Abstract
Objectives
The cross-border transmission of infectious diseases is a worldwide public health issue. Current border screening measures are insufficiently sensitive. The study objectives were to describe the epidemiologic pattern of influenza infection among incoming travellers at Xiamen International Airport during nonpandemic periods and to assess the performance of a rapid influenza diagnostic test in border screening.
Methods
Between May 2015 and May 2016, travellers with influenza-like illnesses entering China at Xiamen International Airport were screened with a rapid test, Flu Dot-ELISA, and the collected specimens were further subjected to virus isolation and phylogenetic analysis.
Results
Of the 1 540 076 incoming travellers, 1224 cases of influenza-like illness were identified; 261 tested positive in the rapid test, and 176 were confirmed to be influenza through virus culture. The sensitivity and specificity of the rapid test were demonstrated to be 96.6% (170/176) and 91.3% (957/1048), respectively, and the positive predictive and negative predictive values were 65.1% (170/261) and 99.4% (957/963), respectively. The epidemiologic study indicated that H3N2 and (H1N1)pdm09 were dominant in 2015 and 2016, respectively. In 2016, an increased number of influenza B isolates and cocirculation of both Victoria and Yamagata lineage influenza B viruses were observed, and mismatches between circulating influenza A(H1N1)pdm09 and influenza B Victoria lineage strains and vaccine strains also occurred.
Conclusions
We demonstrated the suitability and value of a high-sensitivity rapid influenza test in border screening and highlighted the importance of incoming travellers as a source of imported infectious diseases.
Keywords: Border screening, influenza, Phylogenetic analysis, Rapid test, Vaccine mismatch
Introduction
The cross-border transmission of infectious diseases due to the increasing cross-border and cross-continental movement of people has become an important public health issue worldwide [1]. Xiamen, which is located adjacent to the Taiwan Strait, is a well-known tourist port city on the southeast coast of China (Supplementary Fig. 1). A gradually increasing number of international travellers are entering Xiamen, leading to a higher risk of exposure to imported infectious diseases in the area. Experience gained during the severe acute respiratory syndrome and influenza A(H1N1)pdm09 virus pandemics has highlighted the importance of border screening to the understanding of cross-border transmission of infectious diseases [2]. Border screening is therefore an important tool for monitoring infectious diseases carried by incoming travellers at Xiamen International Airport and understanding their effect on the local circulation of infectious diseases.
After the outbreak of pandemic influenza A(H1N1)pdm09 in 2009 [3], entry border screening for suspected cases of influenza in incoming travellers was conducted at the Xiamen airport. This project exposed several problems in the border screening system: (a) screening measures such as visual inspection and thermal scanning have low sensitivity and are ineffective in detecting infected persons; (b) the sensitivity of most currently available rapid antigen tests, which are largely based on the colloidal gold system, is too low to effectively detect infection; and (c) the RT-PCR assay is not suitable for border screening as a result of its high cost and lengthy, labour-intensive protocols. New rapid antigen tests with high sensitivity are the most desirable option for disease surveillance in the border screening setting.
We performed active border screening at Xiamen airport using a new commercial rapid high-sensitivity influenza antigen test. The objectives of this study were to describe the epidemiologic pattern of influenza infection among incoming travellers at the airport during a nonpandemic period and to assess the diagnostic performance of this new rapid test in border screening.
Materials and methods
Study design and participants
This study was designed by the National Institute of Diagnostics and Vaccine Development in Infectious Diseases. Continuous screening for influenza was conducted from May 2015 to May 2016 to determine the incidence of imported influenza viruses carried by incoming travellers with influenza-like illness (ILI) symptoms at the Xiamen airport entry border. Every traveller was screened by infrared thermometer, and travellers with body temperature ≥37.5°C or with the obvious presence of specific symptoms, including cough, rhinorrhoea, sore throat and/or gastrointestinal disturbance, were considered to be likely ILI cases and the patients invited to participate in a consultation to obtain informed consent, basic personal information and a medical history. Nasopharyngeal swab specimens were collected from travellers with ILIs and tested at the airport using a rapid influenza antigen test. Each swab sample was soaked in virus transport medium (total volume of 1.5 mL) and transported to the laboratory within 48 hours of collection for virus isolation through culture in Madin-Darby canine kidney (MDCK) cells. Travellers did not receive any kind of medical certificate, diagnosis or therapeutic intervention in this study. This study was approved by the medical ethics committee at Xiamen University, China.
Rapid antigen test for detection of influenza
The rapid influenza antigen test, Flu Dot-ELISA, is a commercial diagnostic kit (Beijing Wantai Biological Pharmacy, Beijing, China) for the detection of the nucleoprotein of influenza A and B viruses based on horseradish peroxidase signal amplification [4]. The test was performed according to the manufacturer's instructions. Briefly, 200 μL of swab specimen eluate was gently mixed with 400 μL of extraction buffer, vortexed for 10 seconds and then added to the individual wells of the testing device. Results were read visually after 15 minutes' incubation at room temperature, with blue-coloured dots indicating a positive test result.
Virus isolation
Virus isolation in cell culture was performed as described previously [5]. The swab specimens in virus transport medium were vortexed for 10 seconds; then 424 μL of specimen medium was mixed with 520 μL of Dulbecco modified Eagle medium and inoculated into MDCK cells in a 6-well plate. After 48 hours, haemagglutinin (HA) assays and visualization of cytopathic effects were used to identify positive isolates. Viruses in positive isolates were cultured and further subtyped by gene sequencing. Specimens were regarded as negative if HA assays were negative and if there was no sign of cytopathic effects after three serial blind passages.
Virus RNA extraction and analysis of influenza virus isolates
Virus culture supernatant with an HA titer of >64 was prepared and RNA extracted using previously described methods [6], [7]. Reverse transcription was performed at 42°C using two different sets of universal primers (uni12) for influenza A and influenza B viruses. To amplify and sequence HA genes, subtype specific primers were used for influenza A and two pairs of lineage-specific primers for influenza B. The following optimized conditions were used for amplification: 94°C for 5 minutes, followed by 40 cycles at 94°C for 40 seconds, 58°C for 40 seconds, 72°C for 150 seconds and a final extension step at 72°C for 10 minutes. The PCR products were sequenced using the Sanger method. The sequences of the virus isolates were subjected to phylogenetic analysis, as described previously [8]. The nucleotide sequences generated in this study have been submitted to GenBank under accession numbers KY273035–KY273077.
Analysis
The sensitivity, specificity, positive predictive value and negative predictive value of the Flu Dot-ELISA assay were determined using virus culture as the reference standard. Estimation of the 95% confidence interval (CI) was performed using exact binomial methods. Calculations were performed by SPSS 21.0 statistical software (IBM SPSS, Chicago, IL, USA).
Results
Temporal distribution of imported influenza
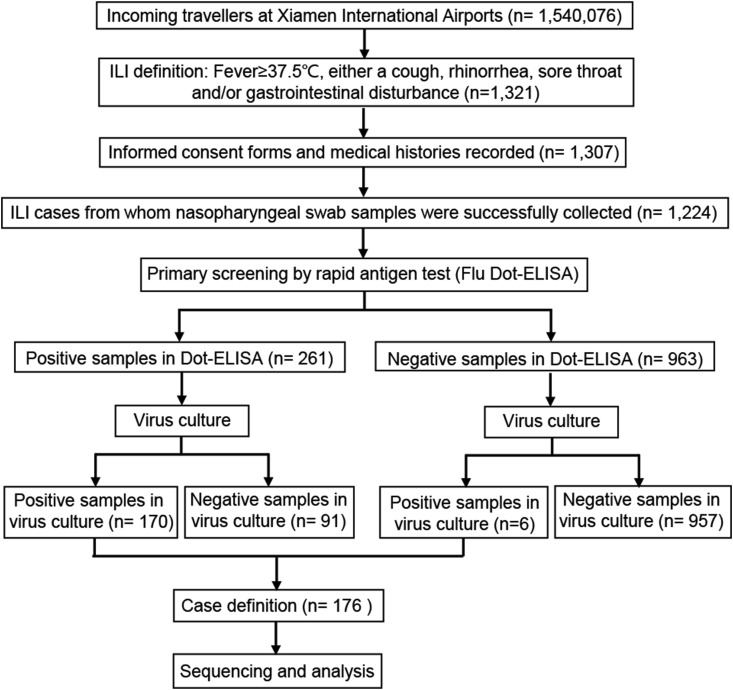
Among the 1 540 076 incoming passengers during this study period, 1321 ILI cases were identified, and 1307 of these provided informed consent (Fig. 1 ). Of the 1224 ILI specimens successfully collected, 176 (14.4%) were confirmed to be influenza by virus culture. Thus, 0.011% of travellers were identified as being infected with influenza in this study.
Fig. 1.
Flowchart of study's active influenza surveillance system process.
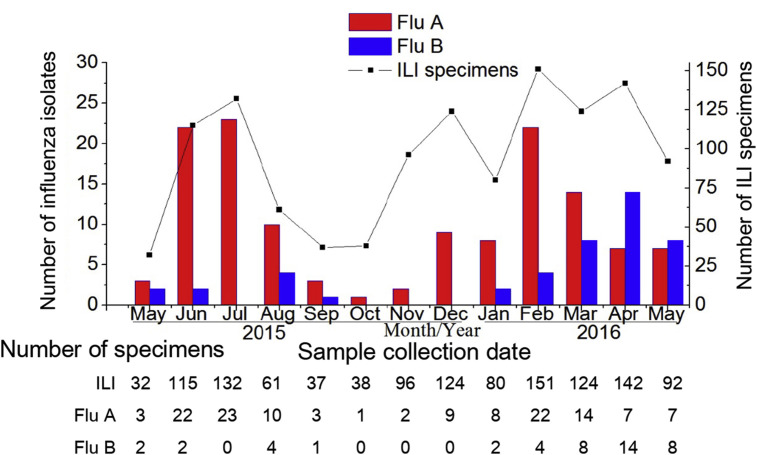
A similar seasonal pattern of ILI and influenza prevalence was observed (Fig. 2 ), with a higher incidence in the summer and winter months, peaking during July 2015 and February 2016, respectively. Interestingly, influenza A viruses were dominant from 2015 to early 2016, but after March 2016, the number of imported influenza B isolates increased substantially, even exceeding the number of influenza A viruses identified in April and May 2016, suggesting that a considerable change in the circulation characteristics of the influenza B virus had occurred.
Fig. 2.
Monthly distribution of influenza viruses isolated from incoming travellers with ILI symptoms at Xiamen International Airport entry border between May 2015 and May 2016. Bars represent number of influenza isolates; plotted line represents number of ILI specimens. Red bars indicate number of influenza isolates; blue bars, number of influenza B isolates. ILI, influenza-like illness.
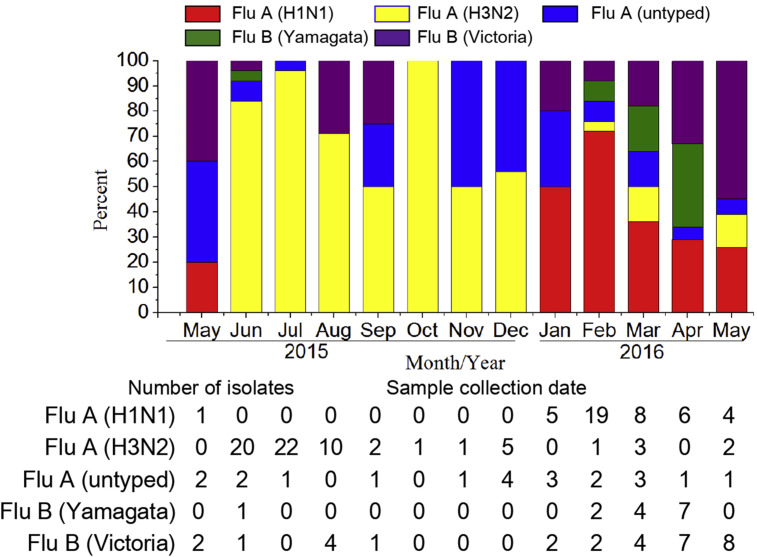
Because the two main subtypes of influenza A virus (H1N1 and H3N2) and the two lineages of influenza B virus (Yamagata and Victoria) cocirculate in humans, we analysed which subtype or lineage of the isolated influenza viruses was more dominant by HA subtype-specific molecular subtyping of all 176 influenza virus isolates (Fig. 3 ). The results indicated that 83.6% (61/73) of the influenza A isolates were H3N2 virus in 2015, whereas 72.4% (42/58) of the influenza A isolates were A(H1N1)pdm09 in 2016, thus suggesting that influenza A(H1N1)pdm09 entered a new endemic phase in 2016, replacing H3N2. Some of the other influenza A viruses were neither H1 or H3 and are designated as untyped. For the influenza B viruses, more Victoria-like virus strains were isolated than Yamagata-like virus strains in this study. Notably, Yamagata-like viruses were seldom isolated in 2015 but became much more common after February 2016. This finding demonstrates the presence of cocirculation of both the Yamagata and Victoria lineages.
Fig. 3.
Monthly distribution of various influenza virus subtypes/lineages. Bars represent percentage prevalence of isolates obtained each month: Flu A/H1N1 (red), Flu A/H3N2 (yellow), Flu A/untyped (blue), Flu B/Yamagata (green), Flu B/Victoria (purple).
Performance of rapid antigen test in entry screening for influenza
A total of 261 (21.3%) of the 1224 ILI specimens showed positive results in the Flu Dot-ELISA (Table 1 ), and 170 were further confirmed to be influenza through virus culturing, the current reference standard, with a sensitivity of approximately 95% (Table 2 ). The other 91 Flu Dot-ELISA–positive samples were negative in virus culture, and six of the 963 Flu Dot-ELISA–negative specimens were later shown to be positive in virus culture. Thus, compared to virus culture, the sensitivity and specificity of the Flu Dot-ELISA for nasopharyngeal swab specimens were 96.6% (170/176, 95% CI 92.7–98.7%) and 91.3% (957/1048, 95% CI 89.4–93.0%), respectively, and the positive predictive and negative predictive values for the border screening were 65.1% (170/261, 95% CI 59.0–70.9) and 99.4% (957/963, 95% CI 98.7–99.8%), respectively.
Table 1.
Results of Flu Dot-ELISA rapid test administered to incoming travellers to Xiamen International Airport with influenza-like illness
| Virus culture |
|||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 170 | 91 | 261 |
| Negative | 6 | 957 | 963 |
| Total | 176 | 1048 | 1224 |
Table 2.
Performance of Flu Dot-ELISA rapid test in border screening of incoming travellers to Xiamen International Airport with influenza-like illness
| Finding | % (95% confidence interval) |
|---|---|
| Sensitivity | 96.6 (92.7–98.7) |
| Specificity | 91.3 (89.4–93.0) |
| Positive predictive value | 65.1 (59.0–70.9) |
| Negative predictive value | 99.4 (98.7–99.8) |
Phylogenetic analysis of the HA gene
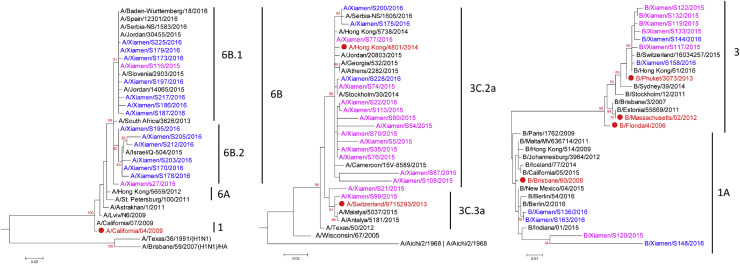
To further understand the genetic diversity and phylogenetic relationships among the influenza viruses isolated in this study, phylogenetic trees of the HA gene were constructed, which also included reference influenza virus strains isolated from around the world (Fig. 4 ).
Fig. 4.
Phylogenetic trees of HA genes of representative influenza viruses isolated in this study. (A) Phylogenetic tree of HA genes of influenza A(H1N1)pdm09 isolates. (B) Phylogenetic tree of haemagglutinin (HA) genes of influenza A(H3N2) isolates. (C) Phylogenetic tree of HA genes of influenza B isolates. Virus names in pink represent strains isolated during 2015; virus names in blue represent strains isolated during 2016. Virus names in red and accompanied by solid red circle indicate influenza vaccine strains recommended by World Health Organization. Virus names in black indicate reference strains representing various subclades and sublineages. Vaccine and reference strain HA sequences were downloaded from GenBank. Data were analysed with MEGA 5.2 software, and maximum likelihood methods were used, with 1000 bootstrap replicates. Subclade or sublineage of each virus is classified according to reference strains that they cluster with.
For influenza A(H1N1)pdm09, all of the virus strains isolated in this study during 2015 and 2016 belonged to genetic group 6B (6B.1 and 6B.2) (Fig. 4A). This finding was consistent with reports that from 2015 to 2016 the dominant group of influenza A(H1N1)pdm09 viruses worldwide changed from genetic group 6C, which had dominated before and during 2014 [9].
For the H3N2 viruses, the phylogenetic tree showed that most of the imported H3N2 isolates belonged to genetic clade 3C.2a, which includes the representative H3N2 vaccine strain A/Hong Kong/4801/2014, with only two imported strains belonging to clade 3C.3a, which includes the previous H3N2 vaccine strain A/Switzerland/9715293/2013 (Fig. 4B). This result further confirms reports from the European Centre for Disease Prevention and Control that clade 3C.2a was the dominant genetic subgroup circulating worldwide in 2015–2016 [10], [11].
Regarding influenza B, the phylogenetic tree showed that the imported influenza B isolates belonged to genetic groups 1A and 3 (Fig. 4C). Some of the Victoria-like influenza B viruses isolated in this study (genetic group 1A) exhibited significant drift relative to the Victoria lineage vaccine strain B/Brisbane/60/2008. The Yamagata-like influenza B virus isolates in this study (genetic group 3) were more similar to the most recent influenza B vaccine strain B/Phuket/3073/2013, which belongs to the Yamagata lineage.
Discussion
In this study, border screening was conducted at Xiamen airport during a nonpandemic period to assess the performance of a commercial rapid influenza antigen test for the detection of influenza infection among incoming travellers and to examine the incidence of imported influenza infection. Endemic trends and vaccine mismatches of currently circulating influenza viruses were also analysed.
This study demonstrated that the Flu Dot-ELISA rapid test is highly sensitive and suitable as a screening tool for influenza surveillance at airports, offering a significant reduction in time and labour demands compared to virus culture and RT-PCR. Because this assessment study was conducted in an active influenza border screening situation, it provides direct experience in the use of the rapid test which will be applicable to future border screening. The epidemiologic study of influenza viruses imported during nonpandemic periods may provide useful information distinct from that obtained through border screening conducted during pandemic periods. This study has some limitations: the probability that some asymptomatic travellers missed detection; the sensitivity of the reference standard, virus culture, is less than 100%, which may have biased the assessment; and the lack of any follow-up of the influenza cases identified.
The sensitivity of currently available rapid influenza antigen tests ranges 11–88%, which is too low for application as screening tools [12], [13], [14], [15]. This study showed that the Flu Dot-ELISA has comparatively high sensitivity in border screening for influenza infection (96.6, 95% CI 92.7–98.7). The higher sensitivity of the assay may be attributed to two aspects: first, the enzyme-induced signal amplification system used confers sensitivity at least ten times higher than that of traditional rapid assays based on the colloidal gold system [16]; and second, the nasopharyngeal specimens we tested were one of the most appropriate sample types for virus detection using rapid tests [17], [18]. Nevertheless, six of the 176 positive influenza isolates were missed by the rapid test; further improvements in sensitivity would be desirable. The specificity of the Flu Dot-ELISA (91.3, 95% CI 89.4–93.0) is acceptable but somewhat lower than the 100% specificity shown in a previous report [15], possibly because of the application strategy we used in our study. To identify as many influenza viruses in incoming travellers with ILIs as possible, we chose to regard even those samples with very weak colour development in the Flu Dot-ELISA as positive, thus leading to a false-positive rate of 8.7% (91/1048).
This study showed that the dominant circulating influenza A virus in 2015 was the H3N2 subtype, but that influenza A(H1N1)pdm09 dominated in 2016. This observation may reflect the circulation trend in the countries from which travellers departed. Phylogenetic analysis of the HA gene showed that H3N2 virus strains isolated in 2015 were phylogenetically diverse and comparatively distantly related to the H3N2 vaccine strain A/Switzerland/9715293/2013 recommended by the World Health Organization (WHO) in 2014, confirming a recently reported similar finding [19]. However, the H3N2 virus strains isolated in both 2015 and 2016 were more closely related to the new H3N2 vaccine strain A/Hong Kong/4801/2014, which was recommended by the WHO in 2015 [20]. These findings strongly suggest that changing the H3N2 vaccine strain for the 2015–2016 influenza season must have contributed to better control of the H3N2 endemic in 2016. In addition, the increasing frequency and phylogenetic differentiation of influenza A(H1N1)pdm09 viruses isolated in 2016 indicates that changing the current vaccine strain A/California/04/2009 should be considered to address this increased antigenic drift and prevent a vaccine mismatch. A similar phenomenon was observed in the surveillance of influenza B viruses. The markedly increased proportion of both influenza B lineages in 2016 indicates that cocirculation of both lineages (Yamagata and Victoria) is occurring. Notably, the more pronounced antigenic drift and mismatch with the Victoria lineage vaccine strain B/Brisbane/60/2008, shown here and indicated in a recent report [21], suggests that not only should the application of the quadrivalent influenza vaccine, which includes both influenza B lineages, be considered but also that the vaccine strain for the Victoria lineage should be changed.
In conclusion, we demonstrated that a high-sensitivity rapid influenza test performed well and may be a suitable tool for border screening. The epidemiologic study of imported influenza viruses also showed that incoming travellers should not be overlooked as a source of imported infectious diseases. Future research should focus on several areas: (a) routine border screening for other common infectious diseases with a rapid higher-sensitivity detection technique should be implemented worldwide to increase understanding of the cross-border transmission of infectious diseases; (b) the effect of imported influenza viruses on locally circulating endemic viruses should be studied by conducting local influenza surveillance; and (c) new highly sensitive rapid tests based on fluorescence or chemiluminescence amplification systems should be developed.
Transparency declaration
Financial support was received via grants from the National Natural Science Foundation of China (31670934, 81202255 and 81371817). All authors report no conflicts of interest relevant to this article.
Acknowledgements
We thank for J. Wai-Kuo Shih for his comments and suggestions and S. Ke for her technical support. We are grateful to J. Rayner for editing the article.
Editor: M. Paul
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.cmi.2017.05.027.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Coker R.J., Hunter B.M., Rudge J.W., Liverani M., Hanvoravongchai P. Emerging infectious diseases in Southeast Asia: regional challenges to control. Lancet. 2011;377:599–609. doi: 10.1016/S0140-6736(10)62004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowling B.J., Lau L.L., Wu P., Wong H.W., Fang V.J., Riley S. Entry screening to delay local transmission of 2009 pandemic influenza A (H1N1) BMC Infect Dis. 2010;10:82. doi: 10.1186/1471-2334-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garten R.J., Davis C.T., Russell C.A., Shu B., Lindstrom S., Balish A. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng X.D., Yuan Q., Yue Q.H., Zheng Q.B., Ma Y.Y., Yang B.C. Evaluation of a new rapid influenza A diagnostic test for detection of pandemic (H1N1) 2009 and seasonal influenza A virus. J Clin Virol. 2011;50:153–155. doi: 10.1016/j.jcv.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Tobita K., Sugiura A., Enomote C., Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975;162:9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann E., Stech J., Guan Y., Webster R.G., Perez D.R. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- 7.Zhou B., Lin X., Wang W., Halpin R.A., Bera J., Stockwell T.B. Universal influenza B virus genomic amplification facilitates sequencing, diagnostics, and reverse genetics. J Clin Microbiol. 2014;52:1330–1337. doi: 10.1128/JCM.03265-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tewawong N., Suwannakarn K., Prachayangprecha S., Korkong S., Vichiwattana P., Vongpunsawad S. Molecular epidemiology and phylogenetic analyses of influenza B virus in Thailand during 2010 to 2014. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skowronski D.M., Chambers C., Sabaiduc S., De Serres G., Winter A.L., Dickinson J.A. Integrated sentinel surveillance linking genetic, antigenic, and epidemiologic monitoring of influenza vaccine–virus relatedness and effectiveness during the 2013–2014 influenza season. J Infect Dis. 2015;212:726–739. doi: 10.1093/infdis/jiv177. [DOI] [PubMed] [Google Scholar]
- 10.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2015. Influenza virus characterisation, summary Europe, May 2015. [Google Scholar]
- 11.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2016. Influenza virus characterisation, summary Europe, May 2016. [Google Scholar]
- 12.Mitamura K., Shimizu H., Yamazaki M., Ichikawa M., Nagai K., Katada J. Clinical evaluation of highly sensitive silver amplification immunochromatography systems for rapid diagnosis of influenza. J Virol Methods. 2013;194:123–128. doi: 10.1016/j.jviromet.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Wunderli W., Thomas Y., Müller D.A., Dick M., Kaiser L. Rapid antigen testing for the surveillance of influenza epidemics. Clin Microbiol Infect. 2003;9:295–300. doi: 10.1046/j.1469-0691.2003.00650.x. [DOI] [PubMed] [Google Scholar]
- 14.Vasoo S., Stevens J., Singh K. Rapid antigen tests for diagnosis of pandemic (swine) influenza A/H1N1. Clin Infect Dis. 2009;49:1090–1093. doi: 10.1086/644743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y., Xu F., Gui X., Yang K., Wu X., Zheng Q. A rapid test for the detection of influenza A virus including pandemic influenza A/H1N1 2009. J Virol Methods. 2010;167:100–102. doi: 10.1016/j.jviromet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Yuan Q., Cheng X.D., Yang B.C., Zheng Q.B., Chen Y.X., Chen Q.R. Differential diagnosis of pandemic (H1N1) 2009 infection by detection of haemagglutinin with an enzyme-linked immunoassay. Clin Microbiol Infect. 2011;17:1574–1580. doi: 10.1111/j.1469-0691.2010.03413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loens K., Van Heirstraeten L., Malhotra-Kumar S., Goossens H., Ieven M. Optimal sampling sites and methods for detection of pathogens possibly causing community-acquired lower respiratory tract infections. J Clin Microbiol. 2009;47:21–31. doi: 10.1128/JCM.02037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agoritsas K., Mack K., Bonsu B.K., Goodman D., Salamon D., Marcon M.J. Evaluation of the Quidel QuickVue test for detection of influenza A and B viruses in the pediatric emergency medicine setting by use of three specimen collection methods. J Clin Microbiol. 2006;44:2638–2641. doi: 10.1128/JCM.02644-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua S., Li X., Liu M., Cheng Y., Peng Y., Huang W. Antigenic variation of the human influenza A (H3N2) virus during the 2014–2015 winter season. Sci China Life Sci. 2015;58:882–888. doi: 10.1007/s11427-015-4899-z. [DOI] [PubMed] [Google Scholar]
- 20.Lewis N.S., Russell C.A., Langat P., Anderson T.K., Berger K., Bielejec F. The global antigenic diversity of swine influenza A viruses. eLife. 2016;5 doi: 10.7554/eLife.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vijaykrishna D., Holmes E.C., Joseph U., Fourment M., Su Y.C., Halpin R. The contrasting phylodynamics of human influenza B viruses. eLife. 2015;4 doi: 10.7554/eLife.05055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.