Abstract
The role of non-invasive ventilation (NIV) in acute respiratory failure caused by viral pneumonia remains controversial. Our objective was to evaluate the use of NIV in a cohort of (H1N1)v pneumonia. Usefulness and success of NIV were assessed in a prospective, observational registry of patients with influenza A (H1N1) virus pneumonia in 148 Spanish intensive care units (ICUs) in 2009–10. Significant variables for NIV success were included in a multivariate analysis. In all, 685 patients with confirmed influenza A (H1N1)v viral pneumonia were admitted to participating ICUs; 489 were ventilated, 177 with NIV. The NIV was successful in 72 patients (40.7%), the rest required intubation. Low Acute Physiology and Chronic Health Evaluation (APACHE) II, low Sequential Organ Failure Assessment (SOFA) and absence of renal failure were associated with NIV success. Success of NIV was independently associated with fewer than two chest X-ray quadrant opacities (OR 3.5) and no vasopressor requirement (OR 8.1). However, among patients with two or more quadrant opacities, a SOFA score ≤7 presented a higher success rate than those with SOFA score >7 (OR 10.7). Patients in whom NIV was successful required shorter ventilation time, shorter ICU stay and hospital stay than NIV failure. In patients in whom NIV failed, the delay in intubation did not increase mortality (26.5% versus 24.2%). Clinicians used NIV in 25.8% of influenza A (H1N1)v viral pneumonia admitted to ICU, and treatment was effective in 40.6% of them. NIV success was associated with shorter hospital stay and mortality similar to non-ventilated patients. NIV failure was associated with a mortality similar to those who were intubated from the start.
Keywords: Influenza A (H1N1), non-invasive ventilation, prognosis, respiratory failure, viral pneumonia
Introduction
In the last decade, two viral pandemics have had a significant impact on worldwide health, resulting mainly in severe acute respiratory failure (ARF). The first was the Severe Acute Respiratory Syndrome (or SARS) in 2003 [1], a virulent clinical entity with a high mortality rate; the second was influenza A (H1N1)v [2, 3, 4, 5, 6] in 2009, which, according to the WHO report, caused more than 18 000 deaths within the first season.
The use of non-invasive ventilation (NIV) in adults has proved effective in treating chronic obstructive pulmonary disease exacerbation, cardiogenic pulmonary oedema, and ARF in immunocompromised patients [7]. In these patients, NIV has achieved significant reductions in the rate of endotracheal intubation and ventilator-associated complications, and has improved survival rates. Nevertheless, some meta-analyses argue against the use of NIV in ARF, because it offers no advantages over conventional ventilation [7, 8]; moreover, delaying intubation in hypoxaemic intubated patients with pneumonia may increase the risk of complications [9, 10].
Early use of NIV in ARDS caused by viral pneumonia is controversial. We tried to assess when clinicians used this technique and whether it was successful, performing a secondary analysis of the GTEI/SEMICYUC (Grupo de Trabajo de Enfermedades Infecciosas/Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias) Registry, a large cohort of patients admitted to intensive care units (ICUs) for respiratory failure caused by 2009 pandemic influenza A (H1N1)v pneumonia. Secondary objectives were to assess if NIV use might increase mortality if intubation was subsequently required and if in some patients NIV could be predicted to be more successful. We hypothesized that the use of NIV in patients with ARF because of pandemic viral pneumonia might be effective in some cases and might avoid the need for invasive mechanical ventilation.
Material and Methods
Data for this study were obtained from a voluntary registry created by node 18 (Director: Jordi Rello) of CIBERES (PCI Neumonia) and recruited by investigators of the GTEI/SEMICYUC Study Group (Coordinator: Rafael Zaragoza), in 2009–10. Inclusion criteria were: adult viral pneumonia patients aged over 18 years, febrile (>38°C) acute illness; respiratory symptoms such as cough, sore throat, myalgia or influenza-like illness; ARF (conventional oxygen therapy ≥0.5 to maintain Spo2 ≥92%) requiring ICU admission; and microbiological confirmation of 2009 pandemic influenza A (H1N1)v by real-time PCR. Data were reported by the attending physician reviewing medical charts and radiological and laboratory records within the first 12 h after ICU admission. Patients with exacerbations of chronic obstructive pulmonary disease, acute pulmonary oedema, acute asthma and those already intubated at ICU admission or using NIV for palliative use or rescue therapy were excluded from the analysis [11]. The study was approved [11] by the institutional review board of Joan XXIII University Hospital, Tarragona (Spain). Patient identification remained anonymous and the requirement for informed consent was waived because of the observational nature of the study. The ICU admission criteria and treatment decisions for all patients, including determination of the need for intubation and type of antibiotic and antiviral therapy administered, were not standardized and were made by the attending physician.
Nasopharyngeal-swab specimens were collected at admission and respiratory secretions were also obtained in intubated patients. Reverse transcription-PCR testing was performed in accordance with the CDC protocol (http://www.cdc.gov/h1n1flu/guidance). H1N1 testing was performed at each institution, or centralized in a reference laboratory when not available. A ‘confirmed case' was defined as an acute respiratory illness with laboratory-confirmed pandemic H1N1 virus infection by real-time reverse transcription-PCR or viral culture [12]. Only ‘confirmed cases' were included in the current study.
The following information was recorded: demographic data, comorbidities, times of illness onset and hospital admission, time to first dose of antiviral delivery, microbiological findings and chest X-ray findings at ICU admission. Intubation and mechanical ventilation requirements (invasive and noninvasive), medical complications during ICU stay and laboratory findings at ICU admission were also recorded. Chronic obstructive pulmonary disease was defined as a disease state characterized by the presence of airflow limitation because of chronic bronchitis or emphysema. The airflow obstruction could be accompanied by airway hyper-reactivity and could be partially reversible [13]. To determine the severity of illness, the Acute Physiology and Chronic Health Evaluation (APACHE) II score [14] was recorded in all patients within 24 h of ICU admission. In addition, organ failure was assessed using the Sequential Organ Failure Assessment (SOFA) scoring system [15].
Definition of community-acquired pneumonia was based on current American Thoracic Society and Infectious Disease Society of America guidelines [16]. Primary viral pneumonia was defined in patients in the acute phase of influenza virus illness who presented with acute respiratory distress and unequivocal alveolar opacities with negative respiratory and blood bacterial cultures. Secondary bacterial pneumonia was considered in patients with confirmation of influenza virus infection who showed recurrence of fever, increase in cough and production of purulent sputum plus positive bacterial respiratory or blood cultures [17]. Respiratory cultures were based on tracheal aspirates obtained immediately after intubation. Acute renal failure was defined as the need for renal replacement therapy, in accordance with the International Consensus Conference criteria [18].
We have defined comorbidities as the pathological antecedents of each patient, and antiviral gap as time of delay between the onset of symptoms and the start of antiviral treatment. Multiple organ dysfunction syndrome (MODS) and shock were defined following international criteria [15, 19]. NIV was always used for ARF at an early stage. Failure of NIV was defined if the patient was intubated and invasively ventilated after an NIV trial (when Spo2 <92% or important respiratory work appeared).
Data were analysed using spss 18.0 software (Chicago, IL, USA). Data are expressed as frequency (percentage) or median (25th–75th interquartile range). For univariate analysis of the qualitative variables, the Chi-squared and Fisher tests were used. Quantitative variables were analysed by comparison of means with the Student's t test. Stepwise multivariate analysis was performed with logistic regression taking the variables which had p values <0.20 in the univariate analysis, or others with a special clinical interest (chronic obstructive pulmonary disease or heart failure), as dependent variables. A p value of 0.05 or less was considered to be statistically significant.
Results
In all, 685 adults with 2009 pandemic influenza A viral pneumonia were admitted to the ICUs. Fig. 1 shows the distribution of the study population depending on the ventilation provided. Baseline characteristics of 177 non-invasively ventilated patients, compared with other subgroups, are briefly described in Table 1 . Compared with non-ventilated subjects, patients with successful NIV presented more comorbidities (70.8 versus 57%; p <0.05), a higher lactate dehydrogenase (758 versus 523 U/L; p <0.05) and creatine kianse (307 versus 133 U/L; p <0.05) levels, as well as longer ICU stay (6 versus 4 days; p <0.05) (Table 1). An NIV trial was successful in 72 patients (40.7%), but failed in 105 (59.3%), who required intubation and invasive mechanical ventilation. Hence, of the 417 patients who underwent intubation, 312 (74.8%) were intubated and ventilated from the beginning, and 105 (25.2%) were intubated and ventilated after NIV failure. Therefore, 14.7% of ventilated patients admitted to the ICU for influenza A (H1N1)v pneumonia benefited from NIV.
FIG. 1.
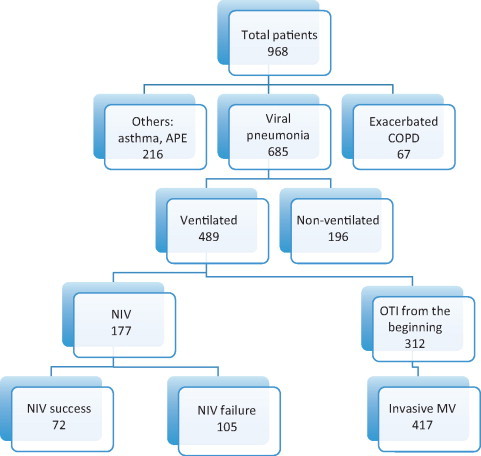
Patients with influenza A (H1N1) virus infection admitted to intensive care units. APE, acute pulmonary oedema; COPD, chronic obstructive pulmonary disease; MV, mechanical ventilation; NIV, non-invasive ventilation; OTI, orotracheal intubation.
TABLE 1.
Baseline characteristics of the patients admitted to intensive care units because of 2009 influenza A (H1N1)v pneumonia, comparing non-ventilated with all the groups of ventilated patients
| Non-invasive ventilation |
|||||
|---|---|---|---|---|---|
| Variables | Non-ventilated (n = 196) | All (n = 177) | Successful (n = 72) | Failed (n = 105) | Initially intubated (n = 312) |
| Gender (male) (%) | 56.2 | 56.5 | 56.9 | 56.2 | 53.4 |
| Age (years) | 41 (32–52) | 44 (33–53) | 45 (34–53) | 44 (32–53) | 43 (32–52) |
| APACHE II | 9 (6–13) | 12 (9–16) | 10 (8–14) | 14 (10–18.2)* | 15 (10–19) |
| SOFA score | 3 (2–4) | 4 (3–7) | 4 (3–4) | 6 (4–8)* | 6 (4–9) |
| Comorbidities (%) | 57 | 74.6 | 70.8+ | 77.1 | 71.2 |
| MODS (%) | 29.1 | 61 | 27.8 | 83.8* | 82.7 |
| CXRqo <2 (%) | 34.6 | 46.2 | 38.8 | 35.3** | 43.2 |
| Shock (%) | 8.4 | 45.2 | 13.9 | 66.7* | 65.8 |
| Obesity >30% BMI (%) | 17.9 | 31.6 | 38.9 | 26.7 | 21.7 |
| Chronic renal failure (%) | 4.2 | 5.6 | 2.8 | 7.6 | 5.2 |
| Asthma (%) | 11.1 | 10.2 | 13.9 | 7.6 | 8.4 |
| COPD (%) | 5.8 | 13.6 | 13.9 | 13.3 | 11.7 |
| Heart failure (%) | 3.2 | 6.2 | 8.3 | 4.8 | 4.9 |
| Leucocyte count (per mm3) | 6250 (3450–10 650) | 5400 (3500–8770) | 5000 (4000–7975) | 6000 (3475–9175) | 6215 (3775–10 200) |
| Platelet count (1000/mm3) | 161.5 (117.5–228.3) | 156 (121.5–226.5) | 158 (115.8–2350) | 155 (124–217) | 150 (110–201.5) |
| LDH (U/L) | 523(313–829) | 781 (441–1116) | 758(441–1006) + | 835 (438–1249) | 787(458–1136) |
| CK (U/L) | 133 (53–365) | 265 (102–580) | 307 (105–618)+ | 251 (100–572) | 22 (95–636) |
| Serum creatinine (mg/dL) | 0.84 (0.6–1) | 0.88 (0.61–1.12) | 0.8 (0.6–1) | 0.9 (0.7–1.2) | 0.9 (0.7–1.3) |
| Antiviral gap (days) | 5 (3–7) | 4 (2–6) | 4 (2–6) | 4 (2–6) | 5 (3–6) |
| Oseltamivir treatment (days) | 7 (5–10) | 10 (7–12.2) | 7 (7–10) | 10 (7–14)** | 11 (10–14) |
| Steroid treatment (%) | 32.3 | 43.5 | 36.7 | 48.5 | 41.4 |
| Days from symptom onset | 5 (3–6) | 4 (3–5) | 4 (2–5) | 5 (3–6) | 4 (3–6) |
| to hospital admission Days from hospital to ICU admission | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) |
| MV days | 7 (3–14) | 3 (2–6) | 12 (5–18.5)* | 13 (8–23) | |
| VAP (%) | 11.1 | 0 | 11.1*** | 19.2 | |
| ICU days | 4 (3–6) | 9 (5–17) | 6 (3–9) | 15 (8–25)* | 17 (10–28.2) |
| Hospital days | 9 (7–13) | 16 (10–24.2) | 11.5 (7–15)+ | 20.5(13.7–32.7)* | 24 (15–37.5) |
| In-hospital mortality (%) | 2.2 | 17.3 | 4.2 | 26.5* | 24.2 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment scoring at ICU admission; MODS, multiple organ dysfunction syndrome; CXRqo, chest X-ray quadrants opacities; BMI, body mass index; COPD, chronic obstructive pulmonary disease; LDH, lactate dehydrogenase; CK, creatine kinase; MV, mechanical ventilation; VAP, ventilation-associated pneumonia; ICU, intensive care unit.
Comorbidities included obesity >30% BMI, chronic renal failure, asthma, COPD and heart failure, and calculated as the percentage of patients having at least one comorbidity. All the laboratory parameters are at ICU admission. Antiviral gap: time from symptom onset to start antiviral treatment. Hospital days: from hospital admission to discharge. +p <0.05 comparing successful NIV with non-ventilated H1N1 pneumonia patients;
p <0.001 (NIV failure versus NIV success);
p <0.01 (NIV failure versus NIV success);
p <0.05 (NIV failure versus NIV success).
Data are expressed as medians (25th–75th interquartile range), or percentage.
Lower APACHE II score (median 10 versus 14, p <0.001), lower SOFA score (median 3.5 versus 6, p <0.001), the presence of fewer than two chest X-ray quadrant opacities, haemodynamic stability (analysed as the absence of the need for vasopressors) and the absence of acute renal failure or MODS were associated with NIV success (Table 2 ). These patients required shorter mechanical ventilation time (median 3 versus 12 days, p <0.001), shorter ICU stay (6 versus 15 days, p <0.001) and shorter hospital stay (11.5 versus 20.5 days, p <0.001) than patients with NIV failure. Multivariate analysis (Table 2) demonstrated that NIV success was associated with the presence of fewer than two chest X-ray quadrant opacities (OR 3.59) and no vasopressor requirement (OR 8.18). Moreover, when we compared the subset of non-invasively ventilated patients with two or more quadrant opacities for those with a SOFA score >7 versus those with a SOFA ≤7, the success rate increased from 5.9% to 40% (OR 10.7, 95% CI 1.3–88.6), whereas when the SOFA score was <3, the success rate became 71% (95% CI 58–84%).
TABLE 2.
Univariate and multivariate analysis of the comorbidities and parameters associated with non-invasive ventilation success (Total of 177 patients)
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Comorbidities | ||||||
| Asthma | 0.51 | 0.19–1.36 | 0.18 | |||
| COPD | 0.95 | 0.39–2.28 | 0.91 | |||
| Heart failure | 0.55 | 0.16–1.87 | 0.34 | |||
| Non-chronic renal failure | 2.88 | 0.59–14.01 | 0.18 | |||
| Pregnancy | 3.28 | 0.68–15.65 | 0.13 | |||
| BMI <30% | 0.57 | 0.30–1.08 | 0.08 | |||
| APACHE <15 | 2.94 | 1.26–6.87 | <0.05 | |||
| SOFA <7 | 15.90 | 3.51–72.06 | <0.01 | |||
| CXRqo <2 | 2.89 | 1.35–6.19 | <0.01 | 3.59 | 1.15–11.19 | <0.05 |
| Haemodynamic | 12.4 | 5.67–27.09 | <0.01 | 8.18 | 2.07–32.30 | <0.01 |
| stability No MODS | 13.45 | 6.47–27.97 | <0.01 | |||
| Normal renal function | 8.42 | 1.86–38.09 | <0.01 | |||
COPD, chronic obstructive pulmonary disease; BMI, body mass index; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment scoring at intensive care unit admission; CXRqo, chest X-ray quadrant opacities; MODS, multiple organ dysfunction syndrome. Haemodynamic stability analysed as absence of vasopressor requirement.
No differences were found comparing invasively ventilated patients after NIV failure with those initially submitted to orotracheal intubation and invasively ventilated, with regard to APACHE II and SOFA scores, radiographic infiltrates, proportion of shock or ventilator-associated pneumonia, ICU and hospital stay or mortality.
To study the possible association of complications with delay in intubation in NIV failure patients, we compared these patients intubated because of NIV failure with those intubated at ICU admission but they did not show significantly different rates of ventilator-associated pneumonia (19.2 versus 11.1%). Over two-thirds of the ventilator-associated pneumonia episodes (68.8%) were caused by Pseudomonas aeruginosa (31.1%), Acinetobacter baumannii (24.4%) or methicillin-resistant Staphylococcus aureus (13.3%). Mortality rates in ICU were similar in patients who failed NIV (26.5%) and in those who were intubated and invasively ventilated from the beginning (24.2%) (OR 1.12; 95% CI 0.67–1.88; p 0.64). Lengths of ICU stay and of hospital stay were also similar in both groups.
Discussion
This is the first large multicentre cohort study to suggest that ARF in some patients with viral 2009 influenza A (H1N1) pneumonia may respond to NIV therapy. Patients with only one radiological quadrant opacity, haemodynamic stability plus a SOFA score <8, were more likely to have a positive response to this treatment. Moreover, in patients with NIV failure, delayed intubation because of a trial of non-invasive mechanical ventilation did not increase mortality.
The use of NIV by clinicians in this large cohort of patients with severe acute respiratory infection is provocative, contrasting with the recommendations in many guidelines. Probably based on a previous study during the severe acute respiratory syndrome pandemic [20], the European Society of Intensive Care Medicine (ESICM) [21], the British Thoracic Society and WHO [22] include NIV as a high-risk procedure for disease transmission in ARF caused by influenza A (H1N1)v, although it has been shown that intubation is associated with a higher risk of viral particle transmission during severe acute respiratory syndrome [23]. Nonetheless, some reports suggest that NIV can be used in the management of some specific clinical cases of respiratory failure [24, 25].
In this cohort, clinicians used NIV in 36.1% of ventilated patients, corresponding to 25.8% of all patients admitted to ICUs with influenza A (H1N1)v pneumonia. These values are comparable to other series with an average of reported cases of around 25% [4, 5, 26, 27] ranging from 19% [27] to 40.7% [4] of all ventilated patients, with and without pneumonia. Overall, these reports include a total of 158 NIV patients. Only three of these series [4, 5, 26], with a total of 94 patients, assessed the efficacy of NIV, reporting it to be around 25% (ranging from 14.6% [5] to 58.8% [26]). None of these studies was designed with the purpose of studying only the role of NIV, so heterogeneity and lack of standardized criteria to start NIV is obviously a weakness.
Treatment decisions were not standardized and were made by the attending physician. NIV was performed in several patients despite possible contra-indications, i.e. haemodynamic instability and MODS (61% of patients). Indeed, the multivariate analysis identified haemodynamic stability and presentation with chest X-ray opacities in fewer than two quadrants as independent factors associated with NIV success. Although, vasopressor use per se is not an absolute contra-indication for NIV, data on vasopressor dosage and lactate levels could have better clarified this aspect. Based on the severity of illness of several patients undergoing NIV, one of the main messages of the present study could be that NIV should not be offered to critically ill patients with acute respiratory failure complicating H1N1 viral pneumonia and other severe acute organ failures.
However, a key question is what happens to patients who fail NIV and must be intubated and invasively ventilated, and whether the delay is really detrimental to their clinical outcome. Some studies have reported an increasing risk of complications and worse prognosis in hypoxaemic patients intubated after NIV failure [9, 10, 28, 29]. In our series, our comparison of invasively ventilated patients after NIV failure with those initially intubated and invasively ventilated presented a similar mortality. We found no differences between the two populations.
The main limitation of this study was its observational design. It is a secondary study of a database in which NIV was not implemented with a standardized protocol. First, no data were provided on the severity of acute respiratory failure in the patients included in the study (for instance: respiratory rate, Pao 2/Fio 2, Paco 2, pH). It is different to use NIV at an early stage of respiratory failure (for instance when the Pao 2/Fio 2 ratio is <250) or a late stage of ARF in patients presenting with intubation criteria. The lack of these data does not allow comparison of this study with previous studies dealing with hypoxaemic respiratory failure. Moreover, ICU admission and intubation criteria were not standardized. Delay time of intubation after NIV failure was not recorded. For the same reasons, NIV techniques and ventilation protocols are not reported. Again, comparison with previous studies is not possible. In addition, we cannot exclude that the lack of statistical difference in incidence of ventilator-associated pneumonia between patients intubated from the beginning (19.2%) and those intubated after NIV failure (11.1%) is the result of a lack of power of the current sample size. In patients at risk of failure with NIV, methods used to optimize NIV are not reported. Another limitation is the self-reporting by sites, which introduces the risk of selection bias. Healthcare workers were not prospectively assessed with follow-up serologies and the safety of the procedure should be investigated. Although no official notification of nosocomially transmitted cases has been reported using this therapy, the technique must be applied using the security measures recommended by the CDC guidelines and many scientific societies. In spite of recommendations against its use and these limitations, clinicians used NIV in nearly half of this large multicentre cohort, and in a significant proportion it was successful, suggesting that patients with severe acute respiratory infection can take benefit from early non-invasive oxygenation techniques. A randomized clinical trial is warranted to determine which patients may benefit from these techniques, to improve prognosis and to help reduce healthcare costs.
In conclusion, clinicians used NIV in 25.8% of patients with influenza A (H1N1)v viral pneumonia admitted to ICU and treatment was effective in 40.6% of them. NIV was successful in patients who presented with fewer than two chest X-ray opacities with haemodynamic stability and SOFA score ≤7. NIV failure was associated with a mortality similar to that in patients who were intubated from the start.
Author Contributions
Drs Masclans and Rello contributed to the conception and design of this study, acquisition of data, analysis and interpretation of data, and manuscript preparation. Both are guarantors of the paper, taking responsibility for the integrity of the work as a whole. Drs Pérez, Socias, Marques, Vidaur, Lorente and Almirall contributed to the acquisition of data, analysis and interpretation of data, and manuscript preparation. Investigators from 148 ICUs of GTEI/SEMYCIUC contributed to the acquisition of data, and are listed in the Appendix. In addition, Dr Perez was specifically responsible for statistical analysis.
Transparency Declaration
The authors have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article. Administrative support, logistics and storage of data were funded by resources assigned to node 18 (Director: Jordi Rello) of CIBERES (Centro de Investigacion Respiratoria en Red en Enfermedades Respiratorias, Instituto de Salud Carlos III, Madrid, Spain), being part of PCI Neumonia-CIBERES and its own research group. Indeed, the generous dedication of Thiago Lisboa, Sandra Trefler, Mireia Llaurado and Rosi Luque (salary funded by node 18 of CIBERES) and Alejandro Rodriguez (salary funded by Institut Catala de la Salut) during the peak of the pandemic is greatly appreciated.
Appendix 1
H1N1 Participating Investigators of GTEI/SEMICYUC Working Group (Coordinator: Rafael Zaragoza. Associated Coordinator: Alejandro Rodriguez)
Andalucía: Pedro Cobo (Hospital Punta de Europa, Algeciras); Javier Martins (Hospital Santa Ana Motril, Granada); Cecilia Carbayo (Hospital Torrecardenas, Almería); Emilio Robles-Musso, Antonio Cárdenas, Javier Fierro (Hospital del Poniente, Almería); Dolores Ocaña Fernãndez (Hospital Huercal – Overa, Almería); Rafael Sierra (Hospital Puerta del Mar, Cádiz); Ma Jesús Huertos (Hospital Puerto Real, Cádiz); Juan Carlos Pozo, R. Guerrero (Hospital Reina Sofía, Córdoba); Enrique Márquez (Hospital Infanta Elena, Huelva); Manuel Rodríguez-Carvajal (Hospital Juan Ramón Jiménez, Huelva); Antonio Jareño, A. Estella (Hospital del SAS de Jerez, Jerez de la Frontera); José Pomares, José Luis Ballesteros (Hospital Universitario San Cecilio, Granada); Yolanda Fernández, Francisco Lobato, José F. Prieto, José Albofedo-Sánchez (Hospital Costa del Sol, Marbella); Pilar Martínez (Hospital Vírgen de la Victoria, Málaga); Miguel Angel Díaz Castellanos (Hospital Santa Ana de Motril, Granada); Guillermo Sevilla (Clínica Sagrado Corazón, Sevilla); José Garnacho-Montero, Rafael Hinojosa, Esteban Fernández (Hospital Virgen del Rocío, Sevilla); Ana Loza, Cristóbal León (Hospital Universitario Nuestra Señora de Valme, Sevilla); Angel Arenzana (Hospital Virgen de la Macarena, Sevilla); Dolores Ocaña (Hospital de la Inmaculada, Sevilla); Inés Navarrete (Hospital Virgen de las Nieves, Granada); Medhi Zaheri Beryanaki (Hospital de Antequera); Ignacio Sánchez (Hospital NISA Sevilla ALJARAFE, Sevilla).
Aragón: Manuel Luis Avellanas, Arantxa Lander, S Garrido Ramírez de Arellano, MI Marquina Lacueva (Hospital San Jorge, Huesca); Pilar Luque (Hospital Lozano Blesa, Zaragoza); Ignacio González (Hospital Miquel Servet, Zaragoza); Jose Ma Montón (Hospital Obispo Polanco, Teruel); Paloma Dorado Regil (Hospital Royo Villanova, Zaragoza).
Asturias: Lisardo Iglesias, Carmen Pascual González (Hospital Universitario Central de Asturias – HUCA, Oviedo); Quiroga (Hospital De Cabueñes, Gijón); Águeda García-Rodríguez (Hospital Valle del Nalón, Langreo).
Baleares: Lorenzo Socias, Pedro Ibánez, Marcío Borges-Sa; A. Socias, Del Castillo A (Hospital Son LLatzer, Palma de Mallorca); Ricard Jordà Marcos (Clínica Rotger, Palma de Mallorca); José M Bonell (USP. Clínica Palmaplanas, Palma de Mallorca); Ignacio Amestarán (Hospital Son Dureta, Palma de Mallorca).
Canarias: Sergio Ruiz-Santana, Juan José Díaz, J Ferrer, Jordi Sole-Violan (Hospital Dr Negrín, Las Palmas de Gran Canaria); Sisón (Hospital Doctor José Molina, Lanzarote); David Hernández, Ana Trujillo, Luis Regalado (Hospital General la Palma, La Palma); Leonardo Lorente (Hospital Universitario de Canarias, Tenerife); Mar Martín (Hospital de la Candelaria, Tenerife); Sergio Martínez, J.J.Cáceres (Hospital Insular de Gran Canaria).
Cantabria: Borja Suberviola, P. Ugarte (Hospital Universitario Marqués de Valdecilla, Santander).
Castilla La Mancha: Fernando García-López (Hospital General, Albacete); Angel Álvaro Alonso, Antonio Pasilla (Hospital General La Mancha Centro, Alcázar de San Juan); Ma Luisa Gómez Grande (Hospital General de Ciudad Real, Ciudad Real); Antonio Albaya (Hospital Universitario de Guadalajara, Guadalajara); Alfonso Canabal, Luis Marina (Hospital Virgen de la Salud, Toledo); Almudena Simón (Hospital Nuestra Señora del Prado, Toledo); José María Añón (Hospital Virgen de la Luz, Cuenca).
Castilla y León: Juan B López Messa (Complejo Asistencial de Palencia, Palencia); Ma Jesús López Pueyo (Hospital General Yagüe, Burgos); Zulema Ferreras (Hospital Universitario de Salamanca, Salamanca); Santiago Macias (Hospital General de Segovia, Segovia); José Ángel Berezo, Jesús Blanco Varela (Hospital Universitario Río Hortega, Valladolid); Andaluz Ojeda A (Hospital Universitario, Valladolid); Antonio Álvarez Terrero (Hospital Virgen de la Concha, Zamora); Fabiola Tena Ezpeleta (Hospital Santa Bárbara, Soria); Zulema Paez; Álvaro García (Hospital Virgen Vega, Salamanca).
Catalunya: Rosa Ma Catalán (Hospital General de Vic, Vic); Miquel Ferrer, Antoni Torres (Hospital Clínic, Barcelona); Sandra Barbadillo (Hospital General de Catalunya – CAPIO, Barcelona); Lluís Cabré (Hospital de Barcelona, Barcelona); Assumpta Rovira (Hospital General de l'Hospitalet, L'Hospitalet); Francisco Álvarez-Lerma, Antonia Vázquez, Joan Nolla (Hospital Del Mar, Barcelona); Francisco Fernández, Joaquim Ramón Cervelló (Centro Médico Delfos, Barcelona); Rafael Mañéz, J. Ballús, Rosa Ma Granada (Hospital de Bellvitge, Barcelona); Jordi Vallés, Marta Ortíz, C. Guía (Hospital de Sabadell, Sabadell); Fernando Arméstar, Joaquim Páez (Hospital Dos De Mayo, Barcelona); Jordi Almirall, Xavier Balanzo (Hospital de Mataró, Mataró); Elena Arnau, Cesar Laborda, Jessica Souto, JR Masclans, Lluis Llopart, Ana Sanchez, Mercedes Palomar (Hospital Vall d'Hebron, Barcelona); Iñaki Catalán (Hospital Sant Joan de Déu, Manresa); Josep Ma Sirvent, Cristina Ferri, Nerea López de Arbina (Hospital Josep Trueta, Girona); Mariona Badía, Montserrat Valverdú-Vidal, Fernando Barcenilla (Hospital Arnau de Vilanova, Lleida); Mònica Magret (Hospital Sant Joan de Reus, Reus); MF Esteban, José Luna (Hospital Verge de la Cinta, Tortosa); Juan Ma Nava, J González de Molina (Hospital Universitario Mutua de Terrassa, Terrassa); Zoran Josic (Hospital de Igualada, Igualada); Francisco Gurri (Hospital Quirón, Barcelona, Jordi Rello, Alejandro Rodríguez, Thiago Lisboa, Diego de Mendoza, Ana Parra, Evelyn Garcia (Hospital Universitario Joan XXIII, Tarragona); Rosa María Díaz (Hospital San Camil. Sant Pere de Ribes, Barcelona); Eduard Mesalles (Hospital Germans Trias i Pujol, Badalona).
Extremadura: Juliá-Narváez José (Hospital Infanta Cristina, Badajóz); Alberto Fernández-Zapata, Teresa Recio, Abilio Arrascaeta, Ma José García-Ramos, Elena Gallego (Hospital San Pedro de Alcántara, Cáceres); Fernándo Bueno (Hospital Virgen del Puerto, Plasencia); Mercedes Díaz (Hospital de Mérida, Mérida).
Galicia: Ma Lourdes Cordero, José A. Pastor, Luis Álvarez – Rocha (CHUAC, A Coruña); Dolores Vila (Hospital Do Meixoeiro, Vigo); Ana Díaz Lamas (Hospital Arquitecto Marcide, Ferrol); Javier Blanco Pérez, M Ortiz Piquer (Hospital Xeral – Calde, Lugo); Eleuterio Merayo, Victor Jose López-Ciudad, Juan Cortez, Eva Vilaboy (Complejo Hospitalario de Ourense, Ourense); Eva Maria Saborido (Hospital Montecelo, Pontevedra); Raul José González (H. Miguel Domínguez, Pontevedra); Santiago Freita (Complejo Hospitalario de Pontevedra, Pontevedra); Ana María López; Julio Canabal, Enrique Ferres (Clinica Universitaria Santiago de Compostela, Santiago).
La Rioja: José Luis Monzón, Felix Goñi (Hospital San Pedro, Logroño).
Madrid: Frutos Del Nogal Sáez, M Blasco Navalpotro (Hospital Severo Ochoa, Madrid); Ma Carmen García-Torrejón (Hospital Infanta Elena, Madrid); César Pérez -Calvo, Diego López (Fundación Jiménez Díaz, Madrid); Luis Arnaiz, S.Sánchez-Alonso, Carlos Velayos (Hospital Fuenlabrada, Madrid); Francisco del Río, Miguel Ángel Gonzalez (Hospital Clínico San Carlos, Madrid); María Cruz Martín, José Ma Molina (Hospital Nuestra Señora de América, Madrid); Juan Carlos Montejo, Mercedes Catalán (Hospital Universitario 12 de Octubre, Madrid); Patricia Albert, Ana de Pablo (Hospital del Sureste, Arganda del rey); José Eugenio Guerrero, Jaime Benitez Peyrat (Hospital Gregorio Marañón, Madrid); Enrique Cerdá, Manuel Alvarez, Carlos Pey (Hospital Infanta Cristina, Madrid); Montse Rodríguez, Eduardo Palencia (Hospital Infanta Leonor, Madrid); Rafael Caballero (Hospital de San Rafael, Madrid); Concepción Vaquero, Francisco Mariscal, Susana García-Plaza (Hospital Infanta Sofía, Madrid); Nieves Carrasco (Hospital Universitario La Princesa, Madrid); Isidro Prieto, A Liétor, R. Ramos (Hospital Ramón y Cajal, Madrid); Beatríz Galván, Juan C. Figueira, M. Cruz Soriano (Hospital La Paz, Madrid); P Galdós; Bárbara Balandin Moreno (Hospital Puerta de Hierro, Madrid); Fernández del Cabo (Hospital Monte Príncipe, Madrid); Cecilia Hermosa, Federico Gordo (Hospital de Henares, Madrid); Alejandro Algora (Hospital Universitario Fundación Alcorcón, Madrid); Amparo Paredes (Hospital Sur de Alcorcón, Madrid); JA Cambronero (Hospital Universitario Príncipe de Asturias, Madrid); Sonia Gómez-Rosado (Hospital de Móstoles, Madrid); Luis Miguel Prado López (Hospital Sanitas La Zarzuela, Madrid).
Murcia: Sofía Martínez (Hospital Santa María del Rosell, Murcia); F. Felices Abad (Hospital Universitario Reina Sofía, Murcia); Mariano Martínez (Hospital Universitario Virgen de la Arrixaca, Murcia); Sergio Manuel Butí, Bernardo Gil Rueda, Francisco García (Hospital Morales Messeguer, Murcia).
Navarra: Enrique Maraví-Poma, I Jimenez Urra, L Macaya Redin, A Tellería (Hospital Virgen del Camino, Pamplona); Josu Insansti (Hospital de Navarra, Pamplona).
Pais Vasco: Nagore González, Pilar Marco, Loreto Vidaur, Emilio Perez-Trallero (Hospital de Donostia, San Sebastián); B. Santamaría (Hospital de Basurto, Bilbao); Juan Carlos Vergara, Jose Ramon Iruretagoyena Amiano (Hospital de Cruces, Bilbao); Alberto Manzano (Hospital Santiago Apóstol, Vitoria); Carlos Castillo Arenal (Hospital Txagorritxu, Vitoria); Pedro María Olaechea (Hospital Galdakao-Usansolo, Vizcaya).
Valencia: José Blanquer (Hospital Clinic Universitari, Valencia); Roberto Reig Valero, A. Belenger, Susana Altaba (Hospital General de Castellón, Castellón); Bernabé Álvarez -Sanchez (Hospital General de Alicante, Alicante); Santiago Alberto Picos (Hospital Torrevieja Salud, Alicante); Ángel Sánchez-Miralles (Hospital San Juan, Alicante); Juan Bonastre, M. Palamo, Javier Cebrian, José Cuñat (Hospital La Fe, Valencia); Belén Romero (Hospital de Manises, Valencia); Rafael Zaragoza (Hospital Dr Peset, Valencia); Virgilio Paricio (Hospital de Requena, Valencia); Asunción Marques, S. Sánchez-Morcillo, S. Tormo (Hospital de la Ribera, Valencia). J. Latour (H.G Universitario de Elche, Valencia); M Ángel García (Hospital de Sagunto, Castellón).
Andorra: Antoli Ribas (Hospital Nuestra Señora de Meritxell, Andorra).
Footnotes
Editor: M. Paul
Article published online: 14 February 2012
References
- 1.Lee N, Hui D, Wu A. A major outbreak of SARS in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 2.Rello J, Rodríguez A, Ibañez P. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13:R148–R156. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain S, Kamimoto L, Bramley AM. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 4.Domínguez‐Cherit G, Lapinsky SE, Macias AE. Critically ill patients with 2009 Influenza A (H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Zarychanski R, Pinto R. Critically ill patients with 2009 Influenza A (H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 6.The ANZIC Influenza Investigators Critical Care Services and 2009 H1N1 Influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 7.Nava S, Hill N. Non‐invasive ventilation in acute respiratory failure. Lancet. 2009;374:250–259. doi: 10.1016/S0140-6736(09)60496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonelli M, Conti G, Moro ML. Predictors of failure on non‐invasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi‐center study. Intensive Care Med. 2001;27:1718–1728. doi: 10.1007/s00134-001-1114-4. [DOI] [PubMed] [Google Scholar]
- 9.Moretti M, Cilione C, Tampieri A, Fracchia C, Marchioni A, Nava S. Incidence and causes of non‐invasive mechanical ventilation failure after initial success. Thorax. 2000;55:819–825. doi: 10.1136/thorax.55.10.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuchi T, Toba S, Sekiguchi Y. Protocol‐based noninvasive positive pressure ventilation for acute respiratory failure. J Anesth. 2011;25:42–49. doi: 10.1007/s00540-010-1051-x. [DOI] [PubMed] [Google Scholar]
- 11.Diaz E, Rodríguez A, Martín‐Loeches I. Impact of obesity in patients infected with 2009 Influenza A (H1N1) Chest. 2011;139:382–386. doi: 10.1378/chest.10-1160. [DOI] [PubMed] [Google Scholar]
- 12.Jamieson DJ, Honein MA, Rasmussen SA. The novel influenza A (H1N1) pregnancy working group. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 13.Rello J, Rodriguez A, Torres A. Implications of COPD in patients admitted to the intensive care unit by community‐acquired pneumonia. Eur Respir J. 2006;27:1210–1216. doi: 10.1183/09031936.06.00139305. [DOI] [PubMed] [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J. The SOFA (sepsis‐related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 16.Mandell LA, Wunderink RG, Anzueto A. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cate TR. Viral pneumonia due to influenza and parainfluenza viruses and adenoviruses. In: Marrie J, editor. Community acquired pneumonia. Kluwer Academic; New York: 2001. pp. 593–616. [Google Scholar]
- 18.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute dialysis quality initiative workgroup: acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of Acute Dialysis Quality Initiative (ADQI) group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy MM, Fink MP, Marshall JC. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 20.Yu IT, Xie ZH, Tsoi KK. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44:1017–1025. doi: 10.1086/512819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprung CL, Cohen R, Bruria A, ESICM Task force Recommendations and standard operating procedures for ICU and hospital preparations for an influenza epidemic or mass disaster. Intensive Care Med. 2010;36:s65–s69. doi: 10.1007/s00134-010-1766-z. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization . CDC protocol of realtime RTPCR for influenza A (H1N1) World Health Organization; Geneva: 2009. Available at: http://www.who.int/csr/resources/publications/swineflu/ (last accessed 21 February 2012). [Google Scholar]
- 23.Fowler RA, Guest CB, Lapinsky SE. Transmission of severe acute respiratory during intubation and mechanical ventilation. Am J Respir Crit Care Med. 2004;169:1198–1202. doi: 10.1164/rccm.200305-715OC. [DOI] [PubMed] [Google Scholar]
- 24.Winck JC, Marinho A. NIV in ARF related to 2009 pandemic influenza A/H1N1v infection. Crit Care. 2010;14:408. doi: 10.1186/cc8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djibre M, Berkane S, Salengro A. Non‐invasive management of ARDS related to influenza A (H1N1)v pneumonia in a pregnant woman. Intensive Care Med. 2010;36:373–374. doi: 10.1007/s00134-009-1684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esquinas A. International survey acute respiratory failure in H1N1 infection. Am J Respir Crit Care Med. 2010;181:A6117. [Google Scholar]
- 27.Estenssoro E, Ríos FG, Apezteguía C. Pandemic 2009 influenza A in Argentina. A study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. 2010;182:41–48. doi: 10.1164/201001-0037OC. [DOI] [PubMed] [Google Scholar]
- 28.Festic E, Grajic O, Limper AH, Aksamit TR. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection. Chest. 2005;128:573–579. doi: 10.1378/chest.128.2.573. [DOI] [PubMed] [Google Scholar]
- 29.Esteban A, Frutos‐Vivar F, Ferguson ND. Non‐invasive positive‐pressure ventilation for respiratory failure. Am J Respir Crit Care Med. 2001;163:283–291. doi: 10.1164/ajrccm.163.1.ats1000. [DOI] [PubMed] [Google Scholar]


