Abstract
A CHO cell clone (CHO-PoIFN-β) with stable porcine IFN-β expression under control of CMV promoter was selected under G418 pressure. In a 25 cm2 cell culture flask (5 ml culture medium), the cumulative protein yield of recombinant PoIFN-β reached 2.3 × 106 IU/ml. This cells clone maintained stable expression for at least 20 generations even in the absence of G418 selection pressure. The expressed recombinant PoIFN-β could induce the expression of porcine Mx protein in PK15 cells, and activate the chicken Mx promoter-controlled luciferase reporter gene expression, confirming that the recombinant PoIFN-β has the biological activity of natural porcine type-I interferon. In addition, the recombinant PoIFN-β fully protected PK15 cells against 1000 TCID50 of porcine transmissible gastroenteritis virus and pseudo-rabies virus infection, demonstrating its high potential in therapeutic applications. This is the first report of establishing a mammalian cell line with stable expression of porcine IFN-β.
Keywords: Porcine interferon-β, CHO-K1 cells, Stable expression, Biological activity, Antiviral activity
1. Introduction
Type-I interferon, an important component of the innate immune system in vertebrates, has gained much attention in modern medical research. Currently, one of the research focuses is the mechanism employed by viruses to evade the innate immune defense such as interferons when infecting the host [1], [2], [3], this in theory provides strong support for effective viral disease prevention and treatment. Viruses such as porcine reproductive and respiratory syndrome virus (PRRSV) [4], [5], pseudo-rabies virus (PRV) [6], [7], porcine arteritis virus (PoAV) [8], swine fever virus [9], [10], [11] and transmissible gastroenteritis virus (TGEV) [12], employ the strategy to evade the host immune system by destroying type-I interferon system when infecting the host.
Type-I interferon has played an important role in the treatment of chronic hepatitis B [13], [14], chronic hepatitis C [15], multiple sclerosis [16], tumor [17], [18] and other diseases [19], [20]. In veterinary medicine, porcine type-I interferon has good prospect in the treatment of common viral diseases such as TGEV [21], swine fever virus [22], PRV [23], [24], etc. Therefore, type-I interferon with high activity is needed whether it is for basic research or clinical application. In addition, pure and stable interferon with high activity per unit mass is needed as the standard for accurate and convenient determination of the interferon biological activity.
Recombinant interferon from mammalian cell expression displays correct folding and glycosylation in comparison to that from other expression systems, best suited for use in therapeutics [25] and as standards [26]. However, Currently, the production of porcine interferon is mainly from the prokaryotic [22], [27], [28], yeast [24], [29], [30], [31], [32] and baculovirus [33] expression systems. It is therefore important for basic and applied research to establish a high level expression system for porcine type-I interferon in mammalian cells. The purpose of this study was to establish highly efficient and stable expression of recombinant porcine IFN-β in CHO-K1 cell line, and further characterize the biological activity of the product.
2. Materials and methods
2.1. Plasmids, cell lines and virus strains
Porcine kidney (PK15) cells and Madin-Darby bovine kidney (MDBK) cells preserved in our laboratory were cultured in the DMEM (Gibco) culture medium containing 5% FBS (Gibco). Chicken embryo fibroblasts (CEFs) were prepared from 10-day-old SPF chicken embryos according to routine method. MDBK-Mxp-luc cell line with stably integrated luciferase reporter gene under chicken Mx promoter was established in this laboratory (unpublished). Transmissible gastroenteritis virus (TGEV) and pseudo-rabies virus (PRV) were preserved in the Harbin Veterinary Research Institute for Swine Diseases of the Chinese Academy of Agricultural Sciences, and determination of TCID50 was performed on PK15 cells. The LaSota strain of Newcastle disease virus (NDV) was preserved in this laboratory, and titrated on primary CEFs. Vesicular stomatitis virus (VSV) was preserved in this laboratory, titrated on MDBK cells. Porcine IFN-α standard were from Pestka Biomedical Laboratories (Piscataway, NJ, USA). Plasmid PET32 (a+) was purchased from Novagen corporation.
2.2. IFN-β gene cloning and plasmid construction
PoIFN-β ORF sequence was obtained from GenBank (GenBank Accession No. S41178). Primers were designed as: the upstream primer 5′-TGCCACCATGGCTAACAAGTGCATC-3′, the downstream primer 5′-AGCCACAGGGGGGAGATGTTCAGT-3′. Kozak sequence was added to the upstream primer before the initiation codon ATG to facilitate expression in the eukaryotic cells.
PK15 cells with about 90% confluence were infected with NDV at a MOI (multiplicity of infection) of 1.0 and harvested after 8 h. Total RNA was extracted using Trizol (Invitrogen) and subjected to RT-PCR using the mentioned primers above. PoIFN-β PCR product was then subcloned into PMD18-T vector (Takara) to generate plasmid pMD18-PoIFN-β and sequenced.
pMD18-PoIFN-β was double-digested with EcoR I/Sal I to obtain the PoIFN-β ORF fragment, which was then subcloned into Xho I/Nhe I double-digested pCAGGS plasmid to generate plasmid pCA-PoIFN-β, or Sma I digested pCI-neo plasmid (Promega) after end-blunted with T4 DNA polymerase (MBI) to generate plasmid pCN-PoIFN-β.
2.3. Antiviral activity titration
Antiviral activity of interferon was titrated as described [34] with modifications. In brief, MDBK cells in 96-well plate were grown to 90–100% confluence and the supernatant was discard, 100 μl of 10-fold serially diluted IFN sample was added to each well (101–108-fold dilution) with two parallel wells set up for each dilution, and the cells were treated at 37 °C and 5% CO2 culture conditions for 24 h. After the supernatant was removed, 100 μl of VSV virus diluted to 30,000 PFU/100 μl in DMEM containing 2% FBS was applied to each well. Three wells of virus control (VC) with virus added but no interferon treatment, and three wells of blank control (BC) with neither interferon nor virus were set up. When cytopathic effect (CPE) in VC-wells reached 100%, the cells were stained with naphthol blue-black and the absorbance at 630 nm was read using a microplate reader (BioRad). Antiviral activity units (IU) were calculated using porcine IFN-α standards as reference [35], [36].
2.4. Transient transfection
pCA-PoIFN-β and pCAGGS were used to transfect BHK-21 cells with Fugene 6 (Roche) according to the manufacturer’s instructions. Medium was changed 16 h after transfection and culture continued for another 48 h. Culture supernatants were collected, named PoIFN-βpCA and mockpCA respectively, and stored in aliquots at −70 °C before use.
2.5. Stable transfection
CHO-K1 cells were transfected with pCN-PoIFN-β using Fugene 6. Twenty-four hours after transfection, the cells were passaged 1:10 and cultured in the pressure selection medium containing 800 μg/ml G418. After 10–14 days, the neomycin-resistant cell colonies were isolated using cloning rings, trypsin digested and culture expanded in 96-, 24- and 6-well plates sequentially. The selected cell clones were seeded into a 6-well plate with 0.5 × 106 cells per well and grown to a density of 100% for 24 h, and then the cell culture supernatants were collected for titration of antiviral activity. The cell clone with the highest level of expression was subjected to single cell cloning using the limited dilution method. The steps above were repeated and the cell clone with the highest expression was selected and freeze preserved according to conventional methods. The cell clone was maintained in the culture medium containing 400 μg/ml G418.
2.6. Polyclonal antibody preparation
Prokaryotic expression of the chicken Mx protein and purification steps were as follows: NDV (MOI = 1.0) was used to infect CEFs of about 100% confluence. When CPE reached 60–80%, the cells were harvested and total RNA were extracted with Trizol. Using the primers in parentheses (upstream primer 5′-GGGGATATCAGCAATCAGATGGCTTTC-3′, introducing restriction site EcoR V; downstream primer 5′-TTTGTCGACTGGGATGACCTCGTTTTG-3′, introducing Sal I restriction site), RT-PCR was performed to amplify the first half of the ORF gene fragment of chicken Mx protein. The PCR product was double-digested with EcoR V/Sal I and cloned into pET32(a+) (Novagen) underwent the same double-digestion. The chicken Mx protein produced from this prokaryotic expression plasmid was a fusion protein with the His tag. The plasmid was transformed into BL21 and induced with 0.5 mM IPTG at 37 °C for 3 h. The fusion protein was purified with Ni–NTA agarose affinity resin (Invitrogen) according to the manufacturer’s instructions and further dialysed to remove urea. Then the recombinant chicken Mx protein was used to immunize BALB/C mice according to conventional method [37], and serum was collected and stored at −20 °C before use.
2.7. Induction of Mx protein expression in PK15 cells
PK15 cells grown in 6-well plates to confluent monolayers were incubated with serially diluted recombinant PoIFN-β in 5% CO2 at 37 °C for 24 h. The cells were digested with trypsin and collected by centrifugation at 3000 rpm/min for 5 min. The cell pellets were then mixed with 100 μl each 1× SDS sample buffer, boiled in water for 20 min, and loaded for SDS–PAGE. The proteins were transferred to a nitrocellulose membrane, blocked with 5% fish skin protein (prepared in PBST) overnight, and then incubated with mouse polyclonal anti-chicken Mx protein antibody 1:100 diluted in PBST, or mouse polyclonal anti-porcine beta-actin protein antibody (Sigma) 1:1000 diluted in PBST as internal reference, followed by horseradish peroxidase-anti-mouse IgG (Sigma) 1:5000 diluted in PBST and color developed in DAB for 3–5 min before termination with deionized water.
2.8. Examination of chicken Mx promoter activation in MDBK-Mxp-luc cells
MDBK-Mxp-luc cells in 24-well plate were grown overnight to approximately 100% confluence, 400 μl of IFN samples 10-fold serially diluted in DMEM containing 5% FBS was added to each well, and culture continued at 37 °C and 5% CO2. After 5 h, intracellular luciferase expression was determined using the Bright-Glo Luciferase Assay System (Promega) according to the manufacturer’s instructions. Two parallel wells were set up for each dilution, and wells with no IFN treatment were set as blank control (BC).
2.9. Evaluation of antiviral capability in vitro
PK15 cells were grown to 90% confluence in 96-well plates; the medium was replaced with 400 μl of fresh culture medium with 10-fold serially diluted PoIFN-βCHO, and incubated in 5% CO2 at 37 °C for 24 h. After removal of the culture medium, 1000 TCID50 (in 20 μl DMEM) of TGEV or PRV was added to each well and incubated for 1 h. The virus solution was then removed and DMEM containing 2% FBS was added and culture continued. Wells receiving virus in the absence of IFN treatment were set up as the virus control (VC), and wells not treated with either virus or IFN were the blank control (BC). When the CPE of the VC-wells reached 90–100%, the inhibitory effect of interferon on the replication of TGEV and PRV was observed under an inverted microscope, and the culture medium in each well were collected to titer TGEV or PRV on PK15 cells in 96-well plates.
3. Results
3.1. Construction of a stable expression plasmid for PoIFN-β
In order to construct a stable expression plasmid for PoIFN-β, it was first examined whether the acquired sequence was right. PoIFN-β fragment amplified by PCR was 587 bp in size by electrophoresis as expected and further confirmed by sequencing to be the complete PoIFN-β gene ORF after cloning into the PMD18-T cloning vector, completely matching the PoIFN-β gene sequence from the GeneBank (GenBank Accession No. S41178).
To examine whether the protein coded by this cloned PoIFN-β gene has antiviral activity, the PoIFN-β gene ORF was subcloned into the eukaryotic expression plasmid pCAGGS to obtain pCA-PoIFN-β. The pCA-PoIFN-β and pCAGGS plasmids were used to transiently transfect BHK-21 cells. The cell culture supernatants harvested were named PoIFN-βpCA and mockpCA, respectively, and antiviral activity was assayed in MDBK cells using VSV. The result showed that the antiviral activity of PoIFN-βpCA was 9.9 × 105 IU/ml, while 10-fold diluted mockpCA had no antiviral activity. Together with the sequencing results, it is confirmed that the correct full ORF of PoIFN-β gene was obtained, and subcloned to pCN to generate a stable recombinant expression plasmid pCN-PoIFN-β.
3.2. Screening of CHO-K1 cell clones with stable recombinant PoIFN-β expression
After pCN-PoIFN-β transfection, CHO cells were selected in the selection medium containing 800 μg/ml G418. The obtained 23 neomycin-resistant cell colonies were harvested with cloning rings and expanded. Based on the antiviral activity of the collected culture supernatant, three cell clones with the highest expressions were selected. The clones were further screened through single cell cloning, once again their expression levels were tested, and the one with the highest level of expression was selected and freeze preserved, named CHO-PoIFN-β. Afterwards CHO-PoIFN-β cells were maintained and passaged in the selection medium containing 400 μg/ml G418.
3.3. Transcription and expression of recombinant PoIFN-β in CHO-PoIFN-β cells
Although the bioactivity of PoIFN-β was detected in the culture medium of CHO-PoIFN-β cells, the RT PCR was carried out to ensure the PoIFN-β gene was transcribed. Total RNA from CHO-PoIFN-β cells was extracted with Trizol, and subjected to RT-PCR to amplify the PoIFN-β gene fragment. The expected size of 587 bp was confirmed in CHO-PoIFN-β cells (Fig. 1 A, lane2) but not in CHO cells (Fig. 1A, lane1). The result showed that PoIFN-β gene was indeed transcribed into mRNA in CHO-PoIFN-β cells.
Fig. 1.
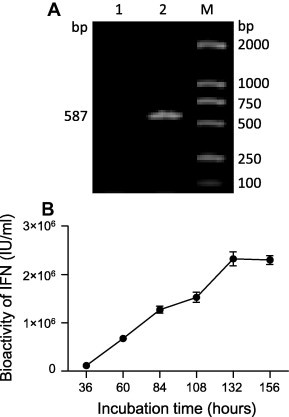
Transcription and expression of recombinant PoIFN-β in CHO-PoIFN-β cells. A. Validation of PoIFN-β gene transcription in CHO-PoIFN-β cells by RT-PCR, and PoIFN-β gene was amplified in CHO-PoIFN-β cells (lane 2), while not in CHO cells (lane1), lane M is DL2000 marker. B. Accumulation of recombinant PoIFN-β in CHO-PoIFN-β culture medium; CHO-PoIFN-β cells were cultured in DMEM/F12 medium with 10% FBS in 25 cm2 cell culture flasks, culture supernatant of 100 μl was sampled at 36, 60, 84, 108, 132 and 156 h respectively, and antiviral activity was determined in MDBK cells using VSV.
In order to get the knowledge of the expression ability of CHO-PoIFN-β cell line, CHO-PoIFN-β cells were cultured in DMEM/F12 culture medium (Gibco) containing 10% FBS, passaged in 1:7 to 25 cm2 cell culture flasks (with 5 ml culture medium in each flask, about 5 × 106 cells when confluent). In 48 h the cells grew into a single contiguous layer; in 156 h the cells began to die. Cell culture supernatant of 100 μl was sampled at 36, 60, 84, 108, 132 and 156 h respectively, and stored at −70 °C before use. Fresh medium of 100 μl was added each time. Antiviral activity of all the samples in the same batch was determined in MDBK cells using VSV. As shown in Fig. 1B, the maximum accumulated yield reached 2.3 × 106 IU/ml. The recombinant PoIFN-β in the culture medium was maintained at about 2.3 × 106 IU/ml afterward and no further increases were observed.
3.4. The effect of passaging and G418 on the stability of PoIFN-β expression by CHO-PoIFN-β cells
CHO-PoIFN-β cells in 25 cm2 flasks were passaged in culture media with or without G418 every 3–4 days; samples were taken from the 0th, 5th, 10th, 15th and 20th generations, respectively. Cell culture was repeated three times under each condition. Prior to sampling, cells were seeded at 105 cells/ml in cell culture flask with 5 ml of culture medium. When cells formed a contiguous layer, the culture was continued for additional 144 h before sampling. The samples were stored at −70 °C, and antiviral activity titration was performed when all the samples were ready. As shown in Fig. 2 , CHO-PoIFN-β cell line maintained a very stable expression of recombinant PoIFN-β for at least 20 generations with or without G418 selection pressure.
Fig. 2.
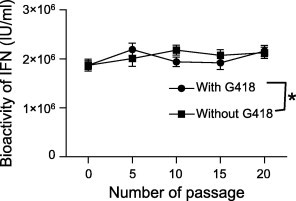
Effect of passaging and G418 on PoIFN-β production by CHO-PoIFN-β cells. CHO-PoIFN-β cells were passaged every 3 or 4 days in 25-cm2 flasks with or without the pressure of G418. Culture supernatants were collected at the time of passaging and assayed for antiviral activity. ∗Statistics analysis showed that the difference is not significant (P > 0.05).
3.5. Activation of Mx promoter by recombinant PoIFN-β
PoIFN-βCHO sample (1.1 × 106 IU/ml) expressed from CHO-PoIFN-β cells was 10-fold serially diluted, and was used to stimulate PK15 cells for 24 h. Cells were harvested, and then subjected to immunoblotting for detection of porcine Mx protein expression using mouse polyclonal antibody against chicken Mx protein and sheep anti-mouse IgG antibody. As shown in Fig. 3 A, using beta-actin protein as internal reference, PoIFN-βCHO induced clearly detectable Mx protein (molecular weight of about 76 kDa) at a threshold of 11.1 IU/ml, and the extent of Mx protein expression is positively correlated with the PoIFN-βCHO unit activity in the range of 11–1.1 × 105 IU/ml. There was no Mx protein expression in cells not stimulated with PoIFN-βCHO (Fig. 3A, lane0).
Fig. 3.
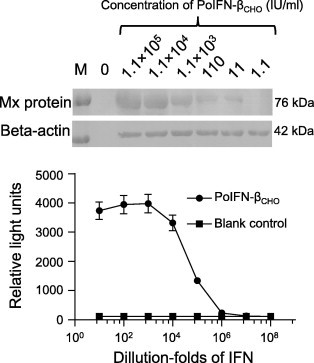
Mx promoter stimulation. A. Dose dependent expression of Mx protein in PK15 cells following stimulation with PoIFN-βCHO; PK15 cells were stimulated for 24 h in the absence (lane 0) or presence of PoIFN-βCHO (1.1 × 105, 1.1 × 104, 1.1 × 103, 110, 11 and 1.1 IU/ml), and then cells were used to analyze the expression of Mx protein by Western blotting using Mx polyclonal antibody, and bête-actin was used as internal reference. Lane M is protein MW marker. B. Activation of Mx promoter in MDBK-Mxp-luc cell line by recombinant PoIFN-βCHO; MDBK-Mxp-luc cells with luciferase reporter gene expression under the control of chicken Mx promoter were grown in a 24-well plate to approximately 100% confluence, 10-fold serially diluted recombinant PoIFN-βCHO (Start from 1.1 × 106 IU/ml) was added, luciferase expression was examined after 5 h of incubation; Blank control is the wells free from interferon.
In parallel experiment, PoIFN-βCHO was 10-fold serially diluted as above and used to stimulate MDBK-Mxp-luc cells (Fig. 3B). The results showed that PoIFN-βCHO as low as 1.1 IU/ml (106-fold dilution) could induce luciferase expression; and the expression level of luciferase was linear to the antiviral activity of PoIFN-βCHO in the range of about 1.1–1100 IU/ml (106–103-fold dilution).
3.6. Antiviral capacity of recombinant PoIFN-β against porcine viruses in vitro
In order to study the antiviral capacity of recombinant PoIFN-β against porcine viruses in vitro, PK15 cells were treated with 10-fold serially diluted PoIFN-βCHO (start from1.1 × 106 IU/ml) for 24 h, and then infected with 1000 TCID50 of TGEV (Fig. 4 A–C) or PRV (Fig. 4F–H). When CPE formed in the virus control wells reached 90–100% (Fig. 4D and I), virus suppression by PoIFN-βCHO was observed and compared with the blank control (Fig. 4E and J). The results showed that: 110 IU/ml of the PoIFN-βCHO completely inhibited TGEV infection in PK15 cells (Fig. 4A); 1100 IU/ml of the PoIFN-βCHO completely inhibited PRV infection in PK15 cells (Fig. 4F).
Fig. 4.
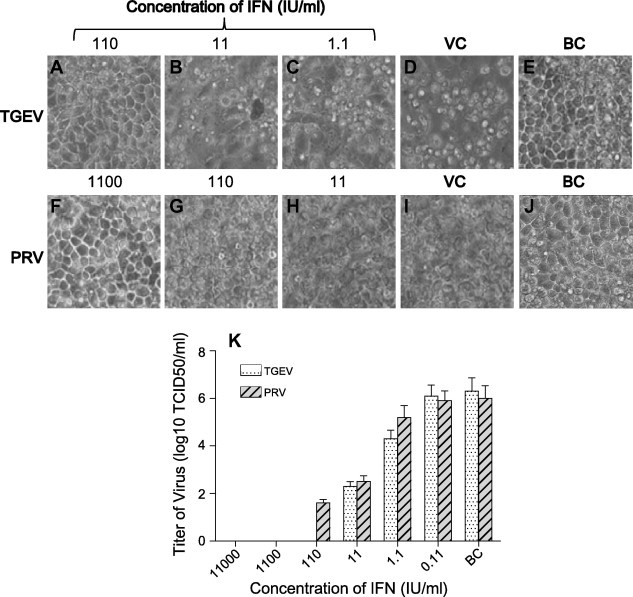
Antiviral activity of recombinant PoIFN-β on PK15 cells. PK15 cells were treated with 10-fold serially diluted PoIFN-βCHO (Start from 1.1 × 106 IU/ml) for 24 h, and then challenged with 103 TCID50 TGEV (A–C) and PRV (F–H), when CPE formed in the virus control wells (D and I) reached 90–100%, the effect of antiviral activity was evaluated under a microscope, and the culture medium in each well were collected to titer TGEV or PRV on PK15 cells in 96-well plates (K). 110 IU/ml of PoIFN-βCHO (A, K) completely protected PK15 cells against the infection of TGEV, but 11 IU/ml (B, K) and 1.1 IU/ml (C, K) did not. While 1100 IU/ml of PoIFN-βCHO (F, K) completely protected against the infection of PRV, but 110 IU/ml (G, K) and 11 IU/ml (H, K) did not. The well free from interferon but addition virus was set as virus control (VC) (D, I and K), the wells free from interferon and virus were set as blank control (BC) (E, J and K).
And the tittering results of TGEV or PRV in supernatant in each well were concordant with the CPE results above (Fig. 4K), no TGEV were detect in 110 IU/ml PoIFN-βCHO treated wells, and no PRV were detect in 1100 IU/ml PoIFN-βCHO treated wells, while virus could be detected in mock wells and lower diluted-PoIFN-βCHO treated wells. Even if a few more days were allowed, TGEV and PRV still could not replicate to generate CPE (data not shown), indicating that PoIFN-βCHO could adequately protect PK15 cells against 1000 TCID50 of TGEV and PRV infection. However, if more than 105 TCID50 of virus were used for infection, even 1.1 × 105 IU/ml of PoIFN-βCHO could not provide complete protection (data not shown).
4. Discussion
Since there is no established standard from World Health Organization (WHO) for porcine IFN-β [38], commercial IFN-α from prokaryotic expression system was used in this study as the standard to determine interferon activity. The CHO cell-derived recombinant IFN-β is very suitable as a standard [26], so that the recombinant PoIFN-β in the present study has potential application as the standard for PoIFN-β products. To acquire a cell line with stable gene integration and stable expression, it is necessary to perform one to two rounds of single cell cloning after the cell clones with high expression were selected after transfection. In this study, after single cell cloning, the expression of CHO-PoIFN-β cell line remained stable after 20 passages regardless of G418 presence in the culture medium, indicating that PoIFN-β expression framework has been integrated into the CHO-K1 cell genome.
In order to verify the biological activity of expressed interferon in this study, both conventional antiviral activity titration and Mx promoter activation were adopted. Chicken Mx promoter is not only suitable for the detection of chicken type I IFN, but also suitable for the detection of biological activity of mammalian type I IFN [14]. The results of antiviral activity titration, Mx protein induction and Mx promoter stimulation are all consistent, indicating that the recombinant PoIFN-β expressed in CHO-PoIFN-β cells has the natural biological activity and functions of type-I interferon. In addition, the expressed recombinant PoIFN-β completely suppressed the 1000 TCID50 of TGEV (110 IU/ml PoIFN-βCHO) and PRV (1100 IU/ml PoIFN-βCHO) infection in PK15 cells in vitro, indicating its potential value in clinical therapeutic applications. The result that the antiviral activity of PoIFN-β needed was 10 times lower against the TGEV (Coronavirus branch, single-stranded RNA virus) than the PRV (Herpesviridae, double-stranded DNA virus) indicates that PoIFN-β is more effective against RNA viruses.
Interferon from prokaryotic expression has lower activity per unit mass [39] and more pyrogens in addition to being more antigenic, thus having more side effects in clinical applications and reducing the therapeutic effect of interferon. In contrast, IFN expressed from mammalian cells has higher activity per unit mass and less pyrogens, is less antigenic, and thus having less side effects and so on. It is worthy to note that IFN from yeast and baculovirus expressions differs significantly with natural IFN in glycosylation, while recombinant IFN-β expressed by mammalian cells displays almost the same properties in terms of glycosylation and folding as its natural counterpart, and correct glycosylation of the IFN-β is essential for its activity and stability [19], [26], [40].
Porcine leucocyte-derived interferon is used as quality standards for veterinary biological products promulgated by the Ministry of Agriculture of China. It is obtained by inducing swine leukocytes using Newcastle disease virus. However, the production process is complicated, the product has a complex composition, and the activity of the final product is about 10,000 IU/ml. In comparison, recombinant PoIFN-β production from CHO cells has great advantages in that the process is simple, the product composition is easy to control, and the protein yield of PoIFN-β in this study is 70 times higher. Human IFN-α expression in mammalian cells could reach the level of 2.4 × 107 IU/ml [41], 10 times the expression of IFN-β in this study. The difference in the expression levels may be due to the differences in screening tags, promoters, cells, IFN activity detection systems and units defined for activity, etc. By optimizing the culture medium and cultivation techniques, it is expected that expression levels can be greatly increased to meet future demand for industrial production. Currently, enrichment and purification of recombinant PoIFN-β are in progress.
In conclusion, research of animal interferon expression in mammalian cells lags far behind that of human interferon. This study reported for the first time internationally to have established a mammalian cell line with stable expression of PoIFN-β, in an exploration of using mammalian cells to express interferon for veterinary use. The CHO-PoIFN-β cell line established in this study expresses the recombinant PoIFN-β that has the same biological function as natural porcine type I IFN. The recombinant PoIFN-β displays a high level of antiviral activity of up to 2.3 × 106 IU/ml, and has a very high activity per unit mass and stability. The recombinant PoIFN-β can be used as a standard for the detection of biological activities of porcine IFN-β, and has good prospect in clinical applications.
Acknowledgements
We thank Dr. Changming Liu for providing the Transmissible gastroenteritis virus and pseudo-rabies virus, and Dr. Yanwu Wei for the help in the part of antiviral experiment in vitro using Transmissible gastroenteritis virus and pseudo-rabies virus.
Contributor Information
Kehe Huang, Email: khhuang@njau.edu.cn.
Zhigao Bu, Email: zgb@hvri.ac.cn.
References
- 1.Sen G.C. Viruses and interferons. Annu Rev Microbiol. 2001;55:255–281. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Weber F., Kochs G., Haller O. Inverse interference. How viruses fight the interferon system. Viral Immunol. 2004;17:498–515. doi: 10.1089/vim.2004.17.498. [DOI] [PubMed] [Google Scholar]
- 3.Stetson D.B., Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Buddaert W., Van Reeth K., Pensaert M. In vivo and in vitro interferon (IFN) studies with the porcine reproductive and respiratory syndrome virus (PRRSV) Adv Exp Med Biol. 1998;440:461–467. doi: 10.1007/978-1-4615-5331-1_59. [DOI] [PubMed] [Google Scholar]
- 5.Lee S.M., Schommer S.K., Kleiboeker S.B. Porcine reproductive and respiratory syndrome virus field isolates differ in in vitro interferon phenotypes. Vet Immunol Immunopathol. 2004;102:217–231. doi: 10.1016/j.vetimm.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dufour V., Chevallier S., Cariolet R., Somasundaram S., Lefevre F., Jestin A. Induction of porcine cytokine mRNA expression after DNA immunization and pseudorabies virus infection. J Interferon Cytokine Res. 2000;20:889–895. doi: 10.1089/10799900050163262. [DOI] [PubMed] [Google Scholar]
- 7.Brukman A., Enquist L.W. Suppression of the interferon-mediated innate immune response by pseudorabies virus. J Virol. 2006;80:6345–6356. doi: 10.1128/JVI.00554-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albina E., Carrat C., Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J Interferon Cytokine Res. 1998;18:485–490. doi: 10.1089/jir.1998.18.485. [DOI] [PubMed] [Google Scholar]
- 9.Ruggli N., Bird B.H., Liu L., Bauhofer O., Tratschin J.D., Hofmann M.A. N(pro) of classical swine fever virus is an antagonist of double-stranded RNA-mediated apoptosis and IFN-alpha/beta induction. Virology. 2005;340:265–276. doi: 10.1016/j.virol.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Rau H., Revets H., Balmelli C., McCullough K.C., Summerfield A. Immunological properties of recombinant classical swine fever virus NS3 protein in vitro and in vivo. Vet Res. 2006;37:155–168. doi: 10.1051/vetres:2005049. [DOI] [PubMed] [Google Scholar]
- 11.Bauhofer O., Summerfield A., Sakoda Y., Tratschin J.D., Hofmann M.A., Ruggli N. Classical swine fever virus Npro interacts with interferon regulatory factor 3 and induces its proteasomal degradation. J Virol. 2007;81:3087–3096. doi: 10.1128/JVI.02032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riffault S., Grosclaude J., Vayssier M., Laude H., Charley B. Reconstituted coronavirus TGEV virosomes lose the virus ability to induce porcine interferon-alpha production. Vet Res. 1997;28:77–86. [PubMed] [Google Scholar]
- 13.Vilar Gomez E., Gra Oramas B., Arus Soler E., Ruenes Domech C., Davila Gonzalez Y. Sequential combination therapy with prednisone, lamivudine and interferon alfa-2b for HBeAg-positive chronic hepatitis B. Gastroenterol Hepatol. 2006;29:534–541. doi: 10.1157/13094348. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L.S. Problems and strategies during interferon therapy for hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2006;14:378–379. [PubMed] [Google Scholar]
- 15.Koyama R., Arase Y., Ikeda K., Suzuki F., Suzuki Y., Saitoh S. Efficacy of interferon therapy in elderly patients with chronic hepatitis C. Intervirology. 2006;49:121–126. doi: 10.1159/000089372. [DOI] [PubMed] [Google Scholar]
- 16.Satoh J. Interferon-beta therapy in multiple sclerosis. Nippon Rinsho. 2006;64:1297–1309. [PubMed] [Google Scholar]
- 17.Yoshida J., Mizuno M., Wakabayashi T. Interferon-beta gene therapy for cancer: basic research to clinical application. Cancer Sci. 2004;95:858–865. doi: 10.1111/j.1349-7006.2004.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyake K., Tsuchida K., Sugino H., Imura S., Morine Y., Fujii M. Combination therapy of human pancreatic cancer implanted in nude mice by oral fluoropyrimidine anticancer agent (S-1) with interferon-alpha. Cancer Chemother Pharmacol. 2007;59:113–126. doi: 10.1007/s00280-006-0250-5. [DOI] [PubMed] [Google Scholar]
- 19.Mahmutovic S., Beslagic E. Significance of the interferon (IFN) in the therapy. Bosn J Basic Med Sci. 2004;4:42–44. doi: 10.17305/bjbms.2004.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kappos L., Hartung H.P. 10 years of interferon beta-1b (Beta feron therapy. J Neurol. 2005;252(Suppl. 3):iii1–iii2. doi: 10.1007/s00415-005-2009-z. [DOI] [PubMed] [Google Scholar]
- 21.Overend C., Mitchell R., He D., Rompato G., Grubman M.J., Garmendia A.E. Recombinant swine beta interferon protects swine alveolar macrophages and MARC-145 cells from infection with Porcine reproductive and respiratory syndrome virus. J Gen Virol. 2007;88:925–931. doi: 10.1099/vir.0.82585-0. [DOI] [PubMed] [Google Scholar]
- 22.Xia C., Dan W., Wen-Xue W., Jian-Qing W., Li W., Tian-Yao Y. Cloning and expression of interferon-alpha/gamma from a domestic porcine breed and its effect on classical swine fever virus. Vet Immunol Immunopathol. 2005;104:81–89. doi: 10.1016/j.vetimm.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Pol J.M., Broekhuysen-Davies J.M., Wagenaar F., La Bonnardiere C. The influence of porcine recombinant interferon-alpha 1 on pseudorabies virus infection of porcine nasal mucosa in vitro. J Gen Virol. 1991;72(Pt 4):933–938. doi: 10.1099/0022-1317-72-4-933. [DOI] [PubMed] [Google Scholar]
- 24.Cao R.B., Zhou G.D., Zhou H.X., Bao J.J., Chen P.Y. Secreted expression of porcine interferon beta in Pichia pastoris and its inhibition effect on the replication of pseudorabies virus. Wei Sheng Wu Xue Bao. 2006;46:412–417. [PubMed] [Google Scholar]
- 25.Loignon M., Perret S., Kelly J., Boulais D., Cass B., Bisson L. Stable high volumetric production of glycosylated human recombinant IFNalpha2b in HEK293 cells. BMC Biotechnol. 2008;8:65. doi: 10.1186/1472-6750-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meager A., Das R.G. Biological standardization of human interferon beta: establishment of a replacement world health organization international biological standard for human glycosylated interferon beta. J Immunol Methods. 2005;306:1–15. doi: 10.1016/j.jim.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y.J., Wu D., Chen R.W., Sun S.H. Cloning, high level expression and purification of porcine IFN gamma. Sheng Wu Gong Cheng Xue Bao. 2001;17:183–186. [PubMed] [Google Scholar]
- 28.Cao R.B., Xu X.Q., Zhou B., Chen D.S., Chen P.Y. Gene modification and high prokaryotic expression of porcine interferon alpha-1. Sheng Wu Gong Cheng Xue Bao. 2004;20:291–294. [PubMed] [Google Scholar]
- 29.Wan J.Q., Wu W.X., Xia C. Expression of porcine interferon-gamma gene in Pichia pastoris and its effect of inhibiting porcine reproductive and respiratory syndrome virus. Sheng Wu Gong Cheng Xue Bao. 2002;18:683–686. [PubMed] [Google Scholar]
- 30.Huang Z.Q., Hu H.Y., Chen X.L., Ren L.M., Lin A.X., Chen Y.F. Secreted expression of porcine interferon-gamma gene in Pichia pastoris. Sheng Wu Gong Cheng Xue Bao. 2005;21:731–736. [PubMed] [Google Scholar]
- 31.Huang H., Xie P., Yu R.S., Liu H.L., Zhang D.F., Cao X.R. High level secretion expression of PoIFNalpha in Pichia pastoris. Yi Chuan. 2005;27:215–220. [PubMed] [Google Scholar]
- 32.Wang Y.B., Wang Z.Y., Chen H.Y., Cui B.A., Zhang H.Y., Wang R. Secretory expression of porcine interferon-gamma in baculovirus using HBM signal peptide and its inhibition activity on the replication of porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2009;132:314–317. doi: 10.1016/j.vetimm.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Qin L.T., Wang X.J., Hu S., Li Z.Z., Chen W.Y., Ge J.Y. Expression of porcine gamma-interferon in recombinant baculovirus and determination of its antiviral activity. Sheng Wu Gong Cheng Xue Bao. 2007;23:386–391. [PubMed] [Google Scholar]
- 34.Meager A. Assays for antiviral activity. Methods Mol Biol. 2004;249:121–134. doi: 10.1385/1-59259-667-3:121. [DOI] [PubMed] [Google Scholar]
- 35.Yeh T.J., McBride P.T., Overall J.C., Jr., Green J.A. Automated, quantitative cytopathic effect reduction assay for interferon. J Clin Microbiol. 1982;16:413–415. doi: 10.1128/jcm.16.2.413-415.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong J.A. Cytopathic effect inhibition assay for interferon: microculture plate assay. Methods Enzymol. 1981;78:381–387. doi: 10.1016/0076-6879(81)78145-x. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y.X., Guo J.Y., Shen L., Chen Y., Zhang Z.C., Zhang Y.L. Get effective polyclonal antisera in one month. Cell Res. 2002;12:157–160. doi: 10.1038/sj.cr.7290122. [DOI] [PubMed] [Google Scholar]
- 38.Meager A. Biological assays for interferons. J Immunol Methods. 2002;261:21–36. doi: 10.1016/s0022-1759(01)00570-1. [DOI] [PubMed] [Google Scholar]
- 39.McCormick F., Trahey M., Innis M., Dieckmann B., Ringold G. Inducible expression of amplified human beta interferon genes in CHO cells. Mol Cell Biol. 1984;4:166–172. doi: 10.1128/mcb.4.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bekisz J., Schmeisser H., Hernandez J., Goldman N.D., Zoon K.C. Human interferons alpha, beta and omega. Growth Factors. 2004;22:243–251. doi: 10.1080/08977190400000833. [DOI] [PubMed] [Google Scholar]
- 41.Rossmann C., Sharp N., Allen G., Gewert D. Expression and purification of recombinant, glycosylated human interferon alpha 2b in murine myeloma NSo cells. Protein Expr Purif. 1996;7:335–342. doi: 10.1006/prep.1996.0050. [DOI] [PubMed] [Google Scholar]


