Abstract
Objectives
Highly pathogenic viruses such as EBOV are a threat to routine laboratory workers. Inactivation procedures with Triton X-100 0.1% and/or heat are currently recommended, but have unknown effects on the accuracy of serological testing. Furthermore, virus inactivation by Triton X-100 0.1% was shown to be ineffective in serum. This study aimed to demonstrate virus inactivation in serum by Triton X-100 1% and maintained accuracy of serological testing.
Methods
A panel of 19 serological tests was run on patient serum samples after treatment with Triton X-100 1%, 0.1%, and 0.1% + heat inactivation at 60°C for 1 h. Mean differences between measurements (bias) were calculated applying the Bland–Altman method. To determine effectiveness of virus inactivation, herpes simplex virus 1 (HSV-1) was spiked into medium containing 90% or 1% serum, and treated with Triton X-100 0.1% or 1%. Infectious titres were then determined on Vero cells.
Results
Serological measurements showed good agreement between controls and samples treated with Triton X-100 0.1% and 1%, with an estimated bias of 0.6 ± 9.2% (n = 258) and –0.1 ± 18.6% (n = 174), respectively. Discordant qualitative results were rare. Conversely, heat inactivation alone and combined with Triton X-100 0.1% triggered a bias of 17.5 ± 66.4% (n = 200) and 37.9 ± 79.8% (n = 160), respectively. Triton X-100 1% completely inactivated HSV-1 in 1% and 90% serum while Triton X-100 0.1% failed to do so in 90% serum.
Conclusions
Unlike heat inactivation, Triton X-100 1% enabled accurate serological testing and completely inactivated HSV-1 in serum. This simple method could allow safe routine serological diagnostics in high-risk patients.
Keywords: Biosafety, Diagnosis of infections, Serological tests, Triton X-100, Virus inactivation
Graphical abstract
Introduction
Highly pathogenic viruses such as EBOV or SARS coronavirus are a continuous threat to clinical laboratory workers. The current epidemic of Lassa virus in Nigeria is one of the latest concerns [1] and European hospitals must be prepared to deal with potentially infected returning travellers. While the microbiological diagnosis of such viruses should be performed under the required biosafety measures in specialized laboratories, blood samples from patients arriving at the emergency rooms are often sent directly to the routine laboratory where biosafety levels are low.
Early symptoms caused by highly pathogenic viruses are usually non-specific and require differential diagnosis of other infectious diseases such as malaria, Dengue fever, HIV, HBV, or CMV infections. In confirmed cases, a potentially long stay in intensive care increases risks of secondary infections such as invasive fungal infections. Diagnosis of these pathologies remains mostly based on serology. Polymerase chain reaction (PCR) is an alternative diagnostic method that can be safely performed on infectious samples [2], [3]; however, it is only appropriate during the viraemic phase.
To inactivate highly pathogenic viruses in serum the World Health Organization (WHO) and the US Center for Diseases Control and Prevention (CDC) recommend chemical inactivation with Triton X-100 0.1%, heat inactivation at 56°C for 30 min, or a combination thereof [4], [5]. Triton X-100 is a non-ionic surfactant which can disrupt the lipid membrane of enveloped viruses without affecting proteins. It is therefore widely used in combination with tri-(N-butyl)-phosphate (TnBP) for solvent–detergent inactivation procedures of plasma products for transfusion [6]. Besides, Triton X-100 0.1% has been successfully evaluated for haematology and clinical chemistry [7], [8], [9], [10] and for malaria rapid diagnostic tests (RDTs) [11], [12]. Accuracy of immunoassays after Triton X-100 treatment of serum was only partially assessed in 1988 and no decrease in sensitivity was reported [13].
Heat inactivation was compatible with detection of EBOV-specific IgG and IgM [14] and other viral antigens [15]. However clinical chemistry studies showed that protein measurements and especially enzymatic activity measurements were mildly to severely affected by heat [8], [16], [17], [18]. Other easily applicable inactivation methods such as SDS treatment unfortunately denature proteins and are not suited for serological or clinical chemistry tests [10], [19].
Efficacy of heat inactivation was demonstrated for several enveloped viruses [20], [21], including haemorrhagic fever viruses [18]. Concerning detergents, complete inactivation of HIV was achieved with 0.2% of Triton X-100 [13] whereas EBOV treatment with 0.1% of Triton X-100 led to incomplete inactivation with a 4 log infectivity reduction [22]. Besides, it has been recently claimed that the inactivation potential of Triton X-100 0.1% was annulled in serum [10]. Inactivation tests were indeed performed on EBOV and herpes simplex virus type 1 (HSV-1) in 0.98%, 9.8%, or 98% serum, and viral inactivation by Triton X-100 only succeeded in medium with less than 10% serum, suggesting that some factors in serum interfere with detergent effects. In practice, Triton X-100 concentration is often increased to 1% to ensure a higher level of safety although no inactivation data support this choice. It thus remains to be determined if a higher concentration of Triton X-100 can overcome efficiency issues in serum.
The objective of our study was to assess the accuracy of a wide range of serological tests after chemical or thermal inactivation of serum. Triton X-100 0.1% or 1% final concentration and/or heat inactivation at 60°C for 60 min were selected as promising inactivation methods. In addition, HSV-1 inactivation tests were performed with Triton X-100 0.1% and 1% in medium containing 1% or 90% serum. We hypothesize that a tenfold increase of Triton X-100 final concentration might allow complete virus inactivation in serum.
Methods
Ethics and sample selection
All human blood samples were obtained for routine patient serological testing at the Institute for Infectious Diseases, University of Bern, Switzerland. There was no suspicion of highly pathogenic virus infection in these patients. Sera used in this study were stored at –20°C for less than 5 years. They were retrospectively identified using the electronic database of the Institute for Infectious Diseases of the University of Bern according to the serological tests originally performed and to the results obtained. Each selected serum sample was anonymized and tested for the same serological test as originally. Informed consent for use of anonymized leftover patient material was waived. This procedure has been approved by the Cantonal Research Ethics Committee of Bern, Switzerland (BASEC-No Req-2018-00288).
Serum inactivation for serological testing
The chemical inactivation procedure consisted of mixing patient serum 10:1 with PBS or Triton X-100 1% or 10% working solution (please see Supplementary Materials). Samples were incubated for 60 min at room temperature. Final concentrations of Triton X-100 were 0.1% and 1% (precisely 0.09% and 0.9%). Some of the samples treated with PBS or Triton X-100 1% were additionally thermally inactivated by incubation at 60°C for 1 h in a table top thermoblock without shaking. Samples were then kept a maximum of 24 h at 4°C before being used for different serological tests.
Serological tests
Pre-treated samples were analysed in parallel with the following methods. HIV, HCV, HBV, EBV, CMV, and toxoplasmosis markers (Ag, total Ig, IgM and/or IgG) were analysed on an Architect® system (Abbott Diagnostics), a fully automated immuno-analyser based on chemiluminescent microparticle immunoassays (CMIAs). Antibodies to Brucella species (IgM and IgG) were tested by indirect chemiluminescent immunoassay (CLIA) on another fully automated analyser (VirClia®, VirCell, Granada, Spain). For aspergillosis, a galactomanan test (PLATELIA™ ASPERGILLUS Ag, Biorad 62794, Hercules, CA, USA) was performed manually. Antibodies to Dengue virus (IgM, IgG) were detected by a manual ELISA (Euroimmun EI 266b-9601).
Reactivity to Francisella tularensis was evaluated by a rapid test (Virapid Tularemia, Viracell Microbiologists VR006) and by a microagglutination test using F. tularensis Ag (Becton Dickinson 241050, Franklin Lakes, NJ, USA). All manual tests were performed according to the manufacturer's instructions.
Please see Supplementary Materials for further details.
HSV-1 inactivation and titration
Concentrated HSV-1 stock was spiked in MEM containing 1% FBS or in pure human serum from an HSV-1 seronegative donor. It was then mixed 10:1 with Triton X-100 working solution and incubated for 1 h at room temperature. Final concentrations of Triton X-100 were 0.1% and 1% (precisely 0.09% and 0.9%). Final concentrations of serum were 90% for human serum and 1% for FBS. Initial viral titre in these samples ranged between 1.4 × 107 and 7.6 × 107 TCID50/mL. Excess detergent was removed from inactivated viral samples (please see Supplementary Materials) and each sample was then titrated on Vero cells (please see Supplementary Materials). Each experiment was repeated twice. Controls containing Triton X-100 without virus were also filtered and titrated to evaluate cytotoxicity caused by residual detergent.
Statistics
Graphpad Prism (version 7.04) was used to plot and analyse data. For serological test results, Bland–Altman plots were constructed to evaluate differences between treated and untreated samples as percentage of differences from the mean (bias). Serological results from chemically inactivated samples were additionally analysed by the Deming linear regression model. Spearman's correlation coefficients were calculated (data did not pass the D'Agostino and Pearson normality test). To assess agreement of qualitative items Cohen's weighted kappa coefficients were calculated with GraphPad QuickCalcs.
HSV-1 titres were log-converted to obtain a near-normal distribution for statistical analysis. One-way analysis of variance (ANOVA) was performed followed by Dunnett's post test to compare multiple groups to a control group. A p value >0.05 was considered not significant (ns); p < 0.001 was considered highly significant (***).
Results
Serological test results of chemically or thermally inactivated patient serum samples
Patient serum samples received for routine serological testing were chemically inactivated with Triton X-100 at a final concentration of 0.1% or 1% and compared with PBS-treated controls by several ELISA-based serological assays (Table 1 ). Samples were carefully selected to cover a wide range of concentrations. Qualitative results were assessed for each sample and a false result rate (FRR) was calculated by computing false-negative and false-positive results compared with controls for each test.
Table 1.
Accuracy of ELISA-based serological tests after chemical and/or thermal inactivation of serum
| Baseline values | Triton X-100 0.1% |
Triton X-100 1% |
Heat |
Triton X-100 0.1% + heat |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % Bias ± SD | 95% LoA | FRR, % (95% CI) | n | % Bias ± SD | 95% LoA | FRR, % (95% CI) | n | % Bias ± SD | 95% LoA | FRR, % (95% CI) | n | % Bias ± SD | 95% LoA | FRR, % (95% CI) | ||
| CMIA | 201 | –0.9 ± 9.4 | –19.4; 17.6 |
0 (0–1.9) |
117 | –2.7 ± 20.8 | –43.4; 38.1 |
0.9 (0–4.7) |
200 | 17.5 ± 66.4 | –112.6; 147.5 |
14.5 (10.3–20) |
160 | 37.9 ± 79.8 | –118.4; 194.2 |
25.6 (19.5–32.9) |
|
| HIV Ag + Ab | 0.2–837.9 S/CO | 17 | 0.3 ± 1.6 | –2.9; 3.5 | 0 (0–18.4) |
10 | 4.2 ± 6 | –7.4; 15.9 | 0 (0–27.8) |
17 | –8.2 ± 34.8 | –76.5; 60 | 5.9 (0.3–27) |
10 | –15.2 ± 44 | –101.4; 70.9 | 10 (0.5–40.4) |
| HCV IgG | 0.2–14.1 S/CO | 17 | –2.2 ± 7.5 | –16.9; 12.4 | 0 (0–18.4) |
10 | –1.2 ± 4.3 | –9.7; 7.2 | 0 (0–27.8) |
17 | 41 ± 62 | –80.6; 162.6 | 11.8 (2.1–34.3) |
11 | 84.8 ± 61.2 | –35.1; 204.8 | 63.6 (35.4–84.8) |
| HBV HBs Ag | 0.22–5410.5 S/CO | 22 | –7.6 ± 10.2 | –27.5; 12.3 | 0 (0–14.9) |
10 | –14.5 ± 34.4 | –81.9; 53 | 0 (0–27.8) |
22 | 10 ± 20.1 | –29.4; 49.4 | 0 (0–14.9) |
15 | 12.4 ± 26.3 | –39.1; 63.9 | 0 (0–20.4) |
| HBV HBs IgG | 0.5–900 mIU/mL | 22 | 2.9 ± 8.9 | –14.6; 20.3 | 0 (0–14.9) |
10 | –11.6 ± 32.2 | –74.7; 51.6 | 0 (0–27.8) |
21 | –12.2 ± 24.7 | –60.7; 36.2 | 4.8 (0.2–22.7) |
8 | –16.9 ± 43.7 | –102.6; 68.8 | 0 (0–32.4) |
| HBV HBc Ig | 0.2–10.1 S/CO | 22 | –1.1 ± 6.3 | –13.4; 11.3 | 0 (0–14.9) |
10 | –16.8 ± 43.9 | –102.7; 69.2 | 10 (0.5–40.4) |
22 | 24.9 ± 47.6 | –68.4; 118.1 | 9.1 (1.6–27.8) |
15 | 66.8 ± 59.7 | –50.2; 183.7 | 26.7 (10.9–52) |
| EBV VCA IgM | 0.2–19.2 S/CO | 15 | 0.2 ± 3.3 | –6.2; 6.6 | 0 (0–20.4) |
9 | 2.5 ± 4.2 | –5.7; 10.7 | 0 (0–29.9) |
15 | –35.7 ± 37.8 | –109.8; 38.4 | 0 (0–20.4) |
15 | –37.5 ± 47.9 | –131.4; 56.5 | 0 (0–20.4) |
| EBV VCA IgG | 0.2–68.8 S/CO | 15 | 2.3 ± 16.5 | –30; 34.7 | 0 (0–20.4) |
9 | –2.7 ± 3.4 | –9.2; 3.9 | 0 (0–29.9) |
15 | 69 ± 83.3 | –94.4; 232.3 | 13.3 (2.4–37.9) |
15 | 84 ± 78.3 | –69.4; 237.5 | 13.3 (2.4–37.9) |
| EBV EBNA IgG | 0.2–21.2 S/CO | 15 | 1.4 ± 3.9 | –6.3; 9.1 | 0 (0–20.4) |
9 | –1.9 ± 4.4 | –10.6; 6.8 | 0 (0–29.9) |
15 | 50.3 ± 55.2 | –57.9; 158.6 | 40 (19.8–64.3) |
15 | 72.1 ± 66.6 | –58.4; 202.5 | 60 (35.7–80.2) |
| CMV IgM | 0.2–8.7 S/CO | 14 | 1.6 ± 4.9 | –8; 11.2 | 0 (0–21.5) |
10 | 4 ± 7.6 | –10.9; 18.9 | 0 (0–27.8) |
14 | –0.8 ± 80.8 | –159.1; 157.5 | 21.4 (7.6–47.6) |
14 | 60.1 ± 72.5 | –81.9; 202.2 | 21.4 (7.6–47.6) |
| CMV IgG | 0.27–225 AU/mL | 14 | –2.8 ± 19.4 | –40.8; 35.2 | 0 (0–21.5) |
10 | 11.8 ± 17.3 | –22; 45.6 | 0 (0–27.8) |
14 | 95.2 ± 92.9 | –86.8; 277.2 | 50 (26.8–73.2) |
14 | 109.3 ± 95.2 | –77.3; 295.9 | 57.1 (32.6–78.6) |
| Toxo IgM | 0.2–19.1 S/CO | 14 | –1.6 ± 4.5 | –10.4; 7.3 | 0 (0–21.5) |
10 | –1.8 ± 6.8 | –15.1; 11.6 | 0 (0–27.8) |
14 | –44.6 ± 33.3 | –109.7; 20.6 | 21.4 (7.6–47.6) |
14 | –49.1 ± 36.5 | –120.6; 22.5 | 21.4 (7.6–47.6) |
| Toxo IgG | 0.2–180 IU/mL | 14 | –2.8 ± 7.7 | –17.9; 12.3 | 0 (0–21.5) |
10 | –3.6 ± 9.8 | –22.8; 15.5 | 0 (0–27.8) |
14 | 33.7 ± 72.8 | –109.1; 176.4 | 14.3 (2.5–39.9) |
14 | 54.4 ± 77.8 | –98.1; 207 | 28.6 (11.7–54.6) |
| CLIA | 26 | 1.3 ± 7.7 | –13.7; 16.4 |
0 (0–12.9) |
26 | 3.4 ± 11.2 | –18.4; 25.3 |
0 (0–12.9) |
|||||||||
| Brucella IgM | 0.2–5.3 index | 13 | 2.4 ± 5.6 | –8.4; 13.3 | 0 (0–22.8) |
13 | 5.5 ± 9.7 | –13.5; 24.6 | 0 (0–22.8) |
||||||||
| Brucella IgG | 0.2–10 index | 13 | 0.2 ± 9.5 | –18.4; 18.7 | 0 (0–22.8) |
13 | 1.3 ± 12.5 | –23.1; 25.7 | 0 (0–22.8) |
||||||||
| Manual ELISA | 31 | 0.1 ± 8.7 | –16.9; 17 |
3.2 (0.2–16.2) |
31 | 6.4 ± 12.2 | –17.6; 30.3 |
6.5 (1.1–20.7) |
|||||||||
| Aspergillus | 0.1–7.9 index | 11 | –1.5 ± 12.7 | –26.4; 23.5 | 0 (0–25.9) |
11 | 5.9 ± 18 | –29.5; 41.2 | 9.1 (0.5–37.7) |
||||||||
| DENV IgM | 0.1–6.4 index | 10 | 2 ± 6.7 | –11.2; 15.1 | 0 (0–27.8) |
10 | 10 ± 8.8 | –7.2; 27.4 | 0 (0–27.8) |
||||||||
| DENV IgG | 0.1–3.9 index | 10 | –0.2 ± 4.2 | –8.5; 8.1 | 10 (0.5–40.4) |
10 | 3.2 ± 5.7 | –8; 14.3 | 10 (0.5–40.4) |
||||||||
| Total | 258 | –0.6 ± 9.2 | –18.6; 17.4 |
0.4 (0–2.2) |
174 | –0.1 ± 18.6 | –36.7; 36.4 |
1.7 (0.5–4.9) |
200 | 17.5 ± 66.4 | –112.6; 147.5 |
14.5 (10.3–20) |
160 | 37.9 ± 79.8 | –118.4; 194.2 |
25.6 (19.5–32.9) |
|
Bias, mean difference (Treated – Control) vs. average in %; LoA, Bland–Altman limits of agreement (mean ± 1.96SD); SD, standard deviation; CI, confidence interval; FRR, false results rate or percentage at which the qualitative results of the treated samples differed from that of the controls; CMIA, chemiluminescent microparticle immunoassays (Architect®, Abbott Diagnostics); CLIA, chemiluminescent immunoassay (VirClia®, VirCell); S/CO, signal-to-cutoff ratio; HIV, human immunodeficiency virus; HCV, hepatitis C virus; HBV, hepatitis B virus; EBV, Epstein–Barr virus; CMV, human cytomegalovirus; Toxo, Toxoplasma gondii; DENV, Dengue virus; Ag, antigen; Ab, antibody.
Control samples were treated with PBS.
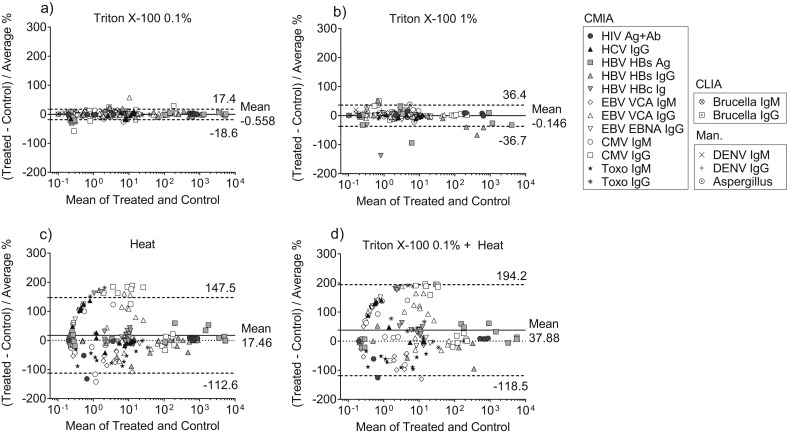
The bias of test results compared with controls (mean differences versus average) was –0.6 ± 9.2% after treatment with 0.1% of Triton X-100 and –0.1 ± 18.6% after treatment with 1% of Triton X-100 (Table 1, Figs. 1 a,b). This good agreement was confirmed by other statistical methods including Deming linear regression with Spearman correlation coefficient >0.99 (Table S1). Automated chemiluminescent assays (CMIA and CLIA) gave similar results to manually performed ELISAs. Only one discordant qualitative result was observed for Dengue IgG after treatment with Triton X-100 0.1% and concerned a sample which was around the borderline cut-off (Table S2). The overall false result rate after treatment with 0.1% of Triton X-100 was 0.4% (0–2.2%, Table 1). Increasing concentration of Triton X-100 to 1% led to a non-significant increase of this false result rate to 1.7% (0.5–4.9%, Table 1). Accordingly, serology results were not substantially altered (Fig. 1b). False results concerned the same borderline Dengue IgG sample: one sample with an Aspergillus test value around the positive cut-off and one sample for which HBV anti-HBc total Ig changed from weak positive in the control to negative in Triton X-100 1% (Table S2).
Fig. 1.
Agreement of serological test results after chemical or thermal inactivation of patient serum samples. Bland–Altman plots showing differences versus average of treated and control samples in percent (bias). Patient serum samples inactivated with Triton X-100 0.1% (a), Triton X-100 1% (b), heat inactivation at 60°C for 1 h (c), or a combination of Triton X-100 0.1% and heat inactivation (d) were used for a panel of ELISA-based serological assays including automated chemiluminescence immunoassays (CMIA and CLIA) and manual ELISAs (Man.) Control samples received an equivalent amount of PBS. Solid lines indicate mean bias, dashed lines indicate 95% limits of agreement (mean ± 1.96 SD), dotted lines indicate 0 (no bias). Abbreviations: HIV, human immunodeficiency virus; HCV, hepatitis C virus; HBV, hepatitis B virus; EBV, Epstein-Barr virus; CMV, human cytomegalovirus; Toxo, Toxoplasma gondii; DENV, Dengue virus; Ag, antigen; Ab, antibody.
In contrast, thermal inactivation by incubation of serum samples at 60°C for 1 h yielded aberrant serological test results with a mean bias of 17.5 ± 66.4%, which further increased to 37.9 ± 79.8% when combined with Triton X-100 0.1% (Table 1, Figs. 1c,d). Since results did not fulfil the assumption of homoscedasticity (Figs. 1c,d), no regression analysis was performed. The most relevant changes were seen for CMV IgG with biases of more than 95% (Table 1). Accordingly, high false result rates were observed for most of the assays with an average of 14.5% (10.3–20.0) after heat inactivation and 25.6% (19.5–32.9) after the combination treatment (Table 1). HIV antigen + antibody (Ag+Ab), HBV HBs Ag, and IgG test results, however, remained relatively unaffected by heat inactivation with minimal bias and no discordant qualitative results (Table 1).
In addition to the ELISA-based assays, we analysed the performances of a rapid test and a microagglutination test for the diagnosis of tularaemia after chemical or thermal inactivation of serum samples (Table 2 ). Triton X-100 0.1% did not have an effect on the accuracy of both tests. Triton X-100 1%, however, yielded acceptable rapid test results but disturbed microagglutination results. End titres were affected and several prozone and partial agglutination effects were observed. Heat inactivation yielded both poor rapid test and poor microagglutination results with many prozone effects.
Table 2.
Effect of inactivation treatments on Tularemia rapid test and microagglutination test
| Patient | Rapid test |
Microagglutination |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | Triton X-100 0.1% | Triton X-100 1% | Heat | Control | Triton X-100 0.1% | Triton X-100 1% | Heat | |
| 1 | – | – | – | – | <10 | <10 | <10a | <10 |
| 2 | 2+ | 2+ | 2+ | 1+ | 2560 | 2560 | 1280b | 1280b |
| 3 | – | – | – | – | <10 | <10 | <10a | <10 |
| 4 | 2+ | 2+ | 2+ | 1+ | 1280 | 1280 | 1280b | 640b |
| 5 | 2+ | 2+ | 1+ | – | 160 | 160 | <10a | <10 |
| 6 | 2+ | 2+ | 1+ | ± | 320 | 320 | 40a | <10 |
| 7 | – | – | – | – | <10 | <10 | 80a | <10 |
| 8 | 2+ | 2+ | 2+ | 1+ | 640 | 640 | 1280b | 320b |
| 9 | 2+ | 2+ | 2+ | ± | 2560 | 2560 | 1280b | 1280b |
| 10 |
2+ |
2+ |
2+ |
1+ |
5120 |
5120 |
10240 |
5120b |
| Weighted kappa | 1.000 | 0.851 | 0.353 | 1.000 | 0.657 | 0.684 | ||
Bold indicates false-qualitative result.
Controls and heat-inactivated samples were treated with an equivalent volume of PBS.
A negative result in the rapid test is indicated by a dash (–), whereas positive test results are indicated by ±, 1+, or 2+ (scale from weakly positive test to strongly positive test).
Partial agglutination.
Prozone effect.
Effect of serum concentration on the chemical inactivation of HSV-1
To evaluate the inactivation potential of Triton X-100 on enveloped viruses we chose HSV-1 as a model virus. HSV is indeed a prototypic enveloped virus that grows easily to high titres and for which rapid and accurate infectivity assays are available [23]. For these reasons it has been widely used for inactivation studies with various detergents [10], [19], [24]. Since it has been recently shown that serum annulled the inactivation effect of Triton X-100 0.1% [10] we performed our assays in 90% human serum or in medium containing 1% serum.
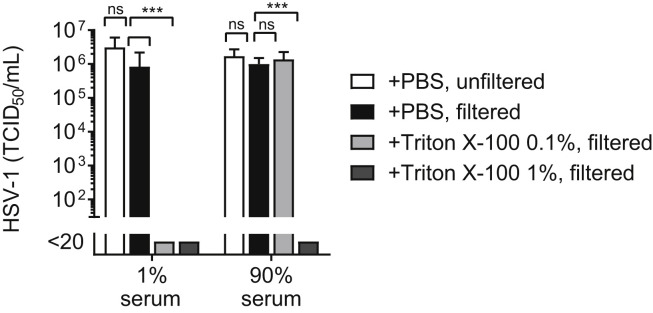
As previously shown, Triton X-100 0.1% could completely inactivate HSV-1 in medium containing 1% serum with a titre reduction of at least 5 log10 but it had no effect on HSV-1 in 90% serum (Fig. 2 ). On the contrary, a Triton X-100 concentration of 1% led to complete HSV-1 inactivation in both 1% and 90% serum with a titre reduction of at least 5 log10 (Fig. 2).
Fig. 2.
Influence of serum concentration on the chemical inactivation of herpes simplex virus type 1 (HSV-1). Reduction in HSV-1 titres in samples containing either 1% fetal bovine serum (FBS) or 90% human serum after treatment with 0.1% or 1% Triton X-100 for 1 h at room temperature. Controls received an equivalent amount of PBS. Before titration, samples were filtrated through 100-kDa membranes to remove residual Triton X-100. Unfiltered PBS controls are shown to account for eventual virus loss through the filter. TCID50/ml titres presented as mean ± SD were determined in three independent experiments with 4-8 replicates per experiment. Abbreviations: HSV-1, herpes simplex virus type 1; TCID50, 50% Tissue Culture Infectious Dose.
Discussion
Recent events including Ebola and Lassa outbreaks in West Africa [1], [25] and two cases of Andes Hantavirus infection in a returning traveller at our hospital [26] drew our attention to issues regarding biosafety for laboratory workers and optimal diagnostics for high-risk patients. In our routine laboratory we noticed a lack of protocols for virus inactivation and a lack of data regarding effect of inactivation on the accuracy of serological tests.
Here we demonstrate that heat inactivation is not a suitable method for the serological diagnosis of infectious diseases. On the contrary routine ELISA-based-serological tests and rapid tests can be accurately performed after a treatment with Triton X-100 0.1% or 1% final concentration. Both quantitative and qualitative results remained relatively unaffected and false results mostly concerned borderline samples. These results should prompt further studies about the effect of Triton X-100 1% on clinical chemistry parameters, haematological tests, and malaria tests for which only Triton X-100 0.1% has been tested so far [7], [8], [9], [10], [11], [12]. As recently published [10], Triton X-100 0.1% is not sufficient to inactivate HSV-1 in high concentrations of serum. Increasing Triton X-100 final concentration to 1% however led to complete inactivation of HSV-1 in 90% serum samples. This is of primordial importance for the safety of laboratory workers, considering that they receive and process undiluted serum samples, which are potentially infectious.
Since Triton X-100 exerts its inactivation effect at the level of the viral envelope only, complete inactivation of HSV-1 with 1% of Triton X-100 suggests that other enveloped viruses such as HIV, influenza, SARS, or EBOV could also be completely inactivated by the same procedure. This remains, however, to be tested especially for EBOV, which is a rather stable virus [2], [3]. Preliminary results of EBOV inactivation with Triton X-100 1% for 20 min are promising [27]. Other proven inactivation methods such as the combination of Triton X-100 and heat against EBOV [2], [14], [28] are unfortunately not compatible with serological diagnosis and should be limited to molecular diagnosis. Moreover, although this combination allowed detection of EBOV-specific IgG and IgM [14], our study suggests that inactivation procedures used for EBOV serology should be further evaluated and compared to inactivation with Triton X-100 alone.
If proven efficient for enveloped viruses in general, this simple inactivation procedure based on a single pipetting step could be implemented in basic microbiology laboratories and in the field diagnostics during outbreaks of highly pathogenic viruses where a safe and rapid diagnosis is of primordial importance [14]. The initial step of adding detergent to the serum sample does however require trained personnel, rigorous safety practices, and a biosafety cabinet of level 2. Triton X-100 has a long history of usage in diagnostic procedures; nevertheless, it is unknown if high concentrations of detergent could potentially affect routine laboratory analysers. Another limitation to the broad usage of Triton X-100 consists in environmental concerns. This reagent will soon be forbidden in Europe because of its endocrine disruption effects in aquatic organisms [29]. Restricted usage for well-defined procedures and appropriate waste treatment solutions will most probably still be possible in future. Ecofriendly alternatives such as lauryldimethylamine N-oxide (LDAO) [30] have been shown to exhibit effective virus inactivation in a broad range of protein concentrations but need further evaluation with different viruses and conditions.
In conclusion, we showed that addition of 1% Triton X-100 to serum samples enables complete inactivation of HSV-1 and is compatible with accurate results of a wide range of routine serological tests. This study provides basis to the development of safe serological assays for the rapid and accurate differential diagnosis of infectious diseases in patients with suspected or confirmed infection with highly pathogenic viruses.
Transparency declaration
The authors declare no conflicts of interest. The authors declare no external funding.
Acknowledgements
We wish to thank the laboratory staff of the Serology and Virology departments of the Institute for Infectious Diseases and especially Richard Kamgang and Sabine Funez for excellent technical assistance. We are also thankful to Johanna Signer from Spiez Laboratory for her technical advices. A special thanks goes to Désirée Mayor who provided HSV-1 seronegative serum. Some icons used for the graphical abstract are protected by a Creative Commons license. A previous version of this data was presented at the 28th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Madrid, Spain on 22 April.
Editor: G. Greub
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2018.10.016.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Roberts L. Nigeria hit by unprecedented Lassa fever outbreak. Science. 2018;359:1201–1202. doi: 10.1126/science.359.6381.1201. [DOI] [PubMed] [Google Scholar]
- 2.Haddock E., Feldmann F., Feldmann H. Effective chemical inactivation of Ebola virus. Emerg Infect Dis. 2016;22:1292–1294. doi: 10.3201/eid2207.160233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smither S.J., Weller S.A., Phelps A., Eastaugh L., Ngugi S., O'Brien L.M. Buffer AVL alone does not inactivate Ebola virus in a representative clinical sample type. J Clin Microbiol. 2015;53:3148–3154. doi: 10.1128/JCM.01449-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan American Health Organization WHO . Regional Office for the Americas of the World Health Organization; Washington, DC: 2015. General procedures for inactivation of potentially infectious samples with Ebola virus and other highly pathogenic viral agents. [Google Scholar]
- 5.Centers for Disease C, Prevention Update: management of patients with suspected viral hemorrhagic fever--United States. MMWR Morb Mortal Wkly Rep. 1995;44:475–479. [PubMed] [Google Scholar]
- 6.Hsieh Y.T., Mullin L., Greenhalgh P., Cunningham M., Goodrich E., Shea J. Single-use technology for solvent/detergent virus inactivation of industrial plasma products. Transfusion. 2016;56:1384–1393. doi: 10.1111/trf.13619. [DOI] [PubMed] [Google Scholar]
- 7.Mifsud A., Peelen D., Brincat P., Abela S., Debattista N., Laspina S. A feasibility study on the effects of Triton X-100 for the in vitro inactivation of Ebola virus on haematological assays. J Clin Pathol. 2016;69:637–642. doi: 10.1136/jclinpath-2015-203331. [DOI] [PubMed] [Google Scholar]
- 8.Hersberger M., Nusbaumer C., Scholer A., Knopfli V., von Eckardstein A. Influence of practicable virus inactivation procedures on tests for frequently measured analytes in plasma. Clin Chem. 2004;50:944–946. doi: 10.1373/clinchem.2004.031666. [DOI] [PubMed] [Google Scholar]
- 9.Tempestilli M., Pucci L., Notari S., Di Caro A., Castilletti C., Rivelli M.R. Diagnostic performances of clinical laboratory tests using Triton X-100 to reduce the biohazard associated with routine testing of Ebola virus-infected patients. Clin Chem Lab Med. 2015;53:1967–1973. doi: 10.1515/cclm-2015-0119. [DOI] [PubMed] [Google Scholar]
- 10.van Kampen J.J.A., Tintu A., Russcher H., Fraaij P.L.A., Reusken C., Rijken M. Ebola virus inactivation by detergents is annulled in serum. J Infect Dis. 2017;216:859–866. doi: 10.1093/infdis/jix401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau R., Wang A., Chong-Kit A., Ralevski F., Boggild A.K. Evaluation of Ebola virus inactivation procedures for Plasmodium falciparum malaria diagnostics. J Clin Microbiol. 2015;53:1387–1390. doi: 10.1128/JCM.00165-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loutfy M.R., Assmar M., Hay Burgess D.C., Kain K.C. Effects of viral hemorrhagic fever inactivation methods on the performance of rapid diagnostic tests for Plasmodium falciparum. J Infect Dis. 1998;178:1852–1855. doi: 10.1086/314524. [DOI] [PubMed] [Google Scholar]
- 13.Ukkonen P., Korpela J., Suni J., Hedman K. Inactivation of human immunodeficiency virus in serum specimens as a safety measure for diagnostic immunoassays. Eur J Clin Microbiol Infect Dis. 1988;7:518–523. doi: 10.1007/BF01962603. [DOI] [PubMed] [Google Scholar]
- 14.Cutts T., Grolla A., Jones S., Cook B.W., Qiu X., Theriault S.S. Inactivation of zaire ebolavirus variant makona in human serum samples analyzed by enzyme-linked immunosorbent assay. J Infect Dis. 2016;214:S218–S221. doi: 10.1093/infdis/jiw289. [DOI] [PubMed] [Google Scholar]
- 15.Saluzzo J.F., Leguenno B., Van der Groen G. Use of heat inactivated viral haemorrhagic fever antigens in serological assays. J Virol Method. 1988 Dec;22:165–172. doi: 10.1016/0166-0934(88)90099-7. [DOI] [PubMed] [Google Scholar]
- 16.Bhagat C.I., Lewer M., Prins A., Beilby J.P. Effects of heating plasma at 56 degrees C for 30 min and at 60 degrees C for 60 min on routine biochemistry analytes. Ann Clin Biochem. 2000 Nov;37:802–804. doi: 10.1258/0004563001899997. [DOI] [PubMed] [Google Scholar]
- 17.Gleeson M., Herd L., Burns C. Effect of heat inactivation of HIV on specific serum proteins and tumour markers. Ann Clin Biochem. 1990;27:592–594. doi: 10.1177/000456329002700611. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell S.W., McCormick J.B. Physicochemical inactivation of Lassa, Ebola, and Marburg viruses and effect on clinical laboratory analyses. J Clin Microbiol. 1984;20:486–489. doi: 10.1128/jcm.20.3.486-489.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howett M.K., Neely E.B., Christensen N.D., Wigdahl B., Krebs F.C., Malamud D. A broad-spectrum microbicide with virucidal activity against sexually transmitted viruses. Antimicrob Agents Chemother. 1999;43:314–321. doi: 10.1128/aac.43.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spire B., Dormont D., Barre-Sinoussi F., Montagnier L., Chermann J.C. Inactivation of lymphadenopathy-associated virus by heat, gamma rays, and ultraviolet light. Lancet. 1985;1:188–189. doi: 10.1016/s0140-6736(85)92026-4. [DOI] [PubMed] [Google Scholar]
- 21.Song H., Li J., Shi S., Yan L., Zhuang H., Li K. Thermal stability and inactivation of hepatitis C virus grown in cell culture. Virol J. 2010;7:40. doi: 10.1186/1743-422X-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colavita F., Quartu S., Lalle E., Bordi L., Lapa D., Meschi S. Evaluation of the inactivation effect of Triton X-100 on Ebola virus infectivity. J Clin Virol. 2017;86:27–30. doi: 10.1016/j.jcv.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Blaho J.A., Morton E.R., Yedowitz J.C. Herpes simplex virus: propagation, quantification, and storage. Curr Protoc Microbiol. 2005 doi: 10.1002/9780471729259.mc14e01s00. Chapter 14: Unit 14E 1. [DOI] [PubMed] [Google Scholar]
- 24.Gong Y., Wen A., Cheung D., Wong M., Sacks S.L. Preclinical evaluation of docusate as protective agent from herpes simplex viruses. Antivir Res. 2001;52:25–32. doi: 10.1016/s0166-3542(01)00156-5. [DOI] [PubMed] [Google Scholar]
- 25.Gire S.K., Goba A., Andersen K.G., Sealfon R.S., Park D.J., Kanneh L. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science. 2014;345:1369–1372. doi: 10.1126/science.1259657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuenzli A.B., Marschall J., Schefold J.C., Schafer M., Engler O.B., Ackermann-Gaumann R. Hantavirus cardiopulmonary syndrome due to imported Andes Hantavirus infection in Switzerland: a multidisciplinary challenge, two cases and a literature review. Clin Infect Dis. 2018 Nov 13;67:1788–1795. doi: 10.1093/cid/ciy443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton J.E., Easterbrook L., Pitman J., Anderson D., Roddy S., Bailey D. The effect of a non-denaturing detergent and a guanidinium-based inactivation agent on the viability of Ebola virus in mock clinical serum samples. J Virol Method. 2017;250:34–40. doi: 10.1016/j.jviromet.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Rosenstierne M.W., Karlberg H., Bragstad K., Lindegren G., Stoltz M.L., Salata C. Rapid bedside inactivation of Ebola virus for safe nucleic acid tests. J Clin Microbiol. 2016;54:2521–2529. doi: 10.1128/JCM.00346-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nimrod A.C., Benson W.H. Environmental estrogenic effects of alkylphenol ethoxylates. Crit Rev Toxicol. 1996;26:335–364. doi: 10.3109/10408449609012527. [Research Support, Non-U.S Gov't] [DOI] [PubMed] [Google Scholar]
- 30.Conley L., Tao Y., Henry A., Koepf E., Cecchini D., Pieracci J. Evaluation of eco-friendly zwitterionic detergents for enveloped virus inactivation. Biotechnol Bioeng. 2017;114:813–820. doi: 10.1002/bit.26209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.