Abstract
Pulmonary dysfunction was evaluated in pigs infected with porcine reproductive and respiratory syndrome virus (PRRSV, isolate VR-2332) and compared to clinical and pathological findings. Infected pigs developed fever, reduced appetite, respiratory distress and dullness at 9 days post-inoculation (dpi). Non-invasive pulmonary function tests using impulse oscillometry and rebreathing of test gases (He, CO) revealed peripheral airway obstruction, reduced lung compliance and reduced lung CO-transfer factor. PRRSV-induced pulmonary dysfunction was most marked at 9–18 dpi and was accompanied by a significantly increased respiratory rate and decreased tidal volume. Expiration was affected more than inspiration. On histopathological examination, multifocal areas of interstitial pneumonia (more severe and extensive at 10 dpi than 21 dpi) were identified as a possible structural basis for reduced lung compliance and gas exchange disturbances.
Keywords: Porcine reproductive and respiratory syndrome virus, Pulmonary function testing, Pathology, Pigs
Introduction
Porcine reproductive and respiratory syndrome virus (PRRSV), a member of the family Arteriviridae, genus Arterivirus, causes porcine reproductive and respiratory syndrome (PRRS), an important cause of production losses in pigs (Rossow, 1998). The consequences of PRRSV infection have been well documented for the reproductive system and for systemic infection (Christianson et al., 1992, Botner et al., 1994, Mengeling et al., 1996, Lager et al., 1997, Kranker et al., 1998). Numerous studies have evaluated the clinical, pathological and immunological features of the respiratory form of PRRS (Labarque et al., 2000, Samsom et al., 2000, Opriessnig et al., 2002), but the effects on pulmonary function have not been investigated, even though respiratory dysfunction may have a significant impact on the clinical outcome of the disease.
Typical clinical signs of respiratory infection with PRRSV are tachypnoea or dyspnoea that can be accompanied by an increased respiratory effort, lethargy, fever and occasionally coughing, sneezing and chemosis (Rossow et al., 1994, Opriessnig et al., 2002). Gross lung lesions include failure of the lungs to collapse, as well as moderately well demarcated, mottled, brown areas of pneumonia (Opriessnig et al., 2002). Microscopic lung lesions are characterised by type II pneumocytic hypertrophy and hyperplasia, septal infiltration with mononuclear cells and alveolar exudates (Opriessnig et al., 2002). No data are currently available on the pathophysiological features and derangements of pulmonary function induced by PRRSV. Since the lungs are important for oxygen supply to all tissues, pulmonary dysfunction might have significant clinical and subclinical systemic consequences.
Pulmonary function testing has been applied to pigs with bacterial respiratory infections by our group (Reinhold et al., 2005, Reinhold et al., 2008). A combination of impulse oscillometry and rebreathing of test gases can be used to evaluate lung ventilation, respiratory mechanics and pulmonary gas exchange in spontaneously breathing pigs. Since both methods are non-invasive and applicable to conscious animals, changes in pulmonary function can be evaluated over time. In this study, pulmonary dysfunction was characterised in relation to clinical signs and pathological changes in pigs with experimental PRRSV infection.
Materials and methods
Animals
Twenty-four German hybrid pigs from a closed specific-pathogen-free herd known to be free of PRRSV were transported to our institute at 24–27 days of age and were enrolled in the study after a quarantine period of 21 days. Pigs were housed according to the guidelines for animal welfare and fed twice daily with a commercial grower diet without antibiotics. Water was supplied ad libitum. Pigs were housed in two groups of 12 animals each, with pens of six pigs being separated from each other.
Virus
VR-2332, the prototype type II PRRSV isolated in 1989 in Minnesota, USA, has been shown to be virulent for sows and piglets (Rossow et al., 1994, Opriessnig et al., 2002, Nielsen et al., 2003). Sixth passage VR-2332, propagated and titrated on MA-104 cells, was prepared at a concentration of 593 log10 50% tissue culture infectious doses/mL for use as the inoculum.
Experimental design
The study had a randomised, negatively controlled design (Table 1 ) and was performed at biosafety level 2. Ethical approval was obtained from the Commission for the Protection of Animals of the State of Thuringia, Germany (registration number 04-001/07).
Table 1.
Study design for PRRSV monitoring, additional microbiological analysis, pulmonary function testing and postmortem examination.
 |
M. hyopneumoniae, Mycoplasma hyopneumoniae; APP, Actinobacillus pleuropneumoniae; PCV-2, porcine circovirus type 2; PRCV, porcine respiratory coronavirus; SIV, swine influenza virus; TGEV, transmissible gastroenteritis virus; PFT, pulmonary function tests (8 pigs per group examined postmortem at 21 dpi).
an = 12 Pigs per group.
bn = 8 Pigs per group (with PFT).
cn = 4 Pigs per group (without PFT).
Twelve pigs were exposed to PRRSV and 12 pigs served as controls. On the day of challenge, pigs exposed to PRRSV had an age of 62.2 ± 1.0 days (mean ± standard deviation, SD) and a body weight of 22 ± 2 kg, while controls were 61.2 ± 0.7 days old and weighed 22 ± 2 kg. Pigs were inoculated intranasally (1 mL per nostril) and intramuscularly (1 mL per pig) with either PRRSV VR-2332 or 0.9% saline (controls). For intranasal inoculation, a 10 mL syringe was connected to a tube (40 cm × 1.7 mm inner diameter feeding tube, Rüsch sterile, Ref 224000, size No. 3. Willy Rüsch GmbH) which had been inserted approximately 3 cm into the nose. One millilitre of virus solution, along with 9 mL air, per syringe was administered into each nostril with manual pressure, followed by IM injection of 1 mL into the gluteal muscle.
Clinical observations were recorded twice daily and included general behaviour, feed intake, appetite, rectal temperature, respiratory rate (RR) and the presence or absence of clinical signs of respiratory disease or diarrhoea. To monitor for the presence of PRRSV by PCR and for seroconversion, blood samples were collected three times before challenge (−11, −5 days and −1 h) and six times after challenge (3, 7, 10, 14, 17 and 21 days post-inoculation, dpi). Blood samples, nasal swabs, rectal swabs and tracheal swabs were also collected to exclude concurrent infections (Table 1).
Pulmonary function tests (PFTs) were performed twice before challenge (−7 and −3 days) and seven times after challenge (2, 4, 6, 9, 12, 15, 18 and 21 dpi) in eight pigs exposed to PRRSV and in eight controls (Table 1). Body weight was determined 1 day before each PFT and at postmortem examination. Four pigs per group (without PFT) were sacrificed at 10 dpi and eight pigs per group (with PFT) were sacrificed at 21 dpi for gross pathological, histopathological and immunohistochemical examination (Table 1).
Quantitative real-time PCR
To quantify the PRRSV load in blood, serum samples were analysed by real-time PCR for PRRSV strain VR-2332 and its attenuated form, Ingelvac MLV. RNA was extracted using QIAamp Viral RNA Mini Kit (Qiagen). Reverse transcription was carried out using the Multiscribe RT Enzyme Kit (Applied Biosystems). Reverse transcription conditions were 25 °C for 10 min, 37 °C for 120 min and 85 °C for 5 s. Amplification was carried out in triplicate using the TaqMan Universal PCR Master Kit (Applied Biosystems) and the PRRS VR-2332-specific primers MLV F1 (5′-GCAGCTCCCATCTACAGCTGATT-3′) and MLV R1 (5′-AGACAATGTGAGTCAAAACGGGAAAGAT-3′). The probe was TET-5′-TTGGCTAGCTAACAAATTTGATTGGGCAGTGGAGAGTTT-3′-TAMRA. Thermal cycling conditions were 50 °C for 2 min, 95 °C for 10 min, then 50 cycles of 94 °C for 15 s and 60 °C for 1 min. PRRSV cDNA quantification was achieved by comparison of the unknown sample with a standard curve derived from known amounts of plasmid DNA.
ELISA
A commercial PRRSV ELISA (HerdCheck PRRS ELISA, IDEXX) was used to detect anti-PRRS antibodies in the blood serum of pigs before and after challenge. Samples with sample-to-positive (S/P) ratio ⩾ 0.4 were considered to be positive for antibodies against PRRSV, as recommended by the manufacturer.
Differential microbiological examinations
Routine bacteriological culture was performed on nasal, tracheal and faecal swabs for Bordetella spp., Pasteurella spp., Haemophilus spp., Actinobacillus pleuropneumoniae (APP) and Salmonella spp. (Table 1). Samples were tested for Mycoplasma spp. by indirect immunofluorescence and for Chlamydia spp. by PCR.
Paired serum samples from each pig (blood collected at the beginning of the study and at postmortem examination) were used for serology (Table 1). Commercial ELISA test kits were used to detect antibodies against Mycoplasma hyopneumoniae (DAKO M. hyo ELISA, Oxoid), APP (Cypress Diagnostics), swine influenza virus (SIV; IDEXX), transmissible gastroenteritis virus (TGEV; SVANOVA) and porcine respiratory coronavirus (PRCV; SVANOVA). Testing for antibodies against porcine circovirus type 2 (PCV-2) was performed by an indirect fluorescent antibody test (Fachinger et al., 2008).
Pulmonary function tests
Approximately 15 min prior to PFT, each pig was sedated with diazepam (1.5–2.0 mg/kg body weight IM; Faustan, Weimer Pharma). The sedated animal was restrained in a canvas sling with openings for the limbs and wore a tightly fitting face mask, allowing spontaneous breathing. After an adaptation period of approximately 5 min, two non-invasive lung function techniques were applied consecutively: (1) impulse oscillometry system (IOS; MasterScreen IOS; Jaeger); and (2) rebreathing system (MasterScreen Diffusion; Jaeger). Both systems were originally produced for human medicine and have been successfully applied to pigs previously (Reinhold et al., 2005, Reinhold et al., 2008).
Impulse oscillometry
IOS was used to measure variables of respiratory mechanics based on a forced oscillation technique, as previously validated for pigs (Klein and Reinhold, 2001, Klein et al., 2003). Externally generated test impulses given by a loudspeaker were superimposed on spontaneous airflow of the breathing animal. Variations in pressure and flow signals were analysed using Fast Fourier Transformation to calculate the complex respiratory impedance, which consists of both respiratory resistance (Rrs) and respiratory reactance (Xrs) (Smith et al., 2005). Airflow was registered during spontaneous breathing using a Lilly-type pneumotachograph with a mesh resistance of 36 Pa/(L/s) and used to calculate spirometric variables of spontaneous breathing (RR and tidal volume, Vt).
Each test per day and animal consisted of three consecutive IOS measurements free of any artefacts (e.g. coughing or irregular breathing pattern). Each measurement lasted for 60 s. Three impulses were generated per second, leading to 180 independent results per min. The sampling rate was set at 200 Hz (period between two sampling points of 5 ms), selecting 32 sampling points after each impulse. Results of the three measures per pig and per time point were averaged for statistical analysis.
The following variables of ventilation and respiratory mechanics were analysed: (1) RR; (2) Vt; (3) volume of minute ventilation (MV); (4) both Vt and MV related to body weight (Vt/kg, MV/kg); (5) Rrs; (6) Xrs, each at 3, 5, 10 and 15 Hz, and separated for inspiration and expiration at each frequency (Rrs,in3Hz … Rrs,in15Hz; Rrs,ex3Hz … Rrs,ex15Hz; Xrs,in3Hz … Xrs,in15Hz; Xrs,ex3Hz … Xrs,ex15Hz); (7) resistance of proximal airways (Rprox); and resistance of distal airways (Rdist).
Rebreathing method
Using different test gases and a multiple breath approach (i.e. steady state method), the rebreathing system permitted the simultaneous measurement of two additional pulmonary function variables. The functional residual capacity (FRC) of the lungs was measured by the helium (He) dilution technique (wash-in). The transfer factor of the lungs for carbon monoxide (TL CO) was determined in order to evaluate the transfer of oxygen from the lungs into the blood.
For assessing both FRC and TL CO, each pig inhaled the test gas mixture (9% He, 0.25% CO in synthetic air) from a reservoir bag. Since animals were growing, the volume filled in the reservoir bag was 4 L in the pre-challenge period and was adjusted for the increasing lung volume with growth to 5 L from 2 to 18 dpi. The time to perform this test (i.e. rebreathing time) increased from 91 ± 17 s (mean ± SD) 1 week before challenge (body weight of 17 ± 1 kg, mean ± SD) to 116 ± 16 s at 18 dpi (body weight of 32 ± 2 kg) due to a significant correlation with increasing body weight (r = 0.25; R 2 = 6.28%; P = 0.003) or increasing FRC (r = 0.29; R 2 = 8.26%; P = 0.0005), respectively.
FRC was calculated per kg body weight (FRC/kg) to avoid body weight being a confounding factor. Data for TL CO were corrected for the individual concentration of haemoglobin in the blood (TL COHb), as determined before each PFT using a haemoxymeter that allows species-specific analysis of haemoglobin fractions for pigs (OSM3, Radiometer). To correct for body weight and metabolism, TL COHb was calculated per kg body weight (TL COHb/kg), as well as per kg metabolic body weight (TL COHb/kg0.75).
Gross pathology and histopathology
Pigs were euthanased by intravenous injection of pentobarbital sodium (Release, WDT) at ⩾600 mg/10 kg body weight. The trachea was exposed, severed distal to the larynx and the lungs were instilled with neutral buffered formalin (NBF) at a pressure of 300 mm water column. After 15 min of intrathoracic fixation, the trachea was closed and the lungs were removed from the thoracic cavity and placed in NBF for 24 h. Samples collected from proximal and distal parts of the cranial and caudal pulmonary lobes and from one area of the accessory lobe were embedded in low-melting paraffin (50–52 °C). Paraffin sections were stained with haematoxylin and eosin for histological examination.
Immunohistochemistry
PRRSV antigen was detected by immunohistochemistry using the alkaline phosphatase anti-alkaline phosphatase (APAAP) method. Paraffin sections collected on charged slides were pretreated with proteinase K (0.05% in phosphate buffered saline, pH 7.4) for 10 min. The monoclonal antibody SR30-A (Rural Technologies) was used as the primary antibody and rabbit anti-mouse Ig (Dianova) was used as the secondary antibody to bind the APAAP complex (Dako). Alkaline phosphatase was visualised using neufuchsin. Slides were counterstained with methylene green. Positive control sections (BioScreen EVDMC) were included with each reaction.
Statistical analysis
Statistical analyses were performed to determine the relationship between PRRSV infection and clinical signs, body weight or lung function variables. For statistical evaluation of clinical signs, the study period from 6 days before inoculation until 21 dpi was separated into nine sub-periods, each of three consecutive days. Within each sub-period, data obtained per day and per animal were averaged. For calculating baseline data obtained by PFT, all measures per pig before inoculation were averaged.
Numeric data are presented as medians, minima and maxima. The Mann–Whitney–Wilcoxon-test (W-test; comparison of medians) was used to determine significant differences between groups at P ⩽ 0.05. For regression analyses a linear model was used (y = a + bx) and both coefficient of linear correlation (r) and coefficient of determination (R 2) were calculated.
Results
PRRSV viraemia and seroconversion
PCR for quantification of viral load and ELISA for antibody detection demonstrated that all pigs were negative for PRRSV before challenge. Controls remained free of PRRSV infection throughout the trial. In pigs exposed to PRRSV, infection was confirmed by increasing viral loads in serum. Viraemia was detected in all pigs at 3 dpi and reached a maximum 10 dpi (Fig. 1 A). Antibody titres against PRRSV were first observed at 7 dpi in one pig and at 14 dpi in all other pigs, reaching a maximum at 17 dpi (Fig. 1B).
Fig. 1.
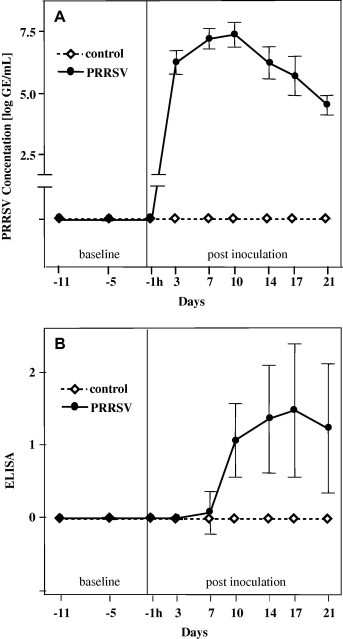
(A) Detection of PRRSV in serum by PCR. (B) Titres of antibodies against PRRSV in serum by ELISA. Data are presented as means ± SD; days −11 to 10: n = 12 per group; days 14–21: n = 8 per group. ELISA: 0, negative (s/p ratio = 0.0–0.39); 1, seropositive 1 (s/p ratio = 0.4–0.999); 2, seropositive 2 (s/p ratio = 1–1.49); 3, seropositive 3 (s/p ratio = 1.5–1.99).
Clinical signs
None of the control pigs exhibited any clinical signs of respiratory disease during the study. Pigs exposed to PRRSV developed clinical signs initially observed at 1–3 dpi, including reduced appetite, dullness and fever. Body temperature was significantly increased in challenged pigs compared to controls from 1 to 15 dpi and this increase showed a biphasic character, with maxima at 1–3 dpi and 7–9 dpi (Fig. 2 A). Most PRRSV challenged pigs remained dull until the end of the trial, whereas reduced appetite and/or diarrhoea was identified only sporadically from 7 to 18 dpi. The daily weight gain was 568 ± 61 g (mean ± SD) in controls and 573 ± 67 g in pigs exposed to PRRSV; there was no significant difference between groups.
Fig. 2.
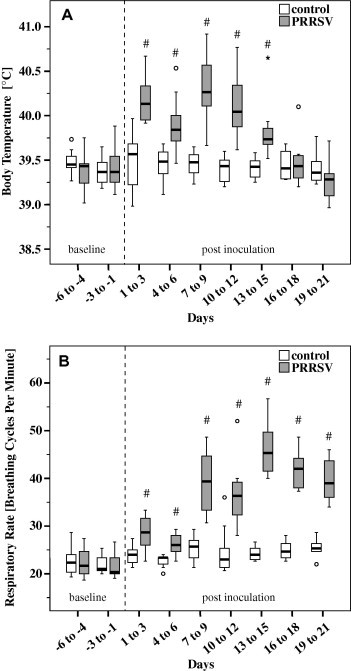
Body temperature (A) and respiratory rate (B) of pigs exposed to PRRSV and controls. Box-and-whisker plot represents median value, 25% and 75% percentiles (box), range, outlier values (o) and extreme values (*). Days −6 to 9: n = 12 per group; Days 10–21: n = 8 per group. # Indicates significant differences between PRRSV challenged pigs and controls (W-test, P ⩽ 0.05).
Coughing, dyspnoea and ocular discharge were detected in pigs exposed to PRRSV. Coughing was present from 1 to 21 dpi in 9/12 pigs and was most prominent from 13 to 15 dpi. RR increased significantly in PRRSV challenged pigs compared to controls starting in the period 1 to 3 dpi and reached maximal values at 13–15 dpi (Fig. 2B). Dyspnoea developed in 8/12 pigs in the period 10–21 dpi, with a maximal intensity at 16–18 dpi. One PRRSV challenged pig exhibited ocular discharge in the period 7–12 dpi. Interestingly, no nasal discharge and no vomiting was observed.
Pulmonary function
Volumes of lung ventilation
Compared to controls, Vt/kg was significantly reduced in pigs exposed to PRRSV after challenge (Fig. 3 A). This decrease was most prominent in the period 9–18 dpi. In contrast, the volume of MV in relation to body weight (MV/kg) increased significantly in PRRSV challenged pigs. Compared to controls, the most marked increase in MV was seen between 6 and 15 dpi (Fig. 3B).
Fig. 3.
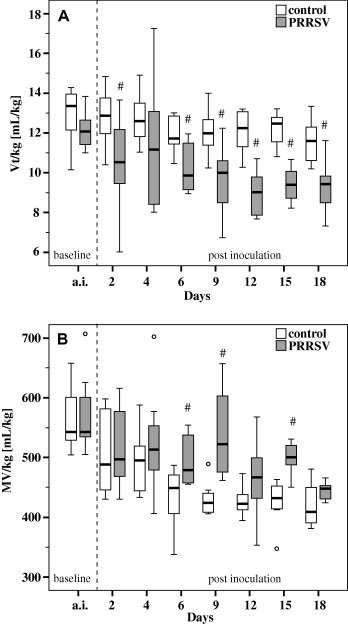
Tidal volume per kg body weight (Vt/kg; A) and minute ventilation per kg body weight (MV/kg; B) of pigs exposed to PRRSV (n = 8) and controls (n = 8) as measured with the impulse oscillometry system. Box-and-whisker plot represents median value, 25% and 75% percentiles (box), range, outlier values (o) and extreme values (*). a.i. Represents data measured prior to inoculation (averaged per pig). # Indicates significant differences between PRRSV challenged pigs and controls (W-test, P ⩽ 0.05).
Respiratory mechanics
In pigs experimentally exposed to PRRSV, marked changes in respiratory impedance occurred at 9–18 dpi; the changes were more prominent during expiration than during inspiration. Spectral curves of Rrs and Xrs within the frequency range of 3–15 Hz at 12 dpi are shown in Fig. 4 . Rrs at 3 and 5 Hz measured during expiration was significantly elevated in comparison with controls, leading to a significantly larger difference between inspiratory and expiratory resistance values. This increase in Rrs,ex was statistically significant on days 12 and 18 after challenge for both Rrs,ex3Hz and Rrs,ex5Hz, as well as for Rrs,ex3Hz alone on days 4 and 9 post-challenge (Supplementary Table 1). In contrast, Rrs at 3 and 5 Hz measured during inspiration was not significantly different between groups (Fig. 4, Supplementary Table 1). Both Rrs,ex and Rrs,in assessed at 10 and 15 Hz were lower in pigs exposed to PRRSV compared to controls (Supplementary Table 1). Due to significantly increased expiratory resistance data at low frequencies (3 and 5 Hz) and significantly lower Rrs,ex data at 15 Hz, the resistance curves during expiration became inversely frequency dependent after PRRSV challenge, i.e. resistance decreased with increasing frequency (Fig. 4).
Fig. 4.
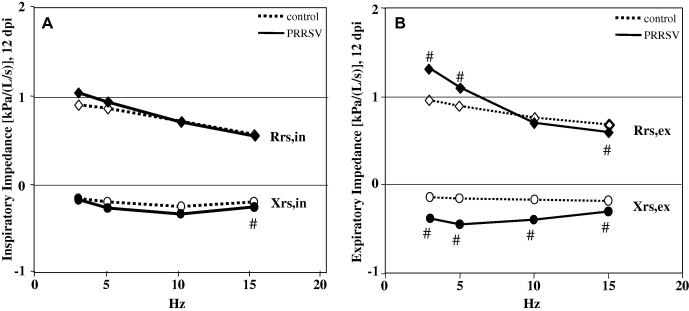
Medians of spectral respiratory impedance within the frequency range of 3–15 Hz separated for respiratory impedance during inspiration (A) and respiratory impedance during expiration (B) of pigs exposed to PRRSV (n = 8) and controls (n = 8) 12 dpi. Rrs, respiratory resistance; Xrs, respiratory reactance, both during expiration (Rrs,ex, Xrs,ex, respectively) and inspiration (Rrs,in, Xrs,in, respectively). # Indicates significant differences between PRRSV challenged pigs and controls (W-test, P ⩽ 0.05).
Xrs at all frequencies (3–15 Hz) was significantly lower in PRRSV infected pigs compared to controls (Fig. 4). This observed negativity of the total Xrs curves was more prominent during expiration compared to inspiration, mainly from 12 to 18 days after exposure (Supplementary Table 2).
Proximal and distal airway resistances are shown in Table 2 . After challenge, pigs exposed to PRRSV had significantly higher distal airway resistance compared to controls. In contrast, Rprox was significantly lower in PRRSV challenged pigs than in control pigs.
Table 2.
Distal airway resistance (Rdist) and proximal airway resistance (Rprox) in pigs exposed to PRRSV (n = 8) and controls (n = 8).
| Day | Control |
PRRSV |
|||
|---|---|---|---|---|---|
| Median | Range min; max | Median | Range min; max | ||
| Rdist in kPa/(L/s) | a.i. | 0.334 | 0.256; 0.456 | 0.434 | 0.311; 0.550 |
| 2 | 0.300 | 0.250; 0.500 | 0.471 | 0.250; 0.617 | |
| 4 | 0.342 | 0.250; 0.400 | 0.558# | 0.233; 0.617 | |
| 6 | 0.292 | 0.250; 0.550 | 0.425 | 0.250; 0.550 | |
| 9 | 0.350 | 0.283; 0.417 | 0.517# | 0.333; 0.750 | |
| 12 | 0.275 | 0.250; 0.400 | 0.600# | 0.417; 0.717 | |
| 15 | 0.334 | 0.250; 0.483 | 0.592# | 0.283; 0.733 | |
| 18 | 0.284 | 0.250; 0.383 | 0.517# | 0.400; 0.717 | |
| Rprox in kPa/(L/s) | a.i. | 0.367 | 0.279; 0.374 | 0.336 | 0.316; 0.368 |
| 2 | 0.365 | 0.271; 0.436 | 0.327 | 0.259; 0.421 | |
| 4 | 0.366 | 0.211; 0.415 | 0.345 | 0.288; 0.407 | |
| 6 | 0.383 | 0.266; 0.460 | 0.329# | 0.298; 0.371 | |
| 9 | 0.388 | 0.308; 0.523 | 0.385 | 0.255; 0.449 | |
| 12 | 0.431 | 0.389; 0.459 | 0.353# | 0.275; 0.447 | |
| 15 | 0.482 | 0.335; 0.523 | 0.363# | 0.289; 0.436 | |
| 18 | 0.517 | 0.399; 0.588 | 0.361# | 0.306; 0.387 | |
a.i. Represents data measured prior to inoculation with PRRSV (averaged per pig).
Indicates significant difference between pigs exposed to PRRSV and controls (W-test, P ⩽ 0.05).
Functional residual capacity
As shown in Table 3 , volumes of FRC increased in growing pigs of both groups during the study (linear correlation between body weight and FRC: control pigs: r = 0.69; R 2 = 47.96%; P ⩽ 0.00001; PRRSV infected pigs: r = 0.44; R 2 = 19.05%; P ⩽ 0.00001). Neither absolute volumes of FRC nor FRC/kg body weight (data not shown) differed significantly between groups at any time point.
Table 3.
Functional residual capacity (FRC) in pigs exposed to PRRSV (n = 8) and controls (n = 8).
| Day | Control |
PRRSV |
|||
|---|---|---|---|---|---|
| Median | Range min; max | Median | Range min; max | ||
| FRC (mL) | a.i. | 140 | 60; 430 | 235 | 50; 370 |
| 2 | 340 | 46; 576 | 366 | 89; 867 | |
| 4 | 368 | 59; 570 | 365 | 206; 770 | |
| 6 | 310 | 192; 414 | 287 | 32; 706 | |
| 9 | 343 | 131; 717 | 367 | 17; 593 | |
| 12 | 544 | 150; 743 | 481 | 149; 603 | |
| 15 | 648 | 271; 950 | 553 | 271; 735 | |
| 18 | 631 | 461; 1048 | 507 | 270; 790 | |
a.i. Represents data measured prior to inoculation (averaged per pig).
Pulmonary gas exchange
In controls, TL COHb ranged from 0.43 mmol/min/kPa (median; minimum-maximum: 0.33–0.95) 7 days before challenge to 1.23 mmol/min/kPa (minimum-maximum: 0.97–3.2) at 18 dpi. Due to the increase in body weight of pigs during the trial (r = 0.51; R 2 = 25.56%; P ⩽ 0.0001) and because gas exchange in the lung physiologically adapts to metabolism, groups were compared on a basis of TL COHb data corrected for either body weight (Fig. 5 A) or metabolic body weight (Fig. 5B). Compared to controls, reduced gas transfer became evident in pigs challenged with PRRSV from 9 dpi until the end of the study. This decrease was statistically significant for TL COHb/kg at 12 and 18 dpi and for TL COHb/kg0.75 at 12 dpi.
Fig. 5.
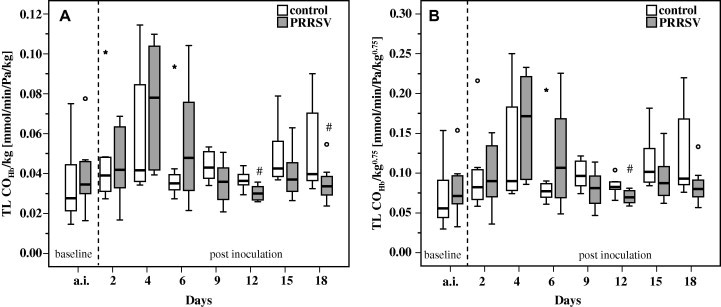
Transfer factor of carbon monoxide corrected for haemoglobin in relation to body weight (TL COHb/kg; A) and metabolic body weight (TL COHb/kg0.75; B) in pigs exposed to PRRSV (n = 8) and controls (n = 8). Box-and-whisker plot represents median value, 25% and 75% percentiles (box), range, outlier values (o) and extreme values (*). a.i. Represents data measured prior to inoculation (averaged per pig). # Indicates significant differences between PRRSV challenged pigs and controls (W-test, P ⩽ 0.05).
Gross pathology
There were no gross changes in the lungs of PRRSV infected or control pigs. Systemic enlargement of lymph nodes (including the tracheobronchial lymph nodes) was evident at 10 and 21 dpi in the PRRSV inoculated group.
Histopathology
Histopathological examination of the lungs of inoculated pigs revealed mild to moderate multifocal interstitial pneumonia characterised by thickening of interalveolar septa, septal infiltration with mononuclear cells, hyperplasia and hypertrophy of type II pneumocytes and alveolar exudates of macrophages, necrotic debris and occasionally multinucleated cells (Fig. 6 A). Pulmonary lesions were more frequent and more severe in cranial lobes compared to caudal lobes and in the proximal part of lobes compared to the distal part. At 10 dpi, mild to moderate interstitial pneumonia was evident in 3/4 pigs. At 21 dpi, lesions were milder, but all eight pigs were affected. Hyperplasia of the tracheobronchial lymph nodes was evident in 7/8 pigs at 21 dpi. In the lungs of control pigs, there were mild to moderate peribronchiolar and perivascular lymphocytic infiltrates, along with mild lymphocytic infiltrates in interalveolar septa and mild multifocal clusters of alveolar macrophages in alveolar spaces. There was no thickening of interalveolar septa (Fig. 6B).
Fig. 6.
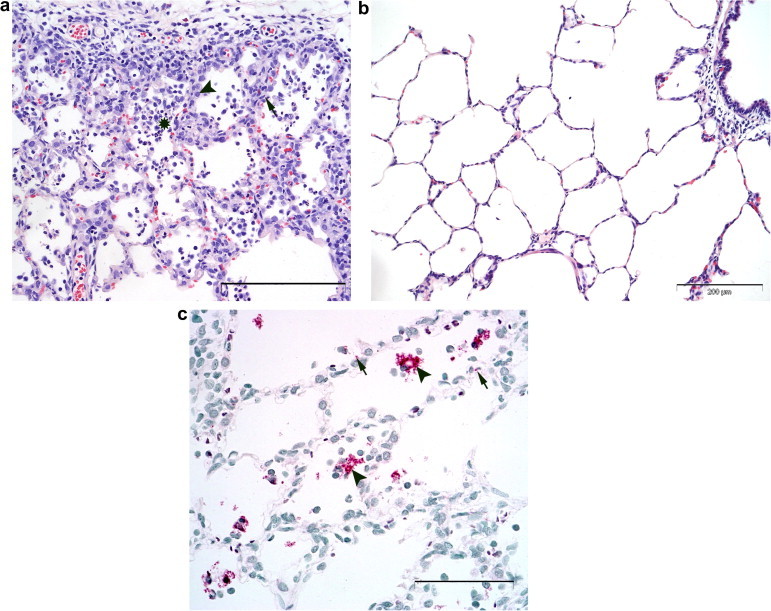
(a) Pulmonary lesions in the right cranial apical lung lobe of a pig 21 days after inoculation with PRRSV isolate VR-2332. Interstitial pneumonia with thickened interalveolar septa, septal infiltration of mononuclear cells (arrow, example), hypertrophy and hyperplasia of alveolar epithelial cells (arrowhead, example) and alveolar exudate (*, example). HE stain, bar = 200 μm. (b) Morphology of unaltered pulmonary tissue in the left cranial pulmonary lobe of a control pig 21 days after sham inoculation. Note open alveolar spaces and thin, even interalveolar septa due to intrapulmonary instillation of fixative. HE stain, bar = 200 μm. (c) Distribution of PRRSV antigen in the right cranial apical lung lobe of a pig 21 days post-inoculation with PRRSV isolate VR-2332. PRRSV antigen as shown by the presence of granular red reaction product is present in alveolar macrophages (arrowheads, examples) and in alveolar walls (arrows, examples). APAAP, bar = 100 μm.
Immunohistochemistry
PRRSV antigen was detected only in pigs inoculated with PRRSV. There was a close correlation between pulmonary lesions and the presence of viral antigen. PRRSV antigen was present in the cytoplasm of alveolar macrophages and occasionally in multinucleate cells within alveoli (Fig. 6C). It was also seen in mononuclear cells within thickened interalveolar septa and in type II alveolar epithelial cells. PRRSV antigen was also detected in one pig which did not develop interstitial pneumonia. At 10 dpi, the highest amount of viral antigen was found in two pigs with moderate pulmonary lesions. There was less PRRSV antigen present in the lungs at 21 dpi compared to 10 dpi.
Other microbiological findings
All pigs were free of APP, Bordetella spp., Mycoplasma spp., Pasteurella spp., SIV and TGEV. Chlamydia spp. were detected in rectal swabs of all pigs. All animals had antibodies against PCV-2 and PRCV. Haemophilus parasuis was detected in a nasal swab from 1/12 pigs in the control group before challenge. Salmonella spp. were detected sporadically in rectal swabs before and after challenge in both groups.
Discussion
This study characterised respiratory dysfunction induced by the well-characterised prototypical NA-type PRRSV isolate VR-2332 (Rossow et al., 1994, Opriessnig et al., 2002, Nielsen et al., 2003). Moderate clinical disease was induced that allowed consecutive pulmonary function testing until 3 weeks after challenge without any spontaneous deaths before the end of the study. This is the first simultaneous within-subject study of clinical, functional and morphological changes in pigs experimentally infected with PRRSV.
In the absence of other major infectious causes of respiratory disease, changes in clinical status, pulmonary dysfunction and pathology in pigs in this study can be attributed to PRRSV. PRRSV viraemia and seroconversion were evident in all challenged pigs. Challenge with VR-2332 induced reproducible and representative respiratory disease consistent with previous studies using the same strain (Rossow et al., 1994, Opriessnig et al., 2002). PRRSV viraemia was detected at 3 dpi and coincided with an increase in rectal temperature. An increase in RR was evident at 7 dpi and lasted until the end of the study, i.e. until at least 21 days after infection. Other clinical signs included dyspnoea and coughing and were most prominent from 15 to 18 dpi. Pulmonary dysfunction was evident earlier (4 dpi) and was most prominent at 12 dpi. Microscopic lung lesions were more severe at 10 dpi than at 21 dpi which is in accordance with Opriessnig et al. (2002).
Some authors have reported coughing as a clinical sign of PRRS (Done et al., 1996, Beyer et al., 2000), whereas others have attributed coughing to secondary infections (e.g. M. hyopneumoniae, B. bronchiseptica, PCV-2) (Thacker et al., 1999, Brockmeier et al., 2000, Thacker and Thanawongnuwech, 2002). We observed a spontaneous dry cough in most of the pigs exposed to PRRSV, but not in controls. Due to the absence of other major respiratory pathogens in the present study and because there was no evidence of tracheitis, bronchiolitis or airway mucus on pathological examination, we interpret the observed cough as being induced by bronchospasms as a component of the respiratory illness caused by PRRSV. This interpretation is supported by the presence of peripheral airway obstruction, as evaluated by pulmonary function testing.
Influence of PRRSV on pattern of breathing
The pattern of ventilation, as indicated by spirometric data determined during spontaneous breathing, includes RR, Vt and MV. In our study, RR increased significantly in pigs exposed to PRRSV, with maximal values at 13–15 dpi (216% compared to baseline data). Increased RR may be a response to hyperthermia, as well as an attempt to compensate for reduced Vt and/or arterial oxygen deficiency caused by reduced gas transfer from the lungs into the blood.
Relative Vt (Vt/kg) was significantly reduced (<10 mL/kg) in pigs exposed to PRRSV, while Vt/kg was always >10 mL/kg in healthy controls. Reduced Vts indicate changes in the pattern of breathing caused by obstructive or restrictive disorders and/or by disorders in gas exchange. Pulmonary function tests in PRRSV challenged pigs indicate both obstructive and restrictive disorders (confirmed by increased Rrs at frequencies ⩽5 Hz and decreased Xrs), as well as disorders in gas exchange (confirmed by decreased TL COHb). The latter correlates with the pathological findings of thickened interalveolar septa and the presence of alveolar exudates.
The observed increases in MV in PRRSV challenged pigs were caused by increases in RR and indicate compensation for reduced Vts. Pigs infected with PRRSV had shorter breathing cycles and shallower inspiration. This pattern of breathing, however, is pathophysiologically linked to a higher percentage of dead space ventilation compared to alveolar ventilation and consequently carries the risk of alveolar hypoventilation. In addition, the energy requirement for breathing increases due to increased effort of respiratory muscles.
Influence of PRRSV on respiratory mechanics
Impulse oscillometry measures complex respiratory impedance, which consists of Rrs and Xrs (Smith et al., 2005). While Rrs reflects mainly airway resistance, Xrs is determined by inertive and capacitive components of the respiratory system. Pressure impulses generated by a loudspeaker in a frequency range of 0–100 Hz are used as test signals. Low frequencies (3–5 Hz) penetrate deep into the respiratory tract and provide a representation of the peripheral airway system, whereas high frequencies (10–15 Hz) provide a representation of the upper and central airway systems. Results at frequencies >15 Hz were disregarded, since they are mainly influenced by mechanical properties of the face mask, which acts as a confounding factor for respiratory impedance measurements in animals (Reinhold et al., 1998, Klein et al., 2003).
In addition to the spectral curves of Rrs and Xrs, two model-derived resistances (i.e. Rdist and Rprox) were analysed to differentiate between the effects of PRRSV on either proximal airways (nasal cavities, larynx and pharynx) or distal airways (lung periphery), respectively. All variables of respiratory mechanics were significantly different in pigs challenged with PRRSV compared to controls. Rrs at 3–5 Hz, Rdist and Xrs are important variables with respect to the lung periphery, including peripheral airways. Rrs at frequencies ⩽5 Hz was significantly elevated in pigs exposed to PRRSV and this increase was only evident during expiration. This phenomenon indicates airflow limitation predominantly in the peripheral airway system, since peripheral airway obstruction affects expiration much more than inspiration.
The presence of peripheral airway obstruction is emphasised by significant increases in model-derived resistance of distal airways (Rdist). Due to the absence of mucus and bronchial wall oedema, peripheral airway obstruction was most likely to have been caused by bronchospasms. Morphological correlates for bronchospasms, however, are lacking, since contractions or spasms of airway muscles do not persist after euthanasia and the fixation method used in this study (intratracheal instillation with formalin) opens airways and fills alveolar regions, irrespective of the ventilation status during normal breathing. Decreases in Xrs indicate reduced compliance of the lung. This might be caused by restrictive disorders due to both stiffness of obstructed airways and pulmonary tissue components. The lungs of pigs challenged with PRRSV had thickened interalveolar septa and septal infiltration with mononuclear cells, which may be responsible for reduced compliance.
Findings with respect to central and/or upper airways are reflected by both Rrs at 10–15 Hz and Rprox. After PRRSV challenge, proximal airway resistance in exposed pigs was significantly lower compared to controls. This phenomenon might be interpreted as ‘upper airway opening’, an attempt to compensate for peripheral airway obstruction by increasing the diameters of upper airways (nasal cavities, larynx and pharynx). Due to the functional character of this phenomenon, no morphological equivalent is visible at postmortem examination. In humans, enlargement of the aperture of the glottis is recognised during a high frequency, low Vt breathing pattern (Stănescu et al., 1972).
A further approach to enlarge the upper airway diameter is reduction of mucosal thickness by changes in blood flow. In rats, cervical sympathetic nerve stimulation induces a reduction in upper airway resistance, which is most likely induced by α-adrenergic vasoconstriction of the upper airway mucosal vasculature, resulting in reduced mucosal thickness (O’Halloran et al., 1998). Stimulation of cervical sympathetic nerves, however, can be caused by systemic hypoxia and hypercapnia (Matsumoto et al., 1987). PRRSV challenged pigs showed a significantly increased partial pressure of CO2 compared to controls in venous blood gas analysis (data not shown).
Other factors contributing mainly to proximal airway resistance are anatomical peculiarities in the upper airways and head position. While performing PFTs, head position was standardised as recommended for pigs (Klein et al., 2003). The anatomical structure of the nasal cavities, however, might contribute to different upper airway resistances between individuals and could explain significantly different Rrs data ⩾10 Hz between groups even before exposure.
Influence of PRRSV on functional residual capacity
FRC represents the volume present in the lung at the end of spontaneous expiration. Therefore, it might be an indicator of ‘trapped air’, hyperinflation or emphysema. In our trial, no significant changes in FRC could be identified, indicating that airway obstruction was not severe enough to induce ‘air trapping’ or obstructive emphysema. This is in accordance with a lack of emphysema on pathological examination in our study, as well as studies of others (Pol et al., 1991, Halbur et al., 1995, Rossow, 1998).
Influence of PRRSV on pulmonary gas exchange
In the present study, both TL COHb in relation to body weight (TL COHb/kg) and metabolic body weight (TL COHb/kg0.75) revealed significant decreases in the diffusion of oxygen from the lungs into the blood in pigs exposed to PRRSV compared to controls. Limiting oxygen diffusion from the alveoli to the blood can be explained by increased diffusion distance due to infiltration of alveolar walls by mononuclear cells. In addition, hyperplasia and hypertrophy of the alveolar epithelial cells, as well as the presence of alveolar exudates, decreases the amount of gas within alveoli. Alveolar hypoventilation due to a rapid and superficial pattern of breathing may also contribute to decreased oxygen exchange. In the pig, which has no collateral airways (McLaughlin et al., 1961), each airway obstruction results in regional heterogeneity of alveolar ventilation (Robinson, 1982). Consequently, obstruction of ventilation in pigs exposed to PRRSV most likely resulted in imbalances between ventilation and perfusion, leading to reduced oxygen transfer from the lungs into the blood. This would result in respiratory acidosis characterised by reduced pH and increased partial CO2 pressure in venous blood, indicating reduced alveolar ventilation.
Conclusions
This is the first study evaluating disorders of lung function caused by PRRSV in pigs. Significant imbalances in respiratory mechanics, lung ventilation and pulmonary gas exchange were correlated with clinical signs and pathological findings. PRRSV isolate VR-2332 alone was capable of inducing respiratory distress for at least 3 weeks after exposure. Studies using other PRRSV strains would be useful to identify differences in the pathogenesis of infections caused by different PRRSV isolates. Evaluation of therapeutic and vaccination strategies will be supported by combining the functional approach of this study with knowledge generated from animal studies focussing on inflammatory markers and the immune response.
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Acknowledgements
The authors are very grateful to Annelie Langenberg, Sylke Stahlberg, Ines Lemser and to all colleagues working in the team of the animal house (FLI Jena, Germany) for their skilful assistance during the study. Furthermore, the authors are thankful to colleagues of the Institute of Bacterial Infections and Zoonoses (IBIZ) in the ‘Friedrich-Loeffler-Institut’ (Jena, Germany), namely Dr. Ulrich Methner and his team for Salmonella spp. diagnosis and Dr. Astrid Raßbach and co-workers for performing bacterial screening for Pasteurella spp., Bordetella spp., Haemophilus spp. and APP. In addition, the authors thank Dr. Konrad Sachse and staff of the OIE Reference Laboratory for Chlamydiosis for Chlamydia spp. testing and Renate Haß for assaying Mycoplasma spp. This work was funded by the non-profit organisation ‘Akademie für Tiergesundheit e.V.’ (Germany), which provided a scholarship for Judith Wagner.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.tvjl.2009.12.022.
Appendix A. Supplementary material
References
- Beyer J., Fichtner D., Schirrmeier H., Polster U., Weiland E., Wege H. Porcine reproductive and respiratory syndrome virus (PRRSV): kinetics of infection in lymphatic organs and lung. Journal of Veterinary Medicine B. 2000;47:9–25. doi: 10.1046/j.1439-0450.2000.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botner A., Nielsen J., Bille-Hansen V. Isolation of porcine reproductive and respiratory syndrome (PRRS) virus in a Danish swine herd and experimental infection of pregnant gilts with the virus. Veterinary Microbiology. 1994;40:351–360. doi: 10.1016/0378-1135(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Brockmeier S.L., Palmer M.V., Bolin S.R. Effects of intranasal inoculation of porcine reproductive and respiratory syndrome virus, Bordetella bronchiseptica, or a combination of both organisms in pigs. American Journal of Veterinary Research. 2000;61:892–899. doi: 10.2460/ajvr.2000.61.892. [DOI] [PubMed] [Google Scholar]
- Christianson W.T., Collins J.E., Benfield D.A., Harris L., Gorcya D.E., Chladek D.W., Morrison R.B., Joo H.S. Experimental reproduction of swine infertility and respiratory syndrome in pregnant sows. American Journal of Veterinary Research. 1992;53:485–488. [PubMed] [Google Scholar]
- Done S.H., Paton D.J., White M.E.C. Porcine reproductive and respiratory syndrome (PRRS): a review, with emphasis on pathological, virological and diagnostic aspects. British Veterinary Journal. 1996;152:153–174. doi: 10.1016/S0007-1935(96)80071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachinger V., Bischoff R., Jedidia S.B., Saalmüller A., Elbers K. The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine. 2008;26:1488–1499. doi: 10.1016/j.vaccine.2007.11.053. [DOI] [PubMed] [Google Scholar]
- Halbur P.G., Paul P.S., Frey M.L., Landgraf J., Eernisse K., Meng X.J., Lum M.A., Andrews J.J., Rathje J.A. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Veterinary Pathology. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- Klein C., Reinhold P. Analysis of respiratory mechanics by impulse oscillometry in non-sedated and diazepam-sedated swine. Research in Veterinary Science. 2001;70:181–189. doi: 10.1053/rvsc.2001.0458. [DOI] [PubMed] [Google Scholar]
- Klein C., Smith H.J., Reinhold P. Respiratory mechanics in conscious swine: effects of face mask, head position and bronchoconstriction evaluated by impulse oscillometry. Research in Veterinary Science. 2003;75:71–81. doi: 10.1016/s0034-5288(03)00037-7. [DOI] [PubMed] [Google Scholar]
- Kranker S., Nielsen J., Bille-Hansen V., Botner A. Experimental inoculation of swine at various stages of gestation with a Danish isolate of porcine reproductive and respiratory syndrome virus (PRRSV) Veterinary Microbiology. 1998;62:21–31. doi: 10.1016/s0378-1135(98)00176-x. [DOI] [PubMed] [Google Scholar]
- Labarque G.G., Nauwynck H.J., Van Reeth K., Pensaert M.B. Effect of cellular changes and onset of humoral immunity on the replication of porcine reproductive and respiratory syndrome virus in the lungs of pigs. Journal of General Virology. 2000;81:1327–1334. doi: 10.1099/0022-1317-81-5-1327. [DOI] [PubMed] [Google Scholar]
- Lager K.M., Mengeling W.L., Brockmeier S.L. Duration of homologous porcine reproductive and respiratory syndrome virus immunity in pregnant swine. Veterinary Microbiology. 1997;53:113–125. doi: 10.1016/s0378-1135(97)00159-4. [DOI] [PubMed] [Google Scholar]
- McLaughlin R.F., Tyler W.S., Canada R.O. A study of the subgross pulmonary anatomy in various mammals. American Journal of Anatomy. 1961;108:149–165. [Google Scholar]
- Matsumoto S., Mokashi A., Lahiri S. Cervical preganglionic sympathetic nerve activity and chemoreflexes in the cat. Journal of Applied Physiology. 1987;62:1713–1720. doi: 10.1152/jappl.1987.62.4.1713. [DOI] [PubMed] [Google Scholar]
- Mengeling W.L., Vorwald A.C., Lager K.M., Brockmeier S.L. Comparison among strains of porcine reproductive and respiratory syndrome virus for their ability to cause reproductive failure. American Journal of Veterinary Research. 1996;57:834–839. [PubMed] [Google Scholar]
- Nielsen H.S., Liu G., Nielsen J., Oleksiewicz M.B., Botner A., Storgaard T., Faaberg K.S. Generation of an infectious clone of VR-2332, a highly virulent North American-type isolate of porcine reproductive and respiratory syndrome virus. Journal of Virology. 2003;77:3702–3711. doi: 10.1128/JVI.77.6.3702-3711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Halloran K.D., Curran A.K., Bradfort A. Influence of cervical sympathetic nerves on ventilation and upper airway resistance in the rat. European Respiratory Journal. 1998;12:177–184. doi: 10.1183/09031936.98.12010177. [DOI] [PubMed] [Google Scholar]
- Opriessnig T., Halbur P.G., Yoon K.-J., Pogranichniy R.M., Harmon K.M., Evans R., Key K.F., Pallares F.J., Thomas P., Meng X.J. Comparison of molecular and biological characteristics of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine (Ingelvac PRRS MLV), the parent strain of the vaccine (ATCC VR-2332), ATCC VR2385, and two recent field isolates of PRRSV. Journal of Virology. 2002;76:11837–11844. doi: 10.1128/JVI.76.23.11837-11844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol J.M.A., Dijk van J.E., Wensvoort G., Terpstra C. Pathological, ultrastructural and immunohistochemical changes by Lelystad virus in experimentally induced infections of mystery swine disease (synonym: porcine epidemic abortion and respiratory syndrome (PEARS)) Veterinary Quarterly. 1991;13:137–143. doi: 10.1080/01652176.1991.9694298. [DOI] [PubMed] [Google Scholar]
- Reinhold P., Smith H.J., Close R., Genicot B., Lekeux P. Validation of impulse oscillometry in Friesian and Blue Belgian calves with respect to changes in extrathoracic upper airway resistance. Research in Veterinary Science. 1998;65:93–101. doi: 10.1016/s0034-5288(98)90158-8. [DOI] [PubMed] [Google Scholar]
- Reinhold P., Jaeger J., Melzer F., Sachse K. Evaluation of lung function in pigs either experimentally or naturally infected with Chlamydiaceae. Veterinary Research Communications. 2005;29:125–150. doi: 10.1007/s11259-005-0843-1. [DOI] [PubMed] [Google Scholar]
- Reinhold P., Kirschvink N., Theegarten D., Berndt A. An experimentally induced Chlamydia suis infection in pigs results in severe lung function disorders and pulmonary inflammation. Veterinary Research. 2008;39:35. doi: 10.1051/vetres:2008012. [DOI] [PubMed] [Google Scholar]
- Robinson N.E. Some functional consequences of species differences in lung anatomy. Advances in Veterinary Science and Comparative Medicine. 1982;26:1–33. [PubMed] [Google Scholar]
- Rossow K.D., Bautista E.M., Goyal S.M., Molitor T.W., Murtaugh M.P., Morrison R.B., Benfield D.A., Collins J.E. Experimental porcine reproductive and respiratory syndrome virus infection in one-, four-, and 10-week-old pigs. Journal of Veterinary Diagnostic Investigation. 1994;6:3–12. doi: 10.1177/104063879400600102. [DOI] [PubMed] [Google Scholar]
- Rossow K.D. Porcine reproductive and respiratory syndrome. Veterinary Pathology. 1998;35:1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- Samsom J.N., de Bruin T.G., Voermans J.J., Meulenberg J.J., Pol J.M., Bianchi A.T. Changes of leukocyte phenotype and function in the broncho-alveolar lavage fluid of pigs infected with porcine reproductive and respiratory syndrome virus: a role for CD8(+) cells. Journal of General Virology. 2000;81:497–505. doi: 10.1099/0022-1317-81-2-497. [DOI] [PubMed] [Google Scholar]
- Smith H.J., Reinhold P., Goldman M.D. Forced oscillation technique and impulse oscillometry. European Respiratory Monograph. 2005;31:72–105. [Google Scholar]
- Stănescu D.C., Clément J., Pattijn J., van de Woestijne K.P. Glottis opening and airway resistance. Journal of Applied Physiology. 1972;32:460–466. doi: 10.1152/jappl.1972.32.4.460. [DOI] [PubMed] [Google Scholar]
- Thacker E.L., Halbur P.G., Ross R.F., Thanawongnuwech R., Thacker B.J. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. Journal of Clinical Microbiology. 1999;37:620–627. doi: 10.1128/jcm.37.3.620-627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker E., Thanawongnuwech R. Porcine respiratory disease complex (PRDC) Thai Journal of Veterinary Medicine. 2002;32:125–134. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


