Abstract
Necrotizing enterocolitis (NEC) is the most common serious, acquired gastrointestinal disorder in the newborn infant. Although many variables are associated with development of NEC, only prematurity has been consistently identified in case-controlled studies. Traditionally, the diving seal reflex has been invoked as the mechanism responsible for ischaemic injury and necrosis. Intestinal ischaemia is likely to be the final common pathway in NEC; however, it is due to the release of vasoconstricting substances, such as platelet activating factor, rather than perinatal asphyxia. Bacteria and/or bacterial toxins are likely to have a key role in the pathogenesis of NEC by fostering production of inflammatory mediators. The role of feeding practices in the pathogenesis of NEC remains controversial. Treatment of infants with NEC generally includes a regimen of bowel rest, gastric decompression, systemic antibiotics and parenteral nutrition. Infants with perforation are generally operated upon; however, there has been recent interest in primary peritoneal drainage as an alternative. Prevention of NEC still remains elusive. Avoidance of preterm birth, use of antenatal steroids and breast-milk feeding are practices that offer the greatest potential benefits. Use of any other strategy should await further trials.
Keywords: Necrotizing enterocolitis, Probiotics, Inflammation, Feeding practices, Bacterial translocation
1. Introduction
Necrotizing enterocolitis (NEC) is the most common serious, acquired gastrointestinal disease in the newborn infant, affecting 1–3% of neonatal intensive care unit (NICU) admissions.1The National Center for Health Statistics and multicentre trials have estimated that there are between 1200 and 9600 cases per year in the USA, resulting in up to 2688 deaths. The term ‘necrotizing enterocolitis’ first appeared in the European literature in the early 1950s in articles by Schmid and Quaiser who described infants dying from necrotic lesions of the gastrointestinal tract.2However, it was not until the 1960s, when Santulli et al. reported a series of preterm infants with NEC at Babies Hospital, that it became recognized as a distinct clinical entity.3Infants with NEC represent some of the sickest infants in the NICU, and exhibit a mortality rate ranging from 20–50%. Furthermore, once an infant is definitively diagnosed with NEC, with the exception of supportive care, there is little one can do to alter the course of the disease. The incidence of NEC may be increasing as more infants weighing less than 1000 g survive. This population of infants is most susceptible to NEC, is more likely to require surgery, and has a greater probability of dying from the disease.
The original report of Santulli et al. described preterm infants who were critically ill. They often had low Apgar scores and commonly required placement of an umbilical arterial catheter for managing respiratory distress syndrome (RDS). Following the institution of formula feeding, affected infants developed abdominal distention and bloody stools. Abdominal radiographs demonstrated the cardinal sign of NEC (pneumatosis intestinalis), and in some cases, there was rapid progression to perforation. Based on clinical observations in infants and studies in experimental animals, three factors were felt to be necessary for NEC to occur: (1) mucosal injury (from asphyxia or catheters); (2) formula feeding (providing a substrate for bacterial fermentation); and (3) the presence of bacteria. The lack of improvement in the mortality rate for NEC over the past 10 years has prompted investigators to re-examine these epidemiologic factors, and search for strategies to prevent the disease. This article will review controversial concepts in the epidemiology and pathogenesis of NEC, and recent clinical trials designed to prevent NEC.
2. Epidemiology
As a result of the many advances in neonatal intensive care, NEC has emerged as a disease of NICU survivors. The overall incidence is 1–3 cases per 1000 live births, with considerable variation observed among institutions and even within an institution. Although 5–25% of cases have been reported in full-term infants, it is a disease predominantly affecting premature infants. The incidence varies inversely with birthweight and gestational age.4, 5Those most susceptible appear to be infants weighing less than 1000 g at birth and under 28 weeks’ gestation.6, 7Data from the National Institute of Child Health and Human Development Neonatal Network (NICHD Neonatal Network) and the Vermont–Oxford Trials Network confirm this increased incidence among very-low-birthweight (VLBW) infants.8, 9The risk of NEC persists until a postconceptual age of at least 36 weeks is reached.
The majority of NEC cases are endemic; however, epidemics may also occur. No seasonal pattern has been reported. In most studies, male and female infants are equally affected. Age at onset is also inversely related to gestational age. In full-term infants, NEC appears to occur at a median age of two days with greater than 40% presenting on the first day of life.5Immature infants continue to be at prolonged risk for NEC; those born at 30 weeks’ gestation or younger may not be symptomatic for several weeks.5
Once NEC has been diagnosed, the clinical presentation appears to be relatively similar among affected infants. The modified Bell staging system for NEC is shown in Table 1 .10Although the most common presentation is abdominal distention, other reported signs include feeding intolerance, haematochezia, lethargy, apnoea, respiratory failure and circulatory instability.11Affected infants also exhibit, to varying degrees, laboratory abnormalities such as leukopenia or leukocytosis, anaemia, thrombocytopenia, hypo- or hyperglycaemia, electrolyte abnormalities and metabolic acidosis. Bacteraemia has been documented in up to 35% of cases. Although no single pathogen has been consistently identified, Escherichia coli, Klebsiella,Enterobacter, Pseudomonas, Salmonella, Clostridium perfringes, C. difficile, C. butyricum, coagulase-negative staphylococci, Enterococcus, coronavirus, rotavirus and enterovirus have been associated with NEC.12The type of organism recovered varies with disease severity. In modified Bell stages I and II NEC, Gram-positive organisms appear to be the predominant pathogens recovered. As NEC worsens, enteric organisms are more commonly isolated.13Radiographically, 70–80% of affected infants display signs of pneumatosis intestinalis, the accumulation of gas produced by gas-forming bacteria in the submucosa or subserosa.14Other signs of intestinal gangrene or impending perforation are portal venous gas (PVG), a fixed distended bowel loop, free intraperitoneal fluid or pneumoperitoneum. PVG is believed to represent either a dissection of gas from the bowel into portal veins or transfer to the portal system via the mesenteric veins; its prognostic importance iscontroversial.15Ultrasonography has been proposed as a useful adjunct for identification of pneumatosis intestinalis, free intraperitoneal fluid and PVG.
Table 1.
Modified Bell staging criteria for necrotizing enterocolitis
| Stage | Classification | System signs | Intestinal signs | Radiological signs |
|---|---|---|---|---|
| IA | Suspected NEC | Temperature instability, apnoea, bradycardia, lethargy | Increased pregavage residuals, mild abdominal distention, emesis, guaiac-positive stool | Normal or intestinal dilation, mild ileus |
| IB | Suspected NEC | Same as above | Bright-red blood from rectum | Same as above |
| IIA | Proven NEC—mildly ill | Same as above | Same as above, plus absent bowel sounds, with or without abdominal tenderness | Intestinal dilation, ileus, pneumatosis intestinalis |
| IIB | Proven NEC—moderately ill | Same as above, plus mild metabolic acidosis and mild thrombocytopenia | Same as above, plus absent bowel sounds, definite abdominal tenderness, with or without abdominal cellulitis or right lower quadrant mass | Same as IIA, plus portal vein gas, with or without ascites |
| IIIA | Advanced NEC—severely ill, bowel intact | Same as IIB, plus hypotension bradycardia, severe apnoea, combined respiratory and metabolic acidosis, disseminated intravascular coagulation, and neutropenia | Same as above, plus signs of generalized peritonitis, marked tenderness, and distention of abdomen | Same as IIB, plus definite ascites |
| IIIB | Advanced NEC—severely ill, bowel perforated | Same as IIIA | Same as IIIA | Same as IIB, plus pneumoperitoneum |
Modified from Ref. 10.
NEC, necrotizing enterocolitis.
Approximately 27–63% of affected infants require surgical intervention. The majority of postoperative complications are related to the stoma or wound. Strictures, found primarily in the colon, may occur in up to 39% of infected infants.14NEC has been reported to recur in up to 6% of infants; however, no consistent association has been shown between recurrence and feeding regimen, anatomical site of injury, or management of initial disease.16Total parenteral nutrition (TPN)-related complications may also occur. Infants with NEC also have extended hospitalizations (22 days in medical NEC and 60 days in surgical NEC beyond their healthy matched controls).17Although VLBW infants with severe disease may be at higher risk of adverse outcomes,18, 19neurodevelopmental outcome measured at school age appears favourable in most infants.20
3. Pathogenesis of necrotizing enterocolitis
Although NEC is an important cause of neonatal morbidity and mortality, its pathogenesis remains incompletely understood. Available theories do not explain the spectrum of observed manifestations of the disease satisfactorily. NEC most likely represents a complex interaction of factors predisposing to mucosal injury and the infant's subsequentresponse.1
Intestinal ischaemia clearly occurs in NEC as evidenced by the histopathologic presence of inflammatory cell infiltration, mucosal oedema, ulceration and coagulative necrosis.21However, it is unclear whether ischaemia is the primary initiator or the end result of intestinal injury. Early observational studies reported a significant association between numerous ischaemic events and the development of NEC. Factors often cited as possible risk factors were perinatal asphyxia, umbilical arterial catheterization, polycythaemia, exchange transfusion, RDS and cyanotic congenital heart disease.4Medications such as indomethacin and methylxanthines, which have been shown to decrease superior mesenteric blood flow, have also been implicated. Many of these perinatal insults were believed to induce the ‘diving seal reflex’ by which blood flow is selectively shunted away from non-vital organs such as the intestine.22, 23However, this physiological mechanism does not fully explain what is seen clinically. Many infants with NEC have no history of perinatal depression at birth, and do not present with signs of NEC until several weeks of life. Recent epidemiologic studies also fail to confirm an association between these hypoxic factors and the development of NEC.4The conflicting data suggest that ischaemia may be a secondary event reflecting a culmination of the various factors described below.
Prematurity is the only factor consistently found in epidemiologic studies to be an independent determinant of NEC. The increased susceptibility is attributed to an immature mucosal barrier and barrier response.1In the presence of low intraluminal gastric acid and proteolytic activity, the incompletely innervated, poorly organized, relatively permeable epithelial barrier is vulnerable to bacterial colonization and pathogenic overgrowth.1, 24, 25In premature infants, the humoral and cellular response to thisovergrowth is impaired. Specifically, secretory IgA deficiency in the lymph-follicle-rich terminal ileum and colon facilitates bacterial translocation, while inadequate T-lymphocyte activity compromises recognition of ensuing membrane alterations.1, 24
Enteral feedings have been implicated as a significant contributor in the development of NEC. Although NEC can occur in infants who have never been fed, 90–95% of cases occur in infants with a history of recent volume advancement or re-initiation of enteral feedings.5The introduction of feedings into the intestinal lumen presumably causes a disruption of mucosal integrity, blood flow and motility. A substantially higher incidence of NEC has been reported in formula-fed infants compared with exclusively breast-fed infants, which has been attributed to a lack of immunoprotective factors.26The timing, initial volume and advancement of feedings are important factors in determining the degree of the mucosal insult that occurs. Since much of the research performed in this area is conflicting, no consensus has been reached regarding the most effective feeding regimen.
The well-documented epidemics of NEC and the improvement in attack rate following the implementation of strict infection control measures validate the role of infection in the pathogenesis of NEC. The role of bacteria is two-fold. Fermentation of carbohydrate substrates by bacteria leads to formation of hydrogen gas (the gas found in pneumatosis intestinalis). Furthermore, as mucosal integrity is compromised, bacteria ‘translocate’ to regional lymph nodes and activate resident macrophages. Colonization of the intestine with bacterial species must precede bacterial translocation.24The physiological growth of intestinal microflora and the pathological modifications of this microflora have been assessed through fecal bacterial measurements. Organisms are introduced into the sterile fetal intestine by contact with maternal vaginal flora. Species such as E. coli, streptococci and Bacteroides are commonly isolated during the immediate neonatal period.24Colonization with aerobic and anaerobic flora normally occurs by 10 days of age.23Over the first few weeks to months, the relative concentrations of E. coli and streptococci decline as the concentrations of lactobacilli and Bacteroides rise.24Introduction of enteral feedings alters this pattern of intestinal colonization. Formula is associated with an early appearance of Enterobacteriaceae such as E. coli and Klebsiella, whereas breast feeding induces an early appearance of Enterobacteriaceae and Bifidobacterium.24, 27Regardless of the choice of feeding, bifidobacteria gradually become the dominant bacteria. In infants requiring neonatal intensive care, colonization occurs more slowly. Following the initiation of feedings in these infants, only a few species are present. If the hospitalization remains uncomplicated, enteric colonization continues to diversify. However, colonization is often delayed or reversed by interruptions in the feeding regimen or by the administration of broad-spectrum antibiotics.23, 24, 28Clinically, delays in colonization appear to roughly correspond to the delay in disease presentation. Substantial alterations in the intestinal flora enable the bacterial overgrowth of a few organisms as a result of the lack of competition. The role of bacterial toxins is less clear. Lawrence et al. hypothesized that bacterial toxin absorption during periods of bacterial overgrowth was the primary mechanism for intestinal damage.23, 29However, the isolation of potent toxins, such as Clostridium toxin, in asymptomatic infants raises questions about the importance of toxin-mediated injury in the evolution of NEC.1, 30
Once bacterial translocation or toxin absorption has occurred, the endogenous production of inflammatory mediators is likely to be a key step in the pathogenesis of NEC. From animal studies, it has been demonstrated that increases in tumour necrosis factor (TNF) and platelet activating factor (PAF) propagate the ongoing injury to the intestinal mucosa, which triggers a cascade of inflammatory events.27, 31This inflammatory response, consisting of leukocyte adhesion and activation, complement activation, and the release of cytokines, reactive oxygen species and nitric oxide, results in areas of focal necrosis.31Clinically, elevated PAF and TNF levels, and reduced levels of PAF acetylhydrolase (the enzyme which degrades PAF) have been observed in infants with NEC. An imbalance of pro- and counter-inflammatory cytokines may partially explain the varying severity of the disease.32, 33In immature enterocytes, this injury may stimulate excessive pro-inflammatory cytokine production.34However, attempts to correlate cytokine levels with the development and progression of NEC have generally been unsuccessful.
4. Treatment of necrotizing enterocolitis
Following the clinical diagnosis of NEC, the mainstay of treatment remains medical stabilization. A regimen consisting of bowel rest, gastric decompression, systemic antibiotics and parenteral nutrition is typically implemented. Initial broad-spectrum antibiotic coverage at our institution consists of ampicillin, gentamicin and clindamycin. With the increasing prevalence of infections from coagulase-negative staphylococcus, vancomycin may be used instead of ampicillin. Amikacin and metronidazole have been substituted for gentamicin and clindamycin by others.11However, antimicrobial choices should be guided by local resistance patterns. Enteral antibiotics are not recommended. Prompt correction of electrolyte abnormalities, persistent metabolic acidosis and coagulopathy are also recommended.
In cases where persistent clinical deterioration or signs of impending perforation or intestinal gangrene are present, operative intervention may be considered. In the absence of pneumoperitoneum, abdominal paracentesis may be helpful in confirming the presence of intestinal gangrene when used in conjunction with other available data.14Currently, intestinal perforation remains the only absolute indication for laparotomy. The cardinal principle of surgical management is excision of grossly necrotic segments and exteriorization of viable ends to allow for continued bowel decompression.14Recently, primary peritoneal drainage (PPD) has been proposed as an alternative to surgical treatment. Ein et al. first described the use of PPD for perforated NEC in VLBW infants in 1977; however, its use was limited to unstable VLBW infants.35Although initially implemented as a temporizing measure, multiple published reports documented the successful use of PPD as adjunctive therapy prior to planned laparotomy (LAP) or as definitive therapy, particularly in extremely-low-birthweight infants less than 1000 g.36, 37, 38, 39A meta-analysis by Moss et al. also demonstrated comparable combined probability of survival for infants with perforated NEC who were treated with either procedure (67% in the LAP group vs 55% in the PPD group, P=0.27) even in the presence of a significant treatment assignment bias favouring the LAP group.40NEC STEPS, a prospective multicentre randomized controlled trial, is currently under way to examine the effectiveness of PPD vs LAP as primary therapy for perforated NEC in VLBW infants stratified by birthweight (<1000 and 1000–1500 g). A potential extension of the use of peritoneal drains in NEC is Moore's ‘patch, drain, and wait’ laparotomy approach, which consists of limited patching of major perforations, gastrostomy tube drainage, bilateral peritoneal drains, and long-term parenteral nutrition.14, 41In cases of isolated or multifocal NEC, some investigators advocate resection and primary anastomosis. When massive pneumatosis intestinalis without definite intestinal gangrene is present, proximal diversion via a high jejunostomy is recommended to minimize bacterial proliferation.14Contrast enemas are typically performed prior to re-initiation of feedings in infants with perforated NEC or when feeding intolerance develops in infants with medical NEC.12Approximately one-quarter of affected infants may develop short gut syndrome.42Some success with intestinal transplantation has been reported in this subset. Although experience is limited, the overall reported one- and three-year survival rates are 60 and 54%, respectively.43
5. Prevention of necrotizing enterocolitis
Strategies for prevention of NEC fall into two major categories: those with probable or proven efficacy (Table 2 ), and those with unproven efficacy or based upon limited data (Table 3 ). When interpreting these studies, it is important to focus on both the overall incidence of NEC in the intervention and control groups, as well as the incidence of each stage. For example, an intervention that decreases the incidence of stage I disease (suspect NEC) is not as relevant as interventions that decrease the incidence of stage II or III disease. Not surprisingly, many of the suggested interventions have been directed at modifying feeding practices, since more than 95% of infants with NEC received milk feedings prior to the onset of the disease. For many years, breast milk has been advocated as a way to prevent NEC.12More than 30 years ago, Barlow et al. demonstrated that breast milk was protective in a rodent model of NEC.44Not surprisingly, that observation engendered a lot of enthusiasm for the use of breast milk to prevent NEC. Unfortunately, the expectation that breast milk would be protective has not been fulfilled. NEC does occur in infants who have been fed exclusively with human milk, especially milk that has been refrigerated, frozen or pasteurized.45Some investigators have suggested that the severity of NEC is less in infants who have been fed human milk. Recent studies by Dvorak et al. lend support to this observation.46Using a rat model of NEC, the investigators demonstrated that rat breast milk reduced the incidence and severity of NEC. It is noteworthy that freezing and thawing did not eliminate the protective effect of maternal milk. In this study, the protective effect of rat breast milk was associated with increased production of the anti-inflammatory cytokine IL-10 at the site of injury. Fresh human milk, however, contains numerous immunoprotective substances (immunoglobulins, lysozyme, lactoferrin, macrophages, lymphocytes and neutrophils) and bioactive factors (Table 4 ).47In addition, human milk contains PAF acetylhydrolase, an enzyme that destroys PAF.48PAF is one of the mediators thought to be important in the pathogenesis of NEC.49Fresh milk also promotes colonization with Lactobacillus bifidus, which may also offer protection (see below). McGuire and Anthonyrecently published a systematic review of studies comparing donor human milk with formula for preventing NEC in preterm infants (Fig. 1 ).50Eleven trials that appeared to be relevant were screened, and four of them fulfilled the inclusion criteria. Although none of the trials showed a statistically significant reduction in the incidence of confirmed NEC, the meta-analysis demonstrated a borderline, statistically significant difference (RR 0.25, 95% CI 0.06, 0.98).51It is important to note that the studies chosen for inclusion were conducted more than 20 years ago, and may not be representative of current NICU practices or populations. There are insufficient data to show that use of preterm human milk (vs preterm formula) reduces the incidence of NEC.
Table 2.
Strategies to prevent necrotizing enterocolitis: probable or proven efficacy
| • Breast feeding |
| • Antenatal steroids |
| • Fluid restriction |
| • Enteral administration of antibioticsa |
Potential for development of resistant flora.
Table 3.
Strategies to prevent necrotizing enterocolitis: unproven efficacy or limited data
| • Cautious advancement of feedingsa |
| • Trophic feedinga |
| • Enteral administration of immunoglobulinb |
| • Supplemental l-argininec |
| • Supplementation of feedings with egg phospholipidsc |
| • Acidification of milk feedingsc |
| • Administration of probioticsc |
Unproven efficacy.
Not efficacious.
Limited data.
Table 4.
Potential effects of selected agents in human milk (HM) on the gastrointestinal tract
| Agent in HM | Epithelial growth | Decreased inflammation | sigA production |
|---|---|---|---|
| EGF | + | ? | − |
| IGF-1 | + | ? | − |
| TGF | + | − | − |
| Erythropoietin | + | ? | − |
| IL-6 | − | ? | + |
| TNF-α | − | − | + |
| IL-10 | − | + | + |
| PAF-AH | − | + | − |
| Lysozyme | − | + | − |
GI, gastrointestinal; sigA, secretory immunoglobulin A; EGF, epithelial growth factor; IGF-1, insulin-like growth factor 1; TGF, transforming growth factor; IL, interleukin; TNF-α, tumour necrosis factor α; PAF-AH, platelet activating factor acetylhydrolase.
Reproduced from Ref. 47, with permission.
Fig. 1.
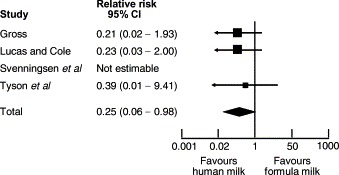
Relative risk of confirmed necrotizing enterocolitis in infants randomized to donor human milk vs formula milk. Reproduced from Ref. 50, with permission.
In 1988, Eibl et al. published the results of a randomized clinical trial demonstrating a reduction in the incidence of NEC in preterm infants fed an oral IgA–IgG preparation.52That report was followed by several other studies investigating whether oral immunoglobulin preparations resulted in a reduction in the incidence of the disease. Foster and Colerecently published a meta-analysis of studies employing that strategy.53Five studies on oral immunoglobulin for prevention of NEC were reviewed and three of them met the inclusion criteria for the meta-analysis. The reviewers concluded that the evidence does not support the administration of oral immunoglobulin forprevention of NEC (RR for definite NEC 0.84, 95% CI 0.57, 1.25). In contrast, a meta-analysis of studies comparing restricted vs liberal water intake in preterm infants (Fig. 2 ) concluded that restricted water intake (without allowing significant dehydration) reduced the incidence of NEC (RR 0.30, CI 0.13, 0.71).54Similarly, a recent meta-analysis of prophylactic enteral antibiotics to prevent NEC (Fig. 3 and 4) demonstrated a significant reduction in the incidence of the disease (RR 0.27, 95% CI 0.28, 0.78) and NEC-related deaths (RR 0.32, 95% CI 0.10, 0.96).55However, because of the potential risk of development of resistant bacteria, that strategy should not be adopted until a large trial investigating all possible harmful effects has beencompleted.
Fig. 2.
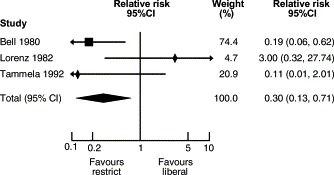
Restricted vs liberal water intake for preventing necrotizing enterocolitis. Modified from Ref. 54.
Fig. 3.
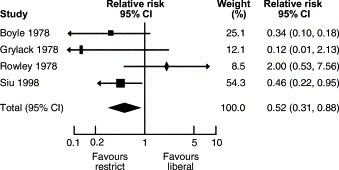
Enteral antibiotics for preventing necrotizing enterocolitis. Modified from Ref. 55.
Fig. 4.
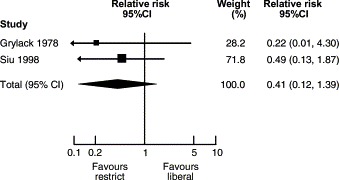
Enteral antibiotics for preventing necrotizing-enterocolitis-related deaths. Modified from Ref. 55.
There have been recent recommendations to limit increments in feeding volumes to decrease the likelihood of developing NEC. Those recommendations were based on retrospective data, which demonstrated a relationship between the rate of feeding advancement and an increased incidence of NEC.13, 56Prospective randomized trials have not reached similar conclusions.13, 57, 58, 59Berseth et al. compared a traditional enteral feeding protocol (using modest feeding advancements of 20 ml/kg/day) with a minimal enteral feeding protocol that was not advanced for 10 days.60The study was terminated after 144 infants were randomized because of a significantly higher incidence of NEC in the feeding advancement group. While feeding advancement may have contributed to an increased incidence of NEC, there is also the possibility that minimal enteral feedings help protect against NEC, and this further supports the use of trophic feedings in critically ill preterm infants.
Probiotics (anaerobic bacterial supplementation with organisms such as bifidobacteria or lactobacilli) have been used for many years to treat a variety of gastrointestinal diseases.61Bifidobacteria are the most common organisms recovered from the gastrointestinal tract of breast-fed infants, and inhibit colonization with other more pathogenic organisms. Furthermore, bifidobacteria fail to activate key mediators in the inflammatory cascade, enhance immune function of peritoneal macrophages, and do not degrade intestinal mucous glycoproteins.62, 63In an animal model of NEC, bifidobacterial supplementation(Bifidobacterium infantis) reduced the incidence of NEC (vs E. coli-treated animals).64In addition, bifidobacteria reduced the plasma concentration of endotoxin and intestinal gene expression of phospholipase A2(an enzyme important in the inflammatory cascade). Similar protective effects of Bifidobacteria have been demonstrated in a quail model of NEC.65In this model, colonization with Bifidobacteria infantis-longum decreased colonization with C. butyricum and reduced the production of butyric acid, a chemical that may be cytotoxic to intestinal epithelium. There are limited data in human newborn infants artificially colonized with bifidobacteria. In a randomized clinical trial to assess potential toxicities, Kitajima et al. administered Bifidobacteria breve to premature infants.66There were no adverse effects attributable to the bacteria, and colonized infants exhibited better weight gain and tolerance of feedings. More recently, Hoyos colonized infants with Lactobacillus acidophilus and B. infantis, and demonstrated a decreased incidence of NEC and NEC-related mortality vs historic controls.67The safety of probiotic supplementation has not been determined in a large prospective trial. Lactobacilli and bifidobacteria are capable of causing infections in humans, especially in immunocompromised hosts.68Therefore, this strategy should not be used until further studies have been completed.
There are a number of other retrospective and prospective studies examining prevention strategies for NEC. As part of a secondary analysis of a randomized trial of feeding preterm infants with a formula that was supplemented or not supplemented with egg phospholipids, Carlson et al. demonstrated significantly lower incidences of stages II and III NEC.69Phospholipids contain arachidonic acid, which is a substrate for intestinal vasodilatory and cytoprotective eicosanoids. Furthermore, Caplan et al. have demonstrated that supplementation of formula with polyunsaturated fatty acids reduced the incidence of death and NEC in rat pups, and decreased indices of inflammation.70In a double-blind placebo-controlled trial, Amin et al. investigated the benefit of supplementing the diet of preterm infants with l-arginine (1.5 mmol/kg/day).71Arginine is a substrate for production of nitric oxide, an important vasodilator and anti-inflammatory mediator. Infants receiving the supplement had a significantly lower incidence of NEC compared with controls. There is a question about the applicability of this study for other NICUs because of the high incidence of NEC (27.3%) in the control group. Lastly, Carrion and Egan in a prospective double-blind study investigated whether acidification of milk (0.01–0.02 ml of 1 N HCl/ml of milk) decreased the incidence of NEC.72Infants in the HCl supplemented group demonstrated a lower gastric pH and a reduced incidence of NEC (including stage I NEC).
In conclusion, very few strategies have proven efficacious for decreasing the incidence of NEC. Avoidance of preterm birth, use of antenatal steroids (for preterm deliveries), breast-milk feeding, and the use of trophic feedings (for all the other proven benefits) seem reasonable. Use of any other preventionstrategy should await the results of further trials.
Practice points.
-
•
Prematurity is the only consistent determinant of NEC; incidence varies inversely with birthweight and gestational age.
-
•
Timing of presentation also varies inversely with gestational age.
-
•
Intestinal ischaemia appears to be the final pathway and not the primary initiator of NEC.
-
•
NEC likely represents an elaborate interaction of factors predisposing to mucosal injury, including the release of vasoconstricting substances and inflammatory mediators.
-
•
A regimen consisting of bowel rest, gastric compression, systemic broad-spectrum antibiotics, and parenteral nutrition is the mainstay of treatment. Intestinal perforation remains the only absolute indication for laparotomy.
-
•
Primary peritoneal drainage may be an alternative to laparotomy in cases of perforated NEC in very-low-birthweight infants.
-
•
Avoidance of preterm birth, judicious use of antenatal steroids in preterm deliveries, breast-milk feedings and trophic feedings may be reasonable strategies in reducing the incidence of NEC.
-
•
Prevention of NEC still remains elusive.
Research directions.
-
•
A better understanding of the risk factors and the underlying pathway leading to NEC.
-
•
Determination of the role of feeding practices in the pathogenesis of NEC.
-
•
Clarification of the efficacy and safety of probiotic supplementation.
-
•
Development of a prediction model for the risk of NEC.
References
- 1.Neu J. Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am. 1996;43:409–432. doi: 10.1016/S0031-3955(05)70413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmid K.O, Quaiser K. Über eine besonders schwer verlaufende Form von Enteritis beim Säugling. Österreichische Zeitschrift für Kinderchirurgie. 1953;8:114. [PubMed] [Google Scholar]
- 3.Santulli T.V, Schullinger J.N, Heird W.C. Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics. 1975;55:376–387. [PubMed] [Google Scholar]
- 4.Covert R.F, Neu J, Elliott M.J. Factors associated with age of onset of necrotizing enterocolitis. Am J Perinatol. 1989;6:455–460. doi: 10.1055/s-2007-999639. [DOI] [PubMed] [Google Scholar]
- 5.Stoll B.J. Epidemiology of necrotizing enterocolitis. Clin Perinatol. 1994;21:205–218. doi: 10.1016/S0095-5108(18)30341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe M.I, Reblock K.K, Kurkchubasche A.G. Necrotizing enterocolitis in the extremely low birth weight infant. J Pediatr Surg. 1994;29:987–990. doi: 10.1016/0022-3468(94)90264-x. [DOI] [PubMed] [Google Scholar]
- 7.Chandler J.C, Hebra A. Necrotizing enterocolitis in infants with very low birth weight. Semin Pediatr Surg. 2000;9:63–72. doi: 10.1016/s1055-8586(00)70018-7. [DOI] [PubMed] [Google Scholar]
- 8.Hack M, Wright L.L, Shankaran S. Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Network, November 1989 to October 1990. Am J Obstet Gynecol. 1995;172:457–464. doi: 10.1016/0002-9378(95)90557-x. [DOI] [PubMed] [Google Scholar]
- 9.The Vermont–Oxford Trials Network Very low birth weight outcomes for 1990. Investigators of the Vermont–Oxford Trials Network Database Project. Pediatrics. 1993;91:540–545. [PubMed] [Google Scholar]
- 10.Walsh M.C, Kliegman R.M. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foglia R.P. Necrotizing enterocolitis. Curr Probl Surg. 1995;32:757–823. doi: 10.1016/s0011-3840(05)80014-0. [DOI] [PubMed] [Google Scholar]
- 12.Kliegman R.M, Fanaroff A.A. Necrotizing enterocolitis. N Engl J Med. 1984;310:1093–1103. doi: 10.1056/NEJM198404263101707. [DOI] [PubMed] [Google Scholar]
- 13.Uauy R.D, Fanaroff A.A, Korones S.B. Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1991;119:630–638. doi: 10.1016/s0022-3476(05)82418-7. [DOI] [PubMed] [Google Scholar]
- 14.Ricketts R.R. Surgical treatment of necrotizing enterocolitis and the short bowel syndrome. Clin Perinatol. 1994;21:365–387. [PubMed] [Google Scholar]
- 15.Molik K.A, West K.W, Rescorla F.J. Portal venous air: the poor prognosis persists. J Pediatr Surg. 2001;36:1143–1145. doi: 10.1053/jpsu.2001.25732. [DOI] [PubMed] [Google Scholar]
- 16.Stringer M.D, Brereton R.J, Drake D.P. Recurrent necrotizing enterocolitis. J Pediatr Surg. 1993;28:979–981. doi: 10.1016/0022-3468(93)90496-8. [DOI] [PubMed] [Google Scholar]
- 17.Bisquera J.A, Cooper T.R, Berseth C.L. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics. 2002;109:423–428. doi: 10.1542/peds.109.3.423. [DOI] [PubMed] [Google Scholar]
- 18.Tobiansky R, Lui K, Roberts S. Neurodevelopmental outcome in very low birthweight infants with necrotizing enterocolitis requiring surgery. J Paediatr Child Health. 1995;31:233–236. doi: 10.1111/j.1440-1754.1995.tb00792.x. [DOI] [PubMed] [Google Scholar]
- 19.Sonntag J, Grimmer I, Scholz T. Growth and neurodevelopmental outcome of very low birthweight infants with necrotizing enterocolitis. Acta Paediatr. 2000;89:528–532. doi: 10.1080/080352500750027790. [DOI] [PubMed] [Google Scholar]
- 20.Stanford A, Upperman J.S, Boyle P. Long-term follow-up of patients with necrotizing enterocolitis. J Pediatr Surg. 2002;37:1048–1050. doi: 10.1053/jpsu.2002.33842. [DOI] [PubMed] [Google Scholar]
- 21.Ledbetter D.J, Juul S.E. Necrotizing enterocolitis and hematopoietic cytokines. Clin Perinatol. 2000;27:697–716. doi: 10.1016/s0095-5108(05)70046-4. [DOI] [PubMed] [Google Scholar]
- 22.Kliegman R.M. Models of the pathogenesis of necrotizing enterocolitis. J Pediatr. 1990;117:2–5. doi: 10.1016/S0022-3476(05)81123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosloske A.M. Epidemiology of necrotizing enterocolitis. Acta Paediatr. 1994;396(Suppl.):2–7. doi: 10.1111/j.1651-2227.1994.tb13232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Camp J.M, Tomaselli V, Coran A.G. Bacterial translocation in the neonate. Curr Opin Pediatr. 1994;6:327–333. [PubMed] [Google Scholar]
- 25.Israel E.J. Neonatal necrotizing enterocolitis, a disease of the immature intestinal mucosal barrier. Acta Paediatr. 1994;396(Suppl.):27–32. doi: 10.1111/j.1651-2227.1994.tb13238.x. [DOI] [PubMed] [Google Scholar]
- 26.Lucas A, Cole T.J. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 27.Claud E.C, Walker W.A. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. Faseb J. 2001;15:1398–1403. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]
- 28.Bennet R, Eriksson M, Nord C.E. Fecal bacterial microflora of newborn infants during intensive care management and treatment with five antibiotic regimens. Pediatr Infect Dis. 1986;5:533–539. doi: 10.1097/00006454-198609000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence G, Bates J, Gaul A. Pathogenesis of neonatal necrotising enterocolitis. Lancet. 1982;1:137–139. doi: 10.1016/s0140-6736(82)90383-x. [DOI] [PubMed] [Google Scholar]
- 30.Scheifele D.W. Role of bacterial toxins in neonatal necrotizing enterocolitis. J Pediatr. 1990;117:S44–S46. doi: 10.1016/s0022-3476(05)81129-1. [DOI] [PubMed] [Google Scholar]
- 31.Hsueh W, Caplan M.S, Tan X. Necrotizing enterocolitis of the newborn: pathogenetic concepts in perspective. Pediatr Dev Pathol. 1998;1:2–16. doi: 10.1007/s100249900002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edelson M.B, Bagwell C.E, Rozycki H.J. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics. 1999;103:766–771. doi: 10.1542/peds.103.4.766. [DOI] [PubMed] [Google Scholar]
- 33.Ng P.C, Li K, Wong R.P. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch Dis Child Fetal Neonatal Ed. 2003;88:F209–F213. doi: 10.1136/fn.88.3.F209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanthakumar N.N, Fusunyan R.D, Sanderson I. Inflammation in the developing human intestine: a possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci U S A. 2000;97:6043–6048. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ein S.H, Marshall D.G, Girvan D. Peritoneal drainage under local anesthesia for perforations from necrotizing enterocolitis. J Pediatr Surg. 1977;12:963–967. doi: 10.1016/0022-3468(77)90607-8. [DOI] [PubMed] [Google Scholar]
- 36.Cheu H.W, Sukarochana K, Lloyd D.A. Peritoneal drainage for necrotizing enterocolitis. J Pediatr Surg. 1988;23:557–561. doi: 10.1016/s0022-3468(88)80368-3. [DOI] [PubMed] [Google Scholar]
- 37.Takamatsu H, Akiyama H, Ibara S. Treatment for necrotizing enterocolitis perforation in the extremely premature infant (weighing less than 1,000 g) J Pediatr Surg. 1992;27:741–743. doi: 10.1016/s0022-3468(05)80105-8. [DOI] [PubMed] [Google Scholar]
- 38.Morgan L.J, Shochat S.J, Hartman G.E. Peritoneal drainage as primary management of perforated NEC in the very low birth weight infant. J Pediatr Surg. 1994;29:310–314. doi: 10.1016/0022-3468(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 39.Azarow K.S, Ein S.H, Shandling B. Laparotomy or drain for perforated necrotizing enterocolitis: who gets what and why? Pediatr Surg Int. 1997;12:137–139. [PubMed] [Google Scholar]
- 40.Moss R.L, Dimmitt R.A, Henry M.C. A meta-analysis of peritoneal drainage versus laparotomy for perforated necrotizing enterocolitis. J Pediatr Surg. 2001;36:1210–1213. doi: 10.1053/jpsu.2001.25764. [DOI] [PubMed] [Google Scholar]
- 41.Moore T.C. Successful use of the ‘patch, drain, and wait’ laparotomy approach to perforated necrotizing enterocolitis: is hypoxia-triggered ‘good angiogenesis’ involved? Pediatr Surg Int. 2000;16:356–363. doi: 10.1007/s003839900337. [DOI] [PubMed] [Google Scholar]
- 42.Patel J.C, Tepas J.J, III, Huffman S.D. Neonatal necrotizing enterocolitis: the long-term perspective. Am Surg. 1998;64:575–579. [PubMed] [Google Scholar]
- 43.Vennarecci G, Kato T, Misiakos E.P. Intestinal transplantation for short gut syndrome attributable to necrotizing enterocolitis. Pediatrics. 2000;105:E25. doi: 10.1542/peds.105.2.e25. [DOI] [PubMed] [Google Scholar]
- 44.Barlow B, Santulli T.V, Heird W.C. An experimental study of acute neonatal enterocolitis—the importance of breast milk. J Pediatr Surg. 1974;9:587–595. doi: 10.1016/0022-3468(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 45.Kliegman R.M, Pittard W.B, Fanaroff A.A. Necrotizing enterocolitis in neonates fed human milk. J Pediatr. 1979;95:450–453. doi: 10.1016/s0022-3476(79)80534-x. [DOI] [PubMed] [Google Scholar]
- 46.Dvorak B, Halpern M.D, Holubec H. Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model. Pediatr Res. 2003;53:426–433. doi: 10.1203/01.PDR.0000050657.56817.E0. [DOI] [PubMed] [Google Scholar]
- 47.Goldman A.S. Modulation of the gastrointestinal tract of infants by human milk. Interfaces and interactions. An evolutionary perspective. J Nutr. 2000;130(2S Suppl.):426S–431S. doi: 10.1093/jn/130.2.426S. [DOI] [PubMed] [Google Scholar]
- 48.Caplan M.S, MacKendrick W. Inflammatory mediators and intestinal injury. Clin Perinatol. 1994;21:235–246. [PubMed] [Google Scholar]
- 49.MacKendrick W, Hill N, Hsueh W. Increase in plasma platelet-activating factor levels in enterally fed preterm infants. Biol Neonate. 1993;64:89–95. doi: 10.1159/000243976. [DOI] [PubMed] [Google Scholar]
- 50.McGuire W, Anthony M.Y. Donor human milk versus formula for preventing necrotising enterocolitis in preterm infants: systematic review. Arch Dis Child Fetal Neonatal Ed. 2003;88:11–14. doi: 10.1136/fn.88.1.F11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGuire W, Anthony M.Y. Formula milk versus term human milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD002971. CD002971. [DOI] [PubMed] [Google Scholar]
- 52.Eibl M.M, Wolf H.M, Furnkranz H. Prevention of necrotizing enterocolitis in low-birth-weight infants by IgA–IgG feeding. N Engl J Med. 1988;319:1–7. doi: 10.1056/NEJM198807073190101. [DOI] [PubMed] [Google Scholar]
- 53.Foster J, Cole M. Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth-weight neonates. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD001816. CD001816. [DOI] [PubMed] [Google Scholar]
- 54.Bell E.F, Acarregui M.J. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000503. CD000503. [DOI] [PubMed] [Google Scholar]
- 55.Bury R.G, Tudehope D. Enteral antibiotics for preventing necrotising enterocolitis in low birthweight or preterm infants. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000405. CD000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown E.G, Sweet A.Y. Preventing necrotizing enterocolitis in neonates. JAMA. 1978;240:2452–2454. [PubMed] [Google Scholar]
- 57.Rayyis S.F, Ambalavanan N, Wright L. Randomized trial of ‘slow’ versus ‘fast’ feed advancements on the incidence of necrotizing enterocolitis in very low birth weight infants. J Pediatr. 1999;134:293–297. doi: 10.1016/s0022-3476(99)70452-x. [DOI] [PubMed] [Google Scholar]
- 58.Kennedy K.A, Tyson J.E, Chamnanvanikij S. Early versus delayed initiation of progressive enteral feedings for parenterally fed low birth weight or preterm infants. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD001970. CD001970. [DOI] [PubMed] [Google Scholar]
- 59.Kennedy K.A, Tyson J.E, Chamnanvanikij S. Rapid versus slow rate of advancement of feedings for promoting growth and preventing necrotizing enterocolitis in parenterally fedlow-birth-weight infants. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD001241. CD001241. [DOI] [PubMed] [Google Scholar]
- 60.Berseth C.L, Bisquera J.A, Paje V.U. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2003;111:529–534. doi: 10.1542/peds.111.3.529. [DOI] [PubMed] [Google Scholar]
- 61.Caplan M.S, Jilling T. Neonatal necrotizing enterocolitis: possible role of probiotic supplementation. J Pediatr Gastroenterol Nutr. 2000;30(Suppl. 2):18–22. [PubMed] [Google Scholar]
- 62.Nicaise P, Gleizes A, Sandre C. Influence of intestinal microflora on murine bone marrow and spleen macrophage precursors. Scand J Immunol. 1998;48:585–591. doi: 10.1046/j.1365-3083.1998.00487.x. [DOI] [PubMed] [Google Scholar]
- 63.Ruseler-van Embden J.G, van Lieshout L.M, Gosselink M.J. Inability of Lactobacillus casei strain GG, L. acidophilus, and Bifidobacterium bifidum to degrade intestinal mucus glycoproteins. Scand J Gastroenterol. 1995;30:675–680. doi: 10.3109/00365529509096312. [DOI] [PubMed] [Google Scholar]
- 64.Caplan M.S, Miller-Catchpole R, Kaup S. Bifidobacterial supplementation reduces the incidence of necrotizingenterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 65.Butel M.J, Roland N, Hibert A. Clostridial pathogenicity in experimental necrotising enterocolitis in gnotobiotic quails and protective role of bifidobacteria. J Med Microbiol. 1998;47:391–399. doi: 10.1099/00222615-47-5-391. [DOI] [PubMed] [Google Scholar]
- 66.Kitajima H, Sumida Y, Tanaka R. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 1997;76:101–107. doi: 10.1136/fn.76.2.f101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoyos A.B. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis. 1999;3:197–202. doi: 10.1016/s1201-9712(99)90024-3. [DOI] [PubMed] [Google Scholar]
- 68.Borriello S.P, Hammes W.P, Holzapfel W. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis. 2003;36:775–780. doi: 10.1086/368080. [DOI] [PubMed] [Google Scholar]
- 69.Carlson S.E, Montalto M.B, Ponder D.L. Lower incidence of necrotizing enterocolitis in infants fed a preterm formula with egg phospholipids. Pediatr Res. 1998;44:491–498. doi: 10.1203/00006450-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 70.Caplan M.S, Russell T, Xiao Y. Effect of polyunsaturated fatty acid (PUFA) supplementation on intestinal inflammation and necrotizing enterocolitis (NEC) in a neonatal rat model. Pediatr Res. 2001;49:647–652. doi: 10.1203/00006450-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 71.Amin H.J, Zamora S.A, McMillan D.D. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J Pediatr. 2002;140:425–431. doi: 10.1067/mpd.2002.123289. [DOI] [PubMed] [Google Scholar]
- 72.Carrion V, Egan E.A. Prevention of neonatal necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 1990;11:317–323. doi: 10.1097/00005176-199010000-00006. [DOI] [PubMed] [Google Scholar]


