Abstract
Pulmonary complications are an important cause for treatment-related morbidity and mortality in hematopoietic cell transplantation (HCT) in children. The aim of this study was to investigate the yield of our pre-HCT pulmonary screening program. We also describe our management guidelines based on these findings and correlate them with symptomatic lung injury after HCT. Since 2008, all patients undergo a dedicated pulmonary screening consisting of pulmonary function test (PFT), chest high-resolution computed tomography (HRCT), and bronchial alveolar lavage (BAL) before HCT. We systematically evaluated the yield during the first 5 years of our screening program. We included 142 consecutive children. In 74% of patients, abnormalities were found. In 66% of patients, 1 or more PFT results were <80% of normal. Chest HRCT showed abnormalities in 55%; 19% of these abnormalities were considered “clinically significant.” BAL was abnormal in 43% of patients; respiratory viruses (PCR) were found in 35 patients, fungi (antigen or culture) in 21, and bacteria (culture) in 22. All 3 screening tests contributed separately to clinically relevant information regarding pulmonary status in these pre-HCT children. In 46 patients (33%), screening results had diagnostic and/or therapeutic implications. We found an association between pre-SCT HRCT findings and lung injury after transplantation. Pre-HCT screening with the combination of 3 modalities, reflecting different domains of respiratory status (function, structure, and microbial colonization), reveals important abnormalities in a substantial number of patients. Whether this improves patient outcome requires further investigation.
Key Words: Pulmonary complications, Screening, Respiratory viruses, Pulmonary function test
Highlights
-
•
Pulmonary screening before hematopoietic cell transplantation reveals important abnormalities.
-
•
Testing for lung function, structure, and microbial colonization contribute separately.
-
•
Therapeutic implications of different findings are described.
Introduction
Hematopoietic cell transplantation (HCT) is a curative treatment for various diseases. Pulmonary complications, both infectious and noninfectious, are frequently seen in patients undergoing HCT. In children, the incidence of pulmonary complications varies from 25% to 74% and is associated with a significantly increased risk for mortality 1, 2, 3. Because of the risk of life-threatening complications of the procedure, patients are routinely screened for HCT eligibility. Lung screening can potentially impact selection of HCT patients as well as affect preemptive treatment and prognosis.
Invasive fungal infections (IFI) are an important cause of morbidity and mortality during HCT. Diagnostic imaging, culturing pathogens, and antigen detection can be helpful to identify patients at high risk for IFI, which may guide therapy [4].
Also, respiratory viruses (RV) may have impact on the overall survival of HCT, either directly as a cause of pneumonitis in the severe immune-compromised patient or indirectly by triggering allo-immunity in the setting of allogeneic transplantation [5].
In 2008, we implemented extensive pre-HCT lung screening, which includes pulmonary function test (PFT), chest high-resolution computed tomography (HRCT), and bronchial alveolar lavage (BAL) in all patients. Here, we evaluate the yield of such an extensive pulmonary screening program and describe our treatment guidelines according to these findings as well as the outcome of patients.
Materials and Methods
All consecutive pediatric patients undergoing a first allogeneic HCT in our center between January 2008 and August 2013 were included. Patients were enrolled in the HCT research protocol after providing written informed consent for data collection and analysis, according to national ethical regulations (Ethical Commission Number 05/143 and 11/063K). Patient characteristics (age, gender, underlying disease), clinical symptoms, results of pulmonary screening tests, and occurrence of symptomatic lung disease after HCT was registered.
Pulmonary Screening
Standard pre-HCT pulmonary screening is performed in the week before transplantation and consists of a PFT, HRCT scan, and BAL.
PFT includes spirometry, whole body plethysmography, and measurement of carbon monoxide diffusion capacity. Measurements are performed in children aged 5 years and older, according to American Thoracic Society/European Respiratory Society criteria, using calibrated pneumotachometer systems (Jaeger, Hochberg, Germany). Values are expressed as percentage of predicted values for age, race, sex, and height-matched controls (The Utrecht data set, Koopman [6]).
Forced expiratory volume in 1 second, forced vital capacity, total lung capacity, and lung diffusion capacity for CO, corrected for hemoglobin and alveolar volume < 80% of predicted values are considered to be abnormal. Residual volume of >25% of total lung capacity is considered to be abnormal and suggestive for trapped air. HRCT scans are acquired using a 16-detector row scanner (Philips Medical Systems, Best, Netherlands). For infants and young children, scans are obtained at 25-cm H2O pressure (inspiration) and 0-cm H2O pressure (expiration). For older children, who were able to cooperate with breath hold instruction, scans were obtained at full inspiration and at end of exhalation. Inspiration images are obtained using fixed 90 kVp and 18 to 60 mAs (depending on bodyweight). For expiration images, we used 90 kVp and 11 mAs. Acquisition was volumetric thin-slice for both inspiratory and expiratory computed tomography.
All HRCT scans were assessed by a pediatric radiologist. Fleischner Society terms for thoracic imaging were used [7]. All abnormalities, as stated in the radiology report, were registered. Those abnormalities with clinical implications, such as antimicrobial treatment, guided lung biopsy or diuretics, were defined as clinically significant.
BAL was performed under general anesthesia.
BAL fluid was cultured and processed in accordance with standard microbiological procedures. Galactomannan (GM) tests are performed using BioRad Platelia Aspergillus EIA. Any positive culture or GM levels > .5 was considered to be abnormal.
Nucleic acids are extracted using the total nucleic acid protocol with the MagNA pure LC nucleic acid isolation system (Roche Diagnostics, Basel, Switzerland). For detection of RNA-viruses cDNA is synthesized by using MultiScribe reverse transcriptase and random hexamers (Applied Biosystems, Foster City, CA). Detection of viral and atypical pathogens was performed in parallel, using real-time PCR assays specific for the following viruses: bocavirus, Human herpesvirus–6, respiratory syncytial virus, influenzavirus A and B, parainfluenzavirus 1 to 4, rhinoviruses, adenoviruses, human coronavirus OC43, NL63 and 229E, human metapneumovirus, and Mycoplasma pneumoniae. Real-time PCR procedures were performed as described previously [8]. Any positive PCR is considered to be abnormal.
The total costs for the pulmonary screening were approximately 900 euro. Chest HRCT costs 300 euro, RV panel PCR 495, bacterial cultures 11 euro, GM 12 euro, PFT (complete) cost 48 euro.
For further analysis, patients were classified according to their risk for pre-HCT pulmonary problems, based on underlying disease, immune competence, infection risk, and pretreatment with potentially lung-toxic therapy. We distinguished 3 groups of patients: those with an inherited immune deficiency, those with a malignant disease and chemotherapy before transplantation, and those with inborn errors of metabolism, mild bone marrow failure, and malignancies without chemotherapeutic treatment.
Standard Antimicrobial Prophylaxis
Antibiotic prophylaxis involved daily ciprofloxacin and fluconazole, from the start of conditioning until the resolution of neutropenia. Additional prophylaxis against Streptococcus viridans was given with cefazoline in the mucositis phase. Empiric antibiotic treatment for febrile neutropenia included vancomycin and ceftazidime. Pneumocystis jeroveci pneumonia prophylaxis was started from 1 month after transplantation as cotrimoxazole 3 times a week. In case of positive serology for herpes simplex virus in all patients, and in case of positive serology for varicella zoster virus in cord blood transplantation recipients, prophylaxis with aciclovir was given. No other antiviral prophylaxis was given. In patients at high risk for IFI, according to our protocol, based on pretreatment, duration of neutropenia, and history of fungal infection, Aspergillus prophylaxis was given with daily voriconazole or twice weekly amphotericin B.
Practical Guidelines according to Findings on Pulmonary Screening
Patients with severely impaired PFT (<50% of normal) were considered to have an unacceptable high risk for treatment-related mortality and were excluded for HCT.
Patients with RV from BAL were considered to have a high risk for alloimmune-mediated lung syndromes. In elective HCT procedures, HCT was postponed until the RV was cleared. In other cases—when the underlying disease did not allow treatment delay—tapering of immune suppression after HCT was adjusted to prevent alloimmune-mediated lung syndromes. In cases with probable fungal disease (positive cultures or GM from BAL), antifungal treatment was considered. Patients with positive bacterial cultures from BAL were not treated, unless pulmonary symptoms developed. Bacterial culture results guide the choice of empirical antibiotic treatment for neutropenic fever after HCT.
In patients with nodular lesions on HRCT, lung biopsy was considered to identify the possible infectious cause and antimicrobial resistance pattern.
In patients with possible or proven IFI based on BAL findings, biopsy results, or HRCT findings, antifungal treatment was started and granulocyte transfusions or haploidentical stem cell support (combined with cord blood grafts) were considered.
Statistical Analysis
Calculation of mean values and standard deviation was done for PFTs. Comparing the results with predicted values for age, race, sex, and height-matched controls was done using t-test (test value 100%). Comparison of the means between the different disease groups was done using ANOVA. The chi-square test was used for comparison of proportions between 2 or more groups. Differences with a P value of < .05 were considered statistically significant. Associations between pre-HCT pulmonary screening findings and clinically manifested lung injury after HCT were analyzed using Cox proportional hazard models. Dichotomous outcomes were used as dependent variables. Univariate predictors with a P value of < .05 were used for multivariate analysis. All statistics were done using SPSS 21.
Results
Patient Cohort
We included 142 consecutive children receiving a first allogeneic HCT. Apart from mild upper respiratory tract symptoms in some, all patients were asymptomatic for lung disease at the time of pre-HCT screening. Patient characteristics are shown in Table 1 .
Table 1.
Patient Characteristics
| Characteristic | Value |
|---|---|
| Age, yr | |
| Median (range) | 7.0 (.2-19.4) |
| Gender | |
| Female | 54 |
| Male | 88 |
| Underlying disease | |
| Immunedeficiency∗ | 27 |
| Leukemia/lymphoma | 60 |
| Bone marrow failure†, bone marrow disease without chemotherapy‡ | 30 |
| Inborn error of metabolism§ | 25 |
Immune deficiencies include combined immune deficiency, severe combined immune deficiency, hemophagocytic lympho histiocytosis, autoimmune lymphoproliferative syndrome, and chronic granulomatous disease. Leukemia includes acute lymphoblastic leukemia and acute myeloid leukemia.
Bone marrow failure includes Fanconi anemia, congenital agranulocytosis, and thallasemia.
Bone marrow diseases not pretreated with chemotherapy include myelodysplastic syndrome and juvenile myelomonocytotic leukemia.
Inborn errors of metabolism includes predominantly Hurler syndrome.
In 3 patients, no pre-SCT lung screening was performed at all; 2 of them were under 4 months of age. In 122 patients (86%), all planned screening modalities could be performed. In 19 children, only some of the tests were done either for logistic reasons (n = 9) or because screening BAL was the only reason for general anesthesia, which was then considered a disproportional invasive procedure (n = 7). In 2 children above the age of 5, PFT was not feasible because of developmental delay. In 1 patient, HRCT was omitted because of the risk of irradiation damage related to underlying disease (Fanconi anemia).
PFT
PFT were performed in 83 patients. This was 94% of all patients in whom we were able to perform these tests, according to age and developmental level. Results are shown in Table 2 . We found PFT patterns of restrictive and obstructive lung disease as well as diffusion abnormalities with forced expiratory volume in 1 second, mean 82.7% (SD 11.5); forced vital capacity, mean 87.8% (SD 14.1); total lung capacity, 86% (SD 10.2); and lung diffusion capacity for CO, corrected for hemoglobin and alveolar volume, 81.9% (SD 18.5). Compared with the reference population with values of 100% (SD 12), all differences were statistically significant; with P values of < .0001. There was no difference in abnormalities in PFT between the different disease categories, see Table 2.
Table 2.
PFT before HCT
| All n = 83 | Mean Difference from Reference Population (1-sided t-test) | Immune-Deficiency n = 13 | Malignant Disease Pretreated with Chemotherapy n = 41 | Bone Marrow Disease, Inborn Errors of Metabolism No Pretreatment n = 29 | Difference between Disease Groups (ANOVA) | |
|---|---|---|---|---|---|---|
| FEV1 (% pred) | 82.7 (SD 11.5) | −17.3 [95% CI, −20.5-14.1; P < .0001] | 83.5 (12.9) | 81.3 (10.1) | 84.3 (12.6) | NS |
| FVC (% pred) | 87.8 (SD 14.1) | −12.2 [95% CI, −16.2-8.2; P < .0001] | 91.5 (14.6) | 86.3 (12.7) | 88.1 (15.7) | NS |
| TLC (% pred) | 86.9 (SD 10.2) | −14.5 [95% CI, −17.9-11.1; P < .0001] | 92.2 (8.3) | 83.8 (10.8) | 84.5 (7.6) | NS |
| RV/TLC | 25.2 (SD 5.7) | 25.4 (5.8) | 23.6 (4.9) | 27.5 (6.0) | NS | |
| TLCO (% pred) | 81.9 (SD 18.5) | −18 [95% CI, −25.8-10.4; P < .0001] | 85.7 (21.6) | 81.0 (19.8) | 81.2 (13.2) | NS |
| Abnormal PFT (<80% pred or RV/TLC > 25) | 8 patients (62%) | 28 patients (68%) | 19 patients (65%) |
FEV1 indicates forced expiratory volume in 1 second; % pred, percentage of predicted value; 95% CI, 95% confidence interval; NS, not significant; FVC, forced vital capacity; TLC, total lung capacity; RV, residual volume; TLCO, lung diffusion capacity for CO.
Mean values and SD in all patients, compared with reference population, mean values and SD per disease group, and comparison between disease groups.
HRCT
Chest HRCT was performed in 137 (96%) patients. In 74 patients (55%), abnormalities were seen; in 63 of 74 patients (85%) these findings were “new” findings. In the group of patients without pretreatment with chemotherapy or immune deficiency, the incidence of HRCT abnormalities before HCT was significantly lower than in the other patients (Figure 1 ). In 18 patients (13%), clinically significant abnormalities were found. Four (3%) had lesions suspect for fungus. Fourteen patients (10%) showed other abnormalities, including bronchiectasis, pleural effusion, consolidations, and aspecific nodules > 1 cm. These were new findings in 8 of 14 patients (57%). Most clinically significant abnormalities were found in the subgroup of patients with immune deficiencies, but this did not reach statistical significance (Figure 1).
Figure 1.
Prevalence of HRCT abnormalities before HCT, per disease group.
BAL
BAL was performed in 127 (90%) patients. Unfortunately, for logistic reasons, it was not always possible to do all microbial tests. Overall, in 47% of tested patients, 1 or more of the microbial tests were positive. Positive PCR for RV was found in 35 (31%) of tested patients. Rhinovirus was the most frequently detected virus (Table 3 ). In 21 (17%) patients, we found microbial evidence of fungal colonization either with positive cultures or GM. In only 2 patients, a positive GM corresponded with a positive culture for Aspergillus; in 1 patient with a positive Aspergillus culture, GM from BAL was negative. The positive findings for the whole cohort are shown in Table 3. In patients under 5 years of age, the incidences of BAL abnormalities in general, RV positivity, and bacteria positivity were significant higher than in older patients (P values of .001, .01, and .0003 respectively).
Table 3.
Results from Screening Tests in BAL before HCT
| Test | Viral PCR (n = 113) | Bacterial Culture (n = 123) | Fungal Culture (n = 123) | GM∗ (n = 119) |
|---|---|---|---|---|
| No. positive, (%) | 35 (31%) | 22 (17%) | 8 (7%) | 17 (14%) |
| 21 (18%)† | ||||
| Rhinovirus: 18; RSV: 3; Influenzavirus: 2; Corona: 2; Adenovirus: 2; Bocavirus: 1; Metapneumovirus: 1; Parainfluenzavirus: 1; mixed viruses: 5 | H. influenza: 7; Streptococci: 3; Mycoplasma Pn: 1; Morganella: 1; Pseudomonas: 1; Stenotrophomonas: 1; Klebsiella: 1; mixed: 7 | Candida species: 2; Aspergillus species: 3; Penicillium species: 3 | ||
Galactomannan in BAL > .5 = positive.
There was overlap between fungal culture results and galactomannan findings in BAL (2 of 3 Aspergillus-positive patients were also galactomannan positive, 1 patient with candida and 1 with penicillium also had positive GM) so 21 patients were considered positive for fungus.
Relationship between Different Tests
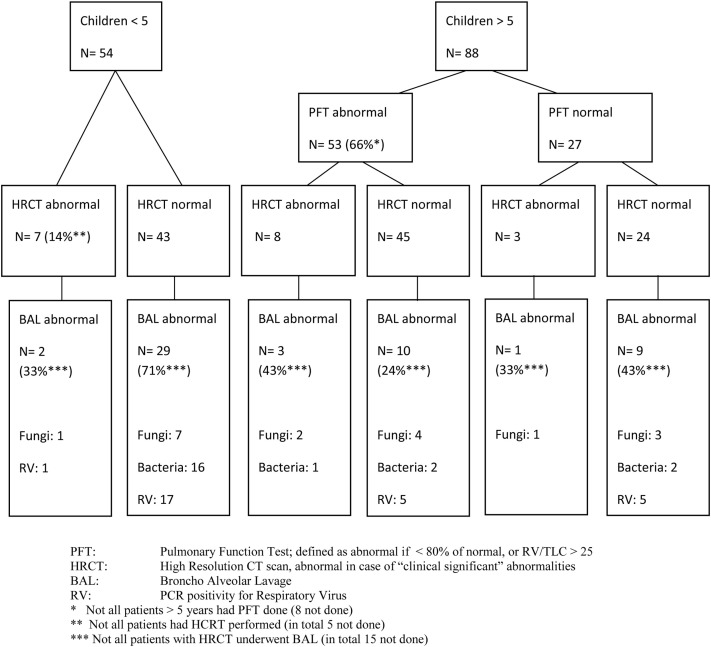
Figure 2 shows the yield of all screening tests. Abnormalities were found in tests of 74% of patients. We found abnormalities in radiological tests among patients with normal and abnormal PFTs, as well as positive microbial test results in patients with normal PFTs and normal HRCT.
Figure 2.
Overview results of pre-HCT screening tests.
Impact on Outcome
In 46 patients, the screening outcome had implications, such as guided BAL/lung biopsy (n = 8), change in antifungal treatment/prophylaxis (n = 12), granulocyte transfusions (n = 2), addition of haploidentical stem cells (n = 3), postponement of HCT (n = 11), or guided tapering of immune suppressive agents (n = 35). These interventions overlapped in some patients.
In 4 patients, the pre-HCT HRCT showed new or progressive signs of infiltrative fungal infection. Antifungal therapy was intensified in 3 based on resistance pattern of the cultured pathogen. In 2 patients, we postponed HCT. In 3 cord blood transplant recipients, we added haploidentical CD34+ cells from a family donor for early myeloid support, and in 2 patients we gave granulocyte transfusions during the period of neutropenia. One patient had graft rejection and showed fatal progression of Aspergillus infection in prolonged neutropenia. The 3 others did not show progression of infection, and therapy could be stopped safely after engraftment.
No patient with RV or bacteria isolated showed progression of pulmonary infection during HCT. None of the patients with isolated positive findings for fungus from BAL, of whom the majority received intensification of fungal prophylaxis/treatment, developed pulmonary fungal disease.
Cox regression analysis did not show a relation between pre-HCT screening findings in BAL or PFT and symptomatic lung injury after HCT. A clinically significant abnormality on chest HRCT before HCT, however, was a predictor for the development of immune-mediated lung injury after HCT (hazard ratio, 3.49; 95% confidence interval, 1.07 to 11.35; P = .037).
Discussion
Our study in 142 pediatric patients shows that pulmonary screening before HCT with PFT, HRCT, and BAL is feasible. We could perform all the tests in the majority of patients (86%). Abnormalities were found in 72% of patients. In 32% of patients, these abnormalities led to supportive/preemptive treatment according to guidelines. Only in patients with clinically significant chest HRCT, abnormalities a higher incidence of lung-injury was noted after HCT. Although not negligible, the costs seem justified in relation to the findings.
It is well known that pulmonary function declines early after HCT 9, 10, and some studies have shown a continuous decline without reaching a plateau during prolonged follow-up [9]. Several studies have demonstrated that impaired PFT before transplantation increases the risk for post-transplantation lung complications and mortality 1, 9, 11, 12, 13, 14. Possible explanations for these observations are that patients can have such marginal lung reserve capacity, endangering a period of critical illness and/or further lung toxic events. Also, in patients with pre-existing lung injury, this organ may be at increased risk for allo-immune phenomena, such as graft-versus-host disease.
We evaluated the yield of HRCT scanning. Omitting HRCT from our screening in this cohort would have missed 7 (5%) children with abnormalities, including 2 of the 4 with infiltrative lesions suspect for fungus. On the other hand, HRCT leads to radiation exposure and may require general anesthesia in children and, therefore, deserves critical appraisal. The relevance of abnormal findings on HRCT are a matter of debate. In the radiological reports in this study, abnormalities were described in 55% of patients. Because the severity of the reported abnormalities varied considerably, we chose to take into account those HRCT findings which had “significant clinical meaning” at time of transplantation, such as consolidations requiring antibiotic or antifungal therapy, bronchiectasis as a risk factor for infections warranting change in prophylaxis, or pleural effusions requiring diuretics. In most patients, a plain chest x-ray was available but showed abnormalities in only 50%, and, of note, did not show any abnormalities in the 4 patients with signs of invasive fungal infection on HRCT (data not shown).
The yield of BAL procedures was high in our study. Omitting BAL would have missed 19 (14%) patients with fungal colonization and 35 (25%) with RV. All these patients had normal HRCT scans and no significant pulmonary symptoms.
Figure 2 illustrates that all tests contribute separately to information regarding pulmonary status in pre-HCT children. This argues that all these screening modalities, reflecting different domains of respiratory status (function, structure, and microbial colonization), should be done in all pediatric pre-HCT patients, if a sensitive pre-HCT screening for pulmonary pathology is desired. The impact of the finding and the invasiveness of the test should guide clinicians in decision-making on whether or not to perform all tests.
As far as we know, this is the first report on such comprehensive pulmonary screening with PFT, HRCT, and BAL in a large cohort of children before HCT. We have shown considerable decrease in pulmonary function, a significant amount of clinical important HRCT findings, and high prevalence of infectious agents. This is most likely because of underlying disease, pretreatment with chemotherapy, and the age distribution of our patients.
In this cohort of patients, there is a significant association between clinically significant HRCT findings before HCT and lung injury after HCT. The findings in BAL and PFT were not related with outcome. This might be due to small numbers. We might also conclude that with current treatment strategies for this group of pulmonary compromised patients, we manage to have comparable outcome.
We conclude that this screening protocol is feasible and provides important information for risk classification with therapeutic consequences. We would advocate all 3 screening methods, as they all contribute separately. Prospective studies are needed to further identify the importance of baseline abnormalities in the risk for pulmonary complications and treatment-related mortality and whether outcome is improved by using intensive screening.
Acknowledgments
Financial disclosure statement: There are no items to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: A.B.V. had full access to all data in the study and takes responsibility for integrity of data and analysis. C.K.E., J.J.B., T.W., P.d.J., and M.B. contributed equally and substantially to study design, interpretation of data, and writing of the manuscript.
Footnotes
Financial disclosure: See Acknowledgments on page 1626.
References
- 1.Kaya Z., Weinier D.J., Yilmaz D. Lung function, pulmonary complications and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant. 2009;15:817–826. doi: 10.1016/j.bbmt.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Gower W.A., Collaco M.C., Mogayzel P.J. Lung function and late pulmonary complications among survivors of hematopoietic stem cell transplantation during childhood. Paediatr Respir Rev. 2010;11:115–122. doi: 10.1016/j.prrv.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Eikenberry M., Bartakova H., Defor T. Natural history of pulmonary complications in children after bone marrow transplantation. Biol Blood Marrow Transplant. 2005;11:56–64. doi: 10.1016/j.bbmt.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Ruhnke M., Bohme A., Buchheidt D. Diagnosis of invasive fungal infections in hematology and oncology- guidelines from the Infectious Disease Working Party in Haematology and Oncology of the German Society for Haematology and Oncology (AGIHO) Ann Oncol. 2012;23:823–833. doi: 10.1093/annonc/mdr407. [DOI] [PubMed] [Google Scholar]
- 5.Versluys A.B., Rossen J.W., van Ewijk B. Strong association between respiratory viral infection early after hematopoietic stem cell transplantation and the development of life-threatening acute and chronic alloimmune lung syndromes. Biol Blood Marrow Transplant. 2010;16:782–791. doi: 10.1016/j.bbmt.2009.12.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koopman M., Zanen P., Kruitwagen C. Reference values for paediatric pulmonary function testing: the Utrecht dataset. Respir Med. 2011;105:15–23. doi: 10.1016/j.rmed.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Hansell D.M., Bankier A.A., MacMahon H. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 8.van de Pol A.C., Wolfs T.F., Jansen N.J. Diagnostic value of real-time polymerase chain reaction to detect viruses in young children admitted to the paediatric intensive care unit with lower respiratory tract infection. Crit Care. 2006;10:R61. doi: 10.1186/cc4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inaba H., Yang J., Pan J. Pulmonary dysfunction in survivors of childhood hematological malignancies after allogeneic hematopoietic stem cell transplantation. Cancer. 2010;116:2020–2030. doi: 10.1002/cncr.24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhlving H.H., Bang C.L., Christensen I.J. Lung function after allogeneic hematopoietic stem cell transplantation in children: a longitudinal study in a population based cohort. Biol Blood Marrow Transplant. 2013;19:1348–1354. doi: 10.1016/j.bbmt.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Chien J.W., Madtes D.K., Clark J.G. Pulmonary function testing prior to hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35:429–435. doi: 10.1038/sj.bmt.1704783. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez-Sarmiento A., Orozco-Levi M., Walter E. Influence of pretransplantation restrictive lung disease on allogeneic hematopoietic cell transplantation outcomes. Biol Blood Marrow Transplant. 2010;16:199–206. doi: 10.1016/j.bbmt.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter E.C., Orozco-Levi M., Ramirez-Sarmiento A. Lung function and long-term complications after hematopoietic cell transplant. Biol Blood Marrow Transplant. 2010;16:53–61. doi: 10.1016/j.bbmt.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsberg J.P., Aplenc R., McDonough J. Pretransplant lung function is predictive of survival following pediatric bone marrow transplantation. Pediatr Blood Cancer. 2010;54:454–460. doi: 10.1002/pbc.22337. [DOI] [PubMed] [Google Scholar]