Abstract
Background: Parallel to its technical development starting in the 1930s, electron microscopy (EM) became an important tool in basic and clinical virology. First utilized in the rapid diagnosis of smallpox, it developed to a diagnostic routine in the early 1960s using the negative staining technique. EM was applied to infected cell-cultures and also to ‘dirty’ specimens including urine, feces, vesicle fluid, liquor. With the implementation of molecular biological and genetic techniques, the use of diagnostic EM decreased. Objectives: (1) To give a perspective on future indications and possible uses by discussing the past and the present of diagnostic EM, (2) To describe the system of External Quality Assessment on EM virus diagnosis (EQA-EMV) established in 1994 by our laboratory and its achievements. Study design: EQA-EMV is run to evaluate, to confirm and to improve the quality of diagnostic EM. Two different types of specimen are sent out: (1) prepared grids to assess and train the diagnostic skills of the participants, (2) stabilized virus particle suspensions to assess preparation efficiency. Results: Diagnostic EM differs from other diagnostic tests in its rapidity and its undirected ‘open view’. To emphasize these advantages, the indications for diagnostic EM are discussed, fundamental for a continuing future adaptation. Besides appropriate techniques, quality control measures are required to achieve and keep high diagnostic standards. The results from 6 years of EQA-EMV are presented. Conclusions: In the history of diagnostic EM in virology, a change in use has been seen. Starting in the 1990s and coincident with the broad introduction of ‘modern’ diagnostic techniques, the number of EM diagnostic labs has decreased considerably—in spite of the obvious advantages of this technique. To guarantee the continuing performance of diagnostic EM in the future, EQA runs have to be performed as with other techniques in the diagnostic armament. The growing number of participants and participating countries indicates an interest in as well as a need for this program.
Keywords: Electron microscopy, Rapid viral diagnosis, Quality control, External quality assessment
Abbreviations: EM, electron microscope, electron microscopy; EQA, external quality assessment scheme; EQA-EMV, external quality assessment scheme on EM virus diagnosis; ICTV, International Committee on the Taxonomy of Viruses; IEM, immune electron microscopy; NAT, nucleic acid amplification techniques; SPIEM, solid phase immune electron microscopy; SRNSV, small round non-structured viruses; SRSV, small round structured viruses
1. Past and present of diagnostic electron microscopy
Knowledge, expertise and capabilities depend on the available detection methods as clearly exemplified by Robert Koch’s achievements in bacteriology. When systematically applying light microscopic techniques to search for and identify pathogens in the second half of the 19th century, Koch soon succeeded in describing precisely a number of medically important agents, e.g. Bacillus anthracis in 1876, Mycobacterium tuberculosis in 1882, and Vibrio cholerae in 1883 (Koch, 1912a, Koch, 1912b, Koch, 1912c). But, like others, he experienced the limits of light microscopy when studying what we know today as viral diseases. Fulfilling Ernst Abbe’s early prophecies (Abbe, 1873), demonstrating sub-lightmicroscopic, ultrafiltrable agents only became possible with the construction of an entirely new type of microscope using monochromatic accelerated electrons instead of visible light for imaging (Knoll and Ruska, 1932, Marton, 1934, von Ardenne, 1940, for historical reviews see c.f. Ruska, 1979, Hawkes, 1985).
Technical development culminated in the late 1930s when EM with its inherently higher resolution became irreplaceable in characterizing submicroscopic structures. Previous research on virus particles depended mainly on indirect analysis, e.g. filtration and sedimentation. By applying EM, virology made a great step forward, as it allowed the direct demonstration of the particulate nature of viruses as well as the description of their size and morphology, shown already by the first electron micrographs of poxviruses (von Borries et al., 1938). Within a short period of time a number of additional viruses and bacteriophages, as well as bacteria, were morphologically characterized (Kausche et al., 1939, Ruska et al., 1939, Ruska, 1940a, Ruska, 1940b, Luria et al., 1943) making insights into structure-function relations and classification of viruses possible. Immune EM techniques were also developed very early, based on specific antibodies as part of the preparative and analytical repertoire (Anderson and Stanley, 1941, von Ardenne et al., 1941).
The value of EM in viral diagnosis soon became apparent. Although lacking today’s contrast techniques, the early osmium smoking methods allowed the differentiation between the chickenpox herpesvirus and the brick-shaped smallpox virus directly from the patients’ vesicle fluid (Nagler and Rake, 1948, van Rooyen and Scott, 1948, Peters et al., 1962; see c.f. Fig. 1 ). Heavy metal shadowing techniques, developed soon afterwards, permitted particle counting and the morphological assessment of purified virus and were of great scientific value (Williams and Wyckhoff, 1945). In virus diagnosis, however, shadowing often obscured viral structures because of the debris usually present in diagnostic specimens. The advent in the 1960s of both the negative staining techniques (Brenner and Horne, 1959; for review see Hayat and Miller, 1990, Bozzola and Russell, 1992, Harris, 1997) and easy-to-handle instruments encouraged a broad application of EM to routine viral diagnosis. In the following years, a great number of clinically important, previously undescribed agents, e.g. adeno-, entero-, myxo-, paramyxo-, and reoviruses, was identified, mostly from diagnostic cell cultures. By extensive sero-epidemiological and virus neutralization studies these isolates could be linked etiologically to respective disease. Scrutinized by EM, many of them were recognized as ‘new’ and morphologically distinct viruses. The differences observed in morphology were used as the criteria for virus classification (Tyrrell and Almeida, 1967).
Fig. 1.
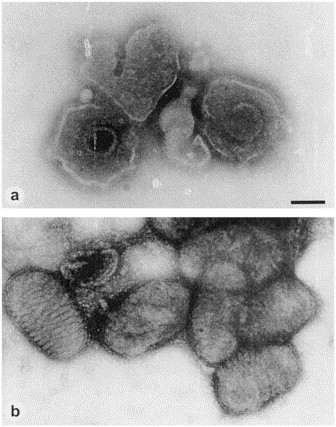
Direct EM of (a) herpesvirus particles from a varicella vesicle and (b) parapoxvirus recovered from the skin ulcers of a diseased seal, demonstrating the principles of morphological diagnosis after negative staining and the advantages of using different stains. The membrane destroying and/or penetrating effect of 2% phosphotungstic acid helps to reveal the 100 nm herpesvirus capsid within the viral envelope. 1% uranyl acetate on the other hand, by its remarkable membrane stabilizing effects, reveals the surface detail of the brick-shaped poxvirus very clearly. Magnification, ×80 000; bar, 100 nm.
In a number of clinically important diseases, however, e.g. hepatitis and non-bacterial gastroenteritis, cell culture isolation techniques failed to discover the causative agents. Though the transmissibility of a disease was clearly shown, some through the use of volunteers experiments (Reimann et al., 1945), sometimes without clear consent (Krugman et al., 1967), the presumed viral agents turned out to be non-cultivable. The hunt for these 'fastidious’ agents went on for more than two decades and became successful only when direct EM was utilized in the 1970s to study also ‘dirty’ specimens like plasma, urine, feces (for overview see c.f. Almeida, 1983, Madeley, 1979a, Madeley, 1995). Hepatitis B and A viruses were then detected in plasma and stool specimens respectively (Dane et al., 1970, Feinstone et al., 1973). In the next few years several etiological agents linked to gastroenteritis were discovered: Using direct EM, rotaviruses were found to be a major cause of endemic acute gastroenteritis in animals and humans (Bishop et al., 1973, Flewett et al., 1973, Bern et al., 1992; for review see Kapikian and Chanock, 1996). Investigating a recent outbreak of diarrhea and vomiting at Norwalk, Ohio, Kapikian and colleagues detected small, round particles by immune aggregation and linked those structures to the outbreak (Kapikian et al., 1972). The capsids of Norwalk and Norwalk-like viruses display only weak surface details (Fig. 2 c), based on their nucleic acid composition, however, they are classified today as members of the Caliciviridae (Murphy et al., 1995, Caul, 1996, Kapikian et al., 1996, Middleton, 1996). Astroviruses were described (Madeley and Cosgrove, 1975, Madeley, 1979b) as causes of endemic gastroenteritis in humans; later they were also found as common pathogens in poultry and mammals (review see c.f. Matsui and Greenberg, 1996). Also particles morphologically resembling the earlier described animal caliciviruses, i.e. showing the deep cup-shaped holes on the capsid (Fig. 2a), have been linked to cases of human diarrhea and vomiting (Flewett et al., 1976, Madeley and Cosgrove, 1976, Vinjé and Koopmans, 1996, Wright et al., 1998; for review see c.f. Kapikian et al., 1996). Due to the lack of molecularly-based diagnostic tests, the majority of these uncultivable small spherical particles were tentatively assigned to one of two morphologically-defined groups (Caul and Appleton, 1982): either small round structured viruses (SRSV; Fig. 2) comprising the astro-, calici-, and Norwalk/Norwalk-like viruses, or to small round non-structured viruses (SRNSV), including the Parvo- or Picornaviridae, e.g. polio or coxsackie viruses, displaying a smooth capsid structure.
Fig. 2.
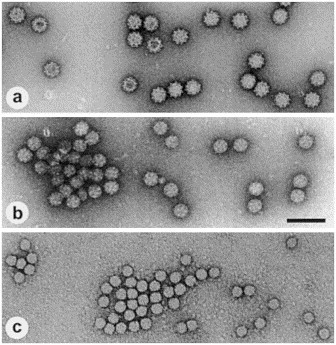
Overview of the morphology of small round viruses, known to be causative agents of gastroenteritis. (a) calicivirus, named according to the very distinct capsid morphology with 32 deep surface indentations (Greek ‘calix’, cup, goblet). (b) astrovirus with their typical, star-like capsid symmetry (Greek ‘aster’, star). (c) Norwalk virus, barely displaying any surface detail, named after the place, where the first outbreak caused by this virus was described. (a)–(c) Negative staining using 1% uranyl acetate. Magnification, ×80 000; bar, 100 nm.
Because of its inherent quantitative and economical limitations, however, EM is not suited for a mass screening of clinical specimens. Therefore, in the following years a number of alternative tests were developed. Today, for many of the ‘new’ viruses ELISAs and/or molecular-genetic techniques are available to detect viral antigen, antiviral antibodies, or nucleic acids. These techniques have taken over much of the diagnostic EM’s previous burden, although they are not a real substitute for and therefore not comparative to EM diagnosis: their objective, e.g. viral antigen or nucleic acids, or antiviral antibodies, differs from that of EM, the virus particle itself.
In conclusion, looking back at the history of diagnostic EM, a dramatic change of paradigms can be seen: During the early phase, EM viral diagnosis was confined mainly to differentiating smallpox from other viruses present in vesicle fluids of skin lesions (Nagler and Rake, 1948, van Rooyen and Scott, 1948, Peters et al., 1962, Nagington, 1964, Cruickshank et al., 1966, Long et al., 1970). During the second phase, so far the broadest application of EM in viral diagnosis, many new viruses were described. When electron microscopists in the 1970s also learned to study body excretous (Fig. 3 ), a number of ‘new’ etiological agents was discovered with many of them being diagnosed exclusively by EM (for review see Mahony and Chernesky, 1991, Madeley, 1995). The approach of direct EM was successful because many of these viruses, e.g. rota-, astro-, and parvoviruses are shed in high concentrations, often reaching particle concentrations of 1011 ml−1. The third phase finally is characterized by a marked reduction of laboratories performing diagnostic EM. Following the rapid development of modern test systems based on both molecular biology and genetics, the use of diagnostic EM has gone out of fashion. This loss of its use as a standard tool in viral diagnosis is more evident in human medicine, while the veterinarians are still thinking of EM as an universal, and therefore not too expensive, diagnostic tool. As a result of this decline in use, the quality of diagnostic EM laboratories lacking the daily routine is often decreased, resulting in further reductions in its use. To assess the future role of EM in viral diagnosis we would like to ask a number of questions, and without satisfactory answers to these questions, the future of EM as a constituent of the viral diagnostic armament appears dim.
Fig. 3.
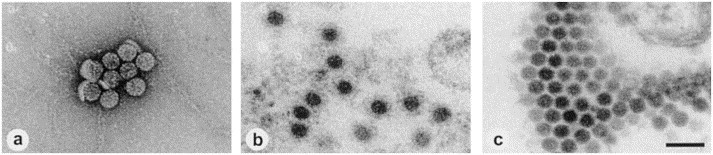
Human polyomavirus from the urine of bone marrow transplant recipients. (a) Negative staining after ultracentrifuge enrichment (100 000 ×g, 1 h, 20°C) using 1% uranyl acetate. (b) Thin section EM of a sediment (see a) embedded in Epon showing an immune aggregate. (c) Thin section EM of diagnostic HEL cell cultures inoculated with urine after ultracentrifuge enrichment. A paracrystalline array of polyoma virus particles is displayed. Magnification, ×80 000; bar, 100 nm.
2. What are the specific benefits of EM in viral diagnosis compared to the alternatives?
Many direct and indirect techniques are in use for detecting virus in clinical specimens. Differing in their objectives and their principles, their efficiencies must be compared with caution, e.g. one infectious unit detectable in cell culture might be equivalent to 10–10 000 ‘physical’ particles detectable by visualization, depending on the particle:infectivity ratio (Table 1 ). It is generally agreed that diagnostic EM with its rapid preparation and evaluation permits a very quick diagnosis (Gardner, 1977, Almeida, 1983). Another principal advantage compared to alternative test systems is the undirected ‘open view’ making this technique a ‘catch-all’ method: using diagnostic EM no prior selection of which virus to be sought is made or is necessary, enabling the detection of even unexpected viral structures (Madeley, 1995, Biel and Gelderblom, 1998). The current ICTV classification describes 75 virus families (Murphy et al., 1995) with 25 and 21 families representing vertebrate and human viruses, respectively. Because the virions of each of the 25 families of animal viruses display distinct morphologies, the particles can be assessed according to size and fine structure and grouped to one specific family out of the 25 a priori by the ICTV defined taxons. Assignment to a particular virus family often fulfills the immediate needs of the clinician. Thus, the present system of virus taxonomy justifies a morphology-based diagnosis. Several excellent atlases and textbooks on the fine structure of viruses and aspects of EM diagnostic virology present detailed information on the structural peculiarities and distinctive criteria (Doane and Anderson, 1987, Madeley and Field, 1988, Palmer and Martin, 1988a, Palmer and Martin, 1988b, Nermut and Stevens, 1989).
Table 1.
Comparison of different techniques for virus detection
| Technique | Detection of | Sensitivity | Specificity | Speed | Multiplex assays available |
|---|---|---|---|---|---|
| Nucleic acid amplification (NAT) | Viral genome, i.e. parts of it | High (10–50 molecules per assay) | Type/group/family | 1–4 h | Yes (usually for different types or groups of the same family) |
| Cell culture detection | Infectivity and cytopathity | High (1 infectious particle per assay) | Group/family | 2–14 d | Yes for cytopathic viruses |
| Electron microscopy Immune EM Type | Virus particles | Low (>107 mL−1) intermediate (>104–105mL−1) | Group/family | 15 min 120–240 min | By virtue of its ‘open view’ it is a ‘catch-all-method’ |
| ELISA | Viral antigen or specific antibodies | Intermediate | Type/group | 2–3 h | No |
| Immune light microscopy (immune fluorescence, APAAP) | Viral antigen or specific antibodies | High | Type/group | 2–3 h | Yes |
The benefits and limitations, e.g. the ‘open view’ and rapidity of the individual EM diagnosis on the one hand and the need for high particle concentrations resulting in relatively low sensitivity of diagnostic EM on the other hand have been discussed in many reports (Gardner, 1977, Hsiung et al., 1979, Almeida, 1979, Kjeldsberg, 1980, Field, 1982, Almeida, 1983, Flewett, 1985, Palmer and Martin, 1988b, Miller and Howell, 1997, Gelderblom et al., 1991, Mahony and Chernesky, 1991, Landry and Hsiung, 1994, Chrystie, 1996, Madeley, 1995, Biel and Gelderblom, 1997). Today, the benefits of diagnostic EM can range from null to remarkably high, depending on the diagnostic problem itself. The performance of diagnostic EM, however, depends on a number of technical and methodological factors.
3. Methods used in diagnostic EM
If one decides to apply EM viral diagnosis, there are a number of methods available. So, what recommendation should be given to a beginner in diagnostic EM? Generally, a transmission EM is used, and the preparative technique most often applied is negative staining (for review see Biel and Gelderblom, 1999). Thin section EM, however, is essential in the further characterization of a virus, e.g. the elucidation of its interaction with the host cell. The instrument used should not necessarily be highly sophisticated, it should allow stable primary routine magnifications of 40 000× to assess fine structural detail and also have data recording means, e.g. a sheet film or digital camera.
Using negative staining, direct EM as the most simple approach should be used. It will allow assessment of the specimen quality and indicate whether enrichment techniques should be used. Immune EM (IEM) may then be applied, if necessary. Several prerequisites for the efficient performance of diagnostic EM are:
(1) The use of stable, hydrophilic carbon-coated grids leads to a an efficient and stable adsorption of particles and a suitable stain distribution on the grid. Hydrophilicity can be achieved by pretreatment of the grids with polycations (MacRae and Srivastava, 1998; for details see c.f. Biel and Gelderblom, 1999) or by a glow discharge (Aebi and Pollard, 1987, Bozzola and Russell, 1992, Harris, 1997).
(2) The parallel application of two negative stains with different staining features, e.g. aqueous solutions of uranyl acetate and phosphotungstic acid, might help to visualize difficult-to-assess structures more convincingly, in particular with unfamiliar specimens (Biel and Gelderblom, 1999).
(3) To increase the concentration of virus particles on the grid, i.e. to lower the detection limit, three principal enrichment techniques are available: agar filtration, sedimentation, and bioaffinity techniques; being part of our diagnostic repertoire they are listed with their respective enrichment factors in Table 2 .
Table 2.
Key data of different particle enrichment techniques for negative staining EM
| Technique | Particle enrichment factor | Particle recovery on grid (%) | Minimal particle concentration | Time required for preparation (min) | Evaluation Method |
|---|---|---|---|---|---|
| Conventional direct EM | =1 | 0.01–0.50 | 107 ml−1 | 5–10 | Measurement of radioactive-labeled viruses |
| Agar filtration | 3–7 | 0.08–1.10 | 5×106 ml−1 | 15–30 | |
| Airfuge® sedimentation | 10–100 | 1.02–5.60 | 105 ml−1 | 10–20 | |
| Ultracentrifuge sedimentation | 50–100 | n.d. | 105 ml−1 | 60–120 | Comparative particle counting |
| SPIEM | 10–100 | n.d. | 105 ml−1 | 30–120 | |
| SPIEM+ultra-centrifugation | 100–800 | n.d. | 104 ml−1 | 90–240 |
Agarfiltration initially was introduced for particle counting in EM (Kellenberger and Bitterli, 1976): while giving cleaner specimens, filtration also enriches particles by a factor of 3–7. Likewise, the pseudoreplica technique leads to an appreciable enrichment (for details see c.f. Hayat and Miller, 1990). Ultracentrifugation permits the sedimentation of virus particles from a large suspension volume into a small sediment, following a preceding low-speed centrifugation for debris removal, if necessary. By resuspending the pellet in a small volume, the virus is concentrated 50–100-fold. Alternatively, particles may be sedimented directly onto the grid using the Airfuge® technique, resulting in similar enrichment factors (Gelderblom and Reupke, 1978, Hammond et al., 1981).
Bioaffinity methods or IEM mainly relies on high-affinity virus-specific antibodies or other specific ligands, e.g. lectins, that may be used in different ways. Using hyper-immune sera directed against a specific virus or paired (acute and reconvalescent) sera, the etiologic role of the agent can be demonstrated by immune-aggregation or -decoration. Early, immune-aggregation was used to clump individual virus particles into larger immune complexes that can be easier detected than individual particles scattered in dilute suspensions (Anderson and Stanley, 1941, von Ardenne et al., 1941). Thus, polyclonal sera are useful in increasing the detectability of dispersed virus particles, while monospecific antibodies help for both, immune-aggregation-enrichment and serotyping by immune-decoration.
The IEM approach can be very successful, as recently exemplified by the delineation of at least nine different serotypes of SRSV (Okada et al., 1990) or three serotypes of Norwalk-like viruses (Lewis, 1990), respectively. As well as direct IEM techniques, indirect immuno-aggregation techniques have also been developed, using species specific anti-IgG antibodies or protein A in addition to enrich, to label, and to type antibody-coated viruses (Valters et al., 1975, Katz et al., 1980).
The most sensitive IEM technique in viral diagnosis is solid phase IEM (SPIEM). Here the specific antibody is bound to the grid and, in a second step, the virus is captured immuno-specifically from the diagnostic suspension. This approach, also known as the ‘fly-paper’ technique and first developed for plant virus quantification (Derrick, 1973) was soon applied also to diagnostic specimens. The sensitivity was further improved by the introduction of protein A as to bind antibody to the grid (Shukla and Gough, 1979, Nicolaieff et al., 1980), and widely applied to clinical specimens (Kjeldsberg and Mortensen-Egnund, 1982, Gerna et al., 1985, Gerna et al., 1987, Humphrey et al., 1990, Lewis, 1990). SPIEM reaches a sensitivity comparable to the equivalent ELISA systems (Kjeldsberg and Mortensen-Egnund, 1982, Svensson et al., 1983, Gerna et al., 1987). Remarkably, it has also been experienced, that even direct EM can reach the sensitivity of commercial ELISAs (Moosai et al., 1985, Cubitt et al., 1999).
Using SPIEM, the sensitivity of diagnostic EM is improved up to 100 times (Kjeldsberg and Mortensen-Egnund, 1982) and reaches detection limits down to values of e.g. 102–103 plaque forming units per milliliter (Giraldo et al., 1982). Compared to nucleic acid amplification techniques (NAT) for SRSVs neither SPIEM nor NAT detect every virus-positive specimen—however, they complement each other (Humphrey et al., 1997, Cubitt et al., 1999).
(4) In general, the use of routine laboratory and reporting protocols following GLP rules is highly recommended (for details see Table 3 ).
Table 3.
GLP requirements for diagnostic EM
| Lab organization | Technical facilities |
|---|---|
| Lab is part of a diagnostic network with efficient informational exchange (e.g. public health network or big clinic) | Working facilities according to safety regulations |
| Regularly trained personnel | Electron microscope with appropriate primary magnification |
| Data recording system | Low speed centrifuge for clearing |
| Controlled lab data flow | Ultracentrifuge for enrichment |
| Stable highly adsorptive grids | |
| Different contrasting ‘stains’ | |
| Panel of high affinity antibodies directed against diagnostic relevant viruses |
4. What are the actual indications for EM viral diagnosis?
A morphological diagnosis uncovers the implicated virus family and often fulfills the clinician’s immediate needs, saving precious time and complex diagnostic efforts. If a complete diagnosis to the specific serotype is required, an early morphological diagnosis will focus the diagnostic efforts onto the relevant virus family, thus again saving diagnostic efforts. Therefore, the decision to utilize EM must be made early in the diagnostic procedure, in parallel or even before routine cell culture inoculations (e.g. the need for rapid viral diagnosis in suspected varicella in a newborn ward or an immuno-compromised patient).
As with other diagnostic means, EM viral diagnosis is not possible in all cases, mainly because the agent possibly involved may vary in its detectability for both quantitative and qualitative reasons. This is exemplified by two recent outbreaks of emergent diseases, e.g. Hantavirus Pulmonary Syndrome (HPS) in the USA in 1993, also known as ‘Four Corner’s Disease’ (Elliott et al., 1994, Khan et al., 1996) and the paramyxovirus infections killing horses and humans and caused by a ‘new’ morbillivirus in Australia in 1995 (Murray et al., 1995). EM was of no help in the direct elucidation of HPS for two reasons: First, the causative agent, a member of the Bunyaviridae, is present only in low concentration in specimens taken directly from the patient or in diagnostic cell cultures. Second, the morphology of the bunyavirus particles is not so distinctive in diagnostic cell culture supernatants to allow a clear-cut and reliable morphological diagnosis.
The situation was different in the elucidation of the equine morbillivirus: negative staining EM of infected diagnostic cell cultures soon revealed typical ribonucleoprotein strands pointing towards a paramyxovirus. This helped to guide more targeted diagnostic efforts three days before any other specific results were available (Nowak, 1995). In the field of emerging virus diseases, diagnosis of filovirus infections will also benefit from EM (Fig. 4 ; Geisbert and Jahrling, 1995, Schmitz, 1998; for review see c.f. Beer and Kurth, 1999).
Fig. 4.
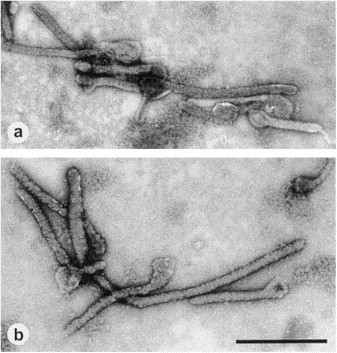
Negative staining of purified cell culture isolates from (a) Ebola virus and (b) Marburg virus. The filamentous structures of Ebola virus generally longer than those shown by Marburg virus. Magnification, ×20 000; bar, 1000 nm.
Arguing, with today’s financial limitations, EM is driven out of many laboratories, and EM viral diagnosis often is only confined to infections with more severe clinical or epidemiological implications. Under suitable circumstances, however—assuming an efficient laboratory organization—EM viral diagnosis can be cost-effective also today (Cubitt et al., 1999): the main cost is buying the instrument in the first place, although it is no more expensive, even new, than some of the sophisticated autoanalysers. Given the microscope, the only other major expense is the microscopist, who can be a senior technician provided there is a proper back-up; the consumables are not important in comparison. If the running expenses are low, EM should be used as much as possible, thus keeping pattern recognition capabilities of the microscopists high and the costs per specimen as low as possible. In addition, spending all the time on what is left over in clinical virology as ‘difficult’ specimens is worrying to the microscopists—the simpler specimens often do not reach the EM. Looking at these calculations, EM properly used is not more expensive than the other techniques, test for test (Madeley, 1999).
In conclusion, if EM diagnostic facilities are run by experienced personnel using state-of-the-art techniques, the possible benefits in viral diagnosis are evident. EM is essential in epidemiological or clinical conditions when a rapid viral diagnosis is required for instant clinical reasons, to abbreviate cell culture diagnosis, and/or when alternative standard diagnostic methods fail to produce reasonable results. In particular it is important as an instrument in the rapid viral diagnosis of suspected emerging virus infections (Table 4 ). A broader application of routine EM diagnosis is often confined to certain clinical syndromes, e.g. the elucidation of epidemic and endemic gastroenteritis.
Table 4.
Indications for the use of diagnostic EM
| Indication | Example |
|---|---|
| Rapid viral diagnosis | Critical epidemiological or clinical conditions, e.g. suspect of emerging viral diseases or need for fast diagnosis in immuno-compromised individuals |
| Abbreviation of classical cell culture diagnosis | Unknown CPE |
| Search for unknown or undetectable viruses | Failure or lack of other diagnostic techniques |
| Need for ‘catch-all-method’ | Lack of clinical data or broad group of suspected agents (e.g. in diarrhea) |
| Quality control in industrial processes | Following GMP rules in the production of biologicals, e.g. search for contaminants in cell culture based vaccine production |
5. Requirements for future EM diagnosis—how can the EM diagnostic labratory be prepared for the future?
As diagnostic virology in general becomes more controlled and standardized, only accredited laboratories will be permitted in the near future to provide any diagnostic services within the public health framework. This development leaves two alternatives: either (1) radically stopping EM-diagnosis as a tool in clinical virology or (2) to join a system of assessment and evaluation provided by national and/or international EQA schemes. These schemes will help diagnostic EM to retain high standards and to accredit them by developing standards for EM quality assurance and control.
Besides appropriate technical facilities and methods, effective organization and continuing educational efforts are required, i.e. the general implementation of GMP rules. External quality control of EM diagnosis is important for a reliable test service, which on the other hand, also determines the future of diagnostic EM. Regular participation in well designed external quality assurance programs is required for accreditation as a diagnostic facility. The first European External Quality Assurance Program (EQA) was started in 1967 by the Public Health Laboratory Services (PHLS) in Great Britain, including both serological and virus isolation procedures (Reed et al., 1985a). Later, in 1977, the PHLS also set up an EQA for EM viral diagnosis (Reed, et al., 1985b). However, this program after 20 distributions was stopped in 1993. To our knowledge, no other EQA for EM diagnosis was available until our program started in 1994. Its aim is to evaluate, to monitor and to improve the quality of diagnostic EM. It is run within the framework of, and supported by, a number of national and international societies, e.g. European Society for Clinical Virology (ESCV), European Society for Veterinary Virology (ESVV), German Society for Electron Microscopy (Deutsche Gesellschaft für Elektronenmikroskopie, DGE), German Society for Hygiene and Microbiology (Deutsche Gesellschaft für Hygiene und Mikrobiologie, DGHM), German Society against Viral Diseases (Deutsche Vereinigung zur Bekämpfung der Viruskrankheiten, DVV), Virological Society (Gesellschaft für Virologie, GfV).
EQA-EMV is run twice a year and is open to all laboratories working in the framework of viral diagnosis (Philipp and Gelderblom, 1995, Biel and Gelderblom, 1998). Two different types of coded specimen are sent out: (a) ready-made specimens (Pioloform coated, carbon reinforced copper grids 300/400 mesh, diameter 3.02 mm; uranyl acetate negative staining) and (b) inactivated and stabilized virus particle suspensions (0.1 ml; formaldehyde 4%, sodium azide 0.02%). Further information can be obtained from the internet at http://www.rki.de/INFEKT/EMQM/EM_MAIN.HTM.
In the first two runs, purified virus was used to prepare the test specimens to assess the diagnostic skills of the participants. The distribution of more realistic, i.e. more ‘clinical’ specimens started with EQA 3. From EQA 4 onwards the specimens have been distributed all over Europe and, since EQA 6 this has been worldwide (Fig. 5 ). Beginning with the sixth distribution, the participants results have been compared with a standard set tested by six selected reference laboratories and representing the 100% correct standard results. The participants are informed of their individual and on the cumulative results and, if required, may receive also a certificate of participation.
Fig. 5.
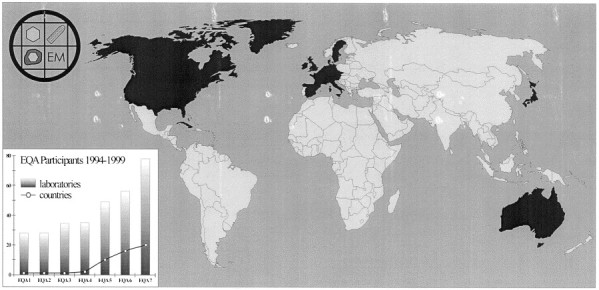
EQA map showing the participating countries in EQA 7 (distributed in February 1999) highlighted in dark gray. The development of the numbers of participants and participating countries, respectively, are shown in the lower left.
The participants are physicians and veterinarians as well as biologists, working in public health services, governmental institutions, universities, industry, at the army; the number of participants has risen from 28 German laboratories to 78 institutions in 20 countries (Fig. 5).
The main emphases of this EQA program are twofold: (1) training the participants’ diagnostic skills, especially the ‘open view’, and (2) with the stabilized virus suspensions, controlling the efficiency of specimen preparation. Other helpful aspects are: being confronted with rare and/or diagnostically difficult viruses, and, finally, because specimens containing dual infections and negative controls are also sent out, the participants learn to follow standard protocols for screening specimens with EM, e.g. screening for defined periods.
The results of EQA 1–6 can be summarized as follows:
-
1.
Though in some countries the number of laboratories performing EM viral diagnosis is still diminishing, there is a considerable number of participants and participating countries (Fig. 5). These data confirm a need for this program.
-
2.
The efficiency of diagnostic EM depends clearly on virus concentration and purity. Some participants have had problems in differentiating between paramyxo- and orthomyxovirus, polyoma- and papillomavirus, reo- and rotavirus, coronavirus and ‘normal’ constituents, as well as SRS(N)Vs and normal constituents. ‘Dual infections’ with widely different particle concentrations are not always detected. However, the educational value of EQA are obvious, as shown by the overall performance index rising as well as the indices for the more problematic viruses (Table 5 ). To retain the capacity and a reasonable good expertise in EM virus diagnosis for future diagnostic needs, regular quality control regimes and educational efforts are necessary. These measures will help to keep EM viral diagnostics within the ‘modern’ diagnostic laboratory.
Table 5.
Summary of EQA 1-6, showing the overall performance as well as for selected viruses
| EQA No | Number of participants | Grids |
Suspensions |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (%) |
Paramyxo (%) |
Adeno (%) |
Rota (%) |
Dual infections (%) |
Overall (%) |
Paramyxo (%) |
Adeno (%) |
Herpes (%) |
Dual infections (%) |
||
| Purified viruses at high particle concentrations | |||||||||||
| 1 | 28 | 82 | 29 | 89 | 96 | – | – | – | – | – | – |
| 2 | 28 | 98 | 93 | – | – | – | 55 | – | – | 96 | – |
| ‘Dirty’ specimens with more realistic particle concentrations | |||||||||||
| 3 | 34 | 47 | – | – | 28 | 24 | – | – | – | – | – |
| 4 | 35 | 61 | 34 | 100 | 100 | 29 | 55 | 20 | 71 | 80 | 17 |
| 5 | 49 | 77 | 90 | 100 | 96 | – | 61 | 31 | 92 | 84 | 29 |
| 6 | 56 | 77 | – | 93 | – | 29 | 71 | 27 | 95 | 89 | 25 |
Acknowledgements
The authors are very grateful to Charles Humphrey, CDC; Atlanta, for sharing his expertise in the EM diagnostic possibilities in outbreaks, to Dick Madeley, Stocksfield, UK, for thoroughly reading our final draft of the manuscript and ‘some’ helpful suggestions, and to Bärbel Jungnickl for her work in the darkroom.
Footnotes
Presented in part during the ESCV-meeting 1998, Hamburg.
References
- Abbe E. Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. M. Schulzes Arch. f. mikrosk. Anat. 1873;9:413–414. [Google Scholar]
- Aebi U., Pollard T.D. A glow discharge unit to render electron microscopic grids and other surfaces hydrophilic. J. Electr. Microsc. Techn. 1987;7:29–33. doi: 10.1002/jemt.1060070104. [DOI] [PubMed] [Google Scholar]
- Almeida J.D. Practical aspects of diagnostic electron microscopy. Yale J. Biol. Med. 1979;53:5–18. [PMC free article] [PubMed] [Google Scholar]
- Almeida J.D. Uses and abuses of diagnostic electron microscopy. Curr. Top. Microbiol. Immun. 1983;104:147–157. doi: 10.1007/978-3-642-68949-9_9. [DOI] [PubMed] [Google Scholar]
- Anderson T.F., Stanley W.M. A study by means of the electron microscope of the reaction between tobacco mosaic virus and its antiserum. J. Biol. Chem. 1941;139:339–344. [Google Scholar]
- Beer B., Kurth R. Characteristics of Filoviridae: Marburg and Ebola viruses. Naturwissenschaft. 1999;86:8–17. doi: 10.1007/s001140050562. [DOI] [PubMed] [Google Scholar]
- Bern C., Martines J., de Zoysa I., Glass R.I. The magnitude of the global problem of diarrhoeic disease: a ten-year update. Bull. WHO. 1992;70:705–714. [PMC free article] [PubMed] [Google Scholar]
- Biel S.S., Gelderblom H.R. Elektronenmikroskopische Erregerdiagnostik—Sinn und Unsinn, Qualitätssicherung und Perspektiven. Eur. J. Cell Biol. 1997;7(Suppl. 74):41. [Google Scholar]
- Biel, S.S., Gelderblom, H.R. (1998) External quality assessment scheme in electron microscopic virus diagnostics. Progress in Clinical Virology IV, Hamburg, Abstract 277, 49.
- Biel, S.S., Gelderblom, H.R. (1999) Electron microscopy of viruses. In: Cann, AJ (Ed.), Cell Culture—A Practical Approach, Oxford University Press, Oxford, in press.
- Bishop R.F., Davidson G.P., Holmes I.H., Ruck B.J. Virus particles in epithelial cells of duodenal mucosa from children with acute nonbacterial gastroenteritis. Lancet. 1973;2:1281–1283. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- Bozzola J.J., Russell, L.D. (1992) Electron Microscopy—Principles and Techniques for Biologists. Jones and Bartlett Publ., Boston
- Brenner S., Horne R.W. A negative staining method for high resolution electron microscopy of viruses. Biochim. Biophys. Acta. 1959;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Caul E.D., Appleton H. The electron microscopical and physical characteristics of small round human fecal viruses: an interim scheme for classification. J. Med. Virol. 1982;9:257–265. doi: 10.1002/jmv.1890090403. [DOI] [PubMed] [Google Scholar]
- Caul E.O. Viral gastroenteritis: small round-structured viruses, caliciviruses and astroviruses. Part I. The clinical and diagnostic perspective. J. Clin. Path. 1996;49:874–880. doi: 10.1136/jcp.49.11.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrystie I.L. Electron Microscopy. In: Mahy B.W.J., Kangro H.O., editors. Virological Methods Manual. Academic Press; London: 1996. pp. 92–106. [Google Scholar]
- Cruickshank J.G., Bedson H.S., Watson D.H. Electron microscopy in rapid diagnosis of smallpox. Lancet. 1966;2:527–530. doi: 10.1016/s0140-6736(66)92882-0. [DOI] [PubMed] [Google Scholar]
- Cubitt W.D., Mitchell D.K., Carter M.J., Willcocks M.M., Holzel H. Application of electron microscopy, enzyme immunoassay, and RT-PCR to monitor an outbreak of astrovirus type 1 in a pediatric bone marrow transplant unit. J. Med. Virol. 1999;57:313–321. doi: 10.1002/(sici)1096-9071(199903)57:3<313::aid-jmv16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Dane D.S., Cameron C.H., Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970;1:695–698. doi: 10.1016/s0140-6736(70)90926-8. [DOI] [PubMed] [Google Scholar]
- Derrick K.S. Quantitative assay for plant viruses using serologically specific electron microscopy. Virology. 1973;56:652–653. doi: 10.1016/0042-6822(73)90068-8. [DOI] [PubMed] [Google Scholar]
- Doane F.W., Anderson N. Diagnostic Virology—A Practical Guide And Atlas. Cambridge University Press; London, NY: 1987. Electron Microscopy. [Google Scholar]
- Elliott L.H., Ksiazek T.G., Rollin P.E., Spiropoulou C.F., Morzunov S., Monroe M., Goldsmith C.S., Humphrey C.D., Zaki S.R., Krebs J.W. Isolation of the causative agent of hantavirus pulmonary syndrome. Am. J. Trop. Med. Hyg. 1994;51:102–108. doi: 10.4269/ajtmh.1994.51.102. [DOI] [PubMed] [Google Scholar]
- Feinstone S.M., Kapikian A.Z., Purcell R.H. Hepatitis A: detection by immune electron microscopy of a virus-like antigen associated with acute illness. Science. 1973;182:1026–1028. doi: 10.1126/science.182.4116.1026. [DOI] [PubMed] [Google Scholar]
- Field A.M. Diagnostic virology using electron microscopic techniques. Adv. Virus Res. 1982;27:1–68. doi: 10.1016/S0065-3527(08)60432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewett T.H., Bryden A.S., Davis H.A. Virus particles in gastroenteritis. Lancet. 1973;2:1497. doi: 10.1016/s0140-6736(73)92760-8. [DOI] [PubMed] [Google Scholar]
- Flewett T.H., Bryden A.S., Davies H. Caliciviruses in man. Lancet. 1976;1:311. doi: 10.1016/s0140-6736(76)91450-1. [DOI] [PubMed] [Google Scholar]
- Flewett T.H. Rapid diagnosis of virus diseases. Br. Med. Bull. 1985;41:315–321. doi: 10.1093/oxfordjournals.bmb.a072070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P.S. Rapid viral diagnosis. J. Gen. Virol. 1977;36:1–29. doi: 10.1099/0022-1317-36-1-1. [DOI] [PubMed] [Google Scholar]
- Geisbert T.W., Jahrling P.B. Differentiation of filoviruses by electron microscopy. Virus Res. 1995;39:129–150. doi: 10.1016/0168-1702(95)00080-1. [DOI] [PubMed] [Google Scholar]
- Gelderblom H, Reupke H. Rapid viral diagnosis using the Airfuge. IV. Internat. Congr. Virol., The Hague, Abstract W50A/9 1976;630.
- Gelderblom H.R., Renz H., Özel M. Negative staining in diagnostic virology. Micron Microscop. Acta. 1991;22:435–447. [Google Scholar]
- Gerna G., Passarini N., Sarasini A., Battaglia M. Characterization of human rotavirus strains by solid-phase immune electron microscopy. J. Inf. Dis. 1985;152:1143–1151. doi: 10.1093/infdis/152.6.1143. [DOI] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Passarini N., Torsellini M., Parea M., Battaglia M. Comparative evaluation of a commercial enzyme-linked immunoassay and solid-phase immune electron microscopy for rotavirus in stool specimens. J. Clin. Microbiol. 1987;25:1137–1139. doi: 10.1128/jcm.25.6.1137-1139.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo G., Beth E., Lee J., de Harven E., Chernesky M. Solid-phase immune electron microscopy-double-antibody technique for rapid detection of papovaviruses. J. Clin. Microbiol. 1982;15:517–521. doi: 10.1128/jcm.15.3.517-521.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G.W., Hazelton P.R., Chuang I., Klisko B. Improved detection of viruses by electron microscopy after direct ultracentrifuge preparation of specimens. J. Clin. Microbiol. 1981;14:210–221. doi: 10.1128/jcm.14.2.210-221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JR. Negative Staining and Cryoelectron Microscopy. Royal Microscopic Society Microscopy Handbooks 35. Oxford: Bios Scientific Publishers, 1997.
- Hawkes PW. The beginnings of electron microscopy. Adv. Electron Phys., Suppl. 16. Orlando: Academic Press, 1995.
- Hayat MA, Miller, SE. Negative Staining. NY: McGraw-Hill 1990.
- Hsiung G.D., Fong C.K.Y., August M.J. The use of electron microscopy for diagnosis of virus infections: an overview. Prog. Med. Virol. 1979;25:133–159. [PubMed] [Google Scholar]
- Humphrey C.D., Cook E.H., Jr, Bradley D.W. Identification of enterically transmitted virus particles by solid phase immune electron microscopy. J. Virol. Meth. 1990;29:177–188. doi: 10.1016/0166-0934(90)90111-r. [DOI] [PubMed] [Google Scholar]
- Humphrey CD, Noel JS, Liu, BL, Rodriguez EM, Lambden PR, Clarke. Morphologic identification of viral gastroenteritis caused by a human calicivirus strain undetected initially by molecular or immunologic methods. In: Dwyer DM, Ando T, Glass RI, Monroe SS, editors. Microscopy and microananylsis. Cleveland/Ohio: 1997: 79.
- Kapikian A.Z., Wyatt R.G., Dolin R. Visualization by immune electron microscopy of a 27 nm particle associated with acute infectious non-bacterial gastroenteritis. J. Virol. 1972;10:1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A.Z., Chanock R.M. Rotaviruses. In: Fields B.N., Knipe D.M., Howley P.M., editors. Virology. 3rd ed. Lippincott-Raven; Philadelphia: 1996. pp. 1657–1708. [Google Scholar]
- Kapikian A.Z., Estes M.K., Chanock R.M. Norwalk group of viruses. In: Fields B.N., Knipe D.M., Howley P.M., editors. Virology. 3rd edn. Lippincott-Raven; Philadelphia: 1996. pp. 783–810. [Google Scholar]
- Katz D., Straussmann Y., Shahar A., Kohn A. Solid-phase immune electron microscopy (SPIEM) for rapid viral diagnosis. J. Immunol. Meth. 1980;38:171–174. doi: 10.1016/0022-1759(80)90341-5. [DOI] [PubMed] [Google Scholar]
- Kausche G.A., Pfankuch E., und Ruska H. Die Sichtbarmachung von pflanzlichem Virus im Übermikroskop. Naturwissenschaften. 1939;27:292–299. [Google Scholar]
- Kellenberger E., Bitterli D. Preparation and counts of particles in electronmicroscopy: application of negative stain in the agarfiltration method. Microsc. Acta. 1976;78:131. [PubMed] [Google Scholar]
- Khan A.S., Khabbaz R.F., Armstrong L.R., Holman R.C., Bauer S.P., Graber J., Strine T., Miller G., Reef S., Tappero J., Rollin P.E., Nichol S.T., Zaki S.R., Bryan R.T., Chapman L.E., Peters C.J., Ksiazek T.G. Hantavirus pulmonary syndrome: the first 100 US cases. J. Infect. Dis. 1996;173:1297–1303. doi: 10.1093/infdis/173.6.1297. [DOI] [PubMed] [Google Scholar]
- Kjeldsberg E. Application of electron microscopy in viral diagnosis. Path. Res. Pract. 1980;167:3–21. doi: 10.1016/S0344-0338(80)80179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsberg E., Mortensen-Egnund K. Comparison of solid-phase immune electron microscopy, direct electron microscopy and enzyme-linked immunosorbent assay for detection of rotavirus in fecal samples. J. Virol. Meth. 1982;4:45–53. doi: 10.1016/0166-0934(82)90053-2. [DOI] [PubMed] [Google Scholar]
- Knoll M., Ruska E. Das Elektronenmikroskop. Z. Physik. 1932;78:318–339. [Google Scholar]
- Koch R. Die Ätiologie der Milzbrand-Krankheit, begründet auf die Entwicklungsgeschichte des Bacillus Anthracis. In: Schwalbe J., editor. Gesammelte Werke von Robert Koch I. Thieme Verlag; Leipzig: 1912. pp. 5–26. [Google Scholar]
- Koch R. Die Ätiologie der Tuberkulose. In: Schwalbe J., editor. Gesammelte Werke von Robert Koch I. Thieme Verlag; Leipzig: 1912. pp. 482–562. [Google Scholar]
- Koch R. Berichte über die Tätigkeit der zur Erforschung der Cholera im Jahre 1883 nach Ägypten und Indien entsandten Kommission. In: Schwalbe J., editor. Gesammelte Werke von Robert Koch II.1. Thieme Verlag; Leipzig: 1912. pp. 1–19. [Google Scholar]
- Krugman S., Giles J.P., Hammond J. Infectious hepatitis: evidence for two distinct clinical, epidemiological and immunological types of infection. J. Am. Med. Soc. 1967;200:365–373. doi: 10.1001/jama.200.5.365. [DOI] [PubMed] [Google Scholar]
- Landry M.L., Hsiung G.D. Isolation and identification by microscopy. In: Webster R.G., Granoff A., editors. Encycl. Virol. Academic Press; NY, London: 1994. pp. 335–343. [Google Scholar]
- Lewis D.C. Three serotypes of Norwalk-like virus demonstrated by solid-phase immune electron microscopy. J. Med. Virol. 1990;30:77–81. doi: 10.1002/jmv.1890300117. [DOI] [PubMed] [Google Scholar]
- Long G.W., Noble J., Jr, Murphy F.A., Herrmann K.L., Lourie B. Experience with electron microscopy in the differential diagnosis of smallpox. Appl. Microbiol. 1970;20:497–504. doi: 10.1128/am.20.3.497-504.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S.E., Delbrück M., Anderson R.F. Electron microscope studies of bacterial viruses. J. Bacteriol. 1943;46:57–67. doi: 10.1128/jb.46.1.57-77.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae J., Srivastava M. Detection of viruses by electron microscopy: an efficient approach. J. Virol. Meth. 1998;72:105–108. doi: 10.1016/s0166-0934(97)00192-4. [DOI] [PubMed] [Google Scholar]
- Madeley C.R., Cosgrove B.P. 28 nm particles in faeces in infantile gastroenteritis. Lancet. 1975;2:451–452. doi: 10.1016/s0140-6736(75)90858-2. [DOI] [PubMed] [Google Scholar]
- Madeley C.R., Cosgrove B.P. Caliciviruses in man. Lancet. 1976;1:199–200. doi: 10.1016/s0140-6736(76)91309-x. [DOI] [PubMed] [Google Scholar]
- Madeley C.R. Viruses in the stools. J. Clin. Pathol. 1979;32:1–10. doi: 10.1136/jcp.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeley C.R. Comparison of the features of astroviruses and caliciviruses seen in samples of feces by electron microscopy. J. Inf. Dis. 1979;139:519–523. doi: 10.1093/infdis/139.5.519. [DOI] [PubMed] [Google Scholar]
- Madeley CR, Field AM (1988). Virus Morphology. 2nd ed. Churchill Livingston, Edinburgh.
- Madeley C.R. Viruses associated with acute diarrhoeal disease. In: Zuckermann A.J., Banatvala J.E., Pattison J.R., editors. Principles and Practice of Clinical Virology. 3rd edn. Wiley; Chichester: 1995. pp. 189–227. [Google Scholar]
- Madeley, CR (1999) Personal communication.
- Mahony J.B., Chernesky M.A. Negative staining in the detection of viruses in clinical specimens. Micron Micros Acta. 1991;22:449–460. [Google Scholar]
- Marton L. Electron microscopy of biological objects. Nature. 1934;133:911. [Google Scholar]
- Matsui S.M., Greenberg H.B. Astroviruses. In: Fields B.N., Knipe D.M., Howley P.M., editors. Virology. 3rd ed. Lippincott-Raven; Philadelphia: 1996. pp. 811–824. [Google Scholar]
- Middleton P.J. Viruses that multiply in the gut and cause endemic and epidemic gastroenteritis. Clin. Diagn. Virol. 1996;6:93–101. doi: 10.1016/0928-0197(96)00231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.E., Howell D.N. Concerted use of immunologic and ultrastructural analyses in diagnostic medicine: immuno-electron microscopy and correlative microscopy. Imunol. Invest. 1997;26:29–38. doi: 10.3109/08820139709048913. [DOI] [PubMed] [Google Scholar]
- Moosai R.B., Alcock R., Bell T.M., Laidler F.R., Peiris J.S., Wyn-Jones A.P., Madeley C.R. Detection of rotavirus by a latex agglutination test, Rotalex: comparison with electron microscopy, immunofluorescence, polyacrylamide gel electrophoresis, and enzyme linked immunosorbent assay. J. Clin. Pathol. 1985;38:694–700. doi: 10.1136/jcp.38.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F.A., Fauquet C.M., Bishop D.H.L., Ghabrial S.A., Jarvis A.W., Martelli G.P., Mayo M.A., Summers M.D. Arch. Virol. Springer-Verlag Wien; NY: 1995. Sixth Report of the International Committee on Taxonomy of Viruses (ICTV)—Classification and Nomenclature of Viruses. [Google Scholar]
- Murray K., Selleck P., Hooper P., Hyatt A., Gold A., Gleeson L., Westbury H., Hiley L., Selvey L., Rodwell B., Ketterer P. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- Nagington J. Electron microscopy in differential diagnosis of poxvirus infections. Br. Med. J. 1964;2:1499–1500. doi: 10.1136/bmj.2.5423.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagler F.P.O., Rake G. The use of electron microscopy in diagnosis of variola, vaccinia, and varicella. J. Bacteriol. 1948;55:45–51. doi: 10.1128/jb.55.1.45-51.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M.V., Stevens A.C. Animal Virus Struct. Elsevier; Amsterdam: 1989. [Google Scholar]
- Nicolaieff A., Obert G., van Regenmortel M.H.V. Detection of rotavirus by serological trapping on antibody-coated electron microscope grids. J. Clin. Microbiol. 1980;12:101–104. doi: 10.1128/jcm.12.1.101-104.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak R. Cause of fatal outbreak in horses and humans traced. Science. 1995;268:32. doi: 10.1126/science.7701338. [DOI] [PubMed] [Google Scholar]
- Okada S., Sekine S., Ando T., Hayashi Y., Murao M., Yabuuchi K., Miki T., Ohashi M. Antigenic characterization of small, round-structured viruses by immune electron microscopy. J. Clin. Microbiol. 1990;28:1244–1248. doi: 10.1128/jcm.28.6.1244-1248.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E.L., Martin M.L. Atlas Mamm. Viruses. CRC Press; Boca Raton, FL: 1988. [Google Scholar]
- Palmer E.L., Martin M.L. Electron Microscopy Viral Diagn. CRC Press; Boca Raton, FL: 1988. [Google Scholar]
- Peters D., Nielsen G., Beyer M.E. Variola: die Zuverlässigkeit der elektronenmikroskopischen Schnelldiagnostik. Dtsch. Med. Wschr. 1962;87:2240–2246. [Google Scholar]
- Philipp S.R., Gelderblom H.R. Standardisierung in der elektronenemikroskopischen Virusdiagnostik (Rapid Viral Diagnosis, Ringversuche) Bundesgesundhbl. 1995;38:55–58. [Google Scholar]
- Reed S.E., Gardner P.S., Stanton J. United Kingdom scheme for external quality assessment in virology—Part II: Specimen distribution, performance assessment, and analyses of participants’ methods in detection of rubella antibody, hepatitis B markers, general virus serology, virus identification, and electron microscopy. J Clin. Pathol. 1985;38:542–553. doi: 10.1136/jcp.38.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S.E., Gardner P.S., Snell J.J.S., Chai O. United Kingdom scheme for external quality assessment in virology—Part I: General method of operation. J. Clin. Pathol. 1985;38:534–541. doi: 10.1136/jcp.38.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann H.A., Hodges J.H., Price A.H. Epidemic diarrhea, nausea, and vomiting of unknown cause. J. Am. Med. Soc. 1945;127:1–6. [Google Scholar]
- Ruska H., von Borries B., und Ruska E. Die Bedeutung der Übermikroskopie für die Virusforschung. Arch. ges. Virusforschg. 1939;1:155–169. [Google Scholar]
- Ruska H. Die Sichtbarmachung der bakteriophagen Lyse im Übermikroskop. Naturwisssenschaft. 1940;28:45–46. [Google Scholar]
- Ruska H. Über ein neues bei der bakteriophagen Lyse auftretendes Formelement. Naturwissenschaft. 1940;29:367–368. [Google Scholar]
- Ruska E. Die frühe Entwicklung der Elektronenlinsen und der Elektronenmikroskopie. Acta Histor. Leopoldina 12, Halle/Saale 1979. [PubMed]
- Schmitz, H (1998) Personal communication.
- Shukla D.D., Gough K.H. The use of protein A, from Staphylococcus aureus, in immune electron microscopy for detecting plant virus particles. J. Gen. Virol. 1979;45:533–536. doi: 10.1099/0022-1317-51-2-415. [DOI] [PubMed] [Google Scholar]
- Svensson L., Grandien M., Pettersson C.A. Comparison of solid-phase immune electron microscopy by use of protein A with direct electron microscopy and enzyme-linked immunosorbent assay for detection of rotavirus in stool. J. Clin. Microbiol. 1983;18:1244–1249. doi: 10.1128/jcm.18.5.1244-1249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D.A.J., Almeida J. Direct electron-microscopy of organ cultures for the detection and characterization of viruses. Arch. Virusforsch. 1967;22:417–425. doi: 10.1007/BF01242962. [DOI] [PubMed] [Google Scholar]
- Valters W.A., Boehm L.G., Edwards E.A., Rosenbaum M.J. Detection of adenovirus in patient specimens by indirect immune electron microscopy. J. Clin. Microbiol. 1975;1:472–475. doi: 10.1128/jcm.1.5.472-475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooyen C.E., Scott G.D. Smallpox diagnosis with special reference to electron microscopy. Can. J. Publ. Health. 1948;39:467–477. [PubMed] [Google Scholar]
- Vinjé J., Koopmans M.P.G. Molecular detection and epidemiology of small round-structured viruses in outbreaks of gastroenteritis in the Netherlands. J. Inf. Dis. 1996;174:610–615. doi: 10.1093/infdis/174.3.610. [DOI] [PubMed] [Google Scholar]
- von Ardenne M. Ergebnisse einer neuen Elektronen-Übermikroskop-Anlage. Naturwissensch. 1940;28:113–127. [Google Scholar]
- von Ardenne M., Friedrich-Freksa H., Schramm G. Elektronenmikroskopische Untersuchungen der Präcipitinreaktion von Tabakmosaikvirus mit Kaninchenantiserum. Arch. ges. Virusforschg. 1941;2:80–86. [Google Scholar]
- von Borries B., Ruska E., Ruska H. Bakterien und Virus in übermikroskopischer Aufnahme. Klin. Wochenschr. 1938;17:921–925. [Google Scholar]
- Williams R.C., Wyckhoff R.W.G. Electron shadow micrography of virus particles. Proc. Soc. Exp. Biol. Med. 1945;58:265–270. [Google Scholar]
- Wright P.J., Gunesekere I.C., Doultree J.C., Marshall J.A. Small round-structured (Norwalk-like) viruses and classical human caliciviruses in southeastern Australia, 1980–1996. J. Med. Virol. 1998;55:312–320. [PubMed] [Google Scholar]


