Abstract
Porcine reproductive and respiratory syndrome (PRRS) virus (PRRSV) impairs local pulmonary immune responses by damaging the mucociliary transport system, impairing the function of porcine alveolar macrophages and inducing apoptosis of immune cells. An imbalance between pro- and anti-inflammatory cytokines, including tumour necrosis factor-α and interleukin-10, in PRRS may impair the immune response of the lung. Pulmonary macrophage subpopulations have a range of susceptibilities to different PRRSV strains and different capacities to express cytokines. Infection with PRRSV decreases the bactericidal activity of macrophages, which increases susceptibility to secondary bacterial infections. PRRSV infection is associated with an increase in concentrations of haptoglobin, which may interact with the virus receptor (CD163) and induce the synthesis of anti-inflammatory mediators. The balance between pro- and anti-inflammatory cytokines modulates the expression of CD163, which may affect the pathogenicity and replication of the virus in different tissues. With the emergence of highly pathogenic PRRSV, there is a need for more information on the immunopathogenesis of different strains of PRRS, particularly to develop more effective vaccines.
Keywords: Porcine reproductive and respiratory syndrome virus, Pathogenesis, Cytokines, Macrophages, Acute phase proteins
Introduction
Porcine reproductive and respiratory syndrome (PRRS) is caused by an arterivirus, PRRS virus (PRRSV) (King et al., 2012). Two genotypes of PRRSV have been identified: (1) European or type 1 genotype (PRRSV-1), the prototype of which is Lelystad virus (LV); and (2) North American or type 2 genotype (PRRSV-2), the prototype of which is the reference strain ATC2332. Significant antigenic and pathogenic differences have been reported between and within genotypes (Stadejek et al., 2006, Stadejek et al., 2008, Darwich et al., 2011), correlated with a lack of cross-protection by vaccines (Geldhof et al., 2012). Highly pathogenic strains of PRRSV (HP-PRRSV) have been identified within both genotypes (Xiao et al., 2010, Hu et al., 2012).
The respiratory form of PRRS primarily affects growing and finishing pigs, causing interstitial pneumonia, which induces respiratory signs and increases susceptibility to infection with other pathogens (Rossow, 1998). Although infection with PRRSV may be subclinical, clinical disease becomes evident when secondary infections are present (Drew, 2000); PRRSV thus contributes to the porcine respiratory disease complex (PRDC) (Opriessnig et al., 2011).
In 1996, the appearance of disease outbreaks caused by atypical or HP-PRRSV strains was reported in the USA (Halbur and Bush, 1997). These outbreaks were characterised by increased abortions (10–50%), sow mortality (5–10%) and preweaning mortality, primarily due to respiratory disease. HP-PRRSV strains have been isolated in China and South East Asia (Tian et al., 2007, Feng et al., 2008) and Eastern Europe (Karniychuk et al., 2010). Infection with HP-PRRSV strains is associated with severe clinical signs, pulmonary lesions and aberrant host immune responses (Xiao et al., 2010, Amarilla et al., 2012, Hu et al., 2012).
This paper reviews some aspects of the immunopathogenesis of PRRS and demonstrates how PRRSV replication affects the host pulmonary microenvironment, leading to opportunistic secondary infections.
Lung defences in PRRS
PRRSV damages the pseudostratified ciliated epithelium of the respiratory tract, impairing the mucociliary transport system and preventing the removal of microorganisms from the respiratory system (Done and Paton, 1995, Halbur et al., 1995). The primary target cells for replication of PRRSV are porcine alveolar macrophages (PAMs) (Duan et al., 1997), which are responsible for phagocytosis of microorganisms in the alveoli (Cheung et al., 2000). Replication of PRRSV in PAMs directly impairs their basic functions, including phagocytosis, antigen presentation and production of cytokines (Thanawongnuwech et al., 2001, De Baere et al., 2012). PRRSV induces necrosis or apoptosis in PAMs (Costers et al., 2008) and also induces apoptosis of lymphocytes and macrophages in the lungs and lymphoid organs, impairing the host immune response (Labarque et al., 2003, Gómez-Laguna et al., 2012b).
PRRSV increases the susceptibility of pigs to secondary bacterial infections and other viral infections (Van Reeth et al., 1996, Thacker et al., 1999, Brockmeier et al., 2000, Drew, 2000, Halbur et al., 2000, Wills et al., 2000, Renukaradhya et al., 2010, Díaz et al., 2012). However, under experimental conditions, secondary infections do not always establish in pigs infected previously with PRRSV (Drew, 2000). Concurrent infection with PRRSV and porcine circovirus type 2 (PCV2) is associated with more severe disease and higher piglet mortality (Allan et al., 2000, Harms et al., 2001, Rovira et al., 2002, Opriessnig et al., 2011). Mycoplasma hyopneumoniae potentiates PRRSV-induced disease (Thacker et al., 1999). There is a high frequency of concurrent infection with PRRSV and Streptococcus suis, M. hyopneumoniae or PCV2 in the field (Segalés et al., 2002).
Factors involved in increased susceptibility of pigs to secondary infections include the pneumovirulence of PRRSV (Martínez-Lobo et al., 2011) and the ability of the virus to persist (>250 days) in lymphoid organs, mainly the tonsils and lymph nodes (Wills et al., 2003). These features are influenced by differences in antigenicity and virulence among PRRSV genotypes, as well as the genetic background of the host. PRRSV-2 exhibits higher pneumovirulence than PRRSV-1, without marked differences in systemic clinical signs, viral load or viral distribution (Martínez-Lobo et al., 2011). However, HP-PRRSV, whether HP-PRRSV-1 or HP-PRRSV-2, is associated with more severe clinical signs, as well as increased severity of lung lesions and viral replication (Amarilla et al., 2012, Hu et al., 2012).
Role of macrophages in PRRS
The mononuclear phagocyte system of the lung consists of pulmonary alveolar macrophages (PAMs), pulmonary interstitial macrophages and, in pigs and some other species, pulmonary intravascular macrophages (PIMs) (Longworth, 1997). PAMs are free in the alveolar spaces, where they phagocytise inhaled particles. PIMs are found within the pulmonary capillaries, adherent to endothelial cells; they remove foreign particles from the blood and are able to migrate to sites of injury. PIMs release pro-inflammatory mediators, such as metabolites of arachidonic acid and cytokines that modulate pulmonary microvascular physiology (Bertram et al., 1988, Chitko-McKown et al., 1991, Carrasco et al., 2002).
PAMs represent the main cellular target for PRRSV replication, although the virus is also able to replicate in other macrophage subpopulations (Duan et al., 1997). In addition, PRRSV replicates in monocyte and bone marrow derived dendritic cells (DCs) in vitro, although such replication has not been demonstrated clearly in vivo (Loving et al., 2007, Wang et al., 2007, Chang et al., 2008, Flores-Mendoza et al., 2008, Gimeno et al., 2011). The main cell surface receptors for PRRSV attachment, internalisation and uncoating are heparan sulphate, sialoadhesin and CD163 (Delputte et al., 2005, Van Gorp et al., 2008).
PRRSV replicates in PAMs and PIMs in vitro, affecting their bactericidal functions (Thanawongnuwech et al., 1997). Since one of the major functions of PIMs is the clearance of bacteria from blood (Winkler and Cheville, 1987, Staub, 1994), viral replication in PAMs and PIMs may affect colonisation of the lungs by secondary viral or bacterial pathogens, as well as haematogenous bacterial dissemination.
PRRSV has a higher tropism for PAMs than for septal macrophages (PIMs and interstitial macrophages) (Gómez-Laguna et al., 2010b, Amarilla et al., 2012). Whereas PRRSV replicates mainly in PAMs, expression of pro-inflammatory cytokines has been observed mainly in septal macrophages, pointing to different roles of PAMs and septal macrophages in PRRS. The main function of PAMs is to provide the first line of phagocytic defence against microbial invasion (Cheung et al., 2000, De Baere et al., 2012). Septal macrophages, which mostly are activated indirectly by the replication of PRRSV in bystander cells, may modulate local inflammatory and immune responses through secretion of cytokines (Gómez-Laguna et al., 2010b). Sialoadhesin, but not CD163, downregulates phagocytosis in PRRSV-infected PAMs (De Baere et al., 2012).
Modulation of the immune response by PRRSV and secondary respiratory infections
PRRSV increases the susceptibility of the host to a wide range of viral and bacterial respiratory pathogens, resulting in increased severity of clinical signs and lung lesions. Concomitant infections with PRRSV and swine influenza virus, porcine respiratory coronavirus (PRCV) or PCV2 are common. More severe clinical signs and growth retardation occur in co-infected pigs than in pigs infected with PRRSV alone (Van Reeth et al., 1996, Harms et al., 2001, Jung et al., 2009, Renukaradhya et al., 2010, Opriessnig et al., 2011). In pigs dually infected with PRRSV and PRCV, exacerbation of clinical signs is associated with an impaired innate immune response in the lungs, specifically a decrease in natural killer (NK) cell-mediated cytotoxicity due to decreased expression of interferon (IFN)-α. The adaptive immune response is also impaired, leading to enhanced apoptosis of PAMs as a consequence of increased concentrations of interleukin (IL)-6 and IL-10 (Jung et al., 2009, Renukaradhya et al., 2010).
Modulation of the innate immune response in PRRSV-infected pigs under field conditions is associated with a decrease in cytotoxicity, but not the percentage, of NK cells (Dwivedi et al., 2012). Functional modulation of NK cells is associated with increased plasma concentrations of IL-4, IL-10 and IL-12, suggesting a role for these cytokines in modulating the host immune response (Dwivedi et al., 2012). Regulatory T cells (Tregs) induced by PRRSV-2, but not PRRSV-1, impair the host immune response (Cecere et al., 2012, Silva-Campa et al., 2009, Silva-Campa et al., 2010, Silva-Campa et al., 2012). Although PRRSV-2 increases the percentage of Tregs, its effect on the immune response in vivo is not yet clear.
The susceptibility of PRRSV-infected pigs to secondary bacterial pathogens has been linked to upregulation of CD14 and lipopolysaccharide (LPS)-binding protein (LBP), which cooperate in the recognition and signalling of LPS. Enhanced expression of these molecules in the lungs increases susceptibility to secondary bacterial infection. Although there is upregulation of CD14 in PRRSV-infected septal macrophages and PAMs, most CD14 enhancement in the lungs of PRRSV-infected pigs is probably due to infiltration of CD14-positive monocytes into the pulmonary interstitium; these may later differentiate into septal macrophages (Van Gucht et al., 2005, Qiao et al., 2011). PRRSV and bacterial endotoxin act synergistically to amplify the inflammatory response of infected macrophages (Qiao et al., 2011). Higher expression of pro-inflammatory cytokines is observed in septal macrophages of PRRSV-infected pigs (Gómez-Laguna et al., 2010b). These findings suggest a role for septal macrophages in the immunopathogenesis of secondary bacterial infections in PRRS through upregulation of CD14 and activation of a cytokine cascade (Fig. 2).
Fig. 2.
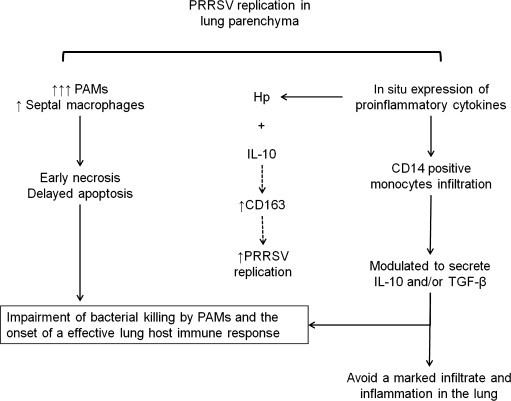
Proposed immunopathogenesis of porcine reproductive and respiratory syndrome virus (PRRSV) and secondary bacterial infection in the lung. PAMs, pulmonary alveolar macrophages; Hp, haptoglobin; IL-10, interleukin-10; TGF-β, transforming growth factor-β.
Acute phase proteins in PRRS
Acute phase proteins (APPs) are involved in the acute phase response and are synthesised mainly by hepatocytes in response to pro-inflammatory cytokines (Eckersall, 2000, Petersen et al., 2004). APPs can be classified as ‘positive’ or ‘negative’, depending on whether their serum concentrations are increased or decreased, respectively, in the acute phase response. Haptoglobin (Hp), C-reactive protein (CRP), serum amyloid A (SAA) and ‘pig-major acute protein’ (Pig-MAP) are the main APPs in pigs (Petersen et al., 2004). APPs induce pro-inflammatory reactions and fever, but overexpression can also induce an anti-inflammatory state (Ceciliani et al., 2002, Petersen et al., 2004).
A lack of significant changes in serum concentrations of pro-inflammatory cytokines in PRRS may point to a strategy by which PRRSV is able to escape from the host immune response, thereby preventing activation of hepatocytes to induce an efficient acute phase response. Early expression of Hp and Pig-MAP is observed in PRRS, whereas serum concentrations of CRP and SAA exhibit a delayed and variable enhancement (Gómez-Laguna et al., 2010c). CRP is involved in complement activation and opsonisation, as well as inducing production of cytokines by macrophages (Ceciliani et al., 2002, Petersen et al., 2004). SAA is chemotactic for monocytes, T cells and polymorphonuclear leucocytes (Ceciliani et al., 2002, Petersen et al., 2004). The late increase in CRP and SAA may contribute to the inefficient early immune response in PRRSV-infected pigs.
Hp modulates the immune response through complex interactions with a range of effectors. In experimental infections with PRRSV, there is an increase in serum concentrations of Hp, along with increased expression of IL-6 and tumour necrosis factor (TNF)-α (Asai et al., 1999, Gómez-Laguna et al., 2010c). Hp interacts with CD163, the receptor for PRRSV, increasing expression of the anti-inflammatory cytokine IL-10 (Díaz et al., 2005). CD163 removes haemoglobin–Hp complexes circulating in the blood (Kristiansen et al., 2001), decreasing the amount of haemoglobin available for bacterial pathogens and reducing oxidative stress. Hp may play a role in the pathogenesis of PRRS through modulating the immune response and inducing anti-inflammatory cytokines, such as IL-10.
Samples of saliva and meat juice are alternatives to serum for measuring and monitoring APPs during PRRSV infection in pigs (Gutiérrez et al., 2009, Gómez-Laguna et al., 2010a). Pen-based oral-fluid samples also represent a reliable, cost-effective approach to PRRSV surveillance in pigs (Prickett et al., 2008, Kittawornrat et al., 2010).
Secretion of pro-inflammatory cytokines in PRRS
In contrast to other viral respiratory diseases of pigs (Carrasco et al., 2002, Khatri et al., 2010), production of pro-inflammatory cytokines is limited in PRRS (Van Reeth et al., 1999). Low expression of pro-inflammatory cytokines, both at mRNA and protein levels, has been reported in pigs infected with PRRSV-1 and PRRSV-2 (Thanawongnuwech et al., 2001, Gómez-Laguna et al., 2010c, Renukaradhya et al., 2010, García-Nicolás et al., 2011). This may be related to the typically mild respiratory and systemic clinical signs in experimental PRRSV infections, in which absence of fever is correlated with development of more severe interstitial pneumonia (Van Reeth et al., 1999, Van Gucht et al., 2003, Gómez-Laguna et al., 2009). There appears to be a correlation between the virulence of the PRRSV strain, the severity of clinical signs and the expression of pro-inflammatory cytokines; in pigs infected with HP-PRRSV, marked inappetence and severe respiratory signs are related to severe interstitial pneumonia and high levels of expression of IL-1α in the lungs in comparison with other classical PRRSV strains (Amarilla et al., 2012, Hu et al., 2012). Table 1 summarises the main pro-inflammatory cytokines that play a role in PRRS.
Table 1.
Cytokines examined in porcine reproductive and respiratory syndrome (PRRS) and their role in the immunopathogenesis of the disease.
| Cytokine | Role in PRRS | References |
|---|---|---|
| IL-1 | Chemotaxis of monocytes and neutrophils | Thanawongnuwech et al. (2001) |
| Early upregulation in BALF and in the lung parenchyma | Labarque et al. (2003) | |
| Poor and delayed enhancement in serum | Van Gucht et al. (2003) | |
| Gómez-Laguna et al., 2010b, Gómez-Laguna et al., 2010c | ||
| Renukaradhya et al. (2010) | ||
| García-Nicolás et al. (2011) | ||
| IL-6 | Induction of acute phase proteins | Asai et al. (1999) |
| Upregulation in situ in the lung parenchyma | Gómez-Laguna et al. (2010b) | |
| TNF-α | Inhibition of PRRSV replication | López-Fuertes et al. (2000) |
| Induction of acute phase proteins | Gómez-Laguna et al. (2010b) | |
| Downregulated in PRRSV-infected PAMs | Albina et al. (1998) | |
| IFN-α | Correlation between virus specific IFNα-SCs and IFNγ-SCs | Van Reeth et al. (1999) |
| Interference with PRRSV replication | Royaee et al. (2004) | |
| Downregulation in PRRSV-infected PAMs and/or PBMCs | ||
| IFN-γ | Inhibition PRRSV replication | Bautista and Molitor, 1999 |
| Enhancement by PRRSV vaccination with IL-12 or IFN-α | Foss et al. (2002) | |
| Delayed onset of PRRSV specific IFNγ-SCs | Meier et al. (2003) | |
| Higher expression with highly virulent PRRSV strains | Thanawongnuwech et al. (2003) | |
| Díaz et al., 2005, Díaz et al., 2006 | ||
| IL-10 | Correlation with the expression of PRRSV in the lung | Díaz et al. (2006) |
| Inhibition of the expression of IFN-γ and other cytokines | Gómez-Laguna et al. (2010b) | |
| TGF-β | Induction of regulatory T cells after PRRSV-2 infection | Silva-Campa et al. (2009) |
| Correlation with the expression of PRRSV in the lung | Gómez-Laguna et al. (2012a) | |
IL, interleukin; BALF, bronchoalveolar lavage fluid; TNF, tumour necrosis factor; PRRSV, porcine reproductive and respiratory syndrome virus; PAMs, porcine alveolar macrophages; IFN, interferon; SC, secreting cells; PBMCs, peripheral blood mononuclear cells; TGF, transforming growth factor; PRRSV-2, porcine reproductive and respiratory syndrome virus type 2.
In studies of other respiratory diseases of pigs, efficient activation of the inflammatory response occurs only locally in the lung, whereas there are no substantial alterations in serum concentrations of pro-inflammatory cytokines (Baarsch et al., 1995, Conn et al., 1995). Increases in expression of IL-1α, IL-6 and TNF-α in the lungs of pigs infected with PRRSV-1 are correlated with the development of interstitial pneumonia (Gómez-Laguna et al., 2010b). As discussed above, PRRSV impairs the host immune response (Darwich et al., 2010). Expression of PRRSV antigens is correlated with expression of regulatory cytokines, such as IL-10 and transforming growth factor (TGF)-β, in the lungs of pigs (Gómez-Laguna et al., 2010b, Gómez-Laguna et al., 2012a; Table 1). The lack of substantial changes in serum concentrations of pro-inflammatory cytokines may reflect a strategy by which PRRSV evades the host immune response. Therefore, samples of blood and/or serum do not necessarily reflect the events occurring in lungs of PRRSV-infected animals at a given time, although these are very accessible samples.
Differential expression of pro-inflammatory cytokines has been observed in lymphoid organs of PRRSV-infected pigs. Whereas a marked increase in expression of TNF-α and IL-1α was observed in the mediastinal lymph nodes, there was a limited increase in expression of these cytokines in the tonsils and retropharyngeal lymph nodes (Barranco et al., 2012).
Pro- and anti-inflammatory responses in PRRS
PRRSV isolates have been classified on the basis of whether they induce TNF-α, IL-10, both of these cytokines or neither of these cytokines (Gimeno et al., 2011). TNF-α plays an important role in the inflammatory response; this cytokine may act as an antiviral cytokine, protecting cells from infection against viruses or enhancing selective elimination of virus-infected cells through an IFN-independent mechanism. Recombinant porcine TNF-α inhibits PRRSV replication in vitro and PRRSV-infected PAMs have reduced expression of TNF-α (López-Fuertes et al., 2000). Several PRRSV strains are weak inducers of TNF-α and also impair TNF-α production by inhibiting the extracellular signal-regulated kinase (ERK) signalling pathway; this mechanism may contribute to the virulence of HP-PRRSV (Hou et al., 2012). Promoter and post-transcriptional downregulation of TNF-α has been associated with PRRSV non-structural proteins (NSPs) 1α, 1β and 2 (Chen et al., 2010, Subramaniam et al., 2010, Subramaniam et al., 2012, Darwich et al., 2011). Specific amino acid residues of NSP1α and NSP1β have been identified that affect TNF-α promoter activity; substitution of specific NSP1β amino acids is potential tool for the generation of attenuated PRRSV vaccines (Subramaniam et al., 2012). The limited expression of TNF-α in experimental infections with some PRRSV strains may be a mechanism by which these strains impair the host immune response and prevent viral clearance (Table 1; Fig. 1 ).
Fig. 1.

Immune regulation associated with the expression and/or inhibition of tumour necrosis factor (TNF)-α and interleukin (IL)-10 during porcine reproductive and respiratory syndrome virus (PRRSV) infection.
IL-10 is a regulatory cytokine that, among other functions, inhibits the synthesis of pro-inflammatory cytokines (Moore et al., 2001). A role for IL-10 in inhibiting or counterbalancing the IFN-γ response and restricting PRRSV replication has been proposed in infections with some PRRSV strains (Bautista and Molitor, 1999, Díaz et al., 2006). Thus, in the same way that IL-10 inhibits the production of IFN-γ, it may also suppress the pro-inflammatory response in PRRSV-infected pigs (Table 1; Fig. 1).
A different behaviour of regulatory cytokines, such as IL-10 or TGF-β, has been observed in PRRSV-infected pigs. There is a significant correlation between PRRSV antigen expression and the expression of regulatory cytokines, such IL-10 and TGF-β, in the lungs, but not in the lymphoid organs, of infected pigs (Barranco et al., 2012, Gómez-Laguna et al., 2010b, Gómez-Laguna et al., 2012a). One hypothesis suggested by these findings is that cells recruited to the pulmonary parenchyma are modulated to secrete IL-10 and/or TGF-β, among other immune mediators. An alternative hypothesis is that the expression of regulatory cytokines plays a key role in the modulation of the inflammatory response within the lung, reducing infiltration and proliferation of inflammatory cells (Spight et al., 2005, Charavaryamath et al., 2006, Backus et al., 2010).
IL-10 is expressed mainly by septal macrophages and, to a lesser extent, PAMs and other cells, whereas TGF-β is expressed mainly in PAMs and secondarily in septal macrophages (Gómez-Laguna et al., 2010b, Gómez-Laguna et al., 2012a). Expression of regulatory cytokines by different subsets of lung macrophages may impair bacterial killing by PAMs and PIMs, as well reduce the efficiency of the local host immune response by septal macrophages. Production of TGF-β occurs in recall responses to some PRRSV-1 strains and is more marked after homologous reinfection, suggesting the presence of TGF-β producing T cell clones dependent on the PRRSV strain (Díaz et al., 2012).
The imbalance between pro- and anti-inflammatory cytokines may modulate the expression of CD163, which is one component of a complex of receptors required for PRRSV entry, including heparan sulphate and sialoadhesin (Delputte et al., 2005, Van Gorp et al., 2008). Expression of CD163 is upregulated by IL-10 and IL-6, promoting PRRSV entry and replication, but downregulated by TNF-α, TGF-β and IFN-γ (Buechler et al., 2000, Sulahian et al., 2000, Pioli et al., 2004, Weaver et al., 2007, Patton et al., 2009). Other mediators may regulate expression of CD163 (Table 2 ). There is differential expression of cytokines by different PRRSV isolates (Gimeno et al., 2011) and within different lymphoid organs (Barranco et al., 2012), indicating that analysis of the expression of pro- and anti-inflammatory cytokines is a useful tool in the study of the immunopathogenesis of different PRRSV strains.
Table 2.
Mediators involved in regulation of CD163 receptor.
| Mediator | Upregulation | Downregulation | References |
|---|---|---|---|
| IL-6 | + | − | Buechler et al. (2000) |
| IL-10 | + | − | Buechler et al. (2000) |
| Sulahian et al. (2000) | |||
| Weaver et al. 2007 | |||
| Patton et al. (2009) | |||
| M-CSF | + | − | Ritter et al. (1999) |
| Hb–Hp complexes | + | − | Ugocsai et al. (2006) |
| Dexamethasone | + | − | Ritter et al. (1999) |
| TNF-α | − | + | Buechler et al. (2000) |
| IFN-γ | − | + | Buechler et al. (2000) |
| Weaver et al. (2007) | |||
| TGF-β | − | + | Pioli et al. (2004) |
| GM-CSF+IL-4 | − | + | Ritter et al. (1999) |
| Lipopolysaccharide | − | + | Buechler et al. (2000) |
| Patton et al. (2009) | |||
| 12-O-tetradecanoylphorbol-13-acetate | − | + | Patton et al. (2009) |
IL, interleukin; M-CSF, macrophage colony-stimulating factor; HB–Hp, haemoglobin–haptoglobin; TNF, tumour necrosis factor; IFN, interferon; TGF, transforming growth factor; GM-CSF, granulocyte macrophage colony-stimulating factor.
Conclusions
PRRSV impairs local immune responses in the lungs of pigs by a range of mechanisms, including impairment of the mucociliary transport system, decreases in the function and number of PAMs, induction of apoptosis of immune cells and creating an imbalance between pro- and anti-inflammatory cytokines, which may allow the virus to persist in the host. PRRSV decreases the bactericidal activity of macrophages, resulting in increased susceptibility to secondary infections. The variability in cytokine profiles induced by different PRRSV strains, as well as the emergence of HP-PRRSV in Asia and Eastern Europe, highlight the need to evaluate differences in immunobiology among PRRSV strains. PRRSV is able to modulate the immune response by increasing the expression of Hp in the early stages of infection, which may interact with the viral receptor CD163 and induce the synthesis of anti-inflammatory mediators, such as IL-10. There is a need for improved knowledge of the immune response in PRRSV infections in order to improve the efficacy of vaccines to control PRRS.
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Acknowledgement
This work was financially supported by the Spanish Ministry of Education and Science (Project Number AGL2009-12438/GAN).
References
- Albina E., Carrat C., Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. Journal of Interferon and Cytokine Research. 1998;18:485–490. doi: 10.1089/jir.1998.18.485. [DOI] [PubMed] [Google Scholar]
- Allan G.M., McNeilly F., Ellis J., Krakowka S., Meehan B., McNair I., Walker I., Kennedy S. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Archives of Virology. 2000;145:2421–2429. doi: 10.1007/s007050070031. [DOI] [PubMed] [Google Scholar]
- Amarilla, P., Morgan, S., Gómez-Laguna, J., Graham, S., Frossard, J.P., Steinbach, F., Carrasco, L., Salguero, F.J., 2012. A virulent Eastern European PRRSV strain induces high levels of IL-1α in the lung. In: Proceeding of the 30th Meeting of the European Society of Veterinary Pathology, León, Spain, 5–8 September 2012, p. 76.
- Asai T., Mori M., Okada M., Uruno K., Yazawa S., Shibata I. Elevated serum haptoglobin in pigs infected with porcine reproductive and respiratory syndrome virus. Veterinary Immunology and Immunopathology. 1999;70:143–148. doi: 10.1016/s0165-2427(99)00069-0. [DOI] [PubMed] [Google Scholar]
- Baarsch M.J., Scamurra R.W., Burger K., Foss D.L., Maheswaran S.K., Murtaugh M.P. Inflammatory cytokine expression in swine experimentally infected with Actinobacillus pleuropneumoniae. Infection and Immunity. 1995;63:3587–3594. doi: 10.1128/iai.63.9.3587-3594.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus G.S., Howden R., Fostel J., Bauer A.K., Cho H.Y., Marzec J., Peden D.B., Kleeberger S.R. Protective role of interleukin-10 in ozone-induced pulmonary inflammation. Environmental Health Perspectives. 2010;118:1721–1727. doi: 10.1289/ehp.1002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barranco I., Gómez-Laguna J., Rodríguez-Gómez I.M., Salguero F.J., Pallarés F.J., Carrasco L. Differential expression of proinflammatory cytokines in the lymphoid organs of porcine reproductive and respiratory syndrome virus-infected pigs. Transboundary and Emerging Diseases. 2012;59:145–153. doi: 10.1111/j.1865-1682.2011.01252.x. [DOI] [PubMed] [Google Scholar]
- Bautista E.M., Molitor T.W. IFN gamma inhibits porcine reproductive and respiratory syndrome virus replication in macrophages. Archives of Virology. 1999;144:1191–1200. doi: 10.1007/s007050050578. [DOI] [PubMed] [Google Scholar]
- Bertram T.A., Overby L.H., Danilowicz R., Eling T.E., Brody A.R. Pulmonary intravascular macrophages produce prostaglandins and leukotrienes in vitro. Chest. 1988;93:82S–84S. doi: 10.1378/chest.93.3_supplement.82s. [DOI] [PubMed] [Google Scholar]
- Brockmeier S.L., Palmer M.V., Bolin S.R. Effects of intranasal inoculation of porcine reproductive and respiratory syndrome virus, Bordetella bronchiseptica, or a combination of both organisms in pigs. American Journal of Veterinary Research. 2000;61:892–899. doi: 10.2460/ajvr.2000.61.892. [DOI] [PubMed] [Google Scholar]
- Buechler C., Ritter M., Orso E., Langmann T., Klucken J., Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. Journal of Leukocyte Biology. 2000;67:97–103. [PubMed] [Google Scholar]
- Carrasco L., Núñez A., Salguero F.J., Díaz San Segundo F., Sánchez-Cordón P., Gómez-Villamandos J.C., Sierra M.A. African swine fever: Expression of interleukin-1 alpha and tumour necrosis factor-alpha by pulmonary intravascular macrophages. Journal of Comparative Pathology. 2002;126:194–201. doi: 10.1053/jcpa.2001.0543. [DOI] [PubMed] [Google Scholar]
- Cecere T.E., Meng X.J., Pelzer K., Todd S.M., Beach N.M., Ni Y.Y., Leroith T. Co-infection of porcine dendritic cells with porcine circovirus type 2a (PCV2a) and genotype II porcine reproductive and respiratory syndrome virus (PRRSV) induces CD4+ CD25+ FoxP3+ T cells in vitro. Veterinary Microbiology. 2012;160:233–239. doi: 10.1016/j.vetmic.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceciliani F., Giordano A., Spagnolo V. The systemic reaction during inflammation: The acute-phase proteins. Protein and Peptide Letters. 2002;9:211–223. doi: 10.2174/0929866023408779. [DOI] [PubMed] [Google Scholar]
- Chang H.C., Peng Y.T., Chang H.L., Chaung H.C., Chung W.B. Phenotypic and functional modulation of bone marrow-derived dendritic cells by porcine reproductive and respiratory syndrome virus. Veterinary Microbiology. 2008;129:281–293. doi: 10.1016/j.vetmic.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Charavaryamath C., Janardhan K.S., Caldwell S., Singh B. Pulmonary intravascular monocytes/macrophages in a rat model of sepsis. The Anatomical Record. Part A, Discoveries in Molecular, Cellular and Evolutionary Biology. 2006;288:1259–1271. doi: 10.1002/ar.a.20401. [DOI] [PubMed] [Google Scholar]
- Chen Z., Zhou X., Lunney J.K., Lawson S., Sun Z., Brown E., Christopher- Hennings J., Knudsen D., Nelson E., Fang Y. Immunodominant epitopes in NSP2 of porcine reproductive and respiratory syndrome virus are dispensable for replication, but play an important role in modulation of the host immune response. Journal of General Virology. 2010;91:1047–1057. doi: 10.1099/vir.0.016212-0. [DOI] [PubMed] [Google Scholar]
- Cheung D.O., Halsey K., Speert D.P. Role of pulmonary alveolar macrophages in defense of the lung against Pseudomonas aeruginosa. Infection and Immunity. 2000;68:4585–4592. doi: 10.1128/iai.68.8.4585-4592.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitko-McKown C.G., Chapes S.K., Brown R.E., Philips R.M., McKown R.D., Blecha F. Porcine alveolar and pulmonary intravascular macrophages: Comparison of immune functions. Journal of Leukocyte Biology. 1991;50:364–372. doi: 10.1002/jlb.50.4.364. [DOI] [PubMed] [Google Scholar]
- Conn C.A., McClellan J.L., Maassab H.F., Smitka C.W., Majde J.A., Kluger M.J. Cytokines and the acute phase response to influenza virus in mice. American Journal of Physiology. 1995;268:R78–R84. doi: 10.1152/ajpregu.1995.268.1.R78. [DOI] [PubMed] [Google Scholar]
- Costers S., Lefebvre D.J., Delputte P.L., Nauwynck H.J. Porcine reproductive and respiratory syndrome virus modulates apoptosis during replication in alveolar macrophages. Archives of Virology. 2008;153:1453–1465. doi: 10.1007/s00705-008-0135-5. [DOI] [PubMed] [Google Scholar]
- Darwich L., Díaz I., Mateu E. Certainties, doubts and hypotheses in porcine reproductive and respiratory syndrome virus immunobiology. Virus Research. 2010;154:123–132. doi: 10.1016/j.virusres.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Darwich L., Gimeno M., Sibila M., Díaz I., de la Torre E., Dotti S., Kuzemtseva L., Martin M., Pujols J., Mateu E. Genetic and immunobiological diversities of porcine reproductive and respiratory syndrome genotype I strains. Veterinary Microbiology. 2011;150:49–62. doi: 10.1016/j.vetmic.2011.01.008. [DOI] [PubMed] [Google Scholar]
- De Baere M.I., Van Gorp H., Delputte P.L., Nauwynck H.J. Interaction of the European genotype porcine reproductive and respiratory syndrome virus (PRRSV) with sialoadhesin (CD169/Siglec-1) inhibits alveolar macrophage phagocytosis. Veterinary Research. 2012;43:47. doi: 10.1186/1297-9716-43-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delputte P.L., Costers S., Nauwynck H.J. Analysis of porcine reproductive and respiratory syndrome virus attachment and internalization: Distinctive roles for heparan sulphate and sialoadhesin. Journal of General Virology. 2005;86:1441–1445. doi: 10.1099/vir.0.80675-0. [DOI] [PubMed] [Google Scholar]
- Díaz I., Darwich L., Pappaterra G., Pujols J., Mateu E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology. 2006;351:249–259. doi: 10.1016/j.virol.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Díaz I., Darwich L., Pappaterra G., Pujols J., Mateu E. Immune responses of pigs after experimental infection with a European strain of porcine reproductive and respiratory syndrome virus. Journal of General Virology. 2005;86:1943–1951. doi: 10.1099/vir.0.80959-0. [DOI] [PubMed] [Google Scholar]
- Díaz I., Gimeno M., Darwich L., Navarro N., Kuzemtseva L., López S., Galindo I., Segalés J., Martín M., Pujols J., Mateu E. Characterization of homologous and heterologous adaptive immune responses in porcine reproductive and respiratory syndrome virus infection. Veterinary Research. 2012;43:30. doi: 10.1186/1297-9716-43-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Done S.H., Paton D.J. Porcine reproductive and respiratory syndrome: Clinical disease, pathology and immunosuppression. Veterinary Record. 1995;136:32–35. doi: 10.1136/vr.136.2.32. [DOI] [PubMed] [Google Scholar]
- Drew T.W. A review of evidence for immunosuppression due to porcine reproductive and respiratory syndrome virus. Veterinary Research. 2000;31:27–39. doi: 10.1051/vetres:2000106. [DOI] [PubMed] [Google Scholar]
- Duan X., Nauwynck H.J., Pensaert M.B. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV) Veterinary Microbiology. 1997;56:9–19. doi: 10.1016/S0378-1135(96)01347-8. [DOI] [PubMed] [Google Scholar]
- Dwivedi V., Manickam C., Binjawadagi B., Linhares D., Murtaugh M.P., Renukaradhya G.J. Evaluation of immune responses to porcine reproductive and respiratory syndrome virus in pigs during early stage of infection under farm conditions. Virology Journal. 2012;9:45. doi: 10.1186/1743-422X-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckersall P.D. Recent advances and future prospects for the use of acute phase proteins as markers of disease in animals. Revue Médecine Vétérinaire. 2000;151:577–584. [Google Scholar]
- Feng Y., Zhao T., Nguyen T., Inui K., Ma Y., Nguyen T.H., Nguyen V.C., Liu D., Bui Q.A., To L.T., Wang C., Tian K., Gao G.F. Porcine respiratory and reproductive syndrome virus variants, Vietnam and China, 2007. Emerging Infectious Diseases. 2008;14:1774–1776. doi: 10.3201/eid1411.071676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Mendoza L., Silva-Campa E., Reséndiz M., Osorio F.A., Hernández J. Porcine reproductive and respiratory syndrome virus infects mature porcine dendritic cells and up-regulates interleukin-10 production. Clinical and Vaccine Immunology. 2008;15:720–725. doi: 10.1128/CVI.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss D.L., Zilliox M.J., Meier W., Zuckermann F., Murtaugh M.P. Adjuvant danger signals increase the immune response to porcine reproductive and respiratory syndrome virus. Viral Immunology. 2002;15:557–566. doi: 10.1089/088282402320914502. [DOI] [PubMed] [Google Scholar]
- García-Nicolás, O., Quereda, J.J., Gómez-Laguna, J., Barranco, I., Rodríguez-Gómez, I.M., Ramis, G., Pallarés, F.J., Muñoz, A., Carrasco, L., 2011. Comparison of pro-inflammatory cytokines gene expression and protein levels in tonsil and serum of PRRSV-infected pigs. In: Proceedings of the 6th Symposium on Emerging and Re-emerging Pig Diseases, Barcelona, Spain, 12–15 June 2011, pp. 200.
- Geldhof M.F., Vanhee M., Van Breedam W., Van Doorsselaere J., Karniychuk U.U., Nauwynck H.J. Comparison of the efficacy of autogenous inactivated porcine reproductive and respiratory syndrome virus (PRRSV) vaccines with that of commercial vaccines against homologous and heterologous challenges. BMC Veterinary Research. 2012;8:182. doi: 10.1186/1746-6148-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno M., Darwich L., Díaz I., de la Torre E., Pujols J., Martín M., Inumaru S., Cano E., Domingo M., Montoya M., Mateu E. Cytokine profiles and phenotype regulation of antigen presenting cells by genotype-I porcine reproductive and respiratory syndrome virus isolates. Veterinary Research. 2011;42:9. doi: 10.1186/1297-9716-42-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Laguna J., Gutiérrez A., Pallarés F.J., Salguero F.J., Cerón J.J., Carrasco L. Haptoglobin and C-reactive protein as biomarkers in the serum, saliva and meat juice of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. The Veterinary Journal. 2010;185:83–87. doi: 10.1016/j.tvjl.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Gómez-Laguna J., Rodríguez-Gómez I.M., Barranco I., Pallarés F.J., Salguero F.J., Carrasco L. Enhanced expression of TGFβ protein in lymphoid organs and lung, but not in serum, of pigs infected with a European field isolate of porcine reproductive and respiratory syndrome virus. Veterinary Microbiology. 2012;158:187–193. doi: 10.1016/j.vetmic.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Laguna J., Salguero F.J., Barranco I., Pallarés F.J., Rodríguez-Gómez I.M., Bernabé A., Carrasco L. Cytokine expression by macrophages in the lung of pigs infected with the porcine reproductive and respiratory syndrome virus. Journal of Comparative Pathology. 2010;142:51–60. doi: 10.1016/j.jcpa.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Laguna J., Salguero F.J., De Marco M.F., Pallarés F.J., Bernabé A., Carrasco L. Changes in lymphocyte subsets and cytokines during European porcine reproductive and respiratory syndrome: Increased expression of IL-12 and IL-10 and proliferation of CD4−CD8high. Viral Immunology. 2009;22:261–271. doi: 10.1089/vim.2009.0003. [DOI] [PubMed] [Google Scholar]
- Gómez-Laguna J., Salguero F.J., Fernández de Marco M., Barranco I., Rodríguez-Gómez I.M., Quezada M., Carrasco L. Type 2 porcine reproductive and respiratory syndrome virus infection mediated apoptosis in B- and T-cell areas in lymphoid organs of experimentally infected pigs. Transboundary and Emerging Diseases. 2012 doi: 10.1111/j.1865-1682.2012.01338.x. [DOI] [PubMed] [Google Scholar]
- Gómez-Laguna J., Salguero F.J., Pallarés F.J., Fernández de Marco M., Barranco I., Cerón J.J., Martínez-Subiela S., Van Reeth K., Carrasco L. Acute phase response in porcine reproductive and respiratory syndrome virus infection. Comparative Immunology, Microbiology and Infectious Disease. 2010;33:e51–e58. doi: 10.1016/j.cimid.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Gutiérrez A.M., Martínez-Subiela S., Soler L., Pallarés F.J., Cerón J.J. Use of saliva for haptoglobin and C-reactive protein quantifications in porcine respiratory and reproductive syndrome affected pigs in field conditions. Veterinary Immunology and Immunopathology. 2009;132:218–223. doi: 10.1016/j.vetimm.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Halbur P.G., Bush E. Update on abortion storms and sow mortality. Swine Health and Production. 1997;5:73. [Google Scholar]
- Halbur P.G., Paul P.S., Frey M.L., Landgraf J., Eernisse K., Meng X.J., Lum M.A., Andrews J.J., Rathje J.A. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Veterinary Pathology. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- Halbur P.G., Thanawongnuwech R., Brown G., Kinyon J., Roth J., Thacker E., Thacker B. Efficacy of antimicrobial treatments and vaccination regimens for control of porcine reproductive and respiratory syndrome virus and Streptococcus suis coinfection of nursery pigs. Journal of Clinical Microbiology. 2000;38:1156–1160. doi: 10.1128/jcm.38.3.1156-1160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms P.A., Sorden S.D., Halbur P.G., Bolin S.R., Lager K.M., Morozov I., Paul P.S. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Veterinary Pathology. 2001;38:528–539. doi: 10.1354/vp.38-5-528. [DOI] [PubMed] [Google Scholar]
- Hou J., Wang L., He W., Zhang H., Feng W.H. Highly pathogenic porcine reproductive and respiratory syndrome virus impairs LPS- and poly(I:C)-stimulated tumor necrosis factor-alpha release by inhibiting ERK signaling pathway. Virus Research. 2012;167:106–111. doi: 10.1016/j.virusres.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Hu S.P., Zhang Z., Liu Y.G., Tian Z.J., Wu D.L., Cai X.H., He X.J. Pathogenicity and distribution of highly pathogenic porcine reproductive and respiratory syndrome virus in pigs. Transboundary and Emerging Diseases. 2012 doi: 10.1111/j.1865-1682.2012.01354.x. [DOI] [PubMed] [Google Scholar]
- Jung K., Renukaradhya G.J., Alekseev K.P., Fang Y., Tang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: Implications for respiratory viral co-infections. Journal of General Virology. 2009;90:2713–2723. doi: 10.1099/vir.0.014001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniychuk U.U., Geldhof M., Vanhee M., Van Doorsselaere J., Saveleva T.A., Nauwynck H.J. Pathogenesis and antigenic characterization of a new East European subtype 3 porcine reproductive and respiratory syndrome virus isolate. BMC Veterinary Research. 2010;6:30. doi: 10.1186/1746-6148-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, A.M.Q., Adams, J.M., Carstens, E.B., Lefkowitz, E.J. (Eds.), 2012. 9th Report of the International Committee on Taxonomy of Viruses. Elsevier, Academic Press, London, UK, pp. 796–805.
- Khatri M., Dwivedi V., Krakowka S., Manickam C., Ali A., Wang L., Qin Z., Renukaradhya G.J., Lee C.W. Swine influenza H1N1 virus induces acute inflammatory immune responses in pig lungs: A potential animal model for human H1N1 influenza virus. Journal of Virology. 2010;84:11210–11218. doi: 10.1128/JVI.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittawornrat A., Prickett J., Chittick W., Wang C., Engle M., Johnson J., Patnayak D., Schwartz T., Whitney D., Olsen C., Schwartz K., Zimmerman J. Porcine reproductive and respiratory syndrome virus (PRRSV) in serum and oral fluid samples from individual boars: Will oral fluid replace serum for PRRSV surveillance? Virus Research. 2010;154:170–176. doi: 10.1016/j.virusres.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Kristiansen M., Graversen J.H., Jacobsen C., Sonne O., Hoffman H.J., Law S.K., Moestrup S.K. Identification of the hemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- Labarque G., Van Gucht S., Nauwynck H., Van Reeth K., Pensaert M. Apoptosis in the lungs of pigs infected with porcine reproductive and respiratory syndrome virus and associations with the production of apoptogenic cytokines. Veterinary Research. 2003;34:249–260. doi: 10.1051/vetres:2003001. [DOI] [PubMed] [Google Scholar]
- Longworth K.E. The comparative pathology of pulmonary intravascular macrophages. Frontiers in Bioscience. 1997;2:232–241. doi: 10.2741/a186. [DOI] [PubMed] [Google Scholar]
- López-Fuertes L., Campos E., Doménech N., Ezquerra A., Castro J.M., Domínguez J., Alonso F. Porcine reproductive and respiratory syndrome (PRRS) virus down-modulates TNF-α production in infected macrophages. Virus Research. 2000;69:41–46. doi: 10.1016/s0168-1702(00)00172-6. [DOI] [PubMed] [Google Scholar]
- Loving C.L., Brockmeier S.L., Sacco R.E. Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology. 2007;120:217–229. doi: 10.1111/j.1365-2567.2006.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Lobo F.J., Díez-Fuertes F., Segalés J., García-Artiga C., Simarro I., Castro J.M., Prieto C. Comparative pathogenicity of type 1 and type 2 isolates of porcine reproductive and respiratory syndrome virus (PRRSV) in a young pig infection model. Veterinary Microbiology. 2011;154:58–68. doi: 10.1016/j.vetmic.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Meier W., Galeota J., Osorio F., Husmann R.J., Schnitzlein W.M., Zuckermann F.A. Gradual development of the interferon-γ response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology. 2003;309:18–31. doi: 10.1016/s0042-6822(03)00009-6. [DOI] [PubMed] [Google Scholar]
- Moore K.W., de Waal Malefyt R., Coffman R.L., O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Opriessnig T., Giménez-Lirola L.G., Halbur P.G. Polymicrobial respiratory disease in pigs. Animal Health Research Reviews. 2011;12:133–148. doi: 10.1017/S1466252311000120. [DOI] [PubMed] [Google Scholar]
- Patton J.B., Rowland R.R., Yoo D., Chang K.O. Modulation of CD163 receptor expression and replication of porcine reproductive and respiratory syndrome virus in porcine macrophages. Virus Research. 2009;140:161–171. doi: 10.1016/j.virusres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Petersen H.H., Nielsen J.P., Heegaard P.M. Application of acute phase protein measurements in veterinary clinical chemistry. Veterinary Research. 2004;35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- Pioli P.A., Goonan K.E., Wardwell K., Guyre P.M. TGF-β regulation of human macrophage scavenger receptor CD163 is Smad3-dependent. Journal of Leukocyte Biology. 2004;76:500–508. doi: 10.1189/jlb.1203617. [DOI] [PubMed] [Google Scholar]
- Prickett J., Simer R., Christopher-Hennings J., Yoon K.J., Evans R.B., Zimmerman J.J. Detection of porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: A longitudinal study under experimental conditions. Journal of Veterinary Diagnostic Investigation. 2008;20:156–163. doi: 10.1177/104063870802000203. [DOI] [PubMed] [Google Scholar]
- Qiao S., Feng L., Bao D., Guo J., Wan B., Xiao Z., Yang S., Zhang G. Porcine reproductive and respiratory syndrome virus and bacterial endotoxin act in synergy to amplify the inflammatory response of infected macrophages. Veterinary Microbiology. 2011;149:213–220. doi: 10.1016/j.vetmic.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Renukaradhya G.J., Alekseev K., Jung K., Fang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus-induced immunosuppression exacerbates the inflammatory response to porcine respiratory coronavirus in pigs. Viral Immunology. 2010;23:457–466. doi: 10.1089/vim.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter M., Buechler C., Langmann T., Orso E., Klucken J., Schmitz G. The scavenger receptor CD163: regulation, promoter structure and genomic organization. Pathobiology. 1999;67:257–261. doi: 10.1159/000028105. [DOI] [PubMed] [Google Scholar]
- Rossow K.D. Porcine reproductive and respiratory syndrome. Veterinary Pathology. 1998;35:1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- Rovira A., Balasch M., Segalés J., García L., Plana-Durán J., Rosell C., Ellerbrok H., Mankertz A., Domingo M. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. Journal of Virology. 2002;76:3232–3239. doi: 10.1128/JVI.76.7.3232-3239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royaee A.R., Husmann R.J., Dawson H.D., Calzada-Nova G., Schnitzlein W.M., Zuckermann F.A., Lunney J.K. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Veterinary Immunology and Immunopathology. 2004;102:199–216. doi: 10.1016/j.vetimm.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalés J., Calsamiglia M., Rosell C., Soler M., Maldonado J., Martín M., Domingo M. Porcine reproductive and respiratory syndrome virus (PRRSV) infection status in pigs naturally affected with post-weaning multisystemic wasting syndrome (PMWS) in Spain. Veterinary Microbiology. 2002;85:23–30. doi: 10.1016/s0378-1135(01)00474-6. [DOI] [PubMed] [Google Scholar]
- Silva-Campa E., Cordoba L., Fraile L., Flores-Mendoza L., Montoya M., Hernández J. European genotype of porcine reproductive and respiratory syndrome (PRRSV) infects monocyte-derived dendritic cells but does not induce Treg cells. Virology. 2010;396:264–271. doi: 10.1016/j.virol.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Silva-Campa E., Mata-Haro V., Mateu E., Hernández J. Porcine reproductive and respiratory syndrome virus induces CD4+CD8+CD25+Foxp3+ regulatory T cells (Tregs) Virology. 2012;430:73–80. doi: 10.1016/j.virol.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Silva-Campa E., Flores-Mendoza L., Reséndiz M., Pinelli-Saavedra A., Mata-Haro V., Mwangi W., Hernández J. Induction of T helper 3 regulatory cells by dendritic cells infected with porcine reproductive and respiratory syndrome virus. Virology. 2009;387:373–379. doi: 10.1016/j.virol.2009.02.033. [DOI] [PubMed] [Google Scholar]
- Spight D., Zhao B., Haas M., Wert S., Denenberg A., Shanley T.P. Immunoregulatory effects of regulated, lung-targeted expression of IL-10 in vivo. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2005;288:L251–L265. doi: 10.1152/ajplung.00122.2004. [DOI] [PubMed] [Google Scholar]
- Stadejek T., Oleksiewicz M.B., Potapchuk D., Podgorska K. Porcine reproductive and respiratory syndrome virus strains of exceptional diversity in Eastern Europe support the definition of new genetic subtypes. Journal of General Virology. 2006;87:1835–1841. doi: 10.1099/vir.0.81782-0. [DOI] [PubMed] [Google Scholar]
- Stadejek T., Oleksiewicz M.B., Scherbakov A.V., Timina A.M., Krabbe J.S., Chabros K., Potapchuk D. Definition of subtypes in the European genotype of porcine reproductive and respiratory syndrome virus: Nucleocapsid characteristics and geographical distribution in Europe. Archives of Virology. 2008;153:1479–1488. doi: 10.1007/s00705-008-0146-2. [DOI] [PubMed] [Google Scholar]
- Staub N.C. Pulmonary intravascular macrophages. Annual Review of Physiology. 1994;56:47–67. doi: 10.1146/annurev.ph.56.030194.000403. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Beura L.K., Kwon B., Pattnaik A.K., Osorio F.A. Amino acid residues in the non-structural protein 1 of porcine reproductive and respiratory syndrome virus involved in down-regulation of TNF-α expression in vitro and attenuation in vivo. Virology. 2012;432:241–249. doi: 10.1016/j.virol.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Kwon B., Beura L.K., Kuszynski C.A., Pattnaik A.K., Osorio F.A. Porcine reproductive and respiratory syndrome virus non-structural protein 1 suppresses tumor necrosis factor-alpha promoter activation by inhibiting NF-κB and Sp1. Virology. 2010;406:270–279. doi: 10.1016/j.virol.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Sulahian T.H., Hogger P., Wahner A.E., Wardwell K., Goulding N.J., Sorg C., Droste A., Stehling M., Wallace P.K., Morganelli P.M., Guyre P.M. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–1321. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- Thacker E.L., Halbur P.G., Ross R.F., Thanawongnuwech R., Thacker B.J. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. Journal of Clinical Microbiology. 1999;37:620–627. doi: 10.1128/jcm.37.3.620-627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanawongnuwech R., Rungsipipat A., Disatian S., Saiyasombat R., Napakanaporn S., Halbur P.G. Immunohistochemical staining of IFN-γ positive cells in porcine reproductive and respiratory syndrome virus-infected lungs. Veterinary Immunology and Immunopathology. 2003;91:73–77. doi: 10.1016/s0165-2427(02)00268-4. [DOI] [PubMed] [Google Scholar]
- Thanawongnuwech R., Young T.F., Thacker B.J., Thacker E.L. Differential production of proinflammatory cytokines: In vitro PRRSV and Mycoplasma hyopneumoniae co-infection model. Veterinary Immunology and Immunopathology. 2001;79:115–127. doi: 10.1016/s0165-2427(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Thanawongnuwech R., Thacker E.L., Halbur P.G. Effect of porcine reproductive and respiratory syndrome virus (PRRSV) (isolate ATCC VR-2385) infection on bactericidal activity of porcine pulmonary intravascular macrophages (PIMs): In vitro comparisons with pulmonary alveolar macrophages (PAMs) Veterinary Immunology and Immunopathology. 1997;59:323–335. doi: 10.1016/s0165-2427(97)00078-0. [DOI] [PubMed] [Google Scholar]
- Tian K., Yu X., Zhao T., Feng Y., Cao Z., Wang C., Hu Y., Chen X., Hu D., Tian X., Liu D., Zhang S., Deng X., Ding Y., Yang L., Zhang Y., Xiao H., Qiao M., Wang B., Hou L., Wang X., Yang X., Kang L., Sun M., Jin P., Wang S., Kitamura Y., Yan J., Gao G.F. Emergence of fatal PRRSV variants: Unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS ONE. 2007;2:e526. doi: 10.1371/journal.pone.0000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugocsai P., Barlage S., Dada A., Schmitz G. Regulation of surface CD163 expression and cellular effects of receptor mediated hemoglobin-haptoglobin uptake on human monocytes and macrophages. Cytometry A. 2006;69:203–205. doi: 10.1002/cyto.a.20235. [DOI] [PubMed] [Google Scholar]
- Van Gorp H., Van Breedam W., Delputte P.L., Nauwynck H.J. Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. Journal of General Virology. 2008;89:2943–2953. doi: 10.1099/vir.0.2008/005009-0. [DOI] [PubMed] [Google Scholar]
- Van Gucht S., Van Reeth K., Pensaert M. Interaction between porcine reproductive-respiratory syndrome virus and bacterial endotoxin in the lungs of pigs: Potentiation of cytokine production and respiratory disease. Journal of Clinical Microbiology. 2003;41:960–966. doi: 10.1128/JCM.41.3.960-966.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gucht S., Van Reeth K., Nauwynck H., Pensaert M. Porcine reproductive and respiratory syndrome virus infection increases CD14 expression and lipopolysaccharide-binding protein in the lungs of pigs. Viral Immunology. 2005;18:116–126. doi: 10.1089/vim.2005.18.116. [DOI] [PubMed] [Google Scholar]
- Van Reeth K., Labarque G., Nauwynck H., Pensaert M. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: Correlations with pathogenicity. Research in Veterinary Science. 1999;67:47–52. doi: 10.1053/rvsc.1998.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K., Nauwynck H., Pensaert M. Dual infections of feeder pigs with porcine reproductive and respiratory syndrome virus followed by porcine respiratory coronavirus or swine influenza virus: A clinical and virological study. Veterinary Microbiology. 1996;48:325–335. doi: 10.1016/0378-1135(95)00145-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Eaton M., Mayer M., Li H., He D., Nelson E., Christopher-Hennings J. Porcine reproductive and respiratory syndrome virus productively infects monocyte-derived dendritic cells and compromises their antigen-presenting ability. Archives of Virology. 2007;152:289–303. doi: 10.1007/s00705-006-0857-1. [DOI] [PubMed] [Google Scholar]
- Weaver L.K., Pioli P.A., Wardwell K., Vogel S.N., Guyre P.M. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. Journal of Leukocyte Biology. 2007;81:663–671. doi: 10.1189/jlb.0706428. [DOI] [PubMed] [Google Scholar]
- Wills R.W., Doster A.R., Galeota J.A., Sur J.H., Osorio F.A. Duration of infection and proportion of pigs persistently infected with porcine reproductive and respiratory syndrome virus. Journal of Clinical Microbiology. 2003;41:58–62. doi: 10.1128/JCM.41.1.58-62.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills R.W., Gray J.T., Fedorka-Cray P.J., Yoon K.J., Ladely S., Zimmerman J.J. Synergism between porcine reproductive and respiratory syndrome virus (PRRSV) and Salmonella choleraesuis in swine. Veterinary Microbiology. 2000;71:177–192. doi: 10.1016/S0378-1135(99)00175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler G.C., Cheville N.F. Postnatal colonization of porcine lung capillaries by intravascular macrophages: An ultrastructural, morphometric analysis. Microvascular Research. 1987;33:224–232. doi: 10.1016/0026-2862(87)90019-7. [DOI] [PubMed] [Google Scholar]
- Xiao S., Mo D., Wang Q., Jia J., Qin L., Yu X., Niu Y., Zhao X., Liu X., Chen Y. Aberrant host immune response induced by highly virulent PRRSV identified by digital gene expression tag profiling. BMC Genomics. 2010;11:544. doi: 10.1186/1471-2164-11-544. [DOI] [PMC free article] [PubMed] [Google Scholar]


