Abstract
The Saudi Arabian Ministry of Health implemented a pro‐active surveillance programme for Middle East respiratory syndrome (MERS) coronavirus (MERS‐CoV). We report MERS‐CoV data from 5065 Kingdom of Saudi Arabia individuals who were screened for MERS‐CoV over a 12‐month period. From 1 October 2012 to 30 September 2013, demographic and clinical data were prospectively collected from all laboratory forms received at the Saudi Arabian Virology reference laboratory. Data were analysed by referral type, age, gender, and MERS‐CoV real‐time PCR test results. Five thousand and 65 individuals were screened for MER‐CoV: hospitalized patients with suspected MERS‐CoV infection (n = 2908, 57.4%), healthcare worker (HCW) contacts (n = 1695; 33.5%), and family contacts of laboratory‐confirmed MERS cases (n = 462; 9.1%). Eleven per cent of persons tested were children (<17 years of age). There were 108 cases (99 adults and nine children) of MERS‐CoV infection detected during the 12‐month period (108/5065, 2% case detection rate). Of 108 cases, 45 were females (six children and 39 adults) and 63 were males (three children and 60 adults). Of the 99 adults with MERS‐CoV infection, 70 were hospitalized patients, 19 were HCW contacts, and ten were family contacts. There were no significant increases in MERS‐CoV detection rates over the 12‐month period: 2.6% (19/731) in July 2013, 1.7% (19/1100) in August 2013, and 1.69% (21/1238) in September 2013. Male patients had a significantly higher MERS‐CoV infection rate (63/2318, 2.7%) than females (45/2747, 1.6%) (p 0.013). MERS‐CoV rates remain at low levels, with no significant increase over time. Pro‐active surveillance for MERS‐CoV in newly diagnosed patients and their contacts will continue.
Keywords: Clinical, coronavirus, demographic, diagnosis, MERS‐CoV, Middle East, real‐time PCR, sample type, SARS, screening, viral load
Introduction
Understanding the natural history, epidemiology and clinical presentation of new killer infectious diseases is dependent on, and influenced by, the WHO recommended surveillance strategies for case detection, which largely focus on severe illness and microbiological testing, coupled with details from case studies. Contact‐tracing activities allow for the detection of confirmed cases with a broader spectrum of illness. For infectious diseases caused by viruses, confirmed cases include only those with a positive PCR test result for viral genetic material, in accordance with the laboratory guidelines. Since the first case report of the novel Middle East respiratory syndrome (MERS) coronavirus (MERS‐CoV) in September 2012 1, the Kingdom of Saudi Arabia (KSA) Ministry of Health (KSA‐MoH) has been working closely with international collaborators and the WHO to better understand and define the epidemiological, demographic, clinical and laboratory features of the new disease. A molecular real‐time PCR diagnostic test was rapidly developed after the first case, and was subsequently the method recommended by the WHO for detecting the presence of the MERS‐CoV infection 2, 3. This test was used in a retrospective analysis on biobanked samples to confirm two cases of MERS from an earlier outbreak of respiratory infection in Jordan in April, 2012 4.
Important steps for the surveillance and control of MERS‐CoV infection are the early detection and isolation of patients with active MERS‐CoV disease, and screening of their contacts. Surveillance studies also help in defining and monitoring transmission rates, case load, and epidemic risk assessment, and assist in instituting infection control measures with new diagnostic methods and treatments. Although MERS‐CoV case detection is critically dependent on the degree of awareness of the attending physician, accurate laboratory testing is also essential in making a diagnosis. Soon after the detection of the first case of MERS in Jeddah in September 2012 1, the KSA‐MoH put in place a proactive surveillance and screening programme for inpatients admitted with respiratory illness suspected of being caused by MERS‐CoV. It also included active screening of contacts of confirmed MERS cases. KSA‐MoH recommendations for MERS‐CoV screening are based on the WHO guidelines on case definition, detection, and contact investigations 5, 6, 7. This led to an increase in the numbers of requests for MERS‐CoV screening from hospitals throughout the KSA. We report these laboratory data on the use of real‐time PCR tests on clinical samples received from 5065 individuals screened for MERS‐CoV during a 12‐month period, commencing from the first case detection in September 2012.
Methods
Selection of individuals for MERS‐CoV screening
The KSA‐MoH has implemented a pro‐active early case detection and surveillance system for MERS‐CoV. It recommends sending respiratory tract samples from critically ill patients admitted to hospitals with fever and lower respiratory tract infection symptoms. Screening for MERS‐CoV is also recommended for family and healthcare worker (HCW) contacts of proven cases of MERS‐CoV infection. All samples are transported to and are processed by the KSA‐MoH virology laboratory in Jeddah, which is accredited and regulated by the Central Board for Accreditation of Health Care Institute. Quality assurance and control for all diagnostic tests is monitored through Internal Policy Procedures and by external quality assurance schemes.
Collection of clinical specimens
Respiratory specimens collected from patients and contacts were: sputum samples; nose and throat (N + T) swabs; nasopharyngeal (NP) swabs; and tracheal aspirate samples. Sputum was collected directly into a sterile, leak‐proof, screw‐capped sputum collection sterile container; NP swabs and N + T swabs were collected with sterile synthetic tip Dacron flocked swabs. For NP specimens, the swabs were inserted through the nostril, parallel to the palate, into the nasopharynx. Swabs were left in place for a few seconds to absorb secretions. For N + T swabs, both nostrils and the throat were swabbed with separate swabs. All swabs were placed immediately into sterile tubes containing 2–3 mL of viral transport medium. For inpatients, lower respiratory tract samples—2–3 mL of bronchoalveolar lavage fluid and tracheal aspirate—were obtained and placed into sterile, leak‐proof, screw‐capped sterile dry containers.
Labelling, storage and transportation of specimens
When there were short periods of transportation (≤48 h) of specimens to the laboratory, specimens were held in a refrigerator at 2–8°C rather than frozen; for periods exceeding 48 h, specimens were shipped on dry ice at −70°C as soon as possible after collection. Each specimen container was labelled with the patient's ID number, the specimen type, and the date on which the sample was collected. All specimens were pre‐packed to prevent breakage and spillage. Specimen containers were sealed with parafilm and placed in zip‐lock bags. Absorbent material to absorb the entire contents of the secondary container (containing the primary container) was placed to separate the primary containers (containing specimen) to prevent breakage.
RNA extraction
Extraction of RNA was performed with Roche MagNa Pure LC (RNA Viral isolation Kit). Sputum samples were pretreated with 2 × lysis buffer for 30 min in a shaking incubator. Swabs were placed in lysis buffer. Two hundred microlitres of each sample was added to the MagNA Pure LC plate, which contains 96 wells. Reaction reagents were then loaded and checked, before the samples were run according to the manufacturer's instructions for nucleic acid extraction in the specimen area.
MERS‐CoV screening test
A biosafety level 2 facility equipped with microbiological safety cabinets was used for the handling of clinical specimens and for extracting RNA for PCR. PCR is the recommended method for detecting the virus. At least three sites in the genome of MERS‐CoV have been identified as suitable targets for the diagnostic test—the upstream E protein gene (upE), open reading frame (ORF) 1A, and ORF 1B—and sequences of the specific primers have been published. Positive controls for the upE screening and the ORF 1A confirmation assays are available. Clinical samples were screened by real‐time PCR, as previously described 6, with amplification targeting both upE and ORF 1A for confirmation—these are standard assay used in the KSA for MERS‐CoV testing. The test result was considered to be positive if both assays gave positive results. In cases of discordance between the first and second assays, or if the result was considered to be a doubtful positive result, another clinical sample was requested and analysed.
Data collection
Data from laboratory forms accompanying clinical samples received at the KSA‐MoH virology laboratory specifically requesting MERS‐CoV testing during the period 1 October 2012 to 30 September 2013 were collected and analysed. Demographic and laboratory PCR test data and MERS‐CoV viral load data were recorded. Where data were missing from the records or where clarification was required, data were obtained through direct communication with attending physicians and other healthcare providers.
Serological testing
No serological testing data were available, because there are no validated accurate serological diagnostic tests for MERS‐CoV available to date.
Statistical analyses
Demographic, clinical and laboratory descriptive data were tabulated. Univariate analysis was performed with binary logistic regression analysis. A p‐value of <0.05 was considered to indicate statistical significance.
Results
A total of 5065 individuals (625 children and 4440 adults) were screened for MERS‐CoV during the 12‐month period: hospitalized patients with suspected MERS‐CoV infection (n = 2908, 57.4%), HCW contacts (n = 1695; 33.5%), and family contacts of laboratory‐confirmed MERS cases (n = 462; 9.1%) (Fig. 1). There were 108 cases (99 adults and nine children) of MERS‐CoV infection detected during the 12‐month period (108/5065, 2% case detection rate) (Table 1). Of 108 MERS cases, 45 were females (six children and 39 adults) and 63 were males (three children and 60 adults). Of the 99 adults with MERS‐CoV infection, 70 were hospitalized patients, 19 were HCW contacts, and ten were family contacts. Of the nine children with MERS‐CoV infection, two were hospitalized patients and seven were family contacts. A significant increase in the number of screened and tested specimens was evident over the study period (Fig. 2a), but there were no significant increases in MERS‐CoV detection rates over the 12‐month period (Fig. 2b). The monthly case detection rates of MERS‐CoV were 2.6% (19/731) in July 2013, 1.7% (19/1100) in August 2013, and 1.69% (21/1238) in September 2013 (Fig. 2b). Male patients had a significantly higher MERS‐CoV infection rate (63/2318, 2.7%) than females (45/2747, 1.6%) (p 0.013) (Table 2).
Figure 1.
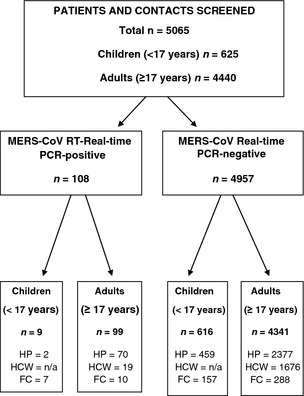
Flow chart: screening of hospitalized patients and contacts. FC, family contacts; HCW, healthcare workers and their contacts; HP, hospital patients; NA, not applicable.
Table 1.
Middle East respiratory syndrome coronavirus (MERS‐CoV) screening by referral type and age group
| Patients and contacts | Children (aged <17 years) | Adults (aged ≥17 years) | All | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MERS‐CoV real‐time PCR | Positive | Total | % Positivea | Positive | Total | % Positivea | Positivea | Total | % Positivea |
| Hospital patients | 2 | 461 | 0.43 | 70 | 2441 | 2.86 | 72 | 2908 | 2.51 |
| HCW contacts | NA | NA | NA | 19 | 1695 | 1.12 | 19 | 1695 | 1.12 |
| Family contacts | 7 | 164 | 4.2 | 10 | 298 | 3.36 | 17 | 462 | 3.6 |
| Total | 9 | 625 | 1.4 | 99 | 4440 | 2.19 | 108 | 5065 | 2.1 |
HCW, healthcare worker; NA, not applicable.
For children: p‐value for positive PCR in hospitalized patients vs. family contacts, 0.0021.
For adults: p‐value for positive PCR in hospitalized patients vs. HCWs, 0.0029; p‐value for hospitalized patients vs. family contacts, 0.46; p‐value for HCWs vs. family contacts, 0.025.
Percentage within each age group.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 2.
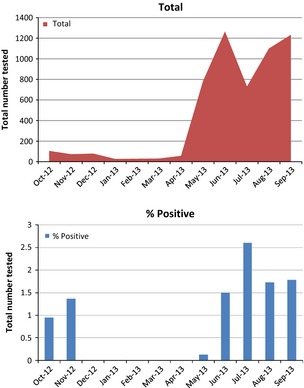
Kingdom of Saudi Arabia Middle East respiratory syndrome coronavirus case screening (a) and detection rates (b) over time.
Table 2.
Distribution of all individuals screened by age, gender and Middle East respiratory syndrome coronavirus (MERS‐CoV) status
| Children | Adults | |||||
|---|---|---|---|---|---|---|
| Number positive | Total screened | % Positive | Number positive | Total screened | % Positive | |
| Female | ||||||
| Family contacts | 5 | 87 | 5.7 | 3 | 147 | 2.04 |
| HCW contacts | 0 | 0 | – | 15 | 1155 | 1.30 |
| Patients | 1 | 207 | 0.48 | 21 | 1151 | 1.82 |
| Total | 6 | 294 | 2 | 39 | 2453 | 1.59a |
| Males | ||||||
| Family contacts | 2 | 77 | 2.5 | 7 | 151 | 4.64 |
| HCW contacts | 0 | 0 | – | 4 | 540 | 0.74 |
| Patients | 1 | 254 | 0.39 | 49 | 1296 | 3.78 |
| Total | 3 | 331 | 0.9 | 60 | 1987 | 3.02a |
HCW, healthcare worker.
Male patients had a significantly higher positive rate for MERS‐CoV than female patients (p 0.013).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Discussion
Since the first KSA case report in September 2012, the KSA‐MoH has recommended mandatory testing for MERS‐CoV of all cases of respiratory illness requiring intensive‐care admission. This is the largest study describing the incidence rates of MERS‐CoV in the KSA over a period of 12 months. The real‐time PCR MERS‐CoV diagnostic test has been in use for detecting new MERS cases in hospitalized patients, and for screening of HCW and family contacts of confirmed MERS cases 7, 8, 9, 10, 11. Six months after MERS‐CoV was discovered, at the end of March 2013, there were only 17 MERS‐CoV cases reported globally, nine of which were from the KSA 12. In light of the infrequent, but continuing, detection of sporadic MERS‐CoV cases in the community and the hospital outbreak at Al‐Hasa 10, the WHO constituted an Emergency Committee under the International Health Regulations to advise the Director‐General on the status of the MERS‐CoV situation 13. The important issue at that time was whether MERS‐CoV was going to progress to cause a major pandemic, as did the SARS epidemic in the early 2000s, which was also caused by a novel coronavirus, SARS‐CoV 14.
Our results show that pro‐active surveillance during the months after the Al‐Hasa outbreak, which occurred in April–May 2013, showed no significant increase in the MERS case detection rates in the ensuing 8 months. The monthly positive rates of MERS‐CoV were 2.7% in July 2013 and then 1.7% in August and September 2013. The initial increase in July 2013 was related to the intensification of the surveillance of MERS‐CoV in the KSA following the healthcare‐related outbreak at Al‐Hasa 10. From our data, three areas of transmission can be focused on for active surveillance and screening.
The first pattern is the occurrence of sporadic cases in communities. The true incidence of the disease in the community is not known, and remains to be defined through case–control serological surveys when accurate, rapid, sensitive and specific serological tests become available. In the community, the asymptomatic cases or those with minimal symptoms are difficult to identify, and are usually missed. Those who become acutely ill in the community present to emergency rooms, where real‐time PCR testing of respiratory samples is performed.
The second pattern of transmission is transmission within families 8. The rate of intrafamilial transmission is not known. Our results provide an estimate of a rate of 3.6% for acquisition of MERS‐CoV from close family contacts. However, this finding is not conclusive, as no serological assays were utilized for screening mild or subclinical cases. However, the finding is in agreement with previous observations of low secondary attack rates among family members or contacts of patients in the KSA and European countries 8, 15, 16, 17, 18, 19, 20, 21, 22.
The third transmission pattern is nosocomial transmission to HCWs. In one study, seven HCWs with MERS‐CoV infection were reported. Two of them were asymptomatic, and five had mild upper respiratory tract symptoms 11. The current study sheds more light on the transmission of MERS‐CoV in healthcare setting. The positivity rate of MERS‐CoV by PCR was only 1.12% of all tested HCWs. Transmission within healthcare settings was retrospectively reported from Jordan 4, and then subsequently reported in France, the KSA, the UK, the United Arab Emirates, and Qatar, and included HCWs treating MERS‐CoV patients 23, 24, 25, 26, 27. These data suggest that the current risk of transmission within healthcare facilities remains small, and that the recommended infection control measures are adequate 6, 9, 13, 20.
Studies of family and hospital case clusters of MERS‐CoV infections in the KSA and other European countries that have reported MERS cases indicate that a spectrum of clinical illness occurs 7, 8, 9, 15, 16. Reports from Tunisia and the UAE of MERS‐CoV infections occurring in siblings whose father's illness was a probable MERS‐CoV infection case, and the case from the UK 8, 24, 25, show that the siblings who are not immunocompromised only manifest mild respiratory illnesses, and do not require hospitalization. In the UK family cluster, among 33 close contacts (20 household and 13 non‐household), there were only two cases (6% attack rate) of confirmed MERS‐CoV infection, one with mild illness and one with severe illness 15. There were no cases of MERS‐CoV infection among 59 HCWs who were in contact with the index case without wearing full personal protective equipment 15.
The fact that we have subsequently identified milder or asymptomatic cases of MERS in HCWs, children and family members of contacts of MERS cases indicates that the severe cases represent only the tip of the iceberg, and there is a spectrum of milder clinical disease that requires definition. Our data indicate that MERS‐CoV affects both genders, and, although a few cases of MERS‐CoV in children have been detected, it remains mainly a disease of adults across all age groups. To date, there is still no evidence of sustained community transmission. Despite extensive investigation and testing of thousands of contacts by the KSA‐MoH, only a few instances of transmission to HCWs or family contacts have been identified. Almost all patients who died or who had been hospitalized had severe disease or other comorbidities 9, 10. The mortality rate and severity of disease are exaggerated to some degree by the detection of such cases. The case‐fatality rate has fallen in recent months, owing to the detection of milder and asymptomatic cases 23, 24, 25, 26, 27. The most critical characteristic of pandemic MERS‐CoV strains would be progression to efficient human‐to‐human transmission. The number of sporadic MERS cases being reported has been small, and indicates that the virus appears to be not readily capable of rapid human‐to‐human transmission. Two million pilgrims from over 180 countries, and 1 million local KSA pilgrims, have very recently visited Makkah and Madinah, KSA to perform the 2013 annual Hajj pilgrimage, and have returned home after stays of between 2 and 8 weeks 28. Millions of others will visit the KSA throughout the year for the mini‐pilgrimage UMRAH, and although MERS‐CoV infection rates in the KSA remain at steady, low levels, and no significant increase in the incidence of MERS‐CoV infection over time is occurring, pro‐active surveillance for MERS‐CoV in newly diagnosed patients and their contacts will continue. The availability of more rapid and accurate serological tests will help to better define the community prevalence of MERS‐CoV.
Transparency Declaration
All authors have no conflict of interest to declare.
Clin Microbiol Infect 2014; 20: 469–474 24460984
References
- 1. Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367: 1814–1820. [DOI] [PubMed] [Google Scholar]
- 2. Corman VM, Eckerle I, Bleicker T et al Detection of a novel human coronavirus by real‐time reverse‐transcription polymerase chain reaction. Euro Surveill 2012; 17: pii: 20285. [DOI] [PubMed] [Google Scholar]
- 3. Costabel U, Timm J, Binger T, Meyer B et al Assays for laboratory confirmation of novel human coronavirus (hCoV‐EMC) infections. Euro Surveill 2012; 17: pii: 20334. [DOI] [PubMed] [Google Scholar]
- 4. Hijawi B, Abdallat M, Sayaydeh A et al Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J 2013; 19 (suppl 1): S12–S18. [PubMed] [Google Scholar]
- 5. World Health Organization . Global Alert and Response. Coronavirus Infections (2012. –2013). Available at: http://wwwwhoint/csr/disease/coronavirus_infections/en/
- 6. World Health Organization . Laboratory testing for novel coronavirus. Interim Recommendations, 21 December 2012. Available at: http://www.who.int/csr/disease/coronavirus_infections/LaboratoryTestingNovelCoronavirus_21Dec12.pdf (last accessed 22 October 2013).
- 7. Albarrak AM, Stephens GM, Hewson R, Memish ZA. Recovery from severe novel coronavirus infection. Saudi Med J 2012; 33: 1265–1269. [PubMed] [Google Scholar]
- 8. Memish ZA, Zumla AI, Al‐Hakeem RF, Al‐Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med 2013; 368: 2487–2494. [DOI] [PubMed] [Google Scholar]
- 9. Assiri A, McGeer A, Perl TM et al Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med 2013; 369: 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Assiri A, Al‐Tawfiq JA, Al‐Rabeeah AA et al Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013; 13: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med 2013; 369: 884–886. [DOI] [PubMed] [Google Scholar]
- 12. Promed refe World_Health_Organization . Middle East respiratory syndrome coronavirus (MERS‐CoV)—update 2 June 2013. 2013. World Health Organization: MERS‐CoV summary and literature update—as of 20 June 2013. Available at: http://www.who.int/csr/disease/coronavirus_infections/update_20130620/en/index.ht.
- 13.Middle East respiratory syndrome coronavirus (MERS‐CoV)‐Alert, response, and capacity building under the international health regulations (IHR). http://www.who.int/ihr/procedures/statements_20130709/en/index.htm
- 14. Peiris J, Yuen KY, Osterhaus AD, Stöhr K. The severe acute respiratory syndrome. N Engl J Med 2003; 349: 2431–2441. [DOI] [PubMed] [Google Scholar]
- 15. Evidence of person‐to‐person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Health Protection Agency (HPA) UK Novel Coronavirus Investigation Team. Euro Surveill 2013; 18: pii: 20427. [DOI] [PubMed] [Google Scholar]
- 16. Bermingham A, Chand MA, Brown CS et al Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill 2012; 17: pii: 20290. [PubMed] [Google Scholar]
- 17. Mailles A, Blanckaert K, Chaud P et al First cases of Middle East respiratory syndrome coronavirus (MERS‐CoV) infections in France, investigations and implications for the prevention of human‐to‐human transmission, France, May 2013. Euro Surveill 2013; 18: pii: 20502. [PubMed] [Google Scholar]
- 18. Guery B, Poissy J, el Mansouf L et al Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: a report of nosocomial transmission. Lancet 2013; 381: 2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drosten C, Seilmaier M, Corman VM et al Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis 2013; 13: 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buchholz U, Muller MA, Nitsche A et al Contact investigation of a case of human novel coronavirus infection treated in a German hospital, October–November 2012. Euro Surveill 2013; 18: pii: 20406. [PubMed] [Google Scholar]
- 21. Puzelli S, Azzi A, Santini MG et al Investigation of an imported case of Middle East respiratory syndrome coronavirus (MERS‐COV) infection in Florence, Italy, May to June 2013. Euro Surveill 2013; 18: pii: 20564. [DOI] [PubMed] [Google Scholar]
- 22. Omrani AS, Matin MA, Haddad Q, Al‐Nakhli D, Memish ZA, Albarrak AM. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis 2013; 17: e668–e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The WHO MERS‐CoV Research group . State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS‐CoV) in humans. The WOO Mers‐Cov Research Group. PLoS Curr 2013; 5: doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Penttinen PM, Kaasik‐Aaslav K, Friaux A et al Taking stock of the first 133 MERS coronavirus cases globally—is the epidemic changing? Euro Surveill 2013; 18: pii: 20596. [DOI] [PubMed] [Google Scholar]
- 25. Memish ZA, Al‐Tawfiq JA, Assiri A. Hospital‐associated Middle East respiratory syndrome coronavirus infections. N Engl J Med 2013; 369: 1761–1762. [DOI] [PubMed] [Google Scholar]
- 26. World Health Organization . Global Alert and Response (GAR): novel coronavirus infection—update (Middle East respiratory syndrome coronavirus). Geneva: World Health Organization; 2013. Available at http://www.who.int/csr/don/2013_05_23_ncov/en/index.html [Google Scholar]
- 27. Centers for Disease Control and Prevention . Update: severe respiratory illness associated with Middle East respiratory syndrome coronavirus (MERS‐CoV)—worldwide, 2012–2013. MMWR 2013; 62: 480–483. [PMC free article] [PubMed] [Google Scholar]
- 28. Rashid H, Azeem MI, Heron L, Haworth E, Booy R, Memish ZA. Has Hajj‐associated MERS‐CoV transmission occurred? The case for effective post‐Hajj surveillance for infection. Clin Microbiol Infect 2013. doi: 10.1111/1469-0691.12492 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


