Abstract
The possible role of some acute phase proteins (APPs) and immunoglobulins in both the pathogenesis and diagnosis of feline infectious peritonitis (FIP) has been investigated. Serum protein electrophoresis and the concentration of haptoglobin (Hp), serum amyloid A (SAA), α1-acid glycoprotein (AGP), IgG and IgM were evaluated in cats exposed to feline coronavirus (FCoV) and in cats with FIP. The highest concentration of APPs was detected in affected cats, confirming the role of these proteins in supporting a clinical diagnosis of FIP. Repeated samplings from both FIP affected and FCoV-exposed cats showed that when FIP appeared in the group, all the cats had increased APP levels. This increase persisted only in cats that developed FIP (in spite of a decrease in α2-globulins) but it was only transient in FCoV-exposed cats, in which a long lasting increase in α2-globulins was observed. These results suggest that changes in the electrophoretic motility of APPs or APPs other than Hp, SAA and AGP might be involved in the pathogenesis of FIP or in protecting cats from the disease.
1. Introduction
Previous studies on serum from cats with experimentally induced feline infectious peritonitis (FIP) have shown an early increase in α-globulins, mainly due to acute phase proteins (APPs) such as haptoglobin (Hp) and α1-acid glycoprotein (AGP), followed by increases in γ-globulins, reflecting the production of antibodies against the feline coronavirus (FCoV) which is responsible for the disease (Stoddart et al., 1988). The balance between these two globulin fractions, together with the degree of lymphopenia, seem to influence the development of lesions (Paltrinieri et al., 2001).
No reports about possible changes of APPs in cats resistant to the infection are available. Furthermore, although the electrophoretic changes strongly support a clinical diagnosis of FIP, they can also be found, under certain environmental or pathophysiological conditions, in cats without FIP (Kristensen and Barsanti, 1977; Kaneko, 1997). The detection of protein markers would be very useful in the diagnosis of FIPV infection as has already been demonstrated for AGP (Duthie et al., 1997). Moreover, changes in APPs in FCoV-exposed cats that do not develop the disease, might also be used as a marker for resistance to FIP.
In the present study, the concentration of some APPs such as Hp, AGP and serum amyloid A (SAA) have been evaluated in cats exposed to FCoV infection and in cats affected by FIP. The aim was to investigate the possible role of these proteins in the pathogenesis and diagnosis of FIP or in protecting cats living with FCoV shedders from the disease. IgG and IgM, were also studied in order to check the protein composition of the different globulin fractions.
2. Materials and methods
This study used on 82 blood samples collected from 67 pet cats (Table 1 ) grouped as follows:
Table 1.
Breed, sex, age and FCoV serology results of the examined cats
| Group 1: Controls | Group 2: FcoV-exposed cats | Group 3: Cats with FIP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Breed | Sex | Age | FS | No. | Breed | Sex | Age | FS | No. | Breed | Sex | Age | FS |
| 1 | Persian | F | 11 y | + | 25* | Persian | F | 3 y | − | 36 | Balines | M | 4 m | + |
| 2 | DSH | M | 12 m | − | 26* | Persian | F | 2 y | + | 37 | DSH | SF | 7 y | − |
| 3 | DSH | M | 9 m | + | 27* | Exotic | M | 3 y | − | 38 | Persian | unk | unk | + |
| 4 | Persian | M | 12 y | − | 28* | Persian | M | 5 y | − | 39 | DSH | F | unk | + |
| 5 | Persian | F | 8 y | + | 29 | DSH | nM | 3 y | − | 40 | Persian | unk | 10 m | + |
| 6 | Persian | F | 8 y | − | 30 | DSH | nM | 12 m | + | 41 | Persian | M | 10 y | + |
| 7 | Persian | F | 6 y | − | 31 | DSH | nM | 18 m | + | 42 | DSH | nM | 13 y | + |
| 8 | Persian | M | 12 m | − | 32 | DSH | nM | 4 y | − | 43 | DSH | nM | 5 y | + |
| 9 | Persian | F | 13 y | − | 33 | Siamese | F | 18 m | − | 44 | DSH | M | 15 m | + |
| 10 | Persian | M | 9 y | − | 34 | Oriental | F | 18 m | + | 45 | unk | unk | unk | + |
| 11 | Persian | F | 11 y | + | 35 | Oriental | M | 18 m | − | 46 | unk | unk | unk | + |
| 12 | Persian | M | 3 y | − | 47 | DSH | F | 12 y | + | |||||
| 13 | Persian | F | 4 y | + | 48 | DSH | F | 7 m | + | |||||
| 14 | Persian | F | 5 y | + | 49 | DSH | M | 12 m | + | |||||
| 15 | Persian | M | 12 m | − | 50 | Persian | M | unk | − | |||||
| 16 | DSH | M | 9 y | − | 51** | Persian | F | 12 m | + | |||||
| 17 | DSH | F | 8 y | − | 52 | unk | unk | 6 m | + | |||||
| 18 | DSH | F | 3 y | − | 53 | Siames | M | 3 m | + | |||||
| 19 | DSH | F | 2 y | − | 54 | unk | unk | unk | + | |||||
| 20 | DSH | F | 2 y | − | 55 | DSH | M | 10 m | + | |||||
| 21 | DSH | M | 12 m | − | 56 | unk | unk | unk | + | |||||
| 22 | DSH | F | 2 y | − | 57*** | DSH | F | 7 m | + | |||||
| 23 | DSH | F | 5 y | − | 58 | unk | unk | unk | + | |||||
| 24 | DSH | M | 9 m | − | 59 | unk | unk | unk | + | |||||
| 60 | DSH | F | 12 m | + | ||||||||||
| 61 | unk | unk | unk | − | ||||||||||
| 62 | unk | unk | unk | + | ||||||||||
| 63 | DSH | M | 8 m | + | ||||||||||
| 64 | DSH | F | 7 m | + | ||||||||||
| 65 | DSH | M | 7 m | + | ||||||||||
| 66 | DSH | M | 7 m | + | ||||||||||
| 67 | DSH | F | 8 m | + | ||||||||||
FS=FCoV serology; +=positive; −=negative; DSH=domestic shorthair; unk=unknown; M=male; nM=neutered male; F=female; sF=spayed female.
Sampled also 28, 53 and 83 days after the basal sampling.
Sampled also 28 and 53 days after the basal sampling.
Sampled also 40 days after the basal sampling.
Group 1—Controls: 24 non-SPF (specific pathogen free) cats without any clinical signs of disease, selected from single-cat environments (n=7) or from catteries with no clinical cases of FIP recorded in the previous five years (n=17).
Group 2—FCoV-exposed cats: 11 cats without clinical signs of FIP, selected from three catteries in which recent cases (1–6 months before the study) of FIP had been diagnosed. In one of these, five non-symptomatic cats were sampled: one (cat No. 51) developed a wet FIP 28 days later and results from this animal were not considered in group 2 and the cat was placed in group 3 and sampled also on days 28 (T 28) and 53 (T 53) after the basal sampling (T 0). The four non-symptomatic cats (Nos. 25–28) remained in group 2 and were also sampled at T 28, T 53 and T 83.
Group 3—FIP affected cats: 32 cats with spontaneously occurring FIP (25 wet and seven dry forms), diagnosed by necropsy, histology and/or by the detection of FCoV in the effusion using a direct immunofluorescence test (Paltrinieri et al., 1999). Cats No. 51 and 57 were also sampled 25 and 40 days after the onset of the symptoms, respectively. Blood (3 mL) was taken from the jugular vein and serum was obtained by centrifugation. Total proteins were measured by a discrete analyzer (EOS-BRAVO, Hospitex) using the biuret colorimetric method. Serum protein eletrophoresis was performed as previously described (Paltrinieri et al., 2001).
Serology for feline immunodeficiency virus (FIV) and feline leukaemia virus (FeLV) was tested using commercially available ELISA kits (IDEXX). On 18 samples (four from group 2 and 14 from group 3) FCoV-serology was performed in our laboratory, using an Indirect Fluorescence Assay (IFA) (VMRD). As suggested by the producer of the kit, cats with antibody titres higher than 1:400 were considered positives. The other 49 cats were already classified as FCoV-positive or negative by the referring veterinarians, based on results obtained using different semiquantitative in-clinic ELISA kits.
Radial immunodiffusion (RID) kits were used to measure immunoglobulins (Ig) G and M (VET-RID, Bethyl Lab) and AGP (Cardiotech Services), according to the manufacturer’s instructions. The concentration of SAA was determined using an ELISA kit (Tridelta Development) based on the method described by McDonald et al. (1991). Hp was measured using a kit (Tridelta Development) based on haemoglobin (Hb) binding method (Eckersall et al., 1999). IgG, IgM, SAA, AGP and Hp were determined on 38, 38, 64, 77 and 82 samples, respectively (see Table 2, Table 3, Table 4 ).
Table 2.
Mean values (± SD) recorded in the three groups of cats
| Controls (C) (n=24) | FCoV-exposed (FE) (n=11) | FIP(F) (n=32) | ANOVA | Tukey HSD test |
Reference values | |||
|---|---|---|---|---|---|---|---|---|
| * | ** | *** | ||||||
| Total proteins (g/dL) | 7.69±0.83 | 7.86±0.67 | 8.27±1.49 | n.s. | 5.4–7.8a | |||
| A/G ratio | 1.03±0.24 | 0.97±0.32 | 0.47±0.25 | *** | F vs. C,FE | 0.45–1.19a | ||
| Albumins (g/dL) | 3.83±0.57 | 3.91±0.99 | 2.43±0.46 | *** | F vs. C, FE | 2.1–3.3a | ||
| Globulins (g/dL) | 3.86±0.74 | 4.29±1.21 | 5.85±1.57 | *** | F vs. FE | F vs. C | 2.6–5.1a | |
| α1-Globulins (g/dL) | 0.55±0.34 | 0.64±0.26 | 0.53±0.28 | n.s. | 0.2–1.1a | |||
| α2-Globulins (g/dL) | 0.71±0.33 | 0.54±0.21 | 1.16±0.43 | *** | F vs. C, FE | 0.4–0.9a | ||
| β-Globulins (g/dL) | 0.76±0.18 | 0.82±0.39 | 1.09±0.51 | ** | F vs. C | 0.9–1.9a | ||
| γ-Globulins (g/dL) | 1.84±0.57 | 2.29±0.87 | 3.07±1.41 | *** | F vs. C | 2.5–4.1b | ||
| Serum amyloid A (μg/mL) | 10.21±8.32 (n=17) | 7.92±7.12 (n=7) | 82.88±50.23 (n=26) | *** | F vs. C, FE | 16.6±11.4c | ||
| Haptoglobin (mg/mL) | 1.30±0.64 | 1.80±0.83 | 2.13±0.73 | *** | F vs. FE | F vs. C | 0.04–3.84d | |
| α1-Acid glycoprotein (mg/mL) | 1.20±0.62 (n=22) | 1.19±0.49 (n=11) | 2.72±1.46 (n=29) | *** | F vs. C, FE | 0.1–0.48d | ||
| Immunoglobulin G (g/dL) | 1.73±1.8 (n=7) | n.d. | 2.25±3.09 (n=30) | n.s. | 0.4–2.00e | |||
| Immunoglobulin M (g/dL) | 0.63±0.63 (n=7) | n.d. | 0.36±0.31 (n=30) | n.s. | 0.03–0.15e | |||
*P<0.05; **P<0.01; ***P<0.001; n.d.=not determined; n.s.=not significant.
From Kaneko (1997).
From Kajikawa et al. (1999).
From Duthie et al. (1997).
From Tizard (2000).
Table 3.
Results of the cats with FIP repeatedly sampled during the course of the disease
| Cat No. 51 |
Cat No. 57 |
||||
|---|---|---|---|---|---|
| T0 | T28a | T53 | T0a | T40 | |
| Total proteins (g/dL) | 9.1 | 7.6 | 9.8 | 9.8 | 12.0 |
| A/G ratio | 0.38 | 0.41 | 0.38 | 0.50 | 0.18 |
| Albumins (g/dL) | 2.50 | 2.20 | 2.70 | 3.25 | 1.81 |
| Globulins (g/dL) | 6.60 | 5.40 | 7.10 | 6.55 | 10.19 |
| α1-Globulins (g/dL) | 0.34 | 0.24 | 0.16 | 0.56 | 0.52 |
| α2-Globulins (g/dL) | 1.05 | 0.65 | 0.30 | 1.36 | 0.86 |
| β-Globulins (g/dL) | 0.77 | 0.86 | 1.14 | 1.41 | 1.15 |
| γ-Globulins (g/dL) | 4.44 | 3.64 | 5.50 | 3.21 | 7.66 |
| Serum amyloid A (μg/mL) | 56.97 | 119.93 | n.d. | 120.22 | 122.05 |
| Haptoglobin (mg/mL) | 2.23 | 2.27 | 1.41 | 2.56 | 2.10 |
| α1-Acid glycoprotein (mg/mL) | 4.06 | 3.12 | 1.65 | 2.68 | 5.41 |
| Immunoglobulin G (g/dL) | n.d. | n.d. | n.d. | 2.089 | 5.67 |
| Immunoglobulin M (g/dL) | n.d. | n.d. | n.d. | 1.21 | 0.74 |
n.d.=not determined; T0=first sample; T28,T40,T53=28,40 and 53 days after T0, respectively.
Appearance of the symptoms.
Table 4.
Results of the four FCoV-exposed cats repeatedly sampled
| T0 | T28a | T53 | T83 | ANOVA | Tukey HSD Test |
||
|---|---|---|---|---|---|---|---|
| * | ** | ||||||
| Total proteins (g/dL) | 8.18±0.43 | 7.45±0.44 | 6.53±0.32 | 7.45±0.54 | ** | T53 vs. T28,T83 | T53 vs. T0 |
| A/G ratio | 1.07±0.19 | 1.03±0.26 | 0.86±0.23 | 0.77±0.07 | n.s. | ||
| Albumins (g/dL) | 4.65±1.06 | 3.73±0.45 | 2.96±0.36 | 3.23±0.07 | * | T0 vs. T53 | |
| Globulins (g/dL) | 4.46±1.34 | 3.72±0.54 | 3.56±0.55 | 4.22±0.49 | n.s. | ||
| α1-Globulins (g/dL) | 0.45±0.16 | 0.44±0.17 | 0.29±0.06 | 0.49±0.41 | n.s. | ||
| α2-Globulins (g/dL) | 0.42±0.18 | 0.45±0.05 | 0.51±0.20 | 0.64±0.16 | n.s. | ||
| β-Globulins (g/dL) | 1.02±0.61 | 0.64±0.08 | 0.71±0.26 | 0.67±0.23 | n.s. | ||
| γ-Globulin (g/dL) | 2.30±0.37 | 2.19±0.40 | 2.06±0.31 | 2.41±0.17 | n.s. | ||
| Serum amyloid A (μg/mL) | 4.97±0.74 | 31.8±0.97 | 15.5±0.87 | 6.83±0.46 | n.s. | ||
| Haptoglobin (mg/mL) | 0.95±0.74 | 1.20±0.97 | 1.29±0.87 | 1.27±0.46 | n.s. | ||
| α1-Acid glycoprotein (mg/mL) | 0.97±0.37 | 1.10±0.48 | 0.76±0.38 | 0.92±0.44 | n.s. |
*P<0.05; **P<0.01; T0=first sample; T28, T53, T83=28,53 and 83 days after T0, respectively; n.s., not significant.
Appearance of the symptoms in another cat of the group.
Statistical analysis used specific software (Statsoft). Results from the three groups were compared with each other using an one-way ANOVA followed by the honest significant differences (HSD) Tukey test. If a normality test showed that data did not have a normal distribution, the non-parametric Kruskal Wallis test was used. ANOVA for repeated measurements or the corresponding non-parametric Friedmann tests were used to compare data obtained in repeated samplings. The percentages of positivity for FCoV in the three groups were compared using the chi-squared test.
3. Results
All cats were FIV- and FeLV-negative, while FCoV-positive cats were present in all groups. The percentage of positive animals was higher (P<0.001) in cats with FIP (90.6%) than in other groups (group 1: 25%; group 2: 36.4%).
Results from FCoV-exposed cats did not significantly differ from those of controls, whereas cats with FIP had increased total globulins and globulin fractions, except for α1-globulins, and increased APPs (Table 2). However, β- and γ-globulins were higher compared to controls, but not to FCoV-exposed cats. The results from cats with wet FIP were not significantly different from those obtained in cats with dry FIP.
After the appearance of clinical symptoms, α1-, α2-globulins and Hp decreased, while γ-globulins increased in both the cats with FIP (Table 3). β-Globulins and AGP were however different in these two cats and SAA did not change in the single cat in which it was measured. Total proteins and albumins significantly decreased at T 53 in FcoV-exposed cats (Table 4). No other significant changes were detectable, in spite of an increase of mean values of α2-globulin from T 0 to T 83 and of the APPs at T 28 and/or a T 53 . In fact after the onset of the disease in the group, transient increases of the APPs were detected in all four cats, but in different samplings (Fig. 1, Fig. 2, Fig. 3 ).
Fig. 1.
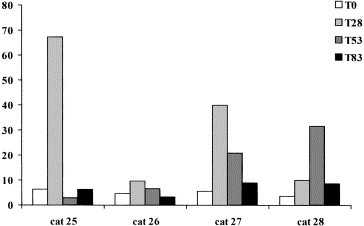
Serum amyloid A concentrations (μg/mL) in the four samples from FCoV-exposed cats.
Fig. 2.
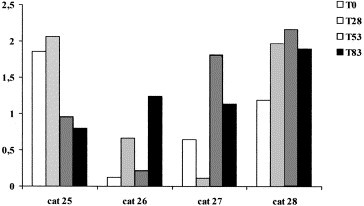
Haptoglobin concentrations (mg/mL) in the four samples from FCoV-exposed cats.
Fig. 3.
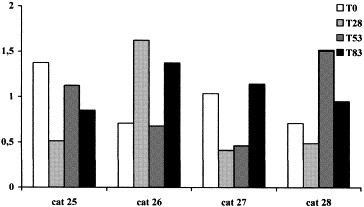
α1-Acid glycoprotein concentrations (mg/mL) in the four samples FCoV-exposed cats.
4. Discussion
Cats with anti-FCoVs antibodies were present in both controls and cats classified as “FCoV exposed”, probably because most of them came from multicat environments, where fluctuating antibody titres could be found (Pedersen, 1995). Nevertheless we considered separately these two groups due to their different incidences of FIP: in fact, although individual cats with clinical disease are not more likely to shed the virus than cats without FIP, the prevalence of FCoV shedding is higher in catteries with a high incidence of clinical cases of FIP (Foley et al., 1997). In contrast, the percentage of seropositivity was higher in cats that developed the disease, in agreement with previous findings (Pedersen, 1995).
Results from our controls were within reference ranges (Duthie et al., 1997; Kajikawa et al., 1999; Kaneko, 1997; Tizard, 2000), except for IgM and AGP. Mean AGP values were slightly higher than those reported by Duthie et al. (1997), but that study was undertaken on SPF cats, while we used cats from multicat environments, where a variety of antigenic stimuli can induce changes in serum proteins even in the absence of clinical signs (Kristensen and Barsanti, 1977). IgG and IgM levels showed a high standard deviation, due to cat No. 1 (2 and 5.7 g/dL, respectively) which did not show any clinical symptoms.
No statistically significant differences were detected between FCoV-exposed cats and controls, most likely due to the high individual variability of group 2. Compared to controls, cats with FIP shared the typical electrophoretic changes (Sparkes et al., 1991), and increased APP levels. In particular, a 10-fold increase of SAA was detectable, thus confirming its role as an APP in cats (Kajikawa et al., 1999). Although no cats with diseases other than FIP were included in this study, the high SAA concentration detected in sick cats suggests that this protein might also be a marker of FIP, as has been reported for Hp and AGP (Duthie et al., 1997; Stoddart et al., 1988). It is interesting to note that the APPs were particularly high at T 0 in cat No. 51, suggesting that an inflammatory reaction was already occurring before the appearance of the symptoms (Kajikawa et al., 1999). Furthermore, cats with FIP had high IgG levels, most likely due to antibody production, but low IgM concentrations probably reflecting the fact that most of the cats were at an advanced stage of disease: in fact, β-globulins, where IgM migrate, increase only in cases of FIP with recent lesions (Paltrinieri et al., 2001). Unfortunately it was not possible to repeat the sampling in the two cats with FIP which were not euthanasized after the diagnosis. The behaviour of β-globulins and of APPs was different in the two cats probably depending on the different clinical forms or on the different times of sampling: in experimentally induced FIP, Hp decreases during the first 20 days post-infection, then increases (Stoddart et al., 1988).
When a clinical form of FIP appeared in a cattery, however, all cats of the group shared a transient increase of SAA and Hp and strong fluctuations of AGP. These changes were not statistically significant because they did not simultaneously occur in the different cats. Nevertheless, they suggest the presence of an acute phase reaction also in cats that did not develop the disease, maybe as a consequence of the spread of mutated FCoVs in the cattery.
IgG and IgM levels were quantitatively lower than the corresponding electrophoretic band (γ- and β-globulins, respectively), in particular in cats with FIP. Non-Ig proteins with γ-motility have been already reported (Paltrinieri et al., 1998). Although APPs with β-motility (e.g., transferrin or CRP) might account for the increase of β-globulins recorded in FCoV-exposed cats, one could speculate that the electrophoretic motility of some APPs might change during FIP, as occurs in humans, maybe due to changes in their glycosylation pattern (Mackiewicz and Mackiewicz, 1995; Ducret et al., 1996). This hypothesis is supported by the finding that AGP concentration was often higher than the total amount of α1-globulins, where AGP should migrate. Moreover the decrease of α2-globulins, observed in both the cats with FIP during the course of the disease, is not due to a quantitatively similar decrease of APPs. SAA concentration was too low (μg/mL) to influence the total α2-globulin concentration, but changes of Hp levels should induce changes in the corresponding electrophoretic band. In contrast, the decrease of α2-globulins in cats with FIP was higher than that of Hp, probably due to the decrease of some other “negative APP” (Kaneko, 1997) or to changes of electrophoretic motility of APPs. The behaviour of α2-globulins needs to be further investigated because it might play a crucial role in the pathogenesis of the disease. This fraction decreased during FIP, but slightly increased in FCoV-exposed cats.
In conclusion, this study confirms the possible diagnostic role for APPs in FIP. After the onset of a clinical form of FIP, the concentration of APPs increased in all the exposed cats in the cattery. These changes were transient in cats that did not develop FIP, but persisted in one cat that developed the clinical form of the disease. Furthermore, α2-globulins increased in FCoV-exposed cats, but decreased in cats that developed the disease. Further studies on APPs might allow us to understand better the pathogenesis and possible mechanisms of resistance or susceptibility to FIP.
Acknowledgements
This work was supported by the Italian fund M.U.R.S.T., ex-60%. The authors are very grateful to Dr. Fabrizio Ceciliani and to Dr. Stefano Comazzi for their support.
References
- Ducret A., Bruun C.F., Bures E.J., Marhaug G., Husby G., Aebersold R. Characterization of human serum amyloid A protein isoforms separated by two-dimensional electrophoresis by liquid chromatography/electrospray ionization tandem mass spectrometry. Electrophoresis. 1996;17:866–876. doi: 10.1002/elps.1150170508. [DOI] [PubMed] [Google Scholar]
- Duthie S., Eckersall P.D., Addie D.P., Lawrence C.E., Jarret O. Value of α1-acid glycoprotein in the diagnosis of feline infectious peritonitis. The Veterinary Record. 1997;141:299–303. doi: 10.1136/vr.141.12.299. [DOI] [PubMed] [Google Scholar]
- Eckersall P.D., Duthie S., Safi S., Moffatt D., Horadagoda N.U., Doyle S., Parton R., Bennett D., Fitzpatrick J.L. An automated biochemical assay for haptoglobin: prevention of interference from albumin. Comparative Haematology International. 1999;9:117–124. [Google Scholar]
- Foley J.E., Poland A., Carlson J., Pedersen N.C. Risk factors for feline infectious peritonitis among cats in multiple-cat environments with endemic feline enteric coronavirus. Journal of American Veterinary Medical Association. 1997;210:1313–1318. [PubMed] [Google Scholar]
- Kajikawa T., Furuta A., Onishi T., Tajima T., Sugii S. Changes in concentrations of serum amyloid A protein, α1-acid glycoprotein, haptoglobin, and C-reactive protein in feline sera due to induced inflammation and surgery. Veterinary Immunology and Immunopathology. 1999;68:91–98. doi: 10.1016/s0165-2427(99)00012-4. [DOI] [PubMed] [Google Scholar]
- Kaneko J.J. Serum proteins and the dysproteinemias. In: Kaneko J.J., Harvey J.W., Bruss M.L., editors. Clinical biochemistry of Domestic Animals. fifth ed. Academic Press; London: 1997. pp. 117–138. [Google Scholar]
- Kristensen F., Barsanti J. Analysis of serum proteins in clinically normal pet and colony cats, using agarose electrophoresis. American Journal of Veterinary Research. 1977;38:399–402. [PubMed] [Google Scholar]
- Mackiewicz A., Mackiewicz K. Glycoforms of serum alpha 1-acid glycoprotein as markers of inflammation and cancer. Glycoconjugate Journal. 1995;12:241–247. doi: 10.1007/BF00731326. [DOI] [PubMed] [Google Scholar]
- McDonald T.L., Weber A., Smith J.W. A monoclonal antibody sandwich immunoassay for serum amyloid A (Saa) protein. Journal of Immunological Methods. 1991;144:149–155. doi: 10.1016/0022-1759(91)90081-p. [DOI] [PubMed] [Google Scholar]
- Paltrinieri S., Cammarata Parodi M., Cammarata G., Comazzi S. Some aspects of humoral and cellular immunity in naturally occurring feline infectious peritonitis. Veterinary Immunology and Immunopathology. 1998;65:205–220. doi: 10.1016/S0165-2427(98)00155-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltrinieri S., Cammarata Parodi M., Cammarata G. In vivo diagnosis of feline infectious peritonitis by comparison of protein content, cytology, and direct immunofluorescence test on peritoneal and pleureal effusions. Journal of Veterinary Diagnostic Investigation. 1999;11:358–361. doi: 10.1177/104063879901100411. [DOI] [PubMed] [Google Scholar]
- Paltrinieri S., Grieco V., Comazzi S., Cammarata Parodi M. Laboratory profiles in cats with different pathological and immunohistochemical findings due to feline infectious peritonitis (FIP) Journal of Feline Medicine and Surgery. 2001;3:149–159. doi: 10.1053/jfms.2001.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. The history and interpretation of feline coronavirus serology. Feline Practice. 1995;23:46–51. [Google Scholar]
- Sparkes A.H., Gruffydd-Jones T.J., Harbour D.A. Feline infectious peritonitis: a review of clinical pathological changes in 65 cases, and a critical assessment of their diagnostic value. The Veterinary Record. 1991;129:209–212. doi: 10.1136/vr.129.10.209. [DOI] [PubMed] [Google Scholar]
- Stoddart M.E., Whicher J.T., Harbour D.A. Cats inoculated with feline infectious peritonitis virus exhibit a biphasic acute phase plasma protein response. The Veterinary Record. 1988;123:621–624. [PubMed] [Google Scholar]
- Tizard I. Veterinary Immunology. An Introduction. sixth ed. W.B. Saunders; Philadelphia: 2000. pp. 139–148. [Google Scholar]


