Abstract

Practical relevance Feline uveitis can be a subtle, insidious, painful, vision-threatening disease with causes that can sometimes be fatal. It is essential that clinicians remain alert to its various clinical presentations, thoroughly diagnose cases once detected, and treat the primary cause whenever possible.
Clinical challenges In the majority of patients, a cause is not found and aggressive immunomodulating therapy of what may become a chronic or recurrent immune-mediated disease must be instigated. As with immune-mediated diseases elsewhere, this involves local or systemic immunosuppression with slow tapering and frequent monitoring. The aim of this review is to aid diagnosis and therapy of uveitis by likening it to inflammation elsewhere (because it is more similar than it is different) while highlighting differences (because these are helpful).
Global importance Feline uveitis is similar in its presentation throughout the world. Although the list of infectious causes may vary in composition or order of likelihood, idiopathic, immune-mediated and neoplastic causes of feline uveitis are universal.
Patient group Patients of either gender and all ages and breeds are affected by uveitis.
Evidence base Despite the fact that feline uveitis is a serious and common disorder, the peer-reviewed literature regarding this disease is somewhat limited. Approximately half the publications are review articles, case reports or case series. The majority of prospective and retrospective research describes epidemiologic surveys of antibodies, antigens and organism DNA in serum and aqueous humor.
Classifying uveitis in clinically useful ways
The uvea contains familiar tissues and cell types (eg, lymphocytes, smooth muscle, blood vessels), is inflamed by familiar antigens (infectious agents, neoplasia, autoantigens) and reacts with the five cardinal signs of inflammation seen elsewhere (heat, pain, swelling, redness and loss of function). There are numerous systems for categorizing uveitis. Those emphasized here permit directed and prioritized diagnostic testing, guide selection of appropriate therapy while test results are pending, and inform predictions regarding visual outcome and, in some cases, prognosis for life.
What parts of the uvea are involved?
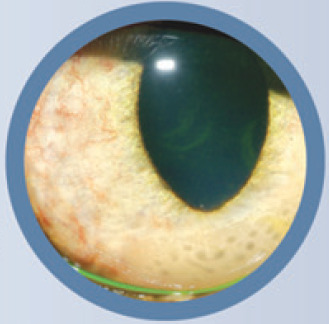
Uveitis is inflammation of the middle vascular (uveal) tunic of the eye, which is located between the outer fibrous (corneoscleral) tunic and the inner neurosensory (retinal) tunic (fig 1). The uvea is composed of just three structures: the choroid posteriorly and the iris and ciliary body anteriorly. These anatomic divisions explain terms frequently used to categorize uveitis. Anterior uveitis (sometimes termed iridocyclitis) is inflammation of the iris and ciliary body Posterior uveitis is inflammation of the choroid; however, because the retina and choroid are so intimately associated anatomically and functionally, retinal inflammation inevitably occurs along with choroiditis and so posterior uveitis is usually called chorioretinitis. Other anatomic classifications include panuveitis (inflammation of the choroid, iris and ciliary body), and pars planitis or intermediate uveitis (inflammation of the posterior ciliary body, or pars plana, and anterior choroid).
Fig 1.
The middle (uveal) tunic of the eye is located between the outer fibrous (corneoscleral) tunic and the inner neurosensory (retinal) tunic and is composed of the choroid posteriorly and the iris and ciliary body anteriorly
Uveitis has few pathognomonic signs and these are notably more subtle in cats than they are in dogs. Therefore, uveitis in cats often goes undetected by owners and untreated by veterinarians until potentially blinding sequelae such as glaucoma, cataracts and retinal detachment or degeneration occur.
While these anatomic divisions are useful, inciting antigens and effector cells and cytokines rarely totally respect them and pure anterior or posterior uveitis should never be assumed. For example, in a patient with hyphema a dilated fundic examination should always be conducted for the presence of chorioretinal hemorrhage. Likewise, a patient with chorioretinitis should always undergo intraocular pressure (IOP) measurement (tonometry) to assess any involvement of the anterior uvea. That said, some diagnostic, therapeutic and prognostic decisions can be made following determination of whether the patient has predominantly anterior and/or posterior uveal involvement.
Diagnostic implications Although many causative agents of uveitis can lead to panuveitis (eg, feline infectious peritonitis [FIP] virus), many tend to produce signs that predominate in either the anterior uvea (such as feline immunodeficiency virus [FIV]) or the posterior uvea (such as the systemic mycoses). Therefore, clinically localizing the uveitis to one or both anatomic sites can assist with ranking differential diagnoses as well as choosing and prioritizing diagnostic tests (Table 1).
Treatment implications Posterior segment disease must be treated via the systemic route while anterior segment inflammation may be treated with topical application of drugs that penetrate the cornea. Hence knowing the extent of uveal involvement is critical before choosing a route of therapy. Additionally, humans report that inflammation of the posterior uvea is not as painful as anterior uveitis. If this is also true in cats, then analgesia may be not be necessary in patients showing no ciliary body involvement.
Prognostic implications Although anterior segment inflammation can be blinding, chorioretinitis is more likely to be associated with vision-threatening outcomes. This reinforces the importance of posterior segment examination before and throughout the course of treatment.
Table 1.
Typical clinical course, predominant location and suggestive signs of common causes of uveitis
| Cause | Clinical course | Typical location | Suggestive signs* |
|---|---|---|---|
| Trauma | Acute | Anterior uveitis | Hyphema; AC fibrin; miosis; aqueous flare; hypotony |
| Reflex uveitis (due to ulcerative keratitis) | Acute | Anterior uveitis | Miosis; aqueous flare; hypopyon (if ulcer is infected); hypotony |
| FIP | Subacute | Panuveitis (anterior uveitis may dominate) | KPs; aqueous flare; AC fibrin; hypopyon; retinal vascular engorgement and increased tortuosity; perivascular chorioretinal granulomas; retinal detachment |
| Lymphoma | Subacute | Anterior uveitis | Hypopyon; hyphema; AC fibrin; aqueous flare; iridal thickening; iridal nodules; rubeosis iridis; iris bombé; secondary glaucoma |
| Systemic mycoses | Subacute | Panuveitis (posterior uveitis dominates) | Hypopyon; hyphema; AC fibrin; aqueous flare; iridal thickening; rubeosis iridis; iris bombé; vitreal debris/infiltrates; secondary glaucoma; chorioretinal granulomas; retinal detachment |
| Lensinduced uveitis | Phacoclastic (acute) | Anterior uveitis | Hypopyon; hyphema; AC fibrin; aqueous flare; iridal thickening; posterior synechiae; ocular hypertension; miosis |
| Phacolytic (chronic) | Aqueous flare; iridal thinning/atrophy; rubeosis iridis; posterior synechiae; mature/hypermature cataract; secondary glaucoma | ||
| Idiopathic | Chronic or recurrent | Anterior or intermediate uveitis | Iridal thinning/atrophy; iridal nodules; rubeosis iridis; aqueous flare; KPs; snow banking; vitreous debris/infiltrates; posterior synechiae; cortical cataract; secondary glaucoma |
| Primary uveal neoplasia | Chronic | Anterior uveitis or chorioretinitis depending on site of tumor | Anteriorly located Hypopyon; hyphema; AC fibrin; aqueous flare; anterior iridal displacement; rubeosis iridis; vitreous debris/infiltrates; secondary glaucoma |
| Posteriorly located Retinal detachment; subretinal neoplasm; vitreous debris/infiltrates | |||
| FIV | Chronic | Intermediate uveitis | Vitreous debris/infiltrates; snow banking; iridal thinning/atrophy; rubeosis iridis; aqueous flare; posterior synechiae; cortical cataract; secondary glaucoma |
AC = anterior chamber, FIP = feline infectious peritonitis, KPs = keratic precipitates, FIV = feline immunodeficiency virus
Note the considerable overlap of signs. No sign is pathognomonic for a given cause, and absence of one or more of the signs listed does not allow a cause to be eliminated. Rather, these features can be used to rank the likelihood of potential causes for further diagnostic testing
What is the likely cause of the uveitis?
The causes of uveitis are diverse and sometimes elusive. Even with very complete diagnostic testing, a cause is not discovered in as many as 70% of cats with uveitis. 1 Therefore, although feline uveitis can be subdivided into infectious, inflammatory, traumatic, neoplastic causes, etc, the most clinically relevant classifications are both two-category systems.
First, uveitis can be categorized (following detailed diagnostic investigation) as idiopathic or as having an identified cause. While this categorization may at first sight seem too broad to be of any clinical value, it actually permits some critical decisions to be made about therapy. For example, if a cause is identified, then therapy may be targeted at prompt and specific reduction or removal of the antigen (organism, neoplasm, etc) that is driving the immune response with minimal non-specific immunosuppression. An example would be a cat diagnosed with systemic cryptococcosis. Specific antifungal therapy with minimal and only local (ocular) immunomodulation is required and, if successful, should lead to resolution of the uveitis, perhaps with some scarring. By contrast, if after thorough diagnostic testing a cause cannot be found, then the antigen cannot be medically reduced or resolved and this patient will require aggressive non-specific immune damping with the expectation that recurrence might be likely. These two scenarios exemplify the fact that elimination of known causes of uveitis that could progress or even become fatal with immunosuppression is critical, and that categorizing patients into those with a known cause and those with idiopathic uveitis is a very important approach to treating these cats.
The second broad etiologic system involves categorizing uveitis as due to endogenous or exogenous causes. 2 Examples of exogenous causes include trauma, lens luxation and ulcerative keratitis; the last produces a so-called ‘reflex uveitis’ via stimulation of the trigeminal nerve. Exogenous causes are usually more obvious than endogenous causes and tend to be associated with other ocular signs, which can be identified during a thorough ophthalmic examination. By contrast, endogenous uveitis includes those cases induced by immune-mediated, infectious or neoplastic disease, as well as idiopathic disease. In cats, endogenous causes tend to be more common, produce more subtle clinical signs, require more diagnostic verification, and be more resistant to treatment than exogenous causes.
How long has the patient been affected?
Classification of uveitis as acute or chronic is very important since exogenous uveitis tends to be acute and self-limiting; cases associated with systemic diseases tend to be subacute; and idiopathic cases tend to become chronic or recurrent (Table 1). Historical data gained through careful interview of the client and from repeat examinations will assist in determining chronicity. Additionally, there are a number of clinical signs (discussed later) seen only with chronicity.
Is the uveitis bilateral or unilateral?
Bilateral uveitis tends to be associated with systemic causes and unilateral uveitis is more consistent with idiopathic disease or exogenous causes. However, this distinction cannot always be relied on and signs of uveitis may be very asymmetric in their magnitude or stage. Therefore, it is critical that both eyes are examined to avoid misdirected diagnostic testing, inadequate therapy, or an inaccurate prognosis with potentially blinding or even fatal consequences.
Categorizing feline uveitis using adequate history-taking and clinical observation as present in a well or systemically ill patient, involving the anterior and/or posterior uvea, endogenous or exogenous, idiopathic or with identified cause, acute or chronic, and unilateral or bilateral will direct diagnostic testing, therapeutic recommendations and prognostic predictions. The remainder of this review will focus on endogenous anterior uveitis for which either a cause is found or which remains idiopathic after appropriate diagnostic testing.
Clinical signs of uveitis: A functional approach
Uveitis has few pathognomonic signs and these are notably more subtle in cats than they are in dogs. Therefore, uveitis in cats often goes undetected by owners and untreated by veterinarians until potentially blinding sequelae such as glaucoma, cataracts and retinal detachment or degeneration occur. For these reasons, clinicians must maintain a high index of suspicion regarding uveitis in all cats with ocular disease and even those with non-specific signs such as lethargy, ‘hiding’, anorexia or fever.
An appreciation of the diversity and importance of normal anterior uveal functions facilitates recognition of the classic clinical signs and devastating consequences of uveitis (Table 2).
Table 2.
Functional approach to uveitis
| Structure | Function | Acute dysfunction (mechanism) | Chronic dysfunction (mechanism) |
|---|---|---|---|
| Iris and pupil | Regulation of retinal illumination | Miosis (spasm); dyscoria/corectopia (iridal mass/infiltration, posterior synechiae) | Miosis/mydriasis/dyscoria/corectopia (posterior synechiae); mydriasis (secondary glaucoma) |
| Ciliary body epithelium | AH formation | Ocular hypotension (decreased production) | Phthisis (chronic hypotony plus scarring) |
| ICA and deeper outflow tract | AH outflow | Ocular hypotension initially (increased uveoscleral outflow); secondary ocular hypertension (‘clogging’ of the ICA with inflammatory debris and swelling of the uveal components of the outflow tract) | Ocular hypertension (peripheral anterior synechiae, scarring of ICA and deeper outflow tract) |
| BAB | Regulation of AH composition | Plasma components in AH (aqueous flare, AC fibrin, hypopyon, hyphema. KPs); posterior or anterior synechiae (fibrinous exudate on iris surface); corneal edema (altered AH composition) | Posterior or anterior synechiae (fibrinous exudate on iris surface); lens luxation (due to enzymatic lysis or phagocytosis of zonules); cataract and chronic keratitis (altered AH composition); phthisis (organization and contraction of fibrin) |
| Lymphoid tissue | Antigen recognition and processing | Exuberant immune response (hypopyon) | Chronic recurrent, indischminate immune activation |
AH = aqueous humor, ICA = iridocorneal angle, BAB = Blood-aqueous barrier, KPs - keratic precipitates, AC = anterior chamber
The anterior uvea is responsible for:
aqueous humor formation by the ciliary body epithelium and therefore nutrition of the avascular cornea and lens;
formation of the blood-aqueous barrier (BAB) by the ciliary body epithelium and vascular endothelium;
determination of retinal illumination due to pupil size;
accommodation due to alteration of lens curvature by the ciliary body musculature; and
regulation of aqueous humor outflow through the iridocorneal angle (conventional outflow) and uveal parenchyma (non-conventional or uveoscleral outflow).
Given this diverse list, it is not surprising that minor disruptions in uveal function during uveitis can have far-reaching and vision-threatening effects within the globe.
The final function of the uveal tract listed in Table 2 (antigen recognition and processing) is critical in the pathogenesis of chronic or recurrent uveitis. Because there are no intraocular lymphatic vessels and therefore no ‘draining’ lymph node for intraocular tissues, the uvea plays a major role in immune surveillance and response within the eye. Its role can be likened to that of a lymph node or an extension of the reticuloendothelial system. In acute uveitis, this function is vital for normal identification, processing and clearance of inciting antigens. However, ongoing unbridled immunologic activity within the uvea, sometimes in response to an autoantigen or unrecognized foreign antigen, is responsible for chronic, low-grade, immune-mediated uveitis and serious sequelae. The extraocular analogy would be a reactive lymph node. Thus uveitis can be thought of as ‘intraocular lymphadenopathy’, since the list of differential considerations is almost identical for lymphadenopathy and uveitis, especially in chronic uveitis. This also introduces the therapeutically critical concept that, in idiopathic uveitis, recurrence is likely as immunomodulation is tapered.
Manifestations of heat, pain, swelling and redness in eyes with uveitis
As mentioned, uveitis manifests as one or a combination of the five cardinal signs of inflammation: heat, pain, swelling, redness and loss of function. Heat is not readily palpated in the eye, as it would be in other soft tissue swellings such as cellulitis. Therefore, particular attention must be paid to evidence of the other four changes.
Intraocular pain is sometimes difficult to interpret in animals, especially cats. As in dogs, uveal pain in cats can manifest as blepharospasm or epiphora; however, cats seem far more likely to show subtle and less localizing signs such as lethargy, anorexia, or reduced interaction with their owner or environment. This seems more akin to humans who complain of symptoms such as photophobia and headache or brow-ache when they have uveitis.
Swelling is an important sign of iridocyclitis. However, since the ciliary body cannot be observed clinically, iridal swelling must be relied on solely for this sign. Observation of iridal swelling requires that the eye is examined using a source of magnification (such as the Optivisor) in association with a bright and focal light source (such as the Finoff transilluminator) directed very obliquely across the globe (fig 2). The normal iris is highly textured, with crypts and ridges across its entire anterior face (fig 3a). In cats with uveitis, these become obscured by iridal swelling and the anterior iris face appears ‘muddy’ or flattened (fig 3b). Cellular infiltration may also be evident as a homogeneous change in color of the iris, particularly in cats with lighter irides, or as nodular swellings.
Fig 2.
Oblique illumination is essential for examination of the anterior iris face. It is achieved by directing a bright and focal light source across the eye from the lateral canthus while viewing the eye from in front using a source of magnification
Fig 3 (a).
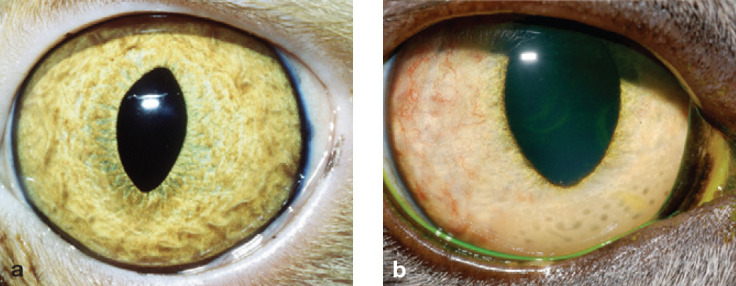
The normal anterior iris face is highly textured when viewed with oblique illumination and a source of magnification. (b) With uveitis, iridal swelling is evident as a ‘muddy’ or flattened iris surface, sometimes in association with nodular swellings. This eye also demonstrates rubeosis iridis (especially laterally) and keratic precipitates as grey spots against the inner cornea ventromedially
Redness is one of the most overt signs of inflammation elsewhere in the body, but scleral injection is very subtle in many cats with uveitis.
It is made even less obvious by the fact that so little of the sclera is visible when the eyelids are in their normal open position. Therefore, feline eyes must be examined particularly carefully and great importance attributed to even subtle congestion of blood vessels present over the sclera. The temptation to diagnose any redness of this region as conjunctivitis must be avoided. Unlike dogs, uveitis in cats tends to produce more subtle vascular engorgement than conjunctivitis does. In addition, uveitis does not tend to produce notable chemosis (conjunctival edema), whereas conjunctivitis frequently does. Finally, it is important to distinguish deep episcleral vessels (which become injected in cases of uveitis, other intraocular diseases and deep corneal disease) from superficial conjunctival vessels (which indicate conjunctivitis or superficial corneal disease; fig 4). Compared with superficial conjunctival vessels, deep episcleral vessels tend to be straighter, less mobile, of larger diameter, and to branch less. They also appear to stop 1–2 mm before they reach the corneoscleral limbus whereas conjunctival vessels proceed to and sometimes loop back at the limbus.
Fig 4.
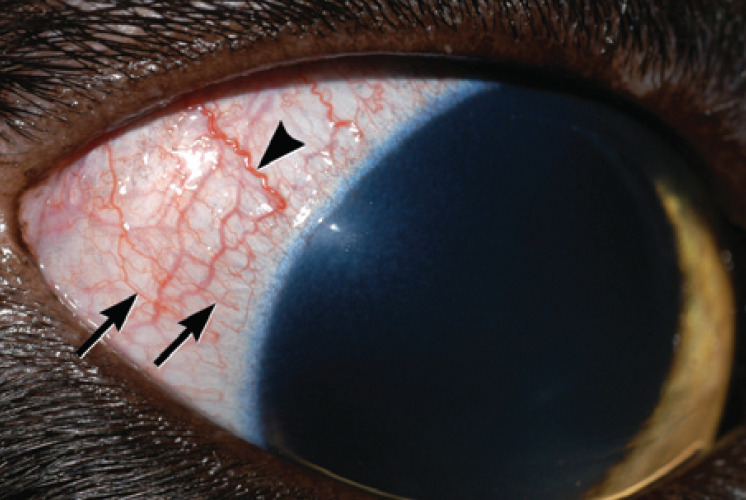
Deep episcleral vessels (arrowhead) should be differentiated from superficial conjunctival vessels (arrows). Where this is difficult, the aim must be to disprove that uveitis exists rather than assume that conjunctivitis exists. Note also the subtle corneal edema adjacent to the lateral limbus in this eye
A minority of cats (in particular brachycephalic individuals) may normally have a single large conjunctival or episcleral blood vessel. This should not confuse the diagnosis since cats with uveitis and episcleral injection will reliably have other evidence of uveitis including engorgement of multiple vessels rather than a single vessel. Congestion of the iridal vessels themselves is typically disguised from the examiner by melanocytes and iridophores. When it is noted, redness of the iris usually indicates neovascularization and not congestion (see later discussion of chronic changes).
Loss of function produces the major clinical signs of uveitis
The final hallmark of inflammation — loss of function — produces most of the pathognomonic or highly characteristic signs of uveitis (Table 2). It is critical to be alert to these signs.
Breakdown of the blood-aqueous barrier
Breakdown of the BAB is a pathognomonic sign of uveitis. Therefore the anterior chamber should be comprehensively examined for the presence of free-floating cells or proteins that would normally remain within the intravascular compartment. This is no different to inflammation elsewhere with exudation of intravascular contents into the interstitial space. In the eye, the aqueous humor is equivalent to the interstitial space such that the extent and nature of exudation can be observed. Hypopyon (white blood cells; fig 5), hyphema (red blood cells; Figs 5 and 6) and fibrin (fig 7) are usually relatively obvious, whereas specialized examination techniques are necessary to detect aqueous flare (albumin and other small proteins suspended in the aqueous humor) and keratic precipitates (KPs) (white blood cells and inflammatory proteins clumped against the corneal endothelial surface; fig 3b).
Fig 5.

A 12-year-old female spayed Manx cat with moderate hyphema and marked dense hypopyon bilaterally. This cat was diagnosed with lymphoma
Fig 6.
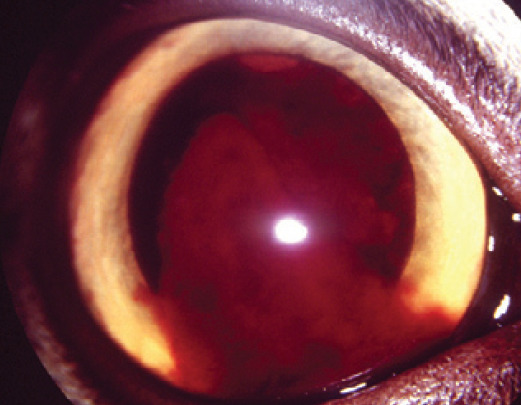
Right eye of a cat with marked, diffuse, unilateral hyphema as a result of recent blunt trauma
Fig 7.
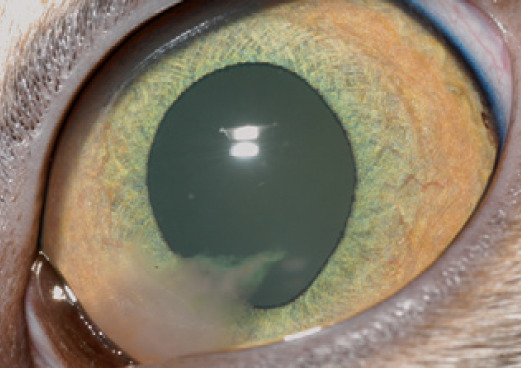
Left eye of a 15-month-old female spayed domestic shorthair cat with a moderate-sized fibrinous clot in the ventromedial anterior chamber. A cause was not found for the uveitis
Aqueous flare is best detected using magnification and a very focal, intense light source in a totally darkened room. A slit lamp is ideal; however, the beam produced by the smallest circular aperture on a direct ophthalmoscope held as closely as possible to the cornea and viewed transversely with magnification will also provide excellent results (fig 8). In the normal eye, a focal reflection is seen where the light strikes the cornea. The beam is then invisible as it traverses the almost protein- and cell-free aqueous humor in the anterior chamber and is visible again as a focal reflection on the anterior lens capsule, and then as a diffuse beam through the body of the normal lens. If uveitis has allowed leakage of serum proteins into the aqueous humor, this will scatter (and make visible) the light beam as it passes through the anterior chamber. A beam of light that is visible traversing the anterior chamber and joining the reflections from the cornea and anterior lens capsule therefore indicates aqueous flare.
Fig 8.

Aqueous flare can be detected by using the beam produced by the smallest circular aperture on a direct ophthalmoscope held as close as possible to the cornea in a completely darkened room and viewed transversely with magnification. Complete pupil dilation may allow aqueous flare to be seen more easily due to the apparent dark space created by the pupil
Keratic precipitates may be observed during routine examination of the cornea (fig 3b) but, when they are very small or few in number, their visualization may be enhanced using retroillumination (see box below). If present, KPs will be evident as multifocal dark spots obscuring the fundic glow (fig 9). Due to gravity, they are often more dense against the ventral cornea and can be missed unless the cat's nose is pointed down towards the floor so that the eye rolls up during the examination.
Fig 9.
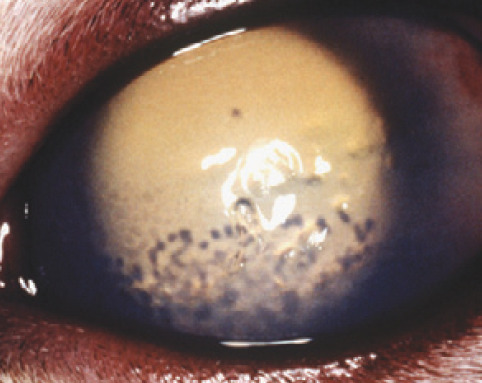
Keratic precipitates highlighted by retroillumination following pupil dilation
As these various figures show, one manifestation of the breakdown of the BAB may predominate, but frequently many such signs coexist.
The nature of the exudate within the anterior chamber provides clues as to the chronicity of the change (KPs suggest a more chronic process than hyphema, hypopyon or flare), as well as the degree of BAB breakdown (albumin is smaller than fibrin and red or white blood cells). These clues, in turn, provide a means of ranking the likely potential causes for further diagnostic testing (Table 1). However, there is considerable overlap and no exudate is pathognomonic for a given diagnosis. Similarly, the absence of one or more of the types of exudate does not allow a cause to be ruled out.
Miosis and hypotony
Other signs of uveal dysfunction (Table 2) are attributable to prostaglandin-mediated spasm of smooth muscle within the iris (evident as miosis) and ciliary body (evident as pain). While the ciliary body cannot be observed during the ophthalmic examination, miosis can. Subtle miosis is best detected using retroillumination (see box) with the transilluminator or direct ophthalmoscope held close to the examiner's eye and directed over the bridge of the patient's nose from at least arm's length so as to equally illuminate each eye. The fundic reflection is used to assess and compare pupil size and shape.
Even if miosis is not immediately evident, subtle iridal sphincter muscle spasm may still be present. This can be detected after applying one drop of tropicamide to each eye to dilate the pupils. The pupil of eyes with uveitis is often resistant to dilation compared with the contralateral normal eye, which should dilate fully within about 15 mins. IOP should also be assessed in all patients with suspected uveitis. In uveitis uncomplicated by glaucoma, the IOP is frequently low due to reduced production by the ciliary body and increased uveoscleral outflow. In subtle uveitis, IOP may be within the normal range (approximately 10–20 mmHg) but notably (> 20%) lower than the normal eye and so can still be classified as hypotony. If uveitis is complicated by impaired aqueous outflow, the IOP will be higher than the opposite eye and sometimes above normal. Coincident secondary glaucoma is a major concern in such cases.
In my experience, tonometry is one of the most sensitive tests for monitoring uveitis during treatment (see later).
Corneal edema
The cornea owes its transparency to a number of factors, among which a state of relative dehydration that is maintained largely by the corneal endothelium is critically important. With breakdown of the BAB and alterations in aqueous outflow, the composition of the aqueous humor is altered. Increased concentrations of inflammatory mediators and consumption of nutrients usually present are associated with dysfunction of the corneal endothelium and subsequent corneal edema. This appears as a bluish discoloration and loss of transparency of the cornea that can be very subtle in cats compared with dogs (fig 4). It is best seen by viewing the cornea from directly in front of the cat while directing a focal light transversely across the cornea.
Retroillumination
Retroillumination is a powerful method for detecting imperfections in any of the transparent ocular media (tear film, cornea, aqueous, lens or vitreous). In a darkened room, a focal light source (Finoff transilluminator or direct ophthalmoscope) is held close to the examiner's eye and directed into the patient's eye from at least arm's length to elicit the fundic reflection. This is usually gold or green in tapetal animals or red in atapetal individuals. Opacities in the ocular media, such as KPs on the inner cornea, will obstruct the fundic reflection.

If KPs cannot be seen initially then retroillumination should be repeated from much closer to the patient after pupillary dilation with one or two drops of tropicamide, at which time an otoscope head without the plastic cone can be used to retroilluminate the eye with added magnification (as pictured here).
Clinical signs associated with chronic uveitis and its sequelae
As with inflammation at other sites of the body, chronic uveitis induces a different set of changes from those seen with acute uveitis. Like elsewhere, these are associated with scarring (fusion of one tissue to another and cicatrization), chronic dysfunction and neovascularization (Table 2). The presence of vascular components within the anterior chamber due to breakdown of the BAB can cause clogging (in the subacute phase) and ultimately scarring (in the chronic stage) of the aqueous outflow tract. This in turn causes raised IOP and ultimately glaucoma. Adhesions may also occur between adjacent tissues; most notably the iris and lens (posterior synechiae) or iris and corneal endothelium (anterior synechiae). Posterior syriechiation is evident as melanotic debris adherent to the anterior lens capsule or changes in pupil shape (dyscoria), position (corectopia) or size (miosis or mydriasis). If posterior synechiae become extensive, accumulation of aqueous humor in the posterior chamber will cause a forward bowing of the iris (iris bombé) and secondary glaucoma. Peripheral anterior synechiae are less visible during ophthalmic examination but further obstruct the aqueous outflow and manifest as glaucoma. If severe enough, scarring within the globe may undergo cicatrization with subsequent contracture of the globe (phthisis) or tractional retinal detachment.
Altered aqueous humor composition and circulation also causes a relative malnutrition of the lens and inner cornea, with resultant cataracts and keratitis (corneal edema, vascularization, fibrosis, etc), respectively, once this becomes chronic. Lens luxation may occur due to enzymatic lysis or phagocytosis of the lens zonules, secondary to cataract development, or as a sequela to buphthalmos due to secondary glaucoma. Neovascularization of the face of the iris (rubeosis iridis) is a pathognomonic sign of subacute or chronic active uveitis.
What is a reasonable diagnostic approach to a cat with uveitis?
Having a high degree of clinical suspicion and performing a targeted clinical examination with appropriate ocular diagnostic testing (retroillumination, assessment of aqueous flare and IOP, and a dilated fundic examination — see box on the right) will ensure that uveitis is diagnosed when present. However, detecting uveitis is the beginning of the diagnostic process — not the end. Confirming or eliminating all suspected etiological diagnoses is the essential next step.
By conducting a thorough general physical and ophthalmic examination as well as gathering a focused history, the initial goal is to categorize the uveitis as present in a well or systemically ill patient; unilateral or bilateral; exogenous or endogenous; acute or chronic; and as involving the anterior uvea, choroid, or both. This will help to prioritize the diagnostic and ultimately the therapeutic approaches. I like to assemble evidence that strongly supports or does not strongly support the need for further diagnostic testing (fig 10). The majority of cases of feline endogenous uveitis are infectious, neoplastic or immune-mediated in origin. Application of the standard ‘DAMNIT-V’ list will identify some less common causes of uveitis.
Fig 10.
By conducting a thorough general physical and ophthalmic examination, as well as gathering a focused history, evidence can be assembled to strongly support or not the need for further diagnostic testing
Since immune-mediated uveitis is a diagnosis of exclusion the emphasis in the following discussion is the diagnostic approach to patients with suspected infectious or neoplastic uveitis.
Diagnostic tests for uveitis
The following tests are essential to detect evidence of uveitis and should be conducted in the order shown
Retroillumination - to look for:
Anisocoria (especially due to miosis)
Dyscoria
Corectopia
Iridal alrophy
Opacities in the clear ocular media (KPs, hypopyon, hyphema, vitreous debris, posterior synechiae, secondary cataract)
Oblique illumination of eye - to look for:
Corneal edema
Iridal swelling/nodules
Iridal thinning/atrophy
Rubeosis iridis
Posterior synechiae
Iris bombe
Hypopyon
Hyphema
Fibrin in the anterior chamber
IOP measurement (tonometry) - to look for:
Decreased or low-normal pressure, suggesting uncomplicated uveitis
Elevated or high-normal pressure, suggesting uveitis complicated by glaucoma Pupil dilation - to look for:
Resistance to dilation
Snow banking
Assessment of aqueous flare - to look for:
Serum proteins in the anterior chamber due to breakdown of the blood-aqueous barrier
Fundic examination - to look for:
Signs of posterior uveitis (retinal detachment/degeneration, chorioretinal granulomas, hemorrhage, edema)
Vitreous debris/infiltrates
Snow banking
Fluorescein staining - to look for:
Corneal ulceration, which suggests exogenous (axonal) uveitis. Corneal ulcers also preclude use of topical corticosteroids
Diagnostic approach for infectious uveitis
An etiologic diagnosis in a case of infectious uveitis is usually reached in one or more of four ways.
Detection of infectious agent within ocular tissues or fluids Detection of the infectious agent directly within the eye is best suited to larger organisms. For example, migrating or aberrant parasitic larvae can be detected without magnification within the anterior chamber or vitreous.3,4 Fungal organisms, protozoa or bacteria 5 may be detected histologically within biopsy tissue or cytologically following paracentesis of the anterior chamber, subretinal space or vitreous body. Culture of these aspirates may also be attempted; however, retrospective studies reveal that this is a low-yield approach.1,6 More recently, polymerase chain reaction (PCR) has been used to detect organism DNA in ocular aspirates or tissue samples. However, due to the extreme sensitivity of this technique certain organisms may be found intraocularly or through surface or blood contamination at the time of sampling in healthy animals, thus lowering the diagnostic sensitivity of this approach. Additionally, since breakdown of the BAB is a hallmark of uveitis, any organism present within the bloodstream can leak into the inflamed eye whether or not it is the cause of the inflammation. Therefore, intraocular detection of such organisms does not prove causation of the uveitis. For these various reasons, false negative and false positive detection of intraocular organisms or their DNA may occur in cats with uveitis.
Serologic evidence of the agent
The value of serology for diagnosing the cause of uveitis varies greatly with the organism. Circulating antibodies suggest exposure to that organism but do not necessarily suggest that exposure was recent, let alone current. For example, immunoglobulin (Ig) G titers to Toxoplasma gondii may remain elevated for many years in healthy animals. 7 IgM titers have been assessed as a means of detecting more recent infection. However, like IgG, detection of this antibody class simply reflects exposure to a given organism and not necessarily causation of the current clinical signs; furthermore, IgM titers may not be elevated in animals undergoing recurrent rather than primary disease. 8 An additional complication is that antibody tests can be cross-reactive with related but sometimes non-pathogenic organisms. An example of this is the failure of serologic assays to discriminate between antibodies directed at the FIP virus and those targeting the feline enteric coronaviruses.
Detecting uveitis as present is the beginning of the diagnostic process — not the end. Confirming or eliminating all suspected etiological diagnoses is the essential next step.
Finally, antibody tests typically do not differentiate natural exposure to the pathogenic organism from vaccinal exposure to a modified, nonpathogenic variant. A long-recognized example of this phenomenon is feline herpesvirus 1 (FHV-1), while the recent release of an FIV vaccine means that this organism must now be added to that list. Together, these shortfalls lead to serious concerns regarding the etiologic diagnosis of feline uveitis based on serologic testing alone. In one study, equivalent numbers of cats with and without uveitis had circulating antibodies to FHV-1, 9 while in another study circulating antibodies specific to Bartonella species were more common and of greater titer in cats without uveitis than in cats with uveitis. 10
Regardless, there are some organisms and situations in which antibody testing is useful. For example, because FIV is not usually cleared by the immune system, detection of circulating antibodies to FIV in an unvaccinated cat is considered confirmation of infection with that virus (although does not confirm that any uveitis present is caused by the virus). Conversely, negative titers can sometimes be used to exclude that organism as a cause, especially in cats with chronic uveitis. Detection of circulating antigens, as is done for feline leukemia virus (FeLV) or Cryptococcus species, is also extremely useful in confirming current infection with those organisms. For most other organisms, though, the major use of serology seems to be large, controlled epidemiologic studies.
Evidence of agent-specific intraocular antibody production
The questionable value of antibody detection in the serum has led to interest in the diagnostic value of intraocular antibody production by lymphocytes within the uvea. However, breakdown of the BAB permits serum antibodies to leak into the eye and these must be differentiated from locally produced antibodies. This is done by calculating the ratio of the antibody of interest to total antibody within the serum and within an intraocular fluid (typically aqueous humor). This is called the Goldmann-Witmer coefficient and is expressed as a C value (see box, page 175). If this ratio is greater intraocularly than in the serum (ie, C value > 1), intraocular antibody production is assumed.
However, the diagnostic utility of the Goldmann-Witmer coefficient is reduced by knowledge that non-specific immune stimulation can cause specific intraocular antibody production. In one study, naive cats and cats previously exposed to T gondii were exposed subcutaneously to inactivated T gondii antigens or to Freud's adjuvant. 8 Previously infected cats produced intraocular antibody (C values > 1) even when stimulated with adjuvant alone. Of even more interest, Toxoplasma-naive cats had C values > 1 when stimulated with inactivated Toxoplasma antigens. These findings have two important implications. First, they confirm that the uvea can behave like a reactive lymph node in response to a distant and sometimes unrelated antigen. Secondly, they suggest that higher C value cut-points might have more clinical utility While this requires further investigation, there is some emerging support for this hypothesis. 9
Detection of suggestive changes outside the eye
In many cats with uveitis the organism may be detected at extraocular sites. This is particularly true for systemic mycoses where cytologic assessment of tissue aspirates, nasal discharge or draining skin tracts may yield evidence of a pathogen known also to cause uveitis. Although not pathognomonic, changes noted on evaluation of a serum biochemistry panel and complete blood count (CBC) can permit prioritizing of differential diagnoses or direct further testing for those organisms that cause systemic changes in conjunction with uveitis.
Diagnostic approach for neoplastic uveitis
Reaching an etiologic diagnosis in a patient with neoplastic uveitis tends to be somewhat easier than for infectious uveitis and relies on cytologic or histologic demonstration of tumor within the eye or at a distant site with signs consistent with metastasis. As such, the diagnostic approach replicates the investigation of neoplasia at other sites.
Intraocular neoplasia tends to manifest in one of three ways — breakdown of the BAB (with hyphema, hypopyon, intraocular fibrin or aqueous flare), ocular dysfunction as a result of a mass effect (dyscoria, anisocoria, corectopia, glaucoma, etc), or as a visible mass within the eye. Uveal masses can often be missed because the majority of the uvea (choroid, ciliary body and posterior iris) is not visible during the customary external ocular examination, and because the ciliary body and posterior iris cannot be examined even after full pupil dilation. Thus, ocular neoplasia, whether it is a discrete mass hidden from view (such as a ciliary body adenoma) or diffuse uveal invasion of a metastatic tumor (such as lymphoma), should always be considered in patients with endogenous uveitis.
Staging of diagnostic testing of cats with uveitis should be based on risk, cost and expected yield (see below). Any tests should always follow and be guided by excellent history-taking, and results of general physical and ocular examinations, and be tailored for the age and lifestyle of the cat, regions of the world that the cat has lived in or visited, and the clinical signs seen.
As for infectious agents, the diagnostic search typically begins outside the eye with a thorough general physical examination, CBC, serum biochemistry profile, urinalysis, and imaging of the abdominal and thoracic cavities. Due to the association with lymphoma, serologic assessment of FeLV and FIV status is wise. Any masses elsewhere, as well as lymph nodes, should always be aspirated. Since cytologic abnormalities are not always associated with lymphadenomegaly, aspiration of palpably normal lymph nodes should be considered; 11 as there is no ‘draining’ lymph node for the eye, any lymph node can be aspirated. If a diagnosis cannot be reached with extraocular testing, then cytologic assessment of an aspirate of aqueous or vitreous humor, sub retinal fluid (if the retina is detached) or an intraocular mass can be undertaken by those trained and experienced in these techniques. 6
What are the most common causes of endogenous anterior uveitis in cats?
The known causes of endogenous anterior uveitis in cats are expanding but still too few to explain the majority of cases. To my knowledge, the most recent reasonably large retrospective analyses of feline uveitis were published in 1991.1, 12 Following very complete clinical examination and clinicopathological, serological and histopathological testing at that time, a cause was discovered in only 30% of 53 cats with uveitis in one of these studies. The most commonly identified agent in these cats was FeLV and the most commonly identified cause of uveitis was lymphosarcoma or melanoma. 1 In the second study, 139 cats (158 globes) with such severe disease that their eyes were enucleated or the cats died or were euthanazed were retrospectively assessed. A cause was found in only 67% of cases; the most common diagnoses were FIP, FeLV-associated lymphosarcoma and trauma. 12
Infectious agents
Table 3 lists the microbiological causes of feline uveitis. Only the more commonly implicated and better understood agents are discussed here and, as with all diagnostic enquiries, clinicians should consider the previous and current geographic locale in which the patient has resided when ranking the likelihood of each agent.
Table 3.
Infectious causes of feline uveitis
| Viral | Bacterial | Fungal/algal | Parasitic | Protozoal |
|---|---|---|---|---|
| FIP15,16 | Bartonella | Cryptococcus neoformans 53 † | Cuterebra 3 | Toxoplasma gondii 52 |
| FeLV47* | species10′28′29 | Histoplasma capsulatum 53 † | Leishmania species 5 | |
| FIV 47 | Mycobacterium | Blastomyces dermatitidis 53 † | ||
| FHV 9 | species48′49 | Candida albicans 54 | ||
| Ehrlichia species 50 ‡ | Coccidioides immitis 55 ‡ | |||
| Borrelia burgdorferi 51 | Aspergillus species 56 |
Via immunosuppression or oncogenesis
Chorioretinitis predominates
Seroprevalence data only (no clinical evidence)
Viral
Perhaps one of the best understood viral causes of uveitis is FIP. Signalment (younger patients often from a multicat environment), 13 clinicopathologic findings (hyperglobulinemia, lymphocytopenia, and seropositivity to feline coronavirus)13,14 as well as a highly cellular panuveitis (KPs, hypopyon, chorioretinal granulomas) often permit FIP to be elevated as a prime differential consideration in affected animals (Table 1). The majority of cases are seen with the non-effusive or ‘dry’ form of the disease and typically ocular signs coexist with other systemic signs such as fever, lethargy, anorexia or neurologic signs15,16 The major differential consideration is lymphoma and therefore this is one of the circumstances where aqueocentesis is worth considering. Although pathognomonic cytologic changes are not seen with FIP, patients with lymphoma may have neoplastic cells within the anterior chamber. Additionally, I have found the positive (89%) and negative (99%) predictive values of the triad of hyperglobulinemia, lymphocytopenia and seropositivity to feline coronavirus reported by Sparkes et al 14 to be particularly useful in diagnosing cats with uveitis believed to be due to FIP.
Aqueous humor samples from affected cats can be tested for the presence of feline corona-virus DNA. However, this does not provide definitive evidence of FIP as being the cause of uveitis because the PCR assay cannot differentiate FIP from enteric coronaviruses, because enteric coronaviruses in white blood cells can be detected with this assay, and because white blood cells are present intraocularly in uveitis resulting from many different causes.17–19
Cats presented with anterior uveitis are sometimes infected with FIV or FeLV; however, the role of these retroviruses is not always directly causal. In fact, FeLV is not a proven cause of anterior uveitis in cats 20 and ocular disease is a very uncommon finding in cats seropositive for this virus.21,22 By contrast, FIV is suggested to be a cause of pars planitis, 23 and one study found 84% of cats with advanced FIV infection to have lymphocytic/plasmacytic iridocyclitis when examined histologically. 24 This is sometimes clinically evident as inflammatory cell accumulation in the anterior vitreous immediately behind the iris. 23 However, this lesion, which is termed 'snow banking’ (fig 11), is not pathognomonic. In fact, it is an incidental finding in some patients with no apparent discomfort or other obvious signs of active uveitis and may represent slower removal of inflammatory cells from the vitreous than from the anterior chamber.
Fig 11.
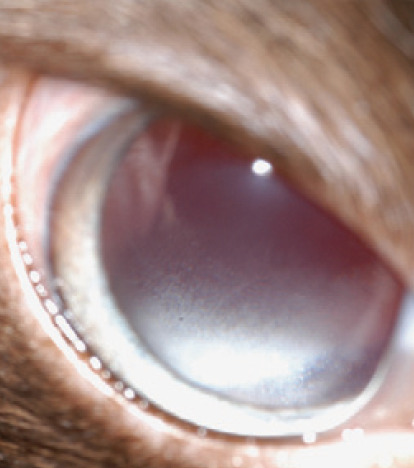
White blood cell accumulation in the anterior vitreous is termed 'snow banking’ and may be seen with oblique examination of the eye following wide pupil dilation. This lesion is seen in patients with pars planitis or intermediate uveitis
Notwithstanding, both retroviruses should be on the list of differential considerations for feline uveitis because they are important causes of lymphoma, which is the most common neoplastic cause of uveitis in cats. In particular, FIV-infected cats develop B-cell lymphoma, 25 which can occur within the eye without lymph node or bone marrow involvement. 26 FIV and FeLV can also permit superinfection with uveitogenic organisms via immunosuppression.
Bacterial
Theoretically, any organism responsible for bacteremia could also cause uveitis, but, in practice, this is extremely rare; perhaps due to the integrity of the normal blood-ocular barrier. However, there is increasing evidence that Bartonella species are associated with and may even cause uveitis in some cats. An initial case report described a cat with uveitis without any other detectable cause. 27 There was notable intraocular production of Bartonella species-specific antibodies (C value = 4.4) but intraocular detection of Bartonella DNA or culture was not attempted. The cat's uveitis was unresponsive to a 4-week course of topical and systemic corticosteroids, but responded promptly and completely when doxycycline was added to the treatment regimen.
Subsequently, a number of epidemiologic studies have revealed that circulating antibodies to Bartonella species are common in experimentally infected cats and cats with uveitis, and that intraocular antibody production and organism DNA are more common in cats with uveitis and in experimentally infected cats than in cats without uveitis.10,28,29 However, neither the presence nor titer magnitude of Bartonella species-specific antibodies in serum are diagnostically reliable.
Fungal/algal
The fungi capable of causing systemic mycosis in cats frequently cause uveitis, albeit the organisms encountered vary somewhat with geographic region. Histoplasma capsulatum and Cryptococcus species tend to be the most commonly reported. Less common causes include Blastomyces dermatitidis and Coccidioides immitis. Other fungi and algae are sometimes seen, particularly in immunocompromised patients. Although all can cause anterior uveitis, fungal elements seem to have a predilection for the posterior segment and so chorioretinitis predominates.
Regardless of ocular location, fungal uveitis tends to be extremely granulomatous, manifesting as ‘greasy’ KPs, a highly cellular and fibrinous hypopyon, and granulomas within the retina and choroid, often with exudative retinal detachments. Most affected cats are systemically ill and fungal elements may be detected at extraocular sites; especially skin tracts, lungs, lymph nodes and bone. In patients with a blind and painful globe the diagnosis may reasonably be achieved by therapeutic enucleation with histopathology since the fungal elements are typically numerous and can be seen on light microscopy. Antigen detection is particularly useful for diagnosing cryptococcosis.
Protozoal
Protozoal species may also cause feline uveitis. Of these, T gondii has been the most extensively studied using techniques such as fulfillment of Koch's postulates,30,31 intraocular detection of organisms, 32 detection of circulating T gondii-specific antibodies33,34 or antigens, 35 response to therapy, 33 and detection within aqueous humor of T gondii-specific intraocular antibody production, 36 antigens, 36 DNA 37 and immune complexes. 38 Taken together, data from these studies make it clear that T gondii is associated with uveitis in some cats; especially chorioretinitis in systemically ill cats during primary exposure to the organism. 32 However, the role of T gondii in the more insidious anterior uveitis of older, otherwise healthy cats and the correct means of diagnosing T gondii as the cause of disease in those cats are not well established. While reactivation of tissue cysts is one proposed mechanism, there is accumulating evidence that the presence of organisms within the eye may not be necessary. Rather, immunopathology due to an autoantigen, molecular mimicry or loss of immunotolerance may be involved.8,39 Unfortunately, no test(s) can currently be recommended to definitively diagnose toxoplasmic uveitis in systemically well cats because many normal cats are seropositive for the organism,33,34 intraocular antibody production can be induced even in T gondii-naive patients, 8 and T gondii DNA can be detected in aqueous humor samples from cats without uveitis. 37 However, lack of evidence of intraocular organisms along with evidence of immunopathology does endorse the use of topical corticosteroids in such patients.
Increasing evidence that Leishmania species can cause uveitis in dogs, 40 as well as case reports of Leishmania amastigotes within the iris and ciliary body of cats with granulomatous panuveitis,5,41 suggest that this organism causes uveitis in cats. Intraocular examination via light microscopy, immunohistochemical assessment and PCR analysis appear to be useful for diagnosis.
Neoplasia
While the most common primary intraocular neoplasm is melanoma, this typically causes little or no uveitis. By sharp contrast, the most common metastatic ocular neoplasm — lymphoma — tends to be associated with marked breakdown of the BAB with hypopyon formation, fibrin exudation into the anterior chamber, and hyphema. Curiously, the degree of pain associated with this disease often appears less than might be expected from the severity of other signs of intraocular inflammation. The exception to this is when secondary glaucoma occurs, which can be quite frequent due to the highly cellular and fibrinous nature of the anterior chamber exudate.
Lymphoma can cause unilateral uveitis, sometimes without extraocular involvement. If the tumor cannot be diagnosed from an extraocular site, detection of neoplastic lymphocytes in an aqueocentesis sample may be helpful. 6 Diagnostic testing aimed at discovering evidence of the tumor elsewhere should be performed first, however, both for staging the disease and because it is less invasive.
Despite the severity of intraocular inflammation in many of these patients, this is often one of the more rewarding forms of uveitis to treat. If the patient's tumor is one that readily undergoes remission when chemotherapy is initiated, then there is usually a dramatic concurrent reduction in the degree of uveitis. Topical application of adjunctive corticosteroids is wise. Radiation is not a realistic option, even in patients where the eye appears to be the sole site, because of its painful and frequently blinding effects on ocular tissue. 42 Mean survival time after diagnosis in one study was 14 months. 43
Therapy for uveitis
Immunomodulatory agents
Corticosteroids are highly potent, available in topical or systemic forms, relatively inexpensive, generally well tolerated by cats, and can be administered at anti-inflammatory or immunosuppressive dosages. For these reasons, they are commonly used for feline uveitis. Their systemic use should be reserved until a definitive cause responsive to corticosteroids has been found or, failing this, causes known to be worsened by glucocorticoids have been adequately eliminated. In particular, systemic mycoses must be ruled out as potential causes. Likewise, patients in which lymphoma is possible and which would benefit from multidrug chemotherapy should not be treated with systemic corticosteroids alone.
By contrast, topical corticosteroids may be used safely even when an infectious or neoplastic cause might prevent systemic administration of the same drugs. This is because systemic effects are insignificant with short-term topical application. It is possible that topically administered corticosteroids may alter cytologic findings and so, if safe, their use should perhaps be delayed until ocular centesis has been performed. Topical corticosteroids should never be used in the face of corneal ulceration because they can be associated with rapid worsening of the ulcer due to superinfection, collagenolysis, local immunosuppression and delayed wound healing. Prednisolone acetate (1% or 0.125%) and dexamethasone (0.1%) will penetrate intact corneal epithelium and reach the anterior uveal tract. Hydrocortisone (as found in many combined antibiotic-corticosteroid ophthalmic preparations) does not penetrate intraocularly and should not be used. The frequency of application should be tailored to the severity of the uveitis, starting as frequently as q 2 h and tapering as a clinical response is noted.
When safe, corticosteroids should be administered systemically for posterior uveitis and when more significant immunomodulation is necessary, or when corneal ulceration prohibits their topical use. Typical doses of prednisolone range from 1 mg/kg q 12 h when notable inflammation is present to 0.5 mg/kg once daily when a more moderate anti-inflammatory effect is desired. As with topical corticosteroids, dose and dose frequency of systemically administered glucocorticoids should be carefully reduced based entirely on clinical evidence of waning disease. In cats with acute/subacute uveitis, this can be fairly rapid. Cats with chronic idiopathic (immune-mediated) uveitis require slow tapering (eg, a halving of dose or dose frequency every 2–3 weeks), with the expectation that inflammation may return below a critical dose. In these patients, returning to the previously effective dose will be necessary. Some cats will suffer herpetic recrudescence when receiving corticosteroids, regardless of route.
Compared with corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs) are not immunosuppressive, and in some countries are more expensive, sold in smaller volumes and may not be available in ointment form. These limitations make them a generally less useful group of drugs than corticosteroids for cats with uveitis. However, they may be preferred in patients with diabetes or other endocrinopathies in which corticosteroid use may not be wise. They can also be administered systemically in place of corticosteroids when systemic infectious disease is suspected or proven, or until lymphosarcoma is eliminated as a differential consideration. As such they may make an excellent choice for initial control of inflammation while likely causes are being ruled in or out. The above general comments regarding dose frequency and route of administration of corticosteroids apply equally to NSAIDs. Also, this class of drugs is known to reactivate human herpesviruses 45 and could do likewise in cats with FHV-1.
Ibibrocycloplegic agents
Parasympatholytic drugs such as atropine have multiple favorable actions in eyes with uveitis and form a critical component of treatment. These drugs paralyze the parasympathetically innervated iris sphincter and ciliary body muscles, causing mydriasis and cycloplegia, respectively.
Pupil dilation has numerous important effects. It reduces leakage of vascular elements into the aqueous humor by causing radial blood vessels within the iris stroma to ‘concertina’ (providing a physiological tamponade); iris surface area is decreased (from which inflammatory mediators and vascular components originate); uveal vascular endothelial permeability is reduced; and the risks and consequences of posterior synechiation are diminished. However, ‘bunching’ of the iris in the periphery does increase the risk of anterior synechiae and potentially obstruction of the iribibrocorneal angle.
Cycloplegia reduces ocular pain but also increases resistance to aqueous outflow. Therefore, pupil dilation and cycloplegia are desirable in all cases of uveitis except those where secondary glaucoma is present or likely. In these patients, the effect of mydriasis on IOP can be tested by a single application of the short acting drug tropicamide followed by tonometry when the pupil is fully dilated. If IOP is increased by tropicamide, atropine should not be administered. If atropine therapy is initiated, IOP should be rechecked regularly and the drug discontinued if IOP increases above normal. An ophthalmic ointment formulation rather than a solution should be used because atropine is bitter and passage down the nasolacrimal duct can cause violent salivation and frothing, which is harmless but disturbing to the cat and its owner. Atropine should be applied to effect. Since cycloplegia cannot be observed, the pupil is used for monitoring dose. Depending on the severity of uveitis, once to twice daily application may be needed for the first day or two to open the pupil. Subsequently, once to twice weekly application will often keep the pupil mydriatic. Posterior synechiae will not be resolved with atropine and will prohibit use of pupil size as an indicator of drug efficacy. However, atropine should still be administered to patients with synechiae since the analgesia resulting from cycloplegia should not be affected.
Autoimmunity
The only common type of uveitis in cats in which autoimmunity is confirmed is lens-induced uveitis. This occurs in two forms: phacolytic uveitis, where there is slow leakage of lens proteins across an intact lens capsule, as occurs with advanced cataracts; and phacoclastic uveitis, which is a more severe form associated with lens capsule rupture and resultant rapid leakage of large amounts of lens proteins. In both forms (phacoclastic particularly so), lens proteins are released in amounts that exceed immunotolerance, leading to a true autoimmune response. Phacolytic uveitis occurs far less frequently in cats than in dogs due to the relatively low incidence of feline cataracts; especially diabetic cataracts with which this syndrome is most commonly seen. By contrast, phacoclastic uveitis represents a significant concern in cats not only due to its severity but because a percentage of cats with traumatic lens injuries develop post-traumatic intraocular sarcoma, which can be fatal, usually through extra-orbital invasion but sometimes due to metastasis. For this reason, eyes with traumatic lens capsule rupture should be aggressively treated and very carefully monitored for the life of the cat. Traumatized eyes that are blind should be enucleated prior to development of this tumor.
KEY POINTS
Anterior uveitis is common, subtle, potentially blinding, and sometimes an indicator of systemic disease.
Consider uveitis as a differential diagnosis early and frequently in sick cats with or without localizing ocular signs.
Run sufficient appropriate diagnostic tests to permit classification of each patient as having idiopathic endogenous uveitis or uveitis of known cause.
Wherever possible, treat the specific cause of uveitis, use anti-inflammatory agents judiciously, and aim for minimal non-specific immunosuppression of the patient.
Idiopathic uveitis must be treated as an immune-mediated disease. Use aggressive and early immunosuppressive therapy and taper slowly according to clinical improvement.
Closely monitor all cases, especially those identified as idiopathic, for recurrence and/or sequelae.
Aside from lens-induced uveitis, many other uveitides are proposed to be immune-mediated although specific autoantigens have not been identified. Various immunological mechanisms are possible including loss or failure of development of immunotolerance to autoantigens usually sequestered within the eye behind the blood-ocular barrier or exposure of autoantigens during breakdown of the blood-ocular barrier due to an unrelated inflammatory insult. These autoantigens remain the target of ongoing immunologic activity after the primary insult has been resolved. Molecular mimicry, whereby autoantigens sufficiently like foreign peptides of infectious agents initiate or perpetuate intraocular immune responses, may also be important in immune-mediated uveitis. This has been shown for other species such as the horse, in which the cornea and lens contain proteins very similar to those found in leptospirosis. 44 Finally, it is possible that normal host proteins within the eye become altered such that they become antigenic.
These hypotheses help explain the common scenario whereby a thorough antigen search is unrewarding and the patient improves with empirical anti-inflammatory therapy, only to deteriorate as this is tapered below a critical level.
What are the major treatment decisions?
Treatment of feline anterior uveitis must be tailored to the individual case based on proven or suspected cause, severity, anatomical location, chronicity, and the presence of systemic or other intraocular disease. Regardless, some general therapeutic guidelines are possible. Optimal treatment relies on identification and removal or reduction of the causative antigen; however, this is rarely possible. Additionally, all patients with uveitis need their intraocular inflammation controlled rapidly and completely, since it is painful and produces vision-threatening sequelae. Thus, immunomodulating drugs form the mainstay of therapy for uveitis. The major decisions are therefore which immunomodulating drugs should be given, via what route and at what dose (see box, page 178).
Potential sequelae and monitoring of treatment
Prompt specific treatment of uveitis with tapering of therapy based on reduction of clinical signs may result in some sequelae but these are usually mild and should be static. If they are not, this suggests chronic or recurrent uveitis and further investigations and treatment are necessary. Classic sequelae include corneal fibrosis, cataract or posterior synechiae. None of these changes should result in pain or, unless severe, vision disturbance. By contrast, more severe, unrecognized, persistent or recurrent uveitis frequently results in a blind and sometimes painful globe.
George, a 16-month-old male castrated domestic shorthair cat, presented with ‘cloudiness’ of both eyes which his owners had observed over the past 24 h.
Pertinent history George was found as a feral kitten. After a short period in a humane shelter, he was adopted by his current owners at about 5 months of age. He now lives indoors with one other indoor cat. His owners state that there is a rat problem in the home but that neither George nor the other cat hunts. Other than a short episode of upper respiratory disease many months ago, which was diagnosed as FHV-1 infection, George has been in good health since they obtained him. Over the past 3–4 weeks George's owners have noticed a reduction in his energy levels: he has been playing less with his favorite toy, and more recently has just been sitting quietly on the couch. Over the past week, George has appeared ‘wobbly’, more lethargic, and his appetite has decreased. Yesterday, they observed the cloudiness in his eyes. He has no history of vomiting, diarrhea, coughing or sneezing, and his thirst and urination patterns appear normal.
Ophthalmic examination On full ophthalmic examination both eyes were open and appeared comfortable, and George behaved as if he was at least partially sighted. There was no overt ocular discharge from either eye. On retroillumination, the fundic reflection from the left eye (OS) was generally reduced or ‘hazy’, especially in the ventral half; no fundic reflection could be elicited from the right eye (OD). No overt anisocoria was noted. Sequential examination of both eyes revealed normal eyelids and third eyelids. The anterior chamber of both eyes (OU) contained a fibrin clot and 1+ (out of 4+) aqueous flare. The anterior chamber OU was notably shallow due to a markedly swollen iris, potentially with iris bombé. There was marked rubeosis iridis OU. The left pupil was displaced medially (corectopia). The direct pupillary light reflex was present OS and a consensual reflex was present from OD to OS. It was not possible to assess movements OD due to the anterior chamber fibrin. Neither pupil dilated after two topical applications of tropicamide. This (along with the anterior chamber fibrin) limited examination of the lens, vitreous and fundus, but no further abnormalities were noted OU. Fluorescein stain was not retained by either cornea and was visible within 2 mins at both nares.
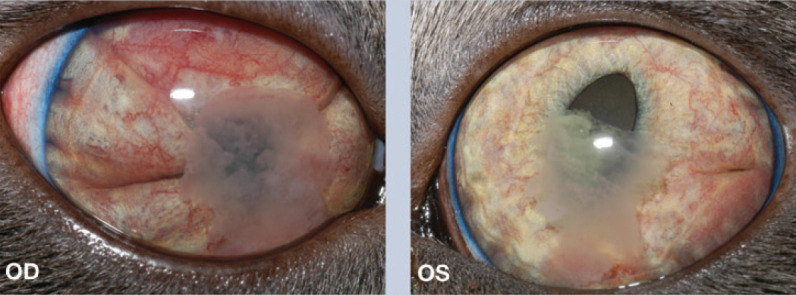
WHAT IS YOUR ASSESSMENT?
What clinical abnormalities do you see in George's eyes?
Based on the clinical description and your observations, what pathognomonic signs of uveitis are present?
Would you characterize George's uveitis as anterior or posterior, endogenous or exogenous, idiopathic or with identified cause, acute or chronic, and unilateral or bilateral? Do you consider George to be a well or systemically ill patient?
How would you proceed?
Measurement of IOP.
General physical examination.
CBC, biochemistry profile and urinalysis.
Retroviral testing.
All of the above.
The fact that George has bilateral chronic, endogenous, fibrinous uveitis and is systemically unwell strongly supports stage 1 testing (see box, page 175). Stage 2 testing could be initiated simultaneously or delayed for prioritization based on stage 1 test results if preferred. George's owners elected to perform all stage 1 and some stage 2 tests.
Measurement of IOP IOP should always be measured in cats with uveitis. George's IOP was 20 mmHg OD and 14 mmHg OS. Given that marked anterior uveitis should be associated with low IOP these pressures are consistent with ocular hypertension/glaucoma OD and suggest it may be beginning OS.
General physical examination George was apparently bright and responsive. He was in good body condition and weighed 6.1 kg. His temperature was 39.2°C and his pulse and respiratory rates were 138 beats per minute and 24 breaths per minute, respectively. No abnormalities were detected on thoracic auscultation; however, the kidneys appeared enlarged and there was a mobile caudal abdominal mass. Lymph nodes were normal on palpation.
GBO, biochemistry profile and urinalysis Hematology revealed lymphopenia and a mature neutrophilia, which was interpreted as a stress leukogram. There were no abnormalities on the serum biochemistry panel and urinalysis.
Retroviral testing FeLV, FIV and coronavirus serology were all negative.
Further diagnostics Abdominal ultrasound revealed bilaterally enlarged kidneys with considerable hypoechoic tissue in the subcapsular space and mild pelvic dilation. A large intramural mass was also noted in the distal jejunum. Several mesenteric lymph nodes were mildly enlarged, hypoechoic and round. There was scant ascites. The intestinal mass and kidneys were aspirated for cytologic assessment.
5 Which of the following best ranks (in decreasing order) the likely differential diagnoses for George's uveitis?
Toxoplasmosis, lymphoma, bartonellosis.
Lymphoma, FIP, metastatic neoplasia.
Idiopathic/immune-mediated, melanoma, FIV.
FeLV, idiopathic/immune-mediated, trauma.
Answers and discussion
Rubeosis iridis OU, iridal swelling OU, fibrin in the anterior chamber OU, corectopia OS, episcleral and conjunctival hyperemia OD
Rubeosis iridis OU, iridal swelling OU, fibrin in the anterior chamber OU, aqueous flare OU
-
George's uveitis is subacute/chronic because rubeosis iridis occurs with more longstanding uveal inflammation. The aqueous flare confirms that there is ongoing breakdown of the BAB.
Ophthalmic examination failed to identify an obvious exogenous cause; no corneal ulcer is evident and trauma would not produce chronic changes. Additionally, with trauma, hyphema would be more likely than pure fibrinous exudation. All this points to anterior uveitis that is chronic, active, endogenous, and bilateral in a systemlcally unwell cat (though panuveitis cannot be ruled out because a complete posterior segment examination cannot be performed).
(e)
Answer and discussion
5 (b). Lymphoma is the most likely diagnosis for a young, systemically unwell cat with bilateral, fibrinous, subacute uveitis, iridal swelling and extraocular (abdominal) masses (see Table 1). This is supported by the essentially normal findings on serology, CBC, serum biochemistry and urinalysis (which make infectious disease less likely). The bilateral kidney enlargement and Intestinal mass are most consistent with lymphoma or other metastatic neoplasia, although fungal disease or FIP should also be considered. Trauma can be ruled out because of the subacute to chronic clinical course of the uveitis. Idiopathic uveitis is typically chronic and mild, seen most commonly in middle-aged to older healthy cats and is usually unilateral (at least at first). Primary melanoma has not been reported as a bilateral disease, is rarely inflammatory, and is typically associated with a pigmented lesion. George's seronegativity to FeLV and FIV (although not absolute) further lower the likelihood of answers (c) and (d).
Case outcome
Cytologic assessment of the ultrasound-guided intestinal and renal aspirates was consistent with lymphoma. At the owners’ request, further staging was not conducted and therapy was initiated with L-asparaginase (3000 IU SC), prednisolone (15 mg PO q 24 h) and prednisolone acetate ophthalmic suspension (1 drop OU q 4 h). Response to therapy was poor and the owners elected euthanasia. On full post mortem examination, George was diagnosed with multicentric large B-cell lymphoma.
The most common sequelae include cataracts (20–36% of eyes), lens luxation (11–18%), glaucoma (16–46%) and enucleation (29%).1,46 Many cats experience more than one of these and thus frequent and careful monitoring of a patient with uveitis is essential. This should be performed as for patients with immune-mediated disease elsewhere, with gradual tapering of medications and re-examination at doubling intervals assuming there is improvement; more often if there is not. Re-examination and tapering of medications should be continued until there is complete resolution of every clinical sign of active uveitis.
I believe that tonometry is the most sensitive test with which to monitor uveitis during treatment because subtle hypotony (sometimes only relative to the contralateral eye) can continue long after other more overt signs have normalized. Ongoing treatment of these patients may prevent or delay the development of sight-threatening ocular complications.
Acknowledgements
I thank all members of the University of California Davis Ophthalmology Service for many of the photos and John Doval for biomedia assistance. In particular, I appreciate Dr Sara Thomasy's willingness to share clinical details and photos used in the case notes.
References
- 1.Davidson MG, Nasisse MP, English RV, Wilcock BP, Jamieson VE. Feline anterior uveitis: A study of 53 cases. J Am Anim Hosp Assoc 1991; 22: 77–83. [Google Scholar]
- 2.Powell CC, Lappin MR. Causes of feline uveitis. Compend Contin Educ Pract Vet 2001; 23: 128–40. [Google Scholar]
- 3.Harris BP, Miller PE, Bloss JR, Pellitteri PJ. Ophthalmomyiasis interna anterior associated with Cuterebra spp in a cat. J Am Vet Med Assoc 2000; 216: 352–55, 345. [DOI] [PubMed] [Google Scholar]
- 4.Stiles J, Rankin A. Ophthalmomyiasis interna anterior in a cat: Surgical resolution. Vet Ophthalmol 2006; 9: 165–68. [DOI] [PubMed] [Google Scholar]
- 5.Leiva M, Lloret A, Peña T, Roura X. Therapy of ocular and visceral leishmaniasis in a cat. Vet Ophthalmol 2005; 8: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olin DD. Examination of the aqueous humor as a diagnostic aid in anterior uveitis. J Am Vet Med Assoc 1977; 171: 557–59. [PubMed] [Google Scholar]
- 7.Dubey JP, Lappin MR, Thulliez P. Long-term antibody responses of cats fed toxoplasma gondii tissue cysts. J Parasitol 1995; 81: 887–93. [PubMed] [Google Scholar]
- 8.Lappin MR, Chavkin MJ, Munana KR, Cooper CM. Feline ocular and cerebrospinal fluid Toxoplasma gondii-specific humoral immune responses following specific and nonspecific immune stimulation. Vet Immunol Immunopathol 1996; 55: 23–31. [DOI] [PubMed] [Google Scholar]
- 9.Maggs DJ, Lappin MR, Nasisse MP. Detection of feline herpes-virus-specific antibodies and DNA in aqueous humor from cats with or without uveitis. Am J Vet Res 1999; 60: 932–36. [PubMed] [Google Scholar]
- 10.Fontenelle JP, Powell CC, Hill AE, Radecki SV, Lappin MR. Prevalence of serum antibodies against Bartonella species in the serum of cats with or without uveitis. J Feline Med Surg 2008; 10: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams LE, Broussard MT, Johnson JL, Neel J. Comparison of results of clinicians' assessments, cytologic examination of fine-needle lymph node aspirates, and flow cytometry for determination of remission status of lymphoma in dogs. J Am Vet Med Assoc 2005; 226: 562–66. [DOI] [PubMed] [Google Scholar]
- 12.Peiffer RL, Jr, Wilcock BP. Histopathologic study of uveitis in cats: 139 cases (1978–1988). J Am Vet Med Assoc 1991; 198: 135–38. [PubMed] [Google Scholar]
- 13.Foley JE, Lapointe JM, Koblik P, Poland A, Pedersen NC. Diagnostic features of clinical neurologic feline infectious peritonitis. J Vet Intern Med 1998; 12: 415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sparkes AH, Gruffydd-Jones TJ, Harbour DA. An appraisal of the value of laboratory tests in the diagnosis of feline infectious peritonitis. J Am Anim Hosp Assoc 1994; 30: 345–50. [Google Scholar]
- 15.Hartmann K. Feline infectious peritonitis. Vet Clin North Am Small Anim Pract 2005; 35: 39–79, vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrew SE. Feline infectious peritonitis. Vet Clin North Am Small Anim Pract 2000; 30: 987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Can-Sahna K, Soydal AV, Pinar D, Oguzoglu TC. The detection of feline coronaviruses in blood samples from cats by mRNA RT-PCR. J Feline Med Surg 2007; 9: 369–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewerchin HL, Cornelissen E, Nauwynck HJ. Replication of feline coronaviruses in peripheral blood monocytes. Arch Virol 2005; 150: 2483–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrewegh AA, de Groot RJ, Cepica A, Egberink HF, Horzinek MC, Rottier PJ. Detection of feline coronavirus RNA in feces, tissues, and body fluids of naturally infected cats by reverse transcriptase PCR. J Clin Microbiol 1995; 33: 684–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heider HJ, Pox C, Loesenbeck G, Egberink H. Ophthalmological findings in association with different virus infections in the cat. Eur J Companion Anim Pract 1998; 8: 35–42. [Google Scholar]
- 21.Brightman AH, Ogilvie GK, Tompkins M. Ocular disease in FeLV-positive cats: 11 cases (1981–1986). J Am Vet Med Assoc 1991; 198: 1049–51. [PubMed] [Google Scholar]
- 22.Swenson CL, Kociba GJ, Mathes LE, et al. Prevalence of disease in nonviremic cats previously exposed to feline leukemia virus. J Am Vet Med Assoc 1990; 196: 1049–52. [PubMed] [Google Scholar]
- 23.English RV, Davidson MG, Nasisse MP, Jamieson VE, Lappin MR. Intraocular disease associated with feline immunodeficiency virus infection in cats. J Am Vet Med Assoc 1990; 196: 1116–119. [PubMed] [Google Scholar]
- 24.Loesenbeck G, Drommer W, Heider HJ. [Findings in the eyes of serologically FIV (feline immunodeficiency virus) positive cats.] Dtsch Tierarztl Wochenschr 1995; 102: 348–51. [PubMed] [Google Scholar]
- 25.English RV, Nelson P, Johnson CM, Nasisse M, Tompkins WA, Tompkins MB. Development of clinical disease in cats experimentally infected with feline immunodeficiency virus. J Infect Dis 1994; 170: 543–52. [DOI] [PubMed] [Google Scholar]
- 26.Gabor LJ, Love DN, Malik R, Canfield PJ. Feline immunodeficiency virus status of Australian cats with lymphosarcoma. Aust Vet J 2001; 79: 540–45. [DOI] [PubMed] [Google Scholar]
- 27.Lappin MR, Black JC. Bartonella spp infection as a possible cause of uveitis in a cat. J Am Vet Med Assoc 1999; 214: 1205–7, 1200. [PubMed] [Google Scholar]
- 28.Lappin MR, Kordick DL, Breitschwerdt EB. Bartonella spp antibodies and DNA in aqueous humour of cats. J Feline Med Surg 2000; 2: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ketring KL, Zuckerman EE, Hardy WD., Jr. Bartonella: A new etiological agent of feline ocular disease. J Am Anim Hosp Assoc 2004; 40: 6–12. [DOI] [PubMed] [Google Scholar]
- 30.Dubey JP, Mattix ME, Lipscomb TP. Lesions of neonatally induced toxoplasmosis in cats. Vet Pathol 1996; 33: 290–95. [DOI] [PubMed] [Google Scholar]
- 31.Davidson MG, Lappin MR, English RV, Tompkins MB. A feline model of ocular toxoplasmosis. Invest Ophthalmol Vis Sci 1993; 34: 3653–60. [PubMed] [Google Scholar]
- 32.Dubey JP, Carpenter JL. Histologically confirmed clinical toxoplasmosis in cats: 100 cases (1952–1990). J Am Vet Med Assoc 1993; 203: 1556–66. [PubMed] [Google Scholar]
- 33.Chavkin MJ, Lappin MR, Powell CC, Roberts SM, Parshall CJ, Reif JS. Seroepidemiologic and clinical observations of 93 cases of uveitis in cats. Progress Vet & Comp Ophth 1992; 2: 29–36. [Google Scholar]
- 34.Lappin MR, Marks A, Greene CE, et al. Serologic prevalence of selected infectious diseases in cats with uveitis. J Am Vet Med Assoc 1992; 201: 1005–9. [PubMed] [Google Scholar]
- 35.Lappin MR, Greene CE, Prestwood AK, Dawe DL, Tarleton RL. Enzyme-linked immunosorbent assay for the detection of circulating antigens of Toxoplasma gondii in the serum of cats. Am J Vet Res 1989; 50: 1586–90. [PubMed] [Google Scholar]
- 36.Lappin MR, Roberts SM, Davidson MG, Powell CC, Reif JS. Enzyme-linked immunosorbent assays for the detection of Toxoplasma gondii-specific antibodies and antigens in the aqueous humor of cats. J Am Vet Med Assoc 1992; 201: 1010–16. [PubMed] [Google Scholar]
- 37.Lappin MR, Burney DP, Dow SW, Potter TA. Polymerase chain reaction for the detection of Toxoplasma gondii in aqueous humor of cats. Am J Vet Res 1996; 57: 1589–93. [PubMed] [Google Scholar]
- 38.Lappin MR, Cayatte S, Powell CC, Gigliotti A, Cooper C, Roberts CM. Detection of Toxoplasma gondii antigen-containing immune complexes in the serum of cats. Am J Vet Res 1993; 54: 415–19. [PubMed] [Google Scholar]
- 39.Nussenblatt RB, Mittal KK, Fuhrman S, Sharma SD, Palestine AD. Lymphocyte proliferative responses of patients with ocular toxoplasmosis to parasite and retinal antigens. Am J Ophthalmol 1989; 107: 632–41. [DOI] [PubMed] [Google Scholar]
- 40.Peña MT, Naranjo C, Klauss G, et al. Histopathological features of ocular leishmaniosis in the dog. J Comp Pathol 2008; 138: 32–39. [DOI] [PubMed] [Google Scholar]
- 41.Hervás J, Chacón-Manrique DL, López J, Moreno J, Guerrero M-J, Gómez-Wlamandos JC. Granulomatous (pseudotumoral) iridodclitis associated with leishmaniasis in a cat. Vet Rec 2001; 149: 624–25. [DOI] [PubMed] [Google Scholar]
- 42.Ching SV, Gillette SM, Powers BE, Roberts SM, Gillette EL, Withrow SJ. Radiation-induced ocular injury in the dog: A histological study. Int J Radiat Oncol Biol Phys 1990; 19: 321–28. [DOI] [PubMed] [Google Scholar]
- 43.Corcoran KA, Peiffer RL, Jr, Koch SA. Histopathologic features of feline ocular lymphosarcoma: 49 cases (1978–1992). Vet Comp Ophthalmol 1995; 5: 35–41. [Google Scholar]
- 44.Parma AE, Sanz ME, Lucchesi PM, Mazzonelli J, Petruccelli MA. Detection of an antigenic protein of Leptospira interrogans which shares epitopes with the equine cornea and lens. Vet J 1997; 153: 75–79. [DOI] [PubMed] [Google Scholar]
- 45.Trousdale MD, Dunkel EC, Nesburn AB. Effect of flurbiprofen on herpes simplex keratitis in rabbits. Invest Ophthalmol Vis Sci 1980; 19: 267–70. [PubMed] [Google Scholar]
- 46.Roze M. Feline uveitis. A clinical and serological study of 44 cases. Eur J Companion Anim Pract 2008; 9: 149–56. [Google Scholar]
- 47.Willis AM. Feline leukemia virus and feline immunodeficiency virus. Vet Clin North Am Small Anim Pract 2000; 30: 971–86. [DOI] [PubMed] [Google Scholar]
- 48.Formston C. Retinal detachment and bovine tuberculosis in cats. J Small Anim Pract 1994; 35: 5–8. [Google Scholar]
- 49.Dietrich U, Arnold P, Guscetti F, Pfyffer GE, Spiess B. Ocular manifestation of disseminated Mycobacterium simiae infection in a cat. J Small Anim Pract 2003; 44: 121–25. [DOI] [PubMed] [Google Scholar]
- 50.Breitschwerdt EB, Abrams-Ogg AC, Lappin MR, et al. Molecular evidence supporting Ehrlichia canis-Yike infection in cats. J Vet Intern Med 2002; 16: 642–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magnarelli LA, Bushmich SL, Ijdo JW, Fikrig E. Seroprevalence of antibodies against Borrelia burgdorferi and Anaplasma phagocytophilum in cats. Am J Vet Res 2005; 66: 1895–99. [DOI] [PubMed] [Google Scholar]
- 52.Davidson MG. Toxoplasmosis. Vet Clin North Am Small Anim Pract 2000; 30: 1051–62. [DOI] [PubMed] [Google Scholar]
- 53.Gionfriddo JR. Feline systemic fungal infections. Vet Clin North Am Small Anim Pract 2000; 30: 1029–50. [DOI] [PubMed] [Google Scholar]
- 54.Gerding PA, Jr, Morton LD, Dye JA. Ocular and disseminated candidiasis in an immunosuppressed cat. J Am Vet Med Assoc 1994; 204: 1635–38. [PubMed] [Google Scholar]
- 55.Greene RT, Troy GC. Coccidioidomycosis in 48 cats: A retrospective study (1984–1993). J Vet Intern Med 1995; 9: 86–91. [DOI] [PubMed] [Google Scholar]
- 56.Tomsa K, Glaus TM, Zimmer C, Greene CE. Fungal rhinitis and sinusitis in three cats. J Am Vet Med Assoc 2003; 222: 1380–84, 1365. [DOI] [PubMed] [Google Scholar]