Highlights
-
•
The immunotherapy is an effective method for SARS-CoV, and MERS-CoV.
-
•
The immunotherapies include vaccines, monoclonal antibody candidates, and etc.
-
•
There is no serious immunotherapy research for COVID-19.
-
•
Similar studies on the coronaviruses showed notable results.
-
•
So immunotherapy for COVID-19 virus can also be a suitable option.
Keywords: 2019-nCoV, COVID-19, Immunotherapy, Monoclonal antibody, Vaccine, Interleukin
Abstract
The novel coronavirus (2019-nCoV) is an emerging pathogen that was first described in late December 2019 and causes a severe respiratory infection in humans. Since the outbreak of COVID-19, international attention has raised to develop treatment and control options such as types of immunotherapies.
The immunotherapy is an effective method for fighting against similar viral infections such as SARS-CoV, and MERS-CoV. These methods include several types of vaccines, monoclonal antibody candidates, and etc.
This systematic review article was designed to evaluate the existing evidence and experience related to immunotherapy for 2019-nCoV. Web of Science (ISI), PubMed, and Scopus databases were used to search for suitable keywords such as 2019-nCoV, novel coronavirus, Immunotherapy, interleukin, vaccine and the related words for relevant publications up to 24.3.2020. The present systematic review was performed based on PRISMA protocol. Data extraction and quality valuation of articles were performed by two reviewers. 51 articles were the results of the search and based on the inclusions and exclusions criteria, 7 articles were included in the final review.
As a conclusion of these studies demonstrated that although no serious research has been done on this subject at the time of writing this article, similar studies on the related viruses showed notable results. So immunotherapy for this virus can also be a suitable option.
1. Background
2019-nCoV a large enveloped virus with a positive sense, single-stranded RNA genome, is the third known coronavirus after SARS-CoV and MERS-CoV that was first identified in late December 2019 and causes severe respiratory illness and pneumonia-like infection in humans [1], [2]. WHO has declared the pandemic outbreak of novel coronavirus (2019-nCoV) as a global health emergency. Immunotherapy is an efficient therapeutic option intervention against viral infections. Most immunotherapy attempts have been successful to fight against similar COVID-19 viruses such as SARS-CoV and MERS-CoV. The main methods in this regard include several vaccines and monoclonal antibody candidates. Furthermore, according to existing evidence in fighting against viral infections such as Ebola, influenza, SARS, and MERS plasma exchange can likely reduce the viral load and disease mortality [3], [4].
In both SARS-CoV and SARS-CoV-2 viruses entry into the host cells is mediated by interaction of the receptor-binding domain (RBD) in S protein on virus outer-membrane and angiotensin-converting enzyme 2 (ACE2) on cell. So, these proteins can be the major potential targets for immunotherapy [1], [5]. The increasing knowledge of MERS-CoV and SARS-CoV immunotherapies in recent years might increase the opportunities to design effective same therapeutics for novel coronavirus [3].
Despite the relatively high identity of RBD in 2019-nCoV and SARS-Co, it is necessary to develop novel monoclonal antibodies that could bind specifically to 2019-nCoV RBD since some of the most potent SARS-CoV-specific neutralizing antibodies (e.g. m396, CR3014) that target the ACE2 binding site of SARS-CoV failed to bind 2019-nCoV spike protein, implying that the difference in the RBD of SARS-CoV and 2019-nCoV [5].
Our purpose in this article is to review immunotherapeutic attempts against 2019-nCoV and to list current investigational immunotherapeutic strategies for 2019-nCoV.
2. Methods
The present systematic review article was performed based on PRISMA protocol [6] (Table S1).
2.1. Search strategy
Web of Science (ISI), PubMed, and Scopus databases were used to search for the proper keywords such as: 2019-nCoV, novel coronavirus, Immunotherapy, interleukin, vaccine and the related words for publications published until 24.3.2020. The search strategies are available in the supplementary data (Table S2). Titles and abstracts were separately reviewed by two authors (A.AJ and S.GH) to detect potentially related articles. The full texts of articles that appeared confusing were evaluated to determine their appropriateness for inclusion.
2.2. Included studies
The studies reviewed in this article were about the categories of immunotherapy (monoclonal antibody, interleukins, vaccines, etc.) for all three coronaviruses, but they must have been mentioned use of immunotherapy for treatment or control of 2019-nCoV.
2.3. Excluded studies
The articles with only abstract were excluded from this review. Some articles were also excluded because they were only about SARS and MERS without any discussion or outlook on the 2019-nCoV.
2.4. Data extraction
Data from articles were extracted independently by two reviewers to fill in table items.
2.5. Quality assessment
Quality of each publication was evaluated by two independent reviewers (A.AJ and S.GH). This review addressed 7 domains: Type of immunotherapy, Dedicated or for other viruses, Treatment/ Interventions, Accompanied by another treatment, Number of patients treated (percent improved), Outcomes, results and clinical outcome.
2.6. Statistical analysis:
It was not possible to conduct a meta-analysis because there was not enough proper research studies on this subject.
3. Results
3.1. Study selection
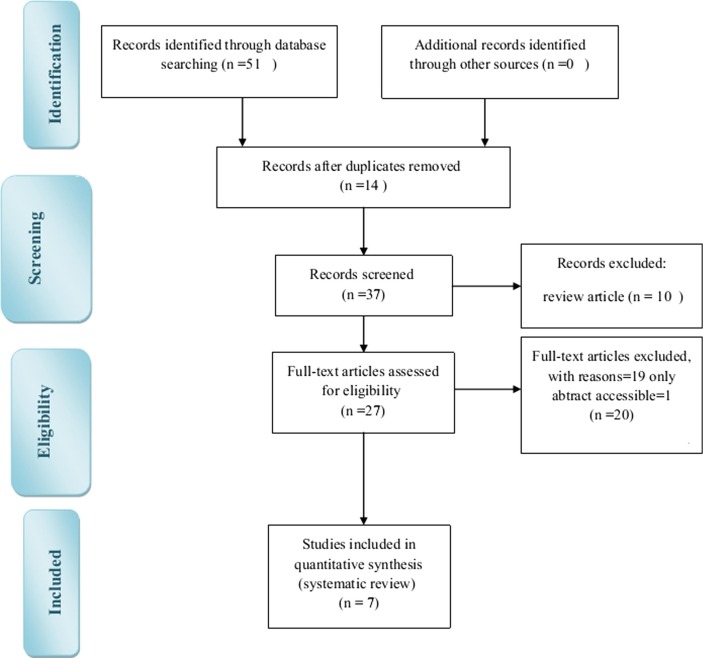
We retrieved a total of 51 potentially eligible articles from initial database searches, 14 articles were excluded because of being duplicate. 30 articles were also excluded because they were review articles or addressed other virusess or their full text were not accessible. Therefore, a total of 7 articles were included in final analysis (Fig. 1 ).
Fig. 1.
Flowchart of the articles selection.
3.2. Study characteristics (Eligibility criteria)
According to inclusion criteria stated in the protocol, articles were included in English language without any date restriction. We updated the search process in PubMed, WOS and Scopus up to 24.3.2020. Endnote library (version X6, Thomson Reuters) was used to import Citations from all databases and duplicate citations were removed. The title and abstract of the remainder of the search results were reviewed by both authors to exclude irrelevant studies. Full texts of the remainder of the citations were collected for further screening and data extraction. Included studies were original articles published until 24.3.2020 and most publications were related to 2020 with the aim to manage and control corona virus pandemic by several types of immunotherapy.
4. Results
According to the extracted data in Table 1 , all immunotherapy attempts for 2019-nCoV until now include polyclonal antibody by Plasma therapy, Polypeptide hormone for maturation of T cells, immunoglubolins, ACE2 immunoadhesin and monoclonal antibody against the interleukin-6. other interventions such as viral-vectors, nanoparticles, inactivated whole virus and DNA as vaccines and monoclonal antibody have been used for SARS-CoV that holds the promise for using in 2019-nCoV.
Table 1.
Main characteristics of included studies: Immunotherapy potentials.
| Treatment by | Specific or for similar viruses | Type of immunotherapy (vaccine and…..) | Treatment/Interventions | Accompanied by another treatment | Number of patients treated (percent improved) | Outcomes, results and relationships | Ref |
|---|---|---|---|---|---|---|---|
| Plasma therapy(Convalescent plasma) | Specific (for COVID-19) | polyclonal antibody immune response(passive antibody) | – | Standard treatment | 300 people in COVID-19 clinical trial | Clinical improvement | [7] |
| Immunoglobulin | Specific (for COVID-19) | – | – | Standard treatment | 80 people in COVID-19 clinical trial | Clinical improvement | [7] |
| Thymosin | Specific (for COVID-19) | Polypeptide hormone for maturation of T cells | – | Camrelizumab (anti-PD-1 immune checkpoint inhibitor), conventional treatment | 120 people in COVID-19 clinical trial | Lung injury score | [7] |
| Tocilizumab | Specific (for COVID-19) | monoclonal antibody against the interleukin-6 receptor (IL-6R) | – | – | 188 people in COVID-19 clinical trial | Cure rate | [7] |
| SARS-CoV RBD-specific antibodies | SARS-CoV-2 | Vaccine | cross-neutralize SARS-CoV-2 | – | – | Experimental test | [8] |
| cytotoxic T lymphocyte (CTL) and B cell epitopes | Specific (for COVID-19) | Vaccine | – | – | – | untested | [9] |
| Immunoglobulin Fc domain | Specific (for COVID-19) | ACE2 immunoadhesin | – | – | – | untested | [10] |
| Viral-vector | MERS-CoV | Vaccine | Vectors that encoding S or S1 protein | – | In transgenic mice or BALB/c mice | acceptable | [11] |
| Viral-vector and Nanoparticle | MERS-CoV | Vaccine | rAd5 and MERS-CoV S nanoparticle | – | in SPF BALB/c mice | induce both Th1 and Th2 immune responses | [11] |
| DNA | MERS-CoV | Vaccine | DNA encoding S or S1 Protein |
– | In animals | acceptable | [11] |
| Inactivated whole-virus | MERS-CoV | vaccine | – | – | mice | In some features is acceptable | [11] |
| Live-attenuated | MERS-CoV mutant | vaccine | Mutation in E and or NSP16 genes | – | transgenic mice | In some features is acceptable | [11] |
| Subunits or nanoparticle | MERS-CoV | vaccine | – | – | mice | In some features are acceptable | [11] |
| CR3022 | SARS-CoV | monoclonal antibody | cross-reactive antibodies | alone or in combination with other neutralizing antibodies (e.g. m396, CR3014) | – | Un-tested | [5] |
| Based T cell epitope | SARS-CoV-2 proteins | vaccine | – | – | – | Only about SARS-CoV | [12] |
Plasma therapy and immunoglobulin intervention on 2019-nCoV infected patients could improve clinical outcome. The vaccines and ACE2 immunoadhesin have not been tested yet. As the preferred immunotherapy method, monoclonal antibody has performed only for SARS; however, this method has not yet been tested. Most articles in this field are review articles recommending immunotherapy for 2019-nCoV with reasons and evidence of previous studies on two other coronaviruses SARS-CoV and MERS-CoV.
There is only one clinical trial study in the field that mentions some of these methods on 2019-nCoV [7].
5. Discussion
As both SARS-CoV and SARS-CoV-2 (2019-nCoV) have the same receptor for virus entry, potential biotherapeutics to prevent SARS entry could be extrapolated to use for SARS-CoV-2. Among immunotherapy approaches to block the virus attachment or entry, monoclonal antibodies are preferred due to their specificity, purity, low risk of blood-borne pathogen contamination and safety compared to serum therapy and intravenous immunoglobulins preparations [3].
The promising results in targeting spike protein in SARS-CoV and MERS-CoV by monoclonal antibodies encourages researchers to use them in fighting against 2019-nCoV. Monoclonal antibody cocktail or the combination of different monoclonal antibodies that recognizes different epitopes on the viral surface may increase the effectiveness of virus neutralizing [3].
Other targets in immunotherapy for COVID-19 that seem to be Promising are cytokines. Among cytokines, specificity of IL-6 in COVID-19 comes from that Elevated IL-6 is correlated with inflammatory cytokine storm severity. Therefore targeting IL-6 and its receptor (IL6R) by Siltuximab and tocilizumab monoclonal antibodies (mAb) could mitigate cytokine storm-related symptoms in severe COVID-19 patients [13].
Despite major progress in the development of monoclonal antibody-based passive immunotherapy for coronavirus infection, there is no marketed monoclonal antibody. What limits the use of antibodies is that large-scale production of monoclonal antibodies for clinical application is laborious, expensive and time-consuming. So designing and developing advanced protein production platforms and expression systems is urgent to provide efficient monoclonal antibodies at the affordable cost in a short time [3].
Although no research has been tested, and there is no approved vaccines or anti-viral therapeutic agents to treat COVID-19 until now, the vaccines can be successful due to the specific structure of the virus. Furthermore, existing evidence on immunotherapy for SARS-CoV and MERS-CoV holds the promise for using in 2019-nCoV.
6. Conclusion
As is evident from this systematic review, immunotherapy is an efficient therapeutic option intervention against COVID-19 and the main methods in this regard such as using immunoglobulins and plasma therapy have improved clinical outcomes in COVID-19 infected patients.
Acknowledgments
Acknowledgments
The authors would like to thank the Shahrekord University of Medical Sciences.
Declaration of Competing of Interest
There is no potential conflict of interest for authors to disclose.
Authors’ contributions
A.AJ and S.GH contributed to conception, literature search, screening, and data extraction and revised the draft; they provided the first draft of the article. Both authors contributed to statistical analysis and data interpretation and critically revised the article. Both authors contributed to the review design and article drafting.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.106455.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Shanmugaraj B. Emergence of novel coronavirus 2019-nCoV: need for rapid vaccine and biologics development. Pathogens. 2020;9(2) doi: 10.3390/pathogens9020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik Y.S. Emerging novel coronavirus (2019-nCoV)—current scenario, evolutionary perspective based on genome analysis and recent developments. Veterinary Quart. 2020;40(1):68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanmugaraj B. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pac. J. Allergy Immunol. 2020 doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 4.Pang J. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J. Clin. Med. 2020;9(3) doi: 10.3390/jcm9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian X. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberati A. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Internal Med. 2009;151(4) doi: 10.7326/0003-4819-151-4-200908180-00136. W-65-W-94. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q. Clinical trial analysis of 2019-nCoV therapy registered in China. J Med. Virol. 2020 doi: 10.1002/jmv.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai W. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020:1–8. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baruah V., Bose S. Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019-nCoV. J. Med. Virol. 2020 doi: 10.1002/jmv.25698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruse R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Res. 2020;9:72. doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yong CY. Recent Advances in the Vaccine Development Against Middle East Respiratory Syndrome-Coronavirus. Front. Microbiol. 2019;10(1781) doi: 10.3389/fmicb.2019.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12(3) doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.T. Liu, et al., The potential role of IL-6 in monitoring coronavirus disease, 2019. Available at SSRN 3548761, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.