, stasis leads to partial or complete obstruction or accumulation of gas within the GI tract (bloat) that can be a life-threatening emergency (discussed later). Clinical signs of GI stasis can include decreased or absent fecal material, anorexia, bruxism, pain with abdominal palpation, decreased GI sounds, dehydration, abdominal distension, gastric tympany, and respiratory or cardiovascular compromise.14 Severely ill rabbits progress to hypovolemic shock with reduced blood pressure and altered mentation.
Diagnosis requires a thorough history, physical examination, and abdominal imaging (radiographs and/or ultrasound). Radiographic studies of the abdomen in 2 views are essential for examining gastric contents, colonic fecal contents, and, most importantly, severe gas/fluid accumulation suggestive of obstruction, which constitutes a surgical emergency.15 Trichobezoars are an uncommon cause of stasis in guinea pigs but also require surgical intervention.16 Laboratory studies (complete blood cell count, biochemistry, and urinalysis) can be helpful for determination of an underlying cause of anorexia, but most have only nonspecific findings of dehydration or possibly elevated hepatic enzymes from developing hepatic lipidosis.17
Routine GI stasis is treated with comprehensive supportive care (aggressive fluid hydration, pain management, and assisted nutrition) best performed in a hospital setting for close monitoring. Animals should be kept warm in a dark, quiet place to minimize stress. Fluid replacement is achieved with warmed fluids (25–35 mL/kg every 8 hours) and can be given orally or subcutaneously [SC]), although animals with severe dehydration require more aggressive intravenous (IV) fluids. Anxiety can be minimized with injectable midazolam (0.25–0.5 mg/kg IV/intramuscular [IM]) and pain controlled with analgesics, such as buprenorphine (0.01–0.05 mg/kg, IM or SC every 4–6 hours), which can later be transitioned to meloxicam after adequate hydration (0.2 mg/kg IM/SC/by mouth every 24 hours in guinea pigs and 0.2–1 mg/kg IM/SC/by mouth every 24 hours in rabbits). Once obstruction has been ruled out, prokinetic agents, including metoclopramide (0.5 mg/kg SC/by mouth every 8–12 hours) and/or cisapride (0.5 mg/kg by mouth every 8–12 hours) can be used. Simethicone (20 mg/kg by mouth every 8–12 hours) can be used to reduce gas distension and ranitidine (2 mg/kg IV every 24 hours, 2–5 mg/kg by mouth every 12 hours) used in cases with prolonged anorexia where gastric ulceration is likely. Nutritional support is essential and can be performed by syringe feeding (15 mL/kg every 8 hours) of an herbivore critical care formulation. Prolonged nutritional support can be provided by nasogastric tube. Antibiotics should only be used in cases complicated by enterotoxemia and bacterial enteritis.17, 18, 19
Dysbiosis and Antibiotic-associated Enterotoxemia
Diarrheal disease is common and frequently results from alterations in the intestinal microflora balance,20 or dysbiosis, ranging from mild changes causing soft stools, to pathogenic bacterial overgrowth with more significant enteritis, to life-threatening enterotoxemia. The gut flora is sensitive to any type of environmental change. Frequent causes of dysbiosis include poor diet, hypomotility, stress, toxins, and antibiotic use. Low-fiber, high-carbohydrate diets are the primary risk factor for several reasons: (1) formation of dense masses that prohibit adequate digestion and sterilization by the low pH gastric juice and enzymes; (2) reduced hindgut motility (discussed previously), delaying clearance of luminal bacteria and fermentation byproducts, causing an altered cecal pH and fermentation environment that favors the growth of pathogenic species; and (3) carbohydrate-rich diets that provide a readily available source of luminal glucose for opportunistic organisms, such as Escherichia coli and Clostridium spp.12, 21 Systemic illness and stress, also acting to reduce motility, can precipitate dysbiosis. Indiscriminate antibiotic use, particularly with narrow-spectrum agents that selectively target beneficial gram-positive bacteria (penicillins, amoxicillin ± clavulanic acid, cephalosporins, ampicillin, clindamycin, and lincomycin), creates optimal conditions for overgrowth of pathogenic species. Chloramphenicol, trimethoprim/sulfas, and fluoroquinolones are least likely to damage the microflora, and there is a decreased risk with parenteral versus oral administration.14, 22
Overgrowth of C spiroforme in rabbits causes an often-fatal enterotoxemia due to elaboration of bacterial toxin. Although adults with dysbiosis can develop enterotoxemia, weanlings are most susceptible due to their poorly established flora and high gastric pH. Newborns can also develop toxemia from toxin secreted into the milk of infected mothers.12 Recently, it has been demonstrated that C spiroforme toxin (binary actin-ADP-ribosylating, iota-like toxin) gains access to enterocytes via the lipolysis-stimulated lipoprotein receptor, which is also used by the C difficile transferase and C perfringes iota toxins, causing a secretory diarrhea.23, 24 In acute infections, rabbits develop watery diarrhea, possibly with blood, that soils the perineum and legs. They become anorexic and decline over 2 to 4 days into a moribund state with hypovolemic shock, leading to death. On necropsy, the cecum, the primary reservoir for bacterial growth, is often covered with petechial and ecchymotic hemorrhage that can spread into the appendix and proximal colon. The mucosa can also contain hemorrhage, thick mucus, gas, or pseudomembranes.25
In young rabbits (7–14 weeks of age), bacterial dysbiosis and resultant cecal hyperacidity can lead to the proliferation of enteric goblet cells with voluminous mucus production, causing a mucoid enteritis.26 Although the exact cause is unclear, the disease is predominantly found in intense breeding colonies and is uncommon in pet rabbits. Affected animals have anorexia and develop lethargy, weight loss, and cecal impaction.25
Clinical symptoms of antibiotic associated enterotoxemia begin 1 to 5 days after antibiotic administration, and include anorexia, dehydration, and hypothermia; diarrhea may or may not be present. Diagnosis is based on clinical history and clinical signs and can be confirmed with polymerase chain reaction or ELISA-based commercial tests for C difficile toxin. Treatment of dysbiosis/enteritis/enterotoxemia involves aggressive supportive care and correction of hypomotility. Correction of dehydration and nutritional deficiencies are critical, as is providing a warm, safe environment with adequate analgesia. For cases of enteritis caused by bacterial overgrowth, fecal bacterial culture and sensitivity can be helpful to guide antiobiotic therapy with broad-spectrum agents, including trimethoprim-sulfamethoxazole (30 mg/kg by mouth every 12 hours) or enrofloxacin (15 mg/kg by mouth every 24 hours). For enterotoxemia, a dual approach of metronidazole (20 mg/kg every 12 hours) and cholestyramine (2 g/20 mL water every 24 hours by gavage) can be used to treat Clostridium infection and bind its toxin, although C spiroforme has widespread intrinsic and acquired antimicrobial resistance.27, 28 Chloramphenicol (50 mg/kg by mouth every 8 hours) can be used to attempt to suppress Clostridial overgrowth.14, 22 Preventing dysbiosis with a high-fiber diet and reducing stress is key to prevention. Attempts to correct the dysbiosis have anecdotal success, including transfaunation and commercial probiotics containing Lactobacillus spp, although controlled studies to document their utility are lacking.29 Exterotoxemia is best avoided by judicious antibiotic use in guinea pigs and rabbits.
Gastrointestinal Disease in the Guinea Pig
Gastric dilation and volvulus
GI stasis can result in the accumulation of gas within the GI tract (bloat), particularly in the stomach and cecum, prompting a surgical emergency. Gastric dilation-volvulus (GDV) is a rare, life-threatening complication of this gas accumulation, with such a high mortality that is frequently diagnosed at necropsy. Although far less common than in other species (human, canine, and swine), several cases have been documented in guinea pigs,30, 31, 32, 33 many involving breeding females in laboratory colonies. The authors have had 3 recent cases of GDV in guinea pigs at their institution (Fig. 1 ).
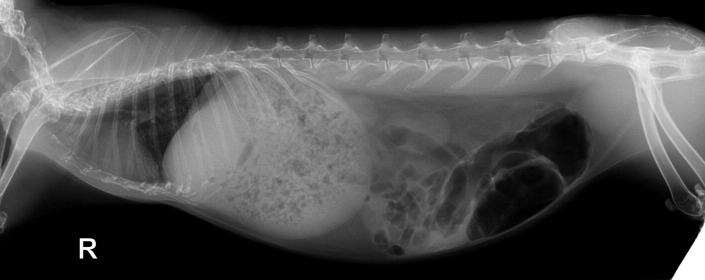
Fig. 1.
Lateral radiographic projection of a guinea with a caudally displaced gastric dilatation volvulus. There is also mineralized material in the caudal region suggestive of urolithiasis. The owners declined emergency surgery and this guinea pig died within 3 hours of instituting supportive care measures.
Clinical signs of gastric dilation are similar to those found with GI stasis, although more severe symptoms, such as dyspnea, cyanosis, tachycardia, and cardiovascular shock, may also be present.30 When gastric tympany is present and/or there is evidence of gastric dilation on imaging, decompression is achieved by passing a large red rubber tube into the stomach through the oral cavity. A needle used as a trocar can be passed percutaneously, although this carries a risk of gastric or cecal rupture or peritonitis. If GDV is suspected, emergent surgical intervention is required to reduce the volvulus. After surgical intervention, medical management per GI stasis is used. Simethicone can be used to enhance gas absorption but only after aggressive hydration to avoid a potential foreign body from dehydrated simethicone.18
Bacterial enteritis
Although dietary factors (low fiber and excess carbohydrates) can cause soft stools in all guinea pigs, diarrhea from bacterial enteritis is usually seen in weanlings, pregnant sows, and immunocompromised/chronically stressed adults. The most common cause is C piliforme (Tyzzer disease), transmitted by fecal-oral route. Infected animals progress rapidly from onset of lethargy, anorexia, and diarrhea to acute death. Antemortem diagnosis is generally not possible because C piliforme is an intracellular bacterium that does not grow in culture. At necropsy, infections are marked by intestinal inflammation and patchy hepatic necrosis. Treatment has not proved beneficial, and prevention is best achieved by good husbandry and stress reduction, especially during weaning.14, 34
Salmonellosis from S typhimurium and S enteritidis are less-frequent causes of bacterial enteritis but are highly lethal with greater than 50% mortality. Transmission is generally from contaminated food or water, although fecal transmission also occurs. Infected animals exhibit anorexia, weight loss, light-colored feces with or without diarrhea, weakness, depression, and poor grooming. Infected pregnant sows have a high incidence of abortion. Physical examination frequently reveals hepatosplenomegaly (with punctate necrotic foci in the viscera at necropsy) and conjunctivitis. Salmonella can be cultured from the feces. Because infected animals can become asymptomatic carriers of this zoonotic disease, treatment is not recommended. Preventive measures include thorough disinfection of the environment and proper storage and washing of all fresh fruits and vegetables offered.14, 35
Other causes of bacterial enteritis include Yersinia pseudotuberculosis, E coli, C perfringes, Pseudomonas aeruginosa, Citrobacter freundii, Listeria monocytogenes, and Lawsonia intracellularis. Y pseudotuberculosis can cause distinct clinical presentations: an acute, rapidly fatal (28–48 h) septicemic form; a chronic diarrheal disease with wasting and death within weeks; and a nonfatal infection marked by abscesses of the head and neck lymph nodes. Y pseudotuberculosis can also cause abscess formation in the ileum, cecum, mesenteric lymph nodes, liver, and omentum. Treatment is not advised, because it can induce an asymptomatic carrier state and promote zoonotic transmission.22, 35 E coli infection/overgrowth is particularly virulent in weanlings and is marked by anorexia, diarrhea, depression, wasting, and death. At necropsy, yellow fluid and gas are found in the intestines, and there is often focal hepatic necrosis.35 L intracellularis is most often found in swine but can also infect rodents, including guinea pigs. The intracellular organism infects enteric epithelial cells, causing a proliferative enteropathy marked by diarrhea, wasting, and eventual death.36
Parasitic disease
GI helminth infections are predominately due to the guinea pig roundworm, Paraspidodera uncinata. The nematode develops and resides in the cecum and colon but does not invade the mucosa. Typical oxyurid eggs are shed in the feces, and ingestion leads to infection within 3 to 5 days. Infections are often mild and subclinical, although heavy infections lead to anorexia, diarrhea, weight loss, and poor coat. Treatment is with ivermectin (0.2 mg/kg SC) and prevention by effective sanitation.22, 34
Cryptosporidium wrairi is a protozoan that targets the small intestine epithelial brush border. Transmission is by ingestion of oocysts from infected food, water, and fomites. Infections are marked by diarrhea, weight loss, rectal prolapse, potbellied appearance, and a greasy coat. Although immunocompetent animals generally recover within 4 weeks and develop resistance, weanlings, juveniles, and immunosuppressed animals often have a more severe course with mortality approaching 50%. Oocysts can be diagnosed on fecal examination, and organisms may be seen on histopathology. Oocysts can be destroyed with 5% ammonia or extremes of temperature. Cryptosporiodosis is potentially zoonotic.
Eimeria caviae is an intestinal coccidian of guinea pigs that is generally nonpathogenic but can cause significant disease in weanlings. Infections are more common in breeding colonies due to overcrowding, poor husbandry, and concurrent disease. Watery diarrhea and pasty stools usually start 10 to 13 days postexposure to oocysts and can last 4 to 5 days. This is accompanied by anorexia and lethargy and is frequently fatal. Fecal analysis can provide a diagnosis, and treatment with sulphonamides is effective when combined with sanitation (10% ammonia kills oocysts) and housing improvements.37 Balantidium caviae, Trichomonas caviae, and Giardia duodenalis are other generally nonpathogenic protozoans found in guinea pigs.
Fecal impaction
Fecal impaction is predominately identified in older guinea pigs, especially boars. The exact cause is unknown, but inguinal gland infections, loss of muscle tone, and reduced coprophagy have all been implicated. Animals present with straining and have a large, foul-smelling impacted mass of feces and sebaceous secretions within a large, flaccid vent opening. Therapeutic interventions include dietary changes to increase fiber, mineral oil, and repeated manual evacuations using a cotton-tipped applicator. Long-term therapy is generally required.14, 22
Hepatic lipidosis
Hepatic lipidosis is a rapidly developing, fatal complication of anorexia, especially in obese guinea pigs. A pathogenic mobilization and uptake of fatty acids for glucose generation is a likely cause, although the mechanisms are unclear. Significant hepatic damage can occur within 48 hours; thus, anorexia for 12 hours or more is an emergency requiring nutritional support.14
Neoplasia
Neoplasms of the GI tract are rare in guinea pigs but can mimic clinical findings of more common GI disorders. Lymphosarcomas, adenocarcinomas of the stomach and cecum, and GI stromal tumors have been reported.38
Gastrointestinal Disease in the Rabbit
Gastric ulceration
Rabbits secrete higher levels of gastric acid and pepsin than do rats and guinea pigs, likely contributing to their higher incidence of gastric ulceration.39 In a review of 1000 rabbit postmortem examinations, Hinton40 reported 7.3% with gastric ulcers. The majority occurred in the fundus and without significant surrounding reaction, suggesting a stress response to other illness. The link between stress and ulcers can be experimentally demonstrated in rabbits by intraperitoneal injections of epinephrine, because rabbits serve as a model organism for ulcers.41 Similarly, hypovolemic shock can induce gastric ulcers in rabbits in a matter of hours.42 In Hinton’s review, 2% of the rabbits had solitary peptic ulcers, the majority of which were perforated and found in females dying in the peripartum period.40
Rabbits with gastric ulcers frequently present with anorexia and evidence of pain, such as bruxism. In more severe or perforated disease there may be clinical signs of anemia and shock. Physical examination may reveal acute abdomen with peritoneal signs. Radiographic imaging or ultrasonography is helpful to eliminate other causes, such as obstruction, neoplasia, or foreign body. Although endoscopy can provide direct visualization of ulcers, many animals are not sufficiently stable for an anesthetized procedure.13 Treatment should be aimed at controlling any underlying diseases, providing hydration and analgesia (discussed previously), mucosal protection with sucralfate, and acid blockade with ranitidine (2 mg/kg IV every 24 hours or 2–5 mg/kg by mouth every 12 hours).
Cecal impaction
Altered cecocolonic motility, as well as diets high in fine-particle indigestible fiber (such as psyllium), can cause dehydration and compaction of cecal and colonic contents into hard lumps, or cecoliths. Cecoliths are the most common cause of lower intestinal obstruction, most frequently in the sacculated colon. Frequently, this is a chronic problem and rabbits have a history of anorexia, abdominal pain, and failure to thrive. Many are also positive for Encephalitozoon cuniculi, suggesting a possible link.12
Clinical presentation correlates with severity of the obstruction and ranges from anorexia and abdominal pain to moribund animals requiring emergent attention. Cecoliths are readily palpable on physical examination, and abdominal imaging is helpful to gauge intestinal obstruction. Treatment involves SC or IV fluid therapy, analgesia (buprenorphine, 0.03–0.05 mg/kg SC/IV every 6–12 hours), and careful enemas to advance fecal contents without destroying the damaged colonic mucosa. Animals should initially be fed foods with a high water content supplemented with grass hay for fiber and can later be supplemented with canned pumpkin (1 tbsp every 12 hours) to boost water content.12
Dysautonomia
Dysautonomia is a rare idiopathic, progressive loss of autonomic system function. It was successfully documented in the rabbit after studies of animals with presumed mucoid enteropathy.43, 44 Clinical features are consistent with autonomic dysfunctions, including dry mucous membranes, mydriasis, urinary incontinence, bradycardia, proprioceptive deficits, cecal impaction, and loss of anal sphincter tone. Anorexia and depression are common. A presumptive diagnosis is made by the clinical findings, and radiography is helpful to document megaesophagus, aspiration pneumonia, and a dilated, impacted colon. Definitive diagnosis requires histologic documentation of chromolytic degeneration of autonomic neurons. Supportive care is provided, although the prognosis is poor in rabbits.
Bacterial enteritis
Bacterial enteritis from enteropathogenic E coli can cause large outbreaks in weaning commercial rabbits but is not reported in pet rabbits. The bacteria attach to cecal and colonic epithelial cells and cause effacement of the surface microvilli, inhibiting colonic absorption and causing watery diarrhea. Severity depends on the age of the rabbit and serotype, although mortality can be more than 50%. On necropsy, the cecal wall may have characteristic longitudinal, paintbrush hemorrhages. A presumptive diagnosis is made by isolation of E coli on fecal cultures, although serotyping is not commercially available. Supportive care as well as antimicrobial therapy with trimethoprim-sulfamethoxazole (30 mg/kg by mouth every 12 hours) or enrofloxacin (10 mg/kg by mouth every 12 hours) pending culture sensitivity results.45, 46
Enteritis caused by C piliforme (Tyzzer disease), a motile gram-variable spore-forming obligate intracellular bacterium, is found in many species of small mammals, including rabbits and guinea pigs. Infected animals, in particular weanlings, progress rapidly from onset of lethargy, anorexia, and watery diarrhea to acute death. Adults may have a more chronic course. Antemortem diagnosis is generally not possible because C piliforme is an intracellular bacterium that does not grow in culture. At necropsy, infections are marked by patchy necrosis in the liver and proximal colon and degenerative lesions of the myocardium. Treatment has not proved beneficial, and prevention is best achieved by disinfection (spores are killed with 0.3% sodium hypochlorite solution or 80°C heat for 30 min), good husbandry, and stress reduction, especially during weaning.12
Proliferative enteritis caused by L intracellularis, an intracellular, gram-negative, curved-to-spiraled bacterium, is most often found in swine but can also be found in rabbits and rodents. The intracellular organism infects enteric epithelial cells, causing a proliferative enteropathy marked by diarrhea and wasting.36 It is most common in weanlings (2–4 months) and can be treated with chloramphenicol (30–50 mg/kg by mouth/SC every 12 hours for 7–14 days) because macrolide antibiotics, used to treat L intracellularis in other species, are not recommended for use in rabbits.12
Viral diseases
Oral papillomatosis
Rabbit oral papillomatosis virus infection seems restricted to laboratory rabbits, especially New Zealand white rabbits, causing benign oral papillomas on the ventral surface of the tongue. Rarely, papillomas occur elsewhere in the mouth, and there is 1 report of a concomitant conjunctival papilloma.47 Papillomas start as small millimeter-sized sessile lesions and can grow into larger (3–5 mm) clusters of pedunculated papules. The lesions are benign and can persist for as long as 145 days.48
Rabbit enteric coronavirus
The virus was discovered as a cause of rapidly fatal enteritis in young (3–10 weeks) laboratory rabbits.49 Infected rabbits develop lethargy, diarrhea, abdominal swelling, pleural effusion, and cardiomyopathy and invariably die within 24 hours. At necropsy, the intestine is fluid-filled and the villi are effaced. The virus has hemagglutination activity and can be detected in the feces. A divergent coronavirus strain has recently been identified in game rabbits in Asia.50
Rotavirus
Rotavirus is highly infectious with a high morbidity and variable (generally low) mortality. Weanling rabbits (2–4 months) are most susceptible, and disease severity is increased with coinfection with another enteric pathogen.51 Antibodies to rotavirus are found in laboratory, commercial, and pet rabbits, indicating it can infect most strains.52 Infection impairs the sodium solute pumps on the enterocyte surface, impairing reabsorption.53 Rotavirus infections are marked by anorexia, dehydration, and green-yellow watery diarrhea. The intestines become distended and congested, with petechial hemorrhages, chronic inflammation, and villous atrophy. Diagnosis requires virus identification, and treatment is with supportive care.
Rabbit hemorrhagic disease virus
This calicivirus of the genus Lagovirus affects only European rabbits. It was first described in China in 1984 and rapidly spread throughout Asia, Australia, and New Zealand and into Europe, with rare outbreaks in the United States and elsewhere.54 Transmission is via direct contact (shed into urine, feces, and respiratory secretions), fomite contamination, and even by intermediate insect vectors. The disease occurs in rabbits over 2 months of age, in part due to its binding to the histo-blood group antigens H, A, and B type 2 oligosaccharides, which are present on the surface of mature respiratory and intestinal epithelial cells.55 The virus replicates in the liver, causing severe hepatic necrosis and eventual death from disseminated intravascular coagulation. The clinical presentation and course varies from a peracute disease lasting only 12 to 36 hours, followed by sudden death, to an acute or subacute febrile illness with anorexia, diarrhea (or constipation), neurologic, and other systemic symptoms, lasting a few days to weeks, to a persistent/latent disease with continued virus shedding.
Laboratory studies demonstrate a worsening lymphopenia and thrombocytopenia, with eventual prolonged prothrombin and thrombin times. At necropsy, there is extensive hepatic necrosis, splenomegaly, pulmonary hemorrhage, and evidence of disseminated intravascular coagulation. The virus cannot be cultured; thus, diagnosis requires molecular testing.54 Rabbit hemorrhagic disease virus is a reportable disease. Vaccination programs using attenuated vaccines have had mixed results. A recombinant vaccine has recently been developed and should assist prevention in endemic areas.56 The virus can be inactivated with 0.5% sodium hypochlorite or 1% formalin.
Parasitic diseases
Coccidiosis
Coccidia are the most common parasites of the rabbit GI tract and, although they cause significant disease in young (<6 months old) rabbits, they can be incidentally found in fecal studies in adult rabbits. Of the 12 species of the genus Eimeria, E stiedae is exclusive to the liver, with the rest causing intestinal disease. Hepatic coccidiosis is ubiquitous in commercial rabbitries and can be fatal in young rabbits by obstructing liver function. Severe disease is marked by anorexia, diarrhea, abdominal bloating, and icterus. Biochemical tests confirm hepatic disease, with aspartate aminotransferase, alanine aminotransferase, bile acids, and total bilirubin elevations. On necropsy, the liver is studded with nodular, encapsulated abscesses. Oocysts can be identified in bile or feces.
Intestinal coccidiosis is common in rabbits of all ages and most often associated with E perforans infection. Subclinical infection is common, and disease severity varies with age (worse under 6 months), species of Eimeria, parasite burden, and condition of the rabbit (stress, poor husbandry, and poor diet). Significant disease is marked by diarrhea with possible mucus or blood, dehydration, and weight loss. Intussusception is a complication of severe disease. Diagnosis depends on histopathology and/or fecal identification. Molecular assays have been developed to identify intestinal Eimeria spp.57 In addition to supportive care, sulfa drugs are most effective at limiting multiplication. Sulfadimethoxine (15 mg/kg by mouth every 12 hours) or trimethoprim-sulfamethoxazole (30 mg/kg by mouth every 12 hours) can be used for 10 days of therapy. Recovering rabbits develop lifelong immunity.12
Cryptosporidiosis
Cryptosporidium parvum infects the small intestine and causes a self-limited diarrheal illness (4–5 days duration) in young rabbits (peak, 30–40 days old). Illness is accompanied by anorexia, depression, and dehydration. The organism can be identified on histopathology. Other than supportive care, there is no effective treatment. Recently, reports of rabbit Cryptosporidium spp causing zoonotic disease in humans have been reported in several countries.58
Nematodes
Passalurus ambiguous, the rabbit pinworm, is found in most rabbits, and even large parasite burdens are not pathogenic. Adult worms reside in the cecum and colon, and transmission is direct by ingestion of eggs during cecotrophy. Diagnosis is often routine by identification of worms or eggs in the feces, although identification should not prompt treatment in most cases. When treatment is necessary, benzimidazoles, such as fenbendazole (10–20 mg/kg by mouth, repeated in 10–14 days), are effective.12
Aflatoxicosis
Aflatoxins produced by the fungi, Aspergillus flavus and Aspergillus parasiticus, cause liver and biliary damage in rabbits. Rabbits are the most sensitive species to these toxins and serve as an animal model for aflatoxicosis.59 Outbreaks occur from contaminated feed and are accompanied by anorexia, depression, and weight loss, progressing to icterus and death within 3 to 4 days.60 On necropsy, livers are congested with periportal and ductal fibrosis, sinusoidal dilation, and hepatocyte degenerative changes. Treatment involves removal of contaminated feed and supportive care.
Neoplasia
GI neoplasms in rabbits include epithelial and smooth muscle tumors. Epithelial tumors include gastric adenocarcinoma, papilloma of the sacculus rotundus, papillomas of the rectal squamocolumnar junctional mucosa, and metastatic tumors, especially uterine adenocarcinoma. Smooth muscle tumors include leiomyoma and leiomyosarcoma of the stomach and intestines. Clinically, these tumors can present as intestinal obstruction.61 Biliary tumors, such as bile duct adenoma and carcinoma, are reported in the rabbit.62
Lead Toxicity
Signs of lead toxicity in rabbits include neurologic presentations, such as seizures, torticollis, and blindness, but more common signs may be nonspecific and include anemia, anorexia, loss of body condition, and GI stasis.63 Lead toxicity should be a differential in rabbits that chew baseboards or paint in older houses and have nucleated red cells or basophilic stippling on blood smears. Lead levels greater than 10 μg/dL are diagnostic for lead poisoning.63 The authors diagnose at least 1 to 2 cases of lead toxicity in rabbits annually and signs usually involve GI stasis or loose stools (Fig. 2 ).64 Affected rabbits can be treated with calcium ethylenediaminetetraacetic acid (30 mg/kg SC every 12 hours for 5–7 days) in addition to supportive care for GI stasis. Debilitated animals should be hospitalized for more intensive supportive care but stable animals can be treated at home with SC injections given by the owner. The source of lead should be determined and eliminated from the rabbit’s environment to prevent further intoxication.
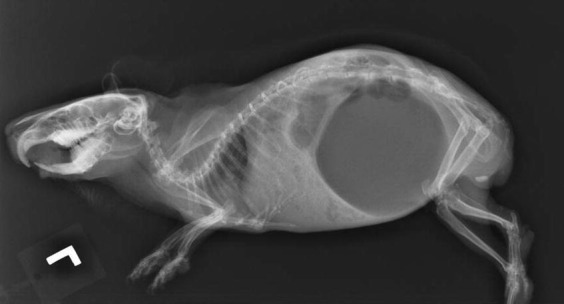
Fig. 2.
Lateral radiographic projection of a rabbit with lead toxicosis (lead >65 μg/dL; normal <10 μg/dL). This rabbit presented with a 2-week history of diarrhea that had been refractory to antibiotic therapy by the referring veterinarian. Note that this radiograph is consistent with nonspecific GI stasis and no metallic densities are seen on the films.
Colonic Entrapment
Partial colonic entrapment and chronic recurring GI stasis may result from adhesions after ovariohysterectomy.64 Practitioners should keep this differential in mind if seeing a recently spayed female rabbit with recurring ileus. A mass effect may be palpated in the region of adhesions and colonic segments cranial to the area of entrapment may be dilated. Radiographs and ultrasound can be helpful to assess these patients and surgical exploratory is indicated in patients with significant disease. Due to the delicate nature of the rabbit intestinal tract, prognosis may be poor if there is significant accompanying colonic pathology.
Liver Lobe Torsion
One of the authors (JG) has documented 16 cases of liver torsion in rabbits at a single referral institution in 5 years.64, 65, 66 Rabbits with this condition present with nonspecific signs of GI stasis and some have cranial abdominal pain or an abnormally placed liver lobe on abdominal palpation. Because signs can be nonspecific, it is advisable to perform blood work on all rabbits presenting with nonspecific signs of GI stasis. If liver enzymes are elevated, abdominal ultrasound is recommended. Ultrasound reveals lack of blood flow in the affected liver lobe on color flow Doppler (Fig. 3 ) and is diagnostic for liver lobe torsion. Prompt surgical removal of the affected lobe is advisable if the patient is stable for surgery.65 If an owner declines surgery, supportive care measures alone (fluids, syringe feeding, prokinetic agents, analgesics, and antibiotics, if indicated) are still indicated. The author (JG) has documented survival in 3 of 6 rabbits with liver lobe torsion treated with supportive care measures alone.
Fig. 3.
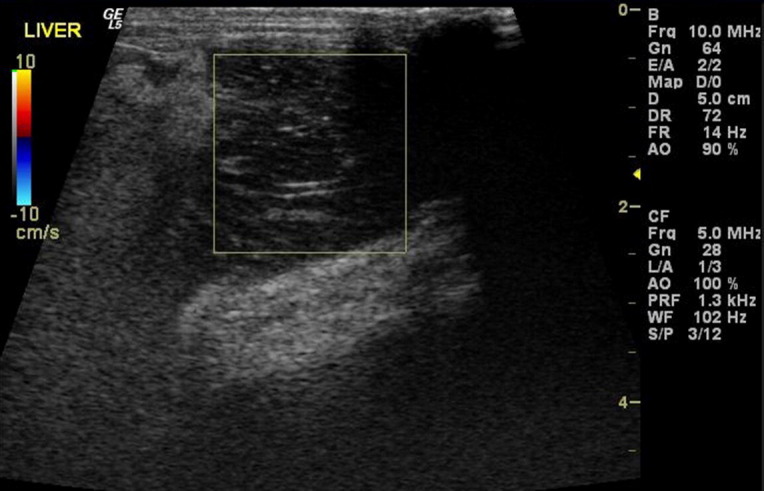
Ultrasound image of a rabbit with a caudate liver lobe torsion. The box surrounds the torsed lobe and demonstrates a lack of blood flow in the affected liver lobe on color flow Doppler. Note the surrounding hyperechoic fat, a common finding seen on ultrasound of liver lobe torsions in rabbits.
Footnotes
The authors have nothing to disclose.
References
- 1.Reiter A.M. Pathophysiology of dental disease in the rabbit, guinea pig, and chinchilla. J Exo Pet Med. 2008;17(2):70–77. [Google Scholar]
- 2.Capello V. Diagnosis and treatment of dental disease in pet rodents. J Exo Pet Med. 2008;17(2):114–123. [Google Scholar]
- 3.Harcourt-Brown F.M. The progressive syndrome of acquired dental disease in rabbits. J Exo Pet Med. 2007;16(3):146–157. [Google Scholar]
- 4.Lennox A.M. Diagnosis and treatment of dental disease in pet rabbits. J Exo Pet Med. 2008;17(2):107–113. [Google Scholar]
- 5.Gracis M. Clinical technique: normal dental radiography of rabbits, guinea pigs, and chinchillas. J Exo Pet Med. 2008;17(2):78–86. [Google Scholar]
- 6.Hernandez-Divers S.J. Clinical technique: dental endoscopy of rabbits and rodents. J Exo Pet Med. 2008;17(2):87–92. [Google Scholar]
- 7.Capello V., Cauduro A. Clinical technique: application of computed tomography for diagnosis of dental disease in the rabbit, guinea pig, and chinchilla. J Exo Pet Med. 2008;17(2):93–101. [Google Scholar]
- 8.Lennox A.M. Clinical technique: small exotic companion mammal dentistry– anesthetic considerations. J Exo Pet Med. 2008;17(2):102–106. [Google Scholar]
- 9.Wenger S. Anesthesia and analgesia in rabbits and rodents. J Exo Pet Med. 2012;21(1):7–16. [Google Scholar]
- 10.Clauss M. Clinical technique: feeding hay to rabbits and rodents. J Exo Pet Med. 2012;21(1):80–86. [Google Scholar]
- 11.Capello V. Clinical technique: treatment of periapical infections in pet rabbits and rodents. J Exo Pet Med. 2008;17(2):124–131. [Google Scholar]
- 12.Olglesbee B.L., Jenkins J.R. Rabbits: gastrointestinal diseases. In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits, and rodents: clinical medicine and surgery. 3rd edition. Saunders Elsevier; St Louis (MO): 2012. pp. 193–204. [Google Scholar]
- 13.Reusch B. Rabbit gastroenterology. Veterinary Clin North Am Exot Anim Pract. 2005;8(2):351–375. doi: 10.1016/j.cvex.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkins M.G., Bishop C.R. Disease problems of guinea pigs. In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits, and rodents: clinical medicine and surgery. 3rd edition. Saunders Elsevier; St Louis (MO): 2012. pp. 295–310. [Google Scholar]
- 15.Harcourt-Brown T.R. Management of acute gastric dilation in rabbits. J Exo Pet Med. 2007;16(3):168–174. [Google Scholar]
- 16.Theus M., Bitterli F., Foldenauer U. Successful treatment of a gastric trichobezoar in a Peruvian guinea pig (cavia aperea porcellus) J Exo Pet Med. 2008;17(2):148–151. [Google Scholar]
- 17.Lichtenberger M., Lennox A. Updates and advanced therapies for gastrointestinal stasis in rabbits. Veterinary Clin North Am Exot Anim Pract. 2010;3(3):525–541. doi: 10.1016/j.cvex.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins M.G., Graham J.E. Emergency and critical care of rodents. Vet Clin North Am Exot Anim Pract. 2007;10(2):501–531. doi: 10.1016/j.cvex.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Tamura Y. Current approach to rodents and patients. J Exo Pet Med. 2010;19(1):36–55. doi: 10.1053/j.jepm.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenthal KL, Harris J, Rankin S. Molecular analysis of the gastrointestinal microbiota of Oryctolagus cuniculus. Proceedings. In Proceedings of the Association of Avian Veterinarians (AAV)/Association of Exotic Mammal Veterinarians (AEMV) Annual Conference. Seattle (WA): AAV/AEMV; 2011. p. 153.
- 21.Johnson DH. The gastrointestinal tract of the rabbit: health and disease (part II). Proceedings. In: Proceeding of the American Board of Veterinary Practitioners (ABVP) Symposium. San Antonio (TX): ABVP; 2012.
- 22.Johnson DH. The gastrointestinal tract of the guinea pig: health and disease. Proceedings. In: Proceedings of the American Board of Veterinary Practitioners (ABVP) Symposium. San Antonio (TX): ABVP; 2012.
- 23.Xia Y., Hu H.Z., Pothoulakis C. Clostridium difficile toxin A excites enteric neurones and suppresses sympathetic neurotransmission in the guinea pig. Gut. 2000;46(4):481–486. doi: 10.1136/gut.46.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papatheodorou P., Wilczek C., Nölke T. Identification of the cellular receptor of Clostridium spiroforme toxin. Infect Immun. 2012;80(4):1418–1423. doi: 10.1128/IAI.06378-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harcourt-Brown F. 1st edition. Alden Press; Oxford (UK): 2002. Textbook of rabbit medicine. p. 284, 285. [Google Scholar]
- 26.Haligur M., Ozmen O., Demir N. Pathological and ultrastructural studies on mucoid enteropathy in new zealand rabbits. J Exo Pet Med. 2009;18(3):224–228. [Google Scholar]
- 27.Lipman N.S., Weischedel A.K., Connars M.J. Utilization of cholestyramine resin as a preventative treatment for antibiotic (clindamycin)-induced enterotoxaemia in the rabbit. Lab Anim. 1992;26:1–8. doi: 10.1258/002367792780809039. [DOI] [PubMed] [Google Scholar]
- 28.Agnoletti F., Ferro T., Guolo A. A survey of Clostridium spiroforme antimicrobial susceptibility in rabbit breeding. Vet Microbiol. 2009;136(1–2):188–191. doi: 10.1016/j.vetmic.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Wasson K., Criley J.M., Clabaugh M.B. Therapeutic efficacy of oral lactobacillus preparation for antibiotic-associated enteritis in guinea pigs. Contemp Top Lab Anim Sci. 2000;39(1):32–38. [PubMed] [Google Scholar]
- 30.Dudley E.S., Boivin G.P. Gastric volvulus in guinea pigs: comparison with other species. J Am Assoc Lab Anim Sci. 2011;50(4):526–530. [PMC free article] [PubMed] [Google Scholar]
- 31.Keith J.C., Jr., Rowles T.K., Warwick K.E. Acute gastric distention in guinea pigs. Lab Anim Sci. 1992;42(4):331–332. [PubMed] [Google Scholar]
- 32.Lee K.J., Johnson W.D., Lang C.M. Acute gastric dilation associated with gastric volvulus in the guinea pig. Lab Anim Sci. 1977;27(5 Pt 1):685–686. [PubMed] [Google Scholar]
- 33.Mitchell E.B., Hawkins M.G., Gaffney P.M. Gastric dilation-volvulus in a guinea pig (Cavia porecllus) J Am Anim Hosp Assoc. 2010;46(3):174–180. doi: 10.5326/0460174. [DOI] [PubMed] [Google Scholar]
- 34.Ward M.L. Rodents: digestive system disorders. In: Keeble E., Meredith A., editors. BSAV manual of rodents and ferrets. British Small Animal Veterinary Association; Gloucester (United Kingdom): 2009. pp. 123–141. [Google Scholar]
- 35.Percy D.H., Barthold S.W. Guinea pig. In: Percy D.H., Barthold S.W., editors. Pathology of laboratory rodents and rabbits. 2nd edition. Blackwell Publishing; Ames (IA): 2001. pp. 209–247. [Google Scholar]
- 36.Lawson G.H., Gebhardt C.J. Proliferative enteropathy. J Comp Pathol. 2000;122:77–100. doi: 10.1053/jcpa.1999.0347. [DOI] [PubMed] [Google Scholar]
- 37.Elsheikha H., Brown P., Skuse A. Death and diarrhea in guinea pigs (Cavia porcellus) Lab Anim. 2009;38(6):189–191. doi: 10.1038/laban0609-189. [DOI] [PubMed] [Google Scholar]
- 38.Jelinek F., Hron P., Hozmanova F. Gastrointestinal stromal tumor in a guinea pig: a case report. Acta Vet Brno. 2009;78:287–291. [Google Scholar]
- 39.Redfern J.S., Lin H.J., McArthur K.E. Gastric acid and pepsin secretion in conscious rabbits. Am J Physiol. 1991;261:G295–G304. doi: 10.1152/ajpgi.1991.261.2.G295. [DOI] [PubMed] [Google Scholar]
- 40.Hinton M. Gastric ulceration in the rabbit. J Comp Pathol. 1980;90:475–481. doi: 10.1016/0021-9975(80)90017-1. [DOI] [PubMed] [Google Scholar]
- 41.Man W.K., Silcocks P.B., Wales R. Histology of experimental stress ulcer: the effects of cimetidine on adrenaline gastric lesions in the rabbit. Br J Exp Pathol. 1981;62(4):411–418. [PMC free article] [PubMed] [Google Scholar]
- 42.Collin B.J. Stress ulcer induced by hypovolemic shock in female rabbit. Anat Histol Embryol Zentral Vet. 1977;6(1):94. [Google Scholar]
- 43.Van der Hage M, Dorrestein GM. Cecal impaction in the rabbit: relationship with dysautonomia. Paper presented at the 6th World Rabbit Conference Lempdes. France, July 9–12, 1996. p. 77–80.
- 44.Whitwell K., Needham J. Mudoid enteropathy in UK rabbits: dysautonomia confirmed. Vet Rec. 1996;139:323–324. [PubMed] [Google Scholar]
- 45.Blanco J.E., Blanco M., Blanco J. Prevalence and characteristics of enteropathogenic Escheria coli with the eae gene in diarrhoeic rabbits. Microbiol Immunol. 1997;41(2):77–82. doi: 10.1111/j.1348-0421.1997.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 46.Swennes A.G., Buckley E.M., Parry N.M. Enzootic enteropathogenic Escheria coli infection in laboratory rabbits. J Clin Microbiol. 2012;50(7):2353–2358. doi: 10.1128/JCM.00832-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munday J.S., Aberdein D., Squires R.A. Persistent conjunctival papilloma due to oral papillomavirus infection in a rabbit in New Zealand. J Am Assoc Lab Anim Sci. 2007;46(5):69–71. [PubMed] [Google Scholar]
- 48.Sundberg J.P., Junge R.E., El Shazly M.O. Oral papillomatosis in New Zealand white rabbits. Am J Vet Res. 1985;46:664–668. [PubMed] [Google Scholar]
- 49.LaPierre J., Marsolais G., Pilon P. Preliminary report on the isolation of a coronavirus in the intestine of the laboratory rabbit. Can J Microbiol. 1980;26:1204–1208. doi: 10.1139/m80-201. [DOI] [PubMed] [Google Scholar]
- 50.Lau S.K., Woo P.C., Yip C.C. Isolation and characterization of a novel Betacoronavirus subgroup A coronavirus, rabbit coronavirus HKU14, from domestic rabbits. J Virol. 2012;86(10):5481–5496. doi: 10.1128/JVI.06927-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciarlet M., Gilger M.A., Barone C. Rotavirus disease, but not infection and development of intestinal histopathological lesions, is age restricted in rabbits. Virology. 1998;251(2):343–360. doi: 10.1006/viro.1998.9406. [DOI] [PubMed] [Google Scholar]
- 52.DiGiacomo R.F., Thouless M.E. Epidemiology of naturally occurring rotavirus infection in rabbits. Lab Anim Sci. 1986;36(2):153–156. [PubMed] [Google Scholar]
- 53.Halaihel N., Lievin V., Alvarado F. Rotavirus infection impairs intestinal brush-border membrane Na+ - solute cotransport activities in young rabbits. Am J Physiol Gastrointest Liver Physiol. 2000;279(3):G587–G596. doi: 10.1152/ajpgi.2000.279.3.G587. [DOI] [PubMed] [Google Scholar]
- 54.Abrantes J., Van Der Loo W., Le Pendu J. Rabbit hemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): a review. Vet Res. 2012;43(12):1–19. doi: 10.1186/1297-9716-43-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nystrom K., Le Gall-Reculé G., Grassi P. Histo-blood group antigens act as attachment factors of rabbit hemorrhagic disease virus infection in a virus strain-dependent manner. PLoS Pathog. 2011;7:e1002188. doi: 10.1371/journal.ppat.1002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X., Qiu L., Hao H. Adenovirus-based oral vaccine for rabbit hemorrhagic disease. Vet Immunol Immunopathol. 2012;145(1–2):277–282. doi: 10.1016/j.vetimm.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 57.Oliveira U.C., Fraga J.S., Licois D. Development of molecular assays for the identification of the 11 Eimeria species of the domestic rabbit (Oryctolagus cuniculus) Vet Parasitol. 2011;176(2–3):275–280. doi: 10.1016/j.vetpar.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 58.Chalmers R.M., Robinson G., Elwin K. Cryptosporidium sp. rabbit genotype, a newly identified human pathogen. Emerg Infect Dis. 2009;15(5):829–830. doi: 10.3201/eid1505.081419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clark J.D., Jain A.V., Hatch R.C. Experimentally induced chronic aflatoxicosis in rabbits. Am J Vet Res. 1980;41(11):1841–1845. [PubMed] [Google Scholar]
- 60.Krishna L., Dawra R.K., Vaid J. An outbreak of aflatoxicosis in Angora rabbits. Vet Hum Toxicol. 1991;33(2):159–161. [PubMed] [Google Scholar]
- 61.Harcourt-Brown F.M. Gastric dilation and intestinal obstruction in 76 rabbits. Vet Rec. 2007;161(12):409–414. doi: 10.1136/vr.161.12.409. [DOI] [PubMed] [Google Scholar]
- 62.DeCubellis J., Kruse A.M., McCarthy R.J. Billiary cystadenoma in a rabbit (Oryctolagus cuniculus) J Exo Pet Med. 2010;19(2):177–182. [Google Scholar]
- 63.Fisher P.G., Carpenter J.W. Neurologic and musculoskeletal diseases. In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits, and rodents: clinical medicine and surgery. 3rd edition. Saunders Elsevier; St Louis (MO): 2012. pp. 245–256. [Google Scholar]
- 64.Graham JE. GI stasis in rabbits: when it’s not just “ileus”. Proceedings. In: Proceedings of the Association of Avian Veterinarians (AAV) Symposium. Louisville (KY): AAV; 2012. p. 71–4.
- 65.Stanke N.J., Graham J.E., Orcutt C.J. Successful outcome of hepatectomy as treatment for liver lobe torsion in four domestic rabbits. J Am Vet Med Assoc. 2011;238(9):1176–1183. doi: 10.2460/javma.238.9.1176. [DOI] [PubMed] [Google Scholar]
- 66.Graham JE. Liver lobe torsion in rabbits. Proceedings. In: Proceedings of the Association of Avian Veterinarians (AAV) Symposium. Louisville (KY): AAV; 2012. p. 83.