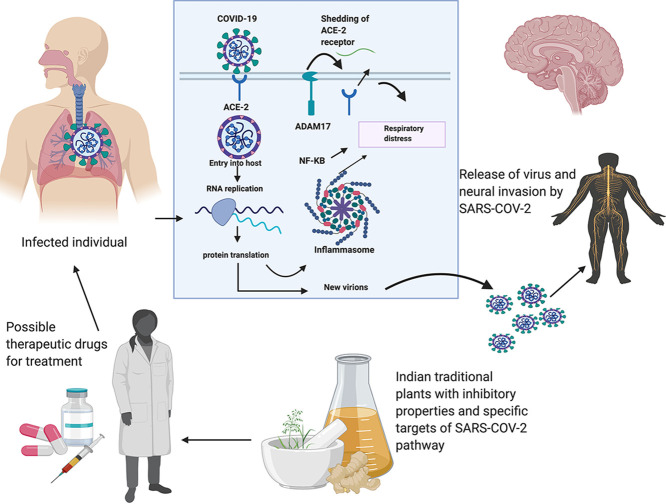
Abstract
The novel Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2, which is the causative agent of a potentially fatal disease that is of great global public health concern. The outbreak of COVID-19 is wreaking havoc worldwide due to inadequate risk assessment regarding the urgency of the situation. The COVID-19 pandemic has entered a dangerous new phase. When compared with SARS and MERS, COVID-19 has spread more rapidly, due to increased globalization and adaptation of the virus in every environment. Slowing the spread of the COVID-19 cases will significantly reduce the strain on the healthcare system of the country by limiting the number of people who are severely sick by COVID-19 and need hospital care. Hence, the recent outburst of COVID-19 highlights an urgent need for therapeutics targeting SARS-CoV-2. Here, we have discussed the structure of virus; varying symptoms among COVID-19, SARS, MERS and common flu; the probable mechanism behind the infection and its immune response. Further, the current treatment options, drugs available, ongoing trials and recent diagnostics for COVID-19 have been discussed. We suggest traditional Indian medicinal plants as possible novel therapeutic approaches, exclusively targeting SARS-CoV-2 and its pathways.
Abbreviations: ACE2, angiotensin-converting enzyme 2; ACE2-Fc, Angiotensin Converting Enzyme 2 Fc; ADAM17, ADAM metallopeptidase domain 17; ARDS, acute respiratory distress syndrome; ASC, Apoptosis-associated speck-like protein containing a CARD; CNS, Central Nervous System; COVID-19, coronavirus disease 2019; ER, endoplasmic reticulum; ExoN, exoribonuclease; FDA, Food and Drug Administration; FP, internal fusion protein; HCoV, Human coronavirus; HIV, human immunodeficiency virus; JAK-STAT, Janus kinase/signal transducer and activator of transcription; JNK, c-Jun N- terminal kinase; MCP-1, Monocyte chemoattractant protein-1; MERS, Middle East respiratory syndrome; MERS-CoV, Middle East respiratory syndrome coronavirus; MHV, mouse hepatitis virus; mRNA, messenger RNA; NF-kB, Nuclear Factor kappa-light-chain-enhancer of activated B-cells; NIH, National Institutes of Health; NLRP3, Nod-like receptor protein 3; ORF, open reading frame; PHEIC, Public Health Emergency of International Concern; PHEV, Porcine Hemagglutinating Encephalomyelitis Virus; RBD, receptor binding domain; RBM, receptor binding motif; RCT, randomized controlled treatment; RdRp, RNA dependent RNA polymerase; RNA, Ribonucleic acid; ROS, Reactive oxygen species; RTC, replicase-transcriptase complex; SARS, Severe acute respiratory syndrome; SARS-COV-2, Severe acute respiratory syndrome coronavirus-2; TM, transmembrane; TMPRSS11a, Transmembrane serine protease 11a; TNFβ, Tumor necrosis factor β; TRAF3, TNF receptor associated factor 3; TRS, transcriptional regulatory sequence; WHO, World Health Organization
Keywords: Coronavirus disease 2019 (COVID-19), SARS-CoV-2, Mechanism of action, Therapeutic approach, Indian traditional medicine
Graphical abstract
1. Introduction
The novel coronavirus disease 2019 (COVID-19), caused by the Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is in the midst of worldwide panic and global health concern since December 2019. As of March 26th, 2020, the World Health Organization (WHO) has reported that 416,686 and 18,589 death cases have been confirmed worldwide, and it has spread to 197 countries (WHO, 2020a). With this emerging battle against this deadly virus, the WHO has strategized to interrupt human-human contact, isolate patients at early stages, identify and reduce transmission from the animal source, address crucial mysteries about the virus and accelerate research, communicate information correctly to the public and minimize the social and economic impact. At this juncture, it is tremendously vital to understand the basic mechanism of the virus to develop specific drugs. Currently, it has been established that SARS-CoV-2 shares sequence homology with the SARS-CoV and a bat coronavirus (Gorbalenya, 2020). Despite its similarity to SARS-CoV, its transmission efficiency and diagnostic methods are rather different. The distinguishing factor is probably the nucleotide changes in the spike (S) protein and its receptor-binding domain (RBD) (Kannan et al., 2020; Coutard et al., 2020; Wan et al., 2020). Currently, the treatments include Lopinavir/Ritonavir and supportive care, as this is primarily dependent on the severity of the illness. From a research standpoint, various drugs are being developed at an extremely quick pace and new targets are being identified every day, and also numerous drugs are also undergoing clinical trials. Researches are very curious about how to provide the best protection to the public before a vaccine can be made available (Balachandar et al., 2020). Indian medicinal herbs are a promising field for treatment of various illnesses (Gomathi et al., 2020). Ayurveda and Siddha practices originated in India and are still widely used among the Indian population. By identifying certain phytocompounds, it is possible to effectively characterize medicinal herbs that could help to alleviate the infection. Hence, by repurposing the Indian medicinal plants, more innovative treatment options can be penned down for their role in defeating this viral transmission. At a time of worldwide anxiety, it is imperative to find long term solutions to prevent the transmission of such pandemics. So, it's time for all the citizens to join hands together to fight against coronavirus by practicing self-hygiene and social distancing (Balachandar et al., 2020). In this review, the structure, immunological influence, mechanism of action of the SARS-CoV-2 infection in the human host cell, the availability of disease-specific drugs, ongoing clinical trials, recent diagnostics and the potential use of certain Indian medicinal herbs for the effective treatment of COVID-19 has been discussed. Through this review, we suggest that the Indian traditional medicinal herbs may be a beneficial step to combat viruses like the SARS-CoV-2.
2. A brief overview of coronavirus
Coronaviruses, having a total of 39 species under the broad realm of Riboviria, belong to the family Coronaviridae, suborder Cornidovirineae and order Nidovirales (Gorbalenya et al., 2020). All the SARS-CoV fall under the species Severe acute respiratory syndrome-related coronavirus and genus Beta-coronavirus. Most of the species under this head are enzootic and only a few of these species infect humans (Schoeman and Fielding, 2019). Currently, seven human CoVs (HCoVs) have been confirmed. Specifically, they are named as Human coronavirus NL63 (HCoV-NL63) and Human coronavirus 229E (HCoV-229E), which belong to the alpha-coronavirus genus; whereas Human coronavirus OC43 (HCoV-OC43), Human coronavirus (HCoV-HKU1), SARS-CoV, SARS-CoV-2 and Middle East respiratory syndrome coronavirus (MERS-CoV), belong to the beta-coronavirus genus. HCoV-229E, HCoV-NL63, HCoV-HKU1 and HCoV-OC43 strains of coronavirus cause mild respiratory diseases in humans. The SARS-CoV-2 is a zoonotic virus that belongs to the Coronaviridae family that can infect human and several animal species (Lu et al., 2020). The SARS-CoV-2 belongs to the subgenus Sarbecovirus and mostly resembles a bat coronavirus, with which it shares 96.2% sequence homology (Chan et al., 2020a). Currently, it is thought that SARS-CoV-2 has been introduced to human by an unidentified intermediary animal and then it has spread from human-to-human.
Human coronaviruses are predominantly concomitant with upper respiratory tract illnesses ranging from mild to moderate including common cold. Most of the people may be infected with one or more of these viruses at some point in their lifetime (Killerby et al., 2018). The SARS-CoV and MERS-CoV are the two major causes of severe pneumonia in human (Song et al., 2019). A comparative analysis of the symptoms among COVID-19, SARS, MERS and common flu has been explained (Table.1 ). The world observed the sudden emergence of COVID-19 in 2019. The exact origin of the virus, continues to remain as a mystery, to researchers worldwide. Investigations need to be carried out to pinpoint the exact source of infection. The WHO, on February 11, 2020, officially named the viral disease COVID-19 (Jiang et al., 2020; Guarner, 2020). The Coronavirus Study Group of the International Committee on Taxonomy of Viruses named the new pathogen as SARS-CoV-2 (Gorbalenya, 2020). The predecessor SARS-CoV first emerged in 2002. During its course of infection from 2002 to 2003, 774 deaths were recorded out of the 8000+ infections spread across 37 countries (Peiris et al., 2004). This was closely followed by the emergence of MERS-CoV at Saudi Arabia in 2012, which caused 858 deaths among the 2494 known infected cases (Zaki et al., 2012). Similar to its antecedents, the SARS-CoV-2 appeared in December 2019 from the animal kingdom and spread to human populations. The COVID-19 is known to show symptoms slowly over an incubation period of around 2 weeks. During this time the virus replicates in the upper and lower respiratory tract, forming lesions (Chan et al., 2020b). The general symptoms observed in the infected individuals are fever, cough, dyspnoea and lesion in the lungs (Huang et al., 2020). In the advanced stage, the symptoms of this virus show pneumonia which progresses to severe pneumonia and acute respiratory distress syndrome (ARDS) which results in to the need for life-support to sustain the patient's life (Heymann and Shindo, 2020).
Table 1.
Symptomatic comparison of COVID-19, SARS, MERS and Common.
| Diseases | Symptoms | Onset of disease | Incubation period | Recovery | Transmission of disease | Complications if any | Treatments if available |
|---|---|---|---|---|---|---|---|
| Novel Coronavirus (COVID-19) | Fever Cough Shortness of Breath Fatigue |
Sudden | 2–14 days after exposure | 2–8 weeks | Human to Human | Acute pneumonia, septic shock, respiratory failure in adverse condition. | No vaccines available, only symptoms can be treated. |
| Severe Acute Respiratory Syndrome (SARS) | Fever Dry Cough Headache Difficulty in breathing Muscle aches Loss of appetite Diarrhoea |
Sudden | 2–7 days after exposure | 5–6 weeks | Human to Human | Heart, Liver and Respiratory failure in adverse condition. | Breathing ventilator to deliver oxygen. Pneumonia treating antibiotics Antiviral medicines Steroids to reduce lung swelling |
| Middle East Respiratory Syndrome (MERS) | Fever Chills Diarrhoea Nausea Vomiting Congestion Sneezing Sore throat |
Sudden | 5–6 days after exposure | 6–7 weeks | Human to Human | Acute pneumonia Kidney failure in adverse condition. | Treatment only for symptoms such as Fluids replacement Oxygen therapy. |
| Common flu | Runny or Stuffy nose Sneezing Sore throat Mild Headache Low grade fever |
Gradual | 2–3 days after exposure | 7–10 days | Human to Human | Extremely rare or None | Symptoms can be treated by medication. |
This table represents the parallel investigation of Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), Novel Coronavirus (COVID-19) and Common Flu with their Symptoms, Onset of Disease, Incubation Period, Recovery, Transmission of Disease, Complications and available treatments.
3. Structural assembly of SARS-CoV-2 virus
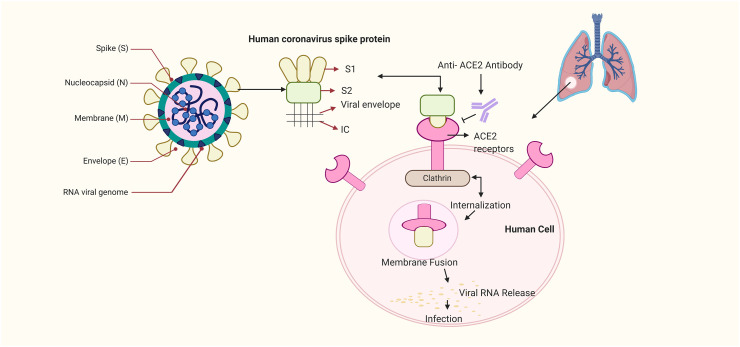
The SARS-CoV-2 belongs to the largest family of the RNA viruses and its genome ranges from 27 to 32 kilobases in size (~125 nm or 0.125 μm). It is a single stranded enveloped RNA virus which possess a positive-sense RNA genome also known as (+ssRNA) with a 5′-cap structure and 3′-poly-A tail (Chen, 2020). The viruses belonging to this category, have a few common characteristics that are applicable to SARS-CoV-2 as well. The virus has four important structural proteins which are (E) the envelope protein (M) the membrane protein (S) the spike protein and (N) the nucleocapsid protein, which are required to regulate the function and viral structure (Schoeman and Fielding, 2019). Among these four proteins the most important ones are N and S, where the former one helps the virus to develop the capsid and the entire viral structure appropriately and the later one helps in the attachment of virus to the host cells (Siu et al., 2008; Walls et al., 2020). The S protein has three major sections which are, the large ectodomain, a single-pass transmembrane anchor and a short intracellular tail. These play a major role in anchoring the host cells. Among these sections the ectodomain has two subunits which are, the S1 receptor-binding subunit and S2 the membrane fusion subunit. These subunits are in the clove-trimeric or crown structure which is the reason coronavirus (corona = crown) got its name (Zumla et al., 2016).
It has been reported that the SARS-CoV and SARS-CoV-2 have similar kind of receptors, especially the receptor binding domain (RBD) and the receptor binding motif (RBM) in the viral genome (Yin and Wunderink, 2018; Zhang et al., 2020; Tai et al., 2020). During the SARS infection, the RBM of the S protein gets directly attached to the Angiotension-Converting Enzyme 2 (ACE2) in the human or the host cells (Phan, 2020). The ACE2 protein is expressed in various organs of the human body mainly in the lungs, kidney and intestine, the prime targets of the coronavirus (Zhao et al., 2020). The ACE1 and ACE2 have gained recognition as significant regulators of the physiology and pathology of the reproductive system (Pan et al., 2013). Although, due to the novel nature of the virus, no study has proven that it will reduce men's fertility or sexual potency but medics in Wuhan have suggested the likelihood that the disease can affect the production of sperm leading to low sperm count and the formation of male sex hormones (low libido). In addition, SARS-CoV-2 infects host cell through ACE2 receptors leading to COVID-19 related pneumonia, while also causing acute myocardial injury and chronic damage to the cardiovascular system (Zheng et al., 2020).
Interestingly, it has also been proposed that SARS-CoV-2 mechanism of action in infection of humans is similar to the SARS. It has been reported that the RBM of the SARS-CoV-2 has a major amino acid residue (Gln493) that favours the attachment and fusion of the viral S protein with virus into the ACE2 protein of the human cell especially the one present in the lungs which results in respiratory infections in humans (Zhao et al., 2020; Yin and Wunderink, 2018). An illustration about the structure and binding of S protein to ACE2 has been depicted (Fig. 1 ). The simplest and most direct approach to combat SARS-CoV-2 would be to neutralize the virus from entering cells as this has been utilized in previous viruses of its kind (Walker and Burton, 2018). The key advantage here is the host ACE2 protein does not change, so there is no fear about advantageous mutations that may hinder drug development (Karakus et al., 2020). These findings suggest that an in-depth knowledge about the receptors and its targets and basis of viral replication would be a stepping stone to find a remedy for the SARS-CoV-2 infection.
Fig. 1.
Structure and binding of COVID-19 virus to ACE2. The above-mentioned figure depicts the structure of the COVID-19 virus. Among the viral structure the S protein has a major role in binding of the virus to the host receptor cells. S protein has two subunits which are the S1 receptor-binding subunit and S2 the membrane fusion subunit; where the earlier one attached itself to the ACE2 receptor of the human host cell and the S2 subunit internalises and creates the membrane fusion among the viral subunit and the ACE2 receptors. This leads to the release of the viral RNA into the host cell and results into respiratory infection.
4. Replication of SARS-CoV-2
After the SARS-CoV-2 virus has entered the human host cells, the next step for its survival is its RNA replication. The viral RNA replication is the most unusual and critical step carried out by the virus for its survival inside the host body. The tools that are required for the process of replication are open reading frames (ORFs), two replicase genes (rep1a and rep1ab), a slippery sequence (5′-UUUAAAC-3′) and two polyproteins (pp1a and pp1ab). Both these polyproteins contain the most important proteins of the virus that are the Nsp proteins (Nsp1–11and Nsp1–16), these proteins are a common occurrence in these virus types (Baranov et al., 2005). Recently, it has been found that, the Nsp 15 protein not only has a vital role in replication but also attacks the immune system of the host during viral replication (Youngchang et al., 2020). Further these Nsp proteins (Nsp1/2, Nsp2/3 and Nsp3/4) assemble to form the replicase-transcriptase complex (RTC) which creates an environment inside the host body suitable for RNA synthesis and replication. Also, these Nsps have various roles in RNA replication of the virus. Nsp12 codes for the RNA-dependent RNA polymerase (RdRP) domain, Nsp13 is encrypted with RNA helicase domain and RNA 5′-triphosphase, Nsp14 encodes exoribonuclease (ExoN) which helps in replication conformity and finally Nsp16 encodes 2′-Omethyltransferase activity. These evidences prove that Nsp protein has a vital role in keeping the virus alive inside the host body by promoting basic synthesis, replication and translation.
The process of replication in the SARS-CoV-2 similar to SARS-CoV virus is multifaceted and needs more understanding (Fehr and Perlman, 2015; Zhang et al., 2020). For replication, the genomic RNA contains a 5′ end region that has the untranslated leader (L) sequence with the transcription regulation sequence (TRS) present at the descending region of the genome (Brian and Baric, 2005). The replicase gene encoded enzymes uses the negative RNA genome as a template to develop a few sets of small, overlapping messenger RNA (mRNA) molecules that further gets translated into the structural proteins viz, (N, M, E and S protein) also known as the building block for the production of new viral particles inside the host body, while the positive stranded RNA genome is used as a template to produce the negative strand. During the replication process inside the human host, the N protein of the virus binds to the genome while the M protein is associated with the membranes of the endoplasmic reticulum (ER). Further with the help of Nsp proteins the RNA gets assembled into a helical twisted structure and buds into the ER lumen. Viral progenies are transferred to the cell membranes by the Golgi bodies and exocytosed into the extracellular space of the human host cell environment. These mechanisms were discovered in the preceding viruses and may have a pivotal role in SARS-CoV-2 as well (Brian and Baric, 2005; de Haan and Rottier, 2005). From the replication process of the SARS-CoV-2 it is evident that targeting Nsp proteins could enable us to develop a strategy to overcome this viral infection. Other than replication, other pathways associated with the virus can also be targeted for drug development.
5. SARS-CoV-2- proposed mechanism
SARS-CoV-2 shares homology with the SARS-CoV but the rate of transmission and infectivity of the SARS-CoV-2 has been remarkable; this accelerated spreading rate may be due to a gain of function mutation, making this novel virus different from the SARS-CoV virus. These changes found in SARS-CoV-2 include, an absent 8a, longer 8b and shorter 3b segments and different Nsp 2 and 3 proteins (Wu et al., 2020a, Wu et al., 2020b; Xu et al., 2020a, Xu et al., 2020b). Nsp 2 of SARS-CoV-2 consists of mutation that is probably associated with the ability of the virus to be more contagious (Angeletti et al., 2020). In addition, the orf8 and orf10 proteins are also different in SARS-CoV-2. It may be beneficial to understand the biological function of these proteins. Further, it has been found that more pathogenic viruses contain a furin like cleavage site in the S protein, which is not present in SARS-CoV but present in the SARS-CoV-2 (Coutard et al., 2020). This may be the reason for increased virulence of SARS-CoV-2. Moreover, SARS-CoV-2 binds the same receptor as SARS-CoV, namely, ACE2 with much higher strength; this could be the reason for the increased transmission rate and its capacity to affect other species with such ease. The S protein has S1 on its N terminal and S2 at its C terminal, and the RBD is present at the S1 region. The S2 domain of the S protein consists of the fusion protein, a second proteolytic site (S2′), followed by an internal fusion peptide (FP) and two heptad-repeat domains preceding the transmembrane domain (TM) and internal FP is identical between SARS-CoV-2 and SARS-CoV (Coutard et al., 2020). From previous studies it was suggested that SARS-CoV-2 might have a similar mechanism as like SARS-CoV to enter the host cell.
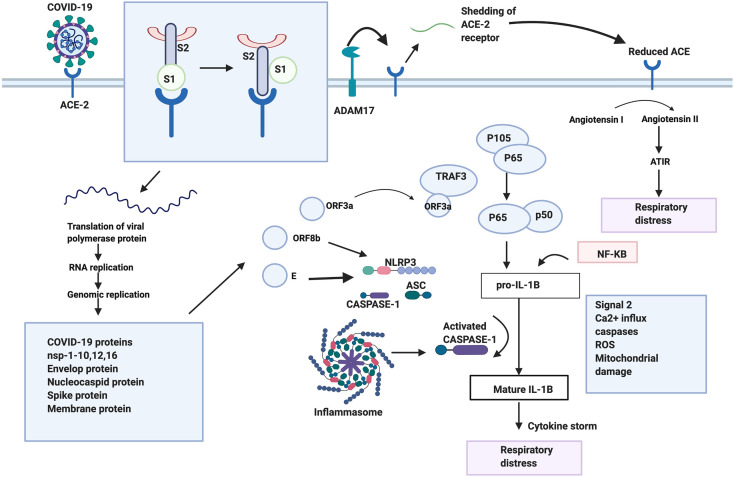
The SARS-CoV-2 like other beta-coronaviruses undergoes a few steps to enter into and affect the host cell. SARS-CoV-2 binds to same ACE2 receptor present in the respiratory epithelium and alveoli of the lungs (Liu et al., 2020a, Liu et al., 2020b). In SARS-CoV, upon binding to the receptor, proteases are recruited to cleave the S protein into S1 and S2 domains. This cleavage induces a conformational change that activates S2, this is followed by the insertion of the FP into the membrane and membrane fusion occurs facilitating the entry of the virus into the cell. Since the nucleotides are conserved in RBD binding motif that is associated with ACE2, it is possible that SARS-CoV-2 utilizes the same mechanism as well. Once the virus enters the cell, ACE2 gets cleaved and shed by ADAM17 into the extra membrane space. Reduced ACE2 has been known to be concomitant with alveoli injury and increases pulmonary vascular permeability (Li and Clercq, 2020). This could be due to the conversion of angiotensin I to angiotensin II by ACE2, which is a negative regulator of the renin-angiotensin pathway. Angiotensin II stimulated ATIR results in the lung pathology associated with respiratory distress (Li and Clercq, 2020). Once the virus translates its proteins in the cell, the ORF3a protein is produced and codes for a Ca2+ ion channel that is similar to SARS-CoV and SARS-CoV-2. It interacts with TRAF3 and activates the transcription of the NF-kB pathway, resulting in the transcription of the pro-IL-1B gene (Siu et al., 2019), ORF3a along with TRAF3 also recruits the inflammasome complex. This complex consists of NLRP3, ASC and caspase 1. A second signal such as Ca2+ influx, caspases activation, ROS production and mitochondrial damage converts pro-IL-1B to IL-B and results in cytokine production. Another, ORF8b protein also activates the inflammasome pathway through NLRP3, and this protein is longer in SARS-CoV-2 (Shi et al., 2019). The extra nucleotides present in this virus need to be further studied to figure out if that has caused an added advantage. The E protein forming an ion channel, is also conserved in the two viruses and is involved in the overproduction of cytokines through the NLRP3 inflammasome pathway (Nieto-Torres et al., 2015). All these pathways combined together cause a cytokine storm resulting in respiratory distress a common symptom of COVID-19. Another pathway involved in SARS-CoV includes the JNK pathway; which is activated by ORF3a, ORF3b and ORF7a which may lead to an increased production of pro-inflammatory factors, escalating lung damage (Liu et al., 2014). The JNK pathway can also be considered as a target for SARS-CoV-2 as it also involves the proteins that are analogous in both viruses.
During the infection of the virus, the most important part is the interaction with the host cell nucleases. It is possible that SARS-CoV-2 may use proteases similar to SARS-CoV such as TMPRSS11a, Trypsin, Plasmin, Cathepsin L and Furin in the cleavage of the spike protein for the virus to enter the cell. These proteases can be used as targets to reduce the symptoms of COVID-19 as proteasomal inhibitors used for HIV treatment are being used in treatment of COVID-19 (Fig. 2 ). A target for the COVID-19 may be advantageous to understand the involvement of the immune system in COVID-19, to explore the possibility of developing specific vaccines for it, as elucidated for previous viruses (Simmons et al., 2013).
Fig. 2.
Possible mechanism of action of SARS-COV-2. Depiction of the binding of SARS-COV-2 to its receptor ACE-2. The S1 and S2 subunits are subsequently cleaved followed by the shedding of ACE-2 by ADAM 17. This resulting in an increased amount of Angiotensin II leading to respiratory distress. Upon binding, the virus fuses with the membrane and enters the cell, followed by translation, and replication of the proteins. ORF3a, ORF8b,E proteins and the NF-KB pathway activates the inflammasome pathway through various means, leading to the activation of cytokine. This results in a cytokine storm, further resulting in respiratory distress.
6. SARS-CoV-2 and the immune system
The HCoVs generally are very long (30,000 bp) positive-sense single-stranded RNA viruses. Two groups of protein characterize HCoVs; the structural proteins, and non-structural proteins such as RNA dependent RNA polymerase (RdRp) (nsp12) (Elfiky, 2020). Coronaviruses such as SARS and MERS are particularly adept at evading immune detection and dampening immune responses. It's not yet clear how SARS-CoV-2 affects the immune system. During viral infection, host factors elicits immune response against the viruses. T cells, particularly CD4+ and CD8+ play a significant antiviral role to combat the pathogens and elevate the risk of developing autoimmunity/inflammation (Cecere et al., 2012). The CD4 + T cells advance the production of viral-specific antibodies by activating T cell-dependent B cells. However, CD8+ T cells are cytotoxic and kill virus infected cells. The CD8+ T cells account for about 80% of total inflammatory cells in the pulmonary interstitium in SARS-CoV infected patients and play a critical role in clearing coronaviruses in infected cells and inducing immune injury (Maloir et al., 2018). In addition, T helper cells make proinflammatory cytokines via NF-kB signaling (Manni et al., 2014). The cytokines, IL-17 recruit monocytes and neutrophils to the infection site showing inflammation and activates other downstream cascades of cytokines and chemokines, including IL-1, IL-6, IL-8, IL-21, TNF-β, and MCP-1 (Bunte and Beikler, 2019). It was observed that, T cell apoptosis was induced by a novel BH3-like region located in the C-terminal cytosolic domain of SARS-CoV protein mediated by Bcl-xL (Yang et al., 2005). From the experimental evidences it was shown that T cell response to S protein and other structural proteins (including the M and N proteins) is long-lasting, persistent and provides evidence for designing new drugs and vaccines for SARS-CoV-2 composed of viral structural proteins, which can induce dominant, effective, and long-term memory cell responses against the virus. However, earlier studies have also reported a crucial role of both CD8+ and CD4+ T cells in SARS-CoV clearance (Chen et al., 2010), while Janice Oh et al. (2012) also observed that development of SARS-CoV specific neutralizing antibodies requires CD4+ T helper cells. Moreover, the ACE2 protein fused to a human immunoglobulin G Fc domain (ACE2-Fc) of SARS-CoV-2 patients may have the benefits of a traditional neutralizing antibody which could be used as a treatment for the infection. Ultimately, there will be a need for clinical trials to delineate any specific side effects of ACE2-Fc treatment (Kruse, 2020). Therefore ACE2-Fc might play an important role in the treatment of SARS-CoV-2, if the function of ACE2-Fc is inhibited (Kruse, 2020). These immunological studies show how crucial it is to understand the basics of the immune responses in these viruses, so these immune cells can be induced to further attack the virus with increased specificity. Besides the immune system, scientists have also found a possible involvement of the COVID-19 in the nervous system.
7. Neuroinvasion of HCoVs
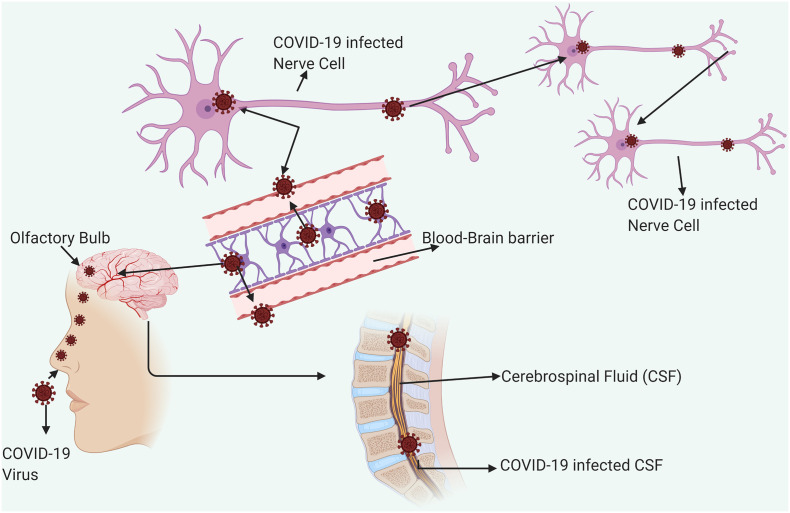
The COVID-19 are not always confined to the respiratory tract, but they also invade the Central Nervous System (CNS) to induce neurological diseases. Coronaviruses with such potential are the beta-coronaviruses, including SARS-CoV (Glass et al., 2004), MERS-CoV (Li et al., 2016), HCoV-229E (Talbot et al., 1994), HCoV-OC43 (Dubé et al., 2018), mouse hepatitis virus (MHV) (Zhou et al., 2017), and Porcine Hemagglutinating Encephalomyelitis Virus (PHEV) (Mengeling et al., 1972). According to previous study, coronaviruses may initially invade peripheral nerves and enter the CNS via the synaptic route, where this trans-synaptic transfer has been documented in HEV67 and avian bronchitis virus (Matsuda et al., 2004). The first coronavirus found to invade the porcine brain was HEV 67 N, and it shares >91% homology with HCoV-OC43 (Li et al., 2016). Therefore, the neuroinvasive propensity has been demonstrated as a common feature of coronaviruses. Since there is a high similarity between SARS-CoV and SARS-CoV-2, it is quite likely that SARS-CoV-2 may also possess an analogous potential. Based on an epidemiological survey, the first symptom is dyspnea which occurs in 5 days, followed by hospital admission at 7 days, and intensive care at 8 days for COVID-19 (Wang et al., 2020a, Wang et al., 2020b, Wang et al., 2020c). This latency period is enough for the virus to enter and destroy the medullary neurons. A possible mechanism about the entry of SARS-CoV-2 inside the CNS has been illustrated (Fig. 3 ). Similarly, Matthew, 2020stated that the symptoms might attribute to respiratory disease is due to the inability of air to get into the lungs, that might actually be the defects in respiration controlled by the nervous system. It has been reported that some COVID-19 patients showed neurologic signs, including headache (about 8%), nausea and vomiting (1%). As the neuroinvasion of SARS-CoV-2 is accompanied by respiratory failure in COVID-19 patients, the entry of the virus into the CNS must be prevented. As an emerging virus, awareness of the possible entry of SARS-CoV-2 into the CNS is significant for prevention and treatment. It is also important to find effective antiviral drugs that can cross the blood-brain barrier (Li et al., 2020). Therefore, more innovative approaches are required to detect this viral infection at an earlier period.
Fig. 3.
COVID-19 entry into CNS. Entry of human Coronavirus in CNS through olfactory bulb upon nasal infection which causes inflammation and demyelination. Further it reaches the whole brain via Blood Brain Barrier and CSF via Blood- CSF barrier in <7 days. The possible entry of SARS-CoV-2 into the Brain and CNS is important to design effective antiviral drugs. Effective drugs that may cross Blood Brain Barrier and Blood CSF barrier may be taken in to consideration while designing and this could be a promising in treatment strategies.
8. Recent diagnostic techniques
During the SARS and MERS outbreaks effective diagnostic tools were developed for accurate detection. Although, useful at that time, it is now essential to develop specific tests for COVID-19. The viral nucleic acid detection is primarily used in SARS-CoV-2 diagnosis (Wang et al., 2020a, Wang et al., 2020b, Wang et al., 2020c). CDC has recommended the collection of upper respiratory nasopharyngeal (NP) swabs for the diagnostic tests (CDC, 2020). The CDC detection assay targets the N region and consists of one test for beta-coronaviruses and two unique probes for SARS-CoV-2. The Charité algorithm comprises of probes for E protein and RA-dependent RNA polymerase (RdRp). Once both are positive, the sample is again tested against specific SARS-CoV-2 RdRp (Loeffelholz and Tang, 2020). Contrastingly, the E protein with RdRp was also detecting SARS-CoV, and so, these assays can be used to test for the SARS-CoV-2 when there are no traces of SARS-CoV (Cordes and Heim, 2020). When the commercially available Real Star kit, Virus + Rox Vial kit and Super Script III One-step RT-PCR System with Platinum TaqDNA Polymerase were compared for their efficiency, the RealStar Kit did not have any unwanted signals and exceeded the other two in its performance (Konrad et al., 2020). These methods can also be compromised due to inadequate sample volume, inaccuracies in methods of testing, not collecting samples at the appropriate time window, and contamination. Similar issues have been identified as potential problems that may diminish the precision of the tests (Lippi et al., 2020). Moreover, these tests are also expensive, hence cheaper alternatives have been developed to track the symptoms of COVID-19 using smart-phone surveillance (Dorigatti et al., 2020). Imaging techniques can also be utilized as a diagnostic method in COVID-19. Additionally, chest CT scans have been facilitated to detect lung abnormalities in this SARS-CoV-2 infection (Shi et al., 2020; Xu et al., 2020a, Xu et al., 2020b). Abnormalities in the CT scans can be concomitant with disease progression and prognosis. But, not all the cases can be perfectly detected with CT scans (Lei et al., 2020). Therefore, it is essential to conduct molecular tests and consider travel history and clinical symptoms of the patient as well. As there are an upsurge of infected people, more efficient, quicker and cheaper diagnostic tools must be developed to effectively identify infected individuals. Hence, the integrated approach of imaging and molecular diagnosis would help in screening and treating COVID-19 effectively. In order to design these specific drugs, it is important to understand the current strategies used to treat this novel COVID-19
9. Classification of pipeline drugs
Though the number of affected individuals is constantly on the rise, there are no FDA approved drugs for COVID-19 yet. At present, treatment provided to the affected individuals are mainly symptom based, and the seriously ill individuals are provided with organ support (Jin et al., 2020; Zumla et al., 2020). It is necessary to invest time and effort in identifying vaccines and drugs for this novel virus. Since the development of drugs specific for COVID-19 will take at least a few months drugs which have been proven to be safe for humans can be repurposed to treat this disease. The vast majority of the drugs used for treatment worldwide falls under any of the following classification of drugs.
9.1. Antiviral drugs
Drugs u4nder this category usually follow either of the following three mechanisms in the virus-viral replication inhibition, ion channel inhibition and serine protease inhibition. Commercially available antiviral drugs mostly target the four major groups of viruses: human immunodeficiency virus (HIV), herpes, hepatitis and influenza (Razonable, 2011). Earlier outbreak episodes of viral infections like SARS-CoV and MERS-CoV as well as hemorrhagic fever viruses like Ebola were treated with this category of drugs (De Clercq, 2007).
9.2. Antimalarial drugs
These drugs also fall under three categories based on their mode of action aryl amino-alcohol compound, antifolate compound and artemisinin. Most of these drugs are eliminated gradually from the body remaining for long periods of time after intake. A disadvantage of this drug is that antimalarial drug resistance develops for any drugs under this category (Edwin et al., 2019).
9.3. Anti-HIV drugs
These drugs are classified into different categories based on their targets reverse transcription, retro-transcription, proteolytic processing, viral-cell fusion, co-receptors interactions and incorporation of proviral DNA into the host genome. Drugs that fall in these categories have been approved by the FDA (Food and Drug Administration) and are now officially used for the treatment of HIV (De Clercq, 2009).
9.4. Anti-inflammatory drugs
Huge inflammatory response is observed in COVID-19. Anti-inflammatory drugs especially JAK-STAT inhibitors, used against rheumatoid arthritis, may be effective against elevated levels of cytokines and useful in inhibiting viral infection. According to recent study, an inflammatory drug, baricitinib when used in combination with anti-viral drugs like Remidesivir, increases the potential of the drug to reduce viral infection (Stebbing et al., 2020).
9.5. Monoclonal antibodies
The virus is known to enter the host cells by binding the S protein to ACE2 receptors. By developing neutralizing antibodies against the receptors, there is a high possibility for reducing the severity of the disease (Zheng and Song, 2020). Currently, only a handful of drugs have been approved for use against SARS-CoV-2.
10. Clinically used drugs
Even before the declaration of COVID-19 as a pandemic by WHO, there was an immense lack of disease specific drugs. Being a rapidly spreading virus, it is essential to provide timely treatment for the affected individuals (Zumla et al., 2016). A list of potential drugs is provided in Table 2 and a few of the commonly used drugs are discussed below;
Table 2.
The detailed report of commercially available drugs in treatment of COVID–19.
| S. no. | Name of drug | Illnesses treated | References |
|---|---|---|---|
| 1. | α-Interferon | Spectrum of respiratory infections, RSV and SARS | (Cinatl et al., 2003; Guerrero et al., 2013; Markland et al., 2000) |
| 2. | Ritonavir and lopinavir | SARS, MERS | (Chu et al., 2004) |
| 3. | Ribavirin | RSV and RSV pneumonia | (Lewinsohn et al., 1996; McIntosh et al., 1984) |
| 4. | Reverse transcriptase inhibitors: zidovudine, didanosine, zalcitabine, stavudine, lamivudine, abacavir and emtricitabine. | SARS | (De Clercq, 2007) |
| 5. | Nucleotide reverse transcriptase inhibitor: tenofovir disoproxil fumarate. | SARS | (De Clercq, 2007) |
| 6. | Non-nucleoside reverse transcriptase inhibitors (NNRTIs): nevirapine, delavirdine and efavirenz. | SARS | De Clercq, 2007 |
| 7. | Protease Inhibitors (PIs): saquinavir, ritonavir, indinavir, nelfinavir, amprenavir, lopinavir, atazanavir and fosamprenavir. | SARS | De Clercq, 2007 |
| 8. | Fusion inhibitor: enfuvirtide. Lamivudine and adefovir dipivoxil. | SARS | De Clercq, 2007 |
| 9. | Umifenovir | ARVI, influenza, rhinovirus, adenovirus, parainfluenza, respiratory syncytial virus, coronavirus, including the causative agent of atypical pneumonia Used in the phase III trials of 2019- nCoV virus, SARS, MERS |
(De Clercq, 2007) |
| 10. | 3-Chymotrypsin-like protease | SARS, MERS | (Chou et al., 2008; Kilianski et al., 2013; Li and Clercq, 2020) |
| 11. | Papain-like protease | SARS, MERS and Human Coronavirus NL63. | (Chen, 2020; Harcourt et al., 2004; Kilianski et al., 2013) |
| 12. | RNA-dependent RNA polymerase | SARS, Murine Coronavirus. | (Imbert et al., 2006; Lu, 2020; Mahy et al., 1983) |
| 13. | Capsid spike glycoprotein (hCoV-EMC) | SARS, Human Coronavirus | (Gierer et al., 2013; Hoffmann et al., 2020; Howard et al., 2008) |
| 14. | Guanosine-analog RNA synthesis inhibitors | Coronavirus | (Beaucourt and Vignuzzi, 2014) |
| 15. | Nitazoxanide | SARS, MERS and Influenza | (Rossignol, 2016) |
| 16. | Influenza drugs | MERS | (De Clercq, 2007) |
| 17. | Remdesivir | COVID-19, SARS, MERS | Agostini et al., 2018; Wang, 2020 |
| 18. | Favipiravir | COVID-19 | (Wang, 2020) |
| 19. | Darunavir | COVID-19 | (Beck et al., 2020; Lin et al., 2020) |
| 20. | Lopinavir | COVID-19, SARS, MERS | Yao et al., 2020 |
| 21. | Alcohol Vaporization or Nebulization Inhalation Therapy | COVID-19 | (Cao, 2020) |
| 22. | Chloroquine | SARS, Human Coronavirus OC43. | (Keyaerts et al., 2009, Keyaerts et al., 2004; Vincent et al., 2005) |
| 23. | ASC09 | ARDS, Respiratory distress syndrome, SARS, MERS | (March and Bogatcheva, 2018, March and Bogatcheva, 2019) |
| 24. | TMPRSS2 inhibitor Camostat mesylate | SARS, MERS, Coronavirus 229E and COVID-19 | (Bertram et al., 2013; Hoffmann et al., 2020; Kawase et al., 2012; Shirato et al., 2014) |
| 25. | Baricitinib | COVID-19 | (Richardson et al., 2020; Stebbing et al., 2020) |
| 26. | Ruxolitinib | COVID-19 | (Stebbing et al., 2020) |
| 27. | Saquinavir | SARS and Feline Coronavirus | (Blanchard et al., 2004; Comper, 2005; Hsieh et al., 2010) |
| 28. | Indinavir | SARS and COVID-19 | (Contini, 2020; Tan et al., 2004) |
| 29. | Carfilzomib | COVID-19 | (Wang, 2020) |
| 30. | Oseltamivir | COVID-19 | (Haagmans et al., 2004; Lu, 2020) |
| 31. | Azvudine | COVID-19 | (Hu et al., 2020) |
| 32. | Baloxavir marboxil | COVID-19 | (Li and Clercq, 2020) |
| 33. | Thymosin α1 | MERS | (Leyva-Grado and Behzadi, 2019) |
| 34. | Methylprednisolone | SARS, MERS | (Kim et al., 2016; Que et al., 2003) |
| 35. | Tocilizumab | COVID-19 | (Diao et al., 2020) |
| 36. | Interferon Subtypes of β-1b, α-n1, α-n3, and human leukocyte interferon α | SARS | (Tan et al., 2004) |
| 37. | Acyclovir | SARS, MERS, Coronavirus 229E and COVID-19 | (Peters et al., 2015) |
| 38. | Cathespin L | SARS | (Simmons et al., 2005) |
This table represents the commercially available drugs used for the treatment of the various forms of coronaviruses. The viral infections discussed in the table are SARS - Severe Acute Respiratory Syndrome, MERS - Middle East Respiratory Syndrome, RSV - Respiratory Syncytial Virus, ARVI - Acute respiratory viral infections.
10.1. Ribavirin
Ribavirin is also a broad-spectrum drug whose therapeutic potential was uncovered during 1972. This antiviral drug is used in the treatment of hepatitis C. It is usually used in combination with interferon α (IFN). This drug, approved by the FDA, competes for the active site of RdRp. Ribavirin scored 109.5 μM of half maximal concentration against SARS-CoV-2 (Elfiky, 2020).
10.2. Sofosbuvir
This drug is also an FDA approved drug against NS5B and acts as a nucleotide polymerase inhibitor used for the treatment of hepatitis C. It was used in combination with interferon or RBV. This drug was previously used for the treatment of Zika virus (Cheema et al., 2019).
10.3. Lopinavir/Ritonavir
Lopinavir is a protease inhibitor which targets the HIV virus. It was identified by 1998 and approved b the FDA by 2000. This drug prevents the formation of viral proteins by disrupting the proteolytic processing by mimicking its structure as a peptide cleaved by HIV protease. This drug along with another flu drug oseltamivir was reported to result in complete recovery after showing signs of COVID-19 related pneumonia (Wu et al., 2020a, Wu et al., 2020b).
10.4. Remidesivir (anti-viral peptide)
This particular drug is an adenosine nucleotide analog, which was used in treatments against Ebola, SARS-CoV and MERS-CoV. It is a promising and potential drug which causes premature termination by entering the nascent viral RNA (Warren et al., 2016). Currently, it is undergoing clinical trials for Ebola treatment (Mulangu et al., 2019). Another recent study has shown that Remidesivir scored 0.77 μM at half maximal concentration against COVID-19 and blocked viral infection (Wang et al., 2020a, Wang et al., 2020b, Wang et al., 2020c).
10.5. Chloroquine
This drug, classified as an anti-malarial drug, has shown potential in the treatment of avian influenza A (Yan et al., 2013). Chloroquine also has shown to have anti-viral as well as immune modulating properties. This drug also showed 1.13 μM at half maximal concentration against SARS-CoV-2 and blocked viral infection by increasing the endosomal pH required for viral fusion (Wang et al., 2020a, Wang et al., 2020b, Wang et al., 2020c; Vincent et al., 2005).
10.6. Favipiravir
This drug is also a broad spectrum anti-viral drug which has obtained approval from Shenzan Health Commission for treating COVID–19 patients (Wu et al., 2020a, Wu et al., 2020b).
10.7. Ongoing clinical trials
Currently, there are numerous companies that have applied for clinical trials to repurpose existing drugs as well as to develop vaccines and drugs to fight against the fast spreading COVID–19 (Rudra et al., 2017). In the case of repurposing the existing drugs, randomized controlled treatment (RCT) are being carried out by various biotechnological companies as well as research organizations such as National Institutes of Health (NIH), USA to identify disease specific drugs. The major drugs undergoing clinical trials that have the potential to treat this viral infection (Table 3 ). More research may be required in traditional medicine to utilize them in the treatment of COVID-19.
Table 3.
Ongoing clinical trials for COVID–19.
| S. no. | Study | Drug | Status | Organization |
|---|---|---|---|---|
| 1. | Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID-19 | Sarilumab | Recruiting | Regeneron Study Site New York, New York, United States |
| 2. | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Severe Coronavirus Disease (COVID-19) | Remdesivir | Recruiting | 1. Hoag Memorial Hospital Presbyterian Newport Beach, California, United States 2.Stanford Hospital, Stanford, California, United States 3. Providence Regional Medical Center Everett, Everett, Washington, United States |
| 3. | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment | Remdesivir | Recruiting | 1. Hoag Memorial Hospital Presbyterian Newport Beach, California, United States 2.Stanford Hospital, Stanford, California, United States 3. Providence Regional Medical Center Everett, Everett, Washington, United States |
| 4. | Fingolimod in COVID-19 | Fingolimod 0.5 mg | Recruiting | Wan-Jin Chen Fuzhou, China |
| 5. | The Clinical Study of Carrimycin on Treatment Patients With COVID-19 | 1.Carrimycin 2. Lopinavir/ritonavir tablets or Arbidol or Chloroquine phosphate |
Not yet recruiting | – |
| 6. | Efficacy and Safety of Corticosteroids in COVID-19 | Methylprednisolone | Recruiting | 1. Hubei province hospital of integrated Chinese & Western Medicine Wuhan, Hubei, China 2. Yichang first people's Hospital Yichang, Hubei, China 3. Renmin Hospital of Wuhan University Wuhan, China |
| 7. | Mild/Moderate 2019-nCoV Remdesivir RCT | Remdesivir | Recruiting | Jin Yin-tan hospital Wu Han, Hubei, China |
| 8. | Adaptive COVID-19 Treatment Trial | Remdesivir | Recruiting | 1.National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Clinical Research Section Bethesda, Maryland, United States 2. University of Nebraska Medical Center - Infectious Diseases Omaha, Nebraska, United States 3. University of Texas Medical Branch - Division of Infectious Disease Galveston, Texas, United States 4. Providence Sacred Heart Medical Center Spokane, Washington, United States |
| 9. | Severe 2019-nCoV Remdesivir RCT | Remdesivir | Recruiting | Bin Cao Beijing, Beijing, China |
| 10. | Nitric Oxide Gas Inhalation for Severe Acute Respiratory Syndrome in COVID-19. | Nitric Oxide Gas | Not yet recruiting | – |
| 11. | Efficacy and Safety of IFN-α2β in the Treatment of Novel Coronavirus Patients | Recombinant human interferon α1β | Not yet recruiting | – |
| 12. | Evaluating and Comparing the Safety and Efficiency of ASC09/Ritonavir and Lopinavir/Ritonavir for Novel Coronavirus Infection | 1.ASC09/ritonavir group 2. Lopinavir/ritonavir group |
Not yet recruiting | – |
| 13. | Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) to Prevent SARS-CoV-2 Infection | mRNA-1273 | Not yet recruiting | Kaiser Permanente Washington Health Research Institute - Vaccines and Infectious Diseases Seattle, Washington, United States |
| 14. | Glucocorticoid Therapy for Novel CoronavirusCritically Ill Patients With Severe Acute Respiratory Failure | Methylprednisolone | Recruiting | Medical ICU,Peking Union Medical College Hospital Beijing, Beijing, China |
| 15. | Lopinavir/Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment | 1. Lopinavir/ritonavir 2. Ribavirin 3. Interferon Beta-1B |
Recruiting | University of Hong Kong, Queen Mary Hospital Hong Kong, Hong Kong |
| 16. | Efficacy of Chloroquine and Lopinavir/Ritonavir in mild/general novel coronavirus (CoVID-19) infections: a prospective, open-label, multicenter randomized controlled clinical study | 1.Chloroquine 2. Lopinavir/Ritonavir |
– | The Fifth Affiliated Hospital Sun Yat-Sen University |
| 17. | A study for the efficacy of hydroxychloroquine for mild and moderate COVID-19 infectious diseases | Hydroxychloroquine | – | The Second Affiliated Hospital of Chongqing Medical University |
| 18. | A prospective, randomized, open-label, parallel controlled trial for the preventive effect of hydroxychloroquine on medical personnel after exposure to COVID-19 | Hydroxychloroquine | – | Renmin Hospital of Wuhan University |
| 19. | The efficacy and safety of carrimycin treatment in patients with novel coronavirus infectious disease (COVID-19): a multicenter, randomized, open- label controlled trial | Carrimycin | – | Beijing You'an Hospital, Capital Medical University |
| 20. | A prospective clinical study for recombinant human interferon alpha 1b spray in the prevention of novel coronavirus (COVID-19) infection in highly exposed medical staffs. | recombinant human interferon alpha 1b | – | Chinese PLA General Hospital |
| 21. | A Pilot Study of Sildenafil in COVID-19 | Sildenafil citrate | Recruiting | Department and Institute of Infectious Disease, Wuhan, Hubei, China |
| 22. | Comparison of Lopinavir/Ritonavir or Hydroxychloroquine in Patients With Mild Coronavirus Disease (COVID-19) |
|
Recruiting | Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea, Republic of Korea |
| 23. | The Efficacy and Safety of Thalidomide Combined With Low-dose Hormones in the Treatment of Severe COVID-19 | Thalidomide | Not yet recruiting | – |
| 24. | Various Combination of Protease Inhibitors, Oseltamivir, Favipiravir, and Chloroquin for Treatment of COVID19: A Randomized Control Trial | Oral | Not yet recruiting | Subsai Kongsaengdao, Bangkok, Thailand |
| 25. | Chloroquine Prevention of Coronavirus Disease (COVID-19) in the Healthcare Setting | Chloroquine | Not yet recruiting | – |
| 26. | Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019 | Favipiravir Combined with Tocilizumab | Recruiting | Anhui Medical University Affiliated First Hospital, Hefei, Anhui, China Guiqiang Wang, Beijing, Beijing, China Peking University First Hospital, Beijing, Beijing, China |
| 27. | Trial of Treatments for COVID-19 in Hospitalized Adults | 1.Remdesivir 2.Lopinavir/ritonavir 3. Interferon Beta-1A |
Not yet recruiting | – |
| 28. | Randomized Controlled Trial of Losartan for Patients With COVID-19 Requiring Hospitalization | Losartan | Not yet recruiting | Hennepin County Medical Center, Minneapolis, Minnesota, United States M Health Fairview University of Minnesota Medical Center, Minneapolis, Minnesota, United States University of Minnesota, Minneapolis, Minnesota, United States |
| 29. | Randomized Controlled Trial of Losartan for Patients With COVID-19 Not Requiring Hospitalization | Losartan | Not yet recruiting | Hennepin County Medical Center, Minneapolis, Minnesota, United States M Health Fairview University of Minnesota Medical Center, Minneapolis, Minnesota, United States University of Minnesota, Minneapolis, Minnesota, United States |
| 30. | Evaluation of Ganovo (Danoprevir) Combined With Ritonavir in the Treatment of Novel Coronavirus Infection | Ganovo with ritonavir +/-Interferon | Recruiting | The Ninth Hospital of Nanchang Nanchang, Jiangxi, China |
| 31. | Eculizumab (Soliris) in Covid-19 Infected Patients | Eculizumab | Initiated | – |
| 32. | Expanded Access Remdesivir (RDV; GS-5734™) | Remdesivir | Initiated | – |
| 33. | Norwegian Coronavirus Disease 2019 Study | Hydroxychloroquine Sulfate | Not yet recruiting | – |
| 34. | Post-exposure Prophylaxis for SARS-Coronavirus-2 | Hydroxychloroquine | Recruiting | University of Minnesota, Minneapolis, Minnesota, United States |
| 35. | The efficacy and safety of pirfenidone capsules in the treatment of severe new coronavirus pneumonia (COVID-19) | Pirfenidone | – | Third Xiangya Hospital of Central South University |
The table represents a list of selected clinical trials for the amelioration of COVID–19 specific drugs and vaccines.
11. Importance of Indian medicine
Indian traditional medicinal systems are considered as one of the oldest treatments in human history and it plays an important role in encountering global health care needs (Ravishankar and Shukla, 2007). Traditional Indian medicinal practices include Ayurveda, Siddha, Unani and Yoga, Naturopathy and Homeopathy, which are successfully practiced for treating various diseases (Gomathi et al., 2020). These practices came into existence 5000 years ago, and these systems have been witnessed and scripted in ancient literature.
Traditional Indian medicine use plants, minerals and animal products for curing human diseases. Traditional knowledge regarding the plant sources and their usage are essential to use them accurately and for the right condition (Tabuti et al., 2003) About 25,000 plant based formulations have been used in folk remedies in Indian medicine (Pundarikakshudu and Kanaki, 2019). Recently, the total number of Indian medicinal plants was estimated to be around 3000, yet, traditional practitioners use around 8000 different species for their practice (Pundarikakshudu and Kanaki, 2019). Traditional medicines are generally ignored in research and development of modern drugs since their translational potentials are often underestimated. Although these medicines are ambiguous, there are wide contexts for their usage in non-Western medical technology (Yuan et al., 2016). A single herb may contain many phytochemical constituents that function alone or in combination with other compounds to produce the desired pharmacological effect (Parasuraman et al., 2014). Due to their use in traditional medicine, many plant molecules have been studied and subsequently modulated into drugs for various diseases (Li-Weber, 2009; Fabricant and Farnsworth, 2001). The search for new compounds with antiviral activity has often been unsatisfactory due to viral resistance along with viral latency and recurrent infection in immune-compromised patients (Sumithira et al., 2012). Among antiviral therapeutic methods, the majority of them are non-specific for viruses (Jiang et al., 2015). The advancements in developing antiviral agents are the major focus in medical research. The antiviral effects of medicinal plants have played a tremendous role at different stages of viral growth (Akram et al., 2018). Plant derived pharmacological formulations marked a major contribution for viral infections (Cragg et al., 1997). Based on the availability of suitable, efficient and rapid bioassay systems, the antiviral compounds have been used for rapid screening from plant extracts and fractions (Scior et al., 2012). Instead of synthetic antiviral drugs, medicinal plants deliver basic raw materials for important antiviral drugs (Moghadamtousi et al., 2015). Synthetic drugs have been replaced by medicinal plants, as life-saving drugs (Gurib-Fakim, 2006) in various viral diseases. Unfortunately, the usage of these medication have been passed down to generations by word of mouth and most of them have been lost over time, due to the lack of proper documentation. Research on these herbs and medicinal plants may help to promote their usage in clinical settings to prevent or treat various illnesses. Since many Indian medicinal plants exhibit antiviral, anti-inflammatory and antioxidant properties, it may be favorable to consider them for the treatment of COVID-19. It is clear that standard clinical trials should be carried out to scientifically prove its efficacy.
12. Indian medicinal plants and their possible effect on COVID-19
Since ancient times, Indian herbs have been used as a treatment and preventive strategy for several diseases, including respiratory viral infections. The benefit of using these herbs in viral respiratory infections is to build immune stimulating and inflammation modulating effects of manage the immune system. Holistic approach of AYUSH systems of medicine gives focus on prevention through lifestyle modification, dietary management, prophylactic interventions for improving the immunity and simple remedies based on presentation of the symptoms (AYUSH, 2020). Indian preventive and prophylactic medicinal plants recommended by AYUSH for COVID-19 (Table 4). Also, other studies on coronavirus using medicinal plants are rather minimal in India, a study has shown anti-mouse coronaviral activity (a surrogate of SARS-CoV) by the plants Indigofera tinctoria (AO), Vitex trifolia, Gymnema sylvestre, Abutilon indicum, Leucas aspera, Cassia alata, Sphaeranthus indicus, Clitoriaternatea, Clerodendruminerme Gaertn, Pergulariadaemi and Evolvulus alsinoides in Tamil Nadu (Vimalanathan et al., 2009). Among them Vitex trifolia and Sphaeranthus indicus have been found to reduce inflammatory cytokines using the NF-kB pathway, a pathway that has been implicated in respiratory distress in SARS-CoV (Alam et al., 2002; Srivastava et al., 2015). Clitoria ternatea has been identified as a metalloproteinase inhibitor, ADAM17, a metalloproteinase that is involved in ACE shredding can be targeted using this plant, as ACE-2 shredding has been associated with an increased formation of viruses (Maity et al., 2012). The plants Glycyrrhiza glabra (Nourazarian, 2015) and Allium sativum (Keyaerts et al., 2007) have been known to target the viral replication of SARS-CoV, arising as promising candidates against SARS-CoV-2. Clerodendrum inerme Gaertn, another herb has been found to have the potential to inactivate the viral ribosome, this can be further investigated for its utility as a drug targeting SARS-CoV-2 protein translation (Olivieri et al., 1996). Similarly, Strobilanthes Cusia (Tsai et al., 2020) blocked the viral RNA genome synthesis and induced papain like protease activity targeting the HCoV. In Asia, Himalayan forests are abundantly flourished with rich medicinal plant species and a study has documented the presence of ethnomedicinal plants against bronchitis (Amber et al., 2017). The study screened the antiviral plant properties against bronchitis, which showed that Hyoscyamus niger, Justicia adhatoda and Verbascum thapsus reduced infections caused by influenza viruses. The molecular mechanism by which these plants target influenza virus can be studied to understand if they attack any molecules overlapping between SARS-CoV-2 and the Influenza viruses. Hyoscyamus niger was found to be a bronchodilator and also had inhibitory effects on Ca2+ channel (Gilani et al., 2008). This could be used to target the orf3a Ca2+channels that trigger various downstream pathways upon viral infection. Most importantly, various medicinal plants have shown inhibitory effects against ACE, and these include Coriandrum sativum (Hussain et al., 2018), Boerhaavia diffusa, Cynara scolymus, Coscinium fenestratum, Punicagranatum Cassia occidentalis and Embeliaribes. Among them, Punicagranatum showed a competitive mode of action while the rest were non-specific inhibitors (Khan and Kumar, 2019; Prathapan et al., 2013). These plants need to be studied further to examine their actual effects on the entry of SARS-CoV-2 into the host cell. One of the tropical species in the Acanthaceae family, Andrographis paniculata (kalmegh) present in South Asia has a strong treating capacity of viral respiratory infections in Ayurvedic and other medicinal systems (Yarnell, 2018; Arora et al., 2011; Coon and Ernst, 2004). It was noted that Andrographis paniculata suppressed increased NOD-like receptor protein 3 (NLRP3), caspase-1, and interleukin-1β molecules which are extensively involved in the pathogenesis of SARS-COV and likely SARS-CoV-2 as well (Liu et al., 2020a, Liu et al., 2020b). Salacia oblonga (He et al., 2011) another plant from Tamil Nadu has also displayed suppressive effects on angiotensin II, AT1 signal, which was related to lung damage. Many plants have also shown inhibitory actions towards HIV proteases, these plants can be promising drugs for COVID-19. They include, Acacia nilotica (Shanti, 2016), Eugenia jambolana (Otake et al., 1995), Euphorbia granulate (Shanti, 2016). Some plants like Ocimum sanctum (Rege and Chowdhary, 2014), Ocimumkilim and scharicum (Thayil Seema and Thyagarajan, 2016), Solanum nigrum (Yu, 2004), Vitex negundo (NAIR, 2012) have been known to target the reverse transcriptase activity of HIV and can be studied for activity against SARS-CoV-2 as well. Further, Sambucus ebulus (Ganjhu et al., 2015) has been known to inhibit the activity of enveloped viruses and can also be used to target this virus. These medicinal plants can be used to ameliorate the symptoms of COVID-19. Though many medicinal plants have been identified, a lot of research has to be carried out for the development of drug specific to SARS-CoV-2. Therefore, it is important to explore the effect of these prescribed traditional medicines on SARS-CoV-2 (Table.5). Various Indian medicinal plants that have been widely used for respiratory diseases have been included (Supplementary Table 1).
Table 4.
AYUSH recommended medicinal plant extracts for treating COVID-19
(Ref: AYUSH Ministry of Health Corona Advisory – D.O. No. S. 16030/18/2019 – NAM; dated: 06th March, 2020).
| Indian medicinal plant | Form of extract | Trade name | Indian traditional medical practice | Preparation | Recommended usage | Effective against |
|---|---|---|---|---|---|---|
| Preventive and prophylactic | ||||||
| Tinospora cordifolia | Aqueous | Samshamani Vati | Ayurveda | Samshamani Vati 500 g with warm water | Twice a day for 15 days | Chronic fever |
| Andrograhis paniculata | Aqueous | Nilavembu kudineer | Siddha | Nilavembu kudineer 60 ml decoction | Twice a day for 14 days | Fever and cold |
|
Cydonia oblonga Zizyphus jujube Cordia myxa |
Aqueous | Behidana Unnab Sapistan |
Unani | Behidana – 3 g Unnab – 5 Nos Sapistan – 9 Nos Boil these 3 in 250 ml water, boil it until it remains half and filter it |
Twice a day for 14 days | Antioxidant, immune-modulatory, anti-allergic, smooth muscle relaxant, anti-influenza activity |
| Arsenicum album 30 | Tablet | Arsenicum album 30 | Homeopathy | – | Daily once in empty stomach for 3 days (Should be repeated after 1 month till the infection persist). | Effective against SARS-CoV-2, immune-modulator. |
| Symptomatic Management for COVID-19 | ||||||
| AYUSH -64 | Tablet | – | Ayurveda | – | 2 tablets twice a day | Respiratory infections |
| Agastya Haritaki | Powder | Agasthya Rasayanam | Ayurveda | 5 g in warm water | Twice a day | Upper respiratory infections |
| Anuthaila | Oil | Sesame oil | Ayurveda | – | 2 drops in each nostril daily morning | Respiratory infections |
| Adathodai Manapagu | Aqueous | Adathodai Manapagu | Siddha | – | 10 ml twice a day | Fever |
| Bryonia alba | Tablet | Bryonia | Homeopathy | – | – | Reduce lung inflammation |
| Rhus toxico dendron | Tablet | Rhus tox | Homeopathy | – | – | Viral infections |
| Atropa belladonna | Tablet | Belladonna | Homeopathy | – | – | Asthma and chronic lung diseases |
| Bignonia sempervirens | Tablet | Gelsemium | Homeopathy | – | – | Asthma |
| Eupatorium perfoliatum | Tablet | Eupatorium perfoliatum | Homeopathy | – | – | Respiratory symptoms |
| Add on interventions to the conventional care | ||||||
| Vishasura kudineer | Tablet | Poly-herbal formulation | Siddha | Decoction 60 ml | Twice a day | Fever |
| Kaba sura kudineer | Tablet | Poly-herbal formulation | Siddha | Decoction 60 ml | Twice a day | Fever, cough, sore throat, shortness of breath |
This table depicts the Indian Medicinal plants and its usage provided by the AYUSH, Government of India as a therapeutic approach for COVID-19.
Table 5.
List of Indian medicinal herbs which might inhibit the HCoVs and other Viruses.
| S. no | Plant source | Mechanism of action | Target | Virus | Reference |
|---|---|---|---|---|---|
| 1. | Acacia nilotica | Inhibition | – | HIV-PR | Mishra et al., 2014 |
| 2. | Allium sativum | Proteolytic and hemagglutinating activity and viral replication | – | SARS | Keyaerts et al., 2004 |
| 3. | Andrographis paniculata | Suppression | NLRP3, capase-1, and IL-1β | SARS-COV and likely SARS-CoV-2 | Liu et al., 2020a, Liu et al., 2020b |
| 4. | Boerhaavia diffusa | Inhibition | ACE | – | Prathapan et al., 2013; Khan and Kumar, 2019 |
| 5. | Clerodendrum inerme Gaertn | Inactivation | Ribosome | SARS-CoV-2 | Olivieri et al., 1996 |
| 6. | Clitoria ternatea | Metalloproteinase inhibitor | ADAM17 | – | Maity et al., 2012 |
| 7. | Coriandrum sativum | Inhibition | ACE | – | Pandey et al., 2011 |
| 8. |
Cynara scolymus Cassia occidentalis Coscinium fenestratum |
Inhibition | ACE | – | Prathapan et al., 2013; Khan and Kumar, 2019 |
| 9. | Embelia ribes | Inhibition | ACE | – | Prathapan et al., 2013; Khan and Kumar, 2019 |
| 10. | Eugenia jambolana | Inhibition | Protease | – | Otake et al., 1995 |
| 11. | Euphorbia granulata | Inhibition | – | HIV-1 PR | Mishra et al., 2014 |
| 12. | Glycyrrhiza glabra | Inhibition of viral replication; Modulation of membrane fluidity | SARS; HIV-1 | Akamatsu et al., 1991; Cinatl et al., 2003; Fiore et al., 2008 | |
| 13. | Gymnema sylvestre | Inhibition of viral DNA synthesis | – | – | Vimalanathan et al., 2009; Arun et al., 2014 |
| 14. | Hyoscyamus niger | Inhibition and Bronchodilator | Ca2+ | – | Gilani et al., 2008 |
| 15. | Ocimum kilimandscharicum | Inhibition | – | HIV-1 | Thayil Seema and Thyagarajan, 2016 |
| 16. | Ocimum sanctum | Inhibition | – | HIV-1 | Rege and Chowdhary, 2014 |
| 17. | Punica granatum | Inhibition | ACE | – | Prathapan et al., 2013; Khan and Kumar, 2019 |
| 18. | Salacia oblonga | Suppression | angiotensin II, AT1 signal | – | He et al., 2011 |
| 19. | Sambucus ebulus | Inhibition | – | Enveloped virus | Ganjhu et al., 2015 |
| 20. | Solanum nigrum | – | – | HIV-1 | Yu, 2004 |
| 21. | Sphaeranthus indicus | Inhibition | – | Mouse corona virus and Herpes virus |
Galani et al., 2010 Tiwari and Khosa, 2009; Vimalanathan et al., 2009 |
| 22. | Strobilanthes callosa | Blocking | – | HCoV-NL63 |
Tsai et al., 2020 Tsai et al., 2020 |
| 23. | Strobilanthes cusia | Blocking | – | HCoV-NL63 |
Tsai et al., 2020 Tsai et al., 2020 |
| 24. | Vitex negundo | Inhibition | – | HIV-1 | Nair, 2012 |
| 25. | Vitex trifolia | Reduction | – | SARS-COV | Liou et al., 2018 |
HIV-1PR: Human Influenza Virus – 1 Protease; SARS: Severe Acute Respiratory Syndrome; SARS-CoV: Severe Acute Respiratory Syndrome – Coranavirus; SARS-CoV-2: Severe Acute Respiratory Syndrome – Coranavirus 2; ACE – Angiotensin converting enzyme; HIV-1: Human Influenza Virus – 1; gp120: Envelope Glycoprotein 120; CD4: Cluster of Differentiation; HCoV-NL63: Human coronavirus NL63; RNA: Ribonucleic acid; MHV-A59: Mouse Hepatitis Virus –A59; CA2+: Calcium ion; NLRP3: NLR Family Pyrin Domain Containing 3; AT1: Angiotensin 1; HCoV-NL63: Human Coranavirus – NL63.
13. COVID-19 - the global challenges
COVID-19 has emerged as the most dangerous pandemic threat through-out the globe since its outbreak during December 2019. It has become a big challenge for the researchers and virologist to find a solution for this deadly disease. This is attributed to the fact that COVID-19 is a viral infection that has been known to have the fastest frequency of recombination or replication in its positive strand resulting in the quick formation of new progeny viral cells inside the host cells. It has also been reported that SARS-CoV-2 has a high rate of mutagenesis and changes in structure, which has created a barrier for both investigations of the disease and therapeutic regimens (American Society for Microbiology, 2020). Recently, few researchers have identified that the SARS-CoV-2 has mainly two types of strains, which are the ‘L’ and ‘S’ strains. Among these strains the L strain is more common and may have evolved from the S strain; additionally, this L strain has a higher rate of replication inside the human host cell, which has resulted in the escalation of the infection in limited time. Hence, it has become a big challenge to analyze the condition and offer therapy at the short time available. Due to the high mutation rate, it has been harder to understand the genomic organization and host interaction of the virus (Habibzadeh and Stoneman, 2020).
The genomic structure of the virus is not the only factor that presents a great challenge to research, its ability to adapt and survive in different environmental conditions make it nearly impossible to identify its mode of survival. It has been earlier reported that the SARS virus can survive at 4 °C with a humidity rate of 20%. The first outbreak of the SARS-CoV-2 was during the peak of winter, where the environmental temperature was around 2 °C to 10 °C. But since then the virus has infected people and survived in countries of completely different climatic conditions, making its demographic association hard to predict. The health care professionals and equipment are limited and are unable to handle the vast number of patients who are infected. Moreover, some of the individuals who are infectious are asymptomatic and continue to travel or gather in social surroundings infecting more people. These factors pose a challenge for scientists, health-care professional and government officials to handle and contain the condition. Government officials in all countries continue to make efforts to minimize human contact by facilitating country wide shutdowns of public places as well as various steps have been initiated to ensure the safety of the people, like social distancing and self-quarantine which limits our social interactions. This will reduce the risk of spreading the COVID-19 to people by breaking the transmission chain and the influx of new COVID-19 cases in a given time period (Balachandar et al., 2020).
14. Concluding remarks
Over the past few decades, there was an urge to discover the root cause of coronavirus infections not only in animals but in humans as well. Currently, COVID-19 has emerged as the most intense and petrifying viral infection to be handled by the human race. According to WHO (2020b), major concern among public health throughout the world and many countries have taken precautionary measures against the virus, and Government officials in all countries continue to make efforts to minimize human contact by facilitating countrywide shutdowns of public places as well as various steps have been initiated to ensure the safety of the people, like social distancing and self-quarantine which limits our social interactions (Balachandar et al., 2020). This will reduce the risk of spreading the COVID-19 to people by breaking the transmission chain and the influx of new COVID-19 cases in a given time period. Total confirmed cases throughout the world are 4,16,686 and total number of confirmed deaths are 18,589 (WHO, 2020a) as on 26th March 26, 2020 and in India 581 cases (ICMR, 2020) have been identified to be positive for this COVID-19 and 11 death cases in India as on 25th March 2020 (20:00 IST). More cases are likely to be identified in the coming days in India. This increase in infection was mainly due to the ability of this virus to recombine, mutate, block the immune system of the host cells and infect multiple species as well as cell types. Moreover, discovering the gene pool of SARS-CoV-2 may help accelerate the production of drugs and vaccines. Further, analyzing and understanding the role of non-structure and accessory proteins encrypted in this virus will aid us in understanding its mechanism of action. Also, acquiring an in-depth framework of its unique RNA replication process will enable us to find a breakthrough point to understand the host immunological response. Our review suggests the importance of a few Indian medicinal plants that have been used for several decades in the treatment of various respiratory conditions. It highlights the pathways that the plant-based medicines may target to reduce the disease burden. Thus, proactive investments in researches based on Indian medicinal plant derived vaccines or drugs to treat COVID-19 would emerge as a source of light to overcome this fatal infection.
15. Recommendations
The cases reported in many parts of China and the outbreaks involve large numbers in Italy, USA, Spain and Germany; hence travel restrictions and quarantine measures have been placed in severely affected areas. The spectrum of symptoms associated with COVID-19 ranges from difficulties in breathing and other respiratory conditions to critical conditions including SARS, kidney failure and sometimes even death. Individuals are likely to be infected by others who have been inflicted with the virus. The disease can spread from person to person via small droplets from nose or mouth when a person with COVID-19 coughs or exhales. These particles in the air, settle on surfaces in the environment further infecting people who breathe these particles or touch these places and then touch their body parts. Hence, it is important to stay >1 m (3 ft) away from a person who is sick (WHO, 2020c). Reports suggest that older persons and persons with pre-existing medical conditions (such as high blood pressure, heart disease, lung disease, cancer or diabetes) appear to develop serious illness more often than others, also pregnant women with the infection had did not pass the infection to their unborn babies (Wu and McGoogan, 2020; Chen et al., 2020). Also it has been reported that some of the Asian populations are more susceptible to acquire this COVID-19 infection when compared to the other races populations (Xu, 2020). Following are the protective measures given by WHO (2020d),
-
a)
Wash hands completely using an alcohol-based hand sanitizer will kill the virus,
-
b)
Avoid touching eyes, nose and mouth when outside.
-
c)
Be updated about the virus.
-
d)
Avoid travelling or gathering in crowded places.
-
e)
Women with infants are encouraged to breastfeed their babies to enhance their immunity.
WHO is coordinating efforts to develop vaccines and medicines to prevent and treat COVID-19 (WHO, 2020d). National Institutes of Health (NIH), has mentioned that SARS-CoV-2 could survive for up to 3 h maximum as aerosols to a maximum of three days on surfaces. Slowing the spread of the COVID-19 cases will significantly reduce the strain on the healthcare system of the country by limiting the number of people who are severely sick by COVID-19 and need hospital care. It will also give researchers more time to develop the vaccine against COVID-19. So, it's time for all the citizens to join hands together to fight against coronavirus by practicing self-hygiene and social distancing.
The following is the supplementary data related to this article.
List of commonly used Indian medicinal plants involved in respiratory diseases.
Authors contribution
Conceptualization: BV; KJ; IM; SMD; Data curation: KJ, IM, SMD, BV, AV; DV, VG, BG; Funding acquisition: BV, SMD; Investigation; IM, KJ; Project administration: BV; Resources; VG, NSK, SGC, SG, BG, BR, AN, PR; Supervision; BV, SGC, NSK, SG, VG, AN; Roles/Writing - original draft: KJ, IM, SMD, BV, AV; DV; HG, KR.
Funding
This work was supported by the Science and Engineering Research Board (SERB), Government of India [ECR/2016/001688]; The Science and Engineering Research Board (SERB), Government of India [ECR/2018/000718]; The Advanced Level State Biotech Hub (BT/04/NE/2009 Dt.29.082014), India; the Expanding Excellence in England, Research England, United Kingdom (E3) scheme.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The author Dr. VB would like to thank Bharathiar University for providing the necessary infrastructure facility and the Science and Engineering Research Board (SERB) (ECR/2016/001688), Government of India, New Delhi for providing necessary help in carrying out this review process in the Neuroinvasive section of the manuscript and Dr.SMD would like to thank the Science and Engineering Research Board (SERB) (ECR/2018/000718), Government of India, New Delhi for providing necessary help in carrying out this review process. The Authors wish to thank the Advanced Level State Biotech Hub (BT/04/NE/2009 Dt.29.082014), Mizoram University, Aizawl sponsored by the Department of Biotechnology (DBT), New Delhi, Government of India for providing the infrastructural support and facilities. P Pattanathu.K.S.M.Rahman thanks Research England for funding support through the Expanding Excellence in England (E3) scheme. The Authors would also like to thank Mr.T.Navaneethakrishnan (Indian Traditional Medicinal plant - based practitioner) Mettupalayam, India for providing valuable support for the preparation of the subtopic based on Indian medicinal plants.
Editor: Damia Barcelo
References
- Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Ray A.S. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9(2) doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamatsu H., Komura J., Asada Y., Niwa Y. Mechanism of anti-inflammatory action of glycyrrhizin: effect on neutrophil functions including reactive oxygen species generation. Planta Med. 1991;57:119–121. doi: 10.1055/s-2006-960045. [DOI] [PubMed] [Google Scholar]
- Akram M., Tahir I.M., Shah S.M.A., Mahmood Z., Altaf A., Ahmad K., Munir N., Daniyal M., Nasir S., Mehboob H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: a systematic review. Phytother. Res. 2018;32:811–822. doi: 10.1002/ptr.6024. [DOI] [PubMed] [Google Scholar]
- Alam G., Wahyuono S., Ganjar I.G., Hakim L., Timmerman H., Verpoorte R. Tracheospasmolytic activity of viteosin-A and vitexicarpin isolated from Vitex trifolia. Planta Med. 2002;68:1047–1049. doi: 10.1055/s-2002-35650. [DOI] [PubMed] [Google Scholar]
- Amber R., Adnan M., Tariq A., Mussarat S. A review on antiviral activity of the Himalayan medicinal plants traditionally used to treat bronchitis and related symptoms. J. Pharm. Pharmacol. 2017;69:109–122. doi: 10.1111/jphp.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society of Microbiology 2020. https://asm.org/PressReleases/2020/COVID-19-Resources
- Angeletti S., Benvenuto D., Bianchi M., Giovanetti M., Pascarella S., Ciccozzi M. COVID-2019: the role of the nsp2 and nsp3 in its pathogenesis. J. Med. Virol. 2020;2020:1–5. doi: 10.1002/jmv.25719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora R., Chawla R., Marwah R., Arora P., Sharma R., Kaushik V., Goel R., Kaur A., Silambarasan M., Tripathi R. Potential of complementary and alternative medicine in preventive management of novel H1N1 flu (Swine flu) pandemic: thwarting potential disasters in the bud. Evid. Based Complement. Alternat. Med. 2011;2011:1–16. doi: 10.1155/2011/586506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun L.B., Arunachalam A.M., Arunachalam K.D., Annamalai S.K., Kumar K.A. In vivo anti-ulcer, anti-stress, anti-allergic, and functional properties of Gymnemic acid isolated from Gymnema sylvestre R Br. BMC Compl. Alternative. Med. 2014;14:70. doi: 10.1186/1472-6882-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandar V., Mahalaxmi I., Kaavya J., Vivek G., Ajithkumar S., Arul N., Singaravelu G., Nachimuthu S.K., Mohana Devi S. COVID-19: emerging protective measures. Eur. Rev. Med. Pharmaco. 2020;24 doi: 10.26355/eurrev_202003_20713. (In Press) [DOI] [PubMed] [Google Scholar]
- Baranov P.V., Henderson C.M., Anderson C.B., Gesteland R.F., Atkins J.F., Howard M.T. Programmed ribosomal frameshifting in decoding the SARS-CoV genome. Virology. 2005;332:498–510. doi: 10.1016/j.virol.2004.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaucourt S., Vignuzzi M. Ribavirin: a drug active against many viruses with multiple effects on virus replication and propagation. Molecular basis of ribavirin resistance. Curr. Opin. Virol. 2014;8:10–15. doi: 10.1016/j.coviro.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B.R., Shin B., Choi Y., Park S., Kang K. Predicting commercially availableantiviral drugs that may act on the novel coronavirus (2019-nCoV), Wuhan, China through a drug-target interaction deep learning model. bioRxiv. 2020 doi: 10.1101/2020.01.31.929547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram S., Dijkman R., Habjan M., Heurich A., Gierer S., Glowacka I., Welsch K., Winkler M., Schneider H., Hofmann-Winkler H. TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium. J. Virol. 2013;87:6150–6160. doi: 10.1128/JVI.03372-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard J.E., Elowe N.H., Huitema C., Fortin P.D., Cechetto J.D., Eltis L.D., Brown E.D. High-throughput screening identifies inhibitors of the SARS coronavirus main proteinase. Chem. Biol. 2004;11:1445–1453. doi: 10.1016/j.chembiol.2004.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian D., Baric R. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 2005;287:1–30. doi: 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunte K., Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int. J. Mol. Sci. 2019;20:3394. doi: 10.3390/ijms20143394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. 2020. Suggestion Using Alcohol Vaporization or Nebulization Inhalation Therapy for Pneumonitis Caused by Coronavirus. (Available at SSRN 3545744) [Google Scholar]
- Cecere T.E., Todd S.M., LeRoith T. Regulatory T cells in arterivirus and coronavirus infections: do they protect against disease or enhance it? Viruses. 2012;4:833–846. doi: 10.3390/v4050833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Prevention and Control (CDC) 2020. https://www.cdc.gov/coronavirus/2019-ncov/index.html (Accessed on 25 March 2020)
- Chan J.F.W., Kok K.H., Zhu Z., Chu H., To K.K.W., Yuan S., Yuen K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.W., Yip C.C.Y., To K.K.W., Tang T.H.C., Wong S.C.Y., Leung K.H., Fung A.Y.F., Ng A.C.K., Zou Z., Tsoi H.W. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema S.U.R., Rehman M.S., Hussain G., Cheema S.S., Gilani N. Efficacy and tolerability of sofosbuvir and daclatasvir for treatment of hepatitis C genotype 1 & 3 in patients undergoing hemodialysis-a prospective interventional clinical trial. BMC Nephrol. 2019;20:438. doi: 10.1186/s12882-019-1631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22:69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R., Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010;84:1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C.Y., Chien C.H., Han Y.S., Prebanda M.T., Hsieh H.P., Turk B., Chang G.G., Chen X. Thiopurine analogues inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biochem. Pharmacol. 2008;75:1601–1609. doi: 10.1016/j.bcp.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Cheng V., Hung I., Wong M., Chan K.H., Chan K.S., Kao R., Poon L., Wong C., Guan Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/s0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comper W. Charged polysaccharides resistant to lysosomal degradation during kidney filtration and renal passage and their use to treat or prevent infection by coronaviruses. 2005. https://europepmc.org/article/pat/wo2004093888
- Contini A. Virtual screening of an FDA approved drugs database on two COVID-19 coronavirus proteins. Chem Rxiv. 2020 doi: 10.26434/chemrxiv.11847381.v1. [DOI] [Google Scholar]
- Coon J.T., Ernst E. Andrographis paniculata in the treatment of upper respiratorytract infections: a systematic review of safety and efficacy. Planta Med. 2004;70:293–298. doi: 10.1055/s-2004-818938. [DOI] [PubMed] [Google Scholar]
- Cordes A.K., Heim A. Rapid random-access detection of the novel SARS-coronavirus-2 (SARS-CoV-2, previously 2019-nCoV) using an open access protocol for the panther fusion. J. Clin. Virol. 2020;125:104305. doi: 10.1016/j.jcv.2020.104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J., Snader K.M. Natural products in drug discovery and development. J. Nat. Prod. 1997;60:52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Three decades of antiviral drugs. Nat. Rev. Drug Discov. 2007;6:941. doi: 10.1038/nrd2485. [DOI] [Google Scholar]
- De Clercq E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int. J. Antimicrob. Agents. 2009;33:307–320. doi: 10.1016/j.ijantimicag.2008.10.010. [DOI] [PubMed] [Google Scholar]
- De Haan C.A., Rottier P.J. Molecular interactions in the assembly of coronaviruses. Adv. Virus Res. 2005;64:165–230. doi: 10.1016/S0065-3527(05)64006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Tan Y., Chen X., Ying Liu, Ning L., Chen L., Li M., Yueping Liu, Wang G. 2020. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorigatti I., Okell L., Cori A., Imai N., Baguelin M., Bhatia S., Boonyasiri A., Cucunubá Z., Cuomo-Dannenburg G., FitzJohn R. Imperial College; 2020. Report 4: Severity of 2019-Novel Coronavirus (nCoV) [DOI] [Google Scholar]
- Dubé M., Le Coupanec A., Wong A.H., Rini J.M., Desforges M., Talbot P.J. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J.Virol. 2018;92:404–418. doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwin G.T., Korsik M., Todd M.H. The past, present and future of anti-malarial medicines. Mala. J. 2019;18:93. doi: 10.1186/s12936-019-2724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore C., Eisenhut M., Krausse R., Ragazzi E., Pellati D., Armanini D., Bielenberg J. Antiviral effects of Glycyrrhiza species. Phytother. Res. 2008;22:141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani V.J., Patel B.G., Rana D.G. Sphaeranthus indicus Linn.: a phytopharmacological review. Int. J. Ayurveda. Res. 2010;1:247–253. doi: 10.4103/0974-7788.76790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganjhu R.K., Mudgal P.P., Maity H., Dowarha D., Devadiga S., Nag S., Arunkumar G. Herbal plants and plant preparations as remedial approach for viral diseases. Virusdisease. 2015;26:225–236. doi: 10.1007/s13337-015-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierer S., Bertram S., Kaup F., Wrensch F., Heurich A., Krämer-Kühl A., Welsch K., Winkler M., Meyer B., Drosten C. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. J. Virol. 2013;87:5502–5511. doi: 10.1128/JVI.00128-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani A.H., Khan A., Raoof M., Ghayur M.N., Siddiqui B.S., Vohra W., Begum S. Gastrointestinal, selective airways and urinary bladder relaxant effects of Hyoscyamus niger are mediated through dual blockade of muscarinic receptors and Ca2+ channels. Fundam. Clin. Pharmacol. 2008;22:87–99. doi: 10.1111/j.1472-8206.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- Glass W.G., Subbarao K., Murphy B., Murphy P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol. 2004;173:4030–4039. doi: 10.4049/jimmunol.173.6.4030. [DOI] [PubMed] [Google Scholar]
- Gomathi M., Padmapriya S., Balachandar V. Drug studies on Rett syndrome: from bench to bedside. J. Autism Dev. Disord. 2020:1–25. doi: 10.1007/s10803-020-04381-y. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A.E. Severe acute respiratory syndrome-related coronavirus–the species and its viruses, a statement of the coronavirus study group. BioRxiv. 2020 doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner J. Three emerging coronaviruses in two decades the story of SARS, MERS, and now COVID-19. Am. J. Clin. Path. 2020;153:420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero E., Vasudevaraju P., Hegde M.L., Britton G., Rao K. Recent advances in α-synuclein functions, advanced glycation, and toxicity: implications for Parkinson’s disease. Mol. Neurobiol. 2013;47:525–536. doi: 10.1007/s12035-012-8328-z. [DOI] [PubMed] [Google Scholar]
- Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F., Van Amerongen G., van Riel D., De Jong T., Itamura S., Chan K.-H. Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibzadeh P., Stoneman E.K. The novel coronavirus: a bird’s eye view. Int. J. Occup. Environ. Med. 2020;11:65. doi: 10.15171/ijoem.2020.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt B.H., Jukneliene D., Kanjanahaluethai A., Bechill J., Severson K.M., Smith C.M., Rota P.A., Baker S.C. Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain-like protease activity. J. Virol. 2004;78:13600–13612. doi: 10.1128/JVI.78.24.13600-13612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Qi Y., Rong X., Jiang J., Yang Q., Yamahara J., Murray M., Li Y. The Ayurvedic medicine Salacia oblonga attenuates diabetic renal fibrosis in rats: suppression of angiotensin II/AT1 signaling. Evid. Based Complement. Alternat. Med. 2011;2011:12. doi: 10.1093/ecam/nep095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann D.L., Shindo N. COVID-19: what is next for public health? Lancet. 2020;395:542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Krueger N., Mueller M.A., Drosten C., Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020 doi: 10.1101/2020.01.31.929042. [DOI] [Google Scholar]
- Howard M.W., Travanty E.A., Jeffers S.A., Smith M., Wennier S.T., Thackray L.B., Holmes K.V. Aromatic amino acids in the juxtamembrane domain of severe acute respiratory syndrome coronavirus spike glycoprotein are important for receptor-dependent virus entry and cell-cell fusion. J. Virol. 2008;82:2883–2894. doi: 10.1128/JVI.01805-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L.E., Lin C.N., Su B.L., Jan T.R., Chen C.M., Wang C.H., Lin D.S., Lin C.T., Chueh L.L. Synergistic antiviral effect of Galanthus nivalis agglutinin and nelfinavir against feline coronavirus. Antivir. Res. 2010;88:25–30. doi: 10.1016/j.antiviral.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F., Jiang J., Yin P. 2020. Prediction of Potential Commercially Inhibitors Against SARS-CoV-2 by Multi-task Deep Model. arXiv:2003.00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain F., Jahan N., Rahman K., Sultana B., Jamil S. Identification of hypotensive biofunctional compounds of Coriandrum sativum and evaluation of their angiotensin-converting enzyme (ACE) inhibition potential. Oxidative Med. Cell. Longev. 2018;3:1–11. doi: 10.1155/2018/4643736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert I., Guillemot J., Bourhis J., Bussetta C., Coutard B., Egloff M., Ferron F., Gorbalenya A.E., Canard B. A second, non-canonical RNA-dependent RNA polymerase in SARS coronavirus. EMBO J. 2006;25:4933–4942. doi: 10.1038/sj.emboj.7601368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indian Council of Medical Research (ICMR) 2020. https://icmr.nic.in/sites/default/files/whats_new/ICMR_website_update_25Mrarch_8PM_IST.pdf
- Janice Oh H.L., Ken-En Gan S., Bertoletti A., Tan Y.J. Understanding the T cell immune response in SARS coronavirus infection. Emerg. Microbes. Infect. 2012;1:1–6. doi: 10.1038/emi.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Kanda T., Nakamoto S., Saito K., Nakamura M., Wu S., Haga Y., Sasaki R., Sakamoto N., Shirasawa H. The JAK2 inhibitor AZD1480 inhibits hepatitis A virus replication in Huh7 cells. Biochem. Biophys. Res. Commun. 2015;458:908–912. doi: 10.1016/j.bbrc.2015.02.058. [DOI] [PubMed] [Google Scholar]
- Jiang S., Shi Z., Shu Y., Song J., Gao G.F., Tan W., Guo D. A distinct name is needed for the new coronavirus. Lancet. 2020;395:949. doi: 10.1016/S0140-6736(20)30419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P., Fang C., Huang D., Huang L.Q., Huang Q. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Military. Med. Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S., Ali P.S.S., Sheeza A., Hemalatha K. COVID-19 (Novel Coronavirus 2019)–recent trends. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2006–2011. doi: 10.26355/eurrev_202002_20378. [DOI] [PubMed] [Google Scholar]
- Karakus U., Pohl M.O., Stertz S. Breaking the convention: sialoglycan variants, coreceptors, and alternative receptors for influenza a virus entry. J. Virol. 2020;94 doi: 10.1128/JVI.01357-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase M., Shirato K., van der Hoek L., Taguchi F., Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J. Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Pannecouque C., Van Damme E., Peumans W., Egberink H., Balzarini J., Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antivir. Res. 2007;75:179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., Li S., Vijgen L., Rysman E., Verbeeck J., Van Ranst M., Maes P. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob. Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.Y., Kumar V. Mechanism & inhibition kinetics of bioassay-guided fractions of Indian medicinal plants and foods as ACE inhibitors. J. Tradit. Complement. Med. 2019;9:73–84. doi: 10.1016/j.jtcme.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilianski A., Mielech A.M., Deng X., Baker S.C. Assessing activity and inhibition of Middle East respiratory syndrome coronavirus papain-like and 3C-like proteases using luciferase-based biosensors. J. Virol. 2013;87:11955–11962. doi: 10.1128/JVI.02105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killerby M.E., Biggs H.M., Haynes A., Dahl R.M., Mustaquim D., Gerber S.I., Watson J.T. Human coronavirus circulation in the United States 2014–2017. J. Clin. Virol. 2018;101:52–56. doi: 10.1016/j.jcv.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., Lee J.E., Kim K.H., Lee S., Lee K., Mok J.H. Successful treatment of suspected organizing pneumonia in a patient with Middle East respiratory syndrome coronavirus infection: a case report. J. Thorac. Dis. 2016;8(10):E1190. doi: 10.21037/jtd.2016.09.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad R., Eberle U., Dangel A., Treis B., Berger A., Bengs K., Fingerle V., Liebl B., Ackermann N., Sing A. Rapid establishment of laboratory diagnostics for the novel coronavirus SARS-CoV-2 in Bavaria, Germany, February 2020. Euro. Surveill. 2020;25:2000173. doi: 10.2807/1560-7917.ES.2020.25.9.2000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Res. 2020;9:72. doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei P., Fan B., Mao J., Wang P. Comprehensive analysis for diagnosis of novel coronavirus disease (COVID-19) infection. J. Inf. Secur. 2020 doi: 10.1016/j.jinf.2020.03.016. [DOI] [Google Scholar]
- Lewinsohn D.M., Bowden R.A., Mattson D., Crawford S.W. Phase I study of intravenous ribavirin treatment of respiratory syncytial virus pneumonia after marrow transplantation. Antimicrob. Agents Chemother. 1996;40:2555–2557. doi: 10.1128/aac.40.11.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Grado V.H., Behzadi M.A. Overview of current therapeutics and novel candidates against influenza, respiratory syncytial virus and Middle East respiratory syndrome coronavirus infections. Fronti. Microbiol. 2019;10:1327. doi: 10.3389/fmicb.2019.01327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:1449–14150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Li Z., He W., Lan Y., Zhao K., Lv X., Lu H., Ding N., Zhang J., Shi J., Shan C. The evidence of porcine hemagglutinating encephalomyelitis virus induced nonsuppurative encephalitis as the cause of death in piglets. Peer. J. 2016;4:2443. doi: 10.7717/peerj.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Bai W., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Shen R., He J., Li X., Guo X. 2020. Molecular Modeling Evaluation of the Binding Effect of Ritonavir, Lopinavir and Darunavir to Severe Acute Respiratory Syndrome Coronavirus 2 Proteases. BioRxiv. [DOI] [Google Scholar]
- Liou C.J., Cheng C.Y., Yeh K.W., Wu Y.H., Huang W.C. Protective effects of casticin from vitex trifolia alleviate eosinophilic airway inflammation and oxidative stress in a murine asthma model. Front. Pharma col. 2018;9:635. doi: 10.3389/fphar.2018.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Simundic A.M., Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0285. [DOI] [PubMed] [Google Scholar]
- Liu D.X., Fung T.S., Chong K.K.-L., Shukla A., Hilgenfeld R. Accessory proteins of SARS-CoV and other coronaviruses. Antivir. Res. 2014;109:97–109. doi: 10.1016/j.antiviral.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.T., Chen H.W., Lii C.K., Jhuang J.H., Huang C.S., Li M.L., Yao H.T. A diterpenoid, 14-deoxy-11, 12-didehydroandrographolide, in Andrographis paniculata reduces steatohepatitis and liver injury in mice fed a high-fat and high-cholesterol diet. Nutrients. 2020;12:523. doi: 10.3390/nu12020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Xiao X., Wei X., Li J., Yang J., Tan H., Zhu J., Zhang Q., Wu J., Liu L. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J. Med. Virol. 2020 doi: 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Loeffelholz M.J., Tang Y.W. Laboratory diagnosis of emerging human coronavirus infections- the state of the art. Emerg. Microbes. Infect. 2020:1–26. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020 doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy B.W., Siddell S., Wege H., Ter Meulen V. RNA-dependent RNA polymerase activity in murine coronavirus-infected cells. J. Gen. Virol. 1983;64:103–111. doi: 10.1099/0022-1317-64-1-103. [DOI] [PubMed] [Google Scholar]
- Maity N., Nema N.K., Sarkar B.K., Mukherjee P.K. Standardized Clitoria ternatea leaf extract as hyaluronidase, elastase and matrix-metalloproteinase-1 inhibitor. Indian. J. pharmacol. 2012;44:584. doi: 10.4103/0253-7613.100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloir Q., Ghysen K., Louis R., Guiot J. Acute respiratory distress revealing antisynthetase syndrome. Rev. Med. Liege. 2018;73:370–375. [PubMed] [Google Scholar]
- Manni M.L., Robinson K.M., Alcorn J.F. A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert. Rev. Respir. Med. 2014;8:25–42. doi: 10.1586/17476348.2014.854167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March K., Bogatcheva N. Use of asc and asc-cm to treat ards, sars, and mers. 2018. http://www.freepatentsonline.com/10143709.html
- March. K., Bogatcheva, N., 2019. Use of asc and asc-cm to treat ards, sars, and mers. United States patent application US 16/177,103. 2019 Apr 25.
- Markland W., McQuaid T., Jain J., Kwong A. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob. Agents Chemother. 2000;44:859–866. doi: 10.1128/aac.44.4.859-866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Park C., Sunden Y., Kimura T., Ochiai K., Kida H., Umemura T. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet. Pathol. 2004;41:101–107. doi: 10.1354/vp.41-2-101. [DOI] [PubMed] [Google Scholar]
- Matthew A. 2020. Lost Smell and Taste Hint COVID-19 Can Target the Nervous System. The Scientist. Published on 24th March, 2020. [Google Scholar]
- McIntosh K., Kurachek S.C., Cairns L.M., Burns J.C., Goodspeed B. Treatment of respiratory viral infection in an immunodeficient infant with ribavirin aerosol. Am. J, Dis. Child. 1984;138:305–308. doi: 10.1001/archpedi.1984.02140410083024. [DOI] [PubMed] [Google Scholar]
- Mengeling W., WL M., AD B., AE R. Characteristics of a coronavirus (strain 67N) of pigs. Am. J. Vet. Res. 1972;33:297–308. [PubMed] [Google Scholar]
- Ministry of Ayush, Government of India Homeopathy for prevention of coronavirus infections. 2020. https://pib.gov.in/PressReleasePage.aspx?PRID=1600895
- Mishra S., Aeri V., Gaur P.K., Jachak S.M. Phytochemical, therapeutic, and ethnopharmacological overview for a traditionally important herb: Boerhaviadiffusa Linn. Biomed. Res. Int. 2014;2014:808302. doi: 10.1155/2014/808302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadamtousi S.Z., Nikzad S., Kadir H.A., Abubakar S., Zandi K. Potential antiviral agents from marine fungi: an overview. Mar. Drugs. 2015;13:4520–4538. doi: 10.3390/md13074520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D., Lusakibanza Manzo M., Nzolo D., Tshomba Oloma A., Ibanda A. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair R. HIV-1 reverse transcriptase inhibition by Vitex negundo L. leaf extract and quantification of flavonoids in relation to anti-HIV activity. J. Cell. Mol. Biol. 2012;10:53–59. [Google Scholar]
- Nieto-Torres J.L., Verdiá-Báguena C., Jimenez-Guardeño J.M., Regla-Nava J.A., Castaño-Rodriguez C., Fernandez-Delgado R., Torres J., Aguilella V.M., Enjuanes L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourazarian A. Effect of root extracts of medicinal herb Glycyrrhiza glabra on HSP90 gene expression and apoptosis in the HT-29 colon cancer cell line. Asian Pac. J. Cancer Prev. 2015;2011:1–16. doi: 10.7314/APJCP.2015.16.18.8563. [DOI] [PubMed] [Google Scholar]
- Olivieri F., Prasad V., Valbonesi P., Srivastava S., Ghosal-Chowdhury P., Barbieri L., Bolognesi A., Stirpe F. A systemic antiviral resistance-inducing protein isolated from Clerodendrum inerme Gaertn. Is a polynucleotide: adenosine glycosidase (ribosome-inactivating protein) FEBS Lett. 1996;396:132–134. doi: 10.1016/0014-5793(96)01089-7. [DOI] [PubMed] [Google Scholar]
- Otake T., Mori H., Morimoto M., Ueba N., Sutardjo S., Kusumoto I.T., Hattori M., Namba T. Screening of Indonesian plant extracts for anti-human immunodeficiency virus—type 1 (HIV-1) activity. Phytother. Res. 1995;9:6–10. [Google Scholar]
- Pan P.P., Zhang Q.T., Le F., Zheng Y.M., Jin F. Angiotensin-converting enzymes play a dominant role in fertility. Int. J. Mol. Sci. 2013;14(10):21071–21086. doi: 10.3390/ijms141021071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A., Bigoniya P., Raj V., Patel K.K. Pharmacological screening of Coriandrum sativum Linn. for hepatoprotective activity. J. Pharm. Bioallied. Sci. 2011;3(3):435. doi: 10.4103/0975-7406.84462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman S., Thing G.S., Dhanaraj S.A. Polyherbal formulation: concept of ayurveda. Pharmacogn. Rev. 2014;8:73. doi: 10.4103/0973-7847.134229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J., Guan Y., Yuen K. Severe acute respiratory syndrome. Nat. Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H.L., Jochmans D., de Wilde A.H., Posthuma C.C., Snijder E.J., Neyts J., Seley-Radtke K.L. Design, synthesis and evaluation of a series of acyclic fleximer nucleoside analogues with anti-coronavirus activity. Bioorg. Med. Chem. Lett. 2015;25:2923–2926. doi: 10.1016/j.bmcl.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T. Novel coronavirus: from discovery to clinical diagnostics. Infect. Genet. Evo. 2020;79:104211. doi: 10.1016/j.meegid.2020.104211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prathapan A., Vineetha V., Abhilash P., Raghu K. Boerhaaviadiffusa L. attenuates angiotensin II-induced hypertrophy in H9c2 cardiac myoblast cells via modulating oxidative stress and down-regulating NF-κβ and transforming growth factor β1. Br. J. Nutr. 2013;110:1201–1210. doi: 10.1017/S0007114513000561. [DOI] [PubMed] [Google Scholar]
- Pundarikakshudu K., Kanaki N.S. Analysis and regulation of traditional Indian medicines (TIM) J. AOAC Int. 2019;102:977–978. doi: 10.5740/jaoacint.18-0376. [DOI] [PubMed] [Google Scholar]
- Que T., Wong V., Yuen K. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399–406. [PubMed] [Google Scholar]
- Ravishankar B., Shukla V. Indian systems of medicine: a brief profile. Afr. J. Trad. Complement. Altern. Med. 2007;4:319–337. doi: 10.4314/ajtcam.v4i3.31226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razonable R.R. Antiviral drugs for viruses other than human immunodeficiency virus. Mayo Clin. Proc. 2011;86:1009–1026. doi: 10.4065/mcp.2011.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege A., Chowdhary A.S. Evaluation of Ocimum sanctum and Tinospora cordifolia as probable HIV protease inhibitors. Int. J. of Pharm. Sci. Rev. Res. 2014;25:315–318. [Google Scholar]
- Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:30–31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol J.-F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J. Infect. Public. Health. 2016;9:227–230. doi: 10.1016/j.jiph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra S., Kalra A., Kumar A., Joe W. Utilization of alternative systems of medicine as health care services in India: evidence on AYUSH care from NSS 2014. PLoS One. 2017;12:e0176916. doi: 10.1371/journal.pone.0176916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virology. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scior T., Bender A., Tresadern G., Medina-Franco J.L., Martínez-Mayorga K., Langer T., Cuanalo-Contreras K., Agrafiotis D.K. Recognizing pitfalls in virtual screening: a critical review. J. Chem. Inf. Model. 2012;52:867–881. doi: 10.1021/ci200528d. [DOI] [PubMed] [Google Scholar]
- Shanti B.M. Perspective of potential plants for medicine from Rajasthan, India, Int. J. Pharm. Res. 2016;7(1):1–6. [Google Scholar]
- Shi C.-S., Nabar N.R., Huang N.-N., Kehrl J.H. SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell. Death. Discov. 2019;5:1–12. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Yu Q., Huang W., Tan C. 2019 novel coronavirus (COVID-19) pneumonia with hemoptysis as the initial symptom: CT and clinical features. Korean J. Radiol. 2020 doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K., Yano T., Senba S., Akachi S., Kobayashi T., Nishinaka T., Notomi T., Matsuyama S. Detection of Middle East respiratory syndrome coronavirus using reverse transcription loop-mediated isothermal amplification (RT-LAMP) Virol. J. 2014;11:139. doi: 10.1186/1743-422X-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. 2005;102(33):11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Zmora P., Gierer S., Heurich A., Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antivir. Res. 2013;100:605–614. doi: 10.1016/j.antiviral.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu Y., Teoh K., Lo J., Chan C., Kien F., Escriou N., Tsao S., Nicholls J., Altmeyer R., Peiris J. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008;82:11318–11330. doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu K.L., Yuen K.S., Castaño-Rodriguez C., Ye Z.W., Yeung M.L., Fung S.Y., Yuan S., Chan C.P., Yuen K.Y., Enjuanes L. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019;33:8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y., Zhu H., Zhao W., Han Y., Qin C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R.A.K., Mistry S., Sharma S. A novel anti-inflammatory natural product from Sphaeranthus indicus inhibits expression of VCAM1 and ICAM1, and slows atherosclerosis progression independent of lipid changes. Nutr. Metab. 2015;12:20. doi: 10.1186/s12986-015-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumithira P., Mangala S., Sophie A., Latha C. Antiviral and antioxidant activities of two medicinal plants. Int J Curr Sci. 2012;256:261. [Google Scholar]
- Tabuti J.R., Lye K.A., Dhillion S. Traditional herbal drugs of Bulamogi, Uganda: plants, use and administration. J. Ethnopharmacol. 2003;88:19–44. doi: 10.1016/S0378-8741(03)00161-2. [DOI] [PubMed] [Google Scholar]
- Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., Zhou Y., Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020:1–8. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P.J., Ékandé S., Cashman N.R., Mounir S., Stewart J.N. Neurotropism of human coronavirus 229E. Coronaviruses. 1994:339–346. doi: 10.1007/978-1-4615-2996-5_52. [DOI] [PubMed] [Google Scholar]
- Tan E.L., Ooi E.E., Lin C.Y., Tan H.C., Ling A.E., Lim B., Stanton L.W. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg. Infect. Dis. 2004;10:581. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayil Seema M., Thyagarajan S. Methanol and aqueous extracts of Ocimum kilimandscharicum (Karpuratulasi) inhibits HIV-1 reverse transcriptase in vitro. Int. J. Pharmacogn. Phytochem. Res. 2016;8:1099–1103. [Google Scholar]
- Tiwari B.K., Khosa R.L. Hepatoprotective and antioxidant effect of Sphaeranthus indicus against acetaminophen-induced hepatotoxicity in rats. J. Pharm. Sci. Res. 2009;1:26–30. [Google Scholar]
- Tsai Y.C., Lee C.L., Yen H.R., Chang Y.S., Lin Y.P., Huang S.H., Lin C.W. Antiviral action of Tryptanthrin isolated from Strobilanthes cusia leaf against human coronavirus NL63. Biomolecules. 2020;10:366. doi: 10.3390/biom10030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimalanathan S., Ignacimuthu S., Hudson J. Medicinal plants of Tamil Nadu (southern India) are a rich source of antiviral activities. Pharm. Biol. 2009;47:422–429. doi: 10.1080/13880200902800196. [DOI] [Google Scholar]
- Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.M., Burton D.R. Passive immunotherapy of viral infections: ‘super-antibodies’ enter the fray. Nat. Rev. Immunol. 2018;18:297. doi: 10.1038/nri.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. S0092-8674(20)30262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J. Virol. 2020 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. 2020. Fast Identification of Possible Drug Treatment of Coronavirus Disease-19 (COVID-19) through Computational Drug Repurposing Study. ChemRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang Y., Ye D., Liu Q. A review of the 2019 novel coronavirus (COVID-19) based on current evidence. Int. J. Antimicrob. Agents. 2020:105948. doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020:1–3. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Laboratory testing of 2019 novel coronavirus (2019-nCoV) in suspected human cases. 2020. https://apps.who.int/iris/handle/10665/330676
- World Health Organization (WHO) Novel coronavirus (2019-nCoV) situation report. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- World Health Organization (WHO) Middle East respiratory syndrome coronavirus (MERS-CoV) – the Kingdom of Saudi Arabia. 2020. https://www.who.int/csr/don/24-february-2020-mers-saudi-arabia/en/
- World Health Organization (WHO) Surveillance case definitions for human infection with novel coronavirus (nCoV) 2020. https://apps.who.int/iris/handle/10665/330376
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. Unveiling the origin and transmission of 2019-nCoV. Trends Microbiol. 2020;28:239–240. doi: 10.1016/j.tim.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L., Wang Y., Guo X. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12:244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Yu C., Qu J., Zhang L., Jiang S., Huang D., Chen B., Zhang Z., Guan W., Ling Z. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. and Mol. Imaging. 2020:1–6. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Zou Z., Sun Y., Li X., Xu K.-F., Wei Y., Jin N., Jiang C. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23:300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xiong Z., Zhang S., Yan Y., Nguyen J., Ng B., Lu H., Brendese J., Yang F., Wang H. Bcl-xL inhibits T-cell apoptosis induced by expression of SARS coronavirus E protein in the absence of growth factors. Biochem. J. 2005;392:135–143. doi: 10.1042/BJ20050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. J. Med. Virol. 2020 doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell E. Herbs for viral respiratory infections. Altern. Complement. Ther. 2018;24:35–43. doi: 10.1089/act.2017.29150.eya. [DOI] [Google Scholar]
- Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngchang K., Robert J., Natalia M., Michael E., Adam G., Karolina M., Andrzej J. 2020. Crystal Structure of Nsp15 Endoribonuclease NendoU from SARS-CoV-2. bioRxiv; p. 968388. [DOI] [Google Scholar]
- Yu Y.-B. The extracts of Solanum nigrum L. for inhibitory effects on HIV-1 and its essential enzymes. Korean. J. Orient. Med. 2004;10:119–126. [Google Scholar]
- Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21:559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020:eabb3405. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv. 2020 doi: 10.1101/2020.01.26.919985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Song L. Novel antibody epitopes dominate the antigenicity of spike glycoprotein in SARS-CoV-2 compared to SARS-CoV. Cell. Mol. Immunol. 2020;17:1–3. doi: 10.1038/s41423-020-0385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Ma Y., Zhang J., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020 doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Huang F., Xu L., Lin Z., de Vrij F., Ayo-Martin A.C., van der Kroeg M., Zhao M., Yin Y., Wang W. Hepatitis E virus infects neurons and brains. J. Infect. Dis. 2017;215:1197–1206. doi: 10.1093/infdis/jix079. [DOI] [PubMed] [Google Scholar]
- Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses—drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Hui D.S., Azhar E.I., Memish Z.A., Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395:35–36. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of commonly used Indian medicinal plants involved in respiratory diseases.